Cancer is the main cause of death in the world and
there is even a view that the world is experiencing a cancer
epidemic (1). In 2018, China had
4.3 million new cancer cases and 2.9 million cancer deaths
(2). The huge economic burden puts
notable pressure on the medical system and patient wellbeing.
Surgical treatment is considered to be the most effective way to
treat tumors; after the advent of chemotherapy in 1940 and targeted
therapy in the late 1990s, immunotherapy is the third important era
of cancer treatment (3). In the
past few decades, anti-cancer immunotherapy has transformed from an
emerging tumor treatment theory to a well-known alternative tumor
therapy. Anti-cytotoxic T-lymphocyte-associated antigen (CTLA)-4
and anti-programmed death receptor 1 (PD)-1/PD-ligand (L) 1, as
immunotherapy, for the treatment of melanoma, colorectal cancer and
breast cancer plays an important role (4–6).
Immunotherapy refers to the method of artificially
enhancing or inhibiting the immune function of the body to cure
diseases. Tumor immunotherapy is based on the immune surveillance
theory proposed by Frank Macfarlane Burnet and Lewis Thomas
(7). The theory of immune
surveillance posits that the system can play a surveillance role to
identify and eliminate foreign components or mutant cells that
express new antigens to maintain the stability of the host
environment. When the immune function is low and cannot effectively
eliminate foreign or mutated cells, tumors may occur (8). Dunn et al (9) put forward the theory of immune
editing, which further improved the framework of tumor immunity.
The immunoediting theory posits that the development of tumors
needs to go through three stages: Immune clearance, balance and
escape (10). Tumor cells that can
escape the immune system may survive natural selection. If tumors
are regarded as immunogens, decades of research have not found
valuable tumor antigen-regulated immune escape theory, and a large
number of experiments have proved that tumor stem cells with
reduced expression of tumor antigens further prove that the search
for tumor-specific antigens or wrong research direction (11,12).
Hypoxia at the tumor site may cause attenuation of tumor immunogens
(13). It can hypothesized that the
immune system recognizes and destroys tumor cells expressing strong
immunogenicity, while tumor cells with weak (or no) immunogenicity
selectively survive and eventually form tumors. Immunotherapy may
become the most advantageous tool to overcome this (14). The relationship between the immune
system and tumors is complicated. In 1891, American doctor William
Coley discovered that postoperative infection of Streptococcus
pyogenes in patients with sarcoma could cause tumor regression.
This discovery provided a new idea for cancer immunotherapy
(15). With the emergence of new
technologies such as humanized antibodies, virus packaging and gene
high-throughput sequencing, tumor therapy has achieved rapid
development. This review summarizes strategies for immunotherapy to
treat cancer (16,17).
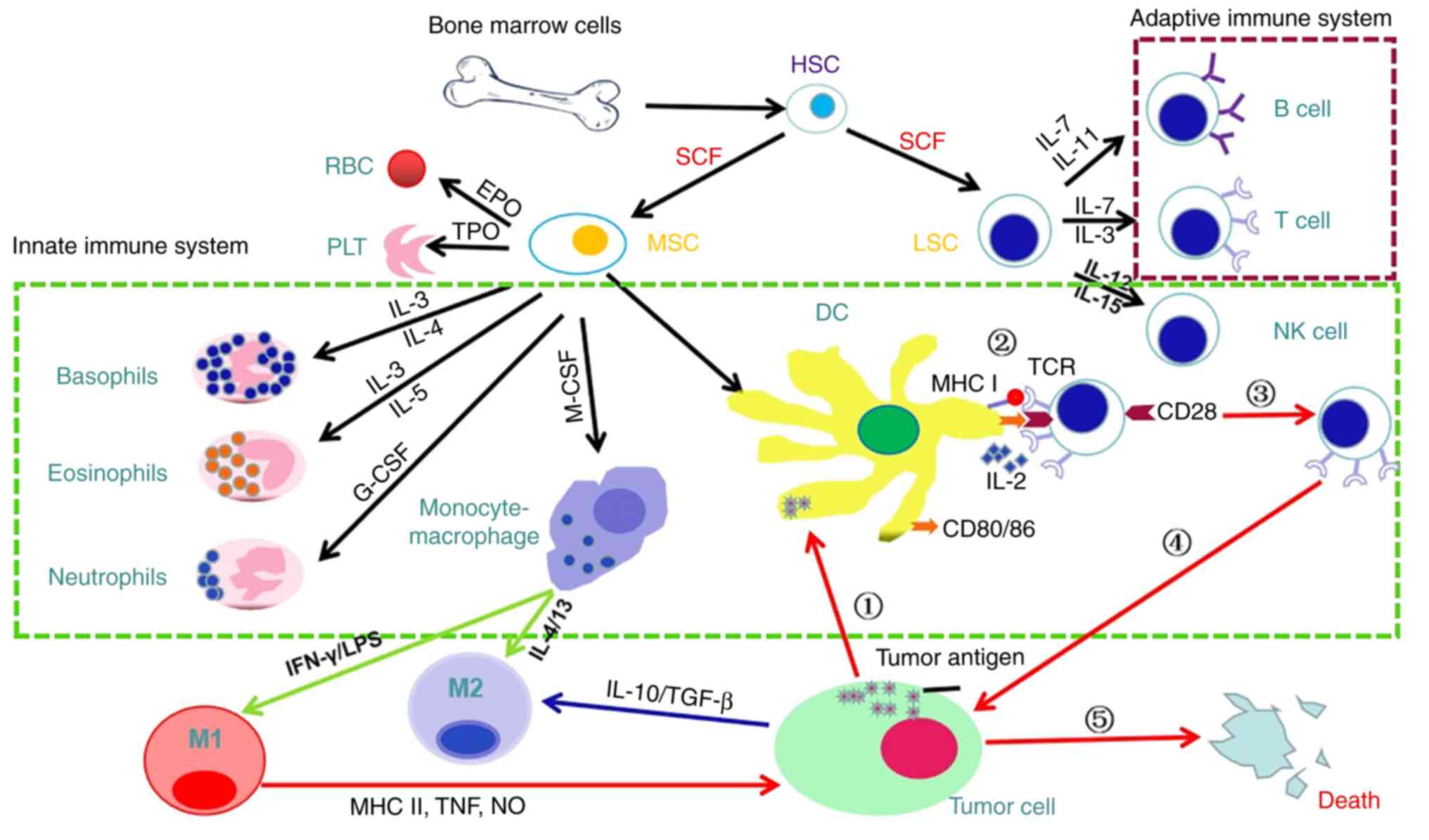
The immune system consists of immune organs (bone
marrow, thymus, spleen, lymph nodes, tonsils, small intestinal
Peyer's lymph nodes, appendix, thymus, etc.), immune cells
(lymphocytes, mononuclear phagocytes, neutrophils, basophils,
eosinophils, etc.) Granulocytes, mast cells, platelets, etc.), and
immune molecules (complement, immunoglobulin, interferon,
interleukin, tumor necrosis factor and other cytokines, etc.)
(18). The immune system recognizes
and eliminates antigenic foreign bodies, coordinating with other
systems of the body, and maintaining the stability of the host
environment and physiological balance (19). Immune organs are be divided into
central (bone marrow and thymus) and peripheral immune organs
(spleen, lymph nodes and tonsils); immune cells occur,
differentiate and mature in central organs and B lymphocytes
colonize and proliferate in peripheral organs, where the immune
response primarily occurs (20,21).
Immune cells comprise innate (dendritic (D) and natural killer (NK)
cells and macrophages) and adaptive immune cells (T and B cells)
(22). Immune molecules comprise
membrane-type (such as T and B cell receptor (CR), adhesion and
major histocompatibility complex (MHC) molecules and cytokine
receptors) and secreted molecules (such as immunoglobulin,
complement and cytokines) (23,24).
The most important function of the innate immune system is to
respond quickly to infection or inflammation and to recruit innate
immune cells or activate complement via cytokines secreted by the
injured site (such as ILs and chemokines) (25). Both B and T cells originate from a
common lymphoid progenitor cell (B cells mature in the bone marrow
and T cells mature in the thymus) and mediate humoral and cellular
immunity, respectively (26). B
cells participate in production of antibodies, and T cells
participate in proliferation of B cells, directly attack pathogens
and regulate immune responses (27). Adaptive immunity is associated with
immune memory and long-term effects of the immune system and serves
a key role in fighting tumors (28).
The tumor antigen-specific T cells produced by
adaptive immunity are considered to be the key factor in killing
tumors (29). This process is
inseparable from the innate immune response and includes the
following steps: Phagocytes engulf and digest tumor cells to
produce tumor antigens; antigen-presenting cell (APC)
cross-presentation of tumor antigens to T cells; initiation and
activation of initial T cells; transport and infiltration of
activated T cells into the tumor microenvironment (TME) and
activated CTL-mediated malignant cell death. The generation of
tumor antigen-specific T cells reflects the coordination between
the innate and the adaptive immune system (30–33).
This dynamic interaction is guided by the phenotype and function of
innate immune cells to influence tumor antigen-specific T cells,
resulting in different biological states (tolerance or
responsiveness) (34). As the APC
in TME, DCs initiate cancer immunity by cross-presenting
tumor-associated antigens to naive T cells (35). Although antigen-loaded DCs are
potent stimulators of T cell activation, DCs activate
antigen-specific CTL expansion through the CD40/CD40L pathway
(36). CD40 is a member of the
tumor necrosis factor (TNF) receptor superfamily that is expressed
in large quantities on the DC membrane. After recognizing its
homologous antigen, CD4+ T helper (Th) cells can express
CD40L and then combine with the complementary CD40. After
activation, the expression of MHC II, CD80and CD86 on the surface
of DCs is increased, which supports T cell activation (37). Conventional DCs (cDCs) in mice are
divided into two lineages with different functions:
CD103+ cDC1 lineage is responsible for the initiation of
CD8+ CTL and CD11b+ cDC2 lineage is
associated with priming CD4+ Th cells (38,39).
In a melanoma mouse model, CD103+ cDC1s promote T cell
recruitment to the TME by releasing the chemokine CXCL9/10
(40). Similar to DCs, macrophages
are key innate immune cells that promote or hinder the activation
of effector T cells (41). This is
because their functional characteristics are affected by signals
from the surrounding microenvironment and the cell phenotype has
strong plasticity. IFN-γ and toll-like receptor agonists induce
differentiation into M1 phenotypes related to anti-tumor activity;
IL-4 and IL-13 induce differentiation into M2, which is associated
with tumor-promoting activity (42,43).
M1 macrophages are mainly involved in the immune response against
foreign pathogens and M2 macrophages help wound healing and secrete
anti-inflammatory cytokines (44).
In tumorigenesis and metastasis, M1 macrophages serve an adaptive
immune surveillance function, while M2 macrophages inhibit the
anti-tumor immune function of T cells (45). M1-like macrophages in TME
phagocytose tumor cells and present tumor antigens to initiate the
anti-tumor activity of CD8+ T cells (46). Macrophages residing in tissues other
than tumors can also transfer phagocytosed tumor antigens to DCs to
trigger an adaptive immune response by inducing CTL cross-reactions
(47). Macrophages are commonly
used by tumors to suppress adaptive immune responses. M2
macrophages inhibit T cell activation by secreting IL-10 to destroy
the TCR (48). In addition,
tumor-infiltrating macrophages express a variety of immune
checkpoint proteins (such as PDL1) that bind to T cell inhibitory
signal receptors to inhibit cell function (49). CD169+ macrophages capture
tumor antigens to prevent them inducing an immune response
(Fig. 1).
Anti-cancer immunotherapy is classified as passive
or active according to the ability to activate the host immune
system against malignant cells. Tumor-targeted monoclonal
antibodies and adoptively transferred T cells are considered
passive forms of immunotherapy because exhibit inherent anti-tumor
activity (50). Anti-cancer
vaccines and immune checkpoint inhibitors that only exert
anti-cancer effects when the host immune system is involved are
classic examples of active immunotherapy (51).
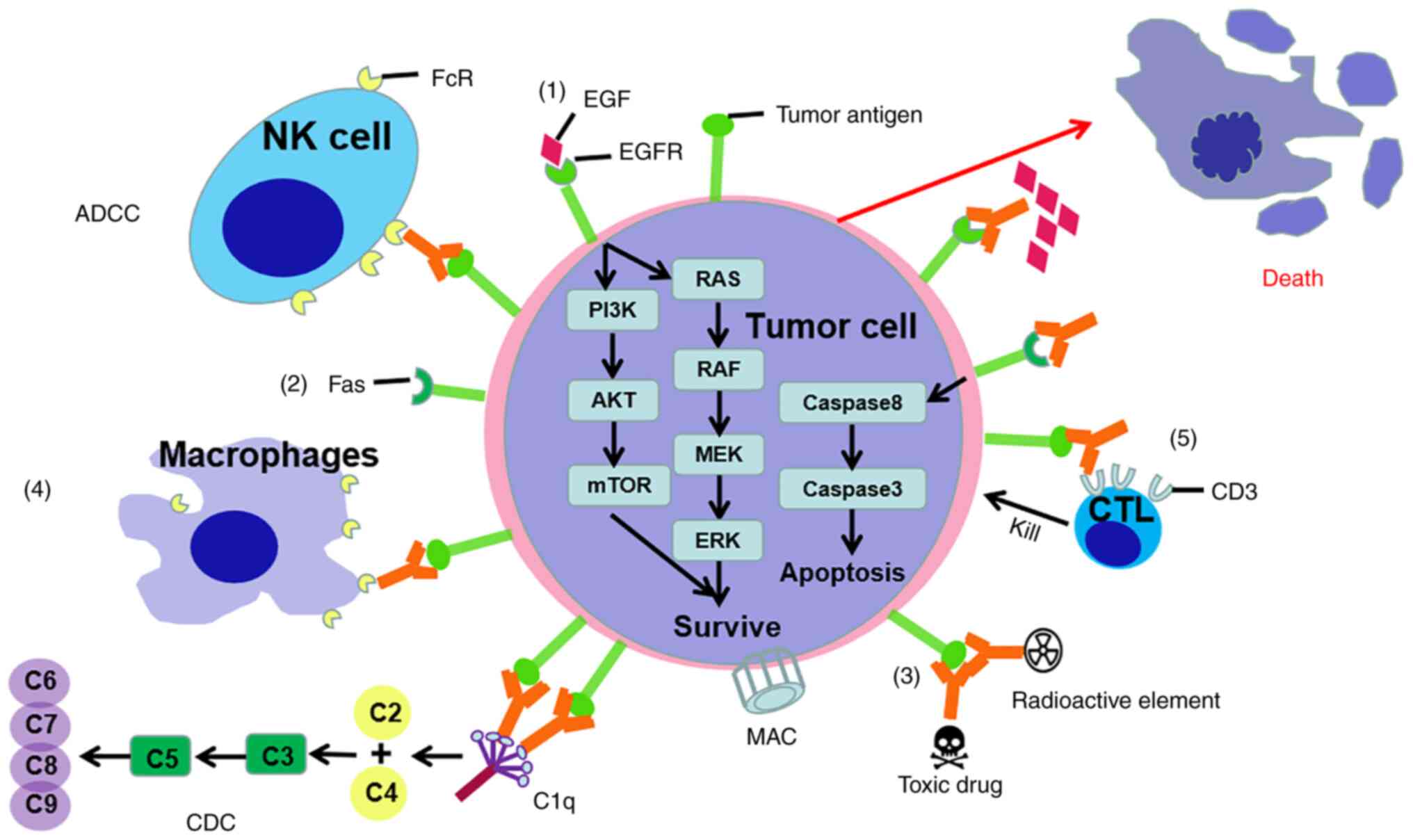
Immunoglobulins, also called antibodies, are the
first molecules involved in specific immune responses (52). Antibodies with unique specificities
that recognize different target molecules have been used to attack
tumor cells that express certain antigens (53). There are five mechanisms for
tumor-targeting antibodies to produce anti-tumor effects.
Therapeutic antibodies, such as epidermal growth factor receptor
(EGFR)-specific monoclonal antibody cetuximab for the treatment of
head and neck and colorectal cancer, inhibit the signaling pathways
required for tumor cell survival or progression. Therapeutic
antibodies, such as tigatuzumab, a monoclonal antibody specific to
TNF receptor superfamily member 10B, activate potentially lethal
receptors expressed on the surface of tumor cells. Immunoconjugates
(tumor antigen-specific antibodies conjugated to toxins or
radionuclides), such as gemtuzumab and ozogamicin (an anti-tumor
cell membrane-expressed CD33 calicheamicin conjugate approved for
use in patients with acute myeloid leukemia), directly kill tumor
cells. Simple antibodies directed against tumor-specific or
-associated antigens (such as rituximab, which is currently
approved for the treatment of chronic lymphocytic leukemia and
non-Hodgkin's lymphoma) work by activating antibody-dependent
cell-mediated cytotoxicity and cellular phagocytosis and
complement-dependent cytotoxicity. Bispecific T cell conjugates are
composed of two monoclonal antibodies from different monoclonal
antibodies (54–59). An artificially modified antibody,
the chimeric protein of its variable region, one targets tumor
cells, and the other specifically targets T cell surface antigens,
shortening the distance between T cells and tumor cells in space,
allowing T cells to directly Kill tumor cells. For example,
blinatumomab, a therapeutic antibody that targets the CD19 molecule
on tumor cells and the CD3 molecule on T cells, is used in the
treatment of Philadelphia chromosome-negative precursor B cell
acute lymphoblastic leukemia (60).
Approved antibodies targeting tumor cells (such as Catumaxoma) and
other antibody drugs (such as Veltuzumab) under development belong
to the IgG class. IgA molecules are also used as anticancer agents.
For example, the anti-EGFR IgA2 containing the variable region of
cetuximab significantly decreases the number of metastases in a
melanoma cell lung metastasis model of transgenic mice expressing
human EGFR (61). This effect of
IgA2 lasts a week longer than the corresponding IgG cetuximab
(62,63). IgE is another antibody class being
explored as a potential cancer treatment (64). The research on the function of using
IgE-mediated immune response against tumor cells in the context of
cancer belongs to the rapidly developing allergoncology field
(Fig. 2) (65).
Cell therapy refers to the transfer of autologous or
allogeneic cell material into the body for medical purposes
(66). Adoptive cell transfer is a
cell-based anti-cancer immunotherapy. The usual practice is to use
immune enhancers to activate blood circulation or
tumor-infiltrating lymphocytes to achieve the purpose of fighting
tumors (67). Other anti-cancer
immunotherapies involve live cell transfusion, such as
hematopoietic stem cell transplantation, to rebuild a healthy,
allogeneic immune system; adoptive cell transfer is the infusion
therapy of immune cells with potential antitumor immune activity
(68). Interventions based on DCs
are different from the aforementioned cell therapies. Infused DCs
do not have anti-cancer activity but can be used as anti-cancer
vaccines to trigger tumor-targeted immune responses (69). The cellular immune response against
tumors primarily depends on T cells. A large number of antigens
have been identified in tumors that are recognized by T cells,
suggesting the potential role of T lymphocytes in anti-tumor immune
responses (70). In certain
patients with melanoma or pancreatic cancer, Epstein-bar
virus-associated malignancy and murine tumor models, functional CTL
have been shown to fight tumor cells that express tumor antigens
(71,72). The permanent establishment of memory
immune T cells serves a key role in preventing tumor recurrence
(73). However, due to the poor
immunogenicity of most tumors, it is difficult to cultivate a
lymphocyte population with sufficient affinity for TCRs, and it is
difficult to introduce engineered surface receptors with enhanced
affinity for a tumor-specific antigen (74). Chimeric antigen receptors (CARs)
consist of antibody-derived antigen recognition domains that are
connected to the internal T cell signaling domain and recognize
antigen targets through a mechanism different from that of
classical TCR (75). Unlike
traditional TCRs that recognize intracellular peptide antigens
presented by MHC molecules, CARs directly recognize antigens
expressed on the surface of tumor cells so are not limited by the
patient HLA subtype, and can recognize a variety of antigen
structures, including proteins, carbohydrates and glycolipids
(60). Gene therapy viral vectors
can transfect genes encoding CAR constructs into T cells to express
high-affinity extracellular antigen-recognition moieties and
membrane proteins derived from monoclonal antibody single-chain
variable fragment-binding TCR signaling domains (76). The internal domain of CAR is
originally derived from the CD3ζ chain of traditional TCR, and
after technical development, it can include one or more
costimulatory domains (most commonly CD28 and 41BB) to enhance the
persistence and cytotoxicity of CAR-expressing cells (77).
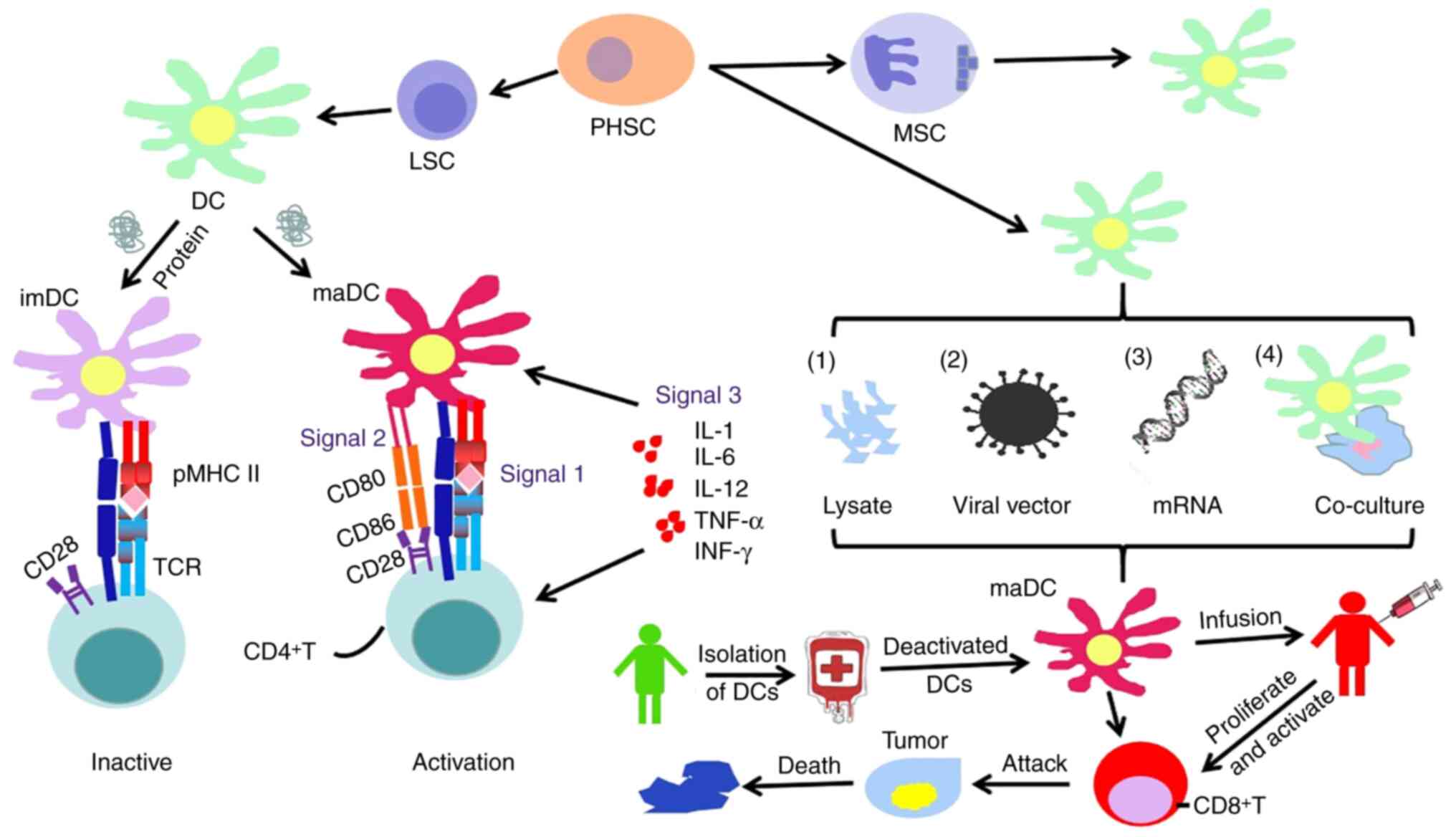
There are several forms of DC-based immunotherapies,
most of which involve isolation of circulating monocytes from
patients or donors and their expansion and differentiation in
vitro to promote the maturation of DCs by cytokines (such as
TNF-α, IL-1β) (78). Immature DCs
have an immunosuppressive function rather than an immune enhancing
function, and the use of macrophage colony-stimulating factor to
stimulate immature DCs to differentiate into mature DCs plays an
important role in their anti-tumor function (88).
For tumor cells with low expression of MHC I, their
tumor antigen presentation ability is weakened and it is difficult
to activate T cells to kill tumor cells (89). To better present tumor antigens to T
cells, DC vaccines introduce tumor-associated antigens (including
proteins, peptides or tumor lysate) from patients into DCs. The
pMHC is expressed on the cell surface to initiate an immune
response. The preparation methods of DC vaccines include directly
sensitizing DCs with tumor antigens and cell lysates to produce
activity; viral vectors encoding tumor associated antigen (TAA)
gene to infect DCs to express the corresponding antigens and Tumor
antigen mRNA is electroporated or chemically transfected into DC
cells or fused with DC cells using a fusion agent with tumor cells
expressing tumor antigens (Fig. 3)
(90,91).
Sensitization of DCs with tumor antigens and cell
lysates is the most common method for preparing DC vaccines
(92). Immature DCs phagocytose
tumor antigens and differentiate into mature DCs in vitro.
These cells carrying antigenic information are returned to the body
to activate the anti-tumor immune response (93). After DCs carrying antigens, such as
carcinoembryonic antigen and melanoma antigen-A1, are returned to
patients with lung cancer, the body can produce specific T
lymphocytes (94). However,
tumor-associated antigens are not unique to tumor cells and it is
difficult to induce a specific immune response against tumor cells.
Therefore, sequencing and mass spectrometry have been used to
analyze and identify neoantigens on the surface of tumor cells
(95). Excised tumor tissue is
lysed by ultrasonic disruption, subjected to repeated freezing and
thawing and used as an active ingredient to prepare DCs that induce
anti-tumor responses (96).
DCVax®-L is a personalized DC vaccine sensitized by
lysate of malignant glioblastoma developed by Northwest
Biotherapeutics in the United Kingdom that infects DCs with viral
vectors to insert the gene encoding TAA into a lentivirus,
recombinant poxvirus or adenovirus vector (97). After the virus infects DCs, it
expresses TAA and maturation is induced. The low efficiency of
virus infecting DC means the development of DC vaccines has been
limited (98). Tumor cells and DCs
are fused under the action of fusion agents, and the fused cells
not only have the function of DCs, but also express tumor antigens
on the cells.
DC vaccines are highly immunogenic and highly
specific. Electrical, viral and chemical fusion are commonly used
methods for preparing DC vaccines. Due to the instability of
electric fusion, this technology is no longer used (99). Viruses commonly used to induce
animal cell fusion include Sendai, Newcastle disease and herpes
virus (100,101). Inactivated Sendai virus is used to
induce cell fusion; the fusion rate is high and it is suitable for
various types of animal cell. Because Sendai virus is unstable, the
preparation process it is cumbersome and may affect the normal
function after entering the cell (102). Polyethylene glycol has good water
solubility and adhesion, and is a commonly used chemical reagent
for the fusion of cells. In DC immunotherapy, the immunosuppressive
nature of the tumor microenvironment inhibits the antigen
presentation ability of DC cells (103), the limited ability of DC cells to
target tumors cannot specifically recognize tumor antigens, and the
specific T cells produced by DCs after presenting antigens Cells
have low affinity for tumor cells (35,93,104).
Due to the multiple roles of DCs in the immune response, DC
vaccines are still a promising treatment.
Cytokines are key biomolecules that communicate with
each other and exert biological functions in immune cells (105). As a family of proteins, they
regulate almost all biological functions of cells via autocrine,
paracrine or endocrine effects. Based on the powerful
immunomodulatory ability, the immunotherapy of cancer with
cytokines has been tried (106,107). The therapeutic effect of IFN is
considered important in cancer immunoediting and has been studied
in many clinical trials (108,109). Type I IFN and IL-2 have been
approved by the US FDA for the treatment of certain types of
malignancy, such as Melanoma, metastatic renal cell carcinoma
(110,111). Granulocyte-macrophage
colony-stimulating factor (GM-CSF) has been approved for adjuvant
therapy of malignant tumors due to its ability to stimulate
proliferation and differentiation of immune cells (112). In addition, GM-CSF promotes
antigen presentation by DCs, making it widely used in tumor
vaccines (113,114). Although the antitumor activity of
cytokines has been observed in many studies (115–117), few cytokines induce complete tumor
regression. To the best of our knowledge, the mechanism of action
of immune-stimulating cytokines has not been fully explored and
some tumor treatment modalities in the clinic may promote cytokine
cascades with unexplained potentially lethal effects (118). Radiotherapy and chemotherapy
trigger a cytokine storm in the tumor stroma, including release of
the pro-tumor cytokines IL-6 and TNFα. Apoptotic tumor cells
activate macrophages to produce pro-inflammatory mediators and
cellular debris can also stimulate anti-tumor immunity; therefore,
dead and dying tumor cells contribute to a TME that may promote
tumor progression (119,120). The anti-tumor function of
cytokines is complex, and studies have shown that their anti-tumor
activity depends on the host immune system (121–123). Cytokines are functionally divided
into pro- and anti-inflammatory. Pro-inflammatory cytokines (such
as IL-1α/β, TNF-α/β, IL-6, IL-11, IL-18 and IFN-γ) upregulate
inflammatory responses and enhance recruitment, infiltration and
resistance of immune cells to tumor site (124–126). Anti-inflammatory cytokines (such
as IL-10, IL-6, TGF-β, IL-27 and IL-35) downregulate inflammatory
responses and promote tissue healing and tumor growth (127,128). Cytokine-induced inflammatory
responses are context-dependent; the same cytokines induce
pro-inflammatory or anti-inflammatory responses depending on
factors such as target cells, dose, and presence of other cytokines
(129). Cytokine classification in
tumor therapy stems from the association between cytokines and T
cell responses. T cells differentiate into cell populations with
different functions, characterized by production of certain
cytokine groups. Th1 cells produce type 1 cytokines such as IL-2,
IL-12 and IFN-γ; Th2 cells produce type 2 cytokines, such as IL-4,
IL-5, IL-6, IL-10 and IL-13; regulatory T cells produce IL-10 and
TGF-β (130). In general, type 1
cytokines mediate the development of strong cellular immune
responses, while type 2 cytokines facilitate strong humoral immune
responses (131). Tumors are often
associated with a tolerant and immunosuppressive microenvironment.
Cytokine-mediated therapy uses type 1 cytokines to stimulate
anticancer immune responses (132). Vaccine-based therapies use type 2
cytokines as adjuvants based on their role in B cell maturation,
while autoimmune diseases may benefit from regulatory cytokines
(133). In many cases, these
distinctions are not sufficient to classify cytokines because their
effects on the immune system are complex. For example, IL-18 can
promote Th2-biased cytokine production by T cells but in the
presence of IL-15 or IL-12, IL-18, leads to potent Th1-biased
cytokine production (134).
Furthermore, type 1 cytokines are not limited to cellular immune
responses, as they contribute to the development of certain
antibody classes and functional differentiation of B cells
(135).
Cytokine storm involves a variety of cytokines such
as TNF-α, IL-1, IL-6, IL-12, IFN-α, IFN-β, IFN-γ, monocyte
chemotactic protein 1 and IL-2 (136). The phenomenon of rapid and massive
production of IL-8 is an important cause of acute respiratory
distress syndrome and multiple organ failure (137). Injected CAR T cells to treat
CD19+ lymphoma induce a cytokine storm, with levels of
IFN-γ and IL-6 exceeding physiological levels, due to high levels
of activated CAR T cells (138).
The cytokines that mediate the cytokine storm are achieved not by
CAR T cells but by macrophages, and their damage to the body can be
mitigated by the use of IL-6 and IL-1 drugs (139). Glucocorticoids and IL-6 inhibitors
are also effective in treating this type of cytokine storm
(140). To the best of our
knowledge, no cases of cytokine storm or elevated IL-6 levels have
been reported in NK cell CAR therapy.
In conclusion, cytokines are potent but complex
immune mediators. Developing cytokine drugs is a challenge that
requires a deep understanding of cytokine biology and contemporary
biotechnology to exploit their antitumor activity, while minimizing
toxicity. In future, how to confine the action of cytokines to the
desired site to avoid systemic pro-inflammatory effects and how to
incorporate these treatments into combination immunotherapy
strategies should be investigated.
Oncolytic viruses refer to non-pathogenic viruses
that specifically infect tumor cells and cause their death.
Oncolytic viruses are an emerging class of antitumor immunotherapy
(141). The effectiveness of
oncolytic viruses depends on sufficient numbers of oncolytic virus
to infect tumor cells. Because oncolytic viruses have intrinsic
anticancer activity, they are considered passive immunotherapy
(142). Although not fully
understood, it is hypothesized that oncolytic viruses mediate
anti-tumor activity through two distinct mechanisms of action:
Selectively replicating within tumor cells, resulting in direct
lytic effects or inducing systemic antitumor immune response
(143). Specifically, oncolytic
virus therapy relies on tumor cell-specific changes associated with
tumor characteristics, including increased receptor expression,
impaired antiviral response and alterations in cellular metabolism;
it is hypothesized that oncolytic virus replication is limited to
the tumor site and healthy tissue is not harmed (144). In addition to directly lysing
tumors, oncolytic viruses induce extracellular matrix remodeling,
thus exerting anti-angiogenic effects (145). In the anti-tumor immune response,
tumor cells release cytoplasmic components such as intracellular
tumor-associated antigens, damage and pathogen-associated molecules
after death to stimulate the body's innate immunity, and a large
number of cytokines and chemokines are produced to promote
subsequent specific immunity of the tumor. Mediate the maturation
of APC and enhance its antigen presentation ability, promote the
initiation, activation, proliferation, transport, memory formation,
cytokine release and cytotoxic activity of polyclonal T cells, and
generate systemic anti-tumor immune response (146). Various oncolytic viruses have been
used to treat different forms of cancer, including adenoviruses,
poxviruses, rhabdoviruses, herpes viruses, paramyxovirus (PV) and
reoviruses. Due to the difference in innate immune response to
virus, anti-tumor mechanisms differ. Herpes viruses are DNA viruses
capable of establishing lytic and latent infection in the host; the
utility of herpes simplex virus 1 (HSV-1) as an oncolytic agent has
been the most widely explored. Using gene editing method to make
HSV-1 express GM-CSF can promote the recruitment of T cells to the
tumor site and enhance the anti-tumor effect (147). PVs are members of the
Paramyxoviridae family of disease-causing viruses in humans and
animals. PV is a strong inducer of IFN and other immunostimulatory
cytokines that activate various immune factors to mount excellent
antitumor innate and adaptive immune responses (148). Mumps virus, which has been proven
to have cytopathic effects, causes infected cells to secrete
various cytokines and IFN pathway-associated genes or receptors to
achieve anti-tumor effects (149).
The first FDA- and European Medicines
Agency-approved oncolytic virus, talimogene laherparepvec (T-VEC),
is a modified HSV virus for the treatment of malignant melanoma
that encodes GM-CSF to enhance antitumor immune responses (150). A recombinant adenovirus
(Oncorine®) was approved by Chinese regulators as early
as November 2005 for the treatment of HNC (in combination with
chemotherapy) and a number of oncolytic viruses are in clinical
development (151,152). The multifunctional properties of
oncolytic viruses in tumor therapy make them highly synergistic
when used in combination with other drugs (153). Currently, a large body of evidence
suggests that oncolytic virus therapy induces tumor cell death by
enhancing the antigenicity of tumor cells or their susceptibility
to immune cells when used in combination with radiotherapy,
chemotherapy and other immunotherapies (154,155). Many naturally occurring viruses,
such as parvovirus, measles virus, reovirus and Newcastle disease
virus, exhibit a natural preference for cancer cells (156). However, other viruses such as
adenovirus, vesicular stomatitis and vaccinia virus and HSV need to
be engineered to be cancer specific (Table I). Four approaches are commonly used
to design oncolytic viruses to selectively target tumor cells. The
first is use of virus-specific receptor-mediated cellular
targeting, such as EGFR and HER-2 (157). The second approach exploits the
rapidly dividing nature of tumor cells to increase the efficiency
of viral replication compared with normal cells. For example,
mutations in tumor drivers or other enzymes such as protein kinase
R increase viral replication in tumor cells (158). Numerous types of tumor cell
exhibit a lack of normal antiviral IFN or TNF responses that
promote selective viral replication (159). The fourth is that normal cells
respond to viral infection by inducing apoptosis or inhibiting
translation, transcription and/or transduction targeting to prevent
cell lysis, which may limit viral spread (160).
The immune response to oncolytic viruses is an
important part of the antitumor effect, but it can be a
double-edged sword. On the one hand, viruses promote immune
responses against tumor cells by increasing tumor antigen
presentation via viral infection. On the other hand, neutralizing
antiviral responses may prevent viral replication and persistent
infection of tumor cells (161).
Therapeutic outcomes depend on the complex interplay between these
opposing forces, and local injection of the tumor can be used to
observe the therapeutic response. To balance this response of the
immune system, methods to optimize current oncolytic viruses or
develop novel viruses to enhance the stimulation of the host immune
response to tumor cells without triggering rapid clearance of
oncolytic viruses (for example, deletion of Herpes simplex virus
protein ICP34.5 and ICP47 in T-VEC) have been investigated
(162). In addition, genes for
cytokines or chemokines can be integrated into the genome to
enhance the therapeutic effect of oncolytic viruses. GM-CSF, an
immune-associated cytokine, can increase APC activation and trigger
systemic antitumor immune responses, which increases oncolytic
virus efficacy (163). TAAs,
immune-associated ligands, or bispecific T-cell engager antibodies
can also be used to modify oncolytic viruses (164).
In addition, it is difficult for some viruses to
obtain extremely high titer products required for clinical doses,
which limits large-scale production of oncolytic viruses due to the
high cost of current technologies (165). The host immune barrier and
antiviral response can inhibit viral replication and lead to
resistance to oncolytic viruses, such as macrophages, which
directly capture viruses in organs such as the liver, thereby
decreasing viral titers in the body and affecting oncolysis
(166). The most common adverse
reactions to oncolytic viruses are fever and local injection site
reactions but can also include chills, nausea and vomiting,
flu-like symptoms, fatigue and pain (167).
In conclusion, although oncolytic viruses have
potential, there are some obstacles to their production and
application. Current molecular biotechnology strategies enhance the
targeting and killing effects of oncolytic viruses, but further
research is needed to develop tumor treatments with higher efficacy
and lower adverse reaction rates. The production technology of
oncolytic virus is imperfect, and there is no uniform standard for
industrial production quality and inspection, which is an obstacle
to its application.
A recent strategy is to make tumors express ABO
blood group antigens and using a mechanism similar to that caused
by blood group incompatibility to activate the immune system to
kill tumor cells (168). This
differs from passive immunotherapy as it does not activate the
immune response systemically; it also removes the need to identify
tumor-associated antigens as it does not require the body to
produce specific anti-tumor CTL cells. Different from the mechanism
of oncolytic viruses, the virus does not directly lyse cells but
serves as a carrier to express blood group antigens on the tumor
cell membrane and is recognized and activated by naturally
occurring blood group antibodies. The complement response produces
cytolysis, which demonstrates the key anti-tumor role of the innate
immune system (169). Although
studies have detected loss of blood group antigen expression in
primary breast tumors and their metastases, loss of blood group
antigen expression may be considered a marker of invasion and half
of proximal colon tumors show loss of antigen expression (170–172). However, this does not simply
activate the immune system by allowing the tumor to express blood
group antigens. If a tumor expresses an antigen, there will be an
antibody that can bind to it naturally in the body, and it will
react with the antigen and antibody, and then activate the immune
system to produce cell lysis. There are similar antigens that can
treat tumors, such as the Rhesus blood group antigen. Expressing
the corresponding antigens on tumor cells of patients with
autoimmune diseases is also a strategy for treating tumors
(173). The advantages of adopting
such a strategy to treat tumors include lack of tumor resistance to
treatment; the lentiviral vector itself has little immunogenicity
and is not easily cleared by the body. Theoretically, as long as
the tumor tissue expresses an antigen that can be recognized by the
body's immune system, tumor cells can be directly recognized by the
immune system and produce a lytic reaction. With the development of
molecular biology, vector viruses can be used to make tumor cells
express any protein. Therefore, this treatment method can solve the
problem of tumor drug resistance. Intratumoral drug injection
therapy is safer than systemic medication and can avoid the failure
of body organs caused by the storm of inflammatory factors caused
by systemic medication. Simple intratumoral injections decrease the
risk and pain associated with surgery and chemotherapy; the
procedure is simple and can be performed by doctors in the primary
hospital. Although the local administration method is safer
compared with systemic administration, the optimal injection dose
still needs to be determined to ensure adequate dispersion of drug
in the tumor tissue and minimize the leakage from the tumor tissue.
As a novel tumor treatment strategy, further research is required
to develop use of naturally occurring antigen-antibody immune
response to treat tumors.
In the past decades, anti-cancer immunotherapy has
changed from a promising treatment method to a reality of clinical
treatment. Many immunotherapy programs that can be used for
patients with cancer have now been approved by the US FDA and
European Medicines Agency and many other treatment programs are
being studied as independent therapeutic interventions or in
combination with clinical routine treatment (174,175). Treatment strategies are no longer
based solely on interfering with metabolism of tumor cells or
whether it is a purely clonal proliferative disease. A number of
studies has shown that the survival of tumor cells depends to a
large extent on the surrounding environment, which contains
abundant and heterogeneous untransformed components, including
stroma and endothelial and immune cells (176–178).
Immunotherapy has become a clinical reality, and an
increasing number of patients with cancer will receive
immunotherapy at some stage. The treatment of tumors by interfering
with immune checkpoints has become an important and effective form
of immunotherapy. Drugs targeting CTLA-4, PD-1 and PD-L1 are the
most widely studied (179).
Numerous studies have also shown that what was previously
classified as passive immunotherapy, including several
tumor-targeting monoclonal antibodies, adoptive T cell transfer,
and oncolytic viruses, may constitute a potent active form of
immunotherapy (115,180,181). Drugs such as immunosuppressive
metabolic inhibitors and PRR agonists have attracted interest not
only as adjuvants to conventional vaccines, but also as therapeutic
measures that may mediate the antitumor effect or enhance the
therapeutic effects of other anticancer drugs (182).
In 2013, the clinical success of immunotherapy was
named ‘Breakthrough of the Year’ by Science (183). Clinical research is also focused
on whether immunotherapy can be used as a stand-alone treatment or
in combination with other antitumor drugs to improve the efficacy
and safety in patients with cancer.
One of the key challenges in developing cancer
vaccines is to identify specific tumor antigens for use as
immunotherapeutic targets. Good target antigens should exhibit high
antigenicity and homologous expression in tumor tissue to overcome
problems caused by tumor heterogeneity. Tumor cells can undergo
antigen modulation, which means that the immune system attacks
tumor cells, resulting in reduction or loss of tumor antigen
epitopes on the surface, thereby escaping recognition and killing
by the immune system (32,184). Studies have also confirmed that
tumor cells exhibit characteristics of stem cells and may actively
decrease expression of antigens (185–187). The development of therapeutic
oncolytic viruses is faced with challenges regarding how to
formulate a reasonable clinical trial design, dosing regimen,
pharmacodynamic analysis and biosafety issues (188). Immunocompromised patients may not
be candidates for oncolytic virus therapy because oncolytic
virus-mediated antitumor immunity may be compromised in these
patients (189). The treatment of
tumors with blood group antigens is different from that of
oncolytic viruses; it actively allows tumors to express naturally
occurring antigens in the body to activate the natural immune
response and treat tumors (190).
Further studies are required to determine clinical feasibility.
In summary, tumor immunotherapy may enhance the
direct killing effect of CTLs on tumors and weaken the
immunosuppressive TME.
Not applicable.
The present study was supported by Inner Mongolia Natural
Science Foundation Project (grant no. 2021MS08060) and Inner
Mongolia Medical University Joint Project (grant no.
YKD2021LH068).
Not applicable.
FZ, DS and LW performed the literature review and
wrote the manuscript. MS, HY, XZ, LC and ZH wrote the manuscript.
LL and LW conceived, reviewed and revised the article. All authors
revised the manuscript. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Not applicable.
Not applicable.
LW is the corresponding author of ABO blood group
antigen therapy: A potential new strategy against solid tumors. The
other authors declare that they have no competing interests.
|
1
|
Lin L, Li Z, Yan L, Liu Y, Yang H and Li
H: Global, regional, and national cancer incidence and death for 29
cancer groups in 2019 and trends analysis of the global cancer
burden, 1990–2019. J Hematol Oncol. 14:1972021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: Good or bad news from the 2018
global cancer statistics? Cancer Commun (Lond). 39:222019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan C, Liu H, Robins E, Song W, Liu D, Li
Z and Zheng L: Next-generation immuno-oncology agents: Current
momentum shifts in cancer immunotherapy. J Hematol Oncol.
13:292020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Jiang L, Wen T, Guo X, Shao X, Qu
H, Chen X, Song Y, Wang F, Qu X and Li Z: Trends in the research
into immune checkpoint blockade by anti-PD1/PDL1 antibodies in
cancer immunotherapy: A bibliometric study. Front Pharmacol.
12:6709002021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Du X, Liu M, Tang F, Zhang P, Ai
C, Fields JK, Sundberg EJ, Latinovic OS, Devenport M, et al:
Hijacking antibody-induced CTLA-4 lysosomal degradation for safer
and more effective cancer immunotherapy. Cell Res. 9:609–627. 2019.
View Article : Google Scholar
|
|
6
|
Smith KM and Desai J: Nivolumab for the
treatment of colorectal cancer. Expert Rev Anticancer Ther.
18:611–618. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonzalez H, Hagerling C and Werb Z: Roles
of the immune system in cancer: From tumor initiation to metastatic
progression. Genes Dev. 32:1267–1284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ribatti D: The concept of immune
surveillance against tumors. The first theories. Oncotarget.
8:7175–7180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: From immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mittal D, Gubin MM, Schreiber RD and Smyth
MJ: New insights into cancer immunoediting and its three component
phases-elimination, equilibrium and escape. Curr Opin Immunol.
27:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Finn OJ: Human tumor antigens yesterday,
today, and tomorrow. Cancer Immunol Res. 5:347–354. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verginelli F, Pisacane A, Gambardella G,
D'Ambrosio A, Candiello E, Ferrio M, Panero M, Casorzo L, Benvenuti
S, Cascardi E, et al: Cancer of unknown primary stem-like cells
model multi-organ metastasis and unveil liability to MEK
inhibition. Nat Commun. 12:24982021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng H, Yang W, Zhou Z, Tian R, Lin L, Ma
Y, Song J and Chen X: Targeted scavenging of extracellular ROS
relieves suppressive immunogenic cell death. Nat Commun.
11:49512020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren Y, Kumar A, Das JK, Peng HY, Wang L,
Balllard D, Xiong X, Ren X, Zhang Y, Yang JM and Song J: Tumorous
expression of NAC1 restrains antitumor immunity through the
LDHA-mediated immune evasion. J Immunother Cancer. 10:e0048562022.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bazett M, Costa AM, Bosiljcic M, Anderson
RM, Alexander MP, Wong SWY, Dhanji S, Chen JM, Pankovich J, Lam S,
et al: Harnessing innate lung anti-cancer effector functions with a
novel bacterial-derived immunotherapy. Oncoimmunology.
7:e13988752017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosewell Shaw A, Porter C, Biegert G,
Jatta L and Suzuki M: HydrAd: A helper-dependent adenovirus
targeting multiple immune pathways for cancer immunotherapy.
Cancers (Basel). 14:27692022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Li Z, Skrzypczynska KM, Fang Q,
Zhang W, O'Brien SA, He Y, Wang L, Zhang Q, Kim A, et al:
Single-cell analyses inform mechanisms of myeloid-targeted
therapies in colon cancer. Cell. 181:442–459.e29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krausgruber T, Fortelny N, Fife-Gernedl V,
Senekowitsch M, Schuster LC, Lercher A, Nemc A, Schmidl C, Rendeiro
AF, Bergthaler A and Bock C: Structural cells are key regulators of
organ-specific immune responses. Nature. 583:296–302. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmad HI, Jabbar A, Mushtaq N, Javed Z,
Hayyat MU, Bashir J, Naseeb I, Abideen ZU, Ahmad N and Chen J:
Immune tolerance vs immune resistance: The interaction between host
and pathogens in infectious diseases. Front Vet Sci. 9:8274072022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bettcher BM, Tansey MG, Dorothée G and
Heneka MT: Peripheral and central immune system crosstalk in
Alzheimer disease-a research prospectus. Nat Rev Neurol.
17:689–701. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morsink MAJ, Willemen NGA, Leijten J,
Bansal R and Shin SR: Immune organs and immune cells on a chip: An
overview of biomedical applications. Micromachines (Basel).
11:8492020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Benci JL, Johnson LR, Choa R, Xu Y, Qiu J,
Zhou Z, Xu B, Ye D, Nathanson KL, June CH, et al: Opposing
functions of interferon coordinate adaptive and innate immune
responses to cancer immune checkpoint blockade. Cell.
178:933–948.e14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gangopadhyay K, Roy S, Sen Gupta S,
Chandradasan AC, Chowdhury S and Das R: Regulating the
discriminatory response to antigen by T-cell receptor. Biosci Rep.
42:BSR202120122022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Druszczyńska M, Godkowicz M, Kulesza J,
Wawrocki S and Fol M: Cytokine receptors-regulators of
antimycobacterial immune response. Int J Mol Sci. 23:11122022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Šket T, Ramuta TŽ, Starčič Erjavec M and
Kreft ME: The role of innate immune system in the human amniotic
membrane and human amniotic fluid in protection against
intra-amniotic infections and inflammation. Front Immunol.
12:7353242021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He L, Valignat MP, Zhang L, Gelard L,
Zhang F, Le Guen V, Audebert S, Camoin L, Fossum E, Bogen B, et al:
ARHGAP45 controls naïve T- and B-cell entry into lymph nodes and
T-cell progenitor thymus seeding. EMBO Rep. 22:e521962021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yiwen Z, Shilin G, Yingshi C, Lishi S,
Baohong L, Chao L, Linghua L, Ting P and Hui Z: Efficient
generation of antigen-specific CTLs by the BAFF-activated human B
Lymphocytes as APCs: A novel approach for immunotherapy.
Oncotarget. 7:77732–77748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nicholson LB: The immune system. Essays
Biochem. 60:275–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vila-Leahey A, MacKay A,
Portales-Cervantes L, Weir GM, Merkx-Jacques A and Stanford MM:
Generation of highly activated, antigen-specific tumor-infiltrating
CD8+ T cells induced by a novel T cell-targeted
immunotherapy. Oncoimmunology. 9:17825742020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schönefeldt S, Wais T, Herling M, Mustjoki
S, Bekiaris V, Moriggl R and Neubauer HA: The diverse roles of γδ T
cells in cancer: From rapid immunity to aggressive lymphoma.
Cancers (Basel). 13:62122021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Leko V and Rosenberg SA: Identifying and
targeting human tumor antigens for T cell-based immunotherapy of
solid tumors. Cancer Cell. 38:454–472. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Fu M, Wang M, Wan D, Wei Y and Wei
X: Cancer vaccines as promising immuno-therapeutics: Platforms and
current progress. J Hematol Oncol. 15:282022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu YT and Sun ZJ: Turning cold tumors
into hot tumors by improving T-cell infiltration. Theranostics.
11:5365–5386. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanimine N, Ohira M, Tahara H, Ide K,
Tanaka Y, Onoe T and Ohdan H: Strategies for deliberate induction
of immune tolerance in liver transplantation: From preclinical
models to clinical application. Front Immunol. 11:16152020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hargadon KM: Tumor microenvironmental
influences on dendritic cell and T cell function: A focus on
clinically relevant immunologic and metabolic checkpoints. Clin
Transl Med. 10:374–411. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elizondo DM, Andargie TE, Kubhar DS,
Gugssa A and Lipscomb MW: CD40-CD40L cross-talk drives fascin
expression in dendritic cells for efficient antigen presentation to
CD4+ T cells. Int Immunol. 29:121–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morris DL, Oatmen KE, Mergian TA, Cho KW,
DelProposto JL, Singer K, Evans-Molina C, O'Rourke RW and Lumeng
CN: CD40 promotes MHC class II expression on adipose tissue
macrophages and regulates adipose tissue CD4+ T cells
with obesity. J Leukoc Biol. 99:1107–1119. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Y, Slone N, Chrisikos TT, Kyrysyuk O,
Babcock RL, Medik YB, Li HS, Kleinerman ES and Watowich SS: Vaccine
efficacy against primary and metastatic cancer with in
vitro-generated CD103+ conventional dendritic cells. J
Immunother Cancer. 8:e0004742020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Krishnaswamy JK, Gowthaman U, Zhang B,
Mattsson J, Szeponik L, Liu D, Wu R, White T, Calabro S, Xu L, et
al: Migratory CD11b+ conventional dendritic cells induce
T follicular helper cell-dependent antibody responses. Sci Immunol.
2:eaam91692017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roselli E, Araya P, Núñez NG, Gatti G,
Graziano F, Sedlik C, Benaroch P, Piaggio E and Maccioni M: TLR3
activation of intratumoral CD103+ dendritic cells
modifies the tumor infiltrate conferring anti-tumor immunity. Front
Immunol. 10:5032019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koh YC, Yang G, Lai CS, Weerawatanakorn M
and Pan MH: Chemopreventive effects of phytochemicals and medicines
on M1/M2 polarized macrophage role in inflammation-related
diseases. Int J Mol Sci. 19:22082018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Castro F, Cardoso AP, Gonçalves RM, Serre
K and Oliveira MJ: Interferon-gamma at the crossroads of tumor
immune surveillance or evasion. Front Immunol. 9:8472018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Song X, Traub B, Shi J and Kornmann M:
Possible roles of interleukin-4 and −13 and their receptors in
gastric and colon cancer. Int J Mol Sci. 22:7272021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Babazadeh S, Nassiri SM, Siavashi V,
Sahlabadi M, Hajinasrollah M and Zamani-Ahmadmahmudi M: Macrophage
polarization by MSC-derived CXCL12 determines tumor growth. Cell
Mol Biol Lett. 26:302021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oshi M, Tokumaru Y, Asaoka M, Yan L,
Satyananda V, Matsuyama R, Matsuhashi N, Futamura M, Ishikawa T,
Yoshida K, et al: M1 Macrophage and M1/M2 ratio defined by
transcriptomic signatures resemble only part of their conventional
clinical characteristics in breast cancer. Sci Rep. 10:165542020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He M, Wang Y, Zhang G, Cao K, Yang M and
Liu H: The prognostic significance of tumor-infiltrating
lymphocytes in cervical cancer. J Gynecol Oncol. 32:e322021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu A, Zhang L, Yuan J, Babikr F, Freywald
A, Chibbar R, Moser M, Zhang W, Zhang B, Fu Z and Xiang J: TLR9
agonist enhances radiofrequency ablation-induced CTL responses,
leading to the potent inhibition of primary tumor growth and lung
metastasis. Cell Mol Immunol. 16:820–832. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Crawshaw A, Kendrick YR, McMichael AJ and
Ho LP: Abnormalities in iNKT cells are associated with impaired
ability of monocytes to produce IL-10 and suppress T-cell
proliferation in sarcoidosis. Eur J Immunol. 44:2165–2174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang YL, Gong Y, Lv Z, Li L and Yuan Y:
Expression of PD1/PDL1 in gastric cancer at different
microsatellite status and its correlation with infiltrating immune
cells in the tumor microenvironment. J Cancer. 12:1698–1707. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weigelin B, Bolaños E, Teijeira A,
Martinez-Forero I, Labiano S, Azpilikueta A, Morales-Kastresana A,
Quetglas JI, Wagena E, Sánchez-Paulete AR, et al: Focusing and
sustaining the antitumor CTL effector killer response by agonist
anti-CD137 mAb. Proc Natl Acad Sci USA. 112:7551–7556. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baik AH, Tsai KK, Oh DY and Aras MA:
Mechanisms and clinical manifestations of cardiovascular toxicities
associated with immune checkpoint inhibitors. Clin Sci (Lond).
135:703–724. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Turula H, Bragazzi Cunha J, Mainou BA,
Ramakrishnan SK, Wilke CA, Gonzalez-Hernandez MB, Pry A, Fava J,
Bassis CM, Edelman J, et al: Natural secretory immunoglobulins
promote enteric viral infections. J Virol. 92:e00826–18. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ehrlich P: Address in pathology, on
chemiotherapy: Delivered before the seventeenth international
congress of medicine. Br Med J. 2:353–359. 1913. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
He Q, Liu Z, Liu Z, Lai Y, Zhou X and Weng
J: TCR-like antibodies in cancer immunotherapy. J Hematol Oncol.
12:992019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Byeon HK, Ku M and Yang J: Beyond EGFR
inhibition: Multilateral combat strategies to stop the progression
of head and neck cancer. Exp Mol Med. 51:1–14. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Santoro V, Jia R, Thompson H, Nijhuis A,
Jeffery R, Kiakos K, Silver AR, Hartley JA and Hochhauser D: Role
of reactive oxygen species in the abrogation of oxaliplatin
activity by cetuximab in colorectal cancer. J Natl Cancer Inst.
108:djv3942015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen KF, Chen HL, Shiau CW, Liu CY, Chu
PY, Tai WT, Ichikawa K, Chen PJ and Cheng AL: Sorafenib and its
derivative SC-49 sensitize hepatocellular carcinoma cells to
CS-1008, a humanized anti-TNFRSF10B (DR5) antibody. Br J Pharmacol.
168:658–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fenwarth L, Fournier E, Cheok M, Boyer T,
Gonzales F, Castaigne S, Boissel N, Lambert J, Dombret H,
Preudhomme C and Duployez N: Biomarkers of gemtuzumab ozogamicin
response for acute myeloid leukemia treatment. Int J Mol Sci.
21:56262020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Boyer-Suavet S, Andreani M, Lateb M,
Savenkoff B, Brglez V, Benzaken S, Bernard G, Nachman PH, Esnault V
and Seitz-Polski B: Neutralizing anti-rituximab antibodies and
relapse in membranous nephropathy treated with rituximab. Front
Immunol. 10:30692020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Strohl WR and Naso M: Bispecific T-Cell
redirection versus chimeric antigen receptor (CAR)-T cells as
approaches to kill cancer cells. Antibodies (Basel). 8:412019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Huo Y, Sheng Z, Lu DR, Ellwanger DC, Li
CM, Homann O, Wang S, Yin H and Ren R: Blinatumomab-induced T cell
activation at single cell transcriptome resolution. BMC Genomics.
22:1452021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Davis SK, Selva KJ, Kent SJ and Chung AW:
Serum IgA Fc effector functions in infectious disease and cancer.
Immunol Cell Biol. 98:276–286. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Brandsma AM, Bondza S, Evers M, Koutstaal
R, Nederend M, Jansen JHM, Rösner T, Valerius T, Leusen JHW and Ten
Broeke T: Potent Fc receptor signaling by IgA leads to superior
killing of cancer cells by neutrophils compared to IgG. Front
Immunol. 10:7042019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Leoh LS, Daniels-Wells TR and Penichet ML:
IgE immunotherapy against cancer. Curr Top Microbiol Immunol.
388:109–149. 2015.PubMed/NCBI
|
|
65
|
Chauhan J, McCraw AJ, Nakamura M, Osborn
G, Sow HS, Cox VF, Stavraka C, Josephs DH, Spicer JF, Karagiannis
SN and Bax HJ: IgE antibodies against cancer: Efficacy and safety.
Antibodies (Basel). 9:552020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
El-Kadiry AE, Rafei M and Shammaa R: Cell
therapy: Types, regulation, and clinical benefits. Front Med
(Lausanne). 8:7560292021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mora Román JJ, Del Campo M, Villar J,
Paolini F, Curzio G, Venuti A, Jara L, Ferreira J, Murgas P,
Lladser A, et al: Immunotherapeutic potential of mollusk
hemocyanins in combination with human vaccine adjuvants in murine
models of oral cancer. J Immunol Res. 2019:70769422019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kurosawa S, Mizuno S, Arai Y, Masuko M,
Kanda J, Kohno K, Onai D, Fukuda T, Ozawa Y, Katayama Y, et al:
Syngeneic hematopoietic stem cell transplantation for acute myeloid
leukemia: A propensity score-matched analysis. Blood Cancer J.
11:1592021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lin J, Wang H, Liu C, Cheng A, Deng Q, Zhu
H and Chen J: Dendritic cells: Versatile players in renal
transplantation. Front Immunol. 12:6545402021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Markov O, Oshchepkova A and Mironova N:
Immunotherapy based on dendritic cell-targeted/-derived
extracellular vesicles-A novel strategy for enhancement of the
anti-tumor immune response. Front Pharmacol. 10:11522019.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Blando J, Sharma A, Higa MG, Zhao H, Vence
L, Yadav SS, Kim J, Sepulveda AM, Sharp M, Maitra A, et al:
Comparison of immune infiltrates in melanoma and pancreatic cancer
highlights VISTA as a potential target in pancreatic cancer. Proc
Natl Acad Sci USA. 116:1692–1697. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cui X and Snapper CM: Epstein barr virus:
Development of vaccines and immune cell therapy for EBV-associated
diseases. Front Immunol. 12:7344712021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Luo XH, Meng Q, Rao M, Liu Z, Paraschoudi
G, Dodoo E and Maeurer M: The impact of inflationary
cytomegalovirus-specific memory T cells on anti-tumour immune
responses in patients with cancer. Immunology. 155:294–308. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pesch T, Bonati L, Kelton W, Parola C,
Ehling RA, Csepregi L, Kitamura D and Reddy ST: Molecular design,
optimization, and genomic integration of chimeric B cell receptors
in murine B cells. Front Immunol. 10:26302019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Stoiber S, Cadilha BL, Benmebarek MR,
Lesch S, Endres S and Kobold S: Limitations in the design of
chimeric antigen receptors for cancer therapy. Cells. 8:4722019.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Kasala D, Yoon AR, Hong J, Kim SW and Yun
CO: Evolving lessons on nanomaterial-coated viral vectors for local
and systemic gene therapy. Nanomedicine (Lond). 11:1689–1713. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Muliaditan T, Halim L, Whilding LM, Draper
B, Achkova DY, Kausar F, Glover M, Bechman N, Arulappu A, Sanchez
J, et al: Synergistic T cell signaling by 41BB and CD28 is
optimally achieved by membrane proximal positioning within parallel
chimeric antigen receptors. Cell Rep Med. 2:1004572021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Verheye E, Bravo Melgar J, Deschoemaeker
S, Raes G, Maes A, De Bruyne E, Menu E, Vanderkerken K, Laoui D and
De Veirman K: Dendritic cell-based immunotherapy in multiple
myeloma: Challenges, opportunities, and future directions. Int J
Mol Sci. 23:9042022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Steinman RM and Cohn ZA: Identification of
a novel cell type in peripheral lymphoid organs of mice. I.
Morphology, quantitation, tissue distribution. J Exp Med.
137:1142–1162. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Steinman RM and Banchereau J: Taking
dendritic cells into medicine. Nature. 449:419–426. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Handy CE and Antonarakis ES: Sipuleucel-T
for the treatment of prostate cancer: Novel insights and future
directions. Future Oncol. 14:907–917. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Artyomov MN, Munk A, Gorvel L, Korenfeld
D, Cella M, Tung T and Klechevsky E: Modular expression analysis
reveals functional conservation between human Langerhans cells and
mouse cross-priming dendritic cells. J Exp Med. 212:743–757. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Barbosa CRR, Barton J, Shepherd AJ and
Mishto M: Mechanistic diversity in MHC class I antigen recognition.
Biochem J. 478:4187–4202. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Oh DS and Lee HK: Autophagy protein ATG5
regulates CD36 expression and anti-tumor MHC class II antigen
presentation in dendritic cells. Autophagy. 15:2091–2106. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rühle PF, Fietkau R, Gaipl US and Frey B:
Development of a modular assay for detailed immunophenotyping of
peripheral human whole blood samples by multicolor flow cytometry.
Int J Mol Sci. 17:13162016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mengos AE, Gastineau DA and Gustafson MP:
The CD14+HLA-DRlo/neg monocyte: An
immunosuppressive phenotype that restrains responses to cancer
immunotherapy. Front Immunol. 10:11472019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Trujillo-Ocampo A, Cho HW, Clowers M,
Pareek S, Ruiz-Vazquez W, Lee SE and Im JS: IL-7 during antigenic
stimulation using allogeneic dendritic cells promotes expansion of
CD45RA−CD62L+CD4+ invariant NKT
cells with Th-2 biased cytokine production profile. Front Immunol.
11:5674062020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mylonas KJ, Anderson J, Sheldrake TA,
Hesketh EE, Richards JA, Ferenbach DA, Kluth DC, Savill J and
Hughes J: Granulocyte macrophage-colony stimulating factor: A key
modulator of renal mononuclear phagocyte plasticity. Immunobiology.
224:60–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shklovskaya E and Rizos H: MHC class I
deficiency in solid tumors and therapeutic strategies to overcome
it. Int J Mol Sci. 22:67412021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pardi N, Hogan MJ, Porter FW and Weissman
D: mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov.
17:261–279. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Coughlan L, Kremer EJ and Shayakhmetov DM:
Adenovirus-based vaccines-a platform for pandemic preparedness
against emerging viral pathogens. Mol Ther. 30:1822–1849. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Guo J, De May H, Franco S, Noureddine A,
Tang L, Brinker CJ, Kusewitt DF, Adams SF and Serda RE: Cancer
vaccines from cryogenically silicified tumour cells functionalized
with pathogen-associated molecular patterns. Nat Biomed Eng.
6:19–31. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Subtil B, Cambi A, Tauriello DVF and de
Vries IJM: The therapeutic potential of tackling tumor-induced
dendritic cell dysfunction in colorectal cancer. Front Immunol.
12:7248832021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Guo L, Sang M, Liu Q, Fan X, Zhang X and
Shan B: The expression and clinical significance of
melanoma-associated antigen-A1, -A3 and -A11 in glioma. Oncol Lett.
6:55–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Gopanenko AV, Kosobokova EN and Kosorukov
VS: Main strategies for the identification of neoantigens. Cancers
(Basel). 12:28792020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tomić S, Petrović A, Puač N, Škoro N,
Bekić M, Petrović ZL and Čolić M: Plasma-activated medium
potentiates the immunogenicity of tumor cell lysates for dendritic
cell-based cancer vaccines. Cancers (Basel). 13:16262021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Polyzoidis S and Ashkan K:
DCVax®-L-developed by northwest biotherapeutics. Hum
Vaccin Immunother. 10:3139–3145. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Mott KR, Allen SJ, Zandian M, Akbari O,
Hamrah P, Maazi H, Wechsler SL, Sharpe AH, Freeman GJ and Ghiasi H:
Inclusion of CD80 in HSV targets the recombinant virus to PD-L1 on
DCs and allows productive infection and robust immune responses.
PLoS One. 9:e876172014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Stewart MP, Langer R and Jensen KF:
Intracellular delivery by membrane disruption: Mechanisms,
strategies, and concepts. Chem Rev. 118:7409–7531. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bello MB, Yusoff K, Ideris A, Hair-Bejo M,
Jibril AH, Peeters BPH and Omar AR: Exploring the prospects of
engineered Newcastle disease virus in modern vaccinology. Viruses.
12:4512020. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Leroy H, Han M, Woottum M, Bracq L,
Bouchet J, Xie M and Benichou S: Virus-mediated cell-cell fusion.
Int J Mol Sci. 21:96442020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Russell CJ and Hurwitz JL: Sendai
virus-vectored vaccines that express envelope glycoproteins of
respiratory viruses. Viruses. 13:10232021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Huang TX, Tan XY, Huang HS, Li YT, Liu BL,
Liu KS, Chen X, Chen Z, Guan XY, Zou C and Fu L: Targeting
cancer-associated fibroblast-secreted WNT2 restores dendritic
cell-mediated antitumour immunity. Gut. 71:333–344. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cao L, Li W, Yang X, Zhang W, Li M, Zhang
H, Qin C, Chen X and Gao R: Inhibition of host Ogr1 enhances
effector CD8+ T-cell function by modulating acidic
microenvironment. Cancer Gene Ther. 28:1213–1224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Aguilar-Cazares D, Chavez-Dominguez R,
Marroquin-Muciño M, Perez-Medina M, Benito-Lopez JJ, Camarena A,
Rumbo-Nava U and Lopez-Gonzalez JS: The systemic-level
repercussions of cancer-associated inflammation mediators produced
in the tumor microenvironment. Front Endocrinol (Lausanne).
13:9295722022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mizui M: Natural and modified IL-2 for the
treatment of cancer and autoimmune diseases. Clin Immunol.
206:63–70. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Glassman CR, Mathiharan YK, Jude KM, Su L,
Panova O, Lupardus PJ, Spangler JB, Ely LK, Thomas C, Skiniotis G
and Garcia KC: Structural basis for IL-12 and IL-23 receptor
sharing reveals a gateway for shaping actions on T versus NK cells.
Cell. 184:983–999.e24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Takeda K, Nakayama M, Hayakawa Y, Kojima
Y, Ikeda H, Imai N, Ogasawara K, Okumura K, Thomas DM and Smyth MJ:
IFN-γ is required for cytotoxic T cell-dependent cancer genome
immunoediting. Nat Commun. 8:146072017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Duncan TJ, Rolland P, Deen S, Scott IV,
Liu DT, Spendlove I and Durrant LG: Loss of IFN gamma receptor is
an independent prognostic factor in ovarian cancer. Clin Cancer
Res. 13:4139–4145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ravaud A, Négrier S, Cany L, Merrouche Y,
Le Guillou M, Blay JY, Clavel M, Gaston R, Oskam R and Philip T:
Subcutaneous low-dose recombinant interleukin 2 and
alpha-interferon in patients with metastatic renal cell carcinoma.
Br J Cancer. 69:1111–1114. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Weinreich DM and Rosenberg SA: Response
rates of patients with metastatic melanoma to high-dose intravenous
interleukin-2 after prior exposure to alpha-interferon or low-dose
interleukin-2. J Immunother. 25:185–187. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wu YN, Zhang L, Chen T, Li X, He LH and
Liu GX: Granulocyte-macrophage colony-stimulating factor protects
mice against hepatocellular carcinoma by ameliorating intestinal
dysbiosis and attenuating inflammation. World J Gastroenterol.
26:5420–5436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Goyal G, Wong K, Nirschl CJ, Souders N,
Neuberg D, Anandasabapathy N and Dranoff G: PPARγ contributes to
immunity induced by cancer cell vaccines that secrete GM-CSF.
Cancer Immunol Res. 6:723–732. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang SR, Nold MF, Tang SC, Bui CB, Nold
CA, Arumugam TV, Drummond GR, Sobey CG and Kim HA: IL-37 increases
in patients after ischemic stroke and protects from inflammatory
brain injury, motor impairment and lung infection in mice. Sci Rep.
9:69222019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jones DS II, Nardozzi JD, Sackton KL,
Ahmad G, Christensen E, Ringgaard L, Chang DK, Jaehger DE,
Konakondla JV, Wiinberg M, et al: Cell surface-tethered IL-12
repolarizes the tumor immune microenvironment to enhance the
efficacy of adoptive T cell therapy. Sci Adv. 8:eabi80752022.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Miyazaki T, Maiti M, Hennessy M, Chang T,
Kuo P, Addepalli M, Obalapur P, Sheibani S, Wilczek J, Pena R, et
al: NKTR-255, a novel polymer-conjugated rhIL-15 with potent
antitumor efficacy. J Immunother Cancer. 9:e0020242021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hamilton JA: GM-CSF in inflammation. J Exp
Med. 217:e201909452020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kumar V: Toll-like receptors in
sepsis-associated cytokine storm and their endogenous negative
regulators as future immunomodulatory targets. Int Immunopharmacol.
89:1070872020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Mercogliano MF, Bruni S, Mauro F, Elizalde
PV and Schillaci R: Harnessing Tumor necrosis factor alpha to
achieve effective cancer immunotherapy. Cancers (Basel).
13:5642021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang TT, Zhao YL, Peng LS, Chen N, Chen W,
Lv YP, Mao FY, Zhang JY, Cheng P, Teng YS, et al: Tumour-activated
neutrophils in gastric cancer foster immune suppression and disease
progression through GM-CSF-PD-L1 pathway. Gut. 66:1900–1911. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Bi E, Ma X, Lu Y, Yang M, Wang Q, Xue G,
Qian J, Wang S and Yi Q: Foxo1 and Foxp1 play opposing roles in
regulating the differentiation and antitumor activity of
TH9 cells programmed by IL-7. Sci Signal.
10:eaak97412017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Alizadeh D, Wong RA, Yang X, Wang D,
Pecoraro JR, Kuo CF, Aguilar B, Qi Y, Ann DK, Starr R, et al: IL15
enhances CAR-T cell antitumor activity by reducing mTORC1 activity
and preserving their stem cell memory phenotype. Cancer Immunol
Res. 7:759–772. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hu HJ, Liang X, Li HL, Du CM, Hao JL, Wang
HY, Gu JF, Ni AM, Sun LY, Xiao J, et al: The armed oncolytic
adenovirus ZD55-IL-24 eradicates melanoma by turning the tumor
cells from the self-state into the nonself-state besides direct
killing. Cell Death Dis. 11:10222020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Park A, Kim S, Jung IH and Byun JH: An
immune therapy model for effective treatment on inflammatory bowel
disease. PLoS One. 15:e02389182020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Berraondo P, Sanmamed MF, Ochoa MC,
Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME,
Ponz-Sarvise M, Castañón E and Melero I: Cytokines in clinical
cancer immunotherapy. Br J Cancer. 120:6–15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Waldmann TA: Cytokines in cancer
immunotherapy. Cold Spring Harb Perspect Biol. 10:a0284722018.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Briukhovetska D, Dörr J, Endres S, Libby
P, Dinarello CA and Kobold S: Interleukins in cancer: From biology
to therapy. Nat Rev Cancer. 21:481–499. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Riley P, Glenny AM, Worthington HV,
Littlewood A, Fernandez Mauleffinch LM, Clarkson JE and McCabe MG:
Interventions for preventing oral mucositis in patients with cancer
receiving treatment: Cytokines and growth factors. Cochrane
Database Syst Rev. 11:CD0119902017.PubMed/NCBI
|
|
130
|
Esquivel-Velázquez M, Ostoa-Saloma P,
Palacios-Arreola MI, Nava-Castro KE, Castro JI and Morales-Montor
J: The role of cytokines in breast cancer development and
progression. J Interferon Cytokine Res. 35:1–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hosokawa H and Rothenberg EV: Cytokines,
transcription factors, and the initiation of T-cell development.
Cold Spring Harb Perspect Biol. 10:a0286212018. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Dayakar A, Chandrasekaran S, Kuchipudi SV
and Kalangi SK: Cytokines: Key determinants of resistance or
disease progression in visceral leishmaniasis: Opportunities for
novel diagnostics and immunotherapy. Front Immunol. 10:6702019.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Wang X, Wong K, Ouyang W and Rutz S:
Targeting IL-10 family cytokines for the treatment of human
diseases. Cold Spring Harb Perspect Biol. 11:a0285482019.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ali MF, Driscoll CB, Walters PR, Limper AH
and Carmona EM: β-Glucan-activated human B lymphocytes participate
in innate immune responses by releasing proinflammatory cytokines
and stimulating neutrophil chemotaxis. J Immunol. 195:5318–5326.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Trottein F and Paget C: Natural killer T
cells and mucosal-associated invariant T cells in lung infections.
Front Immunol. 9:17502018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Isaeva OI, Sharonov GV, Serebrovskaya EO,
Turchaninova MA, Zaretsky AR, Shugay M and Chudakov DM:
Intratumoral immunoglobulin isotypes predict survival in lung
adenocarcinoma subtypes. J Immunother Cancer. 7:2792019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Fajgenbaum DC and June CH: Cytokine storm.
N Engl J Med. 383:2255–2273. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Matthay MA, Zemans RL, Zimmerman GA, Arabi
YM, Beitler JR, Mercat A, Herridge M, Randolph AG and Calfee CS:
Acute respiratory distress syndrome. Nat Rev Dis Primers. 5:182019.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Eskilsson A, Shionoya K, Engblom D and
Blomqvist A: Fever during localized inflammation in mice is
elicited by a humoral pathway and depends on brain endothelial
interleukin-1 and interleukin-6 signaling and central EP3
receptors. J Neurosci. 41:5206–5218. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kang S and Kishimoto T: Interplay between
interleukin-6 signaling and the vascular endothelium in cytokine
storms. Exp Mol Med. 53:1116–1123. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tang XD, Ji TT, Dong JR, Feng H, Chen FQ,
Chen X, Zhao HY, Chen DK and Ma WT: Pathogenesis and treatment of
cytokine storm induced by infectious diseases. Int J Mol Sci.
22:130092021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Hemminki O, Dos Santos JM and Hemminki A:
Oncolytic viruses for cancer immunotherapy. J Hematol Oncol.
13:842020. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Kaufman HL, Kohlhapp FJ and Zloza A:
Oncolytic viruses: A new class of immunotherapy drugs. Nat Rev Drug
Discov. 14:642–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Rahman MM and McFadden G: Oncolytic
viruses: Newest frontier for cancer immunotherapy. Cancers (Basel).
13:54522021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Innao V, Rizzo V, Allegra AG, Musolino C
and Allegra A: Oncolytic viruses and hematological malignancies: A
new class of immunotherapy drugs. Curr Oncol. 28:159–183. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Heidbuechel JPW and Engeland CE: Oncolytic
viruses encoding bispecific T cell engagers: A blueprint for
emerging immunovirotherapies. J Hematol Oncol. 14:632021.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Liu S, Liu F, Zhao M and Zhang J:
Antitumor efficacy of oncolytic herpes virus type 1 armed with
GM-CSF in murine uveal melanoma xenografts. Cancer Manag Res.
12:11803–11812. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Lühl NC, Zirngibl F, Dorneburg C, Wei J,
Dahlhaus M, Barth TF, Meyer LH, Queudeville M, Eckhoff S, Debatin
KM and Beltinger C: Attenuated measles virus controls pediatric
acute B-lineage lymphoblastic leukemia in NOD/SCID mice.
Haematologica. 99:1050–1061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Ammayappan A, Russell SJ and Federspiel
MJ: Recombinant mumps virus as a cancer therapeutic agent. Mol Ther
Oncolytics. 3:160192016. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Bommareddy PK, Patel A, Hossain S and
Kaufman HL: Talimogene laherparepvec (T-VEC) and other oncolytic
viruses for the treatment of melanoma. Am J Clin Dermatol. 18:1–15.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhang R, Cui Y, Guan X and Jiang X: A
recombinant human adenovirus type 5 (H101) combined with
chemotherapy for advanced gastric carcinoma: A retrospective cohort
study. Front Oncol. 11:7525042021. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Omole RK, Oluwatola O, Akere MT, Eniafe J,
Agboluaje EO, Daramola OB, Ayantunji YJ, Omotade TI, Torimiro N,
Ayilara MS, et al: Comprehensive assessment on the applications of
oncolytic viruses for cancer immunotherapy. Front Pharmacol.
13:10827972022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Mardi A, Shirokova AV, Mohammed RN,
Keshavarz A, Zekiy AO, Thangavelu L, Mohamad TAM, Marofi F, Shomali
N, Zamani A and Akbari M: Biological causes of immunogenic cancer
cell death (ICD) and anti-tumor therapy; combination of oncolytic
virus-based immunotherapy and CAR T-cell therapy for ICD induction.
Cancer Cell Int. 22:1682022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ajina A and Maher J: Prospects for
combined use of oncolytic viruses and CAR T-cells. J Immunother
Cancer. 5:902017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Spiesschaert B, Angerer K, Park J and
Wollmann G: Combining oncolytic viruses and small molecule
therapeutics: Mutual benefits. Cancers (Basel). 13:33862021.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Schirrmacher V: Molecular mechanisms of
anti-neoplastic and immune stimulatory properties of oncolytic
Newcastle disease virus. Biomedicines. 10:5622022. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Globerson-Levin A, Waks T and Eshhar Z:
Elimination of progressive mammary cancer by repeated
administrations of chimeric antigen receptor-modified T cells. Mol
Ther. 22:1029–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Chang YH, Lau KS, Kuo RL and Horng JT:
dsRNA binding domain of PKR is proteolytically released by
enterovirus A71 to facilitate viral replication. Front Cell Infect
Microbiol. 7:2842017. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang X, Wang S, Zhu Y, Zhang M, Zhao Y,
Yan Z, Wang Q and Li X: Double-edged effects of interferons on the
regulation of cancer-immunity cycle. Oncoimmunology.
10:19290052021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Ginting TE, Christian S, Larasati YO,
Suryatenggara J, Suriapranata IM and Mathew G: Antiviral
interferons induced by Newcastle disease virus (NDV) drive a
tumor-selective apoptosis. Sci Rep. 9:151602019. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Roulstone V, Mansfield D, Harris RJ,
Twigger K, White C, de Bono J, Spicer J, Karagiannis SN, Vile R,
Pandha H, et al: Antiviral antibody responses to systemic
administration of an oncolytic RNA virus: the impact of standard
concomitant anticancer chemotherapies. J Immunother Cancer.
9:e0026732021. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Cheng JT, Wang YY, Zhu LZ, Zhang Y, Cai
WQ, Han ZW, Zhou Y, Wang XW, Peng XC, Xiang Y, et al: Novel
transcription regulatory sequences and factors of the immune
evasion protein ICP47 (US12) of herpes simplex viruses. Virol J.
17:1012020. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wang G, Kang X, Chen KS, Jehng T, Jones L,
Chen J, Huang XF and Chen SY: An engineered oncolytic virus
expressing PD-L1 inhibitors activates tumor neoantigen-specific T
cell responses. Nat Commun. 11:13952020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Arnone CM, Polito VA, Mastronuzzi A, Carai
A, Diomedi FC, Antonucci L, Petrilli LL, Vinci M, Ferrari F,
Salviato E, et al: Oncolytic adenovirus and gene therapy with
EphA2-BiTE for the treatment of pediatric high-grade gliomas. J
Immunother Cancer. 9:e0019302021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Shi T, Song X, Wang Y, Liu F and Wei J:
Combining oncolytic viruses with cancer immunotherapy: Establishing
a new generation of cancer treatment. Front Immunol. 11:6832020.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Guedan S and Alemany R: CAR-T cells and
oncolytic viruses: Joining forces to overcome the solid tumor
challenge. Front Immunol. 9:24602018. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Chianese A, Santella B, Ambrosino A,
Stelitano D, Rinaldi L, Galdiero M, Zannella C and Franci G:
Oncolytic viruses in combination therapeutic approaches with
epigenetic modulators: Past, present, and future perspectives.
Cancers (Basel). 13:27612021. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Luo Q, Pan M, Feng H and Wang L: ABO blood
group antigen therapy: A potential new strategy against solid
tumors. Sci Rep. 11:162412021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
O'Brien RM, Cannon A, Reynolds JV, Lysaght
J and Lynam-Lennon N: Complement in tumourigenesis and the response
to cancer therapy. Cancers (Basel). 13:12092021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Khalili H, Wolpin BM, Huang ES,
Giovannucci EL, Kraft P, Fuchs CS and Chan AT: ABO blood group and
risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev.
20:1017–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Akin S and Altundag K: Clinical
associations with ABO blood group and rhesus blood group status in
patients with breast cancer: A nationwide retrospective study of
3,944 breast cancer patients in Turkey. Med Sci Monit.
24:4698–4703. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Elkoshi Z: Cancer and autoimmune diseases:
A tale of two immunological opposites? Front Immunol.
13:8215982022. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Kolev M and Markiewski MM: Targeting
complement-mediated immunoregulation for cancer immunotherapy.
Semin Immunol. 37:85–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Makunts T, Saunders IM, Cohen IV, Li M,
Moumedjian T, Issa MA, Burkhart K, Lee P, Patel SP and Abagyan R:
Myocarditis occurrence with cancer immunotherapy across indications
in clinical trial and post-marketing data. Sci Rep. 11:173242021.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Ermer T, Canavan ME, Maduka RC, Li AX,
Salazar MC, Kaminski MF, Pichert MD, Zhan PL, Mase V, Kluger H and
Boffa DJ: Association between food and drug administration approval
and disparities in immunotherapy use among patients with cancer in
the US. JAMA Netw Open. 5:e22195352022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Noman MZ, Hasmim M, Lequeux A, Xiao M,
Duhem C, Chouaib S, Berchem G and Janji B: Improving cancer
immunotherapy by targeting the hypoxic tumor microenvironment: New
opportunities and challenges. Cells. 8:10832019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Kawashima S, Inozume T, Kawazu M, Ueno T,
Nagasaki J, Tanji E, Honobe A, Ohnuma T, Kawamura T, Umeda Y, et
al: TIGIT/CD155 axis mediates resistance to immunotherapy in
patients with melanoma with the inflamed tumor microenvironment. J
Immunother Cancer. 9:e0031342021. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Ma Z, Zhang W, Dong B, Xin Z, Ji Y, Su R,
Shen K, Pan J, Wang Q and Xue W: Docetaxel remodels prostate cancer
immune microenvironment and enhances checkpoint inhibitor-based
immunotherapy. Theranostics. 12:4965–4979. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Lee HT, Lee SH and Heo YS: Molecular
interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in
immuno-oncology. Molecules. 24:11902019. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Capuano C, Pighi C, Battella S, De
Federicis D, Galandrini R and Palmieri G: Harnessing CD16-mediated
NK cell functions to enhance therapeutic efficacy of
tumor-targeting mAbs. Cancers (Basel). 13:25002021. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Li Q, Oduro PK, Guo R, Li R, Leng L, Kong
X, Wang Q and Yang L: Oncolytic viruses: Immunotherapy drugs for
gastrointestinal malignant tumors. Front Cell Infect Microbiol.
12:9215342022. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Burn OK, Prasit KK and Hermans IF:
Modulating the tumour microenvironment by intratumoural injection
of pattern recognition receptor agonists. Cancers (Basel).
12:38242020. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Liu K, Cui JJ, Zhan Y, Ouyang QY, Lu QS,
Yang DH, Li XP and Yin JY: Reprogramming the tumor microenvironment
by genome editing for precision cancer therapy. Mol Cancer.
21:982022. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Sharma VP, Tang B, Wang Y, Duran CL,
Karagiannis GS, Xue EA, Entenberg D, Borriello L, Coste A, Eddy RJ,
et al: Live tumor imaging shows macrophage induction and
TMEM-mediated enrichment of cancer stem cells during metastatic
dissemination. Nat Commun. 12:73002021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Virant-Klun I, Skerl P, Novakovic S,
Vrtacnik-Bokal E and Smrkolj S: Similar population of CD133+ and
DDX4+ VSEL-like stem cells sorted from human embryonic stem cell,
ovarian, and ovarian cancer ascites cell cultures: The real
embryonic stem cells? Cells. 8:7062019. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Yan Y, Chen Y, Yang F, Chen IH, Xiong Z,
Wang J, Lachman LB, Wang H and Yang XF: HLA-A2.1-restricted T cells
react to SEREX-defined tumor antigen CML66L and are suppressed by
CD4+CD25+ regulatory T cells. Int J Immunopathol Pharmacol.
20:75–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Wargo JA, Robbins PF, Li Y, Zhao Y,
El-Gamil M, Caragacianu D, Zheng Z, Hong JA, Downey S, Schrump DS,
et al: Recognition of NY-ESO-1+ tumor cells by engineered
lymphocytes is enhanced by improved vector design and epigenetic
modulation of tumor antigen expression. Cancer Immunol Immunother.
58:383–394. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Yang L, Gu X, Yu J, Ge S and Fan X:
Oncolytic virotherapy: From bench to bedside. Front Cell Dev Biol.
9:7901502021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Han W, Li W, Zhang X, Du Z, Liu X, Zhao X,
Wen X, Wang G, Hu JF and Cui J: Targeted breast cancer therapy by
harnessing the inherent blood group antigen immune system.
Oncotarget. 8:15034–15046. 2017. View Article : Google Scholar : PubMed/NCBI
|