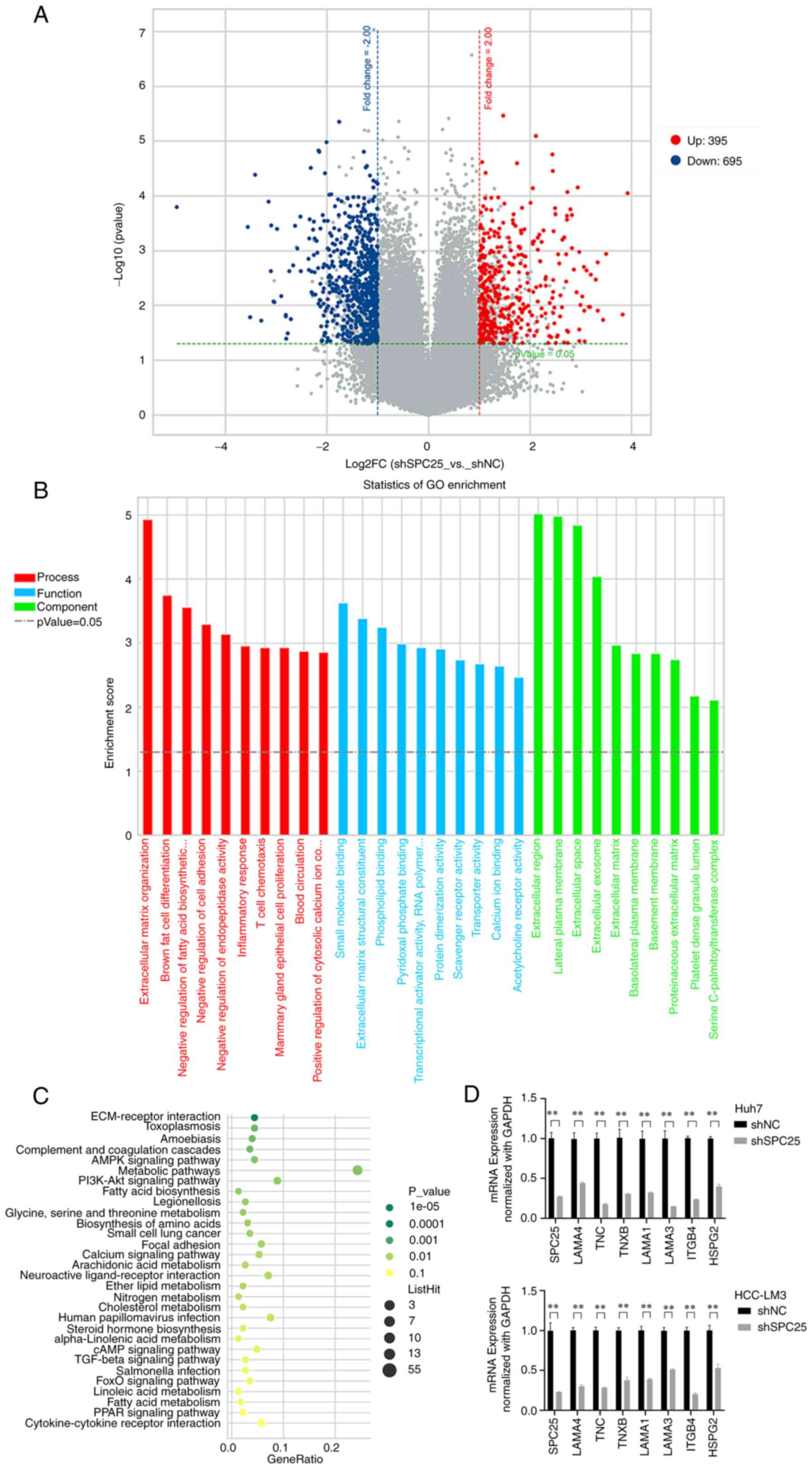
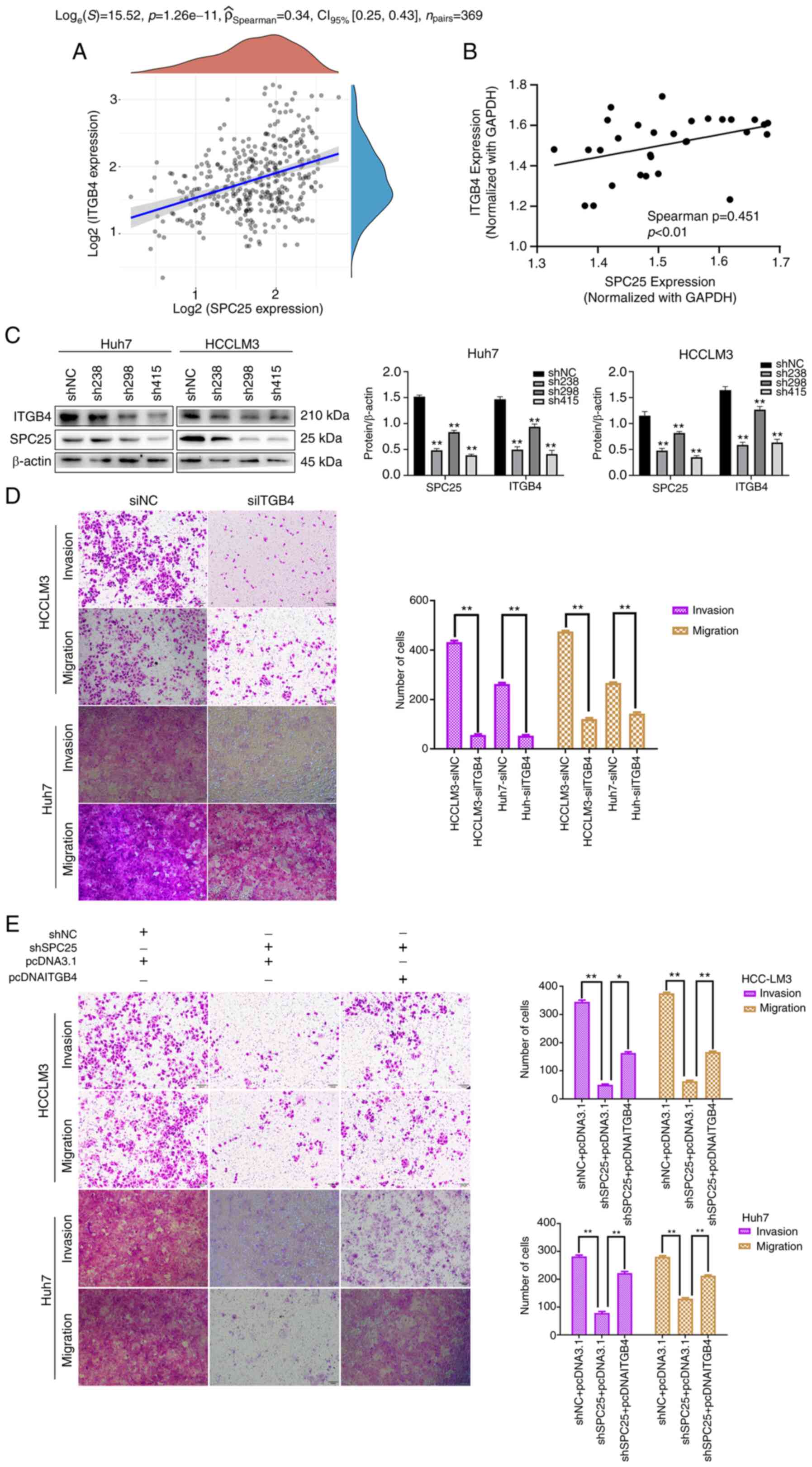
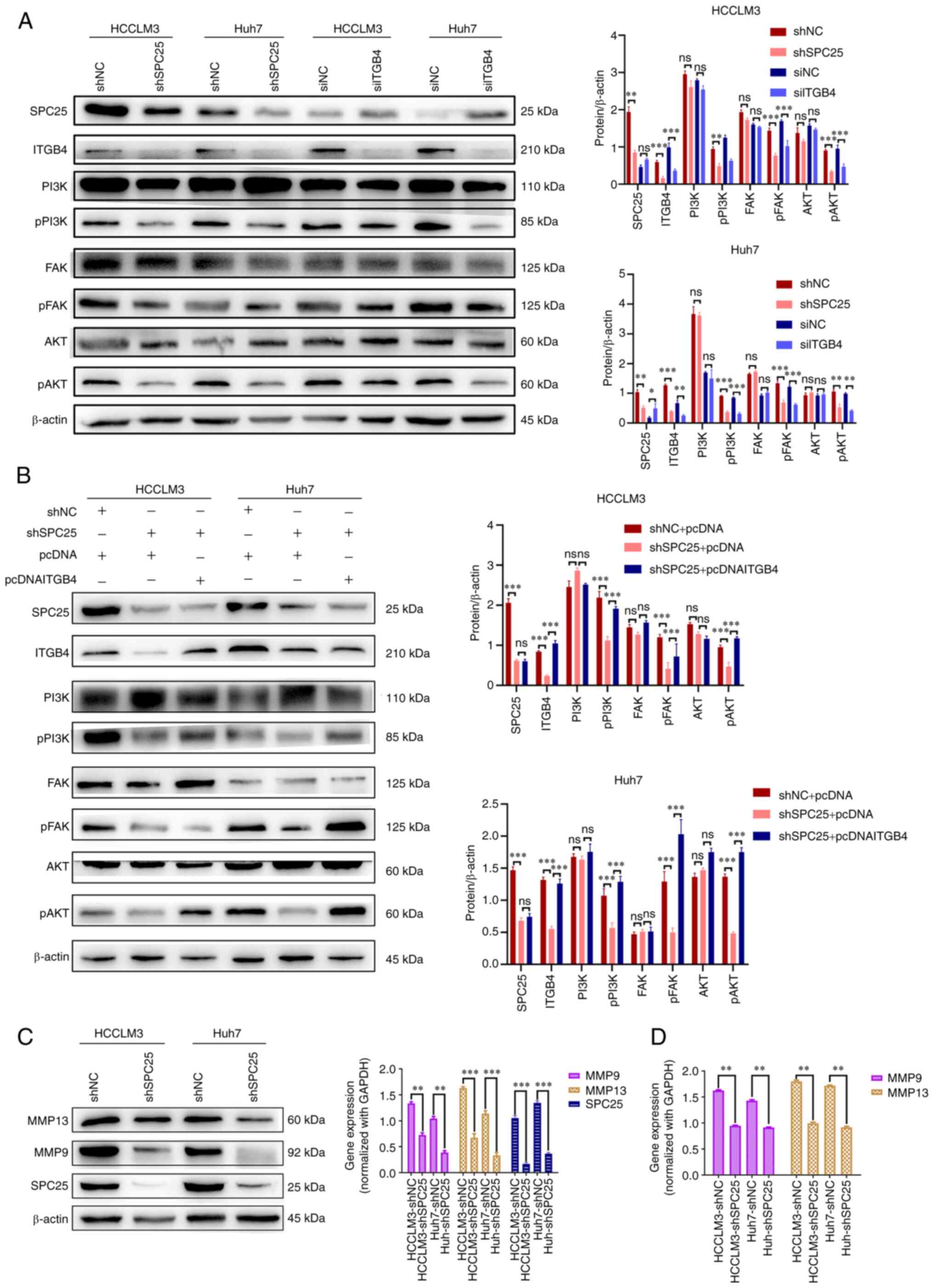
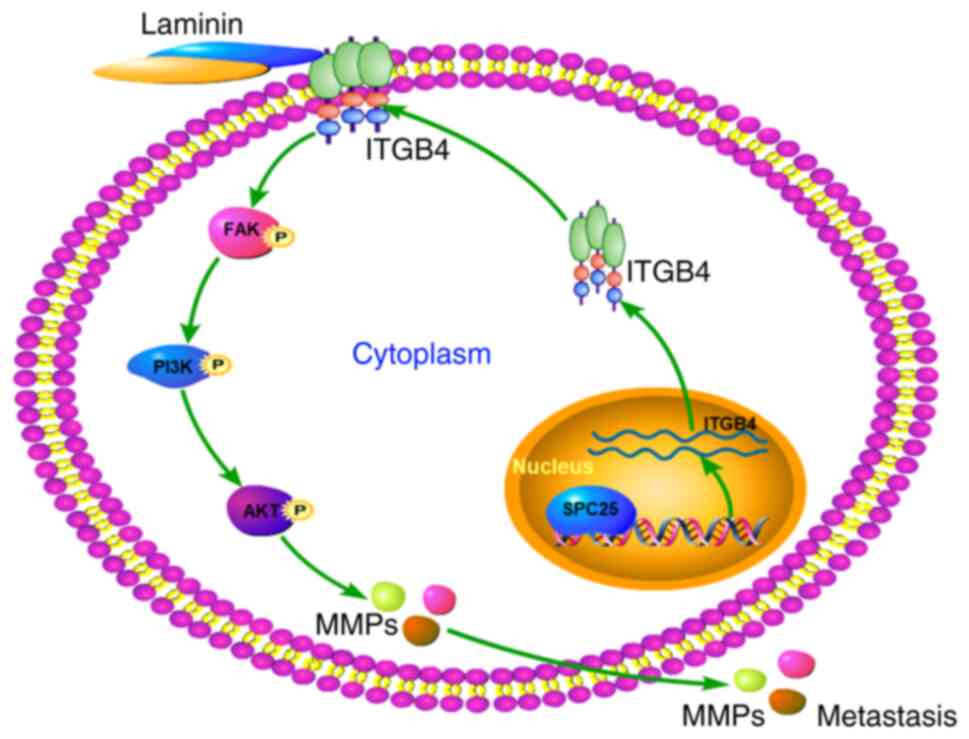
|
1
|
Hindson J: Lenvatinib plus EGFR inhibition
for liver cancer. Nat Rev Gastroenterol Hepatol. 18:6752021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Villanueva A: Hepatocellular carcinoma. N
Engl J Med. 380:1450–1462. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: SHARP investigators study group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J, da Fonseca LG and Reig M:
Insights into the success and failure of systemic therapy for
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
16:617–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Holle AW, Young JL and Spatz JP: In vitro
cancer cell-ECM interactions inform in vivo cancer treatment. Adv
Drug Deliv Rev. 97:270–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitra SK and Schlaepfer DD: Integrin
regulated FAK-Src signaling in normal and cancer cells. Curr Opin
Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SH, Turnbull J and Guimond S:
Extracellular matrix and cell signalling: The dynamic cooperation
of integrin, proteoglycan and growth factor receptor. J Endocrinol.
209:139–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang Y, Chen X, Wu Y, Li J, Zhang S, Wang
K, Guan X, Yang K and Bai Y: LncRNA CASC9 promotes esophageal
squamous cell carcinoma metastasis through upregulating LAMC2
expression by interacting with the CREB-binding protein. Cell Death
Differ. 25:1980–1995. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tai YL, Chu PY, Lai IR, Wang MY, Tseng HY,
Guan JL, Liou JY and Shen TL: An EGFR/Src-dependent β4 integrin/FAK
complex contributes to malignancy of breast cancer. Sci Rep.
5:164082015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leng C, Zhang ZG, Chen WX, Luo HP, Song J,
Dong W, Zhu XR, Chen XP, Liang HF and Zhang BX: An integrin
beta4-EGFR unit promotes hepatocellular carcinoma lung metastases
by enhancing anchorage independence through activation of FAK-AKT
pathway. Cancer Lett. 376:188–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun SC, Lee SE, Xu YN and Kim NH:
Perturbation of Spc25 expression affects meiotic spindle
organization, chromosome alignment and spindle assembly checkpoint
in mouse oocytes. Cell Cycle. 9:4552–4559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aguilera A and García-Muse T: Causes of
genome instability. Annu Rev Genet. 47:1–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Chen H, Yang H and Dai H: SPC25
upregulation increases cancer stem cell properties in non-small
cell lung adenocarcinoma cells and independently predicts poor
survival. Biomed Pharmacother. 100:233–239. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Q, Zhu Y, Li Z, Bu Q, Sun T, Wang H,
Sun H and Cao X: Up-regulation of SPC25 promotes breast cancer.
Aging (Albany NY). 11:5689–5704. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui F, Tang H, Tan J and Hu J: Spindle
pole body component 25 regulates stemness of prostate cancer cells.
Aging (Albany NY). 10:3273–3282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang X, Sun H, Song Y, Yang L and Liu H:
Diagnostic and prognostic values of upregulated SPC25 in patients
with hepatocellular carcinoma. PeerJ. 8:e95352020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang B, Zhou Q, Xie Q, Lin X, Miao W, Wei
Z, Zheng T, Pang Z, Liu H and Chen X: SPC25 overexpression promotes
tumor proliferation and is prognostic of poor survival in
hepatocellular carcinoma. Aging (Albany NY). 13:2803–2821. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen F, Zhang K, Huang Y, Luo F, Hu K and
Cai Q: SPC25 may promote proliferation and metastasis of
hepatocellular carcinoma via p53. FEBS Open Bio. 10:1261–1275.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi WK, Zhu XD, Wang CH, Zhang YY, Cai H,
Li XL, Cao MQ, Zhang SZ, Li KS and Sun HC: PFKFB3 blockade inhibits
hepatocellular carcinoma growth by impairing DNA repair through
AKT. Cell Death Dis. 9:4282018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiyonobu N, Shimada S, Akiyama Y, Mogushi
K, Itoh K, Akahoshi K, Matsumura S, Ogawa K, Ono H, Mitsunori Y, et
al: Fatty Acid Binding Protein 4 (FABP4) overexpression in
intratumoral hepatic stellate cells within hepatocellular carcinoma
with metabolic risk factors. Am J Pathol. 188:1213–1224. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimada S, Mogushi K, Akiyama Y, Furuyama
T, Watanabe S, Ogura T, Ogawa K, Ono H, Mitsunori Y, Ban D, et al:
Comprehensive molecular and immunological characterization of
hepatocellular carcinoma. EBioMedicine. 40:457–470. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun FX, Tang ZY, Lui KD, Ye SL, Xue Q, Gao
DM and Ma ZC: Establishment of a metastatic model of human
hepatocellular carcinoma in nude mice via orthotopic implantation
of histologically intact tissues. Int J Cancer. 66:239–243. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang CH, Zhu XD, Ma DN, Sun HC, Gao DM,
Zhang N, Qin CD, Zhang YY, Ye BG, Cai H, et al: Flot2 promotes
tumor growth and metastasis through modulating cell cycle and
inducing epithelial-mesenchymal transition of hepatocellular
carcinoma. Am J Cancer Res. 7:1068–1083. 2017.PubMed/NCBI
|
|
27
|
Lee JS and Thorgeirsson SS: Functional and
genomic implications of global gene expression profiles in cell
lines from human hepatocellular cancer. Hepatology. 35:1134–1143.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Jiang X, Wang G, Zhai C, Liu Y, Li
H, Zhang Y, Yu W and Zhao Z: ITGB4 is a novel prognostic factor in
colon cancer. J Cancer. 10:5223–5233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu P, Wang Y, Wu Y, Jia Z, Song Y and
Liang N: Expression and prognostic analyses of ITGA11, ITGB4
and ITGB8 in human non-small cell lung cancer. PeerJ.
7:e82992019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wilkinson EJ, Woodworth AM, Parker M,
Phillips JL, Malley RC, Dickinson JL and Holloway AF: Epigenetic
regulation of the ITGB4 gene in prostate cancer. Exp Cell Res.
392:1120552020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhuang H, Zhou Z, Ma Z, Li Z, Liu C, Huang
S, Zhang C and Hou B: Characterization of the prognostic and
oncologic values of ITGB superfamily members in pancreatic cancer.
J Cell Mol Med. 24:13481–13493. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sung JS, Kang CW, Kang S, Jang Y, Chae YC,
Kim BG and Cho NH: ITGB4-mediated metabolic reprogramming of
cancer-associated fibroblasts. Oncogene. 39:664–676. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li XL, Liu L, Li DD, He YP, Guo LH, Sun
LP, Liu LN, Xu HX and Zhang XP: Integrin β4 promotes cell invasion
and epithelial-mesenchymal transition through the modulation of
Slug expression in hepatocellular carcinoma. Sci Rep. 7:404642017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang W, Zuidema A, Te Molder L, Nahidiazar
L, Hoekman L, Schmidt T, Coppola S and Sonnenberg A: Hemidesmosomes
modulate force generation via focal adhesions. J Cell Biol.
219:e2019041372020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walko G, Castañón MJ and Wiche G:
Molecular architecture and function of the hemidesmosome. Cell
Tissue Res. 360:363–378. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu L, Hou Y, Tu G, Chen Y, Du YE, Zhang H,
Wen S, Tang X, Yin J, Lang L, et al: Nuclear Drosha enhances cell
invasion via an EGFR-ERK1/2-MMP7 signaling pathway induced by
dysregulated miRNA-622/197 and their targets LAMC2 and CD82 in
gastric cancer. Cell Death Dis. 8:e26422017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Radisky DC, Levy DD, Littlepage LE, Liu H,
Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et
al: Rac1b and reactive oxygen species mediate MMP-3-induced EMT and
genomic instability. Nature. 436:123–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walen KH: Genomic Instability in cancer I:
DNA-Repair triggering primitive hereditary 4n-Skewed, amitotic
division-system, the culprit in EMT/MET/Metaplasia cancer-concepts.
J Cancer Ther. 9:974–997. 2018. View Article : Google Scholar
|