Introduction
Ovarian cancer (OC) has high morbidity and mortality
rates, and age-standardized mortality is the highest among
gynecological cancers worldwide (1). As patients are often asymptomatic,
>40% of cases are advanced at diagnosis, and the treatment
strategy is debulking surgery followed by chemotherapy (2). First-line chemotherapy is a
combination of carboplatin and paclitaxel (3), and the response to platinum agents
contributes the most to therapeutic efficacy. OC has a very high
recurrence rate and is classified as a platinum-resistant tumor if
it recurs within 6 months of completing platinum-based chemotherapy
(4). In patients with
platinum-resistant tumors, the response rate to other
chemotherapies is 10–15%, and the overall survival is within 12
months (5). In recent years, the
effectiveness of molecular-targeted therapy, including
immunotherapy, for platinum-resistant tumors has been reported, but
treatment strategies have not yet been established (6,7).
Ferroptosis is defined as iron-dependent cell death
due to the accumulation of lipid hydroperoxides, and is
distinguished from apoptosis and necroptosis (8,9).
Accumulating evidence has shown that ferroptosis plays an important
role in pathological conditions and diseases, including
neurodegenerative diseases, ischemic injury and cancers (10–13).
Since certain types of cancers highly express glutathione
peroxidase 4 (GPX4) and/or ferroptosis suppressor protein 1 (FSP1),
leading to an anti-ferroptotic status, it has been proposed that
ferroptosis inducers inhibiting these proteins may be used as
anticancer drugs (14–18). In addition, it has previously been
reported that platinum agents are involved in ferroptosis (19–21).
However, the relationship between resistance to platinum and
ferroptosis remains unclear.
Intracellular iron levels must be strictly regulated
to inhibit ferroptosis. It has been indicated that iron-sulfur
proteins are important in regulating intracellular iron homeostasis
and oxidation-reduction reactions of electron transport in the
mitochondria and other organelles. Ferredoxin1 (Fdx1), an
iron-sulfur protein, plays an important role in the biosynthesis of
iron-sulfur clusters and in steroidogenesis by catalyzing electron
transfer to cytochrome P450 in the mitochondria (22,23).
Since the loss of Fdx1 has detrimental effects on the activities of
iron-sulfur cluster enzymes and intracellular iron homeostasis,
leading to mitochondrial iron overload (24), it can be assumed that Fdx1 may play
a role in preventing cells from undergoing ferroptosis.
Furthermore, it has been reported that Fdx1 may be a possible
immunotherapy and/or prognostic biomarker for cancer treatment
(25,26). However, the function of Fdx1 in
cancer cells and its relationship with ferroptosis remain
elusive.
In the present study, it was revealed that Fdx1
expression is associated with cisplatin resistance in OC by
investigating cell lines and clinical specimens. Treatment of OC
cells with cisplatin resulted in ferroptosis through a drastic
increase in the mitochondrial membrane potential and lipid
peroxidation. It was also found that Fdx1 upregulation in
platinum-resistant cells may play an important role in suppressing
ferroptosis by inhibiting cisplatin-induced mitochondrial membrane
potential and lipid peroxidation. Indeed, the suppressed expression
of Fdx1 in cisplatin-resistant OC cells resulted in enhanced
ferroptosis induced by cisplatin. Considering the relatively higher
expression of Fdx1 in patients with cisplatin-resistant OC than in
those with cisplatin-sensitive OC, these results indicated that the
induction of ferroptosis by inhibiting Fdx1 may provide a new
therapeutic strategy for the treatment of platinum-resistant
OC.
Materials and methods
Cell culture and transfection
The following ovarian endometrioid carcinoma cell
lines were obtained from ECACC (Salisbury, United Kingdom): The
A2780 cell line (parental) and the cisplatin-resistant A2780 cell
line (A2780cis cell line). Another cell line of ovarian
endometrioid carcinoma, OVK18 cells (parental), was obtained from
RIKEN BRC Cell Bank (Ibaraki, Japan). Cisplatin-resistant OVK18
cells (OVK18cis cells) were established by exposing parental OVK18
cells to increasing concentrations of cisplatin (FUJIFILM Wako Pure
Chemical Corporation). In brief, OVK18 cells were exposed to
cisplatin at a final concentration of 0.1 µM as the initial
concentration. Subsequently, the concentration of cisplatin was
increased stepwise for the surviving cells, and the surviving cells
up to a concentration of 6 µM were defined as OVK18cis. A2780cis
and OVK18cis cells were continuously treated with cisplatin (1.5
µM). All cells were cultured in RPMI-1640 (Nacalai Tesque, Inc.)
with 10% (v/v) FBS and incubated at 37°C with 5% CO2 and
90% humidity. The short tandem repeat profiles of these cells were
analyzed (BEX Co., Ltd.), and it was confirmed that these cells
were not contaminated.
Small interfering RNA (siRNA) transfection was
performed as previously described (27). A2780, A2780cis, OVK18 and OVK18cis
cells were transfected with their respective siRNAs using
Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. Briefly, 30 nmol/l of
siRNAs were mixed with RNAiMAX reagent diluted in Opti-MEM (Thermo
Fisher Scientific, Inc.), was incubated for 20 min at room
temperature (20–25°C) and added to cultured cells. The following
target sequences were used: siFdx1#1,
5′-CGAUCUGGCAUAUGGACUA-3′ and 5′-UAGUCCAUAUGCCAGAUCG-3′; and
siFdx1#2, 5′-GCCAAAUCUGUUUGACAAA-3′ and
5′-UUUGUCAAACAGAUUUGGC-3′.
Viability assay
Cell viability was assessed by the WST8 assay using
Cell Counting Kit 8 (CCK-8; Dojindo Laboratories, Inc.) according
to the manufacturer's instructions. Briefly, 1,000-5,000 cells were
seeded into each well of a 96-well plate with 100 µl of culture
medium/well in triplicate. Cells were treated with the respective
drugs (cisplatin: 0.1–64 µM; deferoxamine: 100 µM; penicillamine: 1
µM) for 48–96 h, after which cells were incubated in fresh medium
containing 10% (v/v) CCK-8 reagent for 2 h. The absorbance of the
culture medium from each well was measured at a wavelength of 450
nm.
Superoxide dismutase (SOD) assay
SOD activities were monitored by using SOD Assay
Kit-WST (Dojindo Laboratories, Inc.), according to the
manufacturer's instructions. Briefly, 5,000 cells were seeded into
each well of a 96-well plate with 100 µl culture medium/well in
triplicate. Cells were treated with 1 µM penicillamine 48 h,
thereafter cells were incubated in WST working solution and Enzyme
working solution at 37°C for 20 min. The absorbance from each well
was measured at 450 nm.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
RNA isolation and RT-qPCR were conducted as
previously described (28). Total
RNA was isolated using Sepasol-RNA I SuperG (Nacalai Tesque, Inc.)
and subjected to reverse transcription to synthesize cDNA using a
PrimeScript RT Reagent Kit (Takara Bio, Inc.). qPCR was performed
using SYBR Green (Roche Diagnostics) on a LightCycler 480 II system
(Roche Diagnostics). The amount of mRNA was normalized to that of
18S ribosomal RNA. The following primers were used: Fdx1
forward, 5′-AACAGACAGATCACGGTTGGG-3′ and reverse,
5′-GGTCTTGCCCACATCAATGG-3′; Tmp1 forward,
5′-ACTACCTGCAGTTTTGTGGCT-3′ and reverse, 5′-CTGGTCCGTCCACAAGCAA-3′;
and Usp17l11 forward, 5′-CAGCTCAGAGTGTCCAGCAA-3′ and
reverse, 5′-AGTTAACGTCTTGGAGGCCG-3′.
RNA-sequencing (RNA-Seq) analysis
Total RNA was extracted from A2780, A2780cis OVK18
and OVK18cis cells by using Sepasol-RNA I SuperG (Nacalai Tesque,
Inc.). AmpliSeq libraries were created using the Ion AmpliSeq
Transcriptome Human Gene Expression Kit (Thermo Fisher Scientific,
Inc.), which was designed by amplifying over 20,000 human genes
simultaneously in a single primer pool. The libraries were
amplified using an Ion OneTouch 2 System (Thermo Fisher Scientific,
Inc.). Thereafter, libraries were sequenced using Ion Torrent PGM
(Thermo Fisher Scientific, Inc.) or Ion S5 (Thermo Fisher
Scientific, Inc.). RNA-Seq reads were analyzed using CLC bio
Genomics Workbench Version 12.0 (CLC Bio). Genes with a q-value
<0.001 and fold change >0 between A2780 and A2780cis cells or
OVK18 and OVK18cis cells were defined as differentially expressed
genes (DEGs). RNA-Seq analysis was performed twice, and the common
DEGs were correlated with acquiring cisplatin resistance in A2780
and OVK18 cells.
Flow cytometric analysis
Cells (5×105 cells per well) were seeded
into a 6-cm f dish followed by treatment with the respective drugs
(cisplatin: 2.0 µM; deferoxamine: 100 µM; rotenone: 0.5 µM) or
their combination. To monitor lipid peroxidation or mitochondrial
membrane potential, Liperfluo or MT-1 (both from Dojindo
Laboratories, Inc.) reagent was added to each dish and incubated
for 30 min. Flow cytometric analysis was performed using BD
LSRFortessaTM X-20 (BD Biosciences). The results of flow cytometric
analysis were analyzed by FlowJo (version 10. 7. 1; BD
Biosciences).
Western blotting
Western blotting was performed as previously
described (28). The cells were
solubilized in ice-cold lysis buffer [50 mM HEPES (pH 7.5), 150 mM
NaCl, 1% (v/v) Nonidet P-40, 1 mM EDTA, 10 µg/ml aprotinin, 10
µg/ml leupeptin and 1 mM p-APMSF]. Proteins (10 µg per lane) were
separated using SDS-PAGE (20%) and transferred onto Immobilon-P
membranes (Merck KGaA). Membranes were then blocked by 5% (w/v)
skim milk at room temperature for 30 min, and were immunoblotted
with the following antibodies: anti-Fdx1 antibody (1:1,000; cat.
no. 12592-1-AP; Proteintech Group, Inc.) and anti-α-tubulin
(1:1,000; cat. no. PM054; MBL International Co.). Subsequently, the
membranes were immunoblotted with secondary antibody (1:10,000;
cat. no. 170-6515; Bio-Rad Laboratories, Inc.). Immunoreactive
bands were visualized using ImmunoStar LD (FUJIFILM Wako Pure
Chemical Corporation). The respective band intensities were
measured using ImageJ software (version: v1.53t; National
Institutes of Health).
Immunohistochemical analysis
The OC tissue specimens resected from 45 patients
(date range: 04/2015~03/2019; age distribution: 39~75) at Kobe
University Hospital (Kobe, Japan) were fixed with 10% (v/v)
formalin at room temperature for 48 h and embedded in paraffin.
Cylindrical tissue cores (2 mm) were then extracted from each
paraffin block and re-embedded into a single paraffin block to
create a tissue microarray (TMA) for sectioning. Epithelial OC
cases were selected from the TMA and classified into
platinum-sensitive and platinum-resistant groups according to their
clinical course. The resultant TMA sections were incubated with
antibody against Fdx1 (1:200; cat. no. 12592-1-AP; Thermo Fisher
Scientific, Inc.) overnight at 4°C and then with anti-Rabbit IgG
antibodies conjugated with HRP-labeled polymer (ImmPRESS Reagent
kit Peroxidase; Vector Laboratories, Inc.) for 30 min at room
temperature. Secondary antibodies were visualized using DAB
Chromogen (Dako; Agilent Technologies, Inc.), and nuclei were
counterstained with hematoxylin. The specimens were observed under
a BZ-X700 microscope (Keyence Corporation). Clinical tissue
specimens were obtained by opt-out method and analyzed in
accordance with procedures approved (approval nos. B200076 and
B220122) by the Institutional Review Board of Kobe University
Hospital (Kobe, Japan).
Immunofluorescence analysis
Cells (1×105 cells/well) were seeded into
12-well plates and incubated with MitoTracker® Red
CMXRos (Lonza Group, Ltd.) for 30 min prior to fixation. After
fixation, cells were incubated with antibodies against Fdx1 (1:100;
cat. no. 12592-1-AP; Thermo Fisher Scientific, Inc.) overnight at
4°C followed by treatment with anti-rabbit IgG antibodies (1:500;
cat. no. A11034; Invitrogen; Thermo Fisher Scientific, Inc.) for 30
min at room temperature. Fluorescent images were obtained using a
laser scanning confocal imaging system (LSM710; Carl Zeiss AG).
Statistical analysis
Data were analyzed using BellCurve for Excel (Social
Survey Research Information Co., Ltd.). Paired Student's t-test was
used when two groups were compared, and one-way ANOVA followed by
Tukey's honestly significant difference test was used when three or
more groups were compared. *P<0.05 was considered to indicate a
statistically significant difference.
Results
Upregulated expression of Fdx1 in OC
cell lines is associated with their enhanced resistance against
cisplatin
To identify genes associated with enhanced cisplatin
resistance in OC, gene expression profiles were compared between
cisplatin-sensitive and cisplatin-resistant OC cell lines. The
following ovarian endometrioid carcinoma cell lines were obtained:
the A2780 cell line (parental cells) and the A2780cis cell line
(29). For generality, another
ovarian endometrioid carcinoma cell line, the OVK18 cell line
(parental cells), was obtained, and their derived OVK18cis cells
were established by selecting parental OVK18 cells with exposure to
a stepwise increasing concentration of cisplatin. To confirm
whether the A2780cis and OVK18cis cells showed enhanced cisplatin
resistance compared with their parental A2780 and OVK18 cells, a
cell viability assay after treatment with different concentrations
of cisplatin was performed. A2780cis and OVK18cis cells acquired
enhanced cisplatin resistance compared with their parental cells
[half maximal inhibitory concentration (IC50) of the
respective cells was as follows: 1.4942 µM (A2780), 6.3489 µM
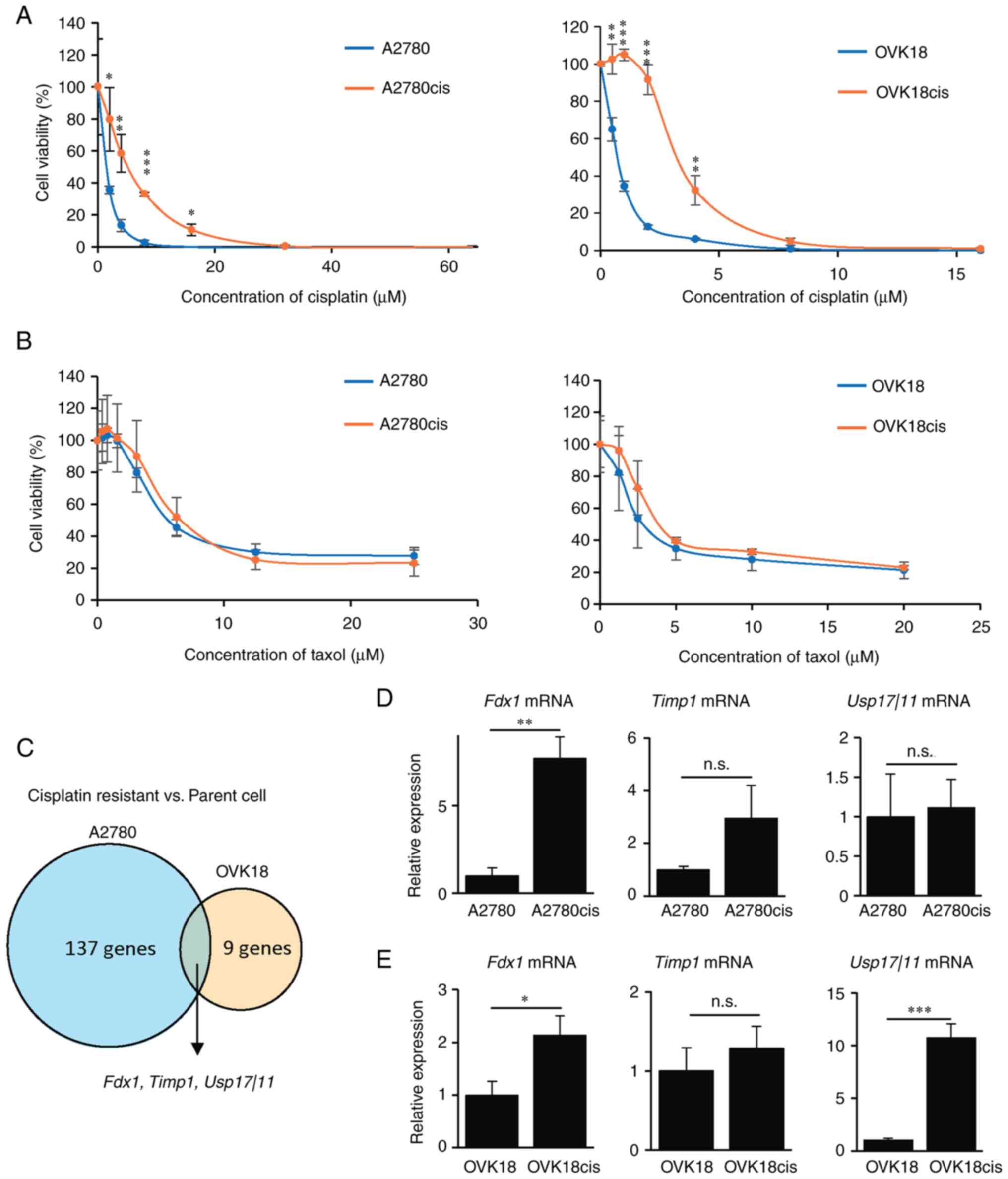
(A2780cis), 0.7006 µM (OVK18) and 3.3703 µM (OVK18cis); Fig. 1A]. Since combined therapy with a
platinum agent and taxol is currently considered the gold standard
for the treatment of OC, whether A2780cis and OVK18cis cells
exhibited resistance to taxol was next investigated. It was found
that A2780cis and OVK18cis cells exhibited sensitivity to taxol
comparable to that of their parental cells [IC50 of the
respective cells was as follows: 5.585 µM (A2780), 6.41644 µM
(A2780cis), 2.8559 µM (OVK18), and 3.90092 µM (OVK18cis); Fig. 1B]. The gene expression profiles
between A2780 and A2780cis cells or OVK18 and OCK18cis cells were
then compared by RNA-seq to extract important candidate genes
associated with enhanced cisplatin resistance. A total of three
candidate gene transcripts (Fdx1, Timp1 and Usp17l11)
were identified that were highly expressed in A2780cis and OVK18cis
cells compared with their parental cells (Fig. 1C). Among these three candidate
genes, expression of Fdx1 in both A2780cis and OVK18cis
cells was significantly higher than that in their parental cells,
as assessed by RT-qPCR analysis; while the expression of
Timp1 in both A2780cis and OVK18cis cells was not
significantly higher than that in their parental cells and the
expression of Usp17l11 was not consistent between A2780 and
OVK18 cells (Fig. 1D and E). Based
on these findings, Fdx1 became the focus to elucidate its role in
cisplatin resistance of OC cells.
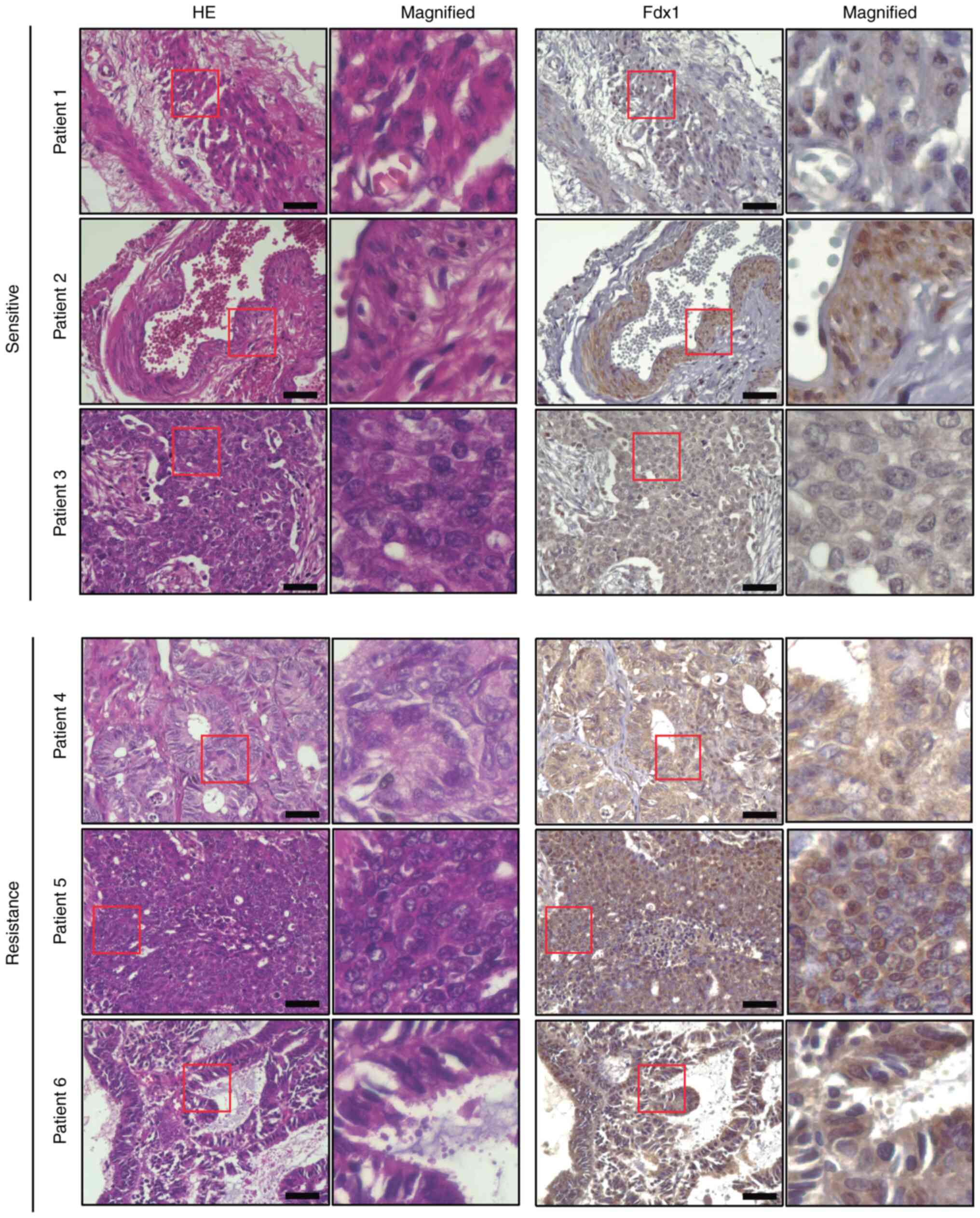
To investigate the expression of Fdx1 in patients
with OC, a TMA including 45 OC specimens was prepared. Since A2780
and OVK18 cells were used; namely, ovarian endometrioid carcinoma
cell lines, in the in vitro experiments, the expression of
Fdx1 in patients with ovarian endometrioid carcinoma was examined
by immunohistochemical analysis of TMA. A total of 13 of the 45
specimens were from patients with ovarian endometrioid carcinoma,
and seven of the 13 specimens were from patients with
cisplatin-resistant ovarian endometrioid carcinoma. The TMA was
then stained with an anti-Fdx1 antibody, and it was identified that
Fdx1 was expressed in ovarian endometrioid carcinoma (Fig. 2). Furthermore, a stronger signal of
Fdx1 was detected in cisplatin-resistant specimens than in
cisplatin-sensitive ones (Fig. 2),
indicating that expression of Fdx1 may be associated with enhanced
cisplatin resistance in ovarian endometrioid carcinoma.
Fdx1 inhibits ferroptosis induced by
cisplatin in OC cells
Since a growing body of evidence has demonstrated
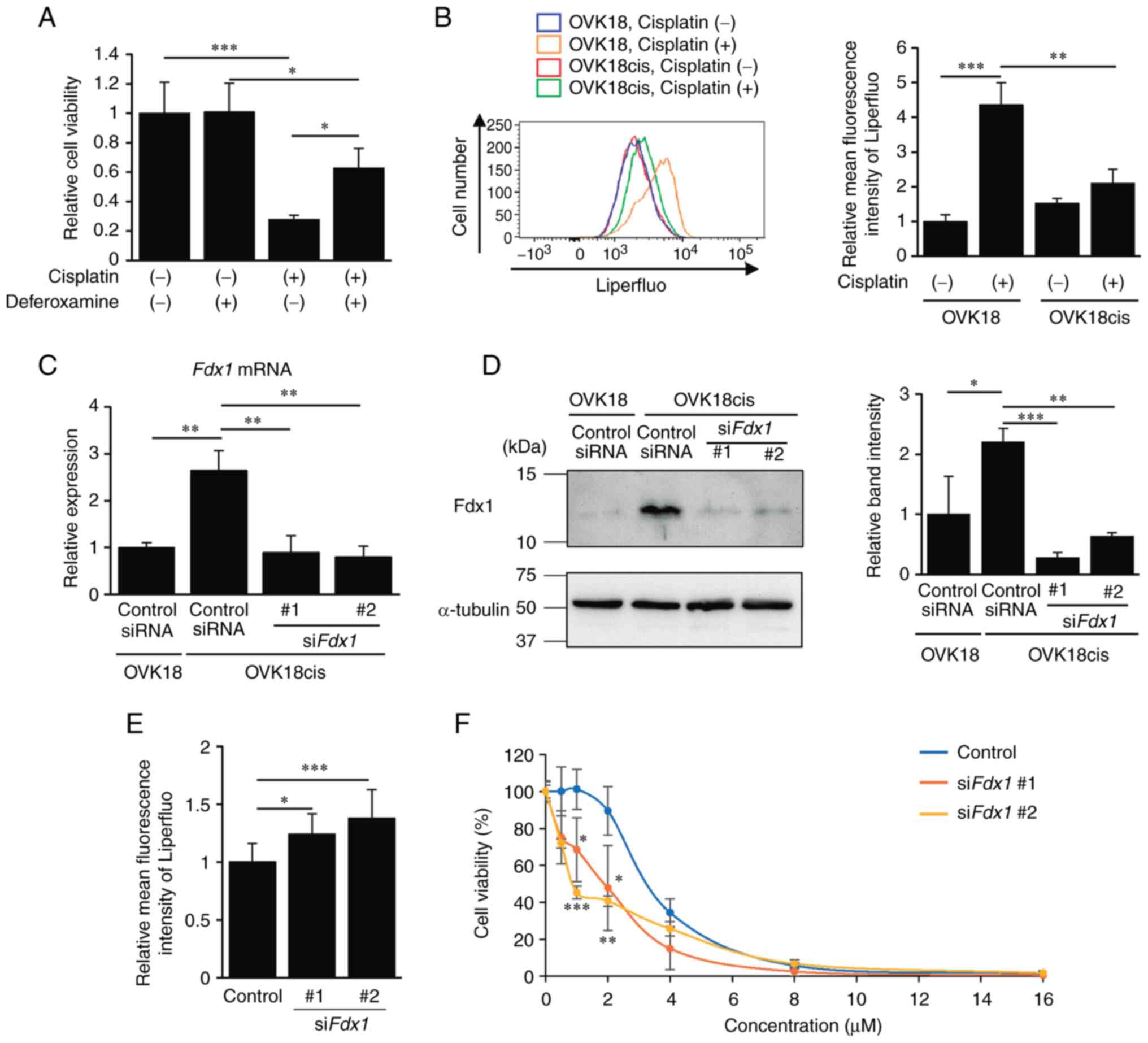
that cisplatin induces ferroptosis in carcinoma cells (13), whether treatment with deferoxamine,
an iron chelator, could inhibit cisplatin-induced death of OC cells
was confirmed. Deferoxamine suppressed cisplatin-induced cell death
in OVK18 and A2780 cells (Figs. 3A
and S1A), indicating that
cisplatin induces iron-dependent cell death; namely, ferroptosis.
Ferroptosis is characterized as iron-dependent cell death
associated with accumulated lipid peroxidation (8,9). Thus,
the extent of lipid peroxidation in OVK18 and OVK18cis cells
treated with cisplatin was examined by flow cytometric analysis
using the fluorescent probe Liperfluo. It was revealed that the
extent of lipid peroxidation was significantly increased by
treatment with cisplatin in OVK18 cells but not in OVK18cis cells
(Fig. 3B). Similarly, a significant
increase in lipid peroxidation was detected in A2780 cells but not
in A2780cis cells (Fig. S1B).
These results suggested that cisplatin induces ferroptosis in
cisplatin-sensitive cells, but fails to induce ferroptosis in
cisplatin-resistant cells. Therefore, it can be assumed that
acquired resistance to ferroptosis may be one of the mechanisms
underlying the enhanced resistance of OC cells to cisplatin.
Accumulating evidence has revealed that resistance
to copper-dependent cell death; namely, cuproptosis, is also
important for the progression of carcinomas (30,31).
Therefore, whether penicillamine, a copper chelator, could inhibit
cisplatin-induced cell death was further examined. It was first
examined whether or not the activities of SOD, copper-dependent
enzyme, in A2780 cells and OVK18 cells could be suppressed by
treatment with penicillamine to confirm penicillamine works well
under the experimental conditions of the present study. As a
result, it was confirmed that penicillamine treatment suppressed
activities of SOD in A2780 cells (Fig.
S2A) and OVK18 cells (Fig.
S2B). It was further revealed that treatment with penicillamine
failed to affect cisplatin-induced cell death (Fig. S2C and D), suggesting that cisplatin
treatment induces ferroptosis rather than cuproptosis.
Since the expression of Fdx1 was found to be
associated with cisplatin resistance in OC cells, whether Fdx1 is
involved in resistance against cisplatin-induced ferroptosis was
next examined. To this end, OVK18cis cells were transfected with
either control siRNA or siRNAs against Fdx1 (siFdx1
#1 or #2), and the mRNA and protein levels of Fdx1 were analyzed by
RT-qPCR and western blotting, respectively, to confirm the
knockdown efficiency of the respective siRNAs (Fig. 3C and D). The effect of
Fdx1-knockdown on cisplatin-induced lipid peroxidation in OVK18cis
cells was examined. Suppressed expression of Fdx1 resulted in
enhanced lipid peroxidation in OVK18cis cells following treatment
with cisplatin (Fig. 3E).
Furthermore, Fdx1-depleted OVK18cis cells exhibited exacerbated
cell survival after cisplatin treatment, particularly at lower
concentrations (Fig. 3F). Similar
results were obtained for the A2780cis cells. When expression of
Fdx1 was suppressed in A2780cis cells with Fdx1 siRNAs
(siFdx1 #1 or #2), as assessed by western blotting (Fig. S3A), suppressed expression of Fdx1
in A2780cis cells resulted in the inhibition of cell viability
after treatment with cisplatin (Fig.
S3B). These results indicated that the expression of Fdx1 in
cisplatin-resistant OC cells may confer resistance against
cisplatin by inhibiting cisplatin-induced ferroptosis.
Fdx1 inhibits ferroptosis by
regulating mitochondrial membrane potential
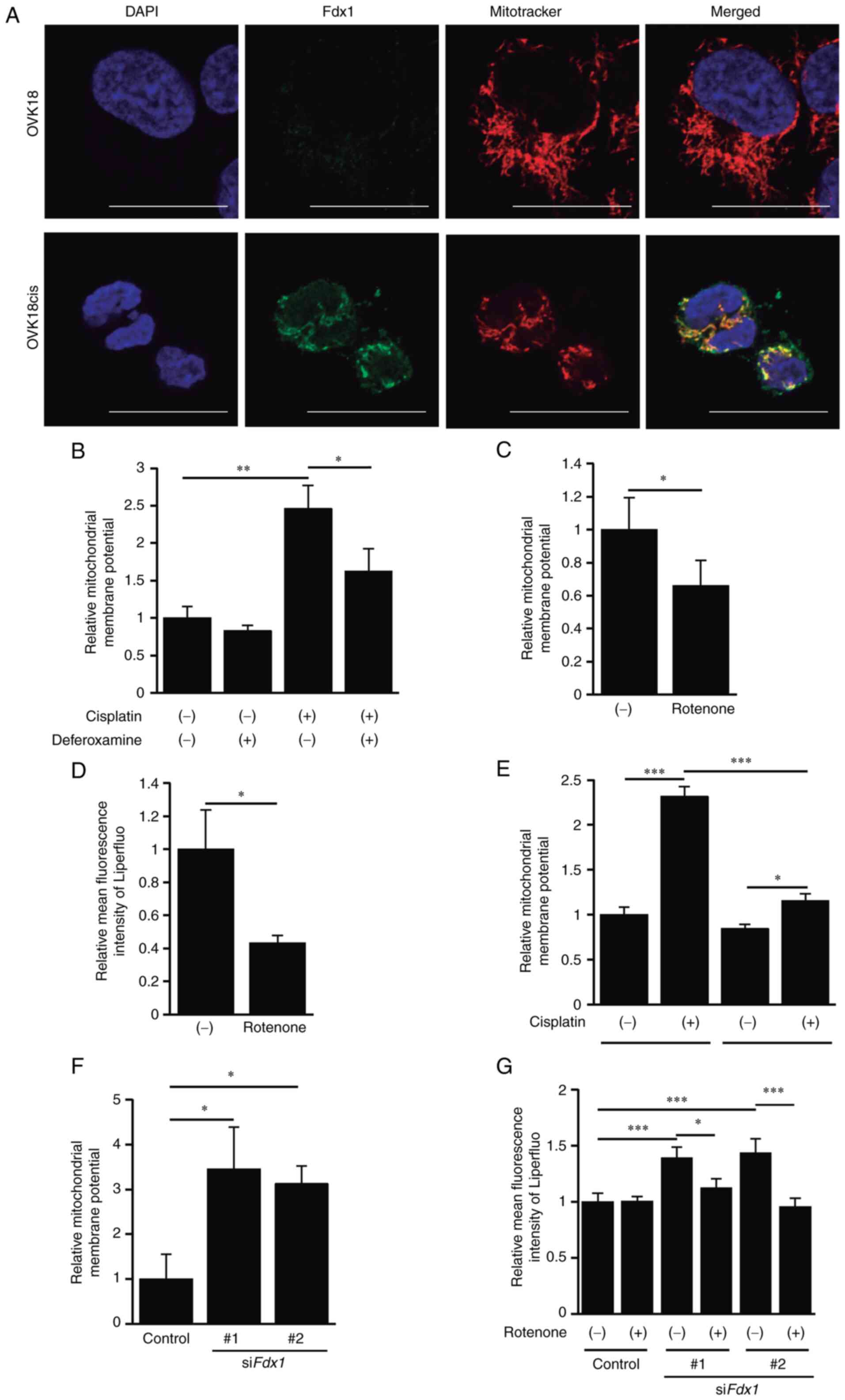
To further investigate the function of Fdx1 in
cisplatin-resistant OC cells, immunofluorescence staining of Fdx1
in OVK18 and OVK18cis cells was first performed to detect its
intracellular localization. Fdx1 was localized predominantly to the
mitochondria of OVK18cis cells, as assessed by its colocalization
with Mitotracker, and its relative expression levels in OVK18cis
cells were obviously higher than those in OVK18 cells (Fig. 4A). Therefore, the possible
relationship between mitochondrial function and Fdx1-mediated
ferroptosis was examined. For this purpose, whether treatment with
cisplatin could affect mitochondrial membrane potential was first
examined by employing an MT-1 MitoMP detection assay. As a result,
the mitochondrial membrane potential in OVK18cis cells was
upregulated by treatment with cisplatin and suppressed by treatment
with deferoxamine (Fig. 4B). Since
it has been reported that an upregulated mitochondrial membrane
potential is involved in ferroptosis (32), whether suppression of the
mitochondrial membrane potential using rotenone, an inhibitor of
the mitochondrial respiratory chain complex I, could inhibit
cisplatin-induced lipid peroxidation in OVK18 cells was next
investigated. Treatment with rotenone suppressed cisplatin-induced
augmentation of the mitochondrial membrane potential of OVK18 cells
(Fig. 4C). It was also found that
cisplatin-induced lipid peroxidation in OVK18 cells was inhibited
by treatment with rotenone (Fig.
4D). Similar results were obtained in A2780 cells, where
upregulated mitochondrial membrane potential and lipid peroxidation
by cisplatin could be suppressed by treatment with rotenone
(Fig. S4A and B). These results
indicated that cisplatin could promote ferroptosis by enhancing the
mitochondrial membrane potential.
Next, the mitochondrial membrane potential in
OVK18cis and A2780cis cells was examined in comparison with that in
their parental OVK18 and A2780 cells after treatment with
cisplatin. Cisplatin-induced drastic upregulation of the
mitochondrial membrane potential was not observed in either
OVK18cis (Fig. 4E) or A2780cis
cells (Fig. S4C), indicating that
the suppression of cisplatin-induced drastic upregulation of the
mitochondrial membrane potential in cisplatin-resistant OC cells
may be attributable to their cisplatin-resistant properties.
Finally, whether upregulated Fdx1 in OVK18cis cells could inhibit
their upregulated mitochondrial membrane potential after treatment
with cisplatin was investigated. As expected, Fdx1 depletion in
OVK18cis cells resulted in a significant increase in mitochondrial
membrane potential after treatment with cisplatin (Fig. 4F). Moreover, rotenone treatment
almost cancelled upregulated lipid peroxidation in Fdx1-depleted
OVK18cis cells treated with cisplatin (Fig. 4G). Collectively, these results
indicated that Fdx1 may play a pivotal role in suppressing both the
upregulated mitochondrial membrane potential and cisplatin-induced
lipid peroxidation, thereby conferring cisplatin-resistant
properties in OC cells.
Discussion
OC is a carcinoma with a poor prognosis that is
often detected at an advanced stage with peritoneal dissemination
that becomes refractory. Patients with OC who had recurrence or
disease progression within 6 months of their platinum-free interval
were defined as being platinum-resistant OC cases, but the
platinum-free interval could have been influenced by the frequency
and types of investigations that a patient received during
follow-up. The definition of platinum resistance is ambiguous since
it is defined only by the period of the platinum-free interval and
does not consider genetic, morphological, or biochemical
alterations. Therefore, an objective and accurate definition of
platinum resistance is required.
Cisplatin is widely used for the treatment of solid
tumors, and its cytotoxic effect is the formation of DNA-DNA
intra-strand adducts that cause single- or double-strand DNA
breaks, leading to cell cycle arrest and apoptosis (33,34).
Accumulating evidence has shown that cisplatin is involved in
ferroptosis. It has been reported that pretreatment with erastin,
an inducer of ferroptosis, enhances the therapeutic effect of
cisplatin (20), and that
concomitant overexpression of ferroptosis suppressors, SLC7A11 and
GPX4, can often be observed in platinum-resistant OC (21). The results of the present study
revealed that cisplatin induced ferroptosis and that
cisplatin-resistant OC cells were resistant to cisplatin-induced
ferroptosis. Therefore, it can be assumed that cisplatin induces
cell death through ferroptosis in addition to DNA breaks, and that
cisplatin-resistant cells may acquire ferroptosis resistance.
However, the mechanism by which cisplatin-resistant cells acquire
ferroptosis resistance remains unclear.
In addition to mutations within a set of cancer
driver genes in various types of cancers, alterations in copy
numbers and/or epigenetic modulations of particular genes are known
to be crucial for the progression of several types of cancers,
including OC (35–37). Thus, identification of candidate
genes that are upregulated or downregulated in OC is important for
understanding their pathological features. Although several studies
have compared gene expression profiles between platinum-sensitive
and -resistant OC cells (38–41), a
consensus on the critical genes upregulated in platinum-resistant
cells has yet to be reached. The present study showed that Fdx1 was
expressed at remarkably higher levels in the two
cisplatin-resistant OC cell lines, A2789cis and OVK18cis, than in
the cisplatin-sensitive parental cell lines, that is, A2780 and
OVK18. It was also found that the expression of Fdx1 was higher in
patients with cisplatin-resistant OC than in those with
cisplatin-sensitive OC. Thus, upregulation of Fdx1 expression may
play a critical role in acquiring cisplatin resistance, and Fdx1
may be a suitable diagnostic and/or prognostic marker for the
treatment of platinum-resistant OC.
Fdx1 is an iron-sulfur protein that plays an
important role in the biosynthesis of iron-sulfur clusters and
steroidogenesis (22,23). Since depletion of Fdx1 results in
dysregulation of iron homeostasis, leading to mitochondrial iron
overload (24), it can be envisaged
that Fdx1 is critically involved in the regulation of ferroptosis.
However, the detailed molecular mechanism by which Fdx1 regulates
ferroptosis remains largely unknown. Accumulating evidence has
demonstrated that upregulation of the mitochondrial membrane
potential is associated with ferroptosis, and inhibitors of the
electron transport chain suppress ferroptosis (32). The findings of the present study
provide pertinent evidence that Fdx1, upregulated in
cisplatin-resistant OC cells, inhibits cisplatin-induced
upregulation of mitochondrial membrane potential and lipid
peroxidation, which are characteristic biochemical events of
ferroptosis, thereby supporting their survival and progression.
Since it is conceivable that mutation, gene amplification, or
epigenetic regulation of the Fdx1 gene may be responsible
for its upregulated expression, further genetic or epigenetic
analyses are required to clarify the molecular mechanism underlying
the upregulated expression of Fdx1 in cisplatin-resistant OC
cells. How Fdx1 regulates mitochondrial membrane potential and
lipid peroxidation in cisplatin-resistant OC cells in the absence
or presence of cisplatin also remains to be elucidated. Therefore,
further studies are required to clarify these issues.
It has recently been reported that cuproptosis is
also critical in several diseases, including cancer (30). Notably, Fdx1 is known to be one of
the key proteins regulating cuproptosis (31). Since the experiments in the present
study with a copper chelator failed to restore cell death of OC
cells induced by cisplatin, it is hypothesized that cisplatin may
induce cell death of the carcinoma cells in an
iron-dependent/copper-independent manner. Further studies are
required to clarify this.
A valid and reliable therapeutic strategy for the
treatment of platinum-resistant OC has not yet been established,
and there is an urgent need to develop and establish an appropriate
treatment. The findings of the present study that ferroptosis in
cisplatin-resistant OC cells can be induced by inhibiting the
expression of Fdx1 may represent a novel therapeutic strategy for
the treatment of platinum-resistant OC. Therefore, it will be of
interest to develop proper clinical methods to induce ferroptosis
in platinum-resistant OC cells by selectively inhibiting the
expression or function of Fdx1.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by JST SPRING (grant no.
JPMJSP2148) and JST (Moonshot R&D) (grant no. JPMJMS2022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The RNA sequence data are available to DNA Data Bank of
Japan (DDBJ) under the accession no. E-GEAD-588 (https://ddbj.nig.ac.jp/public/ddbj_database/gea/experiment/E-GEAD-000/E-GEAD-588).
Authors' contributions
RT, KK and YM designed the experiments, analyzed the
data and edited the manuscript. KY and YT prepared the tissue
microarrays and revised the manuscript. RT and KK performed the
experiments. All authors read and approved the final manuscript. KK
and YM confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval nos.
B200076 and B220122) by the institutional review board of Kobe
University Hospital (Kobe, Japan). Patients were able to opt-out of
having their data included in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
A2780cis cells
|
cisplatin-resistant A2780 cells
|
|
CCK-8
|
Cell Counting Kit-8
|
|
DEG
|
differentially expressed gene
|
|
Fdx1
|
Ferredoxin1
|
|
FSP1
|
ferroptosis suppressor protein 1
|
|
GPX4
|
glutathione peroxidase 4
|
|
OC
|
ovarian cancer
|
|
OVK18cis cells
|
cisplatin-resistant OVK18 cells
|
|
TMA
|
tissue microarray
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bristow RE, Tomacruz RS, Armstrong DK,
Trimble EL and Montz FJ: Survival effect of maximal cytoreductive
surgery for advanced ovarian carcinoma during the platinum era: A
meta-analysis. J Clin Oncol. 20:1248–1259. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bookman MA, Brady MF, McGuire WP, Harper
PG, Alberts DS, Friedlander M, Colombo N, Fowler JM, Argenta PA, De
Geest K, et al: Evaluation of new platinum-based treatment regimens
in advanced-stage ovarian cancer: A phase III trial of the
gynecologic cancer intergroup. J Clin Oncol. 27:1419–1425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilson MK, Pujade-Lauraine E, Aoki D,
Mirza MR, Lorusso D, Oza AM, du Bois A, Vergote I, Reuss A, Bacon
M, et al: Fifth ovarian cancer consensus conference of the
gynecologic cancer Intergroup: Recurrent disease. Ann Oncol.
28:727–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beesley VL, Green AC, Wyld DK, O'Rourke P,
Wockner LF, DeFazio A, Butow PN, Price MA, Horwood KR, Clavarino
AM, et al: Quality of life and treatment response among women with
platinum-resistant versus platinum-sensitive ovarian cancer treated
for progression: A prospective analysis. Gynecol Oncol.
132:130–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marabelle A, Le DT, Ascierto PA, Di
Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M,
Penel N, Hansen AR, et al: Efficacy of pembrolizumab in patients
with noncolorectal high microsatellite instability/mismatch
repair-deficient cancer: Results from the phase II KEYNOTE-158
study. J Clin Oncol. 38:1–10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: Ferroptosis: A regulated cell death nexus linking
metabolism, redox biology, and disease. Cell. 171:273–285. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian
D, Liu D, Zhang F, Ning S, Yao J and Tian X: Ischemia-induced ACSL4
activation contributes to ferroptosis-mediated tissue injury in
intestinal ischemia/reperfusion. Cell Death Differ. 26:2284–2299.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Derry PJ, Hegde ML, Jackson GR, Kayed R,
Tour JM, Tsai AL and Kent TA: Revisiting the intersection of
amyloid, pathologically modified tau and iron in Alzheimer's
disease from a ferroptosis perspective. Prog Neurobiol.
184:1017162020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lang X, Green MD, Wang W, Yu J, Choi JE,
Jiang L, Liao P, Zhou J, Zhang Q, Dow A, et al: Radiotherapy and
immunotherapy promote tumoral lipid oxidation and ferroptosis via
synergistic repression of SLC7A11. Cancer Discov. 9:1673–1685.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C,
Dai X, Li Z and Wu G: Ferroptosis: A novel anti-tumor action for
cisplatin. Cancer Res Treat. 50:445–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun
X, Kang R and Tang D: Ferroptosis: Process and function. Cell Death
Differ. 23:369–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie Z, Hou H, Luo D, An R, Zhao Y and Qiu
C: ROS-dependent lipid peroxidation and reliant antioxidant
ferroptosis-suppressor-protein 1 in rheumatoid arthritis: A covert
clue for potential therapy. Inflammation. 44:35–47. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ikeda Y, Hamano H, Horinouchi Y, Miyamoto
L, Hirayama T, Nagasawa H, Tamaki T and Tsuchiya K: Role of
ferroptosis in cisplatin-induced acute nephrotoxicity in mice. J
Trace Elem Med Biol. 67:1267982021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato M, Kusumi R, Hamashima S, Kobayashi
S, Sasaki S, Komiyama Y, Izumikawa T, Conrad M, Bannai S and Sato
H: The ferroptosis inducer erastin irreversibly inhibits system
xc- and synergizes with cisplatin to increase
cisplatin's cytotoxicity in cancer cells. Sci Rep. 8:9682018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Shen S, Qin J, Fei W, Fan F, Gu J,
Shen T, Zhang T and Cheng X: High co-expression of SLC7A11 and GPX4
as a predictor of platinum resistance and poor prognosis in
patients with epithelial ovarian cancer. BJOG. 129 (Suppl
2):S40–S49. 2022. View Article : Google Scholar
|
|
22
|
Grinberg AV, Hannemann F, Schiffler B,
Müller J, Heinemann U and Bernhardt R: Adrenodoxin: Structure,
stability, and electron transfer properties. Proteins. 40:590–612.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheftel AD, Stehling O, Pierik AJ,
Elsässer HP, Mühlenhoff U, Webert H, Hobler A, Hannemann F,
Bernhardt R and Lill R: Humans possess two mitochondrial
ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis,
heme, and Fe/S cluster biosynthesis. Proc Natl Acad Sci USA.
107:11775–11780. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Y, Ghosh M, Kovtunovych G, Crooks DR
and Rouault TA: Both human ferredoxins 1 and 2 and ferredoxin
reductase are important for iron-sulfur cluster biogenesis. Biochim
Biophys Acta. 1823:484–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Zeng Y, Guo X, Shen H, Zhang J,
Wang K, Ji M and Huang S: Pan-cancer analyses confirmed the
cuproptosis-related gene FDX1 as an immunotherapy predictor and
prognostic biomarker. Front Genet. 13:9237372022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Ma Y, Guo X, Du Y, Zhu Q, Wang X
and Duan C: FDX1 can impact the prognosis and mediate the
metabolism of lung adenocarcinoma. Front Pharmacol. 12:7491342021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okamoto D, Yamauchi N, Takiguchi G,
Nishita M, Kakeji Y, Minami Y and Kamizaki K: Autonomous and
intercellular chemokine signaling elicited from mesenchymal stem
cells regulates migration of undifferentiated gastric cancer cells.
Genes Cells. 27:368–375. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avincsal MO, Kamizaki K, Jimbo N,
Shinomiya H, Nibu KI, Nishita M and Minami Y: Oncogenic E6 and/or
E7 proteins drive proliferation and invasion of human papilloma
virus-positive head and neck squamous cell cancer through
upregulation of Ror2 expression. Oncol Rep. 46:1482021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Behrens BC, Hamilton TC, Masuda H,
Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T, McKoy WM, Young
RC and Ozols RF: Characterization of a
cis-diamminedichloroplatinum(II)-resistant human ovarian cancer
cell line and its use in evaluation of platinum analogues. Cancer
Res. 47:414–418. 1987.PubMed/NCBI
|
|
30
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Target Ther. 7:3782022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363.e3. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ciriello G, Miller ML, Aksoy BA,
Senbabaoglu Y, Schultz N and Sander C: Emerging landscape of
oncogenic signatures across human cancers. Nat Genet. 45:1127–1133.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cancer Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hollis RL, Thomson JP, Stanley B,
Churchman M, Meynert AM, Rye T, Bartos C, Iida Y, Croy I, Mackean
M, et al: Molecular stratification of endometrioid ovarian
carcinoma predicts clinical outcome. Nat Commun. 11:49952020.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Noriega-Rivera R, Rivera-Serrano M,
Rabelo-Fernandez RJ, Pérez-Santiago J, Valiyeva F and Vivas-Mejía
PE: Upregulation of the long noncoding RNA CASC10 promotes
cisplatin resistance in high-grade serous ovarian cancer. Int J Mol
Sci. 23:77372022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Viscarra T, Buchegger K, Jofre I, Riquelme
I, Zanella L, Abanto M, Parker AC, Piccolo SR, Roa JC, Ili C and
Brebi P: Functional and transcriptomic characterization of
carboplatin-resistant A2780 ovarian cancer cell line. Biol Res.
52:132019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun J, Cai X, Yung MM, Zhou W, Li J, Zhang
Y, Li Z, Liu SS, Cheung ANY, Ngan HYS, et al: miR-137 mediates the
functional link between c-Myc and EZH2 that regulates cisplatin
resistance in ovarian cancer. Oncogene. 38:564–580. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Meng Y, Chen CW, Yung MMH, Sun W, Sun J,
Li Z, Li J, Li Z, Zhou W, Liu SS, et al: DUOXA1-mediated ROS
production promotes cisplatin resistance by activating ATR-Chk1
pathway in ovarian cancer. Cancer Lett. 428:104–116. 2018.
View Article : Google Scholar : PubMed/NCBI
|