Introduction
Acute Myeloid Leukaemia (AML) constitutes 20% of all
paediatric leukaemia and is responsible for substantial mortality.
Despite progress made over the past years in diagnosis, risk
stratification and treatment of AML, survival remains suboptimal
with a success rate of 60–70% (1–3),
with relapse being the leading cause of death. Risk stratification
in patients with AML is of paramount importance in order to deliver
tailored therapy, enabling treatment intensification in high-risk
patients (for example, allogenic stem cell transplantation in
high-risk in first complete remission). Moreover, novel therapies
are needed in order to further improve prognosis in these patients.
Among prognostic factors, cytogenetic and molecular abnormalities,
together with Minimal Residual Disease and treatment response, play
a pivotal role in defining AML prognosis and treatment (4,5).
Rearrangements involving Histone-lysine N-methyltransferase 2A
(KMT2A) gene, formerly known as myeloid/lymphoid or
mixed-lineage leukaemia 1 (MLL1) gene, as well as Fms like
tyrosine kinase 3 (FLT3) internal tandem duplications
(FLT3-ITD) represent useful prognostic factors and,
possibly, therapeutic targets. Indeed, they still define an
intermediate to high risk AML (1–3,6). In
particular, FLT3-ITD mutations are associated with higher
risk of relapse and dismal prognosis (1,3,7),
whereas MLL-rearranged AML is an heterogeneous group of
diseases with more than 100 rearrangements being described and with
different outcome largely dependent on the fusion partner (8).
FLT3-ITD and MLL rearrangements have
been functionally linked to dysregulation of expression of
microRNAs (miRNAs or miRs) (9).
miRs are small non-coding RNA molecules (~18-22 nucleotides long),
involved in several cellular processes (10,11).
In cancer, they are implicated both in promoting carcinogenesis
(oncomiRs) and in suppressing tumour transformation (12). Their role in AML have been
extensively investigated over the past years reviling promising
data on diagnosis, prognostic stratification and, possibly,
treatment in AML patients (13–15).
Despite extensive research performed to understand the role of miRs
in AML, the majority of studies are focused on adult patients,
while a precise characterization of miRNAs expression in paediatric
AML is less documented. Moreover, data on the role of different
miRs are conflicting because of the variability of genetic
abnormalities found in AML (16).
In the present study, it was aimed to identify AML specific miR
signatures in a cohort of patients harbouring molecular lesions
(FLT3-ITD and MLL rearrangement), studying the
expression of distinct miR sets in relation to relapse risk.
Epigenetic networks, including histone modification
mechanisms, are involved in the regulation of both miRs expression
and function. An increasing interest in the field of cancer
therapeutic drugs is focused on small molecular compounds targeting
epigenetic regulation (17).
Bromodomain and extra-terminal domain family of
proteins (BET) and histone acetyl transferase (HAT) inhibitors
proteins are the best characterized ones. BET are effective in
preventing Bromodomain Containing protein 4 (BRD4) associated
transcription of several oncogenes, reducing proliferation and
increasing apoptosis in AML (18–22).
BRD4 is a member of BET family proteins, characterized by the
presence of functional structures called bromodomains which bind
specific acetylated residues on histone tails to modulate
transcription of target gene (23–25),
enhancing transcription of several oncogenes (26).
Among BRD4 inhibitors, JQ1 was used as its activity
on modulation of miRs was previously described (27). JQ1
[(S)-tert-butyl-2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate]
is a small molecule belonging to the thienotriazolodiazepine group
and it prevents the binding of BRD4 to acetylated residues on
histone H3 tails, particularly H3AcK14 (20,28–30).
Furthermore, among HAT inhibitors, curcumin, a
natural compound extracted from the root of Curcuma Longa
has been shown to inhibit acetylation of histone tails, blocking
the activity of the HAT p300 even causing its proteasomal
degradation (31). This results in
a global decrease of acetylation on histone tails and a consequent
modulation of gene transcription (32,33).
BRD4 inhibitors have exhibited only moderate results
in clinics and novel ways to increase their antitumour activity are
needed (34). It was therefore
hypothesized that the association with curcumin would increase JQ1
efficacy. The BET family are ‘readers’ of chromatin acetylation
whereas HAT could be classified as a ‘writer’ of histone
acetylation (34) thus offering a
theoretical basis for JQ1 and curcumin synergic activity.
Moreover, it was previously showed that also JQ1,
like curcumin, blocks p300-mediated acetylation (25,35).
Thus, it was investigated in vitro whether a combination of
BRD4 and HAT inhibitors have an effect in terms of modulation of
miRs and antitumour effects on different AML cell-lines harbouring
mutations resembling those present in our patients.
Materials and methods
Samples of patients
A total of 23 patients aged 1 to 18 years, who
received a diagnosis of AML harbouring FLT3-ITD or
MLL rearrangement (Table
SI). Although not mutually exclusive, these rearrangements are
not frequently found together. In the present study, none of the
patients had both the rearrangements.
Bone marrow (BM) samples were collected from January
1st 2010 to December 31st 2016 at Bambino Gesù Children's Hospital
in Rome and at Department of Paediatrics, University in Padua, at
diagnosis and at disease recurrence from the 13 patients who
underwent relapse (REL-D and REL-R groups, respectively) and at
diagnosis from the 10 patients who did not display relapse (NREL
group).
A total of 8 frozen age-matched BM samples from
healthy children (HD) (unused aliquots from healthy BM donors) were
retrieved from the tissue bank at Bambino Gesù Children's Hospital
as a control population. Informed consent was obtained from either
parents or legal guardians according to the Declaration of Helsinki
(2008). The present study was approved by the Institutional Review
Boards of Bambino Gesù Children's Hospital (Rome, Italy).
CD34+ cells isolation
Mononuclear cells were isolated by density gradient
centrifugation at 400 g and 20°C for 30 min, diluted in 90% fetal
bovine serum (FBS) plus 10% dimethyl sulfoxide (DMSO) and stored in
liquid nitrogen. CD34+ cells from BM samples of three
patients randomly selected in our cohort, were magnetically
separated using MACS CD34+ microbead kit (Miltenyi
Biotech GmbH). In particular, the molecular analysis of these
patients revealed a FLT3-ITD with normal karyotype and two
MLL rearrangements [t(9;11) and t(10;11)]. The identity of CD34
cells was validated by flow cytometry using FACSCantoII equipped
with FACSDiva 6.1 CellQuest software (Becton, Dickinson and
Company) using 20 µl of CD34 PerCP antibody (cat. no 340666; BD
Biosciences) with an incubation of 30 min at 4°C.
RNA extraction and miR profiling
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and purified
using RNA Cleanup and Concentration kit according to the
manufacturer's protocol (Norgen Biotek Corp.). RNA quantification
was performed using Nanodrop 2000 at 260 nm wavelength (Thermo
Fisher Scientific, Inc.) and RNA integrity and purity was assessed
with RNA Bioanalyzer kit according to the manufacturer's protocol
(Agilent Technologies, Inc.). miR expression profile was performed
using the nCounter Human v2 miRNA Expression Assay and nCounter
Nanostring platform according to manufacturer's protocol
(NanoString Technologies).
Cell culture methods
AML cell lines THP-1 (MLL-MLLT3; MLL-AF9),
MOLM-13 (MLL-MLLT3; MLL-AF9; FLT3-ITD) and MV-4-11
(MLL-AFF1; MLL-AF4; FLT3-ITD) were obtained from DSMZ and
cultured at 37°C using RPMI-1640 medium (Euroclone SpA)
supplemented with 10% FBS (Thermo Fisher Scientific, Inc.) and 1%
Penicillin-Streptomycin (Thermo Fisher Scientific, Inc.).
Mycoplasma testing was performed for the cell lines used. Cell
lines were treated with 250 nM JQ1 and 10 µM curcumin singularly
alone or in association, for 48 h. JQ1 and Curcumin were obtained
from Selleck Chemicals and resuspended in DMSO, following the
manufacturer's protocol.
Western blot analysis
Whole-cell lysates were prepared with RIPA lysis
buffer (Thermo Fisher Scientific, Inc.) supplemented with protease
and phosphatase inhibitors (Thermo Fisher Scientific, Inc.). Cells
were lysed by sonication, incubated for 30 min at 4°C and then
obtained cells lysates were centrifuged at 13,000 × g for 20 min at
4°C. The protein concentration of the resulting supernatant was
estimated by BCA assay. Then, 40 µg of sample was separated on
Criterion TGX Precast Gels 4–20% (BioRad Laboratories, Inc.) and
transferred to Hybond ECL nitrocellulose membranes (Amersham;
Cytiva). Membranes were blocked at room temperature for 1 h in 5%
non-fat milk in Tris buffered saline and 0,05% Tween-20 (TBS-T).
Membranes were incubated at 4°C overnight with rabbit polyclonal
anti-human CDKN1B (1:500; cat. no. sc-528; Santa Cruz
Biotechnology, Inc.) and 1 h at room temperature with rabbit
monoclonal anti-human GAPDH (1:1,000; cat. no. D16H11; Cell
Signaling Technology, Inc.) primary antibodies. After incubation
they were washed three times in TBS-T, then incubated with
HRP-labelled goat anti-rabbit (1:5,000; cat. no. sc-2004) and goat
anti-mouse (1:5,000; cat. no. sc-2005; both from Santa Cruz
Biotechnology, Inc.) IgG secondary antibodies, respectively at room
temperature for 1 h. Subsequently, they were washed an additional
three times with TBS-T and then developed with ECL reagent (Western
Lightning Plus; PerkinElmer, Inc.).
miR quantitative (q)PCR
Expression levels of hsa-miR-221-5p, hsa-miR-222-5p
and U6 were measured using TaqMan microRNA assays (cat. nos.
000524, 002276 and 001973; Thermo Fisher Scientific, Inc.). Reverse
transcription (RT) primer, preformulated forward/reverse primer and
MGB probes for each assay were provided by the manufacturer. The
TaqMan MiR Reverse Transcription kit was used for cDNA synthesis
from 10 ng total RNA template according to the manufacturer's
protocol. QuantStudio 12K Flex Real Time PCR System (Thermo Fisher
Scientific, Inc.) was used for qPCR reactions with the following
conditions: Enzyme activation 95°C for 20 sec and 40 cycles of
denaturation (95°C for 1 sec) and annealing/extension (60°C for 20
sec) steps. miRNA expression data were normalized to U6 using the
2−ΔΔCq (36) method by
the Relative Quantification module of Thermo Fisher Cloud Data
Analysis Apps. At least two independent amplifications were
performed for each probe on triplicate samples.
Apoptosis assay
Following treatment with 250 nM BRD4 and 10 µM
curcumin, cells were washed twice with ice cold PBS and stained for
15 min at room temperature in calcium-binding buffer with
PE-conjugated Annexin V (AnnV) and 7-Aminoactinomycin D (7-AAD)
using the AnnV apoptosis detection kit (BD Pharmingen; BD
Biosciences) according to the manufacturer's recommendations.
Samples were analysed within 1 h by a fluorescence-activated cell
sorting using a FACSCantoII equipped with FACSDiva 6.1 CellQuest
software (Becton, Dickinson and Company).
Bioinformatics and statistical
analyses
MicroRNA profiling normalization was performed using
the nSolver Analysis Software (NanoString Technologies) as
recommended by NanoString. P-values were calculated using the LIMMA
(v.3.46.0) package (37) from the
Bioconductor R (v.4.0.5) project. The P-values were adjusted for
multiple testing using the Benjamini and Hochberg method to control
the False Discovery Rate. An independent normalization phase for
each comparison was performed, considering only the samples present
in such comparison (for example, NREL vs. HD). Then, the miRNA
expression of the HD group was specifically normalized in the
comparisons in which the HD group was taken into consideration.
Validated targets of miRs were reported in Table I according to miRWalk 2.0 online
software analysis (http://mirwalk.umm.uni-heidelberg.de/). One-way ANOVA
and post hoc comparison using Tukey's HSD Post Hoc or Dunnett's
test were performed using SPSS software v19 (IBM Corp.) and
GraphPad Prism v6 (GraphPad Software, Inc.). The heatmap was
generated by using GenePattern tool (38), with Euclidean and Spearman
correlation distances in columns and rows, respectively. Venn
diagrams were created using web tool (39).
 | Table I.Molecular based miR expression
signature. |
Table I.
Molecular based miR expression
signature.
| FLT3-ITH vs.
HD | Accession
number | FC | Adjusted
P-value |
|---|
| hsa-miR-10a-5p | MIMAT0000253 | 24.38 |
2×10−5 |
| hsa-miR-451a | MIMAT0001631 | 17.06 |
1×10−3 |
|
hsa-miR-196b-5p | MIMAT0001080 | 4.31 |
5×10−4 |
| hsa-miR-34a-5p | MIMAT0000255 | 3.38 |
4×10−3 |
| hsa-let-7b-5p | MIMAT0000063 | 2.49 |
1×10−2 |
| hsa-miR-29c-3p | MIMAT0000681 | 2.12 |
1×10−2 |
|
hsa-miR-520f-3p | MIMAT0002830 | −2.11 |
4×10−2 |
|
hsa-miR-200c-3p | MIMAT0000617 | −2.16 |
4×10−2 |
| hsa-miR-421 | MIMAT0003339 | −2.34 |
2×10−2 |
|
hsa-miR-6724-5p | MIMAT0025856 | −2.39 |
1×10−2 |
|
hsa-miR-151a-3p | MIMAT0000757 | −2.45 |
1×10−2 |
|
hsa-miR-4755-5p | MIMAT0019895 | −2.45 |
3×10−2 |
| hsa-miR-365a-3p
+ | MIMAT0000710 | −2.52 |
2×10−2 |
|
hsa-miR-365b-3p |
|
|
|
| hsa-miR-520d-5p
+ | MIMAT0002855 | −2.73 |
4×10−3 |
| hsa-miR-527 +
hsa-miR-518a-5p |
|
|
|
| hsa-miR-574-5p | MIMAT0004795 | −2.75 |
1×10−2 |
| hsa-miR-342-3p | MIMAT0000753 | −3.99 |
4×10−3 |
|
| FLT3-ITD vs.
MLL | Accession
number | FC | Adjusted
P-value |
|
| hsa-miR-10a-5p | MIMAT0000253 | 34.87 |
1.44×10−7 |
| hsa-miR-99a-5p | MIMAT0000097 | 2.87 |
2.24×10−2 |
| hsa-miR-9-5p | MIMAT0000441 | −7.89 |
1.38×10−3 |
|
| MLL vs.
HD | Accession
number | FC | Adjusted
P-value |
|
| hsa-miR-9-5p | MIMAT0000441 | 10.75 |
9.33×10−3 |
| hsa-miR-34a-5p | MIMAT0000255 | 5.50 |
8.78×10−5 |
|
hsa-miR-196b-5p | MIMAT0001080 | 4.23 |
1.55×10−2 |
| hsa-miR-192-5p | MIMAT0000222 | −2.73 |
3.92×10−2 |
CD34+ cell culture
CD34+ cells were cultured using MethoCult
H4434 methylcellulose medium (Stem Cell Technologies) supplemented
with 250 nM JQ1 and 10 µM curcumin.
Results
NanoString miR profiling reveals
different expression signatures based on patient clinical and
molecular features
A miR profiling analysis was first performed to
verify whether the two distinct molecular subsets of the cohort of
our patients (MLL rearranged and FLT3-ITD) showed
different miR expression fingerprints. Comparing both MLL
rearranged and FLT3-ITD sets with healthy donors (HDs), 4
and 16 significantly deregulated miRs were identified, respectively
(Table I). miR-196b-5p and
miR-34a-5p resulted upregulated in both AML sets. The comparison
between the two molecular AML sets showed 3 differentially
regulated miRs with miR-10a-5p and miR-99a-5p significantly higher
in FLT3-ITD and miR-9a-5p with enhanced expression in
MLL-rearranged sets (Table
I). Other miRs such as miR-451a, miR-520d-5p/527/518a-5p,
miR-574-5p and miR-192-5p were uniquely dysregulated in
FLT3-ITD or MLL sets with respect to HDs (Table I). A miR expression profiling
analysis was then performed based onto clinical outcomes of
patients to identify those miRs associated with relapse.
The hierarchical clustering results of miRNAs
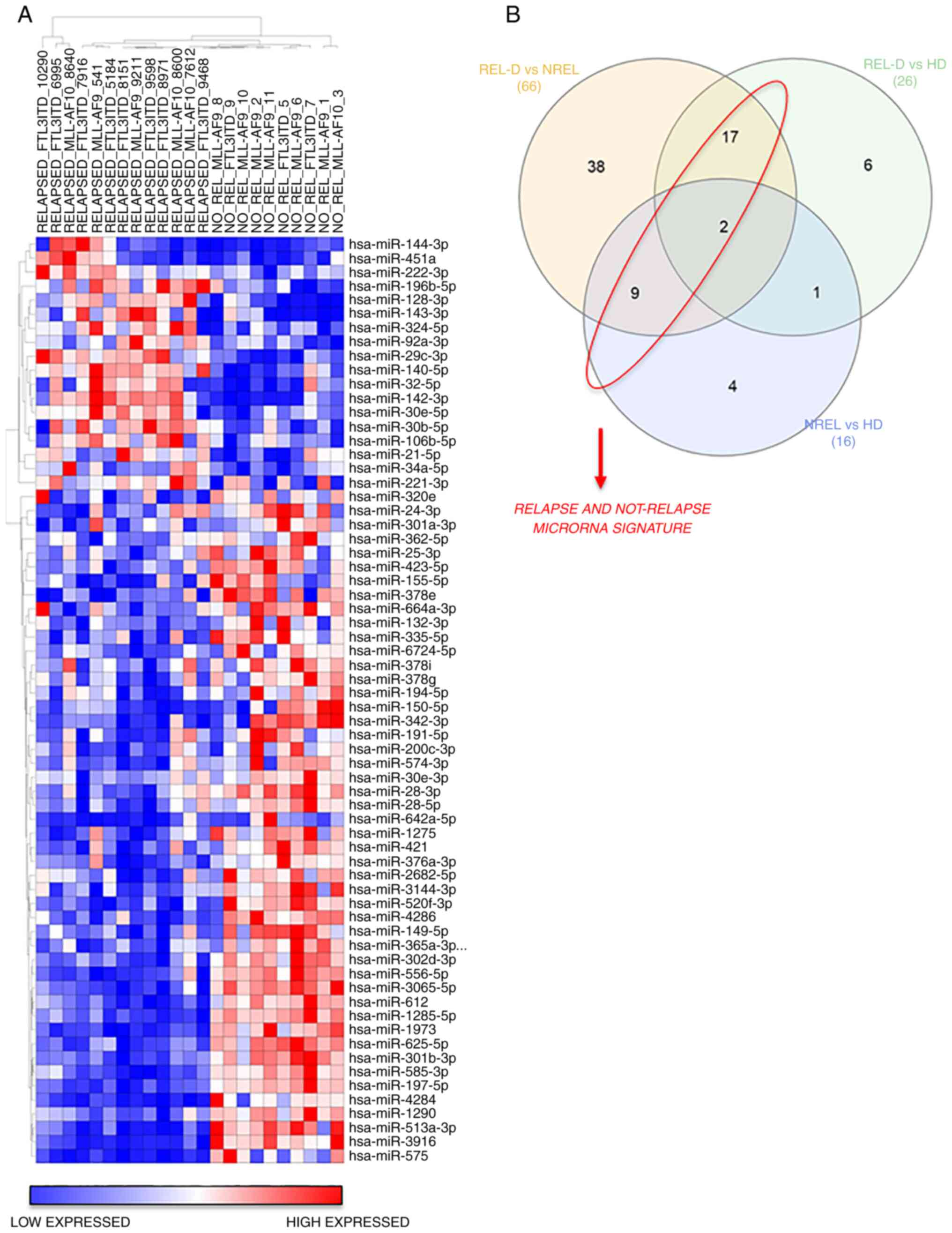
expression of REL-D vs. NREL groups is revealed in Fig. 1. A total of 18 miRs were broadly
upregulated in the REL-D patients set with respect to the NREL set
and 48 miRs displayed the opposite trend (Fig. 1A and Table II). To further identify and refine
a signature that was associated with relapse, among these
differentially expressed 66 miRs, only those shared in the REL-D
vs. HD and NREL vs. HD comparison were subsequently considered
(Fig. 1B and Table II). The resulted signature
associated to relapse and not-relapse is listed in Table III. Validated targets of miRs
were identified using miRWalk 2.0 online software (40) and are reported in Table III. CDKN1B, a key regulator of
cell cycle which has been previously reported to be associated with
prognosis in AML, resulted as primary target of miR-221-3p and
miR-222-3p (41). To evaluate if
the signature of miRs associated with relapse at diagnosis was
maintained over time (if it is present also in the REL-R group),
expression of miRs at diagnosis and at disease recurrence was
compared between those patients who relapsed (REL-R vs. REL-D) and
no significant differences were detected (Table II). Since our goal was to identify
miRs associated to relapse with a significant prognostic value, the
expression level of both miRs resulted overexpressed in REL-D vs.
HD group and REL-D vs. NREL was first evaluated in the AML cells
lines by qPCR (data not shown). High variability was obtained in
the different cell lines. This result prompted the authors to focus
only on hsa-miR-221-3p and hsa-miR-222-3p, presenting the same
trend of expression in all the cell lines, as well as in
vivo in the patients.
 | Table II.Clinical outcome-based miR expression
signature. |
Table II.
Clinical outcome-based miR expression
signature.
| REL-D vs. NREL | Accession
number | FC | Adjusted
P-value |
|---|
| hsa-miR-128-3p | MIMAT0000424 | 8,10 |
4×10−6 |
| hsa-miR-143-3p | MIMAT0000435 | 7,47 |
9×10−4 |
| hsa-miR-142-3p | MIMAT0000434 | 5,63 |
9×10−7 |
| hsa-miR-144-3p | MIMAT0000436 | 3,87 |
4×10−2 |
| hsa-miR-451a | MIMAT0001631 | 3,66 |
3×10−2 |
| hsa-miR-29c-3p | MIMAT0000681 | 3,24 |
4×10−6 |
| hsa-miR-140-5p | MIMAT0000431 | 2,53 |
2×10−5 |
|
hsa-miR-196b-5p | MIMAT0001080 | 2,48 |
7×10−4 |
| hsa-miR-32-5p | MIMAT0000090 | 2,44 |
6×10−3 |
| hsa-miR-221-3p | MIMAT0000278 | 2,04 |
1×10−2 |
| hsa-miR-92a-3p | MIMAT0000092 | 1,93 |
4×10−3 |
| hsa-miR-222-3p | MIMAT0000279 | 1,92 |
5×10−3 |
| hsa-miR-21-5p | MIMAT0000076 | 1,88 |
1×10−2 |
| hsa-miR-324-5p | MIMAT0000761 | 1,84 |
5×10−3 |
| hsa-miR-34a-5p | MIMAT0000255 | 1,77 |
2×10−2 |
| hsa-miR-30b-5p | MIMAT0000420 | 1,67 |
6×10−3 |
| hsa-miR-30e-5p | MIMAT0000692 | 1,67 |
5×10−3 |
|
hsa-miR-106b-5p | MIMAT0000680 | 1,55 |
2×10−2 |
| hsa-miR-30e-3p | MIMAT0000693 | −1,41 |
3×10−2 |
| hsa-miR-378i | MIMAT0019074 | −1,44 |
4×10−2 |
|
hsa-miR-664a-3p | MIMAT0005949 | −1,46 |
2×10−2 |
| hsa-miR-24-3p | MIMAT0000080 | −1,49 |
4×10−2 |
| hsa-miR-25-3p | MIMAT0000081 | −1,54 |
3×10−2 |
|
hsa-miR-2682-5p | MIMAT0013517 | −1,67 |
6×10−2 |
|
hsa-miR-200c-3p | MIMAT0000617 | −1,67 |
4×10−2 |
| hsa-miR-320e | MIMAT0015072 | −1,67 |
5×10−2 |
| hsa-miR-191-5p | MIMAT0000440 | −1,68 |
3×10−2 |
| hsa-miR-423-5p | MIMAT0004748 | −1,69 |
3×10−3 |
| hsa-miR-362-5p | MIMAT0000705 | −1,71 |
2×10−2 |
| hsa-miR-149-5p | MIMAT0000450 | −1,72 |
2×10−3 |
| hsa-miR-194-5p | MIMAT0000460 | −1,75 |
1×10−2 |
|
hsa-miR-6724-5p | MIMAT0025856 | −1,80 |
5×10−3 |
|
hsa-miR-301a-3p | MIMAT0000688 | −1,84 |
3×10−2 |
| hsa-miR-378g | MIMAT0018937 | −1,88 |
5×10−3 |
| hsa-miR-132-3p | MIMAT0000426 | −1,89 |
9×10−3 |
| hsa-miR-28-3p | MIMAT0004502 | −1,97 |
6×10−4 |
| hsa-miR-28-5p | MIMAT0000085 | −2,01 |
9×10−4 |
| hsa-miR-574-3p | MIMAT0003239 | −2,09 |
4×10−3 |
|
hsa-miR-520f-3p | MIMAT0002830 | −2,10 |
5×10−5 |
|
hsa-miR-3144-3p | MIMAT0015015 | −2,11 |
4×10−4 |
| hsa-miR-1290 | MIMAT0005880 | −2,22 |
5×10−5 |
| hsa-miR-421 | MIMAT0003339 | −2,24 |
6×10−4 |
| hsa-miR-1275 | MIMAT0005929 | −2,32 |
2×10−4 |
| hsa-miR-335-5p | MIMAT0000765 | −2,39 |
5×10−4 |
|
hsa-miR-365a/b-3p | MIMAT0000710 | −2,42 |
2×10−4 |
| hsa-miR-625-5p | MIMAT0003294 | −2,67 |
9×10−7 |
| hsa-miR-612 | MIMAT0003280 | −2,70 |
2×10−6 |
|
hsa-miR-302d-3p | MIMAT0000718 | −2,79 |
6×10−6 |
| hsa-miR-150-5p | MIMAT0000451 | −2,84 |
1×10−2 |
|
hsa-miR-301b-3p | MIMAT0004958 | −2,90 |
8×10−7 |
| hsa-miR-155-5p | MIMAT0000646 | −2,95 |
3×10−3 |
| hsa-miR-556-5p | MIMAT0003220 | −3,04 |
8×10−7 |
|
hsa-miR-3065-5p | MIMAT0015066 | −3,07 |
3×10−7 |
|
hsa-miR-376a-3p | MIMAT0000729 | −3,15 |
6×10−4 |
| hsa-miR-342-3p | MIMAT0000753 | −3,16 |
5×10−4 |
| hsa-miR-197-5p | MIMAT0022691 | −3,30 |
1×10−7 |
| hsa-miR-4286 | MIMAT0016916 | −3,30 |
3×10−5 |
| hsa-miR-585-3p | MIMAT0003250 | −3,37 |
8×10−7 |
|
hsa-miR-642a-5p | MIMAT0003312 | −3,41 |
5×10−3 |
|
hsa-miR-513a-3p | MIMAT0004777 | −4,07 |
2×10−7 |
|
hsa-miR-1285-5p | MIMAT0022719 | −4,10 |
1×10−7 |
| hsa-miR-4284 | MIMAT0016915 | −4,82 |
8×10−7 |
| hsa-miR-575 | MIMAT0003240 | −5,55 |
2×10−6 |
| hsa-miR-1973 | MIMAT0009448 | −6,57 |
4×10−8 |
| hsa-miR-3916 | MIMAT0018190 | −6,94 |
9×10−9 |
| hsa-miR-378e | MIMAT0018927 | −9,46 |
5×10−3 |
|
| REL-D vs.
HD | Accession
number | FC | Adjusted
P-value |
|
| hsa-miR-451a | MIMAT0001631 | 10,46 |
1×10−2 |
|
hsa-miR-196b-5p | MIMAT0001080 | 5,70 |
3×10−5 |
| hsa-miR-34a-5p | MIMAT0000255 | 5,29 |
3×10−4 |
| hsa-miR-221-3p | MIMAT0000278 | 2,14 |
2×10−2 |
| hsa-miR-222-3p | MIMAT0000279 | 2,09 |
2×10−2 |
| hsa-miR-29c-3p | MIMAT0000681 | 1,85 |
5×10−2 |
| hsa-miR-941 | MIMAT0004984 | −1,87 |
5×10−2 |
| hsa-miR-194-5p | MIMAT0000460 | −1,94 |
5×10−2 |
|
hsa-miR-200c-3p | MIMAT0000617 | −1,96 |
5×10−2 |
| hsa-miR-192-5p | MIMAT0000222 | −2,01 |
5×10−2 |
| hsa-miR-1290 | MIMAT0005880 | −2,02 |
5×10−2 |
| hsa-miR-612 | MIMAT0003280 | −2,08 |
5×10−2 |
| hsa-miR-575 | MIMAT0003240 | −2,11 |
4×10−2 |
|
hsa-miR-151a-3p | MIMAT0000757 | −2,11 |
4×10−2 |
|
hsa-miR-520f-3p | MIMAT0002830 | −2,11 |
2×10−2 |
| hsa-miR-421 | MIMAT0003339 | −2,16 |
3×10−2 |
|
hsa-miR-6724-5p | MIMAT0025856 | −2,26 |
1×10−2 |
|
hsa-miR-4755-5p | MIMAT0019895 | −2,30 |
3×10−2 |
| hsa-miR-574-5p | MIMAT0004795 | −2,32 |
3×10−2 |
| hsa-miR-363-3p | MIMAT0000707 | −2,38 |
3×10−2 |
|
hsa-miR-1285-5p | MIMAT0022719 | −2,39 |
3×10−2 |
| hsa-miR-4286 | MIMAT0016916 | −2,61 |
2×10−2 |
|
hsa-miR-365a/b-3p | MIMAT0000710 | −3,00 |
3×10−3 |
|
hsa-miR-520d-5p/527/518a-5p | MIMAT0002855 | −3,16 |
7×10−4 |
| hsa-miR-342-3p | MIMAT0000753 | −3,30 |
2×10−2 |
| hsa-miR-150-5p | MIMAT0000451 | −3,96 |
4×10−2 |
|
| NREL vs.
HD | Accession
number | FC | Adjusted
P-value |
|
| hsa-miR-9-5p | MIMAT0000441 | 6,90 |
8×10−3 |
| hsa-miR-1973 | MIMAT0009448 | 3,10 |
5×10−3 |
| hsa-miR-4284 | MIMAT0016915 | 2,82 |
3×10−2 |
| hsa-miR-575 | MIMAT0003240 | 2,72 |
5×10−2 |
| hsa-miR-155-5p | MIMAT0000646 | 2,55 |
3×10−2 |
|
hsa-miR-196b-5p | MIMAT0001080 | 2,37 |
5×10−2 |
| hsa-miR-191-5p | MIMAT0000440 | 2,27 |
2×10−2 |
| hsa-miR-324-5p | MIMAT0000761 | −2,50 |
8×10−3 |
| hsa-miR-140-5p | MIMAT0000431 | −2,53 |
1×10−2 |
|
hsa-miR-450a-5p | MIMAT0001545 | −2,72 |
5×10−2 |
| hsa-miR-142-3p | MIMAT0000434 | −3,35 |
6×10−3 |
| hsa-miR-192-5p | MIMAT0000222 | −4,39 |
6×10−4 |
| hsa-miR-128-3p | MIMAT0000424 | −11,68 |
6×10−4 |
| hsa-miR-422a | MIMAT0001339 | −12,62 |
2×10−3 |
| hsa-miR-143-3p | MIMAT0000435 | −20,19 |
6×10−4 |
| hsa-miR-579-3p | MIMAT0003244 | −20,20 |
8×10−3 |
|
| REL-R vs.
HD | Accession
number | FC | Adjusted
P-value |
|
| hsa-miR-451a | MIMAT0001631 | 18,17 |
1×10−3 |
| hsa-miR-144-3p | MIMAT0000436 | 7,97 |
5×10−3 |
| hsa-miR-34a-5p | MIMAT0000255 | 4,16 |
5×10−3 |
|
hsa-miR-196b-5p | MIMAT0001080 | 4,22 |
1×10−2 |
|
hsa-miR-520d-5p/527/518a-5p | MIMAT0002855 | −2,45 |
3×10−2 |
| hsa-miR-574-5p | MIMAT0004795 | −2,74 |
3×10−2 |
| hsa-miR-342-3p | MIMAT0000753 | −3,27 |
3×10−2 |
|
hsa-miR-6724-5p | MIMAT0025856 | −2,50 |
4×10−2 |
|
| REL-R vs.
REL-D | Accession
number | FC | Adjusted
P-value |
|
| hsa-miR-579-3p | MIMAT0003244 | 3,52 | 1 |
| hsa-miR-143-3p | MIMAT0000435 | 2,90 | 1 |
|
hsa-miR-450a-5p | MIMAT0001545 | 2,30 | 1 |
| hsa-miR-378e | MIMAT0018927 | 2,27 | 1 |
| hsa-miR-145-5p | MIMAT0000437 | 2,19 | 1 |
| hsa-miR-4516 | MIMAT0019053 | 1,61 | 1 |
| hsa-miR-363-3p | MIMAT0000707 | 1,57 | 1 |
|
hsa-miR-365a/b-3p | MIMAT0000710 | 1,46 | 1 |
|
hsa-miR-664a-3p | MIMAT0005949 | −1,35 | 1 |
| hsa-let-7a-5p | MIMAT0000062 | −1,38 | 1 |
| hsa-miR-222-3p | MIMAT0000279 | −1,48 | 1 |
| hsa-let-7c-5p | MIMAT0000064 | −1,59 | 1 |
|
hsa-miR-181a-5p | MIMAT0000256 | −1,60 | 1 |
| hsa-let-7b-5p | MIMAT0000063 | −1,84 | 1 |
| hsa-miR-100-5p | MIMAT0000098 | −1,86 | 1 |
| hsa-miR-1246 | MIMAT0005898 | −2,09 | 1 |
| hsa-miR-9-5p | MIMAT0000441 | −2,13 | 1 |
|
hsa-miR-125b-5p | MIMAT0000423 | −2,20 | 1 |
| hsa-miR-10a-5p | MIMAT0000253 | −2,53 | 1 |
 | Table III.miR expression signatures related to
relapse risk or not-relapse with validated targets. |
Table III.
miR expression signatures related to
relapse risk or not-relapse with validated targets.
| RELAPSE RISK | Validated target
genes | NOT RELAPSE | Validated target
genes |
|---|
| hsa-miR-128-3p |
|
hsa-miR-200c-3p | ZEB1; ERRFI1 |
| hsa-miR-143-3p | KRAS; FNDC3B;
DNMT3A; MAPK7 | hsa-miR-191-5p |
|
| hsa-miR-142-3p |
| hsa-miR-194-5p |
|
| hsa-miR-451a | MIF; ABCB1 |
hsa-miR-6724-5p |
|
| hsa-miR-29c-3p |
|
hsa-miR-520f-3p |
|
| hsa-miR-140-5p | HDAC4; IGFBP5;
VEGFA | hsa-miR-1290 |
|
|
hsa-miR-196b-5p |
| hsa-miR-421 |
|
| hsa-miR-221-3p | BMF; FOS; CDKN1B;
KIT; ESR1; |
hsa-miR-365a/b-3p |
|
|
| CDKN1C; ICAM1;
DDIT4 |
|
|
| hsa-miR-222-3p | CDKN1B; FOS; KIT;
CDKN1C; | hsa-miR-612 |
|
|
| ESR1; MMP1; SOD2;
PPP2R2A |
|
|
| hsa-miR-324-5p | SMO; GLI1 | hsa-miR-150-5p |
|
| hsa-miR-34a-5p | MAP2K1; E2F3;
SIRT1; MYB; CDK6; | hsa-miR-155-5p | JARID2; IKBKE;
ETS1; |
|
| DLL1; CCND1;
NOTCH1; NOTCH2; |
| BACH1; TAB2;
MEIS1; |
|
| JAG1; MET; BCL2;
MYCN; WNT1; |
| MECP2; CEBPB;
FOXO3; |
|
| AXIN2; VEGFA;
MYC |
| FGF7; SOCS1;
INPP5D; |
|
|
|
| AGTR1; SPI1;
CYR61; |
|
|
|
| SMAD2; LDOC1;
MATR3; |
|
|
|
| TM6SF1; RHOA |
|
|
| hsa-miR-342-3p |
|
|
|
| hsa-miR-4286 |
|
|
|
|
hsa-miR-1285-5p |
|
|
|
| hsa-miR-4284 |
|
|
|
| hsa-miR-575 |
|
|
|
| hsa-miR-1973 |
|
Association of JQ1 and curcumin leads
to miR-221-3p and miR-222-3p downregulation and CDKN1B upregulation
in AML cell lines
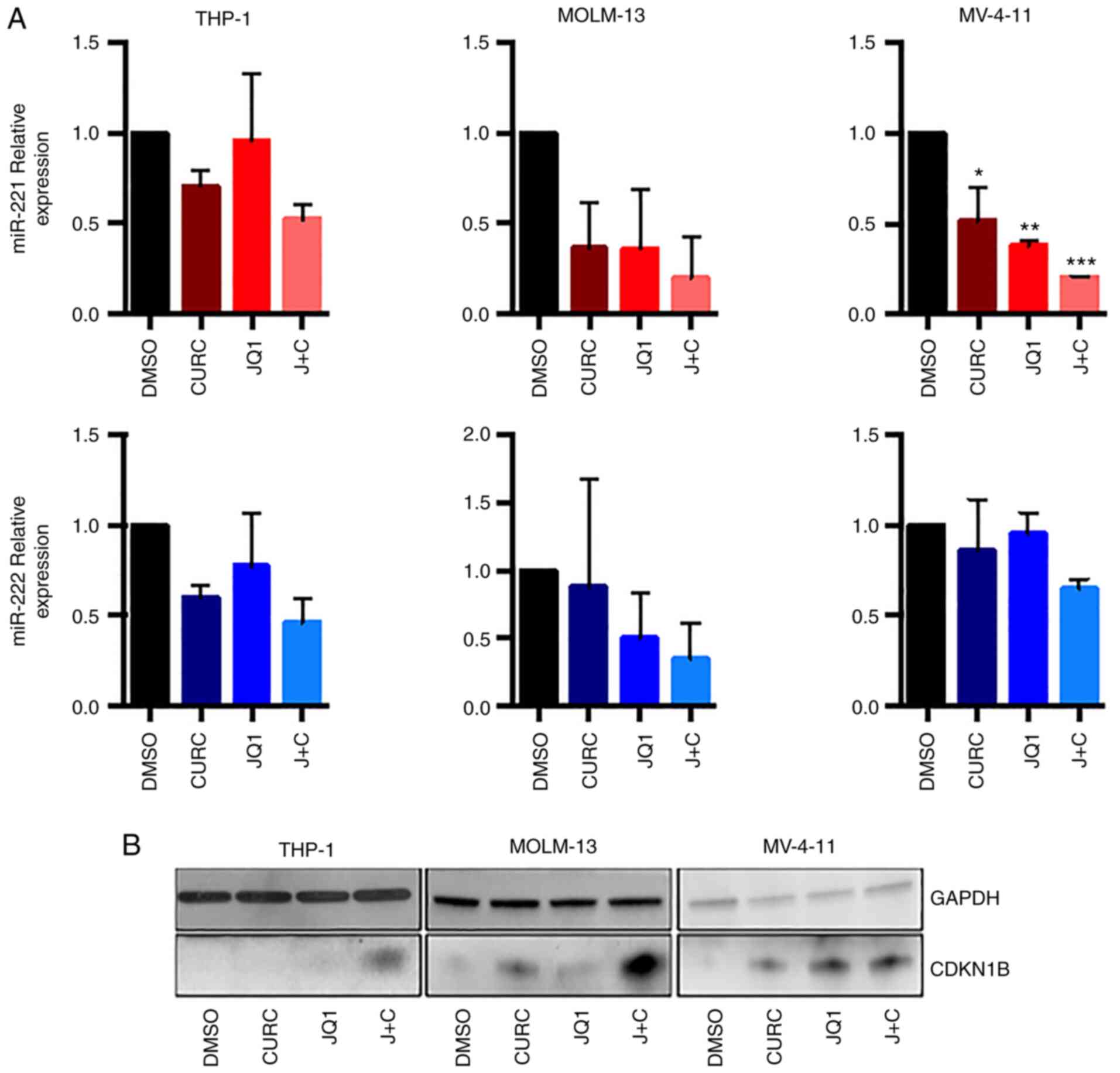
It was first analysed whether the combination of JQ1
and curcumin could modulate miR-221-3p and miR-222-3p expression in
AML cell lines.
RT-qPCR experiments (Fig. 2A), revealed a trend towards
downregulation of the two miRs in all the cell lines, featuring an
enhanced effect with the drug combination. The expression of
miR-221-3p expression showed a clear trend towards downregulation,
reaching statistical significance in MV-4-11 cells when treated
with JQ1, curcumin or the combination of both (Fig. 2A, upper panel). miR-222-3p
demonstrated a trend towards downregulation with coupled treatment
in all cell-lines, but it did not reach statistical significance
(Fig. 2A, lower panel).
It was then analysed whether the combination of JQ1
and curcumin could modulate the CDKN1B protein level, that it is a
miR-221-3p and miR-222-3p target, as before mentioned. As revealed
in Fig. 2B, the treatment
determined an increase in CDKN1B expression both in THP-1 and in
MOLM-13 cells while in MV-411 cells the effect of drug combination
on the upregulation of CDKN1B expression was comparable to that
obtained by single treatment with JQ1.
Association of curcumin and JQ1 causes
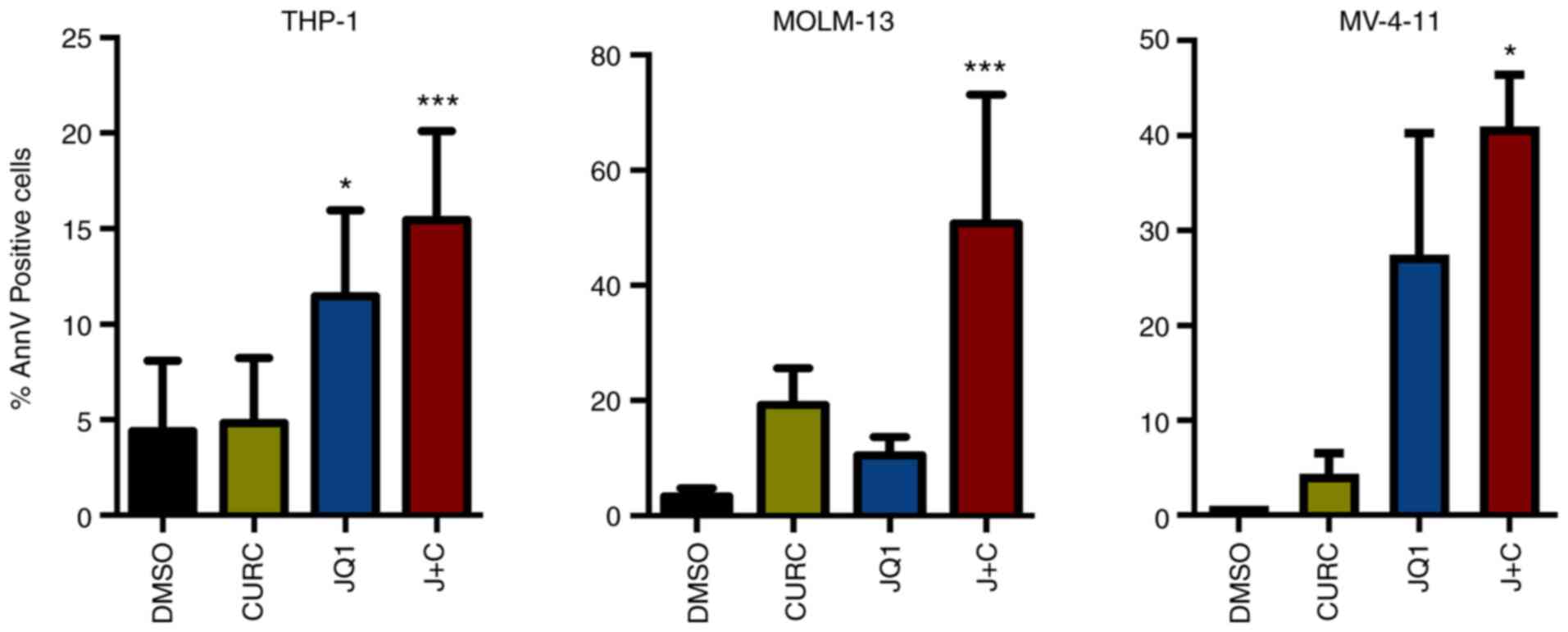
apoptosis
It was assessed whether these treatments could drive
the cell lines toward an apoptotic response. A significant increase
in AnnV positive cell percentage was identified in all the cell
lines while treated with the drug combination compared with DMSO
(Figs. S1 and 3). At a deeper glance, THP-1 and MV-4-11
cells, showing a minor increase in CDKN1B expression, displayed a
milder apoptotic response compared with MOLM-13, characterized
instead by higher changes in term of expression of CDKN1B (Figs. 2 and 3).
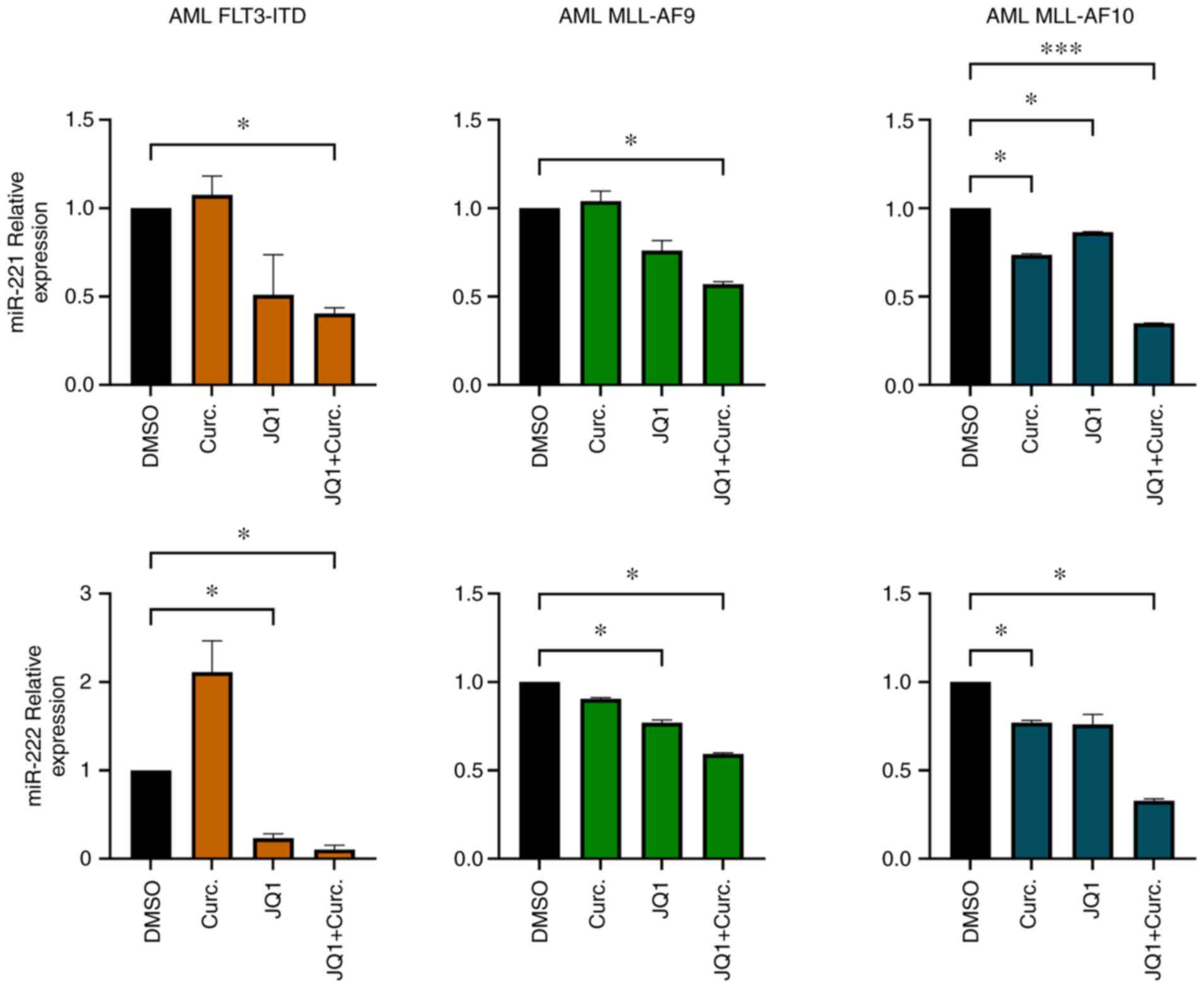
CD34+ primary cells from
AML patients exhibit miR-221-3p and miR-222-3p downregulation after
single and double treatments
miR-221-3p and miR-222-3p expression
was analysed in CD34+ cells isolated from BM samples
from three randomly selected patients from our cohort. The purity
of CD34+ cells after isolation was 89% (Fig. S2). After CD34+ cells
were cultured with MethoCult methylcellulose medium supplemented
with JQ1 and curcumin, RNA was extracted and miR-221-3p, miR-222-3p
expression was evaluated. In all the samples analysed miR-221-3p
and miR-222-3p were modulated when treated with JQ1 and curcumin.
In particular, the double treatment led to a significant
downregulation of their expression (Fig. 4).
Discussion
Despite improvements in the treatment of paediatric
patients affected by AML, novel therapeutic strategies are needed,
particularly for paediatric high-risk AML, characterized by high
relapse incidence. miRs have been extensively studied as potential
biomarkers in adult AML and, recently, expression signatures of
miRs in paediatric samples have been proposed (14,19,42).
Notably, few miRNA-based prognostic models have been
proposed for paediatric cytogenetically normal AML (18,43),
t(8;21) RUNX1-RUNX1T1 AML (44)
and AML without considering the cytogenetics (18,45).
However, conflicting results on the prognostic value of different
miRs were reported, possibly due to the vast variability of miRs
expression among different cytogenetic and molecular subtypes of
AML (15). It was therefore
decided, first, to characterize signature of miRs in patients
harbouring FLT3-ITD and MLL rearrangement to verify
whether distinct AML molecular subsets could affect different miR
expression fingerprints. Moreover, to further narrow our miRs
signature (identifying only AML associated miRs), HDs were used as
a control group. Data on expression of miRs among different AML
molecular subtypes are available (16). In accordance with literature data
(46), miR-9-5p and miR-10a-5p
were upregulated in MLL-rearranged and FLT3-ITD sets,
respectively, even compared with HDs. In addition, miR-99a-5p was
upregulated in FLT3-ITD patients as compared with MLL
patients, and was already described in literature as a potential
oncomiR in paediatric AML (42).
miR-196b-5p and miR-34a-5p were both upregulated in MLL and
FLT3-ITD with respect to HDs and they are possibly involved
in the pathogenesis of both AML variants. miR-34a is a widely
studied miR as a promising target and it has been considered as a
preclinical and clinical model for the treatment of solid tumours,
myeloma and B-cell lymphoma (47,48).
Despite the limited number of patients, to the best
of our knowledge, this is the first study investigating the role of
miRs as predictive biomarker of relapse in paediatric AML patients
with FLT3-ITD or MLL rearrangement. A total of 28
miRs differentially expressed were identified between patients who
relapsed and those who did not, being possibly associated with
prognosis. Among them, the vast majority of miRs have already been
reported as related to cancer. Comparing the signature of our miRs
to those previously reported, a common dysregulation of mir-155 was
identified, but displaying an opposite behaviour with a protective
effect in the present study as opposed to a relapse association in
others (19,49). miR-155 has led to conflicting
results in previous studies, with certain groups reporting an
anti-leukemic role (50) and
others (13,51–53)
reporting a role as oncomiR in AML. This is possibly explained by a
different level of expression of miR-155, acting as a tumour
suppressor when highly expressed and as an oncomiR when
overexpressed to an intermediate level (54). miR-34a-5p expression was positively
correlated with relapse. It was already described that patients
with low miR-34a expression showed shorter overall and
recurrence-free survival (55).
Numerous of the identified miRs, have also been independently
considered as promising tool to develop novel therapies; these
include miR-200c (47,56), which was revealed to be associated
to NREL patients.
miR-221-3p and miR-222-3p, both associated to
relapse, gained the attention of the authors as they have been
broadly reported in literature as oncomiRs both in hematologic
malignancies such as chronic lymphocytic leukemia (57), myelodisplastic syndrome (58), acute lymphoblastic leukemia
(59) and AML (60,61)
as well as in solid tumours. These miRs have as their primary
target CDKN1B, a master regulator of the cell cycle. CDKN1B is a
well-known cyclin-dependent kinase inhibitor which regulates cell
cycle progression at G1 stage, preventing the activation of cyclin
E-CDK2 or cyclin D-CDK4 complexes, resulting in a blockade of cell
division cycle (62).
BET and HAT inhibitors have already been associated
with modulation of miRs in hematological malignances (63,64).
The present findings revealed, for the first time, that JQ1
determines a clear trend towards downregulation of miR-221-3p
expression in both MOLM-13 and MV-4-11AML cell lines with a
synergic effect when associated with curcumin; reaching statistical
significance in the second one. This is interesting also
considering that for FLT3-ITD rearrangement MOLM-13
expresses both mutated and wild-type allele, while MV-4-11
expresses mutated allele only (65). It could be hypothesized that
FLT3-ITD in both mutated alleles renders MV-4-11 cells more
sensitive to the effect of drug on miR-221-3p modulation.
Moreover, following the combined treatment, an
increase was demonstrated in CDKN1B expression and in apoptotic
response in our AML cell lines. The present results were confirmed
in cultures with primary leukemic cells, showing a marked reduction
of miR-221-3p and miR-222-3p expression. These results supported
the idea that BET inhibitors, along with curcumin, could regulate
not only coding RNA transcription, but also non-coding RNA such as
miRs.
Although the combination of JQ1 and curcumin
synergistically reduced miR-221 and miR-222 expression and
increased apoptosis in AML cells, a limitation to the present study
was represented by insufficient patient samples. Direct regulation
of CDKN1B by miR-221-3p and miR-222-3p should be confirmed by
further experiments including western blot analysis to verify
expression levels of CDKN1B in samples of patients and HDs and the
use of inhibitors of miR-221 or miR-222 in a bigger cohort of
patients.
In conclusion, the present study identified
fingerprints of miRs related to relapse and non-relapse in
paediatric FLT3-ITD- or MLL-rearranged AML. Numerous
of these miRs are known to be involved in pathogenetic mechanisms
of several haematological malignancies as well as solid tumours and
represent both good candidates for targeted treatments and
therapeutic tools in different neoplasms. The use of the well-known
BRD4 inhibitor JQ1, as well as novel BRD inhibitors in the care of
leukaemia could be potentiated by epigenetic drugs such as HATs
inhibitors, antagomiR or miR mimic and could expand the therapeutic
arsenal in HR-AMLs, particularly for paediatric patients.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Giuseppe Basso
(1948–2021), MD, Department of Woman's and Children's Health,
University of Padua (Padua, Italy) for his help in the study design
and for providing samples.
Funding
The present study was supported by Ministero della Salute (grant
no. GR-2011-02350175) and by Fondazione Umberto Veronesi (Milano,
Italy).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the GEO repository (accession no.
GSE209871; http://www.ncbi.nlm.nih.gov/gds).
Authors' contributions
PPL, PV, AB, PM and DP designed the study. RR, AB,
PPL and PV conceived and designed the experiments. PPL, PV and VT
performed the experiments and data analyses. GN, DV, PV, PF, MS and
SR performed Nanostring, data and statistical analyses. VP, FS, EM,
AB, DP and PM enrolled patients and collected clinical samples. SR,
PV, PPL, MM and PM contributed to manuscript preparation, editing
and reviewing. GN and PPL confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Boards of Bambino Gesù Children's Hospital (Rome, Italy).
Informed consent was obtained from either parents or legal
guardians according to the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pui CH, Carroll WL, Meshinchi S and Arceci
RJ: Biology, risk stratification, and therapy of pediatric acute
leukemias: An update. J Clin Oncol. 29:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Creutzig U, van den Heuvel-Eibrink MM,
Gibson B, Dworzak MN, Adachi S, de Bont E, Harbott J, Hasle H,
Johnston D, Kinoshita A, et al: Diagnosis and management of acute
myeloid leukemia in children and adolescents: Recommendations from
an international expert panel. Blood. 120:3187–3205. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubnitz JE and Inaba H: Childhood acute
myeloid leukaemia. Br J Haematol. 159:259–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elgarten CW and Aplenc R: Pediatric acute
myeloid leukemia: Updates on biology, risk stratification, and
therapy. Curr Opin Pediatr. 32:57–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buldini B, Rizzati F, Masetti R, Fagioli
F, Menna G, Micalizzi C, Putti MC, Rizzari C, Santoro N, Zecca M,
et al: Prognostic significance of flow-cytometry evaluation of
minimal residual disease in children with acute myeloid leukaemia
treated according to the AIEOP-AML 2002/01 study protocol. Br J
Haematol. 177:116–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Manara E, Basso G, Zampini M, Buldini B,
Tregnago C, Rondelli R, Masetti R, Bisio V, Frison M, Polato K, et
al: Characterization of children with FLT3-ITD acute myeloid
leukemia: A report from the AIEOP AML-2002 study group. Leukemia.
31:18–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masetti R, Vendemini F, Zama D, Biagi C,
Pession A and Locatelli F: Acute myeloid leukemia in infants:
Biology and treatment. Front Pediatr. 3:372015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balgobind BV, Raimondi SC, Harbott J,
Zimmermann M, Alonzo TA, Auvrignon A, Beverloo HB, Chang M,
Creutzig U, Dworzak MN, et al: Novel prognostic subgroups in
childhood 11q23/MLL-rearranged acute myeloid leukemia: Results of
an international retrospective study. Blood. 114:2489–2496. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang X, Huang H, Li Z, Li Y, Wang X,
Gurbuxani S, Chen P, He C, You D, Zhang S, et al: Blockade of
miR-150 maturation by MLL-fusion/MYC/LIN-28 is required for
MLL-associated leukemia. Cancer Cell. 22:524–535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Leva G, Garofalo M and Croce CM:
MicroRNAs in cancer. Annu Rev Pathol. 9:287–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marcucci G, Mrózek K, Radmacher MD, Garzon
R and Bloomfield CD: The prognostic and functional role of
microRNAs in acute myeloid leukemia. Blood. 117:1121–1129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obulkasim A, Katsman-Kuipers JE, Verboon
L, Sanders M, Touw I, Jongen-Lavrencic M, Pieters R, Klusmann JH,
Michel Zwaan C, van den Heuvel-Eibrink MM and Fornerod M:
Classification of pediatric acute myeloid leukemia based on miRNA
expression profiles. Oncotarget. 8:33078–33085. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danen-van Oorschot AA, Kuipers JE,
Arentsen-Peters S, Schotte D, de Haas V, Trka J, Baruchel A,
Reinhardt D, Pieters R, Zwaan CM and van den Heuvel-Eibrink MM:
Differentially expressed miRNAs in cytogenetic and molecular
subtypes of pediatric acute myeloid leukemia. Pediatr Blood Cancer.
58:715–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wallace JA and O'Connell RM: MicroRNAs and
acute myeloid leukemia: Therapeutic implications and emerging
concepts. Blood. 130:1290–1301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu D, Qiu Y, Jiao Y, Qiu Z and Liu D:
Small molecules targeting HATs, HDACs, and BRDs in cancer therapy.
Front Oncol. 10:5604872020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu R, Zhao W, Fan F, Tang L, Liu J, Luo
T, Deng J and Hu Y: A 3-miRNA signature predicts prognosis of
pediatric and adolescent cytogenetically normal acute myeloid
leukemia. Oncotarget. 8:38902–38913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim EL, Trinh DL, Ries RE, Wang J, Gerbing
RB, Ma Y, Topham J, Hughes M, Pleasance E, Mungall AJ, et al:
MicroRNA expression-based model indicates event-free survival in
pediatric acute myeloid leukemia. J Clin Oncol. 35:3964–3977. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brondfield S, Umesh S, Corella A, Zuber J,
Rappaport AR, Gaillard C, Lowe SW, Goga A and Kogan SC: Direct and
indirect targeting of MYC to treat acute myeloid leukemia. Cancer
Chemother Pharmacol. 76:35–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benetatos L and Vartholomatos G: MicroRNAs
mark in the MLL-rearranged leukemia. Ann Hematol. 92:1439–1450.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bretones G, Delgado MD and León J: Myc and
cell cycle control. Biochim Biophys Acta. 1849:506–516. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Donati B, Lorenzini E and Ciarrocchi A:
BRD4 and cancer: Going beyond transcriptional regulation. Mol
Cancer. 17:1642018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang G, Deng W, Liu Y and Wang C: General
mechanism of JQ1 in inhibiting various types of cancer. Mol Med
Rep. 21:1021–1034. 2020.PubMed/NCBI
|
|
25
|
Wu T, Kamikawa YF and Donohoe ME: Brd4′s
bromodomains mediate histone H3 acetylation and chromatin
remodeling in pluripotent cells through P300 and Brg1. Cell Rep.
25:1756–1771. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fiskus W, Sharma S, Qi J, Shah B, Devaraj
SG, Leveque C, Portier BP, Iyer S, Bradner JE and Bhalla KN: BET
protein antagonist JQ1 is synergistically lethal with FLT3 tyrosine
kinase inhibitor (TKI) and overcomes resistance to FLT3-TKI in AML
cells expressing FLT-ITD. Mol Cancer Ther. 13:2315–2327. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mio C, Conzatti K, Baldan F, Allegri L,
Sponziello M, Rosignolo F, Russo D, Filetti S and Damante G: BET
bromodomain inhibitor JQ1 modulates microRNA expression in thyroid
cancer cells. Oncol Rep. 39:582–588. 2018.PubMed/NCBI
|
|
28
|
Schick M, Habringer S, Nilsson JA and
Keller U: Pathogenesis and therapeutic targeting of aberrant MYC
expression in haematological cancers. Br J Haematol. 179:724–738.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen H, Liu H and Qing G: Targeting
oncogenic Myc as a strategy for cancer treatment. Signal Transduct
Target Ther. 3:52018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Delmore JE, Issa GC, Lemieux ME, Rahl PB,
Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, et
al: BET bromodomain inhibition as a therapeutic strategy to target
c-Myc. Cell. 146:904–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marcu MG, Jung YJ, Lee S, Chung EJ, Lee
MJ, Trepel J and Neckers L: Curcumin is an inhibitor of p300
histone acetylatransferase. Med Chem. 2:169–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang SK, Cha SH and Jeon HG:
Curcumin-induced histone hypoacetylation enhances
caspase-3-dependent glioma cell death and neurogenesis of neural
progenitor cells. Stem Cells Dev. 15:165–174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morimoto T, Sunagawa Y, Kawamura T, Takaya
T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T and
Hasegawa K: The dietary compound curcumin inhibits p300 histone
acetyltransferase activity and prevents heart failure in rats. J
Clin Invest. 118:868–878. 2008.PubMed/NCBI
|
|
34
|
Spriano F, Stathis A and Bertoni F:
Targeting BET bromodomain proteins in cancer: The example of
lymphomas. Pharmacol Ther. 215:1076312020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang X, Yang Y, Ren D, Xia Y, He W, Wu Q,
Zhang J, Liu M, Du Y, Ren C, et al: JQ1, a bromodomain inhibitor,
suppresses Th17 effectors by blocking p300-mediated acetylation of
RORγt. Br J Pharmacol. 177:2959–2973. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reich M, Liefeld T, Gould J, Lerner J,
Tamayo P and Mesirov JP: GenePattern 2.0. Nat Genet. 38:500–501.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heberle H, Meirelles GV, da Silva FR,
Telles GP and Minghim R: InteractiVenn: A web-based tool for the
analysis of sets through Venn diagrams. BMC Bioinformatics.
16:1692015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Haferlach C, Kern W, Schindela S, Kohlmann
A, Alpermann T, Schnittger S and Haferlach T: Gene expression of
BAALC, CDKN1B, ERG, and MN1 adds independent prognostic information
to cytogenetics and molecular mutations in adult acute myeloid
leukemia. Genes Chromosomes Cancer. 51:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang H, Luo XQ, Zhang P, Huang LB, Zheng
YS, Wu J, Zhou H, Qu LH, Xu L and Chen YQ: MicroRNA patterns
associated with clinical prognostic parameters and CNS relapse
prediction in pediatric acute leukemia. PLoS One. 4:e78262009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gaur V, Chaudhary S, Tyagi A, Agarwal S,
Sharawat SK, Sarkar S, Singh H, Bakhshi S, Sharma P and Kumar S:
Dysregulation of miRNA expression and their prognostic significance
in paediatric cytogenetically normal acute myeloid leukaemia. Br J
Haematol. 188:e90–e94. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zampini M, Bisio V, Leszl A, Putti MC,
Menna G, Rizzari C, Pession A, Locatelli F, Basso G, Tregnago C and
Pigazzi M: A three-miRNA-based expression signature at diagnosis
can predict occurrence of relapse in children with t(8;21)
RUNX1-RUNX1T1 acute myeloid leukaemia. Br J Haematol. 183:298–301.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu R, Lin W, Zhao W, Fan F, Tang L and Hu
Y: A 4-microRNA signature for survival prognosis in pediatric and
adolescent acute myeloid leukemia. J Cell Biochem. 120:3958–3968.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Y, Cheng Z, Pang Y, Cui L, Qian T,
Quan L, Zhao H, Shi J, Ke X and Fu L: Role of microRNAs, circRNAs
and long noncoding RNAs in acute myeloid leukemia. J Hematol Oncol.
12:512019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beg MS, Brenner AJ, Sachdev J, Borad M,
Kang YK, Stoudemire J, Smith S, Bader AG, Kim S and Hong DS: Phase
I study of MRX34, a liposomal miR-34a mimic, administered twice
weekly in patients with advanced solid tumors. Invest New Drugs.
35:180–188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yan W, Xu L, Sun Z, Lin Y, Zhang W, Chen
J, Hu S and Shen B: MicroRNA biomarker identification for pediatric
acute myeloid leukemia based on a novel bioinformatics model.
Oncotarget. 6:26424–26436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Palma CA, Al Sheikha D, Lim TK, Bryant A,
Vu TT, Jayaswal V and Ma DD: MicroRNA-155 as an inducer of
apoptosis and cell differentiation in acute myeloid leukaemia. Mol
Cancer. 13:792014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Marcucci G, Maharry KS, Metzeler KH,
Volinia S, Wu YZ, Mrózek K, Nicolet D, Kohlschmidt J, Whitman SP,
Mendler JH, et al: Clinical role of microRNAs in cytogenetically
normal acute myeloid leukemia: miR-155 upregulation independently
identifies high-risk patients. J Clin Oncol. 31:2086–2093. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wallace JA, Kagele DA, Eiring AM, Kim CN,
Hu R, Runtsch MC, Alexander M, Huffaker TB, Lee SH, Patel AB, et
al: miR-155 promotes FLT3-ITD-induced myeloproliferative disease
through inhibition of the interferon response. Blood.
129:3074–3086. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
O'Connell RM, Rao DS, Chaudhuri AA, Boldin
MP, Taganov KD, Nicoll J, Paquette RL and Baltimore D: Sustained
expression of microRNA-155 in hematopoietic stem cells causes a
myeloproliferative disorder. J Exp Med. 205:585–594. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Narayan N, Morenos L, Phipson B, Willis
SN, Brumatti G, Eggers S, Lalaoui N, Brown LM, Kosasih HJ, Bartolo
RC, et al: Functionally distinct roles for different miR-155
expression levels through contrasting effects on gene expression,
in acute myeloid leukaemia. Leukemia. 31:808–820. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Huang Y, Zou Y, Lin L, Ma X and Chen H:
Identification of serum miR-34a as a potential biomarker in acute
myeloid leukemia. Cancer Biomark. 22:799–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Chakraborty C, Sharma AR, Sharma G, Doss
CGP and Lee SS: Therapeutic miRNA and siRNA: Moving from bench to
clinic as next generation medicine. Mol Ther Nucleic Acids.
8:132–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Frenquelli M, Muzio M, Scielzo C, Fazi C,
Scarfò L, Rossi C, Ferrari G, Ghia P and Caligaris-Cappio F:
MicroRNA and proliferation control in chronic lymphocytic leukemia:
Functional relationship between miR-221/222 cluster and p27. Blood.
115:3949–3959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hussein K, Theophile K, Büsche G,
Schlegelberger B, Göhring G, Kreipe H and Bock O: Significant
inverse correlation of microRNA-150/MYB and microRNA-222/p27 in
myelodysplastic syndrome. Leuk Res. 34:328–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Moses BS, Evans R, Slone WL, Piktel D,
Martinez I, Craig MD and Gibson LF: Bone marrow microenvironment
niche regulates miR-221/222 in acute lymphoblastic leukemia. Mol
Cancer Res. 14:909–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rommer A, Steinleitner K, Hackl H,
Schneckenleithner C, Engelmann M, Scheideler M, Vlatkovic I,
Kralovics R, Cerny-Reiterer S, Valent P, et al: Overexpression of
primary microRNA 221/222 in acute myeloid leukemia. BMC Cancer.
13:3642013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lee YG, Kim I, Oh S, Shin DY, Koh Y and
Lee KW: Small RNA sequencing profiles of mir-181 and mir-221, the
most relevant microRNAs in acute myeloid leukemia. Korean J Intern
Med. 34:178–183. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
le Sage C, Nagel R and Agami R: Diverse
ways to control p27Kip1 function: miRNAs come into play. Cell
Cycle. 6:2742–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kohnken R, McNeil B, Wen J, McConnell K,
Grinshpun L, Keiter A, Chen L, William B, Porcu P and Mishra A:
Preclinical targeting of MicroRNA-214 in cutaneous T-cell lymphoma.
J Invest Dermatol. 139:1966–1974.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Mensah AA, Cascione L, Gaudio E,
Tarantelli C, Bomben R, Bernasconi E, Zito D, Lampis A, Hahne JC,
Rinaldi A, et al: Bromodomain and extra-terminal domain inhibition
modulates the expression of pathologically relevant microRNAs in
diffuse large B-cell lymphoma. Haematologica. 103:2049–2058. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Quentmeier H, Reinhardt J, Zaborski M and
Drexler HG: FLT3 mutations in acute myeloid leukemia cell lines.
Leukemia. 17:120–124. 2003. View Article : Google Scholar : PubMed/NCBI
|