Introduction
Living organisms require a continuous influx of
energy to perform the various physiological activities necessary
for cell growth and survival. Adenosine triphosphate (ATP), the
energy currency of cells, is produced by glycolysis in the
cytoplasm and oxidative phosphorylation in the mitochondria. Both
processes occur essentially in most cells. However, oxidative
phosphorylation predominates, and glycolysis is increased when
there is insufficient oxygen supply, such as in muscle cells during
exercise. However, tumor cells rely more on glycolysis than
oxidative phosphorylation for ATP production even under adequate
oxygenation, which is called the Warburg effect (1). Depending on the cancer type, a variety
of intracellular and extracellular changes, including mitochondrial
alterations, upregulation of key glycolytic enzymes, intracellular
pH control and hypoxia-induced conversion to anaerobic metabolism,
have been shown to mediate the Warburg effect (2,3).
Metabolic shift to aerobic glycolysis provides intermediates for
various biosynthetic pathways and adapts cancer cells to hypoxic
conditions in solid tumors (4).
Enhanced glycolysis in tumor cells increases lactic acid
accumulation and acidity in the extracellular compartment in tumor,
which significantly contributes to resistance to chemotherapy,
proliferation and metastasis (5).
The phosphatidylinositide 3-kinase (PI3K)/protein
kinase B (Akt) pathway regulates various cellular processes
including cell survival, proliferation, metabolism, motility and
apoptosis (6). It is initiated by
autophosphorylation of the tyrosine residue in the receptor protein
tyrosine kinases, and is found to be frequently activated in human
cancers. In particular, activation of the PI3K/Akt/mammalian target
of rapamycin (mTOR) signaling pathway plays an essential role in
triggering the Warburg effect in tumor cells by increasing the
activity and expression of glucose transporters (GLUTs) and
glycolytic enzymes (7,8) without affecting mitochondrial
oxidative phosphorylation (9). The
PI3K/Akt/mTOR pathway is frequently activated in malignant
mesothelioma (MM), presumably due to reactive oxygen species
(ROS)-mediated inactivation of phosphatase and tensin homolog
(PTEN) (10). However, little is
known about an association of the Warburg effect with the
PI3K/Akt/mTOR pathway in MM. An intricate interplay exists between
the Akt and p53 pathways. Under appropriate survival signals, Akt
negatively modulates the tumor suppressor p53 levels by enhancing
the murine double minute 2 (MDM2)-mediated degradation of p53
(11). p53 antagonizes PI3K/Akt
signaling by inducing the expression of PTEN, enabling cells to
undergo rapid apoptosis (12).
Therefore, mutations that activate the PI3K/Akt signaling pathway
and inactivate the p53 gene are common mechanisms to increase
cancer cell survival and form more malignant clones, and targeting
Akt has become a key strategy in cancer therapy and prevention
(13).
Apigenin (4′,5,7-trihydroxyflavone) not only induces
apoptosis, autophagy, cell cycle arrest, but also inhibits
angiogenesis and metastasis in different types of cancer cells
(14,15). Several signaling pathways including
PI3K/Akt, nuclear factor kappa B (NF-kB), and mitogen-activated
protein kinase/extracellular signal-regulated kinase (MAPK/ERK) are
known to modulate apigenin activity (15). Among them, the importance of the
PI3K/Akt pathway as a major molecular target for the various
anticancer activities of apigenin has been demonstrated in in
vitro and in vivo studies (14,16).
Little is known about the effect of apigenin in MM, but a previous
study revealed that it induces apoptosis by inhibiting the c-Jun
N-terminal kinase (JNK) pathway (17). Recently, it was demonstrated by the
authors that apigenin induces apoptosis and necroptosis of MM cells
with intracellular ROS accumulation, mitochondrial dysfunction and
ATP depletion at a concentration of 30 µM that does not affect
normal mesothelial MeT-5A cells (18).
A variety of evidence indicates that inhibition of
glycolysis in cancer cells results in ATP depletion and cell death,
which is particularly effective in cells with high glycolytic
activity and resistance to anticancer drugs (19,20).
Severe ATP depletion is more likely to cause cell death through
necrosis rather than apoptosis (19,21). A
distinct shift in energy metabolism towards the Warburg profile was
also observed in MM tissues, as characterized by increased
expression of numerous genes involved in glycolysis and oxidative
metabolism and the accumulation of 2-deoxy-2-[fluorine-18]
fluoro-D-glucose in tumor lesions in positron emission tomography
analysis (10,22). Furthermore, MM cell lines, including
MSTO-211H and H2452 cells, have been shown to depend on glucose
metabolism for rapid growth (23).
Together with the authors' previous findings linking
apigenin-induced cell death with mitochondrial dysfunction and ATP
depletion, the results of the aforementioned studies led us to the
hypothesis that the effects of apigenin on energy metabolism are
mediated by targeting glycolysis through inhibition of the PI3K/Akt
pathway.
In the present study, it was aimed to extend the
previous investigation into the anticancer role of apigenin and to
evaluate its effects on energy metabolism and cell death using two
human MM cell lines pre-adapted to lactate. The cell lines were
established by continuously exposing MSTO-211H and H2452 cells to
3.8 µM lactic acid through 4 serial passages for 15 days. The
current data provide mechanistic evidence suggesting a close link
between the PI3K/Akt pathway, p53 and glycolysis in the induction
of apoptosis and necroptosis. The current study deepens our
understanding of the novel role of apigenin in MM cells.
Materials and methods
Cell culture and assays
Human MM cell lines MSTO-211H and H2452 were
purchased from the American Type Culture Collection. Acidic
pre-adapted cells designated as MSTO-211HAcT (cat. no. CRL-2081)
and H2452AcT (cat. no. CRL-5946) were established by continuously
exposing MSTO-211H and H2452 cells, respectively, to 3.8 µM lactic
acid through 4 passages for 15 days. Cells were cultured in in
RPMI-1640 medium (Welgene, Inc.) containing 3.8 µM lactic acid and
5% fetal bovine serum (Welgene, Inc.), and then treated with
increasing concentrations (10, 20, 40, 80 and 160 µM) of
gemcitabine for cell viability assay. Cells were treated with 20 µM
Ly294002 (Merck KGaA) and 30 µM apigenin (MilliporeSigma) alone or
in combination for 48 h. For combination treatment, pretreatment
with Ly294002 for 2 h followed by treatment with apigenin without
removing Ly294002. As a negative control, cells were treated with
0.1% dimethyl sulfoxide. Cell viability was measured by MTT assay
as previously described (18). The
activities of hexokinase (HK) and pyruvate dehydrogenase (PDH) were
determined using an HK Colorimetric Assay Kit (cat. no. K789-100)
and PDH Activity Colorimetric Assay Kit (cat. no. K679-100),
respectively, according to the protocols provided by the
manufacturer (BioVision, Inc.). Glucose consumption was determined
by assessing the glucose content in the culture media according to
the instruction provided in the Glucose Colorimetric Assay Kit
(cat. no. K606-100; Biovision, Inc.). Nuclear morphology of
apoptotic cells, such as nuclear fragmentation and chromosome
condensation, was observed in cells stained with
2′,7′-dichlorodihydrofluorescein iodide (DAPI, 2 µg/ml) for 10 min
in the dark as previously described by Lee et al (24). Intracellular ATP content was
determined by luminescence measurement using the CellTiter-Glo
Luminescent Cell Viability Assay Kit according to the
manufacturer's instructions (Promega Corporation). The data were
normalized by the number of viable cells. Absorbance and
luminescence values were measured by a GloMax-Multi microplate
multimode reader (Promega Corporation).
Western blotting
Total cell lysates were extracted with 1X RIPA
buffer and protein concentration was determined by BCA protein
assay (Thermo Fisher Scientific, Inc.). The extracted proteins (40
µg/well) were separated on 4–12% NuPAGE gels (Thermo Fisher
Scientific, Inc.) and then transferred to polyvinylidene fluoride
membrane (GE Healthcare Life Sciences). The membranes were blocked
with 1X casein solution (cat. no. 37528; Thermo Fisher Scientific,
Inc.) for 2 h at room temperature, incubated overnight at 4°C with
primary antibodies, and then with horseradish-peroxidase
(HRP)-conjugated secondary antibodies for 2 h at room temperature.
Reactive proteins were visualized with an enhanced
chemiluminescence detection kit (Cyanagen Srl) using X-ray film.
TINA 2.09 software (Raytest Isotopenmessgeraete GmbH) was used for
densitometric analysis of protein bands in western blots. The
following antibodies were used to detect each protein. Oxphos human
WB antibody cocktail (cat. no. 45-8199) and antibodies to HK-I
(1:500; cat. no. 2024), HK-II (1:500; cat. no. 2867), PFKP (1:500;
cat. no. 8164), pyruvate dehydrogenase (1:500; cat. no. 3205),
phosphorylated (p)-MLKL (1:500; cat. no. 91689), p-RIP3 (1:500;
cat. no. 93654; 1:500), p-Akt (1:500; cat. no. 9271), Akt (1:500;
cat. no. 9272), PARP (1:500; cat. no. 9542), cleaved PARP (1:500;
cat. no. 9541), caspase-3 (1:500; cat. no. 14220) and cleaved
caspase-3 (1:500; cat. no. 9664) were all purchased from Cell
Signaling Technology, Inc. and used for antigen detection. Goat
anti-rabbit IgG-HRP (1:5,000; cat. no. sc-2004), goat anti-mouse
IgG-HRP (1:5,000; cat. no. sc-2005) and anti-p53 antibody (1:500
cat. no. sc-126) were all purchased from Santa-Cruz Biotechnology,
Inc. The membranes were re-probed using anti-β-actin (1:10,000;
cat. no. A2228; Sigma-Aldrich; Merck KGaA), anti-RIP3 (1:1,000;
cat. no. 13526; Cell Signaling Technology, Inc.), and anti-MLKL
(1:1,000; cat. no. 14993; Cell Signaling Technology, Inc.) as the
loading controls.
Measurement of ROS and mitochondrial
membrane potential
Cells (105 cells/well) were seeded on
six-well culture plates and incubated overnight in lactic
acid-containing RPMI-1640 medium. Cells were treated with or
without 20 µM Ly294002 for 2 h, then 30 µM apigenin was added to
each well without removing Ly294002 and incubated for 48 h. After
trypsinization, cells were harvested by centrifugation at 500 × g
for 7 min, and then resuspended in serum-free RPMI-1640 medium
containing 2′,7′-dichlorodihydrofluorescein diacetate (10 µM) and
rhodamine 123 (30 nM; both from Sigma-Aldrich; Merck KGaA) in the
dark at 37°C for 30 min to measure the levels of ROS and
mitochondrial membrane potential, respectively. The fluorescence
intensity of the cells was measured with a MACSQuant analyzer and
MACSQuantify software version 2.5 (Miltenyi Biotec GmbH).
Annexin V-PE binding assay
Analysis of apoptotic and necrotic cell distribution
was performed according to the instructions provided with the Muse
Annexin V & Dead Cell Assay Kit (cat. no. MCH100105; Merck
KGaA). Briefly, cells were treated with or without 20 µM Ly294002
for 2 h, then 30 µM apigenin was added to each well without
removing Ly294002 and incubated for 48 h. Cells were trypsinized,
and collected in a culture medium supplemented with Muse Annexin V
& Dead Cell reagent. The cells were then analyzed with Muse
cell analyzer (Merck KGaA). Annexin V-phycoerythrin (PE)-positive
apoptotic and 7-AAD-positive necrotic cells were detected using
Annexin V-PE and 7-amino-actinomycin D (7-AAD) double staining.
Cell cycle analysis
Cell cycle distribution at each phase was determined
via propidium iodide (PI) staining as previously described
(18). Briefly, trypsinized cells
were centrifuged at 500 × g at 4°C for 7 min and then fixed with
70% ethanol overnight at −20°C. After washing the cells with 1X
phosphate-buffered saline (PBS), Muse cell cycle reagent (cat. no.
MCH100106; Merck KGaA) containing PI and RNAse was added and
allowed to react for 30 min. Data from 10,000 cells were analyzed
using the MACSQuant analyzer and MACSQuantify software version 2.5
(MiltenyiBiotec GmbH).
Spheroid culture and viability
assay
Spheroid culture was performed in an ultra-low
attachment 96-well plates as previously described (18). Briefly, plates seeded with
104 cells/well were centrifuged at 500 × g for 10 min to
allow cells to cluster in the wells and maintained in complete
RPMI-1640 medium containing lactic acid (final concentration: 3.8
µM) for 5 days. Spheroids were treated with or without 20 µM
Ly294002 for 2 h, then 30 µM apigenin was added to each well
without removing Ly294002 and incubated for 48 h. Green
fluorescence of fluorescein diacetate (FDA; Sigma-Aldrich, 5 µg/ml)
and red fluorescence for PI (Sigma-Aldrich, 10 µg/ml) were used to
detect live and dead cells, respectively. Phase-contrast images
were acquired with a Leica inverted microscope. Spheroids were
visualized using a Leica EL6000 fluorescence microscope (Leica
Microsystems GmbH). Spheroid viability was determined according to
the instructions provided with the Enhanced Cell Viability Assay
Kit (Young In Frontier Co., Ltd.). Briefly, 10 µl of Cellvia
solution was added to each well, kept at room temperature for 1 h,
and then mixed for 1 min to dissolve the formed formazan crystal.
The amount of formazan formed in living cells was measured
spectrophotometrically at 450 nm using a GloMax-Multi microplate
multimode reader (Promega Corporation).
Statistical analysis
SPSS version 17.0 software (SPSS, Inc.) was used for
statistical analysis of experimental data. Statistical analysis was
performed by one-way ANOVA and Tukey's post hoc correction. Data
are presented as the mean ± standard deviation (S.D.) for three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pre-adaptation of MM cells to lactic
acid exhibits enhanced glycolytic activity
To evaluate the effect of pre-adaptation to an
acidic environment, MSTO-211HAcT and H2452AcT cells were cultured
for 24, 48 and 72 h in media containing 3.8 µM lactic acid. During
this period, the proliferative status was measured and compared
with their parental MSTO-211H and H2452 cells. As demonstrated in
Fig. 1A, MSTO-211HAcT and H2452AcT
cells showed significantly faster growth. Pre-adaptation also
increased the tolerance to the anticancer drug gemcitabine at 48 h
treatment (Fig. 1B). In addition,
the levels of several rate-limiting enzymes in glucose metabolism,
including HK-I, HK-II, phosphofructokinase platelet (PFKP) and PDH,
were apparently upregulated in both cell types (Fig. 1C). Consistent with the results of
western blotting, the activities of HK and PDH were also increased
in culture for 48 h (Fig. 1D).
Next, the levels of the five complexes in the mitochondrial
electron transport chain (ETC) were analyzed. A slight increase was
observed in complexes I (NDUFB8, NADH:ubiquinone oxidoreductase
subunit B8), III (UQCRC2, ubiquinone-cytochrome C reductase core
protein 2), and IV (COX II, mitochondrial cytochrome C oxidase
subunit I) in apigenin-treated MSTO-211HAcT and H2452AcT cells when
compared with MSTO-211H and H2452 cells (Fig. 1E). Significant upregulation of p-Akt
and downregulation of p53, which are potential signaling pathways
involved in glycolysis, were observed in MSTO-211HAcT and H2452AcT
cells (Fig. 1F).
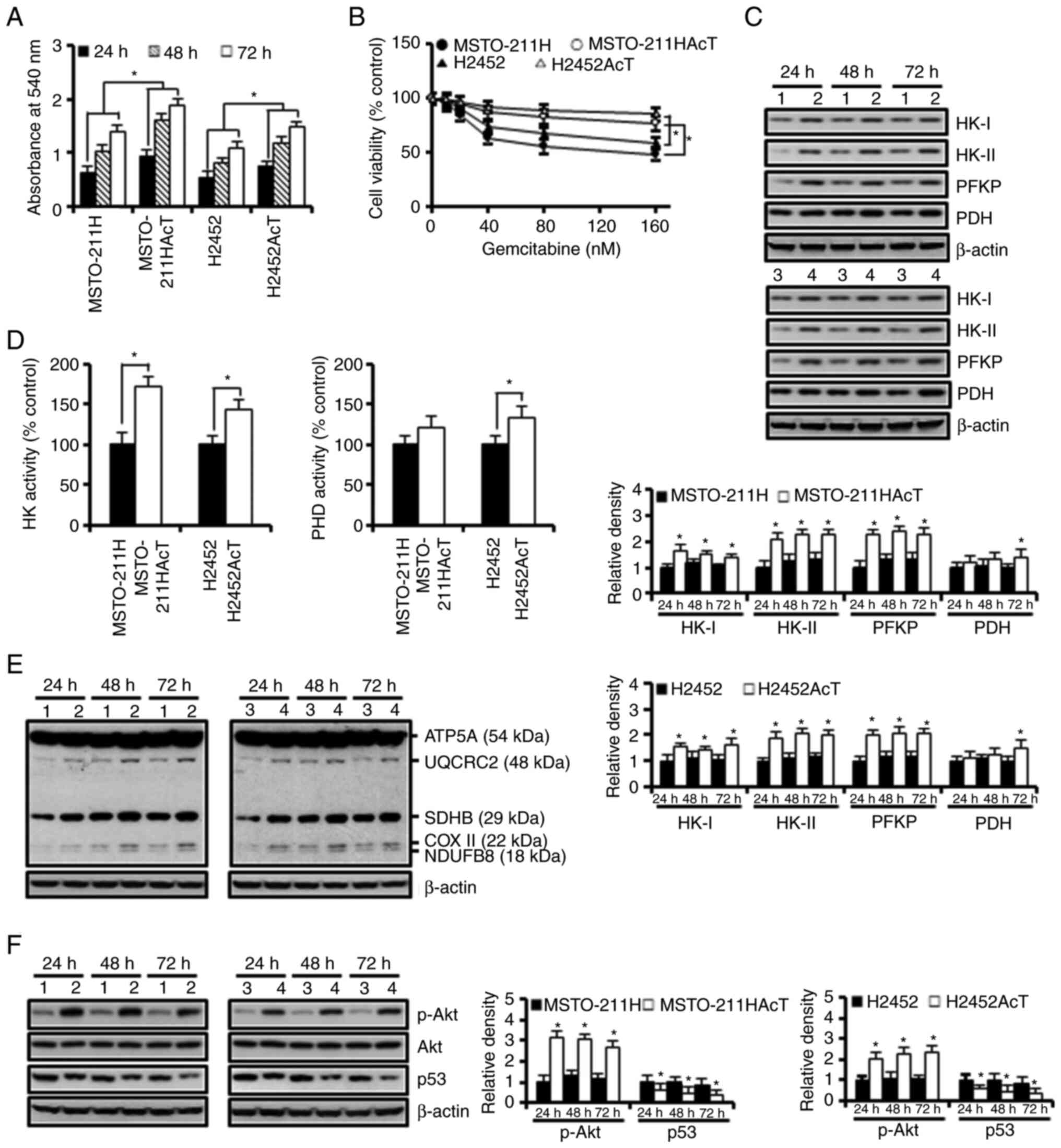 | Figure 1.Enhancement of the Warburg-like
phenotype in MSTO-211HAcT and H2452AcT cells pre-adapted to lactic
acid. Cells were cultured in the complete medium containing 3.8 µM
lactic acid for the indicated times, otherwise 48 h. (A) Cell
viability was measured by MTT assay during cell culture for 72 h.
(B) Cells were treated with increasing concentrations of
gemcitabine, followed by MTT assay to measure cell viability. (C)
The expression levels of rate-limiting enzymes in glucose
metabolism. (D) Activities of hexokinase and pyruvate
dehydrogenase. (E) The expression levels of complexes I–V in
mitochondrial electron transport chain. (F) The levels of p-Akt,
Akt, and p53 proteins. Bar graphs present densitometric analysis of
western blot images normalized to β-actin. *P<0.05 vs.
respective MSTO-211H or H2452 cells. 1, MSTO-211H; 2, MSTO-211HAcT;
3, H2452; 4, H2452AcT; APG, apigenin; HK, hexokinase; PFKP,
phosphofructokinase platelet; PDH, pyruvate dehydrogenase; NDUFB8,
NADH-ubiquinone oxidoreductase subunit B8 (complex I); SDHB,
succinate dehydrogenase complex iron sulfur subunit B (complex II);
UQCRC2, ubiquinone-cytochrome C reductase core protein 2 (complex
III); COX II, mitochondrial cytochrome C oxidase subunit II
(complex IV); ATP5A, ATP synthase F1 subunit alpha (complex V); p-,
phosphorylated. |
Apigenin and/or Ly294002 treatment
induces apoptosis and necroptosis
To determine the role of Akt as an upstream
signaling molecule regulated by apigenin in MSTO-211HAcT and
H2452AcT cells, the additive effect of PI3-kinase/Akt inhibition on
apigenin-induced cell death was investigated after pre-inhibition
of the PI3-kinase using Ly294002 in cultures containing 3.8 µM
lactic acid. An MTT assay was performed to determine the viability
of these cells upon treatment with increasing concentrations of
apigenin for 48 h. As revealed in Fig.
2A, the half maximal inhibitory concentration values for
MSTO-211HAcT and H2452AcT cells were measured to be 47.17 and 56.01
µM, respectively. A concentration of 30 µM, which showed cell
viability in the range of 75–85%, was selected and used for further
experiments. The concentration for Ly294002 treatment was
determined to be 20 µM, which is a concentration that significantly
downregulated p-Akt by western blotting (Fig. S1).
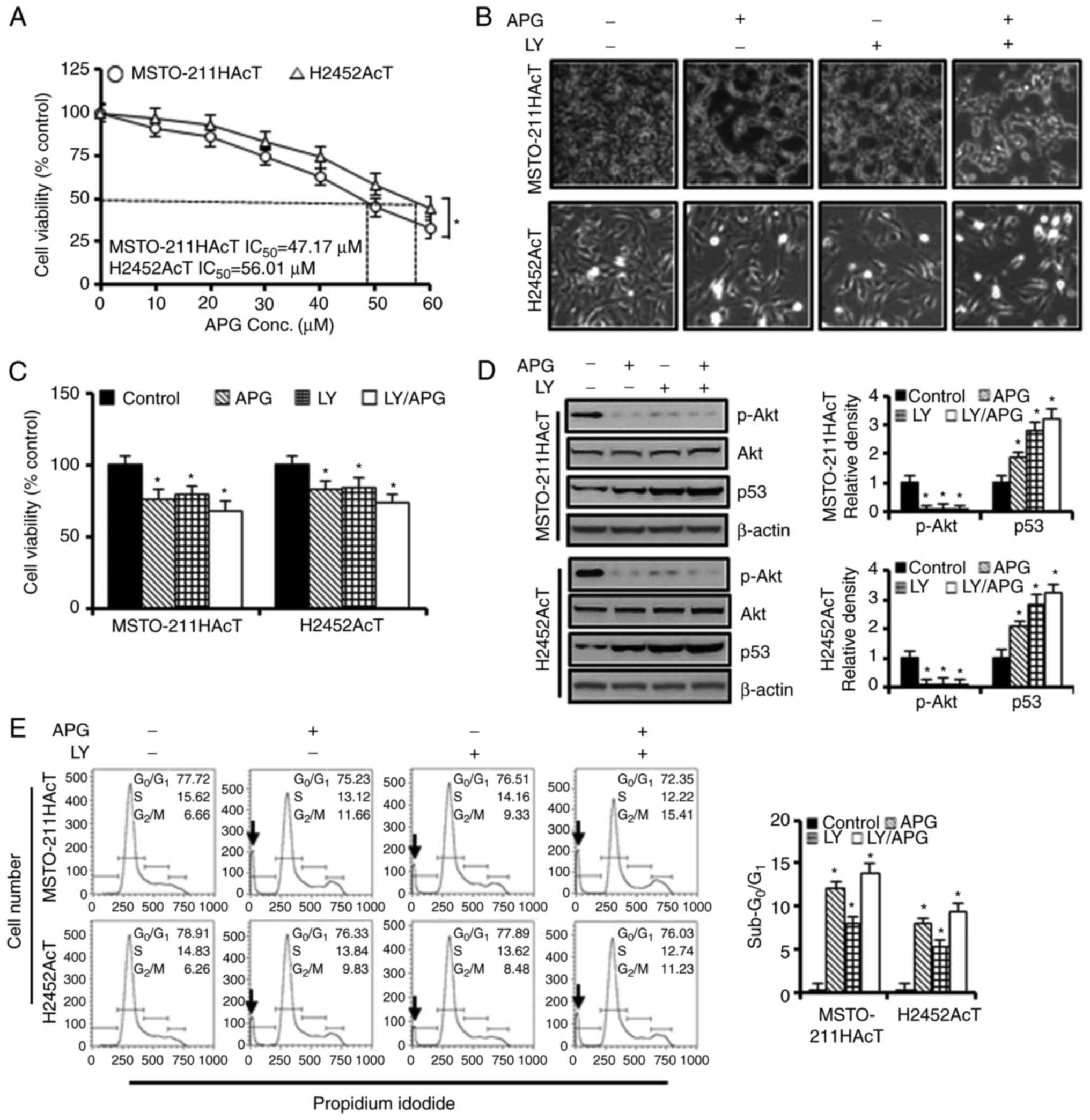 | Figure 2.Cytotoxic effects of apigenin and/or
Ly294002 on MSTO-211HAcT and H2452AcT cells. (A) Cells were treated
with increasing concentrations of apigenin (0, 10, 20, 30, 40, 50
and 60 µM) for 48 h. The percentage of viable cells was determined
by comparison with the results obtained using DMSO-treated control
cells (100%). Cells were treated with or without Ly294002 (20 µM, 2
h) prior to apigenin treatment (30 µM, 48 h) in RPMI-1640 medium
containing 3.8 µM lactic acid. (B) Cell morphology. (C) Percentage
of cell viability. (D) The levels of p-Akt and p53 proteins. (E)
Cell cycle distribution at each phase. *P<0.05 vs. respective
control cells. Arrows indicate sub-G0/G1
peak. APG, apigenin; LY, Ly294002; p-, phosphorylated. |
The majority of control cells adhered to cell
culture plates, but when cells were exposed to Ly294002 and/or
apigenin for 48 h, the cells detached from the surface of the
culture plate, increasing the number of cells floating in the
culture medium (Fig. 2B). Treatment
with apigenin (30 µM) and Ly294002 (20 µM) alone reduced cell
viability to 76.19 and 79.59% in MSTO-211HAcT cells and 82.81 and
83.80% in H2452AcT cells, respectively, compared with control cells
(Fig. 2C). However, the percentage
of cell viability in the combination treatment of apigenin and
Ly294002 did not show a synergistic effect compared with the
treatment with apigenin or Ly294002 alone. Ly294002 and apigenin,
alone or in combination, inhibited Akt phosphorylation and
upregulated p53 levels (Fig. 2D).
In cell cycle analysis, treatment with apigenin and Ly294002 alone
increased the proportion of sub-G0/G1 phase,
indicative of apoptosis, to 12.18 and 8.04% in MSTO-211HAcT cells,
respectively, and to 7.96 and 5.25% in H2452AcT cells,
respectively, as well as the percentage of cell fraction in the
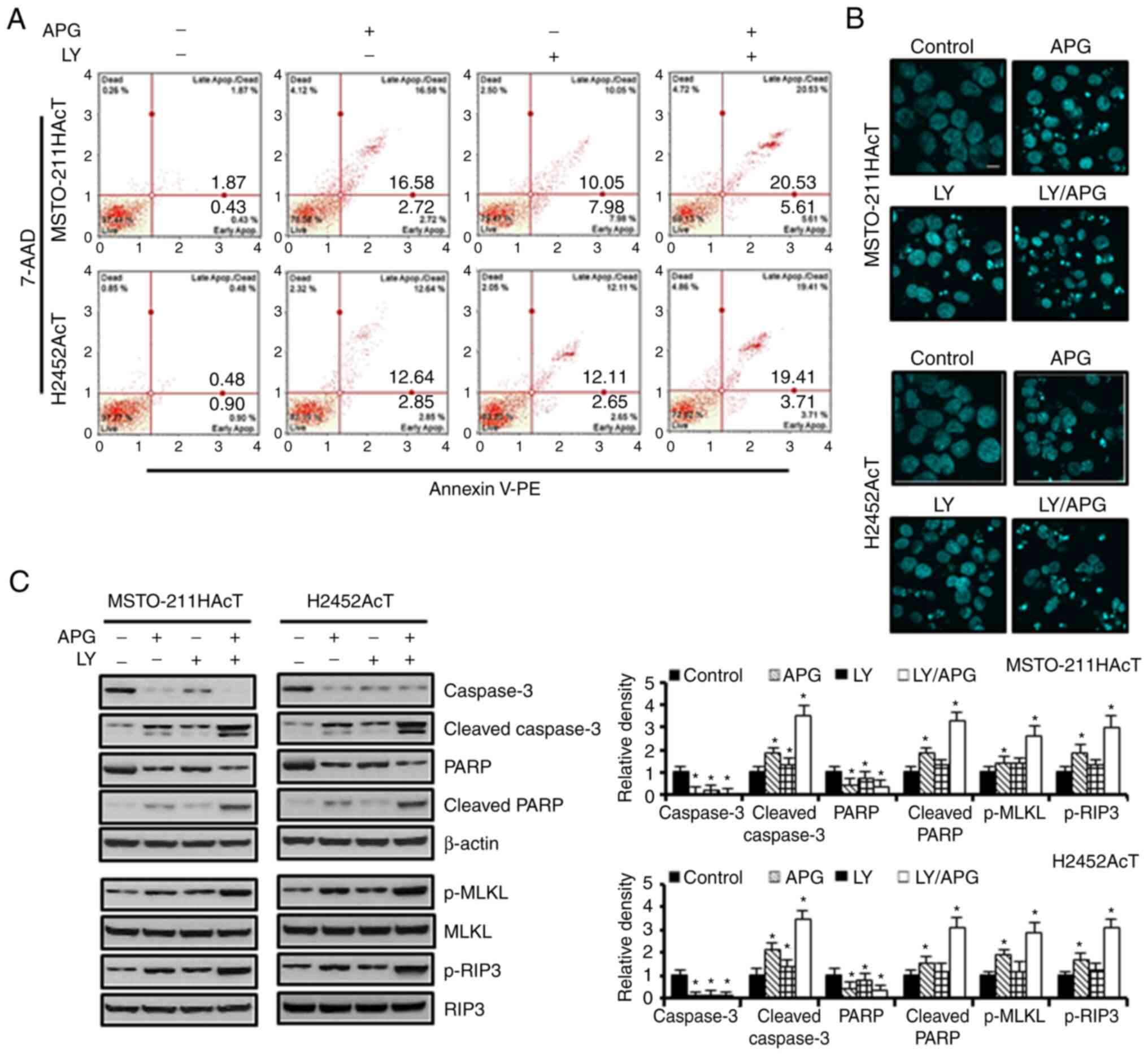
G2/M phase compared with the controls (Fig. 2E). In Annexin V-PE binding assay of
cells treated with apigenin and Ly294002 alone, the percentage of
apoptotic cells, including early and late apoptosis, increased to
19.30 and 18.03% in MSTO-211HAcT cells and 15.49 and 14.76%, in
H2452AcT cells, respectively, compared with the controls (Fig. 3A). Similarly, nuclear staining with
DAPI revealed an increase in the number of cells with the chromatin
condensation and nuclear fragmentation (Fig. 3B). Western blot analysis revealed
that the levels of proteins responsible for apoptosis, including
cleaved caspase-3 and cleaved PARP, and for necroptosis, including
p-MLKL and p-RIP3, were upregulated in Ly294002 or apigenin
treatment compared with the controls, and that these were enhanced
upon combination treatment (Fig.
3C).
Apigenin and/or Ly294002 treatment
inhibits ATP production by targeting glycolysis and oxidative
phosphorylation
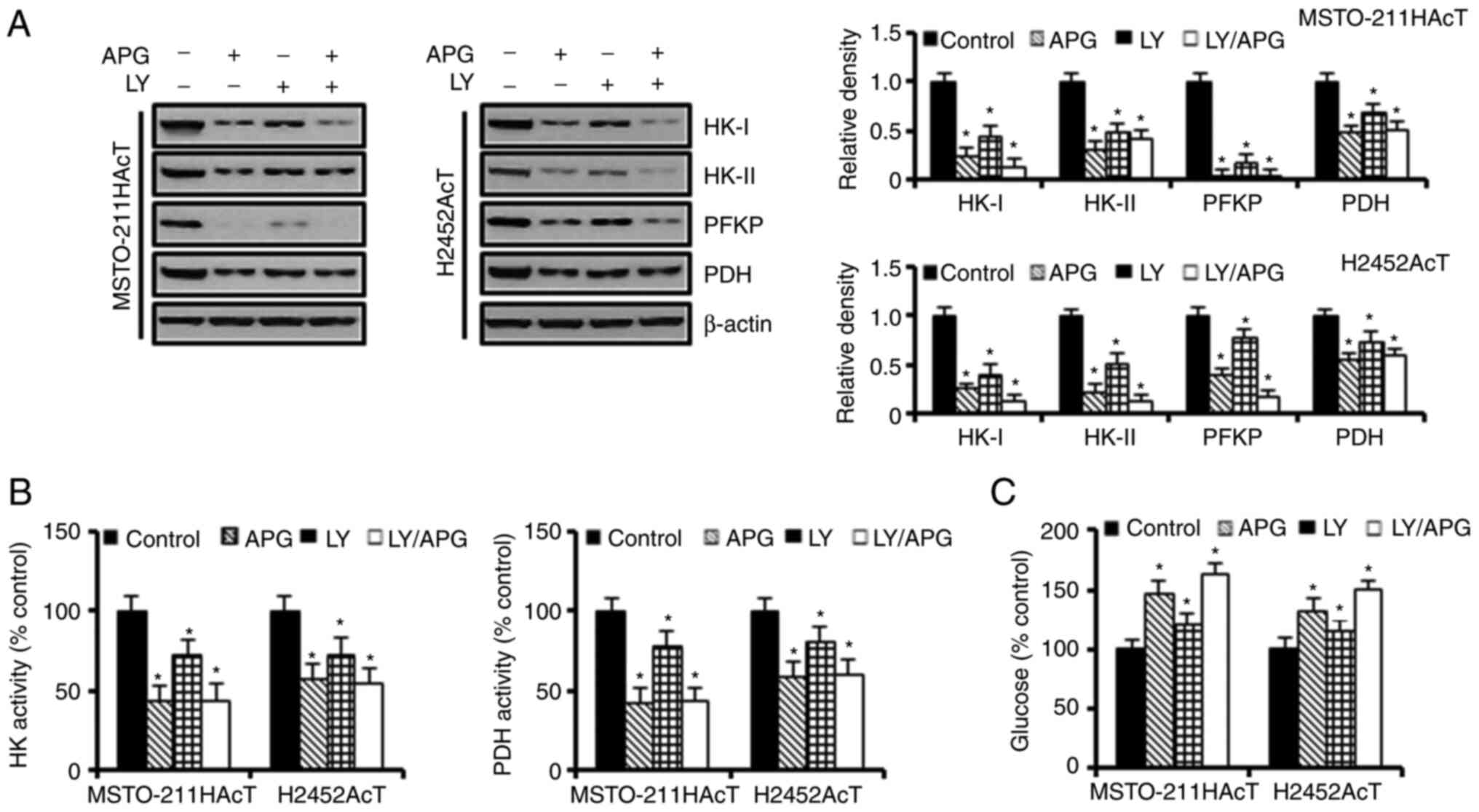
Next, the effect of apigenin-induced inhibition of
the PI3-kinase/Akt pathway on cellular energy metabolism was
investigated. Inhibition of the Akt activity by apigenin and/or
Ly294002 downregulated the expression of HK-I, HK-II, and PFKP
(Fig. 4A) as well as the activities
of HK and PDH (Fig. 4B). Next, it
was investigated how a decrease in the activity and concentration
of these enzymes affected glucose utilization. Treatment with
apigenin and Ly294002 alone increased the glucose concentration in
the culture medium by 46.91 and 20.58%, respectively, in
MSTO-211HAcT cells, and by 32.43 and 14.91%, respectively, in
H2452AcT cells (Fig. 4C). Next, it
was investigated whether Akt inhibition affected mitochondrial
function. After 48-h treatment with apigenin and/or Ly294002, the
fraction of cells with the loss of mitochondrial membrane
potential, indicative of mitochondrial dysfunction, was increased
(Fig. 5A), along with an increase
in intracellular ROS levels (Fig.
5B). During this process, the levels of complexes I–V in ETC
(Fig. 5C) and intracellular ATP
content (Fig. 5D) were
significantly reduced with either apigenin or Ly294002 alone, and
the levels were further decreased with the combination
treatment.
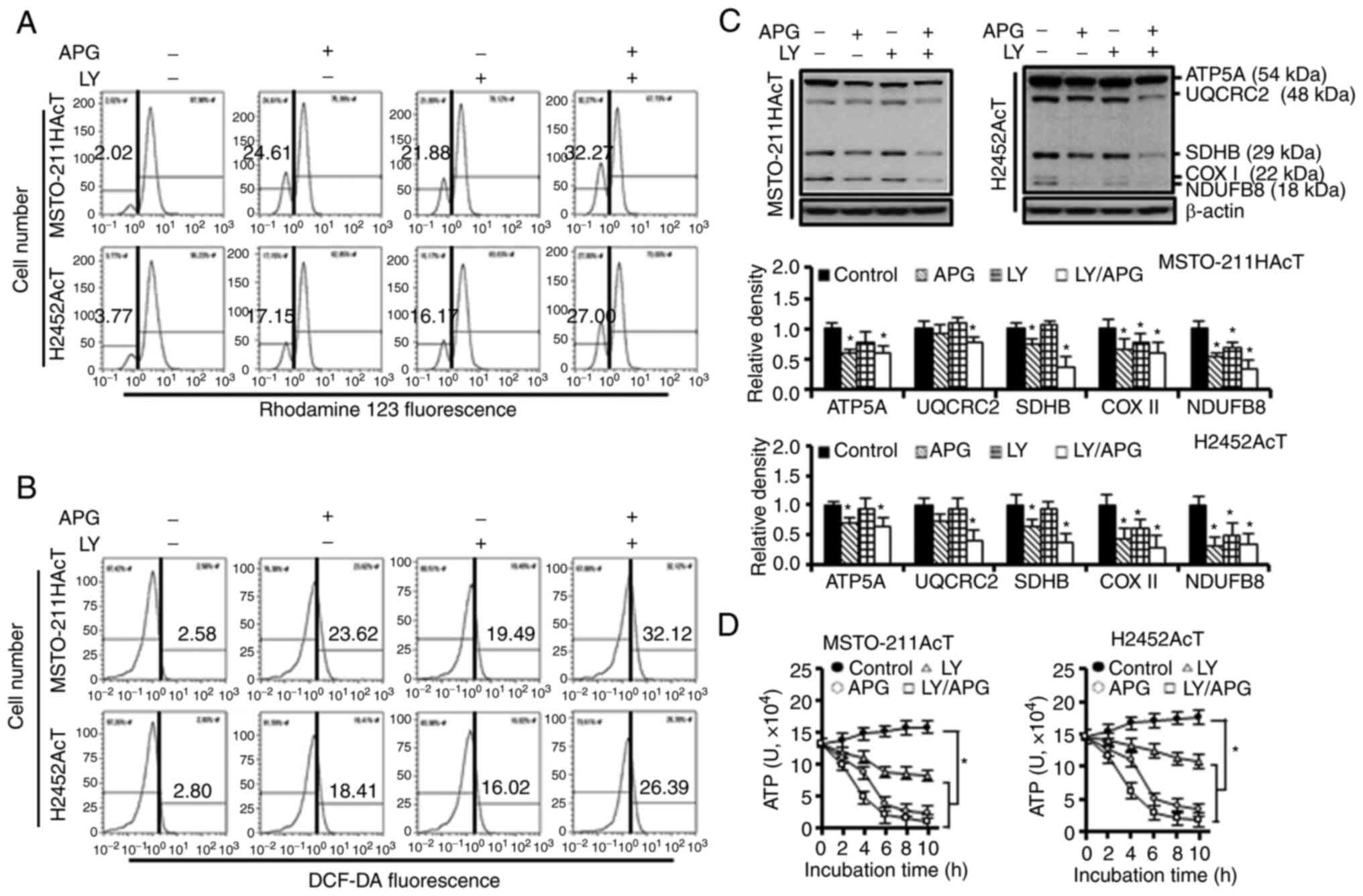 | Figure 5.Effects of apigenin and/or Ly294002
on mitochondrial function in MSTO-211HAcT and H2452AcT cells. Cells
were treated with or without Ly294002 (20 µM, 2 h) prior to
apigenin treatment (30 µM, 48 h) in RPMI-1640 medium containing 3.8
µM lactic acid. (A) Measurement of mitochondrial membrane potential
following staining cells with rhodamine123. (B) Measurement of
intracellular ROS levels following staining cells with DCF-DA (10
µM). (C) The expression levels of complexes I–V in mitochondrial
electron transport chain. Bar graphs present densitometric analysis
of western blot images normalized to β-actin. (D) Intracellular ATP
levels. *P<0.05 vs. respective control cells. APG, apigenin; LY,
Ly294002; NDUFB8, NADH:ubiquinone oxidoreductase subunit B8
(complex I); SDHB, succinate dehydrogenase complex iron sulfur
subunit B (complex II); UQCRC2, ubiquinone-cytochrome C reductase
core protein 2 (complex III); COX II, mitochondrial cytochrome C
oxidase subunit II (complex IV); ATP5A, ATP synthase F1 subunit
alpha (complex V), and DCF-DA, 2′,7′-dichlorodihydrofluorescein
diacetate. |
Apigenin induces anti-glycolytic and
cytotoxic effects in 3D spheroid culture
Based on the results of the 2D monolayer cultures,
the effect of Akt inhibition on the growth of spheroids and the
expression levels of the glycolytic, apoptotic and necroptotic
proteins in 3D spheroid cultures were further investigated. A
two-color fluorescence assay was used to identify live and dead
cells. Cell-permeable FDA is converted into green fluorescent by
esterases within living cells, whereas PI enters the nucleus of
dead or dying cells and emits red fluorescence upon binding to DNA.
As demonstrated in Fig. 6A, Akt
inhibition by apigenin and/or Ly294002 decreased spheroid growth
with an increase in red fluorescence for PI inside the spheroids
indicating dead cells. In addition, the spheroid shape changed from
a compact circle in the control group to a less condensed one with
an irregular surface. The results of apigenin and/or Ly294002
treatment in 3D cultures revealed similar trends to those in 2D
cultures, as shown in reduced spheroid cell viability (Fig. 6B), p-Akt downregulation and p53
upregulation (Fig. 6C), and
upregulation of apoptotic proteins, including cleaved caspase-3 and
cleaved PARP, necroptotic proteins, including p-MLKL and p-RIP3
(Fig. 6D), and downregulation of
key glycolytic enzymes, including HK-I, HK-II, PFKP and PDH
(Fig. 6E) compared with controls.
However, cells cultured in 3D showed less sensitivity to apigenin
and/or Ly294002 compared with those of 2D cultures. Of note,
downregulation of p-Akt and upregulation of p53 in response to
Ly294002 was observed to a lesser extent in 3D than in 2D
cultures.
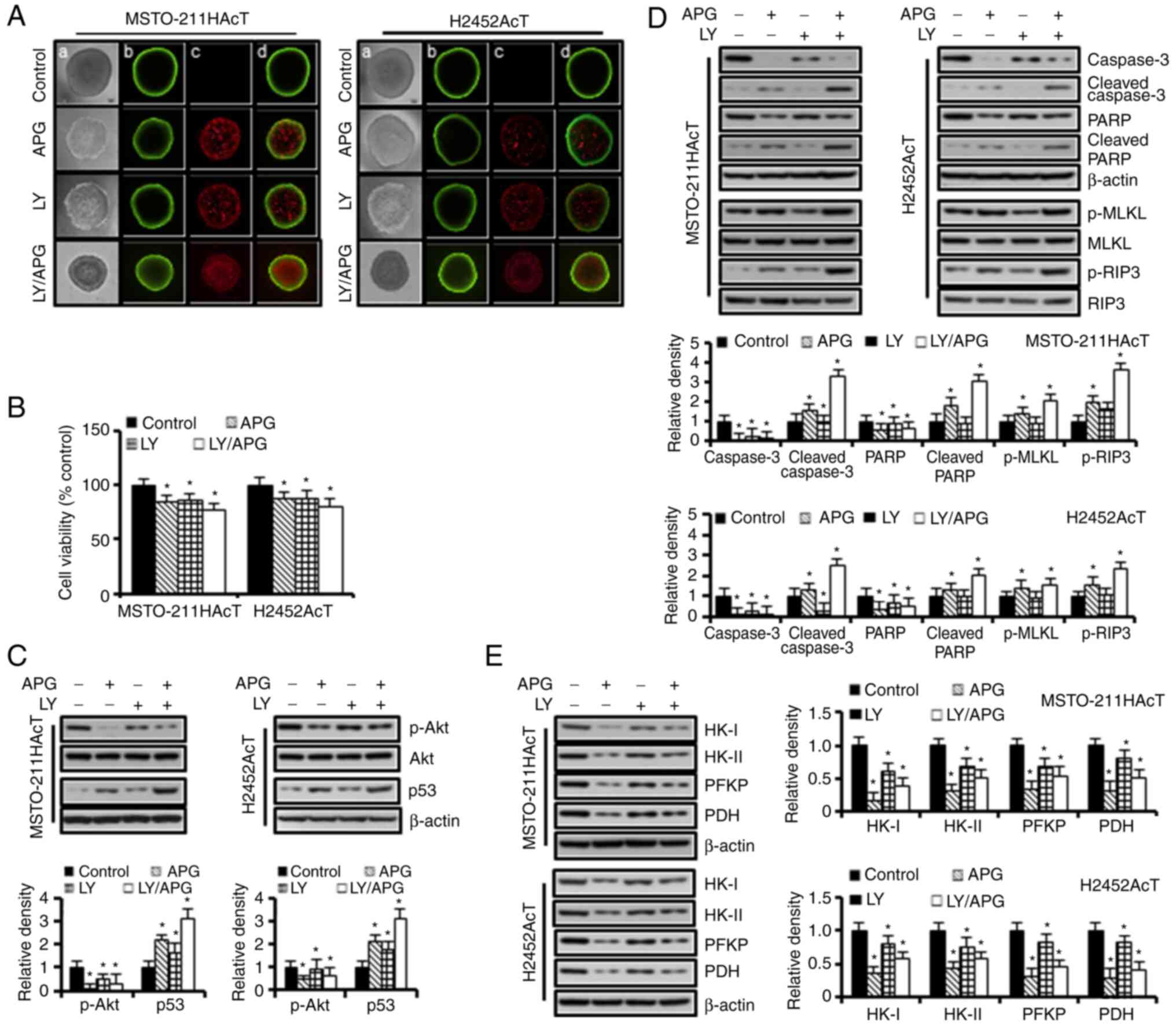 | Figure 6.Anti-glycolytic and cytotoxic effects
of apigenin and Ly294002 in 3D spheroid culture. Spheroids were
cultured in ultralow cluster 96-well plates for 5 days and were
then treated with or without Ly294002 (20 µM, 2 h) prior to
apigenin treatment (30 µM, 48 h) in RPMI-1640 medium containing 3.8
µM lactic acid for 48 h. (A) Vitality staining of spheroids [from
left to right: (a) phase-contrast image, (b) fluorescent images of
fluorescein diacetate(+) living cells in green, (c) propidium
iodide(+) dead cells in red, and (d) merged]. (B) Spheroid
viability. (C) The levels of p-Akt and p53 proteins. (D) The levels
of marker proteins for apoptosis and necroptosis. (E) The levels of
marker proteins for glycolysis. *P<0.05 vs. respective control
cells. Bar graphs present densitometric analysis of western blot
images normalized to β-actin. APG, apigenin; LY, Ly294002; HK,
hexokinase; PFKP, phosphofructokinase platelet; PDH, pyruvate
dehydrogenase; p-, phosphorylated. |
Discussion
To further elucidate the cytotoxic mechanism(s) of
apigenin, it was investigated whether apigenin targeted enzymes
involved in energy metabolism and what the critical upstream
signaling pathway is in this process. In the present study, it was
found that pre-adaptation of MM cells to an acidic medium
containing lactic acid induced a metabolic shift towards enhanced
glycolysis, along with Akt activation and p53 downregulation.
Apigenin and/or Ly294002 treatment increased both apoptosis and
necroptosis of MSTO-211HAcT and H2452AcT cells by downregulating
key enzymes of glucose metabolism and inducing mitochondrial
dysfunction, at least through Akt inactivation and p53
upregulation. These findings were further validated in 3D spheroid
cultures.
Increased cell growth, increased tolerance to the
anticancer drug gemcitabine and upregulation of glycolytic enzymes,
observed in MM cells pre-adapted to lactic acid-containing medium,
suggested the critical role of lactic acid in the metabolic shift
to a Warburg phenotype. Recently, it has been demonstrated that
pre-culturing of human fibroblasts in medium containing lactic acid
promotes the metabolic shift from oxidative phosphorylation to
glycolysis by activating transcription of the glycolytic genes
through ROS-mediated stabilization of hypoxia inducible factor-1α
(HIF-1α) (25). This implies that
an increase in lactate concentration may further enhance the
dependence of cells on aerobic glycolysis for sustained growth and
survival. Enhanced resistance to gemcitabine in MSTO-211HAcT and
H2452AcT cells supported previous findings that aerobic glycolysis
lead to chemoresistance (26,27).
Gemcitabine is an anticancer drug used as standard therapy for MM
in combination with cisplatin. Given that chemoresistance is a
well-known cause of poor prognosis in the treatment of MM, the
development of strategies to reverse resistance to these drugs is
essential to improve the therapeutic efficacy of MM.
Aerobic glycolysis, one of the metabolic features
found in tumor cells, generates an excess of lactate and
H+ in the cytoplasm, which are released extracellularly,
causing local acidification (5).
Lactic acid was traditionally considered a metabolic waste of
anaerobic glycolysis, but has now been described as a key molecule
in tumor cell migration, invasion, growth, angiogenesis,
chemoresistance and immune escape (28). In this process, a number of
signaling pathways and oncogenes, including PI3K/Akt (29), HIF-1 (30) and vascular endothelial growth factor
(31) were activated, which
contribute to cancer progression. PI3K/Akt signaling is an
important regulator of glucose metabolism by enhancing the flux of
glycolysis even in normoxic oxidative tumor cells (29). Activation of PI3K/Akt signaling
pathway promoted glycolysis and cancer growth by increasing the
expression of GLUT-1 and translocation of GLUT-4 to the plasma
membrane, and upregulating the activities of PFK and HK (8). Similarly, PI3K/Akt-mediated
upregulation of HK-II expression enhanced anti-apoptotic and
pro-proliferative effects by promoting the Warburg effect (8,32). As
already mentioned, Akt negatively regulates p53 levels through
crosstalk with each other (11,12).
Given that p53 mediates cell cycle arrest and associated apoptotic
signals, downregulation of p53 may explain part of the potent
anti-apoptotic effect of Akt. In addition to its well-known role as
a tumor suppressor, p53 mediates negative regulation of HIF-1α,
downregulates the expression of GLUT-1, GLUT-4 and HK-II, and
upregulates the expression of TP53-induced glycolysis and
apoptosis regulator, resulting in reduced glycolytic flux (33). Moreover, the p53-deficient cells
could produce a significant higher levels of lactate and exhibited
a metabolic shift from oxidative phosphorylation to glycolysis
(34). Thus, activation of the
PI3K/Akt pathway and downregulation of the p53 protein, observed in
in MM cells pre-adapted to lactic acid, have been shown to drive
metabolic flux towards increased basal glycolysis and enhanced cell
growth rate.
Akt inhibition and p53 upregulation in response to
apigenin in the current study represent an important mechanism
underlying the cytotoxicity of apigenin to MM cells. In line with
this finding, blockade of active Akt using Ly294002 demonstrated
the importance of Akt-p53 network in understanding the role of
apigenin in both targeting glycolysis and inducing apoptosis and
necroptosis of MM cells. Notably, exposure to Ly294002 and
apigenin, alone or in combination, increased cytotoxicity, as
demonstrated by reduced cell viability together with increased
floating cells, increased sub-G0/G1 peak with
a transient delay in the G2/M phase, increased Annexin
V-PE(+) cell fraction, chromatin condensation and nuclear
fragmentation on DAPI staining, and upregulation of apoptosis-and
necroptosis-inducing molecules. However, the combination of
apigenin and Ly294002 further potentiated the apoptotic,
necroptotic, and anti-glycolytic effects compared with apigenin or
Ly294002 alone, with certain synergistic effects. It suggested that
a series of cellular responses to induce apigenin-induced
cytotoxicity are mediated primarily through inhibition of the
PI3K/Akt pathway, but other factors or potential pathways,
including the NF-kB, MAPK/ERK and c-JNK pathways, may also be
involved in this process (15,17).
Apigenin and/or Ly294002 also downregulated HK-I,
HK-II and PFKP, which are rate-limiting enzymes in glycolysis.
Increased glucose concentrations in culture medium of apigenin
and/or Ly294002-treated group indicates a decrease in glucose
utilization, corroborating the results of western blotting showing
inhibition of the glycolytic pathway. Downregulation of oxidative
phosphorylation enzymes and loss of mitochondrial membrane
potential, indicative of mitochondrial dysfunction, limit the ATP
production and consequently induce ATP depletion in cells treated
with apigenin alone or with Ly294002. Mitochondria play a pivotal
role in maintaining cellular redox homeostasis and energy levels
and in regulating various types of cell death, including apoptosis
and necroptosis (35). The
apigenin-induced impairment of mitochondrial function, observed in
the present study, reflects impaired energy metabolism, oxidative
damage, and cell death induced by apigenin. Herein, ROS
accumulation can foster a vicious cycle that can exacerbate
them.
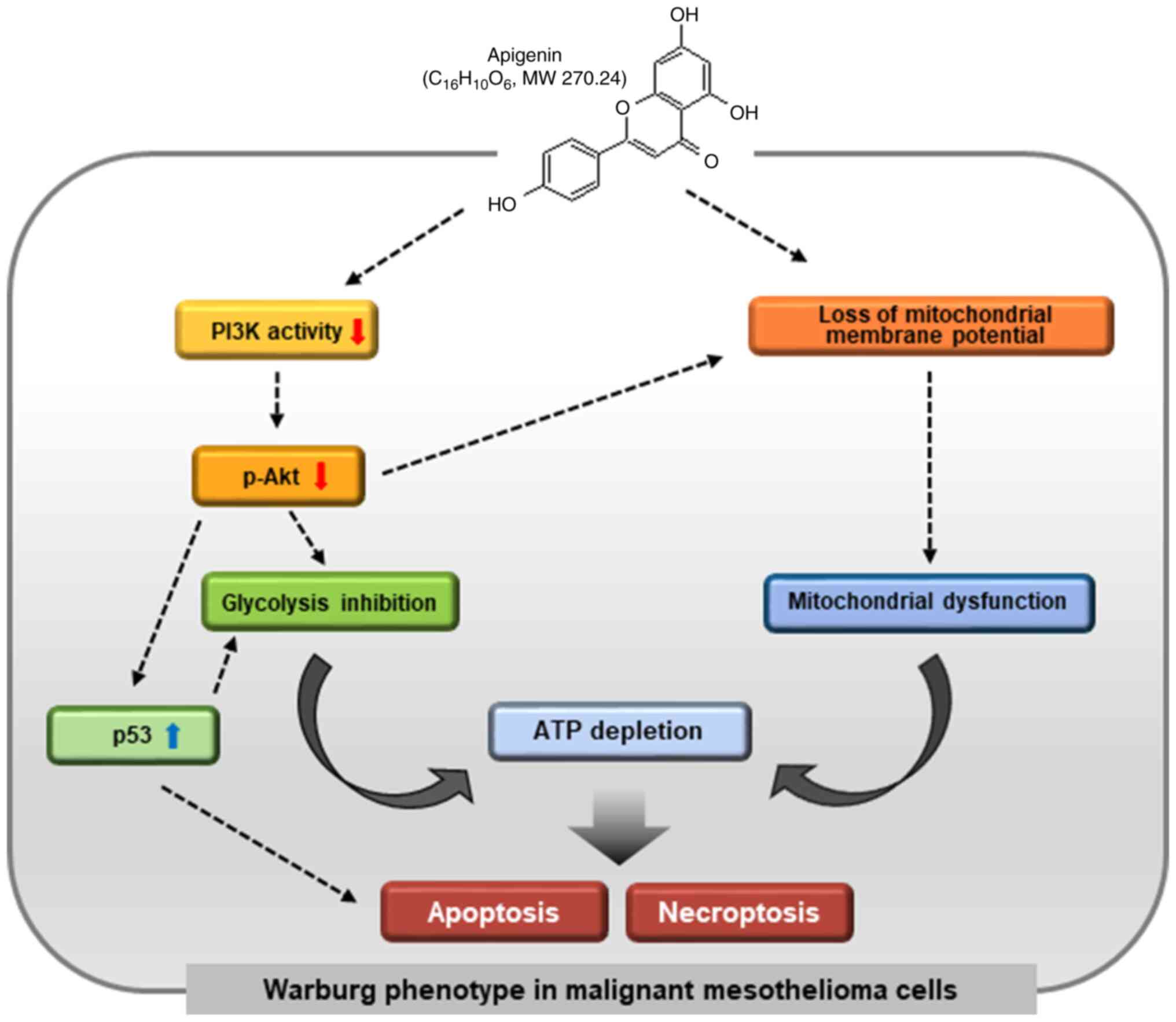
The results of the present study, for the first time
to the best of our knowledge, revealed that apigenin-mediated
inhibition of Akt leads to reduced glycolysis, and thereby the
concurrent induction of apoptosis and necroptosis (Fig. 7). Certain of these results observed
in 2D monolayer cultures were also validated in 3D spheroid
cultures. However, cells cultured in 3D showed less sensitivity to
the effect of apigenin and/or Ly294002 compared with those of 2D
cultures. Decreased drug efficacy in 3D cultures has been reported
by numerous other researchers, possibly due to reduced drug
accessibility to cells due to morphological and/or
pathophysiological differences (e.g., local pH) of 3D spheroid
structure (36,37). No matter how favorable the results
are, in vitro study data alone cannot be a reliable
indicator of the efficacy of drug candidates, and thus the need for
in vivo studies through animal experiments is more
emphasized. In this regard, the lack of in vivo data
demonstrates the limitations of the present study, and further
studies using animal models are needed to confirm the in
vitro effects of apigenin.
As aforementioned, cancer cells rely largely on
aerobic glycolysis rather than efficient oxidative phosphorylation
in glucose metabolism. Given that strategies targeting
cancer-specific energy metabolism provide selective growth
inhibition in rapidly proliferating cancer cells, as a dual
inhibitor targeting both aerobic glycolysis and mitochondrial
function, apigenin offers potential clinical advantage as a
promising therapeutic candidate for MM. Further studies
investigating the metabolic regulation of tumor cells are needed to
deepen our understanding of the mechanism(s) involved in
apigenin-induced necroptosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science Research
Program through the National Research Foundation (NRF) of Korea,
funded by the Ministry of Education (grant no.
NRF-2018R1D1A1B07046129) and by the Soonchunhyang University
Research Fund.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SL and SH conceived the present study. YL, MC and SL
performed the acquisition, analysis and interpretation of data. YL
and KP conducted all the flow cytometric analysis. SL and YL wrote
the original draft. YL and SL confirm the authenticity of all the
raw data. All authors provided critical feedback, read and approved
the final version of the manuscript. SL were in charge of overall
direction and planning.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warburg O: The metabolism of carcinoma
cells. J Cancer Res. 9:148–163. 1925. View Article : Google Scholar
|
|
2
|
Tarrado-Castellarnau M, de Atauri P and
Cascante M: Oncogenic regulation of tumor metabolic reprogramming.
Oncotarget. 7:62726–62753. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eales KL, Hollinshead KER and Tennantm DA:
Hypoxia and metabolic adaptation of cancer cells. Oncogenesis.
5:e1902016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de la Cruz-López KG, Castro-Muñoz LJ,
Reyes-Hernández DO, García-Carrancá A and Manzo-Merino J: Lactate
in the regulation of tumor microenvironment and therapeutic
approaches. Front Oncol. 9:11432019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Courtnay R, Ngo DC, Malik N, Ververis K,
Tortorella SM and Karagiannis TC: Cancer metabolism and the Warburg
effect: The role of HIF-1 and PI3K. Mol Biol Rep. 42:841–851. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Z, Wu J, Zhao Q, Fu S and Jin J:
Emerging roles of aerobic glycolysis in breast cancer. Clin Transl
Oncol. 22:631–646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elstrom RL, Bauer DE, Buzzai M, Karnauskas
R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM and
Thompson CB: Akt stimulates aerobic glycolysis in cancer cells.
Cancer Res. 64:3892–3899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Urso L, Cavallari I, Sharova E, Ciccarese
F, Pasello G and Ciminale V: Metabolic rewiring and redox
alterations in malignant pleural mesothelioma. Br J Cancer.
122:52–61. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abraham AG and O'Neill E:
PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans.
42:798–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naderali E, Valipour B, Khaki AA, Rad JS,
Alihemmati A, Rahmati M and Charoudeh HN: Positive effects of
PI3K/Akt signaling inhibition on PTEN and p53 in prevention of
acute lymphoblastic leukemia tumor cells. Adv Pharm Bull.
9:470–480. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song M, Bode AM, Dong Z and Lee MH: AKT as
a therapeutic target for cancer. Cancer Res. 79:1019–1031. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahmed SA, Parama D, Daimari E, Girisa S,
Banik K, Harsha C, Dutta U and Kunnumakkara AB: Rationalizing the
therapeutic potential of apigenin against cancer. Life Sci.
267:1188142021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Madunić J, Madunić IV, Gajski G, Popić J
and Garaj-Vrhovac V: Apigenin: A dietary flavonoid with diverse
anticancer properties. Cancer Lett. 413:11–22. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong X and Pelling JC: Targeting the
PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer
Agents Med Chem. 13:971–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Masuelli L, Benvenuto M, Mattera R, Di
Stefano E, Zago E, Taffera G, Tresoldi I, Giganti MG, Frajese GV,
Berardi G, et al: In vitro and in vivo anti-tumoral effects of the
flavonoid apigenin in malignant mesothelioma. Front Pharmacol.
8:3732017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee YJ, Park KS, Nam HS, Cho MK and Lee
SH: Apigenin causes necroptosis by inducing ROS accumulation,
mitochondrial dysfunction, and ATP depletion in malignant
mesothelioma cells. Korean J Physiol Pharmacol. 24:493–502. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng
L, Bhalla KN, Keating MJ and Huang P: Inhibition of glycolysis in
cancer cells: A novel strategy to overcome drug-resistance
associated with mitochondrial respiratory defect and hypoxia.
Cancer Res. 65:613–621. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geschwind JF, Ko YH, Torbenson MS, Magee C
and Pedersen PL: Novel therapy for liver cancer: Direct
intraarterial injection of a potent inhibitor of ATP production.
Cancer Res. 62:3909–3913. 2002.PubMed/NCBI
|
|
21
|
Leist M, Single B, Castoldi AF, Kühnle S
and Nicotera P: Intracellular adenosine triphosphate (ATP)
concentration: A switch in the decision between apoptosis and
necrosis. J Exp Med. 185:1481–1486. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singhal S, Wiewrodt R, Malden LD, Amin KM,
Matzie K, Friedberg J, Kucharczuk JC, Litzky LA, Johnson SW, Kaiser
LR and Albelda SM: Gene expression profiling of malignant
mesothelioma. Clin Cancer Res. 9:3080–3097. 2003.PubMed/NCBI
|
|
23
|
Bonelli M, Terenziani R, Zoppi S, Fumarola
C, La Monica S, Cretella D, Alfieri R, Cavazzoni A, Digiacomo G,
Galetti M and Petronini PG: Dual inhibition of CDK4/6 and
PI3K/AKT/mTOR signaling impairs energy metabolism in MPM cancer
cells. Int J Mol Sci. 21:51652020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee YJ and Lee SH: Pro-oxidant activity of
sulforaphane and cisplatin potentiates apoptosis and simultaneously
promotes autophagy in malignant mesothelioma cells. Mol Med Rep.
16:2133–2141. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kozlov AM, Lone A, Betts DH and Cumming
RC: Lactate preconditioning promotes a HIF-1α-mediated metabolic
shift from OXPHOS to glycolysis in normal human diploid
fibroblasts. Sci Rep. 10:83882020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma L and Zong X: Metabolic symbiosis in
chemoresistance: Refocusing the role of aerobic glycolysis. Front
Oncol. 10:52020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He J, Xie G, Tong J, Peng Y, Huang H, Li
J, Wang N and Liang H: Overexpression of microRNA-122 re-sensitizes
5-FU-resistant colon cancer cells to 5-FU through the inhibition of
PKM2 in vitro and in vivo. Cell Biochem Biophys. 70:1343–1350.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pérez-Tomás R and Pérez-Guillén I: Lactate
in the tumor microenvironment: An essential molecule in cancer
progression and treatment. Cancers (Basel). 12:32442020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ruan GX and Kazlauskas A: Lactate engages
receptor tyrosine kinases Axl, Tie2, and vascular endothelial
growth factor receptor 2 to activate phosphoinositide 3-kinase/Akt
and promote angiogenesis. J Biol Chem. 288:21161–21172. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Saedeleer CJ, Copetti T, Porporato PE,
Verrax J, Feron O and Sonveaux P: Lactate activates HIF-1 in
oxidative but not in Warburg-phenotype human tumor cells. PLoS One.
7:e465712012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fukumura D, Xu L, Chen Y, Gohongi T, Seed
B and Jain RK: Hypoxia and acidosis independently up-regulate
vascular endothelial growth factor transcription in brain tumors in
vivo. Cancer Res. 61:6020–6024. 2001.PubMed/NCBI
|
|
32
|
Zhuo B, Li Y, Li Z, Qin H, Sun Q, Zhang F,
Shen Y, Shi Y and Wang R: PI3K/Akt signaling mediated hexokinase-2
expression inhibits cell apoptosis and promotes tumor growth in
pediatric osteosarcoma. Biochem Biophys Res Commun. 464:401–406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simabuco FM, Morale MG, Pavan ICB, Morelli
AP, Silva FR and Tamura RE: p53 and metabolism: From mechanism to
therapeutics. Oncotarget. 9:23780–23823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma W, Sung HJ, Park JY, Matoba S and Hwang
PM: A pivotal role for p53: Balancing aerobic respiration and
glycolysis. J Bioenerg Biomembr. 39:243–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xue C, Gu X, Li G, Bao Z and Li L:
Mitochondrial mechanisms of necroptosis in liver diseases. Int J
Mol Sci. 22:662020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Edmondson R, Broglie JJ, Adcock AF and
Yang L: Three-dimensional cell culture systems and their
applications in drug discovery and cell-based biosensors. Assay
Drug Dev Technol. 12:207–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chitcholtan K, Sykes P and Evans J: The
resistance of intracellular mediators to doxorubicin and cisplatin
are distinct in 3D and 2D endometrial cancer. J Transl Med.
10:382012. View Article : Google Scholar : PubMed/NCBI
|