Introduction
Metastasis is a single event that causes
cancer-related deaths of the majority of patients with cancer,
including melanoma (1). Melanoma
is an aggressive type of skin cancer that originates from
melanocytes, the pigment-producing cells (1). Among all the subtypes, cutaneous
melanoma is the most prevalent, accounting for >90% of all
melanoma cases (1). In 2020, there
were 324,635 cases of melanoma, accounting for 1.7% of all
malignancies and 57,043 deaths, making up 0.6% of all
cancers-related deaths (2).
The development and pathogenesis of melanoma depend
on numerous factors; exposure to ultraviolet radiation (UV),
development of a melanocytic nevi (MN) or changes in molecular
pathways to name a few (3). UV
exposure, including other types of radiation such as ionizing
radiation, is a major risk factor for melanoma (4). Upon exposure to UV light, cAMP is
activated thus expressing MITF which stimulates melanogenesis
(5). Several studies have revealed
that melanogenesis can disrupt the metastatic cascade and that it
can play opposing roles in melanoma metastasis (6–9).
While melanin has been demonstrated to attenuate the aggressiveness
of melanoma, it reduces the efficacy of current pharmaceutical
treatments for this disease (10).
For this reason, the purpose of the present review was to assess
the impact of various components of the melanogenesis pathway on
melanoma metastasis and to determine the potential benefits of
melanin in preventing melanoma metastasis.
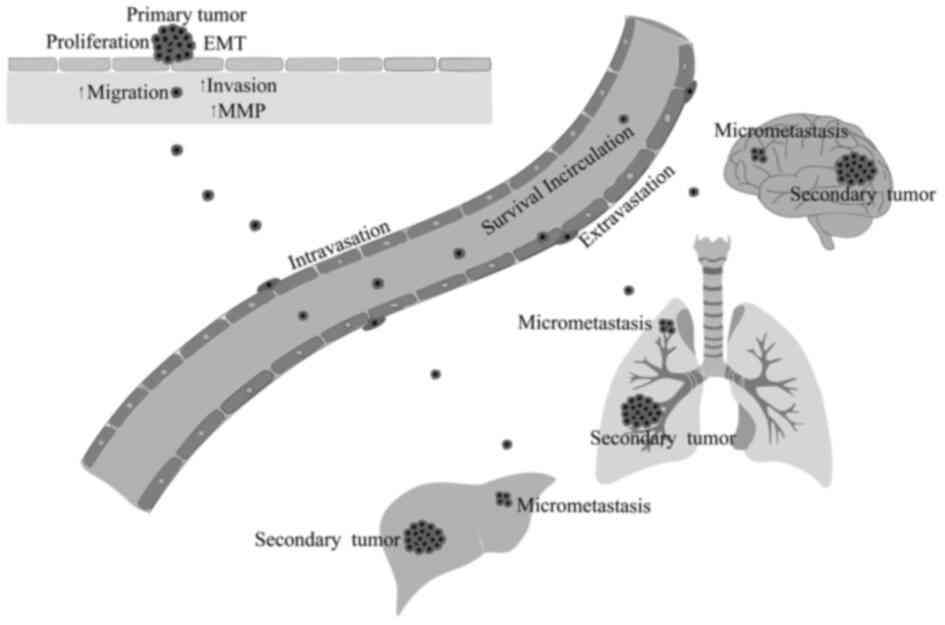
Melanoma metastasis
Metastasis is a process by which cancer cells spread
from its primary site to a secondary site (11). During metastasis, tumour cells
dissociate from the primary site due to loss of cell-to-cell
adhesion and changes in the cell matrix (11). This allows them to invade the
surrounding stroma (11). Tumour
cells also produce new blood vessels by angiogenesis which provides
a route for the cells to enter the circulation and metastasise
(11). Upon reaching a point of
extravasation, tumour cells form a bond with the endothelial cells
through adhesion and then penetrate the endothelium and basement
membranes (11). After
extravasation, tumour cells arrive at the target organ and release
growth factors into the circulation allowing the spread of
micrometastasis (12). These
tumour cells then proliferate to produce macroscopic metastasis
thus ceasing the metastatic cascade (12). Skin, lung, brain, liver, bone, and
intestine are among the most common sites of distant metastases in
melanoma (Fig. 1). Lung metastasis
is the most common location of metastasis, and appears to be the
first clinically visible site of visceral metastasis (13). Abnormal molecular changes in
melanoma cells allow them to become more aggressive or metastatic
(3). These changes include
mutations in genes including v-raf murine sarcoma viral oncogene
homolog B1 (BRAF), neuroblastoma RAS viral oncogene homolog (NRAS),
p53, cyclin-dependent kinase 2A (CDKN2A), cyclin-dependent kinase 4
(CDK4) mutations in c-KIT and also polymorphisms in melanocortin 1
receptor (MC1R) (3). Gene
mutations and molecular changes lead to the activation of a
melanoma metastasis cascade.
According to several studies, metastasis is
triggered by the fusion of cancer cells with macrophages or other
bone marrow-derived migratory cells, which activates regulatory
genes and consequently initiates a variety of pathways (14–16).
The majority of these pathways, including SNAIL, SLUG, SPARC, and
TWIST are associated with epithelial-mesenchymal transition (EMT)
(16,17). EMT is a process where epithelial
cells lose both cell polarity and cell-to-cell adhesion properties,
hence converting into a mesenchymal phenotype (18). Acquisition of a mesenchymal
phenotype allows cells to migrate as a single cell entity (18). Mesenchymal cells are able to move
through matrix-filled space by degrading extracellular matrix (ECM)
proteins with proteases such as matrix metalloproteinases (MMPs)
and urokinase-type plasminogen activator (uPA) thus promoting local
invasion (19,20). In addition, a mesenchymal phenotype
increases both tumorigenicity and metastatic growth of these cells
(20).
Physiologically, EMT is essential to maintain stem
cell properties in order to prevent cell death by apoptosis or
senescence as well as to suppress immune responses. EMT begins when
the epithelial cells lose their cell-to-cell adhesion by the
disassembly of epithelial tight junctions, adherens junctions,
desmosomes, and gap junctions (21). Subsequently, cells lose their
polarity due to the disruption of polarity complexes such as
Crumbs3, partitioning defective (PAR) and Scribble (SCRIB)
(22). At that moment, epithelial
genes (E-cadherin) are suppressed through the expression of
mesenchymal genes (N-cadherin) (22). A change in phenotype from
epithelial to mesenchymal leads to reorganisation of the
cytoskeleton and actin architecture of cells, resulting in the
formation of lamellipodia, filopodia, and invadopodia, which
facilitates movement and invasion (22). Cells also express MMPs that break
the ECM by degrading the ECM extracellular molecules such as
collagen, laminins and proteoglycans, eliminating the physical
barrier, thus increasing invasion (22).
Since melanoma cells are not true epithelial cells,
phenotypic switching is a preferred phrase to elucidate the
EMT-like process that occurs in this type of cancer (23). EMT-inducing transcription factors
(EMT-TFs) regulate phenotype switching in melanoma, which involves
MITFlow and MITFhigh interchangeable states
(23). Phenotype switching
promotes the cadherin switch from E-cadherin to N-cadherin which is
also driven by EMT-TFs such as zinc finger E-box-binding homeobox 1
(ZEB1), TWIST, and SNAIL (23).
The overexpression of N-cadherin causes melanoma cells to lose
contact with neighbouring keratinocytes which allows melanoma cells
to acquire new adhesive properties (24). Expression of MITF is considered as
a key driver of phenotype switching whereby a low MITF expression
was revealed to be correlated with an increased mesenchymal marker
and a high MITF expression was correlated with an increased
epithelial marker (24). Melanomas
undergo the cadherin switch from E-cadherin to N-cadherin typically
driven by EMT-TFs (24).
Role of MITF in melanoma
metastasis
MITF is one of the most essential transcription
factors in the progression of melanoma, and it has been reported to
be upregulated in ~15% of melanoma specimens with BRAFV600E
mutation (25). There are at least
nine different isoforms of MITF (−A, -B, -C, -D, -E, -H, -J, -M,
and -MC) where the M isoform of MITF (MITF-M) is selectively
expressed in melanocytes (25).
MITF-M is responsible for increased pigmentation genes such as
tyrosinase (TYR), tyrosinase-related protein 1 (TRYP1), and
tyrosinase-related protein 2 (TRYP2) as well as increase of melanin
content (25). However, more
research is required to determine the precise significance of the M
isoform in melanoma.
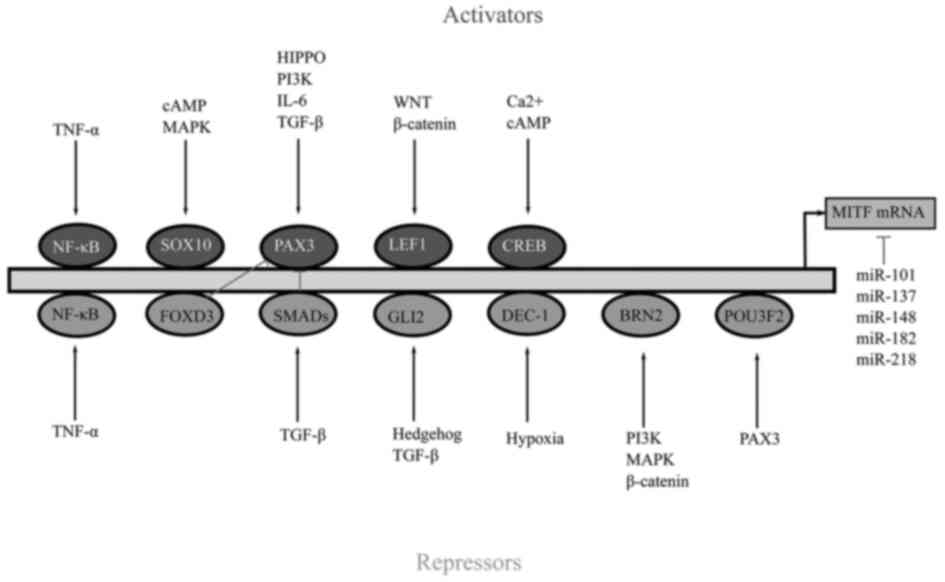
Regulation of MITF gene is controlled by several
activators and repressors (Fig.
2). Sex-determining region Y-box 10 (SOX10) is an MITF
activator which plays a crucial role in regulating MITF (26). SOX10 depletion results in the
reduction of MITF and an increase of SOX9 expression (26). SOX10 along with the cAMP response
element-binding protein (CREB) is activated by p38 signalling and
also by simulation of adenylate cyclase activity (27). Adenylate cyclase activity is
stimulated by the binding of α-melanocyte-stimulating
hormone (α-MSH) to MC1R which activates the cAMP/protein
kinase A (PKA)/CREB pathway (27).
Inhibition of PKA/CREB causes degradation of MITF (28). PKA can also phosphorylate the
serine 675 residue of β-catenin, preventing ubiquitination,
consequently destructing the protein yielded in the accumulation
and activation of β-catenin signalling (28). Nuclear β-catenin/lymphoid
enhancer-binding factor 1 (LEF1) induces the expression of MITF in
actively proliferating melanoma cells (28). Additional MITF activators include
paired box gene 3 (PAX3) and zinc finger E-box-binding homeobox 2
(ZEB2) (29).
There are some transcription factors that suppress
the activity of MITF including GLI family zinc finger 2 (GLI2),
nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB)
and BRN2. GLI2 is a member of the hedgehog pathway which is a
target of the TGF-β gene (30). An inverse association exists
between GLI2 and MITF genes where increased expression of GLI2
decreases MITF expression (30).
Despite the fact that GLI2 inhibits MITF, it increases melanoma
cell invasion and phenotypic plasticity (30). Similar to GL12, NF-κB also
antagonises MITF expression as inhibitors of NF-κB appear to
effectively decrease MITF expression (31). Likewise, BRN2 a POU domain
transcription factor is found to repress MITF expression. However,
previous research has also revealed contradicting evidence
suggesting that BRN2 can also increase the expression of MITF
(32). Additional repressors of
MITF include activating transcription factor 4 (ATF4),
differentially expressed in chondrocytes protein 1 (DEC1), homeobox
A1 (HOXA1), c-MYC and forkhead box D3 (FOXD3) (29).
MITF is key in the development of melanoma. MITF
increases the transcription of pigmentation genes such TYR, TYRP1,
dopachrome tautomeras (DCT), premelanosome protein (PMEL), and
MLANA in melanocytes, promoting melanogenesis (33). In addition, MITF controls the
expression of genes including BCL2 and cyclin-dependent kinase 2
(CDK2) that are important for melanoma survival and proliferation
(33). A number of lysosomal and
metabolic genes are also controlled by MITF (33).
Alterations in the MITF genes as well as alternative
splicing of MITF genes regulate melanoma (34). Changes in the MITF genes include
single base substitutions in the regions encoding its functional
domains (34). MITF
E318K is a recurrent germline mutation which encodes a
protein that inhibits SUMOylation of MITF (34). Generally, SUMOylation reduces the
transcription action of MITF (34). As a result of this, the mutated
MITF E318K enhances transcription regulatory activity
compared to its wild-type (34).
This mutation has been associated with multiple carriers of primary
melanomas and identified as a medium-penetrance melanoma gene
related to melanoma and renal cell carcinoma (34).
In addition, MITF gene splicing aids melanoma
regulation (35). Studies have
described two spliced variants of MITF: MITF (+), which contains an
internal six-amino-acid fragment encoded by exon 6a, and MITF (−),
which does not (35). The
expression of both these variants are controlled by extracellular
signal-regulated kinase (ERK) signaling (35). Among these two variants, MITF (−)
was found to be elevated in 30% of metastatic melanoma tumours in
humans (35).
Despite the fact that MITF is expressed in melanoma,
the data on whether MITF expression levels increase or decrease as
the disease advances remain controversial (36). MITFhigh and
MITFlow cells co-exist in melanoma tumours, according to
single-cell gene expression analysis on 472 cells extracted from
needle biopsies of five primary human melanomas (36). High expression of MITF was
correlated with a proliferative and differentiated phenotype
whereas low expression was correlated with dedifferentiated and
invasive phenotype (36). Hence,
switching between MITFhigh to MITFlow
accounts for melanoma plasticity and intratumor heterogeneity
(37). To further evaluate the
role of MITF in melanoma plasticity, a BRAFV600E
MITFavc7 zebrafish was generated (37). MITFavc7 mutant allele
allows its activity to be altered within an individual animal by
varying the water temperature, as fluctuating the water temperature
can affect endogenous activity (37). First, the temperature of the water
was set to 26°C to stimulate melanoma growth and increased
thereafter to 32°C to cut off activity (37). A total of 12/15 of the very large
tumours had regressed, and tumour sites of six fish were observed
to recover and completely heal over a period of two months.
However, melanomas appeared to recur when the temperature was
lowered to 26°C, thus implying that a subpopulation of melanoma
cells with low activity survived and repopulated the tumour site
(37). Findings from this study
ascertained that while obliterating MITF causes tumour regression,
low levels of activity contribute to melanoma pathology possibly by
maintaining melanoma cells in a progenitor-like state (37).
The role of MITF in melanoma progression appears
contradictory. Bianchi-Smiraglia et al found that MITF
depletion increased the production of invadopodia and matrix
degradation in MITF-depleted SK-Mel-28 and 501Mel cells compared to
control cells, as well as increased melanoma cell invasion by
inhibiting GMPR-dependent suppression of RAC1 activity (38). GMPR is also required for
MITF-dependent reduction of tumorigenicity, and lung colonisation
in C57Bl/6 mice, according to previous findings (38). Conversely, a study carried out by
developing MITF- or BRN2-knockdown cell lines revealed that
reduction of MITF and BRN2 decreased metastasis. Along with MITF,
BRN2 is fundamental in melanoma phenotype switching (39).
Initially, melanoma cell lines that express both
BRN2 and MITF were identified through western blot analysis. C32,
HT144, and MM455 were classified as MITFlow and MM649,
MM96L, and A02 were classified as MITFhigh cells
(39). These cell lines were
transduced with a lentivirus expressing luciferase, tetracycline
(Tet) repressor and shRNA targeting either MITF, BRN2 or lacZ as a
negative control (shNEG), under the control of the CMV/TetO2
promoter (39). MITF depletion
caused a decrease in cell proliferation and cell cycle progression
while BRN2 depletion had no significant effect on the cell
proliferation or cell progression compared to control cells
(39). MITF depletion also reduced
cell invasion and cell migration while BRN2 depletion did not cause
a significant reduction (39).
However, both MITF and BRN2 depletion resulted in impaired growth
of tumours and metastasis in mouse xenografts (39). Similarly, a study carried out by
Swoboda et al, demonstrated that loss of signal transducer
and activator of transcription 3 (STAT3) expression accompanied by
an increase in MITF induced cell proliferation thus increasing the
risk of developing melanoma (40).
Nonetheless, loss of STAT3 was accompanied by a decrease in tumour
invasion and a loss of EMT-like phenotype (40). To summarize, their research
revealed that STAT3 expression enhanced melanoma spread by
suppressing the MITF pathway, although MITF loss not only reduced
cell proliferation but also the risk of developing melanoma
(40).
To understand the role of MITF in melanoma, it is
necessary to explore the role melanin plays in the progression of
melanoma. Melanin is a tyrosine-derived complex polymer, and it is
the pigment responsible for skin, hair and eye colour (41). The central hub of the melanin
synthesis regulation network is the MITF protein (25).
Role of melanin in melanoma
metastasis
Melanogenesis
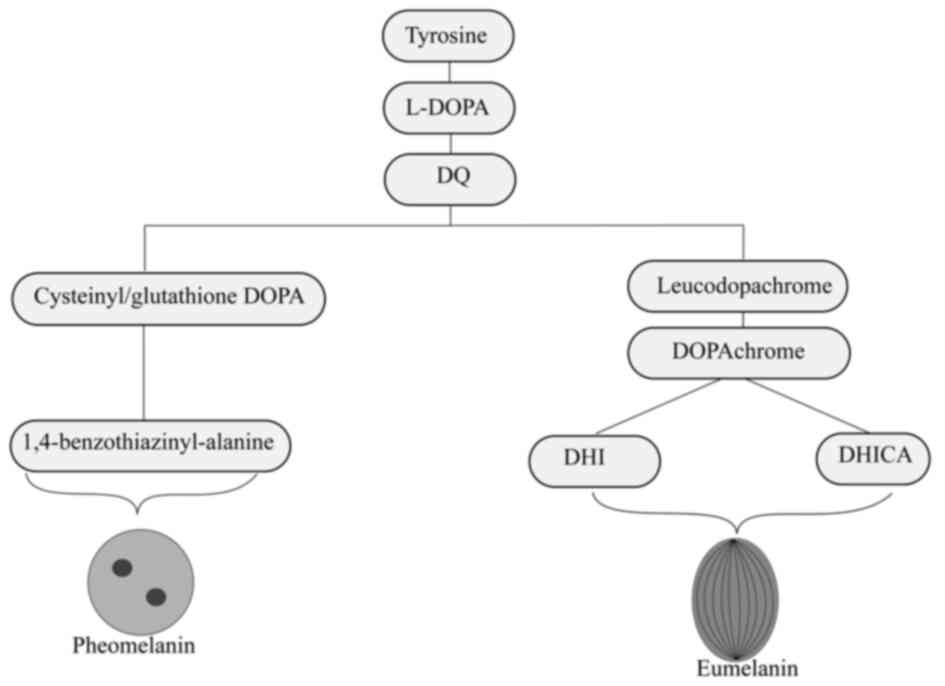
The hydroxylation of tyrosine to
L-3,4-dihydroxyphenylalanine (L-DOPA) begins the synthesis of
melanin, often known as melanogenesis. DOPA is then converted to
DOPAquinone (DQ). Both these reactions are catalysed by the enzyme,
tyrosinase (38). During
melanogenesis, two types of melanin are synthesised, including the
black-brown eumelanin (Fig. 3) and
red-yellow pheomelanin (Fig. 4).
While eumelanin is produced in absence of cysteine or glutathione,
pheomelanin is produced in the presence of cysteine (42).
Synthesis of both eumelanin and pheomelanin begins
with the conversion of tyrosine to dopachrome. After synthesis,
eumelanin matures and appears in an ellipsoidal shape which
contains a specific lattice-like internal structure formed by PMEL
(43). Pheomelanin matures
encompassing numerous internal structures and takes an an oval
shape (43) (Fig. 5). Once produced, both melanin
pigments are mixed together. The ratio of both types of melanin is
determined by genetic differences and race with eumelanin
responsible for black and brown hair and pheomelanin for red hair
(44). Human skin contains
detectable levels of pheomelanin, independent of race, colour, or
skin type. Regardless of pigmentation level, eumelanin is most
prevalent in epidermal melanin, with 74% eumelanin and 26%
pheomelanin in the human epidermis (45). As such, skin colour occurs to be
dictated by the quantity rather than the quality of melanin
generated. Nevertheless, a pH of approximately 5.8-6.3 has been
revealed to increase the production of pheomelanin and suppress
eumelanin (46).
Contribution of melanin to melanoma
metastasis
The role of melanin as a photoprotector has been
established due to the existence of an inverse association between
pigmentation and sun-induced skin cancer. Melanosomes in lightly
pigmented skin can be degraded to ‘melanin dust’ and this reduction
is linked to carcinogenesis, where several studies have explored
this correlation (10,47–50).
Both in vitro and in vivo experiments
demonstrated that melanin can in fact affect the behaviour of
melanoma cells by modifying the nanomechanical and elastic
properties as well as through inhibiting transmigration abilities
of melanoma cells. Most cancer cells usually undergo extensive
cytoskeleton reorganisation during cancer transformation, and the
level of actin cytoskeleton organisation is considered to be the
main contributor to cellular mechanics in both normal and cancer
cells (51). In the case of
melanoma however, research using Bomirski hamster melanoma (BHM)
cells derived from hamster tumours revealed that the effect of
pigmentation on cell elasticity surpassed any effect caused by the
actin cytoskeleton organisation (51). These isolated BHM cells
significantly lost their ability to produce melanin after each time
they were passaged (51). Not only
did they lose the ability to produce melanin, but were also unable
to control cellular proliferation and gained extra elasticity,
allowing them to be more metastatic (51). The elasticity of these cells was
determined by using an elasticity map to evaluate the value of
Young's modulus (the measure of elasticity) (51).
Pigmentation is also able to modify nanomechanical
properties and inhibit the transmigration ability of cells. This
was demonstrated by a study conducted using SKMEL-188 human
melanoma cells with different levels of pigmentations (52). According to the study,
non-pigmented melanoma cells had the lowest elastic deformation
with the highest Young's modulus value compared to pigmented cells
(52). Additional analysis on
BALB/c nude mice showed that the number of metastatic tumours in
mice inoculated with pigmented cells was lower, and the resulted
tumours had a smaller size compared to mice inoculated with
non-pigmented melanoma cells (52). Histological analysis revealed that
tumours produced by pigmented cells had a tight more defined shape
while the tumour formed by the non-pigmented cells had a loose,
less compact shape (52). This
difference in morphology may be caused by the differences of
pigmented and non-pigmented melanoma cells in spreading capacities,
where non-pigmented cells are more capable of spreading than
pigmented cells (52).
As a result, morphological changes in melanoma cells
are due to melanin which is expected to influence the cellular
behaviours such as cell migration, cell invasion and phenotype
switching. The relationship between melanin content and cell
migration as well as invasion of melanoma was analysed by
Netcharoensirisuk et al using two-pore channel 2 (TPC2)
negative MNT-1 melanoma cells (6).
TPC2 resides in lysosome-related organelles such as melanosomes
(6). TPC2 knockout was found to be
associated with an enhanced melanin content in MNT-1 cells via
heightened enzyme tyrosinase activity independent of the MITF
protein (6). Similarly, these
TPC2-ablated cells were found to be less aggressive in migration
and invasion than the wild-type cells, indicating that melanin is
inversely correlated with the aggressiveness of melanoma behaviour
(6). D'Amore et al reported
the same trend where TPC2 knockout in human melanoma cells (CHL1)
and B16 murine melanoma cells exhibited reduced cellular
proliferation, migration and invasion rates which lacked capacity
for angiogenesis compared to their wild-type controls (7). Their research revealed that the
TPC2-knockout cells were more mesenchymal, as mesenchymal markers
such as ZEB1, vimentin and N-cadherin were upregulated compared to
the wild-type control (7). Instead
of reduced cellular proliferation as reported by Netcharoensirisuk
et al (6), an increase in
melanin concentration appears to drive the survivability and
proliferation capacity in both cell lines (7).
Enzymes involved in melanogenesis such as tyrosinase
were associated with heightened invasion and migration in melanoma
(53). Research carried out by
Fürst et al demonstrated that DNp73-dependent tyrosinase
depletion resulted in EMT signalling cascade reactivation, a
mesenchymal-like cell phenotype, and enhanced invasiveness
(53). The forced re-expression of
tyrosinase in both SK-Mel-147 and SK-Mel-103 cells inhibited
invasion and migratory potential, which was confirmed in other
aggressive amelanotic cell lines such A375 M, C8161, and WM793
(53). Further analysis through
immunoblotting revealed that DNp73-dependent tyrosinase depletion
had a reduced expression of E-markers such as E-cadherin and an
increased expression of M-markers fibronectin, N-cadherin,
vimentin, and Slug (53). While
re-expression of tyrosinase in highly invasive cancer cells
SK-Mel-147 and WM793 showed the opposite effect with increased
E-markers and reduced M-markers. Tyrosinase is also known to reduce
ROS, which aids in the reduction of EMT (53). DNp73 overexpression or tyrosinase
downregulation was revealed to increase the level of ROS in
SK-Mel-29 cells (53). These
findings indicated that depletion of tyrosinase by DNp73 caused an
increase in ROS, which led to EMT (53). Thus, it is apparent that enzymes
involved in melanin synthesis played a role in cell migration,
invasion and phenotype switching of melanoma cells.
A previous study by one of authors and other
reasearchers also demonstrated a similar observation in B16 murine
melanoma cells whereby reduction of melanin promoted melanoma
metastasis (8). In this previous
study, a PPARβ/δ antagonist methyl
3-(N-(4-(isopentylamino)-2-methoxyphenyl)
sulfamoyl)-thiophene-2-carboxylate (10 h) was applied to
α-MSH-induced B16F10 cells (8). Other than reduced melanin secretion,
treatment with 10 h led to numerous marked changes in the
morphology of B16F10 cells (8).
Cells appeared in an elongated mesenchymal-like form rather than
their typical ‘cuboidal’ shape (8). In addition, 10 h also promoted cell
motility, cell invasion, MMP-9 expression, and cell adhesion
(8). Extravasation and tumour
burden increased in the C57BL/6 mouse model post 10 h treatment
(8). These findings indicated that
decreasing melanin output can accelerate the spread of melanoma
(8).
In addition to the aforementioned in vitro
and in vivo experiments, clinical correlation studies were
also carried out to determine the role of pigmentation in melanoma.
In one such study, 444 patients with conjunctival melanoma were
observed to determine whether iris and skin colour, as well as
tumour pigmentation played a role in the clinical outcome (Table I) (54). While tumour pigmentation was found
in 327 patients, it was correlated to lighter iris colour. When
comparing patients with low tumour pigmentation to those with high
tumour pigmentation in the cohort study for 56.3 months,
recurrences, metastasis, and even melanoma-related death were all
greater in the low tumour pigmentation group (54). Therefore, low tumour pigmentation
is likely to increase the risk of recurrences, metastasis formation
and death as shown in Table I
(54). A separate study involving
a smaller cohort of 177 patients with primary conjunctival melanoma
(CoM) also demonstrated that primary tumours with low pigmentation
were associated with a greater risk for metastases incidence
(55). It was also discovered that
low tumour pigmentation of the recurrences was found to have a
significant association in the tumour recurrences of 105
individuals (55). As such, low
pigmentation of the primary lesions was significantly correlated
with increased risk of metastasis and melanoma-related death
(55).
 | Table I.Total number of cases and tumour
characterization of 444 cases with conjunctival melanoma. |
Table I.
Total number of cases and tumour
characterization of 444 cases with conjunctival melanoma.
| Parameters | No. of total cases
(%) | No. of cases with
light iris color (%) | No. of cases with
dark iris color (%) | No. of cases with
low pigmentation (%) | No. of cases with
high pigmentation (%) |
|---|
| Total | 444 (100) | 261 (59) | 183 (41) | 130 (40) | 197 (60) |
| Recurrence (% of
total) | 177 (40) | 106 (41) | 71 (39) | 67 (52) | 81 (41) |
| Metastasis (% of
total) | 62 (14) | 34 (13) | 28 (15) | 31 (24) | 23 (12) |
| Melanoma-related
deaths (% of total) | 36 (8) | 20 (8) | 16 (9) | 19 (15) | 13 (7) |
| Exenteration (% of
total) | 50 (11) | 28 (11) | 22 (12) | 24 (19) | 23 (12) |
In addition to conjunctival melanoma, melanoma can
also be found in other organs such as the head, neck, scalp, and
rectum. Progression of melanoma in these organs was also found to
worsen with low pigmentation. In a recent retrospective study
performed by Huayllani et al on 525,271 patients who were
diagnosed with melanoma, 378 patients were diagnosed with
amelanotic melanoma of the head and neck (AMHN) and 69,267 with
common malignant melanoma of the head and neck (CMHN) (56). Evaluation of tumour characteristics
revealed that patients with AMHN had an increased Breslow depth and
greater mitotic count when compared to CMHN (56). Breslow depth measures how deeply
melanomas have spread to the skin while mitotic count is associated
with the rate of metastasis (56).
Another cross-sectional study performed on patients with scalp
melanoma also revealed that invasive scalp melanoma was associated
with amelanosis (57).
Although rare, melanoma may be found in the rectum.
Rectum melanoma is very aggressive and is found to have poor
prognosis while malignancy of primary amelanotic anorectal melanoma
was found to be greater than melanotic melanoma. A clinical
investigation conducted by Liu et al revealed that
paraffin-embedded samples of primary anorectal malignant amelanotic
melanomas exhibited higher vasculogenic mimicry channel formation
than melanotic melanoma (58).
Vasculogenic mimicry is the ability of cancer cells to organise
into vascular-like structures in order to independently obtain
nutrients and oxygen (59). This
is an important process in the early stage of some highly
aggressive and metastatic malignant tumours (59).
It is evident from the majority of the studies that
melanin and pigmentation are pivotal in preventing melanoma
metastasis. In spite of this, findings from a study disputes this
verity. For instance, melanoma metastasis was discovered to be
aided by a tripartite motif-containing protein (TRIM14) which was
also responsible for promoting melanin content in A375 melanoma
cells (9). Overexpression of
TRIM14 in A375 melanoma cells resulted in an increase of melanin
content through the AKT and STAT3 pathways (9). Instead of reduction, an enhanced
TRIM14 expression level drove the behaviour of the cells increasing
EMT, thus improving melanoma cell motility and invading the in
vitro model, and increasing tumorigenesis in the nude mouse
model, all of which were mediated by AKT and STAT3 (9). To elucidate this contention, studies
evaluated both types of melanin in melanoma metastasis in hope that
they may provide more in-depth understanding on the role of melanin
in metastasis.
Role of eumelanin and pheomelanin in
melanoma metastasis
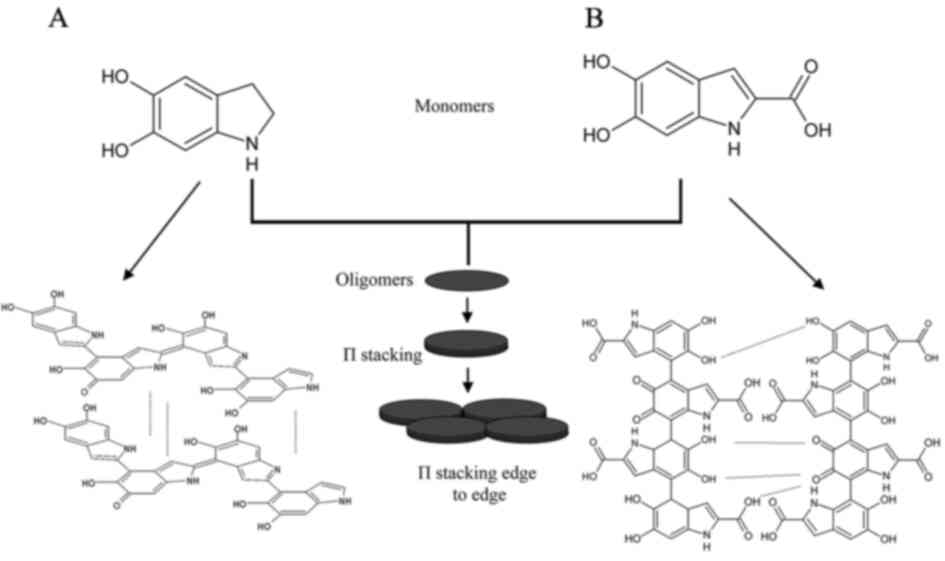
Eumelanin is a heterogeneous polymer consisting of
5,6-dihydroxyindole (DHI) and or 5,6-dihydroxyindole-2-carboxylic
acid (DHICA) (49). The structure
of eumelanin is formed through aggregation of oligomer stacks by
π-to-π interactions and secondary interactions such
as hydrogen bonding and hydrophobic interactions (49). DHI-derived eumelanin oligomers
stack well during the initial stage of assembly while stacking is
restricted in DHICA-derived eumelanin oligomers due to their
twisted form (49). Among the two
types of monomers, higher levels of DHICA-derived eumelanin are
observed in human melanoma due to heightened expression of DCT in
melanoma. DCT is an enzyme which catalyses DHICA-derived eumelanin
biosynthesis (49). As
DHICA-derived eumelanin consists of thin oligomer stacks, they are
more susceptible to oxidative degradation (49). As a result of this, a high
proportion of DHICA-derived eumelanin undergoes structural
dissociation in melanoma, producing small eumelanin fragments
(49). These small eumelanin
fragments increase ROS levels which serve as signalling molecules
to stimulate melanoma proliferation and metastasis (49).
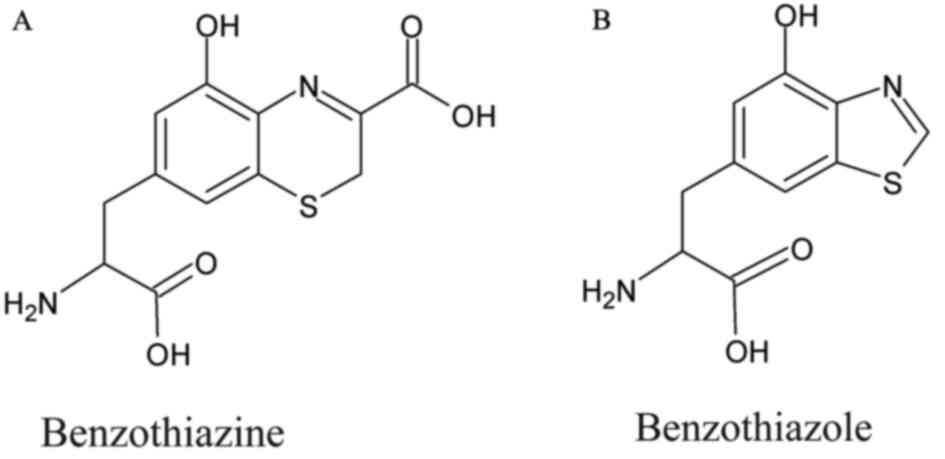
Pheomelanin, which consists of sulphur-containing
benzothiazine and benzothiazole derivatives, is considered to be
photo-unstable and to trigger carcinogenesis. Lighter epidermis
contains pheomelanin with a higher ratio of benzothiazine than
benzothiazole (45,60). In the presence of oxidative stress,
benzothiazine is converted to benzothiazole (45). Morgan et al hypothesised two
potential pathways of pheomelanin which increase the risk of
developing melanoma. The first hypothesis suggests that pheomelanin
can produce ROS which directly damages the DNA (44). This was reported by an experiment
performed on four poultry breeds (61). This study demonstrated that natural
pheomelanin and eumelanin vibrational properties contribute to
feather colour in four poultry breeds with various melanin-based
pigmentation patterns (61).
However, only the vibrational characteristic of pheomelanin has
been linked to ROS production in mitochondrial melanocytes, and
only pheomelanin has been associated to systemic oxidative stress
and damage (61). Another study
carried out on Asian barn swallows (Hirundo rustica
gutturalis) demonstrated that males with more pheomelanin in
their throat feathers were associated with a significantly lower
RGSH/GSSG ratio (higher oxidative stress) (62). The second hypothesis suggests that
synthesis of pheomelanin can cause glutathione (GSH) depletion
(44). GSH is an essential
antioxidant property required to prevent oxidative damage caused by
ROS. Rodríguez-Martínez et al revealed that even
endogenously generated pheomelanin can aid the conversion of
benzothiazine to benzothiazole (63). Benzothiazole is considered to
promote greater GSH depletion and ROS formation under visible UV
light than benzothiazine (63).
Hence, this suggests that benzothiazole can yield pheomelanin to
become cytotoxic (63).
Additionally, purified red human hair with pheomelanin demonstrated
that pheomelanin is a potent pro-oxidant that causes depletion of
antioxidants such as GSH and NADH (64).
Although both types of melanin can contribute to the
progression of melanoma, studies do suggest that pheomelanin causes
more oxidative stress compared to eumelanin. In contrast to
eumelanin, the inclusion of sulphur in the aromatic ring of
pheomelanin lowers its ionisation potential, rendering it unstable
and more prone to free radical formation (60). Mitra et al carried out a
study on C57BL/6 mice to evaluate the role of the
pheomelanin-eumelanin ratio in the development of
BRAFV600E melanoma (65). In this study, wild-type C57BL/6
mice along with mice with premature termination of the MC1R
transcript (MC1Re/e, ‘red’) and mice with an inactivating mutation
in the tyrosinase gene (Tyrc/c, ‘albino’) were used (65). The MC1Re/e variant mimics
individuals with the red hair/fair skin phenotype which had a high
pheomelanin-to-eumelanin ratio while the Tyrc/c, ‘albino’ mimics
individiuals with albinism which had no melanin (65). For each pigmentation phenotype, two
variants were created where melanocytes were observed in the dermis
of one variant and the second variant consisted of stem cell factor
(SCF) expressed under the keratin 14 promoter (K14-SCF) (65). This promotes epidermal melanocyte
localization and mimics SCF expression in human epidermal
keratinocytes (65).
BRAFV600E, the most frequently mutated melanoma
oncogene, was introduced into six groups of mice as a conditional,
melanocyte-targeted allele. Risk of melanoma was greater in the
mice with the red hair/fair skin MC1R polymorphism (65). Compared to albino mouse skin and
the black coat colour mice, the red mice had more oxidative DNA and
lipid damage (65). The
BRAFv600E mutant red mice formed melanoma tumours
spontaneously in the absence of any external chemical carcinogen or
UV exposure (65). This study
detailed the detrimental effects of pheomelanin in the absence of
eumelanin (65).
On the other hand, eumelanin is considered to
produce beneficial effects of melanin by absorbing UV-radiation and
scavenging the UV-generated free radicals. A study carried out by
Nasti and Timares discovered that eumelanin exhibits immune
suppressive properties, because it was revealed that birds with
higher eumelanin content in their feathers responded better to
immune challenges compared to birds with a lower content (60).
Even though oxidative stress caused by fragile and
unstable pheomelanin is detrimental, ROS produced by oxidative
stress is necessary and eminent to control melanoma cells during
the early stages of cancer initiation and development. This was
confirmed by Piskounova et al in NOD-SCID-Il2rg (−/-) (NSG)
mice (66). In this study,
patient-derived melanoma cells (M405, UT10, and M481) were
transplanted into NSG mice subcutaneously (66). These mice were then treated
subcutaneously with antioxidant N-acetyl-cysteine (NAC) at a dose
of 200 mg/kg/day (66). NAC had no
effect on the growth of established subcutaneous tumours (66). However, NAC progressed the disease
in all three groups of mice (M405, UT10, and M481) and
significantly increased the number of circulating melanoma cells in
mice transplanted with two types of melanoma cells (M405 and UT10)
(66). Additionally, it was also
hypothesised that cells which metastasized, successfully underwent
reversible metabolic changes to withstand oxidative stress
(66). One such adaptation was
increased GSH regeneration by increasing production of NADPH. NADPH
is important in the conversion of GSSG to GSH (66). An increased level of NADPH and NADP
was observed in metastatic cells compared to subcutaneous tumours
indicating that metastatic cells generated more NADPH to enhance
their capacity to regenerate GSH in order to cope with oxidative
stress (66). According to this
finding, oxidative stress prevented distant metastasis of melanoma
(66). Consistent with the
previous study, Le Gal et al postulated that antioxidants
aid in increasing melanoma metastasis (67). In this study, NAC and also Trolox,
an analogue of soluble vitamin E, enhanced migration and invasion
of melanoma cells as well as multiplying the number of lymph node
metastases (67). Nevertheless,
antioxidant treatment showed no effect on the number or size of
primary tumours (67).
Despite the fact that certain studies suggest that
oxidative stress aids cancer growth, it has been revealed that
oxidative stress reduces melanoma distant metastasis. Thus, induced
oxidative stress by both pheomelanin and eumelanin can also be
beneficial in the early stages of melanoma progression. As such,
both types of melanin warrant further investigation in melanoma
treatment.
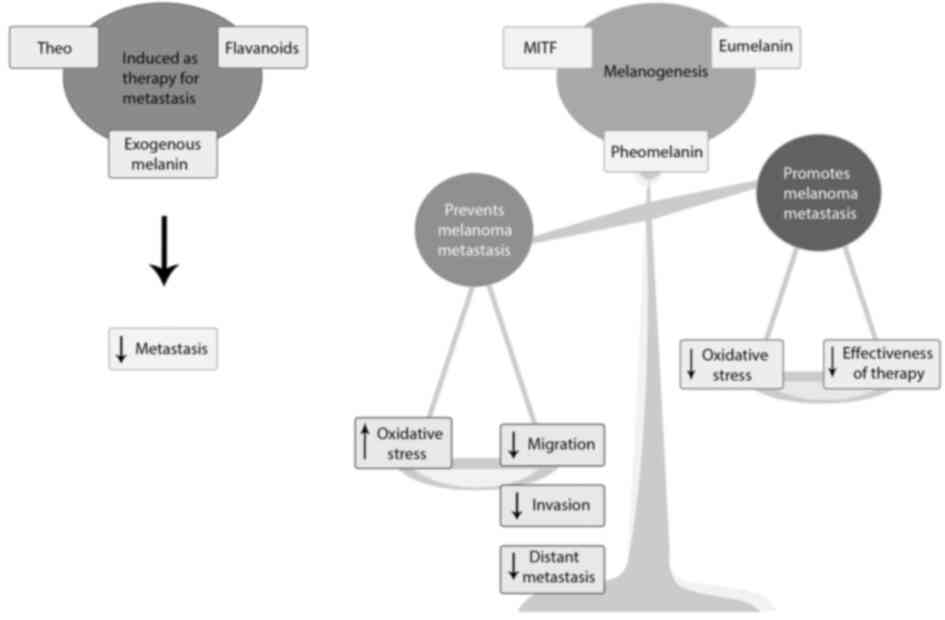
Inducing melanogenesis to prevent melanoma
progression
In light of evidence which highlights the
importance of melanin in regulating melanoma metastasis, it is
vital to recognize the possibility of using compounds or natural
products that increase melanin synthesis, as potential treatment
options to prevent melanoma progression. There are certain natural
products, compounds, and drugs that can stimulate the synthesis of
melanin by interfering with melanogenesis signalling pathways.
In vitro and in vivo experiments revealed that these
compounds that increase melanin content also exhibit antimetastatic
properties.
Theophylline (theo), a standard medication used for
treating chronic obstructive pulmonary disease (COPD) and asthma,
has been reported to boost melanin synthesis (68). Theo at a concentration of 100–500
µM increased melanogenesis in B16F10 cells (68). Western blot analysis revealed that
theo increased the expression of MITF, tyrosinase, and tyrosinase
related protein 1 (TRP-1) (68).
Moreover, Theo was demonstrated to increase the levels of
phosphorylated (p)-ERK and p-glycogen synthase kinase-3 (GSK3),
indicating that theo affects melanogenesis by activating MEK 1/2
and the Wnt/catenin signalling pathways (68). Cordella et al carried out
studies on other melanoma cells such as A375 and SK-MEL-30 and two
patient-derived melanoma-initiating cells (MICs) to determine the
effectiveness of theo in reducing melanoma metastasis due to its
potential in triggering melanogenesis (69). Treatment of A375 and SK-MEL-30
cells with theo particularly at a concentration as low as 2 mM,
reduced melanoma cell proliferation, cell adhesion and migration.
Cells treated with theo acquired a starry-dendritic morphology with
cytoplasmic protrusions, which is typical of melanocytes,
indicating that theo has an effect on membrane/cytoskeleton
dynamics (69). Theo significantly
affected proliferation, decreased migration and even reduced MMP2
activity in MICs (Mel1 and Mel3 cells) (69). In MICs, theo was found to interfere
with cytoskeleton dynamics by specifically expressing DOCK7, a
guanine nucleotide exchange factor, that acts on small Rho GTPases
such as Cdc42, RhoA, and Rac1, which are involved in activities
such as actin cytoskeleton reorganisation. Consistent with the
previous study Cordella et al reported that theo was able to
increase melanin content in MICs by stimulating TYR and DCT
activities (69).
According to an additional study involving natural
products, flavonoids derived from a plant extract were found to
increase melanin production through the upregulation of tyrosinase
(7). An extract prepared from
Dalbergia parviflora (D. parviflora) was used to isolate 44
different flavonoid compounds and the effects of these flavonoid
compounds on melanin production were studied in B16F10 and MNT-1
cells (7). TPC2 inhibitors, UM-9
(a tri-O-methylated isoflavone) and MT-8 (an
O-methylated isoflavone) were discovered to be among the top
five hits. TPC2-dependent increase in melanin was associated with
reduced proliferation, migration, and invasion of melanoma cells
(7). However, inhibiting
melanosomal TPC2 reduced MITF by increasing GSK3-mediated MITF
degradation (7). Thus, flavonoids
that inhibit TPC2 increase melanin production independent of MITF,
concurrently decreasing MITF-driven melanoma progression (7).
Exogenous melanin is used to examine the potential
of melanin in reducing melanoma progression in in vivo
models. Ye et al developed a transdermal microneedle patch
with inactive whole tumour lysate containing 50 µg melanin and
GM-CSF (70). Female C57BL/6J
mice, BALB/cJ mice, CD11c-DTR transgenic mice and
Rag1−/− knockout mice were treated with the microneedle
patch which was applied to the skin at the caudal-dorsal area for
10 min followed by NIR irradiation to the region for another 10 min
each day for five consecutive days (70). Melanin in the patch produced heat,
which aided tumour antigen absorption by dendritic cells when
combined with NIR irradiation (70). Local cytokine release and
infiltration of polarised T-cells were also increased. As a result
of this, in vaccinated C57BL/6J mice, long-term survival was
reported with an 87% tumour rejection rate, and full tumour
protection was observed in mice rechallenged with B16F10 tumour
cells (70). In summary, melanin
through infrared is able to generate local heat, boost T-cell
activities, and promote immune responses against the tumour.
Conclusion
Melanoma is a type of skin cancer that originates
from the pigment-producing cells known as melanocytes. Melanocytes
are responsible for the production of melanin through a process
known as melanogenesis. Melanogenesis, which is controlled by MITF,
produces two forms of melanin: Eumelanin and pheomelanin. The
transcription factor MITF, which is regarded as the key player in
the production of melanin, performs two opposing roles in the
development of melanoma. In contrast to low expression, which was
associated with dedifferentiated and invasive phenotypes, high
expression of MITF was associated with a proliferative and
differentiated phenotype. Melanin pigment also has a dual role in
the development of melanoma in addition to MITF. Melanin has the
ability to both hinder melanoma treatment success while also
slowing the progression of the disease. Studies that report the
antimetastatic effects of melanoma suggest that pigmentation
decreases melanoma metastasis by interfering with different aspects
of the metastatic cascade. However, this reduction of melanoma
progression is dependent on numerous factors such as structure,
stability, and ratio of pheomelanin to eumelanin. Increased
oxidative stress via pheomelanin can induce cellular damage as well
as enhance metastasis during the late stages of melanoma.
Nevertheless, oxidative stress is necessary to impair cancer cell
survival during the early stage of melanoma development and growth.
In summary, there is more evidence supporting the claim that
melanin pigment slows the progression of melanoma. Thus, promoting
melanogenesis and introducing melanin in vitro and in
vivo may possibly reduce the aggressiveness of metastasis.
Natural products and compounds that enhance melanogenesis have been
reported to reduce metastasis. Therefore, these compounds should be
further explored as potential therapeutic interventions against
melanoma progression (Fig. 6).
Acknowledgements
Not applicable.
Funding
This review was supported by the Fundamental Research Grant
Scheme (FRGS) of the Ministry of Education (MOE) of Malaysia (grant
no. 5540566) and by the Putra Grant from the University of Putra
Malaysia (grant no. GP-IPM/2018/9666300).
Availability of data and materials
Not applicable.
Authors' contributions
AS and JCWL conceived the review. AS performed the
literature review and wrote the original draft. JCWL, SRS and JS
reviewed and edited the final manuscript. JCWL supervised and
validated content relevant to melanogenesis and metastasis. SRS
supervised and validated content relevant to the chemistry of
melanin. HSN validated and helped in drafting the figures. JS
supervised and validated content relevant to possible
pharmacological interventions through melanogenesis. All authors
read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Ali Z, Yousaf N and Larkin J: Melanoma
epidemiology, biology and prognosis. EJC Suppl. 11:81–91. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y and Sheikh MS: Melanoma: Molecular
pathogenesis and therapeutic management. Mol Cell Pharmacol.
6:2282014.PubMed/NCBI
|
|
4
|
Shore R: Radiation-induced skin cancer in
humans. Med Pediatr Oncol. 36:549–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu Q, Fung AHY, Xu ML, Poon K, Liu EY,
Kong XP, Yao P, Xiong QP, Dong TTX and Tsim KWK:
Microphthalmia-associated transcription factor up-regulates
acetylcholinesterase expression during melanogenesis of murine
melanoma cells. J Biol Chem. 293:14417–14428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Netcharoensirisuk P, Abrahamian C, Tang R,
Chen CC, Rosato AS, Beyers W, Chao YK, Filippini A, Di Pietro S,
Bartel K, et al: Flavonoids increase melanin production and reduce
proliferation, migration and invasion of melanoma cells by blocking
endolysosomal/melanosomal TPC2. Sci Rep. 11:85152021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Amore A, Hanbashi AA, Di Agostino S,
Palombi F, Sacconi A, Voruganti A, Taggi M, Canipari R, Blandino G,
Parrington J and Filippini A: Loss of two-pore channel 2 (TPC2)
expression increases the metastatic traits of melanoma cells by a
mechanism involving the Hippo signalling pathway and store-operated
calcium entry. Cancers (Basel). 12:23912020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim JCW, Kwan YP, Tan MS, Teo MHY, Chiba
S, Wahli W and Wang X: The role of PPARβ/δ in melanoma metastasis.
Int J Mol Sci. 19:28602018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Huang L, Quan J and Xiang D:
TRIM14 regulates melanoma malignancy via PTEN/PI3K/AKT and STAT3
pathways. Aging (Albany NY). 13:13225–13238. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slominski RM, Zmijewski MA and Slominski
AT: The role of melanin pigment in melanoma. Exp Dermatol.
24:258–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martin T, Ye L, Sanders AJ, Lane J and
Jiang WG: Cancer invasion and metastasis: Molecular and cellular
perspective. In Madame Curie Bioscience Database [Internet].
Jandial R: Landes Bioscience: Austin; TX, USA: pp. 2000–2013.
2013
|
|
12
|
Pachmayr E, Treese C and Stein U:
Underlying mechanisms for distant metastasis-molecular biology.
Visc Med. 33:11–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Damsky WE, Rosenbaum LE and Bosenberg M:
Decoding melanoma metastasis. Cancers (Basel). 3:126–163. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang E, Yan T, Xu Z and Shang Z: Tumour
microenvironment and cell fusion. Biomed Res Int. 2019:50135922019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernandes C, Prabhu P, Juvale K, Suares D
and Yc M: Cancer cell fusion: A potential target to tackle
drug-resistant and metastatic cancer cells. Drug Discov Today.
24:1836–1844. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HF, Xiang W, Xue BZ, Wang YH, Yi DY,
Jiang XB, Zhao HY and Fu P: Cell fusion in cancer hallmarks:
Current research status and future indications (Review). Oncol
Lett. 22:5302021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu MH, Gao X, Luo D, Zhou XD, Xiong W and
Liu GX: EMT and acquisition of stem cell-like properties are
involved in spontaneous formation of tumorigenic hybrids between
lung cancer and bone marrow-derived mesenchymal stem cells. PLoS
One. 9:e878932014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Imodoye SO, Adedokun KA, Muhammed AO,
Bello IO, Muhibi MA, Oduola T and Oyenike MA: Understanding the
complex milieu of epithelial-mesenchymal transition in cancer
metastasis: New insight into the roles of transcription factors.
Front Oncol. 11:7628172021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parri M and Chiarugi P: Rac and Rho
GTPases in cancer cell motility control. Cell Commun Signal.
8:232010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gener P, Seras-Franzoso J, Callejo PG,
Andrade F, Rafael D, Martínez F, Montero S, Arango D, Sayós J,
Abasolo I and Schwartz S Jr: Dynamism, sensitivity, and
consequences of mesenchymal and stem-like phenotype of cancer
cells. Stem Cells Int. 2018:45164542018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ribatti D, Tamma R and Annese T:
Epithelial-mesenchymal transition in cancer: A historical overview.
Transl Oncol. 13:1007732020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vandyck HH, Hillen LM, Bosisio FM, van den
Oord J, Zur Hausen A and Winnepenninckx V: Rethinking the biology
of metastatic melanoma: A holistic approach. Cancer Metastasis Rev.
40:603–624. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dilshat R, Fock V, Kenny C, Gerritsen I,
Lasseur RM, Travnickova J, Eichhoff OM, Cerny P, Möller K,
Sigurbjörnsdóttir S, et al: MITF reprograms the extracellular
matrix and focal adhesion in melanoma. Elife. 10:e630932021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen T, Zhao B, Liu Y, Wang R, Yang Y,
Yang L and Dong C: MITF-M regulates melanogenesis in mouse
melanocytes. J Dermatol Sci. 90:253–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shakhova O, Zingg D, Schaefer SM, Hari L,
Civenni G, Blunschi J, Claudinot S, Okoniewski M, Beermann F,
Mihic-Probst D, et al: Sox10 promotes the formation and maintenance
of giant congenital naevi and melanoma. Nat Cell Biol. 14:882–890.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yun CY, Mi Ko S, Pyo Choi Y, Kim BJ, Lee
J, Mun Kim J, Kim JY, Song JY, Kim SH, Hwang BY, et al: α-Viniferin
improves facial hyperpigmentation via accelerating feedback
termination of cAMP/PKA-signaled phosphorylation circuit in
facultative melanogenesis. Theranostics. 8:2031–2043. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi MH, Jo HG, Yang JH, Ki SH and Shin
HJ: Antioxidative and anti-melanogenic activities of bamboo stems
(phyllostachys nigra variety henosis) via PKA/CREB-mediated MITF
downregulation in B16F10 melanoma cells. Int J Mol Sci. 19:4092018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kaur A, Webster MR and Weeraratna AT: In
the Wnt-er of life: Wnt signalling in melanoma and ageing. Br J
Cancer. 115:1273–1279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Goding CR and Arnheiter H: MITF-the first
25 years. Genes Dev. 33:983–1007. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Javelaud D, Alexaki VI, Pierrat MJ, Hoek
KS, Dennler S, Van Kempen L, Bertolotto C, Ballotti R, Saule S,
Delmas V and Mauviel A: GLI2 and M-MITF transcription factors
control exclusive gene expression programs and inversely regulate
invasion in human melanoma cells. Pigment Cell Melanoma Res.
24:932–943. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fane ME, Chhabra Y, Smith AG and Sturm RA:
BRN2, a POUerful driver of melanoma phenotype switching and
metastasis. Pigment Cell Melanoma Res. 32:9–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kawakami A and Fisher DE: The master role
of microphthalmia-associated transcription factor in melanocyte and
melanoma biology. Lab Invest. 97:649–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghiorzo P, Pastorino L, Queirolo P, Bruno
W, Tibiletti MG, Nasti S and Andreotti V; Genoa Pancreatic Cancer
Study Group, . Paillerets BB and Bianchi Scarrà G: Prevalence of
the E318K MITF germline mutation in Italian melanoma patients:
Associations with histological subtypes and family cancer history.
Pigment Cell Melanoma Res. 26:259–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Primot A, Mogha A, Corre S, Roberts K,
Debbache J, Adamski H, Dreno B, Khammari A, Lesimple T, Mereau A,
et al: ERK-regulated differential expression of the Mitf 6a/b
splicing isoforms in melanoma. Pigment Cell Melanoma Res.
23:93–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ennen M, Keime C, Gambi G, Kieny A,
Coassolo S, Thibault-Carpentier C, Margerin-Schaller F, Davidson G,
Vagne C, Lipsker D and Davidson I: MITF-high and MITF-low cells and
a novel subpopulation expressing genes of both cell states
contribute to intra- and intertumoral heterogeneity of primary
melanoma. Clin Cancer Res. 23:7097–7107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lister JA, Capper A, Zeng Z, Mathers ME,
Richardson J, Paranthaman K, Jackson IJ and Elizabeth Patton E: A
conditional zebrafish MITF mutation reveals MITF levels are
critical for melanoma promotion vs regression in vivo. J Invest
Dermatol. 134:133–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bianchi-Smiraglia A, Bagati A, Fink EE,
Moparthy S, Wawrzyniak JA, Marvin EK, Battaglia S, Jowdy P,
Kolesnikova M, Foley CE, et al: Microphthalmia-associated
transcription factor suppresses invasion by reducing intracellular
GTP pools. Oncogene. 36:84–96. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Simmons JL, Pierce CJ, Al-Ejeh F and Boyle
GM: MITF and BRN2 contribute to metastatic growth after
dissemination of melanoma. Sci Rep. 7:109092017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Swoboda A, Soukup R, Eckel O, Kinslechner
K, Wingelhofer B, Schörghofer D, Sternberg C, Pham HTT, Vallianou
M, Horvath J, et al: STAT3 promotes melanoma metastasis by
CEBP-induced repression of the MITF pathway. Oncogene.
40:1091–1105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
D'Alba L and Shawkey MD: Melanosomes:
Biogenesis, properties, and evolution of an ancient organelle.
Physiol Rev. 99:1–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Slominski A, Zmijewski MA and Pawelek J:
L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators
of melanocyte functions. Pigment Cell Melanoma Res. 25:14–27. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
D'Mello SA, Finlay GJ, Baguley BC and
Askarian-Amiri ME: Signaling pathways in melanogenesis. Int J Mol
Sci. 17:11442016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morgan AM, Lo J and Fisher DE: How does
pheomelanin synthesis contribute to melanomagenesis?: Two distinct
mechanisms could explain the carcinogenicity of pheomelanin
synthesis. Bioessays. 35:672–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Del Bino S, Ito S, Sok J, Nakanishi Y,
Bastien P, Wakamatsu K and Bernerd F: Chemical analysis of
constitutive pigmentation of human epidermis reveals constant
eumelanin to pheomelanin ratio. Pigment Cell Melanoma Res.
28:707–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hida T, Kamiya T, Kawakami A, Ogino J,
Sohma H, Uhara H and Jimbow K: Elucidation of melanogenesis cascade
for identifying pathophysiology and therapeutic approach of
pigmentary disorders and melanoma. Int J Mol Sci. 21:61292020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wakamatsu K, Nagao A, Watanabe M, Nakao K
and Ito S: Pheomelanogenesis is promoted at a weakly acidic pH.
Pigment Cell Melanoma Res. 30:372–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carletti G, Nervo G and Cattivelli L:
Flavonoids and melanins: A common strategy across two kingdoms. Int
J Biol Sci. 10:1159–1170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ju KY, Degan S, Fischer MC, Zhou KC, Jia
X, Yu J and Warren WS: Unraveling the molecular nature of melanin
changes in metastatic cancer. J Biomed Opt. 24:1–13. 2019.
View Article : Google Scholar
|
|
50
|
Premi S: Role of melanin chemiexcitation
in melanoma progression and drug resistance. Front Oncol.
10:13052020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sarna M, Zadlo A, Czuba-Pelech B and
Urbanska K: Nanomechanical phenotype of melanoma cells depends
solely on the amount of endogenous pigment in the cells. Int J Mol
Sci. 19:6072018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sarna M, Krzykawska-Serda M, Jakubowska M,
Zadlo A and Urbanska K: Melanin presence inhibits melanoma cell
spread in mice in a unique mechanical fashion. Sci Rep. 9:92802019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fürst K, Steder M, Logotheti S, Angerilli
A, Spitschak A, Marquardt S, Schumacher T, Engelmann D,
Herchenröder O, Rupp RAW and Pützer BM: DNp73-induced degradation
of tyrosinase links depigmentation with EMT-driven melanoma
progression. Cancer Lett. 442:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Brouwer NJ, Marinkovic M, Luyten GP,
Shields CL and Jager MJ: Lack of tumour pigmentation in
conjunctival melanoma is associated with light iris colour and
worse prognosis. Br J Ophthalmol. 103:332–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brouwer NJ, Marinkovic M, Luyten GPM,
Shields CL and Jager MJ: Pigmentation of conjunctival melanoma
recurrences and outcome. Graefes Arch Clin Exp Ophthalmol.
257:1783–1788. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huayllani MT, Boczar D, Saleem HY,
Spaulding AC, Bagaria SP, Lu X, Kassis S, Perdikis G and Forte AJ:
Amelanotic melanoma of the head and neck: Analysis of tumor
characteristics from the national cancer database. Int J Dermatol.
60:347–351. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Xie C, Pan Y, McLean C, Mar V, Wolfe R and
Kelly JW: Scalp melanoma: Distinctive high risk clinical and
histological features. Australas J Dermatol. 58:181–188. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu G, Wang Y, Fei F, Wang X, Li C, Liu K,
Du J, Cao Y and Zhang S: Clinical characteristics and preliminary
morphological observation of 47 cases of primary anorectal
malignant melanomas. Melanoma Res. 28:592–599. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Fernández-Cortés M, Delgado-Bellido D and
Oliver FJ: Vasculogenic mimicry: Become an endothelial cell ‘but
not so much’. Front Oncol. 9:8032019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nasti TH and Timares L: MC1R, eumelanin
and pheomelanin: Their role in determining the susceptibility to
skin cancer. Photochem Photobiol. 91:188–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Galván I, Jorge A and García-Gil M:
Pheomelanin molecular vibration is associated with mitochondrial
ROS production in melanocytes and systemic oxidative stress and
damage. Integr Biol (Camb). 9:751–761. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Arai E, Hasegawa M, Wakamatsu K and Ito S:
Males with more pheomelanin have a lower oxidative balance in Asian
barn swallows (Hirundo rustica gutturalis). Zoolog Sci.
35:505–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rodríguez-Martínez S, Wakamatsu K and
Galván I: Increase of the benzothiazole moiety content of
pheomelanin pigment after endogenous free radical inducement. Dyes
Pigm. 180:1085162020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Panzella L, Leone L, Greco G, Vitiello G,
D'Errico G, Napolitano A and d'Ischia M: Red human hair pheomelanin
is a potent pro-oxidant mediating UV-independent contributory
mechanisms of melanomagenesis. Pigment Cell Melanoma Res.
27:244–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mitra D, Luo X, Morgan A, Wang J, Hoang
MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, et al: An
ultraviolet-radiation-independent pathway to melanoma
carcinogenesis in the red hair/fair skin background. Nature.
491:449–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Piskounova E, Agathocleous M, Murphy MM,
Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ
and Morrison SJ: Oxidative stress inhibits distant metastasis by
human melanoma cells. Nature. 527:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Le Gal K, Ibrahim MX, Wiel C, Sayin VI,
Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindahl P, Nilsson J
and Bergo MO: Antioxidants can increase melanoma metastasis in
mice. Sci Transl Med. 7:308re82015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang HC, Yen H, Lu JY, Chang TM and Hii
CH: Theophylline enhances melanogenesis in B16F10 murine melanoma
cells through the activation of the MEK 1/2, and Wnt/β-catenin
signaling pathways. Food Chem Toxicol. 137:1111652020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Cordella M, Tabolacci C, Senatore C, Rossi
S, Mueller S, Lintas C, Eramo A, D'Arcangelo D, Valitutti S,
Facchiano A and Facchiano F: Theophylline induces differentiation
and modulates cytoskeleton dynamics and cytokines secretion in
human melanoma-initiating cells. Life Sci. 230:121–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ye Y, Wang C, Zhang X, Hu Q, Zhang Y, Liu
Q, Wen D, Milligan J, Bellotti A, Huang L, et al: A
melanin-mediated cancer immunotherapy patch. Sci Immunol.
2:eaan56922017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hartman ML and Czyz M: MITF in melanoma:
Mechanisms behind its expression and activity. Cell Mol Life Sci.
72:1249–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huijser A, Pezzella A and Sundström V:
Functionality of epidermal melanin pigments: Current knowledge on
UV-dissipative mechanisms and research perspectives. Phys Chem Chem
Phys. 13:9119–9127. 2011. View Article : Google Scholar : PubMed/NCBI
|