Introduction
Oral squamous cell carcinoma (OSCC), characterized
by an increased morbidity and mortality, is one of the main types
of head and neck squamous cell carcinoma (HNSCC) (1). Several mechanisms involved in the
progression of OSCC have been revealed; however, these mechanisms
are not yet fully understood. Thus, the deeper understanding of the
regulatory mechanisms of OSCC is of utmost importance.
Tectonic family member 1 (TCTN1), belonging to the
TCTN family, is a signal-sequence-containing secreted and
transmembrane protein (2). TCTN1
participates in several physiological processes and pathological
processes, including ciliogenesis, Varadi syndrome, Joubert
syndrome and non-motile ciliopathies (3–6).
TCTN1 is involved in the development and progression of various
tumors. The silencing of TCTN1 has been reported to inhibit
proliferation, and induce cell cycle arrest and apoptosis in human
thyroid cancer (7). Furthermore,
it has been revealed that TCTN1 knockdown may significantly inhibit
colon cancer cell growth (8).
Furthermore, TCTN1 is associated with prostate cancer cell growth
and migration (9). Therefore,
TCTN1 has been suggested to be a potential target for cancer
therapy. TCTN1 participates in the cell growth and apoptosis of
esophageal squamous cell carcinoma, a cell type of HNSCC (10). However, although OSCC is a main
type of HNSCC, little is known about TCTN1 involvement in OSCC
progression. Additionally, the mechanisms through which TCTN1
expression is regulated remain largely unknown.
The UALCAN database is a comprehensive web resource
for analyzing cancer OMICS data, which can be used to perform
pan-cancer gene expression analysis and to analyze
clinicopathological feature information (11). In the present study, TCTN1
expression in HNSCC was analyzed and evaluated in each HNSCC tumor
stage and grade. OSCC is a main type of HNSCC, and therefore, it
may be possible that the TCTN1 expression levels in OSCC may be
similar to those of HNSCC. Thus, TCTN1 expression analysis was
evaluated in HNSCC and OSCC.
Transcription is the process through which RNA is
synthesized according to genomic DNA; thus, the genetic information
is transduced from DNA to RNA (12). Transcriptional regulation is an
important part of gene expression regulation. Transcription is very
complex and often requires the assistance of many factors,
including long non-coding RNAs (lncRNAs), microRNAs (miRNAs) and
transcription factors (13–15).
Transcription factors are a group of proteins which can bind to
specific sequences upstream of target genes inducing gene
upregulation or downregulation (16–18).
Currently, there is limited information available on TCTN1
transcriptional regulation. Several software applications may be
used for the prediction of putative transcription factors binding
to target genes, according to the promoter sequence, including
PROMO and JASPAR2020. PROMO is a virtual laboratory for identifying
putative transcription factor binding sites based on DNA sequences
(http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3)
(19). JASPAR2020 software
provides a high-quality transcription factor binding profile
database (http://jaspar.genereg.net/) (20).
In the present study, the association of TCTN1 with
HNSCC tumor stage and grade was first analyzed using bioinformatics
analysis. Subsequently, the regulatory effects of TCTN1 on OSCC
cell proliferation, migration and invasion were evaluated and it
was revealed that an important transcription factor, transcription
factor AP-2 alpha (TFAP2A), may promote TCTN1 expression.
Materials and methods
Cell culture and clinical
specimens
The CAL27 (cat. no. 1101HUM-PUMC000338), SCC15, and
SCC9 cells were purchased from the National Biomedical Laboratory
Cell Bank of China. The SCC15 (cat. no. bio-69136) and SCC9 cells
(cat. no. bio-69190) were purchased from Biobw (https://www.biobw.org/). Tca83 cells were kindly
provided by Professor Ye-Hua Gan of the Stomatological Hospital of
Peking University (21). Normal
human oral keratinocytes (HOK cells) were purchased from ScienCell
Research Laboratories, Inc. (cat. no. 2610). All cell lines were
cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco;
Thermo Fisher Scientific, Inc.), containing 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.) in an incubator
(Thermo Fisher Scientific, Inc.) with 5% CO2 at 37°C.
Trypsin-EDTA reagent (Gibco; Thermo Fisher Scientific, Inc.) was
used for cell digestion. Following digestion, cells were seeded in
a 96- or 12-well plate for cell proliferation, migration and
invasion assay. All seeding numbers for each assay are described
below.
OSCC and adjacent normal tissues were obtained from
21 patients at Liaocheng People's Hospital (Liaocheng). All patient
information is presented in Table
SI. The tissue collection was checked and approved by the
Ethics Committee of Liaocheng People's Hospital (Approval No.
LC202176). In total, 21 paired OSCC and adjacent normal tissues
were used in the present study. All patients provided written
informed consent.
Analysis of the association between
TCTN1 and clinicopathological features
The association between TCTN1 and the patient
clinicopathological features was evaluated using the UALCAN
database (http://ualcan.path.uab.edu/). The UALCAN database is a
comprehensive web resource for analyzing cancer OMICS data, which
can be used for performing pan-cancer gene expression analysis and
analyzing clinicopathological feature information (11).
Reverse transcription-quantitative PCR
(RT-qPCR)
CAL27, SCC15, Tca83 and SCC9 cell RNA, as well as
clinical specimen RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc.). After the concentration
was measured using a ultrafine ultraviolet spectrophotometer
(Thermo Fisher Scientific, Inc.) according to the absorbance at 260
nm, 2 µg RNA was used for cDNA synthesis using the BeyoRT™ II M-MLV
Reverse Transcriptase kit (cat. no. D7160L, Beyotime Institute of
Biotechnology), according to the manufacturer's instructions. qPCR
was performed using a BeyoFast™ SYBR-Green qPCR Mix kit (cat. no.
D7260, Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. All RT-qPCR experiments were performed
on an ABI 7500 Real-Time PCR instrument (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with the following conditions: 95°C, 10
min; 95°C, 15 sec; 60°C, 60 sec for 40 cycles. The primers used for
RT-qPCR are listed in Table SII.
β-actin was used as an internal control. The expression analysis
method used was 2−ΔΔCq (22).
TCTN1 knockdown and TFAP2A
overexpression
TCTN1 knockdown was performed by infecting the CAL27
cells and SCC15 cells with shTCTN1 lentiviral construct which
targets the TCTN1 sequence (5′-GAGAAGGAACTGATGCATCTGAGC-3′); the
cells transfected with the shCon lentiviral construct, which has no
target sequence in cells, was used as a negative control. The
corresponding shRNA sequences were as follows: sh-TCTN1,
5′-GCTCAGATGCATCAGTTCCTTCTCGAGAAGGAACTGATGCATCTGAGCTTTTTT-3′; and
shCon, 5′-AACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTG-3′.
TFAP2A overexpression was performed by infecting the CAL27 cells
and SCC15 cells with the lv-oeTFAP2A lentiviral construct, and the
cells transfected with the lv-oeCon lentiviral construct were used
as negative control. lv-oeTFAP2A was constructed by inserting the
TFAP2A cDNA into the lentivirus; lv-oeCon was constructed by
inserting the fragment with the sequence as TFAP2A cDNA, except
that all the ATG was replaced by AAG to abolish the transcription.
The sequences of the overexpression vector and the control are
presented as supplementary material (Data SI). Both constructs were designed
and synthesized at Genechem, Inc., based on a 3rd generation
lentiviral system. Briefly, 1.5 µg vector and 1.5 µg were
transfected into 293T cells (cat. no. bio-12947, Biobw; http://www.biobw.org/) with Lipofectamine
3000® reagent (cat. no. L3000001; Thermo Fisher
Scientific, Inc.) for 24 h at 37°C, and the medium was then
collected and the virus was harvested by ultracentrifugation at
80,000 × g, 4°C for 2 h in a Optima XPN centrifuge (Beckman,
Coulter, Inc.). For lentivirus transduction, the CAL27 cells and
SCC15 cells were seeded in a 12-well plate at 30,000 cells/well.
Subsequently, at the logarithmic cell growth phase, the lentiviral
constructs (shCon or shTCTN1) with a MOI of 10 were added into the
well with the assistance of 2 µl polybrane reagent (Cat No. H8761,
Beijing Solarbio Science & Technology Co., Ltd.). After 24 h,
the medium was replaced to wipe off the residual constructs. After
the cells were cultured for a further 48 h, cells transfected with
lentiviral constructs were selected using puromycin
(MilliporeSigma). Due to the green fluorescent protein (GFP) gene
inserted in the lentiviral DNA, green fluorescence detected using
an IX53 inverted fluorescence microscope (IX53, Olympus
Corporation) was used to identify whether the cells were
transfected with lentivirus or not. RT-qPCR was used to confirm
whether TCTN1 was effectively knocked down.
Western blot analysis
The CAL27 cells and SCC15 cells were lysed using
RIPA reagent (Applygene). The cells lysates were centrifuged at
12,000 × g at 4°C for 4 h. The supernatants were collected, and the
concentration was measured using a BCA kit (Invitrogen; Thermo
Fisher Scientific, Inc.). Subsequently, 50 µg protein were
subjected to 10% SDS-PAGE electrophoresis and then transferred onto
a nitrocellulose (NC) membrane (MilliporeSigma). After the NC
membrane was blocked in 5% fat-free milk (Applygene) at 20°C for 1
h, the membrane was incubated with the primary antibodies (1:1,000
diluted into TBST) for 12 h at 4°C. The membrane was then incubated
with HRP-conjected goat anti-mouse secondary antibodies (A0216,
Beyotime Institute of Biotechnology) or HRP-conjected goat
anti-rabbit secondary antibodies (A0208, Beyotime Institute of
Biotechnology) (1:10,000 diluted into TBST) for 1 h at 20°C.
Finally, the membrane was immersed in ECL luminous fluid (Beyotime
Institute of Biotechnology) and detected using an automatic
chemiluminescence image analysis system (Tanon4800, Tanon Science
and Technology Co., Ltd.). z. The anti-MMP-9 (#13667), anti-cyclin
D1 antibodies (#2922) and anti-β-actin antibodies (#3700S) were
purchased from Cell Signaling Technology, Inc. β-actin was used as
internal control.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation capacity was evaluated using
CCK-8 assay. The CAL27 and SCC15 cells were seeded in 96-well
plates at 3,000 cells/well, and then cultured in the incubator with
5% CO2 at 37°C. At 0, 24 and 48 h of culture, 10 µl
CCK-8 reagent (Dojindo Molecular Technologies, Inc.) were added to
the cells. Following a 2-h incubation at 37°C, the absorbance at
OD595 nm was measured using a Multiskan
spectrophotometer (Multiskan, Thermo Fisher Scientific, Inc.).
Transwell assay
Transwell assay was performed to evaluate the
migratory and invasive capacity of the CAL27 cells and SCC15 cells.
In order to evaluate migration, 100,000 cells were seeded into the
upper chamber of a Transwell plate (cat. no. 3460, Corning, ME,
USA) with DMEM containing no FBS, while the lower chamber was
supplemented with DMEM containing 15% FBS. Following a 24-h culture
at 37°C, the cells on the upper chamber were wiped away using a
cotton swab, and cells passing through the membrane were stained
with crystal violet (Beyotime Institute of Biotechnology) at 25°C
for 3 min. The cells were photographed, and the cell number was
counted under a T2R inverted microscope (T2R, Nikon Corporation) at
×200 magnification and counted in five randomly selected visual
fields. The protocol for the evaluation of cell invasion was
similar to the migration evaluation protocol, except from the
following differences: The upper chambers were pre-coated with 20
µg extracellular matrix gel (MilliporaSigma) at 37°C for 2 h and
the cells were subsequently seeded at a concentration of 200,000
cells/well.
Construction of TCTN1
promoter-reporter
The human TCTN1 promoter sequence was obtained from
GenBank (https://www.ncbi.nlm.nih.gov/gene/79600). The putative
full-length promoter (−1,500 to +50 bp, transcription starting site
was defined as +1) of human TCTN1 was amplified from the genomic
DNA of CAL27 cells using regular PCR with the following conditions:
95°C, 1 min; 95°C 15 sec; 55°C, 30 sec; 72°C, 1 min for 30 cycles.
The sequences of the primers used are listed in Table SIII. Following an agarose gel
electrophoresis, the promoter segment was extracted using an
Agarose Gel Extraction kit (DH101-01, Biomed; http://www.biomed168.com/) and was cut by two
restriction enzymes, SacI and MluI. Subsequently, the
promoter segment was inserted pGL3-basic vector (Addgene, Inc.)
which was also cut by SacI and MluI. The promoter
reporter was confirmed by Sanger sequencing on an Illumina NextSeq
500 instrument (Illumina) at Sangon Biotech Co., Ltd. and was named
pGL3-TCTN1. The primer used for DNA sequencing was:
5′-CTAGCAAAATAGGCTGTCCC-3′. All the sequences of the TCTN1 promoter
regaions are provided as supplementary material in Data SI.
Construction of deletion mutants for
the TCTN1 promoter
The pGL3-TCTN1 plasmid was used as the template. The
primer sequences were designed alongside the deleted region and are
listed in the Table SIII. The PCR
cycling was performed on an ABI Veriti PCR thermocycle instrument
(Applied Biosystems) with the conditions as follows: 95°C, 1 min;
95°C, 15 sec; 55°C, 30 sec; 72°C, 5 min for 30 cycles; 72°C, 10
min. Subsequently, the product was extracted using an Agarose Gel
Extraction kit (Biomed, http://www.biomed168.com/). Following incubation with
T4 polynucleotide kinase (New England Biolabs, Inc.) at 37°C for 1
h, the product was self-linked using a T4 DNA ligase (New England
Biolabs, Inc.) at 37°C for 1 h and transferred into the top 10
competent bacterial E. coli cells (Biomed) for
amplification. TCTN1 promoter deletion mutants were confirmed by
Sanger sequencing in an Illumina NextSeq 500 instrument (Illumina,
CA, USA) at Sangon Biotech Co., Ltd. The primer used for DNA
sequencing was: 5′-CTAGCAAAATAGGCTGTCCC-3′.
Site-directed mutagenesis
Site-directed mutagenesis was performed according to
a previous study (23). Firstly,
PCR with pGL3-TCTN1 (−550/-491) as the template was performed, by
using a Q5 high-fidelity DNA polymerase (#E0555L, New England
Biolabs, Inc.) in an ABI Veriti PCR thermocycle instrument (Applied
Biosystems) according to the following protocol: 95°C, 1 min; 95°C,
15 sec; 50°C, 30 sec; 72°C, 5 min for 30 cycles; 72°C, 10 min. The
primers used for mutating the TFAP2A-binding site are as follows:
Forward, 5′-ACTGCACTCCAATTGTTTATACAGAGTGAG-3′ and reverse,
5′-CTCACTCTGTATAAACAATTGGAGTGCAGT-3′. The products were digested
with DpnI enzyme (New England Biolabs, Inc.) and then transferred
into the top 10 bacterial E. coli cells (Biomed) for
amplification. Mutations were confirmed by Sanger sequencing.
Plasmid transfection
The TCTN1 promoter plasmid was transfected into the
CAL27 cells or SCC15 cells using Lipofectamine 2000®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. Briefly, the cells were seeded
into a 12-well plate at 30,000 cells/well and after reaching the
logarithmic growth phase, transfection was performed. A plasmid
quantity of 1 µg was added into 100 µl opti-MEM medium (Gibco;
Thermo Fisher Scientific, Inc.); 2 µl Lipofectamine
2000® was then added into 100 µl opti-MEM medium.
Following the plasmid-opti-MEM mixture and Lipofectamine
2000®-opti-MEM mixture incubation at 25°C for 5 min, the
two mixtures were added together and incubated at 25°C for 20 min.
The Lipo2000-plasmid-opti-MEM mixture was then added into the well.
After 4 h, the medium was replaced with DMEM containing 10% FBS.
After 24 h, the cells were used in subsequent experimentation.
Luciferase activity
Luciferase activity was measured as previously
described (21). Briefly, the
CAL27 cells were seeded into a 12-well plate at 30,000 cells/well.
After the cells reached the logarithmic growth phase, 1 µg TCTN1
promoter plasmid was transfected into CAL27 cells or SCC15 cells.
At 24 h following transfection, the activity measurement was
performed. For activity measurement, the cells were lysed in a cell
lysis buffer of the Promega E1500 Luciferase Assay System (Promega
Corporation), and all cell lysates were then incubated with
luciferin of the Promega E1500 Luciferase Assay System. Luciferase
activity was measured with a Berthold SiriusC luminometer
(Titertek-Berthold). Renilla luciferase activity was used
for normalisation.
Analysis of transcription factors
binding to the TCTN1 core promoter
PROMO is a virtual laboratory used for identifying
putative transcription factor binding sites, based on DNA sequences
(19). The core promoter sequence
was submitted to PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3).
The dissimilarity level for predicted factors was set as ≤5. The
putative transcription factor predicted by PROMO was then submitted
to JASPAR2020 software (20)
(http://jaspar.genereg.net/), which
provided a high-quality transcription factor binding profile
database in order to evaluate further the transcription factor
binding to the TCTN1 core promoter. The relative profile score
threshold was set as 80%, and the transcription factor getting the
highest score would be studied further.
Chromatin Immunoprecipitation
(ChIP)
ChIP assay was performed using a ChIP assay kit
(cat. no. P2078, Beyotime Institute of Biotechnology), according to
the manufacturer's instructions. Briefly, crosslink was performed
by incubating the CAL27 and SCC15 cells in 1% formaldehyde
(Beyotime Institute of Biotechnology) and terminsated by 1% glycin
(Beyotime Institute of Biotechnology). The chromatin was fractured
into fragments between 200 and 1,000 bp by ultrasonication at 400 W
for 10 min in a Ultrasonic Cell Disruptor (JY92-IIN, Ningbo Scientz
Biotechnology Co., Ltd.), and was then incubated with the
anti-TFAP2A antibody (ab108311; Abcam) (1:1,000 diluted) at 4°C for
12 h and dismantled by Protein A/G MagBeads (Thermo Fisher
Scientific, Inc.). The chromatin fragments were then incubated with
anti-flag antibody (#14793; Cell Signaling Technology, Inc.)
(1:1,000 diluted) at 4°C for 12 h as a negative control. Following
the cross-linking unfastening with the use of 0.2 µM NaCl at 65°C
for 6 h, the pulled down fragment was subjected to PCR
amplification in an ABI Veriti PCR thermocycle instrument (Applied
Biosystems) according to the following protocol: 95°C, 10 min;
(95°C, 15 sec; 55°C, 15 sec; 72°C, 30 sec for 30 cycles; 72°C, 5
min. The primers for amplifying the fragments containing
TFAP2A-binding sites of the TCTN1 promoter were as follows:
5′-CACGCCTGTAATCCCAACTA-3′ (forward) and 5′-GTGAAGCTGGCGTAAACGAG-3′
(reverse). The PCR products were analyzed on 1.0% agarose gel
electrophoresis and then photographed on an Ultraviolet
luminescence gel imager (Gel Doc XR, Bio-Rad Laboratories,
Inc.).
Statistical analysis
The data of CCK-8 assay, transwell assay, RT-qPCR
and luciferase assay in the present study were performed in
triplicate and the results are presented as the mean ± SD.
Statistical analysis was performed using SPSS 16.0 software (IBM).
An unpaired Student's t-test was used to determine the significance
of differences between two groups which were not equal in numbers.
The significance of the differences in the expression of TCTN1 and
TFAP2A between the OSCC tissues and the paired normal tissues was
determined using a paired t-test. Tukey's post hoc test following
one-way ANOVA was used to evaluate the significance of the
differences among multiple independent groups. The correlation
between TFAP2A expression and TCTN1 was analyzed using Spearman's
rank correlation coefficient analysis with Rho and P-values as
indicated. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of TCTN1 in HNSCC and
OSCC
The association of TCTN1 with the
clinicopathological features of patients with HNSCC was analyzed
using the UALCAN database (http://ualcan.path.uab.edu/index.html). It was
revealed that the TCTN1 expression levels in HNSCC tissues were
increased in comparison with those in normal tissue; however, this
increase was not statistically significant (Fig. 1A). Furthermore, the expression of
TCTN1 in different HNSCC tumor stages was analyzed. The tumor stage
was defined by researchers of the UALCAN database according to
American Joint Committee on Cancer (AJCC) pathological tumor stage
information (24). In total, 443
of the 520 tumor tissues with a defined tumor stage were analyzed.
As depicted in Fig. 1B, the TCTN1
expression levels in TNM stage 2, 3 and 4 tumor tissue groups were
higher than those in the stage 1 group. Tumor grade was attributed
to the tumor tissues according to tumor differentiation by
researchers of the UALCAN database. The TCTN1 expression levels in
the grade 2, 3 and 4 groups were higher than those in the grade 1
tumor group. Additionally, TCTN1 expression levels in the grade 3
and 4 tumor groups were significantly higher than those in the
grade 1 tumor group (Fig. 1C).
These results indicated that TCTN1 was associated with an increased
tumor stage and grade of HNSCC.
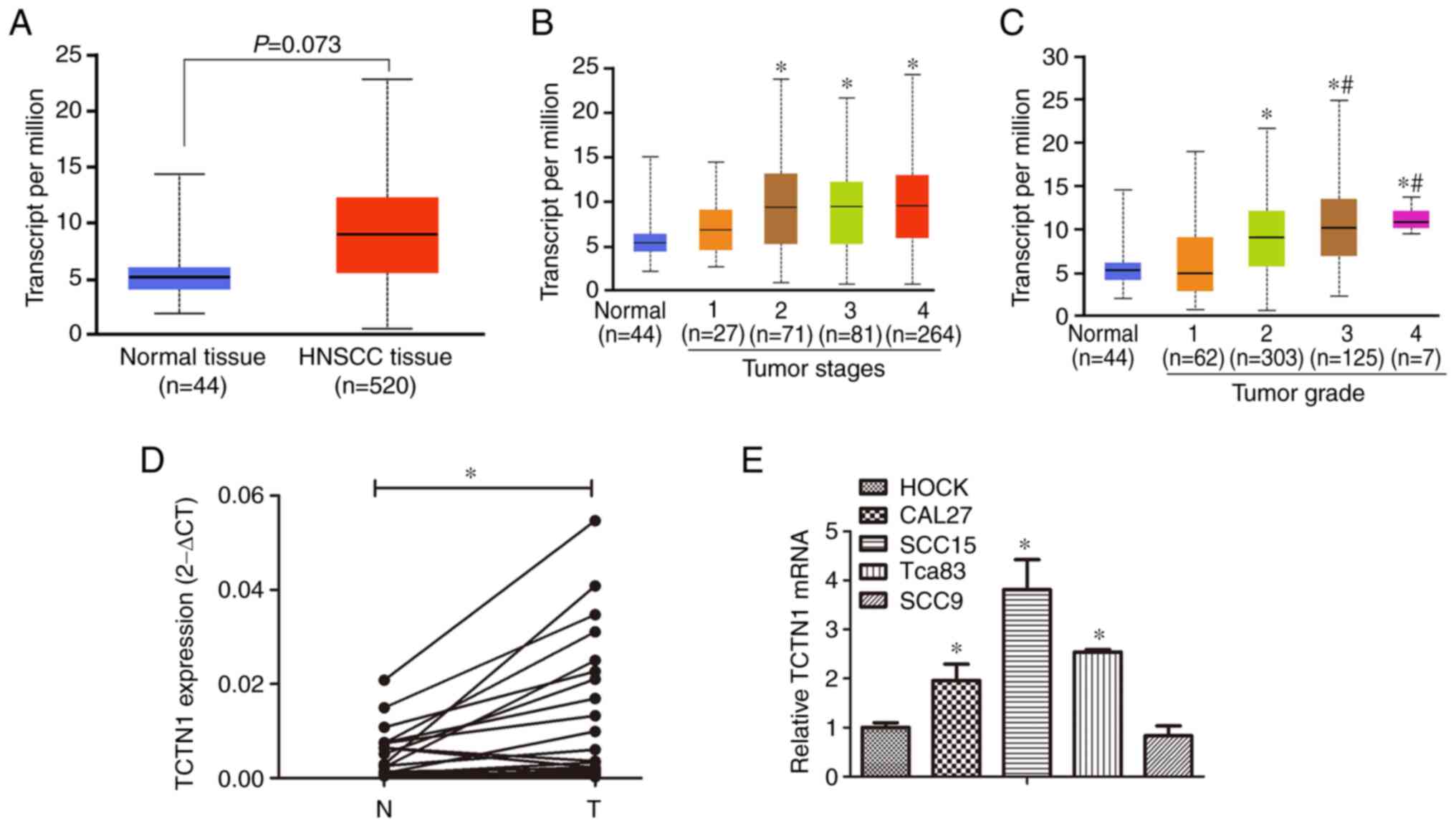 | Figure 1.TCTN1 expression in HNSCC tissues.
(A) TCTN1 expression in normal tissues and HNSCC tissues based on
the UALCAN database. (B) TCTN1 expression in different tumor stages
of HNSCC. *P<0.05 vs. normal. (C) TCTN1 expression in different
tumor grades of HNSCC. *P<0.05 vs. normal, #P<0.05
vs. grade 1. (D) Expression of TCTN1 in 21 OSCC tissues and paired
tumor-adjacent normal tissues was detected by using RT-qPCR.
*P<0.05. N, adjacent normal tissue; T, tumor tissue of OSCC. (E)
Expression of TCTN1 in HOK, CAL27, SCC15, Tca83 and SCC9 cells was
detected using RT-qPCR. *P<0.05. TCTN1, tectonic family member
1; HNSCC, head and neck squamous cell carcinoma; OSCC, Oral
squamous cell carcinoma; RT-qPCR, reverse
transcription-quantitative PCR. |
Considering that OSCC is the main HNSCC subtype, 21
OSCC tissues were obtained and the expression of TCTN1 was also
measured in OSCC tumors. As depicted in Fig. 1D, TCTN1 was overexpressed in the
OSCC tissues compared with the paired normal tissues. Moreover, the
expression of TCTN1 was compared between the HOK cell, a normal
oral epithelial cell line, and four OSCC cells lines. In the CAL27,
SCC15 and Tca83 OSCC cell lines, higher TCTN1 expression levels
were observed in comparison with those in the HOK cells (Fig. 1E). However, in the SCC9 OSCC cell
line, the TCTN1 expression levels appread to be similar to those of
the HOCK cells.
Knockdown of TCTN1 inhibits the
proliferation, migration and invasion of OSCC cells
Since it was observed that TCTN1 was overexpressed
in OSCC tissues, it was therefore hypothesized that TCTN1 may
participate in tumor OSCC progression. TCTN1 expression was knocked
down in the CAL27 and SCC15 OSCC cell lines through Lv-shTCTN1
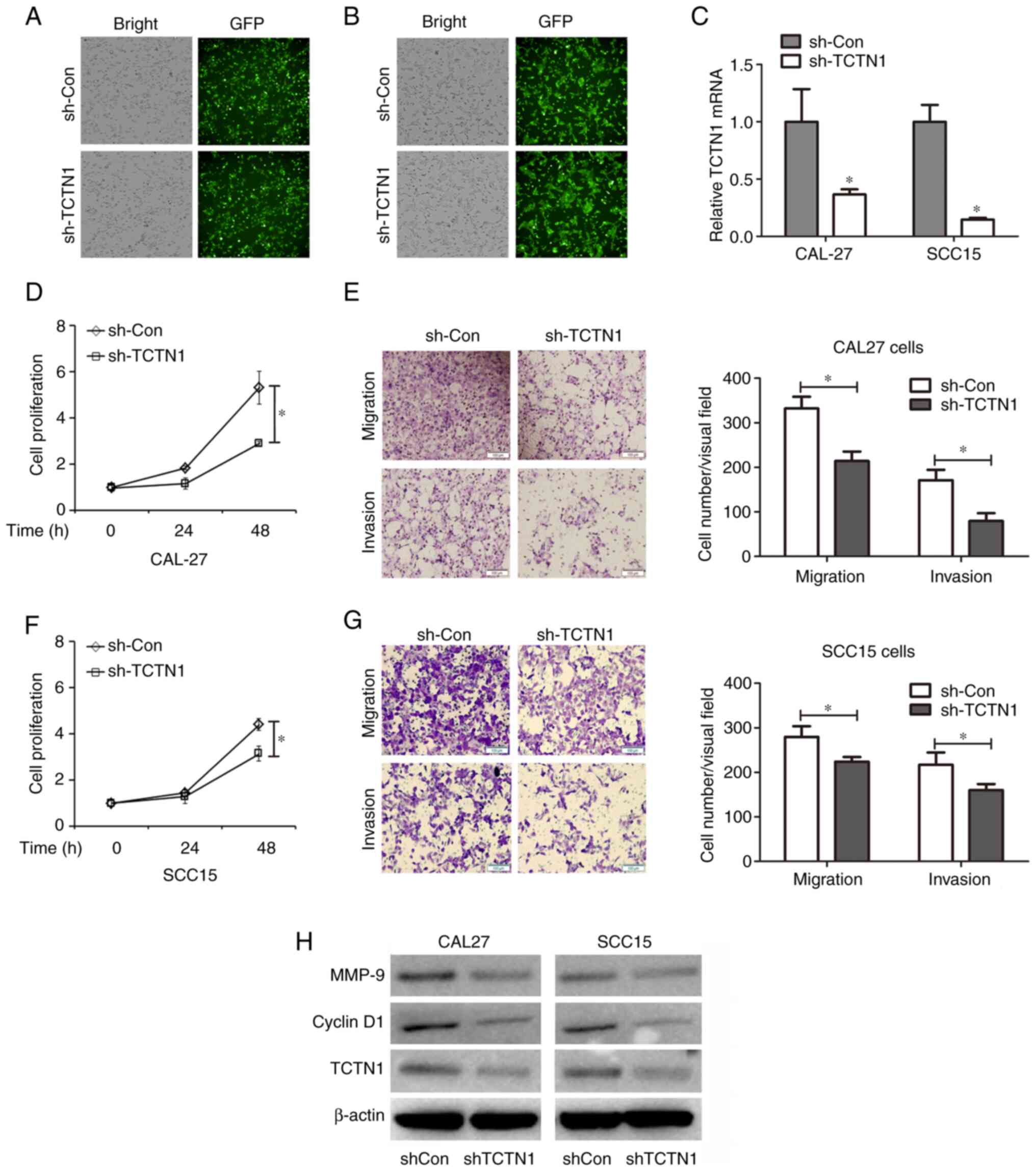
transfection. The green fluorescence indicated that the CAL-27
cells (Fig. 2A) and SCC15 cells
(Fig. 2B) were successfully
transfected with the lentiviral vector. TCTN1 expression was then
determined using RT-qPCR. It was demonstrated in Fig. 2C that TCTN1 mRNA expression was
significantly decreased in the CAL27 cells (62.59% reduction) and
SCC15 cells (85.95% reduction) transfected with Lv-shTCTN1, as
compared with the Lv-shCon-transfected cells. CCK-8 cell assay was
then used to evaluate the cell proliferative capacity. As depicted
in Fig. 2D and F, TCTN1 knockdown
exerted an inhibitory effect on the proliferation of the CAL-27 and
SCC15 cells. Moreover, the cell migratory and invasive capacity was
detected using a Transwell assay. Both cell migration and invasion
were inhibited by TCTN1 knockdown in the CAL27 and SCC15 cells
(Fig. 2E and G). Furthermore,
cyclin D1 and matrix metallopeptidase 9 (MMP-9) expression,
associated with cell proliferation, migration and invasion was
determined. As illustrated in Fig.
2H, the knockdown of TCTN1 decreased the cyclin D1 and MMP-9
protein levels in both the CAL27 and SCC15 cells. These inhibitory
effects of TCTN1 on cell proliferation, migration and invasion
suggested that TCTN1 may be a tumor-promoting gene and that it may
also be a potential target for OSCC therapy; therefore, elaborating
the mechanisms through which TCTN1 is regulated would be of utmost
importance.
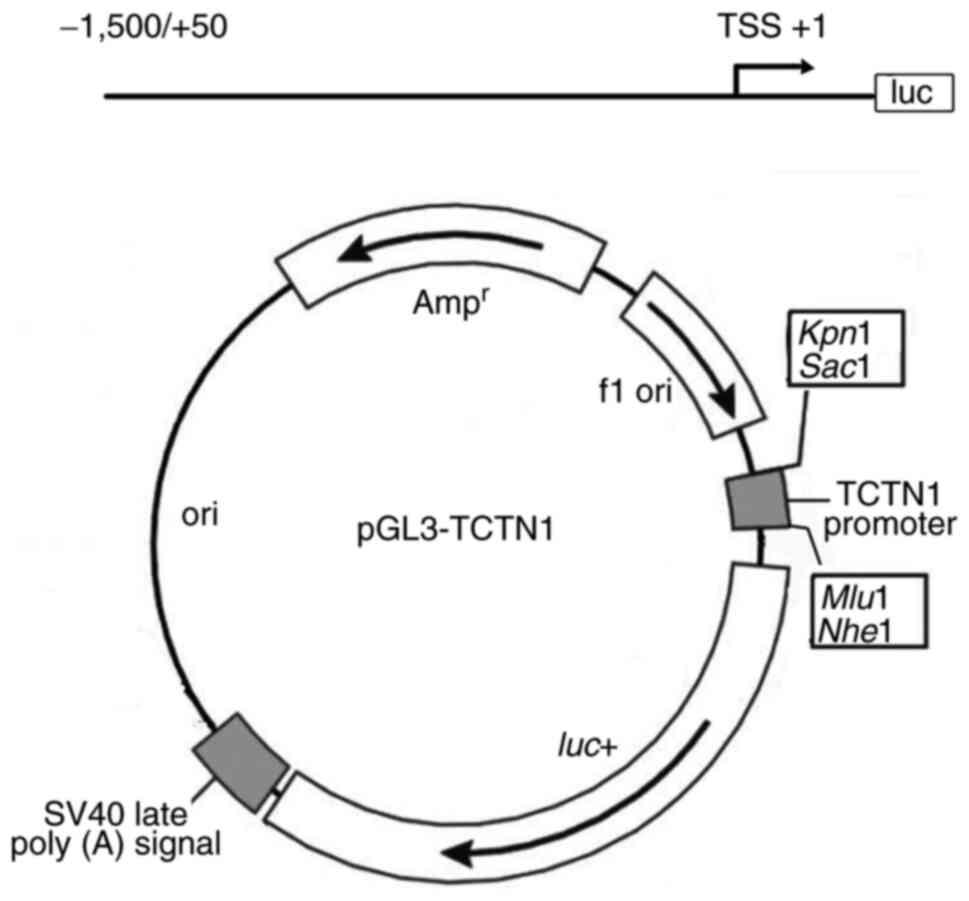
Construction of TCTN1
promoter-reporter
The TCTN1 promoter sequence was obtained from
GenBank (https://www.ncbi.nlm.nih.gov/gene/79600). According to
the sequence, high-fidelity PCR was performed in order to acquire
the TCTN1 promoter fragment (−1,500/+50, transcriptional starting
site was marked as +1). A TCTN1 promoter-reporter was constructed
by inserting the promoter fragment into the pGL3-basic plasmid
between SacI and MluI site, and the reporter was
named pGL3-TCTN1 (Fig. 3).
Identification of the TCTN1 core
promoter
Based on the pGL3-TCTN1, a series of deletion TCTN1
promoter mutants were constructed. These reporters were transfected
into the CAL27 cells, and the promoter activity was measured. As
demonstrated in Fig. 4A, as the
promoter fragment narrowed to −600/-416, there was still almost
71.32% activity compared to the full-length promoter (−1,500/+50),
indicating that the core promoter is located in the region of
−600/-416. The reporter based on pGL3-CDKL1 (−600/-416) was
shortened further and the promoter activity was measured. Even when
the promoter narrowed to −550/-491, there was still 63.81% activity
of the full-length promoter, suggesting the region of −550/-491 is
the core promoter of TCTN1 (Fig.
4B). This was confirmed further in SCC15 cells by comparing the
activity of the full-length promoter (−1,500/+50) and the deletion
mutant (−550/-491) in SCC15 cells. Furthermore, the deletion mutant
(−550/-491) demonstrated ~79.06% the activity of the full-length
promoter (Fig. 4C). The
aforementioned results indicated that the core promoter of TCTN1
may be located in the −550/-491 region.
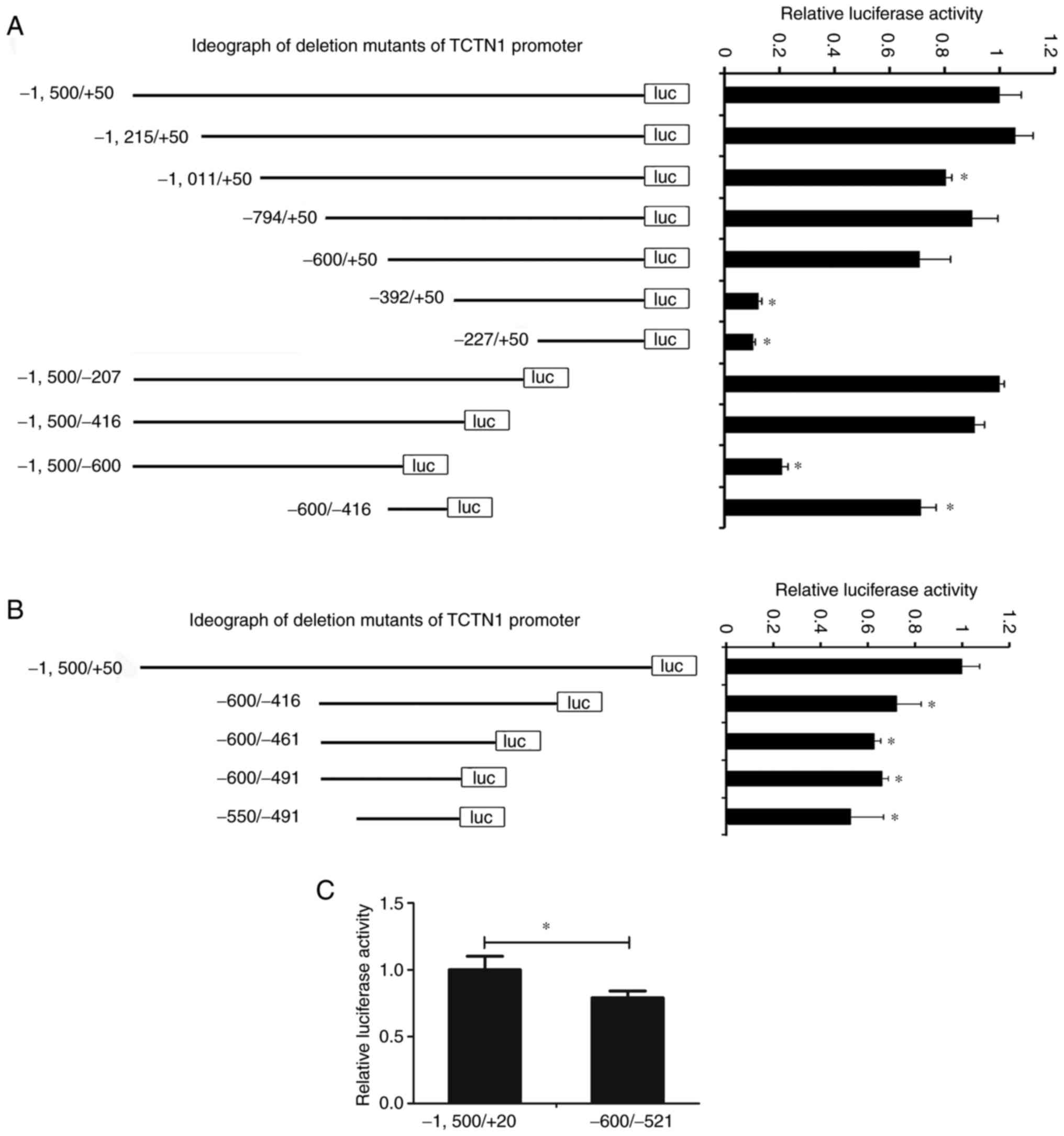 | Figure 4.Identification of TCTN1 core
promoter. (A) Based on the pGL3-TCTN1, a series of deletion mutants
of TCTN1 promoter (−1,215/+50, −1,011/+50, −794/+50, −600/+50,
−392/+50, −227/+50, −1,500/-207, −1500/-416, −1,500/-600 and
−600/-416) were constructed and the luciferase activity was
measured. The left panel shows the ideograph of the deletion
mutants of TCTN1 promoter; the right panel shows the relative
luciferase of each mutant. *P<0.05 vs. the −1,500/+50. (B) Based
on the pGL3-TCTN1 (−600/-416), a series of deletion mutants of
TCTN1 promoter (−600/-461, −600/-491, and −550/-491) were
constructed and the luciferase activity was measured. The left
panel depicts the ideograph of the deletion mutants of TCTN1
promoter; the right panel depicts the relative luciferase of each
mutant. *P<0.05 vs. the −1,500/+50. (C) Promoter activity of
pGL3-TCTN1 (−1,500/+50) and pGL3-TCTN1 (−550/-491). *P<0.05.
TCTN1, tectonic family member 1. |
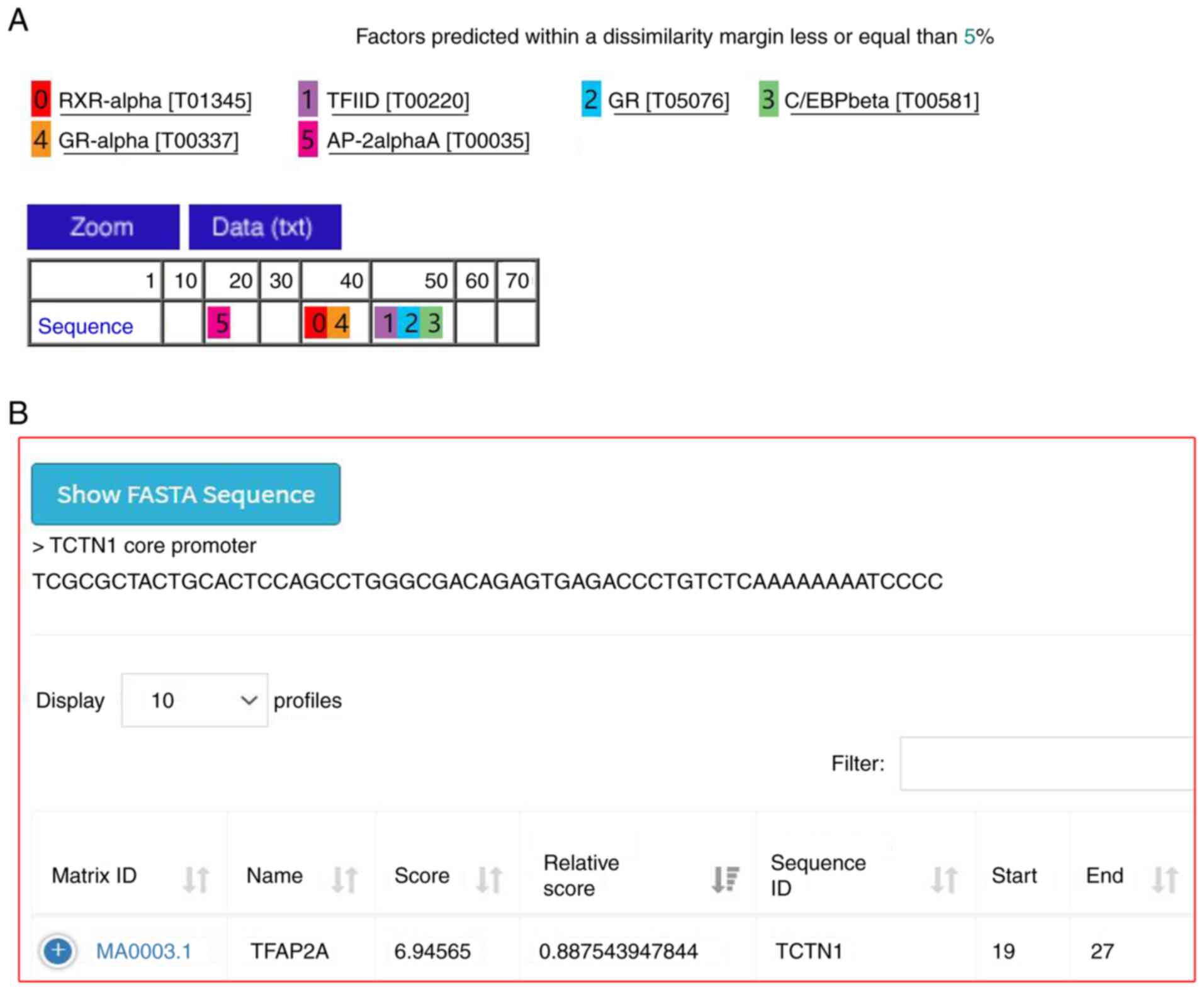
Prediction of putative transcription
factor of TCTN1 core promoter
The core promoter sequence was uploaded onto PROMO
(http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3).
The dissimilarity of predicted factors was set as ≤0. A total of 6
predicted transcriptional factors binding to the TCTN1 core
promoter were detected (Fig. 5A).
The transcription factors were then submitted to JASPAR2020, which
provides access to a high-quality transcription factor binding
profile database. The relative profile score threshold was set as
80%. According to JASPAR2020, only TFAP2A can bind to the TCTN1
core promoter (Fig. 5B).
Therefore, it was hypothesized that TFAP2A may be an important
transcription factor for TCTN1.
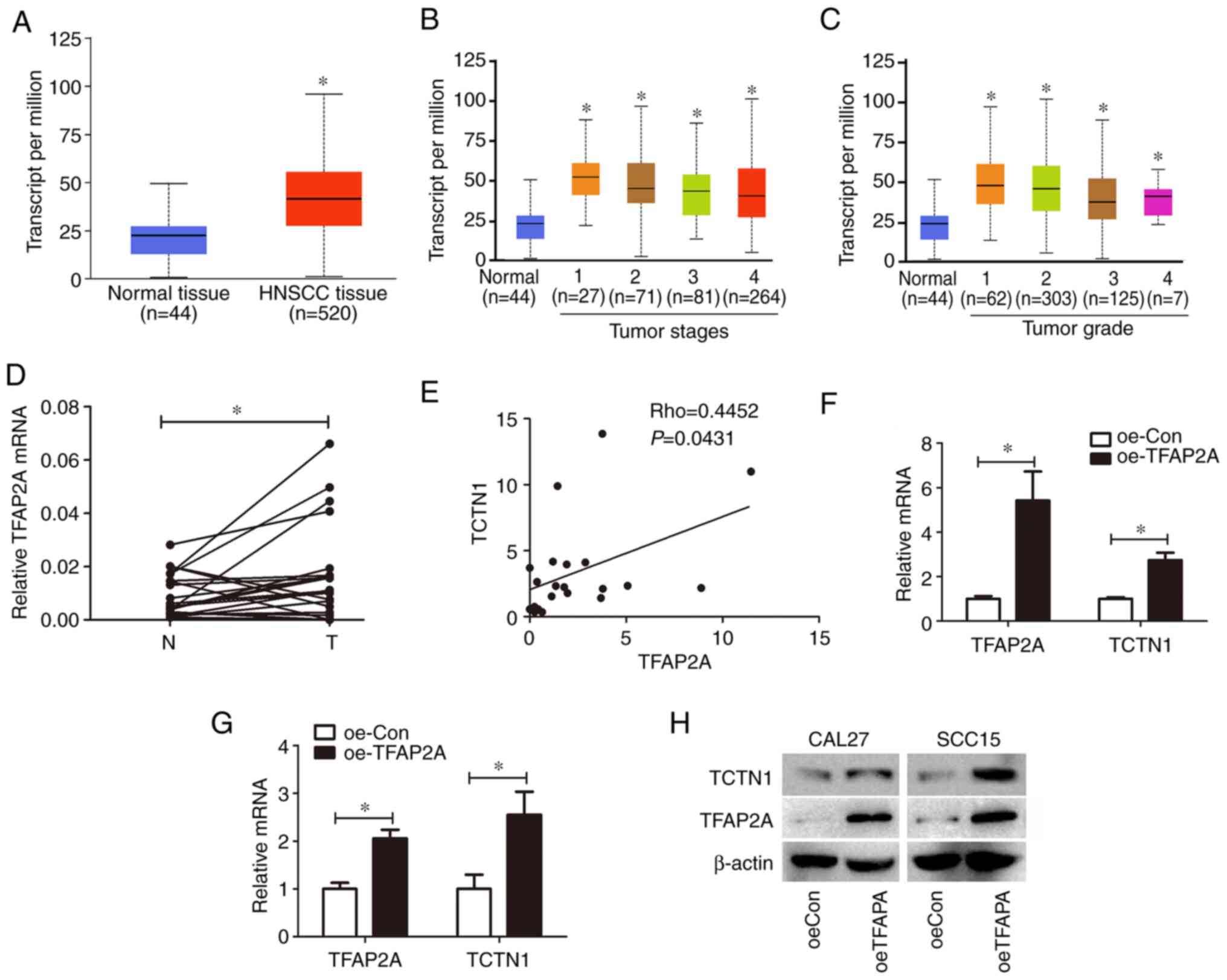
TFAP2A is overexpressed in HNSCC and
regulates TCTN1 expression in OSCC cells
The relevance of TFAP2A with the clinicopathological
features of patients with HNSCC was then analyzed based on the
UALCAN database. The analysis revealed that TFAP2A expression was
significantly higher in HNSCC than in normal tissues (Fig. 6A). Furthermore, TFAP2A expression
was analyzed in relation to different tumor stages and tumor
grades. TFAP2A expression was increased in stage 1, 2, 3 and 4
tumors compared with normal tissues (Fig. 6B); however no differences in TFPA2
expression levels were observed among stage 1, 2, 3 and 4 tumors.
In addition, TFAP2A expression was higher in grade 1, 2, 3 and 4
tumors than in normal tissues; however that between grade 1, 2, 3
and 4 tumors exhibited no difference (Fig. 6C). TFAP2A expression in the 21
paired OSCC tissues and the corresponding adjacent normal tissues
was also confirmed. TFAP2A overexpression was observed in OSCC
tissues in comparison with adjacent normal tissues (Fig. 6D). Additionally, the correlation
between the TFAP2A and TCTN1 expression levels was also analyzed in
the 21 paired tissues and it was demonstrated that TCTN1 expression
positively correlated with the TCTN1 expression levels (Fig. 6E). To confirm whether TFAP2A
regulates TCTN1 expression, TFAP2A overexpression was induced in
the CAL-27 and SCC15 cells, and RT-qPCR was used for TCTN1
expression evaluation. It was observed that TFAP2A was
overexpressed 5.38-fold following a Lv-oeTFAP2A infection in CAL-27
cells, and correspondingly induced the upregulation of TCTN1 in the
CAL-27 cells (Fig. 6F). Moreover,
TFAP2A was overexpressed 2.81-fold with Lv-oeTFAP2A infection, and
correspondingly induced the upregulation of TCTN1 in the SCC15
cells. Furthermore, western blot analysis demonstrated that the
overexpression of TFAP2A protein levels induced the upregulation of
TCTN1 protein levels (Fig.
6H).
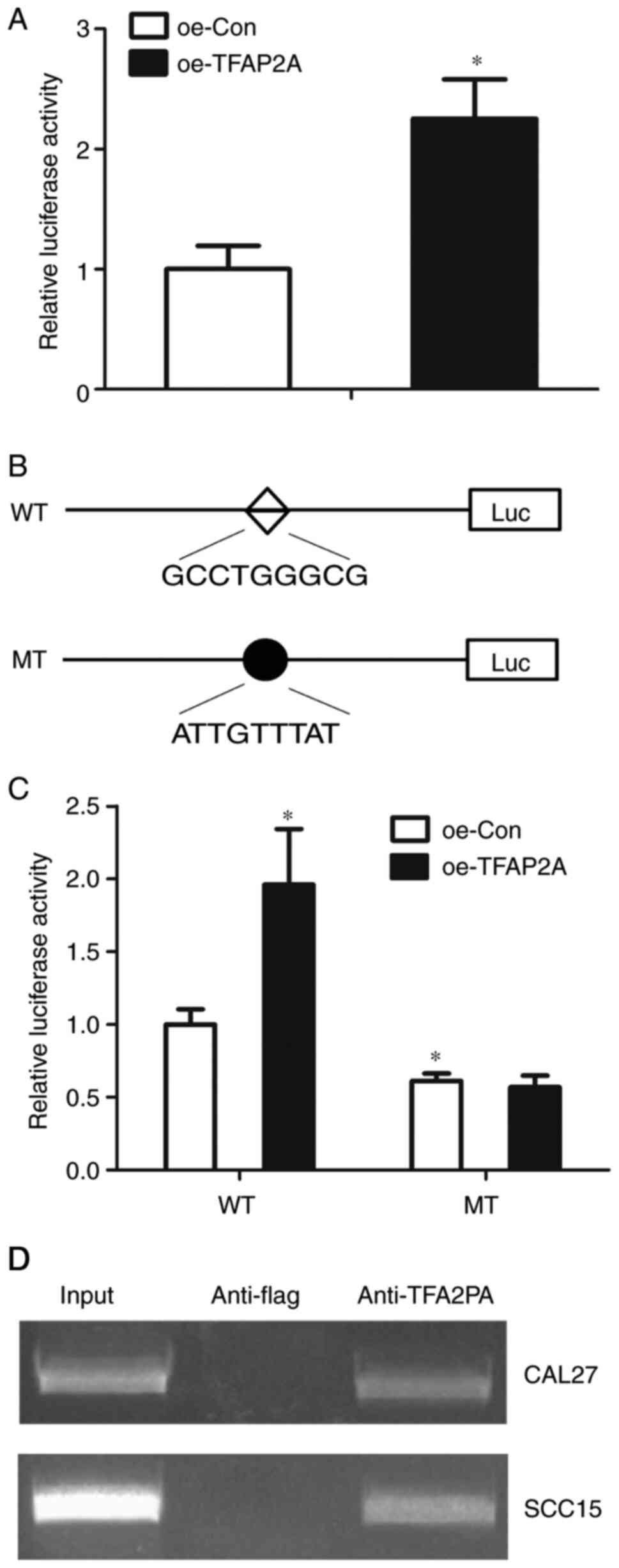
TFAP2A regulates TCTN1 transcription
in OSCC
The present study then detected whether TFAP2A
regulates TCTN1 through the core promoter. TFAP2A was overexpressed
4.912-fold with Lv-oeTFAP2A infection in the CAL-27 cells (Fig. 7A). Subsequently, the core TCTN1
promoter activity in the CAL-27 cells with TFAP2A overexpression
was defined. It was observed that TFAP2A overexpression resulted in
an increased TCTN1 core promoter activity, indicating that TFAP2A
regulated TCTN1 at the transcription level (Fig. 7B). Subsequently, site-directed
mutation was performed, in order to mutate the putative binding
site of TFAP2A at the TCTN1 core promoter. It was then defined
whether the mutations could abolish the effects of TFAP2A
overexpression on the TCTN1 promoter activity. The mutant-type of
TCTN1 core promoter demonstrated a decreased activity in comparison
with the wild-type; additionally, the mutation of the binding site
abolished the effect of TFAP2A on the regulation of the TCTN1 core
promoter activity (Fig. 7C). In
order to confirm that TFAP2A could bind to TCTN1 promoter, ChIP
assay was performed. According to the results, there were visible
bands in the anti-TFAP2A group, whereas in the anti-flag group, no
band was observed, indicating that TFAP2A may bind to the TCTN1
promoter (Fig. 7D).
Discussion
In the present study, the function of TCTN1 in OSCC
cell proliferation, migration and invasion was examined. Moreover,
it was demonstrated that the TFAP2A transcription factor may play a
main role in the regulation of TCTN1 expression.
UALCAN is a comprehensive interactive web resource
for analyzing cancer OMICS data, by which the relevance of a
certain gene with the clinicopathological features of several tumor
types can be analyzed (11). Using
UALCAN, it was revealed that TCTN1 expression was associated with
the tumor stage and grade of HNSCC. Subsequently, it was
hypothesized that TCTN1 overexpression in HNSCC in general may also
possibly occur in the OSCC subtype. In order to verify this, its
expression in OSCC was defined and its function was evaluated. It
was observed that TCTN1 was overexpressed in OSCC tumors, as
compared with adjacent normal tissues. In addition, it was observed
to be overexpressed in three OSCC cells, as compared with the
control cells. It was also noted that the SCC9 cell line did not
exhibit increased TCTN1 levels, in comparison with the HOK control
cell line. This may possibly be attributed to the control cell and
the four OSCC cell line origins from different individuals, thus
gene expression variations possibly exist among them. Therefore,
the effects of alterations of TCTN1 expression on OSCC progression
were examined. TCTN1 knockdown resulted in the inhibition of cell
proliferation, migration and invasion, indicating that TCTN1 may be
a tumor-promoter gene for OSCC progression, suggesting that TCTN1
may be a potential target for OSCC therapy. To the best of our
knowledge, this is the first time the involvement of TCTN1
expression in OSCC has been examined. The aforementioned findings,
concerning TCTN1 in OSCC progression, are in line with those of
previously published studies on other tumor types, including
non-small cell lung cancer (25),
thyroid cancer (7) and colon
cancer cells (8).
Revealing the mechanisms through which
cancer-associated genes are regulated is an important approach
towards the comprehension of the tumor occurrence-related
mechanisms, and the determination of novel therapeutic targets.
Several miRNAs have been reported to regulate TCTN1 through the
binding at the 3′-UTR region (10,26).
Apart from miRNAs, gene expression is also regulated at the
transcriptional level by transcription factors (27). However, the transcriptional
regulation of TCTN1 has not been previously reported, at least to
the best of our knowledge. The core promoter is the smallest
contiguous sequence of DNA in a gene promoter, mediating the
initiation of gene transcription (28). In the present study, a TCTN1
promoter-reporter was constructed as well as a serial-deletion
mutant promoter-reporter. Considering that the region of −550/-491
exhibited almost 63.8% of the full-length promoter activity in the
CAL27 cells and 79.06% of the full-length promoter activity in the
SCC15 cells, also possessing a binding site of transcription factor
IID (TFIID), the core promoter region of TCTN1 was identified,
located at −550/-491. This is the first time, to the best of our
knowledge, that the core promoter of TCTN1 was located,
contributing to the acquisition of key transcriptional factors for
TCTN1.
Several putative transcription factors were
predicted with the use of PROMO and JASPAR2020 and it was also
demonstrated that TFAP2A may be a crucial transcription factor of
TCTN1. TFAP2A, also known as AP2A, AP-2alphaA and AP-2TF, is an
important factor involved in various physiological and pathological
processes, including osteogenic differentiation, epidermal
development, and cancer progression, such as breast cancer and
cervical cancer (29–31). In the present study, it was
revealed that TFAP2A upregulated TCTN1 promoter activity and
expression in OSCC cells. This may be the first finding of TCTN1
being regulated at the transcription level, to the best of our
knowledge.
TFAP2A, a member of the AP-2 family, has been
reported to be vital for gene expression regulation. TFAP2A has
been reported to activate and inhibit the transcription of target
genes (32–34). This transcription factor has been
mentioned to bind to a specific sequence of the target genes and
regulate gene transcription through its interaction with enhancer
elements (34). In the present
study, it was demonstrated that TFAP2A positively regulated TCTN1
expression, a newly identified target gene of TFAP2A, to the best
of our knowledge. TFAP2A plays a crucial role in various crucial
biological processes, including melanoma tumor metastasis (35), cell proliferation and development
(36,37). The function of TFAP2A in tumor
progression has been extensively researched. Different functions of
TFAP2A have been reported in carcinogenesis: In prostate cancer,
melanoma, hepatocellular cancer, gastric cancer and breast cancer
TFAP2A has been associated with a reduced aggressiveness or an
improved clinical survival through the regulation of cell growth,
differentiation or chemotherapy sensitivity; however, in
neuroblastoma, acute myeloid leukemia and several types of squamous
cell carcinomas, such as head and neck squamous cell carcinoma,
TFAP2A has been reported to promote tumor cell growth and
aggressiveness (34).
Subsequently, the putative binding site of TFAP2A in
the TCTN1 core promoter was mutated, as predicted using JASPAR2020
software. It was observed that the mutation of the binding site may
reduce core promoter activity and completely abolish
TFAP2A-regulated TCTN1 core promoter activity, confirming that this
site is the binding site of TFAP2A. Blocking this site may abolish
the regulatory effects of TFAP2A on TCTN1 and may be a promising
OSCC therapeutic target.
A limitation of the present study is that the study
was initiated from a pan-cancer gene expression analysis using
UALCAN, considering that OSCC is a main type of HNSCC.
Subsequently, the association of TCTN1 with OSCC cell
proliferation, migration and invasion was analyzed. It was observed
that TCTN1 was overexpressed in 21 OSCC tissues in comparison with
the paired normal tumor-adjacent tissue. However, the number of
tissues enrolled in the present study was insufficient for the
analysis of the relevance between TCTN1 and the patient
clinicopathological features. Therefore, further clinical analyses
are required in future studies, in order to determine the
associations between TCTN1 and the patient clinicopathological
features. Furthermore, the results were validated in vivo,
in order to demonstrate the role of TCTN1 in OSCC progression and
to further confirm the transcriptional role of TFAP2A in TCTN1
expression. Additionally, another limitation was that the numbers
of patients in the four tumor stage and grade categories differed
markedly. The adjustment of the numbers of patients in each of the
tumor stage and grade categories and the analysis of the
association between TCTN1 expression and tumor stage or grade is
required in future studies. However, UALCAN currently provides only
gene expression data without any adjustments related to group size.
Furthermore, the number of 42 normal tissues in UALCAN is smaller
than the number of 520 HNSCC tissues, possibly inducing bias to the
results. Therefore, in order to reveal more clearly any relevance
between TCTN1 and OSCC, the authors aim to examine a larger cohort
including clinicopathological data in future studies, with the
inclusion of all necessary group number adjustments.
In total, future studies may involve several steps.
Firstly, the relevance between TCTN1 and OSCC may be further
elucidated using a multicenter cohort, to confirm whether TCTN1 is
an appropriate biomarker for OSCC prognosis. Secondly, downstream
factors or signals related to the TCTN1-induced regulation of OSCC
proliferation, migration and invasion may be screened using
bioinformatics tools and subsequently validated using biomedical
laboratory methods. Moreover, TCTN1 was associated with the
clinicopathological features of patients with OSCC; however, it was
only demonstrated whether TCTN1 regulates OSCC cell proliferation,
migration and invasion. In future studies, the possibility of TCTN1
association with other clinicopathological features, including
angiogenesis, immune escape, and therapy resistance may be also
elucidated.
In conclusion, it was demonstrated in the present
study that TCTN1 was overexpressed in OSCC tumors, and was
associated with OSCC tumor stage and tumor grade; additionally, the
core promoter of TCTN1 was identified and it was determined that
TFAP2A may be a crucial transcription factor of TCTN1. The findings
of the present study suggested that TFAP2A/TCTN1 may be potential
therapeutic targets for OSCC treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 81602374), the Medical Science and
Technology Development Project of Shandong Province (grant no.
2015WS0398), and the Natural Science Foundation of Liaocheng
People's Hospital (grant no. LYQN201903).
Availability of data and materials
Raw data from the experimental results have been
uploaded in the Figshare online database (https://figshare.com/s/eda08d428301117387b6). All
remaining datasets used and/or analyzed during the present study
are available from the corresponding author on reasonable
request.
Authors' contributions
PY designed the study. GB performed the cell culture
and western blotting experiments, and drafted the manuscript. NW
collected the tissue samples and performed the RT-qPCR experiments,
and the construction of TCTN1 promoter-reporter. CY revised the
manuscript and was also involved in the western blotting
experiments. FL performed the cell culture experiments. PZ and ZM
performed the statistical analysis. BZ performed the Transwell
assay. YL performed the ChIP and CCK-8 assays. KX and KL performed
the luciferase assay and participated in data analysis. PY and GB
confirmed the authenticity of the raw data. All authors have read
and approved the final manuscript, and agree to be accountable for
all aspects of the present research, ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
OSCC and adjacent normal tissues were collected from
pateints at Liaocheng People's Hospital. The tissue collection was
checked and approved by the Ethics Committee of Liaocheng People's
Hospital (approval no. LC202176). All patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Almangush A, Makitie AA, Triantafyllou A,
de Bree R, Strojan P, Rinaldo A, Hernandez-Prera JC, Suárez C,
Kowalski LP, Ferlito A and Leivo I: Staging and grading of oral
squamous cell carcinoma: An update. Oral Oncol. 107:1047992020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Wang H, Hang C, Fan Y, Ma C and Pan
Y: Lentivirus-mediated knockdown of TCTN1 inhibits glioma cell
proliferation. Appl Biochem Biotechnol. 176:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Li J, Meng Q and Wang B: Three
Tctn proteins are functionally conserved in the regulation of
neural tube patterning and Gli3 processing but not ciliogenesis and
Hedgehog signaling in the mouse. Dev Biol. 430:156–165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Qattan MM, Shaheen R and Alkuraya FS:
Expanding the allelic disorders linked to TCTN1 to include Varadi
syndrome (orofaciodigital syndrome type VI). Am J Med Genet A.
173:2439–2441. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alazami AM, Alshammari MJ, Salih MA,
Alzahrani F, Hijazi H, Seidahmed MZ, Abu Safieh L, Aldosary M, Khan
AO and Alkuraya FS: Molecular characterization of Joubert syndrome
in Saudi Arabia. Hum Mutat. 33:1423–1428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong S, Ji F, Wang B, Zhang Y, Xu X and
Sun M: Tectonic proteins are important players in non-motile
ciliopathies. Cell Physiol Biochem. 50:398–409. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu P, Xia X, Yang Z, Tian Y, Di J and Guo
M: Silencing of TCTN1 inhibits proliferation, induces cell cycle
arrest and apoptosis in human thyroid cancer. Exp Ther Med.
14:3720–3726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai X, Dong M, Yu H, Xie Y, Yu Y, Cao Y,
Kong Z, Zhou B, Xu Y, Yang T and Li K: Knockdown of TCTN1 strongly
decreases growth of human colon cancer cells. Med Sci Monit.
23:452–461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Gao Y, Liu Y, Chen J, Wang J, Gan
S, Xu D and Cui X: Tectonic-1 contributes to the growth and
migration of prostate cancer cells in vitro. Int J Mol Med.
36:931–938. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chai L and Yang G: MiR-216a-5p targets
TCTN1 to inhibit cell proliferation and induce apoptosis in
esophageal squamous cell carcinoma. Cell Mol Biol Lett. 24:462019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandrashekar DS, Bashel B, Balasubramanya
SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BVSK and
Varambally S: UALCAN: A portal for facilitating tumor subgroup gene
expression and survival analyses. Neoplasia. 19:649–658. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paul J: Transcriptional regulation in
mammalian chromosomes. Symp Soc Exp Biol. 25:117–126.
1971.PubMed/NCBI
|
|
13
|
Ramya Devi KT, Karthik D, Mahendran T,
Jaganathan MK and Hemdev SP: Long noncoding RNAs: Role and
contribution in pancreatic cancer. Transcription. 12:12–27. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiao C, Qiao T, Yang S, Liu L and Zheng M:
SNHG17/miR-384/ELF1 axis promotes cell growth by transcriptional
regulation of CTNNB1 to activate Wnt/β-catenin pathway in oral
squamous cell carcinoma. Cancer Gene Ther. Feb 2–2021.(Epub ahead
of print). View Article : Google Scholar
|
|
15
|
Jiang H, Chen H, Wan P, Song S and Chen N:
Downregulation of enhancer RNA EMX2OS is associated with poor
prognosis in kidney renal clear cell carcinoma. Aging (Albany NY).
12:25865–25877. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kou XX, Hao T, Meng Z, Zhou YH and Gan YH:
Acetylated Sp1 inhibits PTEN expression through binding to PTEN
core promoter and recruitment of HDAC1 and promotes cancer cell
migration and invasion. Carcinogenesis. 34:58–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia CP, Pan T, Zhang N, Guo JR, Yang BW,
Zhang D, Li J, Xu K, Meng Z and He H: Sp1 promotes dental pulp stem
cell osteoblastic differentiation through regulating noggin. Mol
Cell Probes. 50:1015042020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan WCW, Tan Z, To MKT and Chan D:
Regulation and role of transcription factors in osteogenesis. Int J
Mol Sci. 22:54452021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Farre D, Roset R, Huerta M, Adsuara JE,
Roselló L, Albà MM and Messeguer X: Identification of patterns in
biological sequences at the ALGGEN server: PROMO and MALGEN.
Nucleic Acids Res. 31:3651–3653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fornes O, Castro-Mondragon JA, Khan A, van
der Lee R, Zhang X, Richmond PA, Modi BP, Correard S, Gheorghe M,
Baranašić D, et al: JASPAR 2020: Update of the open-access database
of transcription factor binding profiles. Nucleic Acids Res.
48:D87–D92. 2020.PubMed/NCBI
|
|
21
|
Gan YH and Zhang S: PTEN/AKT pathway
involved in histone deacetylases inhibitor induced cell growth
inhibition and apoptosis of oral squamous cell carcinoma cells.
Oral Oncol. 45:e150–e154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu K, Meng Z, Xian XM, Deng MH, Meng QG,
Fang W, Zhang D and Long X: LncRNA PVT1 induces chondrocyte
apoptosis through upregulation of TNF-α in synoviocytes by sponging
miR-211-3p. Mol Cell Probes. 52:1015602020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Wan X, Mu Z, Li F, Wang L, Zhao J
and Huang X: MiR-1256 suppresses proliferation and migration of
non-small cell lung cancer via regulating TCTN1. Oncol Lett.
16:1708–1714. 2018.PubMed/NCBI
|
|
26
|
Xu X, Gao F, Wang J, Long C, Tao L, Ding L
and Ji Y: microRNA-216a-5p inhibits the development of gastric
cancer through target combination with TCTN1. Minerva Med. May
29–2020.(Epub ahead of print). View Article : Google Scholar
|
|
27
|
Malik S and Roeder RG: The metazoan
mediator co-activator complex as an integrative hub for
transcriptional regulation. Nat Rev Genet. 11:761–772. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen BS and Hampsey M: Transcription
activation: Unveiling the essential nature of TFIID. Curr Biol.
12:R620–R622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin X, Yang H, Wang L, Li W, Diao S, Du J,
Wang S, Dong R, Li J and Fan Z: AP2a enhanced the osteogenic
differentiation of mesenchymal stem cells by inhibiting the
formation of YAP/RUNX2 complex and BARX1 transcription. Cell
Prolif. 52:e125222019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kousa YA, Fuller E and Schutte BC: IRF6
and AP2A interaction regulates epidermal development. J Invest
Dermatol. 138:2578–2588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orso F, Fassetta M, Penna E, Solero A, De
Filippo K, Sismondi P, De Bortoli M and Taverna D: The AP-2alpha
transcription factor regulates tumor cell migration and apoptosis.
Adv Exp Med Biol. 604:87–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D, Shin TH and Kudlow JE:
Transcription factor AP-2 controls transcription of the human
transforming growth factor-alpha gene. J Biol Chem.
272:14244–14250. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu H, Tan BC, Tseng KH, Chuang CP, Yeh
CW, Chen KD, Lee SC and Yung BY: Nucleophosmin acts as a novel
AP2alpha-binding transcriptional corepressor during cell
differentiation. EMBO Rep. 8:394–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kołat D, Kałuzińska Z, Bednarek AK and
Płuciennik E: The biological characteristics of transcription
factors AP-2α and AP-2γ and their importance in various types of
cancers. Biosci Rep. 39:BSR201819282019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
White JR, Thompson DT, Koch KE, Kiriazov
BS, Beck AC, van der Heide DM, Grimm BG, Kulak MV and Weigel RJ:
AP-2α-mediated activation of E2F and EZH2 drives melanoma
metastasis. Cancer Res. 81:4455–4470. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schorle H, Meier P, Buchert M, Jaenisch R
and Mitchell PJ: Transcription factor AP-2 essential for cranial
closure and craniofacial development. Nature. 381:235–238. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nottoli T, Hagopian-Donaldson S, Zhang J,
Perkins A and Williams T: AP-2-null cells disrupt morphogenesis of
the eye, face, and limbs in chimeric mice. Proc Natl Acad Sci USA.
95:13714–13719. 1998. View Article : Google Scholar : PubMed/NCBI
|