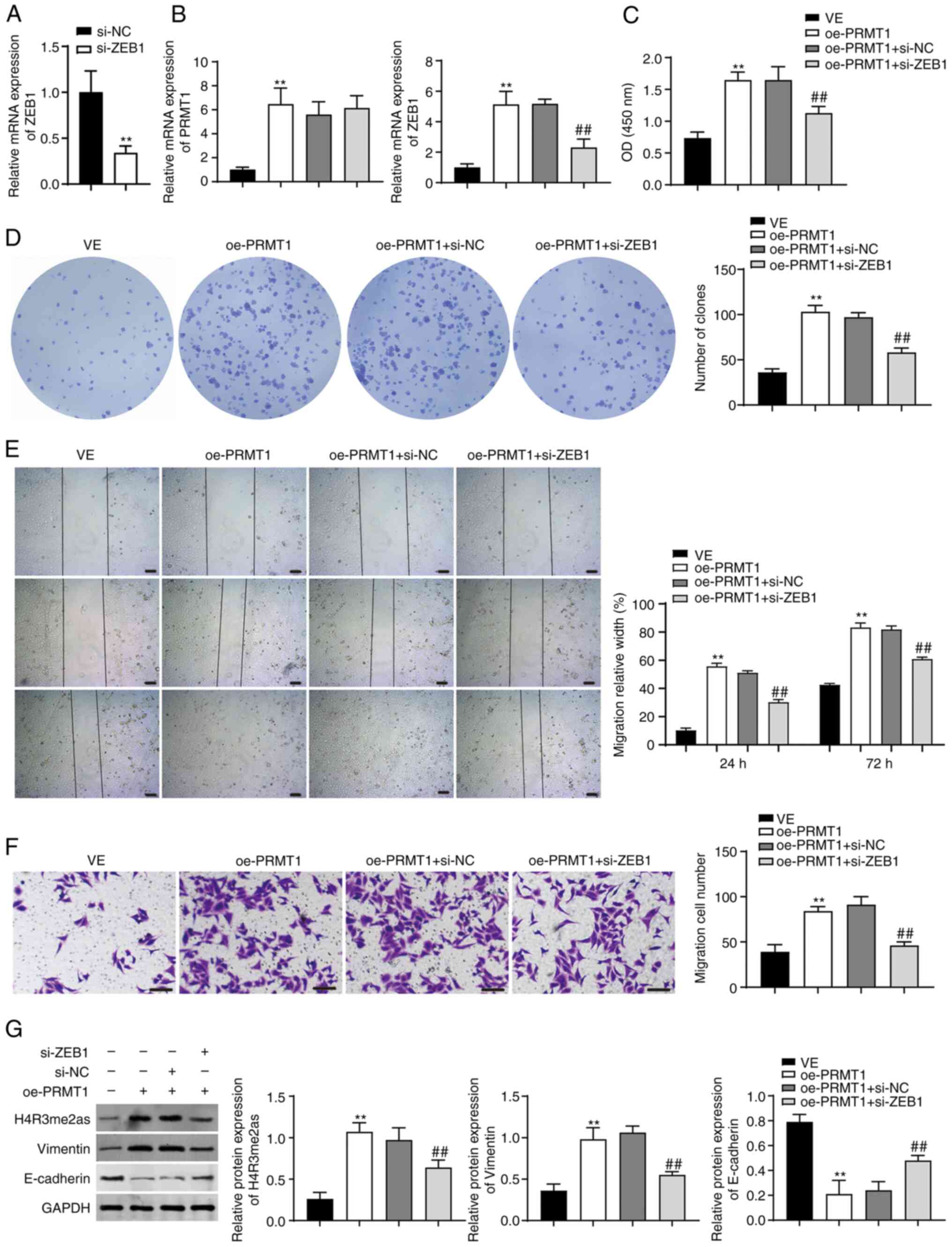
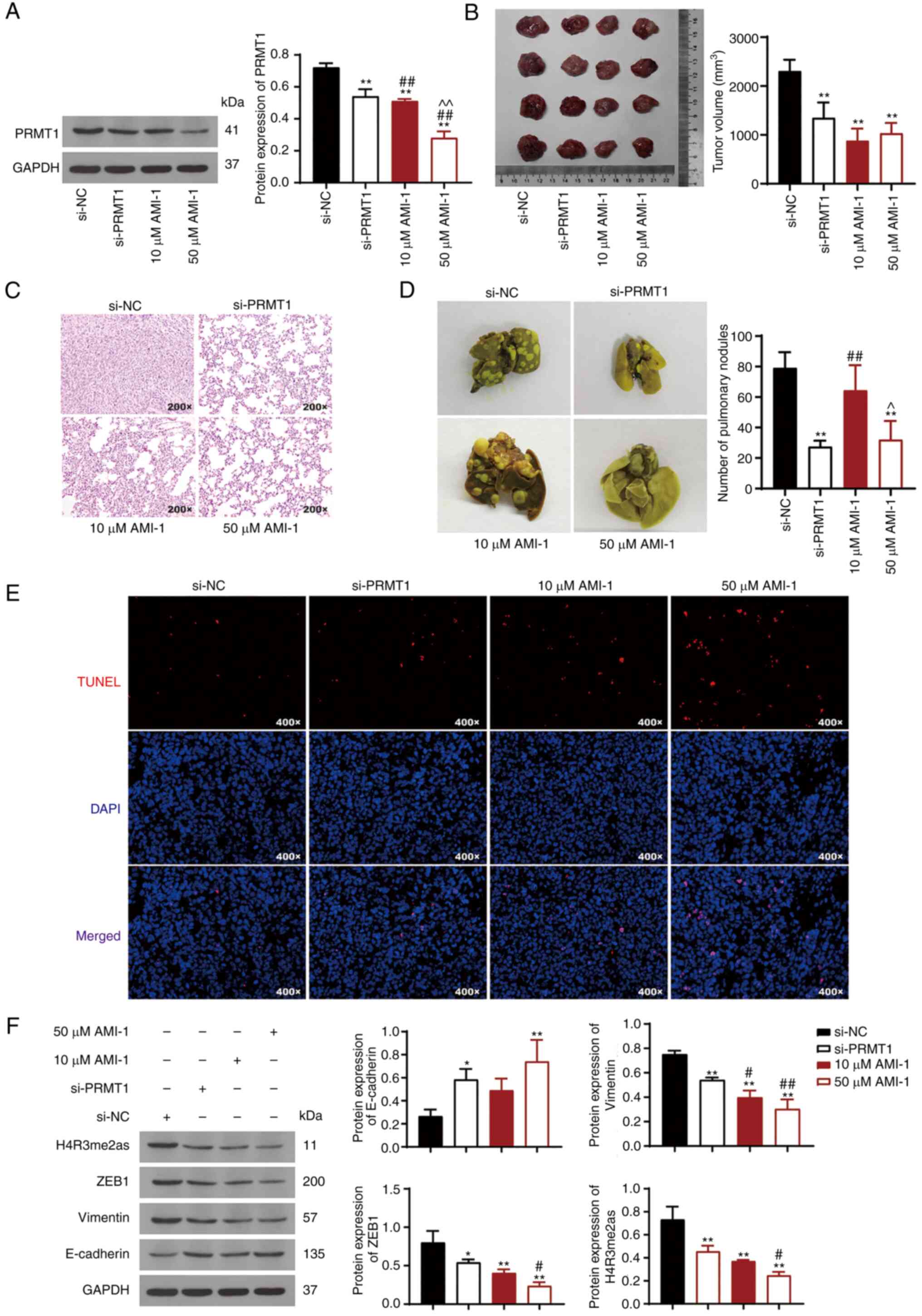
|
1
|
Kleinschmidt-DeMasters B and Marshall C:
Thyroid carcinoma metastases to central nervous system and
vertebrae. Folia Neuropathol. 60:292–300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pan Z, Xu T, Bao L, Hu X, Jin T, Chen J,
Chen J, Qian Y, Lu X, Li L, et al: CREB3L1 promotes tumor growth
and metastasis of anaplastic thyroid carcinoma by remodeling the
tumor microenvironment. Mol Cancer. 21:1902022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deng Y, Li X, Jiang W and Tang J: SNRPB
promotes cell cycle progression in thyroid carcinoma via inhibiting
p53. Open Med (Wars). 17:1623–1631. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Franchini F, Palatucci G, Colao A, Ungaro
P, Macchia PE and Nettore IC: Obesity and thyroid cancer risk: An
update. Int J Environ Res Public Health. 19:11162022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan Y, Wu L, He S, Wu J, Wang T and Zang
H: Identification of hub genes in thyroid carcinoma to predict
prognosis by integrated bioinformatics analysis. Bioengineered.
12:2928–2940. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun R, Yang L, Wang Y, Zhang Y, Ke J and
Zhao D: DNAJB11 predicts a poor prognosis and is associated with
immune infiltration in thyroid carcinoma: A bioinformatics
analysis. J Int Med Res. 49:30006052110537222021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geng X, Sun Y, Fu J, Cao L and Li Y:
MicroRNA-17-5p inhibits thyroid cancer progression by suppressing
Early growth response 2 (EGR2). Bioengineered. 12:2713–2722. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv L, Wang X, Shen J, Cao Y and Zhang Q:
MiR-574-3p inhibits glucose toxicity-induced pancreatic β-cell
dysfunction by suppressing PRMT1. Diabetol Metab Syndr. 14:992022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YC, Wang CW, Lin WC, Tsai YJ, Chang
CP, Lee YJ, Lin MJ and Li C: Identification, chromosomal
arrangements and expression analyses of the evolutionarily
conserved prmt1 gene in chicken in comparison with its vertebrate
paralogue prmt8. PLoS One. 12:e01850422017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao B, Gui T, Zeng X, Deng Y, Wang Z, Wang
Y, Yang D, Li Q, Xu P, Hu R, et al: PRMT1-mediated H4R3me2a
recruits SMARCA4 to promote colorectal cancer progression by
enhancing EGFR signaling. Genome Med. 13:582021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim E, Jang J, Park JG, Kim KH, Yoon K,
Yoo BC and Cho JY: Protein Arginine methyltransferase 1 (PRMT1)
selective inhibitor, TC-E 5003, has anti-inflammatory properties in
TLR4 Signaling. Int J Mol Sci. 21:30582020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsubara H, Fukuda T, Awazu Y, Nanno S,
Shimomura M, Inoue Y, Yamauchi M, Yasui T and Sumi T: PRMT1
expression predicts sensitivity to platinum-based chemotherapy in
patients with ovarian serous carcinoma. Oncol Lett. 21:1622021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Dong L, Zhao Z, Zhang Z, Sun Y, Li
C, Li G, You X, Yang X, Wang H and Hong W: Design and Synthesis of
Novel PRMT1 inhibitors and investigation of their effects on the
migration of cancer cell. Front Chem. 10:8887272022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gu X, He M, Lebedev T, Lin CH, Hua ZY,
Zheng YG, Li ZJ, Yang JY and Li XG: PRMT1 is an important factor
for medulloblastoma cell proliferation and survival. Biochem
Biophys Rep. 32:1013642022.PubMed/NCBI
|
|
15
|
Vezzalini M, Aletta JM, Beghelli S,
Moratti E, Della Peruta M, Mafficini A, Mojica WD, Mombello A,
Scarpa A and Sorio C: Immunohistochemical detection of arginine
methylated proteins (MeRP) in archival tissues. Histopathology.
57:725–733. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Liu X, Liang W, Dean DC, Zhang L
and Liu Y: Expression and Function of ZEB1 in the Cornea. Cells.
10:9252021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Lai Q, He J, Li Q, Ding J, Lan Z,
Gu C, Yan Q, Fang Y, Zhao X and Liu S: LncRNA SNHG6 promotes
proliferation, invasion and migration in colorectal cancer cells by
activating TGF-β/Smad signaling pathway via targeting UPF1 and
inducing EMT via regulation of ZEB1. Int J Med Sci. 16:51–59. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen XJ, Deng YR, Wang ZC, Wei WF, Zhou
CF, Zhang YM, Yan RM, Liang LJ, Zhong M, Liang L, et al:
Hypoxia-induced ZEB1 promotes cervical cancer progression via
CCL8-dependent tumour-associated macrophage recruitment. Cell Death
Dis. 10:5082019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen H, Zhu H, Chen Y, Shen Z, Qiu W, Qian
C and Zhang J: ZEB1-induced LINC01559 expedites cell proliferation,
migration and EMT process in gastric cancer through recruiting
IGF2BP2 to stabilize ZEB1 expression. Cell Death Dis. 12:3492021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Filipović J, Bosić M, Ćirović S, Životić
M, Dunđerović D, Đorđević D, Živković-Perišić S, Lipkovski A and
Marković-Lipkovski J: PRMT1 expression in renal cell
tumors-application in differential diagnosis and prognostic
relevance. Diagn Pathol. 14:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Z, Chen Y, Lin Z, Chen C and Dong Y:
Overexpressing PRMT1 inhibits proliferation and invasion in
pancreatic cancer by inverse correlation of ZEB1. IUBMB Life.
70:1032–1039. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saiselet M, Floor S, Tarabichi M, Dom G,
Hébrant A, van Staveren WC and Maenhaut C: Thyroid cancer cell
lines: An overview. Front Endocrinol (Lausanne). 3:1332012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pozdeyev N, Berlinberg A, Zhou Q, Wuensch
K, Shibata H, Wood WM and Haugen BR: Targeting the NF-κB pathway as
a combination therapy for advanced thyroid cancer. PLoS One.
10:e01349012015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sung BY, Lin YH, Kong Q, Shah PD, Glick
Bieler J, Palmer S, Weinhold KJ, Chang HR, Huang H, Avery RK, et
al: Wnt activation promotes memory T cell polyfunctionality via
epigenetic regulator PRMT1. J Clin Invest. 132:e1405082022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiebaut C, Eve L, Poulard C and Le
Romancer M: Structure, activity, and function of PRMT1. Life
(Basel). 11:11472021.PubMed/NCBI
|
|
27
|
Yin XK, Wang YL, Wang F, Feng WX, Bai SM,
Zhao WW, Feng LL, Wei MB, Qin CL, Wang F, et al: PRMT1 enhances
oncogenic arginine methylation of NONO in colorectal cancer.
Oncogene. 40:1375–1389. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giuliani V, Miller MA, Liu CY, Hartono SR,
Class CA, Bristow CA, Suzuki E, Sanz LA, Gao G, Gay JP, et al:
PRMT1-dependent regulation of RNA metabolism and DNA damage
response sustains pancreatic ductal adenocarcinoma. Nat Commun.
12:46262021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimomura M, Fukuda T, Awazu Y, Nanno S,
Inoue Y, Matsubara H, Yamauchi M, Yasui T and Sumi T: PRMT1
expression predicts response to neoadjuvant chemotherapy for
locally advanced uterine cervical cancer. Oncol Lett. 21:1502021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Wang D, Chen X, Wang W, Wang P, Hou
P, Li M, Chu S, Qiao S, Zheng J and Bai J: PRMT1-mediated EZH2
methylation promotes breast cancer cell proliferation and
tumorigenesis. Cell Death Dis. 12:10802021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan G, Liu Y, Shang L, Zhou F and Yang S:
EMT-associated microRNAs and their roles in cancer stemness and
drug resistance. Cancer Commun (Lond). 41:199–217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang X, Xiang L, Wang B, Hu J, Liu C, Ren
A, Du K, Ye G, Liang Y, Tang Y, et al: CMTM6 promotes migration,
invasion, and EMT by interacting with and stabilizing vimentin in
hepatocellular carcinoma cells. J Transl Med. 19:1202021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Na TY, Schecterson L, Mendonsa AM and
Gumbiner BM: The functional activity of E-cadherin controls tumor
cell metastasis at multiple steps. Proc Natl Acad Sci USA.
117:5931–5937. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Usman S, Waseem NH, Nguyen TKN, Mohsin S,
Jamal A, The MT and Waseem A: Vimentin is at the heart of
epithelial mesenchymal transition (EMT) mediated metastasis.
Cancers (Basel). 13:49852021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wagner S, Weber S, Kleinschmidt MA, Nagata
K and Bauer UM: SET-mediated promoter hypoacetylation is a
prerequisite for coactivation of the estrogen-responsive pS2 gene
by PRMT1. J Biol Chem. 281:27242–27250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao Y, Lu Q, Li C, Wang X, Jiang L, Huang
L, Wang C and Chen H: PRMT1 regulates the tumour-initiating
properties of esophageal squamous cell carcinoma through histone H4
arginine methylation coupled with transcriptional activation. Cell
Death Dis. 10:3592019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ramundo V, Zanirato G and Aldieri E: The
epithelial-to-mesenchymal transition (EMT) in the development and
metastasis of malignant pleural mesothelioma. Int J Mol Sci.
22:122162021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu HT, Zhong HT, Li GW, Shen JX, Ye QQ,
Zhang ML and Liu J: Oncogenic functions of the EMT-related
transcription factor ZEB1 in breast cancer. J Transl Med.
18:512020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jin Z and Chen B: LncRNA ZEB1-AS1
regulates colorectal cancer cells by MiR-205/YAP1 Axis. Open Med
(Wars). 15:175–184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu W, Zheng L, Zhang R, Hou P, Wang J, Wu
L and Li J: Circ-ZEB1 promotes PIK3CA expression by silencing
miR-199a-3p and affects the proliferation and apoptosis of
hepatocellular carcinoma. Mol Cancer. 21:722022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Guan W, Xu X, Wang F, Li X and Xu
G: A novel homeostatic loop of sorcin drives paclitaxel-resistance
and malignant progression via Smad4/ZEB1/miR-142-5p in human
ovarian cancer. Oncogene. 40:4906–4918. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Morillo-Bernal J, Fernández LP and
Santisteban P: FOXE1 regulates migration and invasion in thyroid
cancer cells and targets ZEB1. Endocr Relat Cancer. 27:137–151.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Soleymani L, Zarrabi A, Hashemi F, Hashemi
F, Zabolian A, Banihashemi SM, Moghadam SS, Hushmandi K,
Samarghandian S, Ashrafizadeh M and Khan H: Role of ZEB Family
Members in Proliferation, Metastasis, and Chemoresistance of
Prostate Cancer Cells: Revealing Signaling Networks. Curr Cancer
Drug Targets. 21:749–767. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Iderzorig T, Kellen J, Osude C, Singh S,
Woodman JA, Garcia C and Puri N: Comparison of EMT mediated
tyrosine kinase inhibitor resistance in NSCLC. Biochem Biophys Res
Commun. 496:770–777. 2018. View Article : Google Scholar : PubMed/NCBI
|