Introduction of cancer theragnostics
Cancers, particularly solid tumors, remain a global
concern posing a severe threat to human health (1). Immense efforts have been directed at
exploring new technologies for the diagnosis and treatment of
cancers in laboratory experiments and clinical practice, among
which theragnostics is the newly emerging realm referring to a
single targeting medication that enables both therapy and imaging
diagnosis typically involving radiopharmaceuticals as a major
player in the armamentarium of modernized precision medicine
(2). This novel domain has even
aroused public debates on whether to include the letter ‘g’ in this
new term; the portmanteau word ‘theragnostics’ is more
lexicologically justified (3).
Cancer liquid biopsy with possible
drawbacks
As regards diagnosis in experimental and clinical
oncology, cancer liquid biopsy (CLB) presents an innovative rapidly
advancing high technology. Conventional histopathology remains the
clinical gold standard for cancer diagnosis, of which invasive
tissue biopsy is potentially risky, with limited sample
accessibility and narrowed images for the entire heterogeneous
tumor profiles. On the contrary, CLB presents a non-invasive or
minimally invasive technique for the early detection of circulating
tumor-derived components from the patient (4,5).
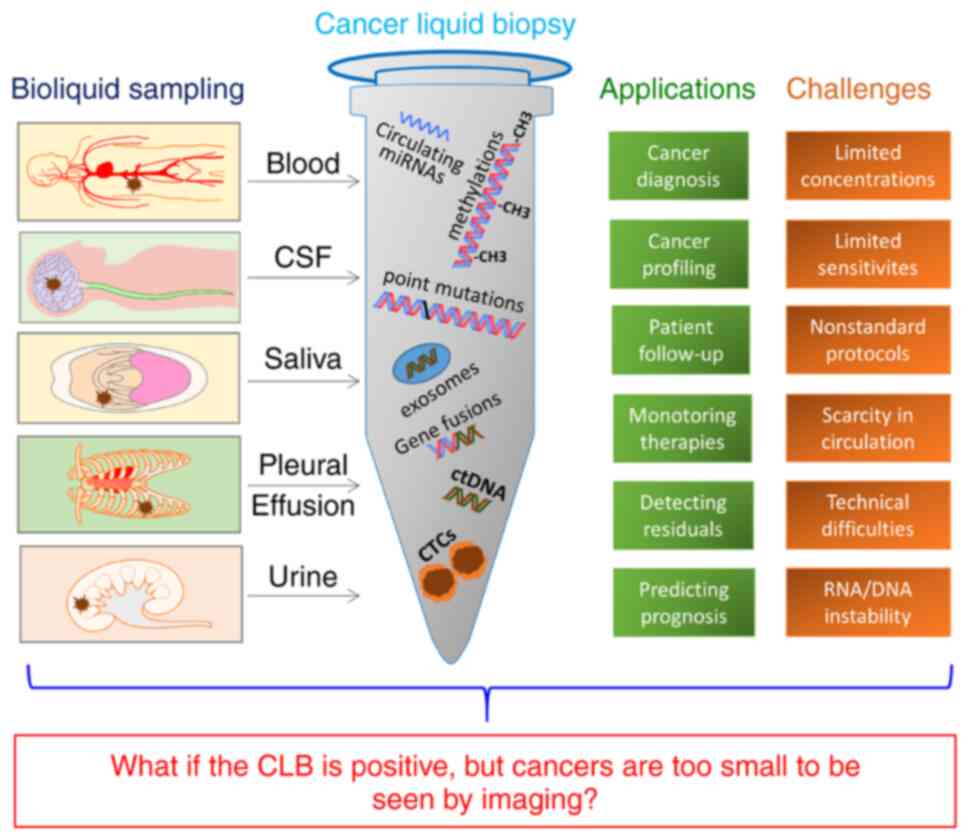
As shown in Fig. 1,
for CLB, the biofluids from the blood, cerebrospinal fluid, saliva,
pleural effusion, urine etc. are sampled and processed to identify
circulating tumor cells, subcellular structures such as
tumor-specific exosomes, circulating micro ribonucleic acid,
circulating tumor deoxyribonucleic acid, and genomic and
transcriptomic alterations including gene fusions, point mutations,
methylations etc. CLB is useful for the screening, diagnosis and
profiling if cancers, monitoring therapies and following-up
patients, as well as for detecting tumor residuals and predicting
prognoses. Current progress regarding CLB suggests that it may have
an impact on the clinical outlook of several tumor types in the
near future (4,5). However, despite increasing evidence
supporting CLB as a valuable oncological tool (4,5),
certain issues such as method sensitivity and substance scarcity or
instability etc., still need to be resolved in order for CLB to be
implemented as a clinical routine. For instance, plausible
discrepancy between positive CLB outcomes and not yet available
clinical countermeasures could lead to complex scenarios, i.e., in
the case that the super-sensitive CLBs clearly indicate the
existence of malignant tumors in patients, but the cancers are too
small to be detected by currently available imaging modalities
(i.e., micro-cancers). This then may alarm, confuse or dishearten
patients and their families, instead of helping or comforting them.
Therefore, it is to address such a possible CLB-related clinical
issue that a potentially revolutionary solution has been proposed
in the present brief overview article. One of the aims of the
present study was to promote multi-institutional verifications on
this straightforward approach with collective expertise and
infrastructure.
OncoCiDia as a novel anticancer theragnostic
strategy
To combat solid cancers, a small molecule
dual-targeting pan-anticancer theragnostic strategy has been
elaborated to exploit the power of the natural cancer targetability
(6,7), as acronymized using ‘OncoCiDia’ where
Onco- stands for cancers, -Ci- for killing and -Dia for imaging
diagnosis (8). This aims to offer
a safe, simple, workable, affordable and generic solution for
diverse types of cancer, and warrants further exploitation. The
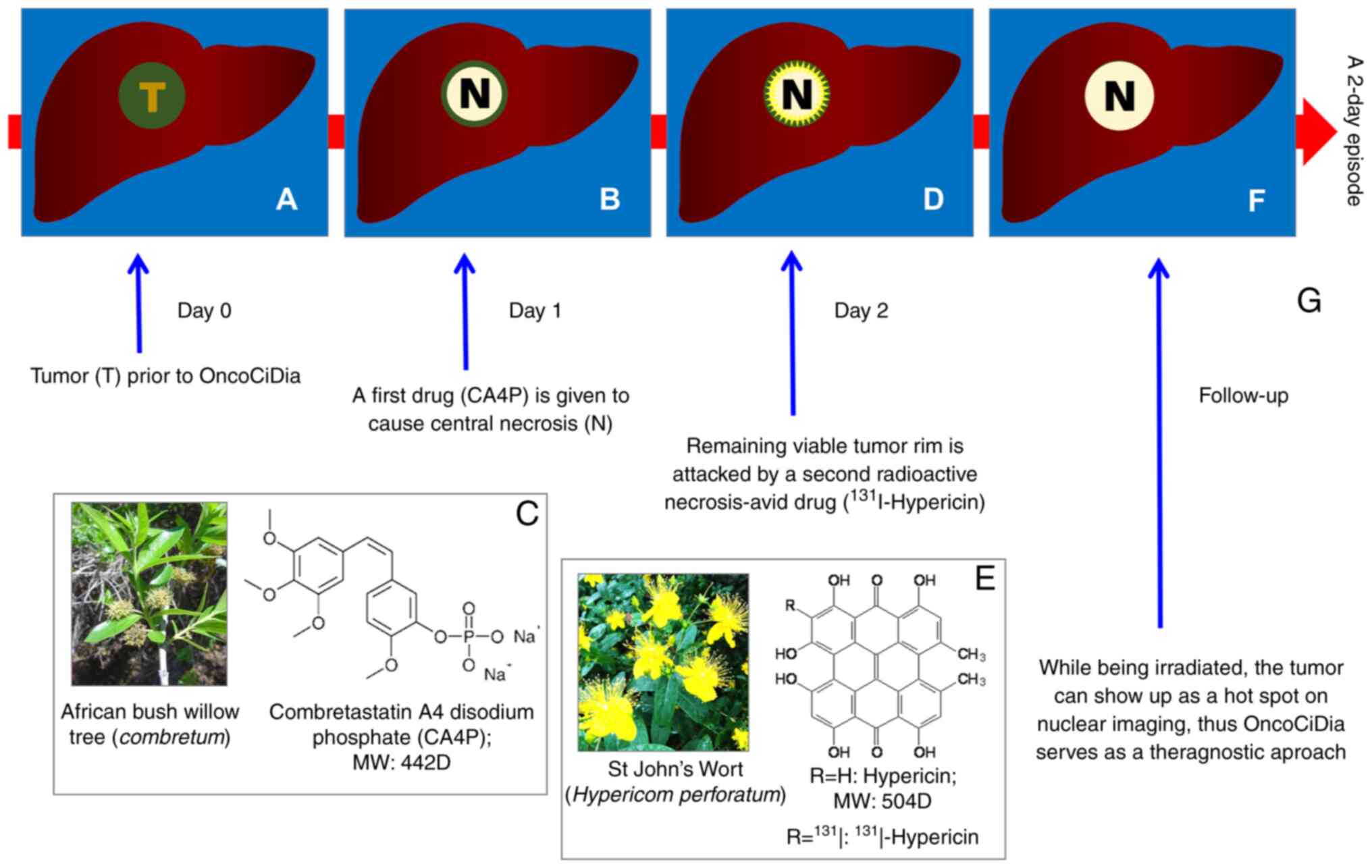
mechanisms of action for OncoCiDia are illustrated in Fig. 2 by the simulation of a liver cancer
(Fig 2A). OncoCiDia consists of
two sequential systemic drug-deliveries accomplished only within
one episode of two consecutive days. On day 1, a cancer-targeting
drug termed vascular disrupting agent (VDA), represented by
combretastatin A4 phosphate (CA4P) that was originally discovered
from African bush willow with a broad native anticancer spectrum,
is intravenously injected to induce massive tumor ischemic necrosis
(Fig. 2B and C) by the selective
shutdown of tumoral blood vessels via depolymerizing the defected
endothelial tubulin cytoskeleton (9).
However, VDA-induced tumor necrosis is only partial
and always leaves residual viable cells behind for cancer relapse
(Fig. 2B), which hinders the
efficacy and authority approval of VDA, despite advanced clinical
trials (9). Nevertheless, such
tumoral necrosis forms an ideal target for the second targeting
compound termed hypericin (Hyp), which is naturally extracted from
St. John's wort (Fig. 2E) and also
chemically synthesizable with several prior known medicinal
applications such as anti-depression, antivirus, photosensitivity,
etc. However, Hyp has been newly found with a strong
necrosis-avidity with exploitable novel utilities (6,10).
Thus, on day 2, the tumouricidal iodine-131 (131I) is
radiochemically labeled onto Hyp to form an
131I-conjugated necrosis avid tracer
(131I-Hyp), which is then intravenously infused to
selectively localize in and strongly bind to VDA-induced necrosis,
while emitting high-energy β−-particles with 2-mm
penetration and an 8-day decaying half-life to exert constant
eradiation on remaining cancer cells by inducing their DNA damage
(Fig. 2D and F). Moreover, its
gamma rays facilitate scintigraphy imaging (Fig. 2G), hence being truly a theragnostic
strategy. The non-necrosis-bond 131I-Hyp can be
eliminated via the hepatobiliary pathway, particularly when aided
by certain safety measures, such as nasobiliary drainage (11,12).
Since both the targets, namely abnormal tumoral vasculature and
intratumoral necrosis, are naturally occurring and generic to all
solid cancers, OncoCiDia has proven to be a truly pan-anticancer
strategy (8).
Internal radiotherapy using radioiodine
(131I) is known to be curative for the majority of
thyroid cancers, even at their metastatic stages (13). OncoCiDia was actually intended to
extend such an excellent efficacy to the treatment of virtually all
solid malignancies, due to its unique dual targetability and
complementary synergy. The anticancer efficacy of systemically
administered internal radiotherapy relies on the cumulative
radiation dose in the target tumor. The potent targetability of
131I-Hyp to the VDA-induced tumoral necrosis in
OncoCiDia could render a therapeutically required cumulative
radiation dose >50 Gy (6,7),
consistent to that shown with curative treatment of differentiated
thyroid cancers by iodine-131 (13). Currently, early clinical trials of
OncoCiDia have been ongoing among both veterinary (https://www.dierenartsenwereld.be/nl/nieuws–n2/ugent–zoekt–honden–met–-kwaadaardige–tumoren–i171/;
accessed on November 28, 2022) and human (OncoCiDia Phase 0 study
(3M150468; https://www.kuleuven.be/onderzoek/portaal/#/projecten/3M150468?hl=en&lang=en;
accessed on December 22, 2022) patients, with curative potentials
revealed in patients after only one episode of OncoCiDia, e.g., in
one patient with a massive inoperable esophageal squamous cell
cancer. Thus, OncoCiDia can most likely solve the bottleneck issues
of the unmet therapeutic demand common for all those VDAs currently
under preclinical and clinical developments.
Serendipitous findings indicative of a
potential cure for early-stage cancers
Although having exhibited extraordinary anticancer
potential, OncoCiDia is still considered largely as a palliative
care for patients with late-stage disease. However, as demonstrated
in Fig. 3, in recent studies,
particularly among those on hypovascular and avascular
micro-cancers (Fig. 3A1-A4 and
B1-B4), it has been serendipitously found that CA4P caused
almost complete necrosis in the majority of micro-cancers in
millimeter scales (Fig. 3C1-C5 and
D1-D5) (14,15), which is contradictory to what has
been known in the literature regarding VDAs, i.e., improved VDA
therapeutic effects were positively associated with the increasing
tumor volume with a larger amount of abnormal tumoral vasculature
as the VDA target (16). It now
appears that those immature vascular structures of CD34 positively
stained endothelium (Fig. 3B5, C5 and
D5) play a crucial role in sustaining the growth of very early
stage solid cancers, which in turn can be effectively targeted and
destroyed by VDAs too, leading to massive proportions of
intratumoral necrosis with merely few layers of viable cancer cells
left at the tumor periphery (Fig.
3C1-C5 and D1-D5) among the observed micro-cancers that are
beyond the resolution of clinical imagers.
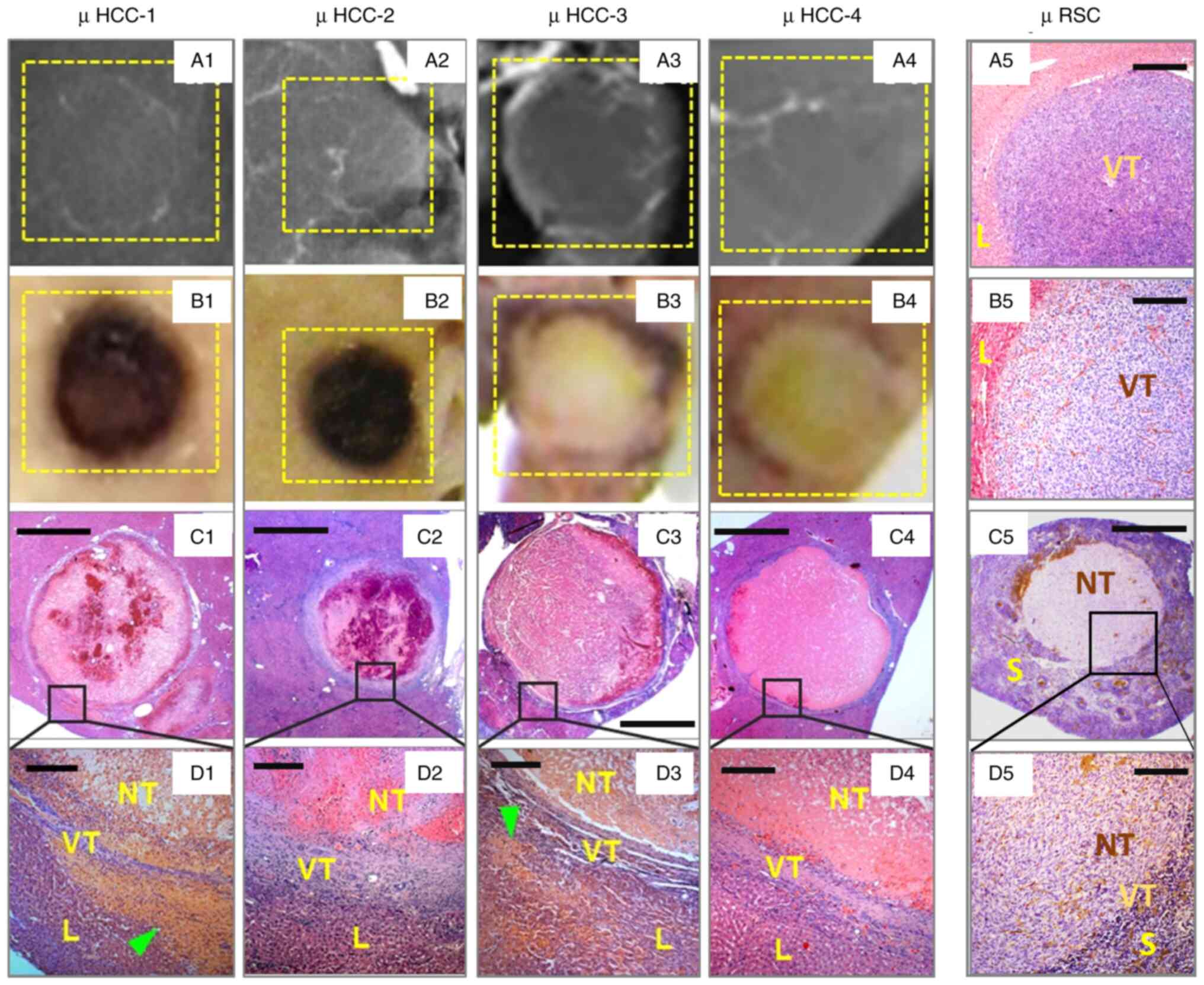 | Figure 3.Incidental findings of surprising VDA
efficacy in hypo- or avascular micro-cancers. Typical rat cases
with (A1-A4) digital radiographic, (B1-B4) macroscopic, (C1-C5) low
magnification and (D1-D5, and A5 and B5) high magnification views
of micro-hepatocellular carcinoma (µHCC-1 to µHCC-4), and a
micro-rhabdomyosarcoma (µRSC) from an untreated control rat with
liver implantation following (A5) hematoxylin and eosin staining
and (B5) endothelial transmembrane glycoprotein
immunohistochemistry or CD34 staining, and from a VDA-treated rat
with the splenic implantation of µRSC following (C5) hematoxylin
and eosin and (D5) CD34 staining, as previously described (14). In general, these micro-tumors are
(A1-A4) hypovascular or avascular, exhibiting typical VDA-induced
(B1 and B2) hemorrhagic or (B3, B4 and C5) coagulative necrosis, as
(D1-D5) microscopically proven, (D1-D4, A5, B5, C5 and D5) lacking
apparent structural tumor blood vessels, (B5, C5 and D5) but
presenting a positively stained intratumoral endothelium. L, liver;
VT, viable tumor; NT, necrotic tumor; S, spleen. (C1-C5) Scale bar,
1 mm; (D1-D5, and A5 and B5) scale bar, 50 µ. Some images have been
adapted from a previous study by the authors (14). |
All naturally occurring malignant tumors undergo the
avascular-hypovascular-hypervascular steps of cancerous
angiogenesis according to Folkman's theory (17), and tumor vascularity can be defined
by the density of tumoral blood vessels or intensity of tumor blood
perfusion, relative to those of the host tissue or organ, as
measured by different imaging modalities (18). Accordingly, almost all
micro-cancers appear avascular or hypovascular (Fig. 3A1-A4) (14,15).
However, Hori et al (19)
made an artificial LY80 micro-tumor model by seeding tumor tissue
into a prior-implanted transparent chamber to better observe in
vivo tumor growth and drug reaction. However, the earlier
chamber-surgery had already stimulated angiogenesis and thus
speeded up the vascularization process of the later implanted tumor
tissue, leading to a strange phenomenon of hypervascular
micro-tumors, as observed in the studies by Hori et al
(19) and Maeda (20) studies on the related topics.
Therefore, such an artificial tumor model is not relevant for the
interpretations of drug reactions in the natural avascular and
hypovascular micro-tumors, and cannot help to predict the efficacy
of VDAs among true avascular and hypovascular micro-tumors in
animals (14,15) and humans.
The aforementioned experimental findings suggest
that the generally palliative OncoCiDia could become a curative
dual-targeting chemo-radio-ablation means with which to eradicate
all solid cancers, particularly at their infancy or ‘micro-tumor’
stages (15,21).
Rationale of OncoCiDia for eradicating
micro-cancers with the combined use of CLB
Given the aforementioned clues that: i) CLB may help
detect the presence of solid malignancies that may be too small to
identified in the body, which may trouble clinical management; and
ii) micro-cancers may well respond to VDAs with possible curative
outcomes once adjuvantly treated with 131I-Hyp, it can
be hypothesized that sub-centimeter cancers could be cured blindly
in animals and humans by OncoCiDia under serial CLB surveillance,
as coined by an international patent application (21). A possible scenario may occur in
which a CLB confirms positive result for a certain type of cancer
that fails to be detected by even a whole-body PET/CT scan from a
patient who is then subjected to an episode of OncoCiDia; a few
weeks later if a follow-up CLB yields a negative result, this
patient has likely been cured of that cancer. To verify this
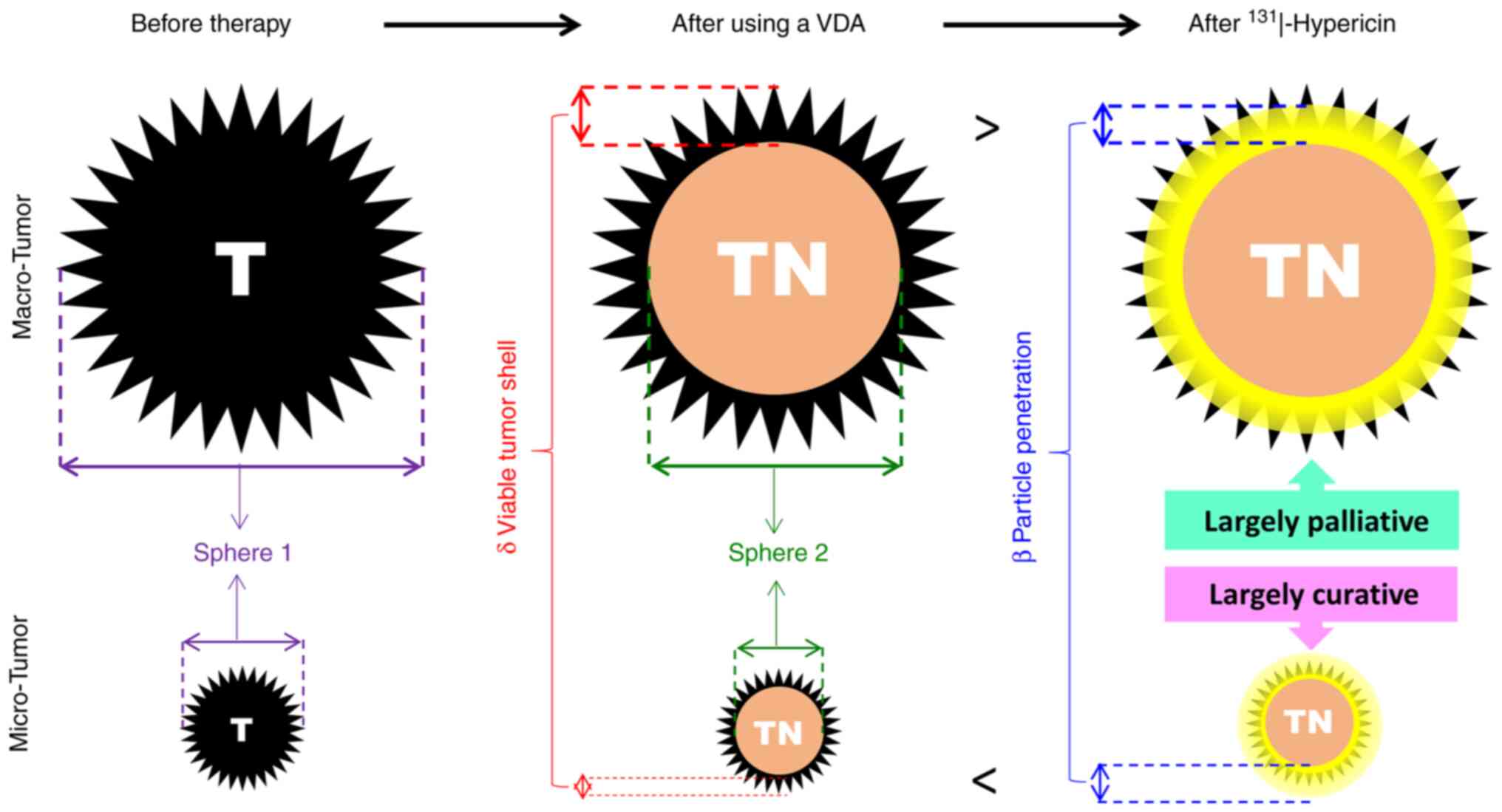
hypothesis, a geometric model has been established by considering
multiple factors of pharmacology, radiophysics, radiobiology and
mathematics, as illustrated in Fig.
4.
In Fig. 4, the left
column is assigned for tumors before therapy, where a large (upper)
and a small (lower) sized thorny sphere 1 simulates a macro and
micro tumor (T), respectively with an invasive growth pattern
characteristic of malignant tumors. The middle column refers to the
same tumors following treatment with a VDA, which is known to
induce massive tumor necrosis (TN) denoted by the inner sphere 2
with a viable shell (δ) at tumor periphery. The right column
simulates the same tumors that are further treated by the
intravenous administration of 131I-Hyp that accumulates
in the central necrosis (TN), particularly at the dead-alive
interface, and emits high energy β particles that penetrate to ~2
mm in depth, which may be shorter (<) than the thickness of the
viable tumor shell (δ), leading to a largely palliative effect in
macro-tumors, but can be surely long enough (>) to cover the
entire remaining cancer cells in all micro-tumors, achieving
virtually total cancer cure. The detailed geometric deductions are
illustrated in the lower part of Fig.
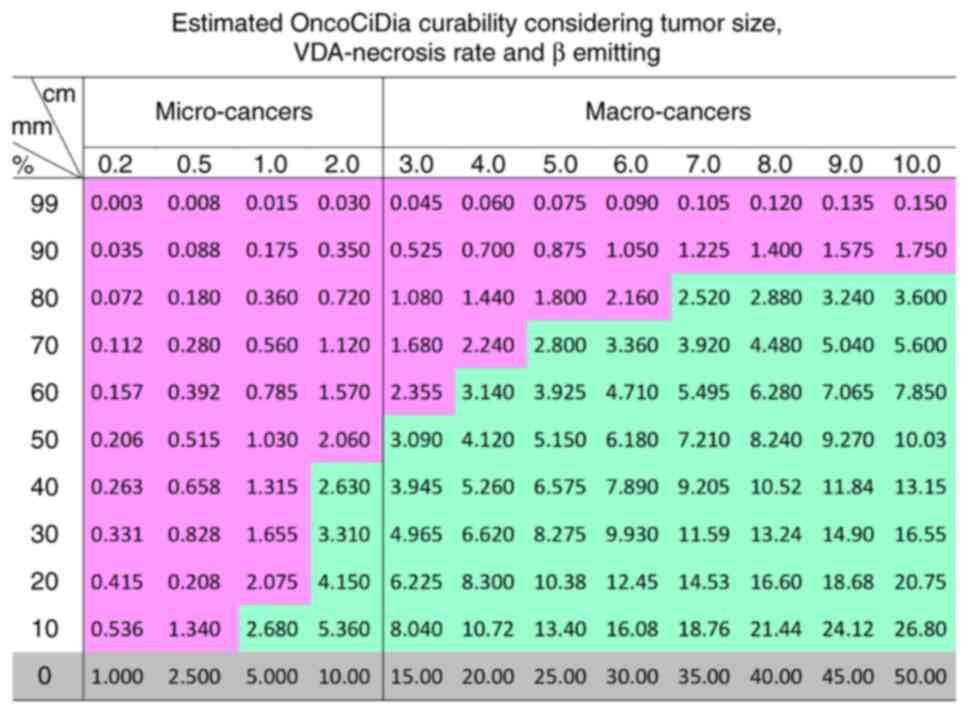
4 with the estimated curability of OncoCiDia among both micro-
and macro-cancers shown in Fig. 5
of a colored comparative table where all micro cancers with >50%
VDA-induced necrosis would be cured, which however have to be first
validated by ongoing animal experiments, then replicated by other
research groups, and eventually further confirmed by clinical
trials in cancer patients.
Distinguishing primary from metastatic tumors poses
currently unsolved experimental and clinical issues, not to mention
differentiation between micro-tumors of metastatic and primary
origins, which are all under intensive multidisciplinary studies
(22–26). However, the simple use of OncoCiDia
combined with CLB to systemically eliminate all hypo- or avascular
micro-tumors (whether primary or metastatic) may help to circumvent
such complex cancer problems.
Conclusive remarks
Complementary to the newly emerging high technology
of CLB, a novel technique OncoCiDia for the dual-targeting
chemo-radio-ablation of generic micro-cancers is proposed by
exploiting the natural existence of pan-anticancer targetability,
efficacy and simplicity. First, this approach closely follows the
natural rules of cancer pathology and mechanisms: i) All solid
cancers rely on the proliferated aberrant neovasculature for
supporting their growth, which leaves a specific defect for
therapeutic intervention by VDAs (8); ii) as recently reported, those
immature ‘prevascular’ endothelial structures observed in
hypovascular and avascular micro-cancers can also be attacked by
VDAs (14,15) (Fig.
3); and iii) tumor necrosis occurs both therapeutically and
spontaneously in all solid cancers, which can be targeted by
necrosis-avid agents that had been upgraded into radionuclide
theragnostics (6–8). Secondly, this approach involves small
molecular therapeutic elements, such as CA4P, Hyp and iodine-131
that are originally derived from the nature with unlimited
economical resources and favorable safety profiles if applied
properly (6–16,). As demonstrated by the preclinical modelling in
the present study (Figs. 4 and
5), the combination of CLB and
OncoCiDia may bring about an alternative cancer cure, particularly
at an early imaging-undetectable stage, which could imply a
paradigm shift in oncology and warrants further investigations to
substantiate and optimize its efficacy, safety and applicability.
Safety-wise: i) CA4P in multiple doses has undergone advanced
clinical trials (9,16), whereas only a single dose is needed
for OncoCiDia; ii) nearly a micro-dose of Hyp is used in OncoCiDia
with negligible chemotoxicity; and iii) the same dose of iodine-131
has been used for over the last half-century in the treatment of
thyroid cancer, hence a relatively safe profile with OncoCiDia.
Acknowledgements
The author would like to acknowledge all the
collaborative colleagues and his postdoctoral and PhD fellows
inside and outside KU Leuven, Belgium, in particular, Dr Yansheng
Jiang for his assistance with the geometric analysis; Dr Yi Miao,
Dr Feng Chen, Dr Yue Li, Dr Humphrey Fonge, Dr Marie Van de Putte,
Dr Huaijun Wang, Dr Marlein Miranda Cona, Dr Junjie Li, Dr Yuanbo
Feng, Dr Yewei Liu, Dr Ting Yin, Dr Stefaan Mulier, Dr Eline Abma,
Dr Shuncong Wang and Dr Lei Chen for their joint productive
research over the past decades; and Dr Jie Yu, who is his wife and
colleague, for her assistance in histopathology during the research
and preparation of the manuscript.
Funding
The author has been entitled a BAYER-Schering Lecture Chair for
two consecutive rounds over a period of 6 years with financial
support, which partially substantiated the research described
herein. Oncocidia Ltd., UK has partially supported preclinical
research on OncoCiDia project.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Author's contributions
YN has made sole contributions to the conception,
design of the work, the acquisition, analysis, and interpretation
of data, has drafted the manuscript and revised it, made the
decision to submit this manuscript, and will be personally
accountable for the author's own contributions and to ensure that
questions related to the accuracy or integrity of any part of the
work, are appropriately investigated, resolved, and the resolution
documented in the literature. The author has read and approved the
final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
All animal experiments were carried out in
compliance with the European and national regulations after
obtaining approval from the KU Leuven Ethics Committee for Animal
Care and Use, with particular codes P161/2014 and P046/2019,
respectively.
Patient consent for publication
Not applicable.
Competing interests
The author YN is a sole inventor of a pending patent
application as cited in ref 21 of this manuscript. The host
institute KU Leuven, Belgium, to which he belongs, is the IP owner
of this patent application, which has now been licensed to
Oncocidia Ltd., London, UK.
Glossary
Abbreviations
Abbreviations:
|
CA4P
|
combretastatin 4 phosphate
|
|
VDAs
|
vascular-disrupting agents
|
|
CLB
|
cancer liquid biopsy
|
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marin JF, Nunes RF, Coutinho AM, Zaniboni
EC, Costa LB, Barbosa FG, Queiroz MA, Cerri GG and Buchpiguel CA:
Theranostics in nuclear medicine: Emerging and Re-emerging
integrated imaging and therapies in the era of precision oncology.
Radiographics. 40:1715–1740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frangos S and Buscombe JR: Why should we
be concerned about a ‘g’? Eur J Nucl Med Mol Imaging. 46:5192019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pinzani P, D'Argenio V, Del Re M,
Pellegrini C, Cucchiara F, Salvianti F and Galbiati S: Updates on
liquid biopsy: Current trends and future perspectives for clinical
application in solid tumors. Clin Chem Lab Med. 59:1181–120. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crosby D: Delivering on the promise of
early detection with liquid biopsies. Br J Cancer. 126:313–315.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cona MM, Oyen R and Ni Y: Necrosis avidity
of organic compounds: A natural phenomenon with exploitable
theragnostic potentials. Curr Med Chem. 22:1829–1849. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Sun Z, Zhang J, Shao H, Cona MM,
Wang H, Marysael T, Chen F, Prinsen K, Zhou L, et al: A dual
targeting anticancer approach: Soil and seed principle. Radiology.
260:799–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ni Y: Oncocidia: A small molecule dual
targeting pan-anticancer theragnostic strategy. Cancer Res.
74:17672014. View Article : Google Scholar
|
|
9
|
Siemann DW, Chaplin DJ and Walicke PA: A
review and update of the current status of the
vasculature-disabling agent combretastatin-A4 phosphate (CA4P).
Expert Opin Investig Drugs. 18:189–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ni Y, Huyghe D, Verbeke K, de Witte PA,
Nuyts J, Mortelmans L, Chen F, Marchal G, Verbruggen AM and Bormans
GM: First preclinical evaluation of mono-[123I]iodohypericin as a
necrosis avid tracer agent. Eur J Nucl Med Mol. 33:595–601. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cona MM, Feng YB, Verbruggen A, Oyen R and
Ni Y: Improve clearance of radioiodinated hypericin as a targeted
anticancer agent by using a duodenal drainage catheter in rats. Exp
Biol Med (Maywood). 238:1437–1449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Jiang C, Jiang X, Sun Z, Cona MM,
Liu W, Zhang J and Ni Y: Biliary and duodenal drainage for reducing
the radiotoxic risk of antineoplastic 131I-hypericin in
rat models. Exp Biol Med (Maywood). 240:1764–1773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verburg FA, Flux G, Giovanella L, van
Nostrand D, Muylle K and Luster M: Differentiated thyroid cancer
patients potentially benefitting from postoperative I-131 therapy:
A review of the literature of the past decade. Eur J Nucl Med Mol
Imaging. 47:78–83. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Yin T, De Keyzer F, Feng YB, Chen
F, Liu JJ, Song SL, Yu J, Vandecaveye V, Swinnen J, et al:
Micro-HCCs in rats with liver cirrhosis: paradoxical targeting
effects with vascular disrupting agent CA4P. Oncotarget.
8:55204–55215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang S, Liu Y, Feng Y, Zhang J, Swinnen J,
Li Y and Ni Y: A review on curability of cancers: More efforts for
novel therapeutic options are needed. Cancers (Basel). 11:17822019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garon EB, Neidhart JD, Gabrail NY, de
Oliveira MR, Balkissoon J and Kabbinavar F: A randomized Phase II
trial of the tumor vascular disrupting agent CA4P (fosbretabulin
tromethamine) with carboplatin, paclitaxel, and bevacizumab in
advanced nonsquamous non-small-cell lung cancer. Onco Targets Ther.
9:7275–7283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Folkman J, Long DM Jr and Becker FF:
Growth and metastasis of tumor in organ culture. Cancer.
16:453–467. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugimoto K, Kim SR, Imoto S, Tohyama M,
Kim SK, Matsuoka T, Yano Y, Kudo M and Hayashi Y: Characteristics
of hypo-vascular versus hypervascular well-differentiated
hepatocellular carcinoma smaller than 2 cm-focus on tumor size,
markers and imaging detectability. Dig Dis. 33:721–727. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hori K, Nishihara M, Shiraishi K and
Yokoyama M: The combretastatin derivative (Cderiv), a vascular
disrupting agent, enables polymeric nanomicelles to accumulate in
microtumors. J Pharm Sci. 99:2914–2925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeda H: Vascular permeability in cancer
and infection as related to macromolecular drug delivery, with
emphasis on the EPR effect for tumor-selective drug targeting. Proc
Jpn Acad Ser B Phys Biol Sci. 88:53–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ni Y: Treatment of avascular or
hypovascular micro-tumors. US Patent 16/628,904, Filed. July 4–2018
issued. January 10–2019.
|
|
22
|
Cacho-Díaz B, García-Botello DR,
Wegman-Ostrosky T, Reyes-Soto G, Ortiz-Sánchez E and
Herrera-Montalvo LA: Tumor microenvironment differences between
primary tumor and brain metastases. J Transl Med. 18:12020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Osako T: How can we better distinguish
metastatic tumors from primary tumors in the breast? Expert Rev
Anticancer Ther. 21:913–916. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim R, Keam B, Kim S, Kim M, Kim SH, Kim
JW, Kim YJ, Kim TM, Jeon YK, Kim DW, et al: Differences in tumor
microenvironments between primary lung tumors and brain metastases
in lung cancer patients: Therapeutic implications for immune
checkpoint inhibitors. BMC Cancer. 19:192021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ikarashi D, Okimoto T, Shukuya T, Onagi H,
Hayashi T, Sinicropi-Yao SL, Amann JM, Nakatsura T, Kitano S and
Carbone DP: Comparison of tumor microenvironments between primary
tumors and brain metastases in patients with NSCLC. JTO Clin Res
Rep. 2:1002302021.PubMed/NCBI
|
|
26
|
Pearce OM, Delaine-Smith RM, Maniati E,
Nichols S, Wang J, Böhm S, Rajeeve V, Ullah D, Chakravarty P, Jones
RR, et al: Deconstruction of a metastatic tumor microenvironment
reveals a common matrix response in human cancers. Cancer Dis.
8:304–319. 2018. View Article : Google Scholar : PubMed/NCBI
|