|
1
|
Grandis JR, Melhem MF, Gooding WE, Day R,
Holst VA, Wagener MM, Drenning SD and Tweardy DJ: Levels of TGF-α
and EGFR protein in head and neck squamous cell carcinoma and
patient survival. J Natl Cancer Inst. 90:824–832. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Comprehensive Cancer Network:
Clinical Practice Guidelines in Oncology. Head and Neck Cancer.
v1:2017.Available from. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site
|
|
3
|
Lo Nigro C, Denaro N, Merlotti A and
Merlano M: Head and neck cancer: Improving outcomes with a
multidisciplinary approach. Cancer Manag Res. 9:363–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
https://www.cancer.net/cancer-types/head-and-neck-cancer/introduction
|
|
5
|
https://www.uptodate.com/contents/epidemiology-and-risk-factors-for-head-and-neck-cancer?search=epidemiology-and-risk-factors-for-head-and-neck-cancer.&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
|
|
6
|
Hukkanen J, Jacob PII and Benowitz NL:
Metabolism and disposition kinetics of nicotine. Pharmacol Rev.
57:79–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warren GW and Singh AK: Nicotine and lung
cancer. J Carcinog. 12:12013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hecht SS: Tobacco carcinogens, their
biomarkers and tobacco-induced cancer. Nat Rev Cancer. 3:733–744.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doll R and Peto R: The causes of cancer:
Quantitative estimates of avoidable risks of cancer in the United
States today. J Natl Cancer Inst. 66:1191–1308. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
US Department of Health and Human
Services, . Reducing the Health Consequences of Smoking: 25 Years
of Progress. A Report of the Surgeon General; Centers for Disease
Control and Prevention; Atlanta, GA: 1989
|
|
11
|
Secretan B, Straif K, Baan R, Grosse Y, El
Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C,
Galichet L, et al: A review of human carcinogens-Part E: Tobacco,
areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol.
10:1033–1034. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
https://www.cancer.net/cancer-types/head-and-neck-cancer/risk-factors-and-prevention
|
|
13
|
US Department of Health and Human
Services, . How Tobacco Smoke Causes Disease: The Biology and
Behavioral Basis for Smoking-attributable Disease. A Report of the
Surgeon General; Centers for Disease Control and Prevention;
Atlanta, GA: 2010
|
|
14
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Smokeless tobacco and some
tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks
Hum. 89:1–592. 2007.PubMed/NCBI
|
|
15
|
Takahashi H, Ogata H, Nishigaki R, Broide
DH and Karin M: Tobacco smoke promotes lung tumorigenesis by
triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell.
17:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
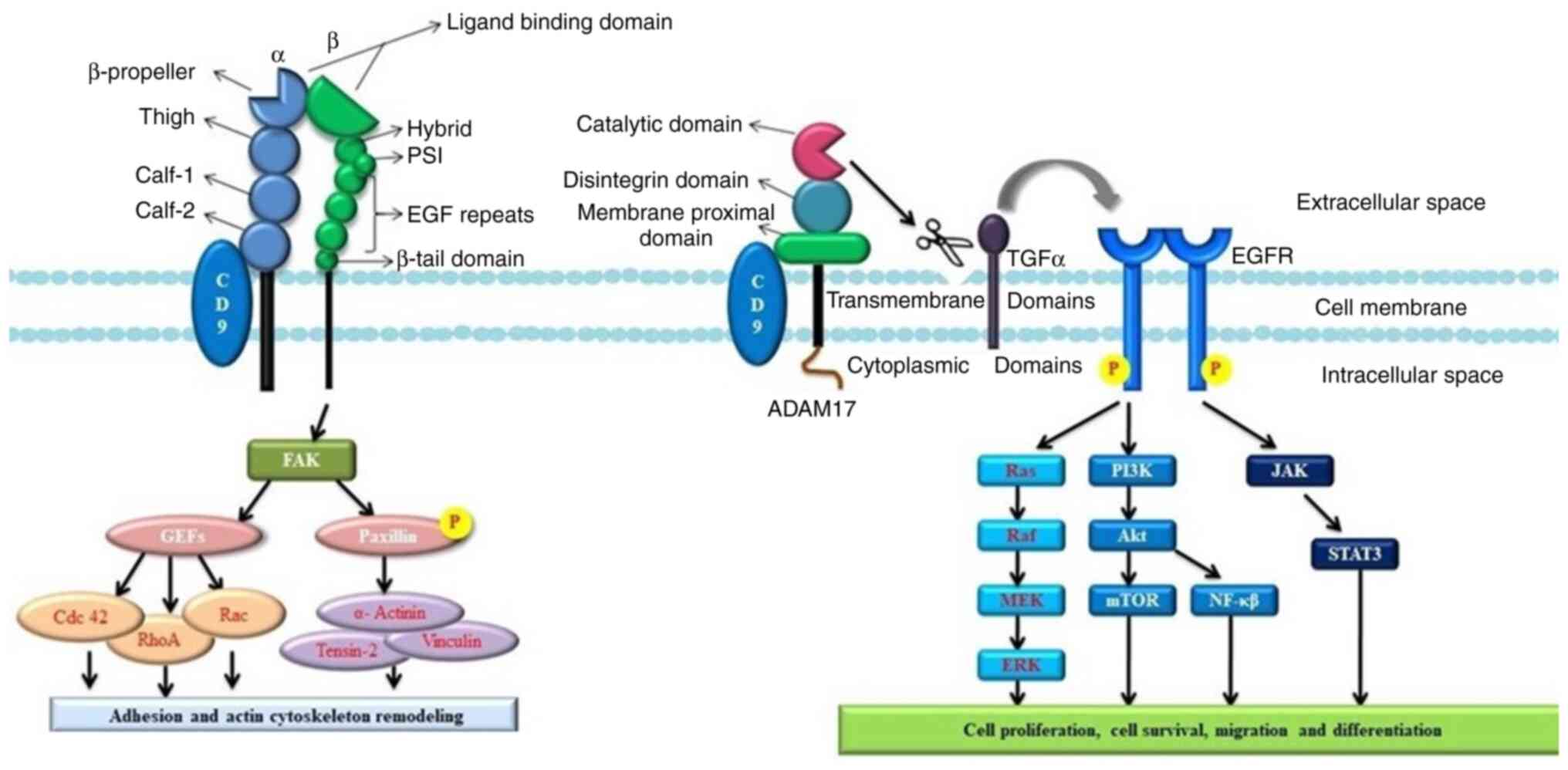
16
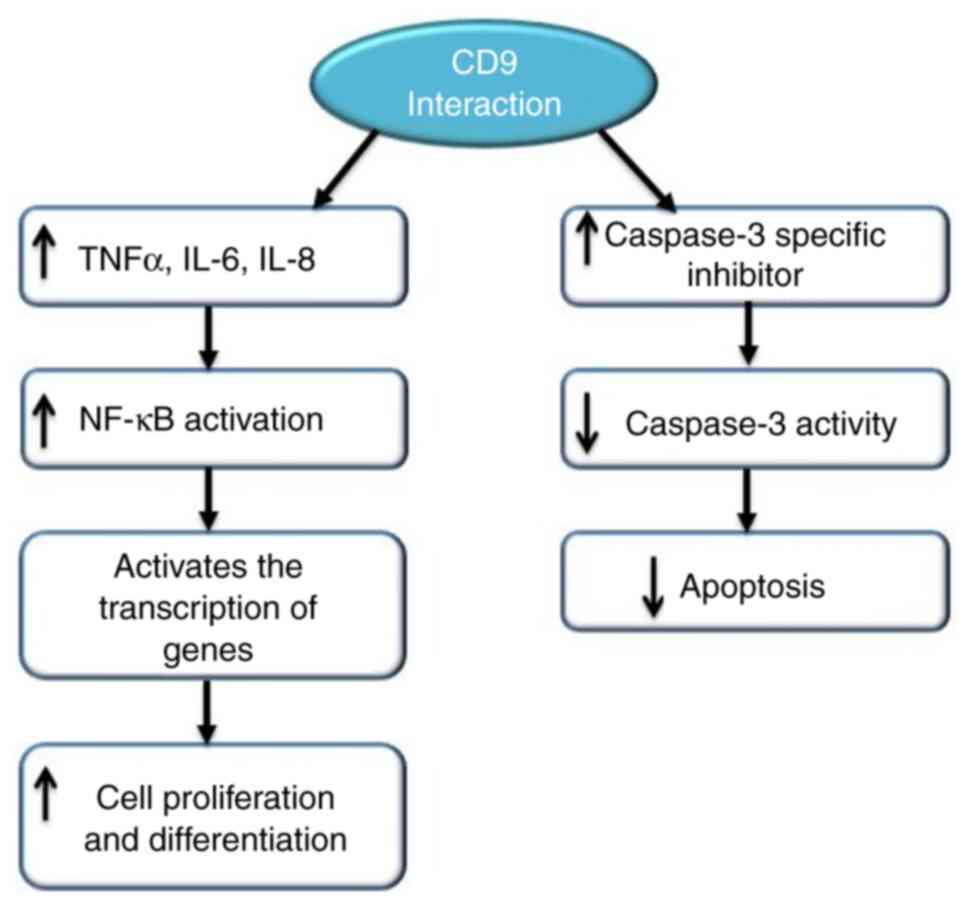
|
Boyland E, Roe FJ and Gorrod JW: Induction
of Pulmonary tumors in mice by nitrosonornicotine, a possible
constituent of tobacco smoke. Nature. 202:11261964. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Tobacco smoke and involuntary
smoking. IARC Monogr Eval Carcinog Risks Hum. 83:1–1438.
2004.PubMed/NCBI
|
|
18
|
Acetaldehyde. IARC Monogr Eval Carcinog
Risk Chem Hum. 36:101–132. 1985.PubMed/NCBI
|
|
19
|
Seitz HK and Stickel F: Molecular
mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer.
7:599–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haorah J, Ramirez SH, Floreani N, Gorantla
S, Morsey B and Persidsky Y: Mechanism of alcohol-induced oxidative
stress and neuronal injury. Free Radic Biol Med. 45:1542–1550.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Yang JL, Yu KK, Xu M, Xu YZ, Chen
L, Lu YM, Fang HS, Wang XY, Hu ZQ, et al: Activation of the NF-κB
pathway as a mechanism of alcohol enhanced progression and
metastasis of human hepatocellular carcinoma. Mol Cancer.
14:102015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shinohara M, Adachi Y, Mitsushita J,
Kuwabara M, Nagasawa A, Harada S, Furuta S, Zhang Y, Seheli K,
Miyazaki H and Kamata T: Reactive oxygen generated by NADPH oxidase
1 (NOX1) contributes to cell invasion by regulating matrix
metalloprotease-9 production and cell migration. J Biol Chem.
285:4481–4488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ha PK, Chang SS, Glazer CA, Califano JA
and Sidransky D: Molecular techniques and genetic alterations in
head and neck cancer. Oral Oncol. 45:335–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suh Y, Amelio I, Guerrero Urbano T and
Tavassoli M: Clinical update on cancer: Molecular oncology of head
and neck cancer. Cell Death Dis. 5:e10182014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leemans CR, Snijders PJF and Brakenhoff
RH: The molecular landscape of head and neck cancer. Nat Rev
Cancer. 18:269–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawakita A, Yanamoto S, Yamada S, Naruse
T, Takahashi H, Kawasaki G and Umeda M: Microrna-21 promotes oral
cancer invasion via the Wnt/β-catenin pathway by targeting DKK2.
Pathol Oncol Res. 20:253–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
IARC Monographs on the Evaluation of
Carcinogenic Risk to Human. Vol 100C. International Agency for
Research on Cancer; Lyon: 2012
|
|
30
|
Bánfalvi G: Heavy metals, trace elements
and their cellular effects. Cellular Effects of Heavy Metals.
Banfalvi G: Springer; Dordrecht: 2011, View Article : Google Scholar
|
|
31
|
Ercal N, Gurer-Orhan H and Aykin-Burns N:
Toxic metals and oxidative stress part I: Mechanisms involved in
metal-induced oxidative damage. Curr Top Med Chem. 1:529–539. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grund SC, Hanusch K and Wolf HU: Arsenic
and arsenic compounds, Ullmann's encyclopedia of industrial
chemistry. Wiley-VCH; Weinheim: 2005
|
|
33
|
Shi H, Shi X and Liu KJ: Oxidative
mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem.
255:67–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flora SJ: Arsenic-induced oxidative stress
and its reversibility. Free Radic Biol Med. 51:257–281. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hartwig A and Schwerdtle T: Interactions
by carcinogenic metal compounds with DNA repair processes:
Toxicological implications. Toxicol Lett. 127:47–54. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mass MJ, Tennant A, Roop BC, Cullen WR,
Styblo M, Thomas DJ and Kligerman AD: Methylated trivalent arsenic
species are genotoxic. Chem Res Toxicol. 14:355–361. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bau DT, Wang TS, Chung CH, Wang AS, Wang
AS and Jan KY: Oxidative DNA adducts and DNA-protein cross-links
are the major DNA lesions induced by arsenite. Environ Health
Perspect. 110 (Suppl 5):S753–S756. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goering PL, Aposhian HV, Mass MJ, Cebrián
M, Beck BD and Waalkes MP: The enigma of arsenic carcinogenesis:
Role of metabolism. Toxicol Sci. 49:5–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wilson K, Yang H, Seo CW and Marshall WE:
Select metal adsorption by activated carbon made from peanut
shells. Bioresour Technol. 97:2266–2270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim HS, Kim YJ and Seo YR: An overview of
carcinogenic heavy metal: Molecular toxicity mechanism and
prevention. J Cancer Prev. 20:232–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dayan AD and Paine AJ: Mechanisms of
chromium toxicity, carcinogenicity and allergenicity: Review of the
literature from 1985 to 2000. Hum Exp Toxicol. 20:439–451. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eastmond DA, MacGregor JT and Slesinski
RS: Trivalent chromium: Assessing the genotoxic risk of an
essential trace element and widely used human and animal
nutritional supplement. Crit Rev Toxicol. 38:173–190. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Katz SA and Salem H: The toxicology of
chromium with respect to its chemical speciation: A review. J Appl
Toxicol. 13:217–224. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khlifi R, Olmedo P, Gil F, Hammami B,
Chakroun A, Rebai A and Hamza-Chaffai A: Arsenic, cadmium, chromium
and nickel in cancerous and healthy tissues from patients with head
and neck cancer. Sci Total Environ. 452:58–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Beddok A, Krieger S, Castera L,
Stoppa-Lyonnet D and Thariat J: Management of fanconi anemia
patients with head and neck carcinoma: Diagnosis and treatment
adaptation. Oral Oncol. 108:1048162020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gasparini G, Longobardi G, Boniello R, Di
Petrillo A and Pelo S: Fanconi anemia manifesting as a squamous
cell carcinoma of the hard palate: A case report. Head Face Med.
2:12006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Swift MR and Hirschhorn K: Fanconi's
anemia. Inherited susceptibility to chromosome breakage in various
tissues. Ann Intern Med. 65:496–503. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Esparza A and Thompson WR: Familial
hypoplastic anemia with multiple congenital anomalies (Fanconi's
syndrome)-report of three cases. Cases presented are of two sisters
and a female cousin with complete clinical and post mortem
findings. R I Med J. 49:103–110. 1966.PubMed/NCBI
|
|
49
|
Mahmood N, Mihalcioiu C and Rabbani SA:
Multifaceted role of the urokinase-type plasminogen activator (uPA)
and its receptor (uPAR): Diagnostic, prognostic, and therapeutic
applications. Front Oncol. 8:242018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pavón MA, Arroyo-Solera I, Céspedes MV,
Casanova I, León X and Mangues R: uPA/uPAR and SERPINE1 in head and
neck cancer: Role in tumor resistance, metastasis, prognosis and
therapy. Oncotarget. 7:57351–57366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ghiso JA, Kovalski K and Ossowski L: Tumor
dormancy induced by downregulation of urokinase receptor in human
carcinoma involves integrin and MAPK signaling. J Cell Biol.
147:89–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ghiso JA: Inhibition of FAK signaling
activated by urokinase receptor induces dormancy in human carcinoma
cells in vivo. Oncogene. 21:2513–2524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liotta LA and Stetler-Stevenson WG:
Metalloproteinases and cancer invasion. Semin Cancer Biol.
1:99–106. 1990.PubMed/NCBI
|
|
55
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shapiro SD: Matrix metalloproteinase
degradation of extracellular matrix: Biological consequences. Curr
Opin Cell Biol. 10:602–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stetler-Stevenson WG, Hewitt R and
Corcoran M: Matrix metalloproteinases and tumor invasion: From
correlation and causality to the clinic. Semin Cancer Biol.
7:147–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stetler-Stevenson WG and Anita EY:
Proteases in invasion: Matrix metalloproteinases. Semin Cancer
Biol. 11:143–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ruokolainen H, Pääkkö P and
Turpeenniemi-Hujanen T: Expression of matrix metalloproteinase-9 in
head and neck squamous cell carcinoma: A potential marker for
prognosis. Clin Cancer Res. 10:3110–3116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Angiero F, Gatta LB, Seramondi R, Berenzi
A, Benetti A, Magistro S, Ordesi P, Grigolato P and Dessy E:
Frequency and role of HPV in the progression of epithelial
dysplasia to oral cancer. Anticancer Res. 30:3435–3440.
2010.PubMed/NCBI
|
|
62
|
Zhang W, Zeng Z, Zhou Y, Xiong W, Fan S,
Xiao L, Huang D, Li Z, Li D, Wu M, et al: Identification of
aberrant cell cycle regulation in Epstein-Barr virus-associated
nasopharyngeal carcinoma by cDNA microarray and gene set enrichment
analysis. Acta Biochim Biophys Sin (Shanghai). 41:414–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
International Agency for Research on
Cancer, . A review of human carcinogens: Arsenic, metals, fibres,
and dusts. IARC Monogr Eval Carcinog Risks Hum. 100:169–211.
2012.PubMed/NCBI
|
|
64
|
Prevention and Control Exchange (PACE)
World Health Organization. Occupational and Environmental Health
Team, . Hazard Prevention and Control in the Work Environment:
Airborne Dust. World Health Organisation. 1999.Available from.
https://apps.who.int/iris/handle/10665/66147
|
|
65
|
Langevin SM, McClean MD, Michaud DS, Eliot
M, Nelson HH and Kelsey KT: Occupational dust exposure and head and
neck squamous cell carcinoma risk in a population-based
case-control study conducted in the greater Boston area. Cancer
Med. 2:978–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Panahi Y, Gholami N, Ghojazadeh M, Moslemi
F, Naghavi-Behzad M, Azami-Aghdash S, Ghaffari A and Piri R:
Complications and carcinogenic effects of mustard Gas-a systematic
review and meta-analysis in Iran. Asian Pac J Cancer Prev.
16:7567–7573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Safarinejad MR: Testicular effect of
mustard gas. Urology. 58:90–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
McClintock SD, Till GO, Smith MG and Ward
PA: Protection from half-mustard-gas-induced acute lung injury in
the rat. J Appl Toxicol. 22:257–262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Thiagarajan A and Iyer NG:
Radiation-induced sarcomas of the head and neck. World J Clin
Oncol. 5:973–981. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ho CM, Lam KH, Wei WI, Lau SK and Lam LK:
Occult lymph node metastasis in small oral tongue cancers. Head
Neck. 14:359–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Spiro RH, Huvos AG, Wong GY, Spiro JD,
Gnecco CA and Strong EW: Predictive value of tumor thickness in
squamous carcinoma confined to the tongue and floor of the mouth.
Am J Surg. 152:345–350. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kawano K and Yanagisawa S: Predictive
value of laminin-5 and membrane type 1-matrix metalloproteinase
expression for cervical lymph node metastasis in T1 and T2 squamous
cell carcinomas of the tongue and floor of the mouth. Head Neck.
28:525–533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Califf RM: Biomarker definitions and their
applications. Exp Biol Med (Maywood). 243:213–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kuropkat C, Plehn S, Herz U, Dunne AA,
Renz H and Werner JA: Tumor marker potential of serum matrix
metalloproteinases in patients with head and neck cancer.
Anticancer Res. 22:2221–2227. 2002.PubMed/NCBI
|
|
75
|
Li Y, St John MA, Zhou X, Kim Y, Sinha U,
Jordan RC, Eisele D, Abemayor E, Elashoff D, Park NH and Wong DT:
Salivary transcriptome diagnostics for oral cancer detection. Clin
Cancer Res. 10:8442–8450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
St John MA, Li Y, Zhou X, Denny P, Ho CM,
Montemagno C, Shi W, Qi F, Wu B, Sinha U, et al: Interleukin-6 and
interleukin-8 as potential biomarkers for oral cavity and
oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck
Surg. 130:929–935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Toyoshima T, Vairaktaris E, Nkenke E,
Schlegel KA, Neukam FW and Ries J: Cytokeratin 17 mRNA expression
has potential for diagnostic marker of oral squamous cell
carcinoma. J Cancer Res Clin Oncol. 134:515–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cohen-Kerem R, Madah W, Sabo E, Rahat MA,
Greenberg E and Elmalah I: Cytokeratin-17 as a potential marker for
squamous cell carcinoma of the larynx. Ann Otol Rhinol Laryngol.
113:821–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Park NJ, Zhou H, Elashoff D, Henson BS,
Kastratovic DA, Abemayor E and Wong DT: Salivary microRNA:
Discovery, characterization, and clinical utility for oral cancer
detection. Clin Cancer Res. 15:5473–5477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Concha-Benavente F, Srivastava RM, Trivedi
S, Lei Y, Chandran U, Seethala RR, Freeman GJ and Ferris RL:
Identification of the cell-intrinsic and -extrinsic pathways
downstream of EGFR and IFNγ that induce PD-L1 expression in head
and neck cancer. Cancer Res. 76:1031–1043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hira-Miyazawa M, Nakamura H, Hirai M,
Kobayashi Y, Kitahara H, Bou-Gharios G and Kawashiri S: Regulation
of programmed-death ligand in the human head and neck squamous cell
carcinoma microenvironment is mediated through matrix
metalloproteinase-mediated proteolytic cleavage. Int J Oncol.
52:379–388. 2018.PubMed/NCBI
|
|
83
|
Yang WF, Wong MC, Thomson PJ, Li KY and Su
YX: The prognostic role of PD-L1 expression for survival in head
and neck squamous cell carcinoma: A systematic review and
meta-analysis. Oral Oncol. 86:81–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Goel R, Moore W, Sumer B, Khan S, Sher D
and Subramaniam RM: Clinical practice in PET/CT for the management
of head and neck squamous cell cancer. Am J Roentgenol.
209:289–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hentschel M, Appold S, Schreiber A,
Abolmaali N, Abramyuk A, Dörr W, Kotzerke J, Baumann M and Zöphel
K: Early FDG PET at 10 or 20 Gy under chemoradiotherapy is
prognostic for locoregional control and overall survival in
patients with head and neck cancer. Eur J Nucl Med Mol Imaging.
38:1203–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mohammed RN, Watson HA, Vigar M, Ohme J,
Thomson A, Humphreys IR and Ager A: L-selectin is essential for
delivery of activated CD8(+) T cells to virus-infected organs for
protective immunity. Cell Rep. 14:760–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Resto VA, Burdick MM, Dagia NM, McCammon
SD, Fennewald SM and Sackstein R: L-selectin-mediated
lymphocyte-cancer cell interactions under low fluid shear
conditions. J Biol Chem. 283:15816–15824. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Longo N, Yáñez-Mó M, Mittelbrunn M, de
la Rosa G, Muñoz ML, Sánchez-Madrid F and Sánchez-Mateos P:
Regulatory role of tetraspanin CD9 in tumor-endothelial cell
interaction during transendothelial invasion of melanoma cells.
Blood. 98:3717–3726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kohmo S, Kijima T, Otani Y, Mori M, Minami
T, Takahashi R, Nagatomo I, Takeda Y, Kida H, Goya S, et al: Cell
surface tetraspanin CD9 mediates chemoresistance in small cell lung
cancer. Cancer Res. 70:8025–8035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Stipp CS, Kolesnikova TV and Hemler ME:
Functional domains in tetraspanin proteins. Trends Biochem Sci.
28:106–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kitadokoro K, Bordo D, Galli G, Petracca
R, Falugi F, Abrignani S, Grandi G and Bolognesi M: CD81
extracellular domain 3D structure: Insight into the tetraspanin
superfamily structural motifs. EMBO J. 20:12–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hemler ME: Specific tetraspanin functions.
J Cell Biol. 155:1103–1107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Clark KL, Oelke A, Johnson ME, Eilert KD,
Simpson PC and Todd SC: CD81 associates with 14-3-3 in a
redox-regulated palmitoylation-dependent manner. J Biol Chem.
279:19401–19406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kovalenko OV, Metcalf DG, degrado WF and
Hemler ME: Structural organization and interactions of
transmembrane domains in tetraspanin proteins. BMC Struct Biol.
5:112005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fitter S, Seldin MF and Ashman LK:
Characterisation of the mouse homologue of CD151 (PETA-3/SFA-1);
genomic structure, chromosomal localisation and identification of 2
novel splice forms. Biochim Biophys Acta. 1398:75–85. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Stipp CS, Kolesnikova TV and Hemler ME:
EWI-2 regulates alpha3beta1 integrin-dependent cell functions on
laminin-5. J Cell Biol. 163:1167–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Seigneuret M, Delaguillaumie A,
Lagaudrière-Gesbert C and Conjeaud H: Structure of the tetraspanin
main extracellular domain. A partially conserved fold with a
structurally variable domain insertion. J Biol Chem.
276:40055–40064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Maecker HT, Todd SC and Levy S: The
tetraspanin superfamily: Molecular facilitators. FASEB J.
11:428–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yanez-Mo M, Mittelbrunn M and
Sanchez-Madrid F: Tetraspanins and intercellular interactions.
Microcirculation. 8:153–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Boucheix C and Rubinstein E: Tetraspanins.
Cell Mol Life Sci. 58:1189–1205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Boucheix C, Benoit P, Frachet P, Billard
M, Worthington RE, Gagnon J and Uzan G: Molecular cloning of the
CD9 antigen. A new family of cell surface proteins. J Biol Chem.
266:117–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ovalle S, Gutiérrez-López MD, Olmo N,
Turnay J, Lizarbe MA, Majano P, Molina-Jiménez F, López-Cabrera M,
Yáñez-Mó M, Sánchez-Madrid F and Cabañas C: The tetraspanin CD9
inhibits the proliferation and tumorigenicity of human colon
carcinoma cells. Int J Cancer. 121:2140–2152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kersey JH, LeBien TW, Abramson CS, Newman
R, Sutherland R and Greaves M: P-24: A human leukemia-associated
and lymphohemopoietic progenitor cell surface structure identified
with monoclonal antibody. J Exp Med. 153:726–731. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wright MD, Moseley GW and van Spriel AB:
Tetraspanin microdomains in immune cell signalling and malignant
disease. Tissue Antigens. 64:533–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hemler ME: Targeting of tetraspanin
proteins-potential benefits and strategies. Nat Rev Drug Discov.
7:747–758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Baek J, Jang N, Choi JE, Kim JR and Bae
YK: CD9 expression in tumor cells is associated with poor prognosis
in patients with invasive lobular carcinoma. J Breast Cancer.
22:77–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zöller M: Tetraspanins: Push and pull in
suppressing and promoting metastasis. Nat Rev Cancer. 9:40–55.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shi W, Fan H, Shum L and Derynck R: The
tetraspanin CD9 associates with transmembrane TGF-alpha and
regulates TGF-alpha-induced EGF receptor activation and cell
proliferation. J Cell Biol. 148:591–602. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hwang JR, Jo K, Lee Y, Sung BJ, Park YW
and Lee JH: Upregulation of CD9 in ovarian cancer is related to the
induction of TNF-α gene expression and constitutive NF-κB
activation. Carcinogenesis. 33:77–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yáñez-Mó M, Alfranca A, Cabañas C,
Marazuela M, Tejedor R, Ursa MA, Ashman LK, de Landázuri MO and
Sánchez-Madrid F: Regulation of endothelial cell motility by
complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-1 with
a3b1 integrin localized at endothelial lateral junctions. J Cell
Biol. 141:791–804. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Okochi H, Kato M, Nashiro K, Yoshie O,
Miyazono K and Furue M: Expression of tetra-spans transmembrane
family (CD9, CD37, CD53, CD63, CD81 and CD82) in normal and
neoplastic human keratinocytes: An association of CD9 with alpha 3
beta 1 integrin. Br J Dermatol. 137:856–863. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nishida M, Miyagawa J, Yamashita S,
Higashiyama S, Nakata A, Ouchi N, Tamura R, Yamamori K, Kihara S,
Taniguchi N and Matsuzawa Y: Localization of CD9, an enhancer
protein for proheparin-binding epidermal growth factor-like growth
factor, in human atherosclerotic plaques: Possible involvement of
juxtacrine growth mechanism on smooth muscle cell proliferation.
Arterioscler Thromb Vasc Biol. 20:1236–1243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Klein-Soyer C, Azorsa DO, Cazenave JP and
Lanza F: CD9 participates in endothelial cell migration during in
vitro wound repair. Arterioscler Thromb Vasc Biol. 20:360–369.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Peñas PF, García-Díez A, Sánchez-Madrid F
and Yáñez-Mó M: Tetraspanins are localized at motility-related
structures and involved in normal human keratinocyte wound healing
migration. J Invest Dermatol. 114:1126–1135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lijen HR, Lupu F, Collen D, Le Nour F and
Boucheix C: CD9 gene deficiency does not affect smooth muscle cell
migration and neointima formation after vascular injury in mice.
Thromb Haemost. 83:956–961. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Erovic BM, Pammer J, Hollemann D,
Woegerbauer M, Geleff S, Fischer MB, Burian M, Frommlet F and
Neuchrist C: Motility-related protein-1/CD9 expression in head and
neck squamous cell carcinoma. Head Neck. 25:848–857. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lagaudrière-Gesbert C, Le Naour F,
Lebel-Binay S, Billard M, Lemichez E, Boquet P, Boucheix C,
Conjeaud H and Rubinstein E: Functional analysis of four
tetraspans, CD9, CD53, CD81, and CD82, suggests a common role in
costimulation, cell adhesion, and migration: Only CD9 upregulates
HB-EGF activity. Cell Immunol. 182:105–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Oren R, Takahashi S, Doss C, Levy R and
Levy S: TAPA-1, the target of an antiproliferative antibody,
defines a new family of transmembrane proteins. Mol Cell Biol.
10:4007–4015. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wice BM and Gordon JI: A tetraspan
membrane glycoprotein produced in the human intestinal epithelium
and liver that can regulate cell density-dependent proliferation. J
Biol Chem. 270:21907–21918. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Buim ME, Lourenço SV, Carvalho KC, Cardim
R, Pereira C, Carvalho AL, Fregnani JH and Soares FA:
Downregulation of CD9 protein expression is associated with
aggressive behavior of oral squamous cell carcinoma. Oral Oncol.
46:166–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Huang CI, Kohno N, Ogawa E, Adachi M, Taki
T and Miyake M: Correlation of reduction in MRP-1/CD9 and KAI1/CD82
expression with recurrences in breast cancer patients. Am J Pathol.
153:973–983. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mhawech P, Herrmann F, Coassin M, Guillou
L and Iselin CE: Motility-related protein 1 (MRP-1/CD9) expression
in urothelial bladder carcinoma and its relation to tumor
recurrence and progression. Cancer. 98:1649–1657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Sauer G, Windisch J, Kurzeder C, Heilmann
V, Kreienberg R and Deissler H: Progression of cervical carcinomas
is associated with down-regulation of CD9 but strong local
re-expression at sites of transendothelial invasion. Clin Cancer
Res. 9:6426–6431. 2003.PubMed/NCBI
|
|
124
|
Kusukawa J, Ryu F, Kameyama T and Mekada
E: Reduced expression of CD9 in oral squamous cell carcinoma: CD9
expression inversely related to high prevalence of lymph node
metastasis. J Oral Pathol Med. 30:73–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang BH, Liu W, Li L, Lu JG, Sun YN, Jin
DJ and Xu XY: KAI1/CD82 and MRP1/CD9 serve as markers of
infiltration, metastasis, and prognosis in laryngeal squamous cell
carcinomas. Asian Pac J Cancer Prev. 14:3521–3526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Miyake M, Koyama M, Seno M and Ikeyama S:
Identification of the motility-related protein (MRP-1), recognized
by monoclonal antibody M31-15, which inhibits cell motility. J Exp
Med. 174:1347–1354. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ikeyama S, Koyama M, Yamaoko M, Sasada R
and Miyake M: Suppression of cell motility and metastasis by
transfection with human motility-related protein (MRP-1/CD9) DNA. J
Exp Med. 177:1231–1237. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Uchida S, Shimada Y, Watanabe G, Li ZG,
Hong T, Miyake M and Imamura M: Motility-related protein
(MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 79:1168–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Higashiyama S, Iwamoto R, Goishi K, Raab
G, Taniguchi N, Klagsbrun M and Mekada E: The membrane protein
CD9/DRAP 27 potentiates the juxtacrine growth factor activity of
the membrane-anchored heparin-binding EGF-like growth factor. J
Cell Biol. 128:929–938. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Nakamura K, Iwamoto R and Mekada E:
Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF)
and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form
a complex with integrin alpha 3 beta 1 at cell-cell contact sites.
J Cell Biol. 129:1691–1705. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hato T, Ikeda K, Yasukawa M, Watanabe A
and Kobayashi Y: Exposure of platelet fibrinogen receptors by a
monoclonal antibody to CD9 antigen. Blood. 72:224–229. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Higashihara M, Takahata K, Yatomi Y,
Nakahara K and Kurokawa K: Purification and partial
characterization of CD9 antigen of human platelets. FEBS Lett.
264:270–274. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hirano C, Nagata M, Noman AA, Kitamura N,
Ohnishi M, Ohyama T, Kobayashi T, Suzuki K, Yoshizawa M, Izumi N,
et al: Tetraspanin gene expression levels as potential biomarkers
for malignancy of gingival squamous cell carcinoma. Int J Cancer.
124:2911–2916. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Nagata M, Fujita H, Ida H, Hoshina H,
Inoue T, Seki Y, Ohnishi M, Ohyama T, Shingaki S, Kaji M, et al:
Identification of potential biomarkers of lymph node metastasis in
oral squamous cell carcinoma by cDNA microarray analysis. Int J
Cancer. 106:683–689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kurokawa A, Nagata M, Kitamura N, Noman
AA, Ohnishi M, Ohyama T, Kobayashi T, Shingaki S and Takagi R;
Oral, Maxillofacial Pathology, Surgery Group, : Diagnostic value of
integrin alpha3, beta4, and beta5 gene expression levels for the
clinical outcome of tongue squamous cell carcinoma. Cancer.
112:1272–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Sugiura T and Berditchevski F: Function of
alpha3beta1-tetraspanin protein complexes in tumor cell invasion.
Evidence for the role of the complexes in production of matrix
metalloproteinase 2 (MMP-2). J Cell Biol. 146:1375–1389. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Huang CL, Ueno M, Liu D, Masuya D, Nakano
J, Yokomise H, Nakagawa T and Miyake M: MRP-1/CD9 gene transduction
regulates the actin cytoskeleton through the downregulation of
WAVE2. Oncogene. 25:6480–6488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kim T, Kim Y and Kwon HJ: Expression of
CD9 and CD82 in papillary thyroid microcarcinoma and its prognostic
significance. Endokrynol Pol. 70:224–231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Murayama Y, Oritani K and Tsutsui S: Novel
CD9-targeted therapies in gastric cancer. World J Gastroenterol.
21:3206–3213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Murayama Y, Shinomura Y, Oritani K,
Miyagawa JI, Yoshida H, Nishida M, Katsube F, Shiraga M, Miyazaki
T, Nakamoto T, et al: The tetraspanin CD9 modulates epidermal
growth factor receptor signaling in cancer cells. J Cell Physiol.
216:135–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wang GP and Han XF: CD9 modulates
proliferation of human glioblastoma cells via epidermal growth
factor receptor signaling. Mol Med Re. 12:1381–1386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Halova I, Dráberová L, Bambousková M,
Machyna M, Stegurová L, Smrž D and Dráber P: Cross-talk between
tetraspanin CD9 and transmembrane adaptor protein non-T cell
activation linker (NTAL) in mast cell activation and chemotaxis. J
Biol Chem. 288:9801–9814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Huang CL, Liu D, Masuya D, Kameyama K,
Nakashima T, Yokomise H, Ueno M and Miyake M: MRP-1/CD9 gene
transduction downregulates Wnt signal pathways. Oncogene.
23:7475–7483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Podergajs N, Motaln H, Rajčević U,
Verbovšek U, Koršič M, Obad N, Espedal H, Vittori M, Herold-Mende
C, Miletic H, et al: Transmembrane protein CD9 is glioblastoma
biomarker, relevant for maintenance of glioblastoma stem cells.
Oncotarget. 7:593–609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Higashiyama M, Taki T, Ieki Y, Adachi M,
Huang CL, Koh T, Kodama K, Doi O and Miyake M: Reduced motility
related protein-1 (MRP-1/CD9) gene expression as a factor of poor
prognosis in non-small cell lung cancer. Cancer Res. 55:6040–6044.
1995.PubMed/NCBI
|
|
146
|
Shi Y, Zhou W, Cheng L, Chen C, Huang Z,
Fang X, Wu Q, He Z, Xu S, Lathia JD, et al: Tetraspanin CD9
stabilizes gp130 by preventing its ubiquitin-dependent lysosomal
degradation to promote STAT3 activation in glioma stem cells. Cell
Death Differ. 24:167–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Funakoshi T, Tachibana I, Hoshida Y,
Kimura H, Takeda Y, Kijima T, Nishino K, Goto H, Yoneda T, Kumagai
T, et al: Expression of tetraspanins in human lung cancer cells:
Frequent downregulation of CD9 and its contribution to cell
motility in small cell lung cancer. Oncogene. 22:674–687. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Yang H, Shen C, Zhang B, Chen H, Chen Z
and Chen J: Expression and clinicopathological significance of CD9
in gastrointestinal stromal tumor. J Korean Med Sci. 28:1443–1448.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Imhof I, Gasper WJ and Derynck R:
Association of tetraspanin CD9 with transmembrane TGF{alpha}
confers alterations in cell-surface presentation of TGF{alpha} and
cytoskeletal organization. J Cell Sci. 121:2265–2274. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Saito Y, Tachibana I, Takeda Y, Yamane H,
He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T, et al:
Absence of CD9 enhances adhesion-dependent morphologic
differentiation, survival, and matrix metalloproteinase-2
production in small cell lung cancer cells. Cancer Res.
66:9557–9565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hashida H, Takabayashi A, Tokuhara T,
Hattori N, Taki T, Hasegawa H, Satoh S, Kobayashi N, Yamaoka Y and
Miyake M: Clinical significance of transmembrane 4 superfamily in
colon cancer. Br J Cancer. 89:158–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ono M, Handa K, Withers DA and Hakomori
SI: Motility inhibition and apoptosis are induced by
metastasis-suppressing gene product CD82 and its analogue CD9, with
concurrent glycosylation. Cancer Res. 59:2335–2339. 1999.PubMed/NCBI
|
|
153
|
Yauch RL, Berditchevski F, Harler MB,
Reichner J and Hemler ME: Highly stoichiometric, stable, and
specific association of integrin alpha3beta1 with CD151 provides a
major link to phosphatidylinositol 4-kinase, and may regulate cell
migration. Mol Biol Cell. 9:2751–2765. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hemler ME, Mannion BA and Barditchevski F:
Association of TM4SF proteins with integrins: Relevance to cancer.
Biochim Biophys Acta. 1287:67–71. 1996.PubMed/NCBI
|
|
155
|
Berditchevski F and Odintsova E:
Characterization of integrin-tetraspanin adhesion complexes: Role
of tetraspanins in integrin signaling. J Cell Biol. 146:477–492.
1999. View Article : Google Scholar : PubMed/NCBI
|