Introduction
Bladder cancer (BC) currently ranks as the 9th most
common malignancy in the world and 13th in terms of
cancer-associated mortality (1).
Clinically, BC can be classified into non-muscle-invasive bladder
cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) (2,3).
NMIBC tends to be confined only to the mucosa or submucosa, but
makes up ~75% of all cases of BC (2). However, the 5-year probability of
postoperative recurrence for NMIBC is at 31–78%, whereas the
probability of postoperative disease progression after 5 years is
0.8-45% (2). Therefore,
postoperative intravesical Bacillus Calmette-Guérin or intravesical
chemotherapy, including mitomycin, epirubicin and gemcitabine
(GEM), are essential for preventing recurrence and progression
(2). In total, ~20–25% of all
cases of BC are MIBC, where ~50% of these patients progress within
5 years after cystectomy (3).
Although combined treatment with GEM and cisplatin has been applied
as the standard chemotherapy regimen for patients with metastatic
BC and MIBC, the disease response rate was reported to be only 49%
(4). In addition, these treatment
strategies may cause severe adverse reactions, including nausea,
vomiting, neutropenia, thrombocytopenia, mucositis and febrile
neutropenia (5), where >50%
patients with BC remain unsuitable for cisplatin treatment due to
the adverse reactions (6).
GEM belongs to a broad-spectrum class of
antimetabolites (7). It is a
cytosine analogue that can replace one of the building blocks of
nucleic acids to induce ‘masked chain termination’, which in turn
inhibits further DNA synthesis and leads to cell death (7). At present, GEM is widely applied for
the treatment of various malignant tumors, such that it is
considered to be an essential component of the first-line
chemotherapy against BC, non-small-cell lung cancer (NSCLC) and
pancreatic cancer (8,9). However, patients remain susceptible
to developing recurrence and drug resistance (8,9). As
a result, a series of drugs have been investigated with the aim of
sensitizing the effects of GEM and reducing side effects. To this
effect, everolimus, sunitinib, vitamin C and vitamin K3 have all
been reported to enhance GEM-induced cytotoxicity in BC cells
(10–12).
Traditional Chinese medicine (TCM) has been utilized
for an extended period of time in China. A growing number of
TCM-derived products and medicinal herbs have been previously shown
to possess bioactive anticancer properties (13,14).
As an isoquinoline alkaloid that can be isolated from Berberis
aquifolium (Oregon grape) and Berberis vulgaris
(barberry) (15), berberine (BER)
has been recently revealed to exert several pharmacological
properties, including anti-inflammatory, cardiovascular-protective,
neuroprotective, anti-hyperglycemic, anti-hyperlipidemic,
anti-hypertensive and antitumor effects (16–18).
Additionally, BER can exert cytotoxicity on numerous types of tumor
cells, including those of human esophageal (19), prostate (20) and ovarian cancers (21). Previous studies have demonstrated
that BER can exert antitumor effects on BC cells by suppressing
proliferation whilst promoting apoptosis and cell cycle arrest
(22), in addition to sensitizing
the response of BC cells to epirubicin (23).
DNA damage normally exerts a pivotal function in
accelerating cell death, and DNA double-strand break (DSB) is one
of the most cytotoxic types of DNA damage (24). Following DSB, the DNA repair system
is swiftly initiated. RAD51 recombinase (Rad51) is a crucial
element for the homologous recombination (HR) repair of DNA and is
considered to mediate an error-free repair mechanism for
maintaining genome integrity (25). High expression levels of Rad51 have
been previously associated with invasiveness and therapeutic
resistance in a number of tumors, including pancreatic
adenocarcinoma (26), lung tumor
(27), breast cancer (28) and ovarian cancer (29), whereas downregulation of Rad51 has
been shown to enhance the efficacy of chemotherapy or radiation
sensitivity (30). Tsai et
al (31) reported that GEM
could induce the upregulation of Rad51 in NSCLC. In another study,
Liu et al (32) previously
reported that BER could sensitize esophageal cancer cells to
radiotherapy by downregulating Rad51 expression.
Therefore, the present study hypothesized that BER
could sensitize BC to GEM by regulating Rad51 expression. The aim
of the present study was to evaluate the potential effects of BER
on GEM-induced cytotoxicity in BC and to explore the possible
underlying mechanisms, which may represent a novel therapeutic
agent or target for BC treatment.
Materials and methods
Cell line and culture
Human BC cell lines 5637 and T24 (The Cell Bank of
Type Culture Collection of The Chinese Academy of Sciences) were
cultured as a monolayer in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 100 mg/ml penicillin-streptomycin in an
incubator at 37°C with a humidified atmosphere of 5% CO2
and 95% air. BER (Beijing Solarbio Science & Technology Co.,
Ltd.) and GEM (MedChemExpress) were dissolved in DMSO. The maximum
concentration (v/v) of DMSO in the final medium was 0.1%. For in
vivo experiments, BER was suspended in water supplemented with
0.5% carboxymethyl cellulose sodium (CMC-Na) and stored at 4°C. All
experiments were conducted during the exponential growth phase of
the cells.
Cell viability assay
Cell viability was examined using a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). In total,
2×103 cells in 100 µl medium/well were seeded into
96-well plates and incubated overnight at 37°C. These cells were
then treated with increasing concentrations of GEM (0, 5, 10, 20,
30, 40, 50 and 60 nM) or BER (0, 1, 5, 10, 20, 40, 80 and 160 µM)
for 48 or 72 h at 37°C. CCK-8 reagent was added to the culture
medium with the ratio 1:10 and incubated for an additional 1 h. An
ELISA plate reader (Bio-Rad Laboratories, Inc.) was used to measure
the optical density in each well at 450 nm. The relative percentage
of surviving cells was used to evaluate the influence of GEM or BER
on cell viability. According to the results, the cells were then
treated with BER (20 µM for 5637 cells and 10 µM for T24 cells) and
GEM (40 nM for 5637 cells and 30 nM for T24 cells) alone or
together for 48 h at 37°C. The cells in the control group were
cultured in RPMI-1640 medium containing 10% FBS without drugs for
48 h at 37°C. The optical density was measured as aforementioned
after CCK-8 was added. PI3K inhibitor (LY294002; 10 µM; Beyotime
Institute of Biotechnology) was used to block PI3K/Akt pathway
activation for 48 h at 37°C.
Cell apoptosis assay
The FITC Annexin V Apoptosis Detection kit (BD
Biosciences) was used to evaluate cell apoptosis. In brief, a total
of 1×105 cells treated with GEM or/and BER were
harvested, trypsinized and centrifuged at room temperature at 1,000
× g for 5 min. After a washing step with PBS, cells were
re-suspended in 0.1 ml binding buffer. Cells were incubated in the
dark for 15 min at room temperature after 5 µl Annexin V-FITC and 5
µl PI were added. A BD FACSCalibur flow cytometer (BD Biosciences)
was used to measure cell apoptosis at 1 h after 0.4 ml binding
buffer was added. Flow cytometry data was analyzed using FlowJo
Software V10 (FlowJo LLC). The apoptotic rate was calculated as the
percentage of early plus late apoptotic cells.
ROS detection
BC cells treated with drugs were incubated with 10
µM 2′,7′-dichlorofluorescein diacetate (DCFH-DA) solution (Beyotime
Institute of Biotechnology) for 30 min at 37°C in the dark. After
incubation, the cells were harvested by trypsinization and washed
with PBS. The fluorescence intensity was detected by flow cytometry
(BD Biosciences).
Wound-healing assay
A total of 5×105 cells were seeded into a
6-well plate and incubated for 24 h at 37°C when the cells had
reached a confluence of >90%, before a sterile 200-µl pipette
tip was used to create a scratch in each well. After washing three
times with PBS, cells were cultured with GEM or/and BER in
serum-free medium for another 24 h. Images were captured at 0 and
24 h using a fluorescence microscope (OLYMPUS IX73; Olympus
Corporation). The wound width was measured and analyzed using
ImageJ software (v1.53, National Institutes of Health). The cell
migration rate was calculated as follows: (initial scratch width -
scratch width at the time of experiment) / initial scratch width
×100%.
Bioinformatics analysis
Rad51 mRNA expression data in BC were gathered from
the Sanchez-Carbayo Bladder 2 datasets in the Oncomine database
(https://www.oncomine.org) (33). Gene Expression Profiling
Interactive Analysis (GEPIA) is an online application that can be
used to analyze the differential expression of genes in cancer and
normal tissues (http://gepia.cancer-pku.cn/) (34). GEPIA is a web-based tool for
analyzing normal and tumor sample RNA sequencing data based on The
Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GETx)
data. In the present study, Rad51 mRNA expression in BC in the
GEPIA database was also observed.
Western blot analysis
The cells were cultured in 6-well plates and then
harvested after 48 h of treatment with GEM and/or BER. The cells
were then washed twice with PBS, and RIPA lysis buffer (Beyotime
Institute of Biotechnology) was used to lyse the cells. After
centrifugation at 10,000 × g for 15 min at 4°C, a BCA Protein Assay
kit (Beyotime Institute of Biotechnology) was used to determine the
concentration of the protein supernatant. In total, 50 µg protein
was fractionated by 8–12% SDS-PAGE and subsequently transferred
onto PVDF membranes. After blocking with 5% non-fat milk in TBS
with 0.05% Tween-20 at room temperature for 2 h, the membranes were
incubated overnight with the respective primary antibodies at 4°C.
At room temperature, the protein membranes were then exposed for an
additional 2 h with secondary antibodies and detected using an ECL
kit (Cytiva). Densitometry was performed by using ImageJ software
(v1.53, National Institutes of Health). The specific antibodies
used for western blot analysis were as follows: Anti-Akt (1:1,000;
cat. no. 4691; Cell Signaling Technology, Inc.);
anti-phosphorylated (p)-Akt (1:2,000; Ser473; cat. no. 4060; Cell
Signaling Technology, Inc.); anti-Rad51 (1:1,000; cat. no. 8875;
Cell Signaling Technology, Inc.); anti-GAPDH (1:1,000; cat. no.
5174; Cell Signaling Technology, Inc.); and the goat anti-rabbit
IgG secondary antibody (1:5,000; cat. no. ab6721; Abcam).
Transfection of expression plasmids
and small interfering RNA (siRNA)
Exponentially growing 5637 and T24 cells were plated
for 18 h in complete RPMI-1640 medium and the cells were
transfected with constitutively active Akt expression plasmid
(pcDNA3.1-myr-Akt; 1 µg plasmid/1×105 cells; Shanghai
Yaji Biotechnology Co., Ltd.), pcDNA3.1(+)-Rad51 plasmid (Rad51 OE;
1 µg plasmid/1×105 cells; Wuhan Yipu Biological
Technology Co., Ltd.) or siRNA targeting Rad51 (200 nM) at 37°C for
24 h using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
The pcDNA3.1(+) empty vector plasmid or negative control scramble
siRNA were used as negative controls. The sense-strand sequences of
siRNA duplexes for Rad51 and scrambled (as a control) (Wuhan Yipu
Biological Technology Co., Ltd.) were 5′-UGUAGCAUAUGCUCGAGCG-3′ and
5′-GCGCGCUUUGUAGGATTCG-3′, respectively. At 48 h post-transfection,
the cells were harvested and used for subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA of BC cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. RNA quality and
concentration were quantified using a NanoDrop™ 2000
Spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.). cDNA was synthesized from 1 µg total RNA at 42°C for 1 h and
70°C for 10 min using a PrimeScript™ RT reagent kit (Takara Bio,
Inc.). qPCR was then performed using a SYBR Premix Ex Taq™ kit
(Takara Bio, Inc.) in an ABI Prism 7500 Sequence Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions for qPCR were as follows: 95°C for 5 min,
followed by 40 cycles at 95°C for 10 sec and 60°C for 30 sec.
β-actin expression was used as the internal control. The relative
gene expression was calculated using the 2−ΔΔCq method
(35). The primer sequences are as
follows: β-actin forward, 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′ and
reverse, 5′-CACCTTCTACAATGAGCTGCGTGTG-3′; and Rad51 forward,
5′-CTTTGGCCCACAACCCATTTC-3′ and reverse,
5′-ATGGCCTTTCCTTCACCTCCAC-3′.
In vivo xenograft experiments
All animal experiments were approved by Jinan
Central Hospital Experimental Animal Welfare Ethics Review
Committee (Jinan, China; approval no. JNCH2021-19) and all
experiments were performed in accordance with the Guide for the
Care and Use of Laboratory Animals (36). In total, 12 BALB/c female nude mice
(age, 5 weeks old; body weight, 14–16 g; Beijing Vital River
Laboratory Animal Technology Co., Ltd.) were kept in specific
pathogen-free conditions and had access to sterilized food and
filtered water freely. The vivarium was maintained at 23–25°C with
a 12-h light/dark cycle and humidity at 40–70%. After 1 week of
adjustable feeding, 5×106 5637 cells suspended in 200 µl PBS were
subcutaneously injected into the flanks of mice. Once tumor masses
became established and palpable, mice were randomized into the
following four groups (three mice per group): i) Control group,
which were orally gavaged with CMC-Na daily and intraperitoneally
injected with PBS weekly; ii) BER group, which were orally gavaged
with BER at 100 mg/kg/day and intraperitoneally injected with PBS
weekly; iii) GEM group, which were orally gavaged with CMC-Na daily
and intraperitoneally injected with GEM dissolved in PBS at 150
mg/kg/week; and iv) BER + GEM group, which were orally gavaged with
BER at 100 mg/kg/day and intraperitoneally injected with GEM at 150
mg/kg/week. After initial detection, tumor volumes were evaluated
every 4 days. Tumor sizes were measured with a digital caliper and
tumor volume in mm3 was calculated using the formula
volume: tumor volume = (width)2 × length × 3.14/6
(37). The maximum tumor diameter
observed in the present study was 12.41 mm. After 4 weeks, mice
were euthanized by CO2 asphyxiation using a 30%
displacement rate of cage volume (30 l)/min before the xenografts
were harvested and weighed.
Immunohistochemical (IHC)
analysis
The xenografts were fixed with 4% paraformaldehyde
for 12 h at room temperature, embedded with paraffin and sectioned
at 4-µm each. Bovine serum albumin (5%; Beyotime Institute of
Biotechnology) was used to block non-specific binding at 37°C for
30 min. IHC staining of Ki67 was performed using a Ventana
Benchmark XT Staining system (Roche Diagnostics) on the sections
with an anti-Ki67 antibody (ready-to-use without further dilution;
cat. no. RMA-0542; Fuzhou Maixin Biotech Co., Ltd.) overnight at
4°C with gentle shaking. Then, the sections were incubated with the
horseradish peroxidase-conjugated goat anti-mouse IgG secondary
antibody (1:50; cat. no. A0216; Beyotime Institute of
Biotechnology) for 2 h at room temperature. Images were captured
using a light microscope (LEICA DM4000; Leica Microsystems, Inc.).
The nuclear expression of Ki67 was manually counted for each tumor
in the area with the highest density of Ki67-positive nuclei (‘hot
spots’).
Statistical analyses
Each experiment was repeated ≥3 times. Data are
presented as the mean ± SD. All data were graphed using GraphPad
Prism 8.0 (GraphPad Software, Inc.) and analysis was performed
using SPSS 19.0 software (IBM Corp.). Unpaired Student's t-test or
one-way ANOVA followed by Tukey's post hoc test were performed to
evaluate differences between or among groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
BER enhances the inhibitory effects of
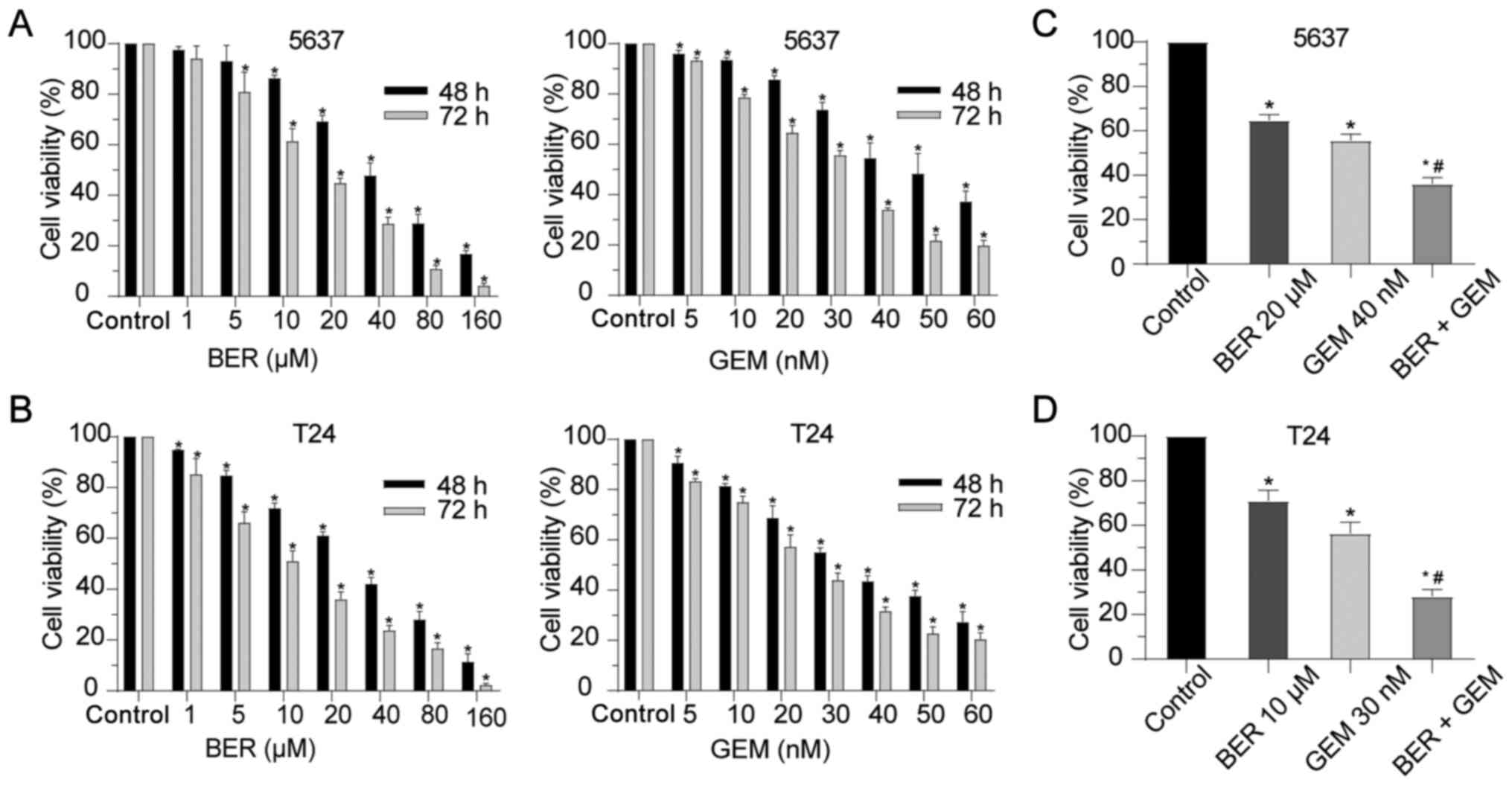
GEM on BC cell viability in vitro
5637 and T24 BC cells were exposed to various
concentrations of BER or GEM for 48 and 72 h. CCK-8 assay was then
performed to assess cell viability. BER or GEM caused time- and
dose-dependent reductions of BC cell viability (Fig. 1A and B). The IC50 of BER
was calculated to be 33.29 µM for 5637 cells and 28.52 µM for T24
cells at 48 h, whilst the IC50 of GEM was calculated to
be 43.69 nM for 5637 cells and 33.59 nM for T24 cells at 48 h.
To further optimize the response of BC cells to GEM
and reduce potential side effects, GEM was combined with BER to
investigate any possible effects on cell viability. According to
results from the CCK-8 assay, the proper concentration of GEM was
set at 40 nM for 5637 cells and 30 nM for T24 cells, whilst that of
BER was set at 20 µM for 5637 cells and 10 µM for T24 cells. The
cells were then exposed to GEM with or without BER for 48 h. As
shown in Fig. 1C and D, cell
viability in the BER + GEM group was significantly lower compared
with that in the GEM group in both cell lines. These data indicated
that BER enhanced the cytotoxic effect of GEM in vitro.
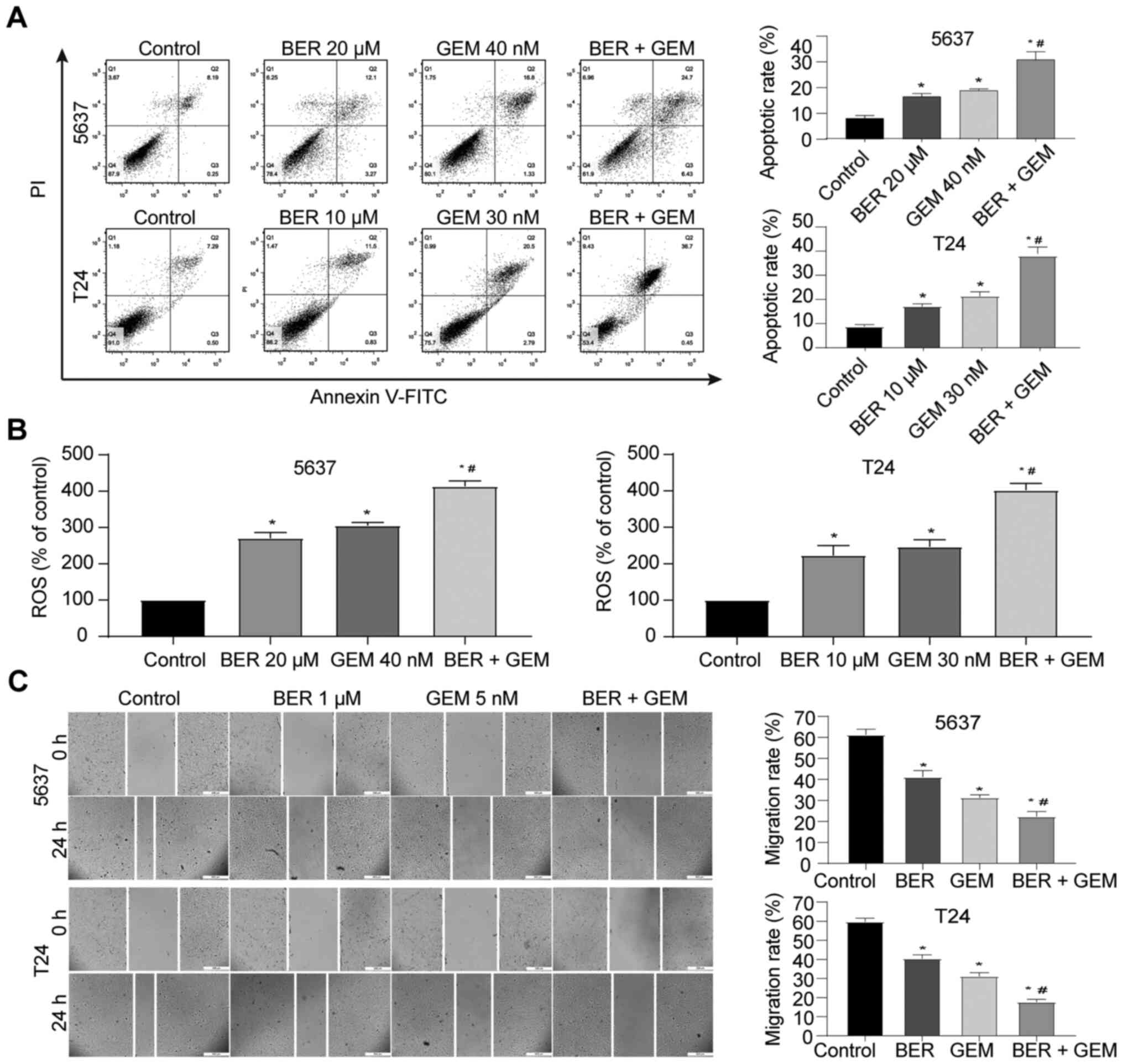
BER enhances GEM-induced apoptosis,
activation of ROS generation and inhibition of migration in
vitro
To further explore the potential mechanism of
action, the apoptotic rate and intracellular ROS were evaluated by
flow cytometry. Although both single-drug treatments increased the
apoptotic rate and the levels of intracellular ROS, BER + GEM
treatment significantly increased apoptosis and ROS compared with
that following GEM treatment alone in both cell lines (Fig. 2A and B). Wound-healing assay
results indicated that single-drug treatment and BER + GEM
treatment inhibited the migratory capacities of 5637 and T24 cells,
where the migration rate in the BER + GEM group was lower compared
with that in the GEM-only group (Fig.
2C). These results suggested that BER enhanced GEM-induced
apoptosis and inhibition of migration.
BER reverses GEM-induced activation of
PI3K/Akt signaling and upregulation of Rad51 expression in BC
cells
The Sanchez-Carbayo Bladder 2 datasets in Oncomine
indicated that Rad51 expression was upregulated in BC compared with
that in normal bladder tissues (Fig.
3A). The same datasets also showed that Rad51 expression was
upregulated in superficial BC (SBC) and infiltrating BC (IBC)
tissues compared with that in normal bladder tissues, but there was
no significant difference in Rad51 expression between SBC and IBC
tissues (Fig. 3B). Consistently,
GEPIA analysis also indicated that Rad51 expression was upregulated
in BC compared with that in normal bladder tissues (Fig. 3C), but there was no difference in
the expression levels among stages II–IV (Fig. 3D).
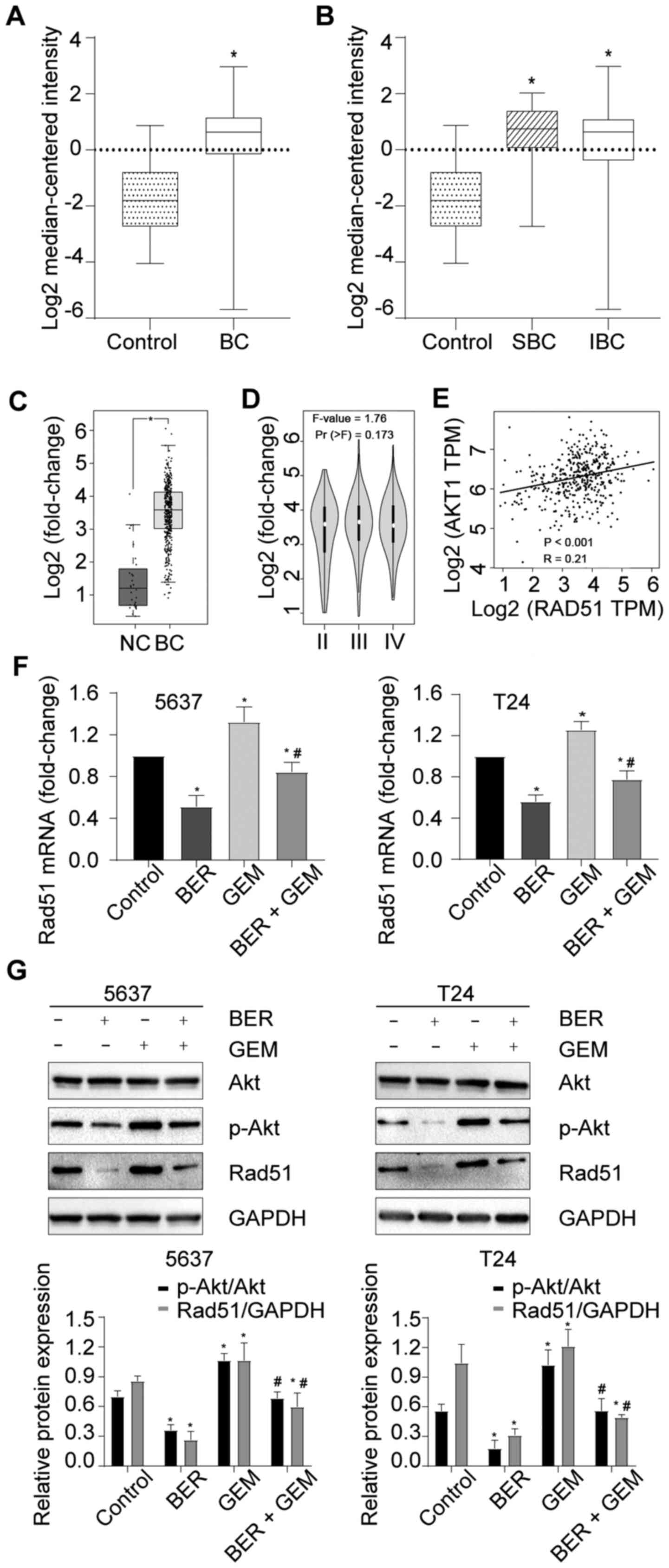 | Figure 3.BER attenuates GEM-induced activation
of PI3K/Akt signaling and upregulation of Rad51 expression in BC.
(A) Rad51 mRNA expression data in BC and control tissues from the
Oncomine database. (B) Rad51 mRNA expression data in SBC, IBC and
control tissues from the Oncomine database. (C) Rad51 mRNA
expression data in BC and normal control tissues as analyzed on
GEPIA. (D) Rad51 mRNA expression data at different stages of BC, as
analyzed using GEPIA. (E) Correlation analysis between Rad51 and
Akt1 expression on GEPIA. (F) mRNA expression levels of Rad51 in BC
cells after treatment with GEM and/or BER. (G) Protein levels of
Rad51, Akt and p-Akt in BC cells after treatment with GEM and/or
BER. *P<0.05 vs. control; #P<0.05 vs. GEM group.
BER, berberine; GEM, gemcitabine; Rad51, RAD51 recombinase; BC,
bladder cancer; SBC, superficial bladder cancer; IBC, infiltrating
bladder cancer; GEPIA, Gene Expression Profiling Interactive
Analysis; p-, phosphorylated; NC, negative control. |
PI3K/Akt signaling has been reported to regulate
Rad51 expression in human NSCLC cells (31,38).
GEPIA analysis also found that there was a weak, but significant
positive correlation between Rad51 and Akt1 expression in BC
(R=0.21; Fig. 3E). Subsequently,
it was found that the expression levels of Rad51 mRNA, p-Akt and
Rad51 protein were all down- and upregulated in the BER and GEM
groups, respectively (Fig. 3F and
G). In addition, significant reductions were also observed in
the BER + GEM group compared with those in the GEM-only group
(Fig. 3F and G). These data
suggested that BER attenuated GEM-induced activation of the
PI3K/Akt pathway and upregulation of Rad51 expression.
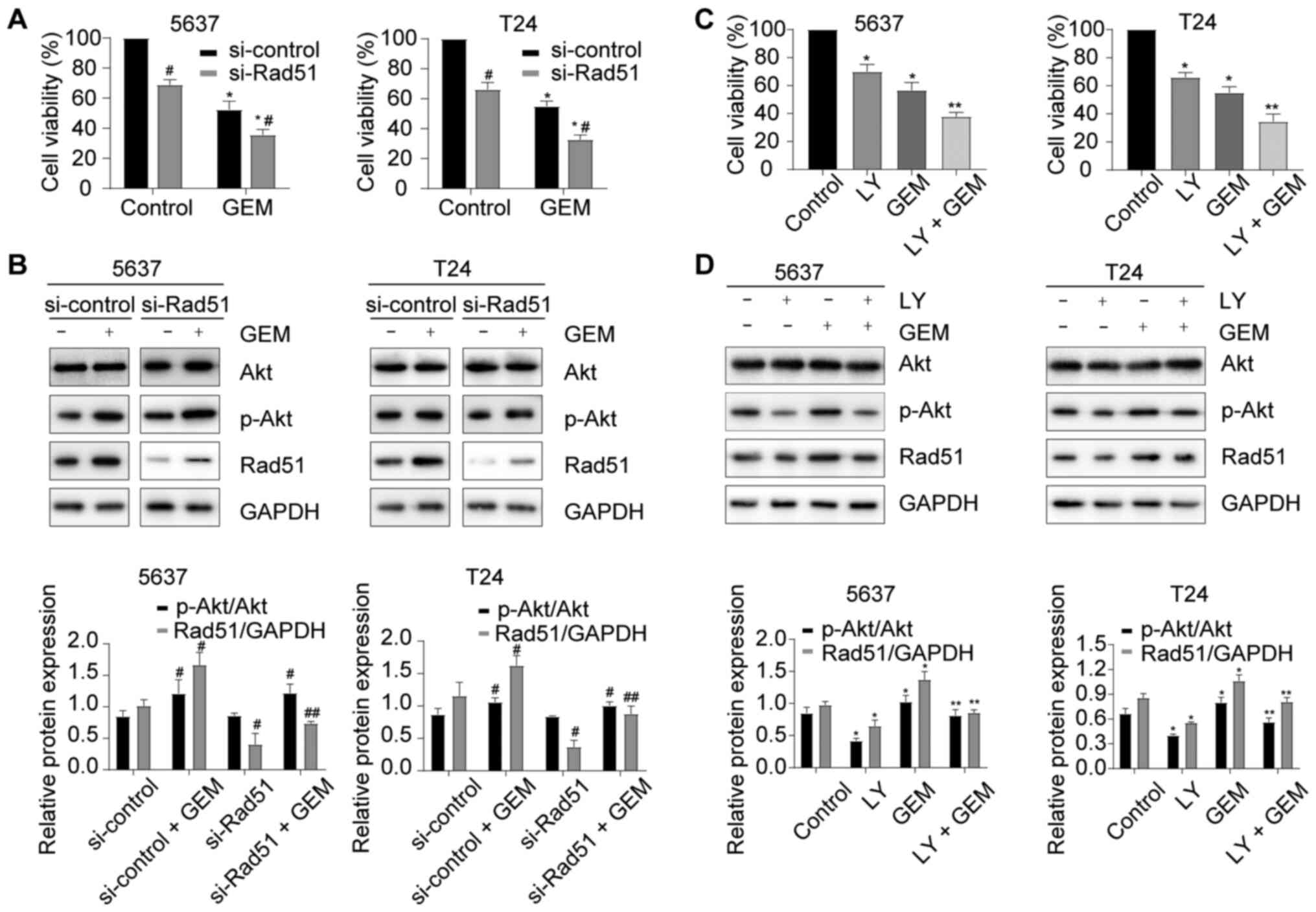
Knockdown of Rad51 enhances
GEM-induced cytotoxicity and inactivation of the PI3K/Akt pathway
enhances GEM-induced cytotoxicity by downregulating Rad51
expression
To explore the role of Rad51 and the PI3K/Akt
pathway in GEM-induced cytotoxicity, Rad51 was knocked down using
si-Rad51. As shown in Fig. 4A and
B, si-Rad51 significantly increased the sensitivity of cells to
GEM compared with the si-control group (P<0.05) and decreased
Rad51 protein expression induced by GEM, but did not interfere with
the GEM-induced activation of the PI3K/Akt pathway. A PI3K
inhibitor (LY294002) was used to block PI3K/Akt pathway activation
in GEM-treated BC cells. LY294002 enhanced GEM-induced cytotoxicity
(Fig. 4C) and decreased the
activation of the PI3K/Akt pathway and Rad51 protein expression
induced by GEM (Fig. 4D). These
results indicated that inactivation of the PI3K/Akt pathway
enhanced GEM-induced cytotoxicity by downregulating Rad51
expression.
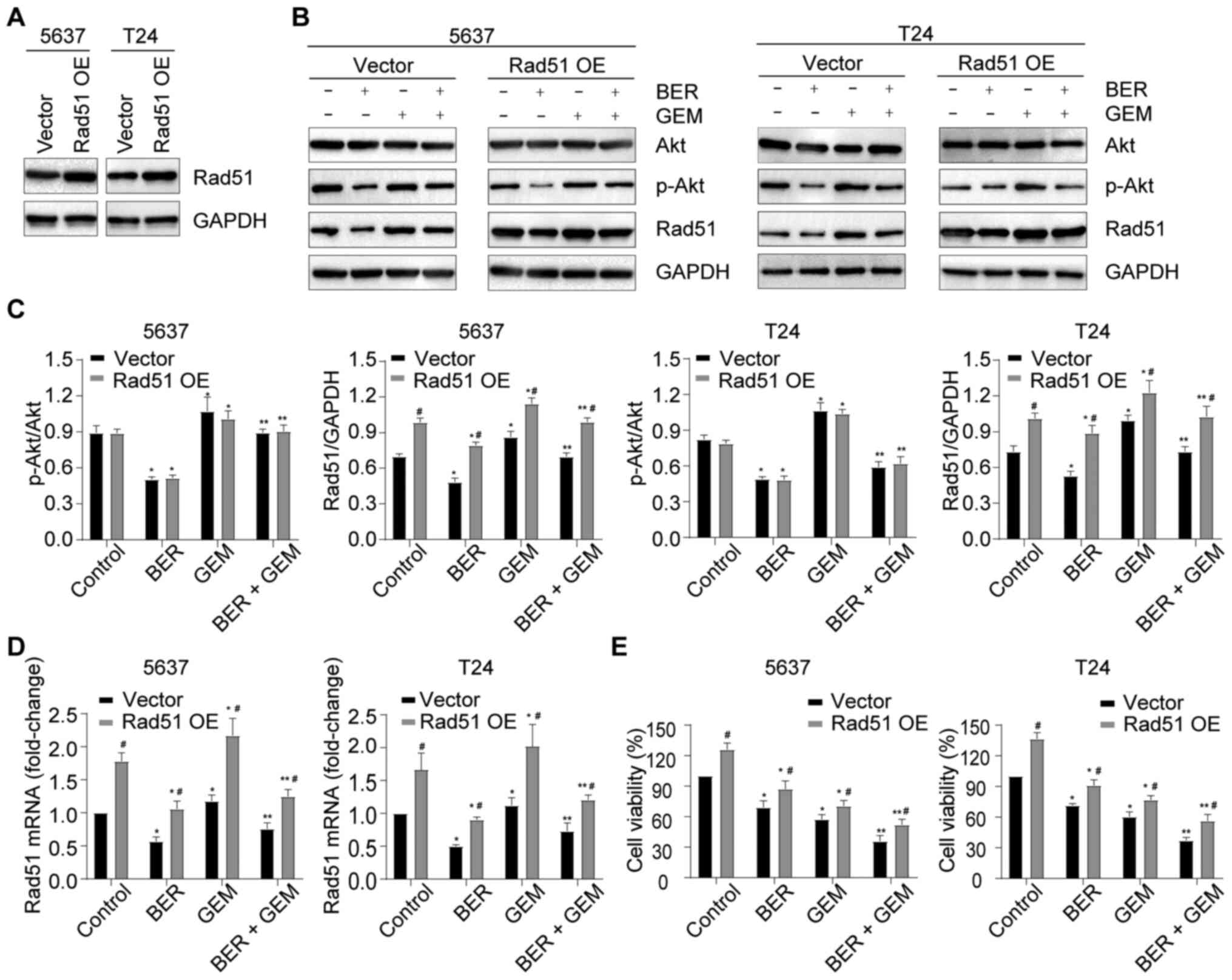
BER enhances GEM-induced cytotoxicity
by downregulating Rad51 expression
To explore the role of Rad51 in the BER-mediated
enhancement of GEM-induced cytotoxicity, 5637 and T24 cells were
transiently transfected with Rad51 OE plasmid, which upregulated
Rad51 protein expression (Fig.
5A). The cells were then treated with GEM or BER either alone
or in combination. It was observed that Rad51 OE reversed the
BER-mediated reduction of Rad51 expression at both the mRNA and
protein levels in addition to restoring the reduction in cell
viability in the BER + GEM group (Fig.
5B-E). However, the ratio of p-Akt/Akt did not change (Fig. 5B and C). Therefore, these findings
suggested that BER potentiated GEM-induced cytotoxicity by
downregulating Rad51 expression, but PI3K/Akt signaling is unlikely
to be regulated by Rad51.
BER enhances GEM-induced cytotoxicity
and downregulates Rad51 expression by inactivating the PI3K/Akt
pathway
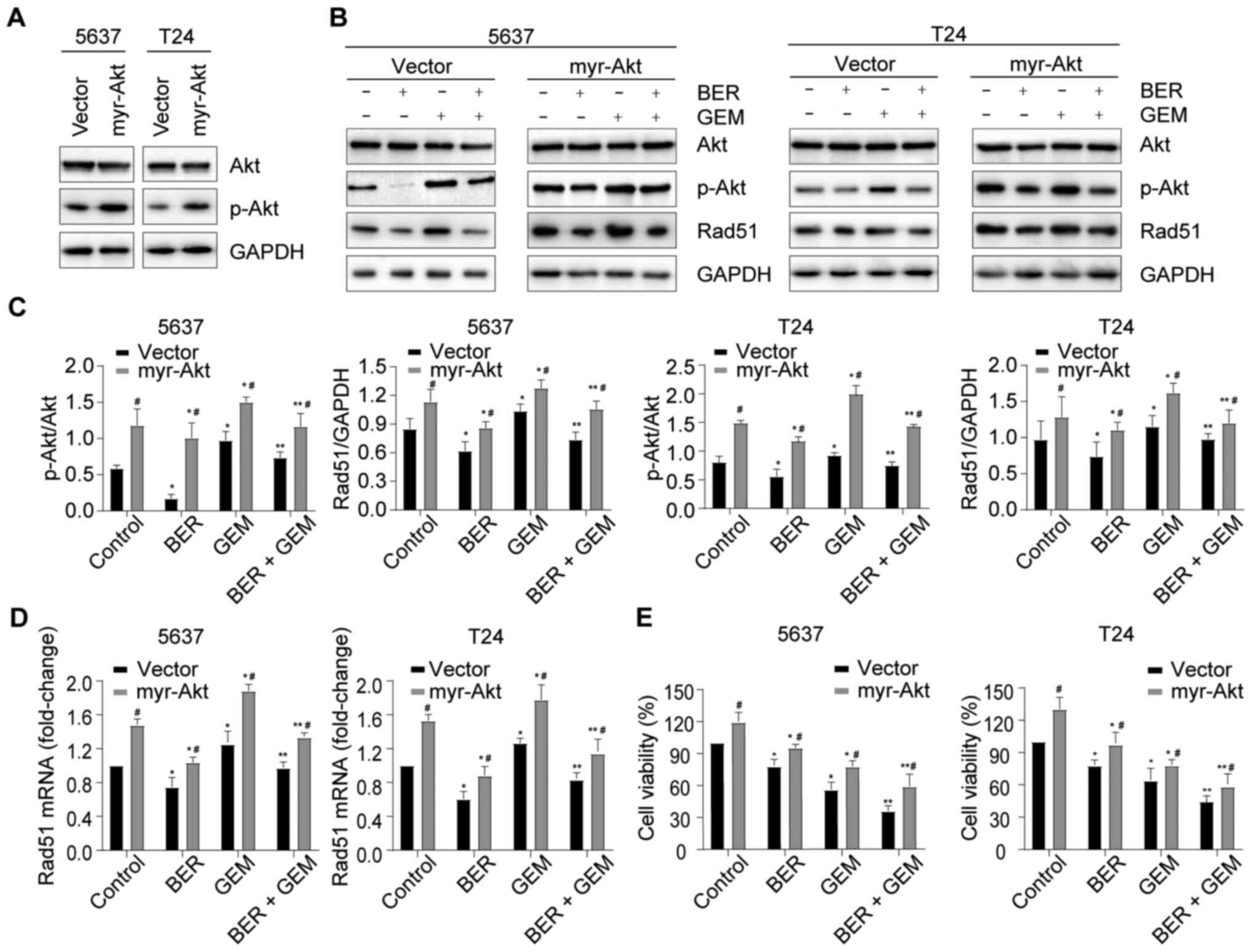
To examine whether the enhancement induced by BER on
GEM-mediated cytotoxicity and BER-induced Rad51 downregulation in
BC cells were mediated by the PI3K/Akt pathway, BC cells were
transiently transfected with myr-AKT plasmid, which upregulated the
phosphorylation of Akt (Fig. 6A).
It was observed that increased Akt phosphorylation upregulated
Rad51 expression at both the protein and mRNA levels in all groups
and reversed the BER-induced decrease in Rad51 expression in the
BER + GEM group (Fig. 6B-D),
suggesting that BER downregulated Rad51 expression by inactivating
the PI3K/Akt pathway. Increased Akt phosphorylation also improved
cell viability in all groups, whilst negating the BER-induced
reduction in cell viability in the BER + GEM group (Fig. 6E), suggesting that BER enhanced
GEM-induced cytotoxicity by inactivating the PI3K/Akt pathway.
These results indicated that BER enhanced GEM-induced cytotoxicity
and downregulated Rad51 expression by inactivating the PI3K/Akt
pathway.
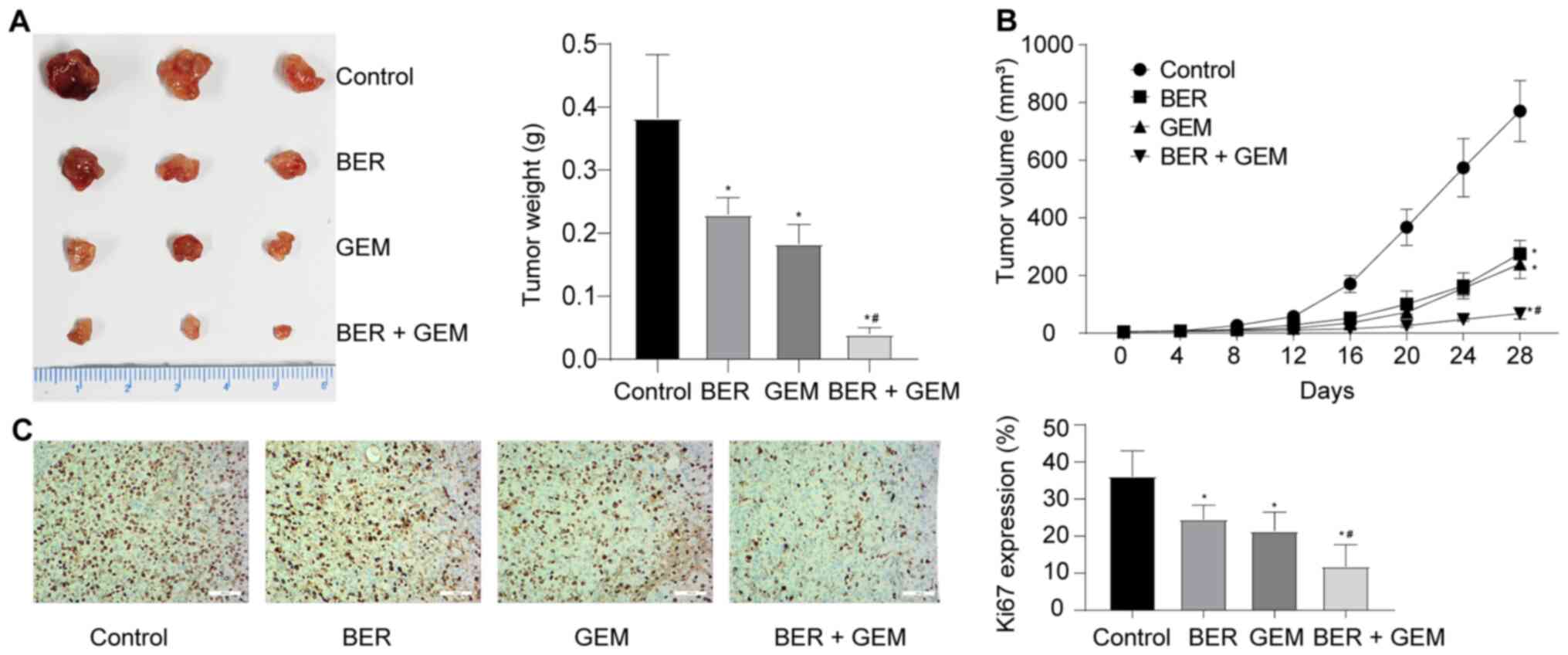
BER enhances the anti-proliferative effects of GEM
in vivo. Results from the xenograft mouse assays in vivo
revealed that BER + GEM treatment significantly reduced the tumor
weight and volume compared with those in mice treated with GEM
(Fig. 7A and B). Cell
proliferation in the tumor was assessed by measuring Ki67 staining
in the xenograft tumor tissues. IHC analysis revealed that the
expression levels of Ki67 in the xenografted tumors were
significantly decreased in the BER + GEM group compared with those
in tissues from mice treated with GEM alone Fig. 7C), suggesting that BER enhanced the
anti-proliferative effects of GEM in vivo.
Discussion
Despite the widespread use of GEM for BC, poor
prognosis and severe adverse reactions underscore the need for
identifying novel therapeutic agents and targets for this disease.
BER has been shown to exhibit a broad spectrum of anticancer
activities on numerous types of human cancers, including BC cells
(22). BER has been previously
demonstrated to sensitize the response of BC cells to epirubicin
(23), indicating that BER may
serve an important role in modulating BC drug sensitivity. The main
objectives of the present study were to determine the effects of
BER on GEM-induced cytotoxicity in human BC cells and if any were
found, to identify the possible molecular signaling pathways
involved.
The present study showed that BER and GEM could
inhibit the viability of 5637 and T24 BC cells in a time- and
dose-dependent manner, such that BER potentiated the inhibitory
effects of GEM on the viability and migration of BC cells.
Suppression of growth is closely associated with apoptosis
(39). Apoptosis is primarily
modulated by specific relevant proteins and is characterized by a
series of intracellular events, including the collapse of
mitochondrial potential, caspase activation and DNA fragmentation
(39). BER and GEM have both been
previously reported to promote the apoptosis of BC cells (22,40,41).
ROS is known to induce apoptosis (42). Takeuchi et al (43) reported that methyl 2-cyano-3,
11-dioxo-18b-olean-1, 12-dien-30-oate induced apoptosis in BC cells
through the induction of ROS. BER and GEM have been reported to
induce ROS (23,44). Therefore, it was hypothesized in
the current study that BER could enhance the pro-apoptotic effects
of GEM by inducing ROS. Flow cytometry results reported that the
apoptotic rates and the levels of intracellular ROS of 5637 and T24
cells in the combination group were increased compared with those
in the GEM-only group.
DSBs are critical cell fate defining events that can
occur in the cell nucleus. As an intercalator, BER can induce DSBs
in the DNA, which in turn activate p53 and ataxia telangiectasia
mutated to activate apoptosis (45). Rad51 is a pivotal molecule in the
process of DNA damage repair and a crucial element for HR (25). Rad51 expression was found to be
upregulated in SBC and IBC tissues compared with that in normal
bladder tissues, according to the data extracted from the Oncomine
database (Sanchez-Carbayo Bladder 2) and analysis by GEPIA in the
present study. In previous studies, Rad51 expression has been found
to be upregulated in pancreatic adenocarcinoma, lung tumors, breast
cancer and ovarian cancer, such that downregulation of Rad51
expression could enhance the sensitivity to radio- or chemotherapy
(26–30). Consistent with previous reports
(31,32), the present study found that GEM
could upregulate the protein and mRNA expression levels of Rad51,
but BER mediated opposite effects. Furthermore, BER attenuated the
GEM-induced upregulation of Rad51 at both the mRNA and protein
levels.
In the present study, it was also found that the
GEM-induced upregulation of PI3K/Akt signaling, represented by Akt
phosphorylation, was attenuated by BER. GEPIA analysis also found a
weak, but significant positive correlation between Rad51 and Akt1
expression in BC. It was therefore hypothesized that the PI3K/Akt
pathway and Rad51 may be involved in the additive physiological
effects of BER on GEM in BC cells. Ko et al (38) previously reported that astaxanthin
downregulated Rad51 expression and Akt phosphorylation, whilst
reducing cell viability in NSCLC, the effects of which were in turn
enhanced by the PI3K inhibitor or Rad51 siRNA transfection.
However, these aforementioned inhibitory effects of astaxanthin
could be suppressed by Akt phosphorylation, suggesting that the
PI3K/Akt pathway can upregulate the expression of Rad51. In another
study, Tsai et al (31)
found that GEM could upregulate the levels of Akt phosphorylation
and Rad51 expression, but treatment with the PI3K inhibitor
attenuated this GEM-induced upregulation of Rad51. By contrast,
inactivation of the PI3K/Akt pathway or knocking down Rad51
expression could significantly increase GEM-induced cytotoxicity in
NSCLC. A recent review documented that BER exerts promising
anticancer effects, mainly by inhibiting the PI3K/Akt pathway
(46). Consistently, the present
study demonstrated that si-Rad51 enhanced GEM-induced cytotoxicity
and inactivation of the PI3K/Akt pathway by LY294002 enhanced
GEM-induced cytotoxicity by downregulating Rad51 expression. Akt
phosphorylation and Rad51 expression were upregulated after GEM
treatment in BC cells, in a manner that could be reversed by BER,
which in turn led to the enhancement of GEM-induced cytotoxicity.
In addition, overexpression of Rad51 attenuated this potentiation
effect BER on GEM-induced cytotoxicity, suggesting that BER
enhanced GEM-induced cytotoxicity by downregulating Rad51
expression. Activation of the PI3K/Akt pathway also upregulated the
expression of Rad51 and increased cell viability in all groups,
whilst reversing BER-induced reduction in Rad51 expression and cell
viability in the BER + GEM group. These observations suggested that
BER regulated Rad51 expression and cell viability via the PI3K/Akt
pathway in GEM-treated BC cells.
Although the present study found that BER and GEM
could inhibit BC cell viability, unlike BER, GEM can also
upregulate the phosphorylation of Akt and Rad51 expression through
other mechanisms that require further study. In addition, Takeuchi
et al (41) reported that
sequential GEM followed by tamoxifen treatment caused the largest
increase in DNA fragmentation in BC cells, meaning that the timing
of adjunct administration is critical for enhancing the effect of
any co-treatment. Due to a lack of time, the present study did not
determine the timing of adjunct administration or verify the
expression of Rad51 in the xenograft tissues in vivo, which
were limitations of this study. Despite these limitations, this
study demonstrated the potentiating effects of BER on GEM in BC and
its underlying mechanism. Nevertheless, further studies are
warranted to determine the downstream mechanism of the PI3K/Akt
pathway in regulating Rad51 expression in BC cells.
In conclusion, the findings in the present study
suggested that BER functioned as a GEM sensitizer for BC
chemotherapy, where Rad51, downstream of the PI3K/Akt signaling
pathway, served a critical role. Therefore, a potential
combinational adjuvant treatment strategy involving BER may restore
the clinical efficacy of current treatment options for BC, such
that Rad51 may represent a potential therapeutic target in patients
with BC.
Acknowledgements
The authors would like to thank Dr Xianguang Meng at
the Department of Dermatology of Jinan Central Hospital (Jinan,
China) and Ms. Xiaowen Xia at the Laboratory Animal Center of Jinan
Central Hospital (Jinan, China) for their experimental
assistance.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant no. 81672522).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG, KY and YF designed the study. XG, DF and XL
carried out the experiments. XG, ZF and JL analyzed the data. XG,
KY and YF confirm the authenticity of all raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Jinan
Central Hospital Experimental Animal Welfare Ethics Review
Committee (Jinan, China; approval no. JNCH2021-19).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder Cancer Incidence and Mortality: A
Global Overview and Recent Trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Burger M, Compérat EM, Gontero
P, Mostafid AH, Palou J, van Rhijn BWG, Rouprêt M, Shariat SF,
Sylvester R, et al: European Association of Urology Guidelines on
Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) -
2019 Update. Eur Urol. 76:639–657. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gakis G: Management of Muscle-invasive
Bladder Cancer in the 2020s: Challenges and Perspectives. Eur Urol
Focus. 6:632–638. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von der Maase H, Hansen SW, Roberts JT,
Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A,
Lippert CM, et al: Gemcitabine and cisplatin versus methotrexate,
vinblastine, doxorubicin, and cisplatin in advanced or metastatic
bladder cancer: Results of a large, randomized, multinational,
multicenter, phase III study. J Clin Oncol. 18:3068–3077. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu C, Hequn C, Jinbo C, Feng Z, Xiongbing
Z and Jian D: Gemcitabine/cisplatin versus
methotrexate/vinblastine/doxorubicin/cisplatin for muscle-invasive
bladder cancer: A systematic review and meta-analysis. J Cancer Res
Ther. 14:1260–1265. 2018.PubMed/NCBI
|
|
6
|
Isono M, Hoffmann MJ, Pinkerneil M, Sato
A, Michaelis M, Cinatl J Jr, Niegisch G and Schulz WA: Checkpoint
kinase inhibitor AZD7762 strongly sensitises urothelial carcinoma
cells to gemcitabine. J Exp Clin Cancer Res. 36:12017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie D, Zhang H and Shang C: Long
non-coding RNA CDKN2B antisense RNA 1 gene inhibits Gemcitabine
sensitivity in bladder urothelial carcinoma. J Cancer. 9:2160–2166.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Foschini F, Formisano L, Marciano R,
Mozzillo E, Carratù A, Napolitano F, Santaniello A, De Placido P,
Cascetta P, Servetto A, et al: FOLFIRINOX after first-line
gemcitabine-based chemotherapy in metastatic pancreatic cancer: A
mono-institutional experience. Ann Oncol. 30 (Suppl 4):iv52–iv53.
2019. View Article : Google Scholar
|
|
9
|
Zhang X, Wang D, Li Z, Jiao D, Jin L, Cong
J, Zheng X and Xu L: Low-Dose Gemcitabine Treatment Enhances
Immunogenicity and Natural Killer Cell-Driven Tumor Immunity in
Lung Cancer. Front Immunol. 11:3312020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kassouf W, Highshaw R, Nelkin GM, Dinney
CP and Kamat AM: Vitamins C and K3 sensitize human urothelial
tumors to gemcitabine. J Urol. 176:1642–1647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pinto-Leite R, Arantes-Rodrigues R,
Palmeira C, Gaivão I, Cardoso ML, Colaço A, Santos L and Oliveira
P: Everolimus enhances gemcitabine-induced cytotoxicity in
bladder-cancer cell lines. J Toxicol Environ Health A. 75:788–799.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoon CY, Lee JS, Kim BS, Jeong SJ, Hong
SK, Byun SS and Lee SE: Sunitinib malate synergistically
potentiates anti-tumor effect of gemcitabine in human bladder
cancer cells. Korean J Urol. 52:55–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng
X, Su J, Zhou Z, Xu Z, Nilsson S, et al: Baicalein inhibits
prostate cancer cell growth and metastasis via the
caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 406:111–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Z, Liu C, Yan K, Liu J, Fang Z and Fan
Y: Huaier Extract Inhibits Prostate Cancer Growth via Targeting
AR/AR-V7 Pathway. Front Oncol. 11:6155682021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang QL, Lai ML, Zhong YF, Wang AM, Su JK
and Zhang MQ: Antinociceptive effect of berberine on visceral
hypersensitivity in rats. World J Gastroenterol. 19:4582–4589.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin X and Zhang N: Berberine: Pathways to
protect neurons. Phytother Res. 32:1501–1510. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imenshahidi M and Hosseinzadeh H:
Berberine and barberry (Berberis vulgaris): A clinical
review. Phytother Res. 33:504–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohammadinejad R, Ahmadi Z, Tavakol S and
Ashrafizadeh M: Berberine as a potential autophagy modulator. J
Cell Physiol. 234:14914–14926. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang SX, Qi B, Yao WJ, Gu CW, Wei XF,
Zhao Y, Liu YZ and Zhao BS: Berberine displays antitumor activity
in esophageal cancer cells in vitro. World J Gastroenterol.
23:2511–2518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian Y, Zhao L, Wang Y, Zhang H, Xu D,
Zhao X, Li Y and Li J: Berberine inhibits androgen synthesis by
interaction with aldo-keto reductase 1C3 in 22Rv1 prostate cancer
cells. Asian J Androl. 18:607–612. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hou D, Xu G, Zhang C, Li B, Qin J, Hao X,
Liu Q, Zhang X, Liu J, Wei J, et al: Berberine induces oxidative
DNA damage and impairs homologous recombination repair in ovarian
cancer cells to confer increased sensitivity to PARP inhibition.
Cell Death Dis. 8:e30702017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan K, Zhang C, Feng J, Hou L, Yan L, Zhou
Z, Liu Z, Liu C, Fan Y, Zheng B, et al: Induction of G1 cell cycle
arrest and apoptosis by berberine in bladder cancer cells. Eur J
Pharmacol. 661:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhuo Y, Chen Q, Chen B, Zhan X, Qin X,
Huang J and Lv X: Berberine promotes antiproliferative effects of
epirubicin in T24 bladder cancer cells by enhancing apoptosis and
cell cycle arrest. Int J Clin Pharmacol Ther. 55:32–40. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Godin SK, Sullivan MR and Bernstein KA:
Novel insights into RAD51 activity and regulation during homologous
recombination and DNA replication. Biochem Cell Biol. 94:407–418.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haber JE: DNA Repair: The Search for
Homology. BioEssays. 40:e17002292018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nagathihalli NS and Nagaraju G: RAD51 as a
potential biomarker and therapeutic target for pancreatic cancer.
Biochim Biophys Acta. 1816:209–218. 2011.PubMed/NCBI
|
|
27
|
Cortez MA, Valdecanas D, Niknam S, Peltier
HJ, Diao L, Giri U, Komaki R, Calin GA, Gomez DR, Chang JY, et al:
In Vivo Delivery of miR-34a Sensitizes Lung Tumors to Radiation
Through RAD51 Regulation. Mol Ther Nucleic Acids. 4:e2702015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong KJ, Hsu MC and Hung WC: RECK impedes
DNA repair by inhibiting the erbB/JAB1/Rad51 signaling axis and
enhances chemosensitivity of breast cancer cells. Am J Cancer Res.
5:2422–2430. 2015.PubMed/NCBI
|
|
29
|
Wang B, Hou D, Liu Q, Wu T, Guo H, Zhang
X, Zou Y, Liu Z, Liu J, Wei J, et al: Artesunate sensitizes ovarian
cancer cells to cisplatin by downregulating RAD51. Cancer Biol
Ther. 16:1548–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JO, Kang MJ, Byun WS, Kim SA, Seo IH,
Han JA, Moon JW, Kim JH, Kim SJ, Lee EJ, et al: Metformin overcomes
resistance to cisplatin in triple-negative breast cancer (TNBC)
cells by targeting RAD51. Breast Cancer Res. 21:1152019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai MS, Kuo YH, Chiu YF, Su YC and Lin
YW: Down-regulation of Rad51 expression overcomes drug resistance
to gemcitabine in human non-small-cell lung cancer cells. J
Pharmacol Exp Ther. 335:830–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Q, Jiang H, Liu Z, Wang Y, Zhao M, Hao
C, Feng S, Guo H, Xu B, Yang Q, et al: Berberine radiosensitizes
human esophageal cancer cells by downregulating homologous
recombination repair protein RAD51. PLoS One. 6:e234272011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sanchez-Carbayo M, Socci ND, Lozano J,
Saint F and Cordon-Cardo C: Defining molecular profiles of poor
outcome in patients with invasive bladder cancer using
oligonucleotide microarrays. J Clin Oncol. 24:778–789. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45((W1)):
W98–W102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. The National Academies Press (US); Washington, DC:
2011
|
|
37
|
Tomayko MM and Reynolds CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ko JC, Chen JC, Wang TJ, Zheng HY, Chen
WC, Chang PY and Lin YW: Astaxanthin down-regulates Rad51
expression via inactivation of AKT kinase to enhance mitomycin
C-induced cytotoxicity in human non-small cell lung cancer cells.
Biochem Pharmacol. 105:91–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jabbarzadeh Kaboli P, Rahmat A, Ismail P
and Ling KH: Targets and mechanisms of berberine, a natural drug
with potential to treat cancer with special focus on breast cancer.
Eur J Pharmacol. 740:584–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yin H, Yang X, Gu W, Liu Y, Li X, Huang X,
Zhu X, Tao Y, Gou X and He W: HMGB1-mediated autophagy attenuates
gemcitabine-induced apoptosis in bladder cancer cells involving JNK
and ERK activation. Oncotarget. 8:71642–71656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takeuchi H, Mmeje CO, Jinesh GG, Taoka R
and Kamat AM: Sequential gemcitabine and tamoxifen treatment
enhances apoptosis and blocks transformation in bladder cancer
cells. Oncol Rep. 34:2738–2744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miller WH Jr, Schipper HM, Lee JS, Singer
J and Waxman S: Mechanisms of action of arsenic trioxide. Cancer
Res. 62:3893–3903. 2002.PubMed/NCBI
|
|
43
|
Takeuchi H, Taoka R, Mmeje CO, Jinesh GG,
Safe S and Kamat AM: CDODA-Me decreases specificity protein
transcription factors and induces apoptosis in bladder cancer cells
through induction of reactive oxygen species. Urol Oncol.
34:337.e11–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang X, Bai Y, Zhang F, Yang Y, Feng D, Li
A, Yang Z, Li D, Tang Y, Wei X, et al: Targeted Inhibition of P4HB
Promotes Cell Sensitivity to Gemcitabine in Urothelial Carcinoma of
the Bladder. OncoTargets Ther. 13:9543–9558. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu Z, Liu Q, Xu B, Wu J, Guo C, Zhu F,
Yang Q, Gao G, Gong Y and Shao C: Berberine induces p53-dependent
cell cycle arrest and apoptosis of human osteosarcoma cells by
inflicting DNA damage. Mutat Res. 662:75–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang J, Feng W, Li S, Tang H, Qin S, Li
W, Gong Y, Fang Y, Liu Y, Wang S, et al: Berberine Exerts
Anti-cancer Activity by Modulating Adenosine Monophosphate-
Activated Protein Kinase (AMPK) and the Phosphatidylinositol
3-Kinase/Protein Kinase B (PI3K/AKT) Signaling Pathways. Curr Pharm
Des. 27:565–574. 2021. View Article : Google Scholar : PubMed/NCBI
|