Head and neck cancer is common in several regions of
the world such as India, Hong Kong and Sri Lanka (1). Head and neck squamous cell carcinomas
(HNSCCs) are a type of epithelial cancer arising in the mucosa of
the upper aerodigestive tract (1).
The oral cavity, hypopharynx, oropharynx and larynx are sites that
have the potential to be affected by this cancer (1). A tetraspanin member, CD9 is found on
the epithelial cells. Hence, it may have a role in the
carcinogenesis of head and neck cancer. HNSCCs are aggressive,
genetically complex and difficult to treat. HNSCCs can develop from
dysplastic or premalignant lesions in the oropharyngeal mucosa that
have occurred due to chronic exposure of the upper aerodigestive
tract to carcinogenic agents (2).
HNSCCs are associated with different types of
epidemiologies, aetiologies and therapies (2). Treatment has to be undertaken by
multidisciplinary teams with training in supportive care that
considers swallowing, nutrition, dental and voice impairment due to
the effects of clinical intervention. In total, 6–90% of patients
at early stages of this cancer show positive responses to local
therapy. Early diagnosis and appropriate treatment results in cure
and survival. The majority of patients with HNSCC who present with
stages III and IV locally advanced head and neck cancer require
multimodality treatment (3).
HNSCCs begin in the flat squamous cells that make up
the thin layer of tissue on the surface of the epithelium in the
head and neck. Directly beneath the epithelium, some areas of the
head and neck have a layer of moist tissue, called the mucosa. A
cancer that is only found in the squamous layer of cells is called
carcinoma in situ. Cancer that has grown beyond the mucosa
and has moved into the deeper tissue is called invasive squamous
cell carcinoma (4). Head and neck
cancer, the sixth most common malignancy, accounts for >650,000
cases and 330,000 deaths annually worldwide (1–3).
Women are less likely to be affected than men, with ratios of 1:2
to 4:1 worldwide thus far. In the Indian subcontinent, mouth and
tongue cancer are more common, whereas nasopharyngeal cancer is
more common in Hong Kong, and pharyngeal and laryngeal cancers are
more common in other populations (5).
The use of tobacco and alcohol are associated with
HNSCC. Consumption of alcohol and long-term use of tobacco are the
main oncogenic drivers and primary risk factors associated with
head and neck cancer (5). Using
alcohol and tobacco together increases this risk even more
(12). Heavy metals, Fanconi
anaemia (FA), the plasminogen activator (PA) system, matrix
metalloprotease (MMP), human papilloma virus (HPV) and Epstein-Barr
virus (EBV) are also etiological factors that are associated with
head and neck cancer.
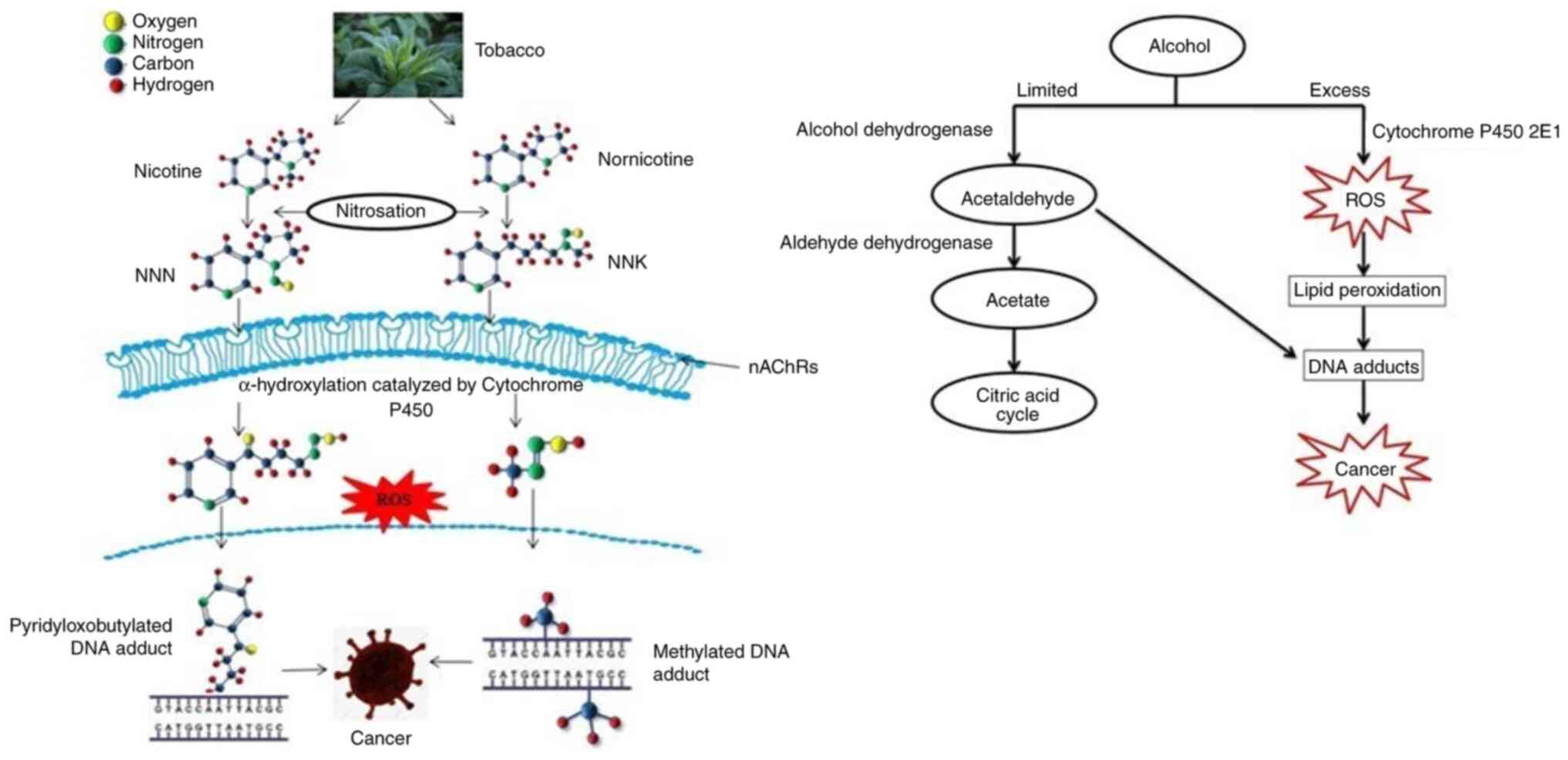
A variety of chemicals, including nicotine and other
carcinogens, are present in tobacco. The type of tobacco products
used and the duration of exposure are two factors that have a major
impact on human health. The main constituent of tobacco products
and smoke is nicotine. As such, nicotine is non-carcinogenic and
addictive, but it has the capacity to activate tumour progression
related to various signalling pathways (13,14).
Nicotine-derived nitrosamines, such as
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and
N'-nitrosonornicotine, can cause cancer in humans through the
formation of DNA adducts and mutations, and they can promote tumour
progression by altering receptor-mediated pathways (7,15–30).
Activation of nicotinic acetylcholine and
β-adrenergic receptors by nicotine and nitrosamines in turn
activates the downstream signal transduction pathways that aid
tumour progression (21).
NNK in tobacco smoke naturally occurs in an inert
form as a procarcinogen, which is converted to DNA reactive forms
by several cytochromes, leading to methylation,
pyridyloxobutylation and pyridylhydroxybutylation of nucleobases in
DNA (22). The other carcinogens
present in tobacco are polycyclic aromatic hydrocarbons, aromatic
amines, aldehydes, phenols, volatile hydrocarbons and
nitrocompounds (15,23) (Fig.
1).
The combination of alcohol consumption with
cigarette smoking increases the risk of head and neck cancer
(24). Alcohol dehydrogenase
converts ethanol into acetaldehyde, which is considered a
carcinogen of the human upper respiratory tract (24). Cytochrome P450 2E1 (CYP2EI) also
has the ability to convert ethanol into acetaldehyde when the
amount of alcohol consumed is high. This leads to the formation of
reactive oxygen species (ROS) (25). Exocyclic DNA adducts are formed
when malonaldehyde and 4-hydroxynonenal, which are the by-products
of lipid peroxidation, accumulate by the action of ROS produced by
CYP2EI (26). The upregulation of
vascular endothelial growth factor and monocyte chemotactic
protein-1, which play an important role in tumour angiogenesis and
growth, is caused by the accumulation of ROS (27). An increase in the expression of
MMPs, such as MMP2 and MMP9, leads to the degradation of the
extracellular matrix (ECM), resulting in cell motility, invasion
and metastases (28) (Fig. 1).
According to the International Agency for Research
on Cancer (IARC), arsenic (As), cadmium (Cd), chromium (Cr) and
nickel (Ni) are category I heavy metals that disrupt tumour
suppressor gene expression (29).
These heavy metals damage the DNA repair process and
metabolism-related enzyme activities (30,31).
As is present in organic and inorganic forms, but the organic form
of As is less toxic when compared with the inorganic form.
Inorganic As compounds are pentavalent and soluble in water and
produce salts, such as arsenate (32). Oxidative stress is the major
mechanism of As-related damage (33,34).
DNA repair processes are inhibited and ROS are the metabolic
products in the spleen and liver of the methylated forms of As
(35,36). ROS accumulation results in abnormal
gene expression and lesions of cellular components that induce cell
death (37). Residues of As bind
to the DNA-binding proteins and increase the risk of carcinogenesis
(38). Cd is an environmental
pollutant that is released from industry and agricultural waste
(39). B cell lymphoma 2
protein-associated X protein and mitogen-activated protein kinase 1
are associated with Cd (40),
which exists in different forms. The trivalent and hexavalent
compounds of Cd are biologically toxic as they can induce oxidative
stress, DNA damage and apoptosis (41–43).
The levels of As, Cd, Cr and Ni have been found to
be significantly high in patients with head and neck cancer
compared with those in healthy individuals (44). This may be due to altered cellular
metabolism during cancer. Occupational or environmental factors
might be the reason for this difference in the concentration of
heavy metals between patients with cancer and healthy individuals
(44).
FA is a genetic disease that is characterised by
alteration in one of the 23 genes of the FS pathway or in the 23rd
FA gene, DNA repair protein RAD51 homolog 1 (45). Genome stability induced by
interstrand DNA crosslink repair in the FA pathway has the
potential to induce tumorigenesis (45). Patients with FA are more prone to
HNSCC and are more sensitive to severe radiation-induced side
effects. Patients with FA who are at higher risk for HNSCC must
abstain from other risk factors, such as tobacco, alcohol and HIV
infections (45). The main
characteristics of this rare autosomal recessive disorder are
congenital malformations, such as abnormal thumbs and arms,
skeletal abnormalities of the hips, ribs or spine, small
reproductive organs in male patients, low body weight at birth,
mental retardation, hyperpigmentation, progressive bone marrow
failure, and the development of solid tumours (46–48).
An extracellular proteolytic enzyme system, the PA
system, comprises various components, such as urokinase-type PA
(uPA), its receptor (uPAR), and PA inhibitor-1 and −2. They have a
major role in cancer progression and metastasis (49). The activation of plasminogen to
plasmin by binding of uPA to uPAR initiates a proteolytic cascade
that degrades ECM components, thus facilitating cancer cell
migration from the site of origin to distant organs (50). uPA/uPAR overexpression increases
tumour cell migration and invasion, playing a key role in
metastasis and conferring poor prognosis of patients with head and
neck cancer (51). It is
associated with focal adhesion kinase 1 and ERK1/2 signalling
activation and an increase in HNSCC tumour growth (51,52).
Activation of plasmin, ECM degradation and indirect activation of
signalling pathways, such as the PI3K-Akt pathway, may be the
reasons for this effect (50).
MMPs are enzymes that degrade the ECM, connective
tissue and the basement membrane collagen, which are crucial in
cancer cell invasion and progression. They require zinc for their
catalytic activity. Type VI collagenase, MMP2 and MMP9 are members
of the MMP family of enzymes (53–59).
In HNSCC, immunohistochemical staining of MMP9 demonstrated that it
has prognostic values that are not dependent on tumour stage.
Patients with extensive positive MMP9 staining had relatively
higher risk of mortality. No correlation has been found between
MMP9 and the stage or grade of the tumour (60).
Inactivation of cellular tumour antigen p53 and
cyclin-dependent kinase inhibitor 2A by cell cycle dysregulation
leads to cell proliferation and inhibition of apoptosis in head and
neck cancer (61). In
oropharyngeal squamous cell carcinoma caused by HPV, the virus
integrates into the host DNA genome, leading to the deregulation of
oncoproteins (E6 and E7), which leads to the p53 and retinoblastoma
tumour suppressor gene product pRb. P16 upregulation is the result
of negative feedback of pRb inactivation. In nasopharyngeal
squamous cell carcinoma caused by EBV, the cell cycle is the most
deregulated pathway. Progression of the G1/S phase is promoted by
the inhibition of p16 expression and pRb upregulation (61,62).
Wood and leather dust are the two types of
occupational dusts that are classified as type 1 carcinogens by
IARC (63). Dusts are small solid
particles present in the air with a size ranging from 1 to 100
μm (64). They are a
heterogenous group of exposures that can be either organic or
inorganic. The carcinogenic effect of dust is exerted through the
induction of chronic inflammation, their intrinsic chemical
properties or they act as carriers of other carcinogenic compounds
(63). Occupational sawdust
exposure has been found to increase the risk of laryngeal carcinoma
(OR, 1.2; 95% CI, 1.0-1.3) and metal dust (OR, 1.2; 95% CI,
1.0-1.4). Exposure to occupational leather dust can increase the
risk of head and neck cancer (OR, 1.5; 95% CI, 1.2-1.9) (65).
1,1-thiobis, also known as sulphur mustard, causes
blisters on contact with the skin and mucous membrane (66). A reactive intermediate, a cyclic
sulfonium ion, is produced as sulphur mustard eliminates a chloride
ion by intramolecular nucleophilic substitution. This intermediate
causes alkylation of guanine nucleotide of DNA that prevents cell
division, which may lead to malignant transformation (67,68).
Radiation is used widely to treat cancers.
Radiation-induced sarcomas are seen in long-term survivors of head
and neck cancer with a risk of up to 0.3% (69). Treatment of head and neck cancer
include surgical eradication, chemotherapy and radiotherapy, which
reduce quality of life (including loss of taste and excessive hair
loss), and are ineffective. Genetic heterogeneity that results in
the loss of function of genes, such as p53 and p16, and the
activation of oncogenes, such as epidermal growth factor receptor
(EGFR) and PIK3CA, plays an important role in HNSCC (70–72).
A biomarker is an objective feature that can be
precisely assessed to determine a specific biological, pathological
or therapeutic development of the host (73). There are several biomarkers for
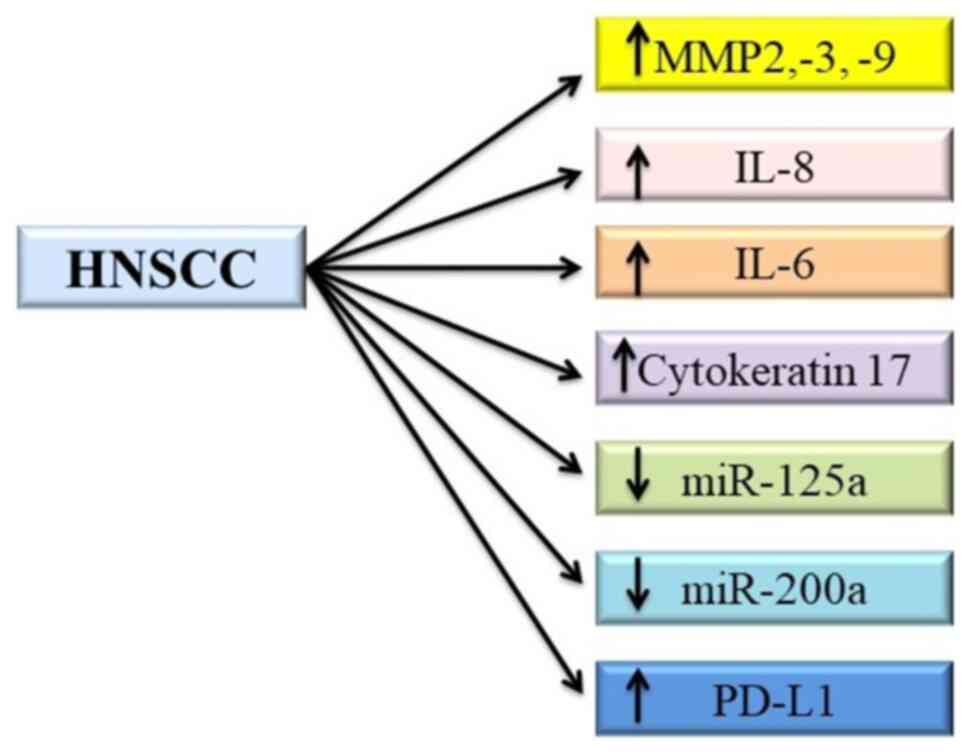
head and neck cancer. MMPs are enzymes that degrade the ECM and
induce cell migration. Serum levels of MMP2, 3 and 9 are elevated
in patients with HNSCC (74).
Inflammatory markers, such as IL-8 and IL-6, are increased in
saliva and serum, respectively (75,76).
Cytokeratin 17 is a cytoskeletal intermediate filament that is
upregulated in oral squamous cell carcinoma (OSCC) when compared
with normal cells, and it has been identified as a
immunohistochemical marker for squamous cell carcinoma of the
larynx (77,78).
MircoRNAs (miRNAs/miRs) are small non-coding
sequences that regulate gene expression after transcription. Levels
of miRNAs, such as miR-125a and miR-200a, are significantly lower
in subjects with OSCC compared with those in normal subjects
(79).
Fluorodeoxyglucose-positron emission tomography is a
powerful imaging tool that can be used to identify cervical node
metastasis and is a standard of care for patients with III and IV
stage HNSCC (84). Patients with
lower ΔSUVmax10/20 showed lower overall survival
compared with those with higher ΔSUVmax10/20 (P=0.02).
The decrease in the SUVmax before and after
chemoradiotherapy acts as a potential prognostic marker in patients
with head and neck cancer (85).
CD62, also known as L-selectin, is a lectin receptor
expressed on leucocytes that regulate the entry of naïve and
central memory T cells into lymph nodes (86). The spread of tumour cells to lymph
nodes is a multistep process that includes invasion of the tumour
cells into the lymphovascular compartment and lodging and growth of
the tumour cell in the new environment. The lymph node is the most
common region of metastasis for head and neck cancer. Head and neck
cancer cells express unrecognized L-selectin that mediates the
binding to lymphocytes and thus aids tumour node metastasis
(87).
Likewise, tetraspanins are one of the markers for
HNSCC. Tetraspanins play a major role in a wide array of cellular
processes, including cell adhesion, motility, intracellular
signalling, cell matrix adhesion and proliferation (88). Of the 33 tetraspanin proteins, CD9
is being extensively studied (89–91).
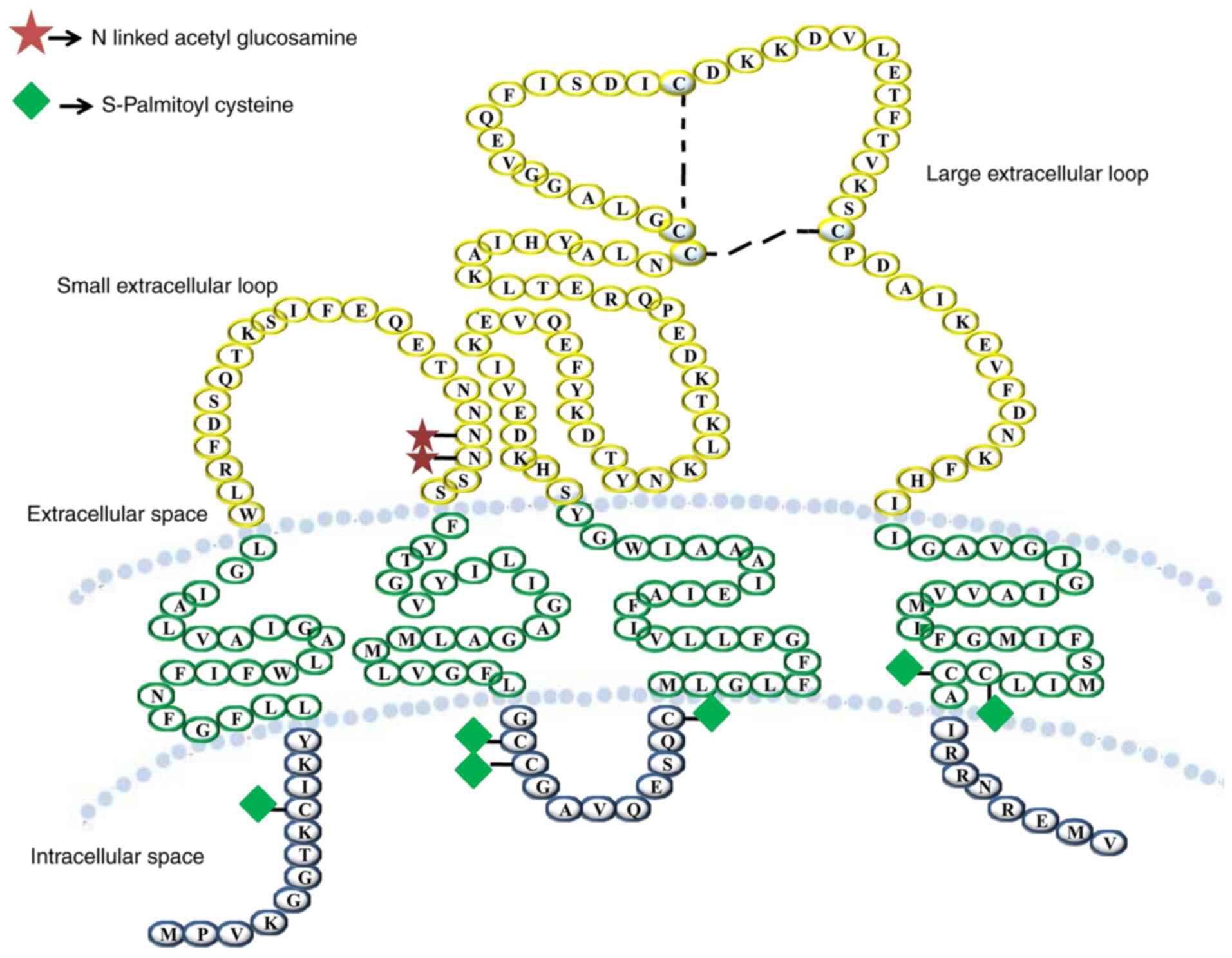
Tetraspanin is a glycoprotein family containing four
transmembrane domains. These proteins form multimeric complexes
with each other and other cell surface proteins, including
integrins, leukocyte antigens and signalling molecules, at
specialized tetraspanin-enriched microdomains (92). They also contain distinct
palmitoylation sites and most members are glycosylated (93).
The large extracellular loop has highly conserved
motifs that aid in the recognition of tetraspanins (94). Cys-Cys-Gly, Phe-X-Ser-Cys and
Glu-Gly-Cys are the conserved motifs of CD9 protein (95–97).
‘Tetraspanin webs’ are formed by the heteromultimerization of
tetraspanins, which are stabilized by the transmembrane domains
(97–99). There are two subdomains in the EC2
domain, a highly conserved subdomain with residue differences and a
subdomain that has variability in size, amino acid sequence and
protein folding for the disulphide bridge (90). The interaction between tetraspanins
and other transmembrane proteins, such as integrins and other
signalling molecules, is regulated by the EC2 domain of the
tetraspanin (90,98–101) (Fig.
3). Tetraspanins recruit cell surface proteins, which stabilize
the functional signalling complexes and act as molecular
facilitators (102).
Among the tetraspanins, CD9 is unusual as it has
only one N-glycosylation site located in its SEL domain, whereas
other tetraspanins have a number of glycosylation sites (105). Critical physiological and
pathological processes, such as sperm-egg fusion, neurite
outgrowth, myotube formation, tumorigenicity and metastasis, are
regulated by CD9 (106–108).
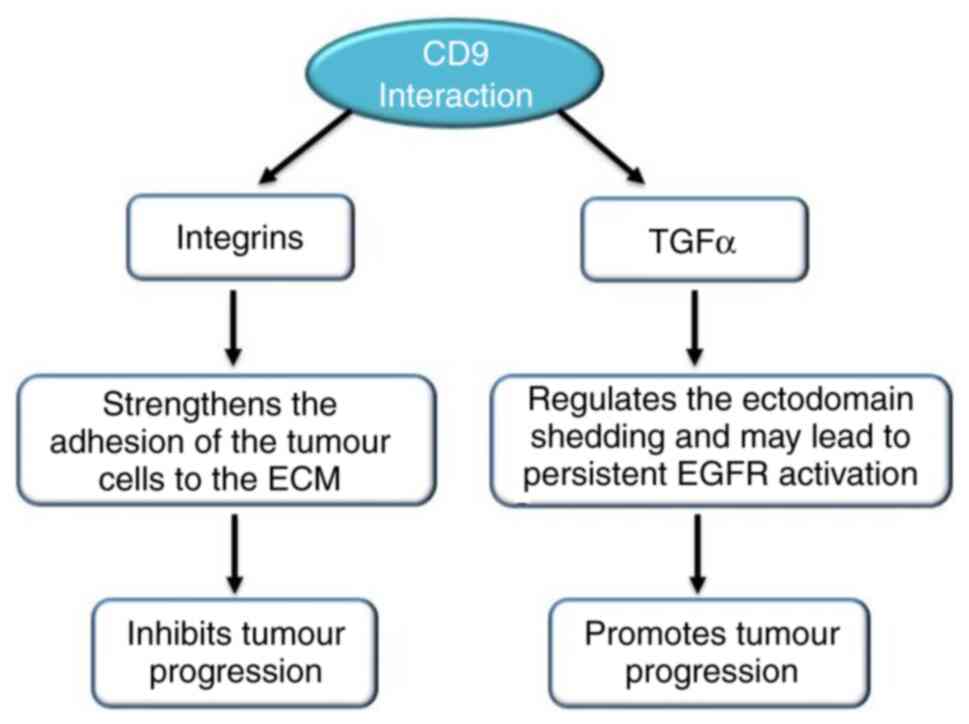
The molecule that interacts with CD9 decides the
role of this tetraspanin in cancer cell motility. The adhesion of
tumour cells to the ECM increases when integrin expression is
upregulated in combination with CD9. Transcription of MMP2 can be
inhibited by CD9 complexes with fibronectin-bound integrins
(109). Increased invasiveness of
tumour cells can be the result of the activation of intracellular
signalling molecules, such as PI4K and Src homology 2, by the
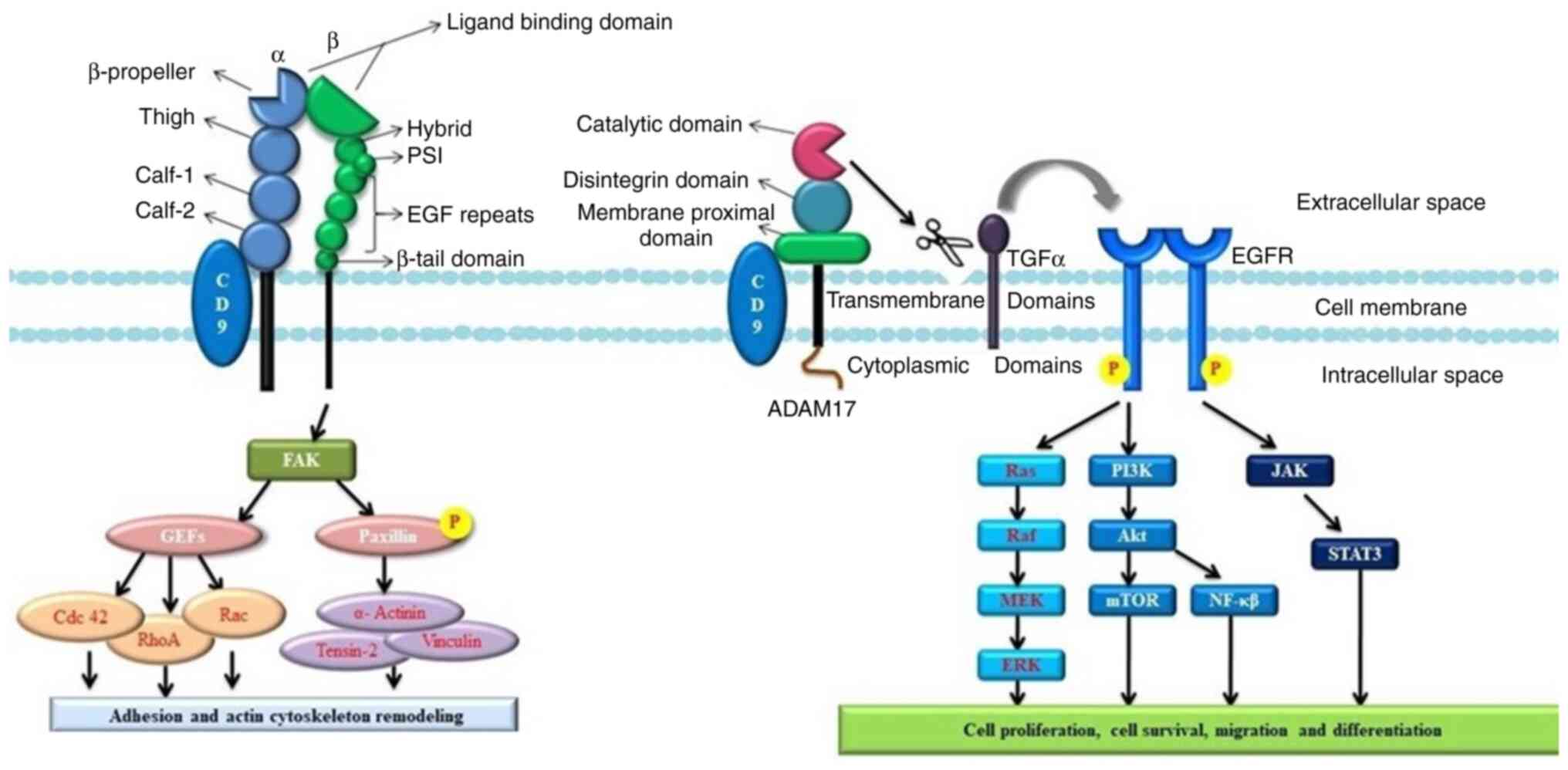
transcription of MMP2 induced by CD9 crosslinking (110). Growth factors of the transforming
growth factor (TGF) family activate the EGFR. Ectodomain shedding
is a process where TGFα is proteolytically cleaved to release an
EGF-core containing ligand. Ectodomain shedding and the release of
TGFα is affected when it interacts with CD9, as it regulates the
cleavage TGFα, which may lead to constant activation of EGFR,
resulting in cell proliferation (110,111) (Fig.
4).
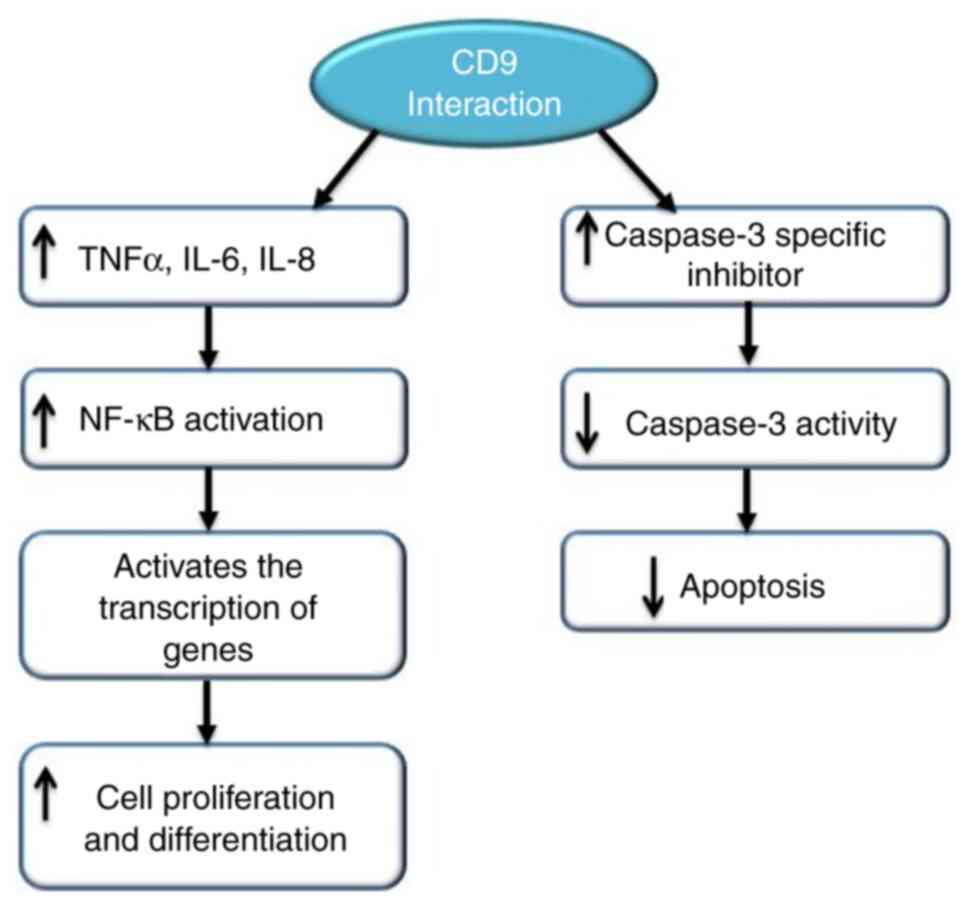
In CD9-overexpressed cells, the NF-κB signalling
pathway has been found to be activated and dependent on CD9
expression. CD9 also induced tumour necrosis factor α (TNFα) gene
expression, which resulted in the increase of IL-6 and IL-8 levels.
NF-κB subunits, upon activation by TNFα, activate the transcription
of genes involved in cell proliferation and differentiation by
translocating into the nucleus. CD9 activates the caspase-3
inhibitor, which reduces the activity of caspase-3. Blockage of CD9
expression with small interfering RNA increases the level of
caspase-3 activity. This shows that CD9 has anti-apoptotic activity
(112) (Fig. 5).
Favourable clinical outcomes have been observed in
HNSCC with elevated CD9 expression. Tetraspanins or α3β1 integrins
show an association with CD9 on the cell-to-cell junctions of human
umbilical vein endothelial cells (109–113). Migration of endothelial cells
during wound repair has been reported to be inhibited by anti-CD9
antibodies (101,114–117), which indicates the stabilizing
effect of CD9 antigen on the integrity of the vascular membranes.
During tumour angiogenesis, downregulation of CD9 proteins may be
linked to vascular supply reorganization (89). CD9 acts by setting up the junctions
between the cell surface and the intercellular matrix via the
formation of a functional signalling complex with other cell
surface proteins (98,118–121). Motility-related protein 1
(MRP-1)/CD9 expression was the only predictive parameter that
seemed to be significant with respect to overall survival
(P>0.049), whereas CD9 expression (P>0.006) and lymph node
status (P>0.007) were significant for prolonged disease-free
survival. Tumour patients with lower CD9 expression survived
shorter periods of time than patients with high CD9 levels in the
overall survival curves estimated by Kaplan-Meier analysis
(P>0.04) (89). The potential
effects of CD9 were confirmed when its expression was observed in
the tumour vessels, indicating the involvement of this protein in
tumour angiogenesis and endothelial cell migration (89).
Patients with positive CD9 tumours show shorter
disease-free survival and overall survival than patients with
negative CD9 expression in OSCC (100). Metastatic lesions have been
reported in patients with lack of expression of these proteins, and
they tended to have poorer prognosis and lower rates of survival
(122–126). The incidence of cervical lymph
node metastasis and survival has been found to be significantly
associated with the abnormal expression of the CD9 protein
(90).
One of the most common cancers in the head and neck
region is laryngeal squamous cell carcinoma (LSCC) (91). The tumour grows in the glottic,
supraglottic and subglottic areas. Death and the patient's quality
of life are influenced by infiltration and metastasis, which have
become the primary factors leading to an increase in the incidence
of LSCC (91). Patients with
negative CD9 protein expression have shorter median survival times
compared with patients with positive CD9 protein expression
(P<0.01) (91). LSCC may
develop due to the combined participation of CD9 and another
tetraspanin protein, CD82 (91).
Infiltration, prognosis of LSCC and metastasis can be determined by
using CD9 as a marker. Patients with TNM stage I–II, which is
well-differentiated and non-metastatic LSCC, show higher CD9
positive expression than patients with TNM stage III–IV, which is
well-differentiated and metastatic LSCC (91). These results show that as the
expression of CD9 decreases, the invasiveness and the metastatic
potential of the cancer cells increase (91).
Overexpression of CD9 by transfection leads to the
suppression of cell motility (127,128). In oesophageal squamous cell
carcinoma, lymph node metastasis may be facilitated by a decrease
in CD9 expression (129). Patient
prognosis can be predicted by the expression status of CD9
(129). A previous study reported
that the cell membranes of normal oesophageal epithelial cells show
positive CD9 expression, whereas CD9 expression is reduced on the
membranes of cancer cells. As the tumours grew deeper, the levels
of reduced CD9 expression significantly increased. As the stage of
cancer advanced, the expression of MRP-1/CD9 was reduced. Lymph
node metastasis and CD9 expression showed a significant inverse
correlation, but there was no correlation between CD9 expression
and distant metastasis. A correlation was found between lymph node
metastases and lymphatic invasion. The 5-year survival rates of
patients with CD9 positive expression were significantly improved
compared with those patients with low or negative CD9 expression
(129). The closest sites to the
primary lesions may be affected by the loss of CD9, leading to
local lymph node metastasis. Hence, there might be an inverse
correlation between CD9 expression and lymphatic invasion (129). The adhesion effects of the
interaction between CD9 and heparin-binding EGF-like growth factor
associated with α3β1 integrin may play an important role in the
initiation of the metastatic cascade (130,131). CD9 antibody activates platelets
and their aggregation, thereby releasing the growth factors that
facilitate tumour activation or growth (127,132).
In total, ~50% of gingival squamous cell carcinoma
(GSCCs) cases show high oral malignant neoplasms and present with
cervical lymph node metastasis (133). The jawbone and its surrounding
tissues, such as nerves, muscles, the nasal cavity and skin, are
invaded by GSCC. Logistic regression analysis with cervical lymph
node metastasis as a target variable has shown that CD9/ACTB
(P=0.013) and CD9/CD82 (P=0.013) have significant association
(133). CD9 is related to the
invasiveness of cancer cells by controlling the function of
integrin receptors (133). Lymph
node metastasis has been shown to be related to an increased level
of the integrin a3 gene and a reduced level of CD9, as indicated in
OSCC gene expression analysis (134,135). A previous in vitro study
demonstrated that the main regulator of cell motility, the
microvilli-like protrusions arising from the cancer cells, had
clusters of tetraspanin-a3 integrin complexes on them. Upon
treating the cells with tetraspanin and integrin antibodies, the
cancer cells had increased invasive potential due to the
stimulation of MMP2 production and elevated long invasive
protrusion formation (136).
Cancer cell motility is negatively influenced by CD9 via actin
cytoskeleton reorganization. There is a negative correlation
between CD9/ACTB gene expression and lymph node metastasis.
Cytoskeleton reconstruction related to elevated ACTB expression may
be associated with a decrease in CD9 expression (137).
In papillary thyroid microcarcinoma, the patients'
age, multifocality and extrathyroidal extension are known factors
that can be used for prognosis (138). CD9 immunostaining intensity has
been found to be higher in patients with lymph node metastasis than
inpatients without metastasis (P=0.002) (138). CD9 intensity is also correlated
with lymph node metastasis, suggesting that CD9 can be considered a
prognostic marker for lymph node metastasis in papillary thyroid
microcarcinoma (138).
Through its association with other partner proteins,
CD9 has various functions and has been identified as a tumour
suppressor (139). CD9 is
involved in and modifies the steps of tumour formation, such as
proliferation, apoptosis, migration, adhesion and angiogenesis, and
the communication with the environment, dissemination and
metastasis (139). Thus, CD9 has
a major role in cancer development and progression. Venous vessel
invasion, metastasis and poor prognosis are related to tetraspanin
CD9 (139). Upon treating
patients with gastric cancer with CD9 antibody, tumour progression
was found to be inhibited by antiproliferative, pro-apoptotic and
anti-angiogenic effects. This indicates that CD9 may be target in
patients with gastric cancer (139).
The EGFR has shown association with CD9. EGFR
amplification is a characteristic of glioblastoma histology,
affecting the signal transduction pathway. CD9 has the ability to
attenuate the ligand-induced activation of the receptor via the
destabilization of the surface expression of EGFR (140). Phosphorylation of EGFR at
specific sites has been shown to be decreased by CD9 (141). Additionally, cell growth and
proliferation pathways, such as EGFR signalling of PI3K/Akt and
MAPK/Erk, can be attenuated by CD9. By contrast, activation of EGFR
signal transduction pathways, including PI3K/Akt and MAPK/Erk, can
be enhanced by the reduction in CD9 expression via small hairpin
RNA-mediated knockdown of CD9. Inhibition of the activity of
PI3K/Akt and MAPK/Erk signalling pathways and phosphorylation of
EGFR maybe the mechanism underlying the CD9-induced suppression of
cell proliferation (141). CD9,
along with other transmembrane proteins, has the ability to
regulate cell migration (142,143).
CD9 has been identified as a glioma stem
cell-enriched protein. In a context-dependent manner, CD9 is
associated with the progression of malignant tumours and plays a
role in pro-tumorigenesis to promote cancer invasion and tumour
growth in glioblastomas (144).
Predicting patient survival using CD9 expression is a potential
prognostic tool (145). According
to previous reports, cell proliferation and tumour formation are
facilitated by CD9 (129,133,144,146).
The progression of solid tumours is associated with
CD9 downregulation. Patients with advanced stages lack these
molecules, and reduced expression is observed less in primary site
tumours than in metastatic tumours. CD9 may contribute to the
highly invasive and metastatic phenotype of small cell lung
carcinoma. Thus, CD9 is an indicator of poor survival (147).
CD9 expression is an independent prognostic factor
of post-operation recurrence-free survival (RFS) for
gastrointestinal stromal tumours (GIST), as shown by the Cox
proportion hazards regression (HR, 0.104; 95% CI, 0.021-0.528;
P=0.006). The RFS of patients with CD9-negative expression was
significantly worse than that of the CD9-positive expression group
(148). CD9 plays a role in the
inhibition of proliferation and metastasis by inhibiting the
activation, degradation and secretion of the Wnt signalling
pathway, TGFα and metalloproteinase (143,149,150). Downregulation of CD9 is
correlated with tumour invasion and metastasis and is a poor
prognostic marker in various cancers, such as like breast, colon,
small cell lung cancer. Malignant behaviour and tumour progression
can be a result of reduced CD9 expression (148). The post-operative three-year RFS
rate of the CD9-negative group was found to be lower than that of
the CD9-positive group (33.3 vs. 78.4%; P<0.001), as shown in
the universal analysis of comparison between the CD-negative and
CD9-positive group (148). RFS
can be predicted independently using CD9 expression via
multivariate analysis (148).
This result showed that CD9 is important in the invasion and
metastasis of GIST, and the risk of metastasis and recurrence
increases as the expression of CD9 decreases. Hence, the aggressive
and progressive behaviour of GIST can be predicted using CD9
expression (148).
The survival rate of patients with colon cancer with
CD9-positive tumours was reported to be significantly higher than
that of patients with CD9-negative tumours (151). Cell motility inhibition and
induction of apoptosis promoted by concurrent GM3 synthesis and
N-glycosylation may be related to the suppression of malignancy by
CD9 (152). The transmembrane 4
superfamily protein CD9 regulates cell motility by acting as a link
between extracellular integrins and intracellular signalling
molecules, such as phosphatidylinositol 4-kinase (153–155).
Increased invasiveness of breast cancer tumour cells
may be the result of activation of intracellular signalling
molecules, such as PI4K and Src homology, by CD9 crosslink-induced
MMP2 transcription (110). In
epithelial cells, cleavage of TGFα is protected by the interaction
with CD9, which leads to the persistent activation of EGFR
(105,106). Patients without CD9 expression
had improved overall survival (P=0.051) and disease-free survival
(P=0.014) compared with patients with CD9 expression (109). The survival of patients with
breast cancer decreased due to altered cellular proliferation
induced by activated EGFR signalling (108).
Not applicable.
Funding: No funding was received.
Not applicable.
SPC wrote the manuscript, and was responsible for
the original draft preparation, research and editing. SSS
supervised, and wrote, reviewed and edited the manuscript. SKN
supervised, validated the research, and wrote, reviewed and edited
the manuscript. PKS and PP made revisions to the manuscript. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Grandis JR, Melhem MF, Gooding WE, Day R,
Holst VA, Wagener MM, Drenning SD and Tweardy DJ: Levels of TGF-α
and EGFR protein in head and neck squamous cell carcinoma and
patient survival. J Natl Cancer Inst. 90:824–832. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Comprehensive Cancer Network:
Clinical Practice Guidelines in Oncology. Head and Neck Cancer.
v1:2017.Available from. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site
|
|
3
|
Lo Nigro C, Denaro N, Merlotti A and
Merlano M: Head and neck cancer: Improving outcomes with a
multidisciplinary approach. Cancer Manag Res. 9:363–371. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
https://www.cancer.net/cancer-types/head-and-neck-cancer/introduction
|
|
5
|
https://www.uptodate.com/contents/epidemiology-and-risk-factors-for-head-and-neck-cancer?search=epidemiology-and-risk-factors-for-head-and-neck-cancer.&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
|
|
6
|
Hukkanen J, Jacob PII and Benowitz NL:
Metabolism and disposition kinetics of nicotine. Pharmacol Rev.
57:79–115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warren GW and Singh AK: Nicotine and lung
cancer. J Carcinog. 12:12013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hecht SS: Tobacco carcinogens, their
biomarkers and tobacco-induced cancer. Nat Rev Cancer. 3:733–744.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doll R and Peto R: The causes of cancer:
Quantitative estimates of avoidable risks of cancer in the United
States today. J Natl Cancer Inst. 66:1191–1308. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
US Department of Health and Human
Services, . Reducing the Health Consequences of Smoking: 25 Years
of Progress. A Report of the Surgeon General; Centers for Disease
Control and Prevention; Atlanta, GA: 1989
|
|
11
|
Secretan B, Straif K, Baan R, Grosse Y, El
Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C,
Galichet L, et al: A review of human carcinogens-Part E: Tobacco,
areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol.
10:1033–1034. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
https://www.cancer.net/cancer-types/head-and-neck-cancer/risk-factors-and-prevention
|
|
13
|
US Department of Health and Human
Services, . How Tobacco Smoke Causes Disease: The Biology and
Behavioral Basis for Smoking-attributable Disease. A Report of the
Surgeon General; Centers for Disease Control and Prevention;
Atlanta, GA: 2010
|
|
14
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Smokeless tobacco and some
tobacco-specific N-nitrosamines. IARC Monogr Eval Carcinog Risks
Hum. 89:1–592. 2007.PubMed/NCBI
|
|
15
|
Takahashi H, Ogata H, Nishigaki R, Broide
DH and Karin M: Tobacco smoke promotes lung tumorigenesis by
triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell.
17:89–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boyland E, Roe FJ and Gorrod JW: Induction
of Pulmonary tumors in mice by nitrosonornicotine, a possible
constituent of tobacco smoke. Nature. 202:11261964. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Tobacco smoke and involuntary
smoking. IARC Monogr Eval Carcinog Risks Hum. 83:1–1438.
2004.PubMed/NCBI
|
|
18
|
Acetaldehyde. IARC Monogr Eval Carcinog
Risk Chem Hum. 36:101–132. 1985.PubMed/NCBI
|
|
19
|
Seitz HK and Stickel F: Molecular
mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer.
7:599–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haorah J, Ramirez SH, Floreani N, Gorantla
S, Morsey B and Persidsky Y: Mechanism of alcohol-induced oxidative
stress and neuronal injury. Free Radic Biol Med. 45:1542–1550.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang F, Yang JL, Yu KK, Xu M, Xu YZ, Chen
L, Lu YM, Fang HS, Wang XY, Hu ZQ, et al: Activation of the NF-κB
pathway as a mechanism of alcohol enhanced progression and
metastasis of human hepatocellular carcinoma. Mol Cancer.
14:102015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shinohara M, Adachi Y, Mitsushita J,
Kuwabara M, Nagasawa A, Harada S, Furuta S, Zhang Y, Seheli K,
Miyazaki H and Kamata T: Reactive oxygen generated by NADPH oxidase
1 (NOX1) contributes to cell invasion by regulating matrix
metalloprotease-9 production and cell migration. J Biol Chem.
285:4481–4488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ha PK, Chang SS, Glazer CA, Califano JA
and Sidransky D: Molecular techniques and genetic alterations in
head and neck cancer. Oral Oncol. 45:335–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suh Y, Amelio I, Guerrero Urbano T and
Tavassoli M: Clinical update on cancer: Molecular oncology of head
and neck cancer. Cell Death Dis. 5:e10182014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leemans CR, Snijders PJF and Brakenhoff
RH: The molecular landscape of head and neck cancer. Nat Rev
Cancer. 18:269–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawakita A, Yanamoto S, Yamada S, Naruse
T, Takahashi H, Kawasaki G and Umeda M: Microrna-21 promotes oral
cancer invasion via the Wnt/β-catenin pathway by targeting DKK2.
Pathol Oncol Res. 20:253–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
IARC Monographs on the Evaluation of
Carcinogenic Risk to Human. Vol 100C. International Agency for
Research on Cancer; Lyon: 2012
|
|
30
|
Bánfalvi G: Heavy metals, trace elements
and their cellular effects. Cellular Effects of Heavy Metals.
Banfalvi G: Springer; Dordrecht: 2011, View Article : Google Scholar
|
|
31
|
Ercal N, Gurer-Orhan H and Aykin-Burns N:
Toxic metals and oxidative stress part I: Mechanisms involved in
metal-induced oxidative damage. Curr Top Med Chem. 1:529–539. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grund SC, Hanusch K and Wolf HU: Arsenic
and arsenic compounds, Ullmann's encyclopedia of industrial
chemistry. Wiley-VCH; Weinheim: 2005
|
|
33
|
Shi H, Shi X and Liu KJ: Oxidative
mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem.
255:67–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Flora SJ: Arsenic-induced oxidative stress
and its reversibility. Free Radic Biol Med. 51:257–281. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hartwig A and Schwerdtle T: Interactions
by carcinogenic metal compounds with DNA repair processes:
Toxicological implications. Toxicol Lett. 127:47–54. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mass MJ, Tennant A, Roop BC, Cullen WR,
Styblo M, Thomas DJ and Kligerman AD: Methylated trivalent arsenic
species are genotoxic. Chem Res Toxicol. 14:355–361. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bau DT, Wang TS, Chung CH, Wang AS, Wang
AS and Jan KY: Oxidative DNA adducts and DNA-protein cross-links
are the major DNA lesions induced by arsenite. Environ Health
Perspect. 110 (Suppl 5):S753–S756. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goering PL, Aposhian HV, Mass MJ, Cebrián
M, Beck BD and Waalkes MP: The enigma of arsenic carcinogenesis:
Role of metabolism. Toxicol Sci. 49:5–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wilson K, Yang H, Seo CW and Marshall WE:
Select metal adsorption by activated carbon made from peanut
shells. Bioresour Technol. 97:2266–2270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim HS, Kim YJ and Seo YR: An overview of
carcinogenic heavy metal: Molecular toxicity mechanism and
prevention. J Cancer Prev. 20:232–240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dayan AD and Paine AJ: Mechanisms of
chromium toxicity, carcinogenicity and allergenicity: Review of the
literature from 1985 to 2000. Hum Exp Toxicol. 20:439–451. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eastmond DA, MacGregor JT and Slesinski
RS: Trivalent chromium: Assessing the genotoxic risk of an
essential trace element and widely used human and animal
nutritional supplement. Crit Rev Toxicol. 38:173–190. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Katz SA and Salem H: The toxicology of
chromium with respect to its chemical speciation: A review. J Appl
Toxicol. 13:217–224. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Khlifi R, Olmedo P, Gil F, Hammami B,
Chakroun A, Rebai A and Hamza-Chaffai A: Arsenic, cadmium, chromium
and nickel in cancerous and healthy tissues from patients with head
and neck cancer. Sci Total Environ. 452:58–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Beddok A, Krieger S, Castera L,
Stoppa-Lyonnet D and Thariat J: Management of fanconi anemia
patients with head and neck carcinoma: Diagnosis and treatment
adaptation. Oral Oncol. 108:1048162020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gasparini G, Longobardi G, Boniello R, Di
Petrillo A and Pelo S: Fanconi anemia manifesting as a squamous
cell carcinoma of the hard palate: A case report. Head Face Med.
2:12006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Swift MR and Hirschhorn K: Fanconi's
anemia. Inherited susceptibility to chromosome breakage in various
tissues. Ann Intern Med. 65:496–503. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Esparza A and Thompson WR: Familial
hypoplastic anemia with multiple congenital anomalies (Fanconi's
syndrome)-report of three cases. Cases presented are of two sisters
and a female cousin with complete clinical and post mortem
findings. R I Med J. 49:103–110. 1966.PubMed/NCBI
|
|
49
|
Mahmood N, Mihalcioiu C and Rabbani SA:
Multifaceted role of the urokinase-type plasminogen activator (uPA)
and its receptor (uPAR): Diagnostic, prognostic, and therapeutic
applications. Front Oncol. 8:242018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pavón MA, Arroyo-Solera I, Céspedes MV,
Casanova I, León X and Mangues R: uPA/uPAR and SERPINE1 in head and
neck cancer: Role in tumor resistance, metastasis, prognosis and
therapy. Oncotarget. 7:57351–57366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ghiso JA, Kovalski K and Ossowski L: Tumor
dormancy induced by downregulation of urokinase receptor in human
carcinoma involves integrin and MAPK signaling. J Cell Biol.
147:89–104. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ghiso JA: Inhibition of FAK signaling
activated by urokinase receptor induces dormancy in human carcinoma
cells in vivo. Oncogene. 21:2513–2524. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liotta LA and Stetler-Stevenson WG:
Metalloproteinases and cancer invasion. Semin Cancer Biol.
1:99–106. 1990.PubMed/NCBI
|
|
55
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: Biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shapiro SD: Matrix metalloproteinase
degradation of extracellular matrix: Biological consequences. Curr
Opin Cell Biol. 10:602–608. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stetler-Stevenson WG, Hewitt R and
Corcoran M: Matrix metalloproteinases and tumor invasion: From
correlation and causality to the clinic. Semin Cancer Biol.
7:147–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Stetler-Stevenson WG and Anita EY:
Proteases in invasion: Matrix metalloproteinases. Semin Cancer
Biol. 11:143–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ruokolainen H, Pääkkö P and
Turpeenniemi-Hujanen T: Expression of matrix metalloproteinase-9 in
head and neck squamous cell carcinoma: A potential marker for
prognosis. Clin Cancer Res. 10:3110–3116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Angiero F, Gatta LB, Seramondi R, Berenzi
A, Benetti A, Magistro S, Ordesi P, Grigolato P and Dessy E:
Frequency and role of HPV in the progression of epithelial
dysplasia to oral cancer. Anticancer Res. 30:3435–3440.
2010.PubMed/NCBI
|
|
62
|
Zhang W, Zeng Z, Zhou Y, Xiong W, Fan S,
Xiao L, Huang D, Li Z, Li D, Wu M, et al: Identification of
aberrant cell cycle regulation in Epstein-Barr virus-associated
nasopharyngeal carcinoma by cDNA microarray and gene set enrichment
analysis. Acta Biochim Biophys Sin (Shanghai). 41:414–428. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
International Agency for Research on
Cancer, . A review of human carcinogens: Arsenic, metals, fibres,
and dusts. IARC Monogr Eval Carcinog Risks Hum. 100:169–211.
2012.PubMed/NCBI
|
|
64
|
Prevention and Control Exchange (PACE)
World Health Organization. Occupational and Environmental Health
Team, . Hazard Prevention and Control in the Work Environment:
Airborne Dust. World Health Organisation. 1999.Available from.
https://apps.who.int/iris/handle/10665/66147
|
|
65
|
Langevin SM, McClean MD, Michaud DS, Eliot
M, Nelson HH and Kelsey KT: Occupational dust exposure and head and
neck squamous cell carcinoma risk in a population-based
case-control study conducted in the greater Boston area. Cancer
Med. 2:978–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Panahi Y, Gholami N, Ghojazadeh M, Moslemi
F, Naghavi-Behzad M, Azami-Aghdash S, Ghaffari A and Piri R:
Complications and carcinogenic effects of mustard Gas-a systematic
review and meta-analysis in Iran. Asian Pac J Cancer Prev.
16:7567–7573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Safarinejad MR: Testicular effect of
mustard gas. Urology. 58:90–94. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
McClintock SD, Till GO, Smith MG and Ward
PA: Protection from half-mustard-gas-induced acute lung injury in
the rat. J Appl Toxicol. 22:257–262. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Thiagarajan A and Iyer NG:
Radiation-induced sarcomas of the head and neck. World J Clin
Oncol. 5:973–981. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ho CM, Lam KH, Wei WI, Lau SK and Lam LK:
Occult lymph node metastasis in small oral tongue cancers. Head
Neck. 14:359–363. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Spiro RH, Huvos AG, Wong GY, Spiro JD,
Gnecco CA and Strong EW: Predictive value of tumor thickness in
squamous carcinoma confined to the tongue and floor of the mouth.
Am J Surg. 152:345–350. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kawano K and Yanagisawa S: Predictive
value of laminin-5 and membrane type 1-matrix metalloproteinase
expression for cervical lymph node metastasis in T1 and T2 squamous
cell carcinomas of the tongue and floor of the mouth. Head Neck.
28:525–533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Califf RM: Biomarker definitions and their
applications. Exp Biol Med (Maywood). 243:213–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kuropkat C, Plehn S, Herz U, Dunne AA,
Renz H and Werner JA: Tumor marker potential of serum matrix
metalloproteinases in patients with head and neck cancer.
Anticancer Res. 22:2221–2227. 2002.PubMed/NCBI
|
|
75
|
Li Y, St John MA, Zhou X, Kim Y, Sinha U,
Jordan RC, Eisele D, Abemayor E, Elashoff D, Park NH and Wong DT:
Salivary transcriptome diagnostics for oral cancer detection. Clin
Cancer Res. 10:8442–8450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
St John MA, Li Y, Zhou X, Denny P, Ho CM,
Montemagno C, Shi W, Qi F, Wu B, Sinha U, et al: Interleukin-6 and
interleukin-8 as potential biomarkers for oral cavity and
oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck
Surg. 130:929–935. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Toyoshima T, Vairaktaris E, Nkenke E,
Schlegel KA, Neukam FW and Ries J: Cytokeratin 17 mRNA expression
has potential for diagnostic marker of oral squamous cell
carcinoma. J Cancer Res Clin Oncol. 134:515–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Cohen-Kerem R, Madah W, Sabo E, Rahat MA,
Greenberg E and Elmalah I: Cytokeratin-17 as a potential marker for
squamous cell carcinoma of the larynx. Ann Otol Rhinol Laryngol.
113:821–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Park NJ, Zhou H, Elashoff D, Henson BS,
Kastratovic DA, Abemayor E and Wong DT: Salivary microRNA:
Discovery, characterization, and clinical utility for oral cancer
detection. Clin Cancer Res. 15:5473–5477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Concha-Benavente F, Srivastava RM, Trivedi
S, Lei Y, Chandran U, Seethala RR, Freeman GJ and Ferris RL:
Identification of the cell-intrinsic and -extrinsic pathways
downstream of EGFR and IFNγ that induce PD-L1 expression in head
and neck cancer. Cancer Res. 76:1031–1043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hira-Miyazawa M, Nakamura H, Hirai M,
Kobayashi Y, Kitahara H, Bou-Gharios G and Kawashiri S: Regulation
of programmed-death ligand in the human head and neck squamous cell
carcinoma microenvironment is mediated through matrix
metalloproteinase-mediated proteolytic cleavage. Int J Oncol.
52:379–388. 2018.PubMed/NCBI
|
|
83
|
Yang WF, Wong MC, Thomson PJ, Li KY and Su
YX: The prognostic role of PD-L1 expression for survival in head
and neck squamous cell carcinoma: A systematic review and
meta-analysis. Oral Oncol. 86:81–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Goel R, Moore W, Sumer B, Khan S, Sher D
and Subramaniam RM: Clinical practice in PET/CT for the management
of head and neck squamous cell cancer. Am J Roentgenol.
209:289–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hentschel M, Appold S, Schreiber A,
Abolmaali N, Abramyuk A, Dörr W, Kotzerke J, Baumann M and Zöphel
K: Early FDG PET at 10 or 20 Gy under chemoradiotherapy is
prognostic for locoregional control and overall survival in
patients with head and neck cancer. Eur J Nucl Med Mol Imaging.
38:1203–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mohammed RN, Watson HA, Vigar M, Ohme J,
Thomson A, Humphreys IR and Ager A: L-selectin is essential for
delivery of activated CD8(+) T cells to virus-infected organs for
protective immunity. Cell Rep. 14:760–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Resto VA, Burdick MM, Dagia NM, McCammon
SD, Fennewald SM and Sackstein R: L-selectin-mediated
lymphocyte-cancer cell interactions under low fluid shear
conditions. J Biol Chem. 283:15816–15824. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Longo N, Yáñez-Mó M, Mittelbrunn M, de
la Rosa G, Muñoz ML, Sánchez-Madrid F and Sánchez-Mateos P:
Regulatory role of tetraspanin CD9 in tumor-endothelial cell
interaction during transendothelial invasion of melanoma cells.
Blood. 98:3717–3726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kohmo S, Kijima T, Otani Y, Mori M, Minami
T, Takahashi R, Nagatomo I, Takeda Y, Kida H, Goya S, et al: Cell
surface tetraspanin CD9 mediates chemoresistance in small cell lung
cancer. Cancer Res. 70:8025–8035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Stipp CS, Kolesnikova TV and Hemler ME:
Functional domains in tetraspanin proteins. Trends Biochem Sci.
28:106–112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kitadokoro K, Bordo D, Galli G, Petracca
R, Falugi F, Abrignani S, Grandi G and Bolognesi M: CD81
extracellular domain 3D structure: Insight into the tetraspanin
superfamily structural motifs. EMBO J. 20:12–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hemler ME: Specific tetraspanin functions.
J Cell Biol. 155:1103–1107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Clark KL, Oelke A, Johnson ME, Eilert KD,
Simpson PC and Todd SC: CD81 associates with 14-3-3 in a
redox-regulated palmitoylation-dependent manner. J Biol Chem.
279:19401–19406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kovalenko OV, Metcalf DG, degrado WF and
Hemler ME: Structural organization and interactions of
transmembrane domains in tetraspanin proteins. BMC Struct Biol.
5:112005. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Fitter S, Seldin MF and Ashman LK:
Characterisation of the mouse homologue of CD151 (PETA-3/SFA-1);
genomic structure, chromosomal localisation and identification of 2
novel splice forms. Biochim Biophys Acta. 1398:75–85. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Stipp CS, Kolesnikova TV and Hemler ME:
EWI-2 regulates alpha3beta1 integrin-dependent cell functions on
laminin-5. J Cell Biol. 163:1167–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Seigneuret M, Delaguillaumie A,
Lagaudrière-Gesbert C and Conjeaud H: Structure of the tetraspanin
main extracellular domain. A partially conserved fold with a
structurally variable domain insertion. J Biol Chem.
276:40055–40064. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Maecker HT, Todd SC and Levy S: The
tetraspanin superfamily: Molecular facilitators. FASEB J.
11:428–442. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yanez-Mo M, Mittelbrunn M and
Sanchez-Madrid F: Tetraspanins and intercellular interactions.
Microcirculation. 8:153–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Boucheix C and Rubinstein E: Tetraspanins.
Cell Mol Life Sci. 58:1189–1205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Boucheix C, Benoit P, Frachet P, Billard
M, Worthington RE, Gagnon J and Uzan G: Molecular cloning of the
CD9 antigen. A new family of cell surface proteins. J Biol Chem.
266:117–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ovalle S, Gutiérrez-López MD, Olmo N,
Turnay J, Lizarbe MA, Majano P, Molina-Jiménez F, López-Cabrera M,
Yáñez-Mó M, Sánchez-Madrid F and Cabañas C: The tetraspanin CD9
inhibits the proliferation and tumorigenicity of human colon
carcinoma cells. Int J Cancer. 121:2140–2152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kersey JH, LeBien TW, Abramson CS, Newman
R, Sutherland R and Greaves M: P-24: A human leukemia-associated
and lymphohemopoietic progenitor cell surface structure identified
with monoclonal antibody. J Exp Med. 153:726–731. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Wright MD, Moseley GW and van Spriel AB:
Tetraspanin microdomains in immune cell signalling and malignant
disease. Tissue Antigens. 64:533–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hemler ME: Targeting of tetraspanin
proteins-potential benefits and strategies. Nat Rev Drug Discov.
7:747–758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Baek J, Jang N, Choi JE, Kim JR and Bae
YK: CD9 expression in tumor cells is associated with poor prognosis
in patients with invasive lobular carcinoma. J Breast Cancer.
22:77–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zöller M: Tetraspanins: Push and pull in
suppressing and promoting metastasis. Nat Rev Cancer. 9:40–55.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shi W, Fan H, Shum L and Derynck R: The
tetraspanin CD9 associates with transmembrane TGF-alpha and
regulates TGF-alpha-induced EGF receptor activation and cell
proliferation. J Cell Biol. 148:591–602. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Hwang JR, Jo K, Lee Y, Sung BJ, Park YW
and Lee JH: Upregulation of CD9 in ovarian cancer is related to the
induction of TNF-α gene expression and constitutive NF-κB
activation. Carcinogenesis. 33:77–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yáñez-Mó M, Alfranca A, Cabañas C,
Marazuela M, Tejedor R, Ursa MA, Ashman LK, de Landázuri MO and
Sánchez-Madrid F: Regulation of endothelial cell motility by
complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-1 with
a3b1 integrin localized at endothelial lateral junctions. J Cell
Biol. 141:791–804. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Okochi H, Kato M, Nashiro K, Yoshie O,
Miyazono K and Furue M: Expression of tetra-spans transmembrane
family (CD9, CD37, CD53, CD63, CD81 and CD82) in normal and
neoplastic human keratinocytes: An association of CD9 with alpha 3
beta 1 integrin. Br J Dermatol. 137:856–863. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nishida M, Miyagawa J, Yamashita S,
Higashiyama S, Nakata A, Ouchi N, Tamura R, Yamamori K, Kihara S,
Taniguchi N and Matsuzawa Y: Localization of CD9, an enhancer
protein for proheparin-binding epidermal growth factor-like growth
factor, in human atherosclerotic plaques: Possible involvement of
juxtacrine growth mechanism on smooth muscle cell proliferation.
Arterioscler Thromb Vasc Biol. 20:1236–1243. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Klein-Soyer C, Azorsa DO, Cazenave JP and
Lanza F: CD9 participates in endothelial cell migration during in
vitro wound repair. Arterioscler Thromb Vasc Biol. 20:360–369.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Peñas PF, García-Díez A, Sánchez-Madrid F
and Yáñez-Mó M: Tetraspanins are localized at motility-related
structures and involved in normal human keratinocyte wound healing
migration. J Invest Dermatol. 114:1126–1135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lijen HR, Lupu F, Collen D, Le Nour F and
Boucheix C: CD9 gene deficiency does not affect smooth muscle cell
migration and neointima formation after vascular injury in mice.
Thromb Haemost. 83:956–961. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Erovic BM, Pammer J, Hollemann D,
Woegerbauer M, Geleff S, Fischer MB, Burian M, Frommlet F and
Neuchrist C: Motility-related protein-1/CD9 expression in head and
neck squamous cell carcinoma. Head Neck. 25:848–857. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lagaudrière-Gesbert C, Le Naour F,
Lebel-Binay S, Billard M, Lemichez E, Boquet P, Boucheix C,
Conjeaud H and Rubinstein E: Functional analysis of four
tetraspans, CD9, CD53, CD81, and CD82, suggests a common role in
costimulation, cell adhesion, and migration: Only CD9 upregulates
HB-EGF activity. Cell Immunol. 182:105–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Oren R, Takahashi S, Doss C, Levy R and
Levy S: TAPA-1, the target of an antiproliferative antibody,
defines a new family of transmembrane proteins. Mol Cell Biol.
10:4007–4015. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wice BM and Gordon JI: A tetraspan
membrane glycoprotein produced in the human intestinal epithelium
and liver that can regulate cell density-dependent proliferation. J
Biol Chem. 270:21907–21918. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Buim ME, Lourenço SV, Carvalho KC, Cardim
R, Pereira C, Carvalho AL, Fregnani JH and Soares FA:
Downregulation of CD9 protein expression is associated with
aggressive behavior of oral squamous cell carcinoma. Oral Oncol.
46:166–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Huang CI, Kohno N, Ogawa E, Adachi M, Taki
T and Miyake M: Correlation of reduction in MRP-1/CD9 and KAI1/CD82
expression with recurrences in breast cancer patients. Am J Pathol.
153:973–983. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mhawech P, Herrmann F, Coassin M, Guillou
L and Iselin CE: Motility-related protein 1 (MRP-1/CD9) expression
in urothelial bladder carcinoma and its relation to tumor
recurrence and progression. Cancer. 98:1649–1657. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Sauer G, Windisch J, Kurzeder C, Heilmann
V, Kreienberg R and Deissler H: Progression of cervical carcinomas
is associated with down-regulation of CD9 but strong local
re-expression at sites of transendothelial invasion. Clin Cancer
Res. 9:6426–6431. 2003.PubMed/NCBI
|
|
124
|
Kusukawa J, Ryu F, Kameyama T and Mekada
E: Reduced expression of CD9 in oral squamous cell carcinoma: CD9
expression inversely related to high prevalence of lymph node
metastasis. J Oral Pathol Med. 30:73–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhang BH, Liu W, Li L, Lu JG, Sun YN, Jin
DJ and Xu XY: KAI1/CD82 and MRP1/CD9 serve as markers of
infiltration, metastasis, and prognosis in laryngeal squamous cell
carcinomas. Asian Pac J Cancer Prev. 14:3521–3526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Miyake M, Koyama M, Seno M and Ikeyama S:
Identification of the motility-related protein (MRP-1), recognized
by monoclonal antibody M31-15, which inhibits cell motility. J Exp
Med. 174:1347–1354. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Ikeyama S, Koyama M, Yamaoko M, Sasada R
and Miyake M: Suppression of cell motility and metastasis by
transfection with human motility-related protein (MRP-1/CD9) DNA. J
Exp Med. 177:1231–1237. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Uchida S, Shimada Y, Watanabe G, Li ZG,
Hong T, Miyake M and Imamura M: Motility-related protein
(MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph
node metastasis in oesophageal squamous cell carcinoma. Br J
Cancer. 79:1168–1173. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Higashiyama S, Iwamoto R, Goishi K, Raab
G, Taniguchi N, Klagsbrun M and Mekada E: The membrane protein
CD9/DRAP 27 potentiates the juxtacrine growth factor activity of
the membrane-anchored heparin-binding EGF-like growth factor. J
Cell Biol. 128:929–938. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Nakamura K, Iwamoto R and Mekada E:
Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF)
and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form
a complex with integrin alpha 3 beta 1 at cell-cell contact sites.
J Cell Biol. 129:1691–1705. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hato T, Ikeda K, Yasukawa M, Watanabe A
and Kobayashi Y: Exposure of platelet fibrinogen receptors by a
monoclonal antibody to CD9 antigen. Blood. 72:224–229. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Higashihara M, Takahata K, Yatomi Y,
Nakahara K and Kurokawa K: Purification and partial
characterization of CD9 antigen of human platelets. FEBS Lett.
264:270–274. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Hirano C, Nagata M, Noman AA, Kitamura N,
Ohnishi M, Ohyama T, Kobayashi T, Suzuki K, Yoshizawa M, Izumi N,
et al: Tetraspanin gene expression levels as potential biomarkers
for malignancy of gingival squamous cell carcinoma. Int J Cancer.
124:2911–2916. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Nagata M, Fujita H, Ida H, Hoshina H,
Inoue T, Seki Y, Ohnishi M, Ohyama T, Shingaki S, Kaji M, et al:
Identification of potential biomarkers of lymph node metastasis in
oral squamous cell carcinoma by cDNA microarray analysis. Int J
Cancer. 106:683–689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Kurokawa A, Nagata M, Kitamura N, Noman
AA, Ohnishi M, Ohyama T, Kobayashi T, Shingaki S and Takagi R;
Oral, Maxillofacial Pathology, Surgery Group, : Diagnostic value of
integrin alpha3, beta4, and beta5 gene expression levels for the
clinical outcome of tongue squamous cell carcinoma. Cancer.
112:1272–1281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Sugiura T and Berditchevski F: Function of
alpha3beta1-tetraspanin protein complexes in tumor cell invasion.
Evidence for the role of the complexes in production of matrix
metalloproteinase 2 (MMP-2). J Cell Biol. 146:1375–1389. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Huang CL, Ueno M, Liu D, Masuya D, Nakano
J, Yokomise H, Nakagawa T and Miyake M: MRP-1/CD9 gene transduction
regulates the actin cytoskeleton through the downregulation of
WAVE2. Oncogene. 25:6480–6488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Kim T, Kim Y and Kwon HJ: Expression of
CD9 and CD82 in papillary thyroid microcarcinoma and its prognostic
significance. Endokrynol Pol. 70:224–231. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Murayama Y, Oritani K and Tsutsui S: Novel
CD9-targeted therapies in gastric cancer. World J Gastroenterol.
21:3206–3213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Murayama Y, Shinomura Y, Oritani K,
Miyagawa JI, Yoshida H, Nishida M, Katsube F, Shiraga M, Miyazaki
T, Nakamoto T, et al: The tetraspanin CD9 modulates epidermal
growth factor receptor signaling in cancer cells. J Cell Physiol.
216:135–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wang GP and Han XF: CD9 modulates
proliferation of human glioblastoma cells via epidermal growth
factor receptor signaling. Mol Med Re. 12:1381–1386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Halova I, Dráberová L, Bambousková M,
Machyna M, Stegurová L, Smrž D and Dráber P: Cross-talk between
tetraspanin CD9 and transmembrane adaptor protein non-T cell
activation linker (NTAL) in mast cell activation and chemotaxis. J
Biol Chem. 288:9801–9814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Huang CL, Liu D, Masuya D, Kameyama K,
Nakashima T, Yokomise H, Ueno M and Miyake M: MRP-1/CD9 gene
transduction downregulates Wnt signal pathways. Oncogene.
23:7475–7483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Podergajs N, Motaln H, Rajčević U,
Verbovšek U, Koršič M, Obad N, Espedal H, Vittori M, Herold-Mende
C, Miletic H, et al: Transmembrane protein CD9 is glioblastoma
biomarker, relevant for maintenance of glioblastoma stem cells.
Oncotarget. 7:593–609. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Higashiyama M, Taki T, Ieki Y, Adachi M,
Huang CL, Koh T, Kodama K, Doi O and Miyake M: Reduced motility
related protein-1 (MRP-1/CD9) gene expression as a factor of poor
prognosis in non-small cell lung cancer. Cancer Res. 55:6040–6044.
1995.PubMed/NCBI
|
|
146
|
Shi Y, Zhou W, Cheng L, Chen C, Huang Z,
Fang X, Wu Q, He Z, Xu S, Lathia JD, et al: Tetraspanin CD9
stabilizes gp130 by preventing its ubiquitin-dependent lysosomal
degradation to promote STAT3 activation in glioma stem cells. Cell
Death Differ. 24:167–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Funakoshi T, Tachibana I, Hoshida Y,
Kimura H, Takeda Y, Kijima T, Nishino K, Goto H, Yoneda T, Kumagai
T, et al: Expression of tetraspanins in human lung cancer cells:
Frequent downregulation of CD9 and its contribution to cell
motility in small cell lung cancer. Oncogene. 22:674–687. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Yang H, Shen C, Zhang B, Chen H, Chen Z
and Chen J: Expression and clinicopathological significance of CD9
in gastrointestinal stromal tumor. J Korean Med Sci. 28:1443–1448.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Imhof I, Gasper WJ and Derynck R:
Association of tetraspanin CD9 with transmembrane TGF{alpha}
confers alterations in cell-surface presentation of TGF{alpha} and
cytoskeletal organization. J Cell Sci. 121:2265–2274. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Saito Y, Tachibana I, Takeda Y, Yamane H,
He P, Suzuki M, Minami S, Kijima T, Yoshida M, Kumagai T, et al:
Absence of CD9 enhances adhesion-dependent morphologic
differentiation, survival, and matrix metalloproteinase-2
production in small cell lung cancer cells. Cancer Res.
66:9557–9565. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hashida H, Takabayashi A, Tokuhara T,
Hattori N, Taki T, Hasegawa H, Satoh S, Kobayashi N, Yamaoka Y and
Miyake M: Clinical significance of transmembrane 4 superfamily in
colon cancer. Br J Cancer. 89:158–167. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ono M, Handa K, Withers DA and Hakomori
SI: Motility inhibition and apoptosis are induced by
metastasis-suppressing gene product CD82 and its analogue CD9, with
concurrent glycosylation. Cancer Res. 59:2335–2339. 1999.PubMed/NCBI
|
|
153
|
Yauch RL, Berditchevski F, Harler MB,
Reichner J and Hemler ME: Highly stoichiometric, stable, and
specific association of integrin alpha3beta1 with CD151 provides a
major link to phosphatidylinositol 4-kinase, and may regulate cell
migration. Mol Biol Cell. 9:2751–2765. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hemler ME, Mannion BA and Barditchevski F:
Association of TM4SF proteins with integrins: Relevance to cancer.
Biochim Biophys Acta. 1287:67–71. 1996.PubMed/NCBI
|
|
155
|
Berditchevski F and Odintsova E:
Characterization of integrin-tetraspanin adhesion complexes: Role
of tetraspanins in integrin signaling. J Cell Biol. 146:477–492.
1999. View Article : Google Scholar : PubMed/NCBI
|