Introduction
The incidence of colorectal adenoma (CRA) increases
with age after the age of 30 and is common in young and middle-aged
individuals over the age of 40, including sporadic and familial
cases (1). The incidence of CRA in
individuals aged >60 is as high as 40%, and the annual rate of
adenoma progression to colorectal cancer (CRC) is ~0.25% (2,3). CRA
is a kind of premalignant lesion. Intestinal malignant lesions
usually develop from focal dysplastic polypoid precursor-adenoma,
which is mainly limited to colorectal mucosa and submucosa. The
occult onset of CRA may be accompanied by positive fecal occult
blood, changes in stool characteristics, abdominal pain, diarrhea
and other non-specific symptoms, which are usually found during
enteroscopy screening. Previous studies suggested that normal
mucosal epithelial cells of the intestine can grow gradually for
10–15 years before undergoing heterogeneous proliferation to
carcinogenesis (4). Under the
influence of multiple factors including genetic susceptibility to
tumors and the immune microenvironment, adenomas further accumulate
genetic mutations and invade the submucosa to become cancerous
(5). Currently, according to the
fifth edition of the World Health Organization classification of
digestive tumors, there are two types of CRA: i) Conventional
adenomas, namely tubular adenomas, villous adenomas, tubular
villous adenomas, conventional serrated adenomas and ii) sessile
serrated lesions (SSL; including sessile serrated adenomas/polyps).
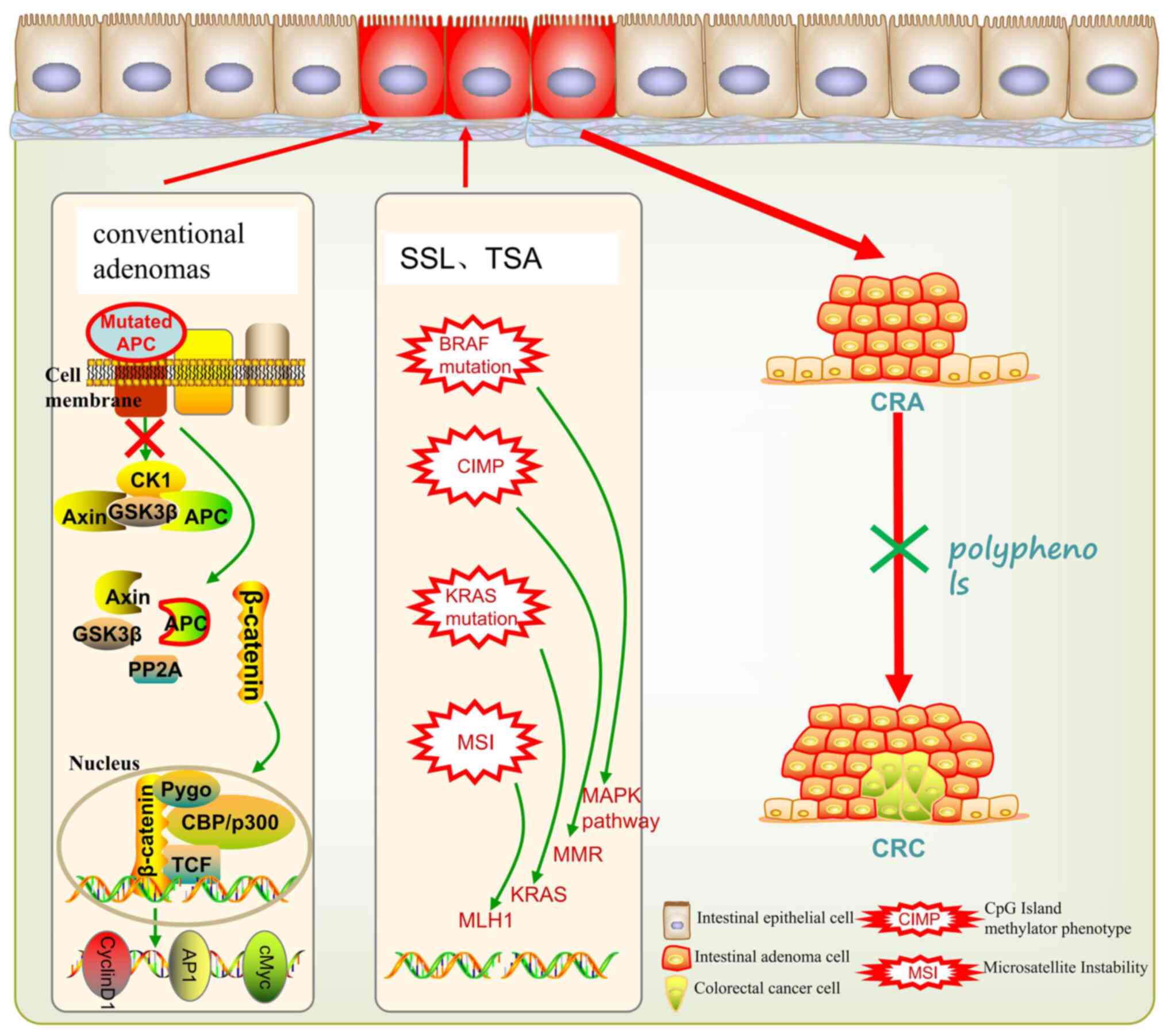
A total of ~70-90% of CRCs develop through the conventional normal
epithelial-adenoma-adenocarcinoma sequence (also known as the
chromosomal instability pathway), usually accompanied by mutations
in the APC gene and excessive activation of the Wnt pathway
(6). And tumors originating in this
pathway usually occur in men and are located in the distal colon.
By contrast, ~10-20% of CRC have a different pathway-serrated
neoplasia pathway. The mechanism of SSLs mainly involves BRAF
mutation, KRAS mutation, CpG island methylation phenotype and
microsatellite instability (7).
SSLs occur in the proximal colon, particularly in the cecum and
ascending colon, and grow significantly faster than tubular
adenomas (8). The possible
mechanisms are exhibited in Fig.
1.
Polyphenols are naturally occurring antioxidant
compounds in plants, and their types and contents vary widely among
fruits, vegetables, leaves and seeds. Polyphenols can reduce the
oxidative damage of enzymes and DNA in cells and tissues (9), and have a variety of physiological
activities, including antibacterial, antioxidant,
anti-inflammatory, antiviral, antitumor and immunomodulatory
functions (10–13). Numerous studies have shown that
polyphenols have inhibitory effects on CRA, and polyphenols are
considered an important source of natural drugs for the treatment
of numerous diseases due to their remarkable efficacy and safety.
In the present review, the progress of experimental studies on
natural polyphenols for the treatment of CRA in the past 10 years
was discussed to provide additional insight for researchers to
study and develop new drugs for the treatment of CRA.
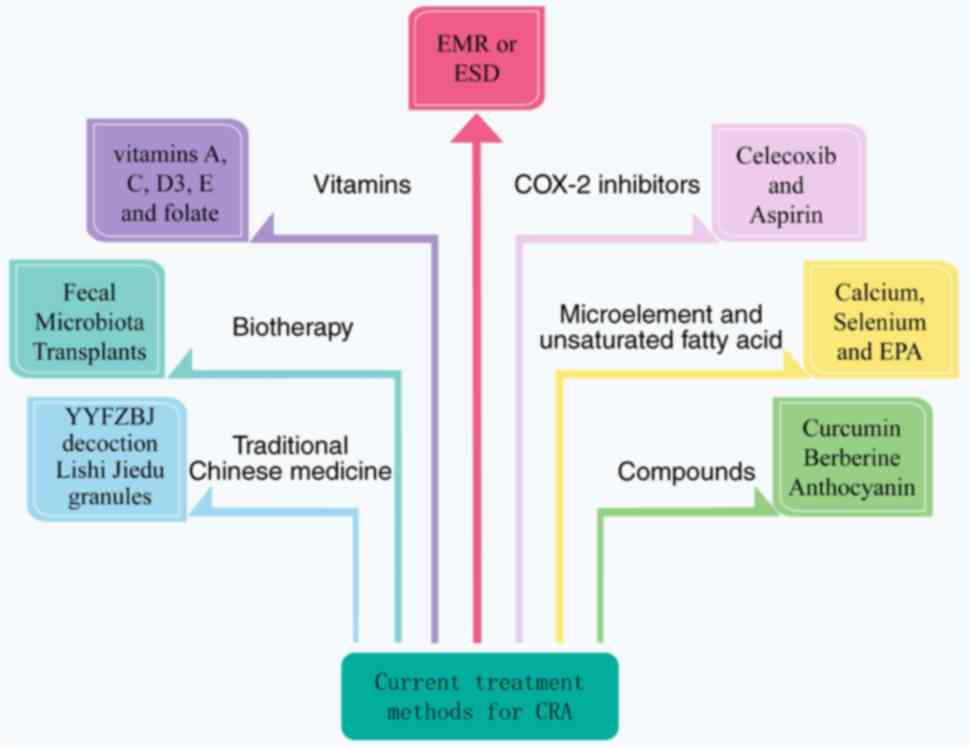
Current treatment and management of CRA
At present, there are few treatment options for CRA.
The conventional treatment of the patient is endoscopic mucosal
resection (EMR) or endoscopic mucosal dissection to remove
adenomas, and the patient is given oral medication to prevent
bleeding from the wound after surgery (14). There is a lack of effective drugs or
treatments to prevent the recurrence and carcinogenesis of CRA in
clinical treatment. Clinical studies have identified that COX-2
inhibitors including celecoxib and aspirin (ASA) may reduce the
recurrence of CRA by reducing intestinal inflammation and
regulating microflora (15,16). In addition, folic acid, calcium,
vitamin D, eicosapentaenoic acid and antioxidants are effective in
the treatment of CRA (17–19). It has been reported that traditional
Chinese medicine (20,21) and natural compounds (22,23)
also have favorable therapeutic effects on CRA. But the efficacy of
these interventions is controversial or has certain side effects
and cannot be used for a long time (24,25).
Among them, those common treatment methods for CRA are exhibited in
Fig. 2.
Classification of polyphenols and their
transformation in the intestine
Polyphenols are found in plants and plant
derivatives, and studies have shown that dietary polyphenols can
reduce the occurrence of numerous diseases, including tumors
(26). According to their chemical
structure, polyphenols can be classified into flavonoids and
non-flavonoids. Flavonoids are particularly abundant in fruits,
vegetables, seeds, spices, vanilla, tea, cocoa and wine (27), and their chemical structure consists
of 15 carbon atoms, with aromatic rings A and B linked by a
three-carbon bridge to form a heterocyclic ring (ring C) (28). Depending on the functional group,
the degree of oxidation of ring C and the connection between ring B
and C, they can be divided into different subgroups (10), which are mainly classified into six
major groups: Flavonols, flavones, anthocyanins, flavanols,
flavanones and isoflavones. Flavonols are the most abundant
flavonoids in plants, mainly found in tea, apples, onions and dark
green vegetables, including quercetin, kaempferol and prunetin;
flavones are relatively low in plants, mainly in celery, coriander
and garlic, including lignan, apigenin and baicalin. Anthocyanins
are also less abundant in plants, mainly found in crimson grapes,
berries and red wine. However, the bioavailability of anthocyanins
appears to be relatively low (29).
Flavanols are mainly found in tea, nuts and grains, including
(−)-epicatechin3-O-gallate (ECG) and
(−)-epigallocatechin-3-O-gallate (EGCG). Oranges, lemons and other
citrus fruits and their juices are rich in flavanones, including
naringin and hesperidin, and isoflavones including genistein and
daidzein mainly exist in soybean and soybean products.
Non-flavonoids are divided into phenolic acids, lignans and
stilbenes according to their different structures. Phenolic acid is
divided into hydroxycinnamic acid and hydroxybenzoic acid, which
mainly exists in fruits and grains. Curcumin (CUR) and caffeic acid
belong to hydroxycinnamic acid, and gallic acid and vanillic acid
belong to hydroxybenzoic acid (30). Lignans are phytoestrogens, similar
in structure to estrogens, and they are mainly found in sesame,
flaxseed, red wine and olive oil, including sesamin, allicin,
magnolol and magnolol. Stilbenes are rarely found in diet and are
found in berries, plums and pine nuts, such as resveratrol
(RSV).
Polyphenols are usually absorbed in the intestine,
combined with glucoside acid, sulfate and/or methyl and metabolized
in the intestinal mucosa and liver. Polyphenols metabolized in the
colon can be widely transformed by intestinal microorganisms, while
polyphenols that reach the liver will undergo furtherstage II
metabolism (31). The main
metabolite of polyphenols, phenolic acids, can enter the systemic
circulation and most of them are excreted in urine and bile within
48 h (32).
Molecular targets of polyphenol for CRA:
Preclinical studies
In vivo and in vitro models, natural
polyphenols show significant antitumor activity, which is
manifested in inhibiting proliferation, inducing apoptosis,
promoting cell cycle arrest, anti-inflammatory, anti-oxidation, and
regulating a variety of signal pathways to play a therapeutic role
on CRA. The therapeutic effects of these polyphenols on tumors are
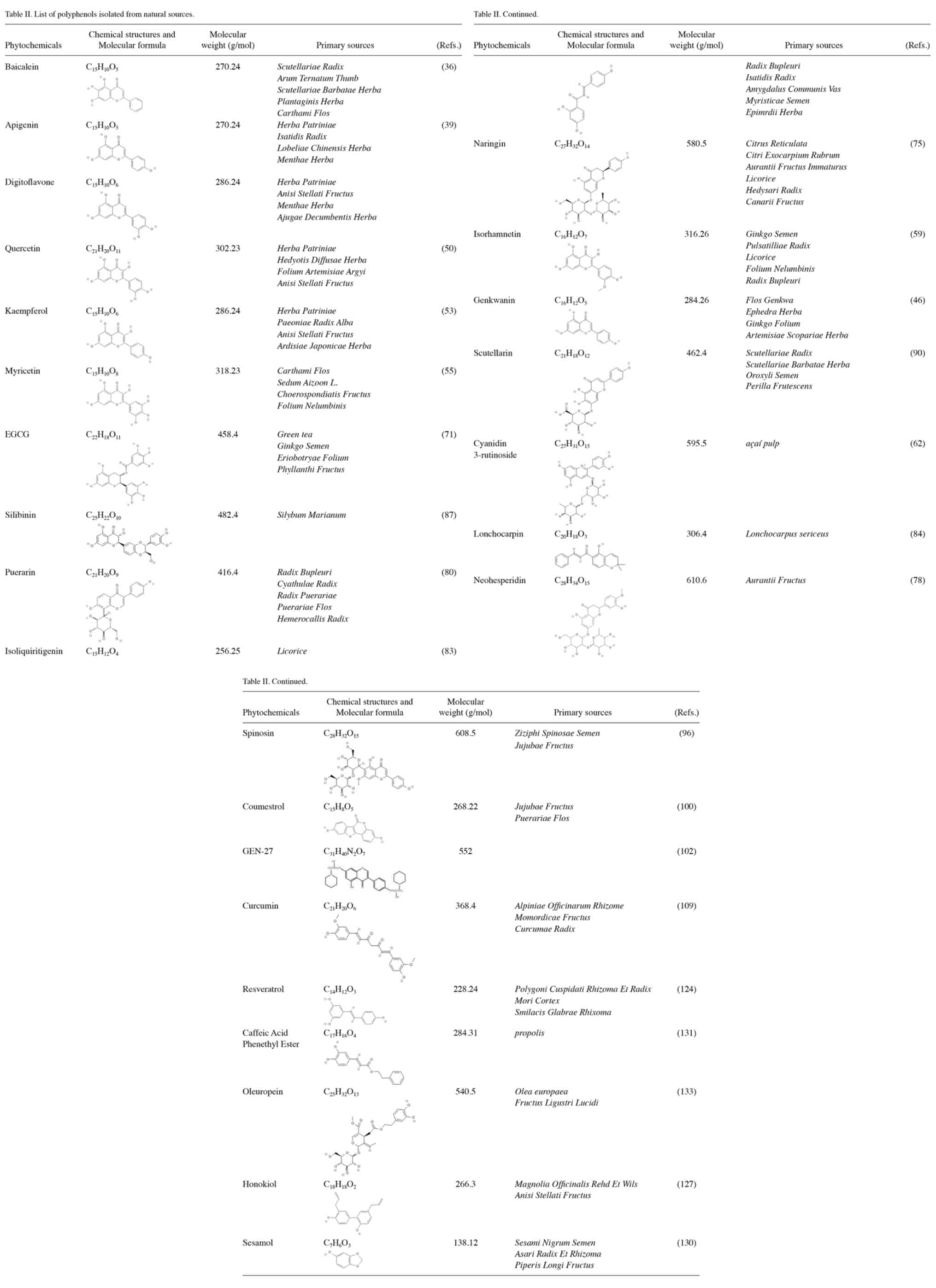
shown in Table I, and detailed
information and sources of polyphenols are shown in Table II.
 | Table I.Summary of the mechanisms of anti-CRA
polyphenols. |
Table I.
Summary of the mechanisms of anti-CRA
polyphenols.
| Phytochemicals | Dose, duration and
testing system | Function | Molecular
targets | (Refs.) |
|---|
| Flavones |
|
|
|
|
| Baicalein | 30 mg/kg/day, In
vivo: ApcMin/+ mice | Decreased the
incidence of tumor | ADP-ribose | (131) |
|
| 1, 5, 10 mg/kg for
16 weeks, In vivo: AOM/DSS | formation with
inflammation | ↑PPARγ |
|
|
| mice | Induces
apoptosis | ↓NF-κB, IL-1CSF,
IL-2, IL-6, IL-10, G-β |
|
|
| 50 µM for 24 h,
In vitro: HCT116 |
| and GM-CSF |
|
| Apigenin | 25, 50 mg/kg for 1
month, In vivo: ApcMin/+mice | Decreases
intestinal adenoma formation | ↑NAG-1, p53,
p21 | (131) |
|
| 1, 10 µM for 72 h,
In vitro: HCT116, HT-29, | Induces
apoptosis | phosphor-p53 |
|
|
| SW480 and LoVo | Arrests cell
cycle |
|
|
| Digitoflavone | 50 mg/kg for 12
weeks, In vivo: AOM/DSS mice | Decreases
intestinal adenoma formation | ↑Nrf2, TR, γ-GCSc,
γ-GCSm, HO-1, GR, | (131) |
|
| 1–20 µM for 8 h,
In vitro: Caco-2, HT-29, HepG2 | Reduces oxidative
stress | NQO-1 |
|
|
| and HEK-293 |
| ↓TNF-α, IL-1β,
IL-6, ROS |
|
| Quercetin | 0. 0.2% (w/w) for
16 weeks, In vivo: ApcMin/+mice | Decreases
intestinal adenoma formation | ↓pSTAT3, IL-6 | (131) |
|
|
| Decreases
macrophage number |
|
|
|
| 25 mg/kg for 3
weeks, In vivo: ApcMin/+mice | Decreases
intestinal adenoma formation |
| (131) |
|
|
| Attenuates the
progression of cancer |
|
|
|
|
| cachexia |
|
|
| Kaempferol | 75, 150 mg/kg for
20 weeks, In vivo: AOM/DSS | Decreases
intestinal adenoma formation | ↑DACT2,
β-catenin, | (131) |
|
| mice | Inhibits cell
proliferation | ↓CTNNB1, LRP6 |
|
|
| (1. 25–150 µM) for
72 h, In vitro: HCT116, HT29 | Reduces cell
migration | At G0/G1 and S
phase |
|
|
| and YB5 | Arrests cell
cycle |
|
|
| Myricetin | 25 mg/kg for 13
weeks, In vivo: ApcMin/+mice | Decreases
intestinal adenoma formation | ↓IL-6, PGE2, Cyclin
D1, PCNA, Bcl-xL, | (131) |
|
|
| Suppresses
cancerization | β-catenin, p-GSK3β,
IL-6, PGE 2, p-JNK, |
|
|
|
| Inhibits cell
proliferation | JNK, p-Erk1/2,
p-p38 MAPK, p-mTOR, |
|
|
|
| Induces
apoptosis | p-Akt, |
|
|
|
| Decreases
inflammation | ↑TUNEL, Bax,
c-Caspase-3, GSK-3β, |
|
|
|
|
| TNF-α |
|
|
| 0 mg/kg for 4
weeks, In vivo: AOM/DSS mice | Decreases
intestinal adenoma formation | TNF-α, IL-1β, IL-6,
NF-κB, p-NF-κB, | (131) |
|
|
| Suppresses
cancerization | COX-2, PCNA, Cyclin
D1 |
|
|
|
| Decreases
inflammation |
|
|
| EGCG | 0. 01% for 2
months, In vivo: ApcMin/+mice | Decreases
intestinal adenoma formation | ↓bFGF | (131) |
|
| 1, 10, 50 µmol/l
for 72 h In vitro: HCT-116 LoVo |
|
|
|
|
| 1% (v/v) for 13
weeks, In vivo: AOM mice | Decreases
intestinal adenoma formation |
↑Bifidobacterium,
Lactobacillus | (131) |
|
|
| Suppresses
cancerization |
|
|
|
|
| Regulates
microbiota |
|
|
| Silibinin | 0. 2% (w/w) for 3
months, In vivo: ApcMin/+mice | Decreases
intestinal adenoma formation | Cdk4 | (131) |
|
|
| Induces
apoptosis | ↓cyclin D1,
Ki67 |
|
Flavonoids
Flavones
Baicalein (5,6,7-trihydroxyflavone) is a flavonoid
mainly derived from the root of Scutellaria baicalensis.
Baicalein is widely used in Chinese herbal medicine for
anti-inflammatory and anticancer treatment (33). Baicalein and baicalin are the
traditional Chinese medicine components of Scutellaria
baicalensis. Studies have shown that baicalin in the extract of
Scutellaria baicalensis can be bio-transformed into
baicalein after culture with intestinal microflora, and exhibits an
antitumor effect in mice (34).
Compared with the model group, baicalein treatment significantly
reduced the number of small intestinal and CRAs, and significantly
reduced the levels of inflammatory cytokines IL-1β, IL-2, IL-6,
IL-10, G-CSF and GM-CSF. A recent study reported that the effect of
baicalein on adenoma is partly achieved by inhibiting intestinal
inflammation (35). In addition,
baicalein significantly reduced the incidence of tumor and
inflammation in azoxymethane
(AOM)/4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) mice, which
may be related to apoptosis induced by morphological changes and
cleavage of poly (ADP-ribose) polymerase and inhibition of NF-κB
activation by PPARγ activation (36).
Apigenin is widely found in numerous plants, and its
extensive antioxidant properties have been reported in a large
number of literature (37).
Apigenin can inhibit tumor by regulating multiple kinase pathways,
including phosphatidylinositol 3-kinase (PI3K), protein kinase
B/Akt, MAPK/ERK, casein kinase II, and arrest cell cycle in G2/M
phase, reducing cyclin B1 in CRC cells (38). Previous studies have shown that
apigenin can significantly reduce the number and size of polyps in
APCMin/+ mice, upregulate the expression of P21 and
specifically phosphorylate p53 protein in intestinal tumor tissues
of mice. In vitro experiments, pro-apoptotic proteins (NAG-1
and p53) and cell cycle inhibitor (p21) were induced in the
presence of apigenin, PKCδ and ataxia telangiectasia mutated play
an important role in activating these proteins (39). Genome integrity is maintained by
blocking cell cycle progression or inducing apoptosis (40). These results indicated that apigenin
affects tumor cell apoptosis in p53-dependent and p53-independent
ways and reduces the occurrence of adenoma (39).
Digitoflavone as a kind of flavonoid in the diet,
including broccoli, carrots, peppers and cabbage, has been used to
treat hypertension, inflammatory diseases and cancer (41). Previous studies have shown that
digitoflavone inhibits tumorigenesis. Yang et al (42) found that digitoflavone significantly
reduced the incidence, number, and size of adenomas in AOM/DSS
mice, and upregulated Nrf2, TR, γ-GCSc, γ-GCSm, and HO-1 protein
expression levels, as well as GR, TR, HO-1, γ-GCSc, γ-GCSm and
NQO-1 mRNA levels. It was proved that the beneficial effect of
digitoflavone on adenoma progression was attributed to the
activation of Nrf2 pathway.
Genkwanin (GKA) is a typical bioactive
non-glycosylated flavonoid isolated from Daphne genkwa and
has significant anti-inflammatory, antibacterial and
immunomodulatory activities (43,44).
GKA has favorable anti-inflammatory activity in lipopolysaccharide
(LPS)-activated macrophages by regulating miR-101/MKP-1/MAPK
pathway (45). Wang et al
(46) found that after oral
administration of 12.5–25 mg/kg/day GKA, abnormal adenomas in
APCMin/+ mice were significantly improved, particularly
for large adenomas, and tumor diversity was reduced. GKA
upregulated IL-2, IL-4, IL-12, IFN-γ, and TNF-α levels and
downregulation of IL-10 levels. The studies showed that GKA's
antitumor activity was partly through enhancing host immunity and
reducing inflammatory cytokines. In another study, the researchers
developed a GKA-loaded self-nanoemulsifying drug delivery system
(GKA-SNEDDS) to improve the solubility of GKA, intestinal
permeability and oral bioavailability. A recent study revealed that
GKA-SNEdDS can induce intestinal tumor cell apoptosis in AOM/DSS
mice to inhibit adenoma carcinogenesis, significantly improve
weight loss and disease activity index (DAI), and lower the
histological score of inflammatory cytokines (47).
Flavonols
Quercetin, a common flavonoid in plants, can induce
G2 cell cycle arrest, inhibit angiogenesis and downregulate NF-κB
expression due to its strong antioxidant and free radical
scavenging properties (48).
Quercetin-rich plants have been used to treat inflammatory
diseases, tumors and hypertension (41). Previous studies showed that
quercetin significantly reduced the number of intestinal adenomas
(>2 mm 69%, 1–2 mm 79%) in APCMin/+ mice (49). Velazquez et al (50) showed that quercetin may reduce the
adenoma load of APCMin/+ mice by inducing
phosphorylation of plasma IL-6 and muscle signal transduction and
transcriptional activator 3. In addition, quercetin significantly
improved the decline of body function and metabolism in mice.
Kaempferol is found in a variety of plants and has
been proven to have anti-inflammatory, antioxidant, antibacterial
and antitumor activities (51).
Kaempferol is associated with reduced incidence of various types of
cancer, including skin, liver and gastric cancer (52). Kaempferol significantly reduces
adenomatosis and tumor load in AOM/DSS mice and increases DACT2
gene expression in tumor tissues. β-catenin, CTNNB1 and LRP6 gene
expression levels in CRC tissues were significantly inhibited by
kaempferol-induced DACT2 repair. Similarly, kaempferol
significantly increased gene expression of DACT2 in HCT116, HT29
and YB5, significantly decreased methylation of DACT2, and
increased unmethylated DACT2 by 13.72 times. Further studies showed
that the proliferation and migration of CRC cells are limited, and
kaempferol could inhibit nuclear β-catenin and inactivates the
Wnt/β-catenin pathway by directly binding DNA methyltransferase
(DNMT) 1 (53).
Myricetin, as a natural dietary flavonoid compound,
has the potential of enhancing immune regulation, inhibiting
cytokine storm and antiviral, and has therapeutic effects on
different types of tumors, inflammatory diseases, atherosclerosis,
thrombosis and diabetes (54).
Previous studies have shown that myricetin can reduce the number of
adenomas in APCMin/+ mice and reduce the number and
degree of dysplasia in adenomas without any side effects. Myricetin
selectively inhibits cell proliferation by downregulating Cyclin D1
and PCNA expression, and induces apoptosis of adenoma cells by
downregulating Bax, c-Caspase-3, which may be related to the
regulation of GSK-3β and Wnt/β-catenin pathways. Myricetin reduces
the concentration of IL-6 and PGE2 in the blood. These results
suggested that the mechanism of myricetin in treating adenoma may
be the downregulation of phosphorylated p38MAPK/Akt/mTOR signaling
pathway (55). Another study showed
that myricetin significantly reduces the size and degree of small
CRAs in AOM/DSS mice. In addition, myricetin significantly reduces
the expression of inflammation factor TNF-α, IL-1β, NF-κB, p-NF-κB
and COX-2 (56).
Isorhamnetin is one of the most important active
components in Ginkgo biloba leaves, with antitumor,
antibacterial, anti-inflammatory and antioxidant effects (57). Clinical studies have shown that
intake of a diet rich in isorhamnetin is associated with a reduced
risk of advanced adenoma recurrence (58), but the mechanism is unclear. A
recent study reported that supplementation with an isorhamnetin
diet significantly reduced the incidence of CRA, tumor number and
load in AOM/DSS mice. Further studies demonstrated that
isorhamnetin reduced DSS-induced inflammatory response, inhibited
C-SRC activation and β-catenin nuclear translocation, and promoted
the expression of c-terminal Src kinase (CSK), a negative regulator
of the Src family of tyrosine kinases, which was also verified
in vitro. In conclusion, the chemoprotective effects of
isorhamnetin in adenomas are associated with anti-inflammatory
activity, inhibition of carcinogenic Src activity, and loss of
nuclear β-catenin (CSK-dependent activity) (59).
Anthocyanins
Anthocyanins are water-soluble flavonoids, whose
stability is affected by pH, light, temperature and other factors
(60). Studies have shown that
anthocyanins can treat a variety of diseases, including
neurodegenerative, cardiovascular and metabolic diseases and
cancer, and regulate oxidative stress and intestinal microbiota
(61). Freeze-dried Acai berry pulp
(AP) is rich in cyanidin 3-rutinoside (C3R). In 1,
2-dimethylhydrazine and 2, 4, 6-trinitrobenzoic acid-induced male
Wistar rats, intake of freeze-dried AP reduced adenoma
proliferation, the incidence of high-grade intraepithelial adenoma,
the total number of aberrant crypt foci (ACF) and ACF multiplicity.
In addition, C3R (25 µM) has the potential to decrease RKO cell
motility in vitro, and AP increases gene expression of
negative cell proliferation regulators including Dlc1 and Akt3
(62). Fernandez et al
(63) found that anthocyanins from
a mixture of dehydrated blackberries and strawberries significantly
reduced the number of CRAs in AOM/DSS mice and increased the total
antioxidant activity of FRAP (plasma iron reduction capacity).
Differences in colon microbiota before and after intervention were
examined by 16S rRNA, which showed that anthocyanins could reduce
the occurrence of adenomas by reducing the abundance of
pro-inflammatory Bilophila wadsworthia population.
Freeze-dried black raspberry (BRB) powder reduced
the rate of adenoma canceration in AOM/DSS mice. The number of
pathogenic bacteria, including Desulfovibrio and
Enterococcus, was significantly increased, while probiotics
such as Eubacterium rectale, Faecalibacterium prausnitzii
and Lactobacillus were significantly decreased in AOM/DSS
mice, but BRB anthocyanin supplementation reversed this imbalance
in the gut microbiome. BRB anthocyanins also cause demethylation of
the SFRP2 gene promoter, resulting in increased SFRP2 expression at
mRNA and protein levels. In addition, BRB anthocyanins
downregulated the expression of DNMT31 and DNMT3B as well as
P-STAT3. These results suggested that BRB anthocyanins may play a
role in reducing the carcinogenesis rate of adenomas by regulating
the composition of intestinal microbiota, changes in inflammation
and methylation status of SFRP2 gene (64).
Another study showed that anthocyanin-containing
purple-fleshed potatoes reduced the number of cells containing
nuclear β-catenin (an indicator of colon CSC) by inducing
mitochondria-mediated apoptosis and reduced adenoma incidence
similar to sulindac in AOM/DSS mice. Further research showed that
anthocyanin-containing baked purple-fleshed potato extracts
inhibited proliferation and promoted apoptosis in colon CSCs in a
p53-independent manner. It also inhibited the levels of Wnt pathway
effector β-catenin (a key regulator of CSC proliferation) and its
downstream proteins (c-myc and cyclin D1) and increased the levels
of Bax and cytochrome C (proteins that mediate mitochondrial
apoptosis) (65).
Anthocyanins from red grape pomace significantly
reduced the adenoma load and the number of adenomas in
APCMin/+ mice, downregulated the expression levels of
Ki-67, Akt and ERK proteins, and upregulated the expression of
pERK, suggesting that Akt and pERK may be suitable biomarkers for
the efficacy of anthocyanin target organs (66).
Kang et al (67) found that anthocyanins from red grape
pomace may reduce the cecal adenoma load in APC Min/+
mice by inhibiting cyclooxygenase, and its inhibitory effect is
more significant than sulindac. In addition, anthocyanins in cherry
diet also significantly inhibited the proliferation of HT29 and
HCT116, indicating that anthocyanins have favorable antitumor
effects.
A previous study demonstrated that anthocyanin-rich
bilberry extract reduces the occurrence of adenomas in AOM/DSS
mice, and decreases inflammatory response and intestinal length
reduction caused by inflammation (68). In addition, it has been revealed
that anthocyanin-enriched Sweet Potatoes significantly reduced the
number of adenomas in APCMin/+ mice (69).
Flavanol
EGCG is the main catechin in green tea and has been
shown to have a variety of pharmacological effects, including
antioxidant, antitumor, anti-inflammatory, anti-microbial,
anti-angiogenic, and cholesterol-lowering effects (70). EGCG was found to inhibit adenoma
formation and reduce adenoma load in APCMin/+ mice. BFGF
protein degrades rapidly in the presence of EGCG, but this
degradation is inhibited by proteasome inhibitors, while EGCG
increases ubiquitin of bFGF and trypsin-like activity of 20S
proteasome, leading to bFGF protein degradation. These results
indicated that EGCG inhibition of adenoma is related to the
decrease of bFGF expression (71).
Similarly, Wang et al (72)
showed that EGCG can reduce the adenoma load of AOM/DSS mice and
reduce the number of ACF to delay the histological progression, and
the intestinal microbiota structure is relatively stable during
EGCG intervention, which may be the potential mechanism of EGCG
inhibiting tumor.
Oligonol is a catechin oligomer extracted from
litchi fruit, consisting of 17.6% of catechin-type monomers and
18.6% of proanthocyanidin dimers and trimers derived from grape
seeds or lychee fruit with antioxidative and anti-inflammatory
properties. A previous study showed that oligonol significantly
inhibited the incidence and diversity of CRAs in AOM/DSS mice, and
inhibited the activation of NF-κB and STAT3, as well as the
expression of COX-2, iNOS and cyclin D1 in DSS-induced mice.
In addition, oligonol prevents oxidative
stress-induced apoptosis of colon epithelial cells by attenuating
lipid peroxidation (malondialdehyde) and protein oxidation
(4-hydroxy-2-nonenal). Further studies showed that oligonol reduced
the expression of IL-1β, TNF-α, IL-6, COX-2 and inosine in
LPS-induced RAW264.7 cells. In another study, Ho-1, NQO-1, TRX 1
and GPX-2 antioxidant genes were upregulated by oligonol in
intestinal epithelial CCD841CoN cells (73).
Flavanones
Naringin, a natural flavonoid, is rich in citrus
fruits and has a wide range of pharmacological activities,
including anti-inflammatory, anticancer, oxidative stress and
genetic damage (74). A study
evaluating the inhibitory effect of naringin on AOM/DSS mice found
that naringin reduced the severity of CRAs by inhibiting
myeloid-derived suppressor cells, pro-inflammatory mediators
GM-CSF/M-CSF, IL-6 and NF-κB/IL-6/ST pathway in CRAs. Moreover,
naringin can prevent ulcerative colitis and canceration induced by
AOM/DSS without obvious side effects. Naringin-treated mice showed
normal colorectal tissue structure and electron microscopic
analysis showed that endoplasmic reticulum (ER) stress-induced
autophagy was inhibited. Naringin inhibits the secretion of ER
transmembrane proteins including GRP78 ATF6, IRE1, and activated
PERK-phosphorylated EIF-2, as well as the autophagosome complexes
ATG3, ATG5, ATG7, ATG12, ATG16 and ATG16L1 in intestinal mucosal
cells. In conclusion, naringin prevents adenoma occurrence and
progression by inhibiting ER stress-induced autophagy of mucosal
cells in CRC (75).
Neohesperidin (NHP) is widely found in citrus fruits
regulating intestinal microbiota (76) and regulating immunity (77). A recent study reported that NHP
inhibits adenomatosis in APCMin/+ mice, induces
apoptosis and blocks angiogenesis in vivo. In the presence
of NHP, the relative abundance of Bacteroidetes decreased,
while the relative abundance of Firmicutes and
Proteobacteria increased. In addition, fecal microbiota
transplantation experiments further demonstrated that intestinal
adenoma was significantly inhibited by feeding with NHP-treated
fecal extract of mice, suggesting that changes in intestinal
microbiota are the cause of NHP-mediated prevention of colorectal
tumor (78).
Isoflavones
Puerarin, a natural isoflavone extracted from
Pueraria lobata, has potential antitumor, antioxidant and
anti-inflammatory activities (79),
but it has low solubility and low bioavailability. In a recent
study, puerarin-loaded alginate microspheres (plams) were prepared
by emulsion/internal gel method for the treatment of adenomas.
Plams not only significantly reduced the inflammatory response by
downregulating the expression of tumor-promoting cytokines TNF-α
and IL-17A but reduced the occurrence of adenoma and carcinogenesis
by inhibiting AOM/DSS-induced EMT in mice (80).
Chalcones
Isoliquiritigenin (ISL), a flavonoid with chalcone
structure extracted from Glycyrrhiza uralensis, can activate
the antioxidant system mediated by nuclear factor erythroid-2
related factor 2 (Nrf2) and negatively regulate nuclear
factors-κB(NF-κB) and nod-like receptor thermal protein domain
associated protein 3 (NLRP3) (81)
and has significant antitumor effect (82). A previous study has shown that ISL
can significantly reduce the incidence of intestinal adenomas and
regulate the intestinal microbiota in AOM/DSS mice. 16S rRNA
detection revealed that the structure of intestinal microbial
community in AOM/DSS mice changed significantly, the level of
Bacteroidetes decreased, and Firmicutes increased.
High dose ISL (150 mg/kg) reversed the imbalance at phylum level
and changed the diversity composition of the intestinal microbiota,
in which the abundance of Helicobacter increased, while the
abundance of Lachnospiraceae and Rikenellaceae
decreased. At the genus level, ISL reduced the abundance of
opportunistic pathogens (E. coli and Enterococcus)
and increased the level of probiotics, particularly
butyrate-producing bacteria (Coccidioides butyricus,
Clostridium and Ruminococcus). In addition, ISL
decreased IL-6, IL-10, TNF-α, IL-1 β and COX-2 expression,
suggesting that ISL may have a synergistic effect with intestinal
microbiota in protecting AOM/DSS mice (83).
Lonchocarpin is a Wnt/β-catenin pathway inhibitor
isolated from Lonchocarpus sericeus, which is cytotoxic to
neuroblastoma and leukemia cell lines (79). A recent study demonstrated that
lonchocarpin could reduce the proliferation of adenoma cells in
AOM/DSS mice and significantly reduce the proportion of Ki-67 and
BrdU positive cells in the tumors of mice without any toxicity
(84). Further studies showed that
lonchocarpin inhibited nuclear localization of β-catenin and
constitutive active form of TCF4-dnTCF4-VP16. Embryological
analysis of Xenopus laevis showed that lonchocarpin plays a
role at the transcriptional level. In addition, lonchocarpin
inhibited cell migration and proliferation in HCT116, SW480 and
DLD-1 CRC cell lines, without any detectable effect on the
non-tumorigenic intestinal cell line IEC-6 (84).
Other flavonoids
Milk thistle, a flavonoid antioxidant compound
mainly composed of silibinin, is a key regulator of CDK4 cell
cycle. When CDK4 becomes active by binding to cyclin D1, it allows
cells to enter the G1 phase of the cell cycle, and enters the S
phase by phosphorylating retinoblastoma protein. APC mutation,
which functions by activating CDK4 pathway, plays a key role in the
early stage of intestinal tumorigenesis (85). A previous study demonstrated that
silibinin inhibits cell proliferation in vivo and in
vitro and leads to G1 arrest of cell cycle (regulated by CDK4
pathway) (86). It was also
identified that silibinin could significantly reduce the size and
number of adenomas in APCMin/+ mice, and significantly
reduce the expression of CDK4 and Ki67 pathway components in the
epithelium (87). In the study of
Barone et al (88), the role
of silybin has been confirmed. Silybin is the main component of
silymarin (SIL). Estrogen receptor beta (ERβ) is considered to be
gradually decreased in human adenoma-cancer sequence, and ERβ
deficiency increases small intestinal tumorigenesis in rodents.
ERβ selective agonists SIL and/or lignin (LIG) were
added to APCMin/+ mice, and even a specific combination
of SIL and LIG significantly counteracted the occurrence of
intestinal adenomas and increased ERβ mRNA and protein levels. Cell
proliferation and apoptosis were rebalanced and cell migration was
accelerated, which was similar to that of wild-type mice (88).
Scutellarin is a flavonoid isolated from
Scutellaria baicalensis, which has the effects of
anti-inflammation, antioxidation and regulating immunity (89). A recent study has shown that
scutellarin inhibits adenoma carcinogenesis and alleviates
pathological symptoms in AOM/DSS mice. Scutellarin decreased the
concentration of serum TNF-α and IL-6, increased the expression of
Bax and decreased the level of Bcl-2 in adenoma by downregulating
the cascade of Wnt/β-catenin signal, which was verified in
vitro. It was suggested that scutellarin can prevent the
carcinogenesis of adenoma by reducing the cascade of Wnt/β-catenin
signal (90).
Radix Tetrastigma hemsleyani flavone (RTHF)
is the main component of the root of Radix Astragali, which
has been shown to have anti-inflammatory, analgesic, antiviral and
immunomodulatory effects (91). Wu
et al (92) found that RTHF
can reduce the number and size of intestinal adenomas and prevent
adenoma carcinogenesis in AOM/DSS mice, and RTHF can reduce cell
proliferation, induce cell cycle arrest in G0/G1 phase and inhibit
epithelial-mesenchymal transition in a dose-dependent manner. In
addition, RTHF inhibits the expression of Lgr5, c-Myc and Cyclin D1
in tumor tissues. In conclusion, the inhibition of CRA by RTHF is
related to the decrease of the activity of Wnt/β-catenin pathway
and the downregulation of target gene expression (92).
Spinosin exists widely in Ziziphi Spinosae
semen (ZSSP), which has the effects of anti-inflammation and
antioxidant stress, and has a favorable curative effect on colitis,
anxiety and sleep disorder (93,94).
The water-soluble polyphenols extracted from ZSSP have significant
anti-colorectal tumor activity, including inhibiting cell
proliferation and promoting cell apoptosis (95). In AOM/DSS mice, ZSSP reduced the
number and volume of adenomas in a dose-dependent manner, inhibited
the carcinogenesis of adenomas and reduced AOM/DSS-induced organ
damage in mice. Immunohistochemical analysis demonstrated that ZSSP
could downregulate the expression of COX-2, EMR1 and Ki67. Further
studies showed that spinosin was identified as an antitumor
component of ZSSP by the macroporous resin of SP207 and
RP-HPLC-MS/MS (96).
Polymethoxyflavones (PMF), a family of natural
compounds containing nobiletin, hesperidin, heptamethoxyflavonoids
and tetramethoxyflavonoids, are found almost exclusively in citrus
plants (97). PMF is higher in
pericarp than in other edible parts of fruit and has a wide range
of biological activities, including antioxidant, antitumor and
anti-inflammatory (98). A previous
study reported that PMF significantly inhibits the formation of
BaP/DSS-induced CRAs by regulating BaP metabolism and reducing BaP
mutagenic metabolites and DNA adducts, and downregulates the
expression levels of IL-6, IL-10, TNF-α, COX-2, MCP1 and CXCL-1 to
alleviate inflammation. The results of RNA-sequencing (RNA-seq)
showed that PMFs improved the abnormal molecular mechanism induced
by BaP/DSS, including activating inflammation, downregulating
antioxidant targets and inducing transfer genes. Autophagy defects
caused by BaP/DSS-induced tumorigenesis were improved by PMF. In
addition, PMFs also changed the composition of intestinal
microbiota, increased butyrate-producing probiotics and reduced
tumor-related bacteria (99).
Coumestrol is a kind of phytoestrogen and belongs to
flavonoids. Previous studies have shown that ovariectomy increased
the number of intestinal adenomas in APCMin/+ mice by
77%. Replacing estradiol (E2) in ovariectomized APCMin/+
mice reduced the number of adenomas to baseline levels and
upregulated the expression of estrogen receptor beta (ERβ). A
previous study revealed that coumestrol significantly inhibited the
volume of intestinal adenomas in ovariectomized APCMin/+
mice. Moreover, coumestrol improved the damage of E-cadherin and
β-catenin and upregulated the protein expression of ERβ (100).
Genistein, an isoflavone found in soybean, can
inhibit inflammation, promote apoptosis and regulate steroid
hormone receptors (101). It has
been found that genistein reduces the number of adenomas at the
same level as non-ovariectomized mice (100). Genistein 27 (GEN-27), a derivative
of genistein, protects mice from adenoma carcinogenesis induced by
AOM/DSS, reduces the number and volume of adenomas and reduces the
mortality of mice. Furthermore, GEN-27 increased the expression of
APC and AXIN2 and decreased the nuclear localization of β-catenin,
which was due to the inhibition of nuclear localization of
NF-κB/p65 and upregulation of CDX2. Moreover, GEN-27 decreased the
binding of p65 to CDX2 silent region and increased the binding of
CDX2 to APC and AXIN2 promoter regions, thus inhibiting the
activation of β-catenin induced by TNF-α. In addition, GEN-27
significantly decreased the secretion of proinflammatory cytokines
and macrophage infiltration. The results showed that the
anti-proliferation effect of GEN-27 is mediated by
p65-CDX2-β-catenin pathway through the inhibition of β-catenin
target gene (102).
Bergamot is a typical plant produced in Italy, and
its fruit is mainly used to extract essential oil. It was
previously identified that flavonoid-rich extract from bergamot
juice (BJe) has antitumor effects (103), which is related to the fact that
BJe can interfere with intracellular signaling pathways related to
tumor initiation, promotion and progression (104). It has been found that Bje has
antioxidant and anti-inflammatory activities in vitro and
in vivo (105). BJe (35–70
mg/kg) significantly reduced the number of colonic and small
intestinal adenomas in Apc mutant rats, and the mucin depletion
foci of colon premalignant lesions showed a significant
dose-related decrease.
It should be noted that BJe of 70 mg/kg
significantly downregulated inflammation-related genes (COX-2,
iNOS, IL-1 β, IL-6, IL-10 and Arginase1), induced apoptosis,
significantly upregulated p53 and survivin, and downregulated p21
gene expression in rat colon tumors. It is suggested that the
inhibitory effect of Bje on adenoma is partly due to its
pro-apoptotic and anti-inflammatory effects (106).
Daphne genkwa Sieb. et Zucc. is a famous
medicinal plant, and total flavonoids of Daphne genkwa
(TFDG) are the active ingredient. In ApcMin/+mice, the
number and diversity of colonic adenomas decreased significantly
after TFDG treatment, and the life span of mice was significantly
prolonged. TFDG significantly downregulated the expression of
IL-1α, IL-1β, IL-6, IL-10 and G-CSF in intestinal tissue and serum,
and downregulated the expression of IL-2, IL-12, IFN-γ and TNF-α in
serum. These results suggested that the effect of TFDG may be
related to its ability to regulate immunity and inhibit the
production of inflammatory cytokines (107).
Phenolic acids
CUR is a bioactive component derived from the
rhizome of turmeric. It has been shown to exert anti-inflammatory
and antitumor properties by interacting with several molecular
targets, including transcription factors, enzymes, cyclins,
cytokines, receptors and adhesion molecules (108). Girardi et al (109) showed that a diet rich in CUR could
reduce the total number and average size of adenomas in
APCMin/+ mice. In the high CUR diet group, the number of
atypical hyperplasia and CRC decreased significantly, and CUR
significantly upregulated caspase3 and downregulated the level of
Cyclin D1 (109). COX2 is one of
the most important molecules involved in tumorigenesis. High
expression of COX-2 can be detected in adenomas (110,111). CUR can reduce the size and number
of adenomas by significantly inhibiting the mRNA and protein
expression of COX2, but does not inhibit the expression of COX1
(112,113). In addition, CUR may play a role in
the treatment of adenoma by inhibiting the expression of
pro-inflammatory cytokines IL-1 β, IL-6 and Cox-2 and
downregulating Axin2 in Wnt/β-catenin signal pathway (114). McFadden et al (115) showed that CUR increased the
abundance of intestinal bacteria in AOM mice, prevented the
decrease of age-related alpha diversity, increased the relative
abundance of Lactobacillus, and reduced or eliminated the
load of CRAs, indicating that the beneficial effect of CUR on
tumorigenesis is related to the maintenance of more diverse
intestinal microecology. CUR has the disadvantage of being
insoluble in water due to its high hydrophobicity. Adachi et
al (116) used surface control
technology to form submicron particles of CUR, which significantly
improved its water solubility, and this derivative was named
Theracurmin. Treatment with 500 ppm Theracurmin for 8 weeks
inhibited the development of intestinal adenoma and did not change
the transcriptional activity of NF-κB promoter, but inhibited the
expression of MCP-1 and IL-6 mRNA, the downstream target of NF-κB
(116). Seiwert et al
(117) also confirmed the
therapeutic effect of CUR on adenomas. Compared with the control
group, the number of adenomas in mice treated with micellar CUR
(mCUR) decreased significantly. No side effects were observed in
animals that received mCur daily for at least three months
(117).
Turmerone (TUR) is another compound of turmeric,
which is composed of five structure-related sesquiterpenes
including ar-TUR. A study of combination therapy showed that both
TUR and CUR could inhibit the expression of iNOS and COX-2 in
AOM/DSS mice induced by LPS. Tracing experiments with actinomycin D
showed that CUR accelerated the decay of iNOS and COX-2 mRNA and
prevented LPS-induced HuR translocation, which indicated that there
was a post-transcriptional mechanism. In AOM/DSS mice, TUR
significantly inhibited the diversity of adenomas, the combination
of CUR and TUR eliminated tumor formation, and oral TUR
significantly inhibited colorectal shortening by 52–58% (118).
Another study on the treatment of AOM/DSS mice with
CUR combined with ASA showed that CUR significantly inhibited the
occurrence and development of adenoma in a dose-dependent manner
compared with ASA. RNA-seq revealed that compared with the single
treatment, the low-dose combination of CUR and ASA could
downregulate the protein expression of Alb and Mfap4, and
upregulate the mRNA expression of Krt36, Tacstd2, Hoxd10 and
Hoxd13. These differentially expressed genes are located in several
classical pathways in adenomatous inflammatory carcinogenesis and
liver metastasis of CRC (119).
Another study of 3,3-diindolylmethane (DIM) combined with CUR in
the treatment of tumors in F344/NTac-Apcam1137 Pirc rats
showed that DIM and CUR significantly decreased the number and
severity of CRAs and increased apoptosis in mucosa. In addition, a
slight decrease in Survivin-Birc5 expression was observed in all
treatments compared with the control group (120). Lev-Ari et al (121) have shown that physiological dose
of celecoxib (5 µmol/l) inhibits cell proliferation and promotes
apoptosis through COX-2-dependent and independent pathways in the
presence of 10–15 µmol CUR, thus inhibiting the growth of CRAs.
Stilbenes
RSV (3,4,5-Trihydroxy-trans-stilbene) is a kind of
natural polyphenol with antioxidant, anti-inflammatory and
anticancer properties, which is well absorbed in vivo and
metabolized rapidly and widely (122). Studies have shown that RSV
significantly reduces the number of adenomas in AOM/DSS mice
(123). RSV combined with grape
seed extract (RSV-GSE) significantly inhibited the occurrence of
intestinal adenoma in AOM/DSS mice without any gastrointestinal
toxicity. RSV-GSE reduced the number of crypts containing nuclear
β-catenin by inducing apoptosis. In vitro, RSV-GSE inhibited
Wnt/β-catenin pathway, c-Myc and cyclin D1 protein levels, but
sulindac did not. RSV-GSE also induces mitochondrial-mediated
apoptosis in colonic CSC, which is characterized by p53, increased
Bax/Bcl-2 ratio and PARP cleavage. In addition, short hairpin
RNA-mediated knockdown of p53 (a tumor suppressor gene) in colonic
CSC did not change the efficacy of RSV-GSE (124). Saud et al (125) constructed
APCCKO/Krasmut mice to study the antitumor
effect of RSV. They found that adding 150 or 300 ppm RSV to mice
inhibits 60% of tumorigenesis. In 40% of the mice with tumors, the
expression of Kras was lost and the expression of miR-96 was
increased. MiR-96 is a miRNA previously shown to regulate Kras
translation. The results showed that RSV could prevent the
formation and growth of colorectal tumors by downregulating the
expression of Kras.
Lignans
Honokiol (HNK) is a lignan that inhibits the
proliferation, invasion and survival of numerous human cancer cell
lines. HNK exerts its antitumor effect by regulating the main
checkpoints, including NF-κB, STAT3, m-TOR and EGFR (126). A recent study showed that HNK
decreased the number and volume of adenomas in AOM/DSS mice, and
significantly decreased the expression of YAP1, TEAD1 and stem cell
marker proteins. In vitro experiments revealed that HNK
could inhibit the proliferation and colony formation of tumor cells
and induce apoptosis. In addition, HNK inhibited the activity of
DCLK1 kinase and the expression of LGR5, CD44, and inhibited Ser127
phosphorylation in YAP1 (127).
Another study demonstrated that the therapeutic effect of HNK on
APCMin/+ mice is highly related to citric acid cycle,
pentose phosphate pathway, pyrimidine, tyrosine, arginine, proline
and glutathione metabolism (128).
Sesamol is a common lignan in sesame, which can
mediate anti-inflammatory effects by downregulating the
transcription of inflammatory markers including cytokines, redox
status, protein kinases and pro-inflammatory enzymes. Sesamol also
induces apoptosis and activates caspase cascades through
mitochondria and receptors (129).
Shimizu et al (130) found
that sesamol could reduce the number of polyps in the middle small
intestine of APCMin/+ mice, and sesamol downregulated
the expression levels of COX-2, cPGES, mPGES2, EP1 and EP2 genes in
polyps. In addition, sesamol did not inhibit the expression levels
of COX-1 and EP4 mRNA in non-polyps and intestinal polyps. In the
aforementioned study, in vitro experiments demonstrated that
the basal cyclooxygenase-2 transcriptional activity decreased by
50% under 100 µM sesamol through the β-galactosidase reporter gene
system, suggesting that sesamol may play an antitumor role as a
cyclooxygenase-2 inhibitor (130).
Catechols
Caffeic acid phenethyl ester (CAPE) is a phenolic
antioxidant extracted from propolis (bee resin) and a kind of
catechol. It has been reported that CAPE has strong
anti-inflammatory activity, which can significantly reduce the
size, number and load of adenomas in AOM/DSS mice, alleviate
intestinal atrophy and increase the length of colon in a
dose-dependent manner. CAPE decreased the activation of NLRP3
inflammatory bodies in BMDMs and THP-1 cells. Further studies have
shown that CAPE regulates NLRP3 at the post-transcriptional level
by inhibiting the production of ROS. However, CAPE does not affect
the transcription of NLRP3 or IL-1β, but enhances the binding of
NLRP3 to ubiquitin molecules and promotes the ubiquitin of NLRP3.
Notably, CAPE suppressed the interaction between NLRP3 and CSN5,
but enhanced the interaction between NLRP3 and Cullin1 (131).
Oleuropein, the main phenolic active ingredient of
Olea europaea (Oleaceae), belongs to catechins. It
has been proven to have the effects of anti-oxidation, antitumor,
anti-inflammation, neuroprotection and liver protection. Oleuropein
can effectively inhibit the progression of esophageal, gastric,
lung and liver cancer (132).
A previous study has shown that oleuropein can
inhibit the occurrence and diversity of CRA, improve adverse
symptoms, and DAI score, downregulate the expression of IL-6,
IFN-γ, TNF-α and IL-17A, and reduce the expression of COX-2 and
PCNA. In addition, oleuropein significantly downregulated NF-κ B,
Wnt/β-catenin, P3IK/Akt and STAT3, and decreased the expression of
CD4 (+) Roγ t (+) IL-17 (+) IFN-γ (+) T cell subsets and IL-17A and
IFN-γ in lamina propria (133).
Other polyphenols
Tea polyphenols (TPs)
TPs are the main components isolated from tea and
are known to have antioxidant, anti-inflammatory and antitumor
activities (134). Previous
studies have identified that TPs can play an important role in the
treatment of most cancers by causing cell cycle arrest in G0/G1
phase and inhibiting angiogenesis, and has fewer side effects than
traditional drugs (135,136). A previous study has shown that TPs
can inhibit the development of adenoma induced by AOM in male
Fischer rats and F344 rats, reduce the incidence and average number
of adenomas in rats, and reduce the occurrence of ACF, but there is
no dose-dependent relationship in male Fischer rats (137). In addition, TPs can regulate MAPK,
PI3K/Akt, Wnt/β-catenin, NF-κB, JAK/STAT and 67 kDa-laminin
receptor pathway (137,138). In another study, green tea rich in
TPs significantly reduced the number of small adenomas (diameters
≤1 mm) in ApcMin/+ mice, downregulated the levels of
β-catenin and its downstream target cyclin D1, and increased
Retinoid X receptor α (RXR α) mRNA and protein expression. Colonic
DNA was treated with bisulfite, and then 24 CpG sites in the
promoter region of RXR α gene were sequenced with pyrophosphate.
The results revealed that CpG methylation decreased significantly.
It has been suggested that low concentration of TPs is sufficient
to desilence RXR α and inhibit intestinal tumorigenesis in
ApcMin/+ mice (139).
Bioactive components of polyphenol-rich
cranberry extracts
Cranberry contains a variety of ingredients related
to human health. Studies have shown that cranberry polyphenol
extract significantly decreased the incidence of adenoma and
canceration rate induced by AOM/DSS, reduced the size of adenoma
and downregulated the expression of IL-1β and IL-6, TNF-α in mice
(140). Walnut phenolic extract
significantly reduced the development of adenoma induced by
AOM/DSS, reduced IκB phosphorylation/degradation and NF-κB DNA
binding activity induced by TNF-α, and decreased NF-κB signal
transduction in colon (141).
Polyphenols extracted from Annurca significantly reduced the
number of adenomas in colon and small intestine, slowed down weight
loss and severe rectal bleeding in ApcMin/+ mice,
increased antioxidant activity and upregulated DNA methylation in
mice (142).
Discussion
This is the first review devoted to the study of
the therapeutic effect of natural polyphenols on CRA. Although
natural polyphenols are considered as possible tumor inhibitors,
their potential effects, mechanisms and safety remain to be solved.
As a highly malignant tumor, the current treatment of CRC is
limited, thus the prevention of CRC is particularly important. It
has been reported that the 10-year cumulative canceration rates of
tubular adenoma, tubular villous adenoma and villous adenoma are
2.7, 5.1 and 8.6% respectively, while serrated polyps are 2.5%
(143). As a premalignant lesion
of CRC, early intervention of CRA is very important to reduce the
morbidity and mortality of CRC (144).
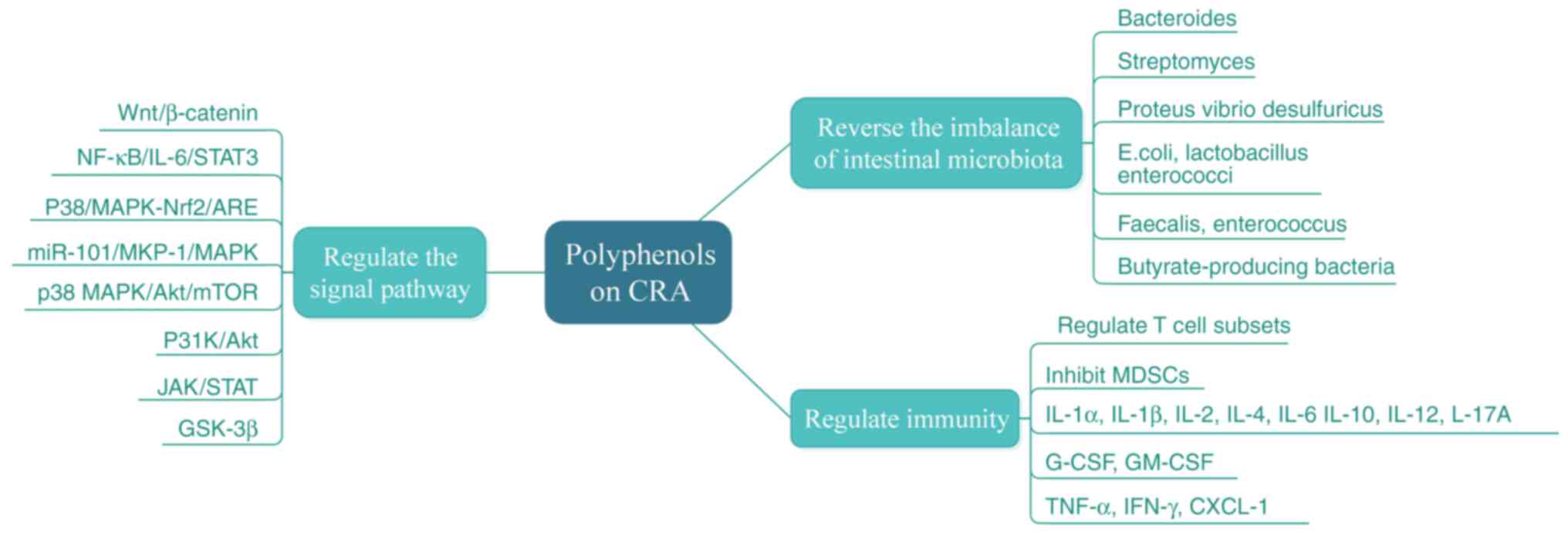
Natural polyphenols have unique advantages in the
treatment of CRA due to their multi-targets and high safety, and
certain polyphenols have been proven to play a potential role in
preventing the occurrence and development of adenomas. The results
showed that natural polyphenols significantly reduced adenoma and
carcinogenesis in animal models by inhibiting intestinal cell
proliferation, inducing apoptosis, inhibiting inflammation and
oxidative stress, regulating intestinal microbiota and inducing
intestinal barrier function recovery. These beneficial effects are
mainly related to the inhibition of activation of multiple signal
pathways, including Wnt/β-catenin, NF-κB, GSK-3β, MAPK, STAT3 and
PI3K/Akt/mTOR. In addition, numerous studies suggested that the
bioavailability of natural polyphenols is low, and it is suggested
that the metabolites generated in the intestine by the resident
microbiota may contribute to the cancer prevention properties
(145). All of the aforementioned
evidence suggests that these natural polyphenols are effective and
promising candidates for the treatment of adenomas. However, the
potential molecular mechanism or toxicological tests of most
natural polyphenols need to be further studied. At present, the
actual efficacy of a few polyphenols in patients with CRA has been
confirmed in clinical studies, including CUR, RSV and anthocyanins.
Most polyphenols have not been confirmed in clinical trials.
Therefore, their molecular mechanism, long-term efficacy and safety
should be further clarified in clinical trials.
Conclusion and prospects
The present study reviewed the protective effect of
natural polyphenols on CRA and shows its potential antitumor
mechanism (Fig. 3). Natural
polyphenols have great potential in the treatment of colorectal
tumors. In future research, the role of natural substances in
preventing colorectal tumors is significant. Research should focus
on the molecular targets of natural polyphenols and their in-depth
mechanistic studies, metabolism and toxicology in vivo to
determine the safe dose for human studies. Given the low
bioavailability of natural polyphenols and the critical role of
intestinal microbiota in tumor development, the synergistic
interaction of drugs, the development of phytochemical derivatives,
synthetic analogs or nanoparticles, the metabolism of compounds by
intestinal microflora cannot be ignored, to promote these
polyphenols to play a greater role in the treatment of tumors in
the future.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Shanghai Science and
Technology Committee (grant no. 20ZR1450400), the Plan of Clinical
Specialty Construction in Shanghai Putuo District Health System
(grant no. 2020tszk02), the Distant effect of anti-PD-1 antibody on
local microwave palliative ablation of advanced lung cancer (grant
no. 20214Y0495), the National Key Research and Development Program
of China (grant no 2019YFC1316000) and Discount for the use of
funds for teaching projects (grant no. Y-445) for their financial
support.
Availability of data and materials
Not applicable.
Authors' contributions
FQM and YHL participated in the writing of the
manuscript. ZHN contributed to the editing of the manuscript. XBW
and TC served as scientific advisors. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Versace VL, Forsyth AD, Vaughan R, Morrice
MG and Morphett BJ: Evidence of elevated colorectal cancer and
adenoma rates for regional National Bowel Cancer Screening Program
participants. Aust J Rural Health. 26:63–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhiqiang F, Jie C, Yuqiang N, Chenghua G,
Hong W, Zheng S, Wanglin L, Yongjian Z, Liping D, Lizhong Z and
DeJian Z: Analysis of population-based colorectal cancer screening
in Guangzhou, 2011–2015. Cancer Med. 8:2496–2502. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conteduca V, Sansonno D, Russi S and
Dammacco F: Precancerous colorectal lesions (Review). Int J Oncol.
43:973–984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brennan CA and Garrett WS: Gut microbiota,
inflammation, and colorectal cancer. Annu Rev Microbiol.
70:395–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang B, Mao L, Li Y, Li Q, Li X, Zhang Y
and Zhai Z: β-catenin, leucine-rich repeat-containing G
protein-coupled receptor 5 and GATA-binding factor 6 are associated
with the normal mucosa-adenoma-adenocarcinoma sequence of
colorectal tumorigenesis. Oncol Lett. 15:2287–2295. 2018.PubMed/NCBI
|
|
7
|
Murakami T, Kurosawa T, Fukushima H,
Shibuya T, Yao T and Nagahara A: Sessile serrated lesions:
Clinicopathological characteristics, endoscopic diagnosis, and
management. Dig Endosc. 34:1096–1109. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lazarus R, Junttila OE, Karttunen TJ and
Makinen MJ: The risk of metachronous neoplasia in patients with
serrated adenoma. Am J Clin Pathol. 123:349–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cirillo G, Curcio M, Vittorio O, Iemma F,
Restuccia D, Spizzirri UG, Puoci F and Picci N: Polyphenol
conjugates and human health: A perspective review. Crit Rev Food
Sci Nutr. 56:326–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fantini M, Benvenuto M, Masuelli L,
Frajese GV, Tresoldi I, Modesti A and Bei R: In vitro and in vivo
antitumoral effects of combinations of polyphenols, or polyphenols
and anticancer drugs: Perspectives on cancer treatment. Int J Mol
Sci. 16:9236–9282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marzocchella L, Fantini M, Benvenuto M,
Masuelli L, Tresoldi I, Modesti A and Bei R: Dietary flavonoids:
Molecular mechanisms of action as anti-inflammatory agents. Recent
Pat Inflamm Allergy Drug Discov. 5:200–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Focaccetti C, Izzi V, Benvenuto M, Fazi S,
Ciuffa S, Giganti MG, Potenza V, Manzari V, Modesti A and Bei R:
Polyphenols as immunomodulatory compounds in the tumor
microenvironment: Friends or Foes? Int J Mol Sci. 20:17142019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mattera R, Benvenuto M, Giganti MG,
Tresoldi I, Pluchinotta FR, Bergante S, Tettamanti G, Masuelli L,
Manzari V, Modesti A and Bei R: Effects of polyphenols on oxidative
stress-mediated injury in cardiomyocytes. Nutrients. 9:5232017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaltenbach T, Anderson JC, Burke CA,
Dominitz JA, Gupta S, Lieberman D, Robertson DJ, Shaukat A, Syngal
S and Rex DK: Endoscopic removal of colorectal lesions:
Recommendations by the US multi-society task force on colorectal
cancer. Am J Gastroenterol. 115:435–464. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Veettil SK, Nathisuwan S, Ching SM,
Jinatongthai P, Lim KG, Kew ST and Chaiyakunapruk N: Efficacy and
safety of celecoxib on the incidence of recurrent colorectal
adenomas: A systematic review and meta-analysis. Cancer Manag Res.
11:561–571. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brennan CA, Nakatsu G, Gallini Comeau CA,
Drew DA, Glickman JN, Schoen RE, Chan AT and Garrett WS: Aspirin
modulation of the colorectal cancer-associated microbe
fusobacterium nucleatum. mBio. 12:e00547–21. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu H, Flanders WD, Ahearn TU, Daniel CR,
Gonzalez-Feliciano AG, Long Q, Rutherford RE and Bostick RM:
Effects of calcium and vitamin D3 on transforming growth factors in
rectal mucosa of sporadic colorectal adenoma patients: A randomized
controlled trial. Mol Carcinog. 54:270–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bonelli L, Puntoni M, Gatteschi B, Massa
P, Missale G, Munizzi F, Turbino L, Villanacci V, De Censi A and
Bruzzi P: Antioxidant supplement and long-term reduction of
recurrent adenomas of the large bowel. A double-blind randomized
trial. J Gastroenterol. 48:698–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
West NJ, Clark SK, Phillips RK, Hutchinson
JM, Leicester RJ, Belluzzi A and Hull MA: Eicosapentaenoic acid
reduces rectal polyp number and size in familial adenomatous
polyposis. Gut. 59:918–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sui H, Zhang L, Gu K, Chai N, Ji Q, Zhou
L, Wang Y, Ren J, Yang L, Zhang B, et al: YYFZBJS ameliorates
colorectal cancer progression in ApcMin/+ mice by
remodeling gut microbiota and inhibiting regulatory T-cell
generation. Cell Commun Signal. 18:1132020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu H, Huang Y, Yang L, Su K, Tian S, Chen
X, Li S and Liu W: Effects of Jianpi Lishi Jiedu granules on
colorectal adenoma patients after endoscopic treatment: Study
protocol for a randomized, double-blinded, placebo-controlled
clinical trial. Trials. 23:3452022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cruz-Correa M, Hylind LM, Marrero JH,
Zahurak ML, Murray-Stewart T, Casero RA Jr, Montgomery EA,
Iacobuzio-Donahue C, Brosens LA, Offerhaus GJ, et al: Efficacy and
safety of curcumin in treatment of intestinal adenomas in patients
with familial adenomatous polyposis. Gastroenterology. 155:668–673.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Briata IM, Paleari L, Rutigliani M,
Petrera M, Caviglia S, Romagnoli P, Libera MD, Oppezzi M, Puntoni
M, Siri G, et al: A presurgical study of curcumin combined with
anthocyanin supplements in patients with colorectal adenomatous
polyps. Int J Mol Sci. 22:2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dulai PS, Singh S, Marquez E, Khera R,
Prokop LJ, Limburg PJ, Gupta S and Murad MH: Chemoprevention of
colorectal cancer in individuals with previous colorectal
neoplasia: Systematic review and network meta-analysis. BMJ.
355:i61882016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szeto CC, Sugano K, Wang JG, Fujimoto K,
Whittle S, Modi GK, Chen CH, Park JB, Tam LS, Vareesangthip K, et
al: Non-steroidal anti-inflammatory drug (NSAID) therapy in
patients with hypertension, cardiovascular, renal or
gastrointestinal comorbidities: Joint
APAGE/APLAR/APSDE/APSH/APSN/PoA recommendations. Gut. 69:617–629.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scalbert A, Manach C, Morand C, Remesy C
and Jimenez L: Dietary polyphenols and the prevention of diseases.
Crit Rev Food Sci Nutr. 45:287–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin H, Leng Q and Li C: Dietary flavonoid
for preventing colorectal neoplasms. Cochrane Database Syst. Rev:
CD009350. 2012.doi: 10.1002/14651858.CD009350.pub2. View Article : Google Scholar
|
|
28
|
Beecher GR: Overview of dietary
flavonoids: Nomenclature, occurrence and intake. J Nutr.
133:3248S–3254S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Manach C, Williamson G, Morand C, Scalbert
A and Remesy C: Bioavailability and bioefficacy of polyphenols in
humans. I. Review of 97 bioavailability studies. Am J Clin Nutr.
81:230S–242S. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wicinski M, Gebalski J, Mazurek E,
Podhorecka M, Sniegocki M, Szychta P, Sawicka E and Malinowski B:
The influence of polyphenol compounds on human gastrointestinal
tract microbiota. Nutrients. 12:3502020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Monagas M, Urpi-Sarda M, Sanchez-Patan F,
Llorach R, Garrido I, Gomez-Cordoves C, Andres-Lacueva C and
Bartolome B: Insights into the metabolism and microbial
biotransformation of dietary flavan-3-ols and the bioactivity of
their metabolites. Food Funct. 1:233–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Manach C, Scalbert A, Morand C, Remesy C
and Jimenez L: Polyphenols: Food sources and bioavailability. Am J
Clin Nutr. 79:727–747. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tuli HS, Aggarwal V, Kaur J, Aggarwal D,
Parashar G, Parashar NC, Tuorkey M, Kaur G, Savla R, Sak K and
Kumar M: Baicalein: A metabolite with promising antineoplastic
activity. Life Sci. 259:1181832020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang CZ, Zhang CF, Chen L, Anderson S, Lu
F and Yuan CS: Colon cancer chemopreventive effects of baicalein,
an active enteric microbiome metabolite from baicalin. Int J Oncol.
47:1749–1758. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang CZ, Zhang CF, Luo Y, Yao H, Yu C,
Chen L, Yuan J, Huang WH, Wan JY, Zeng J, et al: Baicalein, an
enteric microbial metabolite, suppresses gut inflammation and
cancer progression in ApcMin/+ mice. Clin Transl Oncol.
22:1013–1022. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim DH, Hossain MA, Kang YJ, Jang JY, Lee
YJ, Im E, Yoon JH, Kim HS, Chung HY and Kim ND: Baicalein, an
active component of Scutellaria baicalensis Georgi, induces
apoptosis in human colon cancer cells and prevents AOM/DSS-induced
colon cancer in mice. Int J Oncol. 43:1652–1658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fidelis QC, Faraone I, Russo D, Aragao
Catunda-Jr FE, Vignola L, de Carvalho MG, de Tommasi N and Milella
L: Chemical and Biological insights of Ouratea hexasperma (A.
St.-Hil.) Baill.: A source of bioactive compounds with
multifunctional properties. Nat Prod Res. 33:1500–1503. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Imran M, Aslam Gondal T, Atif M, Shahbaz
M, Batool Qaisarani T, Hanif Mughal M, Salehi B, Martorell M and
Sharifi-Rad J: Apigenin as an anticancer agent. Phytother Res.
34:1812–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong Y, Krisanapun C, Lee SH, Nualsanit
T, Sams C, Peungvicha P and Baek SJ: Molecular targets of apigenin
in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur
J Cancer. 46:3365–3374. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agarwal ML, Taylor WR, Chernov MV,
Chernova OB and Stark GR: The p53 network. J Biol Chem. 273:1–4.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Harborne JB and Williams CA: Advances in
flavonoid research since 1992. Phytochemistry. 55:481–504. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang Y, Cai X, Yang J, Sun X, Hu C, Yan Z,
Xu X, Lu W, Wang X and Cao P: Chemoprevention of dietary
digitoflavone on colitis-associated colon tumorigenesis through
inducing Nrf2 signaling pathway and inhibition of inflammation. Mol
Cancer. 13:482014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nasr-Bouzaiene N, Sassi A, Bedoui A, Krifa
M, Chekir-Ghedira L and Ghedira K: Immunomodulatory and cellular
antioxidant activities of pure compounds from Teucrium ramosissimum
Desf. Tumour Biol. 37:7703–7712. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lucarini R, Tozatti MG, Silva ML, Gimenez
VM, Pauletti PM, Groppo M, Turatti IC, Cunha WR and Martins CH:
Antibacterial and anti-inflammatory activities of an extract,
fractions, and compounds isolated from Gochnatia pulchra aerial
parts. Braz J Med Biol Res. 48:822–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gao Y, Liu F, Fang L, Cai R, Zong C and Qi
Y: Genkwanin inhibits proinflammatory mediators mainly through the
regulation of miR-101/MKP-1/MAPK pathway in LPS-activated
macrophages. PLoS One. 9:e967412014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang X, Song ZJ, He X, Zhang RQ, Zhang CF,
Li F, Wang CZ and Yuan CS: Antitumor and immunomodulatory activity
of genkwanin on colorectal cancer in the APC(Min/+) mice. Int
Immunopharmacol. 29:701–707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yin HF, Yin CM, Ouyang T, Sun SD, Chen WG,
Yang XL, He X and Zhang CF: Self-Nanoemulsifying drug delivery
system of genkwanin: A novel approach for anti-colitis-associated
colorectal cancer. Drug Des Devel Ther. 15:557–576. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cai X, Lu W, Ye T, Lu M, Wang J, Huo J,
Qian S, Wang X and Cao P: The molecular mechanism of
luteolin-induced apoptosis is potentially related to inhibition of
angiogenesis in human pancreatic carcinoma cells. Oncol Rep.
28:1353–1361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Murphy EA, Davis JM, McClellan JL and
Carmichael MD: Quercetin's effects on intestinal polyp multiplicity
and macrophage number in the Apc(Min/+) mouse. Nutr Cancer.
63:421–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Velazquez KT, Enos RT, Narsale AA, Puppa
MJ, Davis JM, Murphy EA and Carson JA: Quercetin supplementation
attenuates the progression of cancer cachexia in ApcMin/+ mice. J
Nutr. 144:868–875. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Calderon-Montano JM, Burgos-Moron E,
Perez-Guerrero C and Lopez-Lazaro M: A review on the dietary
flavonoid kaempferol. Mini Rev Med Chem. 11:298–344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Imran M, Salehi B, Sharifi-Rad J, Aslam
Gondal T, Saeed F, Imran A, Shahbaz M, Tsouh Fokou PV, Umair Arshad
M, Khan H, et al: Kaempferol: A key emphasis to its anticancer
potential. Molecules. 24:22772019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lu L, Wang Y, Ou R, Feng Q, Ji L, Zheng H,
Guo Y, Qi X, Kong AN and Liu Z: DACT2 Epigenetic stimulator exerts
dual efficacy for colorectal cancer prevention and treatment.
Pharmacol Res. 129:318–328. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Song X, Tan L, Wang M, Ren C, Guo C, Yang
B, Ren Y, Cao Z, Li Y and Pei J: Myricetin: A review of the most
recent research. Biomed Pharmacother. 134:1110172021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Y, Cui SX, Sun SY, Shi WN, Song ZY,
Wang SQ, Yu XF, Gao ZH and Qu XJ: Chemoprevention of intestinal
tumorigenesis by the natural dietary flavonoid myricetin in
APCMin/+ mice. Oncotarget. 7:60446–60460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang MJ, Su H, Yan JY, Li N, Song ZY,
Wang HJ, Huo LG, Wang F, Ji WS, Qu X J and Qu MH: Chemopreventive
effect of Myricetin, a natural occurring compound, on colonic
chronic inflammation and inflammation-driven tumorigenesis in mice.
Biomed Pharmacother. 97:1131–1137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gong G, Guan YY, Zhang ZL, Rahman K, Wang
SJ, Zhou S, Luan X and Zhang H: Isorhamnetin: A review of
pharmacological effects. Biomed Pharmacother. 128:1103012020.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bobe G, Sansbury LB, Albert PS, Cross AJ,
Kahle L, Ashby J, Slattery ML, Caan B, Paskett E, Iber F, et al:
Dietary flavonoids and colorectal adenoma recurrence in the Polyp
Prevention Trial. Cancer Epidemiol Biomarkers Prev. 17:1344–1353.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Saud SM, Young MR, Jones-Hall YL, Ileva L,
Evbuomwan MO, Wise J, Colburn NH, Kim YS and Bobe G:
Chemopreventive activity of plant flavonoid isorhamnetin in
colorectal cancer is mediated by oncogenic Src and β-catenin.
Cancer Res. 73:5473–5484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mattioli R, Francioso A, Mosca L and Silva
P: Anthocyanins: A comprehensive review of their chemical
properties and health effects on cardiovascular and
neurodegenerative diseases. Molecules. 25:38092020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Khoo HE, Azlan A, Tang ST and Lim SM:
Anthocyanidins and anthocyanins: Colored pigments as food,
pharmaceutical ingredients, and the potential health benefits. Food
Nutr Res. 61:13617792017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fragoso MF, Romualdo GR, Vanderveer LA,
Franco-Barraza J, Cukierman E, Clapper ML, Carvalho RF and Barbisan
LF: Lyophilized acai pulp (Euterpe oleracea Mart) attenuates
colitis-associated colon carcinogenesis while its main anthocyanin
has the potential to affect the motility of colon cancer cells.
Food Chem Toxicol. 121:237–245. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fernandez J, Garcia L, Monte J, Villar CJ
and Lombo F: Functional Anthocyanin-rich sausages diminish
colorectal cancer in an animal model and reduce Pro-inflammatory
bacteria in the intestinal microbiota. Genes (Basel). 9:1332018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen L, Jiang B, Zhong C, Guo J, Zhang L,
Mu T, Zhang Q and Bi X: Chemoprevention of colorectal cancer by
black raspberry anthocyanins involved the modulation of gut
microbiota and SFRP2 demethylation. Carcinogenesis. 39:471–481.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Charepalli V, Reddivari L, Radhakrishnan
S, Vadde R, Agarwal R and Vanamala JK: Anthocyanin-containing
purple-fleshed potatoes suppress colon tumorigenesis via
elimination of colon cancer stem cells. J Nutr Biochem.
26:1641–1649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cai H, Marczylo TH, Teller N, Brown K,
Steward WP, Marko D and Gescher AJ: Anthocyanin-rich red grape
extract impedes adenoma development in the Apc(Min) mouse:
Pharmacodynamic changes and anthocyanin levels in the murine
biophase. Eur J Cancer. 46:811–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kang SY, Seeram NP, Nair MG and Bourquin
LD: Tart cherry anthocyanins inhibit tumor development in Apc(Min)
mice and reduce proliferation of human colon cancer cells. Cancer
Lett. 194:13–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lippert E, Ruemmele P, Obermeier F,
Goelder S, Kunst C, Rogler G, Dunger N, Messmann H, Hartmann A and
Endlicher E: Anthocyanins prevent colorectal cancer development in
a mouse model. Digestion. 95:275–280. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Asadi K, Ferguson LR, Philpott M and
Karunasinghe N: Cancer-preventive Properties of an
Anthocyanin-enriched Sweet Potato in the APC(MIN) mouse model. J
Cancer Prev. 22:135–146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yang CS: Inhibition of carcinogenesis by
tea. Nature. 389:134–135. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sukhthankar M, Yamaguchi K, Lee SH,
McEntee MF, Eling TE, Hara Y and Baek SJ: A green tea component
suppresses posttranslational expression of basic fibroblast growth
factor in colorectal cancer. Gastroenterology. 134:1972–1980. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wang X, Ye T, Chen WJ, Lv Y, Hao Z, Chen
J, Zhao JY, Wang HP and Cai YK: Structural shift of gut microbiota
during chemo-preventive effects of epigallocatechin gallate on
colorectal carcinogenesis in mice. World J Gastroenterol.
23:8128–8139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yum HW, Zhong X, Park J, Na HK, Kim N, Lee
HS and Surh YJ: Oligonol inhibits dextran sulfate sodium-induced
colitis and colonic adenoma formation in mice. Antioxid Redox
Signal. 19:102–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen R, Qi QL, Wang MT and Li QY:
Therapeutic potential of naringin: An overview. Pharm Biol.
54:3203–3210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang YS, Wang F, Cui SX and Qu XJ:
Natural dietary compound naringin prevents azoxymethane/dextran
sodium sulfate-induced chronic colorectal inflammation and
carcinogenesis in mice. Cancer Biol Ther. 19:735–744. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lu JF, Zhu MQ, Zhang H, Liu H, Xia B, Wang
YL, Shi X, Peng L and Wu JW: Neohesperidin attenuates obesity by
altering the composition of the gut microbiota in high-fat diet-fed
mice. FASEB J. 34:12053–12071. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhao T, Hu S, Ma P, Che D, Liu R, Zhang Y,
Wang J, Li C, Ding Y, Fu J, et al: Neohesperidin suppresses
IgE-mediated anaphylactic reactions and mast cell activation via
Lyn-PLC-Ca2+ pathway. Phytother Res. 33:2034–2043. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gong Y, Dong R, Gao X, Li J, Jiang L,
Zheng J, Cui S, Ying M, Yang B, Cao J and He Q: Neohesperidin
prevents colorectal tumorigenesis by altering the gut microbiota.
Pharmacol Res. 148:1044602019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wei SY, Chen Y and Xu XY: Progress on the
pharmacological research of puerarin: A review. Chin J Nat Med.
12:407–414. 2014.PubMed/NCBI
|
|
80
|
Deng XQ, Zhang HB, Wang GF, Xu D, Zhang
WY, Wang QS and Cui YL: Colon-specific microspheres loaded with
puerarin reduce tumorigenesis and metastasis in colitis-associated
colorectal cancer. Int J Pharm. 570:1186442019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang
S, Deng X, Xie Z and Zheng S: Isoliquiritigenin alleviates early
brain injury after experimental intracerebral hemorrhage via
suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome
activation by promoting Nrf2 antioxidant pathway. J
Neuroinflammation. 14:1192017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Yadav VR, Prasad S, Sung B and Aggarwal
BB: The role of chalcones in suppression of NF-κB-mediated
inflammation and cancer. Int Immunopharmacol. 11:295–309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wu M, Wu Y, Deng B, Li J, Cao H, Qu Y,
Qian X and Zhong G: Isoliquiritigenin decreases the incidence of
colitis-associated colorectal cancer by modulating the intestinal
microbiota. Oncotarget. 7:85318–85331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Predes D, Oliveira LFS, Ferreira LSS, Maia
LA, Delou JMA, Faletti A, Oliveira I, Amado NG, Reis AH, Fraga CAM,
et al: The Chalcone Lonchocarpin Inhibits Wnt/β-catenin signaling
and suppresses colorectal cancer proliferation. Cancers (Basel).
11:19682019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang T, Nanney LB, Luongo C, Lamps L,
Heppner KJ, DuBois RN and Beauchamp RD: Concurrent overexpression
of cyclin D1 and cyclin-dependent kinase 4 (Cdk4) in intestinal
adenomas from multiple intestinal neoplasia (Min) mice and human
familial adenomatous polyposis patients. Cancer Res. 57:169–175.
1997.PubMed/NCBI
|
|
86
|
Hogan FS, Krishnegowda NK, Mikhailova M
and Kahlenberg MS: Flavonoid, silibinin, inhibits proliferation and
promotes cell-cycle arrest of human colon cancer. J Surg Res.
143:58–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Karim BO, Rhee KJ, Liu G, Zheng D and Huso
DL: Chemoprevention utility of silibinin and Cdk4 pathway
inhibition in Apc(−/+) mice. BMC Cancer. 13:1572013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Barone M, Tanzi S, Lofano K, Scavo MP,
Pricci M, Demarinis L, Papagni S, Guido R, Maiorano E, Ingravallo
G, et al: Dietary-induced ERbeta upregulation counteracts
intestinal neoplasia development in intact male ApcMin/+ mice.
Carcinogenesis. 31:269–274. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhao TT, Xu YQ, Hu HM, Gong HB and Zhu HL:
Isoliquiritigenin (ISL) and its Formulations: Potential Antitumor
Agents. Curr Med Chem. 26:6786–6796. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zeng S, Chen L, Sun Q, Zhao H, Yang H, Ren
S, Liu M, Meng X and Xu H: Scutellarin ameliorates
colitis-associated colorectal cancer by suppressing Wnt/β-catenin
signaling cascade. Eur J Pharmacol. 906:1742532021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Feng Z, Hao W, Lin X, Fan D and Zhou J:
Antitumor activity of total flavonoids from Tetrastigma
hemsleyanum Diels et Gilg is associated with the inhibition of
regulatory T cells in mice. Onco Targets Ther. 7:947–956.
2014.PubMed/NCBI
|
|
92
|
Wu X, Yu N, Zhang Y, Ye Y, Sun W, Ye L, Wu
H, Yang Z, Wu L and Wang F: Radix Tetrastigma hemsleyani
flavone exhibits antitumor activity in colorectal cancer via
Wnt/β-catenin signaling pathway. Onco Targets Ther. 11:6437–6446.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu Y, Zhao X, Lin T, Wang Q, Zhang Y and
Xie J: Molecular mechanisms of polysaccharides from Ziziphus
jujuba Mill var. spinosa seeds regulating the
bioavailability of spinosin and preventing colitis. Int J Biol
Macromol. 163:1393–1402. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu J, Zhai WM, Yang YX, Shi JL, Liu QT,
Liu GL, Fang N, Li J and Guo JY: GABA and 5-HT systems are
implicated in the anxiolytic-like effect of spinosin in mice.
Pharmacol Biochem Behav. 128:41–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xu MY, Lee SY, Kang SS and Kim YS:
Antitumor activity of jujuboside B and the underlying mechanism via
induction of apoptosis and autophagy. J Nat Prod. 77:370–376. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shan S, Xie Y, Zhang C, Jia B, Li H and Li
Z: Identification of polyphenol from Ziziphi spinosae semen
against human colon cancer cells and colitis-associated colorectal
cancer in mice. Food Funct. 11:8259–8272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Matsumoto S, Tominari T, Matsumoto C,
Yoshinouchi S, Ichimaru R, Watanabe K, Hirata M, Grundler FMW,
Miyaura C and Inada M: Effects of polymethoxyflavonoids on bone
loss induced by estrogen deficiency and by LPS-dependent
inflammation in mice. Pharmaceuticals (Basel). 11:72018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu JC, Tsai ML, Lai CS, Lo CY, Ho CT, Wang
YJ and Pan MH: Polymethoxyflavones prevent benzo[a]pyrene/dextran
sodium sulfate-induced colorectal carcinogenesis through modulating
xenobiotic metabolism and ameliorate autophagic defect in ICR mice.
Int J Cancer. 142:1689–1701. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Javid SH, Moran AE, Carothers AM, Redston
M and Bertagnolli MM: Modulation of tumor formation and intestinal
cell migration by estrogens in the Apc(Min/+) mouse model of
colorectal cancer. Carcinogenesis. 26:587–595. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mukund V, Mukund D, Sharma V, Mannarapu M
and Alam A: Genistein: Its role in metabolic diseases and cancer.
Crit Rev Oncol Hematol. 119:13–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Du Q, Wang Y, Liu C, Wang H, Fan H, Li Y,
Wang J, Zhang X, Lu J, Ji H and Hu R: Chemopreventive activity of
GEN-27, a genistein derivative, in colitis-associated cancer is
mediated by p65-CDX2-β-catenin axis. Oncotarget. 7:17870–17884.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Cirmi S, Maugeri A, Ferlazzo N, Gangemi S,
Calapai G, Schumacher U and Navarra M: Anticancer potential of
citrus juices and their extracts: A systematic review of both
preclinical and clinical studies. Front Pharmacol. 8:4202017.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Cirmi S, Ferlazzo N, Lombardo GE, Maugeri
A, Calapai G, Gangemi S and Navarra M: Chemopreventive agents and
inhibitors of cancer hallmarks: May citrus offer new perspectives?
Nutrients. 8:6982016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ferlazzo N, Cirmi S, Calapai G,
Ventura-Spagnolo E, Gangemi S and Navarra M: Anti-Inflammatory
activity of citrus bergamia derivatives: Where do we stand?
Molecules. 21:12732016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Navarra M, Femia AP, Romagnoli A, Tortora
K, Luceri C, Cirmi S, Ferlazzo N and Caderni G: A flavonoid-rich
extract from bergamot juice prevents carcinogenesis in a genetic
model of colorectal cancer, the Pirc rat
(F344/NTac-Apcam1137). Eur J Nutr. 59:885–894. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Du WJ, Yang XL, Song ZJ, Wang JY, Zhang
WJ, He X, Zhang RQ, Zhang CF, Li F, Yu CH, et al: Antitumor
activity of total flavonoids from Daphne genkwa in
colorectal cancer. Phytother Res. 30:323–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Shishodia S, Sethi G and Aggarwal BB:
Curcumin: Getting back to the roots. Ann N Y Acad Sci.
1056:206–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Girardi B, Pricci M, Giorgio F, Piazzolla
M, Iannone A, Losurdo G, Principi M, Barone M, Ierardi E and Di Leo
A: Silymarin, boswellic acid and curcumin enriched dietetic
formulation reduces the growth of inherited intestinal polyps in an
animal model. World J Gastroenterol. 26:1601–1612. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Eberhart CE, Coffey RJ, Radhika A,
Giardiello FM, Ferrenbach S and DuBois RN: Up-regulation of
cyclooxygenase 2 gene expression in human colorectal adenomas and
adenocarcinomas. Gastroenterology. 107:1183–1188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Elder DJ, Baker JA, Banu NA, Moorghen M
and Paraskeva C: Human colorectal adenomas demonstrate a
size-dependent increase in epithelial cyclooxygenase-2 expression.
J Pathol. 198:428–434. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Oshima M, Dinchuk JE, Kargman SL, Oshima
H, Hancock B, Kwong E, Trzaskos JM, Evans JF and Taketo MM:
Suppression of intestinal polyposis in Apc delta716 knockout mice
by inhibition of cyclooxygenase 2 (COX-2). Cell. 87:803–809. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Jacoby RF, Seibert K, Cole CE, Kelloff G
and Lubet RA: The cyclooxygenase-2 inhibitor celecoxib is a potent
preventive and therapeutic agent in the min mouse model of
adenomatous polyposis. Cancer Res. 60:5040–5044. 2000.PubMed/NCBI
|
|
114
|
Hao J, Dai X, Gao J, Li Y, Hou Z, Chang Z
and Wang Y: Curcumin suppresses colorectal tumorigenesis via the
Wnt/β-catenin signaling pathway by downregulating Axin2. Oncol
Lett. 21:1862021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
McFadden RM, Larmonier CB, Shehab KW,
Midura-Kiela M, Ramalingam R, Harrison CA, Besselsen DG, Chase JH,
Caporaso JG, Jobin C, et al: The role of curcumin in modulating
colonic microbiota during colitis and colon cancer prevention.
Inflamm Bowel Dis. 21:2483–2494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Adachi S, Hamoya T, Fujii G, Narita T,
Komiya M, Miyamoto S, Kurokawa Y, Takahashi M, Takayama T, Ishikawa
H, et al: Theracurmin inhibits intestinal polyp development in
Apc-mutant mice by inhibiting inflammation-related factors. Cancer
Sci. 111:1367–1374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Seiwert N, Fahrer J, Nagel G, Frank J,
Behnam D and Kaina B: Curcumin administered as micellar solution
suppresses intestinal inflammation and colorectal carcinogenesis.
Nutr Cancer. 73:686–693. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Murakami A, Furukawa I, Miyamoto S, Tanaka
T and Ohigashi H: Curcumin combined with turmerones, essential oil
components of turmeric, abolishes inflammation-associated mouse
colon carcinogenesis. Biofactors. 39:221–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Guo Y, Su ZY, Zhang C, Gaspar JM, Wang R,
Hart RP, Verzi MP and Kong AN: Mechanisms of colitis-accelerated
colon carcinogenesis and its prevention with the combination of
aspirin and curcumin: Transcriptomic analysis using RNA-seq.
Biochem Pharmacol. 135:22–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Femia AP, Soares PV, Luceri C, Lodovici M,
Giannini A and Caderni G: Sulindac, 3,3′-diindolylmethane and
curcumin reduce carcinogenesis in the Pirc rat, an Apc-driven model
of colon carcinogenesis. BMC Cancer. 15:6112015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Lev-Ari S, Strier L, Kazanov D,
Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, Marian B, Lichtenberg D
and Arber N: Celecoxib and curcumin synergistically inhibit the
growth of colorectal cancer cells. Clin Cancer Res. 11:6738–6744.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Meng T, Xiao D, Muhammed A, Deng J, Chen L
and He J: Anti-Inflammatory action and mechanisms of resveratrol.
Molecules. 26:2292021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zheng Z, Chen Y, Huang J, Deng H, Tang X
and Wang XJ: Mkp-1 is required for chemopreventive activity of
butylated hydroxyanisole and resveratrol against colitis-associated
colon tumorigenesis. Food Chem Toxicol. 127:72–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Reddivari L, Charepalli V, Radhakrishnan
S, Vadde R, Elias RJ, Lambert JD and Vanamala JK: Grape compounds
suppress colon cancer stem cells in vitro and in a rodent model of
colon carcinogenesis. BMC Complement Altern Med. 16:2782016.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Saud SM, Li W, Morris NL, Matter MS,
Colburn NH, Kim YS and Young MR: Resveratrol prevents tumorigenesis
in mouse model of Kras activated sporadic colorectal cancer by
suppressing oncogenic Kras expression. Carcinogenesis.
35:2778–2786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Rauf A, Patel S, Imran M, Maalik A, Arshad
MU, Saeed F, Mabkhot YN, Al-Showiman SS, Ahmad N and Elsharkawy E:
Honokiol: An anticancer lignan. Biomed Pharmacother. 107:555–562.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Subramaniam D, Ponnurangam S, Ramalingam
S, Kwatra D, Dandawate P, Weir SJ, Umar S, Jensen RA and Anant S:
Honokiol affects stem cell viability by suppressing oncogenic YAP1
function to inhibit colon tumorigenesis. Cells. 10:16072021.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Chen X, Shi BL, Qi RZ, Chang X and Zheng
HG: Ultra-Performance liquid Chromatography/mass spectrometry-based
metabolomics for discovering potential biomarkers and metabolic
pathways of colorectal cancer in mouse model (ApcMin/+) and
revealing the effect of honokiol. Front Oncol. 11:6710142021.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Bosebabu B, Cheruku SP, Chamallamudi MR,
Nampoothiri M, Shenoy RR, Nandakumar K, Parihar VK and Kumar N: An
appraisal of current pharmacological perspectives of sesamol: A
review. Mini Rev Med Chem. 20:988–1000. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Shimizu S, Fujii G, Takahashi M, Nakanishi
R, Komiya M, Shimura M, Noma N, Onuma W, Terasaki M, Yano T and
Mutoh M: Sesamol suppresses cyclooxygenase-2 transcriptional
activity in colon cancer cells and modifies intestinal polyp
development in Apc (Min/+) mice. J Clin Biochem Nutr. 54:95–101.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Dai G, Jiang Z, Sun B, Liu C, Meng Q, Ding
K, Jing W and Ju W: Caffeic acid phenethyl ester prevents
colitis-associated cancer by inhibiting NLRP3 inflammasome. Front
Oncol. 10:7212020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zheng Y, Liu Z, Yang X, Liu L and Ahn KS:
An updated review on the potential antineoplastic actions of
oleuropein. Phytother Res. 36:365–379. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Giner E, Recio MC, Rios JL, Cerda-Nicolas
JM and Giner RM: Chemopreventive effect of oleuropein in
colitis-associated colorectal cancer in c57bl/6 mice. Mol Nutr Food
Res. 60:242–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yang J, Chen B and Gu Y: Pharmacological
evaluation of tea polysaccharides with antioxidant activity in
gastric cancer mice. Carbohydr Polym. 90:943–947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ahmad N, Cheng P and Mukhtar H: Cell cycle
dysregulation by green tea polyphenol epigallocatechin-3-gallate.
Biochem Biophys Res Commun. 275:328–334. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Rashidi B, Malekzadeh M, Goodarzi M,
Masoudifar A and Mirzaei H: Green tea and its anti-angiogenesis
effects. Biomed Pharmacother. 89:949–956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Xiao H, Hao X, Simi B, Ju J, Jiang H,
Reddy BS and Yang CS: Green tea polyphenols inhibit colorectal
aberrant crypt foci (ACF) formation and prevent oncogenic changes
in dysplastic ACF in azoxymethane-treated F344 rats.
Carcinogenesis. 29:113–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wang ST, Cui WQ, Pan D, Jiang M, Chang B
and Sang L X: Tea polyphenols and their chemopreventive and
therapeutic effects on colorectal cancer. World J Gastroenterol.
26:562–597. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Volate SR, Muga SJ, Issa AY, Nitcheva D,
Smith T and Wargovich MJ: Epigenetic modulation of the retinoid X
receptor alpha by green tea in the azoxymethane-Apc Min/+ mouse
model of intestinal cancer. Mol Carcinog. 48:920–933. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wu X, Xue L, Tata A, Song M, Neto CC and
Xiao H: Bioactive components of polyphenol-rich and
non-polyphenol-rich cranberry fruit extracts and their
chemopreventive effects on colitis-associated colon cancer. J Agric
Food Chem. 68:6845–6853. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Koh SJ, Choi YI, Kim Y, Kim YS, Choi SW,
Kim JW, Kim BG and Lee KL: Walnut phenolic extract inhibits nuclear
factor kappaB signaling in intestinal epithelial cells, and
ameliorates experimental colitis and colitis-associated colon
cancer in mice. Eur J Nutr. 58:1603–1613. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Fini L, Piazzi G, Daoud Y, Selgrad M,
Maegawa S, Garcia M, Fogliano V, Romano M, Graziani G, Vitaglione
P, et al: Chemoprevention of intestinal polyps in ApcMin/+ mice fed
with western or balanced diets by drinking annurca apple polyphenol
extract. Cancer Prev Res (Phila). 4:907–915. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Song M, Emilsson L, Bozorg SR, Nguyen LH,
Joshi AD, Staller K, Nayor J, Chan AT and Ludvigsson JF: Risk of
colorectal cancer incidence and mortality after polypectomy: A
Swedish record-linkage study. Lancet Gastroenterol Hepatol.
5:537–547. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Keum N and Giovannucci E: Global burden of
colorectal cancer: Emerging trends, risk factors and prevention
strategies. Nat Rev Gastroenterol Hepatol. 16:713–732. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Sankaranarayanan R, Valiveti CK, Kumar DR,
Van Slambrouck S, Kesharwani SS, Seefeldt T, Scaria J, Tummala H
and Bhat GJ: The Flavonoid metabolite 2,4,6-trihydroxybenzoic acid
is a CDK inhibitor and an anti-proliferative agent: A potential
role in cancer prevention. Cancers (Basel). 11:4272019. View Article : Google Scholar : PubMed/NCBI
|