Introduction
Ovarian cancer is the second most lethal gynecologic
malignancy, with an estimated 206,839 mortalities associated with
the disease in 2022 according to the recent global cancer
statistics (1). The data from the
American Cancer Society in 2024 indicated that there has been no
improvement in the mortality/incidence rate of 62% of ovarian
cancers in the last 30 years (2).
More than 80% of patients with ovarian cancer experience recurrence
and eventually develop treatment resistance after surgery and
platinum-based chemotherapy (3,4). The
mainstay of treatment for platinum-resistant ovarian cancer (PROC),
which progresses within 6 months of completing the platinum
treatment, has been the sequential use of cytotoxic drugs,
including pegylated liposomal doxorubicin (PLD), gemcitabine,
paclitaxel and topotecan, in recent years (4). However, the effective proportion of
these drugs is only 10–15%, and the expected survival time is ~12
months (5,6). The ongoing research on developing new
drugs and identifying new therapeutic targets is constantly
evolving.
ADCs selectively deliver cytotoxic payloads to tumor
cells, representing a rapidly evolving cancer therapy (6–8). At
present, 15 ADCs have been approved by the US Food and Drug
Administration for tumor treatment, and the development of these
drugs for PROC has gained interest. In November 2022, mirvetuximab
soravtansine (MIRV; ELAHERE®) was approved by the US
Food and Drug Administration (FDA) as the first ADC for ovarian
cancer (9). MIRV targets folate
receptor α (FRα) on the surface of tumor cells to induce tumor cell
killing, thereby displaying promising clinical activity in patients
with FRα-positive ovarian cancer (10). Other ADCs are still under active
development in the treatment of PROC. In addition, studies on using
ADCs in combination therapy with various anticancer drugs are
underway (NCT05887609, NCT06660511, NCT05941507).
The present review aimed to describe the structure
and mechanism of action of ADCs, present an overview of the
progress in the effectiveness of various types of ADCs in treating
PROC in preclinical studies and clinical trials, and to discuss the
recent advances in combining ADCs with chemotherapeutic agents,
targeted agents and immunotherapies for PROC treatment. The present
study also analyzed the toxicity characteristics and management of
ADCs and finally considered the future development of ADCs for
ovarian cancer.
Literature search
A literature search was performed using the Web of
Science (https://www.webofscience.com/wos/) and PubMed
(https://pubmed.ncbi.nlm.nih.gov/)
databases. ‘ADC’, ‘monotherapy’, ‘combination therapy’,
‘platinum-resistant ovarian cancer’ and ‘research progress’ were
used as the core search terms. Each core search term was searched
separately, and then Boolean logical operators ‘AND’ and ‘OR’ were
applied for combined searches, for example, ‘(ADC AND monotherapy
AND platinum-resistant ovarian cancer) OR (ADC AND combination
therapy AND platinum-resistant ovarian cancer)’. The search fields
were limited to the title, abstract and keywords to pinpoint
relevant literature. The inclusion criteria were as follows: i)
Studies focused on ADC monotherapy or combination therapy for PROC;
ii) clinical research, basic research or review articles; iii)
language limited to English; and iv) published within the last 10
years (January 1, 2014 to December 1, 2024) to obtain the latest
research developments. The literature needed for the writing
process was also included where appropriate. The exclusion criteria
were as follows: i) Duplicate publications or highly similar
content; ii) non-research literature such as conference abstracts,
reviews, letters, news reports and so forth; and iv) literature
with weak relevance or missing key data.
The quality of clinical studies was assessed by
evaluating randomization methods, implementation of blinding, and
descriptions of loss of visits and withdrawals; the assessment for
the quality of basic research covered dimensions such as
rationality of experimental design, sample size and statistical
methods of data; and that of review articles focused on the
comprehensiveness of their literature coverage, depth of analysis
and logic. Two professionals performed independent assessment, and
a third expert was asked to adjudicate in case of disagreement.
Structure and mechanism of action of
ADCs
Structure and mechanism of action
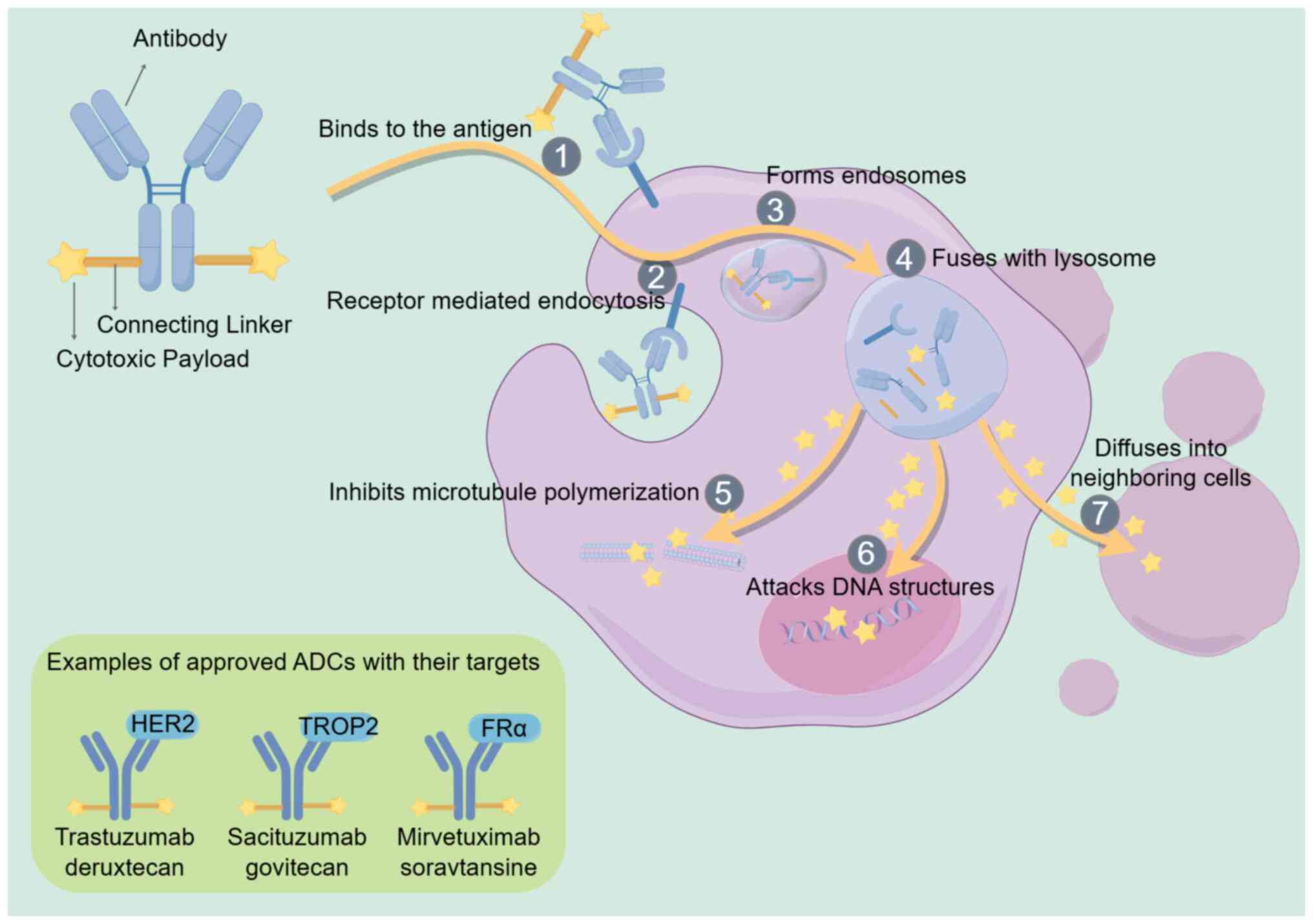
ADCs are complexes comprising an antibody, a
cytotoxic payload and a connecting linker. They specifically
recognize tumor cell surface antigens, induce endocytosis and
release cytotoxic drugs into tumor cells, ultimately leading to the
death of tumor cells (6,11). The connection of cytotoxic drugs
with antibodies was first realized in the 1950s (12). Researchers conducted clinical trials
on the mouse immunoglobulin G (IgG)-based ADCs in the 1980s
(13). Following a long and slow
development, the field has become active again in the last decade,
with ADCs entering the global market at a much faster pace.
Usually, ADCs are administered intravenously. Then, the antibodies
of ADCs bind to the antigen on cancer cells, penetrate the cells
via receptor-mediated endocytosis to form endosomes and fuse with
lysosomes. The cytotoxic payload detaches from the antibody and
diffuses into the cell in the presence of various lysosome enzymes,
resulting in tumor cell death by disrupting DNA structures or
inhibiting microtubule polymerization (8,14,15).
In addition, the payload can penetrate the extracellular matrix and
surrounding cells, resulting in a bystander effect (16). Fig.
1 shows a schematic representation of the structure and
mechanism of action of conventional ADCs.
The most commonly used antibody backbone in ADCs is
IgG. The antibody-binding antigen should be highly expressed on the
surface of tumor cell membranes with minimal expression on normal
tissues (17,18) to enhance the effectiveness of the
drug by delivering it to specific targets, protect the non-target
tissues from the damage caused by chemotherapy, and reduce the
systemic toxicity. FRα, human epidermal growth factor receptor 2
(HER2), trophoblast cell surface antigen-2 (TROP2), mesothelin,
sodium-dependent phosphate transporter 2b (NaPi2b) and CDH6 are
usually overexpressed in epithelial ovarian cancer, making them the
most commonly used conjugated antigens (19).
The covalent binding of antigen-targeting antibodies
to cytotoxic payloads requires connecting linkers (20). Linkers have two main functions; the
primary one is maintaining stability in the bloodstream, keeping
the cytotoxic payload attached to the antibody when it moves
through the plasma; the other aspect is to efficiently release the
payload into the tumor (8). The
linkers should keep this attachment stable and unchanged in the
bloodstream, with the drug being released only after
antigen-antibody binding (21).
Linkers are cleavable or non-cleavable depending on their chemical
characteristics. Cleavable linkers are chemically unstable
structures that can deliver drugs extracellularly and induce the
killing of nearby tumor cells (22,23).
The non-cleavable linker releases the drug solely when the antibody
undergoes internalization and degradation within the lysosomes of
the target cells. However, the cytotoxic payloads are released
after the apoptosis of tumor cells, which may also result in the
non-specific killing of surrounding tumor cells (24).
The cytotoxic payloads in ADCs now encompass a range
of DNA-targeting agents, microtubule-binding proteins and some
topoisomerase 1 inhibitors. The agents targeting microtubules are
the most frequently used payloads, targeting the medenosine and
periwinkle alkaloid sites. They interfere with the kinetics of
microtubule protein polymerization and maintain the cell cycle in
the G2/M phase, leading to cell death (25,26).
Not all tumor types are sensitive to a given type of payload and,
therefore, diversifying payloads is critical to expanding the
indications of ADCs.
ADCs in tumor therapy
Alternative strategies based on the use of ADCs are
highly effective in treating cancer. The use of ADCs in clinical
oncology has increased with the advent of novel technologies and
the discovery of new targets (9,27). To
date, 15 ADCs have received marketing approval from the FDA, and
hundreds more are undergoing preclinical and clinical evaluation
(28). Besides hematological
tumors, ADCs have been approved for use in breast, gastric, lung,
uroepithelial, cervical, ovarian and head-and-neck squamous cell
carcinomas. Several previous studies have described the ADC drugs
approved for oncology treatment (29–31).
Solid tumors offer a broad opportunity for ADC
development. The treatment choices for advanced or metastatic
recurrent solid tumors are limited, and current immunotherapies
rarely cure the disease (9).
Therefore, ADCs have immense potential and unique advantages in a
wide range of solid tumors.
Efficacy of ADC in PROC treatment
ADC monotherapy for PROC
Targeting FRα
FRα, one of the four members of the FR family, is a
glycosylphosphatidylinositol-anchored membrane protein (32) that binds to folic acid and related
compounds and transports them into the cell through
receptor-mediated endocytosis (33,34).
FRα is seldom expressed in normal tissues but is specifically
expressed in epithelial tumors, including ovarian cancer,
endometrial cancer, triple-negative breast cancer and
non-small-cell lung cancer (35,36),
making FRα a promising target for tumor therapy.
In November 2022, MIRV was approved by the FDA as
the first ADC for ovarian cancer (37). Targeting FRα, MIRV comprises an
antibody, a cleavable linker and a small-molecule microtubule
inhibitor DM4 (a maytansine derivative) (38). MIRV is endocytosed by tumor cells
and transferred to lysosomes, where it is degraded to release
lysine-DM4. Lysine-DM4 produces metabolites that can inhibit
tubulin polymerization and microtubule assembly, causing cell cycle
arrest and leading to cell death (39). Further, the disintegration of tumor
cells leads to the release of catabolic metabolites, which may
induce bystander killing (40–42).
Several recent clinical studies have been conducted
on the efficacy and safety of MIRV in ovarian cancer (43–45). A
phase II trial (NCT04296890) including 106 patients with PROC
showed a reduction in tumor size in 71.4% of patients, an objective
response rate (ORR) of 32.4% and a median duration of remission
(DOR) of 6.9 months (43). This
result supports the clinically meaningful efficacy of MIRV in PROC
expressing FRα, regardless of prior therapy or sequencing.
Recently, a phase III trial (NCT04209855) compared MIRV with
chemotherapeutic drugs (paclitaxel, PLD or topotecan) (45). The results showed a median
progression-free survival (PFS) of 5.62 months in the MIRV group
and 3.98 months in the chemotherapy group. The ORR of MIRV (42.3%)
was significantly higher compared with that of chemotherapy
(15.9%). The median overall survival of MIRV (16.46 months) was
also significantly longer compared with that of chemotherapy (12.75
months). Fewer adverse events also demonstrated the safety of MIRV
over chemotherapy. These results support the use of MIRV for
treating platinum-resistant epithelial ovarian cancer, providing a
new alternative for treating PROC (46). Some ongoing clinical trials are
assessing the efficacy and adverse effects of MIRV in PROC
(NCT06365853, NCT06682988 and NCT05622890).
Besides MIRV, luveltamab tazevibulin (STRO-002) is
the second ADC drug targeting FRα. STRO-002 acts as a tumor killer
by releasing the tubulin-targeting cytotoxin 3-aminophenyl
hemiasterlin (SC209) and reducing the potential for drug efflux
(47). STRO-002 is still in phase
I–III trials (NCT03748186, NCT05200364, NCT06238687 and
NCT05870748), both as a monotherapy or in combination with
bevacizumab in a variety of solid tumors, including PROC.
Farletuzumab ecteribulin (MORAb-202) is another ADC
that targets FRα-expressing tumor cells. MORAb-202 initially
inhibited tumor growth in a patient-derived xenograft (PDX) model
of triple-negative breast cancer, demonstrating durable efficacy
proportional to FRα expression (48). In clinical trials, MORAb-202 showed
promising antitumor activity and good tolerability in a phase I
study (NCT03386942) of FRα-positive solid tumors, including PROC
(49). Also, two phase I/II trials
of MORAb-202 for treating PROC (NCT05613088 and NCT04300556) are
underway.
Targeting HER2
HER2 is another promising target that can enhance
cell proliferation, differentiation and migration and inhibit
apoptosis (50). HER2 is
upregulated in a range of solid tumors, including breast, gastric
and gynecological tumors (51).
However, HER2-targeted therapies are not yet approved for use in
diseases other than breast, lung, gastric and colorectal cancers
(52). Trastuzumab deruxtecan
(T-DXd) targets HER2 and contains a topoisomerase I inhibitor
payload (53). T-DXd is approved
for treating HER2-expressing breast and gastric cancers and
HER2-mutated non-small-cell lung cancer (50). Preclinical studies demonstrated the
antitumor activity of T-DXd against primary and metastatic ovarian
tumors overexpressing HER2 (54). A
global multicenter phase II study (NCT04482309) was conducted to
evaluate the efficacy and safety of T-DXd in patients with
HER2-expressing solid tumors across seven cohorts, including three
major gynecological tumors. T-DXd treatment exhibited robust
clinical efficacy, conferring sustained clinical benefits for
patients with HER2-expressing solid tumors (55). Among all studied tumor types, the
highest ORR was observed in the three major gynecological tumor
cohorts, with an ORR of 57.5% for endometrial cancer, 50.0% for
cervical cancer and 45.0% for ovarian cancer. Among the ovarian
cancer cohort, 35.0% of the patients had previously received five
or more lines of treatment and the median overall survival time was
13.2 months; by contrast, the median overall survival time for
patients with high HER2 expression (IHC 3+) increased to 20.0
months (55). These findings
indicate that T-DXd is a promising drug for HER2-expressing
recurrent PROC (56).
Targeting TROP2
TROP2 is another tumor target highly expressed in
ovarian cancer. This is a type I cell surface glycoprotein first
discovered in human trophoblasts (57). It is upregulated in a variety of
malignant tumors and plays an important role in tumor development,
invasion and metastasis (58).
Datopotamab deruxtecan (Dato-DXd; DS-1062a), an effective
topoisomerase I inhibitor, is an ADC that targets TROP2. Dato-DXd
specifically binds to TROP2, transports it intracellularly to
lysosomes and releases DXd. In preclinical experiments, Dato-DXd
induced DNA damage and apoptosis of tumor cells in vitro and
displayed antitumor activity in vivo in xenograft tumors
with high TROP2 expression (59).
When mixed with TROP2 (IHC 3+) tumor cells, Dato-DXd showed a
significant bystander-killing effect on tumor cells with low TROP2
expression, thus prolonging the survival time of epithelial ovarian
cancer xenograft models and reducing toxicity (60). Most clinical trials are conducted on
breast cancer. A phase II clinical trial (NCT05489211) is currently
evaluating Dato-DXd for treating advanced/metastatic solid tumors,
including ovarian cancer (61). The
efficacy of Dato-DXd in ovarian cancer expressing TROP2 deserves
continued attention.
Targeting mesothelin
Mesothelin represents a promising potential target
for ovarian cancer. High expression of mesothelin is associated
with chemotherapeutic resistance and poor prognosis in epithelial
ovarian cancer (62). Anetumab
ravtansine exhibits a high degree of affinity for mesothelin, using
the microtubule inhibitor DM4 as a payload. It has displayed high
antitumor activity and good tolerability as a monotherapy in the
preclinical models of ovarian cancer (63). A phase I study (NCT02751918)
determined the antitumor activity, safety and pharmacokinetics of
anetumab ravtansine in PROC-expressing mesothelin. The result
showed an ORR of 27.7%, DOR of 7.6 months and PFS of 5.0 months
(64). This result suggests that
targeting mesothelin is an effective and well-tolerated treatment
option in patients with PROC. The development of other combination
therapy regimens for anetumab ravtansine (65) and other ADCs targeting mesothelin
(BMS-986148) (66) has also
provided preliminary evidence of clinical activity and tolerable
adverse reactions and toxicity in ovarian cancer.
Targeting NaPi2b
NaPi2b is another promising target for ADC, which is
expressed in 95% of ovarian cancers (67). Lifastuzumab vedotin (LIFA) targeting
NaPi2b displayed activity and acceptable safety in phase I studies
(68,69). A phase II study compared the
efficacy of LIFA and polyethylene glycol liposome doxorubicin in
patients with PROC. The results showed that LIFA was
well-tolerated, and ORR increased (34 vs. 15%; P=0.03) (70). Moreover, XMT-1536 and TUB-040 are
other ADCs targeting NaPi2b. Currently, the safety and efficacy of
these drugs in PROC are under evaluation in clinical trials
(NCT06517485, NCT03319628, NCT06517433 and NCT06303505).
Other ADCs
New ADCs for ovarian cancer are constantly being
developed. Raludotatug deruxtecan (R-DXd) targets cadherin 6 (CDH6)
and was effective and safe in a preclinical study using PDX tumor
models of serous ovarian cancer expressing CDH6 (71). B7-H4-directed ADC, using a
pyrrolobenzodiazepine-dimer payload, displayed antitumor activity
in a PARP inhibitor and platinum-resistant PDX model of high-grade
serous ovarian cancer (72).
The findings of several newly completed clinical
trials examining the use of ADCs in ovarian cancer (NCT06517485 and
NCT06517433) are yet to be published. Table I lists the clinical trials
investigating ADC monotherapy for ovarian cancer that deserve
continued attention in the future.
 | Table I.Ongoing clinical trials of ADCs
monotherapy in ovarian cancer. |
Table I.
Ongoing clinical trials of ADCs
monotherapy in ovarian cancer.
| NCT identifier | ADC | Target | Ovarian cancer | Phase | Completion
time |
|---|
| NCT06303505 | TUB-040 | NaPi2b | Platinum-resistant,
high-grade ovarian cancer | I/II | 2027-01 |
| NCT06457997 | PHN-010 | - | Advanced/metastatic
serous, endometroid, or clear-cell epithelial ovarian cancer | I | 2027-07 |
| NCT06390995 | Mirvetuximab
Soravtansine (TAK-853) | FRα | FRα-positive
advanced ovarian cancer | I/II | 2026-09-30 |
| NCT04152499 | SKB264 | TROP2 | Locally advanced
unresectable/metastatic epithelial ovarian cancer | I/II | 2026-07-16 |
| NCT06234423 | CUSP06 | CDH6 |
Platinum-refractory/resistant ovarian
cancer | I | 2027-08-31 |
| NCT06003231 | Disitamab vedotin
(DV) | HER2 | Previously treated,
locally-advanced, unresectable or metastatic ovarian neoplasms | II | 2028-05-31 |
| NCT06173037 | RC88 | Mesothelin | Platinum-resistant
recurrent epithelial ovarian cancer | II | 2026-12-31 |
| NCT05103683 | TORL-1-23 | CLDN6 | Advanced ovarian
cancer | I | 2025-11-15 |
| NCT06523803 | ZW171 | Mesothelin |
Mesothelin-expressing advanced or
metastatic ovarian cancer | I | 2027-12 |
| NCT05527184 | IMGN151 | FRα | Recurrent, HGS
epithelial ovarian cancer | I | 2025-12-30 |
| NCT06014190 | HS-20089 | B7-H4 | Recurrent or
metastatic ovarian cancer | II | 2027-12-31 |
| NCT05613088 | Farletuzumab
Ecteribulin (MORAb-202) | FRα | Platinum-resistant
HGS ovarian cancer | II | 2026-10-11 |
| NCT06466187 | SGN-MesoC2 | - | Advanced ovarian
neoplasms | I | 2028-11-01 |
| NCT04300556 | MORAb-202 | FRα | Platinum-resistant
HGS epithelial ovarian cancer | I/II | 2024-10-30 |
| NCT06084481 | ABBV-400 | c-Met | Platinum resistant,
high grade epithelial ovarian cancer | I | 2026-07-01 |
| NCT06238479 | LY4101174 | Nectin-4 | Recurrent, advanced
or metastatic ovarian cancer | I | 2027-03-04 |
| NCT06465069 | LY4052031 | Nectin 4 | Advanced or
metastatic ovarian cancer | I | 2027-05 |
| NCT06545617 | BAT8006 | FRα | Platinum-resistant
epithelial ovarian cancer | I/II | 2028-01-31 |
Combination therapy using ADCs and
other agents
Combination therapy using chemotherapeutic
agents
Chemotherapeutic agents such as platinum, which can
damage DNA, target the S phase of the cell cycle and induce the
G2/M phase. Preclinical studies described below have
shown that these chemotherapeutic agents can bind effectively to
ADCs containing microtubule-disrupting payloads. MIRV has been
demonstrated to arrest the cell cycle and enhance DNA damage. The
combination of MIRV with carboplatin, Adriamycin or doxorubicin has
been found to inhibit proliferation synergistically in ovarian
cancer cell lines and PDX models (73). The combination therapy of
carboplatin and STRO-002 further improved the efficacy of STRO-002
(47). The combination of anetumab
ravtansine with carboplatin or PLD demonstrated enhanced efficacy
in vivo and in vitro (in ovarian cancer cell line and
PDX models) compared with monotherapy in ovarian cancer (74). MIRV combined with carboplatin in
early clinical trials achieved an ORR of 71%, with only minor
adverse events (75), making it a
highly active therapy for ovarian cancer.
Combination therapy with the
molecularly targeted drug bevacizumab
A prevalent issue in solid tumors is inadequate
blood flow and hypoxia within the vasculature (76). Using drugs such as anti-vascular
endothelial growth factor antibodies (e.g., bevacizumab) can
modulate angiogenesis and vascular porosity, thereby altering the
tumor vascular system. Bevacizumab, the first targeted drug
indicated for ovarian cancer, has been approved for use in
combination with chemotherapy (77). Administering bevacizumab in
combination with platinum-based chemotherapy after disease
progression still improved PFS (78). ADCs have also shown safety and
efficacy in combination with bevacizumab, particularly in PROC.
Preclinical studies demonstrated that MIRV combined with
bevacizumab resulted in rapid destruction of the tumor
microvascular system and extensive areas of necrosis (73,74).
Moreover, it was highly effective in a PDX model of PROC, causing
significant regression and complete remission (73). Anetumab ravtansine combined with
bevacizumab also demonstrated enhanced antitumor efficacy (74). These results emphasized the superior
biological activity of the combination and further advanced the
combination into clinical trials.
In clinical trials, the combination of MIRV and
bevacizumab demonstrated efficacy and was well-tolerated in
patients with PROC (79). In
another cohort from the same study (NCT02606305), 94 patients with
PROC treated with MIRV and bevacizumab had an ORR of 44%, an median
duration of remission (mDOR) of 9.7 months, and an median
progression-free survival (mPFS) of 8.2 months (80). Furthermore, incorporating MIRV into
dual therapy with platinum and bevacizumab demonstrated enhanced
efficacy and safety profiles in platinum-sensitive ovarian cancer
(81). All these results point to
the importance of ADCs combined with bevacizumab. Currently, a
number of ongoing trials are investigating several additional
combinations of different ADCs with bevacizumab (NCT05445778,
NCT05200364 and NCT03587311).
Combination therapy with
immunotherapy
Other than directly inducing cancer cell death using
cytotoxic payloads, ADC also has antitumor immune activity
(8,82,83).
The related mechanisms, including Fc-mediated effector function,
immune cell death, dendritic cell maturation, enhancement of T-cell
infiltration and enhancement of immune memory, among others, have
been the subject of discussion (84–87).
ADCs have been shown to possess considerable immunomodulatory
capacity in animal models (88).
Combination of ADCs with immunotherapy in refractory tumors is a
promising strategy for clinical treatment; its improved antitumor
effects have been initially demonstrated in many preclinical
studies and early clinical trials.
Immune checkpoint inhibitors (ICIs) combined with
ADCs have synergistic effects in triple-negative breast cancer
(89); the rationale behind this
combination is that ADCs activate the immune system, whereas ICIs
remove the brakes, allowing the immune system to regain its ability
to recognize and kill tumor cells (90). Durvalumab combined with T-DXd
(NCT03742102) exhibited an ORR of 100% and was found to be safe in
a small group of patients with low expression of HER2. The interim
results of T-DXd alone and combined with patulizumab (NCT04538742)
showed ORRs of 77.3 and 82.0% and 12-month PFSs of 77.3 and 89.4%,
respectively, demonstrating promising efficacy and controllable
safety. The clinical benefits of ADCs combined with immunotherapy
have been observed in clinical trials on both uroepithelial cancer
(NCT03288545 and NCT04264936) and lung cancer (NCT02099058),
besides breast cancer (91). MIRV
combined with other agents, including pembrolizumab, was evaluated
in FRα-positive PROC. Preliminary data showed good tolerability and
therapeutic activity, with an ORR of 43%, mDOR of 6.9 months, and
mPFS of 5.2 months for MIRV combined with pembrolizumab
(NCT02606305). The synergistic effect of ADCs and immunotherapy can
overcome treatment resistance, displaying encouraging efficacy and
safety in tumor treatment. However, large, randomized phase III
clinical trials are needed to test their efficacy against
conventional therapy.
Table II lists the
ongoing clinical trials on ADC combination therapy for ovarian
cancer.
 | Table II.Ongoing clinical trials of ADCs
combination therapy in ovarian cancer. |
Table II.
Ongoing clinical trials of ADCs
combination therapy in ovarian cancer.
| NCT Identifier | ADC combination
therapy | Target | Ovarian cancer | Phase | Completion
time |
|---|
| NCT05887609 | MIRV in combination
with Olaparib | FRα | Recurrent
platinum-sensitive ovarian cancer | II | 2027-12 |
| NCT06660511 | Disitamab vedotin
in combination with anlotinib hydrochloride | HER2 | HER-2-expressing
recurrent platinum-resistant ovarian cancer | I | 2025-10-15 |
| NCT05941507 | LCB84 single agent
and in combination with an anti-PD-1 Ab | TROP2 | Advanced ovarian
cancer | I/II | 2027-05 |
| NCT04606914 | MIRV in combination
with carboplatin | FRα | FRα-positive
advanced-stage ovarian cancer | II | 2028-05-31 |
| NCT05797168 | Saruparib
(AZD5305), bevacizumab, carboplatin | - | Advanced ovarian
cancer | I/II | 2028-01-06 |
| NCT05293496 | Vobramitamab
duocarmazine (MGC018) in combination with lorigerlimab
(MGD019) | B7-H3 | Advanced epithelial
ovarian cancer | I | 2026-03 |
| NCT05445778 | MIRV in combination
with bevacizumab | FRα | FRα-high recurrent
platinum-sensitive epithelial ovarian cancer | III | 2029-04 |
| NCT05489211 | Dato-DXd as
monotherapy and in combination with anticancer agents | TROP2 | Advanced/metastatic
ovarian cancer | II | 2026-08-19 |
Toxicity characteristics and management of
ADCs
Adverse reactions and toxicity
ADC has fewer side effects compared with
conventional chemotherapy due to its ability to target tumor cells.
The toxicity of ADCs arises primarily from the payload and
secondarily from linker and bystander effects (92).
A common treatment-related adverse event (TRAE)
associated with MIRV is ocular toxicity. A systematic review and
meta-analysis showed that the most common TRAEs in patients treated
with MIRV were blurred vision (all grades, 45%; grade III, 2%; no
grade IV), nausea (all grades, 42%; grade III, 1%; no grade IV) and
diarrhea (all grades, 42%; grade III, 2%; no grade IV) (93). In a previously mentioned phase II
trial of MIRV (NCT04296890), the most common TRAEs were blurred
vision (all grades, 41%; grade III, 6%; no grade IV), keratoconus
(all grades, 29%; grade III, 8%; grade IV, 1%) and nausea (all
grades, 29%; no grade III or grade IV) (43). Further, ocular events requiring dose
reductions occurred in 12 patients (11%). One patient required
discontinuation of therapy.
T-DXd is associated with interstitial lung disease
(ILD)/pneumonia due to the expression of HER2 in lung epithelial
cells (94). It is mainly localized
to alveolar macrophages; the incidence and severity of
ILD/pneumonia depend on the T-DXd dose and frequency of
administration, suggesting that this ILD/pneumonia may be caused by
cytotoxic lung injury (95). It is
mild in most cases and can be effectively treated, but may be fatal
in some cases (96). Respiratory
disease (pneumonia) has been the most common cause of
treatment-related mortalities in all types of ADC therapy (97). Other TRAEs include nausea and
diarrhea, which are effectively controlled using antiemetic and
antidiarrheal medications.
Management and prevention
Toxicity management includes implementing supportive
measures, suspension of treatment, dosage adjustments, or permanent
discontinuation of therapy. Most ocular adverse events are mild and
reversible; they improve or subside upon discontinuation or therapy
improvement (98). Scheduling
regular eye examinations to monitor early signs and symptoms,
detecting ocular adverse effects in an early stage and prompting
pharmacological intervention, promoting the prophylactic use of
corticosteroids and lubricating eye drops, adjusting ADC dosage
when needed, and maintaining clear communication with the
ophthalmologist can help relieve symptoms before the vision is
affected, ultimately helping the patient to continue treatment
(98,99).
New guidelines for T-DXd-associated ILD/pneumonia
toxicity have been published. A multidisciplinary team and timely
management with steroids are recommended (100). Careful monitoring by a
multidisciplinary team facilitates early detection [e.g., grade I
(100)] of ILD/pneumonia, leading
to timely discontinuation of medication and initiation of steroids,
which prevents the development of fatal ILD/pneumonia.
Challenges in ADC development
The development of ADCs has some challenges. The
target expression is not uniform in tumors due to the heterogeneity
of tumor cells, and ADCs have limited effects on tumor cells with
low or no target expression. Normal tissues expressing the target
are attacked by the drug, limiting the drug dose. When combining
drugs, the optimal dose of each drug and the order of
administration also need to be re-explored. Other challenges
include tumor cells developing resistance to ADCs through multiple
mechanisms, the need to identify potential biomarkers for patient
selection and limitations in current clinical trial designs.
Mechanisms of ADC resistance
ADC resistance can be caused by various mechanisms,
including change in antigen expression, failure of ADC
transportation, failure of ADC internalization, change in tumor
sensitivity, exocytosis of ADC payload and activation of signaling
pathways (101–103). Some of these mechanisms are not
fully understood.
First, alterations occur in target antigen
expression, including target downregulation, loss or mutations in
target genes. Some tumor cells may evade ADC recognition and
binding by reducing or completely losing target expression.
Mutations in the target gene may also alter the structure and
function of the target, thus affecting the binding affinity of the
ADC to the target (101,102).
Second, the abnormal expression or defective
function of endocytosis-related proteins, such as cell surface grid
proteins, in some tumor cells can lead to the inability of ADC to
enter the cell effectively during the internalization and
processing of ADC. ADCs that enter the cell are usually transported
to the lysosome for degradation, releasing cytotoxic drugs to play
a role. If tumor cells enhance the stability of lysosomes or change
the microenvironment of lysosomes, ADCs cannot release drugs in
lysosomes, resulting in drug resistance (103,104).
Furthermore, a resistance mechanism related to drug
metabolism and clearance involves high expression of drug efflux
pumps (e.g., drug transporter proteins such as multidrug resistance
protein 1 and multidrug resistance-associated protein 1), which can
pump ADCs or their released cytotoxic drugs out of the cell,
decreasing the drug concentration in the cell, thus leading to drug
resistance (104). In breast
cancer, acquired resistance to ADC developed in two different
cancer cells after months of drug treatment, primarily due to
increased expression of the drug efflux protein ABCC1 or decreased
expression of HER2 antigen (105).
Finally, the alterations in multiple signaling
pathways in tumor cells are also closely related to ADC resistance.
For example, the activation of the PI3K/Akt/mTOR signaling pathway
can promote the proliferation, survival and metabolism of tumor
cells, making them resistant to the cytotoxic effects of ADCs. The
abnormal activation of the JAK/STAT signaling pathway may also lead
to the development of ADC resistance in tumor cells (106). Alterations in some signaling
pathways involved in regulating the cell cycle and apoptosis (such
as aberrant activation of STAT3) can also lead to the development
of ADC resistance (106).
Selection of potential biomarkers
The selection of potential biomarkers for ADC drug
development is also challenging. Normal tissues and cells may also
partially express ADC targets, and therefore ADC payloads may have
serious side effects on these normal cells (15). The biomarker should preferably be
present only on tumor cells or tumor tissue and not expressed in
normal cells and tissues. Uniform criteria to determine the
expression level that accurately predicts ADC efficacy are lacking.
For example, in the case of gosatuzumab, the TROP-2 ADC drug,
although TROP-2 expression is associated with efficacy, the exact
threshold of expression needs to be further defined with more
sufficient evidence. In addition, the biomarker expression of
different cells within the same tumor may vary or the biomarker
expression levels of the same types of tumors originating from
different patients may be different due to the existence of
intra-tumor and inter-tumor heterogeneity. All of these increase
the difficulty in finding universal biomarkers (107), making the selection of specific
targets highly challenging. Moreover, the tumor cells lacking a
specific target can proliferate rapidly in the presence of ADCs,
leading to a new round of recurrence and drug resistance (108), again demonstrating the importance
of finding tumor-specific targets.
Limitations of clinical trials
Current clinical trials are mostly limited to the
efficacy of ADC monotherapy. The information on the safety and
toxicity of monotherapy and the efficacy of combination therapy is
still in the preliminary stages. First, like-for-like comparisons
of newer ADC drugs with standard-of-care or other comparable ADC
drugs in clinical trials are scarce, limiting their status and
superiority in their class and creating difficulties in dosing
choices for clinicians and patients. Second, the maximum tolerated
dose is usually derived from safety data in phase I trials.
However, the cumulative toxicity of a drug may not be apparent
until after multiple courses of therapy, which needs further
investigation. In addition, the complexity of the design of
sequencing, dosage and duration of combination therapy, as well as
the lack of established theoretical and practical guidance, makes
the design and implementation of clinical trials more
difficult.
Practical challenges in clinical
practice
A number of other novel approaches to tumor
treatment are currently available, which include tumor cell
therapy, chimeric antigen receptor T-cell therapy (109), T-cell receptor engineered T-cell
therapy (110), tumor-infiltrating
lymphocyte therapy (111), ICI
therapies, therapies using gene editing techniques such as
CRISPR-Cas9 (112) and oncolytic
virotherapy (113). ADCs have
shown good efficacy in treating various hematological malignancies
and solid tumors, with relatively good safety, controllable adverse
effects and fewer serious adverse effects such as cytokine release
syndrome (114). The precise
delivery mechanism, excellent therapeutic effect and safety of ADC
indicate its great developmental potential in the field of tumor
therapy and wide recognition in the tumor therapy market.
However, the development of ADCs faces several
practical challenges in clinical practice. First, comprehensive
patient stratification from both clinical and molecular
characteristics is needed for specific clinical applications.
Besides determining the molecular typing of the patient's tumor
cells to select sensitive ADC drugs, the patient's general
conditions, such as age, physical status and comorbidities, and
other tumor-related characteristics, such as tumor site, size,
stage and metastasis, need to be comprehensively considered. For
example, the tolerability of ADC therapy may need to be more
carefully evaluated in patients with advanced stage and poor
physical status. Further, patient tumor samples can be
comprehensively analyzed using multi-omics technologies, such as
genomics, transcriptomics, proteomics and metabolomics, to mine
highly specific and sensitive biomarker combinations for
individualized and precise treatment. Second, as ADCs are
expensive, a study suggested a reduction in total drug acquisition
costs by at least 50% to make it a cost-effective strategy
(115). The present study
recommends using advanced computer simulation and artificial
intelligence techniques to improve drug design and screening
efficiency, optimize research and development processes, and
enhance production by increasing coupling efficiency and product
purity, ultimately reducing research and development and
manufacturing costs.
Future perspectives of ADC therapy
Treatment of PROC remains a major challenge. Despite
the development of systemic therapies for ovarian cancer,
bevacizumab, PARP inhibitors and ICIs still fail to meet the
therapeutic needs of patients with PROC. ADC-specific delivery
payload reduces side effects and has been shown to be superior to
conventional monotherapy in PROC, resulting in patient benefits
(116). ADCs combined with
bevacizumab have displayed superior activity and tolerability in
PROC. Therefore, ADCs may become the preferred combination with
bevacizumab, providing a superior treatment option for patients
with PROC. However, the results from applications in other cancers
have shown that ADCs can eventually allow disease progression due
to the emergence of drug resistance. These ADC-resistant cells
remain sensitive to standard chemotherapeutic agents or other ADCs,
which suggests that ADC resistance can be overcome by the
appropriate use of other alternative drugs.
Several possible ways to overcome ADC resistance
have been proposed, such as the use of cytotoxic agents with poor
efflux potential and combinations with other drugs (117,118). With technological advancement,
dual-targeting ADCs and dual-drug ADCs have been further developed.
Dual-targeting ADCs can recognize and bind to two different targets
at the same time and also bind to tumor cells more precisely and
effectively, thus overcoming the therapeutic challenges posed by
tumor heterogeneity. These strategies are expected to reduce the
likelihood of tumor cells becoming resistant to treatment and
extend the effective duration of drug therapy (119). Dual-drug ADCs, on the contrary,
use two different cytotoxic drugs as payloads; more significant
antitumor activity can be obtained, and the occurrence of drug
resistance can be reduced by precisely controlling the ratio of the
two drugs (120). Enhancing the
bystander-killing effect by converting non-cleavable linkers into
cleavable linkers is also a way to overcome drug resistance
(105). Moreover, ADCs release
tumor-associated antigens while killing tumor cells, thus
activating the antitumor immune response, making ADCs synergistic
with ICIs (121). The combination
of the two can enhance the killing effect on tumor cells, thus
overcoming the drug resistance of ADCs. For example, one study has
shown that combining ADCs and ICIs can improve the ORR of patients,
prolong PFS and also have certain therapeutic effects on some
patients resistant to ADC in triple-negative breast cancer
(NCT03742102). These studies are expected to provide additional
insights into the optimal use of these agents.
The optimization of the ADC drug structure and
innovative developments are expected to further improve its
therapeutic efficacy and safety. Consequent to the ongoing
advancement of science and technology, the search for ADCs more
precisely targeting tumor cells and more stable in the circulation
may represent a prominent research focus in the future. The
continuous discovery and validation of new drug targets can propel
the advancement of ADCs, thereby broadening the range of ADC
applications in clinical settings. This may have a significant and
far-reaching impact on the landscape of cancer treatment. Combining
ADCs with various antitumor agents is a promising strategy that
needs further exploration and optimization to improve efficacy and
mitigate adverse effects. The current ADCs in PROC are primarily
directed toward high-grade plasmacytoid ovarian cancer. Further
studies may include other histological subtypes.
The safety and resistance after long-term drug use
needs to be further evaluated as research continues. At present,
the research on the discovery, related mechanisms and solutions of
drug resistance in ADC mainly focuses on breast cancer, lung cancer
and gastric cancer. The corresponding data on ovarian cancer is
still lacking. ADC resistance may be discovered in the future due
to reduced FRα expression. Therefore, inspiration should be drawn
from the studies on other cancers and actively address potential
drug resistance by developing novel ADC designs or implementing
appropriate alternative and combination therapies.
Conclusions
ADCs have demonstrated potent clinical activity in
PROC by selectively delivering cytotoxic drugs to tumor cells,
improving therapeutic efficacy and reducing toxicity. The approval
of MIRV for treating FRα-positive PROC has led to the rapid
evolution of ADC monotherapy and combination therapies, which
offers hope for patients with PROC. The gradual use of more
effective ADCs for treating PROC may be observed in the future.
The development of novel drugs that can more
accurately target tumor cells and are more stable in the
circulation will be the future pursuit of researchers. The possible
emergence of ADC resistance and the mechanism behind it during
clinical application should be considered, so as to more
effectively use ADCs as a weapon to optimize the therapeutic
regimens for patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KS and SY were responsible for conceptualization,
data curation and writing the original draft. NS and FT
participated in the topic selection and were responsible for data
collection and analysis. SR and HaW were responsible for software
and validation, managing the literature and editing the table
section of the manuscript. YZ and YW were responsible for creating
the figure and participated in drafting the manuscript. HoW and HG
were responsible for reviewing and editing the manuscript. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
St Laurent J and Liu J: Treatment
approaches for platinum-resistant ovarian cancer. J Clin Oncol.
42:127–133. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis A, Tinker AV and Friedlander M:
‘Platinum resistant’ ovarian cancer: What is it, who to treat and
how to measure benefit? Gynecol Oncol. 133:624–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beck A, Goetsch L, Dumontet C and Corvaïa
N: Strategies and challenges for the next generation of
antibody-drug conjugates. Nat Rev Drug Discov. 16:315–337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuchikama K, Anami Y, Ha SYY and Yamazaki
CM: Exploring the next generation of antibody-drug conjugates. Nat
Rev Clin Oncol. 21:203–223. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Drago JZ, Modi S and Chandarlapaty S:
Unlocking the potential of antibody-drug conjugates for cancer
therapy. Nat Rev Clin Oncol. 18:327–344. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dumontet C, Reichert JM, Senter PD,
Lambert JM and Beck A: Antibody-drug conjugates come of age in
oncology. Nat Rev Drug Discov. 22:641–661. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonzalez-Ochoa E, Veneziani AC and Oza AM:
Mirvetuximab soravtansine in platinum-resistant ovarian cancer.
Clin Med Insights Oncol. 17:117955492311872642023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joubert N, Beck A, Dumontet C and
Denevault-Sabourin C: Antibody-Drug conjugates: The last decade.
Pharmaceuticals (Basel). 13:2452020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathe G, Tran BL and Bernard J: Effect on
mouse leukemia 1210 of a combination by diazo-reaction of
amethopterin and gamma-globulins from hamsters inoculated with such
leukemia by heterografts. C R Hebd Seances Acad Sci. 246:1626–1628.
1958.(In French). PubMed/NCBI
|
|
13
|
Perez HL, Cardarelli PM, Deshpande S,
Gangwar S, Schroeder GM, Vite GD and Borzilleri RM: Antibody-drug
conjugates: Current status and future directions. Drug Discov
Today. 19:869–881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khongorzul P, Ling CJ, Khan FU, Ihsan AU
and Zhang J: Antibody-drug conjugates: A comprehensive review. Mol
Cancer Res. 18:3–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Samantasinghar A, Sunildutt NP, Ahmed F,
Soomro AM, Salih ARC, Parihar P, Memon FH, Kim KH, Kang IS and Choi
KH: A comprehensive review of key factors affecting the efficacy of
antibody drug conjugate. Biomed Pharmacother. 161:1144082023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Staudacher AH and Brown MP: Antibody drug
conjugates and bystander killing: Is antigen-dependent
internalisation required? Br J Cancer. 117:1736–1742. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dubowchik GM and Walker MA:
Receptor-mediated and enzyme-dependent targeting of cytotoxic
anticancer drugs. Pharmacol Ther. 83:67–123. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doronina SO, Toki BE, Torgov MY,
Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee
TD, Siegall CB, et al: Development of potent monoclonal antibody
auristatin conjugates for cancer therapy. Nat Biotechnol.
21:778–784. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee EK and Liu JF: Antibody-drug
conjugates in gynecologic malignancies. Gynecol Oncol. 153:694–702.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teicher BA and Chari RV: Antibody
conjugate therapeutics: Challenges and potential. Clin Cancer Res.
17:6389–6397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jain N, Smith SW, Ghone S and Tomczuk B:
Current ADC linker chemistry. Pharm Res. 32:3526–3540. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bargh JD, Isidro-Llobet A, Parker JS and
Spring DR: Cleavable linkers in antibody-drug conjugates. Chem Soc
Rev. 48:4361–4374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kovtun YV, Audette CA, Ye Y, Xie H,
Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blättler WA and
Goldmacher VS: Antibody-drug conjugates designed to eradicate
tumors with homogeneous and heterogeneous expression of the target
antigen. Cancer Res. 66:3214–3221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsuchikama K and An Z: Antibody-drug
conjugates: Recent advances in conjugation and linker chemistries.
Protein Cell. 9:33–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dumontet C and Jordan MA:
Microtubule-binding agents: A dynamic field of cancer therapeutics.
Nat Rev Drug Discov. 9:790–803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen H, Lin Z, Arnst KE, Miller DD and Li
W: Tubulin inhibitor-based antibody-drug conjugates for cancer
therapy. Molecules. 22:12812017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuentes-Antrás J, Genta S, Vijenthira A
and Siu LL: Antibody-drug conjugates: In search of partners of
choice. Trends Cancer. 9:339–354. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Riccardi F, Dal Bo M, Macor P and Toffoli
G: A comprehensive overview on antibody-drug conjugates: From the
conceptualization to cancer therapy. Front Pharmacol.
14:12740882023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu Z, Li S, Han S, Shi C and Zhang Y:
Antibody drug conjugate: The ‘biological missile’ for targeted
cancer therapy. Signal Transduct Target Ther. 7:932022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaplon H, Crescioli S, Chenoweth A,
Visweswaraiah J and Reichert JM: Antibodies to watch in 2023. MAbs.
15:21534102023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yao P, Zhang Y, Zhang S, Wei X, Liu Y, Du
C, Hu M, Feng C, Li J, Zhao F, et al: Knowledge atlas of
antibody-drug conjugates on CiteSpace and clinical trial
visualization analysis. Front Oncol. 12:10398822022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luhrs CA and Slomiany BL: A human
membrane-associated folate binding protein is anchored by a
glycosyl-phosphatidylinositol tail. J Biol Chem. 264:21446–21449.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen C, Ke J, Zhou XE, Yi W, Brunzelle JS,
Li J, Yong EL, Xu HE and Melcher K: Structural basis for molecular
recognition of folic acid by folate receptors. Nature. 500:486–489.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao R, Min SH, Wang Y, Campanella E, Low
PS and Goldman ID: A role for the proton-coupled folate transporter
(PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol
Chem. 284:4267–4274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheung A, Opzoomer J, Ilieva KM, Gazinska
P, Hoffmann RM, Mirza H, Marlow R, Francesch-Domenech E, Fittall M,
Rodriguez DD, et al: Anti-Folate receptor alpha-directed antibody
therapies restrict the growth of triple-negative breast cancer.
Clin Cancer Res. 24:5098–5111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ledermann JA, Canevari S and Thigpen T:
Targeting the folate receptor: Diagnostic and therapeutic
approaches to personalize cancer treatments. Ann Oncol.
26:2034–2043. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Heo YA: Mirvetuximab soravtansine: First
approval. Drugs. 83:265–273. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dilawari A, Shah M, Ison G, Gittleman H,
Fiero MH, Shah A, Hamed SS, Qiu J, Yu J, Manheng W, et al: FDA
approval summary: Mirvetuximab soravtansine-gynx for FRα-positive,
platinum-resistant ovarian cancer. Clin Cancer Res. 29:3835–3840.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oroudjev E, Lopus M, Wilson L, Audette C,
Provenzano C, Erickson H, Kovtun Y, Chari R and Jordan MA:
Maytansinoid-antibody conjugates induce mitotic arrest by
suppressing microtubule dynamic instability. Mol Cancer Ther.
9:2700–2713. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moore KN, Martin LP, O'Malley DM,
Matulonis UA, Konner JA, Vergote I, Ponte JF and Birrer MJ: A
review of mirvetuximab soravtansine in the treatment of
platinum-resistant ovarian cancer. Future Oncol. 14:123–136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tomao F, D'Incalci M, Biagioli E,
Peccatori FA and Colombo N: Restoring platinum sensitivity in
recurrent ovarian cancer by extending the platinum-free interval:
Myth or reality? Cancer. 123:3450–3459. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ab O, Whiteman KR, Bartle LM, Sun X, Singh
R, Tavares D, LaBelle A, Payne G, Lutz RJ, Pinkas J, et al:
IMGN853, a folate receptor-α (FRα)-targeting antibody-drug
conjugate, exhibits potent targeted antitumor activity against
FRα-expressing tumors. Mol Cancer Ther. 14:1605–1613. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matulonis UA, Lorusso D, Oaknin A, Pignata
S, Dean A, Denys H, Colombo N, Van Gorp T, Konner JA, Marin MR, et
al: Efficacy and safety of mirvetuximab soravtansine in patients
with platinum-resistant ovarian cancer with high folate receptor
alpha expression: Results from the SORAYA study. J Clin Oncol.
41:2436–2445. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martin LP, Konner JA, Moore KN, Seward SM,
Matulonis UA, Perez RP, Su Y, Berkenblit A, Ruiz-Soto R and Birrer
MJ: Characterization of folate receptor alpha (FRα) expression in
archival tumor and biopsy samples from relapsed epithelial ovarian
cancer patients: A phase I expansion study of the FRα-targeting
antibody-drug conjugate mirvetuximab soravtansine. Gynecol Oncol.
147:402–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Moore KN, Angelergues A, Konecny GE,
García Y, Banerjee S, Lorusso D, Lee JY, Moroney JW, Colombo N,
Roszak A, et al: Mirvetuximab soravtansine in FRα-positive,
platinum-resistant ovarian cancer. N Engl J Med. 389:2162–2174.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sidaway P: Mirvetuximab soravtansine
superior to chemotherapy in platinum-resistant epithelial ovarian
cancer. Nat Rev Clin Oncol. 21:832024. View Article : Google Scholar
|
|
47
|
Li X, Zhou S, Abrahams CL, Krimm S, Smith
J, Bajjuri K, Stephenson HT, Henningsen R, Hanson J, Heibeck TH, et
al: Discovery of STRO-002, a novel homogeneous ADC targeting folate
receptor alpha, for the treatment of ovarian and endometrial
cancers. Mol Cancer Ther. 22:155–167. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Furuuchi K, Rybinski K, Fulmer J, Moriyama
T, Drozdowski B, Soto A, Fernando S, Wilson K, Milinichik A, Dula
ML, et al: Antibody-drug conjugate MORAb-202 exhibits long-lasting
antitumor efficacy in TNBC PDx models. Cancer Sci. 112:2467–2480.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shimizu T, Fujiwara Y, Yonemori K, Koyama
T, Sato J, Tamura K, Shimomura A, Ikezawa H, Nomoto M, Furuuchi K,
et al: First-in-human phase 1 study of MORAb-202, an antibody-drug
conjugate comprising farletuzumab linked to eribulin mesylate, in
patients with folate receptor-α-positive advanced solid tumors.
Clin Cancer Res. 27:3905–3915. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Najjar MK, Manore SG, Regua AT and Lo HW:
Antibody-Drug conjugates for the treatment of HER2-positive breast
cancer. Genes (Basel). 13:20652022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yan M, Schwaederle M, Arguello D, Millis
SZ, Gatalica Z and Kurzrock R: HER2 expression status in diverse
cancers: Review of results from 37,992 patients. Cancer Metastasis
Rev. 34:157–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Oh DY and Bang YJ: HER2-targeted
therapies-a role beyond breast cancer. Nat Rev Clin Oncol.
17:33–48. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ogitani Y, Aida T, Hagihara K, Yamaguchi
J, Ishii C, Harada N, Soma M, Okamoto H, Oitate M, Arakawa S, et
al: DS-8201a, A novel HER2-targeting ADC with a novel DNA
topoisomerase I inhibitor, demonstrates a promising antitumor
efficacy with differentiation from T-DM1. Clin Cancer Res.
22:5097–5108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mutlu L, McNamara B, Bellone S, Manavella
DD, Demirkiran C, Greenman M, Verzosa MSZ, Buza N, Hui P, Hartwich
TMP, et al: Trastuzumab deruxtecan (DS-8201a), a HER2-targeting
antibody-drug conjugate, demonstrates in vitro and in vivo
antitumor activity against primary and metastatic ovarian tumors
overexpressing HER2. Clin Exp Metastasis. 41:765–775. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Meric-Bernstam F, Makker V, Oaknin A, Oh
DY, Banerjee S, González-Martín A, Jung KH, Ługowska I, Manso L,
Manzano A, et al: Efficacy and safety of trastuzumab deruxtecan in
patients With HER2-expressing solid tumors: Primary results from
the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 42:47–58.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Erickson BK, Zeybek B, Santin AD and Fader
AN: Targeting human epidermal growth factor receptor 2 (HER2) in
gynecologic malignancies. Curr Opin Obstet Gynecol. 32:57–64. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lipinski M, Parks DR, Rouse RV and
Herzenberg LA: Human trophoblast cell-surface antigens defined by
monoclonal antibodies. Proc Natl Acad Sci USA. 78:5147–5150. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Liu X, Deng J, Yuan Y, Chen W, Sun W, Wang
Y, Huang H, Liang B, Ming T, Wen J, et al: Advances in
Trop2-targeted therapy: Novel agents and opportunities beyond
breast cancer. Pharmacol Ther. 239:1082962022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Okajima D, Yasuda S, Maejima T, Karibe T,
Sakurai K, Aida T, Toki T, Yamaguchi J, Kitamura M, Kamei R, et al:
Datopotamab deruxtecan, a novel TROP2-directed antibody-drug
conjugate, demonstrates potent antitumor activity by efficient drug
delivery to tumor cells. Mol Cancer Ther. 20:2329–2340. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
McNamara B, Greenman M, Bellone S, Santin
LA, Demirkiran C, Mutlu L, Hartwich TMP, Yang-Hartwich Y, Ratner E,
Schwartz PE and Santin AD: Preclinical activity of datopotamab
deruxtecan, a novel TROP2 directed antibody-drug conjugate
targeting trophoblast cell-surface antigen 2 (TROP2) in ovarian
carcinoma. Gynecol Oncol. 189:16–23. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Janjigian YY, Oaknin A, Lang JM, Ciombor
KK, Ray-Coquard IL, Oza AM, Yonemori K, Xu RH, Zhao J, Gajavelli S,
et al: TROPION-PanTumor03: Phase 2, multicenter study of
datopotamab deruxtecan (Dato-DXd) as monotherapy and in combination
with anticancer agents in patients (pts) with advanced/metastatic
solid tumors. J Clin Oncol. 41:TPS31532023. View Article : Google Scholar
|
|
62
|
Cheng WF, Huang CY, Chang MC, Hu YH,
Chiang YC, Chen YL, Hsieh CY and Chen CA: High mesothelin
correlates with chemoresistance and poor survival in epithelial
ovarian carcinoma. Br J Cancer. 100:1144–1153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Golfier S, Kopitz C, Kahnert A, Heisler I,
Schatz CA, Stelte-Ludwig B, Mayer-Bartschmid A, Unterschemmann K,
Bruder S, Linden L, et al: Anetumab ravtansine: A novel
mesothelin-targeting antibody-drug conjugate cures tumors with
heterogeneous target expression favored by bystander effect. Mol
Cancer Ther. 13:1537–1548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Santin AD, Vergote I, González-Martín A,
Moore K, Oaknin A, Romero I, Diab S, Copeland LJ, Monk BJ, Coleman
RL, et al: Safety and activity of anti-mesothelin antibody-drug
conjugate anetumab ravtansine in combination with
pegylated-liposomal doxorubicin in platinum-resistant ovarian
cancer: Multicenter, phase Ib dose escalation and expansion study.
Int J Gynecol Cancer. 33:562–570. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lheureux S, Alqaisi H, Cohn DE, Chern JY,
Duska LR, Jewell A, Corr B, Winer IS, Girda E, Crispens MA, et al:
A randomized phase II study of bevacizumab and weekly anetumab
ravtansine or weekly paclitaxel in platinum-resistant or refractory
ovarian cancer NCI trial#10150. J Clin Oncol. 40:55142022.
View Article : Google Scholar
|
|
66
|
Rottey S, Clarke J, Aung K, Machiels JP,
Markman B, Heinhuis KM, Millward M, Lolkema M, Patel SP, de Souza
P, et al: Phase I/IIa trial of BMS-986148, an anti-mesothelin
antibody-drug conjugate, alone or in combination with nivolumab in
patients with advanced solid tumors. Clin Cancer Res. 28:95–105.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Levan K, Mehryar M, Mateoiu C, Albertsson
P, Bäck T and Sundfeldt K: Immunohistochemical evaluation of
epithelial ovarian carcinomas identifies three different expression
patterns of the MX35 antigen, NaPi2b. BMC Cancer. 17:3032017.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gerber DE, Infante JR, Gordon MS, Goldberg
SB, Martín M, Felip E, Garcia MM, Schiller JH, Spigel DR, Cordova
J, et al: Phase Ia study of anti-NaPi2b antibody-drug conjugate
lifastuzumab vedotin DNIB0600A in patients with non-small cell lung
cancer and platinum-resistant ovarian cancer. Clin Cancer Res.
26:364–372. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Moore KN, Birrer MJ, Marsters J, Wang Y,
Choi Y, Royer-Joo S, Lemahieu V, Armstrong K, Cordova J, Samineni
D, et al: Phase 1b study of anti-NaPi2b antibody-drug conjugate
lifastuzumab vedotin (DNIB0600A. in patients with
platinum-sensitive recurrent ovarian cancer. Gynecol Oncol.
158:631–639. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Banerjee S, Oza AM, Birrer MJ, Hamilton
EP, Hasan J, Leary A, Moore KN, Mackowiak-Matejczyk B, Pikiel J,
Ray-Coquard I, et al: Anti-NaPi2b antibody-drug conjugate
lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal
doxorubicin in patients with platinum-resistant ovarian cancer in a
randomized, open-label, phase II study. Ann Oncol. 29:917–923.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Suzuki H, Nagase S, Saito C, Takatsuka A,
Nagata M, Honda K, Kaneda Y, Nishiya Y, Honda T, Ishizaka T, et al:
Raludotatug deruxtecan, a CDH6-targeting antibody-drug conjugate
with a DNA topoisomerase I inhibitor DXd, is efficacious in human
ovarian and kidney cancer models. Mol Cancer Ther. 23:257–271.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gitto SB, Whicker M, Davies G, Kumar S,
Kinneer K, Xu H, Lewis A, Mamidi S, Medvedev S, Kim H, et al: A
B7-H4-targeting antibody-drug conjugate shows antitumor activity in
PARPi and platinum-resistant cancers with B7-H4 expression. Clin
Cancer Res. 30:1567–1581. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ponte JF, Ab O, Lanieri L, Lee J, Coccia
J, Bartle LM, Themeles M, Zhou Y, Pinkas J and Ruiz-Soto R:
Mirvetuximab soravtansine (IMGN853), a folate receptor
alpha-targeting antibody-drug conjugate, potentiates the activity
of standard of care therapeutics in ovarian cancer models.
Neoplasia. 18:775–784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Quanz M, Hagemann UB, Zitzmann-Kolbe S,
Stelte-Ludwig B, Golfier S, Elbi C, Mumberg D, Ziegelbauer K and
Schatz CA: Anetumab ravtansine inhibits tumor growth and shows
additive effect in combination with targeted agents and
chemotherapy in mesothelin-expressing human ovarian cancer models.
Oncotarget. 9:34103–34121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Moore KN, O'Malley DM, Vergote I, Martin
LP, Gonzalez-Martin A, Malek K and Birrer MJ: Safety and activity
findings from a phase 1b escalation study of mirvetuximab
soravtansine, a folate receptor alpha (FRalpha)-targeting
antibody-drug conjugate (ADC), in combination with carboplatin in
patients with platinum-sensitive ovarian cancer. Gynecol Oncol.
151:46–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Haunschild CE and Tewari KS: Bevacizumab
use in the frontline, maintenance and recurrent settings for
ovarian cancer. Future Oncol. 16:225–246. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Pignata S, Lorusso D, Joly F, Gallo C,
Colombo N, Sessa C, Bamias A, Salutari V, Selle F, Frezzini S, et
al: Carboplatin-based doublet plus bevacizumab beyond progression
versus carboplatin-based doublet alone in patients with
platinum-sensitive ovarian cancer: A randomised, phase 3 trial.
Lancet Oncol. 22:267–276. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
O'Malley DM, Matulonis UA, Birrer MJ,
Castro CM, Gilbert L, Vergote I, Martin LP, Mantia-Smaldone GM,
Martin AG, Bratos R, et al: Phase Ib study of mirvetuximab
soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug
conjugate (ADC), in combination with bevacizumab in patients with
platinum-resistant ovarian cancer. Gynecol Oncol. 157:379–385.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gilbert L, Oaknin A, Matulonis UA,
Mantia-Smaldone GM, Lim PC, Castro CM, Provencher D, Memarzadeh S,
Method M, Wang J, et al: Safety and efficacy of mirvetuximab
soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug
conjugate (ADC), in combination with bevacizumab in patients with
platinum-resistant ovarian cancer. Gynecol Oncol. 170:241–247.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Richardson DL, Moore KN, Vergote I,
Gilbert L, Martin LP, Mantia-Smaldone GM, Castro CM, Provencher D,
Matulonis UA, Stec J, et al: Phase 1b study of mirvetuximab
soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug
conjugate, in combination with carboplatin and bevacizumab in
patients with platinum-sensitive ovarian cancer. Gynecol Oncol.
185:186–193. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Nicolò E, Giugliano F, Ascione L,
Tarantino P, Corti C, Tolaney SM, Cristofanilli M and Curigliano G:
Combining antibody-drug conjugates with immunotherapy in solid
tumors: current landscape and future perspectives. Cancer Treat
Rev. 106:1023952022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gerber HP, Sapra P, Loganzo F and May C:
Combining antibody-drug conjugates and immune-mediated cancer
therapy: What to expect? Biochem Pharmacol. 102:1–6. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Vafa O, Gilliland GL, Brezski RJ, Strake
B, Wilkinson T, Lacy ER, Scallon B, Teplyakov A, Malia TJ and
Strohl WR: An engineered Fc variant of an IgG eliminates all immune
effector functions via structural perturbations. Methods.
65:114–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bauzon M, Drake PM, Barfield RM, Cornali
BM, Rupniewski I and Rabuka D: Maytansine-bearing antibody-drug
conjugates induce in vitro hallmarks of immunogenic cell death
selectively in antigen-positive target cells. Oncoimmunology.
8:e15658592019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Müller P, Martin K, Theurich S, Schreiner
J, Savic S, Terszowski G, Lardinois D, Heinzelmann-Schwarz VA,
Schlaak M, Kvasnicka HM, et al: Microtubule-depolymerizing agents
used in antibody-drug conjugates induce antitumor immunity by
stimulation of dendritic cells. Cancer Immunol Res. 2:741–755.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Iwata TN, Ishii C, Ishida S, Ogitani Y,
Wada T and Agatsuma T: A HER2-targeting antibody-drug conjugate,
trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a
mouse model. Mol Cancer Ther. 177:1494–1503. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Junttila TT, Li G, Parsons K, Phillips GL
and Sliwkowski MX: Trastuzumab-DM1 (T-DM1. retains all the
mechanisms of action of trastuzumab and efficiently inhibits growth
of lapatinib insensitive breast cancer. Breast Cancer Res Treat.
128:347–356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wu S, Ge A, Deng X, Liu L and Wang Y:
Evolving immunotherapeutic solutions for triple-negative breast
carcinoma. Cancer Treat Rev. 130:1028172024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Bardia A, Mayer IA, Diamond JR, Moroose
RL, Isakoff SJ, Starodub AN, Shah NC, O'Shaughnessy J, Kalinsky K,
Guarino M, et al: Efficacy and safety of anti-trop-2 antibody drug
conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated
patients with metastatic triple-negative breast cancer. J Clin
Oncol. 35:2141–2148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Camidge DR, Barlesi F, Goldman JW,
Morgensztern D, Heist R, Vokes E, Angevin E, Hong DS, Rybkin II,
Barve M, et al: A phase 1b study of telisotuzumab vedotin in
combination with nivolumab in patients with NSCLC. JTO Clin Res
Rep. 3:1002622022.PubMed/NCBI
|
|
92
|
Anastasio MK, Shuey S and Davidson BA:
Antibody-drug conjugates in gynecologic cancers. Curr Treat Options
Oncol. 25:1–19. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang Y, Liu L, Jin X and Yu Y: Efficacy
and safety of mirvetuximab soravtansine in recurrent ovarian cancer
with FRa positive expression: A systematic review and
meta-analysis. Crit Rev Oncol Hematol. 194:1042302024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kumagai K, Aida T, Tsuchiya Y, Kishino Y,
Kai K and Mori K: Interstitial pneumonitis related to trastuzumab
deruxtecan, a human epidermal growth factor receptor 2-targeting
Ab-drug conjugate, in monkeys. Cancer Sci. 111:4636–4645. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Swain SM, Nishino M, Lancaster LH, Li BT,
Nicholson AG, Bartholmai BJ, Naidoo J, Schumacher-Wulf E, Shitara
K, Tsurutani J, et al: Multidisciplinary clinical guidance on
trastuzumab deruxtecan (T-DXd)-related interstitial lung
disease/pneumonitis-Focus on proactive monitoring, diagnosis, and
management. Cancer Treat Rev. 106:1023782022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhu Y, Liu K, Wang K and Zhu H:
Treatment-related adverse events of antibody-drug conjugates in
clinical trials: A systematic review and meta-analysis. Cancer.
129:283–295. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Eaton JS, Miller PE, Mannis MJ and Murphy
CJ: Ocular adverse events associated with antibody-drug conjugates
in human clinical trials. J Ocul Pharmacol Ther. 31:589–604. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kim SK, Ursell P, Coleman RL, Monk BJ and
Vergote I: Mitigation and management strategies for ocular events
associated with tisotumab vedotin. Gynecol Oncol. 165:385–392.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kubo K, Azuma A, Kanazawa M, Kameda H,
Kusumoto M, Genma A, Saijo Y, Sakai F, Sugiyama Y, Tatsumi K, et
al: Consensus statement for the diagnosis and treatment of
drug-induced lung injuries. Respir Investig. 51:260–277. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Abelman RO, Wu B, Spring LM, Ellisen LW
and Bardia A: Mechanisms of resistance to antibody-drug conjugates.
Cancers (Basel). 15:12782023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Khoury R, Saleh K, Khalife N, Saleh M,
Chahine C, Ibrahim R and Lecesne A: Mechanisms of resistance to
antibody-drug conjugates. Int J Mol Sci. 24:96742023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sung M, Tan X, Lu B, Golas J, Hosselet C,
Wang F, Tylaska L, King L, Zhou D, Dushin R, et al:
Caveolae-mediated endocytosis as a novel mechanism of resistance to
trastuzumab emtansine (T-DM1). Mol Cancer Ther. 17:243–253. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Loganzo F, Sung M and Gerber HP:
Mechanisms of resistance to antibody-drug conjugates. Mol Cancer
Ther. 15:2825–2834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Loganzo F, Tan X, Sung M, Jin G, Myers JS,
Melamud E, Wang F, Diesl V, Follettie MT, Musto S, et al: Tumor
cells chronically treated with a trastuzumab-maytansinoid
antibody-drug conjugate develop varied resistance mechanisms but
respond to alternate treatments. Mol Cancer Ther. 14:952–963. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Collins DM, Bossenmaier B, Kollmorgen G
and Niederfellner G: Acquired resistance to antibody-drug
conjugates. Cancers (Basel). 11:3942019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yan T, Zhu L and Chen J: Current advances
and challenges in CAR T-Cell therapy for solid tumors:
tumor-associated antigens and the tumor microenvironment. Exp
Hematol Oncol. 12:142023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lin L and Lin DC: biological significance
of tumor heterogeneity in esophageal squamous cell carcinoma.
Cancers (Basel). 11:11562019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Mitra A, Barua A, Huang L, Ganguly S, Feng
Q and He B: From bench to bedside: The history and progress of CAR
T cell therapy. Front Immunol. 14:11880492023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Norberg SM and Hinrichs CS: Engineered T
cell therapy for viral and non-viral epithelial cancers. Cancer
Cell. 41:58–69. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Paijens ST, Vledder A, de Bruyn M and
Nijman HW: Tumor-infiltrating lymphocytes in the immunotherapy era.
Cell Mol Immunol. 18:842–859. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Schlabach MR, Lin S, Collester ZR,
Wrocklage C, Shenker S, Calnan C, Xu T, Gannon HS, Williams LJ,
Thompson F, et al: Rational design of a SOCS1-edited
tumor-infiltrating lymphocyte therapy using CRISPR/Cas9 screens. J
Clin Invest. 133:e1630962023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Muthukutty P and Yoo SY: Oncolytic virus
engineering and utilizations: Cancer immunotherapy perspective.
Viruses. 15:16452023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yang C, Nguyen J and Yen Y: Complete
spectrum of adverse events associated with chimeric antigen
receptor (CAR)-T cell therapies. J Biomed Sci. 301:892023.
View Article : Google Scholar
|
|
115
|
Diaby V, Adunlin G, Ali AA, Zeichner SB,
de Lima Lopes G, Kohn CG and Montero AJ: Cost-effectiveness
analysis of 1st through 3rd line sequential targeted therapy in
HER2-positive metastatic breast cancer in the United States. Breast
Cancer Res Treat. 160:187–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Sato S, Shoji T, Jo A, Otsuka H, Abe M,
Tatsuki S, Chiba Y, Takatori E, Kaido Y, Nagasawa T, et al:
Antibody-drug conjugates: The new treatment approaches for ovarian
cancer. Cancers (Basel). 16:25452024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
García-Alonso S, Ocaña A and Pandiella A:
Resistance to antibody-drug conjugates. Cancer Res. 78:2159–2165.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cabaud O, Berger L, Crompot E, Adélaide J,
Finetti P, Garnier S, Guille A, Carbuccia N, Farina A, Agavnian E,
et al: Overcoming resistance to anti-nectin-4 antibody-drug
Conjugate. Mol Cancer Ther. 21:1227–1235. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Andreev J, Thambi N, Bay AE, Delfino F,
Martin J, Kelly MP, Kirshner JR, Rafique A, Kunz A, Nittoli T, et
al: Bispecific antibodies and antibody-drug conjugates (ADCs)
bridging HER2 and prolactin receptor improve efficacy of HER2 ADCs.
Mol Cancer Ther. 16:681–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yamazaki CM, Yamaguchi A, Anami Y, Xiong
W, Otani Y, Lee J, Ueno NT, Zhang N, An Z and Tsuchikama K:
Antibody-drug conjugates with dual payloads for combating breast
tumor heterogeneity and drug resistance. Nat Commun. 12:35282021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang AJ, Gao Y, Shi YY, Dai MY and Cai HB:
A review of recent advances on single use of antibody-drug
conjugates or combination with tumor immunology therapy for
gynecologic cancer. Front Pharmacol. 13:10936662022. View Article : Google Scholar : PubMed/NCBI
|