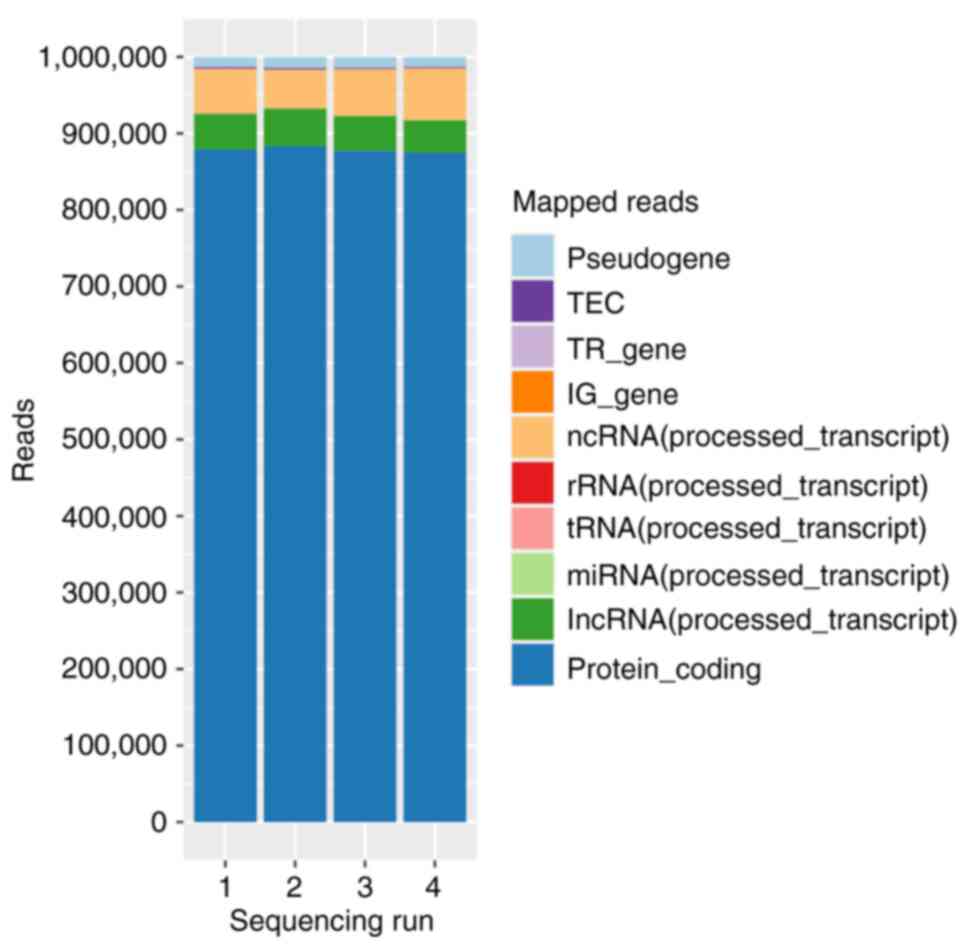
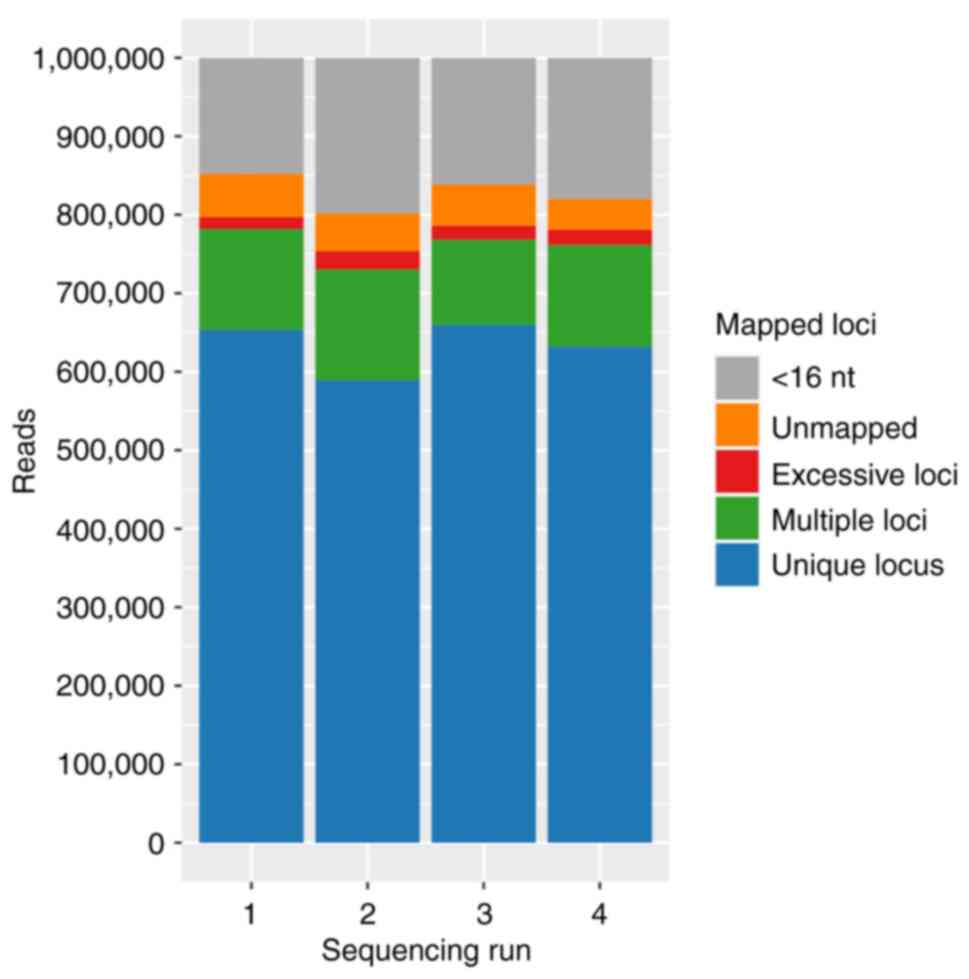
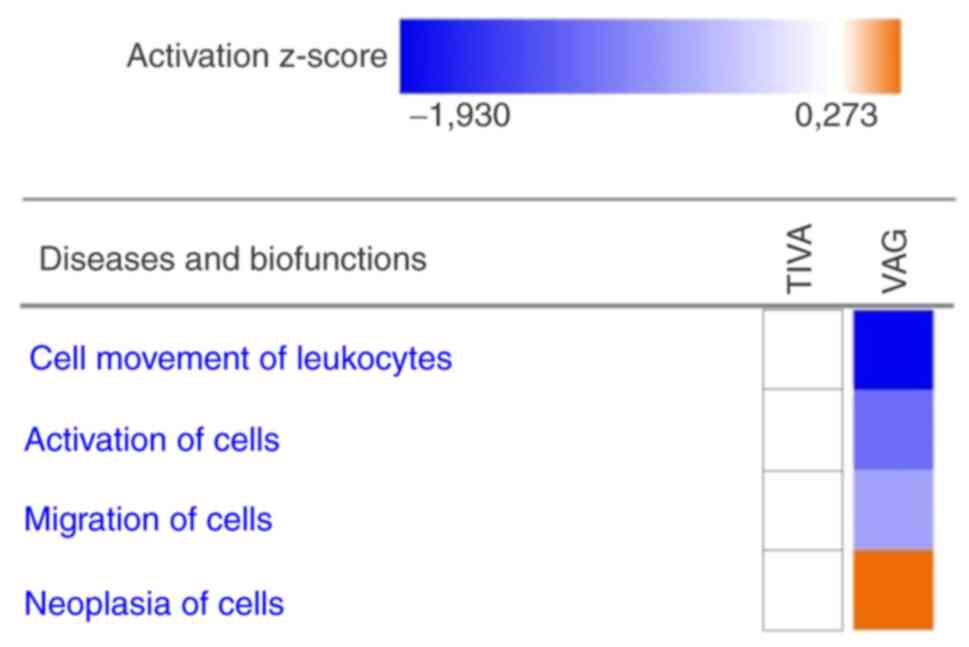
|
1
|
Araghi M, Soerjomataram I, Jenkins M,
Brierley J, Morris E, Bray F and Arnold M: Global trends in
colorectal cancer mortality: Projections to the year 2035. Int J
Cancer. 144:2992–3000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Werner J and Heinemann V: Standards and
challenges of care for colorectal cancer today. Visc Med.
32:156–157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demicheli R, Retsky MW, Hrushesky WJ, Baum
M and Gukas ID: The effects of surgery on tumor growth: A century
of investigations. Ann Oncol. 19:1821–1828. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tohme S, Simmons RL and Tsung A: Surgery
for cancer: A trigger for metastases. Cancer Res. 77:1548–1552.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin Z, Li R, Liu J and Lin J: Long-term
prognosis after cancer surgery with inhalational anesthesia and
total intravenous anesthesia: A systematic review and
meta-analysis. Int J Physiol Pathophysiol Pharmacol. 11:83–94.
2019.PubMed/NCBI
|
|
6
|
Makito K, Matsui H, Fushimi K and Yasunaga
H: Volatile versus total intravenous anesthesia for cancer
prognosis in patients having digestive cancer surgery.
Anesthesiology. 133:764–773. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guerrero Orriach JL, Raigon Ponferrada A,
Malo Manso A, Herrera Imbroda B, Escalona Belmonte JJ, Ramirez
Aliaga M, Ramirez Fernandez A, Diaz Crespo J, Soriano Perez AM,
Fontaneda Heredia A, et al: Anesthesia in combination with propofol
increases disease-free survival in bladder cancer patients who
undergo radical tumor cystectomy as compared to inhalational
anesthetics and opiate-based analgesia. Oncology. 98:161–167. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jun IJ, Jo JY, Kim JI, Chin JH, Kim WJ,
Kim HR, Lee EH and Choi IC: Impact of anesthetic agents on overall
and recurrence-free survival in patients undergoing esophageal
cancer surgery: A retrospective observational study. Sci Rep.
7:140202017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng X, Wang Y, Dong L, Zhao S, Wang L,
Chen H, Xu Y and Wang G: Effects of propofol-based total
intravenous anesthesia on gastric cancer: A retrospective study.
Onco Targets Ther. 11:1141–1148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS,
Lin KT, Lou YS, Lin C, Chang YC and Lai HC: Propofol-based total
intravenous anesthesia is associated with better survival than
desflurane anesthesia in colon cancer surgery. Anesthesiology.
129:932–941. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Fan Y, Liu K and Wang Y: Effects
of different general anaesthetic techniques on immune responses in
patients undergoing surgery for tongue cancer. Anaesth Intensive
Care. 42:220–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan T, Zhang GH, Wang BN, Sun L and Zheng
H: Effects of propofol/remifentanil-based total intravenous
anesthesia versus sevoflurane-based inhalational anesthesia on the
release of VEGF-C and TGF-β and prognosis after breast cancer
surgery: A prospective, randomized and controlled study. BMC
Anesthesiol. 18:1312018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abel F, Giebel B and Frey UH: Agony of
choice: How Anesthetics affect the composition and function of
extracellular vesicles. Adv Drug Deliv Rev. 175:1138132021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ecimovic P, McHugh B, Murray D, Doran P
and Buggy DJ: Effects of sevoflurane on breast cancer cell function
in vitro. Anticancer Res. 33:4255–4260. 2013.PubMed/NCBI
|
|
15
|
Iwasaki M, Zhao H, Jaffer T, Unwith S,
Benzonana L, Lian Q, Sakamoto A and Ma D: Volatile anaesthetics
enhance the metastasis related cellular signalling including CXCR2
of ovarian cancer cells. Oncotarget. 7:26042–26056. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kvolik S, Glavas-Obrovac L, Bares V and
Karner I: Effects of inhalation anesthetics halothane, sevoflurane,
and isoflurane on human cell lines. Life Sci. 77:2369–2383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Müller-Edenborn B, Roth-Z'graggen B,
Bartnicka K, Borgeat A, Hoos A, Borsig L and Beck-Schimmer B:
Volatile anesthetics reduce invasion of colorectal cancer cells
through down-regulation of matrix metalloproteinase-9.
Anesthesiology. 117:293–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Forrest ME, Saiakhova A, Beard L, Buchner
DA, Scacheri PC, LaFramboise T, Markowitz S and Khalil AM: Colon
cancer-upregulated long non-coding RNA lincDUSP Regulates cell
cycle genes and potentiates resistance to apoptosis. Sci Rep.
8:73242018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buschmann D, Brandes F, Lindemann A,
Maerte M, Ganschow P, Chouker A, Schelling G, Pfaffl MW and
Reithmair M: Propofol and sevoflurane differentially impact
MicroRNAs in circulating extracellular vesicles during colorectal
cancer resection: A pilot study. Anesthesiology. 132:107–120. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dinger ME, Pang KC, Mercer TR and Mattick
JS: Differentiating protein-coding and noncoding RNA: Challenges
and ambiguities. PLoS Comput Biol. 4:e10001762008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bánfai B, Jia H, Khatun J, Wood E, Risk B,
Gundling WE Jr, Kundaje A, Gunawardena HP, Yu Y, Xie L, et al: Long
noncoding RNAs are rarely translated in two human cell lines.
Genome Res. 22:1646–1657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han
Z, Sui H, Tang Y, Wang Y, Liu N, et al: Long non-coding RNA MALAT1
promotes tumour growth and metastasis in colorectal cancer through
binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2
complex. Br J Cancer. 111:736–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Siddiqui H, Al-Ghafari A, Choudhry H and
Al Doghaither H: Roles of long non-coding RNAs in colorectal cancer
tumorigenesis: A review. Mol Clin Oncol. 11:167–172.
2019.PubMed/NCBI
|
|
27
|
Thiele JA, Hosek P, Kralovcova E, Ostasov
P, Liska V, Bruha J, Vycital O, Rosendorf J, Opattova A, Horak J,
et al: lncRNAs in Non-Malignant tissue have prognostic value in
colorectal cancer. Int J Mol Sci. 19:26722018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin M: Cutadapt removes adapter
sequences from high-throughput sequencing reads. EMBnet. J.
17:10–12. 2011.
|
|
29
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li B and Dewey CN: RSEM: Accurate
transcript quantification from RNA-Seq data with or without a
reference genome. BMC Bioinformatics. 12:3232011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Y, Liu T, Cui T, Wang Z, Zhang Y, Tan
P, Huang Y, Yu J and Wang D: RNAInter in 2020: RNA interactome
repository with increased coverage and annotation. Nucleic Acids
Res. 48(D1): D189–D197. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang C, Yang L, Zhou M, Xie H, Zhang C,
Wang MD and Zhu H: LncADeep: An ab initio lncRNA identification and
functional annotation tool based on deep learning. Bioinformatics.
34:3825–3834. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanehisa M and Sato Y: KEGG Mapper for
inferring cellular functions from protein sequences. Protein Sci.
29:28–35. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: A model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perkins JR, Dawes JM, McMahon SB, Bennett
DL, Orengo C and Kohl M: ReadqPCR and NormqPCR: R packages for the
reading, quality checking and normalisation of RT-qPCR
quantification cycle (Cq) data. BMC Genomics. 13:2962012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng X, Bi Q, Chen S, Chen X, Li S, Zhong
Z, Guo W, Li X, Deng Y and Yang Y: Identification of a
Five-Autophagy-Related-lncRNA signature as a novel prognostic
biomarker for hepatocellular carcinoma. Front Mol Biosci.
7:6116262021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lin C, Zhang S, Wang Y, Wang Y, Nice E,
Guo C, Zhang E, Yu L, Li M, Liu C, et al: Functional role of a
novel long noncoding RNA TTN-AS1 in esophageal squamous cell
carcinoma progression and metastasis. Clin Cancer Res. 24:486–498.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Li D, Lu J, Chen L, Zhang S, Qi W,
Li W and Xu H: Long noncoding RNA TTN-AS1 facilitates tumorigenesis
and metastasis by maintaining TTN expression in skin cutaneous
melanoma. Cell Death Dis. 11:6642020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jia Y, Duan Y, Liu T, Wang X, Lv W, Wang
M, Wang J and Liu L: LncRNA TTN-AS1 promotes migration, invasion,
and epithelial mesenchymal transition of lung adenocarcinoma via
sponging miR-142-5p to regulate CDK5. Cell Death Dis. 10:5732019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hong MEI, Changen LI, Liang Y and Yingfei
GAO: Expression of lncRNA LINC01001 in breast cancer and its effect
on proliferation of MCF-7 cells. Chin J Cancer Biother. 25:158–162.
2018.
|
|
44
|
Zhou M, Guo M, He D, Wang X, Cui Y, Yang
H, Hao D and Sun J: A potential signature of eight long non-coding
RNAs predicts survival in patients with non-small cell lung cancer.
J Transl Med. 13:2312015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lv M, Cao D, Zhang L, Hu C, Li S, Zhang P,
Zhu L, Yi X, Li C, Yan A, et al: METTL9 regulates N1-histidine
methylation of zinc transporters to promote tumor growth. bioRxiv.
2021.2004.2020.440582. 2021.
|
|
46
|
Wang W, Zhao Z, Yang F, Wang H, Wu F,
Liang T, Yan X, Li J, Lan Q, Wang J and Zhao J: An immune-related
lncRNA signature for patients with anaplastic gliomas. J
Neurooncol. 136:263–271. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kutilin DS, Gusareva MA, Kosheleva NG,
Zinkovich MS, Gvaramiya AK, Gappoeva MA, Fatkina NB, Krokhmal JN,
Vasilieva EO, Karnauhova EA, et al: Differential expression of long
noncoding RNAs in patients with metastatic and nonmetastatic
colorectal cancer. J Clin Oncol. 39 (15_suppl):e150232021.
View Article : Google Scholar
|
|
48
|
Gourvest M, Brousset P and Bousquet M:
Long noncoding RNAs in acute myeloid leukemia: Functional
characterization and clinical relevance. Cancers (Basel).
11:16382019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen C, Wang P, Mo W, Zhang Y, Zhou W,
Deng T, Zhou M, Chen X, Wang S and Wang C: lncRNA-CCDC26, as a
novel biomarker, predicts prognosis in acute myeloid leukemia.
Oncol Lett. 18:2203–2211. 2019.PubMed/NCBI
|
|
50
|
Ma X, Li Y, Song Y and Xu G: Long
Noncoding RNA CCDC26 promotes thyroid cancer malignant progression
via miR-422a/EZH2/Sirt6 Axis. Onco Targets Ther. 14:3083–3094.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hu Y, Zhang Y, Ding M and Xu R: LncRNA
LINC00511 Acts as an oncogene in colorectal cancer via sponging
miR-29c-3p to upregulate NFIA. Onco Targets Ther. 13:13413–13424.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang J, Sui S, Wu H, Zhang J, Zhang X, Xu
S and Pang D: The transcriptional landscape of lncRNAs reveals the
oncogenic function of LINC00511 in ER-negative breast cancer. Cell
Death Dis. 10:5992019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lu G, Li Y, Ma Y, Lu J, Chen Y, Jiang Q,
Qin Q, Zhao L, Huang Q, Luo Z, et al: Long noncoding RNA LINC00511
contributes to breast cancer tumourigenesis and stemness by
inducing the miR-185-3p/E2F1/Nanog axis. J Exp Clin Cancer Res.
37:2892018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Terashima M, Ishimura A, Wanna-Udom S and
Suzuki T: MEG8 long noncoding RNA contributes to epigenetic
progression of the epithelial-mesenchymal transition of lung and
pancreatic cancer cells. J Biol Chem. 293:18016–18030. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Salama EA, Adbeltawab RE and El Tayebi HM:
XIST and TSIX: Novel cancer immune biomarkers in
PD-L1-Overexpressing breast cancer patients. Front Oncol.
9:14592020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jing L, Gong M, Lu X, Jiang Y, Li H and
Cheng W: LINC01127 promotes the development of ovarian tumors by
regulating the cell cycle. Am J Transl Res. 11:406–417.
2019.PubMed/NCBI
|
|
57
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long Non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Feng H, Zhang X, Lai W and Wang J: Long
non-coding RNA SLC16A1-AS1: Its multiple tumorigenesis features and
regulatory role in cell cycle in oral squamous cell carcinoma. Cell
Cycle. 19:1641–1653. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li F, Li Q and Wu X: Construction and
analysis for differentially expressed long non-coding RNAs and
MicroRNAs mediated competing endogenous RNA network in colon
cancer. PLoS One. 13:e01924942018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhao X, Fan Y, Lu C, Li H, Zhou N, Sun G
and Fan H: PCAT1 is a poor prognostic factor in endometrial
carcinoma and associated with cancer cell proliferation, migration
and invasion. Bosn J Basic Med Sci. 19:274–281. 2019.PubMed/NCBI
|
|
61
|
Zhang Q, Ding Z, Wan L, Tong W, Mao J, Li
L, Hu J, Yang M, Liu B and Qian X: Comprehensive analysis of the
long noncoding RNA expression profile and construction of the
lncRNA-mRNA co-expression network in colorectal cancer. Cancer Biol
Ther. 21:157–169. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang YF, Li CS, Zhou Y and Lu XH: Effects
of propofol on colon cancer metastasis through STAT3/HOTAIR axis by
activating WIF-1 and suppressing Wnt pathway. Cancer Med.
9:1842–1854. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ren YL and Zhang W: Propofol promotes
apoptosis of colorectal cancer cells via alleviating the
suppression of lncRNA HOXA11-AS on miRNA let-7i. Biochem Cell Biol.
98:90–98. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Krämer A, Green J, Pollard J Jr and
Tugendreich S: Causal analysis approaches in ingenuity pathway
analysis. Bioinformatics. 30:523–530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Sombroek D and Hofmann TG: How cells
switch HIPK2 on and off. Cell Death Differ. 16:187–194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hofmann TG, Möller A, Sirma H, Zentgraf H,
Taya Y, Dröge W, Will H and Schmitz ML: Regulation of p53 activity
by its interaction with homeodomain-interacting protein kinase-2.
Nat Cell Biol. 4:1–10. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ozaki T and Nakagawara A: Role of p53 in
cell death and human cancers. Cancers (Basel). 3:994–1013. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Puca R, Nardinocchi L, Givol D and D'Orazi
G: Regulation of p53 activity by HIPK2: Molecular mechanisms and
therapeutical implications in human cancer cells. Oncogene.
29:4378–4387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Park WS, Kim HJ, Lee GK, Son HS and Bae Y:
Anti-adhesive functions of CD43 expressed on colon carcinoma cells
through the modulation of integrins. Exp Mol Pathol. 92:82–89.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lara R, Adinolfi E, Harwood CA, Philpott
M, Barden JA, Di Virgilio F and McNulty S: P2X7 in cancer: From
molecular mechanisms to therapeutics. Front Pharmacol. 11:7932020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hirano T, Yoshikawa R, Harada H, Harada Y,
Ishida A and Yamazaki T: Long noncoding RNA, CCDC26, controls
myeloid leukemia cell growth through regulation of KIT expression.
Mol Cancer. 14:902015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Peng W and Jiang A: Long noncoding RNA
CCDC26 as a potential predictor biomarker contributes to
tumorigenesis in pancreatic cancer. Biomed Pharmacother.
83:712–717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hardwick JM and Soane L: Multiple
functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol.
5:a0087222013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Chiou SK, Rao L and White E: Bcl-2 blocks
p53-dependent apoptosis. Mol Cell Biol. 14:2556–2563. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Blagih J, Buck MD and Vousden KH: p53,
cancer and the immune response. J Cell Sci. 133:jcs2374532020.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bronte V, Cingarlini S, Marigo I, De Santo
C, Gallina G, Dolcetti L, Ugel S, Peranzoni E, Mandruzzato S and
Zanovello P: Leukocyte infiltration in cancer creates an
unfavorable environment for antitumor immune responses: A novel
target for therapeutic intervention. Immunol Invest. 35:327–357.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Garziera M and Toffoli G: Inhibition of
host immune response in colorectal cancer: Human leukocyte
antigen-G and beyond. World J Gastroenterol. 20:3778–3794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th Edition.
Wiley-Blackwell; New Jersey: pp. pp2722016
|