Introduction
Cancer is a prominent cause of morbidity and
mortality worldwide, and hepatocellular carcinoma (HCC) is the
third leading cause of cancer-related mortality (1,2).
Autophagy is generally understood to be a process that serves to
carry cytoplasmic cargo to lysosomes for degradation, which plays a
major role in eukaryotic cells and mammalian survival, as well as
in cellular homeostasis, development, tumorigenesis and infection
(3,4). There are three main types of
autophagy: Microautophagy, macroautophagy and chaperone-mediated
autophagy (5). Among the three
types of autophagy, macroautophagy, generally referred to as
‘autophagy’, is the most critical and most extensively studied
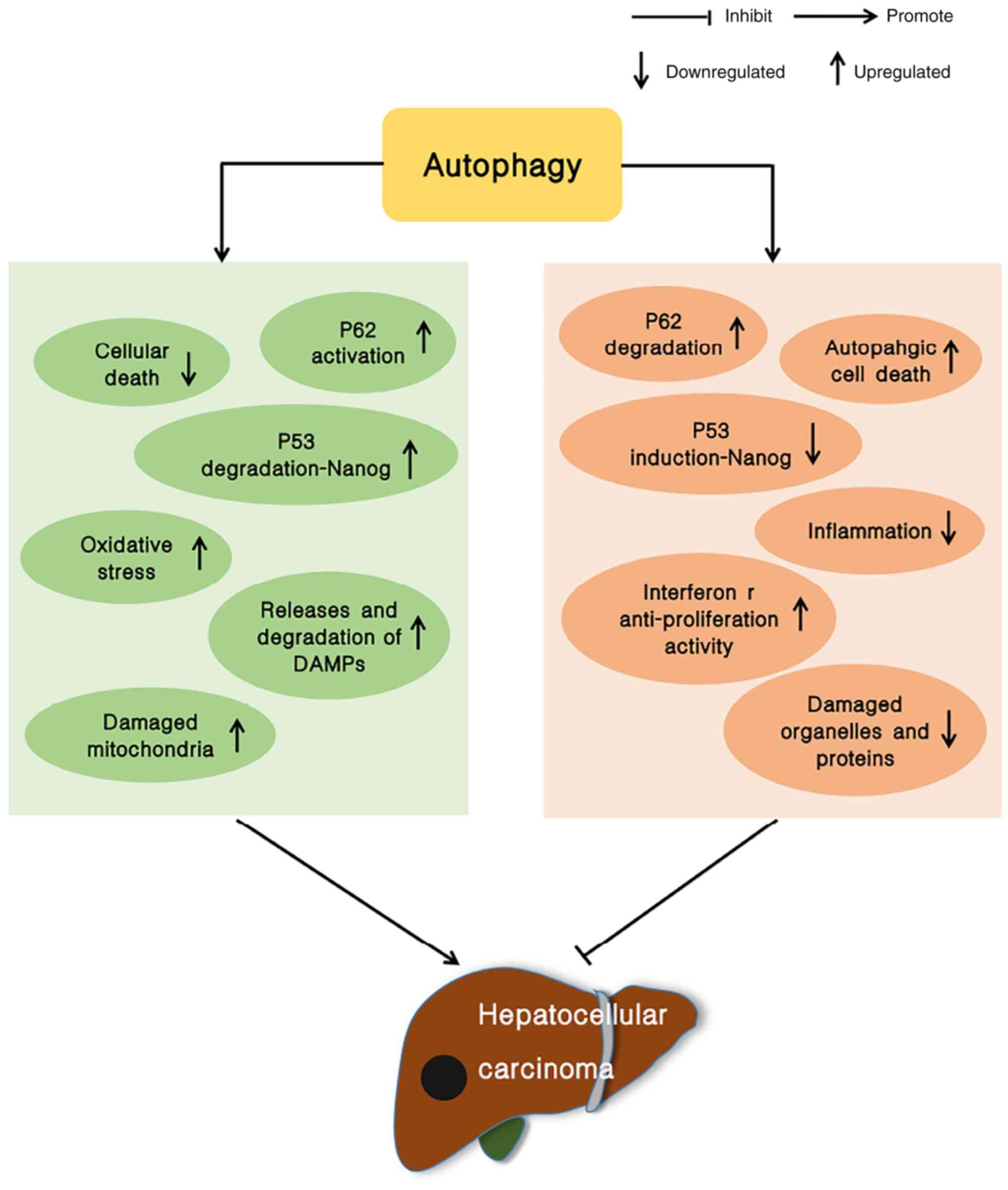
form. There is evidence to indicate that autophagy plays a role in
inhibiting the growth of tumors, particularly in the liver. In
addition to the typical function of autophagy as an inhibitor of
tumor development in non-tumor cells and in early-stage tumor
development, autophagy also enhances tumor cell survival once the
tumor has formed (5–8) (Fig.
1). Therefore, the inhibition of autophagy has become a novel
strategy for anticancer therapeutics (9).
RNAs are transcription products of DNA, and they
encompass non-coding RNAs (ncRNAs) and coding RNAs. Coding RNAs
include messenger RNAs (mRNAs), which serve as a template for
protein biosynthesis (10). ncRNAs
are transcripts without protein-coding potential that have multiple
biological functions, and they modulate gene expression at multiple
levels, affecting processes such as RNA processing, transcription
and translation (11). ncRNAs can
be categorized into small ncRNAs (sncRNAs), circular RNAs
(circRNAs) and lncRNAs (12). The
classification of RNAs and their respective roles are summarized in
Table I.
 | Table I.Classifications and functions of
RNAs. |
Table I.
Classifications and functions of
RNAs.
|
Classifications | Functions | (Refs.) |
|---|
| Coding RNAs |
|
|
|
mRNAs | Acting as template
for protein biosynthesis | (10) |
| ncRNAs |
|
|
|
lncRNAs | Modulating protein
localization, mRNA translation and stability in the cytoplasm,
taking part in chromatin modification, transcription and
post-transcriptional adjustment of gene expression, regulating
tumor growth, metastasis and invasion in vitro | (18–20) |
|
circRNAs | Specifically
binding miRNA to increase the expression level of target genes,
regulating transcription, promoting or inhibiting the occurrence of
cancer | (123–125) |
|
sncRNAs |
|
|
|
snRNAs | Catalyzing the
splicing of precursor mRNA in the spliceosome, participating in the
maturation of mRNA, promoting the development of malignant
tumors | (126,127) |
|
snoRNAs | Regulating
post-transcriptional modification and processing of ribosomal RNA
(rRNA) and other RNAs, improving the fidelity and efficiency of
translation, promoting or inhibiting tumorigenesis in various types
of cancer | (126,128–130) |
|
siRNAs | Regulating gene
silencing and mRNA degradation, inhibiting transcription | (131,132) |
|
miRNAs | Negatively
regulating gene expression, modulating apoptosis, proliferation,
survival and metastasis of cancer cells | (13,15) |
|
piRNAs | Silencing
transposons, promoting or inhibiting tumorigenesis in tumor
tissues | (126,133,134) |
|
Transfer RNAs | Involving in
protein translation, promoting the development of cancer | (126,135) |
As a type of sncRNA of ~19–25 nucleotides in length,
microRNAs (miRNAs/miRs), have been found to be involved in the
negative regulation of gene expression by base pairing with the 3′
untranslated region (UTR) of mRNAs. miRNAs are usually abnormally
expressed in tumor cells and can control the apoptosis,
proliferation, survival and metastasis of cancer cells (13–15).
lncRNAs are RNAs >200 nucleotides in length (16), and the majority of lncRNAs are
transcribed by RNA Pol II (17). A
myriad of functional roles have been attributed to lncRNAs, such as
for example, acting in the cytoplasm to modulate protein
localization and stability and mRNA translation (18), affecting chromatin modification, the
transcription and post-transcriptional processing of transcribed
genes (19), and regulating tumor
growth, metastasis and invasion in vitro (20). As evidenced by recent discoveries,
some miRNAs and lncRNAs have been linked to the occurrence,
development, migration, invasion and resistance of HCC cells, due
to their association with autophagy, suggesting their potential to
modulate autophagy in HCC. For example, miR-26b has been shown to
enhance the sensitivity of HCC cells to doxorubicin (Dox) by
inhibiting Dox-induced autophagy (21). miR-181a has been found to promote
tumor growth and reduce the apoptosis of HCC cells by inhibiting
autophagy (22). The lncRNA
neighbor of BRCA1 gene 2 (NBR2) suppresses HCC cell proliferation
by inhibiting Beclin-1-dependent autophagy (23). miR-30a accelerates the metastasis
and recurrence of HCC by promoting autophagy (24). Therefore, miRNAs and lncRNAs play
essential roles in the progression of HCC. The present review
summarizes the mechanisms and roles of related miRNAs and lncRNAs
in regulating autophagy in HCC in an aim to provide insight into
their potential role as therapeutic targets for HCC.
miRNAs involved in the regulation of
autophagy in HCC
The diverse functions of miRNAs include mediating
HCC cell growth, metastasis, autophagy and resistance to drugs, and
the association between certain miRNAs and the prognosis of HCC
patients is significant. Some miRNAs are abnormally expressed in
HCC, and this abnormal expression results in various effects. Some
miRNAs involved in regulatory processes and the relevant mechanisms
of action are summarized below (Table
II). This information will hopefully aid the identification of
novel targets for HCC treatment.
 | Table II.Role of miRNAs in the regulation of
autophagy in HCC. |
Table II.
Role of miRNAs in the regulation of
autophagy in HCC.
| miRNAs | Expression level in
HCC | Pathway of action
or targets | Regulation of
autophagy | Function in
HCC | (Refs.) |
|---|
| miR-541 | Low | ATG2A and
RAB1B | Promotes | Promoting
proliferation, migration and invasion | (28) |
| miR-490-3p | Low | ATG7 | Promotes | Promoting
proliferation | (31) |
| miR-142-3p | Low | ATG5 and
ATG16L1 | Promotes | Reducing
sensitivity to sorafenib | (33) |
| miR-223 | Low | FOXO3a | Promotes | Reducing
sensitivity to adriamycin | (39) |
| miR-375 | Low | ATG7 | Promotes | Inhibiting
apoptosis | (42) |
| miR-26 | Low | ULK1 | Promotes | Inhibiting
apoptosis, increasing resistance to doxorubicin | (45) |
| miR-101 | Low | RAB5A, STMN1 and
ATG4D | Promotes | Promoting
resistance to cisplatin | (49) |
| miR-101 | Low | EZH2 | Promotes | Promoting
proliferation, inhibiting apoptosis and promoting resistance to
chemotherapy drugs | (48) |
| miR-7 | Low | ATG5 | Promotes | Promoting invasion
and migration | (52) |
| miR-30a | Low | Beclin 1 and
ATG5 | Promotes | Promoting
recurrence and migration | (24) |
| miR-559 | Low | PARD3 | Promotes | Promoting
proliferation | (55) |
| miR-513b-5p | Low | PIK3R3 | Promotes | Promoting
proliferation | (56) |
| miR-125b | Low | EVA1A | Promotes | Promoting cell
proliferation, invasion and EMT, increasing resistance to
oxaliplatin | (58) |
| miR-34a | Low | BACH1 | Inhibits | Promoting the
metastasis and invasion | (59) |
| miR-199a-5p | Low | ATG7 | Promotes | Increasing
resistance to cisplatin | (61) |
| miR-26b | Low | USP9X/P53 | Promotes | Reducing
sensitivity to doxorubicin | (21) |
| miR-1307 | High | CALR-OSTC
endoplasmic reticulum protein folding pathway | Inhibits | Promoting
proliferation | (65) |
| miR-181a | High | ATG5 | Inhibits | Promoting
proliferation and inhibiting apoptosis | (22) |
| miR-193a-3p | High | TGF-β2 | Inhibits | Promoting
apoptosis | (67) |
| miR-25 | High | FBXW7 | Promotes | Increasing
resistance to sorafenib | (68) |
| miR-4790-3p | High | ZNF225 | Promotes | Inhibiting
apoptosis | (69) |
Low expression of miRNAs in HCC
miR-541
miR-541, a newly identified miRNA cluster, lies in a
gene containing a large number of miRNAs (Mirg) within the DLK-DIO3
locus (25). The proliferation,
invasion and migration of osteosarcoma and squamous cell lung
cancer cells has been shown to be decreased with the increased the
expression of miR-541 (26,27). Furthermore, miR-541 limits HCC
occurrence and the autophagy of HCC cells by downregulating
autophagy-related gene (ATG)2A and Ras-related protein (RAB)1B
(28). As previously demonstrated,
low levels of miR-541 increase autophagy and promote proliferation,
invasion and migration, and a low expression of miR-541 is
associated with a poor prognosis of patients with HCC. Similarly, a
high expression of miR-541 indicates the superior sensitivity of
HCC to sorafenib treatment (28).
miR-490-3p
miR-490-3p is located on chromosome 7q33 in the
second intron of CHRM2 and consists of 22 nucleotides. Research has
indicated that miR-490-3p can decrease the metastasis and growth of
lung adenocarcinoma cells and gastric cancer cells (29,30).
In HCC, miR-490-3p can suppress autophagy in HCC cells by targeting
ATG7, thus decreasing proliferation and stalling the cell cycle,
and an increased miR-490-3p expression indicates a good prognosis
(31).
miR-142-3p
miR-142-3p is located on human chromosome 17q22 and
is a member of the miR-142 family (32,33).
miR-142-3p inhibits the tumorigenesis of colorectal cancer by
targeting β-catenin and suppresses the occurrence of breast cancer
(34,35). A previous study confirmed that the
decreased expression of miR-142-3p accelerated sorafenib-induced
HCC cell autophagy through the upregulation of ATG5 and ATG16L1,
accordingly reducing HCC cell sensitivity to sorafenib. Conversely,
the increased expression of miR-142-3p enhanced the sensitivity of
HCC cells to sorafenib (33).
miR-223
The miR-223 gene, positioned on Xq12, is regulated
by transcription factors, including NFI-A, PU.1 and C/EBPs. miR-233
is a pivotal factor influencing the evolution and homeostasis of
the immune system, as for example, modulating specific inflammatory
reactions (36,37). In addition, it can also increase the
proliferation of breast cancer cells, induce carcinogenic effects
in gastric cancer, and promote metastasis and drug resistance in
gastric cancer (38). A low
expression of miR-223 has been shown to promote the
doxorubicin-induced autophagy of HCC cells by targeting FOXO3a,
leading to the reduced sensitivity of HCC cells to doxorubicin.
However, the overexpression of miR-223 has been shown to enhance
the efficacy of doxorubicin in HCC treatment (39).
miR-375
miR-375 is an originally described β-cell-specific
miRNA that has a multifunctional regulatory role in immunity and
inflammation (40). Moreover,
miR-375 is considered to function as a tumor inhibitor in the
majority of cancer types, such as in gastric and colon cancer, and
it inhibits cancer occurrence and metastasis (40,41).
The overexpression of miR-375 has been found to suppress autophagy
under hypoxic conditions by preventing the conversion of LC3I into
LC3II in HCC cells, and reducing ATG7 expression, leading to a
reduction in HCC cell viability (42).
miR-26
The miR-26 family is a group of widely conserved
small RNAs with the same sequence in the seed region. Previous
studies have illustrated that the target genes of miR-26 have
several roles, including modulating cell metabolism, apoptosis,
differentiation, proliferation, metastasis and invasion (43,44).
miR-26 knockout facilitates autophagy by augmenting the expression
of the autophagy promoter, unc-51 like autophagy activating kinase
1, represses cell apoptosis in vivo, and induces HCC
tolerance to Dox (45).
miR-101
miR-101 is located on chromosomes 1 and 9. It serves
critical functions in proliferation, drug resistance, angiogenesis,
apoptosis, metastasis and invasion in multiple cancer types
(46,47). miR-101 inhibits HCC progression by
targeting enhancer of Zeste homolog 2 in HCC tissues and sensitizes
HCC cells to chemotherapeutic drugs (48). Furthermore, miR-101 overexpression
inhibits autophagy by exerting effects on RAB5A, stathmin 1, ATG4D
and other targets, and induces the apoptosis of HepG2 cells in
cooperation with cisplatin, suggesting that miR-101 enhances HepG2
cell sensitivity to cisplatin. A low miR-101 expression has the
opposite effects (48,49).
miR-7
miR-7 is an ancient miRNA (50), that is encoded by three genomic loci
(9q21, 19q13 and 15q26) (51). It
mainly serves as a tumor inhibitor and regulates diverse signaling
pathways, for example, inhibiting cancer cell proliferation,
survival and migration, but stimulating apoptosis by downregulating
the PI3K and MAPK pathways (50).
In HCC tissues, the low expression of miR-7 upregulates ATG5
expression, leading to accelerated autophagy, and the resulting
response promotes the metastasis and invasion of HCC cells
(52).
miR-30a
As a tumor inhibitor, miR-30a is an intronic class
miRNA seated on chromosome 6 (53).
miR-30a has been found to modulate numerous biological processes
related to apoptosis, proliferation, metastasis, invasion and drug
sensitivity (54). miR-30a is
related to vascular infiltration, metastatic potential and disease
recurrence in HCC, and its decreased expression promotes autophagy
by modulating Beclin-1 and ATG5, thereby promoting the metastasis
and recurrence of HCC (24).
miR-559
The low expression of miR-559 can inhibit HCC
processes. Par-3 family cell polarity regulator (PARD3) regulates
cell metastasis and proliferation in a number of cancer types.
Research has indicated that miR-559 can suppress the growth of HCC
by suppressing PARD3 expression to inhibit autophagy, thus
demonstrating that miR-559 has potential as a target for HCC
treatment (55).
miR-513b-5p
miR-513b-5p is an miRNA that is downregulated in HCC
cells, and phosphoinositide-3-kinase regulatory subunit 3 (PIK3R3)
is an oncogene. miR-513b-5p inhibits PIK3R3 expression by targeting
it, thereby inhibiting autophagy during HCC malignant progression.
Therefore, miR-513b-5p may be a potential therapeutic target for
HCC (56).
miR-125b
miR-125b is located on chromosome 21q21 and is part
of the miR-125 family; it plays a crucial role in cancer occurrence
and development (57). Research has
suggested that miR-125b is expressed in low levels in
oxaliplatin-resistant HCC cells, and miR-125b overexpression
inhibits invasion, proliferation and epithelial-mesenchymal
transition (EMT), indicating that miR-125b may enhance the
sensitivity of cells to oxaliplatin. Mechanistically, miR-125b
inhibits EMT and autophagy by downregulating Eva-1 homolog A,
thereby reducing resistance to oxaliplatin in patients with liver
cancer (58).
miR-34a
miR-34a, a member of the miR-34 family, is located
on chromosome 1q36.22 (59).
Ten-eleven translocation 1, a DNA demethylase, can catalyze miR-34a
demethylation, thereby activating miR-34a. miR-34a suppresses BTB
domain and CNC homology 1 levels, thus activating the p53 pathway,
ultimately promoting autophagy in and repressing metastasis and
invasion of HCC cells (59).
miR-199a-5p
miR-199a-5p belongs to the miR-199a family and
functions as a tumor inhibitor in lung cancer (60). miR-199a-5p expression has been found
to be markedly decreased in patients with HCC receiving cisplatin
chemotherapy. Cisplatin-induced miR-199a-5p downregulation
activates autophagy by targeting ATG7, thus promoting HCC
resistance to cisplatin (61).
miR-26b
miR-26b is encoded in 9p21.3, a vulnerable site in
the genome (21). miR-26b
expression has been shown to be decreased in HCC tissues treated
with Dox. miR-26b promotes p53 degradation by reducing
ubiquitin-specific protease-9 expression and inhibiting autophagy
induced by Dox, thereby enhancing HCC sensitivity to Dox (21).
High expression of miRNAs in HCC
miR-1307
miR-1307, a gene on human chromosome 10 (62), can regulate ovarian cancer
resistance to chemotherapy and increase the proliferation of
prostate cancer cells (63,64). miR-1307 suppresses HCC cell
autophagy and accelerates the malignant progression of HCC through
the Calr-OSTC endoplasmic reticulum protein folding pathway
(65).
miR-181a
Research has demonstrated that miR-181a can repress
autophagy in a variety of cancer types. In HCC, miR-181a expression
is high. Luciferase analysis has suggested that ATG5 is a target of
miR-181a. miR-181a can suppress autophagy in HCC cells by targeting
ATG5, which reduces HCC cell apoptosis and increases tumor growth
(22).
miR-193a-3p
miR-193a-3p is located on chromosome 17 and
functions as a tumor suppressor gene in the majority of cancer
types (66). In HCC, miR-193a-3p is
regulated by mitogen-inducible gene 6 (Mig-6), a tumor inhibitor
gene. TGF-β2 is a target of miR-193a-3p. Mig-6 decreases the TGF-β2
level by positively modulating miR-193a-3p and thus promotes
apoptosis and suppresses autophagy in HCC (67).
miR-25
miR-25 expression is upregulated in HCC tissues and
is associated with the clinical stage, lymph node metastasis and
pathological grade. miR-25 promotes HCC resistance to sorafenib by
reducing F-box and WD repeat domain containing 7 protein expression
to activate autophagy. Therefore, miR-25 may be a novel target for
HCC therapy (68).
miR-4790-3p
At present, there are few studies available on
miR-4790-3p. As previously demonstrated, in patients with HCC
treated with a combination of everolimus and Ku0063794, miR-4790-3p
expression is markedly decreased, and the expression of zinc finger
protein 225 (ZNF225), which is a target of miR-4790-3p, is
significantly increased. The downregulation of miR-4790-3p
suppresses autophagy by promoting ZNF225 expression, thereby
reducing HCC cell survival (69).
lncRNAs involved in the regulation of
autophagy in HCC
Previous studies have demonstrated that lncRNAs are
vital for processes related to the occurrence and development of
HCC [e.g., autophagy, drug resistance, malignant progression and
hypoxia/reoxygenation (H/R) damage in HCC cells] (70–73).
lncRNAs can also serve as biomarkers for predicting the survival
and recurrence rates of various types of cancer. lncRNAs have
different expression levels in HCC and thus play differential
roles. Below, the mechanisms through which some lncRNAs are
modulated in HCC and their mechanisms are summarized, providing
insight into the prevention and treatment of HCC (Table III).
 | Table III.Role of lncRNAs in the regulation of
autophagy in HCC. |
Table III.
Role of lncRNAs in the regulation of
autophagy in HCC.
| miRNAs | Expression level in
HCC | Pathway of action
or targets | Regulation of
autophagy | Function in
HCC | (Refs.) |
|---|
| MEG3 | Low | PI3K/Akt/mTOR | Promote | Inhibiting
apoptosis | (75,77) |
| RP11-295G20.2 | High | PTEN | Inhibit | Promoting
proliferation | (79) |
| NEAT1 | High | miR-204/ATG3 | Promote | Increasing
resistance to sorafenib | (81) |
| DCST1-AS1 | High | AKT/mTOR signaling
pathway | Inhibit | Promoting
proliferation and invasion, inhibiting apoptosis | (70) |
| HCG11 | High |
miR-26a-5p/ATG12 | Promote | Promoting
proliferation and metastasis, inhibiting apoptosis | (85) |
| CCAT1 | High |
miR-181a-5p/ATG7 | Promote | Promoting
proliferation | (87) |
| MCM3AP-AS1 | High | miR-455/EGFR | Promote | Promoting
metastasis | (89) |
| SNHG1 | High | SLC3A2/Akt
pathway | Inhibit | Promoting
resistance to sorafenib | (92) |
| LINC00160 | High | miR-132/PIK3R3 | Promote | Inhibiting
apoptosis and promoting drug resistance | (71) |
| PVT1 | High | miR-365/ATG3 | Promote | Promoting
proliferation | (97) |
| HAGLROS | High | miR-5095 | Promote | Promoting
proliferation, inhibiting apoptosis | (101) |
| HULC | High |
miR-383-5p/VAMP2 | Promote | Promoting
proliferation, inhibiting apoptosis and chemotherapy sensitivity to
oxaliplatin | (103) |
| HULC | High | USP22/Sirt1 | Promote | Inhibiting
chemotherapy sensitivity | (104) |
| SNHG16 | High |
miR-23b-3p/EGR1 | Promote | Maintaining
resistance to sorafenib | (107) |
| H19 | High | PI3K-Akt-mTOR
pathway | Promote | Inducing
hypoxia/reoxygenation (H/R) injury | (73) |
| LINC00665 | High |
miR-186-5p/MAP4K3 | Inhibit | Promoting
proliferation, inhibiting apoptosis | (110) |
| HIF1A-AS1 | High | HIF-1α/mTOR | Inhibit | Promoting
proliferation | (111) |
| HNF1A-AS1 | High | miR-30b/ATG5 | Promote | Promoting
proliferation, inhibiting apoptosis | (113) |
| DANCR | High |
miR-222-3p/ATG7 | Promote | Promoting
proliferation | (115) |
| ATB | High | ATG5 | Promote | Promoting
proliferation | (117) |
| MALAT1 | High | miR-146a/PI3K | Inhibit | Promoting
proliferation, inhibiting apoptosis | (118) |
| CCAT2 | High | miR-4496/ATG5 | Promote | Promoting migration
and invasion | (120) |
| MALAT1 | High | miR-216b | Promote | Increasing MDR | (119) |
| NEAT1v1 | High | GABARAP | Promote | Increasing
radiation resistance | (82) |
| BANCR | High |
miR-590-5P/OLR1 | Promote | Decreasing
sensitivity to sorafenib | (122) |
| NBR2 | Low | ERK/JNK | Promote | Promoting
proliferation | (23) |
Low expression of lncRNAs in HCC
Maternally expressed gene 3 (MEG3)
As a novel tumor suppressor, MEG3 is an imprinted
gene located on chromosome 14q32. Interleukin enhancer-binding
factor 3 (ILF3) is a MEG3 conjugate protein. Compared with normal
liver cells, HCC cells have a markedly lower expression of MEG3.
Adenosine is a nucleotide metabolite with significant cytotoxicity
that can induce apoptosis and reduce cell viability and migration.
In addition, adenosine inhibits autophagy in HepG2 cells and
stimulates MEG3 expression. The overexpression of MEG3 decreases
the expression of ILF3 in HepG2 cells, and the downregulation of
ILF3 can also inhibit autophagy by decreasing Beclin-1 expression.
In general, the overexpression of MEG3 can activate the
PI3K/Akt/mTOR pathway by downregulating ILF3 and inactivating the
Beclin-1 signaling pathway to inhibit autophagy, thus increasing
the cytotoxic effects against HCC cells (74–77).
NBR2
NBR2, a long intergenic ncRNA, is located near the
BRCA1 gene on human chromosome 17q21 and affects the biological
functions and drug resistance of various types of cancer (78). It has been reported that the higher
the expression of NBR2, the lower the malignant degree of HCC
cells. The lower expression of lncRNA NBR2 can promote HCC cell
proliferation by inducing Beclin 1-dependent autophagy (23). It is thus clear that NBR2 could
serve as a therapeutic target for HCC.
High expression of lncRNAs in HCC
RP11-295G20.2
RP11-295G20.2, which is 465 nucleotides in length,
is primarily located in the cytoplasm with very small coding
potential in HCC cells and functions as an oncogene in HCC and
other types of cancers (79).
RP11-295G20.2 inhibits autophagy to fuel HCC cell proliferation
in vitro and in vivo, and is associated with
recurrence in patients (79).
Phosphatase and tensin homolog (PTEN) is a key tumor suppressor.
RP11-295G20.2 and the N-terminus of PTEN can be conjugated to
promote the interaction between p62 and PTEN, and this interaction
induces lysosomal degradation and changes PTEN expression in HCC
cells, ultimately resulting in the transcription of ATGs downstream
of the PTEN/Akt/FOXO3a signaling pathway (79).
Nuclear enriched abundant transcript 1
(NEAT1)
NEAT1 is upregulated in several types of human
cancer. Accumulating evidence suggests that NEAT1 promotes cell
growth, invasion and migration, whereas it inhibits apoptosis
(80). The overexpression of NEAT1
facilitates autophagy and increases HCC resistance to sorafenib by
modulating miR-204 to increase ATG3 expression (81). NEAT1 variant 1 (NEAT1v1), a variant
of NEAT1, participates in maintaining cancer stem cells (CSCs) in
HCC. CSCs play a crucial role in drug resistance. Evidence has
illustrated that NEAT1v1 promotes autophagy through
gamma-aminobutyric acid receptor-associated protein, thereby
conferring radioresistance to HCC cells (82).
DCST1-AS1
DCST1-AS1, as a lncRNA, has the capacity to
accelerate the migration and invasion of triple-negative breast
cancer cells (83). The increased
expression of DCST1-AS1 is also associated with a poor prognosis of
patients with HCC. Functioning via the Akt/mTOR signaling pathway,
DCST1-AS1 not only promotes the proliferation and invasion of HCC
cells, but also represses their apoptosis and autophagy (70).
HLA complex group 11 (HCG11)
HCG11 is an HCG gene located on chromosome 6p22.2
upstream of MHC I. HCG11 promotes or inhibits the migration,
proliferation, apoptosis and invasion and cell cycle progression of
tumor cells (84). HCG11 expression
is increased in HCC tissues and cells, and the higher the HCG11
expression is, the poorer the prognosis of HCC patients. HCG11 is
required for the metastasis, proliferation and autophagy of HCC
cells, and suppresses apoptosis by enhancing ATG12 expression via
miR-26a-5p in HCC tissues (85).
Colon cancer-associated transcript 1
(CCAT1)
CCAT1 is located on chromosome 8q24.2 and is 2,628
nucleotides in length. In addition to influencing tumor cell
proliferation, migration, proliferation and apoptosis, CCAT1 is
related to chemotherapeutic resistance (86). In HCC, CCAT1 functions as a sponge
for miR-181A-5p to modulate ATG7 expression and thus promote the
autophagy and proliferation of HCC cells (87).
MCM3AP-AS1
MCM3AP-AS1 is located on chromosome 21 and can
promote or inhibit tumor progression (88). When the MCM3AP-AS1 gene is knocked
down, miR-455 expression is sharply upregulated in HCC cells;
furthermore, miR-455 targets epidermal growth factor receptor
(EGFR) and modulates autophagy. MCM3AP-AS1 overexpression decreases
miR-455 expression, subsequently affecting EGFR expression and
increasing autophagy, thus promoting the metastasis of HCC cells
(89).
Small nucleolar RNA host gene 1
(SNHG1)
SNHG1, a gene on chromosome 11q12.3, has 11 exons
(90). SNHG1 can regulate tumor
cell proliferation, apoptosis, invasion and migration, as well as
other intracellular functions (91). The overexpression of SNHG1 activates
the Akt pathway by regulating solute carrier family 3 member 2,
thereby restraining autophagy and increasing HCC resistance to
sorafenib (92).
LINC00160
Detection of subcellular localization using
fluorescence in situ hybridization has suggested that long
intergenic non-protein coding RNA 00160 (LINC00160) is localized in
the cytoplasm (71) and can mediate
chemotherapeutic drug resistance in breast cancer cells and renal
cell carcinoma (93,94). The overexpression of LINC00160
activates autophagy by affecting miR-132 targeting of PIK3R3, and
increases the viability and drug resistance but inhibits the
apoptosis of HCC cells (71).
Plasmacytoma variant translocation 1
(PVT1)
PVT1 is located in the 8q24 chromosome band
(95); it is related to the
occurrence and development of cancers and may be a prognostic
biomarker (96). PVT1 induces the
proliferation and autophagy of HCC cells, as it upregulates ATG3
expression through miR-365 (97).
HAGLROS
HAGLROS is a lncRNA (699 bp) encoding only one
transcript that is associated with the malignant progression of
gastric, lung and nasopharyngeal cancer (98–100).
The overexpression of HAGLROS increases autophagy by markedly
increasing the total quantity of autolysosomes and Beclin-1,
LC3II/LC3I and LC3II levels, and decreasing p62 expression levels.
Mechanistically, HAGLROS modulates ATG12 expression in a
miR-5095-dependent manner in Huh7 cells, ultimately increasing cell
proliferation and autophagy and inhibiting apoptosis (101).
Highly upregulated in liver cancer
(HULC)
The HULC gene is located on chromosome 6p24.3 and is
~500 nucleotides in length. HULC overexpression is found in many
cancer types and is linked to metastasis, increased tumor size and
poor prognosis. (102) HULC
functions as a factor regulating HCC cell autophagy, subsequently
promoting malignant progression of HCC, and decreased HCC cell
sensitivity to oxaliplatin can be induced by increasing
LC3II-dependent silent information regulator 1 expression in human
HCC (72,103–105).
Small nucleolar RNA host gene 16
(SNHG16)
SNHG16, located on 17q25.1, is a member of the
lncRNA SNHG family; it contains four exons and has 13 splice
variants. SNHG16 plays a major role in cell migration,
proliferation and invasion in multiple types of cancer, including
lung, prostate and breast cancer (106). The poor prognosis of patients with
HCC is associated with the increased expression of SNHG16. The
overexpression of SNHG16 inhibits miR-23b-3p expression by
upregulating early growth response 1, increasing the viability and
autophagy of Hep3B/So cells, and inhibiting apoptosis to maintain
resistance to sorafenib (107).
H19
H19, a 2.7-kb gene located near the telomere region
of chromosome 11p15.5, is expressed by maternal and paternal cell
lines, and tumor formation and tumor cell proliferation and
migration are related to H19 (108). H19 is highly expressed in HCC
cells (HepG2 and HCCLM3). The function of H19 in eliciting H/R
injury to HCC cells is mainly based on the upregulation of
autophagy induced by activating the PI3K/Akt/mTOR pathway (73).
LINC00665
Long intergenic non-protein coding RNA 665
(LINC00665), located on chromosome 19q13.12, is dysregulated in
various types of cancer and influences the proliferation, apoptosis
and metastasis of cancer cells (109). LINC00665 expression is increased
in HCC and is negatively associated with overall survival (OS).
Patients with higher LINC00665 levels have a shorter OS than those
with lower LINC00665 levels. Moreover, the silencing of LINC00665
inhibits tumor growth, and induces autophagy and apoptosis via the
miR-186-5p/MAP4K3 axis (110).
HIF1A-AS1
lncRNA HIF1A-AS1, located on the antisense strand of
the hypoxia inducible factor 1α (HIF-1α) gene, is highly expressed
in HCC and is associated with lymph node metastasis, tumor size,
TNM stage and OS. In a previous study, the OS was shorter in the
higher HIF1A-ASP1 expression group than in the lower HIF1A-ASP1
expression group. HIF1A-AS1 can promote the progression of HCC by
reducing HIF-1α/mTOR-mediated autophagy (111).
HNF1A-AS1
HNF1A-AS1, located on chromosome 12, is considered
to be a prognostic and diagnostic marker in multiple types of
cancer (112). HNF1A-AS1 is often
upregulated in HCC, and a high expression of HNF1A-AS1 is
associated with tumor size, poor differentiation, multiple tumors
and an advanced TNM stage. HNF1A-AS1 facilitates HCC cell growth
and inhibits apoptosis via the induction of Bcl-2 expression by
inhibiting miR-30b. ATG5 is targeted by miR-30b, and HNF1A-AS1 can
promote autophagy by inhibiting miR-30b targeting by ATG5 (113).
Differentiation antagonizing
nonprotein coding RNA (DANCR)
DANCR, located on chromosome 4, is a
tumor-associated lncRNA (114).
DANCR expression is high in HCC and miR-222-3p expression is low.
DANCR increases ATG7-induced autophagy and cell proliferation by
inhibiting miR-222-3p. To a certain extent, the higher DANCR
expression is, the poorer the prognosis of patients with HCC
(115).
ATB
ATB is located on chromosome 14 and affects
biological functions in a variety of cancer types (116). In HCC tissues, ATB expression is
high, and ATB expression is positively associated with TNM stage,
survival rate and tumor size in patients with HCC. ATB promotes
autophagy by activating YAP and increasing ATG5 expression, and the
overexpression of ATB increases HCC cell proliferation (117). However, whether the effects of ATB
on the proliferation of HCC cells are mediated through autophagy
remains unclear.
Metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1)
MALAT1 can promote the malignant progression of
cancers, including HCC. MALAT1 expression is upregulated in HCC.
MALAT1 induces PI3K expression by downregulating miR-146a
expression, thereby activating downstream Akt and mTOR, and
ultimately promoting HCC cell proliferation, but inhibiting
autophagy and apoptosis (118). In
addition, MALAT1 can be upregulated by HIF-2α, thereby reducing the
expression of miR-216b to promote autophagy and increase the
multidrug resistance of HCC cells (119).
CCAT2
The locus of CCAT2 is located on chromosome 8q24.21.
In HCC tissues, the expression of CCAT2 is markedly increased, and
an increased expression of CCAT2 is associated with an advanced
stage, as well as with venous infiltration. CCAT2 exerts
differential effects in the nucleus and cytoplasm. In the
cytoplasm, CCAT2 affects HCC cell invasion and migration by
regulating the miR-4496/ATG5 axis. In the nucleus, CCAT2 increases
ELAVL1 RNA expression and thus inhibits autophagy, thereby
promoting the progression of HCC (120).
BRAF-activated nonprotein coding RNA
(BANCR)
BANCR is located between 9q21.11 and q21.12.
Although BANCR expression varies, BANCR plays a crucial role in
regulating biological functions in various types of cancer
(121). BANCR expression in HCC
tissues is markedly increased, and its expression can be attenuated
by rutin. It has been illustrated that BANCR can downregulate
miR-590-5P expression, while miR-590-5P targets oxidized
low-density lipoprotein receptor 1 (OLR1) to decrease OLR1
expression. Mechanistically, rutin may inhibit autophagy through
the BANCR/miRNA-590-5P/OLR1 axis, thereby attenuating sorafenib
resistance in HCC cells (122).
The classification and functions of RNAs are
summarized in Table I (123–135), and the roles of miRNAs and lncRNAs
in autophagy in HCC are summarized in Tables II and IIII.
Mechanisms of miRNAs and lncRNAs in the
regulation of autophagy in HCC
Autophagy is a multistep process in which multiple
ATGs participate. These ATG proteins take part in various stages of
autophagy, including the initiation of phagocytosis, nucleation,
elongation, closure of autophagosomes, the fusion of autophagosomes
with lysosomes and degradation of decomposition products (5) (Fig.
2).
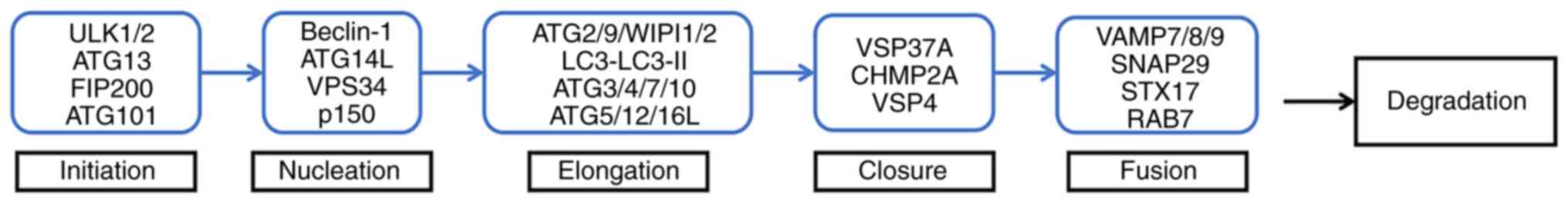 | Figure 2.Pattern diagram of the autophagy
process. Initiation: ULK complex (ULK1 or ULK2, ATG13, ATG101 and
FIP200/RB1CC1) initiates phagocyte formation. Nucleation: ULK
complex can actively regulate the activity of Ptdlns3k complex
(Beclin1/BECN1, ATG14L, p150 and VPS34), and participate in the
nucleation of the autophagosome prestructure together with this
complex. Elongation: ATG9 acts synergistically with ATG2 and
WIPI1/2; together with ATG5-ATG12-ATG16L1 complex and LC3 (in which
ATG3/4/7/10 participates in the formation of ATG5-ATG12-ATG16L1
complex and the conversion of LC3 to LC3-II), ATG3/4/7/10
participates in the prolongation of autophagosome membrane and the
formation of autophagosome. Closure: VSP37A, CHMP2A and VSP4 are
involved in autophagosome closure. Fusion: VAMP7/8/9, SNAP29, STX17
and RAB7 are involved in the fusion of autophagosomes and
lysosomes. Degradation: The resulting autophagy is degraded by
hydrolase and lipase. ULK1/2, unc-51 like autophagy activating
kinase 1 or 2; ATG, autophagy-related gene; FIP200, FAK family
kinase-interacting protein of 200 kDa; LC3, microtubule-associated
protein 1A/1B-light chain 3; VPS37A, vacuolar protein sorting 37
homolog A; CHMP2A, charged multivesicular body protein 2A; VPS4,
vacuolar protein sorting 4; SNAP29, synaptosomal-associated protein
29; STX17, syntaxin 17; RAB7, RAS-related GTP-binding protein. |
Some miRNAs and lncRNAs participate in the
regulation of autophagy by targeting related mRNAs or signaling
pathways and can regulate HCC cell metastasis, proliferation, drug
resistance and apoptosis by promoting or inhibiting autophagy. Some
miRNAs and lncRNAs modulate autophagy by targeting ATGs to
influence the biological functions of HCC cells. For example,
miR-26/ULK1 (45) affects the
initiation of autophagy. miR-541/ATG2A (28), miR-490-3p (31), miR-375 (42), miR-199a-5p/ATG7 (61), miR-7 (52), miR-181a/ATG5 (22), miR-142-3p/ATG5, ATG16L1 (33) and miR-101/ATG4D (49) affect the prolongation of
autophagosomes. miR-30a/Beclin-1 and ATG5 (24) affect the nucleation and prolongation
of autophagosomes. Among the lncRNAs, NEAT1/miR-204/ATG3 (81), PVT1/miR-365/ATG3 (97), HCG11/miR-26a-5p/ATG12 (85), CCAT1/miR-181a-5p/ATG7 (87), DANCR/miR-222-3p/ATG7 (115), HNF1A-AS1/miR-30b/ATG5 (113), ATB/ATG5 (117) and CCAT2/miR-4496/ATG5 (120) affect the prolongation of
autophagosomes. In conclusion, miRNAs and lncRNAs participate in
autophagy regulation in HCC via three different mechanisms: i)
miRNAs target mRNAs or regulate signaling pathways to regulate
autophagy; ii) lncRNAs target mRNAs or regulate signaling pathways
to regulate autophagy; and iii) lncRNAs function as competing
endogenous RNAs (ceRNAs) of miRNAs to regulate the expression of
miRNAs and thus affect the level of mRNAs, regulating autophagy. It
is worth noting that some lncRNAs [DCST1-AS1 (70), HCG11 (85), HAGLROS (101), HULC (104), LINC00160 (71), LINC00665 (110), HNF1A-AS1 (113) and MALAT1 (118)] and miRNAs [miR-541 (28), miR-125b (58), miR-181a (22), miR-26 (45) and miR-101 (48)] can affect various biological
functions in HCC while regulating autophagy (Fig. 3).
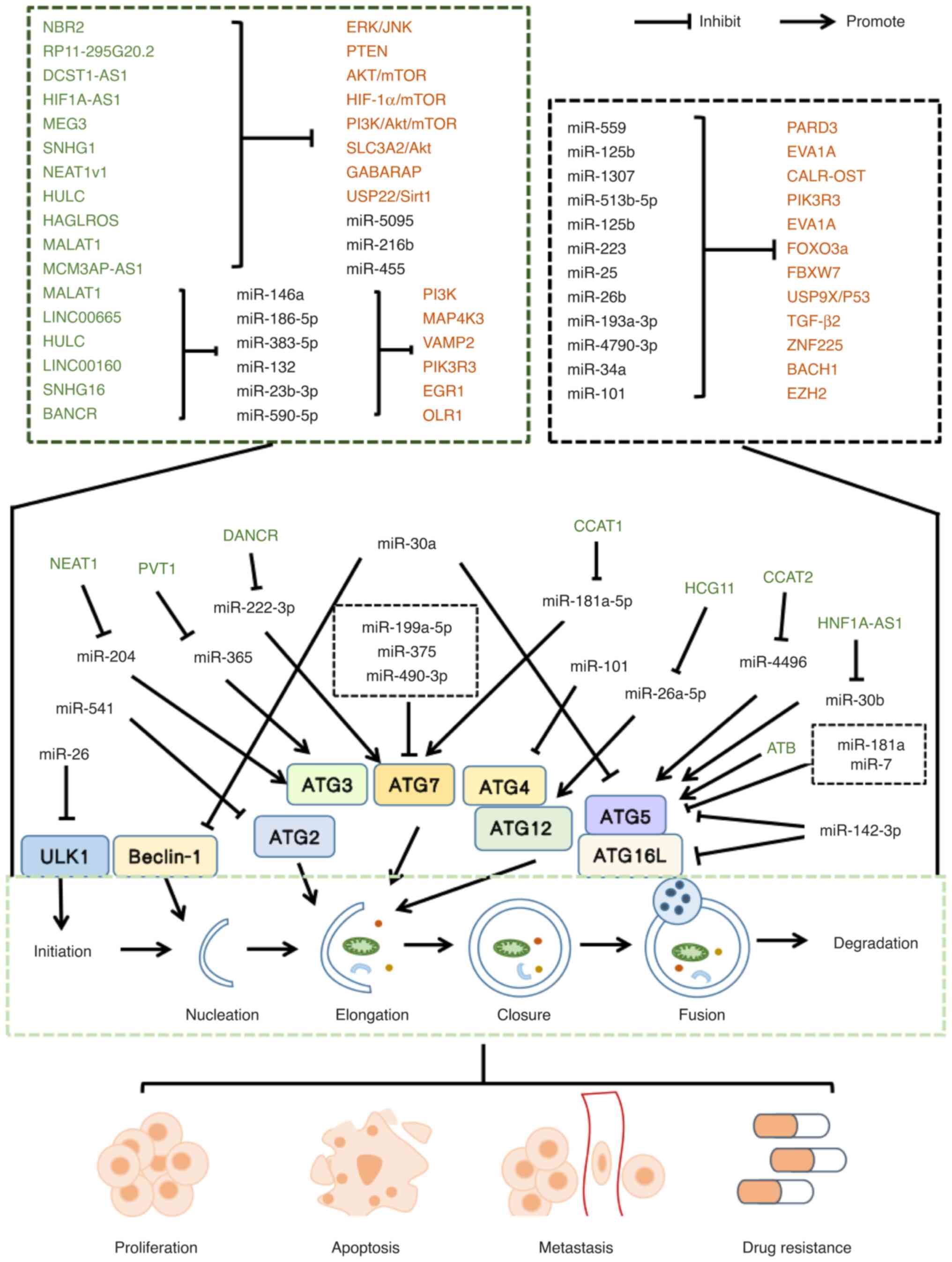 | Figure 3.Schematic diagram of the role of
lncRNAs and miRNAs in autophagy in HCC. miR, microRNA; ATG2A,
autophagy-related gene 2A; RAB1B, Ras-related protein Rab-1B; EZH2,
enhancer of zeste homolog 2; NBR2, neighbor of BRCA1 gene 2;
HIF-1α, hypoxia inducible factor 1α; MALAT1, metastasis-associated
lung adenocarcinoma transcript 1; PVT1, plasmacytoma variant
translocation 1; DANCR, differentiation antagonizing non-protein
coding RNA; HCG11, HLA complex group 11; CCAT1, Colon cancer
associated transcript 1; LINC00665, Long intergenic non-protein
coding RNA 665; MAP4K3, mitogen activated protein kinase kinase
kinase kinase 3; HULC, highly upregulated in liver cancer; VAMP2,
vesicle-associated membrane protein-2; USP9X, ubiquitin-specific
protease-9; ATG16L1, autophagy-related 16-like 1; RAB5A, RAB GTPase
5A; STMN1, stathmin 1; ATG4D, autophagy-related protein 4D; EZH2,
enhancer 1 of zeste homolog 2; USP22, ubiquitin-specific peptidase
22; Sirt1, silent information regulator 1; NEAT1v1, nuclear
enriched abundant transcript 1 variant 1; GABARAP,
gamma-aminobutyric acid receptor-associated protein; SNHG1, small
nucleolar RNA host gene 1; SLC3A2, solute carrier family 3 member
2; MALAT1, metastasis associated lung adenocarcinoma transcript 1;
NEAT1, nuclear enriched abundant transcript 1; LINC00160, long
intergenic non-protein coding rna 00160; PIK3R3,
phosphoinositide-3-kinase regulatory subunit 3; SNHG16, small
nucleolar RNA host gene 16; BANCR, BRAF-activated non-protein
coding RNA; OLR1, oxidized low-density lipoprotein receptor 1;
ZNF225, zinc finger protein225; MEG3, maternally expressed gene 3;
BACH1, BTB domain and CNC homology 1; CCAT2, colon
cancer-associated transcript 2; EGFR, epidermal growth factor
receptor. |
Conclusions and future perspectives
HCC is a major cause of cancer-related mortality
worldwide. The incidence and mortality rate of HCC have been
increasing, and with the survival rate decreasing, it is critical
to identify strategies or targets to suppress the tumorigenesis,
development, metastasis and invasion of HCC, which will lead to
novel methods for the treatment and prognosis of HCC. Autophagy
involves the transportation of heterogeneous intracellular
materials to lysosomes, and it modulates a number of pathological
processes. Autophagy plays differential roles in different stages
of HCC. It has been demonstrated that ncRNAs (miRNAs and lncRNAs)
play a critical role in regulating HCC cell autophagy. In addition,
circRNAs can regulate HCC cell autophagy by regulating miRNA
expression. Circ-SPECC1 negatively regulates the expression of
miR-33a to regulate autophagy and promote HCC tumorigenesis
(136). CircCBFB inhibits
miR-424-5p and upregulates ATG14, thereby promoting HCC cell
proliferation and autophagy (137). Although the role of other ncRNAs
in HCC cell autophagy has not yet been extensively studied, it has
been shown that N7 methylguanosine tRNA modification promotes the
development of esophageal squamous cell carcinoma through the
RPTOR/ULK1/autophagy axis (138).
Whether this modification plays a role in HCC has not yet been
determined. Additional ncRNAs may play a role in autophagy in HCC,
and further studies are warranted.
The majority of the miRNAs and lncRNAs mentioned
herein can affect the growth, apoptosis, metastasis and drug
resistance of HCC cells by regulating autophagy. However, whether
miRNAs such as miR-193a-3p, miR-34a, miR-541 and miR-1307, and
lncRNAs such as LINC00665, HNF1A-AS1, DANCR, MALAT1, DCST1-AS1,
LINC00160, RP11-295G20.2, HCG11, CCAT1, SNHG1, PVT1 and HAGLROS
play a biological role by regulating autophagy in HCC remains to be
determined. In terms of mechanisms, lncRNAs and miRNAs can directly
target mRNAs or signaling pathways to regulate biological functions
in HCC. lncRNAs can also function as ceRNAs of miRNAs, regulating
their expression, and thus affecting mRNA expression. Therefore,
targeting relevant miRNAs and lncRNAs may enable the modulation of
multiple biological functions in HCC, providing a novel direction
for HCC treatment. However, challenges blocking clinical
applications remain.
It is known that miRNAs regulate autophagy in HCC.
Thus, it is necessary to study upstream regulatory factors of
miRNAs in the future. In addition to lncRNAs, circRNAs are also
critical. However, as each miRNA can have multiple targets,
different targets can have diverse effects. Therefore, achieving
target specificity will be a challenge.
In addition, miRNA and lncRNA knockout animal models
are lacking in previous studies, but are necessary to reveal the
functions of miRNAs and lncRNAs. Several possible lncRNA knockout
methods have emerged, such as the complete deletion of lncRNA
genes, the deletion of lncRNA promoters, and the integration of a
premature polyadenylation cassette (139). Whether these methods can be used
to generate miRNA knockouts is unknown. Moreover, lncRNAs have low
sequence similarity across species; thus, translating data from
animal models into humans poses a substantial challenge (140). Finally, the efficacy and safety of
clinical applications employing miRNAs and lncRNAs remain to be
determined. Further research is required to elucidate these
issues.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science
Foundation of China (grant nos. 81673728 and 82274260).
Availability of data and materials
Not applicable.
Authors' contributions
JW acquired the data and wrote the manuscript. YZ
conceived the study. QC and QX contributed to the revisions. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lamb CA, Yoshimori T and Tooze SA: The
autophagosome: Origins unknown, biogenesis complex. Nat Rev Mol
Cell Biol. 14:759–774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levine B and Kroemer G: Biological
functions of autophagy genes: A disease perspective. Cell.
176:11–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian H, Chao X, Williams J, Fulte S, Li T,
Yang L and Ding WX: Autophagy in liver diseases: A review. Mol
Aspects Med. 82:1009732021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ke PY: Diverse functions of autophagy in
liver physiology and liver diseases. Int J Mol Sci. 20:3002019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yazdani HO, Huang H and Tsung A:
Autophagy: Dual response in the development of hepatocellular
carcinoma. Cells. 8:912019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Xiong H, Liu D, Hill C, Ertay A,
Li J, Zou Y, Miller P, White E, Downward J, et al: Autophagy
inhibition specifically promotes epithelial-mesenchymal transition
and invasion in RAS-mutated cancer cells. Autophagy. 15:886–899.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shirokikh NE: Translation complex
stabilization on messenger RNA and footprint profiling to study the
RNA responses and dynamics of protein biosynthesis in the cells.
Crit Rev Biochem Mol Biol. 57:261–304. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cech TR and Steitz JA: The noncoding RNA
revolution-trashing old rules to forge new ones. Cell. 157:77–94.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bella ED, Koch J and Baerenfaller K:
Translation and emerging functions of non-coding RNAs in
inflammation and immunity. Allergy. 77:2025–2037. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang Y, Lin J and Tsung A: Manipulation
of autophagy by MIR375 generates antitumor effects in liver cancer.
Autophagy. 8:1833–1834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geisler S and Coller J: RNA in unexpected
places: Long non-coding RNA functions in diverse cellular contexts.
Nat Rev Mol Cell Biol. 14:699–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Statello L, Guo C, Chen L and Huarte M:
Gene regulation by long non-coding RNAs and its biological
functions. Nat Rev Mol Cell Biol. 22:96–118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mercer TR and Mattick JS: Structure and
function of long noncoding RNAs in epigenetic regulation. Nat
Struct Mol Biol. 20:300–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhamija S and Diederichs S: From junk to
master regulators of invasion: lncRNA functions in migration, EMT
and metastasis. Int J Cancer. 139:269–280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen E, Li E, Liu H, Zhou Y, Wen L, Wang
J, Wang Y, Ye L and Liang T: miR-26b enhances the sensitivity of
hepatocellular carcinoma to doxorubicin via USP9X-dependent
degradation of p53 and regulation of autophagy. Int J Biol Sci.
17:781–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, He Y, Zhai N, Ding S, Li J and
Peng Z: MicroRNA-181a inhibits autophagy by targeting Atg5 in
hepatocellular carcinoma. Front Biosci (Landmark Ed). 23:388–396.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheng JQ, Wang MR, Fang D, Liu L, Huang
WJ, Tian DA, He XX and Li PY: LncRNA NBR2 inhibits tumorigenesis by
regulating autophagy in hepatocellular carcinoma. Biomed
Pharmacother. 133:1110232021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu XT, Shi YH, Zhou J, Peng YF, Liu WR,
Shi GM, Gao Q, Wang XY, Song K, Fan J and Ding ZB: MicroRNA-30a
suppresses autophagy-mediated anoikis resistance and metastasis in
hepatocellular carcinoma. Cancer Lett. 412:108–117. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martins M, Galfrè S, Terrigno M,
Pandolfini L, Appolloni I, Dunville K, Marranci A, Rizzo M,
Mercatanti A, Poliseno L, et al: A eutherian-specific microRNA
controls the translation of Satb2 in a model of cortical
differentiation. Stem Cell Reports. 16:1496–1509. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu C and Yi X: miR-541 serves as a
prognostic biomarker of osteosarcoma and its regulatory effect on
tumor cell proliferation, migration and invasion by targeting
TGIF2. Diagn Pathol. 15:962020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu L, Du B, Lu QJ, Fan XW, Tang K, Yang L
and Liao WL: miR-541 suppresses proliferation and invasion of
squamous cell lung carcinoma cell lines via directly targeting
high-mobility group AT-hook 2. Cancer Med. 7:2581–2591. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu WP, Liu JP, Feng JF, Zhu CP, Yang Y,
Zhou WP, Ding J, Huang CK, Cui YL, Ding CH, et al: miR-541
potentiates the response of human hepatocellular carcinoma to
sorafenib treatment by inhibiting autophagy. Gut. 69:1309–1321.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Jiang D and Yang S: MiR-490-3p
inhibits the malignant progression of lung adenocarcinoma. Cancer
Manag Res. 12:10975–10984. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen J, Xiao Z, Wu WKK, Wang MH, To KF,
Chen Y, Yang W, Li MSM, Shin VY, Tong JH, et al: Epigenetic
silencing of miR-490-3p reactivates the chromatin remodeler SMARCD1
to promote Helicobacter pylori-induced gastric carcinogenesis.
Cancer Res. 75:754–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ou Y, He J and Liu Y: MiR-490-3p inhibits
autophagy via targeting ATG7 in hepatocellular carcinoma. IUBMB
Life. 70:468–478. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fernández C, Bellosillo B, Ferraro M,
Seoane A, Sánchez-González B, Pairet S, Pons A, Barranco L, Vela
MC, Gimeno E, et al: MicroRNAs 142-3p, miR-155 and miR-203 are
deregulated in gastric MALT lymphomas compared to chronic
gastritis. Cancer Genomics Proteomics. 14:75–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang K, Chen J, Zhou H, Chen Y, Zhi Y,
Zhang B, Chen L, Chu X, Wang R and Zhang C: PU.1/microRNA-142-3p
targets ATG5/ATG16L1 to inactivate autophagy and sensitize
hepatocellular carcinoma cells to sorafenib. Cell Death Dis.
9:3122018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu P, Cao F, Sui J, Hong Y, Liu Q, Gao X,
Gong H, Hao L, Lou Z and Zhang W: MicroRNA-142-3p inhibits
tumorigenesis of colorectal cancer via suppressing the activation
of Wnt Signaling by directly targeting to β-catenin. Front Oncol.
10:5529442021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mansoori B, Duijf PHG, Mohammadi A,
Safarzadeh E, Ditzel HJ, Gjerstorff MF, Cho WC and Baradaran B:
MiR-142-3p targets HMGA2 and suppresses breast cancer malignancy.
Life Sci. 276:1194312021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rodriguez AE, Hernández JÁ, Benito R,
Gutiérrez NC, García JL, Hernández-Sánchez M, Risueño A, Sarasquete
ME, Fermiñán E, Fisac R, et al: Molecular characterization of
chronic lymphocytic leukemia patients with a high number of losses
in 13q14. PLoS One. 7:e484852012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan S, Wu Q, Wang Z, Che Y, Zheng S, Chen
Y, Zhong X and Shi F: miR-223: An immune regulator in infectious
disorders. Front Immunol. 12:7818152021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Favero A, Segatto I, Perin T and Belletti
B: The many facets of miR-223 in cancer: Oncosuppressor, oncogenic
driver, therapeutic target, and biomarker of response. Wiley
Interdiscip Rev RNA. 12:e16592021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Y, Chen E, Tang Y, Mao J, Shen J,
Zheng X, Xie S, Zhang S, Wu Y, Liu H, et al: miR-223 overexpression
inhibits doxorubicin-induced autophagy by targeting FOXO3a and
reverses chemoresistance in hepatocellular carcinoma cells. Cell
Death Dis. 10:8432019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Wang Q, Wen J, Wu Y and Man C:
MiR-375: A novel multifunctional regulator. Life Sci.
275:1193232021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei J, Lu Y, Wang R, Xu X, Liu Q, He S,
Pan H, Liu X, Yuan B, Ding Y and Zhang J: MicroRNA-375: Potential
cancer suppressor and therapeutic drug. Biosci Rep.
41:BSR202114942021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chang Y, Yan W, He X, Zhang L, Li C, Huang
H, Nace G, Geller DA, Lin J and Tsung A: miR-375 inhibits autophagy
and reduces viability of hepatocellular carcinoma cells under
hypoxic conditions. Gastroenterology. 143:177–187.e8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li C, Li Y, Lu Y, Niu Z, Zhao H, Peng Y
and Li M: miR-26 family and its target genes in tumorigenesis and
development. Crit Rev Oncol Hematol. 157:1031242021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Calin GA, Sevignani C, Dumitru CD, Hyslop
T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M
and Croce CM: Human microRNA genes are frequently located at
fragile sites and genomic regions involved in cancers. Proc Natl
Acad Sci USA. 101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jin F, Wang Y, Li M, Zhu Y, Liang H, Wang
C, Wang F, Zhang CY, Zen K and Li L: MiR-26 enhances
chemosensitivity and promotes apoptosis of hepatocellular carcinoma
cells through inhibiting autophagy. Cell Death Dis. 8:e25402017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mourelatos Z, Dostie J, Paushkin S, Sharma
A, Charroux B, Abel L, Rappsilber J, Mann M and Dreyfuss G: miRNPs:
A novel class of ribonucleoproteins containing numerous microRNAs.
Genes Dev. 16:720–728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang CZ, Deng F, Li H, Wang DD, Zhang W,
Ding L and Tang JH: MiR-101: A potential therapeutic target of
cancers. Am J Transl Res. 10:3310–3321. 2018.PubMed/NCBI
|
|
48
|
Xu L, Beckebaum S, Iacob S, Wu G, Kaiser
GM, Radtke A, Liu C, Kabar I, Schmidt HH, Zhang X, et al:
MicroRNA-101 inhibits human hepatocellular carcinoma progression
through EZH2 downregulation and increased cytostatic drug
sensitivity. J Hepatol. 60:590–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu Y, An Y, Wang Y, Zhang C, Zhang H,
Huang C, Jiang H, Wang X and Li X: miR-101 inhibits autophagy and
enhances cisplatin-induced apoptosis in hepatocellular carcinoma
cells. Oncol Rep. 29:2019–2024. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Korać P, Antica M and Matulić M: MiR-7 in
cancer development. Biomedicines. 9:3252021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhao J, Tao Y, Zhou Y, Qin N, Chen C, Tian
D and Xu L: MicroRNA-7: A promising new target in cancer therapy.
Cancer Cell Int. 15:1032015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yuan J, Li Y, Liao J, Liu M, Zhu L and
Liao K: MicroRNA-7 inhibits hepatocellular carcinoma cell invasion
and metastasis by regulating Atg5-mediated autophagy. Transl Cancer
Res. 9:3965–3972. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rodriguez A, Griffiths-Jones S, Ashurst JL
and Bradley A: Identification of mammalian microRNA host genes and
transcription units. Genome Res. 14:1902–1910. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jiang LH, Zhang HD and Tang JH: MiR-30a: A
novel biomarker and potential therapeutic target for cancer. J
Oncol. 2018:51678292018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang C, Li C and Hao R: miR-559 inhibits
proliferation, autophagy, and angiogenesis of hepatocellular
carcinoma cells by targeting PARD3. Mediators Inflamm.
2022:31214922022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jin W, Liang Y, Li S, Lin G, Liang H,
Zhang Z, Zhang W and Nie R: MiR-513b-5p represses autophagy during
the malignant progression of hepatocellular carcinoma by targeting
PIK3R3. Aging (Albany NY). 13:16072–16087. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang Y, Tan J, Wang L, Pei G, Cheng H,
Zhang Q, Wang S, He C, Fu C and Wei Q: MiR-125 family in
cardiovascular and cerebrovascular diseases. Front Cell Dev Biol.
9:7990492021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ren WW, Li DD, Chen X, Li XL, He YP, Guo
LH, Liu LN, Sun LP and Zhang XP: MicroRNA-125b reverses oxaliplatin
resistance in hepatocellular carcinoma by negatively regulating
EVA1A mediated autophagy. Cell Death Dis. 9:5472018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun X, Zhu H, Cao R, Zhang J and Wang X:
BACH1 is transcriptionally inhibited by TET1 in hepatocellular
carcinoma in a microRNA-34a-dependent manner to regulate autophagy
and inflammation. Pharmacol Res. 169:1056112021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Meng W, Li Y, Chai B, Liu X and Ma Z:
miR-199a: A tumor suppressor with noncoding RNA network and
therapeutic candidate in lung cancer. Int J Mol Sci. 23:85182022.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F
and Xia Q: Cisplatin-induced downregulation of miR-199a-5p
increases drug resistance by activating autophagy in HCC cell.
Biochem Biophys Res Commun. 423:826–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Y, Gu X and Liu Y: The effect of
dexmedetomidine on biological behavior of osteosarcoma cells
through miR-1307 expression. Am J Transl Res. 13:4876–4883.
2021.PubMed/NCBI
|
|
63
|
Zhou Y, Wang M, Shuang T, Liu Y, Zhang Y
and Shi C: MiR-1307 influences the chemotherapeutic sensitivity in
ovarian cancer cells through the regulation of the CIC
transcriptional repressor. Pathol Res Pract. 215:1526062019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Qiu X and Dou Y: miR-1307 promotes the
proliferation of prostate cancer by targeting FOXO3A. Biomed
Pharmacother. 88:430–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xie S, Jiang X, Qin R, Song S, Lu Y, Wang
L, Chen Y and Lu D: miR-1307 promotes hepatocarcinogenesis by
CALR-OSTC-endoplasmic reticulum protein folding pathway. iScience.
24:1032712021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Khordadmehr M, Shahbazi R, Sadreddini S
and Baradaran B: miR-193: A new weapon against cancer. J Cell
Physiol. 234:16861–16872. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Qu L, Tian Y, Hong D, Wang F and Li Z:
Mig-6 inhibits autophagy in HCC cell lines by modulating
miR-193a-3p. Int J Med Sci. 19:338–351. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Feng X, Zou B, Nan T, Zheng X, Zheng L,
Lan J, Chen W and Yu J: MiR-25 enhances autophagy and promotes
sorafenib resistance of hepatocellular carcinoma via targeting
FBXW7. Int J Med Sci. 19:257–266. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Choi HJ, Park JH, Kim OH, Kim KH, Hong HE,
Seo H and Kim SJ: Combining everolimus and Ku0063794 promotes
apoptosis of hepatocellular carcinoma cells via reduced autophagy
resulting from diminished expression of miR-4790-3p. Int J Mol Sci.
22:28592021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li J, Zhai D, Huang Q, Chen HL, Zhang Z
and Tan QF: LncRNA DCST1-AS1 accelerates the proliferation,
metastasis and autophagy of hepatocellular carcinoma cell by
AKT/mTOR signaling pathways. Eur Rev Med Pharmacol Sci.
23:6091–6104. 2019.PubMed/NCBI
|
|
71
|
Zhang W, Liu Y, Fu Y, Han W, Xu H, Wen L,
Deng Y and Liu K: Long non-coding RNA LINC00160 functions as a
decoy of microRNA-132 to mediate autophagy and drug resistance in
hepatocellular carcinoma via inhibition of PIK3R3. Cancer Lett.
478:22–33. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Xin X, Wu M, Meng Q, Wang C, Lu Y, Yang Y,
Li X, Zheng Q, Pu H, Gui X, et al: Long noncoding RNA HULC
accelerates liver cancer by inhibiting PTEN via autophagy
cooperation to miR15a. Mol Cancer. 17:942018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cui C, Li Z and Wu D: The long non-coding
RNA H19 induces hypoxia/reoxygenation injury by up-regulating
autophagy in the hepatoma carcinoma cells. Biol Res. 52:322019.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhou Y, Zhang X and Klibanski A: MEG3
noncoding RNA: A tumor suppressor. J Mol Endocrinol. 48:R45–R53.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Braconi C, Kogure T, Valeri N, Huang N,
Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T:
microRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yu S, Hou D, Chen P, Zhang Q, Lv B, Ma Y,
Liu F, Liu H, Song EJ, Yang D and Liu J: Adenosine induces
apoptosis through TNFR1/RIPK1/P38 axis in colon cancer cells.
Biochem Biophys Res Commun. 460:759–765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pu Z, Wu L, Guo Y, Li G, Xiang M, Liu L,
Zhan H, Zhou X and Tan H: LncRNA MEG3 contributes to
adenosine-induced cytotoxicity in hepatoma HepG2 cells by
downregulated ILF3 and autophagy inhibition via regulation
PI3K-AKT-mTOR and beclin-1 signaling pathway. J Cell Biochem.
120:18172–18185. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang T, Li Z, Yan L, Yan F, Shen H and
Tian X: Long non-coding RNA neighbor of BRCA1 gene 2: A crucial
regulator in cancer biology. Front Oncol. 11:7835262021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liang L, Huan L, Wang J, Wu Y, Huang S and
He X: LncRNA RP11-295G20.2 regulates hepatocellular carcinoma cell
growth and autophagy by targeting PTEN to lysosomal degradation.
Cell Discov. 7:1182021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Li K, Yao T, Zhang Y, Li W and Wang Z:
NEAT1 as a competing endogenous RNA in tumorigenesis of various
cancers: Role, mechanism and therapeutic potential. Int J Biol Sci.
17:3428–3440. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Li X, Zhou Y, Yang L, Ma Y, Peng X, Yang
S, Li H and Liu J: LncRNA NEAT1 promotes autophagy via regulating
miR-204/ATG3 and enhanced cell resistance to sorafenib in
hepatocellular carcinoma. J Cell Physiol. 235:3402–3413. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sakaguchi H, Tsuchiya H, Kitagawa Y,
Tanino T, Yoshida K, Uchida N and Shiota G: NEAT1 confers
radioresistance to hepatocellular carcinoma cells by inducing
autophagy through GABARAP. Int J Mol Sci. 23:7112022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tang L, Chen Y, Chen H, Jiang P, Yan L, Mo
D, Tang X and Yan F: DCST1-AS1 promotes TGF-β-induced
epithelial-mesenchymal transition and enhances chemoresistance in
triple-negative breast cancer cells via ANXA1. Front Oncol.
10:2802020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yuan X, Zhao Q, Zhang Y and Xue M: The
role and mechanism of HLA complex group 11 in cancer. Biomed
Pharmacother. 143:1122102021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Li M, Zhang Y and Ma L: LncRNA HCG11
accelerates the progression of hepatocellular carcinoma via
miR-26a-5p/ATG12 axis. Eur Rev Med Pharmacol Sci. 23:10708–10720.
2019.PubMed/NCBI
|
|
86
|
Liu Z, Chen Q and Hann SS: The functions
and oncogenic roles of CCAT1 in human cancer. Biomed Pharmacother.
115:1089432019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Guo J, Ma Y, Peng X, Jin H and Liu J:
LncRNA CCAT1 promotes autophagy via regulating ATG7 by sponging
miR-181 in hepatocellular carcinoma. J Cell Biochem.
120:17975–17983. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yu X, Zheng Q, Zhang Q, Zhang S, He Y and
Guo W: MCM3AP-AS1: An indispensable cancer-related LncRNA. Front
Cell Dev Biol. 9:7527182021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zhang H, Luo C and Zhang G: LncRNA
MCM3AP-AS1 regulates epidermal growth factor receptor and autophagy
to promote hepatocellular carcinoma metastasis by interacting with
miR-455. DNA Cell Biol. 38:857–864. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang M, Wang W, Li T, Yu X, Zhu Y, Ding
F, Li D and Yang T: Long noncoding RNA SNHG1 predicts a poor
prognosis and promotes hepatocellular carcinoma tumorigenesis.
Biomed Pharmacother. 80:73–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Thin KZ, Tu JC and Raveendran S: Long
non-coding SNHG1 in cancer. Clin Chim Acta. 494:38–47. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li W, Dong X, He C, Tan G, Li Z, Zhai B,
Feng J, Jiang X, Liu C, Jiang H and Sun X: LncRNA SNHG1 contributes
to sorafenib resistance by activating the Akt pathway and is
positively regulated by miR-21 in hepatocellular carcinoma cells. J
Exp Clin Cancer Res. 38:1832019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wu H, Gu J, Zhou D, Cheng W, Wang Y, Wang
Q and Wang X: LINC00160 mediated paclitaxel- and
doxorubicin-resistance in breast cancer cells by regulating TFF3
via transcription factor C/EBPβ. J Cell Mol Med. 24:8589–8602.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cheng G, Liu Y, Liu L, Ruan H, Cao Q, Song
Z, Bao L, Xu T, Xiong Z, Liu J, et al: LINC00160 mediates sunitinib
resistance in renal cell carcinoma via SAA1 that is implicated in
STAT3 activation and compound transportation. Aging (Albany NY).
12:17459–17479. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Huppi K, Pitt JJ, Wahlberg BM and Caplen
NJ: The 8q24 gene desert: An oasis of non-coding transcriptional
activity. Front Genet. 3:692012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Traversa D, Simonetti G, Tolomeo D, Visci
G, Macchia G, Ghetti M, Martinelli G, Kristensen LS and Storlazzi
CT: Unravelling similarities and differences in the role of
circular and linear PVT1 in cancer and human disease. Br J Cancer.
126:835–850. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yang L, Peng X, Jin H and Liu J: Long
non-coding RNA PVT1 promotes autophagy as ceRNA to target ATG3 by
sponging microRNA-365 in hepatocellular carcinoma. Gene.
697:94–102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang W, Zhang Y and Xi S: Upregulation of
lncRNA HAGLROS enhances the development of nasopharyngeal carcinoma
via modulating miR-100/ATG14 axis-mediated PI3K/AKT/mTOR signals.
Artif Cells Nanomed Biotechnol. 47:3043–3052. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen JF, Wu P, Xia R, Yang J, Huo XY, Gu
DY, Tang CJ, De W and Yang F: STAT3-induced lncRNA HAGLROS
overexpression contributes to the malignant progression of gastric
cancer cells via mTOR signal-mediated inhibition of autophagy. Mol
Cancer. 17:62018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang WL, Yu DJ and Zhong M: LncRNA HAGLROS
accelerates the progression of lung carcinoma via sponging
microRNA-152. Eur Rev Med Pharmacol Sci. 23:6531–6538.
2019.PubMed/NCBI
|
|
101
|
Wei H, Hu J, Pu J, Tang Q, Li W, Ma R, Xu
Z, Tan C, Yao T, Wu X, et al: Long noncoding RNA HAGLROS promotes
cell proliferation, inhibits apoptosis and enhances autophagy via
regulating miR-5095/ATG12 axis in hepatocellular carcinoma cells.
Int Immunopharmacol. 73:72–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yu X, Zheng H, Chan MTV and Wu WKK: HULC:
An oncogenic long non-coding RNA in human cancer. J Cell Mol Med.
21:410–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li P, Li Y and Ma L: Long noncoding RNA
highly upregulated in liver cancer promotes the progression of
hepatocellular carcinoma and attenuates the chemosensitivity of
oxaliplatin by regulating miR-383-5p/vesicle-associated membrane
protein-2 axis. Pharmacol Res Perspect. 9:e008152021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Xiong H, Ni Z, He J, Jiang S, Li X, He J,
Gong W, Zheng L, Chen S, Li B, et al: LncRNA HULC triggers
autophagy via stabilizing Sirt1 and attenuates the chemosensitivity
of HCC cells. Oncogene. 36:3528–3540. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Wang C, Jiang X, Li X, Song S, Meng Q,
Wang L, Lu Y, Xin X, Pu H, Gui X, et al: Long noncoding RNA HULC
accelerates the growth of human liver cancer stem cells by
upregulating CyclinD1 through miR675-PKM2 pathway via autophagy.
Stem Cell Res Ther. 11:82020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ghafouri-Fard S, Khoshbakht T, Taheri M
and Shojaei S: A review on the role of small nucleolar RNA host
gene 6 long non-coding RNAs in the carcinogenic processes. Front
Cell Dev Biol. 9:7416842021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Jing Z, Ye X, Ma X, Hu X, Yang W, Shi J,
Chen G and Gong L: SNGH16 regulates cell autophagy to promote
sorafenib resistance through suppressing miR-23b-3p via sponging
EGR1 in hepatocellular carcinoma. Cancer Med. 9:4324–4338. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Raveh E, Matouk IJ, Gilon M and Hochberg
A: The H19 long non-coding RNA in cancer initiation, progression
and metastasis-a proposed unifying theory. Mol Cancer. 14:1842015.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang C, Xu SN, Li K, Chen JH, Li Q and
Liu Y: The biological and molecular function of LINC00665 in human
cancers. Front Oncol. 12:8860342022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Shan Y and Li P: Long intergenic
non-protein coding RNA 665 regulates viability, apoptosis, and
autophagy via the MiR-186-5p/MAP4K3 axis in hepatocellular
carcinoma. Yonsei Med J. 60:842–853. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Hong F, Gao Y, Li Y, Zheng L, Xu F and LI
X: Inhibition of HIF1A-AS1 promoted starvation-induced
hepatocellular carcinoma cell apoptosis by reducing
HIF-1α/mTOR-mediated autophagy. World J Surg Oncol. 18:1132020.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
ZHANG Y, Shi J, Luo J, Liu C and Zhu L:
Regulatory mechanisms and potential medical applications of
HNF1A-AS1 in cancers. Am J Transl Res. 14:4154–4168.
2022.PubMed/NCBI
|
|
113
|
Liu Z, Wei X, Zhang A, Li C, Bai J and
Dong J: Long non-coding RNA HNF1A-AS1 functioned as an oncogene and
autophagy promoter in hepatocellular carcinoma through sponging
hsa-miR-30b-5p. Biochem Biophys Res Commun. 473:1268–1275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang X and Zhu Y: Research progress on
regulating LncRNAs of hepatocellular carcinoma stem cells. Onco
Targets Ther. 14:917–927. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Wang X, Cheng ML, Gong Y, Ma WJ, Li B and
Jiang YZ: LncRNA DANCR promotes ATG7 expression to accelerate
hepatocellular carcinoma cell proliferation and autophagy by
sponging miR-222-3p. Eur Rev Med Pharmacol Sci. 24:8778–8787.
2020.PubMed/NCBI
|
|
116
|
Xiao H, Zhang F, Zou Y, Li J, Liu Y and
Huang W: The function and mechanism of long non-coding RNA-ATB in
cancers. Front Physiol. 9:3212018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang CZ, Yan GX, Dong DS, Xin H and Liu
ZY: LncRNA-ATB promotes autophagy by activating Yes-associated
protein and inducing autophagy-related protein 5 expression in
hepatocellular carcinoma. World J Gastroenterol. 25:5310–5322.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Peng N, He J, LI J, Huang H, Huang W, Liao
Y and Zhu S: Long noncoding RNA MALAT1 inhibits the apoptosis and
autophagy of hepatocellular carcinoma cell by targeting the
microRNA-146a/PI3K/Akt/mTOR axis. Cancer Cell Int. 20:1652020.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yuan P, Cao W, Zang Q, Li G, Guo X and Fan
J: The HIF-2α-MALAT1-miR-216b axis regulates multi-drug resistance
of hepatocellular carcinoma cells via modulating autophagy. Biochem
Biophys Res Commun. 478:1067–1073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shi J, Guo C and Ma J: CCAT2 enhances
autophagy-related invasion and metastasis via regulating miR-4496
and ELAVL1 in hepatocellular carcinoma. J Cell Mol Med.
25:8985–8996. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hussen BM, Azimi T, Abak A, Hidayat HJ,
Taheri M and Ghafouri-Fard S: Role of lncRNA BANCR in human
cancers: An updated review. Front Cell Dev Biol. 9:6899922021.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhou M, Zhang G, Hu J, Zhu Y, Lan H, Shen
X, Lv Y and Huang L: Rutin attenuates sorafenib-induced
chemoresistance and autophagy in hepatocellular carcinoma by
regulating BANCR/miRNA-590-5P/OLR1 axis. Int J Biol Sci.
17:3595–3607. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Shang Q, Yang Z, Jia R and Ge S: The novel
roles of circRNAs in human cancer. Mol Cancer. 18:62019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Li Z, Huang C, Bao C, Chen L, Lin M, Wang
X, Zhong G, Yu B, Hu W, Dai L, et al: Exon-intron circular RNAs
regulate transcription in the nucleus. Nat Struct Mol Biol.
22:256–264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang Z, Zhang J, Diao L and Han L: Small
non-coding RNAs in human cancer: Function, clinical utility, and
characterization. Oncogene. 40:1570–1577. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Morais P, Adachi H and Yu YT: Spliceosomal
snRNA epitranscriptomics. Front Genet. 12:6521292021. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Matera AG, Terns RM and Terns MP:
Non-coding RNAs: Lessons from the small nuclear and small nucleolar
RNAs. Nat Rev Mol Cell Biol. 8:209–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Janin M, Coll-SanMartin L and Esteller M:
Disruption of the RNA modifications that target the ribosome
translation machinery in human cancer. Mol Cancer. 19:702020.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Liang J, Wen J, Huang Z, Chen XP, Zhang BX
and Chu L: Small nucleolar RNAs: Insight into their function in
cancer. Front Oncol. 9:5872019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Cuciniello R, Filosa S and Crispi S: Novel
approaches in cancer treatment: Preclinical and clinical
development of small non-coding RNA therapeutics. J Exp Clin Cancer
Res. 40:3832021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Novina CD and Sharp PA: The RNAi
revolution. Nature. 430:161–164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ozata DM, Gainetdinov I, Zoch A, O'Carroll
D and Zamore PD: PIWI-interacting RNAs: small RNAs with big
functions. Nat Rev Genet. 20:89–108. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Liu Y, Dou M, Song X, Dong Y, Liu S, Liu
H, Tao J, Li W, Yin X and Xu W: The emerging role of the piRNA/piwi
complex in cancer. Mol Cancer. 18:1232019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Su Z, Wilson B, Kumar P and Dutta A:
Noncanonical roles of tRNAs: tRNA fragments and beyond. Annu Rev
Genet. 54:47–69. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhang B, Liu Z, Cao K, Shan W, Liu J, Wen
Q and Wang R: Circ-SPECC1 modulates TGFβ2 and autophagy under
oxidative stress by sponging miR-33a to promote hepatocellular
carcinoma tumorigenesis. Cancer Med. 9:5999–6008. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zhao Z, He J and Feng C: CircCBFB is a
mediator of hepato-cellular carcinoma cell autophagy and
proliferation through miR-424-5p/ATG14 axis. Immunol Res.
70:341–353. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Han H, Yang C, Ma J, Zhang S, Zheng S,
Ling R, Sun K, Guo S, Huang B, Liang Y, et al:
N7-methylguanosine tRNA modification promotes esophageal
squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy
axis. Nat Commun. 13:14782022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Li L and Chang HY: Physiological roles of
long noncoding RNAs: Insight from knockout mice. Trends Cell Biol.
24:594–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Pang KC, Frith MC and Mattick JS: Rapid
evolution of noncoding RNAs: Lack of conservation does not mean
lack of function. Trends Genet. 22:1–5. 2006. View Article : Google Scholar : PubMed/NCBI
|