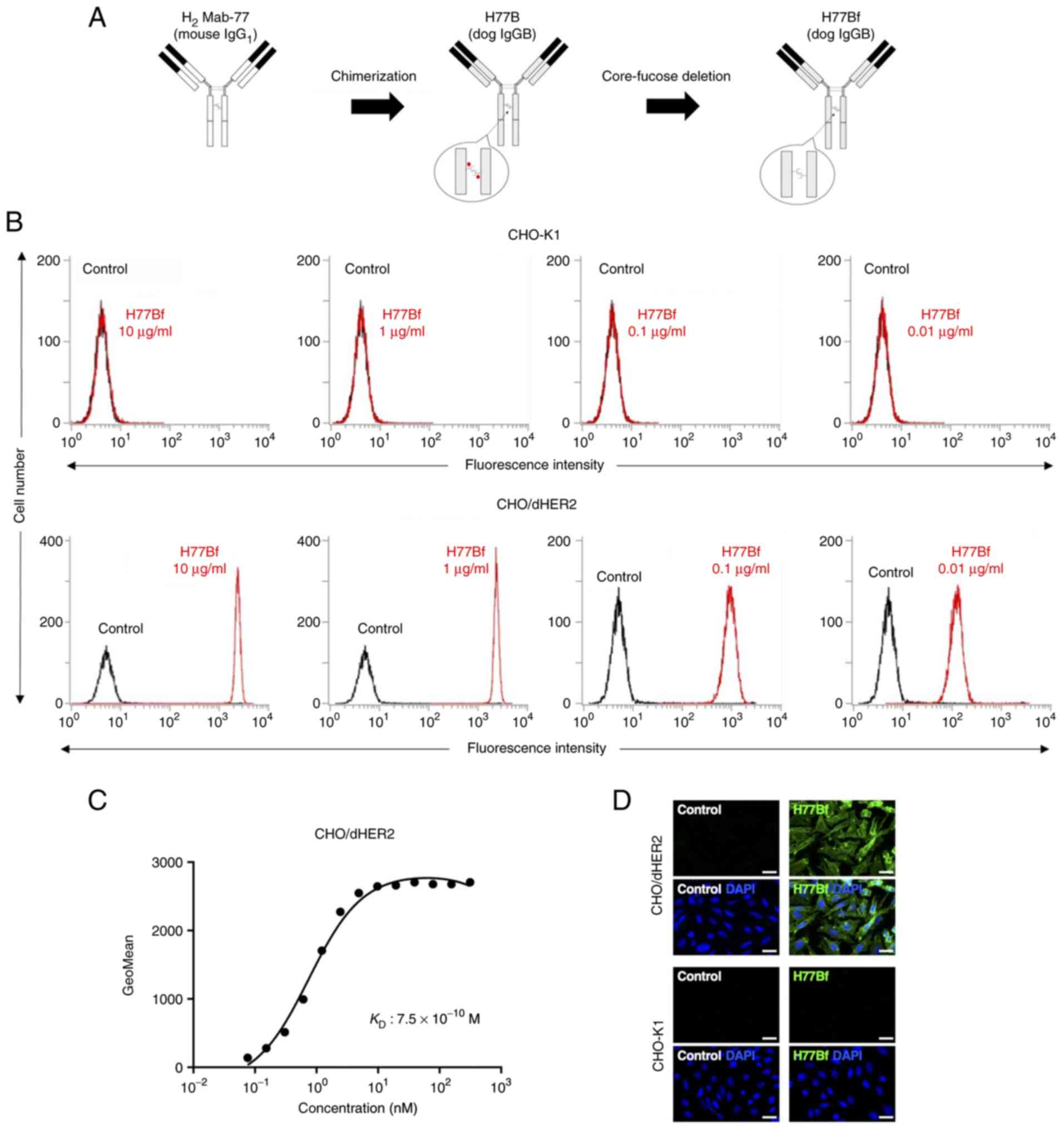
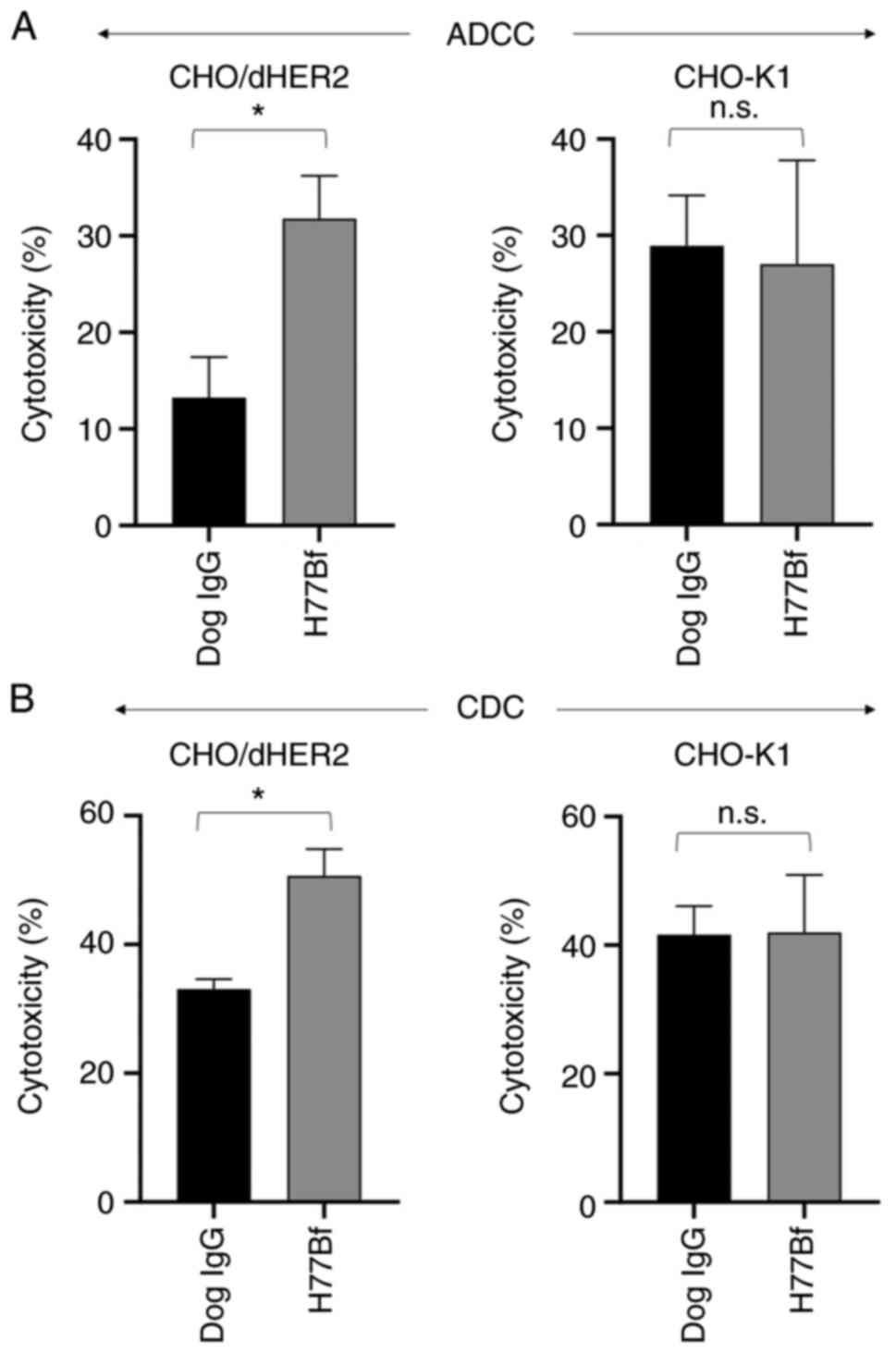
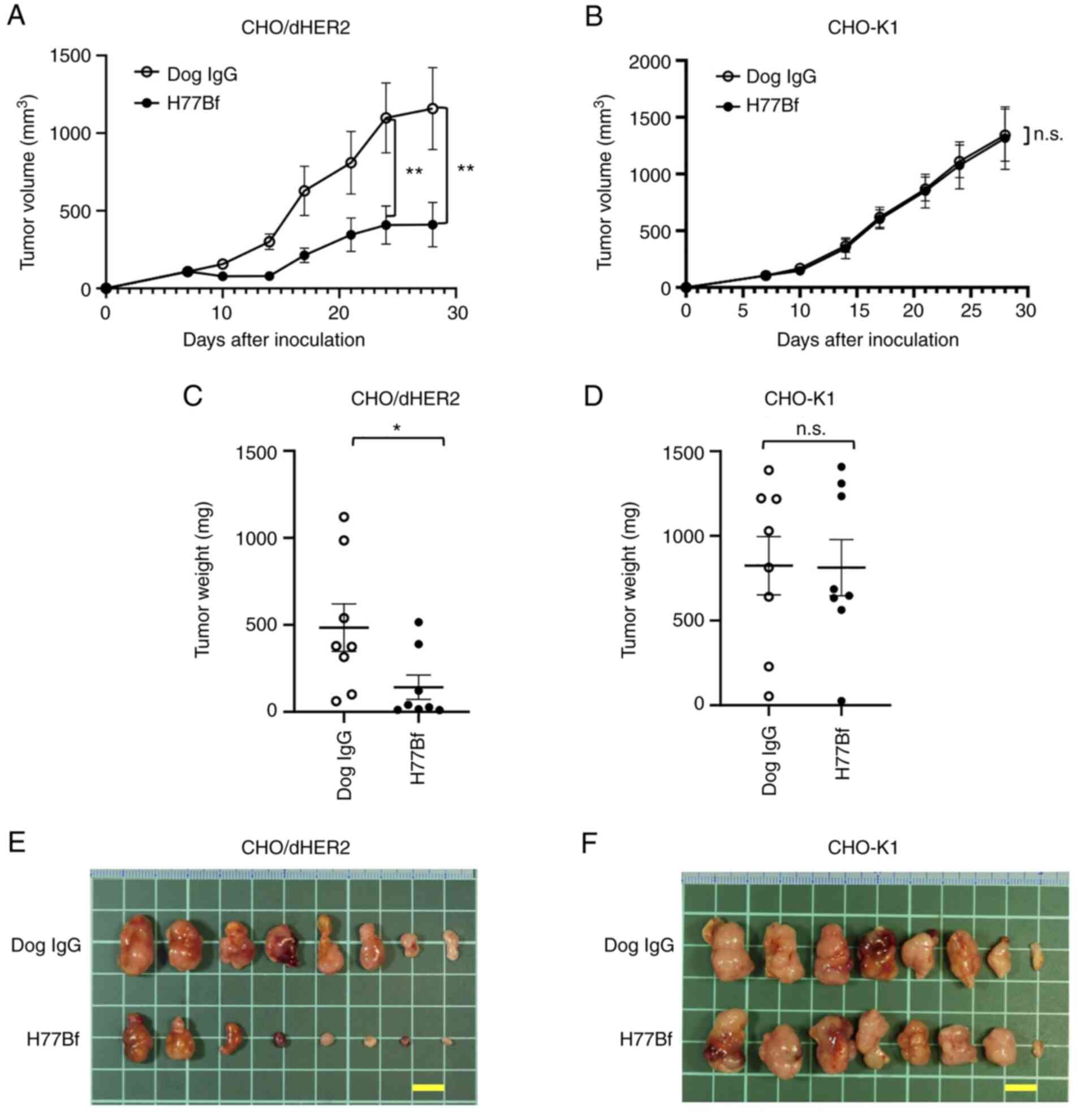
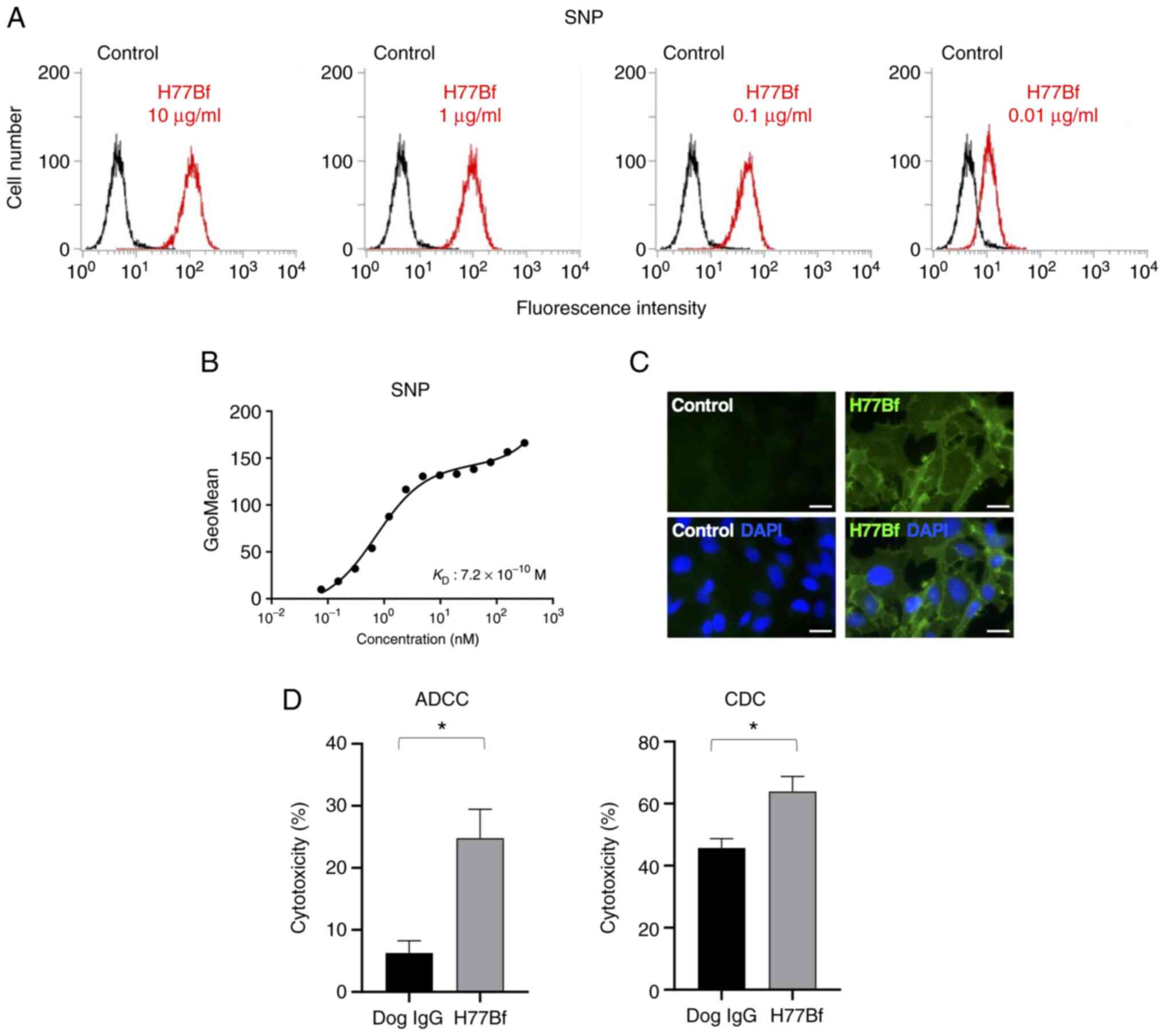
|
1
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
4
|
Moasser MM and Krop IE: The evolving
landscape of HER2 targeting in breast cancer. JAMA Oncol.
1:1154–1161. 2015. View Article : Google Scholar
|
|
5
|
Moasser MM: Two dimensions in targeting
HER2. J Clin Oncol. 32:2074–2077. 2014. View Article : Google Scholar
|
|
6
|
Weigelt B, Lo AT, Park CC, Gray JW and
Bissell MJ: HER2 signaling pathway activation and response of
breast cancer cells to HER2-targeting agents is dependent strongly
on the 3D microenvironment. Breast Cancer Res Treat. 122:35–43.
2010. View Article : Google Scholar
|
|
7
|
Le XF, Pruefer F and Bast RC Jr:
HER2-targeting antibodies modulate the cyclin-dependent kinase
inhibitor p27Kip1 via multiple signaling pathways. Cell Cycle.
4:87–95. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yakes FM, Chinratanalab W, Ritter CA, King
W, Seelig S and Arteaga CL: Herceptin-induced inhibition of
phosphatidylinositol-3 kinase and Akt Is required for
antibody-mediated effects on p27, cyclin D1, and antitumor action.
Cancer Res. 62:4132–4141. 2002.PubMed/NCBI
|
|
9
|
Konecny GE, Pegram MD, Venkatesan N, Finn
R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, et
al: Activity of the dual kinase inhibitor lapatinib (GW572016)
against HER-2-overexpressing and trastuzumab-treated breast cancer
cells. Cancer Res. 66:1630–1639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rusnak DW, Lackey K, Affleck K, Wood ER,
Alligood KJ, Rhodes N, Keith BR, Murray DM, Knight WB, Mullin RJ
and Gilmer TM: The effects of the novel, reversible epidermal
growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on
the growth of human normal and tumor-derived cell lines in vitro
and in vivo. Mol Cancer Ther. 1:85–94. 2001.PubMed/NCBI
|
|
11
|
Maadi H, Soheilifar MH, Choi WS,
Moshtaghian A and Wang Z: Trastuzumab mechanism of action; 20 years
of research to unravel a dilemma. Cancers (Basel). 13:35402021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsao LC, Force J and Hartman ZC:
Mechanisms of therapeutic antitumor monoclonal antibodies. Cancer
Res. 81:4641–4651. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Musolino A, Gradishar WJ, Rugo HS,
Nordstrom JL, Rock EP, Arnaldez F and Pegram MD: Role of Fcγ
receptors in HER2-targeted breast cancer therapy. J Immunother
Cancer. 10:e0031712022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nordstrom JL, Gorlatov S, Zhang W, Yang Y,
Huang L, Burke S, Li H, Ciccarone V, Zhang T, Stavenhagen J, et al:
Anti-tumor activity and toxicokinetics analysis of MGAH22, an
anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding
properties. Breast Cancer Res. 13:R1232011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McAndrew NP: Updates on targeting human
epidermal growth factor receptor 2-positive breast cancer: What's
to know in 2021. Curr Opin Obstet Gynecol. 34:41–45. 2022.
View Article : Google Scholar
|
|
16
|
Rugo HS, Im SA, Cardoso F, Cortés J,
Curigliano G, Musolino A, Pegram MD, Wright GS, Saura C,
Escrivá-de-Romaní S, et al: Efficacy of margetuximab vs trastuzumab
in patients with pretreated ERBB2-Positive advanced breast cancer:
A phase 3 randomized clinical trial. JAMA Oncol. 7:573–584. 2021.
View Article : Google Scholar
|
|
17
|
Golay J and Taylor RP: The role of
complement in the mechanism of action of therapeutic anti-cancer
mAbs. Antibodies (Basel). 9:582020. View Article : Google Scholar
|
|
18
|
Reis ES, Mastellos DC, Ricklin D,
Mantovani A and Lambris JD: Complement in cancer: Untangling an
intricate relationship. Nat Rev Immunol. 18:5–18. 2018. View Article : Google Scholar
|
|
19
|
Salas Y, Márquez A, Diaz D and Romero L:
Epidemiological study of mammary tumors in female dogs diagnosed
during the period 2002–2012: A growing animal health problem. PLoS
One. 10:e01273812015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gray M, Meehan J, Martínez-Pérez C, Kay C,
Turnbull AK, Morrison LR, Pang LY and Argyle D: Naturally-occurring
canine mammary tumors as a translational model for human breast
cancer. Front Oncol. 10:6172020. View Article : Google Scholar
|
|
21
|
Kaszak I, Ruszczak A, Kanafa S, Kacprzak
K, Król M and Jurka P: Current biomarkers of canine mammary tumors.
Acta Vet Scand. 60:662018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gama A, Alves A and Schmitt F:
Identification of molecular phenotypes in canine mammary carcinomas
with clinical implications: Application of the human
classification. Virchows Arch. 453:123–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flint AF, U'Ren L, Legare ME, Withrow SJ,
Dernell W and Hanneman WH: Overexpression of the erbB-2
proto-oncogene in canine osteosarcoma cell lines and tumors. Vet
Pathol. 41:291–296. 2004. View Article : Google Scholar
|
|
24
|
Millanta F, Impellizeri J, McSherry L,
Rocchigiani G, Aurisicchio L and Lubas G: Overexpression of HER-2
via immunohistochemistry in canine urinary bladder transitional
cell carcinoma-A marker of malignancy and possible therapeutic
target. Vet Comp Oncol. 16:297–300. 2018. View Article : Google Scholar
|
|
25
|
Yoshimoto S, Kato D, Kamoto S, Yamamoto K,
Tsuboi M, Shinada M, Ikeda N, Tanaka Y, Yoshitake R, Eto S, et al:
Detection of human epidermal growth factor receptor 2
overexpression in canine anal sac gland carcinoma. J Vet Med Sci.
81:1034–1039. 2019. View Article : Google Scholar
|
|
26
|
Campos LC, Silva JO, Santos FS, Araújo MR,
Lavalle GE, Ferreira E and Cassali GD: Prognostic significance of
tissue and serum HER2 and MUC1 in canine mammary cancer. J Vet
Diagn Invest. 27:531–535. 2015. View Article : Google Scholar
|
|
27
|
Brunetti B, Bacci B, Sarli G, Pancioni E
and Muscatello LV: Immunohistochemical screening of HER2 in canine
carcinomas: A preliminary study. Animals (Basel). 11:10062021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mason NJ, Gnanandarajah JS, Engiles JB,
Gray F, Laughlin D, Gaurnier-Hausser A, Wallecha A, Huebner M and
Paterson Y: Immunotherapy with a HER2-targeting listeria induces
HER2-Specific immunity and demonstrates potential therapeutic
effects in a phase I trial in canine osteosarcoma. Clin Cancer Res.
22:4380–4390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Itai S, Fujii Y, Kaneko MK, Yamada S,
Nakamura T, Yanaka M, Saidoh N, Chang YW, Handa S, Takahashi M, et
al: H2Mab-77 is a sensitive and specific Anti-HER2 monoclonal
antibody against breast cancer. Monoclon Antib Immunodiagn
Immunother. 36:143–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Osaki T, Sunden Y, Sugiyama A, Azuma K,
Murahata Y, Tsuka T, Ito N, Imagawa T and Okamoto Y: Establishment
of a canine mammary gland tumor cell line and characterization of
its miRNA expression. J Vet Sci. 17:385–390. 2016. View Article : Google Scholar
|
|
31
|
Tateyama N, Asano T, Ohishi T, Takei J,
Hosono H, Nanamiya R, Tanaka T, Sano M, Saito M, Kawada M, et al:
An Anti-HER2 monoclonal antibody H2Mab-41 exerts antitumor
activities in mouse xenograft model using dog HER2-overexpressed
cells. Monoclon Antib Immunodiagn Immunother. 40:184–190. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asano T, Kaneko MK and Kato Y: RIEDL tag:
A novel pentapeptide tagging system for transmembrane protein
purification. Biochem Biophys Rep. 23:1007802020.PubMed/NCBI
|
|
33
|
Asano T, Kaneko MK and Kato Y: Development
of a novel epitope mapping system: RIEDL insertion for epitope
mapping method. Monoclon Antib Immunodiagn Immunother. 40:162–167.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Asano T, Kaneko MK, Takei J, Tateyama N
and Kato Y: Epitope mapping of the Anti-CD44 monoclonal antibody
(C44Mab-46) using the REMAP Method. Monoclon Antib Immunodiagn
Immunother. 40:156–161. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nanamiya R, Sano M, Asano T, Yanaka M,
Nakamura T, Saito M, Tanaka T, Hosono H, Tateyama N, Kaneko MK and
Kato Y: Epitope mapping of an anti-human epidermal growth factor
receptor monoclonal antibody (EMab-51) using the RIEDL insertion
for epitope mapping method. Monoclon Antib Immunodiagn Immunother.
40:149–155. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sano M, Kaneko MK, Aasano T and Kato Y:
Epitope mapping of an antihuman EGFR monoclonal antibody (EMab-134)
Using the REMAP method. Monoclon Antib Immunodiagn Immunother.
40:191–195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li G, Ohishi T, Kaneko MK, Takei J, Mizuno
T, Kawada M, Saito M, Suzuki H and Kato Y: Defucosylated mouse-dog
chimeric Anti-EGFR antibody exerts antitumor activities in mouse
xenograft models of canine tumors. Cells. 10:35992021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mizuno T, Kato Y, Kaneko MK, Sakai Y,
Shiga T, Kato M, Tsukui T, Takemoto H, Tokimasa A, Baba K, et al:
Generation of a canine anti-canine CD20 antibody for canine
lymphoma treatment. Sci Rep. 10:114762020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takei J, Kaneko MK, Ohishi T, Hosono H,
Nakamura T, Yanaka M, Sano M, Asano T, Sayama Y, Kawada M, et al: A
defucosylated anti-CD44 monoclonal antibody 5-mG2a-f exerts
antitumor effects in mouse xenograft models of oral squamous cell
carcinoma. Oncol Rep. 44:1949–1960. 2020.PubMed/NCBI
|
|
40
|
Takei J, Ohishi T, Kaneko MK, Harada H,
Kawada M and Kato Y: A defucosylated anti-PD-L1 monoclonal antibody
13-mG2a-f exerts antitumor effects in mouse xenograft models of
oral squamous cell carcinoma. Biochem Biophys Rep.
24:1008012020.PubMed/NCBI
|
|
41
|
Tateyama N, Nanamiya R, Ohishi T, Takei J,
Nakamura T, Yanaka M, Hosono H, Saito M, Asano T, Tanaka T, et al:
Defucosylated anti-epidermal growth factor receptor monoclonal
antibody 134-mG2a-f exerts antitumor activities in mouse
xenograft models of dog epidermal growth factor
receptor-overexpressed cells. Monoclon Antib Immunodiagn
Immunother. 40:177–183. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kato Y, Mizuno T, Yamada S, Nakamura T,
Itai S, Yanaka M, Sano M and Kaneko MK: Establishment of P38Bf, a
core-fucose-deficient mouse-canine chimeric antibody against dog
podoplanin. Monoclon Antib Immunodiagn Immunother. 37:218–223.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Asano T, Ohishi T, Takei J, Nakamura T,
Nanamiya R, Hosono H, Tanaka T, Sano M, Harada H, Kawada M, et al:
AntiHER3 monoclonal antibody exerts antitumor activity in a mouse
model of colorectal adenocarcinoma. Oncol Rep. 46:1732021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tanaka T, Ohishi T, Asano T, Takei J,
Nanamiya R, Hosono H, Sano M, Harada H, Kawada M, Kaneko MK and
Kato Y: An antiTROP2 monoclonal antibody TrMab6 exerts antitumor
activity in breast cancer mouse xenograft models. Oncol Rep.
46:1322021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hosono H, Ohishi T, Takei J, Asano T,
Sayama Y, Kawada M, Kaneko MK and Kato Y: The anti-epithelial cell
adhesion molecule (EpCAM) monoclonal antibody EpMab-16 exerts
antitumor activity in a mouse model of colorectal adenocarcinoma.
Oncol Lett. 20:3832020. View Article : Google Scholar
|
|
46
|
Kaneko MK, Ohishi T, Takei J, Sano M,
Nakamura T, Hosono H, Yanaka M, Asano T, Sayama Y, Harada H, et al:
AntiEpCAM monoclonal antibody exerts antitumor activity against
oral squamous cell carcinomas. Oncol Rep. 44:2517–2526. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaneko MK, Ohishi T, Nakamura T, Inoue H,
Takei J, Sano M, Asano T, Sayama Y, Hosono H, Suzuki H, et al:
Development of core-fucose-deficient humanized and chimeric
anti-human podoplanin antibodies. Monoclon Antib Immunodiagn
Immunother. 39:167–174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hosono H, Takei J, Ohishi T, Sano M, Asano
T, Sayama Y, Nakamura T, Yanaka M, Kawada M, Harada H, et al:
AntiEGFR monoclonal antibody 134mG2a exerts antitumor effects in
mouse xenograft models of oral squamous cell carcinoma. Int J Mol
Med. 46:1443–1452. 2020.PubMed/NCBI
|
|
49
|
Ohishi T, Kato Y, Kaneko MK, Ohba SI,
Inoue H, Harakawa A and Kawada M: Anti-metastatic activity of an
anti-EGFR monoclonal antibody against metastatic colorectal cancer
with KRAS p.G13D mutation. Int J Mol Sci. 21:60372020. View Article : Google Scholar
|
|
50
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: H2Mab-19, an anti-human epidermal growth
factor receptor 2 monoclonal antibody exerts antitumor activity in
mouse oral cancer xenografts. Exp Ther Med. 20:846–853. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kato Y, Ohishi T, Takei J, Nakamura T,
Sano M, Asano T, Sayama Y, Hosono H, Kawada M and Kaneko MK: An
anti-human epidermal growth factor receptor 2 monoclonal antibody
H2Mab-19 exerts antitumor activity in mouse colon cancer
xenografts. Monoclon Antib Immunodiagn Immunother. 39:123–128.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Takei J, Kaneko MK, Ohishi T, Kawada M,
Harada H and Kato Y: A novel anti-EGFR monoclonal antibody
(EMab-17) exerts antitumor activity against oral squamous cell
carcinomas via antibody-dependent cellular cytotoxicity and
complement-dependent cytotoxicity. Oncol Lett. 19:2809–2816.
2020.
|
|
53
|
Itai S, Ohishi T, Kaneko MK, Yamada S, Abe
S, Nakamura T, Yanaka M, Chang YW, Ohba SI, Nishioka Y, et al:
Anti-podocalyxin antibody exerts antitumor effects via
antibody-dependent cellular cytotoxicity in mouse xenograft models
of oral squamous cell carcinoma. Oncotarget. 9:22480–22497. 2018.
View Article : Google Scholar
|
|
54
|
Kato Y, Ohishi T, Yamada S, Itai S, Takei
J, Sano M, Nakamura T, Harada H, Kawada M and Kaneko MK: Anti-Human
epidermal growth factor receptor 2 monoclonal antibody H2Mab-41
exerts antitumor activity in a mouse xenograft model of colon
cancer. Monoclon Antib Immunodiagn Immunother. 38:157–161. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Singer J, Weichselbaumer M, Stockner T,
Mechtcheriakova D, Sobanov Y, Bajna E, Wrba F, Horvat R, Thalhammer
JG, Willmann M and Jensen-Jarolim E: Comparative oncology: ErbB-1
and ErbB-2 homologues in canine cancer are susceptible to cetuximab
and trastuzumab targeting. Mol Immunol. 50:200–209. 2012.
View Article : Google Scholar
|
|
56
|
Collins DM, O'Donovan N, McGowan PM,
O'Sullivan F, Duffy MJ and Crown J: Trastuzumab induces
antibody-dependent cell-mediated cytotoxicity (ADCC) in
HER-2-non-amplified breast cancer cell lines. Ann Oncol.
23:1788–1795. 2012. View Article : Google Scholar
|
|
57
|
Klingemann H: Immunotherapy for dogs:
Still running behind humans. Front Immunol. 12:6657842021.
View Article : Google Scholar
|
|
58
|
Bergeron LM, McCandless EE, Dunham S,
Dunkle B, Zhu Y, Shelly J, Lightle S, Gonzales A and Bainbridge G:
Comparative functional characterization of canine IgG subclasses.
Vet Immunol Immunopathol. 157:31–41. 2014. View Article : Google Scholar
|
|
59
|
Shinkawa T, Nakamura K, Yamane N,
Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M,
Yamasaki M, et al: The absence of fucose but not the presence of
galactose or bisecting N-acetylglucosamine of human IgG1
complex-type oligosaccharides shows the critical role of enhancing
antibody-dependent cellular cytotoxicity. J Biol Chem.
278:3466–3473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kubota T, Niwa R, Satoh M, Akinaga S,
Shitara K and Hanai N: Engineered therapeutic antibodies with
improved effector functions. Cancer Sci. 100:1566–1572. 2009.
View Article : Google Scholar
|
|
61
|
Mizuno T, Takeda Y, Tsukui T and Igase M:
Development of a cell line-based assay to measure the
antibody-dependent cellular cytotoxicity of a canine therapeutic
antibody. Vet Immunol Immunopathol. 240:1103152021. View Article : Google Scholar
|
|
62
|
Scott AM, Wolchok JD and Old LJ: Antibody
therapy of cancer. Nat Rev Cancer. 12:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zahavi D and Weiner L: Monoclonal
antibodies in cancer therapy. Antibodies (Basel). 9:342020.
View Article : Google Scholar
|
|
64
|
Takegawa N, Nonagase Y, Yonesaka K, Sakai
K, Maenishi O, Ogitani Y, Tamura T, Nishio K, Nakagawa K and
Tsurutani J: DS-8201a, a new HER2-targeting antibody-drug conjugate
incorporating a novel DNA topoisomerase I inhibitor, overcomes
HER2-positive gastric cancer T-DM1 resistance. Int J Cancer.
141:1682–1689. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-Positive Breast
Cancer. N Engl J Med. 382:610–621. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li BT, Smit EF, Goto Y, Nakagawa K,
Udagawa H, Mazières J, Nagasaka M, Bazhenova L, Saltos AN, Felip E,
et al: Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung
Cancer. N Engl J Med. 386:241–251. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Shitara K, Bang YJ, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive gastric
cancer. N Engl J Med. 382:2419–2430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kaneko MK, Honma R, Ogasawara S, Fujii Y,
Nakamura T, Saidoh N, Takagi M, Kagawa Y, Konnai S and Kato Y:
PMab-38 recognizes canine podoplanin of squamous cell carcinomas.
Monoclon Antib Immunodiagn Immunother. 35:263–266. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ito A, Ohta M, Kato Y, Inada S, Kato T,
Nakata S, Yatabe Y, Goto M, Kaneda N, Kurita K, et al: A real-time
near-infrared fluorescence imaging method for the detection of oral
cancers in mice using an indocyanine green-labeled podoplanin
antibody. Technol Cancer Res Treat. 17:15330338187679362018.
View Article : Google Scholar
|
|
70
|
Kato Y, Ohishi T, Kawada M, Maekawa N,
Konnai S, Itai S, Yamada S and Kaneko MK: The mouse-canine chimeric
anti-dog podoplanin antibody P38B exerts antitumor activity in
mouse xenograft models. Biochem Biophys Rep. 17:23–26.
2019.PubMed/NCBI
|
|
71
|
Kato Y, Ito Y, Ohishi T, Kawada M,
Nakamura T, Sayama Y, Sano M, Asano T, Yanaka M, Okamoto S, et al:
Antibody-drug conjugates using mouse-canine chimeric anti-dog
podoplanin antibody exerts antitumor activity in a mouse xenograft
model. Monoclon Antib Immunodiagn Immunother. 39:37–44. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pantelyushin S, Ranninger E, Guerrera D,
Hutter G, Maake C, Markkanen E, Bettschart-Wolfensberger R, Rohrer
Bley C, Läubli H and Vom Berg J: Cross-reactivity and functionality
of approved human immune checkpoint blockers in dogs. Cancers
(Basel). 13:7852021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Maekawa N, Konnai S, Nishimura M, Kagawa
Y, Takagi S, Hosoya K, Ohta H, Kim S, Okagawa T, Izumi Y, et al:
PD-L1 immunohistochemistry for canine cancers and clinical benefit
of anti-PD-L1 antibody in dogs with pulmonary metastatic oral
malignant melanoma. NPJ Precis Oncol. 5:102021. View Article : Google Scholar
|
|
74
|
Klingemann H: Immunotherapy for dogs:
Running behind humans. Front Immunol. 9:1332018. View Article : Google Scholar
|
|
75
|
Muhammadnejad A, Keyhani E, Mortazavi P,
Behjati F and Haghdoost IS: Overexpression of her-2/neu in
malignant mammary tumors; translation of clinicopathological
features from dog to human. Asian Pac J Cancer Prev. 13:6415–6421.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ressel L, Puleio R, Loria GR, Vannozzi I,
Millanta F, Caracappa S and Poli A: HER-2 expression in canine
morphologically normal, hyperplastic and neoplastic mammary tissues
and its correlation with the clinical outcome. Res Vet Sci.
94:299–305. 2013. View Article : Google Scholar
|
|
77
|
Kim TM, Yang IS, Seung BJ, Lee S, Kim D,
Ha YJ, Seo MK, Kim KK, Kim HS, Cheong JH, et al: Cross-species
oncogenic signatures of breast cancer in canine mammary tumors. Nat
Commun. 11:36162020. View Article : Google Scholar : PubMed/NCBI
|