The CME is an extremely special environment that
favors cancer progression. The CME is characterized by various
conditions, such as hypoxia and angiogenesis, and contains several
components, such as cancer-associated fibroblasts (CAFs),
infiltrated immune cells, and cancer stem cells (CSCs) (1–3).
These conditions and components affect each other, establishing a
cancer-specific environment. The actual conditions of the CME are
complex and multifactorial. Hypoxia is considered to be an
important factor in the CME. Hypoxia regulates various factors and
conditions of the CME. The signaling pathways and molecules that
are not activated under normoxia may be activated under hypoxia.
Cancer may induce a malignant phenotype through hypoxia-activated
signaling pathways, such as the Hedgehog (HH) signaling pathway,
and molecules. HH signaling is involved in the CME and is activated
under hypoxic conditions (4).
Moreover, the contribution of HH signaling to cancer shifts from
gene mutation to a ligand-dependent paracrine manner via
surrounding conditions such as the CME (5). Therefore, the importance of the CME
in HH signaling activation has received significant attention.
Treating cancers using only a single therapeutic method is
considered difficult. Various processes in cancer progression that
are observed in the CME may be involved in this refractory
mechanism. It is considered that an in-depth understanding of the
various conditions observed in the CME and measures to prevent
these conditions will contribute to the development of new
effective cancer therapies for the next generation. To understand
the CME, the individual factors that constitute the CME should be
first elucidated.
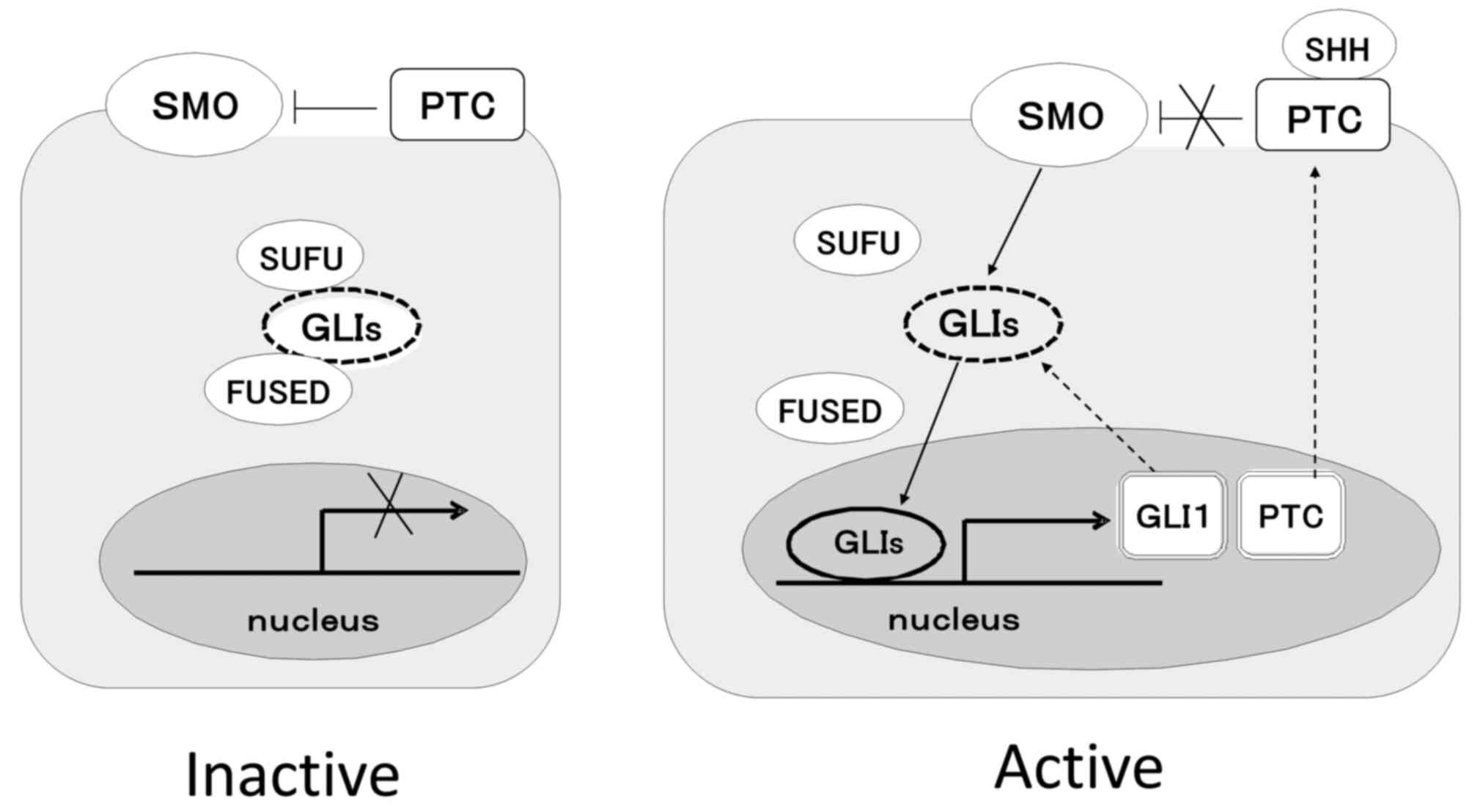
The HH signaling pathway is a morphogenesis
signaling pathway that plays a pivotal role in growth and pattern
during the embryonic period (6).
However, it may be reactivated beyond the embryonic period in
certain cancers, which acquire a malignant phenotype via HH
signaling. Core components of HH signaling that are emphasized in
the present review are the 12-transmembrane and negative regulatory
receptors, Patch (PTC), 7-transmembrane protein and Smoothened
(SMO), 3 Hh ligands including sonic HH (SHH), Indian HH (IHH) and
desert HH (DHH), serine-threonine kinase, FUSED, suppressor of
FUSED (SUFU), and the 3 transcriptional factors, glioma-associated
oncogene (GLI)1, GLI2 and GLI3. In the absence of HH ligands, PTC
inhibits SMO and GLIs form huge complexes with FUSED and SUFU.
Therefore, GLIs cannot translocate to the nucleus, and the signal
does not transduce. In contrast, in the presence of HH ligands, SMO
is released from the inhibition of PTC, and then, GLIs can be
released from the complex. Thereafter, GLIs can translocate to the
nucleus, and signaling is successfully transduced. Target genes of
HH signaling include GLI1 and PTC1. Therefore, GLI1 is considered
to be an index of HH signaling activation (7). Fig.
1 shows an outline of HH signal transduction. The mechanism of
reactivation of HH signaling in cancers is considered to be gene
mutation. For example, there are certain reports of gene mutations
in HH signaling in basal cell carcinoma (8), medulloblastoma (9), rhabdomyosarcoma (10) and glioblastoma (11). After 2003, ligand-dependent HH
signaling activation, but not gene mutation, has been reported. For
example, SHH secreted in an autocrine or paracrine manner from the
CME activates HH signaling in pancreatic cancer (12), colon cancer (13), hepatocellular carcinoma (HCC)
(14), lung (15), ovarian (16), gastric (17) and prostate cancer (18). Previously, it was revealed that
SHH, from monocytes that exist in pancreatic cancer stroma,
activates HH signaling in pancreatic cancer to induce proliferation
(19). HH signaling activation by
SHH secreted from the adjacent tissue is a more severe problem than
gene mutation from the viewpoint of the high probability of
induction of HH signaling activation. This may also be a reason why
determining the association between the HH signaling pathway and
the CME is important.
Cancer hypoxia is an important characteristic of the
CME. Hypoxia is ordinally investigated under 20% O2
conditions, however, 20% O2 conditions do not exist
in vivo. The O2 saturation of all human tissues
is ~1% O2, and cancer tissue is particularly hypoxic
(O2 saturation, ~0.1%) (20). The molecules and signaling pathways
that are not activated under normoxic conditions may be activated
under hypoxic conditions. To determine the cancer phenotype under
hypoxic cancer conditions, 1% O2 is used in experiments.
It has been previously reported that activation of HH signaling is
upregulated under hypoxic conditions (4). In the present study, SMO
transcription increased under hypoxic conditions. A similar result
was reported by Lei et al (21). In the analysis of the mechanism
underlying the increase in SMO expression under hypoxia, the
upstream molecules of SMO were analyzed and two molecules,
recombination signal binding protein for immunoglobulin-kappa-J
region (RBPJ) and mastermind-like 3 (MAML3), were detected
(22). RBPJ and MAML3 have
recently been found to be a transcriptional factor and a
coactivator of Notch signaling, respectively (23). The Notch signaling pathway is also
a morphogenetic signaling pathway. Our previous study on pancreatic
cancer cell lines revealed that hypoxia increases the expression of
RBPJ and MAML3 and contributes to the transcription of SMO
(22). This RBPJ/MAML3/SMO
signaling pathway is also activated in small-cell lung cancer
(24). The RBPJ/MAML3/SMO
signaling may be a comprehensive therapeutic target for
morphogenesis signaling. Hypoxia-inducible factor (HIF)-1a is an
important transcriptional factor that plays a pivotal role in
various cell functions such as cell proliferation, survival,
apoptosis, and angiogenesis under hypoxia. No correlation or
crosstalk was observed in RBPJ/MAML3/SMO signaling in our previous
study (22). However, numerous
studies have shown a correlation between HIF-1α and HH signaling.
Considering that HIF-1α regulates HH signaling as an upstream
mediator, it was demonstrated that fibroblast growth factor
receptor-like-1 (FGFRL1) expression is induced by HIF-1α and that
it promotes tumor progression by crosstalk with HH signaling in
ovarian cancer (25).
Mitochondrial glutamic pyruvate transaminase was revealed to
promote tumorigenesis and stemness of breast cancer cells by
activating HH signaling via HIF-1α (26). In addition, it has been reported
that natural agents contribute to the interaction between HIF-1α
and HH signaling. Resveratrol, which is extracted from various
plants, decreased HIF-1α expression and inhibited HH signaling to
decrease invasiveness in gastric cancer cells (27). Oroxylin A, a bioactive flavonoid,
induced HIF-1α degradation and led to the inactivation of HH
signaling to increase the sensitivity of glioma cells to
temozolomide (28). Curcumin has
an inhibitory effect on HIF-1α, decreasing proliferation in breast
cancer (29), and curcumin was
revealed to suppress hypoxia-induced endothelial-mesenchymal
transition (EMT) by inhibiting HH signaling in pancreatic cancer
cells (30). HIF-1a protects
cancer cells from radiation-induced apoptosis (31). Furthermore, curcumin has been shown
to increase the efficiency of g-irradiation in glioma by
suppressing HH signaling (32).
This suggests that the curcumin-induced decrease of HIF-1α may lead
to inactivation of HH signaling and consequently suppression of
cancer cell function. Conversely, HH signaling has been reported to
regulate HIF-1α. In a previous study, inhibition of HH signaling
suppressed hepatic stellate cells through inhibition of HIF-1α and
heat shock protein 90 (33).
Other morphogenesis signaling and HH signaling
pathways have been associated to the CME. The correlation between
hypoxia and Wnt/b-catenin signaling has been well elucidated. Among
the three subunits of HIF (HIF-1α, 2α and 3α), the contribution of
HIF-2α in tumor progression is well reported in Wnt/β-catenin
signaling (34). Inhibition of
HIF-2α leads to decreased expression of β-catenin and SMAD4 and
suppresses the progression to high-grade mPanINs (35). However, the precise contribution of
hypoxia to Notch signaling activation has not been clearly
reported. As previously described, hypoxia induces the expression
of RBPJ and MAML3 (22).
Considering that RBPJ is a transcriptional factor for Notch
signaling and MAML3 is a transcriptional mediator of Notch
signaling, Notch signaling should be activated under hypoxia.
Moreover, RBPJ and MAML3 may regulate HH signaling and Notch
signaling concurrently. RBPJ and MAML3 could be new comprehensive
therapeutic targets for morphogenesis signaling. In another study,
activated Notch1 markedly increased the transcriptional activity of
HIF-1 (36). In choriocarcinoma
cells, it has been shown that HIF-1α promotes invasiveness through
Notch signaling activation (37).
These results explain the association between hypoxia and Notch
signaling. FGF signaling is also a type of morphogenesis signaling.
Although a direct correlation between hypoxia and FGF signaling has
yet to be demonstrated, hypoxia is involved in the activation of
different signaling pathways in FGF-2-stimulated human
microvascular endothelial cells, which may contribute to
hypoxia-induced angiogenesis (38). FGF signaling is also a key pathway
in HCC (39). Therefore, FGF
signaling plays an important role in the progression of cancer in
the CME.
Acidosis and ROS are among the most characterized
properties of the CME. Hypoxic conditions are considered to induce
ROS generation and cause acidosis. Acidosis also induces ROS
generation (40). ROS contribute
to transformation, survival, proliferation, invasion and metastasis
of cancer cells (41). Although
the correlation between acidosis and HH signaling has not been
fully elucidated, it has been shown that ROS promotes HIF-1α
stabilization to induce HH signaling activated-cancer cell
proliferation (42). Other studies
have revealed that ROS inhibitors block GLI1-dependent EMT and
invasion under hypoxia (43) and
that resveratrol suppresses hypoxia-induced ROS-mediated
invasiveness and migration in pancreatic cancer via inhibition of
HH signaling (44).
Reoxygenation is an important process in the CME. It
is considered to be the process by which cancer cells detach from
hypoxic cancers and metastasize to secondary tissues through the
bloodstream. HH signaling may contribute to cancer progression
during reoxygenation. In a previous study, pancreatic cancer cells
increased proliferation and invasion during the reoxygenation
process through HH signaling activation using the chronic
hypoxia-resistant pancreatic cancer cells that were generated
(45). Consistent with this
result, it has been reported that the activation of HH signaling
protects cell apoptosis and cell viability from reoxygenation
stress in noncancerous H9C2 myocardial cells and HK-2 cells in
experiments assuming the clinical situation of ischemia (46,47).
Therefore, reoxygenation-induced HH signaling activation may be
required for tissue repair. Cancer may utilize this nature of HH
signaling during the reoxygenation process.
Cancer adapts to hypoxic conditions by inducing the
formation of new blood vessels, which is called angiogenesis.
Angiogenesis is implicated in hypoxia. Angiogenesis-related genes
include vascular endothelial growth factor (VEGF), VEGF receptor,
basic FGF (bFGF), platelet-derived growth factor (PDGF),
insulin-like growth factor (IGF), adrenomedullin, and epidermal
growth factor (EGF); these genes are targets of HIF (48).
HH signaling contributes to vasculature development,
differentiation, and maintenance during the embryonic period
(49). Canonical HH signaling has
been reported to regulate hepatic stellate cell-induced
angiogenesis in liver fibrosis (28). Yang et al (50) have revealed that HH signaling,
prospero-related homeobox 1, and HIF-1α contribute to liver
sinusoidal endothelial cell angiogenesis. Considering this fact, HH
signaling appears to affect vasculature development even in cancer
tissues. The association between HH signaling and VEGF has been
reported in several types of cancers, such as HCC (51) and colorectal cancer (52). The association between HH signaling
and bFGF has been reported (53),
and it may also be related to cancer fibrosis induced by HH
signaling, as described below. The association between HH signaling
and PDGF (54), IGF (55), and EGF (56) has also been reported. Bausch et
al (57) have shown that SHH
stimulates angiogenesis indirectly through other pathways,
including the reduction of antiangiogenic thrombospondin 2 and
tissue inhibitor of metalloproteinase 2 in stromal cells in
pancreatic cancer. Thus, angiogenesis, hypoxia, and HH signaling
are well correlated.
Cancer fibrosis is an important process and a
complication in which cancer acquires the refractory phenotype. The
association between HH signaling and fibrosis has been implicated
in chronic lung fibrosis in 2003 (58) and biliary fibrosis in chronic
cholecystitis in 2008 (59).
Fibrosis is marked in pancreatic cancer, and desmoplasia has been
investigated. HH signaling has been reported to promote desmoplasia
in pancreatic cancer in 2008 (60). Recently, it was demonstrated that
the increased secretion of SHH through HIF-1α signaling is
responsible for the cancer fibrosis or the stroma-rich environment
in pancreatic cancer (61,62). A severe case of cancer fibrosis in
the CME may block the circulation of chemotherapeutic agents and
infiltration of immune cells. Olive et al (63) have revealed that inhibition of HH
signaling enhances the delivery of chemotherapy in a pancreatic
cancer mouse model. In our xenograft experiments using pancreatic
cancer cell lines and CAFs, inhibition of cancer fibrosis by HH
inhibition led to an increase in tissue-infiltrating lymphocytes
and an enhancement of the effect of immune checkpoint inhibitors
(64). However, Steele et
al (65) have shown that
inhibition of HH signaling reduces myofibroblastic CAFs and
increases inflammatory CAFs to decrease cytotoxic T-cell
infiltration and expand regulatory T (Treg) cells. There are few
studies on infiltration of dendritic cells (DCs) and macrophages
related to cancer fibrosis. However, some researchers have shown
that macrophage infiltration induces fibrosis. Xue et al
(66) revealed that macrophages
promote pancreatic fibrosis in chronic pancreatitis, and Ueshima
et al (67) demonstrated
that macrophage-secreted transforming growth factor (TGF)-1
contributes to fibroblast activation. Cancer fibrosis consists of
CAFs and an extracellular matrix that secretes various cytokines.
One of the most important cytokines is TGF-β. Fibrosis is a typical
pathological condition of TGF-β-driven disease (68). The TGF-β/SMAD cascade is considered
to be a potent inducer of GLI2 (69). Therefore, in the presence of TGF-β,
it may induce cancer fibrosis and activate HH signaling, which may
lead to more fibrosis. Zhou et al (70) showed that HH signaling and TGF-β1
contribute to the progression of fibrosis in nonalcoholic
steatohepatitis. A previous study revealed the association among
TGF-β, fibrosis, and HH signaling, particularly in liver fibrosis,
and GANT61, a GLI inhibitor, has been shown to be effective for
liver fibrosis (71). Both HH
signaling and TGF-β in the CME may play an important role in cancer
fibrosis.
As aforementioned, the CME is closely correlated
with hypoxia and HH signaling activation. Therefore, the functions
of immune cells such as lymphocytes, macrophages, DCs,
myeloid-derived suppressor cells (MDSCs), and Treg cells that
infiltrate local cancer sites should be considered with regard to
these factors.
HH signaling contributes to the function of
activated lymphocytes, such as migration, proliferation and
cytotoxicity (73). T-cell
receptor activation triggers the expression of HH signaling
components, and HH signaling is required for cytotoxic T lymphocyte
(CTL) killing (85). Conversely,
certain researchers have shown that HH signaling promotes
tumor-associated macrophage polarization to inhibit
tumor-infiltrated CD8 T-cell recruitment (86) and that HH signaling promotes Th2
differentiation in naive human CD4 T cells (87). Other researchers have shown that
GLI1 induces the polarization of invading myeloid cells to MDSCs
(88). These results indicated
that HH signaling is required for lymphocyte function and immune
response in both activation and inhibition. HH signaling is also
involved in the functions of DCs, including induction, migration,
chemotaxis, phagocytosis, maturation, and IL-12 p40 or p70
secretion and the allogeneic lymphocyte stimulation activity of
monocyte-derived DCs (89). The
association between hypoxia and HH signaling may determine the
functions of immune cells.
Previously, the concept of immune checkpoints has
received significant attention. There are patients who are not
eligible to receive standard therapy due to their drug tolerance
and achieve complete response by immune checkpoint inhibitor
treatment (90). Thus, studies on
the PD-1/PD-L1 axis are considered important. It has been shown
that PD-L1 is a direct target of HIF-1α (91). Hypoxia-induced PD-L1/PD-1 crosstalk
impairs T-cell function (92).
Tumors may escape immune cells by regulating the PD-1/PD-L1 axis
under hypoxic conditions. However, it has been shown that HH
signaling induces PD-L1 expression in gastric cancer (93) and that HH inhibition leads to a
decrease in PD-L1 expression under hypoxia in pancreatic cancer
(94). Previously, it has been
shown that soluble PD-1/PD-L1 or exosomal PD-L1 plays an important
regulatory role in antitumor immunity (95,96).
Development of a measure against the enhanced PD-1/PD-L1 axis
should be the next strategy for cancer therapy. With respect to the
other factors of the CME, lymphocytes secrete INF-γ, which induces
PD-L1 when lymphocytes infiltrate the cancer tissue (97). A previous study has shown that
PD-L1 expression is associated with tumor-infiltrating lymphocytes
in squamous cell cervical carcinoma (98). However, it is unclear whether
lymphocyte infiltration into cancer tissue is the cause or result
of PD-L1 expression. In addition, although PD-L1 is considered to
be an exhaustion marker (99), the
significance of PD-L1 expression as a biomarker for immunotherapy
is controversial.
Autophagy is a cellular self-degradation process
that maintains homeostasis using this system. Autophagy is involved
in the initiation, progression, and drug resistance of cancers
(100); therefore, autophagy is
considered a target for cancer therapy. Hypoxia and metabolic
stress upregulate autophagy (101). Autophagy and hypoxia-upregulated
HH signaling appear to be correlated, and the association between
autophagy and HH signaling has been well elucidated (102,103). However, it is unclear whether HH
signaling inhibits or upregulates cancer autophagy. The SMO
antagonist vismodegib was demonstrated to trigger marked autophagy
in non-small cell lung cancer (104), while the GLI1/2 inhibitor GANT61
induced autophagy in HCC (105).
Milla et al (102) have
shown that the HH antagonist cyclopamine prevents autophagy.
Further, Gagné-Sansfaçon et al (106) have revealed that loss of HH
signaling leads to a decrease in autophagy in the intestinal ileum.
Therefore, it is deemed that the contribution of HH signaling to
autophagy warrants further investigation, considering the fact that
there is crosstalk between HH signaling and other signaling
pathways.
Increasing evidence suggests that the host
microenvironment plays a pivotal role in CSC status (107). For example, hypoxia promotes
stem-like properties of laryngeal cancer cells (108) and is closely associated with the
resistance of CSCs to chemotherapy and radiotherapy (109). Hypoxia enhances the expression of
the CSC transcription factors NANOG, Oct4, SOX2 and CD133 (110). Multiple secreted cytokines and
growth factors in the CME induce the enrichment of CSCs in ovarian
cancer (111). As with other CME
factors, nutritional stress in the microenvironment induces
increased expression of glioblastoma CSC-specific biomarkers with
higher invasiveness and angiogenesis through Wnt/HH signaling
(112). In addition, numerous
studies have shown that morphogenesis signaling is important for
the maintenance of CSCs. For example, in an experiment on breast
cancer, the HH signaling pathway was activated in the
CD24−/low CD44+ CSC population, but not in
the CD24+ CD44+ non-CSC population, and HH
signaling inhibition in the CD24−/low CD44+
CSC population attenuated tumor proliferation (113). Notch signaling contributes to
endocrine resistance in breast cancer through the promotion of the
CSC phenotype (114). Notch
inhibitors increase the chemotherapy effect through
CD133+ CSC inhibition in endometrial cancer (115). Inhibition of Wnt/β-catenin
signaling is considered to decrease the aggressiveness of breast
cancer through CSC inhibition (116). Wnt/β-catenin signaling
contributes to CSC-initiated HCC (117). Bone morphogenetic protein
(BMP)/TGF-β signaling, which is a morphogenesis signaling pathway,
contributes to the homeostasis of neural and glioma stem cells
(118). The correlation between
Notch signaling and BMP/TGF-β signaling has also been reported
(119).
Exosomes are extracellular microvesicles measuring
30–100 nm in diameter, are actively secreted through an exocytosis
pathway by various cell types (120,121), and comprise a nucleic acid and
protein derived from secreted cells. Exosomes are significantly
rigid and resistant to enzymatic degradation; therefore, they are
considered to play a pivotal role in cell-to-cell interactions in
the CME. Deep and Panigrahi (122) have reported that exosomes mediate
tumor microenvironment remodeling, such as angiogenesis, EMT,
metastasis, survival, proliferation, metabolism, stemness, and
therapeutic resistance under hypoxic conditions through several
signaling pathways, including the HH signaling pathway. Even during
the morphogenesis period, exosomes are required for the
distribution of morphogenes, such as HH ligands (123). In relation to HH signaling and
CSC, it has been shown that exosomes derived from human bone marrow
mesenchymal stem cells promote the growth of osteosarcoma and
gastric cancer through HH signaling (124). CSC-derived exosomes contain
stemness-specific proteins, self-renewal-promoting miRNAs, and
survival factors, and they play a significant role in tumor
heterogeneity and tumor progression (125). HH pathway proteins, including
PTC1, SMO, and SHH, are exported to the cervical cancer cell line
(126). SHH is highly expressed
in CAFs, and CAF-derived exosomes contribute to the augmentation of
growth and progression in esophageal squamous cell carcinoma
(127). With respect to the
association between exosomes and CME, Wada et al (128) have shown that TGF-β1 expressed on
the surface of cancer ascites-derived exosomes is involved in the
maintenance of the number and suppressive function of Treg cells.
Matsumoto et al (129)
have shown that dendritic cell-derived exosome supports CD4+ T cell
survival. Taken together, exosomes play a pivotal role in the
maintenance of CME.
Local cancer sites often arise from inflammation.
Inflammation is closely related to the CME and is required for the
initiation of immune cell activation. Nuclear transcription factor
(NF)-κB is an important transcriptional factor that regulates
inflammation (130). The
association between SHH and NF-κB has been mainly reported. In a
previous study, NF-kB was shown to contribute to the initiation of
chronic pancreatitis and be involved in cancer initiation through
SHH expression in pancreatic cancer (131). A similar finding was reported by
Kasperczyk et al (132).
The correlation between SHH and NF-κB has been revealed in multiple
myeloma (133). SHH is secreted
by tumor-infiltrated macrophages through the NF-κB pathway and
induces proliferation in a paracrine manner in pancreatic cancer
(19). The contribution of NF-κB
to cancer-infiltrated lymphocytes has also been reported (134). Collectively, NF-κB significantly
contributes to the CME.
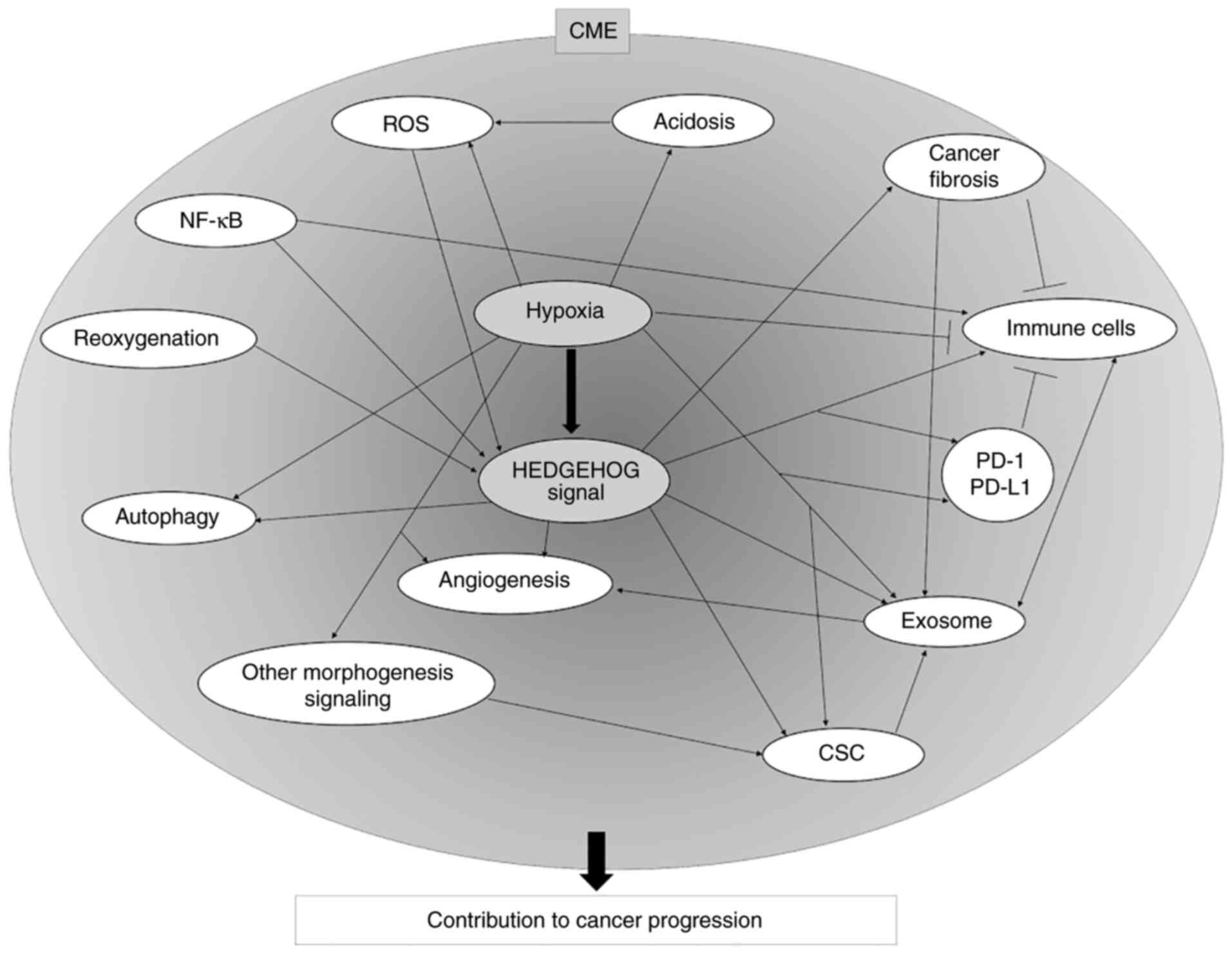
In the present review, the individual factors that
constitute the CME have been described, focusing on hypoxia and HH
signaling. As previously described, these factors are correlated
and form the CME (Fig. 2).
Inhibitors of each factor have been developed, and the mechanisms
involved should be understood considering the complex correlation
among the factors. Fosko et al (135) have revealed that vismodegib
exhibited 60% response in basal cell carcinoma regardless of the
histopathologic subtype. On the other hand, a phase 2 trial using
the SMO inhibitor vismodegib with gemcitabine and nab-paclitaxel in
patients with untreated metastatic pancreatic adenocarcinoma did
not show a significant effective result (136). This trial had difficulties in
analyzing the specimens before and after chemotherapy; the cause of
the failure was not clear. Previous studies have shown that SMO
mutation in cancer cells affects the effects of vismodegib
(137,138). Thus, although HH inhibitors have
exhibited a significant tumor suppressive effect in vitro
(139), this effect has not
always been observed in vivo. It was hypothesized that this
discrepancy may be due to the difficulty in obtaining similar
results with human CME as in in vitro and in vivo
mouse experiments.
The therapy that targets only one CME factor may not
be sufficient for cancer treatment. If the correlation among these
CME factors can be substantiated, each CME inhibitor can be used
effectively for cancer therapy. In Fig. 2, an overview of the correlation
among hypoxia, HH signaling and other CME factors is demonstrated.
Each factor individually plays a pivotal role in the formation of
the CME. The correlated factors constitute the CME and contribute
to cancer progression.
The authors would like to thank Ms. Emi Onishi
(Department of Cancer Therapy and Research, Graduate School of
Medical Sciences, Kyushu University) for her skillful technical
assistance.
The present study was supported by the Japan Society for the
Promotion of Science KAKENHI (grant nos. JP19K22662, JP19K09124,
JP19K09047 and JP21K08712).
The datasets used and/or analyzed are available from
the corresponding author on reasonable request.
HO, KatN and KY wrote the manuscript. SN, KazN and
YO designed the manuscript. AF, KO and AY selected the references.
All authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Balkwill FR, Capasso M and Hagemann T: The
tumor microenvironment at a glance. J Cell Sci. 125:5591–5596.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rakhshandehroo T, Smith BR, Glockner HJ,
Rashidian M and Pandit-Taskar N: Molecular immune targeted imaging
of tumor microenvironment. Nanotheranostics. 6:286–305. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu
Y, Gong Z, Zhang S, Zhou J, Cao K, et al: Role of tumor
microenvironment in tumorigenesis. J Cancer. 8:761–773. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onishi H, Kai M, Odate S, Iwasaki H,
Morifuji Y, Ogino T, Morisaki T, Nakashima Y and Katano M: Hypoxia
activates the hedgehog signaling pathway in a ligand-independent
manner by upregulation of Smo transcription in pancreatic cancer.
Cancer Sci. 102:1144–1150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Onishi H and Katano M: Hedgehog signaling
pathway as a therapeutic target in various types of cancer. Cancer
Sci. 102:1756–1760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: Paradigms and principles. Genes
Dev. 15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Low JA and de Sauvage FJ: Clinical
experience with hedgehog pathway inhibitors. J Clin Oncol.
28:5321–5326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gailani MR and Bale AE: Developmental
genes and cancer: Role of patched in basal cell carcinoma of the
skin. J Natl Cancer Inst. 89:1103–1109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zurawel RH, Allen C, Chiappa S, Cato W,
Biegel J, Cogen P, de Sauvage F and Raffel C: Analysis of
PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes
Cancer. 27:44–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tostar U, Malm CJ, Meis-Kindblom JM,
Kindblom LG, Toftgård R and Undén AB: Deregulation of the hedgehog
signalling pathway: A possible role for the PTCH and SUFU genes in
human rhabdomyoma and rhabdomyosarcoma development. J Pathol.
208:17–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kinzler KW, Bigner SH, Binger DD, Trent
JM, Law ML, O'Brien SJ, Wong AJ and Vogelstein B: Identification of
an amplified, highly expressed gene in a human glioma. Science.
236:70–73. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thayer SP, di Magliano MP, Heiser PW,
Nielson CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Castillo CF,
Yajnik V, et al: Hedgehog is an early and late mediator of
pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qualtrough D, Buda A, Gaffield W, Williams
AC and Paraskeva C: Hedgehog signalling in colorectal tumour cells:
Induction of apoptosis with cyclopamine treatment. Int J Cancer.
110:831–837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng WT, Xu K, Tian DY, Zhang ZG, Liu LJ
and Chen Y: Role of Hedgehog signaling pathway in proliferation and
invasiveness of hepatocellular carcinoma cells. Int J Oncol.
34:829–836. 2009.PubMed/NCBI
|
|
15
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, Horiuchi A, Kikuchi N, Osada R,
Yoshida J, Shiozawa T and Konishi I: Hedgehog signal pathway is
activated in ovarian carcinomas, correlating with cell
proliferation: It's inhibition leads to growth suppression and
apoptosis. Cancer Sci. 98:68–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma X, Chen K, Huang S, Zhang X, Adegboyega
PA, Evers BM, Zhang H and Xie J: Frequent activation of the
hedgehog pathway in advanced gastric adenocarcinomas.
Carcinogenesis. 26:1698–1705. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan L, Pepicelli CV and Dibble CC:
Hedgehog signaling promotes prostate xenograft tumor growth.
Endocrinology. 145:3961–3970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamasaki A, Kameda C, Xu R, Tanaka H,
Tasaka T, Chikazawa N, Suzuki H, Morisaki T, Kubo M, Onishi H, et
al: Nuclear factor kappaB-activated monocytes contribute to
pancreatic cancer progression through the production of Shh. Cancer
Immunol Immunother. 59:675–686. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hockel S, Schlenger K, Vaupel P and Hockel
M: Association between host tissue vascularity and the
prognostically relevant tumor vascularity in human cervical cancer.
Int J Oncol. 19:827–832. 2001.PubMed/NCBI
|
|
21
|
Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan
W, Sun Q, Xu J, Wu Z and Wu E: Hedgehog signaling regulates hypoxia
induced epithelial to mesenchymal transition and invasion in
pancreatic cancer cells via a ligand-independent manner. Mol
Cancer. 12:662013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onishi H, Yamasaki A, Kawamoto M, Imaizumi
A and Katano M: Hypoxia but not normoxia promotes Smoothened
transcription through upregulation of RBPJ and Mastermind-like 3 in
pancreatic cancer. Cancer Lett. 371:143–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ables JL, Breunig JJ, Eisch AJ and Rakic
P: Not(ch) just development: Notch signalling in the adult brain.
Nat Rev Neurosci. 12:269–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Onishi H, Ichimiya S, Yanai K, Umebayashi
M, Nakamura K, Yamasaki A, Imaizumi A, Nagai S, Murahashi M, Ogata
H and Morisaki T: RBPJ and MAML3: Potential therapeutic targets for
small cell lung cancer. Anticancer Res. 38:4543–4547. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tai H, Wu Z, Sun S, Zhang Z and Xu C:
FGFRL1 promotes ovarian cancer progression by crosstalk with
hedgehog signaling. J Immunol Res. 2018:74386082018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao Y, Lin SH, Wang Y, Chin YE, Kang L and
Mi J: Gultamic pyruvate transaminase GPT2 promotes tumorigenesis of
breast cancer cells by activating sonic hedgehog signaling.
Theranostics. 7:3021–3033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu QH, Xiao Y, Li XQ, Fan L, Zhou CC,
Cheng L, Jiang ZD and Wang GH: Resveratrol counteracts
hypoxia-induced gastric cancer invasion and EMT through hedgehog
pathway suppression. Anticancer Agents Med Chem. 20:1105–1114.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei M, Ma R, Huang S, Liao Y, Ding Y, Li
Z, Guo Q, Tan R, Zhang L and Zhao L: Oroxylin A increases the
sensitivity of temozolomide on glioma cells by hypoxia-inducible
factor 1α/hedgehog pathway under hypoxia xia. J Cell Physiol.
234:17392–17404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sarighieh MA, Montazeri V, Shadboorestan
A, Ghahremani MH and Ostad SN: The inhibitory effect on hypoxia
inducer (Hifs) as a regulatory factor in the growth of tumor cells
in breast cancer stem-like cells. Drug Res. 70:512–518. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao L, Xiao X, Lei J, Duan W, Ma Q and Li
W: Curcumin inhibits hypoxia-induced epithelial-mesenchymal
transition in pancreatic cancer cells via suppression of the
hedgehog signaling pathway. Oncol Rep. 35:3728–3734. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fu Z, Chen D, Cheng H and Wang F:
Hypoxia-inducible factor-1α protects cervical carcinoma cells from
apoptosis induced by radiation via modulation of vascular
endothelial growth factor and p53 under hypoxia. Med Sci Monit.
21:319–325. 2015.
|
|
32
|
Meng X, Cai J, Liu J, Han B, Gao F, Gao W,
Zhang Y, Zhang J, Zhao Z and Jiang C: Curcumin increases efficiency
of γ-irradiation in gliomas by inhibiting Hedgehog signaling
pathway. Cell Cycle. 16:1181–1192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang F, Hao M, Jin H, Yao Z, Lian N, Wu
L, Shao J, Chen A and Zheng S: Canonical hedgehog signalling
regulates hepatic stellate cell-mediated angiogenesis in liver
fibrosis. Br J Pharmacol. 175:409–423. 2017. View Article : Google Scholar
|
|
34
|
Zhang Q, Lou Y, Zhang J, Fu Q, Wei T, Sun
X, Chen Q, Yang J, Bai X and Liang T: Hypoxia-inducible factor-2α
promotes tumor progression and has crosstalk with Wnt/β-catenin
signaling in pancreatic cancer. Mol Cancer. 16:1192017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Criscimanna A, Duan LJ, Rhodes JA,
Fendrich V, Wickline E, Hartman DJ, Monga SP, Lotze MT, Gittes GK,
Fong GH and Esni F: PanIN-specific regulation of Wnt signaling by
HIF2α during early pancreatic tumorigenesis. Cancer Res.
73:4781–4790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moriyama H, Moriyama M, Ozawa T, Ttsuruta
D, Iguchi T, Tamada S, Nakatani T, Nakagawa K and Hayakawa T: Notch
signaling enhances stemness by regulating metabolic pathways
through modifying p53, NF-κB, and HIF-1α. Stem Cells Dev.
27:935–947. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tian Q, Xue Y, Zheng W, Sun R, Ji W, Wang
X and An R: Overexpression of hypoxia-inducible factor 1α induces
migration and invasion through Notch signaling. Int J Oncol.
47:728–738. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kroon ME, Koolwijk P, van der Vecht B and
van Hinsbergh VW: Hypoxia in combination with FGF-2 induces tube
formation by human microvascular endothelial cells in a fibrin
matrix: Involvement of at least two signal transduction pathways. J
Cell Sci. 114:825–833. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Le TBU, Vu TC, Ho RZW, Prawira A, Wang L,
Goh BC and Huynh H: Bevacizumab augments the antitumor efficacy of
infigratinib in hepatocellular carcinoma. Int J Mol Sci.
21:94052020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gupta SC, Singh R, Pochampally R, Watabe K
and Mo YY: Acidosis promotes invasiveness of vreast cancer cells
through ROS-AKT-NF-kB pathway. Oncotarget. 5:12070–12082. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eyrich NW, Potts CR, Robinson MH, Maximov
V and Kenney AM: Reactive oxygen species signaling promotes
hypoxia-inducible factor 1 α stabilization in sonic hedgehog-driven
cerebellar progenitor cell proliferation. Mol Cell Biol.
39:e00268–18. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Z, Tu K, Wang Y, Yao B, Li Q, Wang L,
Dou C, Liu Q and Zheng X: Hypoxia accelerates aggressiveness of
hepatocellular carcinoma cells involving oxidative stress,
epithelial-mesenchymal transition and non-canonical hedgehog
signaling. Cell Physiol Biochem. 44:1856–1868. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li W, Cao L, Chen Y, Lei J and Ma Q:
Resveratrol inhibits hypoxia-driven ROS-induced invasive and
migratory ability of pancreatic cancer cells via suppression of the
hedgehog signaling pathway. Oncol Rep. 35:1718–1726. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Morifuji Y, Onishi H, Iwasaki H, Imaizumi
A, Nakano K, Tanaka M and Katano M: Reoxygenation from chronic
hypoxia promotes metastatic processes in pancreatic cancer through
the Hedgehog signaling. Cancer Sci. 105:324–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang RY, Qiao ZY, Liu HJ and Ma JW: Sonic
hedgehog signaling regulates hypoxia/reoxygenation-induced H9C2
myocardial cell apoptosis. Exp Ther Med. 16:4193–4200.
2018.PubMed/NCBI
|
|
47
|
Fang Q, Zhang Y, Siang DS and Chen Y:
Hydroxytyosol inhibits apoptosis in ischemia/reperfusion-induced
acute kidney injury via activating sonic edgehog signaling pathway.
Eur Rev Med Pharmacol Sci. 24:12380–12388. 2020.PubMed/NCBI
|
|
48
|
Emami Nejad A, Najafgholian S, Rostami A,
Sistani A, Shojaeifar S, Esparvarinha M, Nedaeinia R, Haghjooy
Javanmard S, Taherian M, Ahmadlou M, et al: The role of hypoxia in
the tumor microenvironment and development of cancer stem cell: A
novel approach to developing treatment. Cancer Cell Int. 21:622021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chapouly C, Guimbal S, Hollier PL and
Renault MA: Role of hedgehog signaling in vasculature development,
differentiation, and maintenance. Int J Mol Sci. 20:30762019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang X, Wang Z, Kai J, Wang F, Jia Y, Wang
S, Tan S, Shen X, Chen A, Shao J, et al: Curcumol attenuates liver
sinusoidal endothelial cell angiogenesis via regulating
Glis-PROX1-HIF-1 α in liver fibrosis. Cell Prolif. 53:e127622020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pinter M, Sieghart W, Schmid M, Dauser B,
Prager G, Dienes HP, Trauner M and Peck-Radosavljevic M: Hedgehog
inhibition reduces angiogenesis by downregulation of tumoral VEGF-A
expression in hepatocellular carcinoma. United European
Gastroenterol J. 1:265–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhu XQ, Yang H, Lin MH, Shang HX, Peng J,
Chen WJ, Chen XZ and Lin JM: Qingjie fuzheng granules regulates
cancer cell proliferation, apoptosis and tumor angiogenesis in
colorectal cancer xenograft mice via sonic hedgehog pathway. J
Gastrointest Oncl. 11:1123–1134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu ZX, Sun CC, Zhu YT, Wang Y, Wang T,
Chi LS, Cai WH, Zheng JY, Zhou X, Cong WT, et al: Hedgehog
signaling contributes to basic fibroblast growth factor-regulated
fibroblast migration. Exp Cell Res. 355:83–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yao Q, Renault MA, Chapouly C,
Vandierdonck S, Belloc I, Jaspard-Vinassa B, Daniel-Lamaziere JM,
Laffargue M, Merched A, Desgranges C and Gadeau AP: Sonic hedgehog
mediates a novel pathway of PDGF-BB-dependent vessel maturation.
Blood. 123:2529–2437. 2014. View Article : Google Scholar
|
|
55
|
Hsieh A, Ellsworth R and Hsieh D:
Hedgehog/GLI1 regulates IGF dependent malignant behaviors in glioma
stem cells. J Cell Physiol. 226:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Maroufy V, Shah P, Asghari A, Deng N, Le
RNU, Ramirez JC, Yaseen A, Zheng WJ, Umetami M and Wu H: Gene
expression dynamic analysis reveals co-activation of sonic hedgehog
and epidermal growth factor followed by dynamic silencing.
Oncotarget. 11:1358–1372. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bausch D, Fritz S, Bolm L, Wellner UF,
Fernandez-Del-Castillo C, Warshaw AL, Thayer SP and Liss AS:
Hedgehog signaling promotes angiogenesis directly and indirectly in
pancreatic cancer. Angiogenesis. 23:479–492. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Stewart GA, Hoyne GF, Ahmad SA, Jarman E,
Wallace WAH, Harrison DJ, Haslett C, Lamb JR and Howie SEM:
Expression of the developmental sonic hedgehog (Shh) signalling
pathway is up-regulated in chronic lung fibrosis and the Shh
receptor patched 1 is present in circulating T lymphocytes. J
Pathol. 199:488–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Omenetti A, Porrello A, Jung Y, Yang L,
Popov Y, Choi SS, Witek RP, Alpini G, Venter J, Vandongen HM, et
al: Hedgehog signaling regulates epithelial-mesenchymal transition
during biliary fibrosis in rodents and humans. J Clin Invest.
118:3331–3342. 2008.PubMed/NCBI
|
|
60
|
Bailey JM, Swanson BJ, Hamada T, Eggers
JP, Singh PK, Caffery TC, Ouellette MM and Hollingsworth MA: Sonic
hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer
Res. 14:5995–6004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Spivak-Kroizman TR, Hostetter G, Posner R,
Ariz M, Hu C, Demeure MJ, Hoff DV, Hingorani SR, Palculict TB, Izzo
J, et al: Hypoxia triggers hedgehog-mediated tumor-stromal
interactions in pancreatic cancer. Cancer Res. 73:3235–3247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Katagiri T, Kobayashi M, Yoshimura M,
Morinibu A, Itasaka S, Hiraoka M and Harada H: HIF-1 maintains a
functional relationship between pancreatic cancer cells and stromal
fibroblasts by upregulating expression and secretion of sonic
hedgehog. Oncotarget. 9:10525–10535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of hedgehog signaling
enhances delivery of chemotherapy in mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Oyama Y, Onishi H, Koga S, Murahashi M,
Ichimiya S, Nakayama K, Fujimura A, Kawamoto M, Imaizumi A,
Umebayashi M, et al: Patched 1-interacting peptide represses
fibrosis in pancreatic cancer to augment the effectiveness of
immunotherapy. J Immunother. 43:121–133. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Steele NG, Biffi G, Kemp SB, Zhang Y,
Drouillard D, Syu L, Hao Y, Oni TE, Brosnan E, Elyada E, et al:
Inhibition of hedgehog signaling alters fibroblast composition in
pancreatic cancer. Clin Cancer Res. 27:2023–2037. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xue J, Jsharma V, Hsieh MH, Chawla A,
Murali R, Pandol SJ and Habtezion A: Alternatively activated
macrophages promote pancreatic fibrosis in chronic pancreatitis.
Nat Commun. 6:71582015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ueshima E, Fujimori M, Kodama H, Felsen D,
Chen J, Duack JC, Solomon SB, Coleman JA and Srimathveeravalli G:
Macrophage-secreted TGF-β 1 contributes to fibroblast activation
and ureteral stricture after ablation injury. Am J Physiol Renol
Physiol. 317:F52–F64. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Javelaud D, Pierrat MJ and Mauviel A:
Crosstalk between TGF-β and hedgehog signaling in cancer. FEBS
Lett. 586:2016–2025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dennler S, Andre J, Alexaki I, Li A,
Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F and Mauviel A:
Induction of sonic hedgehog mediators by transforming growth
factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression
in vitro and in vivo. Cancer Res. 67:6981–6986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhou X, Wang P, Ma Z, Li M, Teng X, Sun L,
Wan G, Li Y, Guo L and Liu H: Novel interplay between sonic
hedgehog and transforming growth factor-β1 in human nonalcoholic
steatohepatitis. Appl Immunohistochem Mol Morphol. 28:154–160.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jiayuan S, Junyan Y, Xiangzhen W, Zuping
W, Jian N, Baowei H and Lifang J: Gant61 ameliorates CCl4-induced
liver fibrosis by inhibition of hedgehog signaling activity.
Toxicol Appl Pharmcol. 387:1148532020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Noman MZ, Hasmim M, Messai Y, Terry S,
Kieda C, Janji B and Chouaib S: Hypoxia: A key player in antitumor
immune response. A review in the theme: Cellular responses to
hypoxia. Am J Physiol Cell Physiol. 309:C569–C579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Onishi H, Morisaki T, Kiyota A, Koya N,
Tanaka H, Umebayashi M and Katano M: The Hedgehog inhibitor
cyclopamine impairs the benefits of immunotherapy with activated T
and NK lymphocytes derived from patients with advanced cancer.
Cancer Immunol Immunother. 62:1029–1039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Winning S and Fandrey J: Dendritic cells
under hypoxia: How oxygen shortage affects the linkage between
innate and adaptive immunity. J Immunol Res. 2016:51343292016.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ogino T, Onishi H, Suzuki H, Morisaki T,
Tanaka M and Katano M: Inclusive estimation of complex antigen
presentation functions of monocyte-derived dendritic cells
differentiated under normoxia and hypoxia conditions. Cancer
Immunol Immunother. 61:409–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bosco MC, Pierobon D, Blengio F, Raggi F,
Vanni C, Gattorno M, Eva A, Novelli F, Cappello P, Giovarelli M and
Varesio L: Hypoxia modulates the gene expression profile of
immunoregulatory receptors in human mature dendritic cells:
Identification of TREM-1 as a novel hypoxic marker in vitro and in
vivo. Blood. 117:2625–2639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pierobon D, Bosco MC, Blengio F, Raggi F,
Eva A, Filippi M, Musso T, Novelli F, Cappello P, Varesio L and
Giovarelli M: Chronic hypoxia reprograms human immature dendritic
cells by inducing a proinflammatory phenotype and TREM-1
expression. Eur J Immunol. 43:949–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu B and Wei C: Hypoxia induces
overexpression of CCL28 to recruit Treg cells to enhance
angiogenesis in lung adenocarcinoma. J Environ Pathol Toxicol
Oncol. 40:65–74. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Westendorf AM, Skibbe K, Adamczyk A, Buer
J, Geffers R, Hansen W, Pastille E and Jendrossek V: Hypoxia
enhances immunosuppression by inhibiting CD4+ Effector T cell
function and promoting treg activity. Cell Physiol Biochem.
41:1271–1284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chiu DK, Xu IM, Lai RK, Tse AP, Wei LL,
Koh HY, Li LL, Lee D, Lo RC, Wong CM, et al: Hypoxia induces
myeloid-derived suppressor cell recruitment to hepatocellular
carcinoma through chemokine (C-C motif) ligand 26. Hepatology.
64:797–813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Elia AR, Cappello P, Puppo M, Fraone T,
Vanni C, Eva A, Musso T, Novelli F, Varesio L and Giovarelli M:
Human dendritic cells differentiated in hypoxia down-modulate
antigen uptake and change their chemokine expression profile. J
Leukoc Biol. 84:1472–1482. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Burke B, Giannoudis A, Corke KP, Gill D,
Wells M, Ziegler-Heitbrock L and Lewis CE: Hypoxia-induced gene
expression in human macrophages: Implications for ischemic tissues
and hypoxia-regulated gene therapy. Am J Pathol. 163:1233–1243.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fingleton B, Vargo-Gogola T, Crawford HC
and Matrisian LM: Matrilysin [MMP-7] expression selects for cells
with reduced sensitivity to apoptosis. Neoplasia. 3:459–468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sureshbabu SK, Chaukar D and Chiplunkar
SV: Hypoxia regulates the differentiation and anti-tumor effector
functions of γδT cells in oral cancer. Clin Exp Immunol. 201:40–57.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
de la Roche M, Ritter AT, Angus KL,
Dinsmore C, Earnshaw CH, Reiter JF and Griffiths GM: Hedgehog
signaling controls T cell killing at the immunological synapse.
Science. 342:1247–1250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Petty AJ, Li A, Wang X, Dai R, Heyman B,
Hsu D, Huang X and Yang YJ: Hedgehog signaling promotes
tumor-associated macrophage polarization to suppress intratumoral
CD8+ T cell recruitment. Clin Invest. 129:5151–5162. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yánez DC, Lau CI, Chawda MM, Ross S,
Furmanski AL and Crompton TJ: Hedgehog signaling promotes T H 2
differentiation in naive human CD4 T cells. Allergy Clin Immunol.
144:1419–1423.e1. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Merchant JL and Ding L: Hedgehog signaling
links chronic inflammation to gastric cancer precursor lesions.
Cell Mol Gastroenterol Hepatol. 3:201–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Onishi H, Morisaki T, Kiyota A, Koya N,
Tanaka H, Umebayashi M and Katano M: The Hedgehog inhibitor
suppresses the function of monocyte-derived dendritic cells from
patients with advanced cancer under hypoxia. Biochem Biophys Res
Commun. 436:53–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ichimiya S, Fujimura A, Masuda M, Masuda
S, Yasumatsu R, Umebayashi M, Tanaka H, Koya N, Nakagawa S,
Yoshimura S, Onishi H, Nakamura M, Nakamura Y and Morisaki T:
Contribution of pre-existing neoantigen-specific T cells to durable
complete responses after tumor-pulsed dendritic cell vaccine plus
nivolumab therapy in a patient with metastatic salivary duct
carcinoma. Immunol Invest. Sep 5–2021.(Epub ahead of print). doi:
10.1080/08820139.2021.1973491. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under ypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cubillos-Zapata C, Avendaño-Ortiz J,
Hernandez-Jimenez E, Toledano V, Casas-Martin J, Varela-Serrano A,
Torres M, Almendros I, Casitas R, Fernández-Navarro I, et al:
Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in
sleep apnoea. Eur Respir J. 50:17008332017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chakrabarti J, Holokai L, Syu L, Steele
NG, Chang J, Wang J, Ahmed S, Dlugosz A and Zavros Y: Hedgehog
signaling induces PD-L1 expression and tumor cell proliferation in
gastric cancer. Oncotarget. 9:37439–37457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Onishi H, Fujimura A, Oyama Y, Yamasaki A,
Imaizumi A, Kawamoto M, Katano M, Umebayashi M and Morisaki T:
Hedgehog signaling regulates PDL-1 expression in cancer cells to
induce anti-tumor activity by activated lymphocytes. Cell Immunol.
310:199–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhu X and Lang J: Soluble PD-1 and PD-L1:
Predictive and prognostic significance in cancer. Oncotarget.
8:97671–97682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhou K, Guo S, Li F, Sun Q and Liang G:
Exosomal PD-L1: New insights into tumor immune escape mechanisms
and therapeutic strategies. Front Cell Dev Biol. 8:5692192020.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mühlbauer M, Fleck M, Schütz C, Weiss T,
Froh M, Blank C, Schölmerich J and Hellerbrand C: PD-L1 is induced
hepatocytes by viral infection and interferon-alpha and -gamma and
mediates T cell apoptosis. J Hepatol. 45:520–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
D'Alessandris N, Palaia I, Pernazza A,
Tomao F, Di Pinto A, Musacchio L, Leopizzi M, Di Maio V, Pecorella
I, Benedetti Panici P, et al: PD-L1 expression is associated with
tumor infiltrating lymphocytes that predict response to NACT in
squamous cell cervical cancer. Virchows Arch. 478:517–525. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Blank C and Mackensen A: Contribution of
the PD-L1/PD-1 pathway to T-cell exhaustion: An update on
implications for chronic infections and tumor evasion. Cancer
Immunol Immunother. 56:739–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zeng X and Ju D: Hedgehog signaling
pathway and autophagy in cancer. Int J Mol Sci. 19:22792018.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yamasaki A, Yanai K and Onishi H: Hypoxia
and pancreatic ductal adenocarcinoma. Cancer Lett. 484:9–15. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Milla LA, González-Ramírez CN and Palma V:
Sonic hedgehog in cancer stem cells: A novel link with autophagy.
Biol Res. 45:223–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wu X, Won H and Rubinsztein DC: Autophagy
and mammalian development. Biochem Soc Trans. 41:1489–1494. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fan J, Ju D, Li Y, Wang S and Wang Z: A
novel approach to overcome non-small-cell lung cancer:
Co-inhibition of autophagy and Hedgehog pathway. Ann Oncol.
26:vii106–vii151. 2015. View Article : Google Scholar
|
|
105
|
Wang Y, Han C, Lu L, Magliato S and Wu T:
Hedgehog signaling pathway regulates autophagy in human
hepatocellular carcinoma cells. Hepatology. 58:995–1010. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gagné-Sansfaçon J, Allaire JM, Jones C,
Boudreau F and Perreault N: Loss of Sonic hedgehog leads to
alterations in intestinal secretory cell maturation and autophagy.
PLoS One. 9:e987512014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Albini A, Cesana E and Noonan DM: Cancer
stem cells and the tumor microenvironment: Soloists or choral
singers. Curr Pharm Biotechnol. 12:171–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wu CP, Du HD, Gong HL, Li DW, Tao L, Tian
J and Zhou L: Hypoxia promotes stem-like properties of laryngeal
cancer cell lines by increasing the CD133+ stem cell fraction. Int
J Oncol. 44:1652–1660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Sun X, Lv X, Yan Y, Zhao Y, Ma R, He M and
Wei M: Hypoxia-mediated cancer stem cell resistance and targeted
therapy. Biomed. Pharmacother. 130:1106232020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Bhuria V, Xing J, Scholta T, Bui KC,
Nguyen MLT, Malek NP, Bozko P and Plentz RR: Hypoxia induced Sonic
Hedgehog signaling regulates cancer stemness,
epithelial-to-mesenchymal transition and invasion in
cholangiocarcinoma. Exp Cell Res. 385:1116712019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Raghavan S, Snyder CS, Wang A, McLean K,
Zamarin D, Buckanovich RJ and Mehta G: Carcinoma-associated
mesenchymal stem cells promote chemoresistance in ovarian cancer
stem cells via PDGF signaling. Cancers. 12:20632020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mondal S, Bhattacharya K and Mandal C:
Nutritional stress reprograms dedifferention in glioblastoma
multiforme driven by PTEN/Wnt/Hedgehog axis: A stochastic model of
cancer stem cells. Cell Death Discov. 4:1102018. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tanaka H, Nakamura M, Kameda C, Kubo M,
Sato N, Kuroki S, Tanaka M and Katano M: The Hedgehog signaling
pathway plays an essential role in maintaining the CD44+CD24-/low
subpopulation and the side population of breast cancer cells.
Anticancer Res. 29:2147–2157. 2009.PubMed/NCBI
|
|
114
|
Bai JW, Wei M, Li JW and Zhang GJ: Notch
signaling pathway and endocrine resistance in breast cancer. Front
Pharmacol. 11:9242020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Shang C, Lang B and Meng LR: Blocking
NOTCH pathway can enhance the effect of EGFR inhibitor through
targeting CD133+ endometrial cancer cells. Cancer Biol Ther.
19:113–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Castagnoli L, Tagliabue E and Pupa SM:
Inhibition of the Wnt signalling pathway: An avenue to control
breast cancer aggressiveness. Int J Mol Sci. 21:90692020.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Pandit H, Li Y, Li X, Zhang W, Li S and
Martin RCG: Enrichment of cancer stem cells via β-catenin
contributing to the tumorigenesis of hepatocellular carcinoma. BMC
Cancer. 18:7832018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Gargiulo G, Cesaroni M, Serresi M, de
Vries N, Hulsman D, Bruggeman SW, Lancini C and van Lohuizen M: In
vivo RNAi screen for BMI1 targets identifies TGF-β/BMP-ER stress
pathways as key regulators of neural- and malignant glioma-stem
cell homeostasis. Cancer Cell. 23:660–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ader T, Norel R, Levoci L and Rogler LE:
Transcriptional profiling implicates TGFbeta/BMP and Notch
signaling pathways in ductular differentiation of fetal murine
hepatoblasts. Mech Dev. 123:177–194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Schartz NE, Chaput N, André F and Zitvogel
L: From the antigen-presenting cell to the antigen-presenting
vesicle: The exosomes. Curr Opin Mol Ther. 4:372–381.
2002.PubMed/NCBI
|
|
121
|
Mignot G, Roux S, Thery C, Segura E and
Zitvogel L: Prospects for exosomes in immunotherapy of cancer. J
Cell Mol Med. 10:376–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Deep G and Panigrahi GK: Hypoxia-induced
signaling promotes prostate cancer progression: Exosomes role as
messenger of hypoxic response in tumor microenvironment. Crit Rev
Oncog. 20:419–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gradilla AC, González E, Seijo I, Andrés
G, Bischoff M, González-Mendez L, Sánchez V, Callejo A, Ibáñez C,
Guerra M, et al: Exosomes as Hedgehog carriers in cytoneme-mediated
transport and secretion. Nat Commun. 5:56492014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Qi J, Zhou Y, Jiao Z, Wang X, Zhao Y and
Li Y, Chen H, Yang L, Zhu H and Li Y: Exosomes derived from human
bone marrow mesenchymal stem cells promote tumor growth through
hedgehog signaling pathway. Cell Physiol Biochem. 42:2242–2254.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sharma A: Role of stem cell derived
exosomes in tumor biology. Int J Cancer. 142:1086–1092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Bhat A, Sharma A and Bharti AC: Upstream
Hedgehog signaling components are exported in exosomes of cervical
cancer cell lines. Nanomedicine. 13:2127–2138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhao G, Li H, Guo Q, Zhou A, Wang X, Li P
and Zhang S: Exosomal Sonic Hedgehog derived from cancer-associated
fibroblasts promotes proliferation and migration of esophageal
squamous cell carcinoma. Cancer Med. 9:2500–2513. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wada J, Onishi H, Suzuki H, Yamasaki A,
Nagai S, Morisaki T and Katano M: Surface-bound TGF-beta1 on
effusion-derived exosomes participates in maintenance of number and
suppressive function of regulatory T-cells in malignant effusions.
Anticancer Res. 30:3747–3757. 2010.PubMed/NCBI
|
|
129
|
Matsumoto K, Morisaki T, Kuroki H, Kubo M,
Onishi H, Nakamura K, Nakahara C, Kuga H, Baba E, Nakamura M, et
al: Exosomes secreted from monocyte-derived dendritic cells support
in vitro naïve CD4+ T cell survival through NF-(kappa)B activation.
Cell Immunol. 231:20–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Onishi H, Kuroki H, Matsumoto K, Baba E,
Sasaki N, Kuga H, Tanaka M, Katano M and Morisaki T:
Monocyte-derived dendritic cells that capture dead tumor cells
secrete IL-12 and TNF-alpha through IL-12/TNF-alpha/NF-kappaB
autocrine loop. Cancer Immunol Immunother. 53:1093–1100. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Nakashima H, Nakamura M, Yamaguchi H,
Yamanaka N, Akiyoshi T, Koga K, Yamaguchi K, Tsuneyoshi M, Tanaka M
and Katano M: Nuclear factor-kappaB contributes to hedgehog
signaling pathway activation through sonic hedgehog induction in
pancreatic cancer. Cancer Res. 66:7041–7049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Kasperczyk H, Baumann B, Debatin KM and
Fulda S: Characterization of sonic hedgehog as a novel NF-kappaB
target gene that promotes NF-kappaB-mediated apoptosis resistance
and tumor growth in vivo. FASEB J. 23:21–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cai K, Na W, Guo M, Xu R, Wang X, Qin Y,
Wu Y, Jiang J and Huang H: Targeting the cross-talk between the
hedgehog and NF-κB signaling pathways in multiple myeloma. Leuk
Lymphoma. 60:772–781. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Nakayama K, Onishi H, Fujimura A, Imaizumi
A, Kawamoto M, Oyama Y, Ichimiya S, Koga S, Fujimoto Y, Nakashima K
and Nakamura M: NFκB and TGFβ contribute to the expression of PTPN3
in activated human lymphocytes. Cell Immunol. 358:1042372020.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Fosko SW, Chu MB, Armbrecht E, Galperin T,
Potts GA, Mattox A, Kurta A, Polito K, Slutsky JB, Burkemper NM, et
al: Efficacy, rate of tumor response, and safety of a short course
(12–24 weeks) of oral vismodegib in various histologic subtypes
(infiltrative, nodular, and superficial) of high-risk or locally
advanced basal cell carcinoma, in an open-label, prospective case
series clinical trial. J Am Acad Dermatol. 82:946–954. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
De Jesus-Acosta A, Sugar EA, O'Dwyer PJ,
Ramanathan RK, Von Hoff DD, Rasheed Z, Zheng L, Begum A, Anders R,
Maitra A, et al: Phase 2 study of vismodegib, a hedgehog inhibitor,
combined with gemcitabine and nab-paclitaxel in patients with
untreated metastatic pancreatic adenocarcinoma. Br J Cancer.
122:498–505. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yauch RL, Dijkgraaf GJ, Alicke B, Januario
T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, et
al: Smoothened mutation confere resistance to a Hedgehog pathway
inhibitor in medulloblastoma. Science. 326:572–574. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Dijkgraaf GJ, Alicke B, Weinmann L,
Januario T, West K, Modrusan Z, Burdick D, Goldsmith R, Robarge K,
Sutherlin D, et al: Small molecule inhibition of GDC-0499
refractory smoothened mutants and downstream mechanisms of drug
resistance. Cancer Res. 71:435–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Onishi H and Katano M: The Hedgehog
signaling pathway as a new therapeutic target in pancreatic cancer.
World J Gastroenterol. 20:2335–2342. 2014. View Article : Google Scholar : PubMed/NCBI
|