Introduction
Cancer is defined as uncontrolled cell growth in any
part of the body. It accounts for ~10 million deaths in 2020
(1). However, researchers are
striving to establish effective cancer treatments, and microRNAs
(miRNAs or miRs) and the hippo signaling pathway have just been
discovered in this regard. The Hippo signaling pathway is a
mechanism that regulates the organ's size in mammals and humans.
The size of the organ is regulated by mediating cell growth,
division and death (2). Any
dysregulation in the hippo pathway disrupts mediation, leading to
the activation of the transcriptional co-activators, that is, YAP
and TAZ. Their elevated levels result in escalated cell
proliferation and reduced cell death, leading to tumorigenesis
(3).
miRNAs play a regulatory role and work by targeting
and regulating a certain mRNA. miRNAs have oncogenic and
tumor-suppressive characteristics, and their altered levels are
detected in cancer. A previous study has revealed that miRNAs can
function as a positive or negative regulator to modulate the core
components of the hippo pathway (4). Furthermore, YAP and TAZ interact with
the components involved in the miRNA biogenesis, and the
inactivation of the Hippo pathway or constitutively expression of
YAP can result in reduced miRNA biogenesis (5). Understanding the interlinkage between
the hippo pathway and miRNAs is crucial for figuring out the root
cause of cancer. The dysregulated miRNAs and hippo pathway
contribute to uncontrolled growth by regulating each other and even
conferring resistance to anticancer treatments. Previous studies
have highlighted the mechanism through which miRNAs and hippo
regulate each other (2,4). This provides a ray of hope for
advancing treatment strategies for patients with cancer. At
present, the miRNAs therapeutics are in pre-clinical and clinical
trials. These therapeutics would pave the way for cancer treatment.
Furthermore, it would even break the resistance developed against
the anticancer drugs, thereby boosting the efficiency of the
existing treatments (4).
Recapitulation of the Hippo pathway
In mammals, the Hippo signaling pathway is a
mechanism that maintains the size of the organ by controlling cell
proliferation and cell death (6).
Previous studies revealed that the hippo pathway is linked to
several cancer-like traits, including increased cell proliferation
and the development of drug resistance (7,8). The
mammalian hippo signaling pathway is described as a cascade
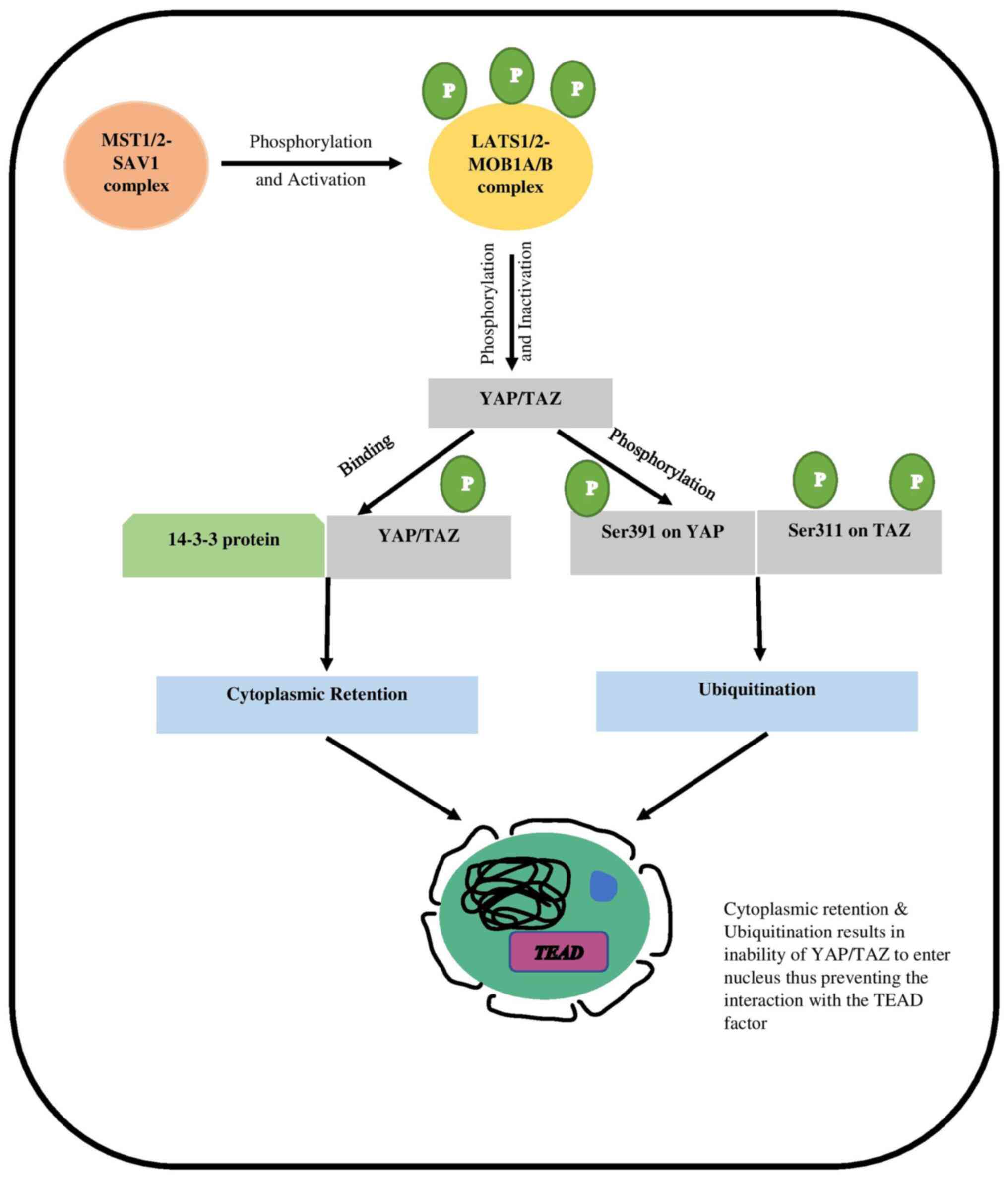
mechanism, including the four key tumor suppressors, which are the
Mammalian sterile 20-like kinase 1/2 (MST1/2), Salvador Homolog 1
(SAV1), Large tumor suppressor1/2 (LATS1/2), and MOB kinase
activator 1A/B (MOB 1A/B). The cascade is initiated when the
activated MST1/2-SAV1 complex phosphorylates the LATS1/2-MOB1A/B
complex, resulting in its activation (9). When the LATS1/2-MOB1A/B complex is
activated, it causes the transcriptional co-activators YAP and TAZ
to be downregulated, thus inactivating them (10). The LATS1/2-MOB1A/B complex is
activated and works by phosphorylating YAP/TAZ at various
locations. When the LATS1/2-MOB1A/B complex is activated, it
phosphorylates YAP/TAZ at numerous sites. The phosphorylation
causes YAP/TAZ to have a higher binding affinity for 14-3-3
protein. This interaction aids the cytoplasmic localization of the
YAP/TAZ. When Ser127 and Ser397 on YAP and Ser89 and Ser311 on TAZ
are phosphorylated, the activity of both proteins is significantly
reduced (11–13). Phosphorylated Ser397 on YAP and
Ser311 on TAZ cause ubiquitination and proteasomal degradation of
YAP/TAZ (12) (Fig. 1).
The absence of phosphorylation due to the
dysregulated hippo pathway leads to the YAP/TAZ upregulation. The
activated YAP/TAZ then moves toward the nucleus, where it interacts
with Transcriptional Enhanced Associate Domain (TEAD), a
transcriptional factor. YAP/TAZ and TEAD interact and bind to
activate the expression of subsequent genes. Increased cell
proliferation and decreased apoptosis are two cancer-like
properties induced by the activated genes (10,14).
In conclusion, as the hippo pathway modulates the
activation of YAP/TAZ, any disruption would elevate the levels of
YAP/TAZ, thereby promoting tumorigenesis.
Hippo pathway: regulation and cancer
development
The hippo signaling system regulates organ size in
mammals by controlling cell growth, division, survival and
apoptosis (15). As a result, the
dysregulation in the hippo pathway can cause YAP/TAZ levels to
rise. Additionally, these altered levels stimulate the activation
and overexpression of associated genes such as CYR61, CTGF, MYC,
and AREG, increasing cell proliferation and tumorigenesis
(16,17). The functioning and activity of the
hippo pathway involve regulation by various molecules and at
different levels of the kinetic cascade (18). Upstream regulators include
molecules like KIBRA, RASSFs, Merlin, and hEx. They are responsible
for promoting and modulating the MST1/2 activity. The Ajuba
molecule, on the other hand, is classified as a negative regulator
since it inhibits the phosphorylation of YAP/TAZ by downregulating
the LATS1/2 by interfering with its function (4). α-catenin, ZO-2, 14-3-3, and AMOT are
molecules that promote the retention of YAP/TAZ in the cytoplasm
(18). Their action aids in the
regulation of YAP/TAZ levels.
Apart from the molecular regulation, the hippo
signaling pathway can be crosslinked with other signaling pathways,
resulting in hippo pathway modulation and hence malignant
situations. The Wnt and AMPK pathways are responsible for YAP
protein downregulation. In the case of the Wnt pathway, the
scaffold protein DVL acts as a link between the hippo and the Wnt
signaling pathway. DVL protein comprises the nuclear export
signals, which promote YAP cytoplasmic translocation (19). Since it increases the
phosphorylation of YAP at numerous locations, the AMPK pathway can
affect the hippo pathway. AMPK also phosphorylates AMOTL1, and
phosphorylated AMOTL1 stimulates the upregulation of the LATS1/2,
according to a previous study (20). Increased LATS1/2 activity causes
YAP inactivation, which is accomplished by phosphorylation. In
addition, TAZ interacts with the heteromeric Smad2/3-Smad4
complexes in the TGF-pathway, proving it to be a positive
regulator. The binding results in the sustainability of
Smad2/3-Smad4 complexes accumulating in the nucleus (21). TAZ phosphorylation and inactivation
reduce the ability of the Smad2/3-Smad4 complexes to accumulate in
the nucleus, thereby altering the transcription process. TGF
signaling is inhibited in the absence of TAZ, which impacts neural
epithelial development (22). In
KRAS signaling, the protein sends signals to the cells directing
the cell to grow and divide, but the KRAS gene is identified
as the oncogene, meaning that upon mutation, it results in the
uncontrolled growth of the cells (23). Studies have shown that the YAP can
be understood as the rescuer of the K-Ras4B-inhibited cells
(24,25). The YAP, along with β-catenin, can
induce resistance by managing the advancement of the cells to the S
phase in the cell cycle (16). In
MAPK/ERK signaling, the YAP protein can promote the development of
resistance against the MAPK/ERK kinase (MEK)-targeted inhibitor
therapy (26). The increased
effect of the active YAP directly affects the MEK inhibitors,
thereby predicting the therapeutic effect.
The process of hippo pathway regulation is modulated
by various molecules and signaling pathways, either directly or
indirectly. Therefore, any sought of disruption responsible for the
altered YAP/TAZ levels would result in uncontrolled cell growth and
reduced apoptosis due to activation of subsequent genes, thereby
developing cancer-like properties (27).
Understanding the miRNAs
miRNAs belong to the class of non-coding endogenous
RNA, also characterized as small single-stranded nucleotides which
play a key regulatory role in various biological processes in
plants and animals (28,29). miRNAs range from 21 to 25
nucleotides in length and work by targeting the specific mRNA by
translational inhibition, degradation, or mRNA destabilization to
regulate the target (29,30). The concept of miRNAs emerged back
in 1993 when the miRNAs were first discovered in Caenorhabditis
elegans (C. elegans) by Ambros and Ruvkun groups (31,32).
Previous studies showed that in protein LIN-14, the expression was
regulated by a non-coding RNA, and this modulation resulted in the
defected development in Caenorhabditis elegans. The protein
LIN-14 is coded by the heterochronic gene lin-14, which
controls the developmental timing in C. elegans (33). The discovery of miRNAs was
advantageous for researchers working in the molecular biology
domain. The miRNAs detected are known to be highly conserved and
mainly play the regulatory role across the species (34,35).
Researchers are still exploring the miRNAs and their role in gene
regulation.
The majority of the miRNAs interact with the target
mRNA through the 3′ untranslated region (UTR), referred to as
3′(UTR), which promotes the suppression of expression, and the
targeted mRNA is degraded (36). A
previous study has shown that apart from 3′UTR, miRNAs also
interact with the other regions of mRNA, such as coding sequence,
gene promoters, and 5′UTR (29).
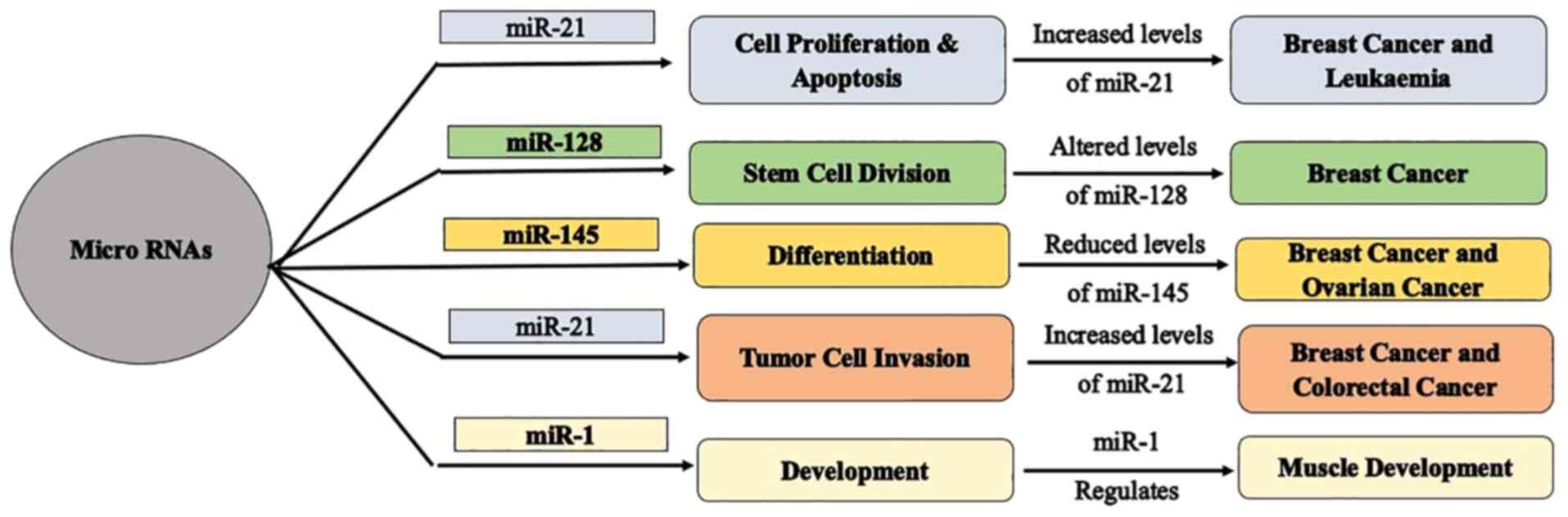
MicroRNAs hold significance in mediating cell proliferation and
apoptosis due to their regulatory functioning (37), play a vital role in stem cell
division (38), and regulate
differentiation, development, and tumor cell invasion (28) (Fig.
2). Since miRNAs mediate cell growth and cell death, any
dysregulation in miRNAs pattern would result in uncontrolled growth
of the affected cells, thereby depicting cancer-like properties
(tumorigenesis).
Biogenesis of miRNAs
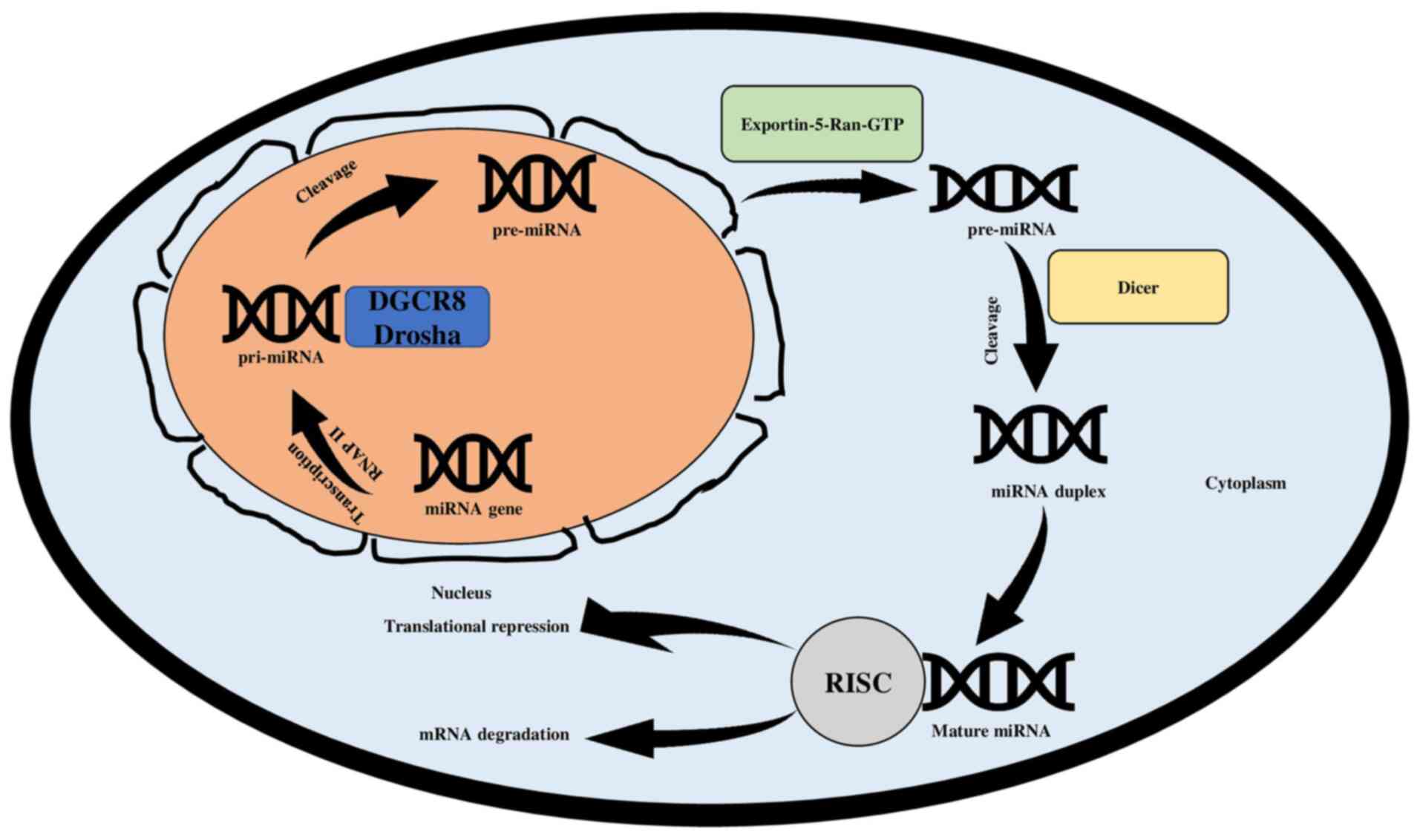
The biogenesis of miRNAs is a convoluted process.
The synthesis of the miRNAs involves a two-step process wherein the
first step occurs in the nucleus and the second one takes place in
the cytoplasm (36). The process
initiates with the formation of primary miRNA (pri-miRNA), which is
achieved through gene transcription. The transcription is performed
by RNA polymerase II but occasionally can also be performed by RNA
polymerase III (39,40). The primary miRNA transcript
structures are 5′ capped and 3′ polyadenylation (41). The cleavage of pri-miRNA is
conducted through a microprocessor complex. The microprocessor
complex incorporates Drosha, an RNase III enzyme that is
responsible for cleaving the pri-miRNA in the nucleus, and DGCR8, a
RNA binding protein (3,42). The microprocessor complex is
responsible for cleaving the pri-miRNA as the DGCR8 recognizes the
ssRNA-dsRNA junction found on pri-mi RNA. This dictates Drosha to
give rise to a 60-nucleotide hairpin-like precursor miRNA
(pre-miRNA) (41,43). The transfer of pre-miRNA from the
nucleus to the cytoplasm is aided by Exportin-5-Ran-GTP. Dicer
ribonuclease, which is another RNase III enzyme, cleaves the
pre-miRNA in the cytoplasm. When pre-miRNA is cleaved, a miRNA
duplex of 21–23 nucleotides is formed, which includes the
complementary strand and mature miRNA strand (4,25).
The mature miRNA is then integrated into the RNA-induced silencing
complex (RISC), a protein complex that also includes the Argonaute
(44). The integration targets the
3′UTR of mRNAs, lowering their post-transcriptional or
translational levels through mRNA degradation or translational
suppression (45,46). (Fig.
3).
miRNAs: Regulation and cancer
development
MicroRNAs are known to regulate gene expression in
both plants and animals. According to a previous study, the number
of miRNAs in cancer varies depending on the processes and
microenvironment (47). The
synthesis of miRNAs is controlled at several levels, including
transcription and transportation. It has been identified that SMAD
proteins and DEAD-box RNA helicases play a role in miRNA maturation
mediated by Drosha (48,49). Methyltransferase-like 3 methylates
pri-miRNAs, allowing DGCR8 to recognize and process them, making
them the biogenesis regulator (50). KSRP also controls miRNA biogenesis
by functioning as a complement to Drosha and Dicer (51). Any disruption in these regulators
may cause fluctuations in miRNA levels, resulting in changes in
miRNA expression.
The altered levels of miRNAs are one cause leading
to cells developing cancer-like properties as numerous miRNAs act
as either tumor suppressors or tumor promoters. Thus, the altered
levels of these miRNAs result in tumorigenesis. For instance,
miRNA-21 and miRNA-155 are identified as miRNAs whose increased
expression is observed in malignant tumors. On the other hand, the
downregulation of these miRNAs has been revealed to cause
controlled cell proliferation, thereby resulting in reduced tumor
growth (52,53). Let-7 is another miRNA that acts as
a tumor suppressor, and its levels are highly downregulated in case
of cancer (54).
Deciphering the interlinkage of miRNAs and
the Hippo pathway
The hippo signaling pathway is primarily responsible
for regulating the size of the organ, which is achieved by
mediating cell proliferation, apoptosis and regeneration (55). Downregulation of the hippo pathway
causes YAP/TAZ to be activated, resulting in escalated cell
proliferation and reduced cell-cell adhesion leading to
tumorigenesis. The miRNAs are small non-coding RNA and play a
regulatory role in gene expression by targeting the specific
miRNAs. In cancer, altered miRNAs level are observed due to
dysregulated miRNA expression. The dysregulation of expression of
miRNAs occurs due to the defective miRNAs biogenesis or the
fluctuating levels of the miRNAs genes (30).
The interlinkage between miRNAs and the hippo
signaling pathway, which leads to cancer, has been proven through
advances in research. The YAP and TAZ, two critical components of
the hippo pathway, have been demonstrated to influence miRNA
biogenesis directly, implying that they play an important role in
cancer progression. On the other hand, various miRNAs can regulate
key elements of the hippo pathway, resulting in cancer (5). As a result, it is critical to figure
out how the hippo pathway and miRNAs are linked and how they
interact to figure out how to treat cancer with therapeutic
approaches.
The Hippo pathway as a regulator of
miRNAs
The research conducted to understand the crosslinks
between the hippo and miRNAs suggested that the hippo pathway
functions as a regulator for miRNA synthesis in a cell
density-dependent manner, implying that the regulation of the hippo
pathway contributes to carcinogenesis. It operates by interfering
with the microprocessor complex that forms pre-miRNA from
pri-miRNA. Furthermore, because it is sensitive to cell density and
disruption of the hippo pathway is a prominent hallmark of tumors,
studies have revealed that it is a regulator of cell
density-dependent miRNA synthesis (5,55,56).
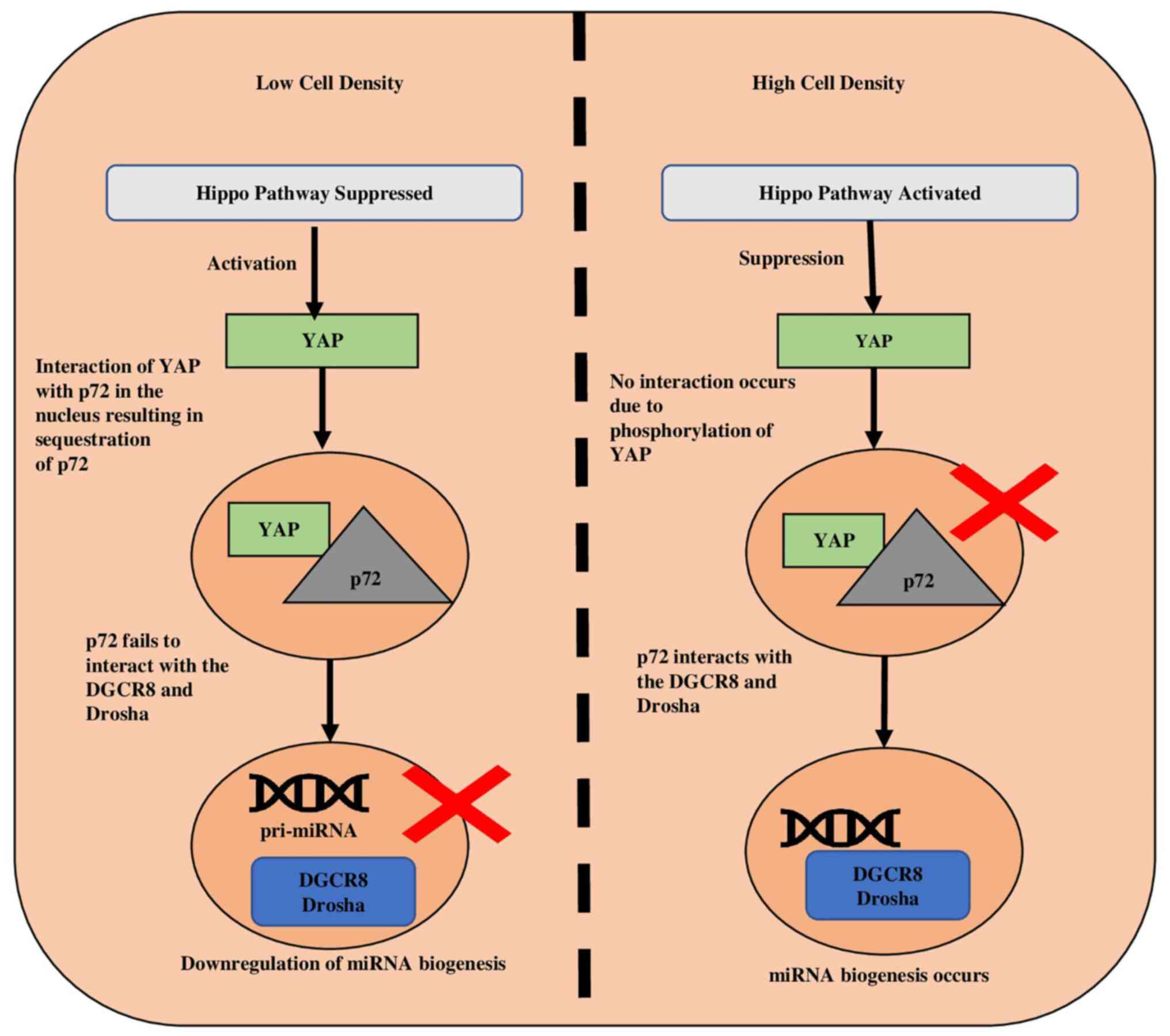
In case of lower cell density, the suppression of
the hippo pathway, leads to activation of YAP, and the activated
YAP moves towards the nucleus. This set of events causes the
activation of subsequent genes accountable for elevated cell
growth, thereby suppressing the biogenesis of miRNAs (8). The cell density increases with the
increase in proliferation, and this promotes the phosphorylation
and cytoplasmic retention of YAP by adherens such as E-cadherin
(55) and α-catenin (57). p72 (DDX17) is an accessory protein
that forms part of the DROSHA-containing complex and plays a role
in the pri-miRNA processing by interacting with DROSHA and DGCR8
(50,58). Since p72 detects the VCAUCH
sequence, which is present in the 3′ flanking region of pri-miRNA,
its interaction with the microprocessor enhances biogenesis
(5). In low density, p72 interacts
with YAP rather than DROSHA and DGCR8, resulting in YAP's retention
of p72 in the nucleus and reduced miRNA biosynthesis (59). (Fig.
4).
miRNAs as a regulator of the the Hippo
signaling pathway
Depending on their environment, miRNAs are known to
act as either oncogenic or tumor suppressors in certain
malignancies (60). According to
previous findings, miRNAs positively and negatively affect the
hippo pathway, implying that some oncogenic miRNAs adversely
regulate the hippo pathway, resulting in cancer. By contrast,
certain miRNAs regulate the hippo pathway positively, thereby
maintaining the hippo pathway.
miR-31 and miR-135b are identified as oncogenic
miRNAs, and elevated expression is observed in the case of cancer
(61). Overexpression of these
miRNAs is one cause that results in the reduced levels of LATS1/2,
which is a core component of the hippo pathway. Downregulation of
LATS1/2 promotes the activation of YAP, which travels towards the
nucleus and, upon reaching, interacts with the TEAD. The
interaction between YAP and TEAD is the activation of the
subsequent genes, thereby increasing cell proliferation and
apoptosis (62). miR-130 works in
a feedback loop with YAP, and its increased levels are found in
various types of cancer including oesophageal squamous cell
carcinoma, gastric and bladder cancer (63,64).
miR-130 is known to play a regulatory role in PTEN
expression and Akt phosphorylation in cancerous cells, and the
increased levels of miRNA-130 is induced by the YAP (4). The miR-130 negatively regulates the
VGLL4, which is responsible for the downregulation of the
YAP-TEAD complex in the nucleus. The consequence of the
downregulation of VGLL4 has increased YAP activity, thereby
promoting carcinogenesis (4,65).
miR-3910 is another miRNA exhibiting oncogenic properties. It
promotes cell proliferation and cell migration, and in mice, its
overexpression has resulted in the formation of hepatocellular
carcinoma (HCC) (66). The
miR-3910 negatively regulates the MST1, and its increased levels
lead to the downregulation of MST1. As MST1 negatively regulates
YAP, its reduced levels increase YAP levels. The absence of
phosphorylation of YAP promotes the expression of the genes such as
CTGF, MYC and BMI1, thereby inhibiting apoptosis and
promoting tumor formation (66).
The tumor suppressor miRNAs work as a regulator for
cell proliferation and apoptosis, thereby regulating the hippo
pathway by targeting either YAP or TAZ (4). For example, miRNA-375 is known as a
tumor suppressor, and its levels are found to be reduced in HCC. In
addition, 3′ UTR of YAP1 is targeted by miRNA-375, which
suppresses the YAP1 levels, thereby regulating the hippo
pathway (67). miR-186 also acts
as the regulator of YAP expression by acting on the YAP1
gene, which suppresses YAP (68).
miR-137 and miR-9 are known to regulate the YAP by
promoting the activity of LATS1 negatively. The increased activity
of LATS1 results in the phosphorylation of YAP, thereby
downregulating the expression of ARE, CYR61 and CTGF,
which are responsible for causing gastric cancer (69). miR-9-3p, which is formed by
processing the 3′ arm of miR-9, has been shown to downregulate TAZ,
thus regulating the pathway (70).
The tumor suppressor miR-129-5p has shown a direct relationship
with the hippo pathway as its downregulation causes the activation
of YAP/TAZ, followed by the interaction with the TEAD (71). A previous study has shown the
relation between the elevated miR-195 levels and YAP activity. The
reduced levels of miR-195 in cancer cells show high levels of YAP,
indicating the direct regulation of YAP by miR-195 (72). The regulation of YAP by miR-195
demonstrates that the miRNAs regulate the hippo pathway, and the
regulation of the biogenesis of miRNAs by the hippo pathway is
interlinked. Together this contributes to the development of
cancer-like properties and tumorigenesis.
miRNAs therapeutics for the treatment of
cancer
Apart from all the treatment methods available, such
as chemotherapy, the use of therapeutics to eliminate cancer cells
proves to be advantageous for the treatment of cancer (3). Since miRNAs play a regulatory role in
gene expression and their altered levels are found in various
cancers, studies are at present diverted towards the therapeutics
miRNAs in order to destroy the cancerous cells, as the miRNAs play
a vital role in tumor formation and expansion (37,73).
The miRNAs can target various proteins by interacting with the
various target mRNA, therefore, demonstrating to be a suitable
candidate for the treatment of cancer (74). Because miRNAs have both oncogenic
and tumor-suppressive roles in organisms, scientists are working on
therapies that consist of miRNA mimics and anti-miRNAs (75). Mimic miRNAs mimic tumor-suppressive
miRNAs and raise their levels in situations where tumor-suppressive
miRNAs are low. They are non-naturally occurring oligonucleotide
duplexes that mimic the function of tumor-suppressive miRNAs
(75). Anti-miRNAs are miRNAs that
serve as antagonists against oncogenic miRNAs. They have
complementary sequences to the targeted miRNAs, blocking oncogenic
miRNAs and lowering their overexpressed levels in malignancies
(76). miRNAs therapeutics are
introduced in the pre-clinical models so as to improve the efficacy
of therapeutics via viral vectors (77), nanoparticles (78) and liposome delivery (79).
Researchers have been working on miRNA therapies for
years, but only a few have progressed to the stage of clinical
trials. Due to the variability of miRNA expression, the most
significant challenge is to identify the miRNA targets and creat a
delivery mechanism that is not hazardous (75). miRNA therapies are now being
examined and could be beneficial to patients with cancer in the
future. In addition, because miRNAs have been proven to regulate
the hippo pathway, researchers can employ treatments to break the
resistance to anticancer therapies induced by the dysregulated
hippo pathway, demonstrating application in carcinogenesis. A few
miRNAs therapeutics under pre-clinal and clinical trials are listed
in Table I (75,80).
 | Table I.miRNA therapeutics under pre-clinical
and clinical trials. |
Table I.
miRNA therapeutics under pre-clinical
and clinical trials.
| Drug Name | Targeted miRNA | Category of
therapeutics | Target cancer | Stage of
trials | Clinical trial
number | (Refs.) |
|---|
| - | miR-10b | Anti-miRNA | Glioblastoma,
breast cancer | Trails in
pre-clinical models | - | (75) |
| - | miR-200 family | Mimic-miRNA | Lung cancer, Breast
cancer, Ovarian cancer | Trails in
pre-clinical models | - | (75) |
| MRX34 | miR-34 | Mimic-miRNA | Various solid
tumors such as liver cancer, lymphoma | Phase-I:
Terminated | NCT01829971 | (75,80) |
|
|
|
|
| Phase I:
Withdrawn | NCT02862145 |
|
| MesomiR-1 | miR-16 | Mimic-miRNA | Lung cancer | Phase I:
Completed | NCT02369198 | (75,80) |
| Miravirsen | miR-122 | Anti-miRNA | Lung cancer | Phase I: | NCT01646489 | (75,80) |
|
|
|
|
| Status:
Complete |
|
|
|
|
|
|
| Phase II: | NCT02508090 |
|
|
|
|
|
| Status:
Complete |
|
|
|
|
|
|
| Phase II: | NCT02452814 |
|
|
|
|
|
| Status:
Complete |
|
|
|
|
|
|
| Phase II: | NCT01200420 |
|
|
|
|
|
| Status:
Complete |
|
|
|
|
|
|
| Phase II:
Unknown | NCT01872936 |
|
|
|
|
|
| Phase II:
Unknown | NCT01727934 |
|
miRNAs, Hippo and resistance to anticancer
drugs
miRNAs are accountable for regulating gene
expression, and the altered levels of miRNAs are detected in
patients who have cancer. Since miRNAs also regulate the genes that
directly influence the response of cells when anticancer drugs are
administered, the dysregulated levels result in the development of
resistance against the anticancer drugs, thereby affecting the
therapeutic effect of the drug (81). The component of the Hippo pathway,
that is, the YAP and TAZ, are involved in imparting resistance
against the anticancer drugs. The elevated levels of YAP/TAZ result
in the altered levels of the various proteins and pathways, thereby
affecting the normal cascade. This alteration results in the
development of resistance to anticancer drugs. For example, the
elevated levels of TAZ result in increased multidrug resistance
protein, which results in the development of resistance against
Paclitaxel (an anticancer drug) (3).
Previous studies have also highlighted the role of
the Hippo pathway in the therapeutic effect, as the dysregulated
pathway results in resistance against the drugs (3,8). As
the miRNAs mediate the hippo pathway, it is crucial to deeply
analyze the crosslinking between the hippo pathway and miRNAs and
how this can affect the resistance against the anticancer drugs to
develop effective treatment strategies. The miRNAs, its target
hippo component, and which drug's therapeutic action is affected in
specific cancer (82) are listed
in Table II (83–87).
 | Table II.miRNA and Hippo Pathway components
affecting therapeutics in different cancers. |
Table II.
miRNA and Hippo Pathway components
affecting therapeutics in different cancers.
| miRNA | Expression in
tumour | Affected component
of the Hippo pathway | Dysregulation of
the Component | Resistance to
Drug | Cancer | (Refs.) |
|---|
| miR-21 | Increased | YAP | Upregulation | Doxorubicin,
Trastuzumab | Breast cancer | (83,84) |
| miR-135a/b | Increased | LATS1/2 | Downregulation | CDDP | Lung cancer | (85) |
|
|
| YAP | Upregulation |
|
|
|
| miR-9 | Decreased | LATS1/2 | Upregulation | Doxorubicin,
CDDP | Ovarian cancer | (86) |
|
|
| YAP | Downregulation |
|
|
|
| miR-338-3p | Decreased | TAZ | Downregulation | Sorafenib | Hepatocellular
cancer | (87) |
miR-21
miR-21 is an oncogenic miRNA, and its increased
levels are observed in different types of cancer, including breast,
cervical and colon cancer (88).
Doxorubicin is a therapeutic drug administered to treat various
types of cancer. It promotes cell apoptosis by binding with
topoisomerase II thereby inhibiting its enzymatic activity
(89). Studies have shown that in
the case of breast cancer, the increased levels of miR-21 promote
resistance against the anticancer drug as the miR-21 downregulates
the PTEN expression (83,84). Furthermore, increased miR-21 levels
result in the downregulation of RUNX1. As RUNX1
inhibits the YAP expression, its downregulation results in the YAP
activation. The upregulation of YAP promotes the partial actuation
of the MAPK pathway, which results in tumorigenesis (3). This, along with altered levels of
YAP, results in the development of resistance against doxorubicin.
By administering anti-miRNAs, the levels of miR-21 would be
regulated, breaking the doxorubicin resistance. Studies have
indicated that trastuzumab resistance has a similar sequence of
events, apart from doxorubicin resistance. Trastuzumab is a
chemotherapeutic medication that inhibits cancer cell growth by
binding to the HER2 protein (90).
Resistance to the anticancer medicine trastuzumab develops due to
overexpression of miRNA and decreased expression of PTEN.
Overexpression of YAP and TEAD enhances treatment resistance in
breast cancer, and increased miRNA levels boost YAP activation
(91). Trastuzumab resistance can
be minimized by using antisense miRNA-21 oligonucleotides to
maintain the levels of miR-21, which regulates the levels of YAP
and so reduces resistance against trastuzumab to some extent
(84).
miR-135a/b
miRNAs fall into the category of both oncogenic
miRNAs and tumor suppressor miRNAs. The research has highlighted
that increased levels of miR-135a/b are detected in the case of
lung cancer (92). Cisplatin is
identified as a therapeutic drug utilized to treat various types of
cancer. It works by binding to the DNA upon entry into the cell and
causing damage to the DNA leading to apoptosis (93). The increased levels of miR-135a/b
result in the progression of resistance against cisplatin as the
miR-135b targets Mcl1, which leads to the hampering of the
apoptosis process (94). miR-135
is also known to regulate the hippo pathway negatively by
downregulating the LATS1/2, which results in increased levels of
the YAP, leading to cancer-like properties. The upregulation of YAP
also aids in the development of resistance and its progression
against cancer drugs. Regulation of miR-135a/b through
therapeutics, that is, by anti-miRNAs, would help maintain the
miR-135a/b levels, which would, in turn, maintain the YAP levels
leading to a reduction in the chemoresistance.
miR-9
MiR-9 has been identified as a tumor suppressor,
with lower levels reported in ovarian and breast cancer. The
medications doxorubicin and cisplatin, which function by destroying
DNA, are routinely used to treat ovarian cancer. Certain DNA damage
repair enzymes, on the other hand, fix the damage, allowing the
cell to survive (81). The
downregulation of miR-9 is one of the ways malignant cells develop
resistance. In ovarian cancer, miR-9 targets DNA damage
repair-related enzyme genes such as BRCA1, preventing BRCA1 action
(86). In addition, the miR-9
stimulates the phosphorylation of YAP by upregulating the LATS1/2,
acting as a positive regulator of the hippo pathway. As a result,
miR-9 levels are lowered in patients with cancer. The reduced miR-9
levels can decrease LATS1/2, which ultimately leads to the
increased expression of cancer-promoting factors, that is, YAP and
TAZ, and can result in tumorigenesis (69). The hyperactivation of YAP/TAZ is
also one cause resulting in resistance to anticancer drugs. The
regulation of miR-9 levels via therapeutics would result in the
dephosphorylation of YAP, leading to increased efficacy of the
anticancer drugs.
miR-338-3p
miR-338-3p works as a tumor suppressor and its
levels are detected to be reduced in case of HCC. Sorafenib is
identified as a kinase inhibitor and works by targeting and
blocking the enzymes and proteins found in and on the surface of
cancerous cells to inhibit their growth (95). The miR-338-3p inhibits the
Hypoxia-inducible factor-1 (HIF-1), which is the mediator of
the hypoxia signaling pathway, which results in increased cell
apoptosis (88). The lower levels
of miR-338-3p result in the upregulation of HIF-1α, which results
in tumor growth and the development of sorafenib resistance
(96–98). miR-338-3p also directly acts on the
TAZ and inhibits it, reducing its levels. The increased levels of
YAP and TAZ promote resistance to anticancer drugs. The maintained
levels of miR-338-3p would help to break the resistance against
sorafenib, thereby increasing the efficiency.
Conclusion
Researchers have been able to broaden horizons in
the field of cancer thanks to their understanding of miRNAs. The
role of microRNAs in cancer progression and carcinogenesis has been
identified. The Hippo pathway and miRNA have been identified to be
interconnected, and their dysregulation may contribute to
carcinogenesis.
It was attempted to establish the interlinkage of
miRNAs and the hippo pathway using potential information in the
present review and relate it to treatment resistance in cancer.
Researchers may be able to manage difficult-to-treat drug
resistance linked with cancer with an improved understanding of the
crosslink. The studies and clinical trials involving miRNA and the
hippo pathway remain in their early phases, and there is markedly
more to learn. The relationship between miRNAs and the hippo
pathway is complicated and requires further research. Researchers
will be able to unravel the origin of cancer and design successful
treatment techniques in the near future with a thorough
understanding of the underlying molecular process and study of
crosstalks.
Acknowledgements
Not applicable.
Funding
The researcher(s) would like to thank the Deanship of Scientific
Research, Qassim University for funding the publication of this
project.
Availability of data and materials
Not applicable.
Authors' contributions
MZN, TA, MAK, SMA and SK collaboratively helped in
curating the recent studies. AAA, MZN and S guided in analyzing the
curated data. SA, VMS and AAE prepared the figures and tables.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. Apr 5–2021.(Epub ahead of
print). View Article : Google Scholar
|
|
2
|
Zygulska AL, Krzemieniecki K and
Pierzchalski P: Hippo pathway-brief overview of its relevance in
cancer. J Physiol Pharmacol. 68:311–335. 2017.PubMed/NCBI
|
|
3
|
Zeng R and Dong J: The Hippo signaling
pathway in drug resistance in cancer. Cancers (Basel). 13:3182021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li N, Xie C and Lu N: Crosstalk between
Hippo signaling and miRNAs in tumor progression. FEBS J.
284:1045–1055. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mori M, Triboulet R, Mohseni M,
Schlegelmilch K, Shrestha K, Camargo FD and Gregory RI: Hippo
signaling regulates microprocessor and links cell-density-dependent
miRNA biogenesis to cancer. Cell. 156:893–906. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pfleger CM: The Hippo pathway: A master
regulatory network important in development and dysregulated in
disease. Curr Top Dev Biol. 123:181–228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dey A, Varelas X and Guan KL: Targeting
the Hippo pathway in cancer, fibrosis, wound healing and
regenerative medicine. Nat Rev Drug Discov. 19:480–494. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaur S, Najm MZ, Khan MA, Akhter N,
Shingatgeri VM, Sikenis M, Sadaf and Aloliqi AA: Drug-resistant
breast cancer: Dwelling the Hippo pathway to manage the treatment.
Breast Cancer (Dove Med Press). 13:691–700. 2021.PubMed/NCBI
|
|
9
|
Praskova M, Xia F and Avruch J:
MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell
proliferation. Curr Biol. 18:311–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng Y and Pan D: The Hippo signaling
pathway in development and disease. Dev Cell. 50:264–282. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nguyen-Lefebvre AT, Selzner N, Wrana JL
and Bhat M: The hippo pathway: A master regulator of liver
metabolism, regeneration, and disease. FASEB J. 35:e215702021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lei QY, Zhang H, Zhao B, Zha ZY, Bai F,
Pei XH, Zhao S, Xiong Y and Guan KL: TAZ promotes cell
proliferation and epithelial-mesenchymal transition and is
inhibited by the hippo pathway. Mol Cell Biol. 28:2426–2436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Najm MZ, Sadaf, Shingatgeri VM, Saha H,
Bhattacharya H, Rath A, Verma V, Gupta A, Aloliqi AA, Kashyap P and
Parveen F: Hippo pathway in cancer: Examining its potential. J Curr
Oncol. 4:115–120. 2021. View Article : Google Scholar
|
|
15
|
Badouel C and McNeill H: SnapShot: The
hippo signaling pathway. Cell. 145:484.e12011.PubMed/NCBI
|
|
16
|
Huang YT, Lan Q, Lorusso G, Duffey N and
Rüegg C: The matricellular protein CYR61 promotes breast cancer
lung metastasis by facilitating tumor cell extravasation and
suppressing anoikis. Oncotarget. 8:9200–9215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niu J, Ma J, Guan X, Zhao X, Li P and
Zhang M: Correlation between Doppler ultrasound blood flow
parameters and angiogenesis and proliferation activity in breast
cancer. Med Sci Monit. 25:70352019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu FX and Guan KL: The Hippo pathway:
Regulators and regulations. Genes Dev. 27:355–371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han Y: Analysis of the role of the Hippo
pathway in cancer. J Transl Med. 17:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mo JS: The role of extracellular
biophysical cues in modulating the Hippo-YAP pathway. BMB Rep.
50:71–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Varelas X, Sakuma R, Samavarchi-Tehrani P,
Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW and Wrana JL:
TAZ controls Smad nucleocytoplasmic shuttling and regulates human
embryonic stem-cell self-renewal. Nat Cell Biol. 10:837–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beyer TA, Weiss A, Khomchuk Y, Huang K,
Ogunjimi AA, Varelas X and Wrana JL: Switch enhancers interpret
TGF-β and Hippo signaling to control cell fate in human embryonic
stem cells. Cell Rep. 5:1611–1624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Kang R and Tang D: The KRAS-G12C
inhibitor: Activity and resistance. Cancer Gene Ther. 2021 Sep
1;(Epub ahead of print). View Article : Google Scholar
|
|
24
|
Shen Z and Stanger BZ: YAP regulates
S-phase entry in endothelial cells. PLoS One. 10:e01175222015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benham-Pyle BW, Pruitt BL and Nelson WJ:
Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin
activation to drive cell cycle entry. Science. 348:1024–1027. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kapoor A, Yao W, Ying H, Hua S, Liewen A,
Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, et al: Yap1 activation
enables bypass of oncogenic Kras addiction in pancreatic cancer.
Cell. 158:185–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shibata M, Ham K and Hoque MO: A time for
YAP1: Tumorigenesis, immunosuppression and targeted therapy. Int J
Cancer. 143:2133–2144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mytsyk Y, Dosenko V, Skrzypczyk MA, Borys
Y, Diychuk Y, Kucher A, Kowalskyy V, Pasichnyk S, Mytsyk O and
Manyuk L: Potential clinical applications of microRNAs as
biomarkers for renal cell carcinoma. Cent European J Urol.
71:295–303. 2018.PubMed/NCBI
|
|
29
|
O'Brien J, Hayder H, Zayed Y and Peng C:
Overview of microRNA biogenesis, mechanisms of actions, and
circulation. Front Endocrinol (Lausanne). 9:4022018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bushati N and Cohen SM: MicroRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wightman B, Ha I and Ruvkun G:
Post-transcriptional regulation of the heterochronic gene lin-14 by
lin-4 mediates temporal pattern formation in C. elegans. Cell.
75:855–862. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hong Y, Lee RC and Ambros V: Structure and
function analysis of LIN-14, a temporal regulator of postembryonic
developmental events in Caenorhabditis elegans. Mol Cell Biol.
20:2285–2295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Croce CM and Calin GA: MiRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hatfield SD, Shcherbata HR, Fischer KA,
Nakahara K, Carthew RW and Ruohola-Baker H: Stem cell division is
regulated by the microRNA pathway. Nature. 435:974–978. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Borchert GM, Lanier W and Davidson BL: RNA
polymerase III transcribes human microRNAs. Nat Struct Mol Biol.
13:1097–1101. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek
SH and Kim VN: MicroRNA genes are transcribed by RNA polymerase II.
EMBO J. 23:4051–4060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
MacFarlane LA and R Murphy P: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pong SK and Gullerova M: Noncanonical
functions of microRNA pathway enzymes-Drosha, DGCR 8, Dicer and Ago
proteins. FEBS Lett. 592:2973–2986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Han J, Lee Y, Yeom KH, Kim YK, Jin H and
Kim VN: The Drosha-DGCR8 complex in primary microRNA processing.
Genes Dev. 18:3016–3027. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wong CM, Tsang FH and Ng IO: Non-coding
RNAs in hepatocellular carcinoma: Molecular functions and
pathological implications. Nat Rev Gastroenterol Hepatol.
15:137–151. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Valinezhad Orang A, Safaralizadeh R and
Kazemzadeh-Bavili M: Mechanisms of miRNA-mediated gene regulation
from common downregulation to mRNA-specific upregulation. Int J
Genomics. 2014:9706072014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang HN, Xu QQ, Thakur A, Alfred MO,
Chakraborty M, Ghosh A and Yu XB: Endothelial dysfunction in
diabetes and hypertension: Role of microRNAs and long non-coding
RNAs. Life Sci. 213:258–268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Romano G and Kwong LN: MiRNAs, melanoma
and microenvironment: An intricate network. Int J Mol Sci.
18:23542017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fukuda T, Yamagata K, Fujiyama S,
Matsumoto T, Koshida I, Yoshimura K, Mihara M, Naitou M, Endoh H,
Nakamura T, et al: DEAD-box RNA helicase subunits of the Drosha
complex are required for processing of rRNA and a subset of
microRNAs. Nat Cell Biol. 9:604–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Davis BN, Hilyard AC, Lagna G and Hata A:
SMAD proteins control DROSHA-mediated microRNA maturation. Nature.
454:56–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alarcón CR, Lee H, Goodarzi H, Halberg N
and Tavazoie SF: N6-methyladenosine marks primary microRNAs for
processing. Nature. 519:482–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Trabucchi M, Briata P, Garcia-Mayoral M,
Haase AD, Filipowicz W, Ramos A, Gherzi R and Rosenfeld MG: The
RNA-binding protein KSRP promotes the biogenesis of a subset of
microRNAs. Nature. 459:1010–1014. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dinami R, Ercolani C, Petti E, Piazza S,
Ciani Y, Sestito R, Sacconi A, Biagioni F, le Sage C, Agami R, et
al: MiR-155 drives telomere fragility in human breast cancer by
targeting TRF1. Cancer Res. 74:4145–4156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li L, Li C, Wang S, Wang Z, Jiang J, Wang
W, Li X, Chen J, Liu K, Li C and Zhu G: Exosomes derived from
hypoxic oral squamous cell carcinoma cells deliver miR-21 to
normoxic cells to elicit a prometastatic phenotype. Cancer Res.
76:1770–1780. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu C, Kelnar K, Vlassov AV, Brown D, Wang
J and Tang DG: Distinct microRNA expression profiles in prostate
cancer stem/progenitor cells and tumor-suppressive functions of
let-7. Cancer Res. 72:3393–3404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fu V, Plouffe SW and Guan KL: The Hippo
pathway in organ development, homeostasis, and regeneration. Curr
Opin Cell Biol. 49:99–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schlegelmilch K, Mohseni M, Kirak O,
Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J,
Brummelkamp TR and Camargo FD: Yap1 acts downstream of α-catenin to
control epidermal proliferation. Cell. 144:782–795. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gregory RI, Yan KP, Amuthan G, Chendrimada
T, Doratotaj B, Cooch N and Shiekhattar R: The Microprocessor
complex mediates the genesis of microRNAs. Nature. 432:235–240.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chang TC, Yu D, Lee YS, Wentzel EA, Arking
DE, West KM, Dang CV, Thomas-Tikhonenko A and Mendell JT:
Widespread microRNA repression by Myc contributes to tumorigenesis.
Nat Genet. 40:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yu T, Ma P, Wu D, Shu Y and Gao W:
Functions and mechanisms of microRNA-31 in human cancers. Biomed
Pharmacother. 108:1162–1169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu X, Sempere LF, Ouyang H, Memoli VA,
Andrew AS, Luo Y, Demidenko E, Korc M, Shi W, Preis M, et al:
MicroRNA-31 functions as an oncogenic microRNA in mouse and human
lung cancer cells by repressing specific tumor suppressors. J Clin
Invest. 120:1298–1309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu Y, Li M, Lin J and Hu C: Hippo/TEAD4
signaling pathway as a potential target for the treatment of breast
cancer. Oncol Lett. 21:3132021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Egawa H, Jingushi K, Hirono T, Ueda Y,
Kitae K, Nakata W, Fujita K, Uemura M, Nonomura N and Tsujikawa K:
The miR-130 family promotes cell migration and invasion in bladder
cancer through FAK and Akt phosphorylation by regulating PTEN. Sci
Rep. 6:205742016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Duan J, Zhang H, Qu Y, Deng T, Huang D,
Liu R, Zhang L, Bai M, Zhou L, Ying G and Ba Y: Onco-miR-130
promotes cell proliferation and migration by targeting TGFβR2 in
gastric cancer. Oncotarget. 7:44522–44533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang Y, Shen H, Withers HG, Yang N,
Denson KE, Mussell AL, Truskinovsky A, Fan Q, Gelman IH, Frangou C
and Zhang J: VGLL4 selectively represses YAP-dependent gene
induction and tumorigenic phenotypes in breast cancer. Sci Rep.
7:61902017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cheng L, Wang H and Han S: MiR-3910
promotes the growth and migration of cancer cells in the
progression of hepatocellular carcinoma. Dig Dis Sci. 62:2812–2820.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu AM, Poon RT and Luk JM: MicroRNA-375
targets Hippo-signaling effector YAP in liver cancer and inhibits
tumor properties. Biochem Biophys Res Commun. 394:623–627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ruan T, He X, Yu J and Hang Z:
MicroRNA-186 targets Yes-associated protein 1 to inhibit Hippo
signaling and tumorigenesis in hepatocellular carcinoma. Oncol
Lett. 11:2941–2945. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Deng J, Lei W, Xiang X, Zhang L, Lei J,
Gong Y, Song M, Wang Y, Fang Z, Yu F, et al: Cullin 4A (CUL4A), a
direct target of miR-9 and miR-137, promotes gastric cancer
proliferation and invasion by regulating the Hippo signaling
pathway. Oncotarget. 7:10037–10050. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Higashi T, Hayashi H, Ishimoto T, Takeyama
H, Kaida T, Arima K, Taki K, Sakamoto K, Kuroki H, Okabe H, et al:
MiR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in
hepatocellular carcinoma cells. Br J Cancer. 113:252–258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tan G, Cao X, Dai Q, Zhang B, Huang J,
Xiong S, Zhang Yy, Chen W, Yang J and Li H: A novel role for
microRNA-129-5p in inhibiting ovarian cancer cell proliferation and
survival via direct suppression of transcriptional co-activators
YAP and TAZ. Oncotarget. 6:8676–8686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yu S, Jing L, Yin XR, Wang MC, Chen YM,
Guo Y, Nan KJ and Han LL: MiR-195 suppresses the metastasis and
epithelial-mesenchymal transition of hepatocellular carcinoma by
inhibiting YAP. Oncotarget. 8:99757–99771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Abd-Aziz N, Kamaruzman NI and Poh CL:
Development of microRNAs as potential therapeutics against cancer.
J Oncol. 2020:80297212020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang V and Wu W: MicroRNA-based
therapeutics for cancer. BioDrugs. 23:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shah V and Shah J: Recent trends in
targeting miRNAs for cancer therapy. J Pharm Pharmacol.
72:1732–1749. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lu PY, Xie F and Woodle MC: In vivo
application of RNA interference: From functional genomics to
therapeutics. Adv Genet. 54:117–142. 2005.PubMed/NCBI
|
|
78
|
Abbas-Terki T, Blanco-Bose W, Deglon N,
Pralong W and Aebischer P: Lentiviral-mediated RNA interference.
Hum Gene Ther. 13:2197–2201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Tong AW: Small RNAs and non-small cell
lung cancer. Curr Mol Med. 6:339–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hanna J, Hossain GS and Kocerha J: The
potential for microRNA therapeutics and clinical research. Front
Genet. 10:4782019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Si W, Shen J, Zheng H and Fan W: The role
and mechanisms of action of microRNAs in cancer drug resistance.
Clin Epigenetics. 11:252019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Samji P, Rajendran MK, Warrier VP, Ganesh
A and Devarajan K: Regulation of Hippo signaling pathway in cancer:
A MicroRNA perspective. Cell Signal. 78:1098582021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wang ZX, Lu BB, Wang H, Cheng ZX and Yin
YM: MicroRNA-21 modulates chemosensitivity of breast cancer cells
to doxorubicin by targeting PTEN. Arch Med Res. 42:281–290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen
J, Su F, Yao H and Song E: Up-regulation of miR-21 mediates
resistance to trastuzumab therapy for breast cancer. Biol Chem.
286:19127–19137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou L, Qiu T, Xu J, Wang T, Wang J, Zhou
X, Huang Z, Zhu W, Shu Y and Liu P: miR-135a/b modulate cisplatin
resistance of human lung cancer cell line by targeting MCL1. Pathol
Oncol Res. 19:677–683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sun C, Li N, Yang Z, Zhou B, He Y, Weng D,
Fang Y, Wu P, Chen P, Yang X, et al: miR-9 regulation of BRCA1 and
ovarian cancer sensitivity to cisplatin and PARP inhibition. J Natl
Cancer Inst. 105:1750–1758. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Xu H, Zhao L, Fang Q, Sun J, Zhang S, Zhan
C, Liu S and Zhang Y: MiR-338-3p inhibits hepatocarcinoma cells and
sensitizes these cells to sorafenib by targeting hypoxia-induced
factor 1α. PLoS One. 9:e1155652014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Feng YH and Tsao CJ: Emerging role of
microRNA-21 in cancer. Biomed Rep. 5:395–402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Thorn CF, Oshiro C, Marsh S,
Hernandez-Boussard T, McLeod H, Klein TE and Altman RB: Doxorubicin
pathways: Pharmacodynamics and adverse effects. Pharmacogenet
Genomics. 21:440–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tai W, Mahato R and Cheng K: The role of
HER2 in cancer therapy and targeted drug delivery. J Control
Release. 146:264–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
González-Alonso P, Zazo S, Martín-Aparicio
E, Luque M, Chamizo C, Sanz-Álvarez M, Minguez P, Gómez-López G,
Cristóbal I, Caramés C, et al: The hippo pathway transducers
YAP1/TEAD induce acquired resistance to trastuzumab in
HER2-positive breast cancer. Cancers (Basel). 12:11082020.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lin CW, Chang YL, Chang YC, Lin JC, Chen
CC, Pan SH, Wu CT, Chen HY, Yang SC, Hong TM and Yang PC:
MicroRNA-135b promotes lung cancer metastasis by regulating
multiple targets in the Hippo pathway and LZTS1. Nat Commun.
4:18772013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Mandati V, Del Maestro L, Dingli F,
Lombard B, Loew D, Molinie N, Romero S, Bouvard D, Louvard D,
Gautreau AM, et al: Phosphorylation of Merlin by Aurora A kinase
appears necessary for mitotic progression. J Biol Chem.
294:12992–13005. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gauthier A and Ho M: Role of sorafenib in
the treatment of advanced hepatocellular carcinoma: An update.
Hepatol Res. 43:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wu XZ, Xie GR and Chen D: Hypoxia and
hepatocellular carcinoma: The therapeutic target for hepatocellular
carcinoma. J Gastroenterol Hepatol. 22:1178–1182. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tak E, Lee S, Lee J, Rashid MA, Kim YW,
Park JH, Park WS, Shokat KM, Ha J and Kim SS: Human carbonyl
reductase 1 upregulated by hypoxia renders resistance to apoptosis
in hepatocellular carcinoma cells. J Hepatol. 54:328–339. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|