Introduction
Lung cancer is currently the leading cause of
cancer-related deaths. The GLOBOCAN 2020 estimates of cancer
incidence and mortality prepared by the International Agency for
Research on Cancer reported an estimated 1.8 million deaths from
lung cancer, representing 18% of all cancer-related deaths, in 2020
worldwide (1). Lung cancer staging
is currently performed using the 8th edition of the
tumor-node-metastasis (TNM) classification (2). The World Health Organization (WHO)
classification of tumors was revised in 2021 (3) prior to the 9th edition of the TNM
classification.
Lung adenocarcinoma, a form of non-small cell lung
cancer (NSCLC), one of the main subtypes of lung cancer, consists
of adenocarcinoma in situ, minimally invasive adenocarcinoma
(MIA), invasive mucinous adenocarcinoma (IMA) and invasive
non-mucinous adenocarcinoma (INMA). INMA is classified into three
histological grades. Various agents have been developed for the
treatment of NSCLC (4–8). For patients with very early-stage
NSCLC, reduced surgery such as segmentectomy or partial resection
is performed (9–11). However, recurrence is a challenge in
these patients, and treatment of cases with recurrence is limited.
Therefore, the identification of prognostic markers to predict
recurrence is critical.
Several biomarkers for predicting therapeutic
efficacy in patients with lung cancer have been identified, such as
driver gene mutations for various molecular-targeted drugs and
programmed death-ligand 1 (PD-L1) and tumor proportion score (TPS)
for programmed death-1 (PD-1) antibody therapy. Loss of function
alterations in RB1, TP53 and STK11/LKB1 have also
previously attracted attention as prognostic biomarkers for drug
therapy (12,13). However, the development of
biomarkers for recurrence in surgically resected NSCLC has not
progressed. The most accurate prognostic factor for surgically
resected early-stage NSCLC is the TNM classification. The
International Association for the Study of Lung Cancer Pathology
Committee proposed histological grade as a prognostic factor in
INMA. The combination of predominant and worst histological
patterns significantly improved patient outcome prediction in
early-stage resected lung adenocarcinomas, and it was superior to
mitotic count, nuclear grade, cytological grade, tumor spread
through air spaces and necrosis (14). While in breast cancer, for example,
genomic assays are used to predict recurrence and determine the
administration of postoperative adjuvant therapy (15), treatment decisions in lung cancer
depend on the TNM classification.
Casein kinase 2 (CK2) is a serine/threonine kinase
that is essential for eukaryote cell survival. CK2α is the
catalytic subunit of CK2. The first study linking CK2 and
malignancy was in CK2α transgenic mice, which were reported to
develop T lymphomas (16). CK2 is
considered one of the driver kinases of carcinogenesis, and
overexpression of CK2α has been reported in various types of cancer
(17–19). Elevated nuclear CK2α protein levels
were observed in squamous cell carcinoma of the head and neck
(20) and breast cancer (21), and its high expression was
associated with poor clinical outcomes. CK2 regulates various
hallmarks of cancer (22),
particularly via its association with nuclear transcription factors
and its involvement in ribosomal gene transcription during rRNA
synthesis (23,24). Therefore, its localization in the
nucleus, especially in the nucleolus, is considered to be important
for its function (23,24). It was previously found that the
nucleolar localization of CK2α was a potential poor prognostic
factor in invasive breast carcinoma (25). Nucleolar CK2α is involved in
inflammatory pathways (26).
Cancers are also characterized by an increased inflammatory burden
(27,28). Thus, studying nucleolar CK2α in lung
cancer is pertinent in the present study. CK2 is known to play a
critical role in both innate and adaptive immune cells (29). CK2 is involved in i) activating
PI3K/AKT/mTOR pathway by phosphorylating AKT at S129 to induce
proliferation and cancer metastasis (30,31);
ii) activating the NF-kB signaling pathway by phosphorylating p65
at S529 (32); and iii) activating
the JAK/STAT pathway by phosphorylating JAK, to induce inflammation
and immune response (33–35). CK2 inhibition by using low molecular
weight inhibitor demonstrated potent antitumor effects in
combination with immunotherapy. The inhibitor resulted in a
decrease of tumor-associated macrophages and polymorphonuclear
myeloid-derived suppressor cells in the tumor microenvironment
(36). In pan-cancer analysis, CK2
alpha protein 1 expression had positive correlations with
M1-macrophages and fibroblasts, and negative correlations with
CD8+ T cells and NK cells (37).
In the present study, the relationship between CK2α
nucleolar localization and patient prognosis was examined. The
subcellular localization of CK2α in surgically resected lung
adenocarcinomas was determined by immunohistochemistry. Focus was
addressed on adenocarcinoma, which is the same histological type as
the breast carcinoma in our previous study (25) and the most frequent type of
NSCLC.
Materials and methods
Antibody generation
For the production of recombinant human protein
kinase CK2α (gene name CSKN2A1), the cDNA was subcloned into
the pGEX-4T plasmid (Amersham; Cytiva). The GST fusion protein was
expressed in Escherichia coli strain BL21(DE3) and then
purified as a GST tag-free protein to the single band level
(24). A total of four BALB/c BDF1
female mice (6 weeks old) which were housed (20°C, auto-fresh
ventilation of 14–15 times/h, 12/12-h light/dark cycle) at
Immuno-Biological Laboratories Co., Ltd., according to the
Guideline and the Law for the Human Treatment and Management of
Animals, were immunized with 50 µg of full-length CK2α five times
in weekly intervals. Sequential screening of mouse hybridoma clones
was performed by enzyme-linked immunosorbent assay coated with
serial dilution of recombinant full-length human CK2α or CK2α', and
then western blotting by using 20 µg of cultured 293 cell cytosolic
lysates with or without exogenously expressed human CK2α, which
were solubilized by the lysis buffer containing 10 mM Hepes (pH
7.4), 20 mM NaCl, 25 mM β-glycerophosphate, 1.5 mM
MgCl2, 0.5 mM Na3VO4, 1 µg/ml of
aprotinin, 0.5 mM PMSF, separated by 10% SDS-PAGE gels and
transferred to PVDF membrane. Briefly, the detection of antigen,
CK2α, was evaluated as follows: PVDF membrane was blocked with 5%
BSA in Tris-HCl (pH 7.4) containing 150 mM NaCl for 30 min at room
temperature; then primary anti-CK2α monoclonal antibody as purified
IgG was used at the concentration of 0.1 µg/ml for 1 h at room
temperature, followed by incubation with secondary anti-mouse
IgG-peroxidase conjugated antibody (1:2,000; cat. no. 6789; Abcam)
for 30 min, and detected by Chemiluminescent Detection Kit (cat.
no. 32209; Thermo Fisher Scientific, Inc.) as previously described
(24). A total of >6 clones with
high affinity and specificity to CK2α both in vitro and
in vivo that did not cross-react with CK2α' were selected
(Fig. S1). The protein A-purified
IgG fraction derived from the hybridoma clone 6A3, subclass mouse
IgG2b κ, was used in the present study.
Patients
A total of 118 patients (64 males and 54 females)
with lung adenocarcinoma who had undergone pulmonary lobectomy as
complete resection between January 2014 and December 2018 at
Fukushima Medical University Hospital (Fukushima, Japan) were
enrolled. Median age was 69.5 (range; 40–86) years old. The
patients did not receive neoadjuvant chemotherapy, radiotherapy, or
immunotherapy before surgery. Pathological staging was evaluated
using the International Staging System for Lung Tumors, 8th edition
(2,38). Up to 2016, patients were
re-diagnosed by pathologists using the 8th edition of the TNM
classification. All patients were pathologically reclassified by
pathologists following the WHO Classification of Tumors: Thoracic
Tumors 5th Edition (3). For INMA,
histological grade was evaluated and categorized by pathologists as
follows: Grade 1, well-differentiated; grade 2, moderately
differentiated; and grade 3, poorly differentiated (3,14). The
present study was conducted according to the guidelines of the
Declaration of Helsinki and was approved (approval no. 30113;
August 30, 2022) by the institutional Ethics Committee of Fukushima
Medical University (Fukushima, Japan). Verbal informed consent was
obtained from all subjects involved in the study.
Immunohistochemistry
Paraffin-embedded tumor specimens were cut into 4-µm
thick sections. For rehydration, Tissue-Tek Prisma® Plus
was used (Sakura FineTek Japan Co. Ltd.) following the
manufacturer's protocol. Briefly, by descending concentration of
ethanol from 99.5, 95, to 80% in every 3 min. After rehydration and
antigen retrieval, the sections were autoclaved at 121°C for 10 min
in 10 mM citrate-Na buffer at pH 8.0. Following incubation with
1:200 normal serum (Vector Laboratories, Inc.) for 30 min at room
temperature, the sections were incubated at 4°C with primary
monoclonal antibody against CK2α (6A3) overnight at a concentration
of 0.1 µg/ml in phosphate-buffered saline containing 1% bovine
serum albumin (cat. no. A8531; MilliporeSigma). The primary
antibody was detected using the avidin-biotin-peroxidase complex
method. Goat anti-mouse biotinylated IgG (H + L; 1:250; cat. no.
BA-9200; Vector Laboratories, Inc.) was incubated at room
temperature for 30 min, followed by VECTASTAIN ABC-HRP Kit (cat.
no. PK-6100; Vector Laboratories Inc.) according to the
manufacturer's protocol. The sections were not counterstained with
hematoxylin to avoid false positive staining of nucleoli. After
incubation with diaminobenzidine (Dojindo Laboratories, Inc.) for
40–80 sec, the sections were mounted on glass slides. The
immunoreactivity of each specimen was scored independently by two
pathologists using a light microscope based on the random selection
of at least three tumor areas. CK2 staining of each specimen was
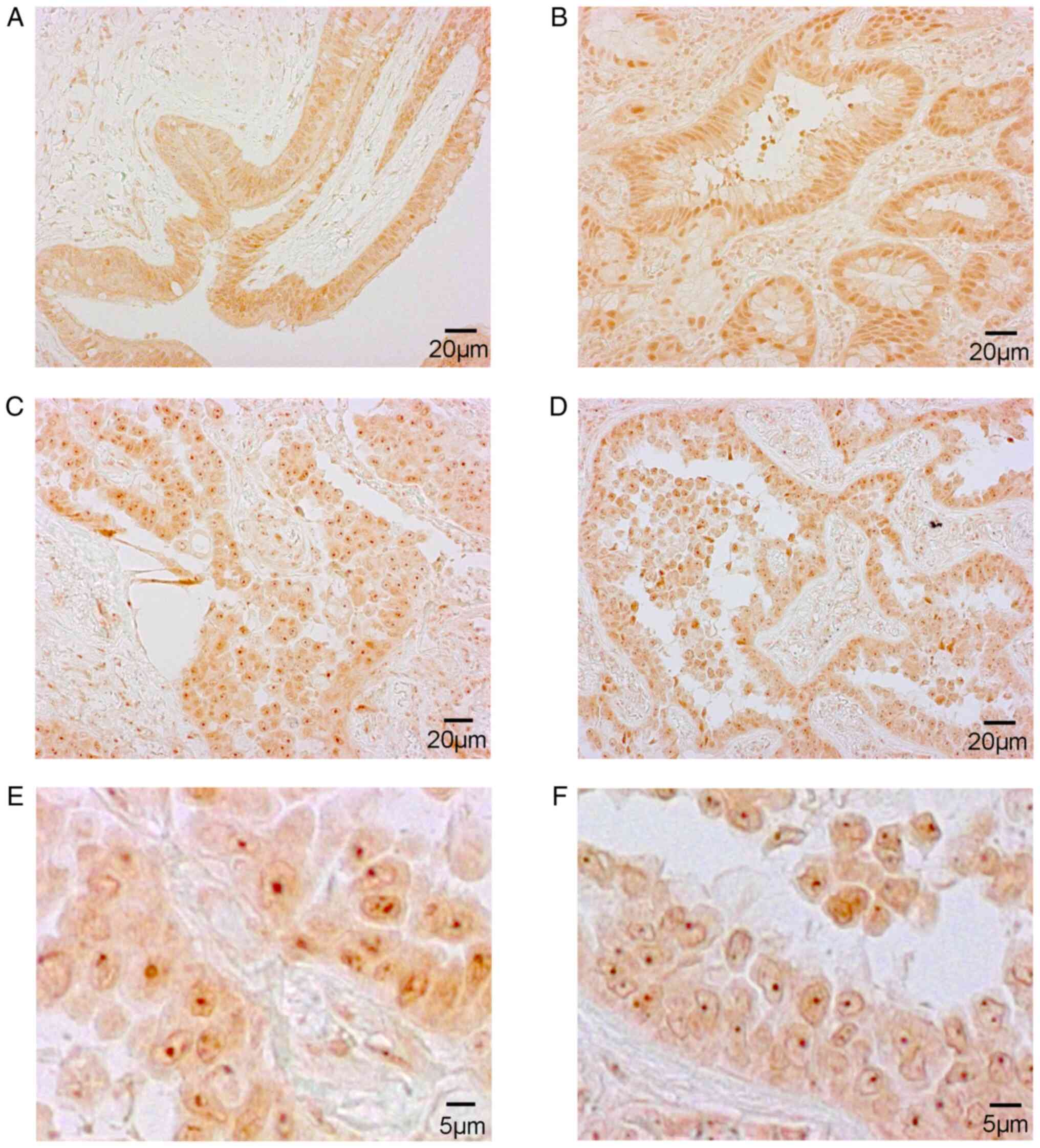
evaluated as follows (25): I,
nuclear staining was not visible, but cell bodies were stained; II,
nuclear staining was more obvious compared with cytosolic staining;
III, nuclear staining was more intense than in category II; IV,
positive nucleolar staining was evident and nuclear staining was
observed; and V, staining was mostly confined to nucleoli, but
without intense staining of the nucleoplasm.
In some analyses, patients were categorized into two
groups: Patients with nucleolar CK2α staining (categories IV and V)
and those without nucleolar CK2α staining (categories I–III).
The EML4-ALK fusion protein was evaluated in 68
patients using the Nichirei Histofine ALK iAEP Kit (Nichirei
Biosciences Inc.) (39). PD-L1 TPS
was evaluated in 43 patients using a PD-L1 IHC 22C3 pharmDx
immunohistochemistry assay on the Dako Autostainer Link 48 at SRL,
Inc. PD-L1 TPS was defined as the percentage of viable tumor cells
with partial or complete membrane staining for PD-L1 (40). EGFR mutations were evaluated
in surgically resected tissue from 82 patients using the cobas EGFR
Mutation Test v2 at SRL, Inc (41).
These 68, 43 and 82 patients were randomly selected from the 118
patients.
Statistical analysis
The associations between nucleolar CK2α expression
and pathological parameters (pathological stage, histological type
and histological grade) were examined. Survival curves were created
using the Kaplan-Meier method and analyzed in patients with and
without nucleolar CK2α staining using the log-rank test which was
performed using GraphPad Prism software v8.4.3 (GraphPad Software,
Inc.; Dotmatics). Recurrence-free survival (RFS) and overall
survival (OS) were defined as the time from surgery to relapse and
the time from surgery to death from any cause, respectively. The
Cox proportional regression model using the forward stepwise
likelihood ratio method was performed to identify prognostic
factors of survival using SPSS software v29 (IBM Corp.). The JMP
Pro v17.0 platform (JMP Statistical Discovery LLC) was used to
examine the relationship between nucleolar CK2α expression and
recurrence in early-stage patients.
Results
CK2 localization in nuclei of cancer
cells in lung adenocarcinoma
The characteristics of the 118 lung adenocarcinoma
patients included in the present study are included in Table I. The intracellular localization of
CK2 in tumor samples was categorized as aforementioned.
Representative images of the five categories of CK2α expression are
shown in Fig. 1.
 | Table I.Patient characteristics (N=118). |
Table I.
Patient characteristics (N=118).
|
Characteristics | Value |
|---|
| Median age, years
(range) | 69.5 (40–86) |
| Sex
(male/female) | 64/54 |
| Smoking status |
|
|
Never | 50 |
| Former
or current | 68 |
| Pathological stage
(8th) |
|
| 0 | 15 |
|
IA1 | 35 |
|
IA2 | 21 |
|
IA3 | 14 |
| IB | 13 |
|
IIA | 2 |
|
IIB | 10 |
|
IIIA | 8 |
| Histological
type |
|
|
Adenocarcinoma in
situ | 15 |
|
Minimally invasive
adenocarcinoma | 16 |
|
Invasive mucinous
adenocarcinoma | 5 |
|
Invasive non-mucinous
adenocarcinoma | 82 |
| Histological
grade |
|
| 1 | 11 |
| 2 | 42 |
| 3 | 29 |
| Epidermal growth
factor receptor mutation |
|
| Ex 19
del | 18 |
| Ex 21
L858R | 20 |
| Ex 19
del + T790M | 1 |
| Ex 21
L858R + T790M | 1 |
| Ex 21
L861Q | 1 |
| Ex 21
insertion | 1 |
| ND | 40 |
| NA | 36 |
| Anaplastic lymphoma
kinase rearrangement |
|
|
Positive | 2 |
| ND | 66 |
| NA | 50 |
| Programmed
death-ligand 1 tumor proportion score |
|
|
<1% | 16 |
|
1–49% | 18 |
|
≥50% | 9 |
| NA | 75 |
CK2α staining was localized to the nucleoli of
cancer cells (category IV and V) in 60 of 118 lung adenocarcinoma
tumors (50.8%; Table SI). There
were no category I specimens in the patient group. The relationship
between CK2α staining status, nucleolar CK2α status and
histopathological diagnosis is summarized in Table II. There were no apparent
associations between CK2α staining status or nucleolar CK2α status
and pathological stage, histological type and histological grade in
INMA, the main subtype of lung adenocarcinoma.
 | Table II.Relationship between CK2α staining
status and nucleolar CK2α status with histopathological
diagnosis. |
Table II.
Relationship between CK2α staining
status and nucleolar CK2α status with histopathological
diagnosis.
|
| Pathological
stage | Histological
type |
|---|
|
|
|
|
|---|
|
| 0 | I | II | III | AIS | MIA | IMA | INMA |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
| Histological
grade |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
| 1 | 2 | 3 |
|---|
| CK2α staining |
|
|
|
|
|
|
|
|
|
|
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1 | 6 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 3 |
| 3 | 7 | 37 | 2 | 4 | 7 | 7 | 3 | 5 | 16 | 12 |
| 4 | 5 | 28 | 5 | 1 | 5 | 6 | 0 | 2 | 18 | 8 |
| 5 | 2 | 12 | 5 | 2 | 2 | 2 | 1 | 3 | 7 | 6 |
| Nucleolar CK2α |
|
|
|
|
|
|
|
|
|
|
| – | 8 | 43 | 2 | 5 | 8 | 8 | 4 | 6 | 17 | 15 |
| + | 7 | 40 | 10 | 3 | 7 | 8 | 1 | 5 | 25 | 14 |
Nucleolar CK2 is associated with poor
prognosis in surgically resected early-stage lung
adenocarcinoma
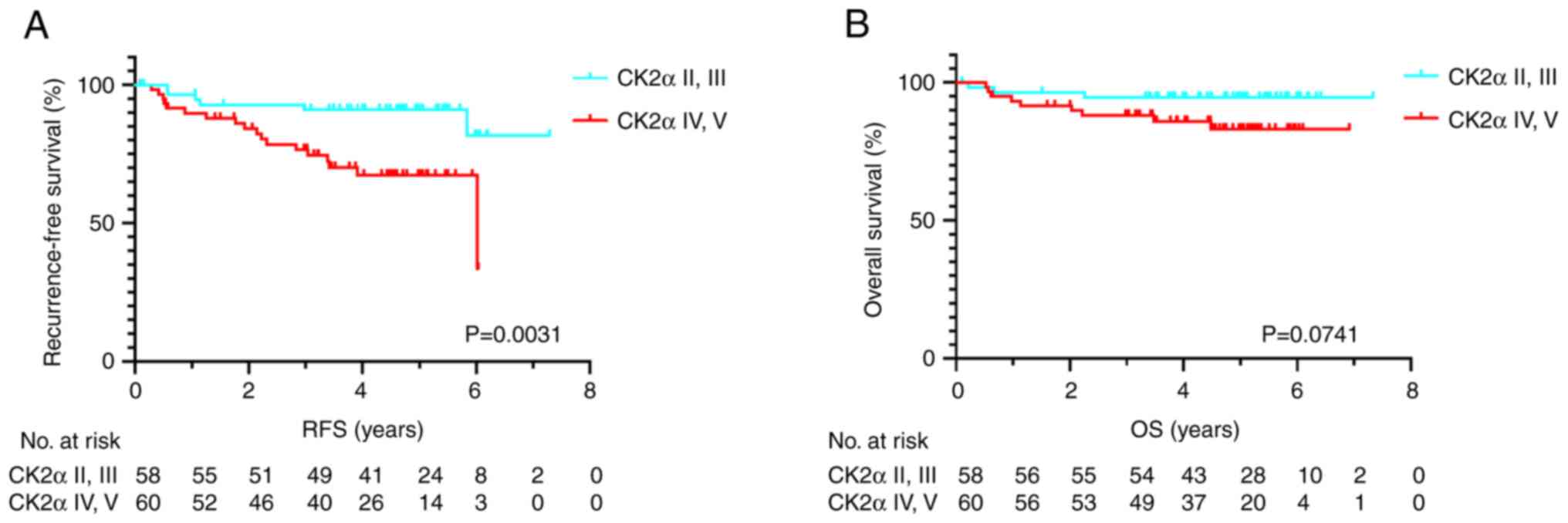
Nucleolar CK2α staining in relation to RFS and OS
was next investigated. Among the 118 patients, 24 (20.3%)
experienced lung cancer recurrence and 12 (10.2%) patients
succumbed to any cause. The RFS time was significantly shorter in
the positive nucleolar CK2α staining group compared with the
negative group according to the log-rank test (P=0.0031; Fig. 2A). The OS time tended to be shorter
in the positive nucleolar CK2α staining group than the negative
group but without statistical significance (P=0.0741; Fig. 2B). The median RFS and OS were not
reached in all groups.
The sites of first recurrence in the 24 recurrent
cases were the lung (n=11), bone (n=8), mediastinal lymph nodes
(n=4), and hilar lymph node, pleural dissemination, brain, and
kidney (n=1 each). The sites of first recurrence in the
CK2α-positive cases were the lung (n=9), bone (n=5), mediastinal
lymph nodes (n=4), and hilar lymph node, pleural dissemination, and
brain (n=1 each).
Multivariate analysis revealed that lymph node
metastasis and positive nucleolar CK2α staining were poor
prognostic factors for RFS (Table
III). Lymphatic invasion was the only poor prognostic factor
for OS (Table IV).
 | Table III.Univariate and Cox regression
multivariable stepwise procedure of recurrence-free survival in all
patients (N=118). |
Table III.
Univariate and Cox regression
multivariable stepwise procedure of recurrence-free survival in all
patients (N=118).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | No. | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥70
years) | 59 | 1.004 | 0.469–2.324 | 0.916 |
|
|
|
| Sex (male) | 64 | 1.732 | 0.737–4.072 | 0.208 |
|
|
|
| Lymph node
metastasis (positive) | 14 | 13.382 | 5.724–31.284 | <0.001 | 11.448 | 4.890–26.803 | <0.001 |
| p-stage
(II–III) | 20 | 10.745 | 4.565–25.292 | <0.001 |
|
|
|
| Lymphatic invasion
(positive) | 18 | 7.470 | 3.287–16.977 | <0.001 |
|
|
|
| Microscopic
vascular invasion (positive) | 25 | 4.148 | 1.829–9.411 | 0.001 |
|
|
|
| Histological type
(invasive mucinous adenocarcinoma) | 5 | 1.718 | 0.231–12.789 | 0.597 |
|
|
|
| Epidermal growth
factor receptor gene mutation (Neg and NE) | 76 | 0.617 | 0.276–1.382 | 0.241 |
|
|
|
| Anaplastic lymphoma
kinase gene translocation (Neg and NE) | 116 | 20.746 |
<0.001–1000< | 0.649 |
|
|
|
| Nucleolar casein
kinase 2 alpha (positive) | 60 | 3.745 | 1.470–9.541 | 0.006 | 3.038 | 1.178–7.836 | 0.022 |
 | Table IV.Univariate and Cox regression
multivariable stepwise procedure of overall survival in all
patients (N=118). |
Table IV.
Univariate and Cox regression
multivariable stepwise procedure of overall survival in all
patients (N=118).
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | No. | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≥70) | 59 | 2.105 | 0.634–6.991 | 0.224 |
|
|
|
| Sex (male) | 64 | 4.455 | 0.976–20.340 | 0.054 |
|
|
|
| Lymph node
metastasis (positive) | 14 | 5.787 | 1.835–18.250 | 0.003 |
|
|
|
| p-stage
(II–III) | 20 | 5.217 | 1.681–16.188 | 0.004 |
|
|
|
| Lymphatic invasion
(positive) | 18 | 6.240 | 2.009–19.378 | 0.002 | 6.240 | 2.009–19.378 | 0.002 |
| Microscopic
vascular invasion (positive) | 25 | 2.874 | 0.910–9.071 | 0.072 |
|
|
|
| Histological type
(invasive mucinous adenocarcinoma) | 5 | 2.706 | 0.348–21.021 | 0.341 |
|
|
|
| Epidermal growth
factor receptor gene mutation (Neg and NE) | 76 | 2.747 | 0.601–12.555 | 0.192 |
|
|
|
| Anaplastic lymphoma
kinase gene translocation (Neg and NE) | 116 | 20.653 |
<0.001–1000< | 0.754 |
|
|
|
| Nucleolar casein
kinase 2 alpha (positive) | 60 | 3.097 | 0.838–11.449 | 0.090 |
|
|
|
Patients with adenocarcinoma in situ and MIA
have a favorable prognosis, and patients with IMA have a worse
prognosis relative to patients with INMA (2,3). Thus,
focus was next addressed on invasive non-mucinous patients.
Multivariate analysis of RFS showed that among patients with INMA,
lymph node metastasis and nucleolar CK2α staining positivity were
independent poor prognostic factors (Table SII). Age ≥70 years, lymph node
metastasis and lymphatic invasion were poor prognostic factors for
OS (Table SIII).
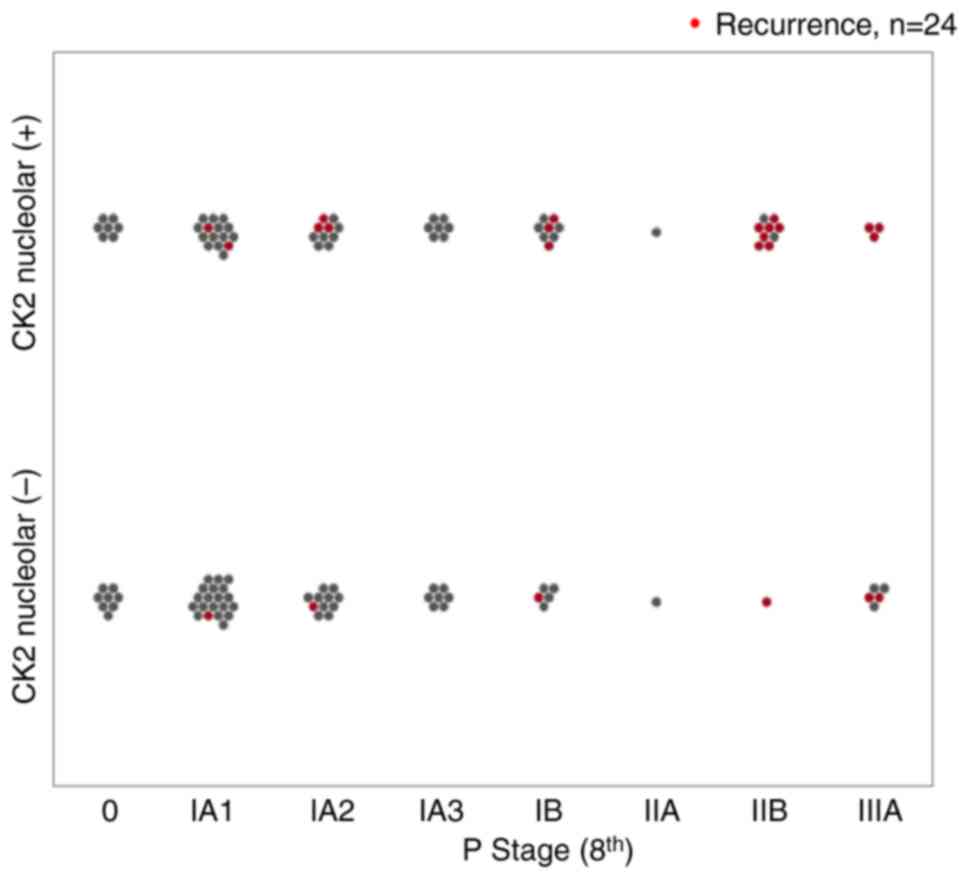
The relationship between nucleolar CK2α staining and
recurrence by stage in all cases is demonstrated in Fig. 3. Recurrence was more frequent in
patients with positive nucleolar CK2α staining, regardless of
pathological stage. The percentages of recurrent cases positive and
negative for nucleolar CK2α staining were 20% (8/40) and 7% (3/43)
in stage I, and 77% (10/13) and 43% (3/7) in stage II–III,
respectively.
Recurrence in stage I was more frequent among
nucleolar CK2α-positive cases. Positive nucleolar CK2α staining
tended to be a poor prognostic factor for RFS even in patients in
stage IA1 to IA2 (Fig. S2).
Discussion
Cancer is characterized by the accumulation of
heterogeneous genetic mutations as it proliferates, and treatment
is generally more difficult after recurrence as the tumors continue
to grow as a non-monoclonal cancer cell population. Therefore, it
is critical to identify patients at risk of recurrence as early as
possible to administer treatment to prevent future recurrence.
The present findings identified that CK2α in the
nucleolus of cancer cells in patients with early-stage lung
adenocarcinoma was associated with poor prognosis. Patients with
positive CK2α staining in nucleoli had significantly worse RFS
after surgical resection compared with patients with negative
staining (P=0.0031). The positive staining of CK2α in nucleoli was
independent of pathological stage, histological type and
histological grade (Table II).
Multivariate analysis revealed that positive CK2α staining in
nucleoli was an independent poor prognostic factor of RFS (Table III). This finding indicates that
positive CK2α staining in cancer cell nucleoli is a novel poor
prognostic factor in patients with early-stage lung adenocarcinoma.
Moreover, CK2α positive staining in the nucleolus may be a useful
marker for predicting future recurrence even in patients with stage
I lung adenocarcinoma, as shown in Fig.
3. Nucleolus-positive staining associated with recurrence.
Positive CK2α staining in the nucleolus may be a potential marker
that can be identified in 2D histopathological images in cases in
which there are extremely small lymphatic or venous invasions that
are difficult to determine on pathological sections.
In INMA of the lung (3,14),
nucleolus CK2α staining may improve the prediction of recurrence
combined with histological grade. In a previous study of invasive
breast carcinoma, positive CK2α staining in the nucleolus was
independent of luminal type, human epidermal growth factor receptor
2, or the triple negative type and a poor prognostic factor
(25). The absence of significant
differences in the OS of patients with and without CK2α nucleolar
staining in the present study may be because of the small number of
events. A longer observation period may also be necessary to
compare OS in patients with surgically resected early-stage NSCLC
because of the influence of treatment after recurrence.
The present findings suggest the potential value of
CK2α nucleolar staining to predict prognosis in surgically resected
early-stage NSCLC. Currently, there are no clear prognostic markers
in NSCLC other than TNM. While the International Association for
the Study of Lung Cancer Pathology Committee proposed histological
grade as a prognostic factor in surgically resected early-stage
INMA (14), the results of the
present study showed that CK2α staining in nucleoli is a prognostic
factor independent of this histological grade. In recent years,
limited resection approaches such as segmentectomy or partial
resection for very early-stage NSCLC have become a standard
treatment (9–11). CK2α staining of the nucleoli may be
worth considering as a biomarker in such patients with very
early-stage NSCLC to determine whether limited resection or
lobectomy should be performed. Rapid immunostaining can be useful
to make this decision intraoperatively (42). In breast cancer, the biological type
determined from genetic analysis is used to predict prognosis and
determine the indication for adjuvant therapy (43–45).
In the present study, adjuvant chemotherapy was administered to
eligible patients, making it difficult to consider the indication
for this on the basis of CK2α nucleolar staining. Nevertheless,
CK2α nucleolar staining could be used to identify those patients
likely to benefit from treatment with adjuvant chemotherapy,
including patients with early-stage non-small lung cancer.
Previous studies reported that CK2 is associated
with lung cancer metastasis (46),
and that chemical inhibitors of CK2 improve drug resistance
(47–49). The relationship between CK2 and
tumor immunity is also gaining attention (50,51). A
previous study reported that CK2 activated NF-E2-related factor 2
(Nrf2) by degrading Kelch-like ECH associated protein 1 (Keap1) and
activating AMP-activated protein kinase in human cancer cells
(52). Mutations in the Keap1-Nrf2
pathway are common in NSCLC and have been associated with poor
prognosis (53). Some clinical
trials of CX-4945, a low-molecular weight inhibitor of CK2α, for
various cancers are now underway. The current study included
patients with NSCLC who underwent surgical resection, and future
studies should be conducted in patients who have received drug
therapy. Studies examining the efficacy of CK2 inhibitors in
adjuvant therapy for surgically resected early-stage NSCLC patients
are also required.
A couple of limitations of the present study are
that it was a single-center, retrospective study, and future
validation at multiple centers is needed. Additionally, future
studies should investigate whether CK2α staining in nucleoli is
related to the efficacy of drug therapy in NCSLC, including
adjuvant therapy; these findings would indicate whether CK2α
staining in nucleoli could be developed into a useful biomarker for
treatment selection in addition to its utility as a prognostic
factor. In normal cells, CK2 is mostly localized in the cytoplasm.
The current results showed CK2 accumulation in the nucleolus in
human cancer tissues. Whether this accumulation of CK2 in the
nucleolus is predictive biomarker of a future recurrence should be
confirmed in future studies. The CK2 complex in MCF-7 breast cancer
cells is associated with protein synthesis (25), and it was previously reported that
CK2 interacts with chromatin in the cell nucleus to enhance gene
expression and is involved in rRNA synthesis (24). The molecular mechanisms underlying
the association of nucleolar CK2α with recurrence in lung
adenocarcinoma are yet to be determined.
In summary, the current findings indicated that CK2α
staining in nucleoli may be a useful marker for poor prognosis in
patients with surgically resected early-stage lung adenocarcinoma.
Positive staining of CK2α in nucleoli was independent of
pathological stage, histological type and histological grade.
Combining CK2α with TNM and histological grade may more accurately
predict recurrence in surgically resected early-stage lung
adenocarcinoma. Rapid evaluation by immunostaining of CK2α in
nucleoli could also be used to identify patients with early-stage
lung adenocarcinoma in whom limited surgery may be appropriate. In
patients with surgically resected nucleolar CK2α-positive lung
adenocarcinoma, CK2 inhibitors may reduce the risk of recurrence
when administered as adjuvant therapy after surgery. With the
global clinical development of CK2α inhibitors now underway, CK2α
is also a promising therapeutic target in lung adenocarcinoma,
including advanced disease, and should be studied in squamous cell
lung cancer.
In conclusion, surgically resected early-stage lung
adenocarcinoma patients with positive nucleolar CK2α staining had
significantly worse RFS compared with patients with negative
staining. Positive staining of CK2α in the nucleoli was independent
of pathological stage, histological type and histological grade,
and was an independent poor prognostic factor in the multivariate
analysis of RFS. The findings of the present study indicated that
nucleolar CK2α may be a prognostic factor and promising therapeutic
target for lung adenocarcinoma. These results require validation in
a multicenter setting with a larger number of patients.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors are grateful to Ms Yukiko Kikuta, Ms Moe
Muramatsu and Ms Junko Yamaki for technical support. The authors
would like to thank Dr Gabrielle White Wolf for editing a draft of
this manuscript.
Funding
This present study was supported by Japan Agency for Medical
Research and Development to MKH (AMED; grant nos. 20lm0203006j0004
and 22ym00126808j0001).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SM, MKH, YH and HS conceptualized the study. SM, YK
and MKH conducted investigation. SM, YK and MKH confirm the
authenticity of all the raw data. SM, MKH, YO, MW, NO and KH
acquired data. SM, YK and MKH analyzed and validated data. SM and
MKH prepared the original draft of the manuscript. MKH and HS
wrote, reviewed and edited the manuscript. SM visualized data. YH
and HS supervised the study. SM and MKH performed project
administration. MKH acquired funding. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and was approved
(approval no. 30113; August 30, 2022) by the institutional Ethics
Committee of Fukushima Medical University (Fukushima, Japan).
Verbal informed consent was obtained from all subjects involved in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALK
|
anaplastic lymphoma kinase
|
|
CK2α
|
casein kinase 2 alpha
|
|
EGFR
|
epidermal growth factor receptor
|
|
Keap1
|
Kelch-like ECH associated protein
1
|
|
Nrf2
|
NF-E2-related factor 2
|
|
PD-1
|
programmed death-1
|
|
PD-L1
|
programmed death-ligand 1
|
|
TPS
|
tumor proportion score
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
UICC International Union Against Cancer, .
TNM Classification of Malignant Tumours. 8th ed. Wiley Blackwell;
2016
|
|
3
|
WHO Classification of Tumours Editorial
Board, . Thoracic Tumours. WHO Classification of Tumours; 5th
Edition. 2021
|
|
4
|
Attili I, Corvaja C, Spitaleri G, Del
Signore E, Trillo Aliaga P, Passaro A and de Marinis F: New
generations of tyrosine kinase inhibitors in treating NSCLC with
oncogene addiction: Strengths and limitations. Cancers (Basel).
15:50792023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felip E, Altorki N, Zhou C, Csőszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB-IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase 3 trial.
Lancet. 398:1344–1357. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant Nivolumab plus Chemotherapy in Resectable
Lung Cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heymach JV, Harpole D, Mitsudomi T, Taube
JM, Galffy G, Hochmair M, Winder T, Zukov R, Garbaos G, Gao S, et
al: Perioperative durvalumab for resectable Non-Small-cell lung
cancer. N Engl J Med. 389:1672–1684. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu S, Wu L, Zhang W, Zhang P, Wang W, Fang
W, Xing W, Chen Q, Mei J, Yang L, et al: Perioperative toripalimab
+ platinum-doublet chemotherapy vs. chemotherapy in resectable
stage II/III non-small cell lung cancer (NSCLC): Interim event-free
survival (EFS) analysis of the phase III Neotorch study. J Clin
Oncol. 41:4251262023. View Article : Google Scholar
|
|
9
|
Altorki N, Wang X, Kozono D, Watt C,
Landrenau R, Wigle D, Port J, Jones DR, Conti M, Ashrafi AS, et al:
Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell
Lung Cancer. N Engl J Med. 388:489–498. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saji H, Okada M, Tsuboi M, Nakajima R,
Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, et al:
Segmentectomy versus lobectomy in small-sized peripheral
non-small-cell lung cancer (JCOG0802/WJOG4607L): A multicentre,
open-label, phase 3, randomised, controlled, non-inferiority trial.
Lancet. 399:1607–1617. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aokage K, Suzuki K, Saji H, Wakabayashi M,
Kataoka T, Sekino Y, Fukuda H, Endo M, Hattori A, Mimae T, et al:
Segmentectomy for ground-glass-dominant lung cancer with a tumour
diameter of 3 cm or less including ground-glass opacity (JCOG1211):
A multicentre, single-arm, confirmatory, phase 3 trial. Lancet
Respir Med. 11:540–549. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Offin M, Chan JM, Tenet M, Rizvi HA, Shen
R, Riely GJ, Rekhtman N, Daneshbod Y, Quintanal-Villalonga A,
Penson A, et al: Concurrent RB1 and TP53 alterations define a
subset of EGFR-Mutant lung cancers at risk for histologic
transformation and inferior clinical outcomes. J Thorac Oncol.
14:1784–1793. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Farooq H, Bien H, Chang V, Becker D, Park
YH and Bates SE: Loss of function STK11 alterations and poor
outcomes in non-small-cell lung cancer: Literature and case series
of US Veterans. Semin Oncol. 49:319–325. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moreira AL, Ocampo PSS, Xia Y, Zhong H,
Russell PA, Minami Y, Cooper WA, Yoshida A, Bubendorf L, Papotti M,
et al: A grading system for invasive pulmonary adenocarcinoma: A
proposal from the international association for the study of lung
cancer pathology committee. J Thorac Oncol. 15:1599–1610. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varga Z, Sinn P and Seidman AD: Summary of
head-to-head comparisons of patient risk classifications by the
21-gene Recurrence Score® (RS) assay and other genomic
assays for early breast cancer. Int J Cancer. 145:882–893. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seldin DC and Leder P: Casein Kinase II α
Transgene-Induced murine lymphoma: Relation to theileriosis in
cattle. Science. 267:894–897. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fleuren EDG, Zhang L, Wu J and Daly RJ:
The kinome ‘at large’ in cancer. Nat Rev Cancer. 16:83–98. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chua MMJ, Lee M and Dominguez I:
Cancer-type dependent expression of CK2 transcripts. PLoS One.
12:e01888542017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strum SW, Gyenis L and Litchfield DW:
CSNK2 in cancer: Pathophysiology and translational applications. Br
J Cancer. 126:994–1003. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gapany M, Faust RA, Tawfic S, Davis A,
Adams GL, Leder P and Ahmed K: Association of elevated protein
kinase CK2 activity with aggressive behavior of squamous cell
carcinoma of the head and neck. Mol Med. 1:659–666. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Landesman-Bollag E, Romieu-Mourez R, Song
DH, Sonenshein GE, Cardiff RD and Seldin DC: Protein kinase CK2 in
mammary gland tumorigenesis. Oncogene. 20:3247–3257. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Firnau MB and Brieger A: CK2 and the
hallmarks of cancer. Biomedicines. 10:19872022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Homma MK, Shibata T, Suzuki T, Ogura M,
Kozuka-Hata H, Oyama M and Homma Y: Role for protein kinase CK2 on
cell proliferation: Assessing CK2 complex components in the nucleus
during the cell cycle progression. In Protein Kinase CK2 Cellular
Function in Normal and Disease States. Ahmed K, Issinger OG and
Szyszka R: Springer International Publishing; Cham: pp. 197–226.
2015, View Article : Google Scholar
|
|
24
|
Homma MK, Nakato R, Niida A, Bando M,
Fujiki K, Yokota N, Yamamoto S, Shibata T, Takagi M, Yamaki J, et
al: Cell cycle-dependent gene networks for cell proliferation
activated by nuclear CK2α complexes. Life Sci Alliance.
7:e2023020772023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Homma MK, Kiko Y, Hashimoto Y, Nagatsuka
M, Katagata N, Masui S, Homma Y and Nomizu T: Intracellular
localization of CK2α as a prognostic factor in invasive breast
carcinomas. Cancer Sci. 112:619–628. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Korsensky L, Chorev D, Saleem H,
Heller-Japheth R, Rabinovitz S, Haif S, Dahan N, Ziv T and Ron D:
Regulation of stability and inhibitory activity of the tumor
suppressor SEF through casein-kinase II-mediated phosphorylation.
Cell Signal. 86:1100852021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sit M, Aktas G, Ozer B, Kocak MZ, Erkus E,
Erkol H, Yaman S and Savli H: Mean platelet volume: An overlooked
herald of malignant thyroid nodules. Acta Clin Croat. 58:417–420.
2019.PubMed/NCBI
|
|
28
|
Atak BM, Bakir Kahveci G, Bilgin S,
Kurtkulagi O and Kosekli MA: Platelet to lymphocyte ratio in
differentiation of benign and malignant thyroid nodules. Exp Biomed
Res. 4:148–153. 2021. View Article : Google Scholar
|
|
29
|
Hong H and Benveniste EN: The immune
regulatory role of protein kinase CK2 and its implications for
treatment of cancer. Biomedicines. 9:19322021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Di Maira G, Salvi M, Arrigoni G, Marin O,
Sarno S, Brustolon F, Pinna LA and Ruzzene M: Protein kinase CK2
phosphorylates and upregulates Akt/PKB. Cell Death Differ.
12:668–677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Maira G, Brustolon F, Pinna LA and
Ruzzene M: Dephosphorylation and inactivation of Akt/PKB is
counteracted by protein kinase CK2 in HEK 293T cells. Cell Mol Life
Sci. 66:3363–3373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D, Westerheide SD, Hanson JL and
Baldwin AS Jr: Tumor necrosis factor alpha-induced phosphorylation
of RelA/p65 on Ser529 is controlled by casein kinase II. J Biol
Chem. 275:32592–32597. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liongue C, O'Sullivan LA, Trengove MC and
Ward AC: Evolution of JAK-STAT pathway components: Mechanisms and
role in immune system development. PLoS One. 7:e327772012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Manni S, Brancalion A, Mandato E, Tubi LQ,
Colpo A, Pizzi M, Cappellesso R, Zaffino F, Di Maggio SA, et al:
Protein kinase CK2 inhibition down modulates the NF-κB and STAT3
survival pathways, enhances the cellular proteotoxic stress and
synergistically boosts the cytotoxic effect of bortezomib on
multiple myeloma and mantle cell lymphoma cells. PLoS One.
8:e752802013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng Y, Qin H, Frank SJ, Deng L,
Litchfield DW, Tefferi A, Pardanani A, Lin FT, Li J, Sha B and
Benveniste EN: A CK2-dependent mechanism for activation of the
JAK-STAT signaling pathway. Blood. 118:156–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hashimoto A, Gao C, Mastio J, Kossenkov A,
Abrams SI, Purandare AV, Desilva H, Wee S, Hunt J, Jure-Kunkel M
and Gabrilovich DI: Inhibition of casein kinase 2 disrupts
differentiation of myeloid cells in cancer and enhances the
efficacy of immunotherapy in Mice. Cancer Res. 78:5644–5655. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu R, Tang W, Qiu K, Li P, Li Y, Li D and
He Z: An Integrative Pan-cancer analysis of the prognostic and
immunological role of casein kinase 2 alpha Protein 1 (CSNK2A1) in
human cancers: A study based on bioinformatics and
immunohistochemical analysis. Int J Gen Med. 14:6215–6232. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
American Joint Committee on Cancer, . AJCC
Cancer Staging Manual. 8th edition. Springer; 2017
|
|
39
|
Seto T, Kiura K, Nishio M, Nakagawa K,
Maemondo M, Inoue A, Hida T, Yamamoto N, Yoshioka H, Harada M, et
al: CH5424802 (RO5424802) for patients with ALK-rearranged advanced
non-small-cell lung cancer (AF-001JP study): A single-arm,
open-label, phase 1–2 study. Lancet Oncol. 14:590–598. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roach C, Zhang N, Corigliano E, Jansson M,
Toland G, Ponto G, Dolled-Filhart M, Emancipator K, Stanforth D and
Kulangara K: Development of a companion diagnostic PD-L1
immunohistochemistry assay for pembrolizumab therapy in
non-small-cell lung cancer. Appl Immunohistochem Mol Morphol.
24:392–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jänne PA, Yang JCH, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Terata K, Saito H, Nanjo H, Hiroshima Y,
Ito S, Narita K, Akagami Y, Nakamura R, Konno H, Ito A, et al:
Novel rapid-immunohistochemistry using an alternating current
electric field for intraoperative diagnosis of sentinel lymph nodes
in breast cancer. Sci Rep. 7:28102017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sotiriou C and Pusztai L: Gene-expression
signatures in breast cancer. N Engl J Med. 360:790–800. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Harris LN, Ismaila N, McShane LM, Andre F,
Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC,
Mennel RG, et al: Use of biomarkers to guide decisions on adjuvant
systemic therapy for women with early-stage invasive breast cancer:
American Society of Clinical Oncology clinical practice guideline.
J Clin Oncol. 34:1134–1150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Giuliano AE, Connolly JL, Edge SB,
Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ and
Hortobagyi GN: Breast Cancer-Major changes in the American Joint
Committee on Cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:290–303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Y, Amin EB, Mayo MW, Chudgar NP,
Bucciarelli PR, Kadota K, Adusumilli PS and Jones DR: CK2α, drives
lung cancer metastasis by targeting brms1 nuclear export and
degradation. Cancer Res. 76:2675–2686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yang B, Yao J, Li B, Shao G and Cui Y:
Inhibition of protein kinase CK2 sensitizes non-small cell lung
cancer cells to cisplatin via upregulation of PML. Mol Cell
Biochem. 436:87–97. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
So KS, Rho JK, Choi YJ, Kim SY, Choi CM,
Chun YJ and Lee JC: AKT/mTOR down-regulation by CX-4945, a CK2
inhibitor, promotes apoptosis in chemorefractory non-small cell
lung cancer cells. Anticancer Res. 35:1537–1542. 2015.PubMed/NCBI
|
|
49
|
Jin C, Song P and Pang J: The CK2
inhibitor CX4945 reverses cisplatin resistance in the A549/DDP
human lung adenocarcinoma cell line. Oncol Lett. 18:3845–3856.
2019.PubMed/NCBI
|
|
50
|
Zhao X, Wei Y, Chu YY, Li Y, Hsu JM, Jiang
Z, Liu C, Hsu JL, Chang WC, Yang R, et al: Phosphorylation and
stabilization of PD-L1 by CK2 suppresses dendritic cell function.
Cancer Res. 82:2185–2195. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Husain K, Williamson TT, Nelson N and
Ghansah T: Protein kinase 2 (CK2): A potential regulator of immune
cell development and function in cancer. Immunol Med. 44:159–174.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jang DE, Song J, Park JW, Yoon SH and Bae
YS: Protein kinase CK2 activates Nrf2 via autophagic degradation of
Keap1 and activation of AMPK in human cancer cells. BMB Rep.
53:72–277. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hellyer JA, Padda SK, Diehn M and Wakelee
HA: Clinical Implications of KEAP1-NFE2L2 Mutations in NSCLC. J
Thorac Oncol. 16:395–403. 2021. View Article : Google Scholar : PubMed/NCBI
|