Introduction
With the development of science and technology, much
progress has been made over the past decade in the development of
novel treatments, including surgery, radiation, chemotherapy,
immunotherapy, hormone therapy, and targeted therapy, but the
overall therapeutic effects are still not satisfactory (1–3).
Thus, clarifying the mechanisms of cancer pathogenesis and
tumorigenesis remain important for identifying novel tumor
treatments.
Cell division cycle 45 (CDC45), which contains 21
exons located at 22q11.21, is a critical component of the
eukaryotic DNA replisome. CDC45 plays an essential role in the
replicative helicase holoenzyme CDC45-MCM-GINS complex, activating
MCM2-7 and resulting in DNA replication and unwinding (4–7).
Knocking down CDC45 suppresses DNA replication and inhibits cell
proliferation in human cells (8).
A previous study demonstrated that the protein levels of CDC45 are
highly expressed in human cancer-derived cells compared to primary
cells (9). In addition, high
expression of CDC45 recapitulates all c-Myc-induced replication and
damage phenotypes, and CDC45 and GINS both function downstream of
Myc (10).
Recently, several bioinformatics studies have
demonstrated the role of CDC45 in different cancers, such as
colorectal cancer (11), non-small
cell lung cancer (12,13), esophageal squamous cell carcinoma
(14), and hepatocellular
carcinoma (15–17), but only one experimental study has
examined the roles of CDC45 in tumorigenesis (18). Currently, there is no pan-cancer
analysis of the function of CDC45 across different cancers.
In the present study, multiple databases were
searched, including The Cancer Genome Atlas (TCGA), Genotype-Tissue
Expression (GTEx), ONCOMINE, Tumor Immune Estimation Resource
(TIMER), tumor-immune system interactions database (TISIDB), Human
Protein Atlas (HPA), MEXPRESS, Gene Expression Profiling
Interactive Analysis (GEPIA), UALCAN, cBioPortal, RNA
Epitranscriptome Collection (REPIC),
N6-methyladenosine (m6A)-Atlas and
Search Tool for the Retrieval of Interacting Genes/Proteins
(STRING), to explore the function of CDC45 across 33 types of
cancer. The RNA and protein expression levels of CDC45 were first
analyzed in different cancers, followed by the examination of the
association between CDC45 expression and overall survival (OS) and
disease-free survival (DFS). Second, the genetic mutations of CDC45
were explored. The association between CDC45 and immunity,
including tumor mutation burden (TMB), microsatellite instability
(MSI), and immune subtype were also explored. Subsequently, DNA
methylation, m6A and receiver operating characteristic
curve (ROC) were examined across 33 cancers. In addition,
co-expressed proteins were searched for and Gene Ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were
performed. Finally, Cell Counting Kit-8 (CCK-8) assays,
5-ethynyl-2′-deoxyuridine (EdU) proliferation assays and cell cycle
analysis were performed to validate the roles of CDC45 in
vitro. The present results indicated that CDC45 plays an
important role in tumorigenesis across 33 different cancer types,
which may represent a new strategy for tumor prognosis and
treatment.
Materials and methods
TCGA
TCGA is a comprehensive, freely accessible database
that contains over 20,000 primary cancer and matched normal samples
across 33 cancer types (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga).
It provides high dimensionality and quality data, including
genomic, epigenomic, transcriptomic, and proteomic data. The
details of the data include clinical, gene expression, copy number
variation, mutation, methylation and protein (19).
Genotype-tissue expression (GTEx)
GTEx (https://www.gtexportal.org/) contains genotype data of
nearly 17,382 RNA-seq samples across 54 tissue sites (from 948
postmortem donors) and 2 cell lines. Full gene expression datasets
are available for free. This portal established a comprehensive
catalog of genetic variants that affect gene expression across
multiple tissues, for the research community, to evaluate
tissue-specific gene expression and regulation in numerous
different tissues.
Oncomine database
Oncomine (https://www.oncomine.org) is a public database that
can easily obtain microarray data across different cancers. The
mRNA levels of CDC45 were compared between cancer and control
tissues using the following threshold: P<0.05; fold change,
>2; gene rank, Top 10%.
TISIDB database
TISIDB (http://cis.hku.hk/TISIDB/index.php) is an online
website that focuses on the interactions between cancer and the
immune system and contains multiple heterogeneous data types
(20). The association between
CDC45 expression and the grade or stage of different cancers from
TCGA was investigated. In addition, it was also used to analyze the
interaction between CDC45 levels in different immune subtypes,
including C1 (wound healing), C2 (IFN-γ dominant), C3
(inflammatory), C4 (lymphocyte depleted), C5 (immunologically
quiet), and C6 (TGF-β dominant).
TIMER
TIMER (http://timer.cistrome.org/) is a comprehensive online
website to explore tumor immunological, clinical and genomic
features (21). Moreover, gene
correlation analysis was also performed using TIMER across 32
cancers. The ‘Expression’ module was searched using the keyword
‘CDC45’ for CDC45 expression in different cancers and matched
normal samples from TCGA. Similarly, the ‘Immune’ module was used
to investigate the correlation between CDC45 and different immune
cells, including CD8+ T cells, CD4+ T cells,
neutrophils, myeloid dendritic cells, macrophages, and B cells,
across all TCGA cancers. Moreover, the ‘Gene-Corr’
module was adopted to determine the correlation between CDC45 and
m6A-related genes.
HPA
HPA (https://www.proteinatlas.org/) has collected the
entire human proteome and characterized it using
immunohistochemistry and immunocytochemistry (22). It is also used to detect protein
localization and expression in human tissues and cells. From this
program, immunohistochemical images of different cancers and
matched normal samples were downloaded.
cBioPortal
The cBioPortal (https://www.cbioportal.org/) is an open source that
contains gene data, including mutation and putative copy number
alterations (CNVs) (23). The
‘TCGA Pan Cancer Atlas Studies’ in ‘Quick select’ was searched
using the keyword ‘CDC45’ for gene mutations and CNVs across 32
cancers. Then, the summary results in the ‘Cancer Types Summary’
module were obtained. In addition, the details of mutated
information of CDC45 are displayed in the diagram of protein
structure or 3D graphics. Furthermore, the correlation between
CDC45 mutation and clinical outcomes was explored using the
‘Comparison/Survival’ module.
GEPIA
GEPIA is an interactive website for exploring
RNA-seq expression from TCGA and GTEx based on the normalized
processing pipeline (http://gepia2.cancer-pku.cn/#index). It provides
differential expression analysis, cancer type, pathological grade,
survival analysis, similar gene detection and correlation analysis
(24). By searching different
modules, the correlation between CDC45 expression and cancer stage,
OS and DFS across 33 cancers was analyzed using the following CDC45
cutoff value: High, 50%; low, 50%. Similar genes for GO and KEGG
analyses were also downloaded.
UALCAN
The UALCAN (http://ualcan.path.uab.edu/index.html) database was
used to analyze the DNA methylation of CDC45 between different
cancer and matched normal samples from TCGA (25). Herein, we compared the methylation
level of the CDC45 promoter region between cancer and matched
control tissues and found that five cancer types exhibited
significant differences, including bladder urothelial carcinoma
(BLCA), breast invasive carcinoma (BRCA), testicular germ cell
tumors (TGCTs), lung adenocarcinoma (LUAD) and head and neck
squamous cell carcinoma (HNSC).
MEXPRESS
MEXPRESS (https://mexpress.be) is an online website that allows
researchers to explore DNA methylation from TCGA (26). Herein, we searched the DNA
methylation of BLCA, BRCA, TGCT, LUAD and HNSC to verify the
results obtained from UALCAN, and promoter region probes were
obtained.
m6A-Atlas
m6A-Atlas (http://180.208.58.66/m6A-Atlas/index.html) is a
comprehensive online tool for revealing the m6A
epitranscriptome (27). By
selecting ‘Human gene’ and ‘CDC45’, detailed results, including
m6A records, overall distribution pattern of
m6A sites, and the distribution of all m6A
sites and RNA binding proteins (RBPs) were obtained.
REPIC
The REPIC (https://repicmod.uchicago.edu/repic) database is an
online source that records numerous data retrieved from the Gene
Expression Omnibus (GEO) and the Sequence Read Archive (SRA) of
m6A-seq and MeRIP-seq using their unified pipeline
(28). This database is used to
query m6A modification sites by specific cell lines or
tissue types. The m6A modification sites in different
cell lines or tissues of CDC45 were explored.
STRING
The STRING database (http://string-db.org) is an online server that focuses
on protein-protein interactions (PPIs) and functional protein
networks (29). By querying
‘CDC45’ in this database and setting up parameters as follows:
Minimum required interaction score [‘low confidence (0.150)’],
meaning of network edges (‘evidence’), max number of interactors to
show (‘no more than 50 interactors’ in 1st shell) and active
interaction sources (‘experiments, database, co-expression’), the
top 50 proteins that bind with CDC45 were obtained.
BioGRID, HIPPIE and HitPredict
BioGRID (https://thebiogrid.org/), HIPPIE (http://cbdm-01.zdv.uni-mainz.de/~mschaefer/hippie/)
and HitPredict (http://www.hitpredict.org/) databases were searched
for PPI analysis.
LinkedOmics
LinkedOmics (http://linkedomics.org/login.php) is a publicly
available portal that provides a unique platform for biologists and
clinicians to access, analyze and compare cancer multi-omics data
within and across tumor types.
R software
To analyze TCGA and GTEx data, R (version 3.6.3)
software (https://cran.r-project.org/bin/windows/base/old/3.6.3/)
was used. With regard to certain cancers without control samples or
those with highly limited normal tissues, the corresponding samples
from GTEx were extracted as control groups, and the ‘ggplot2
package’ was used to visualize gene expression. The ‘ggradar’ and
‘ggplot2’ packages were used to reveal the MSI and neoantigens of
immunity. The ‘timeROC’ package was used to compare the predictive
accuracy of CDC45 and the risk score, and the log-rank test was
also used to compare the survival difference between the two
groups. The ‘clusterProfiller’ and ‘org.Hs.eg.db’ packages were
used for GO and KEGG analyses. P<0.05 was considered to indicate
a statistically significant difference.
Cell culture
Two human GBM cell lines, U251 and glioblastoma of
unknown origin U87, were purchased from American Type Culture
Collection. Cells were cultured in high-glucose Dulbecco's modified
Eagle's medium (DMEM; Corning, Inc.) supplemented with 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) at 37°C and 5%
CO2. These cell lines underwent Mycoplasma tests
and were authenticated by Short Tandem Repeat (STR) analysis
(Beijing Microread Genetics Co., Ltd).
Small interfering RNA (siRNA)
sequences
The sequences of CDC45 siRNA (Hanbio Biotechnology
Co., Ltd.) were as follows: Negative control (NC),
5′-UUCUCCGAACGUGUCACGU-3′ (sense) and 5′-ACGUGACACGUUCGGAGAA-3′
(antisense); siCDC45-1, 5′-GCGUGCAGACUUUCAGCAUTT-3′ (sense) and
5′-AUGCUGAAAGUCUGCACGCTT-3′ (antisense); and siCDC45-2,
5′-GCAAGACAAGAUCACUCAA-3′ (sense)and 5′-UUGAGUGAUCUUGUCUUGC-3′
(antisense).
Cell transfection
U87 and U251 cells were seeded into 24- and 96-well
plates and transfected 48 h later using Lipofectamine 3000 (Life
Technologies Corporation; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. Briefly, for 24-well plates, 1 µl
siRNA (20 µM) was transfected into target cells using 1.5 µl
Lipofectamine 3000 at room temperature. For 96-well plates, 0.2 µl
siRNA (20 µM) was transfected into target cells using 0.3 µl
Lipofectamine 3000 at room temperature. Following 48 h of
incubation at 37°C and 5% CO2, subsequent experiments
were conducted.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol. cDNA was synthesized using the GoScript Reverse
Transcription System (Promega Corporation) and 1 µg of RNA
according to the manufacturer's protocol. qPCR was performed using
a SYBR Green PCR kit (Bimake.com) according to the manufacturer's
instructions on an Applied Biosystems QuantStudio instrument
(Thermo Fisher Scientific, Inc.). Comparative quantification was
performed using the 2−ΔΔCq (30) method with GAPDH as the endogenous
control, The PCR system was 20 µl, and the thermocycling conditions
were as follows: 40 cycles were operated at 95°C for 3 min, 95°C
for 15 sec and 60°C for 1 min. The dissolution curve program was as
follows: 95°C for 15 sec, 60°C for 1 min, and 95°C for 1 sec. The
primer sequences were as follows: CDC45 forward,
5′-TTCGTGTCCGATTTCCGCAAA-3′ and reverse,
5′-TGGAACCAGCGTATATTGCAC-3′; GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
CCK-8 assay
Following transfection, U87 and U251 cells were
divided into 96-well plates at 3×103 cells per well. At
24, 48, 72, 96, 120, and 144 h, CCK-8 solution was added (10 µl;
Beyotime Institute of Biotechnology), and the cells were then
incubated for 2 h at 37°C and 5% CO2. The optical
density (OD) at 450 nm (OD450) was then assessed using a
microplate reader (Biotek Instruments, Inc.).
Cell-Light EdU proliferation
assay
U87 and U251 cells (5×103/well) were
inoculated into 24-well plates. Following 48 h of incubation at
37°C and 5% CO2, an EdU In Vitro Kit (cat. no.
C10310-1; Guangzhou RiboBio Co., Ltd.) was employed based on the
manufacturer's protocol. Briefly, the EdU medium mixture (50 µM)
was discarded, and 4% paraformaldehyde was added to fix cells at
room temperature for 30 min. The cells were then washed with
glycine (2 mg/ml) for 5 min on a shaker, and 0.2% Triton X-100 was
added for 10 min. The cells were then washed twice with PBS, and
click reaction buffer (Tris-HCl, pH 8.5, 100 mM; CuSO4, 1 mM;
Apollo 550 fluorescent azide, 100 µM; and ascorbic acid, 100 mM)
was added for 10–30 min while protecting from light. Subsequently,
the cells were washed three times with 0.5% Triton X-100, and
stained with Hoechst (5 µg/ml) for 30 min at room temperature.
Finally, the cells were washed five times with 0.5% Triton X-100,
and then 150 µl PBS was added. Images were obtained using a
fluorescence microscope (magnification, ×4; cat. no. IX81; Olympus
Corporation).
Cell cycle analysis
The cell cycle was measured using flow cytometry and
a detection reagent kit (Nanjing KeyGen Biotech Co., Ltd.)
according to the manufacturer's protocols. Briefly,
2×106/well cells were seeded into 6-well plates and
cultured for 48 h at 37°C and 5% CO2. Next, cells were
collected using trypsin (Beijing Solarbio Science & Technology
Co., Ltd.), washed with PBS (centrifuged at 396 × g for 5 min at
4°C) three times, and fixed in 70% ice-cold ethanol at 4°C
overnight. After washing with ice-cold PBS (centrifuged at 742 × g
for 5 min at 4°C) twice, cells were stained with 500 µl propidium
iodide containing RNase A buffer for 30 min at room temperature
away from light. The percentage of cells was calculated and
analyzed using a BD FACSAria flow cytometer (BD Biosciences), and
the results were interpreted using FlowJo version 10 (BD
Biosciences).
Statistical analysis
The experiments were repeated three times
independently. Results were analyzed using GraphPad software 8.0
(GraphPad Software, Inc.), and all quantitative data are presented
as the means ± SDs. Unpaired Student's t-test was performed to
compare differences between two different groups with parametric
variables, while one-way ANOVA was used to analyze the significance
of multiple group comparisons. Tukey's post hoc test was performed
following ANOVA. Spearman's rank correlation coefficient was
performed to analyze the strength and direction of association
between two ranked variables. The Pearson correlation coefficient
was used to assess a linear correlation. All statistical analyses
were two-sided, and P<0.05 was considered to indicate a
statistically significant difference. Variance was similar between
the groups being statistically compared.
Results
CDC45 expression in pan-cancer
To analyze CDC45 expression in different cancers,
the Oncomine database, was first searched. Compared with
noncancerous samples, levels of CDC45 were markedly elevated in
various cancers (Fig. 1A). Next,
the relative CDC45 expression across different cancers of TCGA were
explored using TIMER 2.0. CDC45 expression was higher in BLCA,
BRCA, cervical squamous cell carcinoma and endocervical
adenocarcinoma (CESC), cholangiocarcinoma (CHOL), colon
adenocarcinoma (COAD), esophageal carcinoma (ESCA), glioblastoma
multiforme (GBM), HNSC, kidney chromophobe (KICH), kidney renal
clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma
(KIRP), liver hepatocellular carcinoma (LIHC), LUAD, lung squamous
cell carcinoma (LUSC), pancreatic adenocarcinoma (PAAD),
pheochromocytoma and paraganglioma (PCPG), prostate adenocarcinoma
(PRAD), rectum adenocarcinoma (READ), stomach adenocarcinoma
(STAD), thyroid carcinoma (THCA) and uterine corpus endometrial
carcinoma (UCEC) (Fig. 1B) than in
matched control samples. With regard to certain cancers without
control samples or those with highly limited normal tissues,
including adrenocortical carcinoma (ACC), lymphoid neoplasm diffuse
large B-cell lymphoma (DLBC), acute myeloid leukemia (LAML), brain
lower grade glioma (LGG), ovarian serous cystadenocarcinoma (OV),
sarcoma (SARC), skin cutaneous melanoma (SKCM), TGCT, thymoma
(THYM), and uterine carcinosarcoma (UCS), GTEx data were utilized
as control groups, allowing the further investigation of CDC45
expression between various cancers and their matched control
groups. The results revealed that CDC45 was significantly increased
in almost all cancers, except for LAML, SARC, mesothelioma (MESO)
and uveal melanoma (UVM) (Fig.
1C). The association between CDC45 expression and different
cancer stages was further explored in each cancer, and it was
determined that CDC45 expression was positively associated with
ACC, KICH, KIRC, KIRP, LIHC, LUAD and UCEC, while COAD, OV and READ
exhibited negative associations (Fig.
1D). In addition, association between CDC45 expression and
different pathological grades of each cancer was evaluated, and the
results demonstrated that levels of CDC45 were positively
associated with CESC, HNSC, KIRC, LGG, LIHC PAAD and UCEC (Fig. 1E).
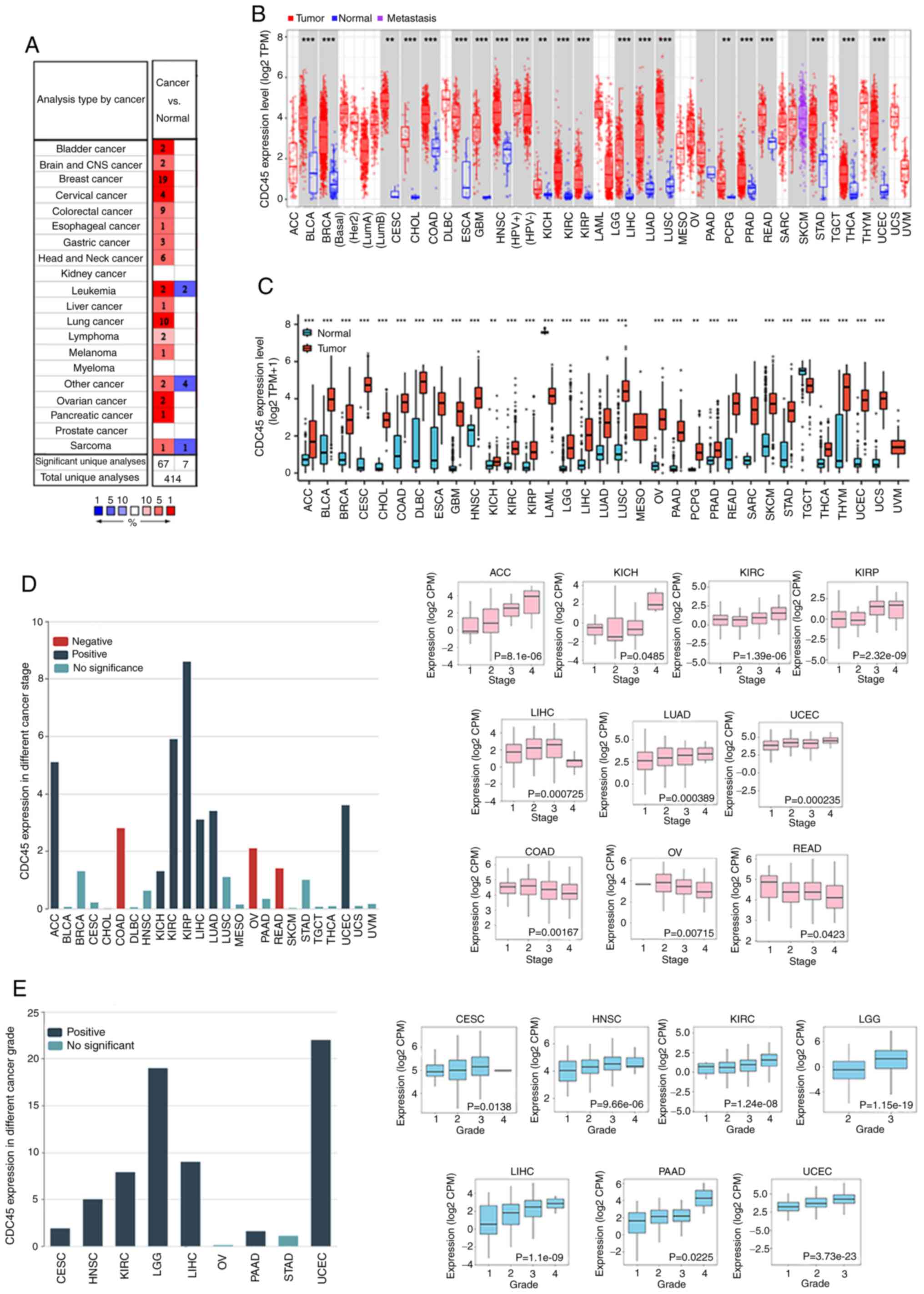 | Figure 1.Levels of CDC45 expression in
different cancers. (A) CDC45 expression in various cancers compared
with control tissues in the Oncomine database. (B) CDC45 expression
in different cancers compared with adjacent control tissues from
TCGA by TIMER 2.0. (C) CDC45 expression in different cancers
between tumors (TCGA) and corresponding control tissues (GTEx). (D)
The association between CDC45 expression and different cancer
stages in ACC, KICH, KIRC, KIRP, LIHC, LUAD, UCEC, COAD, OV and
READ. Red bar, negative association; blue bar, positive
association; and cyan bar, no significance. (E) CDC45 expression of
different pathological stages in CESC, HNSC, KIRC, LGG, LIHC, PAAD
and UCEC. Blue bar, positive association; cyan bar, no
significance. **P<0.01 and ***P<0.001. CDC45, cell division
cycle 45; TCGA, The Cancer Genome Atlas; TIMER, Tumor Immune
Estimation Resource; GTEx, Genotype-Tissue Expression; ACC,
adrenocortical carcinoma; KICH, kidney chromophobe; KIRP, kidney
renal papillary cell carcinoma; LIHC, liver hepatocellular
carcinoma; LUAD, lung adenocarcinoma; UCEC, uterine corpus
endometrial carcinoma; COAD, colon adenocarcinoma; OV, ovarian
serous cystadenocarcinoma; READ, rectum adenocarcinoma; CESC,
cervical squamous cell carcinoma and endocervical adenocarcinoma;
HNSC, head and neck squamous cell carcinoma; LGG, brain lower grade
glioma; PAAD, pancreatic adenocarcinoma. |
As proteins are the substances that execute
biological processes, the protein levels of CDC45 across different
cancers were next analyzed. Based on HPA, it was revealed that
CDC45 protein levels were increased in most cancer tissues compared
with matched control samples (Fig.
S1).
Prognostic value of CDC45 in various
cancers
Given that CDC45 is highly expressed in various
cancers, the survival association between CDC45 and different
cancers was next analyzed using the TCGA project. The results
indicated that high levels of CDC45 were negatively associated with
the overall survival in patients with ACC, KICH, KIRC, KIRP, LAML,
LGG, LIHC, LUAD, MESO, PAAD, SARC, SKCM and UVM, while CESC showed
a positive correlation with OS (Fig.
2). Finally, to further explore the association between CDC45
expression and the predictive ability of OS in different cancers,
the raw counts of RNA-sequencing data and clinical information of
different cancers from TCGA were downloaded, and time ROC analysis
was also performed to compare the predictive accuracy of each gene
and the risk score. The area under the ROC curve (AUC) of each
cancer in the 1-, 3-, and 5-year survival rates conveyed a high
predictive value, especially in ACC (1-year 0.834, 3-year 0.954,
and 5-year 0.847), MESO (1-year 0.800, 3-year 0.805, and 5-year
0.856), KICH (1-year 0.983, 3-year 0.758, and 5-year 0.844) and
KIRP (1-year 0.739, 3-year 0.769, and 5-year 0.661). These results
indicated that CDC45 expression may be a biomarker for the
prognosis of these cancers (Fig.
3).
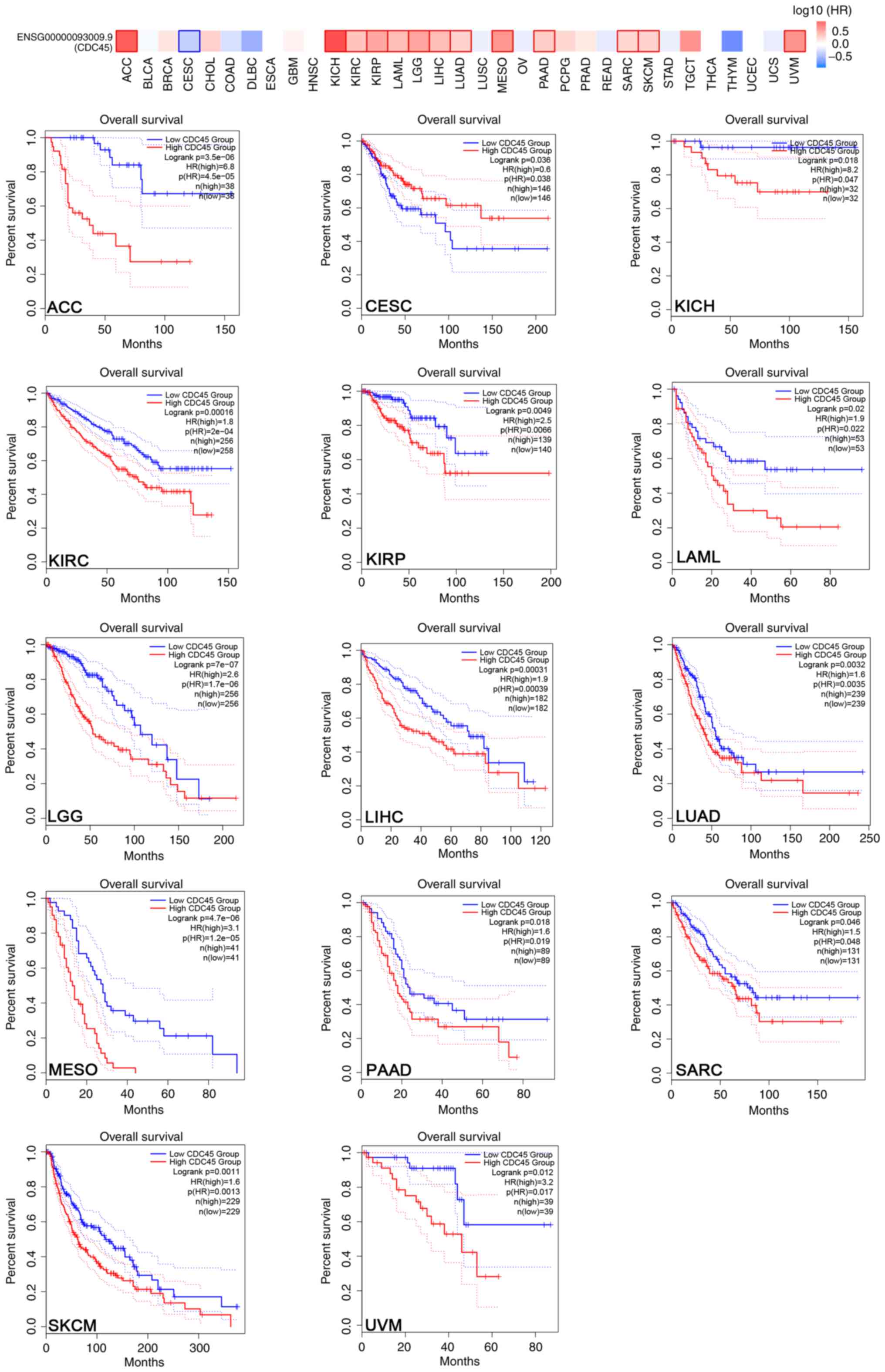 | Figure 2.Prognostic value of CDC45 in various
cancers. Overall survival analysis of CDC45 expression in different
cancers compared with adjacent control tissues from TCGA and GTEx
using GEPIA2. CDC45, cell division cycle 45; TCGA, The Cancer
Genome Atlas; GTEx, Genotype-Tissue Expression; GEPIA, Gene
Expression Profiling Interactive Analysis; ACC, adrenocortical
carcinoma; CESC, cervical squamous cell carcinoma and endocervical
adenocarcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear
cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LAML,
acute myeloid leukemia; LGG, brain lower grade glioma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; MESO,
mesothelioma; PAAD, pancreatic adenocarcinoma; SARC, sarcoma; SKCM,
skin cutaneous melanoma; UVM, uveal melanoma. |
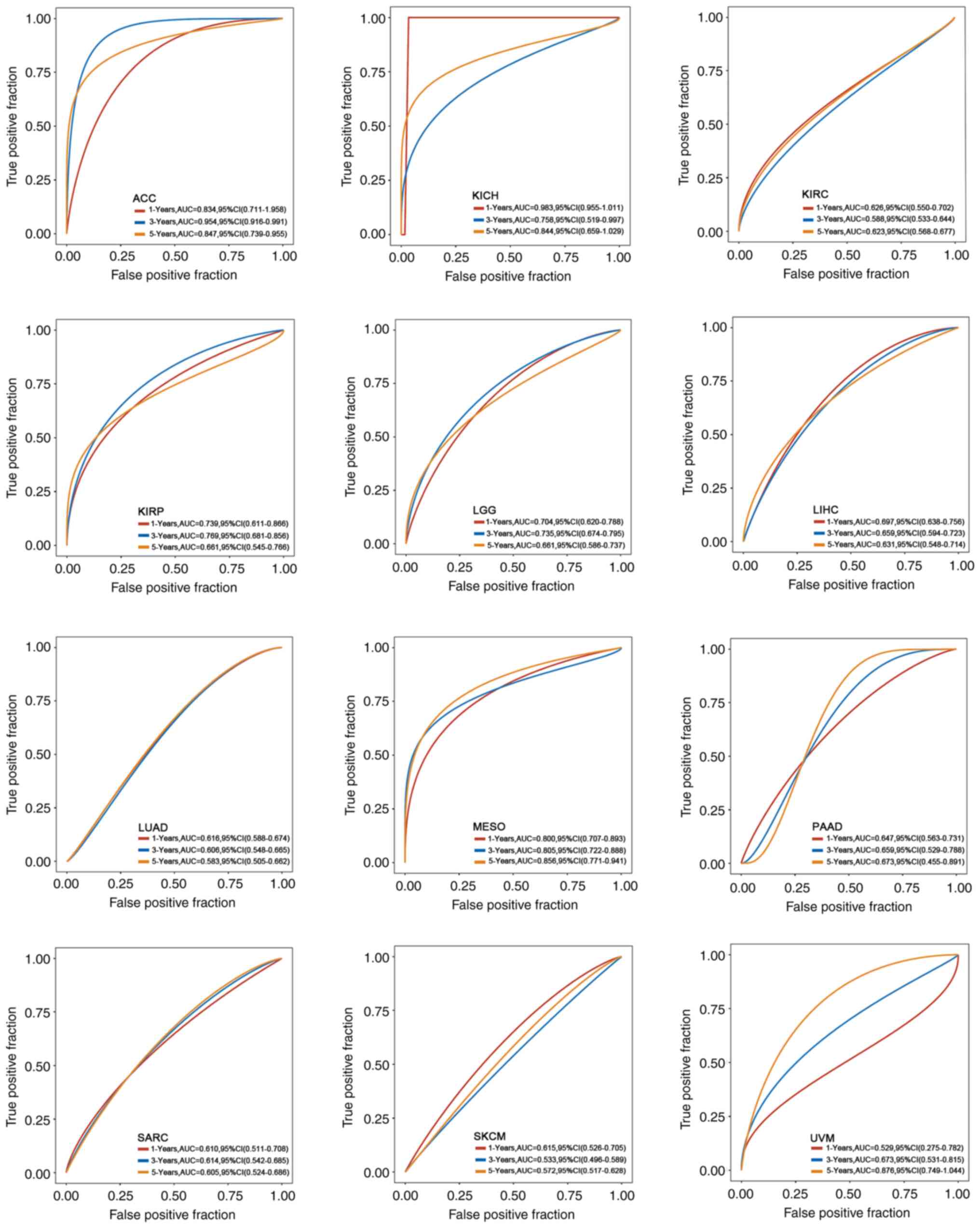 | Figure 3.Association between CDC45 expression
and the predictive ability of overall survival in different
cancers. The raw counts of RNA-sequencing data and clinical
information of different cancers from TCGA were downloaded, and
timeROC analysis was also performed to compare the predictive
accuracy of each gene and the risk score. The AUC of each cancer in
the 1-, 3-, and 5-year survival rates are presented. CDC45, cell
division cycle 45; TCGA, The Cancer Genome Atlas; ROC, receiver
operating characteristic; AUC, area under the ROC curve; ACC,
adrenocortical carcinoma; KICH, kidney chromophobe; KIRC, kidney
renal clear cell carcinoma; KIRP, kidney renal papillary cell
carcinoma; LGG, brain lower grade glioma; LIHC, liver
hepatocellular carcinoma; LUAD, lung adenocarcinoma; MESO,
mesothelioma; PAAD, pancreatic adenocarcinoma; SARC, sarcoma; SKCM,
skin cutaneous melanoma; UVM, uveal melanoma. |
CDC45 alteration analysis in various
cancers
Next, CDC45 alterations in various cancers from the
TCGA database were obtained. As revealed in Fig. S2A, the most frequent genetic
alteration was ‘mutation’ in UCS (7.02%), which was entirely
composed of the ‘amplification’ type, and the same statistical
trends occurred in TGCT (1.34%), MESO (1.15%), PCPG (0.56%) and
THCA (0.4%). The details of ‘mutation’ were also explored using a
diagram (Fig. S2B), including
sites, types and case numbers. Missense was the most common
mutation type of CDC45 mutation (83/101), followed by truncation
(10/101), splice (6/101), in frame (1/101) and SV/fusion (1/101).
R175 and X163 had 3 mutations, and the mutation type of R175 is
presented in the 3D structure of the CDC45 protein (Fig. S2C).CDC45 and DNA methylation in
various cancers. Subsequently, the DNA promoter methylation
levels between different cancers and matched control tissues were
investigated using UALCAN. It was observed that the beta values
were higher in normal samples than in cancer tissues in BLCA, BRCA,
TGCT, LUAD and HNSC (Fig. 4;
images on the left). In addition, potential probes of DNA
methylation across different cancers were searched using MEXPRESS.
As revealed in Fig. 4 (images on
the right), all promoter probes were negatively correlated with
CDC45 DNA methylation, indicating that DNA promoter methylation is
reduced in BLCA, BRCA, TGCT, LUAD and HNSC. These results are
consistent with the data obtained from UALCAN, suggesting that the
promoter methylation level of CDC45 may participate in the
processes of tumorigenesis in BLCA, BRCA, TGCT, LUAD and HNSC.
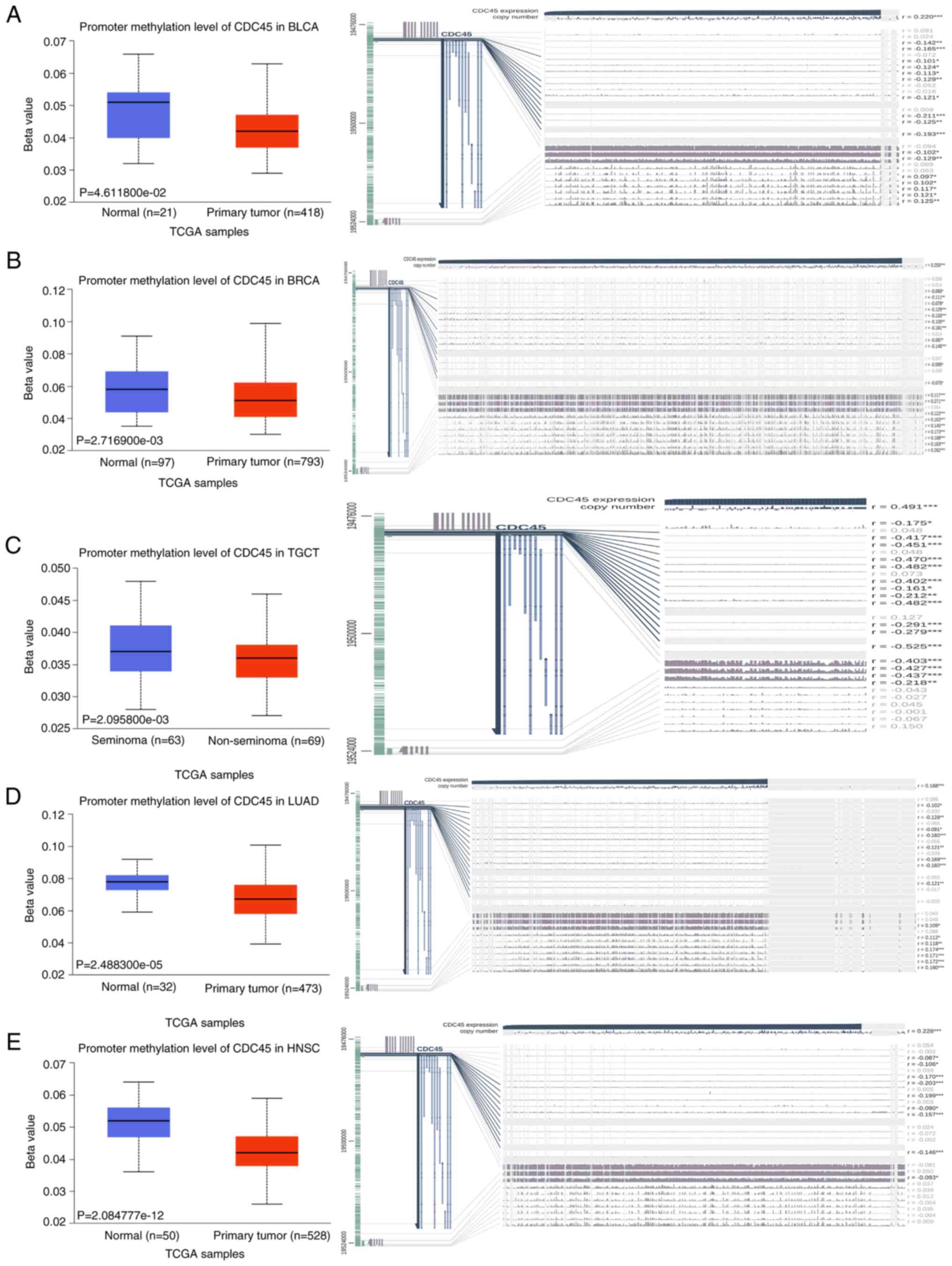 | Figure 4.DNA promoter methylation levels of
CDC45 between tumors and matched control tissues were determined
using UALCAN (left) and details (right) in (A) BLCA, (B) BRCA, (C)
TGCT, (D) LUAD and (E) HNSC. *P<0.05, **P<0.01 and
***P<0.001. CDC45, cell division cycle 45; BLCA, bladder
urothelial carcinoma; BRCA, breast invasive carcinoma; TGCT,
testicular germ cell tumors; LUAD, lung adenocarcinoma; HNSC, head
and neck squamous cell carcinoma. |
CDC45 and immunology in various
cancers
In recent years, immunotherapy has shown great
potential in various cancers. It was next examined whether there
were associations between cancers and immunology with respect to
CDC45. First, the association between CDC45 and different immune
cells, including CD8+ T cells, CD4+ T cells,
neutrophils, myeloid dendritic cells, macrophages, and B cells,
were analyzed across all TCGA cancers in the TIMER2.0 database. The
data revealed that CDC45 was almost positively associated with
these immune cells in KIRC, LIHC, THCA and THYM but negatively
associated with LUAD, LUSC, and GBM (Fig. 5A). Considering the important role
of tumor MSI, which establishes a significant association with the
sensitivity to immune checkpoint inhibitors, Fig. 5B revealed that CDC45 was positively
associated with MSI in 11 cancers, including HNSC (P=0.017), THCA
(P=0.0071), KIRC (P=6.7e-06), STAD (P=0.0013), COAD (P=2.3e-12),
BRCA (P=0.012), SARC (P=2.8e-07), LIHC (P=0.01), UCEC (P=4.3e-0.5),
PRAD (P=6.3e-06) and LUAD (P=0.0098). Subsequently, the association
between CDC45 expression and neoantigens in different cancers was
explored, and the data (Fig. 5C)
illustrated that CDC45 expression was positively associated with
neoantigens in LUAD (P=0.0045), BRCA (P=3.5e-13), UCEC (P=0.00023),
STAD (P=0.0011), BLCA (P=0.011), and PRAD (P=9.1e-05). In addition,
the association between CDC45 expression and Estimation of stromal
and immune cells in malignant tumor tissues using expression data
(ESTIMATE) and checkpoints was analyzed (Fig. S3). Moreover, the different immune
subtypes were also analyzed across various cancers using TISIDB,
including C1 (wound healing), C2 (IFN-γ dominant), C3
(inflammatory), C4 (lymphocyte depleted), C5 (immunologically
quiet) and C6 (TGF-β dominant). A significant difference in CDC45
expression was observed in ACC, BLCA, BRCA, COAD, ESCA, KIRC, KIRP,
LGG, LIHC, LUAD, LUSC, MESO, OV, PAAD, PRAD, SARC, STAD, TGCT,
THCA, UCEC, and UCS. Taking COAD as an example, the C2 subtype
exhibited the highest CDC45 expression while the C6 subtype
exhibited low CDC45 levels (Fig.
5D). These results indicated that CDC45 expression is
associated with the immunology of various tumors.
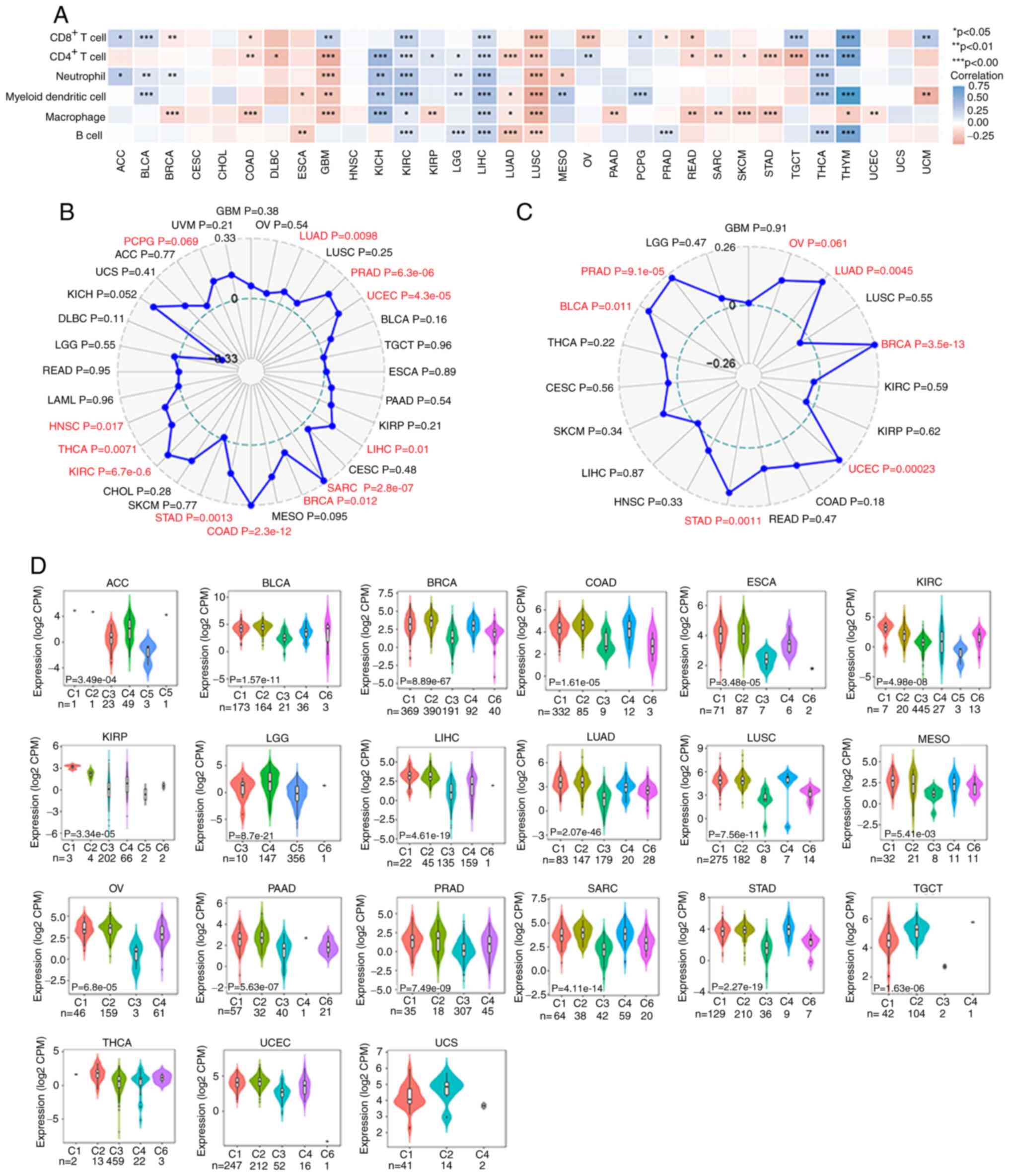 | Figure 5.Association between CDC45 expression
and immunity in various cancers. (A) The association between CDC45
and CD8+ T cells, CD4+ T cells, neutrophils,
myeloid dendritic cells, macrophages, and B cells across all TCGA
cancers in the TIMER2.0 database. (B) The association between CDC45
and MSI and between CDC45 and neoantigens (C) in various cancers.
(D) The association between CDC45 and different immune subtypes
across various cancers via TISIDB. *P<0.05, **P<0.01 and
***P<0.001. CDC45, cell division cycle 45; TCGA, The Cancer
Genome Atlas; TIMER, Tumor Immune Estimation Resource; TISIDB,
tumor-immune system interactions database; MSI, microsatellite
instability GBM, glioblastoma; OV, ovarian serous
cystadenocarcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous
cell carcinoma; PRAD, prostate adenocarcinoma; UCEC, uterine corpus
endometrial carcinoma; BLCA, bladder urothelial carcinoma; TGCT,
testicular germ cell tumors; ESCA, esophageal carcinoma; PAAD,
pancreatic adenocarcinoma; KIRP, kidney renal papillary cell
carcinoma; LIHC, liver hepatocellular carcinoma; CESC, cervical
squamous cell carcinoma and endocervical adenocarcinoma; SARC,
sarcoma; BRCA, breast invasive carcinoma; MESO, mesothelioma; COAD,
colon adenocarcinoma; STAD, stomach adenocarcinoma; SKCM, skin
cutaneous melanoma; CHOL, cholangiocarcinoma; KIRC, kidney renal
clear cell carcinoma; THCA, thyroid carcinoma; HNSC, head and neck
squamous cell carcinoma; LAML, acute myeloid leukemia; READ, rectum
adenocarcinoma; LGG, brain lower grade glioma; DLBC, diffuse large
B-cell lymphoma; KICH, kidney chromophobe; UCS, uterine
carcinosarcoma; ACC, adrenocortical carcinoma; PCPG,
pheochromocytoma and paraganglioma; UVM, uveal melanoma. |
CDC45 and m6A in various
cancers
To further explore the potential roles of CDC45, it
was hypothesized that CDC45 levels are related to m6A
methylation, which is the most abundant modification in eukaryotic
cells (19). According to the
literature, the core genes of m6A methylation, including
m6A methyltransferase ‘writers’ (CBLL1, KIAA1429,
METTL14, METTL3, RBM15, RBM15B, and WTAP), ‘readers’ (HNRNPA2B1,
HNRNPC, IGF2BP1, IGF2BP2, IGF2BP3, RBMX, YTHDC1, YTHDC2, YTHDF1,
YTHDF2, YTHDF3, and ZNF217) and ‘erasers’ (ALKBL5 and FTO)
(20–23) were summarized; hence, the
correlation between CDC45 expression and those genes was analyzed.
The results revealed that CDC45 expression was primarily positively
correlated with ‘writers’ and ‘readers’ in various cancers but
negatively correlated with the ‘reader’ in THYM (Fig. 6A and B). However, the correlation
between CDC45 levels and ‘eraser’ varied across different cancers
(Fig. 6C). The REPIC database was
then searched, and the data showed that CDC45 may be regulated by
m6A posttranslational modification (Fig. S4). These results were further
demonstrated using the m6A-Atlas database, where a total
of 9 studies elucidated the m6A positions of CDC45 in
different cell lines and tissues (HeLa, HepG2, 293T, 293, ESC, and
HCT116) using different techniques (Fig. 6D). The distribution of all the
m6A sites of CDC45 located on chr 22 (GSE63753)
(31) are as follows: 19468535,
19470283, 19484961, 19491689, 19492898, 19495817, 19496177,
19496202, 19502339, 19504159, 19508041, 19508071, respectively
(Fig. 6E and F), and 27 RBPs were
obtained, including HNRNPC, RBM15 and YTHDC1 (Fig. 6G), which may be immediately
regulated through the m6A site. These results indicated
that CDC45 participates in tumorigenesis via m6A
posttranslational modification in tumorigenesis.
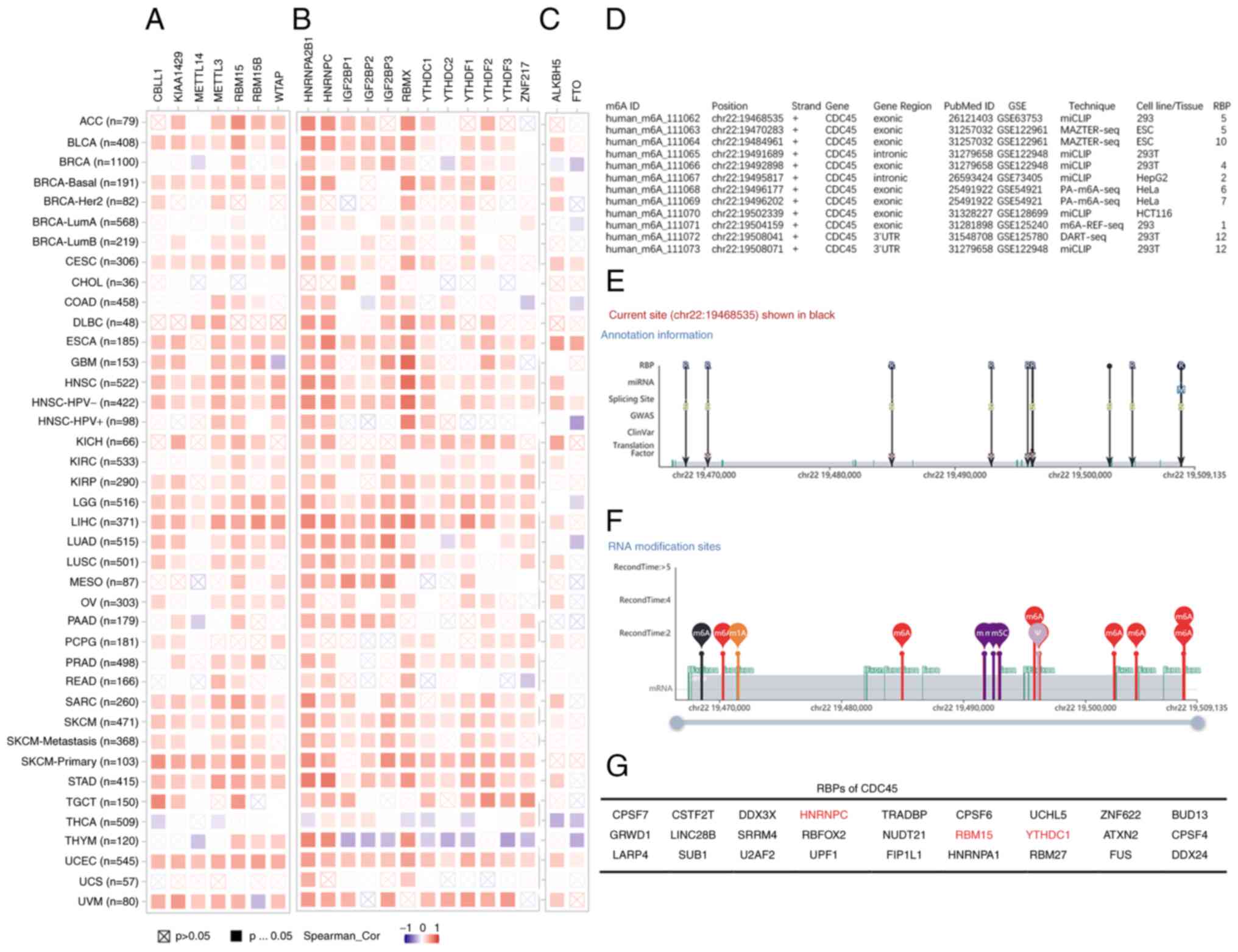 | Figure 6.CDC45 expression and m6A
in various cancers. The correlation between CDC45 expression and
m6A (A) ‘writers’, (B) ‘readers’ and (C) ‘erasers’ was
determined using TIMER 2.0. (D) Summary of CDC45 and m6A
posttranslational modification by m6A-Atlas. (E and F)
The landscape of all the m6A sites and RNA modification
sites of CDC45 from GSE63753. (G) The RBPs of CDC45 collected from
m6A-Atlas. CDC45, cell division cycle 45;
m6A, N6-methyladenosine; RBPs, RNA
binding proteins; ACC, adrenocortical carcinoma; BLCA, bladder
urothelial carcinoma; BRCA, breast invasive carcinoma; CESC,
cervical squamous cell carcinoma and endocervical adenocarcinoma;
CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; DLBC, diffuse
large B-cell lymphoma; ESCA, esophageal carcinoma; GBM,
glioblastoma; HNSC, head and neck squamous cell carcinoma; KICH,
kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP,
kidney renal papillary cell carcinoma; LGG, brain lower grade
glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung
adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO,
mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD,
pancreatic adenocarcinoma; PCPG, pheochromocytoma and
paraganglioma; PRAD, prostate adenocarcinoma; READ, rectum
adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD,
stomach adenocarcinoma; TGCT, testicular germ cell tumors; THCA,
thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial
carcinoma; UCS, uterine carcinosarcoma; UVM, uveal melanoma. |
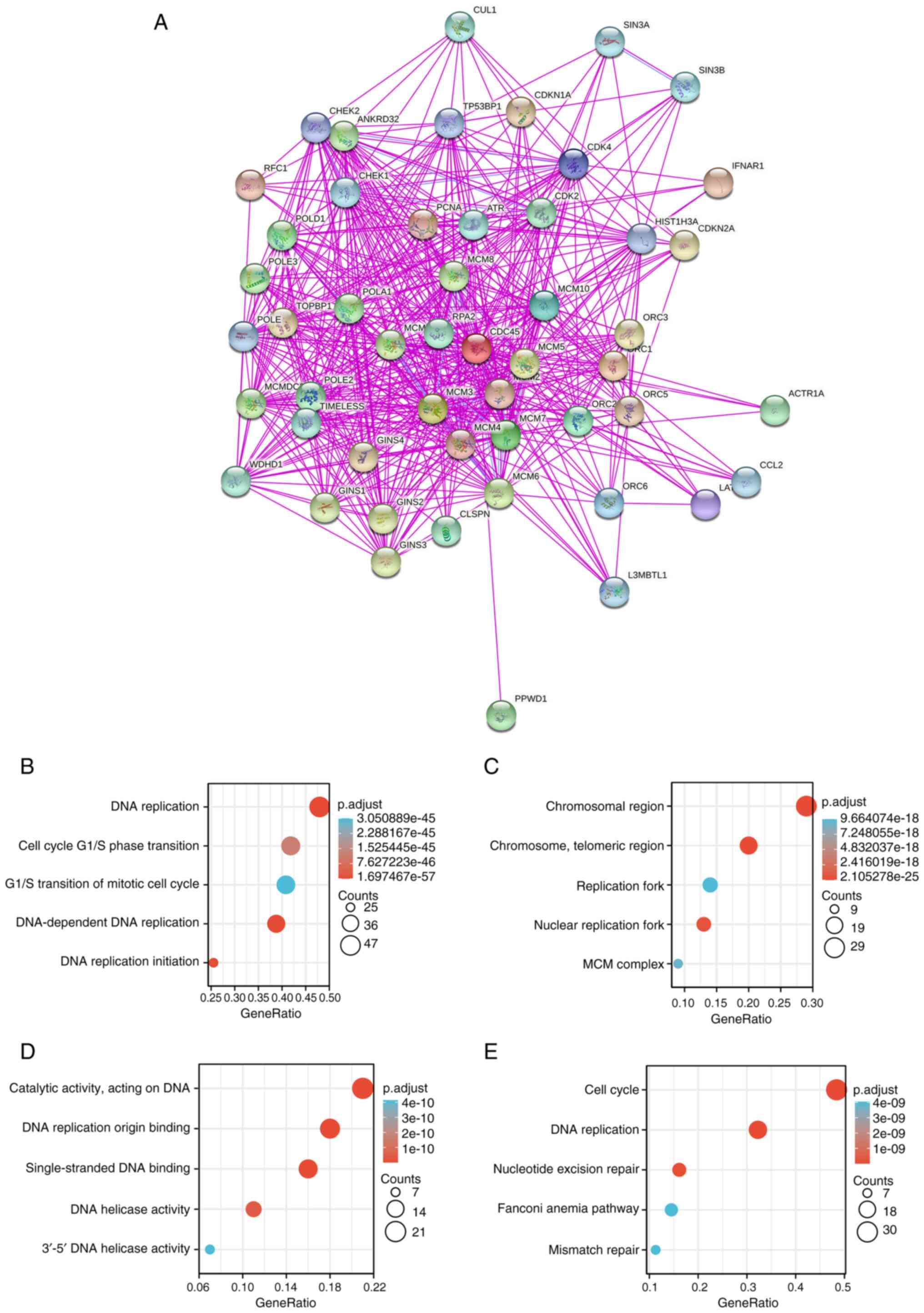
CDC45 and GO/KEGG analysis
To further explore the biological significance of
CDC45 in tumorigenesis in various cancers, gene co-expression
analysis was first performed, and the results showed that CDC45 was
significantly correlated with various cancers (Fig. S5). Next, CDC45-binding proteins
were screened using the STRING database. Their interaction networks
are shown in Fig. 7A, and a total
of 50 interactive genes validated by experimental studies were
obtained for further research. Another database, GEPIA2, was
searched, and the top 100 genes co-expressed with CDC45 were
obtained. Subsequently, the data of those genes was combined to
perform the GO and KEGG analysis, and the identified biological
process (BP) was linked to ‘DNA replication’, ‘cell cycle G1/S
phase transition’, ‘G1/S transition of mitotic cell cycle’,
‘DNA-dependent DNA replication’ and ‘DNA replication initiation’
(Fig. 7B), while the cellular
component (CC) showed that those genes were primarily enriched in
‘chromosomal region’, ‘chromosome telomeric region’, ‘replication
fork’, ‘nuclear replication fork’ and ‘MCM complex’ (Fig. 7C). Finally, the molecular function
(MF) of those genes included ‘catalytic activity acting on DNA’,
‘DNA replication origin binding’, ‘single-strand DNA binding’, ‘DNA
helicase activity’ and ‘3’-5’ DNA helicase activity’ (Fig. 7D). The KEGG pathway analysis
indicated that CDC45 was involved in the ‘cell cycle’, ‘DNA
replication’, ‘nucleotide excision repair’, ‘Fanconi anemia
pathway’ and ‘mismatch repair’ in the tumorigenesis of various
cancers (Fig. 7E). These results
were further supported by three other databases BioGRID, HIPPIE and
HitPredict (Fig. S6).
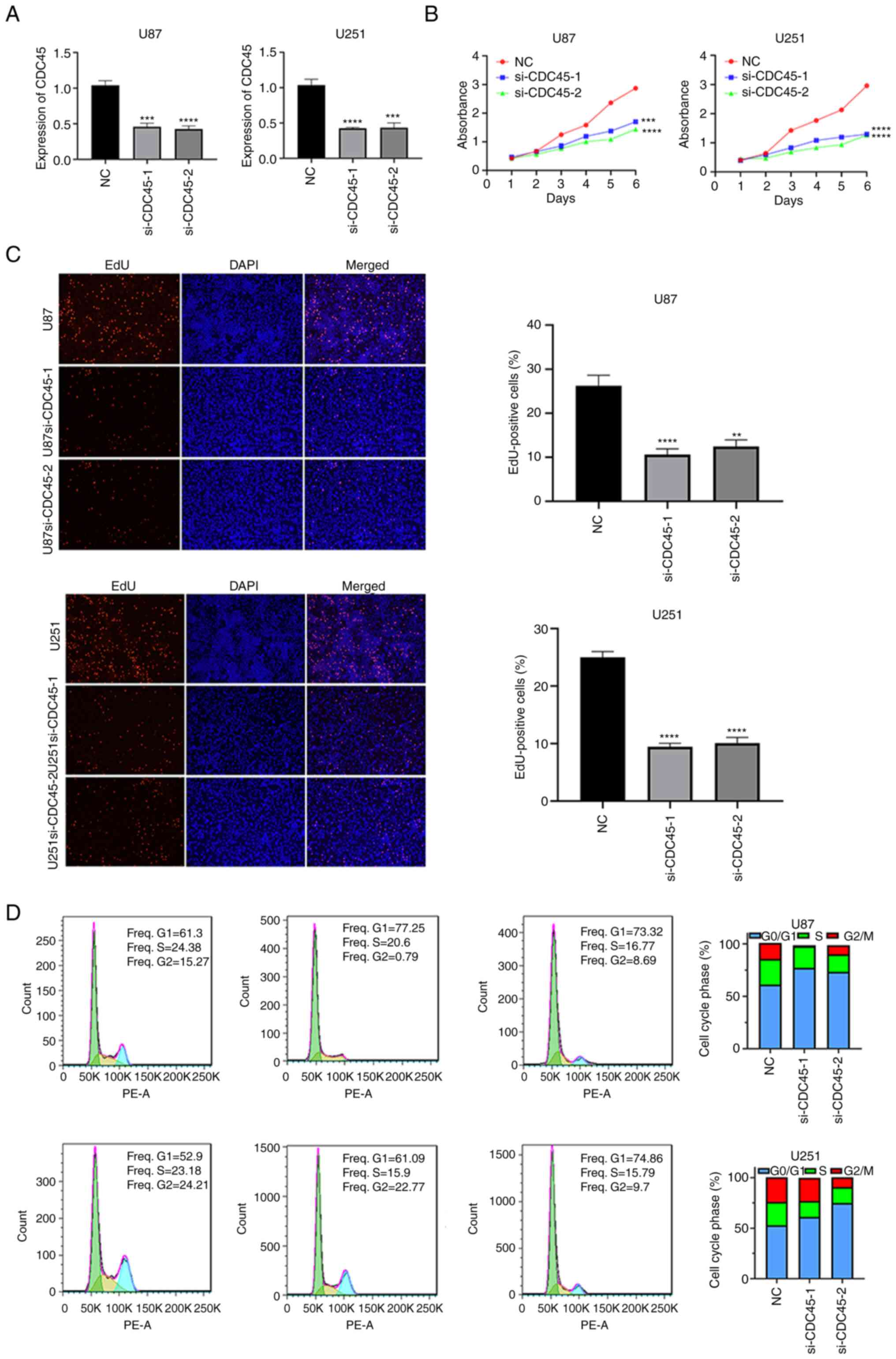
Biological functions of CDC45 in
vitro
Considering the biases of bioinformatics analysis,
in vitro experiments were next performed to validate the
biological functions of CDC45. Following transfection with siRNA,
CDC45 expression in U87 and U251 cells was assessed by RT-qPCR
(Fig. 8A). CCK-8 assays were then
performed, and the results revealed that knockdown of CDC45
inhibited the proliferation of U87 and U251 cells (Fig. 8B). These results were further
supported by the EdU proliferation assay, which revealed that
EdU-positive cells were higher in the control group than in the
CDC45-knockdown group (Fig. 8C).
Finally, cell cycle analysis was also conducted. After knocking
down CDC45, cell cycle progression was primarily arrested at the G1
phase (Fig. 8D). These results
revealed that CDC45 represents an oncogene in GBM.
Discussion
CDC45, which is composed of 650 amino acids, was
first identified as having a key role in chromosomal DNA
replication in budding yeast (24). Thereafter, accumulating studies
have explored the functions of CDC45 in various conditions, and it
has been demonstrated that CDC45 is part of the helicase complex
(CDC45-MCM2-7/GINS), which regulates the initiation of DNA
replication (25–27). Although several studies have
demonstrated that CDC45 serves as an oncogene (9,15,18),
whether CDC45 can play a role in the pathogenesis of different
tumors through certain common molecular mechanisms remains to be
answered. Through a literature search, no publication was retrieved
with a pan-cancer analysis of CDC45 from the perspective of overall
tumors. Thus, the CDC45 gene was comprehensively examined in a
total of 33 different tumors based on the data of different
databases and the molecular features of gene expression, genetic
alteration, DNA methylation, immunology, or GO/KEGG analysis. Thus,
the comprehensive analysis of CDC45 in various cancers was
conducted.
In the present study, CDC45 expression was first
analyzed between various cancers and their matched control tissues,
and the data revealed that CDC45 expression was highly expressed in
most cancers. Moreover, overexpressed CDC45 levels conveyed poor OS
and DFS prognosis in 13 cancer types, while CESC and OV exhibited
the opposite trend, indicating that CDC45 may be an oncogene.
Unfortunately, only one experimental study has been performed
focusing on THCA, however the result of this study was consistent
with our finding (18).
Fundamental studies in other cancers are still required.
Given that gene mutations may participate in
numerous processes of tumorigenesis (1,28),
it was then explored whether mutations of CDC45 participated in the
development of cancers. The cBioPortal database, which contains
TCGA data, was used to analyze the CDC45 mutations, and the results
showed that the most frequent genetic alteration was the
‘amplification’ type, followed by ‘mutation’, ‘deep deletion’ and
‘structure variant’ types. Unfortunately, the original data for the
survival analyses was not retrieved, and thus, the absence of such
data is a limitation of the present study.
DNA promoter methylation participates in a number of
biological processes. Numerous studies have demonstrated the
mechanisms of DNA methylation and histone modification among
different cancers, especially the CpG island promoter
hypermethylation of tumor suppressor genes (29,32,33).
Therefore, methylation in CDC45 promoter regions was explored in
the present study. The UALCAN database was adopted and identified
five types of cancers that exhibited low methylation levels
compared with matched normal samples, including BLCA, BRCA, TGCT,
LUAD and HNSC (all P<0.05), indicating that the low methylation
levels in those cancers lead to CDC45 overexpression. Another
database, MEXPRESS (26), was also
searched to verify this result, and the data confirmed low
methylation levels in those cancers. These results indicated that
CDC45 methylation may participate in the processes of
tumorigenesis.
Due to the important roles of immunotherapy in the
treatment of cancers (34,35), potential correlations between CDC45
levels and different immune cells across various cancers were
investigated. The MSI, neoantigen and immune subtypes were also
explored in different cancers. CDC45 was positively correlated with
the immune cells in KIRC, LIHC, THCA and THYM but negatively
correlated with those in LUAD, LUSC, and GBM. However, none of
these studies (8–18) focused on CDC45 expression or
immunology across different cancers. The findings of the present
study, are the first to the best of our knowledge, to suggest
correlations between CDC45 expression and tumor immunity in certain
cancers.
Accumulating evidence has illustrated the functions
and roles of m6A methylation in different cancers. There
are three types of enzymes known as ‘readers’, ‘writers’ and
‘erasers’ (20–22,36).
These genes were collected and a correlation analysis between CDC45
expression and these genes was performed. The data revealed that
CDC45 was primarily positively correlated with ‘writers’ and
‘erasers’, and these results were further supported by data from
the m6A-Atlas database (21,31,37).
All RBPs in different GSE data were also downloaded and it was
determined that HNRNPC, RBM15 and YTHDC1 may be directly regulated
through m6A sites. Data from the REPIC database was
consistent with these results, and additional experimental evidence
is required for the potential association between CDC45 and
m6A in various cancers.
Subsequently, GO and KEGG analyses were performed.
GO contains three categories: CC, MF and BP, which can aid in
better understanding the functions of these genes (38). The results revealed that CDC45 may
have a potential impact on ‘chromosomal region’, ‘DNA replication’,
‘catalytic activity, acting on DNA’ and ‘cell cycle’ pathways, and
the cell cycle analysis revealed that these results were consistent
with previous studies (39,40).
Finally, the association between CDC45 expression
and the predictive ability of OS in different cancers was assessed.
The results demonstrated that CDC45 expression was negatively
associated with the survival probability in ACC, KICH, KIRC, KIRP,
LGG, LIHC, LUAD, MESO, PAAD, PCPG, SARC, READ, and UCEC (all
P<0.05) but positively associated with DLBC (P=0.011). The ROC
curve of CDC45 expression showed a high predictive value for ACC,
MESO, KICH and KIRP. These results indicated that CDC45 expression
may be a biomarker for the prognosis of those cancers, and
additional clinical studies are required to clarify these potential
predictive values in different cancers. This research, however, is
subject to several limitations. First, the present study focused on
the possible mechanisms of CDC45 in 33 cancers, and the specific
mechanism of CDC45 for certain cancers was not explored. Second,
in vivo experiments were not performed to investigate the
biological functions of CDC45. Therefore, further fundamental
studies are still required to verify the mechanisms of CDC45.
Finally, as single-cell analysis aids in better understanding and
characterizing cell types and their functions regarding
pathophysiological processes based on molecular signatures, it has
emerged as a powerful tool for resolving intratumor heterogeneity,
delineating stromal cell types, and detecting rare subpopulations
(Fig. S7).
In summary, CDC45 expression and survival analysis
was first explored in multiple cancer types. The potential
mechanisms of CDC45 were then explored, including gene alterations,
DNA promoter methylation, different immune cells, m6A,
GO and KEGG enrichment analysis. The potential predictive values of
CDC45 in different cancers were also identified. The results
revealed that CDC45 is an oncogene and provide a potential target
in multiple cancers.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 81572490 and 81172405), the Science
and Technology Fund of Tianjin Binhai New Area Health and Family
Planning Commission (grant nos. 2018BWKZ002 and 2018BWKZ003), and
the Tianjin Science and Technology Committee (grant no.
18JCZDJC98600).
Availability of data and materials
The data that support the present findings of this
study are available from TCGA and GTEx online databases.
Authors' contributions
YL, XC and FL performed the experiments and compiled
the manuscript. HY and YZ contributed to the design of the study
and analyzed data. KD and YN conducted the experiments and analyzed
the data. QH contributed to the conception and design of the
present study and revised the manuscript. YL and XC confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boshuizen J and Peeper DS: Rational cancer
treatment combinations: An urgent clinical need. Mol Cell.
78:1002–1018. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mun EJ, Babiker HM, Weinberg U, Kirson ED
and Von Hoff DD: Tumor-treating fields: A fourth modality in cancer
treatment. Clin Cancer Res. 24:266–275. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ilves I, Petojevic T, Pesavento JJ and
Botchan MR: Activation of the MCM2-7 helicase by association with
Cdc45 and GINS proteins. Mol Cell. 37:247–258. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boos D, Frigola J and Diffley JF:
Activation of the replicative DNA helicase: Breaking up is hard to
do. Curr Opin Cell Biol. 24:423–430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka S and Araki H: Helicase activation
and establishment of replication forks at chromosomal origins of
replication. Cold Spring Harb Perspect Biol. 5:a0103712013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Owens JC, Detweiler CS and Li JJ: CDC45 is
required in conjunction with CDC7/DBF4 to trigger the initiation of
DNA replication. Proc Natl Acad Sci USA. 94:12521–12526. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng D, Tu Z, Wu W and Liang C: Inhibiting
the expression of DNA replication-initiation proteins induces
apoptosis in human cancer cells. Cancer Res. 63:7356–7364.
2003.PubMed/NCBI
|
|
9
|
Pollok S, Bauerschmidt C, Sänger J,
Nasheuer HP and Grosse F: Human Cdc45 is a proliferation-associated
antigen. FEBS J. 274:3669–3684. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Srinivasan SV, Dominguez-Sola D, Wang LC,
Hyrien O and Gautier J: Cdc45 is a critical effector of
myc-dependent DNA replication stress. Cell Rep. 3:1629–1639. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Y, Wang L, Li Z, Wan Z, Shao M, Wu S
and Wang G: Potential prognostic and diagnostic values of CDC6,
CDC45, ORC6 and SNHG7 in colorectal cancer. Onco Targets Ther.
12:11609–11621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang J, Li Y, Lu Z, Che Y, Sun S, Mao S,
Lei Y, Zang R, Li N, Zheng S, et al: Analysis of functional hub
genes identifies CDC45 as an oncogene in non-small cell lung
cancer-a short report. Cell Oncol (Dordr). 42:571–578. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Piao J, Sun J, Yang Y, Jin T, Chen L and
Lin Z: Target gene screening and evaluation of prognostic values in
non-small cell lung cancers by bioinformatics analysis. Gene.
647:306–311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ke Y, Guo W, Huang S, Li Y, Guo Y, Liu X,
Jin Y and Ma H: RYBP inhibits esophageal squamous cell carcinoma
proliferation through downregulating CDC6 and CDC45 in G1-S phase
transition process. Life Sci. 250:1175782020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu HP, Du XF, Li JD, Huang SN, He RQ, Wu
HY, Li MF, Wu WZ, Chen JT, Mo WJ and Chen G: Expression of cell
division cycle protein 45 in tissue microarrays and the CDC45 gene
by bioinformatics analysis in human hepatocellular carcinoma and
patient outcomes. Med Sci Monit. 27:e9288002021.PubMed/NCBI
|
|
16
|
Sang L, Wang XM, Xu DY and Zhao WJ:
Bioinformatics analysis of aberrantly methylated-differentially
expressed genes and pathways in hepatocellular carcinoma. World J
Gastroenterol. 24:2605–2616. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiang XH, Yang L, Zhang X, Ma XH, Miao RC,
Gu JX, Fu YN, Yao Q, Zhang JY, Liu C, et al:
Seven-senescence-associated gene signature predicts overall
survival for asian patients with hepatocellular carcinoma. World J
Gastroenterol. 25:1715–1728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun J, Shi R, Zhao S, Li X, Lu S, Bu H and
Ma X: Cell division cycle 45 promotes papillary thyroid cancer
progression via regulating cell cycle. Tumour Biol.
39:10104283177053422017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng M, Brägelmann J, Schultze JL and
Perner S: Web-TCGA: An online platform for integrated analysis of
molecular cancer data sets. BMC Bioinformatics. 17:722016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ru B, Wong CN, Tong Y, Zhong JY, Zhong SS,
Wu WC, Chu KC, Wong CY, Lau CY, Chen I, et al: TISIDB: An
integrated repository portal for tumor-immune system interactions.
Bioinformatics. 35:4200–4202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res. 48:W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thul PJ and Lindskog C: The human protein
atlas: A spatial map of the human proteome. Protein Sci.
27:233–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Z, Kang B, Li C, Chen T and Zhang Z:
GEPIA2: An enhanced web server for large-scale expression profiling
and interactive analysis. Nucleic Acids Res. 47:W556–W560. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chandrashekar DS, Bashel B, Balasubramanya
SA, Creighton CJ, Ponce-Rodriguez I, Chakravarthi BV and Varambally
S: UALCAN: A portal for facilitating tumor subgroup gene expression
and survival analyses. Neoplasia. 19:649–658. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koch A, Jeschke J, Van Criekinge W, van
Engeland M and De Meyer T: MEXPRESS update 2019. Nucleic Acids Res.
47:W561–W565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Y, Chen K, Song B, Ma J, Wu X, Xu Q,
Wei Z, Su J, Liu G, Rong R, et al: m6A-Atlas: A comprehensive
knowledgebase for unraveling the N6-methyladenosine (m6A)
epitranscriptome. Nucleic Acids Res. 49:D134–D143. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Zhu A, He C and Chen M: REPIC: A
database for exploring the N6-methyladenosine methylome. Genome
Biol. 21:1002020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Linder B, Grozhik AV, Olarerin-George AO,
Meydan C, Mason CE and Jaffrey SR: Single-nucleotide-resolution
mapping of m6A and m6Am throughout the transcriptome. Nat Methods.
12:767–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Eng J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Esteller M: Aberrant DNA methylation as a
cancer-inducing mechanism. Annu Rev Pharmacol Toxicol. 45:629–656.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fesnak AD, June CH and Levine BL:
Engineered T cells: The promise and challenges of cancer
immunotherapy. Nat Rev Cancer. 16:566–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu Z, Ott PA and Wu CJ: Towards
personalized, tumour-specific, therapeutic vaccines for cancer. Nat
Rev Immunol. 18:168–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He L, Li H, Wu A, Peng Y, Shu G and Yin G:
Functions of N6-methyladenosine and its role in cancer. Mol Cancer.
18:1762019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Garcia-Campos MA, Edelheit S, Toth U,
Safra M, Shachar R, Viukov S, Winkler R, Nir R, Lasman L, Brandis
A, et al: Deciphering the ‘m6A Code’ via antibody-independent
quantitative profiling. Cell. 178:731–747.e16. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gene Ontology Consortium: Gene ontology
consortium: Going forward. Nucleic Acids Res. 43:D1049–D1056. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Broderick R and Nasheuer HP: Regulation of
Cdc45 in the cell cycle and after DNA damage. Biochem Soc Trans.
37:926–930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Köhler C, Koalick D, Fabricius A, Parplys
AC, Borgmann K, Pospiech H and Grosse F: Cdc45 is limiting for
replication initiation in humans. Cell Cycle. 15:974–985. 2016.
View Article : Google Scholar : PubMed/NCBI
|