In tumors, certain genes related to proliferation
and invasion are upregulated in expression (1). In neurodegenerative diseases, there
are also a bulk of genes with abnormal expression, including genes
related to the autophagy-lysosomal pathway (2). These abnormally expressed genes may
not only serve as biomarkers for disease diagnosis, but also as
targets for treatment. Human endogenous retroviruses (HERVs) are
derived from ancient retroviruses, which infect and insert viral
genes into germline cells. HERVs constitute an estimated 8% of the
human genome (3). In general, a
complete HERV includes 5′ long terminal repeats (5′ LTR), a
primer-binding site, group-specific antigen gene (gag), a protease
gene, a polymerase gene (pol), an envelope gene and 3′ LTR elements
(4). Paternally expressed imprinted
gene 10 (PEG10) is an evolutionarily conserved HERV gene (5) belonging to the Ty3/Gypsy family of
retrotransposons. Evolutionarily, PEG10 is a therian-specific gene
(6,7), and differentially methylated regions
of PEG10 have emerged in the therian ancestor at least 160 million
years ago (8). During the evolution
of the placenta in mammals, PEG10 was inserted into the genome, as
it exists in marsupials but not in egg-laying monotreme species
(9). PEG10 is a paternally
expressed imprinting gene, which was reported to be located at
human chromosome 7q21 in 2001 for the first time (7). Importantly, PEG10 can be used for RNA
delivery (10–13). As an imprinting gene, PEG10 is
crucial for placental development and plays a key role in mouse
embryonic stem cell and trophoblast stem cell differentiation
(14–16). Ono et al (17) have reported that PEG10 maintains
placental function, and deletion of PEG10 in mice can lead to
embryonic lethality. Traditionally, PEG10 expression is limited to
the testes, adrenal gland and skin after adulthood (18). During the past few years, knowledge
of PEG10 being highly expressed in tumors and neurodegenerative
diseases has emerged. Thus, the present article reviewed the recent
findings on the roles of PEG10 in these diseases.
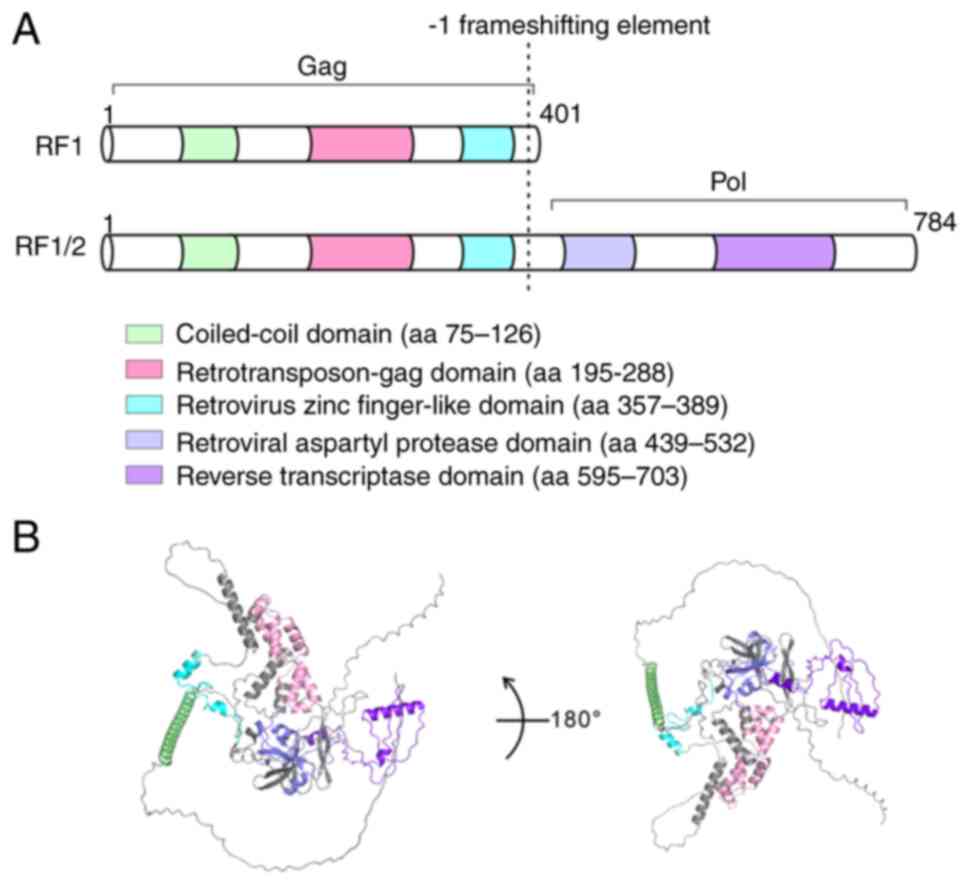
PEG10 contains 2 overlapping open reading frames
(RF1 and RF1/2), and the mRNA of PEG10 utilizes a typical
retroviral frameshift mechanism to encode two proteins: RF1
(encoding gag-like protein) and RF1/2 (encoding gag-pol-like
polyprotein) (19,20). The frameshifting efficiency of PEG10
is ~22% (19) and a study has
identified that the frameshift of PEG10 can be suppressed by a
small-molecule compound (21). The
ribosomal frameshift element of PEG10 consists of ‘slippery’ and
‘pseudoknot’. The slippery sequence is GGGAAAC, while pseudoknot is
composed of multiple stem-loop structures. When ribosomes encounter
the pseudoknot, the A- and P-site tRNAs detach from the zero frame
codons GGA-AAC, shifting back one nucleotide to GGG-AAA, causing
the original encoding of glycine-asparagine to change to
glycine-lysine (22). PEG10 has
multiple evolutionary conserved functional domains, including a
coiled-coil domain, retrotransposon-gag domain, retrovirus zinc
finger-like domain, retroviral asparytyl protease domain and
reverse transcriptase domain (5,7,23–26).
The structure of PEG10 is shown in Fig.
1.
LncRNA is a type of ncRNA with a length exceeding
200 nucleotides. LncRNAs are involved in the occurrence,
development and progression of cancer. PEG10 is an upregulated
lncRNA in patients with lymphoma (27). In diffuse large B-cell lymphoma
(DLBCL), lncRNA PEG10 inhibits microRNA (miR)-101-3p, while
miR-101-3p inhibits kinesin family member 2A (KIF2A). Therefore,
overexpression of PEG10 leads to an increase in KIF2A expression,
promoting DLBCL growth (28,29).
LncRNA PEG10 inhibits miR-449a, increases ribosomal protein S2
expression and increases the proliferation, invasion and migration
of neuroblastoma cells (30).
LncRNA PEG10 and H19 are mutual upstream and downstream regulatory
factors, and PEG10 levels are constitutively associated with a high
lymph node ratio in gastric cancer (31). Furthermore, knockout of PEG10 causes
an increase in miR-3200 and inhibits the proliferation, migration
and invasion of gastric cancer cells (32) and glioma cells (33). LncRNA PEG10 inhibits miR-33a in
melanoma, thus inhibiting the PI3K/AKT and mTOR pathways (34). Furthermore, overexpression of lncRNA
PEG10 also has an essential role in promoting proliferation and
invasion in esophageal cancer cells (35) and hypopharyngeal squamous cell
carcinoma (36).
PEG10 contains 2 overlapping open reading frames,
RF1 and RF2. During embryonic development, PEG10-RF1 and
PEG10-RF1/2 proteins are expressed at different stages, suggesting
that PEG10-RF1 and PEG10-RF1/2 may have different functions.
PEG10-RF1/2 have an aspartate protease domain and new fragments can
be generated through self-cutting (19,22).
PEG10 is found in multiple cellular components, including the
nucleus (24), extracellular
vesicles and stress granules (25).
PEG10-RF1 has nuclear and cytoplasmic localization and does not
enter stress granules under stress conditions. However, PEG10-RF1/2
is only localized in the cytoplasm and enters stress granules under
stress conditions (25). PEG10-RF1
contains a retroviral zinc finger domain and is considered a
transcription factor, regulates the transcription of genes
participates in the progression of cancers and neurodegenerative
diseases; these genes include C9orf72-SMCR8 complex subunit,
doublecortin like kinase 1, plexin A4, semaphorin 5B, slit guidance
ligand 3 and Wnt family member 3A (24).
In addition, PEG10 is a secreted protein produced by
Dental-derived mesenchymal stem cells and bone marrow stem cells,
which is associated with adipose differentiation. PEG10 expression
is generally observed at the immediate early stage of adipocyte
differentiation (37).
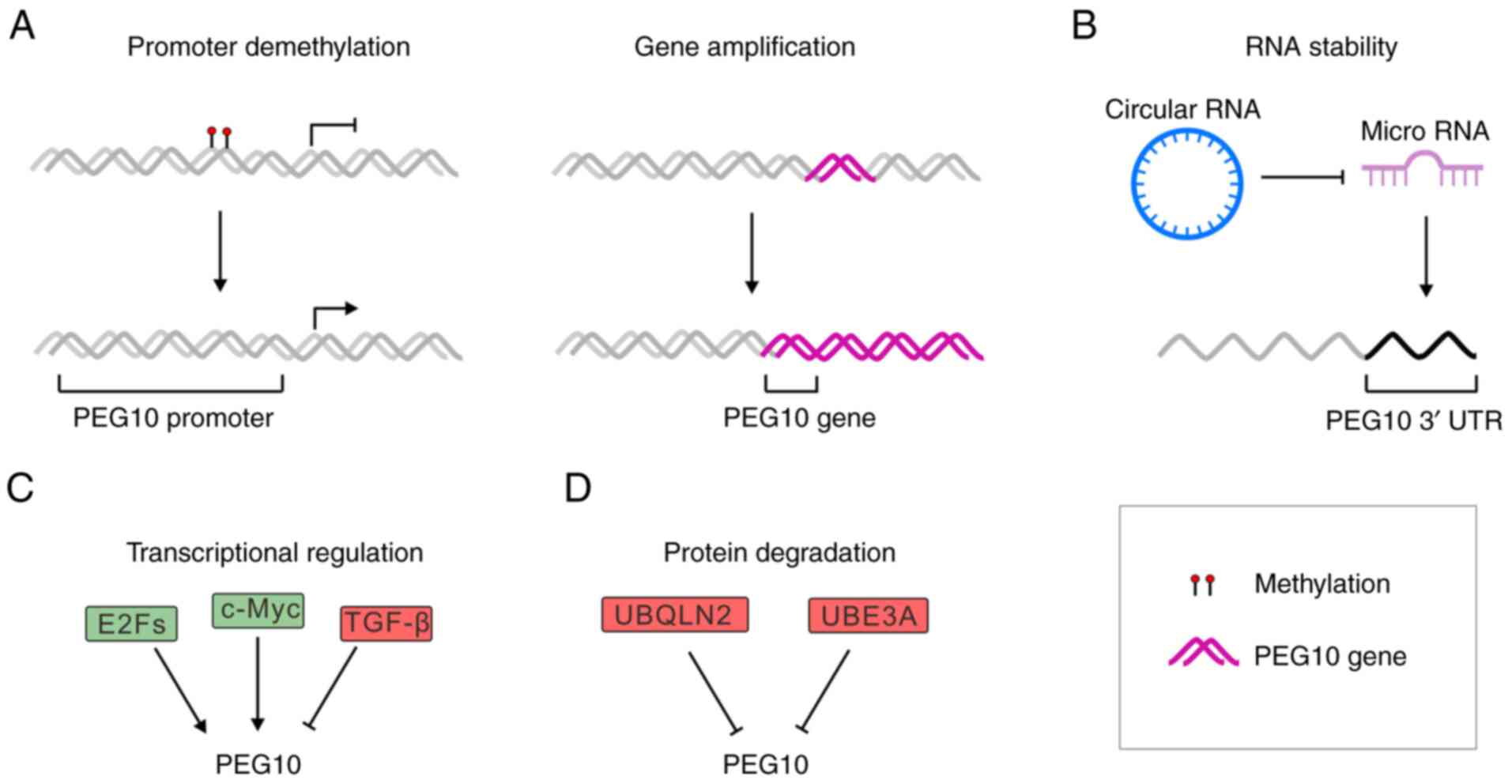
The increase in PEG10 protein may be caused by
various ways, including DNA demethylation, gene duplication,
extended RNA half-life, transcriptional activation and inhibition
of protein degradation (Fig.
2).
In the mammalian genome, DNA methylation is an
important approach to govern gene expression (38,39).
DNA methylation usually inhibits gene expression by recruiting
repression proteins or inhibiting transcription factors to DNA. In
human parthenogenetic embryonic stem cells, activating
transcription factor 7 interacting protein increases PEG10
methylation and inhibits its transcription (40). In KrasG12D-induced T-cell
neoplasms, imprinting control regions (ICRs) of PEG10 are
significantly hypermethylated. Increased DNA methylation at the
ICRs of PEG10 is the earliest detectable change in lymphocytic
T-cell thymic lymphoma (41). Tet
methylcytosine dioxygenase 1 (TET1) is a maintenance DNA
demethylase, which promotes PEG10 expression by inhibiting DNA
methylation, and deleting TET1 results in an increase of
5-hydroxymethylcytosine in PEG10, leading to a decrease in PEG10
expression (42). Histone
methylation is another way to regulate PEG10 expression, and in
hepatocellular carcinoma (HCC), menin/mixed-lineage leukemia 1
(MLL) interaction inhibitor MI-503 prevents the display of the
menin-MLL1 complex from binding to the PEG10 promoter, reduces the
modification of trimethylated H3 lysine 4 in the promoter region
and inhibits PEG10 transcription (43).
Traditionally, gene amplification was identified as
one aspect of the genetic instability strongly associated with
malignantly transformed cells. Cancer cells use this mechanism to
mediate overexpression of certain oncogenes to promote
proliferation, anti-apoptosis and resistance to anticancer drugs
(44). For instance, genomic
amplification of PEG10 at the 7q21.3 locus was found in HCC samples
(45,46). In hepatitis B virus-associated HCC,
the increase in DNA copy number leads to an increased expression of
PEG10 (47).
The stability of mRNA plays an important role in the
control of gene expression (48).
PEG10 is a direct target of miRNA-574-5p (30). MiR-138-5p binds to the 3′UTR of
PEG10 mRNA, shortening the half-life of PEG10 mRNA (49). In regulating retinoblastoma (RB),
the circular RNA Circ:0075804 promotes PEG10 expression by
inhibiting miR-138-5p (49).
N6-methyladenosine residues on the PEG10 3′UTR are recognized and
bind by insulin like growth factor 2 mRNA binding protein 1 to
recruit poly (A) Binding Protein Cytoplasmic 1 and enhance the
stability of PEG10 mRNA, thereby increasing the protein content of
PEG10 (50). In neural progenitor
cells, TET3 increases the DNA methylation levels of PEG10 and
maintains neural stem cell identity (51). In HCC, miR-122 binds to the PEG10
3′UTR and inhibits PEG10 expression (52). LncRNA SNAI3 antisense RNA 1
increases PEG10 expression through competing endogenous RNA
spreading of miR-27a-3p and miR-34a-5p, thereby promoting cancer
cell proliferation (53). In human
colon cancer cells, miR-491 binds to the 3′UTR of PEG10 mRNA and
inhibits PEG10 expression (54). In
colorectal cancer, NOTCH1-associated lncRNA in T-cell acute
lymphoblastic leukemia 1 increases PEG10 expression to promote
tumor progression by sponging miR-574-5p (55).
Transcription factors can bind to the promoter
region of PEG10 and activate its transcription. Myc promotes
tumor-cell proliferation by activating PEG10 transcription
(56,57). In addition, the E2F family of
transcription factors (E2Fs) are transcription factors that promote
the transcription of PEG10 and thereby enhance the proliferation of
HCC cells (58). Glycogen synthase
kinase 3β can phosphorylate and activate E2F transcriptional factor
1 (E2F1). Phosphorylated E2F1 binds to ubiquitin specific peptidase
11, leading to reduced degradation of E2F1 due to deubiquitination.
E2F-1 has also been recently shown to be crucial for PEG10
activation in pancreatic cancer, and to further promote cell
proliferation, migration and invasion (59). Similarly, in HCC, the expression of
PEG10 is positively correlated with lymph node metastasis.
Overexpression of PEG10 promotes epithelial-mesenchymal transition,
and the expression of PEG10 is influenced by the transforming
growth factor β (TGF-β) signaling (60). Furthermore, transcription factor
CTR9 homolog, Paf1/RNA polymerase II complex component promotes
PEG10 transcription (61).
Although TGF-β promotes PEG10 expression in HCC
tissues, in other cancer tissues, the expression of PEG10 is
inhibited by TGF-β, e.g. in chondrosarcoma cells (62). Furthermore, the upregulated PEG10
activity in turn inhibits TGF-β and bone morphogenetic protein
signaling (63). The transcription
factor one cut homeobox 2 binds to the PEG10 promoter and increases
the mRNA levels of PEG10 in lethal prostate cancer (64,65),
and recent studies demonstrated that androgen receptor (AR)
inhibits the transcription of PEG10 (66–68).
RB inhibits PEG10 transcription by inhibiting E2F1 activity
(26,58). Interestingly, the expression of
PEG10 can be regulated by small molecule compounds. For instance,
curcumin inhibits the expression of PEG10 in an unknown mechanism,
thereby inhibiting the growth of breast cancer cells (69). Exposure to cadmium (Cd) can cause a
decrease in the expression of Cd-exposed placentas PEG10 (70).
PEG10 has a C-terminal polyproline repeat domain,
which may be recognized by human ubiquilin 2 (UBQLN2) and
facilitates its proteasomal degradation (24,71).
The deficiency of UBQLN2 leads to an increase in PEG10 protein.
Furthermore, the ubiquitin protein ligase E3A (UBE3A) is another
PEG10 regulating gene. UBE3A inhibits the expression of PEG10 via
the proteasome (25)
Nuclear localized PEG10 functions as a transcription
factor, mediating the transcription of numerous downstream target
genes. Kruppel-like factor 2 (KLF2) is an inhibitory factor of
NF-κB and PEG10 activates NF-κB signaling by inhibiting KLF2
expression (23). Upregulated PEG10
expression activates NOTCH signaling and promotes metastatic cancer
stem cell self-renewal (72). PEG10
was reported to bind to TGF-β receptor ALK1 and to inhibit ALK1 as
well as ALK5 signaling (73). In
HCC, TSG101 binds to PEG10 to prevent its degradation, thereby
enhancing PEG10 expression and downstream target genes p53, p21 and
matrix metalloproteinases (MMPs), leading to cell proliferation,
invasion and migration (74). After
overexpression of PEG10 in human colon cancer cells, the
Wnt1/β-catenin pathway is activated, promoting cell proliferation
and inhibiting cell apoptosis (54). In Burkitt's lymphoma cells, PEG10
promotes tumor cell invasion and metastasis by upregulating the
expression of MMP-2 and −9 (18,26,59,75).
PEG10 promotes the proliferation of endometrial cancer cells by
inhibiting the transcription of p16 and p18 genes. PEG10 tends to
bind to the TGGGAYTACA and CTCNGCCTCC motifs (50). Of note, it was recently found that
PEG10 is an RNA binding protein, promoting trophoblast stem cell
differentiation into placental lineages (14).
PEG10 is considered an oncogene and PEG10 protein
has been found to be significantly increased in various cancer
types to date. Exogenous PEG10 expression leads to increased
cellular proliferation and increased cell viability (76).
The relationship between PEG10 and cancer was first
studied in liver cancer. PEG10 is strongly expressed in HCC
(77). A machine learning study
found that PEG10 is highly expressed in patients unresponsive to
transarterial chemotherapy (78).
Expression of PEG10 is significantly correlated with poor survival
and tumor recurrence in HCC, making PEG10 an independent predictor
of early recurrence of HCC (79).
However, other studies found that PEG10 has an inhibitory effect on
tumor cell growth by activating immune response. For instance,
transduction of dendritic cells with PEG10 recombinant adenovirus
induced anti-tumor immunity against HCC (80). Another study also identified that
androgens activate PEG10 (81).
Thereby, PEG10 is the potential biomarker of HCC (82,83).
An increase in PEG10 content is closely related to
the prognosis of lung cancer; therefore, PEG10 is a diagnostic and
prognostic gene for lung adenocarcinoma and squamous cell carcinoma
(84). E2Fs activate MMPs by
upregulating PEG10, leading to lung cancer progression, prognosis
and metastasis (85,86). E2F1 activates PEG10 gene
transcription, promoting proliferation of lung epithelial cells
(87). Furthermore, transcription
termination factor 1 activates receptor tyrosine kinase like orphan
receptor 1 (ROR1), which activates PEG10 transcription. Inhibition
of ROR1 by small inhibitory (si)RNA in the human lung cancer cell
line PC-9 leads to a decrease in the transcription level of PEG10
(88). On the contrary, the
expression of PEG10 is also inhibited by certain transcription
factors. The expression of PEG10 is inhibited by PI3K/AKT, which
leads to a decrease in PEG10 protein levels in lung cancer cells
(89).
The expression of PEG10 in prostate cancer is
usually suppressed, as AR inhibits the transcription of PEG10
(26). However, during the
treatment of prostate cancer, AR inhibitors drive cancer cells to
evolve into neuroendocrine prostate cancer (NEPC) and the
expression of PEG10 gradually increases during the transition from
adenocarcinoma to NEPC. Therefore, PEG10 can be used as a
predictive indicator for biochemical recurrence of prostate cancer
(90). As a characteristic gene of
NEPC, silencing PEG10 inhibits the in vitro growth of
prostate cancer cells (91). In AR
activated adenocarcinoma type prostate cancer, full-length RF1/2 is
the dominant form, while in NEPC cells, RF1 and RF1/2 are both
highly expressed (26). Full-length
PEG10 (RF1/2) drive the proliferation of NEPC, while short-length
PEG10 (RF1) promotes invasion (26). The expression of PEG10 is associated
with short survival of patients with prostate adenocarcinoma
(92). Importantly, studies have
highlighted the crucial role of PEG10 in promoting the malignant
transformation of the highly lethal AR-negative phenotype prostate
cancer (93). Silencing PEG10
reduced the expression of neuroendocrine markers and inhibited cell
proliferation (94).
Driven by genomic gains and promoter demethylation,
PEG10 is highly expressed and is associated with poor patient
prognosis in mycosis fungoides-large-cell transformation (23). Furthermore, PEG10 overexpression was
observed in B-cell acute lymphoblastic leukemia and B-cell chronic
lymphocytic leukemia (95,96). Reducing PEG10 expression leads to a
decrease in cell volume and a weakened colony-formation ability in
large transformed cutaneous T-cell lymphoma cells; consequently,
PEG10 inhibition may be a promising treatment for advanced invasive
T-cell lymphoma (23). In thymoma,
the DNA methylation level of the promoter region of PEG10 is higher
than that of T-cell lymphoblastic leukemia (97,98).
Abnormal DNA methylation in PEG10 ICRs is expected to become a
prognostic marker for T-cell neoplasm and B-cell chronic
lymphocytic leukemia (41,99).
To date, PEG10 has been shown to be crucial in
several types of cancer, such as Ewing sarcoma (ES), esophageal
squamous cell carcinoma (ESCC) and metastatic thymic
adenocarcinoma. In ES, PEG10 inhibits the TGF-β pathway and reduces
cell growth. In the human skin epidermoid carcinoma cell line A431,
miR-145, miR-432 and miR-1972 inhibit PEG10 expression (100). Besides, genetic mutations can also
affect PEG10 activity. In metastatic thymic adenocarcinoma, PEG10
was found to have a somatic p.R207H mutation (101). LncRNA PEG10 is elevated in serum
exosomes of patients with ESCC (102). Higher PEG10 levels are associated
with unfavorable overall and progression-free survival (103). Therefore, PEG10 can serve as a
marker of carcinogenesis, progression and poor prognosis, as well
as a putative drug target in ovarian cancer (72,104,105), rectal adenocarcinoma (106), early-onset colorectal cancer
(107), neuroendocrine
muscle-invasive bladder cancer (108), bladder cancer (108), adenosquamous carcinomas (109), gallbladder adenocarcinoma
(110), breast cancer (111,112), adenocarcinoma (113), oral squamous cell carcinoma
(114) and glioma (115).
ALS is a chronic neurodegenerative disease that
mainly causes damage to upper and lower motor neurons. Mutations in
the ubiquitin-adaptor protein UBQLN2 is considered one of the
causes of ALS (116,117). PEG10 is a substrate of UBQLN2
(118); UBQLN2 binds to the
C-terminus of PEG10 and initiates its degradation (24).
Angelman syndrome is caused by UBE3A defect;
patients with Angelman syndrome often experience developmental
delay, balance disorders, limb incoordination and gait instability.
The UBE3A mutation causes PEG10 aggregation, alters neuronal
migration and ultimately leads to Angelman syndrome (25).
Although no targeted small-molecule inhibitors to
block PEG10 have been developed, there are still numerous ways to
inhibit PEG10, including inhibitors that target its transcription
factors, siRNAs, shRNAs or miRNAs that target PEG10 mRNA, as well
as using PEG10 as an antigen to develop vaccines (119). PEG10-related treatment methods are
listed in Table I. The functional
diversity of PEG10 poses multiple challenges to drug development,
and ensuring target specificity is key in drug development to avoid
unnecessary damage to embryo development or normal tissue
function.
Not applicable.
This work was supported by the National Science Foundation of
Sichuan Province (grant no. 2023NSFSC1555), the National Clinical
Research Center for Geriatrics, West China Hospital, Sichuan
University (grant no. Z20192008), the special fund in West China
Hospital, Sichuan University (grant no. 008420415031) and the 1·3·5
project for disciplines of excellence, West China Hospital, Sichuan
University (grant no. ZYJC18003).
Not applicable.
DM, SB and XB were involved in the conceptualization
of the study. SW and YC wrote the original draft. YW and YD
prepared the figures. MT and XT reviewed and revised the
manuscript. All authors reviewed the manuscript and have read and
approved the final version. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee MS, Kim JW, Park DG, Heo H, Kim J,
Yoon JH and Chang J: Autophagic signatures in peripheral blood
mononuclear cells from Parkinson's disease patients. Mol Cells.
48:1001732024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dopkins N and Nixon DF: Activation of
human endogenous retroviruses and its physiological consequences.
Nat Rev Mol Cell Biol. 25:212–222. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jakobsson J and Vincendeau M: SnapShot:
Human endogenous retroviruses. Cell. 185:400–400.e1. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Youngson NA, Kocialkowski S, Peel N and
Ferguson-Smith AC: A small family of sushi-class
retrotransposon-derived genes in mammals and their relation to
genomic imprinting. J Mol Evol. 61:481–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iwasaki S, Suzuki S, Pelekanos M, Clark H,
Ono R, Shaw G, Renfree MB, Kaneko-Ishino T and Ishino F:
Identification of a novel PNMA-MS1 gene in marsupials suggests the
LTR retrotransposon-derived PNMA genes evolved differently in
marsupials and eutherians. DNA Res. 20:425–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ono R, Kobayashi S, Wagatsuma H, Aisaka K,
Kohda T, Kaneko-Ishino T and Ishino F: A retrotransposon-derived
gene, PEG10, is a novel imprinted gene located on human chromosome
7q21. Genomics. 73:232–237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Renfree MB, Suzuki S and Kaneko-Ishino T:
The origin and evolution of genomic imprinting and viviparity in
mammals. Philos Trans R Soc Lond B Biol Sci. 368:201201512013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki S, Ono R, Narita T, Pask AJ, Shaw
G, Wang C, Kohda T, Alsop AE, Marshall Graves JA, Kohara Y, et al:
Retrotransposon silencing by DNA methylation can drive mammalian
genomic imprinting. PLoS Genet. 3:e552007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Segel M, Lash B, Song J, Ladha A, Liu CC,
Jin X, Mekhedov SL, Macrae RK, Koonin EV and Zhang F: Mammalian
retrovirus-like protein PEG10 packages its own mRNA and can be
pseudotyped for mRNA delivery. Science. 373:882–889. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang R, Guo L, Wei T, Chen T, Yang H, Ye
H, Lin F, Zeng Y, Yu H, Cai Z and Liu X: Engineering PEG10
assembled endogenous virus-like particles with genetically encoded
neoantigen peptides for cancer vaccination. Elife. 13:RP985792024.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Campodonico W, Mohan HM, Huynh PT, Black
HH, Lau CI, Paulson HL, Sharkey LM and Whiteley AM: The gag-like
gene RTL8 antagonizes PEG10-mediated virus like particles. PLoS
One. 19:e03109462024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Liu Z, Wang D, Ye J, Shi Z, Pan C,
Zhang Q, Ju R, Zheng Y and Liu Y: Intraocular mRNA delivery with
endogenous MmPEG10-based virus-like particles. Exp Eye Res.
243:1098992024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abed M, Verschueren E, Budayeva H, Liu P,
Kirkpatrick DS, Reja R, Kummerfeld SK, Webster JD, Gierke S,
Reichelt M, et al: The Gag protein PEG10 binds to RNA and regulates
trophoblast stem cell lineage specification. PLoS One.
14:e02141102019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pollard KS, Serre D, Wang X, Tao H,
Grundberg E, Hudson TJ, Clark AG and Frazer K: A genome-wide
approach to identifying novel-imprinted genes. Hum Genet.
122:625–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smallwood A, Papageorghiou A, Nicolaides
K, Alley MK, Jim A, Nargund G, Ojha K, Campbell S and Banerjee S:
Temporal regulation of the expression of syncytin (HERV-W),
maternally imprinted PEG10, and SGCE in human placenta. Biol
Reprod. 69:286–293. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ono R, Nakamura K, Inoue K, Naruse M,
Usami T, Wakisaka-Saito N, Hino T, Suzuki-Migishima R, Ogonuki N,
Miki H, et al: Deletion of Peg10, an imprinted gene acquired from a
retrotransposon, causes early embryonic lethality. Nat Genet.
38:101–106. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie T, Pan S, Zheng H, Luo Z, Tembo KM,
Jamal M, Yu Z, Yu Y, Xia J, Yin Q, et al: PEG10 as an oncogene:
Expression regulatory mechanisms and role in tumor progression.
Cancer Cell Inte. 18:1122018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clark MB, Jänicke M, Gottesbühren U,
Kleffmann T, Legge M, Poole ES and Tate WP: Mammalian gene PEG10
expresses two reading frames by high efficiency-1 frameshifting in
embryonic-associated tissues. J Biol Chem. 282:37359–37369. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manktelow E, Shigemoto K and Brierley I:
Characterization of the frameshift signal of Edr, a mammalian
example of programmed-1 ribosomal frameshifting. Nucleic Acids Res.
33:1553–1563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cardno TS, Shimaki Y, Sleebs BE, Lackovic
K, Parisot JP, Moss RM, Crowe-McAuliffe C, Mathew SF, Edgar CD,
Kleffmann T and Tate WP: HIV-1 and human PEG10 frameshift elements
are functionally distinct and distinguished by novel small molecule
modulators. PLoS One. 10:e01390362015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lux H, Flammann H, Hafner M and Lux A:
Genetic and molecular analyses of PEG10 reveal new aspects of
genomic organization, transcription and translation. PLoS One.
5:e86862010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu F, Gao Y, Xu B, Xiong S, Yi S, Sun J,
Chen Z, Liu X, Li Y, Lin Y, et al: PEG10 amplification at 7q21.3
potentiates large-cell transformation in cutaneous T-cell lymphoma.
Blood. 139:554–571. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Black HH, Hanson JL, Roberts JE, Leslie
SN, Campodonico W, Ebmeier CC, Holling GA, Tay JW, Matthews AM, Ung
E, et al: UBQLN2 restrains the domesticated retrotransposon PEG10
to maintain neuronal health in ALS. Elife. 12:e794522023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pandya NJ, Wang C, Costa V, Lopatta P,
Meier S, Zampeta FI, Punt AM, Mientjes E, Grossen P, Distler T, et
al: Secreted retrovirus-like GAG-domain-containing protein PEG10 is
regulated by UBE3A and is involved in Angelman syndrome
pathophysiology. Cell Rep Med. 2:1003602021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akamatsu S, Wyatt AW, Lin D, Lysakowski S,
Zhang F, Kim S, Tse C, Wang K, Mo F, Haegert A, et al: The
placental gene PEG10 promotes progression of neuroendocrine
prostate cancer. Cell Rep. 12:922–936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J and Wang X: Role of long non-coding
RNAs in lymphoma: A systematic review and clinical perspectives.
Crit Rev Oncol Hematol. 141:13–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao J, Su L and Jiang J: Long Non-coding
RNA paternally expressed imprinted gene 10 (PEG10) elevates diffuse
large B-Cell lymphoma progression by regulating kinesin family
member 2A (KIF2A) via targeting MiR-101-3p. Med Sci Monit.
26:e9228102020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng W, Fan H, Wu G, Wu J and Feng J:
Upregulation of long noncoding RNA PEG10 associates with poor
prognosis in diffuse large B cell lymphoma with facilitating
tumorigenicity. Clin Exp Med. 16:177–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Liu W, Ji P and Zhang Y:
Silencing of long chain noncoding RNA paternally expressed gene
(PEG10) inhibits the progression of neuroblastoma by regulating
microRNA-449a (miR-449a)/ribosomal protein S2 (RPS2) axis.
Bioengineered. 13:6309–6322. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishii S, Yamashita K, Harada H, Ushiku H,
Tanaka T, Nishizawa N, Yokoi K, Washio M, Ema A, Mieno H, et al:
The H19-PEG10/IGF2BP3 axis promotes gastric cancer progression in
patients with high lymph node ratios. Oncotarget. 8:74567–74581.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Chu XQ, Zhang D and Kong DF:
Knockdown of long non-coding RNA PEG10 inhibits growth, migration
and invasion of gastric carcinoma cells via up-regulating miR-3200.
Neoplasma. 65:769–778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao H, Ding N, Liao H, Yao Z, Cheng X,
Zhang J and Zhao M: Prediction of relapse and prognosis by
expression levels of long noncoding RNA PEG10 in glioma patients.
Medicine (Baltimore). 98:e175832019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fu Y, Bi Y, Wang F, Chen X and Liu H:
Declination of long noncoding RNA paternally expressed gene 10
inhibits A375 cells proliferation, migration, and invasion via
mediating microRNA-33a. J Cell Biochem. 120:19868–19877. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zang W, Wang T, Huang J, Li M, Wang Y, Du
Y, Chen X and Zhao G: Long noncoding RNA PEG10 regulates
proliferation and invasion of esophageal cancer cells. Cancer Gene
Ther. 22:138–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao M, Sun D, Li X, Xu Y, Zhang H, Qin Y
and Xia M: Overexpression of long noncoding RNA PEG10 promotes
proliferation, invasion and metastasis of hypopharyngeal squamous
cell carcinoma. Oncol Lett. 14:2919–2925. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar A, Kumar V, Rattan V, Jha V and
Bhattacharyya S: Secretome proteins regulate comparative osteogenic
and adipogenic potential in bone marrow and dental stem cells.
Biochimie. 155:129–139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jung S and Lee JS: Single-cell genomics
for investigating pathogenesis of inflammatory diseases. Mol Cells.
46:120–129. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu YL, Lin ZJ, Li CC, Lin X, Shan SK, Guo
B, Zheng MH, Li F, Yuan LQ and Li ZH: Epigenetic regulation in
metabolic diseases: Mechanisms and advances in clinical study.
Signal Transduct Target Ther. 8:982023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bar S, Vershkov D, Keshet G, Lezmi E,
Meller N, Yilmaz A, Yanuka O, Nissim-Rafinia M, Meshorer E,
Eldar-Geva T and Benvenisty N: Identifying regulators of parental
imprinting by CRISPR/Cas9 screening in haploid human embryonic stem
cells. Nat Commun. 12:67182021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bretz CL, Langohr IM, Lee S and Kim J:
Epigenetic instability at imprinting control regions in a
Kras(G12D)-induced T-cell neoplasm. Epigenetics. 10:1111–1120.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamaguchi S, Shen L, Liu Y, Sendler D and
Zhang Y: Role of Tet1 in erasure of genomic imprinting. Nature.
504:460–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kempinska K, Malik B, Borkin D, Klossowski
S, Shukla S, Miao H, Wang J, Cierpicki T and Grembecka J:
Pharmacologic inhibition of the Menin-MLL interaction leads to
transcriptional repression of PEG10 and blocks hepatocellular
carcinoma. Mol Cancer Ther. 17:26–38. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shoshani O, Brunner SF, Yaeger R, Ly P,
Nechemia-Arbely Y, Kim DH, Fang R, Castillon GA, Yu M, Li JSZ, et
al: Chromothripsis drives the evolution of gene amplification in
cancer. Nature. 591:137–141. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dong H, Zhang H, Liang J, Yan H, Chen Y,
Shen Y, Kong Y, Wang S, Zhao G and Jin W: Digital karyotyping
reveals probable target genes at 7q21.3 locus in hepatocellular
carcinoma. BMC Med Genomics. 4:602011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tsuji K, Yasui K, Gen Y, Endo M, Dohi O,
Zen K, Mitsuyoshi H, Minami M, Itoh Y, Taniwaki M and Tanaka S:
PEG10 is a probable target for the amplification at 7q21 detected
in hepatocellular carcinoma. Cancer Genet Cytogenet. 198:118–125.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang J, Sheng HH, Shen T, Hu YJ, Xiao HS,
Zhang Q, Zhang QH and Han ZG: Correlation between genomic DNA copy
number alterations and transcriptional expression in hepatitis B
virus-associated hepatocellular carcinoma. FEBS Lett.
580:3571–3581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kwon HC, Bae Y and Lee SV: The role of
mRNA quality control in the aging of caenorhabditis elegans. Mole
Cells. 46:664–671. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang Y, Dou X, Kong Q, Li Y and Zhou X:
Circ_0075804 promotes the malignant behaviors of retinoblastoma
cells by binding to miR-138-5p to induce PEG10 expression. Int
Ophthalmol. 42:509–523. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang L, Wan Y, Zhang Z, Jiang Y, Gu Z, Ma
X, Nie S, Yang J, Lang J, Cheng W and Zhu L: IGF2BP1 overexpression
stabilizes PEG10 mRNA in an m6A-dependent manner and promotes
endometrial cancer progression. Theranostics. 11:1100–1114. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Santiago M, Antunes C, Guedes M, Iacovino
M, Kyba M, Reik W, Sousa N, Pinto L, Branco MR and Marques CJ: Tet3
regulates cellular identity and DNA methylation in neural
progenitor cells. Cell Mol Life Sci. 77:2871–2883. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shyu YC, Lee TL, Lu MJ, Chen JR, Chien RN,
Chen HY, Lin JF, Tsou AP, Chen YH, Hsieh CW and Huang TS:
miR-122-mediated translational repression of PEG10 and its
suppression in human hepatocellular carcinoma. J Transl Med.
14:2002016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Y, Guo D, Lu G, Mohiuddin Chowdhury
ATM, Zhang D, Ren M, Chen Y, Wang R and He S: LncRNA SNAI3-AS1
promotes PEG10-mediated proliferation and metastasis via decoying
of miR-27a-3p and miR-34a-5p in hepatocellular carcinoma. Cell
Death Dis. 11:6852020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li B, Shi C, Li B, Zhao JM and Wang L: The
effects of Curcumin on HCT-116 cells proliferation and apoptosis
via the miR-491/PEG10 pathway. J Cell Biochem. 119:3091–3098. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ye M, Zhao L, Zhang L, Wu S, Li Z, Qin Y,
Lin F and Pan L: LncRNA NALT1 promotes colorectal cancer
progression via targeting PEG10 by sponging microRNA-574-5p. Cell
Death Dis. 13:9602022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jiménez Martín O, Schlosser A, Furtwängler
R, Wegert J and Gessler M: MYCN and MAX alterations in Wilms tumor
and identification of novel N-MYC interaction partners as biomarker
candidates. Cancer Cell Int. 21:5552021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li CM, Margolin AA, Salas M, Memeo L,
Mansukhani M, Hibshoosh H, Szabolcs M, Klinakis A and Tycko B:
PEG10 is a c-MYC target gene in cancer cells. Cancer Res.
66:665–672. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang C, Xiao Y, Hu Z, Chen Y, Liu N and Hu
G: PEG10 directly regulated by E2Fs might have a role in the
development of hepatocellular carcinoma. FEBS Lett. 582:2793–2798.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Peng YP, Zhu Y, Yin LD, Zhang JJ, Wei JS,
Liu X, Liu XC, Gao WT, Jiang KR and Miao Y: PEG10 overexpression
induced by E2F-1 promotes cell proliferation, migration, and
invasion in pancreatic cancer. J Exp Clin Cancer Res. 36:302017.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang M, Sui C, Dai B, Shen W, Lu J and
Yang J: PEG10 is imperative for TGF-β1-induced
epithelial-mesenchymal transition in hepatocellular carcinoma.
Oncol Rep. 37:510–518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang B, Liu ZY, Wu R, Zhang CM, Cao K,
Shan WG, Liu Z, Ji M, Tian ZL, Sethi G, et al: Transcriptional
regulator CTR9 promotes hepatocellular carcinoma progression and
metastasis via increasing PEG10 transcriptional activity. Acta
Pharmacol Sin. 43:2109–2118. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yahiro Y, Maeda S, Shinohara N, Jokoji G,
Sakuma D, Setoguchi T, Ishidou Y, Nagano S, Komiya S and Taniguchi
N: PEG10 counteracts signaling pathways of TGF-β and BMP to
regulate growth, motility and invasion of SW1353 chondrosarcoma
cells. J Bone Miner Metab. 37:441–454. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shinohara N, Maeda S, Yahiro Y, Sakuma D,
Matsuyama K, Imamura K, Kawamura I, Setoguchi T, Ishidou Y, Nagano
S and Komiya S: TGF-β signalling and PEG10 are mutually exclusive
and inhibitory in chondrosarcoma cells. Sci Rep. 7:134942017.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rotinen M, You S, Yang J, Coetzee SG,
Reis-Sobreiro M, Huang WC, Huang F, Pan X, Yáñez A, Hazelett DJ, et
al: ONECUT2 is a targetable master regulator of lethal prostate
cancer that suppresses the androgen axis. Nat Med. 24:1887–1898.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chatterjee A, Gallent B, Katiki M, Qian C,
Harter MR, Silletti S, Komives EA, Freeman MR and Murali R: The
homeodomain regulates stable DNA binding of prostate cancer target
ONECUT2. Nat Commun. 15:90372024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Akamatsu S, Inoue T, Ogawa O and Gleave
ME: Clinical and molecular features of treatment-related
neuroendocrine prostate cancer. Int J Urol. 25:345–351. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Feng H, Cheng AS, Tsang DP, Li MS, Go MY,
Cheung YS, Zhao GJ, Ng SS, Lin MC, Yu J, et al: Cell cycle-related
kinase is a direct androgen receptor-regulated gene that drives
β-catenin/T cell factor-dependent hepatocarcinogenesis. J Clin
Invest. 121:3159–3175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Qin J, Liu M, Ding Q, Ji X, Hao Y, Wu X
and Xiong J: The direct effect of estrogen on cell viability and
apoptosis in human gastric cancer cells. Mol Cell Biochem.
395:99–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kreutz D, Sinthuvanich C, Bileck A, Janker
L, Muqaku B, Slany A and Gerner C: Curcumin exerts its antitumor
effects in a context dependent fashion. J Proteomics. 182:65–72.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Xu P, Wu Z, Yang W and Wang L:
Dysregulation of DNA methylation and expression of imprinted genes
in mouse placentas of fetal growth restriction induced by maternal
cadmium exposure. Toxicology. 390:109–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wu JJ, Cai A, Greenslade JE, Higgins NR,
Fan C, Le NTT, Tatman M, Whiteley AM, Prado MA, Dieriks BV, et al:
ALS/FTD mutations in UBQLN2 impede autophagy by reducing
autophagosome acidification through loss of function. Proc Natl
Acad Sci USA. 117:15230–15241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhao H, Gao Y, Miao J, Chen S, Li J, Li Z,
Yin C and Yue W: Single-cell RNA-seq highlights a specific
carcinoembryonic cluster in ovarian cancer. Cell Death Dis.
12:10822021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lux A, Beil C, Majety M, Barron S,
Gallione CJ, Kuhn HM, Berg JN, Kioschis P, Marchuk DA and Hafner M:
Human retroviral gag- and gag-pol-like proteins interact with the
transforming growth factor-beta receptor activin receptor-like
kinase 1. J Biol Chem. 280:8482–8493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu Z, Tian Z, Cao K, Zhang B, Wen Q, Zhou
X, Yang W, Wang T, Shi H and Wang R: TSG101 promotes the
proliferation, migration and invasion of hepatocellular carcinoma
cells by regulating the PEG10. J Cell Mol Med. 23:70–82. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Xiong J, Qin J, Zheng Y, Peng X, Luo Y and
Meng X: PEG10 promotes the migration of human Burkitt's lymphoma
cells by up-regulating the expression of matrix metalloproteinase-2
and −9. Clin Invest Med. 35:E117–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Golda M, Mótyán JA, Mahdi M and Tőzsér J:
Functional study of the Retrotransposon-Derived human PEG10
Protease. Int J Mol Sci. 21:24242020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Okabe H, Satoh S, Furukawa Y, Kato T,
Hasegawa S, Nakajima Y, Yamaoka Y and Nakamura Y: Involvement of
PEG10 in human hepatocellular carcinogenesis through interaction
with SIAH1. Cancer Res. 63:3043–3048. 2003.PubMed/NCBI
|
|
78
|
Tang Y, Wu Y, Xue M, Zhu B, Fan W and Li
J: A 10-Gene signature identified by machine learning for
predicting the response to transarterial chemoembolization in
patients with hepatocellular carcinoma. J Oncol. 2022:38227732022.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Bang H, Ha SY, Hwang SH and Park CK:
Expression of PEG10 is associated with poor survival and tumor
recurrence in hepatocellular carcinoma. Cancer Res Treat.
47:844–852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Peng W, Zhao G, Ma Y, Yu H and Wang X:
Dendritic cells transfected with PEG10 recombinant adenovirus
elicit anti-tumor immune response in vitro and in vivo. Vaccine.
29:3501–3506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jie X, Lang C, Jian Q, Chaoqun L, Dehua Y,
Yi S, Yanping J, Luokun X, Qiuping Z, Hui W, et al: Androgen
activates PEG10 to promote carcinogenesis in hepatic cancer cells.
Oncogene. 26:5741–5751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jia HL, Ye QH, Qin LX, Budhu A, Forgues M,
Chen Y, Liu YK, Sun HC, Wang L, Lu HZ, et al: Gene expression
profiling reveals potential biomarkers of human hepatocellular
carcinoma. Clin Cancer Res. 13:1133–1139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ip WK, Lai PB, Wong NL, Sy SM, Beheshti B,
Squire JA and Wong N: Identification of PEG10 as a progression
related biomarker for hepatocellular carcinoma. Cancer Lett.
250:284–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wu X, Wang L, Feng F and Tian S: Weighted
gene expression profiles identify diagnostic and prognostic genes
for lung adenocarcinoma and squamous cell carcinoma. J Int Med Res.
48:3000605198938372020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Deng X, Hu Y, Ding Q, Han R, Guo Q, Qin J,
Li J, Xiao R, Tian S, Hu W, et al: PEG10 plays a crucial role in
human lung cancer proliferation, progression, prognosis and
metastasis. Oncol Rep. 32:2159–2167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Sinha A, Zou Y, Patel AS, Yoo S, Jiang F,
Sato T, Kong R, Watanabe H, Zhu J, Massion PP, et al: Early-stage
lung adenocarcinoma MDM2 genomic amplification predicts clinical
outcome and response to targeted therapy. Cancers (Basel).
14:7082022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang D, Zhao J, Li S, Wei J, Nan L,
Mallampalli RK, Weathington NM, Ma H and Zhao Y: Phosphorylated
E2F1 is stabilized by nuclear USP11 to drive Peg10 gene expression
and activate lung epithelial cells. J Mol Cell Biol. 10:60–73.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Nakagawa N, Miyake N, Ochi N, Yamane H,
Takeyama M, Nagasaki Y, Ikeda T, Yokota E, Fukazawa T, Nakanishi H,
et al: Targeting ROR1 in combination with osimertinib in EGFR
mutant lung cancer cells. Exp Cell Res. 409:1129402021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
De Marco C, Laudanna C, Rinaldo N,
Oliveira DM, Ravo M, Weisz A, Ceccarelli M, Caira E, Rizzuto A,
Zoppoli P, et al: Specific gene expression signatures induced by
the multiple oncogenic alterations that occur within the
PTEN/PI3K/AKT pathway in lung cancer. PLoS One. 12:e01788652017.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Xing Q, Liu S, Luan J, Wang Y and Ma L: A
novel 13 RNA binding proteins (RBPs) signature could predict
prostate cancer biochemical recurrence. Pathol Res Pract.
225:1535872021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lundin-Ström KB, Biloglav A, Lazarevic V,
Behrendtz M, Castor A and Johansson B: Parental origin of monosomy
7 in acute leukaemia. Br J Haematol. 192:e132–e135. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yoshie H, Sedukhina AS, Minagawa K, Oda K,
Ohnuma S, Yanagisawa N, Maeda I, Takagi M, Kudo H, Nakazawa R, et
al: A bioinformatics-to-clinic sequential approach to analysis of
prostate cancer biomarkers using TCGA datasets and clinical
samples: A new method for precision oncology? Oncotarget.
8:99601–99611. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shapovalova M, Lee JK, Li Y, Vander Griend
DJ, Coleman IM, Nelson PS, Dehm SM and LeBeau AM: PEG10
Promoter-driven expression of reporter genes enables molecular
imaging of lethal prostate cancer. Cancer Res. 79:5668–5680. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kim S, Thaper D, Bidnur S, Toren P,
Akamatsu S, Bishop JL, Colins C, Vahid S and Zoubeidi A: PEG10 is
associated with treatment-induced neuroendocrine prostate cancer. J
Mol Endocrinol. 63:39–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hu C, Xiong J, Zhang L, Huang B, Zhang Q,
Li Q, Yang M, Wu Y, Wu Q, Shen Q, et al: PEG10 activation by
co-stimulation of CXCR5 and CCR7 essentially contributes to
resistance to apoptosis in CD19+CD34+ B cells from patients with B
cell lineage acute and chronic lymphocytic leukemia. Cell Mol
Immunol. 1:280–294. 2004.PubMed/NCBI
|
|
96
|
Wu H, Luo H, Wang M, Du Y and Li J: NAP1L5
promotes epithelial-mesenchymal transition by regulating PEG10
expression in acute myeloid leukaemia. Leuk Res. 148:1076232025.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Haider Z, Landfors M, Golovleva I,
Erlanson M, Schmiegelow K, Flægstad T, Kanerva J, Norén-Nyström U,
Hultdin M and Degerman S: DNA methylation and copy number variation
profiling of T-cell lymphoblastic leukemia and lymphoma. Blood
Cancer J. 10:452020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xiong S, Liu F, Sun J, Gao S, Wong CCL, Tu
P and Wang Y: Abrogation of USP9X is a potential strategy to
decrease PEG10 levels and impede tumor progression in cutaneous
T-cell lymphoma. J Invest Dermatol. 144:2778–2788.e9. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kainz B, Shehata M, Bilban M, Kienle D,
Heintel D, Krömer-Holzinger E, Le T, Kröber A, Heller G,
Schwarzinger I, et al: Overexpression of the paternally expressed
gene 10 (PEG10) from the imprinted locus on chromosome 7q21 in
high-risk B-cell chronic lymphocytic leukemia. Int J Cancer.
121:1984–1993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Alanazi I, Hoffmann P and Adelson DL:
MicroRNAs are part of the regulatory network that controls EGF
induced apoptosis, including elements of the JAK/STAT pathway, in
A431 cells. PLoS One. 10:e01203372015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Lee Y, Park S, Lee SH and Lee H:
Characterization of genetic aberrations in a single case of
metastatic thymic adenocarcinoma. BMC Cancer. 17:3302017.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yan S, Du L, Jiang X, Duan W, Li J, Xie Y,
Zhan Y, Zhang S, Wang L, Li S and Wang C: Evaluation of serum
exosomal lncRNAs as diagnostic and prognostic biomarkers for
esophageal squamous cell carcinoma. Cancer Manag Res. 12:9753–9763.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ge H, Yan Y, Wu D, Huang Y and Tian F:
Prognostic value of PEG10 in Asian solid tumors: A meta-analysis.
Clin Chim Acta. 483:197–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sumitani N, Ishida K, Sawada K, Kimura T,
Kaneda Y and Nimura K: Identification of malignant cell populations
associated with poor prognosis in High-grade serous ovarian cancer
using Single-Cell RNA sequencing. Cancers (Basel). 14:35802022.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gov E: Co-expressed functional
module-related genes in ovarian cancer stem cells represent novel
prognostic biomarkers in ovarian cancer. Syst Biol Reprod Med.
66:255–266. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hua Y, Ma X, Liu X, Yuan X, Qin H and
Zhang X: Identification of the potential biomarkers for the
metastasis of rectal adenocarcinoma. APMIS. 125:93–100. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Watson KM, Gardner IH, Byrne RM, Ruhl RR,
Lanciault CP, Dewey EN, Anand S and Tsikitis VL: Differential
expression of PEG10 contributes to aggressive disease in early
versus Late-onset colorectal cancer. Dis Colon Rectum.
63:1610–1620. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kawai Y, Imada K, Akamatsu S, Zhang F,
Seiler R, Hayashi T, Leong J, Beraldi E, Saxena N, Kretschmer A, et
al: Paternally expressed gene 10 (PEG10) promotes growth, invasion,
and survival of bladder cancer. Mol Cancer Ther. 19:2210–2220.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu Z, Yang Z, Liu D, Li D, Zou Q, Yuan Y,
Li J, Liang L, Chen M and Chen S: TSG101 and PEG10 are prognostic
markers in squamous cell/adenosquamous carcinomas and
adenocarcinoma of the gallbladder. Oncol Lett. 7:1128–1138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu DC, Yang ZL and Jiang S:
Identification of PEG10 and TSG101 as carcinogenesis, progression,
and poor-prognosis related biomarkers for gallbladder
adenocarcinoma. Pathol Oncol Res. 17:859–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Li X, Xiao R, Tembo K, Hao L, Xiong M, Pan
S, Yang X, Yuan W, Xiong J and Zhang Q: PEG10 promotes human breast
cancer cell proliferation, migration and invasion. Int J Oncol.
48:1933–1942. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Katuwal NB, Kang MS, Ghosh M, Hong SD,
Jeong YG, Park SM, Kim SG, Sohn J, Kim TH, Moon YW, et al:
Targeting PEG10 as a novel therapeutic approach to overcome CDK4/6
inhibitor resistance in breast cancer. J Exp Clin Cancer Res.
42:3252023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tang FH, Chang WA, Tsai EM, Tsai MJ and
Kuo PL: Investigating novel genes potentially involved in
endometrial adenocarcinoma using Next-generation sequencing and
bioinformatic approaches. Inte J Med Sci. 16:1338–1348. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sharan Singh S, Kumar R, Singh Kushwaha V,
Bhatt MLBB, Singh A, Mishra A, Ram H, Parmar D and Gupta R:
Expression of radioresistant gene PEG10 in OSCC patients and its
prognostic significance. Asian Pac J Cancer Prev. 18:1513–1518.
2017.PubMed/NCBI
|
|
115
|
Liang J, Liu N and Xin H: Knockdown long
non-coding RNA PEG10 inhibits proliferation, migration and invasion
of glioma cell line U251 by regulating miR-506. Gen Physiol
Biophys. 38:295–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Deng HX, Chen W, Hong ST, Boycott KM,
Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, et al:
Mutations in UBQLN2 cause dominant X-linked juvenile and
adult-onset ALS and ALS/dementia. Nature. 477:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kim SH, Nichols KD, Anderson EN, Liu Y,
Ramesh N, Jia W, Kuerbis CJ, Scalf M, Smith LM, Pandey UB and
Tibbetts RS: Axon guidance genes modulate neurotoxicity of
ALS-associated UBQLN2. Elife. 12:e843822023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Whiteley AM, Prado MA, de Poot SAH, Paulo
JA, Ashton M, Dominguez S, Weber M, Ngu H, Szpyt J, Jedrychowski
MP, et al: Global proteomics of Ubqln2-based murine models of ALS.
J Biol Chem. 296:1001532021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Huber F, Arnaud M, Stevenson BJ, Michaux
J, Benedetti F, Thevenet J, Bobisse S, Chiffelle J, Gehert T,
Müller M, et al: A comprehensive proteogenomic pipeline for
neoantigen discovery to advance personalized cancer immunotherapy.
Nat Biotechnol. October 11–2024.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Tang Q and Khvorova A: RNAi-based drug
design: Considerations and future directions. Nat Rev Drug Discov.
23:341–364. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Hill CH and Brierley I: Structural and
functional insights into viral programmed ribosomal frameshifting.
Annu Rev Virol. 10:217–242. 2023. View Article : Google Scholar : PubMed/NCBI
|