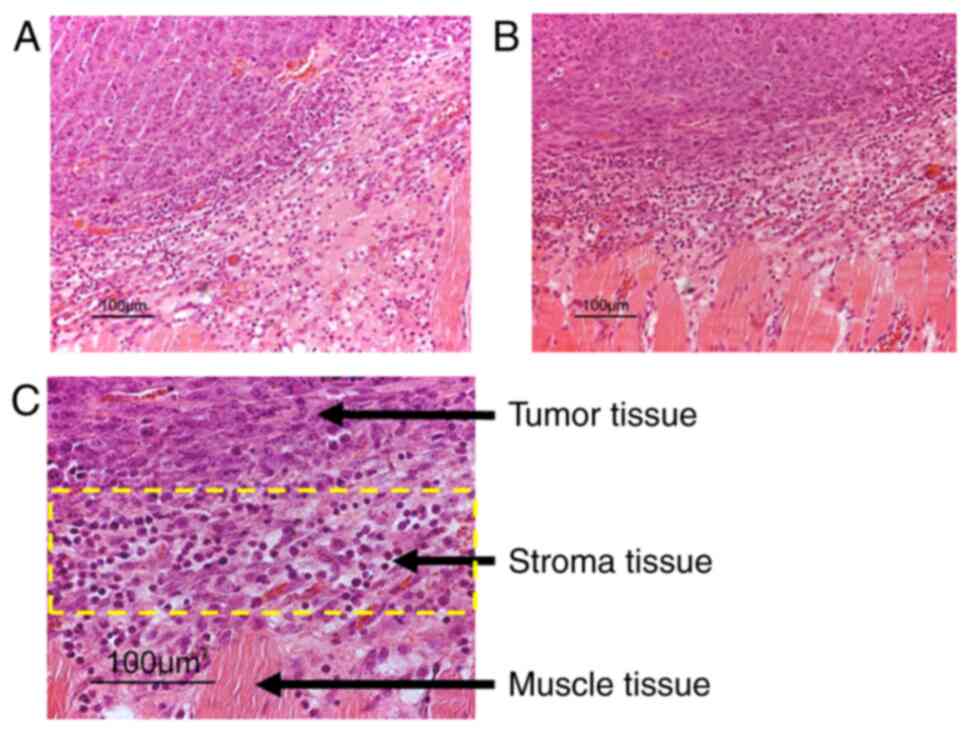
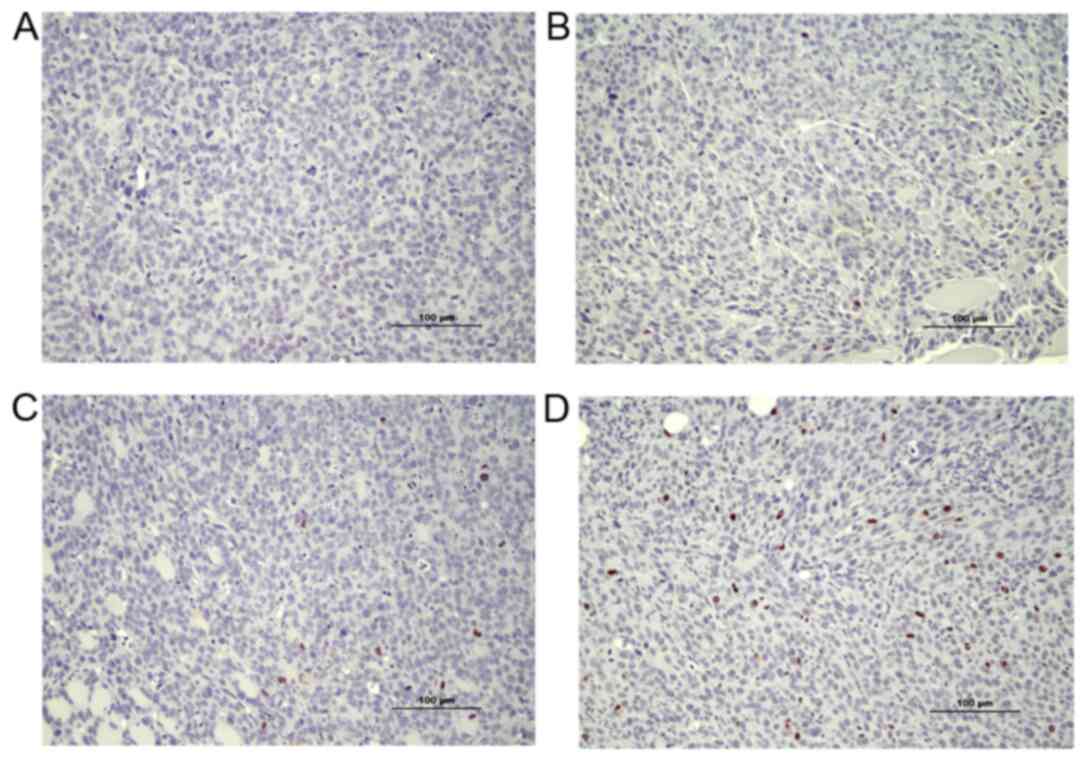
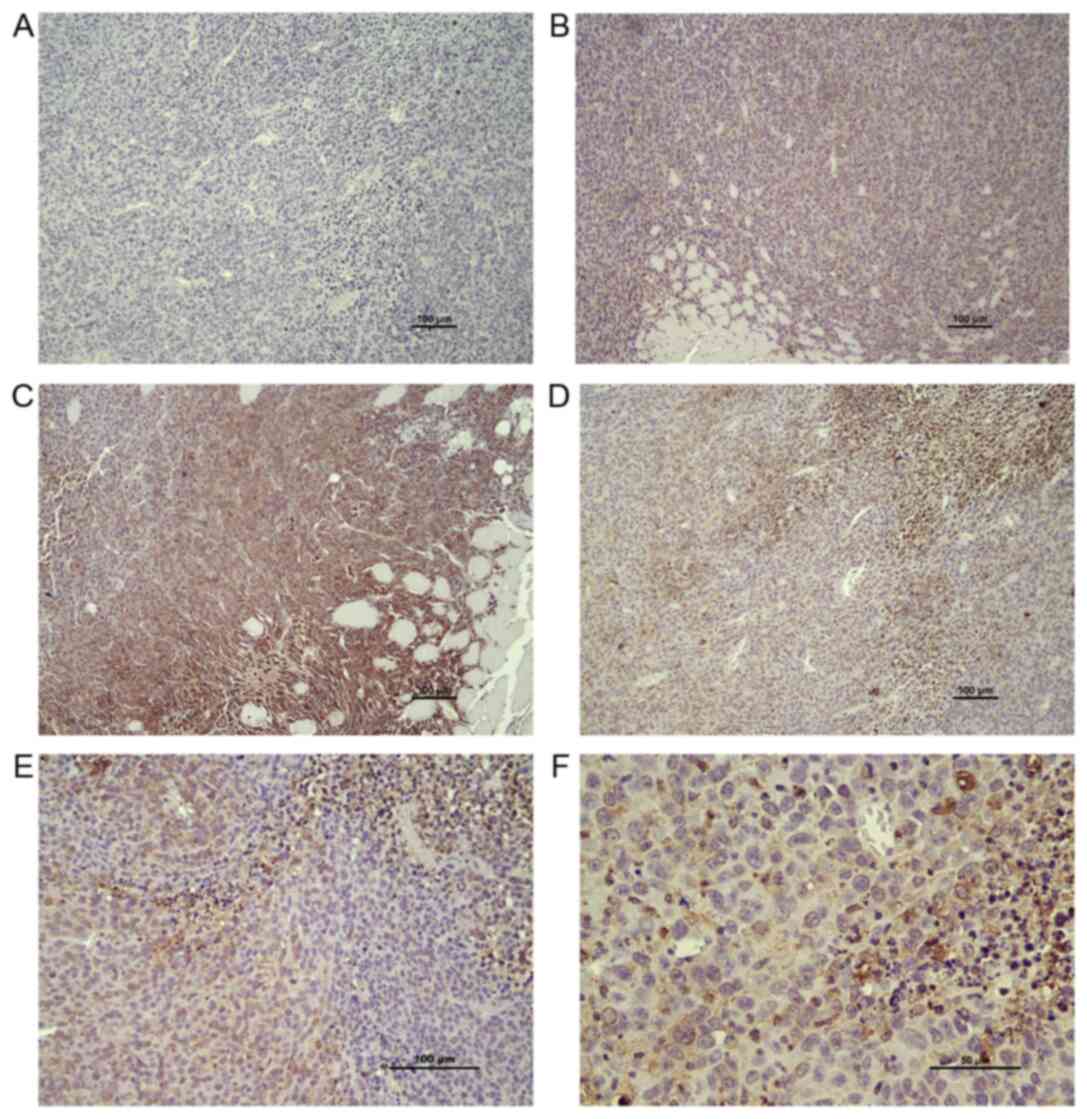
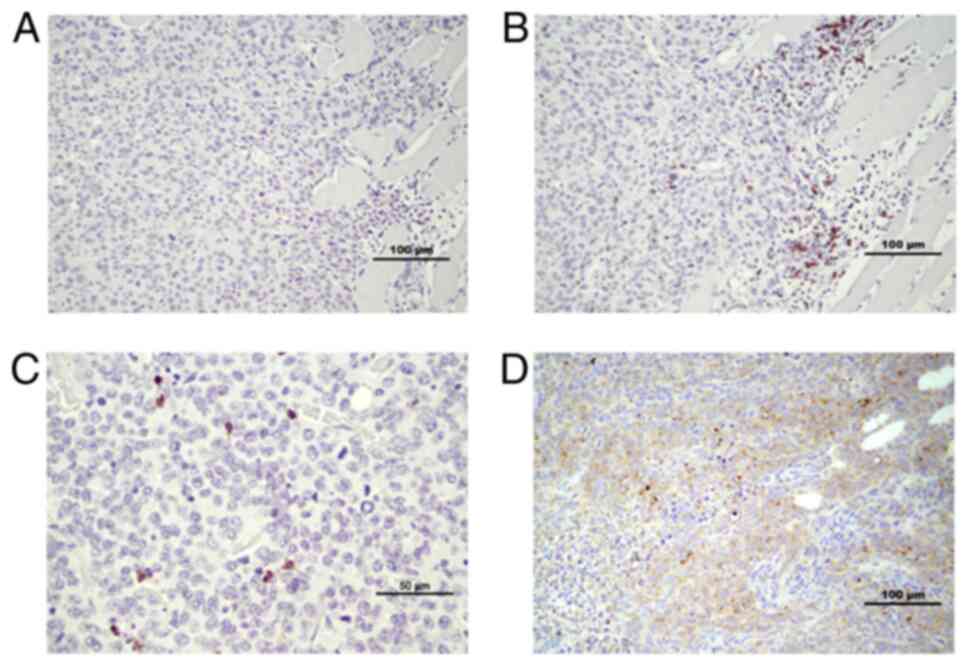
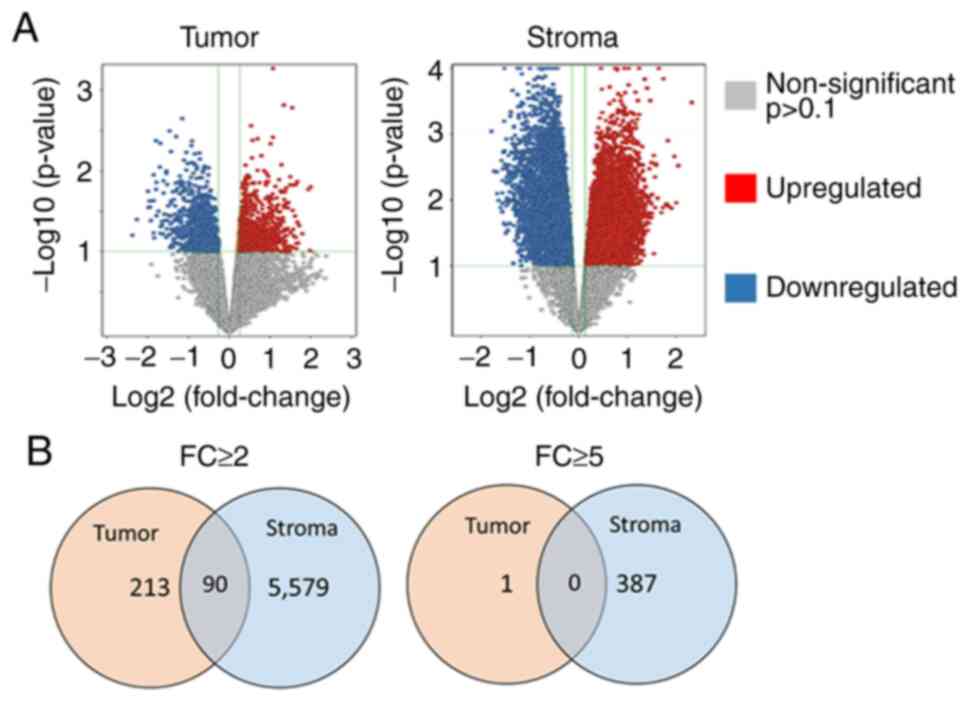
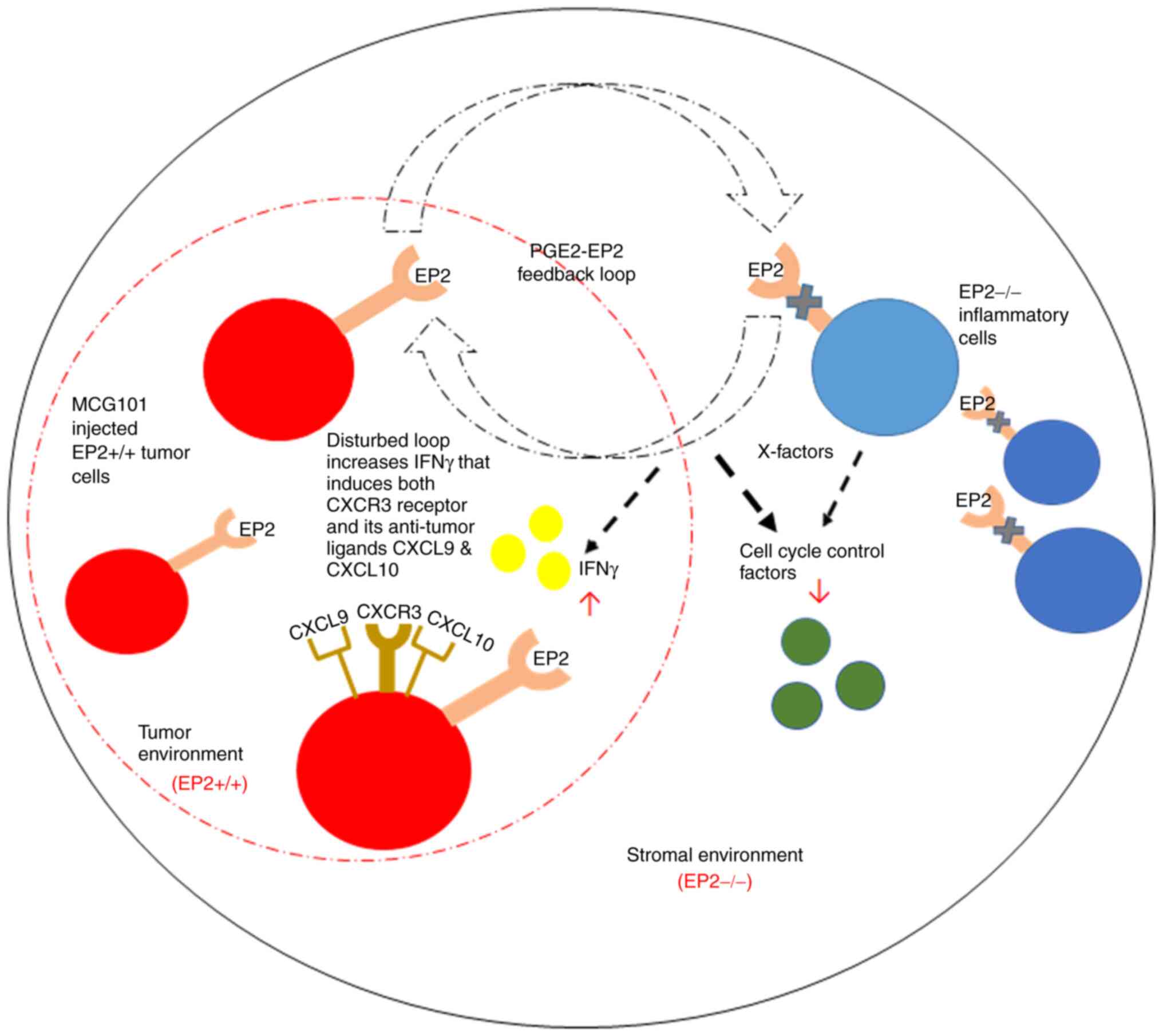
|
1
|
Wang D and DuBois RN: Role of prostanoids
in gastrointestinal cancer. J Clin Invest. 128:2732–2742. 2018.
View Article : Google Scholar
|
|
2
|
Lundholm K, Gelin J, Hyltander A, Lönnroth
C, Sandström R, Svaninger G, Körner U, Gülich M, Kärrefors I, Norli
B, et al: Anti-inflammatory treatment may prolong survival in
undernourished patients with metastatic solid tumors. Cancer Res.
54:5602–5606. 1994.PubMed/NCBI
|
|
3
|
Aoki T and Narumiya S: Prostaglandin
E2-EP2 signaling as a node of chronic inflammation in
the colon tumor microenvironment. Inflamm Regen. 37:42017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gustafsson A, Hansson E, Kressner U,
Nordgren S, Andersson M, Wang W, Lönnroth C and Lundholm K: EP1-4
subtype, COX and PPAR gamma receptor expression in colorectal
cancer in prediction of disease-specific mortality. Int J Cancer.
121:232–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gustafsson A, Hansson E, Kressner U,
Nordgren S, Andersson M, Lönnroth C and Lundholm K: Prostanoid
receptor expression in colorectal cancer related to tumor stage,
differentiation and progression. Acta Oncol. 46:1107–1112. 2007.
View Article : Google Scholar
|
|
6
|
Sonoshita M, Takaku K, Sasaki N, Sugimoto
Y, Ushikubi F, Narumiya S, Oshima M and Taketo MM: Acceleration of
intestinal polyposis through prostaglandin receptor EP2 in
Apc(Delta 716) knockout mice. Nat Med. 7:1048–1051. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu J, Li Q, Bell KA, Yao X, Du Y, Zhang
E, Yu JJ, Yu Y, Shi Z and Jiang J: Small-molecule inhibition of
prostaglandin E receptor 2 impairs cyclooxygenase-associated
malignant glioma growth. Br J Pharmacol. 176:1680–1699. 2019.
View Article : Google Scholar
|
|
8
|
Yang L, Yamagata N, Yadav R, Brandon S,
Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP and
Breyer RM: Cancer-associated immunodeficiency and dendritic cell
abnormalities mediated by the prostaglandin EP2 receptor. J Clin
Invest. 111:727–735. 2003. View Article : Google Scholar
|
|
9
|
Iresjö BM, Wang W, Nilsberth C, Andersson
M, Lönnroth C and Smedh U: Food intake, tumor growth, and weight
loss in EP2 receptor subtype knockout mice bearing PGE2-producing
tumors. Physiol Rep. 3:e124412015. View Article : Google Scholar
|
|
10
|
Asting AG, Iresjö BM, Nilsberth C, Smedh U
and Lundholm K: Host knockout of E-prostanoid 2 receptors reduces
tumor growth and causes major alterations of gene expression in
prostaglandin E2-producing tumors. Oncol Lett.
13:476–482. 2017. View Article : Google Scholar
|
|
11
|
Kennedy CR, Zhang Y, Brandon S, Guan Y,
Coffee K, Funk CD, Magnuson MA, Oates JA, Breyer MD and Breyer RM:
Salt-sensitive hypertension and reduced fertility in mice lacking
the prostaglandin EP2 receptor. Nat Med. 5:217–220. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lonnroth C, Svaninger G, Gelin J, Cahlin
C, Iresjo B, Cvetkovska E, Edstrom S, Andersson M, Svanberg E and
Lundholm K: Effects related to indomethacin prolonged survival and
decreased tumor-growth in a mouse-tumor model with cytokine
dependent cancer cachexia. Int J Oncol. 7:1405–1413. 1995.
|
|
13
|
Lundholm K, Edström S, Karlberg I, Ekman L
and Scherstén T: Relationship of food intake, body composition, and
tumor growth to host metabolism in nongrowing mice with sarcoma.
Cancer Res. 40:2516–2522. 1980.PubMed/NCBI
|
|
14
|
Axelsson H, Lönnroth C, Andersson M and
Lundholm K: Mechanisms behind COX-1 and COX-2 inhibition of tumor
growth in vivo. Int J Oncol. 37:1143–1152. 2010.
|
|
15
|
Andersson C, Gelin J, Iresjö BM and
Lundholm K: Acute-phase proteins in response to tumor growth. J
Surg Res. 55:607–614. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang W, Andersson M, Lönnroth C, Svanberg
E and Lundholm K: Anorexia and cachexia in prostaglandin EP1 and
EP3 subtype receptor knockout mice bearing a tumor with high
intrinsic PGE2 production and prostaglandin related cachexia. J Exp
Clin Cancer Res. 24:99–107. 2005.PubMed/NCBI
|
|
17
|
Svaninger G, Lundberg PA and Lundholm K:
Thyroid hormones and experimental cancer cachexia. J Natl Cancer
Inst. 77:555–561. 1986.
|
|
18
|
McKinney EF, Cuthbertson I, Harris KM,
Smilek DE, Connor C, Manferrari G, Carr EJ, Zamvil SS and Smith
KGC: A CD8+ NK cell transcriptomic signature associated
with clinical outcome in relapsing remitting multiple sclerosis.
Nat Commun. 12:6352021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hansen T: Inhibition of T-cell responses
by CD4 on an antigen-presenting cell. APMIS. 114:32–38. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lönnroth C, Andersson M, Arvidsson A,
Nordgren S, Brevinge H, Lagerstedt K and Lundholm K: Preoperative
treatment with a non-steroidal anti-inflammatory drug (NSAID)
increases tumor tissue infiltration of seemingly activated immune
cells in colorectal cancer. Cancer Immun. 8:52008.
|
|
21
|
Woodward DF, Jones RL and Narumiya S:
International union of basic and clinical pharmacology. LXXXIII:
Classification of prostanoid receptors, updating 15 years of
progress. Pharmacol Rev. 63:471–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Axelsson H, Lönnroth C, Wang W, Svanberg E
and Lundholm K: Cyclooxygenase inhibition in early onset of tumor
growth and related angiogenesis evaluated in EP1 and EP3 knockout
tumor-bearing mice. Angiogenesis. 8:339–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mizuno R, Kawada K and Sakai Y:
Prostaglandin E2/EP signaling in the tumor microenvironment of
colorectal cancer. Int J Mol Sci. 20:62542019. View Article : Google Scholar
|
|
24
|
Tokunaga R, Zhang W, Naseem M, Puccini A,
Berger MD, Soni S, McSkane M, Baba H and Lenz HJ: CXCL9, CXCL10,
CXCL11/CXCR3 axis for immune activation-a target for novel cancer
therapy. Cancer Treat Rev. 63:40–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bronger H, Singer J, Windmüller C, Reuning
U, Zech D, Delbridge C, Dorn J, Kiechle M, Schmalfeldt B, Schmitt M
and Avril S: CXCL9 and CXCL10 predict survival and are regulated by
cyclooxygenase inhibition in advanced serous ovarian cancer. Br J
Cancer. 115:553–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bronger H, Kraeft S, Schwarz-Boeger U,
Cerny C, Stöckel A, Avril S, Kiechle M and Schmitt M: Modulation of
CXCR3 ligand secretion by prostaglandin E2 and cyclooxygenase
inhibitors in human breast cancer. Breast Cancer Res. 14:R302012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kistner L, Doll D, Holtorf A, Nitsche U
and Janssen KP: Interferon-inducible CXC-chemokines are crucial
immune modulators and survival predictors in colorectal cancer.
Oncotarget. 8:89998–90012. 2017. View Article : Google Scholar
|
|
28
|
Dufour JH, Dziejman M, Liu MT, Leung JH,
Lane TE and Luster AD: IFN-gamma-inducible protein 10 (IP-10;
CXCL10)-deficient mice reveal a role for IP-10 in effector T cell
generation and trafficking. J Immunol. 168:3195–3204. 2002.
View Article : Google Scholar
|
|
29
|
Angiolillo AL, Sgadari C, Taub DD, Liao F,
Farber JM, Maheshwari S, Kleinman HK, Reaman GH and Tosato G: Human
interferon-inducible protein 10 is a potent inhibitor of
angiogenesis in vivo. J Exp Med. 182:155–162. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hartner JC, Walkley CR, Lu J and Orkin SH:
ADAR1 is essential for the maintenance of hematopoiesis and
suppression of interferon signaling. Nat Immunol. 10:109–115. 2009.
View Article : Google Scholar
|
|
31
|
Domingo-Gil E and Esteban M: Role of
mitochondria in apoptosis induced by the 2-5A system and mechanisms
involved. Apoptosis. 11:725–738. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Castelli JC, Hassel BA, Maran A, Paranjape
J, Hewitt JA, Li XL, Hsu YT, Silverman RH and Youle RJ: The role of
2′-5′ oligoadenylate-activated ribonuclease L in apoptosis. Cell
Death Differ. 5:313–320. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koeffler HP: Peroxisome
proliferator-activated receptor gamma and cancers. Clin Cancer Res.
9:1–9. 2003.PubMed/NCBI
|
|
34
|
Houseknecht KL, Cole BM and Steele PJ:
Peroxisome proliferator-activated receptor gamma (PPARgamma) and
its ligands: A review. Domest Anim Endocrinol. 22:1–23. 2002.
View Article : Google Scholar
|
|
35
|
Han S, Inoue H, Flowers LC and Sidell N:
Control of COX-2 gene expression through peroxisome
proliferator-activated receptor gamma in human cervical cancer
cells. Clin Cancer Res. 9:4627–4635. 2003.PubMed/NCBI
|
|
36
|
James SY, Lin F, Kolluri SK, Dawson MI and
Zhang XK: Regulation of retinoic acid receptor beta expression by
peroxisome proliferator-activated receptor gamma ligands in cancer
cells. Cancer Res. 63:3531–3538. 2003.PubMed/NCBI
|
|
37
|
Han S and Roman J: Suppression of
prostaglandin E2 receptor subtype EP2 by PPARgamma ligands inhibits
human lung carcinoma cell growth. Biochem Biophys Res Commun.
314:1093–1099. 2004. View Article : Google Scholar : PubMed/NCBI
|