Introduction
Lung cancer has been the most commonly diagnosed
type of cancer worldwide over the past several decades (1,2). The
most common histological type of lung cancer is adenocarcinoma,
consisting of the lepidic, acinar, papillary, micropapillary (MIP)
and solid subtypes (3,4). Among these, MIP adenocarcinoma has
been associated with frequent lymph node metastasis, lymphatic
invasion, vascular invasion and a poor prognosis (5,6).
This subtype was only recently proposed by the International
Association for the Study of Lung Cancer, American Thoracic
Society, and European Respiratory Society (IASLC/ATS/ERS) and
formally added as a new subtype in the 2015 WHO classification
(fourth edition) (4,7).
The MIP adenocarcinoma subtype is characterized by
tumor cells growing in papillary tufts and forming florets that
lack fibrovascular cores, detaching from and connecting to the
alveolar walls. Generally, these tumor cells with ring-like
glandular structures float within the alveolar spaces. However, the
mechanism of the alveolar space localization of the tumor cells
with ring-like glandular structures and its association with a poor
prognosis of patients with MIP adenocarcinoma have not yet been
clarified. Furthermore, information on the pathophysiology of MIP
adenocarcinoma and the development of therapeutic methods are
limited due to the lack of sufficient research materials, such as
cell lines, compared with other types of lung cancer.
The authors recently established the KU-Lu-MPPt3
cell line, the first established cell line of MIP adenocarcinoma,
to the best of our knowledge (8).
In the present study, a possible association of the characteristics
of MIP adenocarcinoma with its malignancy was investigated by
examining the KU-Lu-MPPt3 cells.
Materials and methods
Ethics statement
The present study was carried out in accordance with
the Declaration of Helsinki and was approved by the Ethics
Committee of Kitasato University Medical Ethics Organization
(approval no. KME B09-33). The patient agreed to participate in the
study and provided written informed consent, as in a previous study
by the authors (8).
Cells and cell culture
The KU-Lu-MPPt3 MIP adenocarcinoma cell line was
previously established in the authors' laboratory at Kitasato
University (8). The KU-Lu-MPPt3
cells were cultured in Roswell Park Memorial Institute (RPMI)-1640
(Nacalai Tesque, Inc.) medium supplemented with 10% fetal bovine
serum (FBS) on type I collagen-coated dishes (AGC Techno Glass Co.,
Ltd.) at 37°C with 5% CO2. The cells initially exhibited
adherent cell morphologies at their establishment. Some cells then
formed tufty aggregates and floated away from the adherent cells.
The aggregated floating cells were collected and separately
cultured from the adherent cells in tissue culture dishes. The
KU-Lu-MPPt3 adherent cells were classified as KU-Lu-MPPt3 adhesive
(AD) cells, and the aggregated floating cells were classified as
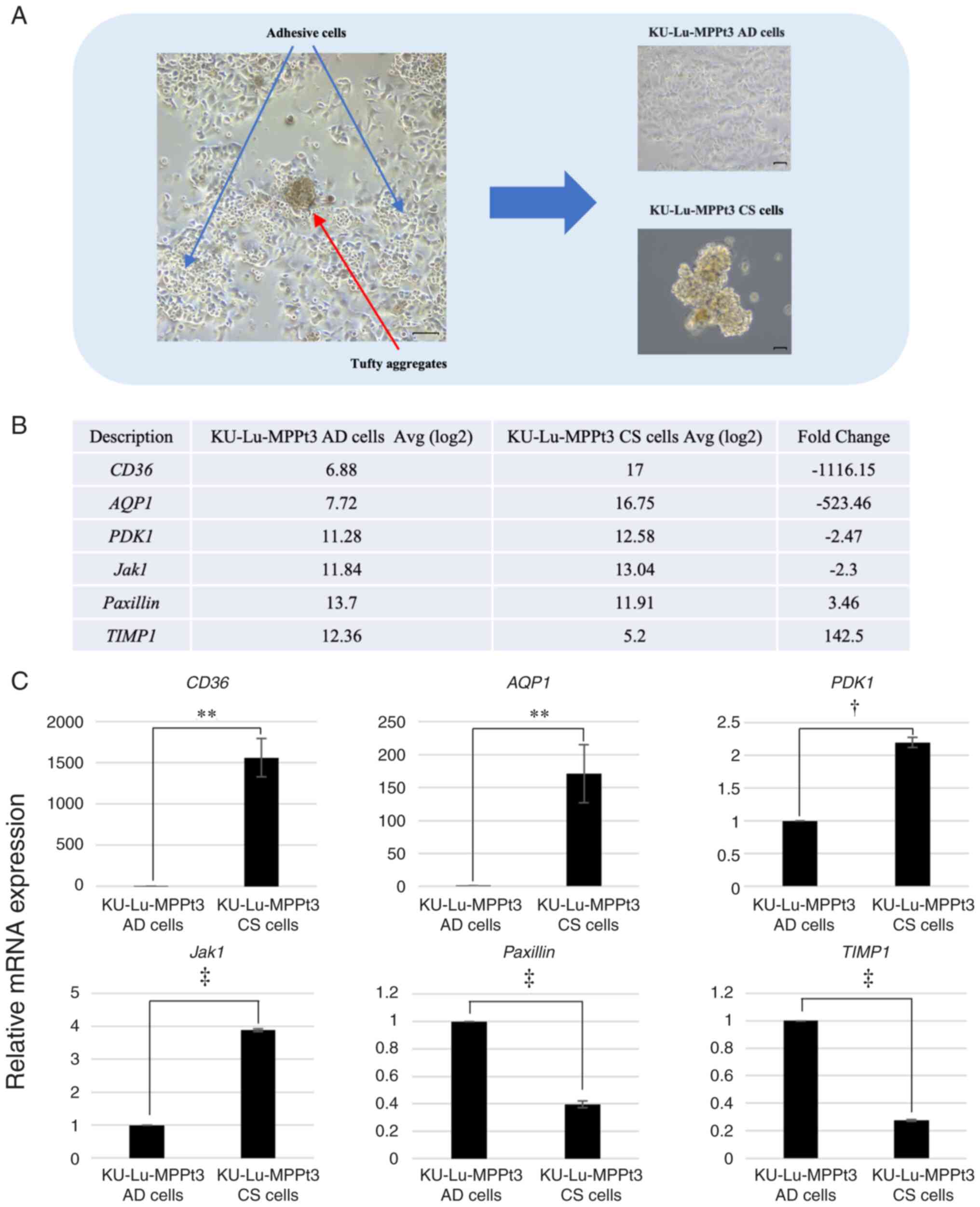
KU-Lu-MPPt3 clumpy and suspended (KU-Lu-MPPt3 CS) cells (Fig. 1A). The ratio of KU-Lu-MPPt3 AD to
KU-Lu-MPPt3 CS cells was not clear. However, the number of
KU-Lu-MPPt3 CS cells was less than that of the KU-Lu-MPPt3 AD cells
when some cells formed tufty aggregates and floated away from the
adherent cells and became KU-Lu-MPPt3 CS cells. Mycoplasma testing
was conducted for these cell lines and confirmed to be negative for
mycoplasma infection (data not shown).
Microarray analysis
Total RNA was isolated from the KU-Lu-MPPt3 AD and
CS cells using the RNeasy Mini kit (Qiagen GmbH) according to the
manufacturer's protocol. Quality checks analysis on the RNA samples
was performed using the Human Clariom D array (cat. no. 902915;
Thermo Fisher Scientific, Inc.) gene chip. All results were
analyzed using the Transcriptome Analysis Console 4.0.2 software
(Thermo Fisher Scientific, Inc.). Gene expression profiles between
the KU-Lu-MPPt3 AD and CS cells were compared. The fold change in
expression was defined as the ratio of expression in KU-Lu-MPPt3 AD
cells to that in KU-Lu-MPPt3 CS cells. Genes with ratios ≥2 were
considered to be significantly differentially expressed.
Bioinformatics analysis
The DAVID 6.8 database (https://david.ncifcrf.gov/tools.jsp) was used to
perform Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment analysis. The species was limited to Homo
sapiens.
Antibodies and inhibitors
The following reagents were purchased commercially:
Anti-CD36 D8L9T (14347; Cell Signaling Technology, Inc.),
anti-aquaporin 1 (AQP1) B-11 (cat. no. sc-25287; Santa Cruz
Biotechnology, Inc.), anti-phosphorylated (p-)Akt (Ser473; cat. no.
9271; Cell Signaling Technology, Inc.), anti-Akt 40D4 (cat. no.
2920; Cell Signaling Technology, Inc.), anti-focal adhesion kinase
(FAK) (H-1; cat. no. sc-1688; Santa Cruz Biotechnology, Inc.),
anti-p-FAK (Tyr397; cat. no. 44–624G; Thermo Fisher Scientific,
Inc.), anti-actin I-19 (cat. no. sc-1616; Santa Cruz Biotechnology,
Inc.), Alexa Fluor-phalloidin (cat. no. A12379; Thermo Fisher
Scientific, Inc.), Akt inhibitor X (cat. no. sc-203811; Santa Cruz
Biotechnology, Inc.) and FAK inhibitor (PF-00562271; AdooQ
BioScience).
Immunohistochemistry
The KU-Lu-MPPt3 AD cells treated with TrypLE Express
Enzyme (Thermo Fisher Scientific, Inc.) and the CS cells were
collected, embedded in iPGell (GenoStaff Co., Ltd.), and fixed with
10% formaldehyde neutral buffer solution at 4°C overnight following
the manufacturer's instructions. The lung cancer tissues were
collected from the patient from whose tissue the KU-Lu-MPPt3 cells
were established, and were paraffinized and cut into 4-µm-thick
sections. All slides were subjected to autoclaving pretreatment for
antigen retrieval using sodium citrate buffer (pH 6.0) and Tris-HCl
buffer (pH 9.0). They were then blocked with Dako X0909 Protein
Block, Serum-Free (Dako; Agilent Technologies, Inc.) at room
temperature for 10 min, washed with PBS three times, and incubated
with anti-CD36 (1:200 dilution), anti-AQP1 (1:100 dilution),
anti-p-FAK (1:50 dilution) and anti-p-Akt (1:50 dilution)
antibodies overnight at 4°C. Subsequently, the cells were washed
with PBS three times and incubated with secondary antibodies
(MAX-PO MULTI, cat. no. 424151; Nichirei Biosciences Inc.) for 30
min at room temperature. The slides were then washed with PBS three
times and stained with stable DAB (Thermo Fisher Scientific, Inc.)
at room temperature (anti-CD36, 6 min; anti-AQP1, 10 min;
anti-p-FAK, 5 min; anti-p-Akt, 6 min). The slides were washed once
again and counterstained with hematoxylin (cat. no. 115938;
Sigma-Aldrich, Inc.) for nuclear staining at 4°C for 5 min. The
slides were observed using an Olympus BX51 microscope (Olympus
Corporation), and images were acquired using Olympus DP2-BSW
software.
Immunofluorescence staining
The KU-Lu-MPPt3 CS cells were cultured on round
coverslips coated with 1.8 mg/ml type I collagen (Koken Co., Ltd.)
for 9 days, fixed in 10% formaldehyde neutral buffer solution for
10 min, washed with PBS three times, and permeabilized with 0.1%
(v/v) Triton X-100 in PBS for 10 min at room temperature. After
rinsing with PBS three times, the cells were blocked with 5% BSA at
room temperature for 1 h. The cells were then incubated with
anti-p-FAK (1:50 dilution) antibody overnight at 4°C. The cells
were then washed with PBS three times and incubated with secondary
antibodies (cat. no. A11035; Thermo Fisher Scientific, Inc.) (1:200
dilution) and phalloidin (1:500 dilution) for 1 h at room
temperature. After washing with PBS once again, the cells were
labeled with 4,6-diamidino-2-phenylindole (DAPI) (cat. no. D9542;
Sigma-Aldrich, Inc.) for nuclear staining (1:1,000 dilution). A
laser scanning confocal imaging system (LSM700; Carl Zeiss AG) was
used to acquire fluorescence images. Three dimensional images were
reconstituted using IMARIS software (Bitplane AG).
Western blot analysis
The cells were lysed in RIPA buffer (16488; Nacalai
Tesque, Inc.) supplemented with a protease inhibitor cocktail
(25955; Nacalai Tesque, Inc.) and phosphatase inhibitor cocktail
solution II (FUJIFILM Wako Pure Chemical Corporation), to prepare
whole-cell lysates. Protein concentrations were determined using a
BCA protein assay kit (Thermo Fisher Scientific, Inc.). A total of
15 µg protein lysate was subjected to SDS-polyacrylamide gel
electrophoresis (SDS-PAGE) using 10% SDS gel (cat. no. 414954;
Cosmo Bio Co., Ltd.). The separated proteins were transferred onto
a PVDF membrane and the membrane was blocked for 1 h at room
temperature using blocking buffer (05999-84; Nacalai Tesque, Inc.),
and incubated overnight at 4°C with primary antibodies against
p-Akt (1:1,000 dilution), p-FAK (1:1,000 dilution), Akt (1:1,000
dilution), FAK (1:200 dilution) and actin (1:200 dilution). The
membrane was then washed with PBST three times and incubated with
secondary antibodies (anti-rabbit IgG: cat. no. 1706515; Bio-Rad
Laboratories, Inc., anti-mouse IgG: cat. no. 1706516; Bio-Rad
Laboratories, Inc., anti-goat: cat. no. A16005; Thermo Fisher
Scientific, Inc.) (1:10,000 dilution) for 1 h at room temperature.
Proteins were visualized and analyzed using an Image Quant LAS 4000
system with Image Quant TL7.0 analysis software imaging system
(Cytiva). All western blot bands were semi-quantified using ImageJ
1.53i software (National Institutes of Health).
Cell proliferation assay
The KU-Lu-MPPt3 CS cells were cultured on
collagen-coated 96-well plates (AGC Techno Glass Co., Ltd.) with or
without inhibitors (Akt inhibitor X and FAK inhibitor PF-00562271)
and incubated for 4 days at 37°C. The cells were then incubated
with WST-8 (CCK-8; Dojindo Molecular Technologies, Inc.) at a final
concentration of 10% (v/v), for 150 min at 37°C. The absorbances of
the culture supernatants were measured at 450 nm using a SpectraMax
M2 multifunctional microplate reader (Molecular Devices, LLC).
Cell adhesion assay
The KU-Lu-MPPt3 CS cells were plated onto
collagen-coated 96-well plates with or without inhibitors (Akt
inhibitor X and FAK inhibitor PF-00562271) and cultured for 3 days
at 37°C in RPMI medium with 10% FBS. In preparation for the
measurement of the numbers of attached cells, the wells were washed
twice with PBS and filled with fresh medium containing 10% (v/v)
WST-8. In preparation for the measurement of the total viable cell
numbers, WST-8 was added without washing to the wells at a final
concentration of 10% (v/v). Plates were then incubated for 150 min
at 37°C, and the absorbances of the culture supernatants were
measured at 450 nm using a SpectraMax M2 multifunctional microplate
reader. The proportions of adherent cells were defined as the
attached cell number/total cell number.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the KU-Lu-MPPt3 AD and
CS cells using Sepasol-RNA I super G (Nacalai Tesque, Inc.) and
subjected to reverse transcription to synthesize cDNA using the
iScript™ cDNA Synthesis Kit (cat. no. 1708890; Bio-Rad
Laboratories, Inc.). The reverse transcription conditions were as
follows: 25°C for 5 min, 42°C for 30 min, 85°C for 5 sec, holding
at 4°C. Thereafter, qPCR was performed using SYBR-Green (cat. no.
1725271; Bio-Rad Laboratories, Inc.) on a CFX96 Touch Real-time PCR
Detection System (Bio-Rad Laboratories, Inc.) and β-actin
was used as the internal reference gene. The PCR cycles were
performed as follows: Initial denaturation at 95°C for 30 sec and
then 40 cycles of 95°C for 10 sec and 60°C for 30 sec. A
calibration curve (standard curve) was created and analyzed using
the analysis was conducted with the 2−ΔΔCq
quantification method (9). The
following primers were used: CD36 forward,
5′-TGGGACCATTGGTGATGAG-3′ and reverse, 5′-GCAACAAACATCACCACACC-3′;
AQP1 forward, 5′-TTTCTGTTTCCTGGCCTCAG-3′ and reverse,
5′-TCCACAACTTCAAGGGAGTG-3′; 3-phosphoinositide-dependent protein
kinase-1 gene (PDK1) forward, 5′-GAAGATGAGTGACCGAGGAGG-3′
and reverse, 5′-GTAAAGACGTGATATGGGCAATC-3′; Janus kinase 1 gene
(Jak1) forward, 5′-GGTCAGCATTAACAAGCAGGACAA-3′ and reverse,
5′-AGCCATCTACCAGGGACACAAAG-3′; TIMP metallopeptidase inhibitor 1
(TIMP1) forward, 5′-TATCCATCCCCTGCAAACTG-3′ and reverse,
5′-TTTTCAGAGCCTTGGAGGAG-3′; Paxillin forward,
5′-ACAGTCGCCAAAGGAGTC-3′ and reverse, 5′-TGGTAGTGCACCTCACAGTA-3′;
and β-actin forward, 5′-TCACCCACACTGTGCCCATCTACGA-3′ and
reverse, 5′-CAGCGGAACCGCTCATTGCCAATGG-3′.
Statistical analysis
All values are presented as the mean ± standard
error, and all error bars represent the standard error of the mean.
Statistical analysis was performed using JMP Pro 16 software (SAS
Institute, Inc.). All results were analyzed using the unpaired
Student's t-test and the one-way ANOVA followed by Dunnett's
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of MIP adenocarcinoma
histopathological specimens and KU-Lu-MPPt3 cells
The KU-Lu-MPPt3 cell line was recently established
by the authors as the first established MIP adenocarcinoma cell
line, at least to the best of our knowledge (8). Although the cells adhere to and grow
on collagen-coated dishes, some cells aggregate and float away from
adherent cells during culture (Fig.
1A). To compare the characteristics of the KU-Lu-MPPt3 CS and
AD cells, their gene expression profiles were examined, using DNA
microarray analysis. In total, 8,054 genes were differentially
expressed >2-fold, of which 3,882 and 4,172 genes were
upregulated and downregulated, respectively (Table SI), in the KU-Lu-MPPt3 CS cells in
comparison with the KU-Lu-MPPt3 AD cells. The most prominently
differentially expressed genes are shown in Fig. 1B.
Among these genes, CD36, which encodes a
fatty acid receptor (10), and
AQP1, which has been reported to encode a water channel
protein involved in the progression, invasion and metastasis of
lung adenocarcinoma tumors (11),
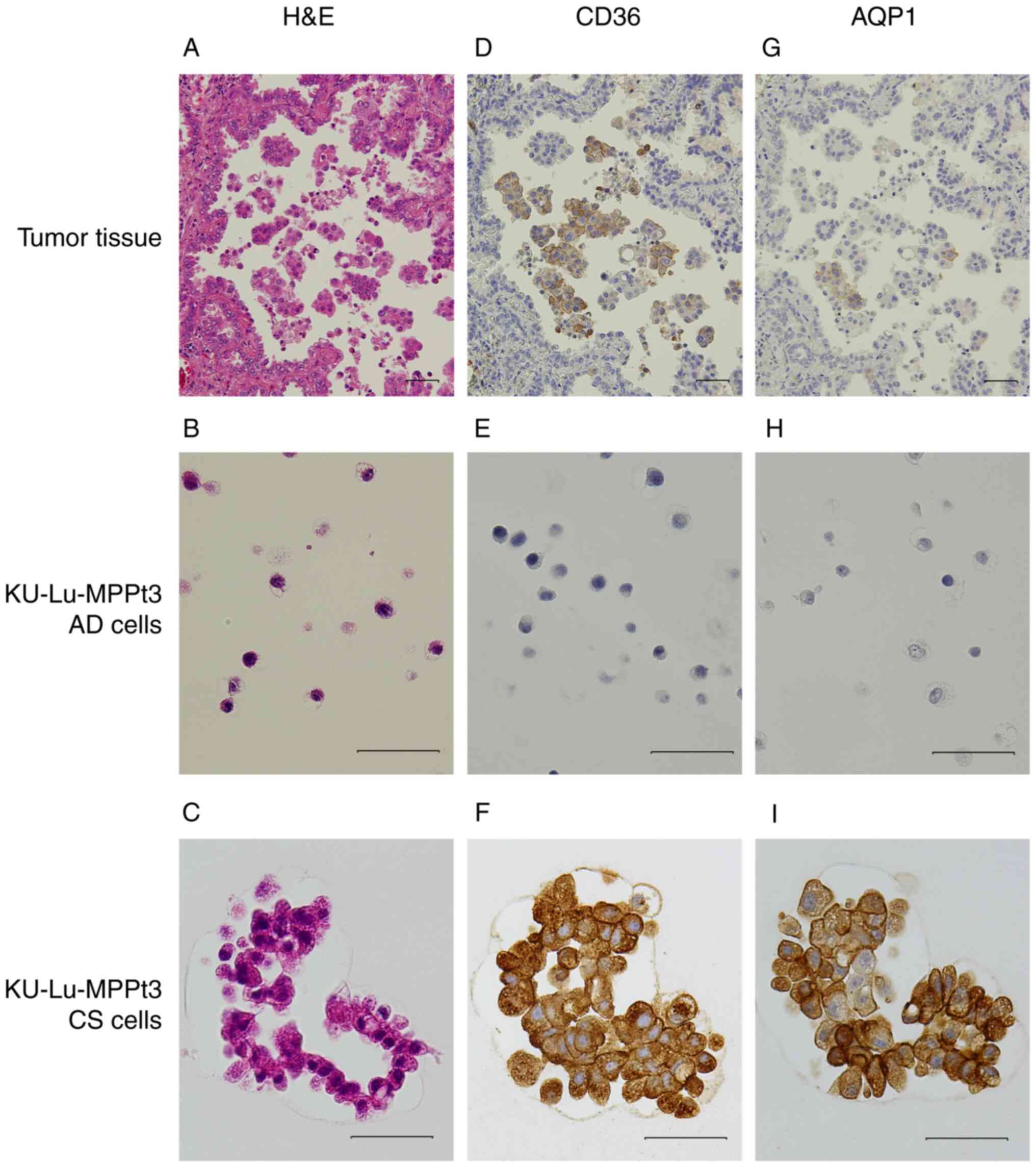
were highly expressed in the KU-Lu-MPPt3 CS cells (Fig. 1C). The CD36 and AQP1
differential expression levels at the protein level were then
confirmed using immunohistochemistry. Their expression patterns
were also compared with those of histopathological specimens from
the patient with MIP adenocarcinoma. It was revealed that the
KU-Lu-MPPt3 CS cells were positive for both CD36 and AQP1 proteins;
however, the KU-Lu-MPPt3 AD cells were not (Fig. 2E, F, H and I). Several MIP
component cells of the alveolar spaces in MIP adenocarcinoma
specimens were positive for these proteins (Fig. 2D and G). Therefore, the KU-Lu-MPPt3
CS cells may retain properties similar to those of the
characteristic tumor cells discovered in the alveolar spaces of
patients with MIP adenocarcinoma. The tumor cells detected in the
alveolar spaces have often been discovered in only a section of MIP
adenocarcinoma. Fewer KU-Lu-MPPt3 CS than KU-Lu-MPPt3 AD cells
existing when certain cells are being separated from adherent cells
and transforming into KU-Lu-MPPt3 CS cells is consistent with this
clinical feature.
All differentially expressed genes identified in the
microarray analysis were subjected to KEGG pathway enrichment
analysis. Cell adhesion molecules (CAMs) and the Akt signaling
pathway were among the enriched pathways. Among the genes
identified using the microarray analysis, genes related to CAMs and
the Akt signaling pathway were examined. CD36, PDK1 and Jak1, which
were highly expressed in the KU-Lu-MPPt3 CS cells and have been
associated with the activation of the Akt pathway (10,12–16),
and paxillin and TIMP1, which were highly expressed in the
KU-Lu-MPPt3 AD cells and are associated with the activation of the
FAK pathway (17–20), were detected (Fig. 1B). Therefore, these genes were
confirmed to be differentially expressed between the two cell types
using RT-qPCR (Fig. 1C).
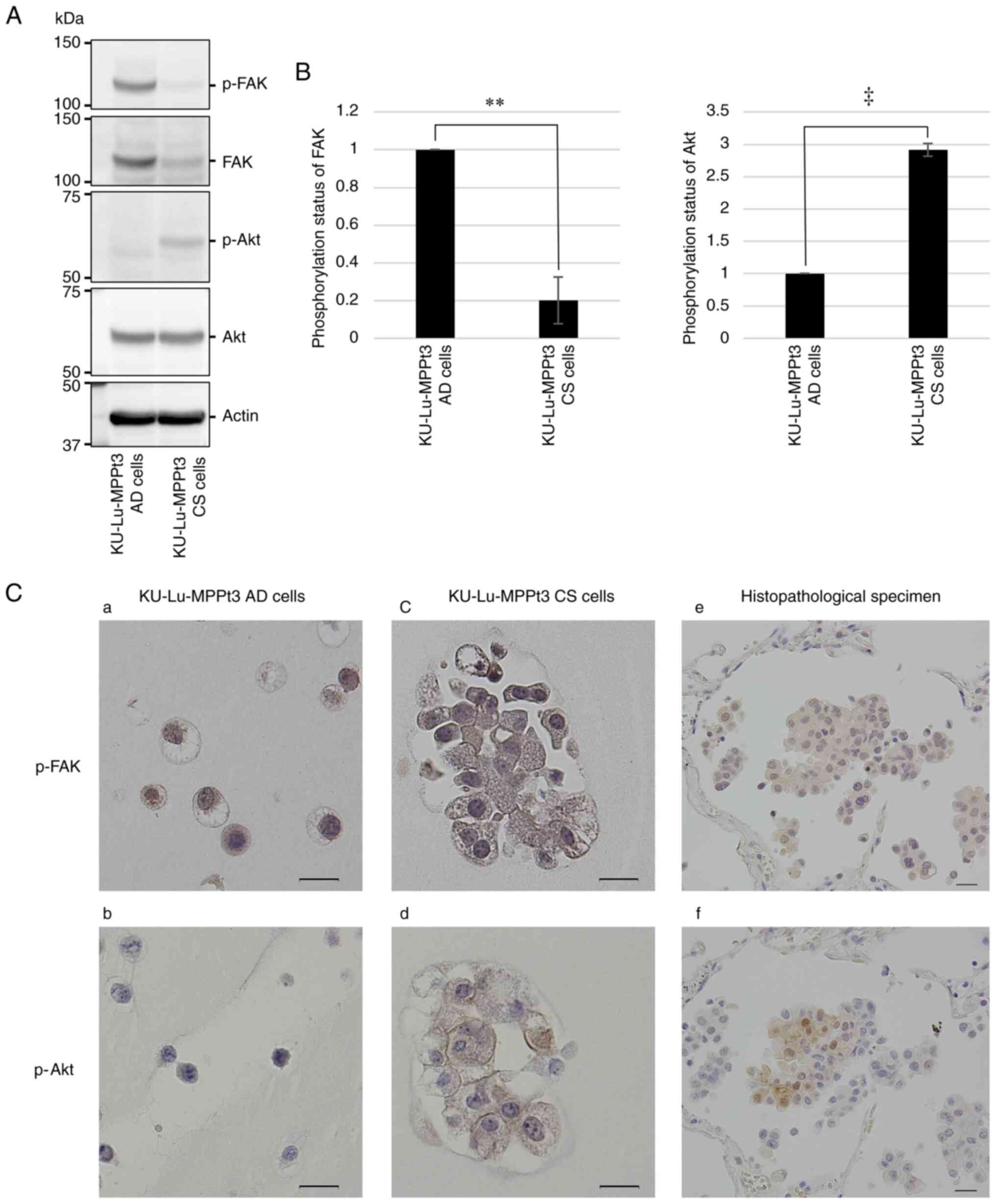
It was then examined whether Akt and FAK were
differentially activated between the two cell types by performing
western blot analysis with phospho-specific antibodies. It was
demonstrated that the expression of total FAK was decreased in the
KU-Lu-MPPt3 CS cells (Fig. 3A).
Apart from this difference in total FAK levels, the p-FAK levels
normalized to the total FAK levels were significantly decreased in
the KU-Lu-MPPt3 CS cells (Fig.
3B). By contrast, whereas the total Akt levels were almost
comparable between the KU-Lu-MPPt3 AD and CS cells (Fig. 3A), the normalized p-Akt levels were
significantly increased in the KU-Lu-MPPt3 CS cells (Fig. 3B). Thus, the KU-Lu-MPPt3 CS cells
exhibited a decreased and increased activity of FAK and Akt,
respectively (Fig. 3A and B).
In the histopathological specimens, p-Akt was
detected primarily in the MIP component cells in the alveolar
space, being consistent with the results obtained for the
KU-Lu-MPPt3 CS cells (Fig. 3C-d
and -f). By contrast, p-FAK was detected in all tumor cells in the
histopathological specimens, regardless of location (Fig. 3C-e). Since p-FAK was detected in
both the KU-Lu-MPPt3 AD and CS cells at comparable levels (Fig. 3C-a and -c), the immunostaining
performed herein may have failed to accurately quantify the levels
of p-FAK. Furthermore, in addition to CD36, the expression of stem
cell markers, such as SOX2, CD44, C-X-C chemokine receptor type 4
(CXCR4) and aldehyde dehydrogenase 1 family, member A1 (ALDH1a) was
higher in the KU-Lu-MPPt3 CS cells than in the KU-Lu-MPPt3 AD cells
in the microarray analysis, and similar results were obtained using
RT-qPCR (unpublished data). The microarray data were uploaded in
the National Center for Biotechnology Information Gene Expression
Omnibus (GEO) database; GEO accession number GSE184883 (https://www.ncbi.nlm.nih.Gov/geo/query/acc.cgi?acc=GSE184883).
Morphological changes in the
KU-Lu-MPPt3 CS cells following attachment to type I collagen
The KU-Lu-MPPt3 CS cells slowly proliferated in
suspension when cultured in tissue culture dishes; however, when
cultured on type I collagen, some cells changed their morphologies,
resembling KU-Lu-MPPt3 AD cells within 9 days (Fig. 4A). Subsequently, several cells
formed tufty aggregates with rising edges, of which specific cells
detached and reverted to the KU-Lu-MPPt3 CS cell morphology (data
not shown).
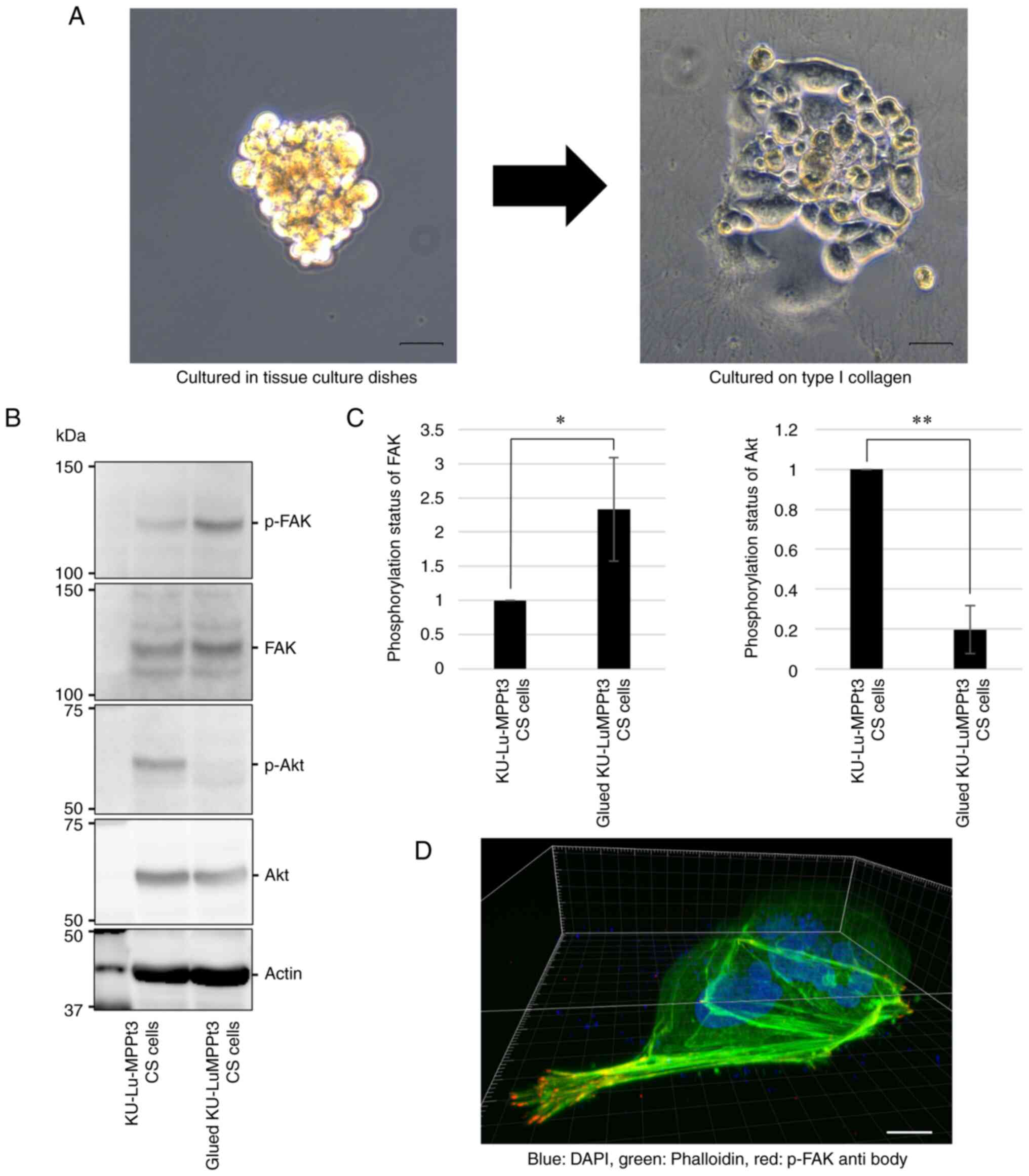 | Figure 4.Changes in phosphorylation levels of
Akt and FAK, following the attachment of KU-Lu-MPPt3 CS cells. (A)
Phase-contrast images of KU-Lu-MPPt3 CS cells, cultured in tissue
culture dishes (left panel) and culture dishes coated with type I
collagen for 9 days (right panel). Scale bars, 100 µm. (B)
Evaluation of p-Akt and p-FAK expression levels in glued
KU-Lu-MPPt3 CS cells using western blot analysis. (C) Relative
quantification of p-FAK/total FAK and p-Akt/total Akt levels. Data
are expressed as the mean ± SE of three independent experiments.
(D) Representative image of the glued KU-Lu-MPPt3 CS cells,
cultured on round coverslips coated with type I collagen for 9 days
and stained by anti-p-FAK antibody (red), phalloidin (green), and
DAPI (blue). Scale bar, 20 µm. *P<0.05 and **P<0.01;
determined using the t-test. FAK, focal adhesion kinase; p-FAK,
phosphorylated FAK; p-Akt, phosphorylated Akt; CS, clumpy and
suspended. |
Thus, the Akt and FAK phosphorylation states between
the KU-Lu-MPPt3 CS and transformed AD cells (hereafter referred to
as glued KU-Lu-MPPt3 CS cells) were compared, following incubation
on type I collagen-coated dishes at 37°C for 9 days. Consistent
with the cell morphology, the p-Akt and p-FAK levels were decreased
and increased, respectively, in the glued KU-Lu-MPPt3 CS cells
(Fig. 4B and C). Confocal
microscopic analysis revealed that these cells formed
lamellipodia-like protrusions with accumulated p-FAK (Fig. 4D). These findings suggest that the
adhesiveness of the KU-Lu-MPPt3 cells was associated with their Akt
and FAK activities.
Involvement of Akt and FAK in the
adhesion and proliferation of KU-Lu-MPPt3 CS cells
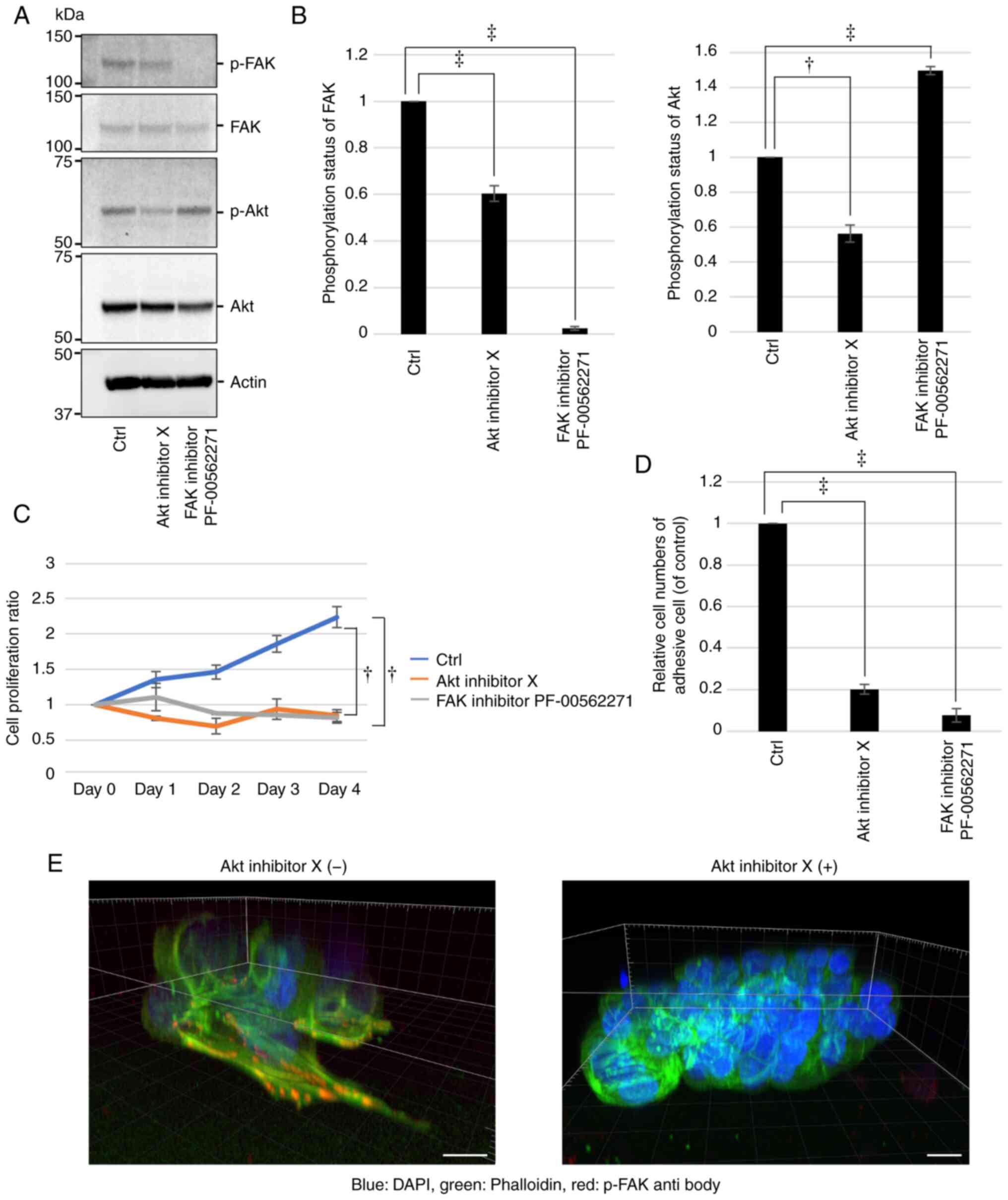
It was further confirmed using western blot analysis
that Akt and FAK inhibitors respectively decreased the p-Akt and
p-FAK levels significantly in the KU-Lu-MPPt3 CS cells (Fig. 5A and B). Of note, it was also
demonstrated that the Akt inhibitor decreased the p-FAK levels,
whereas the FAK inhibitor increased the p-Akt levels (Fig. 5A and B), suggesting a possible
signaling crosstalk between Akt and FAK in the KU-Lu-MPPt3 cells.
In order to investigate the involvement of Akt and FAK activities
in the proliferation and adhesion of the KU-Lu-MPPt3 CS cells, the
effects of Akt and FAK inhibitors on their proliferation and
adhesion to type I collagen-coated plates were evaluated using the
WST-8 assay. Although the untreated cells proliferated in type I
collagen-coated plates, they failed to proliferate in the presence
of either Akt or FAK inhibitors, presenting a significant decrease
in cell numbers (Fig. 5C).
Furthermore, both inhibitors markedly inhibited cell adhesion
(Fig. 5D). The formation of
lamellipodia-like protrusions with accumulated p-FAK was inhibited
by the Akt inhibitor (Fig. 5E).
These results suggested that the activities of both Akt and FAK are
required for the adhesion and proliferation of KU-Lu-MPPt3 CS
cells.
Discussion
MIP adenocarcinoma is a high-grade malignant tumor,
presenting high rates of lymphatic invasion, visceral pleural
invasion and lymph node metastases (5,6); it
is also associated with a poor prognosis even in the early stages
of the disease (6,21), and a high recurrence rate (22). Furthermore, patients with
MIP-positive tumors tend to relapse locoregionally after limited
resection (23,24). However, the biological mechanism
underlying the progression of MIP adenocarcinoma has not yet been
fully elucidated. Furthermore, there is no clear consensus on the
mechanism and malignant effects of the characteristic ring-like
glandular structure of tumor cells floating within alveolar spaces
in MIP adenocarcinoma. By using immunohistochemistry, serial
sectioning and electron microscopy, Kamiya et al (25) revealed that the micropapillary
tufts appeared to float in alveolar spaces or spaces encased by
connective tissues in histopathological sections. Furthermore,
according to the serial sectioning in this previous study, most
tufts were continuous with other tufts and the main tumor (25). In addition, it has also been
reported that due to histopathological findings demonstrating no
vascularity in micropapillary tufts, the route of nourishment for
their constitutive cells was uncertain (25). On the other hand, another unique
feature of MIP adenocarcinoma is the presence of non-integrated
free tumor clusters, located away from the main tumors (26,27).
However, this unique feature does not appear to be associated with
the continuity of the identified tufts.
In the present study, it was observed that the
cultured KU-Lu-MPPt3 CS cells shared morphological and
immunohistological characteristics with the ring-like glandular
structure of tumor cells floating within alveolar spaces. These
cells appeared to survive in suspended states, ensuring nourishment
from their surroundings. In addition, immunostaining of the
pathological tissues revealed that the staining properties of the
ring-like glandular structure of tumor cells floating within
alveolar spaces were heterogeneous. Some cells demonstrated high
levels of p-Akt, in similarity to the high levels of p-Akt in the
KU-Lu-MPPt3 CS cells. These results suggested that two types of
cells may exist in the ring-like glandular structure of tumor cells
floating within alveolar spaces: One that maintains continuity with
the alveolar wall and another one that can survive in a floating
state without continuity with the alveolar wall, similar to the
KU-Lu-MPPt3 CS cells. Non-integrated free tumor clusters may have
been derived from floating tumors moving within the alveolar
spaces, including KU-Lu-MPPt3 CS cells. It was demonstrated in the
present study that the KU-Lu-MPPt3 CS cells not only survived in a
suspended state, but also adhered and changed their morphology in
the presence of appropriate substrates, in support of the clinical
findings.
Of note, the KU-Lu-MPPt3 CS cells demonstrated a
high p-Akt expression in suspended states, and Akt phosphorylation
was observed to be a pre-requisite for adhesion. However, in the
process of adhering and transforming into AD-like cells, the
expression of p-FAK increased and expression of p-Akt decreased.
Furthermore, by inhibiting the phosphorylation of Akt, the levels
of p-FAK and the adhesive ability decreased. Furthermore, by
inhibiting FAK phosphorylation, the p-Akt levels increased.
High levels of MIP adenocarcinoma malignancy may be
attributed to the characteristic tumor cells within the alveolar
spaces with the ring-like glandular structures. Furthermore, it may
be attributable to its ability of directly infiltrating and
changing morphology, in order to allow adhesion under appropriate
conditions. Since Akt and FAK may be involved in this process, Akt
or FAK activity inhibition may block the biological and
pathological functions of MIP adenocarcinoma cells, including their
growth.
In addition, several genes, including CD36, CD44,
SOX2, CXCR4 and ALDH1a were upregulated in the KU-Lu-MPPt3 CS cells
compared with the KU-Lu-MPPt3 AD cells, as determined using
microarray analysis and RT-qPCR. These genes are known as cancer
stem cell markers (28–34). Therefore, the KU-Lu-MPPt3 CS cells may
have stem cell properties and this feature may also contribute to
the malignancy of MIP adenocarcinoma. In future studies, the
authors aim to evaluate the cancer stem cell like properties of the
KU-Lu-MPPt3 CS cells.
Previous clinical trials have demonstrated that Akt
inhibitors may be effective in the treatment of breast cancer
(35,36). In addition, previously performed
clinical trials on FAK inhibitors in patients with solid tumors
have confirmed their safety and effectiveness, allowing the
inhibitor application to proceed to clinical development (37,38).
Thus, Akt and FAK inhibitors may be also useful therapeutic agents
for the treatment of MIP adenocarcinoma. However, further
assessment for these potential treatments is required.
A limitation of the present study is that in the
microarray analysis, only one sample per cell was analyzed and the
sample size was small. Therefore, the false discovery rate could
not be determined. The number of samples will be increased and
analysis will be performed in future studies.
In conclusion, the KU-Lu-MPPt3 CS cells, which share
characteristics with MIP adenocarcinoma cells in the alveolar
space, demonstrated high levels of p-Akt. Furthermore, it was
revealed that their morphology may be altered under appropriate
conditions, which was prevented by the administration of an FAK or
Akt inhibitor. These morphological and biological features may be
associated with the malignancy of MIP adenocarcinoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant from Parents'
Association Grant of Kitasato University, School of Medicine, a
grant-in-aid for Scientific Research (C) (20K07575) from MEXT, JST
(Moonshot R&D) (JPMJMS2022), the Japan Agency for Medical
Research and Development (AMED) (18gm5010001s0901), the Setsuro
Fujii Memorial Osaka Foundation for Promotion of Fundamental
Medical Research, and a grant-in-aid for Scientific Research (C)
(19K09312) from MEXT.
Availability of data and materials
All microarray data were uploaded in the National
Center for Biotechnology Information Gene Expression Omnibus (GEO)
database; GEO accession number GSE184883 (https://www.ncbi.nlm.nih.Gov/geo/query/acc.cgi?acc=GSE184883).
All remaining datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DS, KK, MN and YMi designed the experiments,
analyzed the data and edited the manuscript. DS performed the
majority of the experiments and assembled the data. KK, YMa and KA
performed the immunofluorescence staining and microarray analysis.
MM and KY analyzed the data and revised the manuscript. YS was
involved in the conception and design of the experiments and
supervised the whole experimental work and revised the manuscript.
DS and KK confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present investigation was approved by the ethics
committee of Kitasato University Medical Ethics Organization
(Approval No. KME B09-33). The patient agreed to participate in the
study and provided written informed consent as per a previous study
by the authors (8), and we did not
use clinical specimen from anyone other than this patient in this
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AQP1
|
aquaporin 1
|
|
CD36
|
cluster of differentiation 36
|
|
FAK
|
focal adhesion kinase
|
|
Jak1
|
Janus kinase 1
|
|
MIP
|
micropapillary
|
|
PDK1
|
phosphoinositide-dependent protein
kinase 1
|
|
TIMP1
|
tissue inhibitor of
metalloproteinase-1
|
|
AD
|
adhesive
|
|
CS
|
clumpy and suspended
|
|
CAMs
|
cell adhesion molecules
|
|
CXCR4
|
C-X-C chemokine receptor type 4
|
|
ALDH1a
|
aldehyde dehydrogenase 1 family member
A1
|
References
|
1
|
American Cancer Society: Global Cancer
Facts & Figures. 4th edition. American Cancer Society.
(Atlanta, GA). 2018.
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inamura K: Clinicopathological
characteristics and mutations driving development of early lung
adenocarcinoma: Tumor initiation and progression. Int J Mol Sci.
19:12592018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung Cancer/American thoracic Society/European respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida Y, Nitadori JI, Shinozaki-Ushiku
A, Sato J, Miyaji T, Yamaguchi T, Fukayama M and Nakajima J:
Micropapillary histological subtype in lung adenocarcinoma of 2 cm
or less: Impact on recurrence and clinical predictors. Gen Thorac
Cardiovasc Surg. 65:273–279. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miyoshi T, Satoh Y, Okumura S, Nakagawa K,
Shirakusa T, Tsuchiya E and Ishikawa Y: Early-stage lung
adenocarcinomas with a micropapillary pattern, a distinct
pathologic marker for a significantly poor prognosis. Am J Surg
Pathol. 27:101–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
World Health Organization: WHO
Classification of Tumors of the Lung, Pleura, Thymus and Heart.
Travis WD, Brambilla E, Burke AP, Marx A and Nicholson AG: WHO/IARC
Classification of Tumours. Lyon. (IARC Publications). 2015.
|
|
8
|
Matsuo Y, Shiomi K, Sonoda D, Mikubo M,
Naito M, Matsui Y, Yoshida T and Satoh Y: Molecular alterations in
a new cell line (KU-Lu-MPPt3) established from a human lung
adenocarcinoma with a micropapillary pattern. J Cancer Res Clin
Oncol. 144:75–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo X, Zheng E, Wei L, Zeng H, Qin H,
Zhang X, Liao M, Chen L, Zhao L, Ruan XZ, et al: The fatty acid
receptor CD36 promotes HCC progression through activating
Src/PI3K/AKT axis-dependent aerobic glycolysis. Cell Death Dis.
12:3282021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellezza G, Vannucci J, Bianconi F, Metro
G, Del Sordo R, Andolfi M, Ferri I, Siccu P, Ludovini V, Puma F, et
al: Prognostic implication of aquaporin 1 overexpression in
resected lung adenocarcinoma. Interact Cardiovasc Thorac Surg.
25:856–861. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cifarelli V, Appak-Baskoy S, Peche VS,
Kluzak A, Shew T, Narendran R, Pietka KM, Cella M, Walls CW,
Czepielewski R, et al: Visceral obesity and insulin resistance
associate with CD36 deletion in lymphatic endothelial cells. Nat
Commun. 12:33502021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gan G, Shi Z, Shangguan C, Zhang J, Yuan
Y, Chen L, Liu W, Li B, Meng S, Xiong W and Mi J: The kynurenine
derivative 3-HAA sensitizes hepatocellular carcinoma to sorafenib
by upregulating phosphatases. Theranostics. 11:6006–6018. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elsayed AM, Bayraktar E, Amero P, Salama
SA, Abdelaziz AH, Ismail RS, Zhang X, Ivan C, Sood AK,
Lopez-Berestein G, et al: PRKAR1B-AS2 long noncoding rna promotes
tumorigenesis, survival, and chemoresistance via the PI3K/AKT/mTOR
pathway. Int J Mol Sci. 22:18822021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Hou W, Perera A, Bettler C, Beach
JR, Ding X, Li J, Denning MF, Dhanarajan A, Cotler SJ, et al:
Targeting EphA2 suppresses hepatocellular carcinoma initiation and
progression by dual inhibition of JAK1/STAT3 and AKT signaling.
Cell Rep. 34:1087652021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Subotički T, Mitrović Ajtić O,
Beleslin-Čokić BB, Bjelica S, Djikić D, Diklić M, Leković D, Gotić
M, Santibanez JF, Noguchi CT and Čokić VP: IL-6 stimulation of DNA
replication is JAK1/2 mediated in cross-talk with hyperactivated
ERK1/2 signaling. Cell Biol Int. 43:192–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deramaudt TB, Dujardin D, Noulet F, Martin
S, Vauchelles R, Takeda K and Rondé P: Altering FAK-paxillin
interactions reduces adhesion, migration and invasion processes.
PLoS One. 9:e920592014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song M, Hu J and Quan HY: Abnormal
expression of FAK and paxillin correlates with oral cancer invasion
and metastasis. Acta Biochim Pol. 68:317–323. 2021.PubMed/NCBI
|
|
19
|
Tang J, Kang Y, Huang L, Wu L and Peng Y:
TIMP1 preserves the blood-brain barrier through interacting with
CD63/integrin β 1 complex and regulating downstream FAK/RhoA
signaling. Acta Pharm Sin B. 10:987–1003. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song G, Xu S, Zhang H, Wang Y, Xiao C,
Jiang T, Wu L, Zhang T, Sun X, Zhong L, et al: TIMP1 is a
prognostic marker for the progression and metastasis of colon
cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer
Res. 35:1482016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song Z, Zhu H, Guo Z, Wu W, Sun W and
Zhang Y: Prognostic value of the IASLC/ATS/ERS classification in
stage I lung adenocarcinoma patients-based on a hospital study in
China. Eur J Surg Oncol. 39:1262–1268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshizawa A, Motoi N, Riely GJ, Sima CS,
Gerald WL, Kris MG, Park BJ, Rusch VW and Travis WD: Impact of
proposed IASLC/ATS/ERS classification of lung adenocarcinoma:
Prognostic subgroups and implications for further revision of
staging based on analysis of 514 stage I cases. Mod Pathol.
24:653–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nitadori JI, Bograd AJ, Kadota K, Sima CS,
Rizk NP, Morales EA, Rusch VW, Travis WD and Adusumilli PS: Impact
of micropapillary histologic subtype in selecting limited resection
vs lobectomy for lung adenocarcinoma of 2 cm or smaller. J Natl
Cancer Inst. 105:1212–1220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsubokawa N, Mimae T, Sasada S, Yoshiya T,
Mimura T, Murakami S, Ito H, Miyata Y, Nakayama H and Okada M:
Negative prognostic influence of micropapillary pattern in stage IA
lung adenocarcinoma. Eur J Cardiothorac Surg. 49:293–299. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kamiya K, Hayashi Y, Douguchi J,
Hashiguchi A, Yamada T, Izumi Y, Watanabe M, Kawamura M, Horinouchi
H, Shimada N, et al: Histopathological features and prognostic
significance of the micropapillary pattern in lung adenocarcinoma.
Mod Pathol. 21:992–1001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Watanabe M, Yokose T, Tetsukan W, Imai K,
Tsuboi M, Ito H, Ishikawa Y, Yamada K, Nakayama H and Fujino S:
Micropapillary components in a lung adenocarcinoma predict stump
recurrence 8 years after resection: A case report. Lung Cancer.
80:230–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morimoto J, Nakajima T, Suzuki H, Nagato
K, Iwata T, Yoshida S, Fukuyo M, Ota S, Nakatani Y and Yoshino I:
Impact of free tumor clusters on prognosis after resection of
pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 152:64–72.e1.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Erhart F, Blauensteiner B, Zirkovits G,
Printz D, Soukup K, Klingenbrunner S, Fischhuber K, Reitermaier R,
Halfmann A, Lötsch D, et al: Gliomasphere marker combinatorics:
Multidimensional flow cytometry detects
CD44+/CD133+/ITGA6+/CD36+
signature. J Cell Mol Med. 23:281–292. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghoneum A, Gonzalez D, Abdulfattah AY and
Said N: Metabolic plasticity in ovarian cancer stem cells. Cancers
(Basel). 12:12672020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yin Y, Xie CM, Li H, Tan M, Chen G, Schiff
R, Xiong X and Sun Y: The FBXW2-MSX2-SOX2 axis regulates stem cell
property and drug resistance of cancer cells. Proc Natl Acad Sci
USA. 116:20528–20538. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boumahdi S, Driessens G, Lapouge G, Rorive
S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E,
et al: SOX2 controls tumour initiation and cancer stem-cell
functions in squamous-cell carcinoma. Nature. 511:246–250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maréchal R, Demetter P, Nagy N, Berton A,
Decaestecker C, Polus M, Closset J, Devière J, Salmon I and Van
Laethem JL: High expression of CXCR4 may predict poor survival in
resected pancreatic adenocarcinoma. Br J Cancer. 100:1444–1451.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luo Y, Dallaglio K, Chen Y, Robinson WA,
Robinson SE, McCarter MD, Wang J, Gonzalez R, Thompson DC, Norris
DA, et al: ALDH1A isozymes are markers of human melanoma stem cells
and potential therapeutic targets. Stem Cells. 30:2100–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chefetz I, Grimley E, Yang K, Hong L,
Vinogradova EV, Suciu R, Kovalenko I, Karnak D, Morgan CA,
Chtcherbinine M, et al: A Pan-ALDH1A inhibitor induces necroptosis
in ovarian cancer stem-like cells. Cell Rep. 26:3061–3075.e6. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SB, Dent R, Im SA, Espié M, Blau S,
Tan AR, Isakoff SJ, Oliveira M, Saura C, Wongchenko MJ, et al:
Ipatasertib plus paclitaxel versus placebo plus paclitaxel as
first-line therapy for metastatic triple-negative breast cancer
(LOTUS): A multicentre, randomised, double-blind,
placebo-controlled, phase 2 trial. Lancet Oncol. 18:1360–1372.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martorana F, Motta G, Pavone G, Motta L,
Stella S, Vitale SR, Manzella L and Vigneri P: AKT inhibitors: New
weapons in the fight against breast cancer? Front Pharmacol.
12:6622322012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Soria JC, Gan HK, Blagden SP, Plummer R,
Arkenau HT, Ranson M, Evans TR, Zalcman G, Bahleda R, Hollebecque
A, et al: A phase I, pharmacokinetic and pharmacodynamic study of
GSK2256098, a focal adhesion kinase inhibitor, in patients with
advanced solid tumors. Ann Oncol. 27:2268–2274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mohanty A, Pharaon RR, Nam A, Salgia S,
Kulkarni P and Massarelli E: FAK-targeted and combination therapies
for the treatment of cancer: An overview of phase I and II clinical
trials. Expert Opin Investig Drugs. 29:399–409. 2020. View Article : Google Scholar : PubMed/NCBI
|