Introduction
The Hippo signaling pathway is an evolutionarily
conserved signal pathway that was first identified in Drosophila
and plays an important role in the regulation of cell
proliferation, apoptosis and tissue and organ growth (1,2).
Yes-associated protein 1 (YAP1), a pivotal component of the Hippo
signaling pathway, is encoded by the YAP1 gene located on human
chromosome 11q22 and functions as an oncogenic protein (Fig. 1) (3). When the Hippo pathway is disrupted,
mammalian STE20-like protein kinase 1/2 (Mst1/2) kinases become
phosphorylated and activated, which in turn activates downstream
large tumor suppressor kinase 1 and 2 ((LATS1/2) kinases. These
activated LATS1/2 kinases phosphorylate YAP1, leading to its
degradation in the cytoplasm (4).
Conversely, dephosphorylated YAP1 translocates to the nucleus,
binds to the TEA domain (TEAD) and promotes the transcription of
downstream genes (for example, CTGF and Cyr61) (5,6).
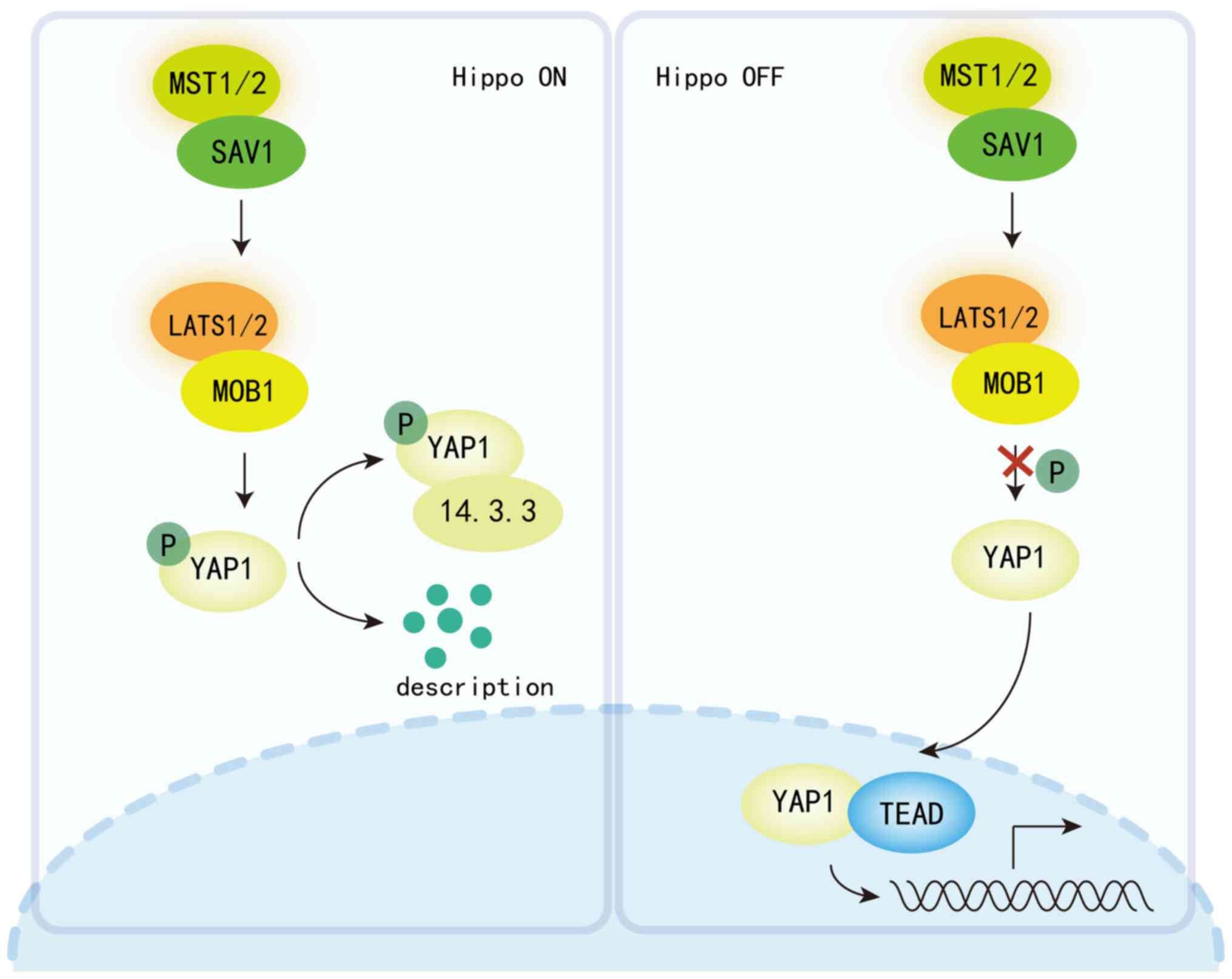 | Figure 1.Hippo signaling pathway is a key
regulator of cellular and systemic metabolism, maintaining organ
size and tissue homeostasis by controlling cell proliferation,
survival and regeneration. When the Hippo signaling pathway is
activated (in the ‘ON’ state), MST1/2 kinases phosphorylate
LATS1/2, which in turn promotes the phosphorylation of YAP/TAZ.
This leads to the retention of YAP/TAZ in the cytoplasm and/or
their degradation via the proteasome pathway, thereby inhibiting
their transcriptional co-activator activity. Conversely, when the
Hippo signaling pathway is inactivated (in the ‘OFF’ state),
dephosphorylated YAP/TAZ can freely translocate to the nucleus,
where they bind to the TEAD family of transcription factors and
activate the expression of a range of genes involved in cell
growth, proliferation, and survival. MST, macrophage-stimulating;
LATS1/2, large tumor suppressor kinase 1/2; YAP, Yes-associated
protein; TAZ, transcriptional co-activator with PDZ-binding motif;
TEAD, TEA domain; SAV1, Salvador family WW domain-containing
protein 1; MOB1, MOB kinase activator 1; P, phosphorylated. |
YAP1/transcriptional co-activator with PDZ-binding
motif (TAZ) play essential roles in regulating cell proliferation
and are crucial for organ regeneration, development and stem cell
self-renewal (7). They are
influenced by various factors, including the microenvironment and
extracellular signals. Additionally, YAP1 can activate survival
pathways to inhibit apoptosis and interacts with multiple signaling
networks (8), contributing to
cancer development and progression (9). Elevated YAP1 expression is associated
with malignant tumor recurrence, metastasis, reduced overall
survival and chemotherapy resistance (10). In human cancers, YAP/TAZ target
genes are associated with poor prognosis and increased treatment
resistance (11). YAP1 is highly
expressed in various cancers and linked to adverse outcomes,
including bladder, colon, pancreatic, hepatocellular,
gastric/esophageal, ovarian, brain, breast and lung cancers
(12–17).
Bladder cancer is the most prevalent malignancy
within the urinary system and is anticipated to rank as the ninth
most common cancer in the United States by 2023 (18). Previous years have seen an
increasing incidence of bladder cancer, with a trend toward younger
patients (19). While the etiology
remains incompletely understood, risk factors include smoking,
aging and occupational exposures (20). Genetic mutations and altered RNA
expression levels also contribute to bladder cancer risk. YAP1 is
notably upregulated in bladder cancer and is associated with poor
prognosis (21). Research indicates
that single nucleotide polymorphisms (SNPs) in YAP1, a key molecule
in the Hippo pathway, are linked to bladder cancer development
(22). BCa is a cancer that
frequently exhibits mutations. A study has indicated that in human
bladder smooth muscle, Gene Ontology annotation analysis shows that
differential genes are enriched in positive regulation of cellular
processes, metabolism, protein binding and signal transduction
processes. Kyoto Encyclopedia of Genes and Genomes enrichment
analysis highlights genes in the MAPK and PI3K/Akt signaling
pathways (23). Additionally, a
next-generation sequencing study suggests that BCa is primarily
associated with a high mutation burden in genes related to the cell
cycle and chromatin regulation. Dysfunctional Hippo signaling may
contribute to the development of bladder cancer, although it is not
an independent driver (24). The
present study reviews the carcinogenic mechanisms of YAP1 in
bladder cancer and evaluates its potential as a therapeutic
target.
Regulation of YAP1 activity in bladder
cancer
YAP1 activity is controlled by its gene expression
levels. Knockdown of this gene in bladder cancer cell lines
significantly reduces YAP1 protein activity (21). Furthermore, the primary method of
regulating YAP1 activity involves modulation of its phosphorylation
status. When phosphorylated, YAP1 is retained in the cytoplasm and
thus is unable to translocate to the nucleus to perform its
transcriptional activation functions. 4-hydroxynonenal induces
phosphorylation of YAP1 at serine residues 127 and 387, leading to
reduced YAP1 expression and consequently decreases bladder cancer
cell proliferation (25).
Ubiquitination is regulated by deubiquitinases (DUBs), which are
specialized Ub proteases that process Ub chains in various ways to
regulate ubiquitination (26).
MINDY1, a DUB specific to YAP1, removes the K48-linked ubiquitin
chain from YAP1, thereby preventing its proteasomal degradation.
This action enhances the stability and abundance of the YAP1
protein, consequently boosting its transcriptional activation
capability and facilitating the progression of bladder cancer
(Fig. 2) (27).
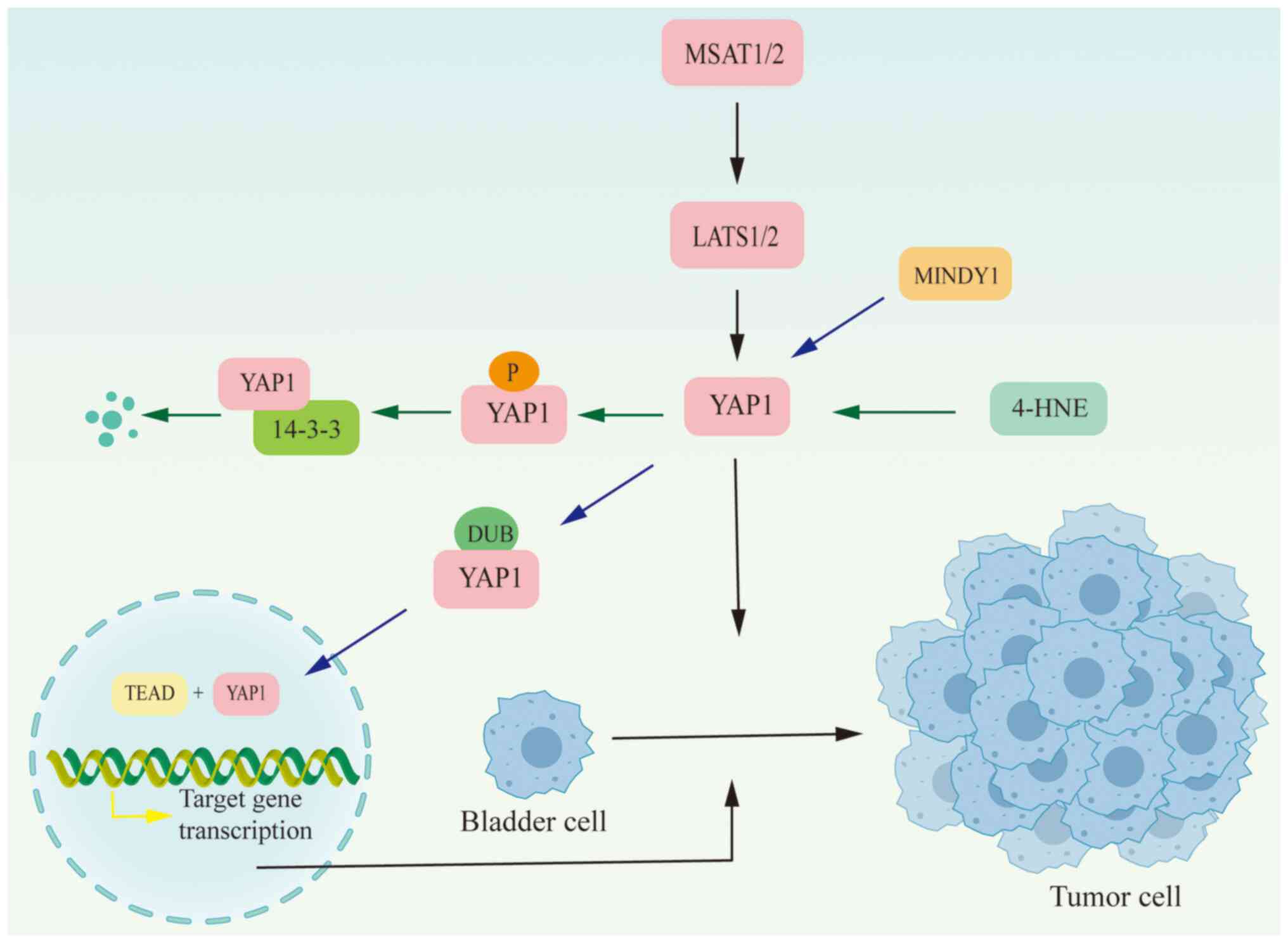 | Figure 2.In bladder cancer, YAP1 activity is
regulated by YAP1 gene expression, post-transcriptional mRNA
modifications, and the activity of the Hippo signaling pathway.
YAP1 protein modifications also affect its activity and function.
4-HNE regulates YAP1 activity through phosphorylation, accelerating
its degradation, while MINDY enhances its stability through
deubiquitination. Activated YAP1 will enter the nucleus and bind to
TEAD to regulate downstream target genes. 4-HNE, 4-hydroxynonenal;
YAP, Yes-associated protein; LATS1/2, large tumor suppressor kinase
1/2; DUB, deubiquitination; TEAD, TEA domain. |
YAP1 participates in the progression of
bladder cancer
Tumor cell proliferation and migration is a dynamic
and intricate process that contributes to the advancement of
numerous diseases. Abnormal cell proliferation often leads to tumor
formation. Once tumor cells acquire the capability to migrate and
detach from the primary site, they can significantly accelerate
tumor dissemination and metastasis. Phosphorylated YAP1 not only
directly influences tumor proliferation but also collaborates with
various molecules to promote both tumor proliferation and
migration. For instance, The drosophile Mask (multiple ankyrin
repeats single KH domain) whose mammalian homologues MASK1 (also
known as ANKHD1) and MASK2 (also known as GTAR or ANKRD17) are a
novel cofactor of YAP1. In bladder cancer cell lines overexpressing
YAP1, MASK2interacts with YAP1 to modulate its downstream target
genes, consequently promoting the proliferation and migration of
bladder cancer cells (28).
The MAPK pathway, ubiquitously present in organisms,
potentially facilitates the onset and progression of cancer when
its signaling becomes dysregulated (29). YAP1 serves as both an upstream
regulator and an activator of the MAPK pathway. Its stimulation of
the MAPK/ERK pathway synergistically promotes the proliferation and
invasion of bladder cancer cells (30). Research demonstrates that YAP1
modulates the transcription of both long non-coding RNA (lncRNA)
and mRNA (31). Specifically,
lncRNA H19, a downstream target of YAP1, is upregulated by YAP1,
influencing the proliferation and migration of bladder cancer cells
(32). Furthermore, in bladder
cancer, lncRNA H19 interacts with microRNA (miRNA/miR)-29b-3p,
impacting epithelial-mesenchymal transition (EMT) and thereby
facilitating cancer metastasis (33).
Furthermore, YAP1 can collaborate synergistically
with miRNA to facilitate the progression of bladder cancer. MiRNAs,
serving as critical regulatory factors in tumor development, are
intricately linked to tumor formation, metastasis, cellular
apoptosis and chemoresistance when expressed abnormally (34). A study by Luo (35) identified a cross-network interaction
between the Hippo and TGF-β signaling pathways, where the
YAP1-Smad3 complex plays a pivotal role. MiR-497 and miR-195
disrupt the formation of the YAP1/SMAD3 complex, thereby
interfering with the interactions between the Hippo/YAP1 and
TGF-β/Smad pathways. This disruption diminishes their influence on
the shared target gene, connective tissue growth factor (CTGF),
ultimately reducing bladder cancer cell proliferation (36). Furthermore, the overexpression of
hypermethylated in cancer 1 suppresses CTGF activity, limits
interactions between YAP1 and the TEAD, and inhibits proliferation,
migration and invasion of bladder cancer cells (37). Additionally, miR-599 curtails
proliferation and invasion, while enhancing apoptosis in bladder
urothelial carcinoma cells by modulating YAP1 expression (38).
Exosomes, a type of extracellular vesicle, are
secreted by virtually all cell types. These vesicles support a wide
array of cellular functions, encompassing intercellular
communication, cell differentiation and proliferation,
angiogenesis, stress responses and immune signaling (39,40).
Research demonstrates a distinct relationship between exosomes and
YAP1, where exosomal miR-217 from normal human bladder stromal
cells is significantly elevated in bladder cancer cell lines, and
by modulating YAP1 and its downstream targets, cysteine-rich
angiogenic inducer 61 (CYR61), CTGF and ankyrin repeat
domain-containing protein 1 miR-217 mimics or inhibitors can
notably influence the proliferation, migration, and apoptosis of
bladder cancer cells (41).
EMT is a critical process in tumor metastasis and a
hallmark of malignant tumors. Additionally, EMT is integral to
embryogenesis and wound healing (42,43).
In a study, high-glucose culture media induces YAP1 and TAZ
expression in bladder cancer cells, resulting in the upregulation
of wave protein, N-cadherin, and fibronectin, alongside the
downregulation of E-cadherin to promote EMT, while also regulating
glucose metabolism through glucose transporter type 1 activity,
thereby highlighting the Hippo signaling pathway as a crucial
factor in the dysregulation of glucose metabolism in bladder cancer
(Table I) (44).
 | Table I.In bladder cancer cell lines, YAP1
and its downstream signaling pathways influence various biological
behaviors of bladder cancer. |
Table I.
In bladder cancer cell lines, YAP1
and its downstream signaling pathways influence various biological
behaviors of bladder cancer.
| Signaling
network | Major outcomes | Cell line
used/bladder subtype | (Refs.) |
|---|
| YAP1/Mask2 | Overexpression of
YAP1 can promote the growth and migration of bladder cancer cells.
The YAP1/Mask2 complex controls the growth and migration of bladder
cancer cells by regulating the expression of target genes in the
Hippo pathway. | 5637 | (28) |
| YAP1/MAPK | YAP1 overexpression
activates proteins and their phosphorylated forms in the MAPK
pathway, including ERK, p38 and JNK, thereby enhancing the cellular
proliferative capacity and invasiveness of bladder cancer
cells. |
5637/UMUC-3/J82 | (30) |
| YAP1/H19 | YAP1 expression is
significantly upregulated in bladder cancer and facilitates cancer
cell proliferation and migration by increasing the expression of
the long non-coding RNA H19. | 5637/UMUC-3 | (32) |
|
YAP1/TGF-β/Smad | MiRNAs miR-497 and
miR-195 inhibit the expression and interaction of YAP1 and Smad3,
thereby diminishing the activity of the YAP1-Smad3 complex and
consequently suppressing the progression of bladder cancer. | 5637/T24 | (36) |
| HIC1/YAP1 | Overexpression of
HIC1 reduces the binding of YAP1 to TEAD, inhibits the activity of
the YAP1/TEAD pathway, and thereby suppresses the progression of
bladder cancer. | J28/T24 | (37) |
| YAP1/COX2/PGE2 | YAP1 and the
COX2/PGE2 signaling pathway synergistically activate SOX2, thereby
promoting cancer stem cell properties and enhancing chemotherapy
resistance. |
BFTC905/BFTC909/5637/T24 | (53) |
Stem cells (SCs) are multipotent cells with the
capacity for self-renewal. Provided specific conditions, these
cells can differentiate into a variety of functional cell types
(45,46). Cancer SCs (CSCs) are characterized
by their capacity for self-renewal and differentiation. These cells
significantly contribute to tumor progression, treatment resistance
and disease recurrence by continuously proliferating, invading
normal tissues and exhibiting resistance to conventional cancer
therapies (47,48).
YAP1 is pivotal in regulating several key processes
in CSCs, including self-renewal, multilineage differentiation,
tumorigenesis, treatment resistance and metastatic invasion
(49,50). In embryonic, neural and
hematopoietic stem cells, elevated expression of the YAP1-TEAD2
complex sustains stem cell potency and serves as an indicator of
stemness (51). YAP1, a regulator
of stem cell functions, is notably upregulated in bladder cancer
stem cells. The overexpression of YAP significantly enhances the
self-renewal ability of these cells (52). YAP1 collaborates with the
pro-inflammatory cyclooxygenase 2 (COX2)/prostaglandin E2 (PGE2)
signaling pathway to enhance the proliferation of bladder
urothelial carcinoma stem-like cells and influence tumor
chemoresistance. As the COX2/PGE2 pathway induces methylation of
the let-7 promoter, this results in reduced let-7 expression and
subsequent upregulation of SRY-box transcription factor 2 (SOX2),
which is further amplified by YAP1′s binding to the SOX2 enhancer,
thereby increasing SOX2 expression (53). The interaction between the COX2 and
YAP1 pathways may significantly influence the recurrence of bladder
cancer.
Furthermore, the miR-146a rs2910164 SNP genotype
C>G significantly affects the prognosis of bladder cancer
patients by modulating key targets within the Hippo and COX2
signaling pathways, leading to the suppression of YAP1 and COX2 at
both mRNA and protein levels, which results in the downregulation
of YAP1 and aldehyde dehydrogenase 1 family, member A1, along with
reduced expression of COX2 and SOX2, all of which are closely
associated with bladder cancer recurrence (54). Simultaneously, a study has
demonstrated that YAP1 is involved in its own recurrence process.
During the postoperative wound healing phase in bladder cancer, it
has been observed that the extracellular matrix regulates the
transport of YAP1 to the nucleus through integrin-FAK-YAP1
signaling. This regulation contributes to the progression and
recurrence of bladder cancer (55).
This implies that YAP1 overexpression not only
affects bladder cancer progression through interactions with
multiple signaling pathways but also contributes to this
progression by regulating the stemness of tumor stem cells.
Consequently, targeting YAP1 and its associated regulatory network
could provide novel therapeutic approaches for bladder cancer.
Therapeutic potential of YAP1-targeting in
bladder cancer
Bladder urothelial carcinoma is classified into two
main categories based on clinical staging, each with different
clinical outcomes and treatment options: Muscle invasive bladder
cancer (MIBC) and non-MIBC (NMIBC). Treatment decisions are
primarily based on clinical factors. Besides surgery, common
treatments currently include cisplatin chemotherapy, immunotherapy
and targeted therapy. Despite these options, the treatment outlook
for bladder cancer remains unsatisfactory, with a high risk of
recurrence. Although the targeting of cancer treatment signaling
pathways has increased over the past few decades, bladder cancer
requires the exploration of potentially more effective treatments
(Table II).
 | Table II.Main mechanism and mode of action of
YAP1 in the treatment of bladder cancer. |
Table II.
Main mechanism and mode of action of
YAP1 in the treatment of bladder cancer.
| Treatment
method | Mechanism of
action | Functions | (Refs.) |
|---|
| Chemotherapy:
Resistance | YAP1 contributes to
chemoresistance and antioxidant response in bladder cancer cells by
regulating the expression of Nrf2 and FOXM1 and modulating
intracellular oxidative stress levels. | YAP1 maintains
cellular antioxidant status by regulating the intracellular
antioxidant system, specifically through the modulation of GSH
synthesis and utilization. | (65) |
|
| YAP1 increases the
chemoresistance of bladder urothelial carcinoma cells to drugs such
as cisplatin by inhibiting apoptosis and promoting cell survival
signaling pathways. | Activation of YAP1
decreases cellular sensitivity to DNA-damaging drugs and inhibits
the apoptotic pathway by reducing the activation of
apoptosis-related proteins caspase 3 and PARP induced by
chemotherapy. | (71) |
| Immunotherapy | Knockdown of YAP1
reduces cell invasiveness and migratory ability, likely due to the
involvement of YAP1-regulated genes such as CTGF and CYR61, which
play crucial roles in extracellular matrix remodeling and cell
migration. | YAP1 plays a
critical role in the proliferation, colony formation, invasiveness
and migratory ability of bladder cancer cells by regulating
multiple molecules and signaling pathways involved in cell cycle
progression, migration, invasion and gene expression. | (80) |
| Molecular Targeted
Therapy: | Verteporfin
inhibits bladder cancer cell growth and invasion by suppressing the
expression of downstream genes in the Hippo signaling pathway
activated by YAP1. | The function of
YAP1 is inhibited, which in turn suppresses the expression of
target genes in the Hippo signaling pathway, thereby inhibiting the
growth and invasion of bladder cancer cells. | (85) |
YAP1 and chemotherapy resistance in
bladder cancer
Chemotherapy remains a cornerstone of treatment for
malignant tumors, with cisplatin used across a spectrum of cancers,
including bladder cancer (56–58).
Yet, the emergence of cisplatin resistance has notably diminished
its therapeutic effectiveness (59,60).
Moreover, the redox state, crucial in the adaptation of cancer
cells to therapeutic stresses, significantly influences
chemoresistance (61). Research
demonstrates that YAP1 activation plays a pivotal role in
conferring resistance across targeted therapy, chemotherapy,
radiotherapy and possibly immunotherapy, thus establishing it as a
critical mediator of chemoresistance in diverse cancers (62,63).
The YAP1/TEAD complex is known to regulate Forkhead
box protein M1(FOXM1) expression, which in turn affects nuclear
factor erythroid 2-related factor 2 (Nrf2) expression (64). Both Nrf2 and YAP1 contribute to the
antioxidative capacity of bladder cancer cells, with a positive
association observed between the expression levels of YAP1 and
Nrf2. Targeted inhibition of YAP1 and Nrf2, aimed at decreasing
cell viability, inducing apoptosis and curbing cancer cell
migration, has been shown to substantially enhance the sensitivity
of these cells to cisplatin (66).
The transcription factor Nrf2 is a pivotal regulator of antioxidant
defenses and cellular protection mechanisms, playing a significant
role in mediating cisplatin resistance in bladder cancer (66). Emerging research based on the
interaction between YAP1 and Nrf2 indicates that Ailanthone (Aila)
can potentially serve as an effective therapeutic for
cisplatin-resistant bladder cancer. Notably, Aila consistently
suppresses Nrf2 expression while concurrently inhibiting YAP1 and
c-Myc, thereby sustaining a robust anti-proliferative effect on
cisplatin-resistant cells (67).
Additionally, Aila markedly decreases the expression of the Nrf2
target glutathione S-transferase A4 and the YAP1/TEAD target
survivin. In cisplatin-resistant bladder cancer cells, levels of
intracellular oxidative stress are notably lower compared with
those in cisplatin-sensitive cells. The post-translational
downregulation of Nrf2 and YAP1 proteins can effectively reduce
cancer cell proliferation and migration, while simultaneously
increasing oxidative stress in chemotherapy-resistant bladder
cancer cell lines (68).
Decitabine, a DNA methyltransferase inhibitor, demonstrates
cytotoxic effects at high doses by directly inducing cell death. At
lower doses, it reactivates silenced genes via demethylation of
promoter regions and is extensively employed in the treatment of
diseases such as leukemia (69).
In bladder cancer cells, decitabine reactivates the
Hippo signaling pathway through the restoration of Ras association
domain-containing protein 1, concurrently inhibiting YAP1
expression and reducing the expression of its oncogenic downstream
targets, including CTGF and CYR61. Furthermore, decitabine enhances
the cytotoxic effects of cisplatin and doxorubicin in these cells
(70). YAP1 expression and
activation are inversely correlated with cisplatin sensitivity both
in vitro and in vivo. The YAP1 inhibitor verteporfin
has been demonstrated to inhibit tumor cell proliferation and
enhance cisplatin sensitivity in bladder cancer cells. Inhibition
of YAP1 expression increases DNA damage and promotes apoptosis
accumulation, thereby sensitizing urothelial carcinoma cells to
radiochemotherapy. Notably, nuclear YAP1 expression is linked to
adverse outcomes in patients with urothelial carcinoma undergoing
perioperative chemotherapy (71).
The YAP1/TEAD1/PDGF-BB/PDGFR autocrine loop plays a pivotal role in
sustaining the characteristics of tumor stem-like cells. Inhibition
of YAP1 has been shown to significantly reduce cisplatin resistance
in OV6-positive bladder cancer stem-like cells, a finding
corroborated by animal model studies.
YAP1 and bladder cancer
immunotherapy
Immunotherapy is a treatment method aimed at
enhancing the body's immune system or modifying the ongoing cancer
defense mechanisms to combat cancer (72,73).
In recent years, immunotherapy has gained prominence in cancer
treatment and has undergone rapid development (74). In bladder cancer, Bacillus
Calmette-Guérin (BCG) remains the preferred immunotherapy for
NMIBC, effectively stimulating immune responses and modulating the
immune system to produce antitumor effects, thereby significantly
reducing recurrence rates and prolonging the interval between
recurrences (75,76). Nevertheless, ~1/3 of patients with
NMIBC do not respond to BCG therapy. A previous study indicates
that combining programmed cell death protein 1 (PD-1) inhibitors
with BCG treatment is a safe and potentially effective strategy for
these non-responders (77).
Additionally, immune checkpoint inhibitors (ICIs) such as PD-1 and
programmed death-ligand 1 (PD-L1) antibodies are approved for
bladder cancer treatment. These ICIs can directly interact with the
urothelium or malignant cells on the bladder mucosa, initiating
active immune responses (78). Baek
et al (79) revealed three
molecular subtypes of advanced urothelial carcinoma through gene
expression analysis, which have potential clinical application
value in predicting responses to immune checkpoint inhibitor
therapy and patient prognosis. Among these, the inactivation of the
YAP/TAZ pathway in the third subtype is associated with the
activation of cell cycle and DNA damage response pathways. This
suggests that in patients with the third subtype, the inactivation
of the YAP/TAZ pathway might be related to a good response to ICI
therapy and improved prognosis (79). Further research by Baek et al
(80) categorized YAP1 activation
into three distinct subtypes based on differential gene expression
and integrated this classification with immunotherapy prognostic
analysis for patients. This analysis suggests that NMIBC subgroups
with YAP1 activation might derive significant benefit from ICI
treatments. Additionally, the activation status of YAP1 in NMIBC is
not only associated with the biological behavior of the disease but
also has the potential to serve as a crucial biomarker for
predicting treatment response and patient prognosis (80).
YAP1 and targeted therapy for bladder
cancer
Based on platinum-based chemotherapy, which serves
as the first-line treatment for patients with NMIBC, survival rates
are improved but still remain relatively low (81). Advances in targeted therapies, which
have transformed treatment paradigms in various cancers, are only
beginning to make inroads into bladder cancer (82,83).
Approved treatments such as PD-1/PD-L1 inhibitors, the fibroblast
growth factor receptor inhibitor Erdafitinib, and Enfortumab
vedotin (an antibody-drug conjugate directed at Nectin-4 that
comprises a fully human, monoclonal antibody and the
microtubule-disrupting agent, monomethyl auristatin E) have shown
promise (84). Notably, Enfortumab
vedotin has achieved a remission rate of ~8% in patients with
locally advanced or metastatic bladder cancer previously treated
with platinum-based and anti-PD-1/PD-L1 therapies (85). Furthermore, the YAP1-specific
inhibitor, Verteporfin (VP), demonstrates potential as a targeted
therapeutic by inhibiting YAP1-induced gene expression within the
Hippo pathway and reducing the proliferation and invasion of
bladder cancer cells in a dose-dependent manner (86). Dual treatment involving Crizotinib
and VP in canine bladder cancer organoids significantly reduce cell
vitality by inducing apoptosis, underscoring the potential of
combining Crizotinib with VP (87).
An additional study has explored the synergistic effects of
Verteporfin and the RhoA inhibitor Simvastatin, which counteract
the proliferative and metastatic activities induced by PLAGL2.
Protein kinase membrane-associated tyrosine/threonine 1, another
direct target of YAP1/TEAD1, is implicated in activating the
YAP1/TAZ pathway, further highlighting the complex regulatory
mechanisms in bladder cancer pathogenesis (88).
Limitations and prospects
YAP1, recognized as an oncogene (89), plays critical roles in cell
proliferation, migration, epithelial-mesenchymal transition,
stemness and chemotherapy resistance across various malignancies,
including bladder cancer. However, research on YAP1 in bladder
cancer mainly focuses on bladder transitional cell carcinoma, and
there is a lack of exploration regarding the expression and
mechanisms of YAP1 in other subtypes of bladder cancer (such as
bladder squamous cell carcinoma, bladder adenocarcinoma and bladder
sarcoma). Despite its established significance in the molecular
mechanisms underlying bladder cancer, in vivo experimental
validation remains limited, and the regulatory mechanisms of the
upstream factors of YAP1 are poorly understood. Enhanced
exploration of the HIPPO signaling pathway's interactions in
bladder cancer could revolutionize diagnostic and therapeutic
approaches. Currently, post-translational modifications of YAP1 are
extensively studied in various human solid tumors (90–92).
Ubiquitination modification of YAP1 has been confirmed to be of
significant importance for tumor progression in multiple tumors
(14,93).
Additionally, glycosylation modification of YAP1 can
increase its stability, promoting the malignant development of
chronic kidney disease (94).
Previous research findings suggest that metabolic pathways play a
role in regulating YAP/TAZ activity. Glycolysis, energy stress and
mevalonate biosynthesis have been identified as regulators of
YAP/TAZ activity (95). Currently,
glucose metabolism is a hotspot in tumor research. At present,
metabolic diagnostics can improve accuracy and affordability
(96). YAP1 is also involved in the
tumor glycolysis process and is considered a glucose metabolism
regulator. YAP1 mediates cardiac hypertrophy induced by pressure
overload through aerobic glycolysis (97). In thyroid tumors and liver tumors,
high glucose levels regulate YAP1 activity and promote tumor
progression (98,99). In bladder tumors, research has shown
that extracellular glucose levels regulate YAP1 and promote the
progression of bladder cancer EMT (44). In bladder cancer, YAP1 is not only
related to glucose metabolism but also acts as an oncogene and
glycoprotein, making it a promising tumor marker for bladder
cancer. Currently, research combines composite materials with
metabolic analysis to screen for potential biomarkers (100). YAP1 is expected to become a
biomarker in bladder cancer; however, extensive clinical data is
still needed to clarify the sensitivity and specificity of
YAP1.
YAP1, known as a cancer stem cell marker,
contributes to the progression of bladder cancer. However, while a
previous study has examined YAP1′s interactions with other
molecular pathways affecting cancer stem cell dynamics, the exact
mechanisms of YAP1′s involvement in these processes remain poorly
defined (49). Research indicates
that YAP1/TAZ may be a new decisive factor in ferroptosis (101). Activation of ferroptosis can
specifically target and kill certain cancer cells, leading to
improved survival rates. Ferroptosis is currently a major focus in
tumor research. Meanwhile, a single study suggests that
nanomaterials and copper ions could offer new approaches for tumor
treatment (102). However, there
are currently no reports on the interaction between YAP1 and
ferroptosis in bladder cancer.
Although YAP1-targeted inhibitors have demonstrated
efficacy in various cancers, research specifically addressing these
inhibitors in bladder cancer is sparse, and their molecular
mechanisms of action are not well understood. Additionally, YAP1
expression is closely linked to the occurrence, size and stage of
bladder cancer, highlighting its potential as a biomarker. Despite
the high recurrence rate of bladder cancer, research into the
fundamental mechanisms driving this recurrence is inadequate.
Notably, the expression of YAP1 is associated with recurrence
rates, but detailed investigations into how YAP1 contributes to
bladder cancer recurrence are still required.
Conclusion
In summary, there is a critical need for
comprehensive studies in bladder cancer focusing on several key
areas: i) The interactions of the Hippo signaling pathway with
YAP1, particularly its post-translational modifications and their
impact on tumor progression; ii) the dynamics between YAP1 and
glucose metabolism; iii) the role of YAP1 in bladder cancer stem
cell behavior; iv) the effectiveness of YAP1-targeted inhibitors in
clinical treatment; and v) the specific mechanisms through which
YAP1 contributes to bladder cancer recurrence. Given its
significant influence on various cancer-related processes, YAP1
holds great promise as a novel biomarker and therapeutic target for
bladder cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science
Foundation of China (grant no. 81860524).
Availability of data and materials
Not applicable.
Authors' contributions
TH contributed to the creation of figures, drafted
the manuscript and conducted research. LF contributed to table
creation and drafted the manuscript. JT contributed to revision of
the manuscript. SC contributed to drafting the original manuscript.
GD contributed to drafting manuscript. ZN contributed to the
conceptual design, provided resources, oversaw the project and
reviewed and edited the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Misra JR and Irvine KD: The Hippo
signaling network and its biological functions. Annu Rev Genet.
52:65–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma S, Meng Z, Chen R and Guan KL: The
Hippo pathway: Biology and pathophysiology. Annu Rev Biochem.
88:577–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lorenzetto E, Brenca M, Boeri M, Verri C,
Piccinin E, Gasparini P, Facchinetti F, Rossi S, Salvatore G,
Massimino M, et al: YAP1 acts as oncogenic target of 11q22
amplification in multiple cancer subtypes. Oncotarget. 5:2608–2621.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu Z and Guan KL: Hippo signaling in
embryogenesis and development. Trends Biochem Sci. 46:51–63. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pocaterra A, Romani P and Dupont S:
YAP/TAZ functions and their regulation at a glance. J Cell Sci.
133:jcs2304252020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP/TAZ at the roots of cancer. Cancer Cell. 29:783–803. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong Z, Jiao Z and Yu FX: The Hippo
signaling pathway in development and regeneration. Cell Rep.
43:1139262024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morciano G, Vezzani B, Missiroli S,
Boncompagni C, Pinton P and Giorgi C: An updated understanding of
the role of YAP in driving oncogenic responses. Cancers (Basel).
13:31002021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abylkassov R and Xie Y: Role of
Yes-associated protein in cancer: An update. Oncol Lett.
12:2277–2282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Galsky MD, Stensland KD, Moshier E,
Sfakianos JP, McBride RB, Tsao CK, Casey M, Boffetta P, Oh WK,
Mazumdar M and Wisnivesky JP: Effectiveness of adjuvant
chemotherapy for locally advanced bladder cancer. J Clin Oncol.
34:825–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo J, Deng L, Zou H, Guo Y, Tong T, Huang
M, Ling G and Li P: New insights into the ambivalent role of
YAP/TAZ in human cancers. J Exp Clin Cancer Res. 42:1302023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu M, Hu Y, Lan T, Guan KL, Luo T and Luo
M: The Hippo signalling pathway and its implications in human
health and diseases. Signal Transduct Target Ther. 7:3762022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao X, Fu J, Hu B, Chen L, Wang J, Fang
J, Ge C, Lin H, Pan K, Fu L, et al: Serine metabolism regulates YAP
activity through USP7 in colon cancer. Front Cell Dev Biol.
9:6391112021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu H, Yan F, Yuan T, Qian M, Zhou T, Dai
X, Cao J, Ying M, Dong X, He Q and Yang B: USP10 promotes
proliferation of hepatocellular carcinoma by deubiquitinating and
stabilizing YAP/TAZ. Cancer Res. 80:2204–2216. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eibl G and Rozengurt E: KRAS, YAP, and
obesity in pancreatic cancer: A signaling network with multiple
loops. Semin Cancer Biol. 54:50–62. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JY, Chang JK, Dominguez AA, Lee HP,
Nam S, Chang J, Varma S, Qi LS, West RB and Chaudhuri O:
YAP-independent mechanotransduction drives breast cancer
progression. Nat Commun. 10:18482019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Driskill JH and Pan D: The Hippo pathway
in liver homeostasis and pathophysiology. Annu Rev Pathol.
16:299–322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder cancer: A review. JAMA. 324:1980–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu JY, Li YH, Lin HX, Liao YJ, Mai SJ,
Liu ZW, Zhang ZL, Jiang LJ, Zhang JX, Kung HF, et al:
Overexpression of YAP 1 contributes to progressive features and
poor prognosis of human urothelial carcinoma of the bladder. BMC
Cancer. 13:3492013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Z, Wang X, Ma L, Guo Z, Liu H, Du M,
Chu H, Wang M, Wang Z and Zhang Z: Genetic variations in Hippo
pathway genes influence bladder cancer risk in a Chinese
population. Arch Toxicol. 94:785–794. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Di X, Xiang L and Jian Z: YAP-mediated
mechanotransduction in urinary bladder remodeling: Based on RNA-seq
and CUT&Tag. Front Genet. 14:11069272023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong T, Huang M, Yan B, Lin B, Yu J, Teng
Q, Li P and Pang J: Hippo signaling modulation and its biological
implications in urological malignancies. Mol Aspects Med.
98:1012802024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cucci MA, Compagnone A, Daga M, Grattarola
M, Ullio C, Roetto A, Palmieri A, Rosa AC, Argenziano M, Cavalli R,
et al: Post-translational inhibition of YAP oncogene expression by
4-hydroxynonenal in bladder cancer cells. Free Radic Biol Med.
141:205–219. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q and Jiang J: Regulation of
hedgehog signal transduction by ubiquitination and
deubiquitination. Int J Mol Sci. 22:133382021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo Y, Zhou J, Tang J, Zhou F, He Z and
Liu T and Liu T: MINDY1 promotes bladder cancer progression by
stabilizing YAP. Cancer Cell Int. 21:3952021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong L, Lin F, Wu W, Huang W and Cai Z:
Transcriptional cofactor Mask2 is required for YAP-induced cell
growth and migration in bladder cancer cell. J Cancer. 7:2132–2138.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rovida E and Tusa I: Targeting MAPK in
cancer 2.0. Int J Mol Sci. 23:57022022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiu D, Zhu Y and Cong Z: YAP triggers
bladder cancer proliferation by affecting the MAPK pathway. Cancer
Manag Res. 12:12205–12214. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Wang H, Zhang Y, Zhen N, Zhang L,
Qiao Y, Weng W, Liu X, Ma L, Xiao W, et al: Mutual inhibition
between YAP and SRSF1 maintains long non-coding RNA, Malat1-induced
tumourigenesis in liver cancer. Cell Signal. 26:1048–1059. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Yu Z, Chen SS, Li F, Lei CY, Chen
XX, Bao JM, Luo Y, Lin GZ, Pang SY and Tan WL: The YAP1 oncogene
contributes to bladder cancer cell proliferation and migration by
regulating the H19 long noncoding RNA. Urol Oncol. 33:427.e1–e10.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv M, Zhong Z, Huang M, Tian Q, Jiang R
and Chen J: lncRNA H19 regulates epithelial-mesenchymal transition
and metastasis of bladder cancer by miR-29b-3p as competing
endogenous RNA. Biochim Biophys Acta Mol Cell Res. 1864:1887–1899.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jafri I: MiRNA a new insight in metabolic
and human diseases: A review. Cell Mol Biol (Noisy-le-grand).
69:102–110. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo K: Signaling cross talk between
TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect
Biol. 9:a0221372017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhuang C, Liu Y, Fu S, Yuan C, Luo J,
Huang X, Yang W, Xie W and Zhuang C: Silencing of lncRNA MIR497HG
via CRISPR/Cas13d induces bladder cancer progression through
promoting the crosstalk between Hippo/Yap and TGF-β/Smad signaling.
Front Mol Biosci. 7:6167682020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou X, Zhang P, Han H, Lei H and Zhang X:
Hypermethylated in cancer 1 (HIC1) suppresses bladder cancer
progression by targeting yes-associated protein (YAP) pathway. J
Cell Biochem. 120:6471–6481. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang J and Deng X: Effects of miR-599
targeting YAP1 on proliferation, invasion and apoptosis of bladder
urothelial carcinoma cells. Exp Mol Pathol. 118:1045992021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hade MD, Suire CN and Suo Z: Mesenchymal
stem cell-derived exosomes: Applications in regenerative medicine.
Cells. 10:19592021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang ZM, Wang H and Ji ZG: Bladder
mesenchymal stromal cell-derived exosomal miRNA-217 modulates
bladder cancer cell survival through Hippo-YAP pathway. Inflamm
Res. 70:959–969. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schmitz-Dräger BJ, Droller M, Lokeshwar
VB, Lotan Y, Hudson MA, van Rhijn BW, Marberger MJ, Fradet Y,
Hemstreet GP, Malmstrom PU, et al: Molecular markers for bladder
cancer screening, early diagnosis, and surveillance: The WHO/ICUD
consensus. Urol Int. 94:1–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li S, Zhu H, Chen H, Xia J, Zhang F, Xu R
and Lin Q: Glucose promotes epithelial-mesenchymal transitions in
bladder cancer by regulating the functions of YAP1 and TAZ. J Cell
Mol Med. 24:10391–10401. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zakrzewski W, Dobrzyński M, Szymonowicz M
and Rybak Z: Stem cells: Past, present, and future. Stem Cell Res
Ther. 10:682019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fuchs E and Blau HM: Tissue stem cells:
Architects of their niches. Cell Stem Cell. 27:532–556. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang T, Song X, Xu D, Tiek D, Goenka A,
Wu B, Sastry N, Hu B and Cheng SY: Stem cell programs in cancer
initiation, progression, and therapy resistance. Theranostics.
10:8721–8743. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nassar D and Blanpain C: Cancer stem
cells: Basic concepts and therapeutic implications. Annu Rev
Pathol. 11:47–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Driskill JH and Pan D: Control of stem
cell renewal and fate by YAP and TAZ. Nat Rev Mol Cell Biol.
24:895–911. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Luo J and Li P: Context-dependent
transcriptional regulations of YAP/TAZ in stem cell and
differentiation. Stem Cell Res Ther. 13:102022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Damkham N, Issaragrisil S and
Lorthongpanich C: Role of YAP as a mechanosensing molecule in stem
cells and stem cell-derived hematopoietic cells. Int J Mol Sci.
23:146342022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao AY, Dai YJ, Lian JF, Huang Y, Lin JG,
Dai YB and Xu TW: YAP regulates ALDH1A1 expression and stem cell
property of bladder cancer cells. Onco Targets Ther. 11:6657–6663.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ooki A, Del Carmen Rodriguez Pena M,
Marchionni L, Dinalankara W, Begum A, Hahn NM, VandenBussche CJ,
Rasheed ZA, Mao S, Netto GJ, et al: YAP1 and COX2 coordinately
regulate urothelial cancer stem-like cells. Cancer Res. 78:168–181.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang T, Yang Y, Wang Z, Zhang X, Li D and
Wei J: A SNP of miR-146a is involved in bladder cancer relapse by
affecting the function of bladder cancer stem cells via the
miR-146a signallings. J Cell Mol Med. 24:8545–8556. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ghasemi H, Mousavibahar SH, Hashemnia M,
Karimi J, Khodadadi I, Mirzaei F and Tavilani H: Tissue stiffness
contributes to YAP activation in bladder cancer patients undergoing
transurethral resection. Ann N Y Acad Sci. 1473:48–61. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gwak HS, Youn SM, Kwon AH, Lee SH, Kim JH
and Rhee CH: ACNU-cisplatin continuous infusion chemotherapy as
salvage therapy for recurrent glioblastomas: Phase II study. J
Neurooncol. 75:173–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gentilin E: New advancements in
cisplatin-based treatments. Int J Mol Sci. 24:59202023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gómez-Ruiz S, Maksimović-Ivanić D,
Mijatović S and Kaluđerović GN: On the discovery, biological
effects, and use of Cisplatin and metallocenes in anticancer
chemotherapy. Bioinorg Chem Appl. 2012:1402842012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wenmaekers S, Viergever BJ, Kumar G,
Kranenburg O, Black PC, Daugaard M and Meijer RP: A potential role
for HUWE1 in modulating cisplatin sensitivity. Cells. 10:12622021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rebucci M and Michiels C: Molecular
aspects of cancer cell resistance to chemotherapy. Biochem
Pharmacol. 85:1219–1226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Nguyen CDK and Yi C: YAP/TAZ Signaling and
resistance to cancer therapy. Trends Cancer. 5:283–296. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim MH and Kim J: Role of YAP/TAZ
transcriptional regulators in resistance toanti-cancer therapies.
Cell Mol Life Sci. 74:1457–1474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Fan Q, Cai Q and Xu Y: FOXM1 is a
downstream target of LPA and YAP oncogenic signaling pathways in
high grade serous ovarian cancer. Oncotarget. 6:27688–27699. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ciamporcero E, Daga M, Pizzimenti S,
Roetto A, Dianzani C, Compagnone A, Palmieri A, Ullio C, Cangemi L,
Pili R and Barrera G: Crosstalk between Nrf2 and YAP contributes to
maintaining the antioxidant potential and chemoresistance in
bladder cancer. Free Radic Biol Med. 115:447–457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hayden A, Douglas J, Sommerlad M, Andrews
L, Gould K, Hussain S, Thomas GJ, Packham G and Crabb SJ: The Nrf2
transcription factor contributes to resistance to cisplatin in
bladder cancer. Urol Oncol. 32:806–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Daga M, Pizzimenti S, Dianzani C, Cucci
MA, Cavalli R, Grattarola M, Ferrara B, Scariot V, Trotta F and
Barrera G: Ailanthone inhibits cell growth and migration of
cisplatin resistant bladder cancer cells through down-regulation of
Nrf2, YAP, and c-Myc expression. Phytomedicine. 56:156–164. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cucci MA, Grattarola M, Dianzani C, Damia
G, Ricci F, Roetto A, Trotta F, Barrera G and Pizzimenti S:
Ailanthone increases oxidative stress in CDDP-resistant ovarian and
bladder cancer cells by inhibiting of Nrf2 and YAP expression
through a post-translational mechanism. Free Radic Biol Med.
150:125–135. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Dhillon S: Decitabine/cedazuridine: First
approval. Drugs. 80:1373–1378. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Khandelwal M, Anand V, Appunni S, Seth A,
Singh P, Mathur S and Sharma A: Decitabine augments cytotoxicity of
cisplatin and doxorubicin to bladder cancer cells by activating
hippo pathway through RASSF1A. Mol Cell Biochem. 446:105–114. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ciamporcero E, Shen H, Ramakrishnan S, Yu
Ku S, Chintala S, Shen L, Adelaiye R, Miles KM, Ullio C, Pizzimenti
S, et al: YAP activation protects urothelial cell carcinoma from
treatment-induced DNA damage. Oncogene. 35:1541–1553. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hong WX, Haebe S, Lee AS, Westphalen CB,
Norton JA, Jiang W and Levy R: Intratumoral immunotherapy for
early-stage solid tumors. Clin Cancer Res. 26:3091–3099. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Demaria O, Gauthier L, Debroas G and
Vivier E: Natural killer cell engagers in cancer immunotherapy:
Next generation of immuno-oncology treatments. Eur J Immunol.
51:1934–1942. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rui R, Zhou L and He S: Cancer
immunotherapies: Advances and bottlenecks. Front Immunol.
14:12124762023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Han J, Gu X, Li Y and Wu Q: Mechanisms of
BCG in the treatment of bladder cancer-current understanding and
the prospect. Biomed Pharmacother. 129:1103932020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Donin NM, Lenis AT, Holden S, Drakaki A,
Pantuck A, Belldegrun A and Chamie K: Immunotherapy for the
treatment of urothelial carcinoma. J Urol. 197:14–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gill J and Prasad V: Pembrolizumab for
non-muscle-invasive bladder cancer-a costly therapy in search of
evidence. JAMA Oncol. 7:501–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Song D, Powles T, Shi L, Zhang L,
Ingersoll MA and Lu YJ: Bladder cancer, a unique model to
understand cancer immunity and develop immunotherapy approaches. J
Pathol. 249:151–165. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Baek SW, Jang IH, Kim SK, Nam JK, Leem SH
and Chu IS: Transcriptional profiling of advanced urothelial cancer
predicts prognosis and response to immunotherapy. Int J Mol Sci.
21:18502020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Baek SW, Mun JY, Jang IH, Yang GE, Jeong
MS, Kim SK, Nam JK, Chu IS and Leem SH: YAP1 activation is
associated with the progression and response to immunotherapy of
non-muscle invasive bladder cancer. EBioMedicine. 81:1040922022.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Grossman H, Natale RB, Tangen CM, Speights
VO, Vogelzang NJ, Trump DL, deVere White RW, Sarosdy MF, Wood DP
Jr, Raghavan D and Crawford ED: Neoadjuvant chemotherapy plus
cystectomy compared with cystectomy alone for locally advanced
bladder cancer. N Engl J Med. 349:859–866. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Joo WD, Visintin I and Mor G: Targeted
cancer therapy-are the days of systemic chemotherapy numbered?
Maturitas. 76:308–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Pérez-Herrero E and Fernández-Medarde A:
Advanced targeted therapies in cancer: Drug nanocarriers, the
future of chemotherapy. Eur J Pharm Biopharm. 93:52–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Rouprêt M, Seisen T, Birtle AJ, Capoun O,
Compérat EM, Dominguez-Escrig JL, Gürses Andersson I, Liedberg F,
Mariappan P, Hugh Mostafid A, et al: European association of
urology guidelines on upper urinary tract urothelial carcinoma:
2023 Update. Eur Urol. 84:49–64. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yu EY, Petrylak DP, O'Donnell PH, Lee JL,
van der Heijden MS, Loriot Y, Stein MN, Necchi A, Kojima T,
Harrison MR, et al: Enfortumab vedotin after PD-1 or PD-L1
inhibitors in cisplatin-ineligible patients with advanced
urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2
trial. Lancet Oncol. 22:872–882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Dong L, Lin F, Wu W, Liu Y and Huang W:
Verteporfin inhibits YAP-induced bladder cancer cell growth and
invasion via Hippo signaling pathway. Int J Med Sci. 15:645–652.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Elbadawy M, Sato Y, Mori T, Goto Y,
Hayashi K, Yamanaka M, Azakami D, Uchide T, Fukushima R, Yoshida T,
et al: Anti-tumor effect of trametinib in bladder cancer organoid
and the underlying mechanism. Cancer Biol Ther. 22:357–371. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen H, Yang W, Li Y and Ji Z: PLAGL2
promotes bladder cancer progression via RACGAP1/RhoA GTPase/YAP1
signaling. Cell Death Dis. 14:4332023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sudol M: YAP1 oncogene and its eight
isoforms. Oncogene. 32:39222013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fang L, Teng H, Wang Y, Liao G, Weng L, Li
Y, Wang X, Jin J, Jiao C, Chen L, et al: SET1A-mediated
mono-methylation at K342 regulates YAP activation by blocking its
nuclear export and promotes tumorigenesis. Cancer Cell.
34:103–118.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan
F, Meng S, Wang Y, Yuan Z and Bi W: SIRT1 regulates YAP2-mediated
cell proliferation and chemoresistance in hepatocellular carcinoma.
Oncogene. 33:1468–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yan F, Qian M, He Q, Zhu H and Yang B: The
posttranslational modifications of Hippo-YAP pathway in cancer.
Biochim Biophys Acta Gen Subj. 1864:1293972020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wu L, Ou Z, Liu P, Zhao C, Tong S, Wang R,
Li Y, Yuan J, Chen M, Fan B, et al: ATXN3 promotes prostate cancer
progression by stabilizing YAP. Cell Commun Signal. 21:1522023.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xu TH, Sheng Z, Li Y, Qiu X, Tian B and
Yao L: OGT knockdown counteracts high phosphate-induced vascular
calcification in chronic kidney disease through autophagy
activation by downregulating YAP. Life Sci. 261:1181212020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Santinon G, Pocaterra A and Dupont S:
Control of YAP/TAZ activity by metabolic and nutrient-sensing
pathways. Trends Cell Biol. 26:289–299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Wang R, Yang S, Wang M, Zhou Y, Li X, Chen
W, Liu W, Huang Y, Wu J, Cao J, et al: A sustainable approach to
universal metabolic cancer diagnosis. Nat Sustain. 7:602–615. 2024.
View Article : Google Scholar
|
|
97
|
Kashihara T, Mukai R, Oka SI, Zhai P,
Nakada Y, Yang Z, Mizushima W, Nakahara T, Warren JS, Abdellatif M
and Sadoshima J: YAP mediates compensatory cardiac hypertrophy
through aerobic glycolysis in response to pressure overload. J Clin
Invest. 132:e1505952022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li X, Wu Z, He J, Jin Y, Chu C, Cao Y, Gu
F, Wang H, Hou C, Liu X and Zou Q: OGT regulated O-GlcNAcylation
promotes papillary thyroid cancer malignancy via activating YAP.
Oncogene. 40:4859–4871. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu
X, Zhu G, Zhao Y, Chen Y, Yu Y, et al: The essential role of YAP
O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat
Commun. 8:152802017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Pei C, Wang Y, Ding Y, Li R, Shu W, Zeng
Y, Yin X and Wan J: Designed concave octahedron heterostructures
decode distinct metabolic patterns of epithelial ovarian tumors.
Adv Mater. 35:22090832023. View Article : Google Scholar
|
|
101
|
Sun T and Chi JT: Regulation of
ferroptosis in cancer cells by YAP/TAZ and Hippo pathways: The
therapeutic implications. Genes Dis. 8:241–249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wei C and Fu Q: Cell death mediated by
nanotechnology via the cuproptosis pathway: A novel horizon for
cancer therapy. VIEW. 4:202300012023. View Article : Google Scholar
|
















