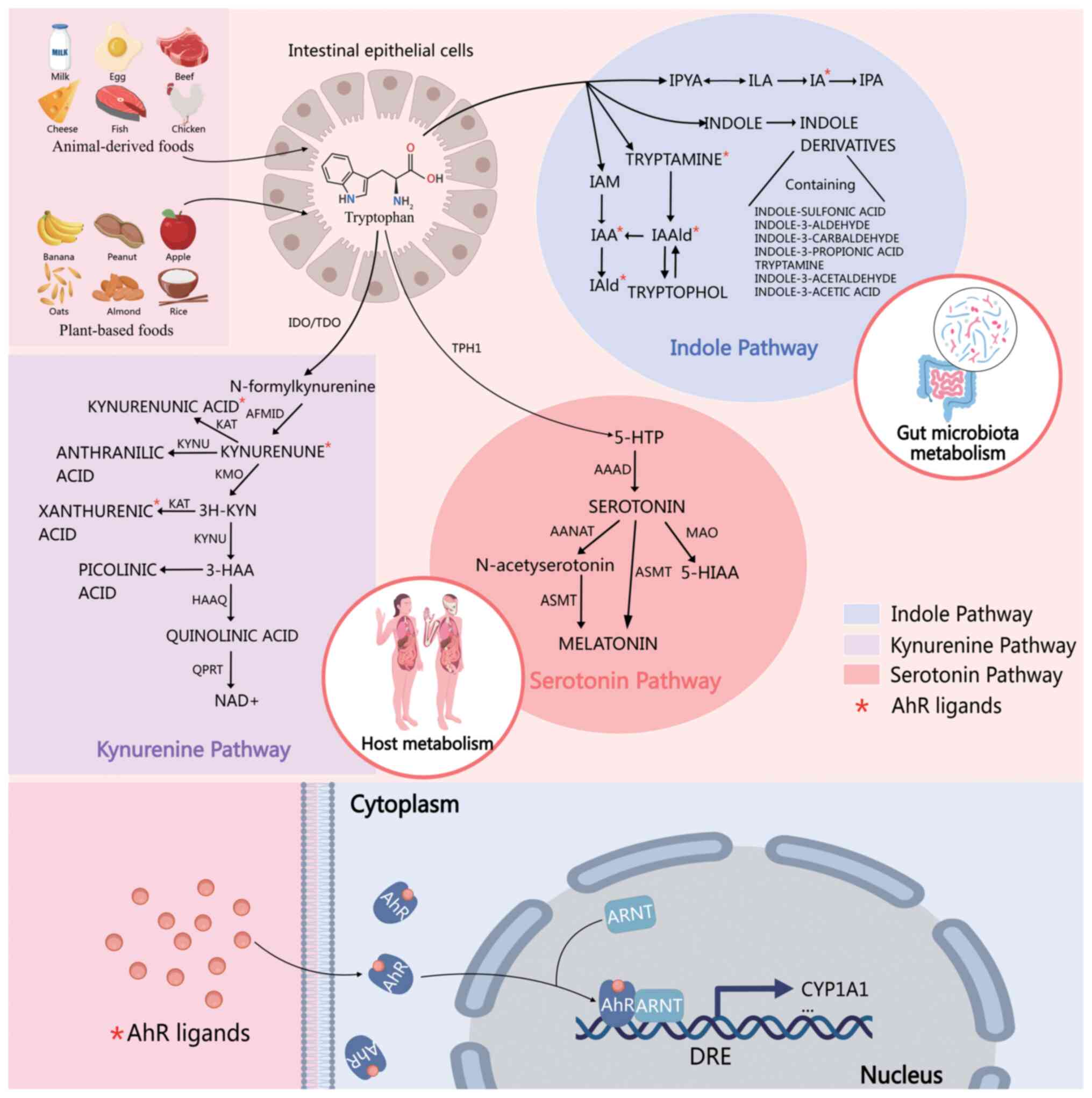
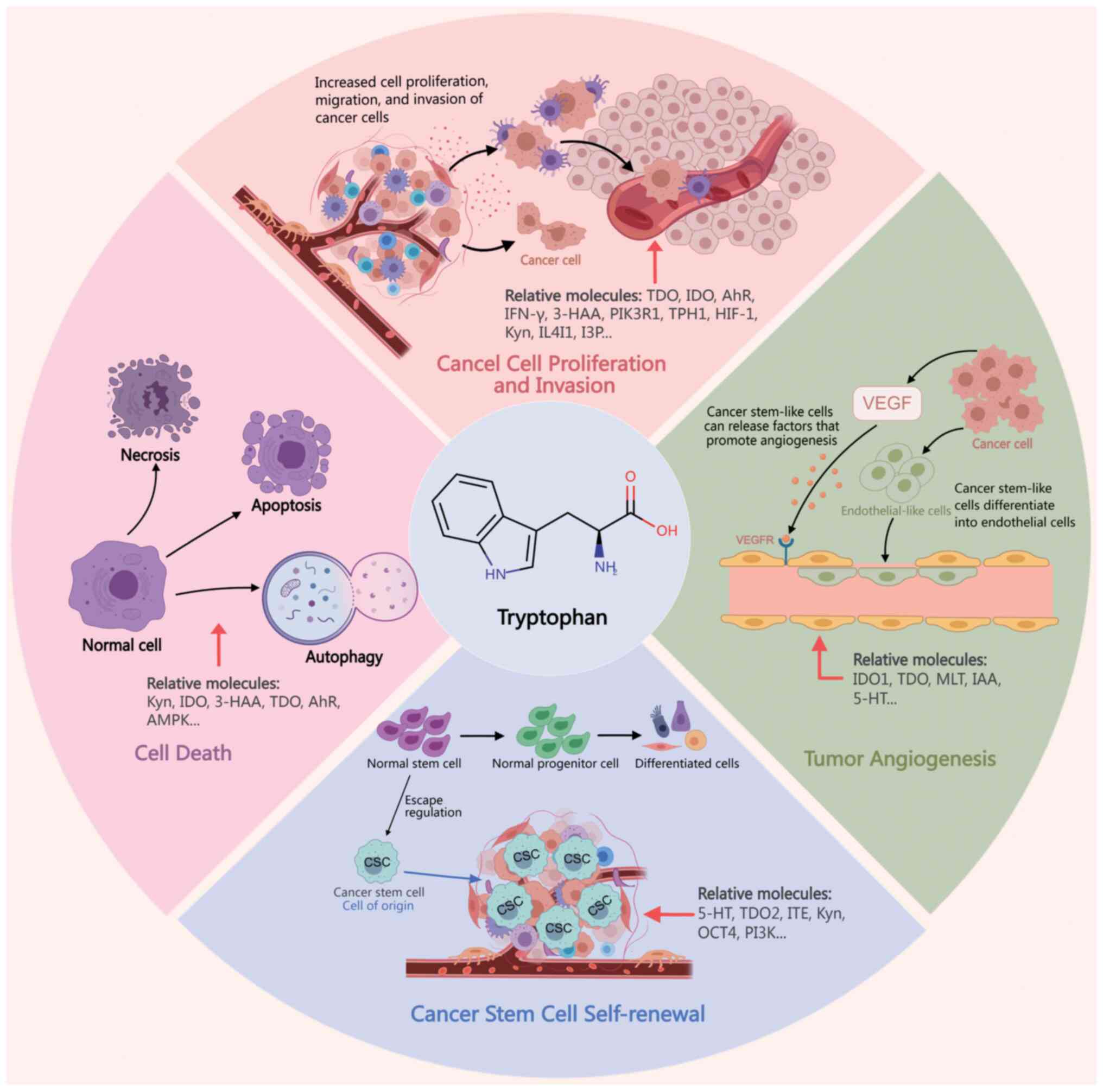
|
1
|
Barik S: The uniqueness of tryptophan in
biology: Properties, metabolism, interactions and localization in
proteins. Int J Mol Sci. 21:87762020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palego L, Betti L, Rossi A and Giannaccini
G: Tryptophan biochemistry: Structural, nutritional, metabolic, and
medical aspects in humans. J Amino Acids. 2016:89525202016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kałużna-Czaplińska J, Gątarek P,
Chirumbolo S, Chartrand MS and Bjørklund G: How important is
tryptophan in human health? Crit Rev Food Sci Nutr. 59:72–88. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarcz R, Bruno JP, Muchowski PJ and Wu
HQ: Kynurenines in the mammalian brain: When physiology meets
pathology. Nat Rev Neurosci. 13:465–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma L and Riva A: Intestinal barrier
function in health and disease - Any role of SARS-CoV-2?
Microorganisms. 8:17442020.https://doi.org/10.3390/microorganisms8111744
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu XH and Zhai XY: Role of tryptophan
metabolism in cancers and therapeutic implications. Biochimie.
182:131–139. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan J, Chen D, Ye Z, Zhu X, Li X, Jiao H,
Duan M, Zhang C, Cheng J, Xu L, et al: Molecular mechanisms and
therapeutic significance of Tryptophan Metabolism and signaling in
cancer. Mol Cancer. 23:2412024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez-Castro L, Garcia R, Venkateswaran N,
Barnes S and Conacci-Sorrell M: Tryptophan and its metabolites in
normal physiology and cancer etiology. FEBS J. 290:7–27. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HL, Zhang AH, Miao JH, Sun H, Yan
GL, Wu FF and Wang XJ: Targeting regulation of tryptophan
metabolism for colorectal cancer therapy: A systematic review. RSC
Adv. 9:3072–3080. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badawy AA: Kynurenine pathway of
tryptophan metabolism: Regulatory and functional aspects. Int J
Tryptophan Res. 10:11786469176919382017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stone TW and Darlington LG: Endogenous
kynurenines as targets for drug discovery and development. Nat Rev
Drug Discov. 1:609–620. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lugo-Huitrón R, Ugalde Muñiz P, Pineda B,
Pedraza-Chaverrí J, Ríos C and Pérez-de la Cruz V: Quinolinic acid:
An endogenous neurotoxin with multiple targets. Oxid Med Cell
Longev. 2013:1040242013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandarano M, Orecchini E, Bellezza G,
Vannucci J, Ludovini V, Baglivo S, Tofanetti FR, Chiari R, Loreti
E, Puma F, et al: Kynurenine/tryptophan ratio as a potential
blood-based biomarker in non-small cell lung cancer. Int J Mol Sci.
22:44032021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwiatkowska I, Hermanowicz JM,
Przybyszewska-Podstawka A and Pawlak D: Not only immune escape-the
confusing role of the TRP metabolic pathway in carcinogenesis.
Cancers (Basel). 13:26672021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki Y, Suda T, Furuhashi K, Suzuki M,
Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H and Chida K:
Increased serum kynurenine/tryptophan ratio correlates with disease
progression in lung cancer. Lung Cancer. 67:361–365. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crotti S, D'Angelo E, Bedin C, Fassan M,
Pucciarelli S, Nitti D, Bertazzo A and Agostini M: Tryptophan
metabolism along the kynurenine and serotonin pathways reveals
substantial differences in colon and rectal cancer. Metabolomics.
13:1482017. View Article : Google Scholar
|
|
17
|
Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa
S, Omura Y, Wada M, Tarumi R, Plitman E, Moriguchi S, et al:
Kynurenine pathway in depression: A systematic review and
meta-analysis. Neurosci Biobehav Rev. 90:16–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maddison DC and Giorgini F: The kynurenine
pathway and neurodegenerative disease. Semin Cell Dev Biol.
40:134–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwarcz R and Pellicciari R: Manipulation
of brain kynurenines: Glial targets, neuronal effects, and clinical
opportunities. J Pharmacol Exp Ther. 303:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walther DJ and Bader M: A unique central
tryptophan hydroxylase isoform. Biochem Pharmacol. 66:1673–1680.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel PD, Pontrello C and Burke S: Robust
and tissue-specific expression of TPH2 versus TPH1 in rat raphe and
pineal gland. Biol Psychiatry. 55:428–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalinichenko LS, Kornhuber J, Sinning S,
Haase J and Müller CP: Serotonin signaling through lipid membranes.
ACS Chem Neurosci. 15:1298–1320. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy DL and Lesch KP: Targeting the
murine serotonin transporter: Insights into human neurobiology. Nat
Rev Neurosci. 9:85–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi X, Zhao G, Li H, Zhao Z, Li W, Wu M
and Du YL: Hydroxytryptophan biosynthesis by a family of
heme-dependent enzymes in bacteria. Nat Chem Biol. 19:1415–1422.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung KH, LoRusso P, Burris H, Gordon M,
Bang YJ, Hellmann MD, Cervantes A, Ochoa de Olza M, Marabelle A,
Hodi FS, et al: Phase I study of the indoleamine 2,3-dioxygenase 1
(IDO1) inhibitor navoximod (GDC-0919) administered with PD-L1
inhibitor (Atezolizumab) in advanced solid tumors. Clin Cancer Res.
25:3220–3228. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ebata T, Shimizu T, Fujiwara Y, Tamura K,
Kondo S, Iwasa S, Yonemori K, Shimomura A, Kitano S, Koyama T, et
al: Phase I study of the indoleamine 2,3-dioxygenase 1 inhibitor
navoximod (GDC-0919) as monotherapy and in combination with the
PD-L1 inhibitor atezolizumab in Japanese patients with advanced
solid tumours. Invest New Drugs. 38:468–477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gomes B, Driessens G, Bartlett D, Cai D,
Cauwenberghs S, Crosignani S, Dalvie D, Denies S, Dillon CP, Fantin
VR, et al: Characterization of the selective indoleamine
2,3-dioxygenase-1 (IDO1) catalytic inhibitor EOS200271/PF-06840003
Supports IDO1 as a critical resistance mechanism to PD-(L)1
blockade therapy. Mol Cancer Ther. 17:2530–2542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crosignani S, Bingham P, Bottemanne P,
Cannelle H, Cauwenberghs S, Cordonnier M, Dalvie D, Deroose F, Feng
JL, Gomes B, et al: Discovery of a novel and selective indoleamine
2,3-dioxygenase (IDO-1) inhibitor
3-(5-Fluoro-1H-indol-3-yl)pyrrolidine-2,5-dione
(EOS200271/PF-06840003) and Its characterization as a potential
clinical candidate. J Med Chem. 60:9617–9629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim M and Tomek P: Tryptophan: A rheostat
of cancer immune escape mediated by immunosuppressive enzymes IDO1
and TDO. Front Immunol. 12:6360812021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roager HM and Licht TR: Microbial
tryptophan catabolites in health and disease. Nat Commun.
9:32942018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Madella AM, Van Bergenhenegouwen J,
Garssen J, Masereeuw R and Overbeek SA: Microbial-derived
tryptophan catabolites, kidney disease and gut inflammation. Toxins
(Basel). 14:6452022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Medvedev A and Buneeva O: Tryptophan
metabolites as mediators of microbiota-gut-brain communication:
Focus on isatin. Front Behav Neurosci. 16:9222742022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghiboub M, Boneh RS, Sovran B, Wine E,
Lefèvre A, Emond P, Verburgt CM, Benninga MA, de Jonge WJ and Van
Limbergen JE: Sustained diet-induced remission in pediatric Crohn's
disease is associated with kynurenine and serotonin pathways.
Inflamm Bowel Dis. 29:684–694. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vanholder R, Nigam SK, Burtey S and
Glorieux G: What if not all metabolites from the uremic toxin
generating pathways are toxic? A hypothesis. Toxins (Basel).
14:2212022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spaepen S and Vanderleyden J: Auxin and
plant-microbe interactions. Cold Spring Harb Perspect Biol.
3:a0014382011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye Z, Yue L, Shi J, Shao M and Wu T: Role
of IDO and TDO in cancers and related diseases and the therapeutic
implications. J Cancer. 10:2771–2782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jochems C, Fantini M, Fernando RI, Kwilas
AR, Donahue RN, Lepone LM, Grenga I, Kim YS, Brechbiel MW, Gulley
JL, et al: The IDO1 selective inhibitor epacadostat enhances
dendritic cell immunogenicity and lytic ability of tumor
antigen-specific T cells. Oncotarget. 7:37762–37772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Someya S, Tohyama S, Kameda K, Tanosaki S,
Morita Y, Sasaki K, Kang MI, Kishino Y, Okada M, Tani H, et al:
Tryptophan metabolism regulates proliferative capacity of human
pluripotent stem cells. iScience. 24:1020902021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Q and Li W: Correlation between
amino acid metabolism and self-renewal of cancer stem cells:
Perspectives in cancer therapy. World J Stem Cells. 14:267–286.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Munro MJ, Wickremesekera SK, Peng L, Tan
ST and Itinteang T: Cancer stem cells in colorectal cancer: A
review. J Clin Pathol. 71:110–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bishnupuri KS, Alvarado DM, Khouri AN,
Shabsovich M, Chen B, Dieckgraefe BK and Ciorba MA: IDO1 and
kynurenine pathway metabolites activate PI3K-Akt signaling in the
neoplastic colon epithelium to promote cancer cell proliferation
and inhibit apoptosis. Cancer Res. 79:1138–1150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thaker AI, Rao MS, Bishnupuri KS, Kerr TA,
Foster L, Marinshaw JM, Newberry RD, Stenson WF and Ciorba MA: IDO1
metabolites activate β-catenin signaling to promote cancer cell
proliferation and colon tumorigenesis in mice. Gastroenterology.
145:416–425.e1-e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, Shao R, Li F, Monteiro M, Liu JP,
Xu ZP and Gu W: PI 3K/Akt/mTOR pathway dual inhibitor BEZ 235
suppresses the stemness of colon cancer stem cells. Clin Exp
Pharmacol Physiol. 42:1317–1326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vermeulen L, De Sousa E, Melo F, Van Der
Heijden M, Cameron K, De Jong JH, Borovski T, Tuynman JB, Todaro M,
Merz C, et al: Wnt activity defines colon cancer stem cells and is
regulated by the microenvironment. Nat Cell Biol. 12:468–476. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu P, Lu T, Chen Z, Liu B, Fan D, Li C,
Wu J, He L, Zhu X, Du Y, et al: 5-hydroxytryptamine produced by
enteric serotonergic neurons initiates colorectal cancer stem cell
self-renewal and tumorigenesis. Neuron. 110:2268–2282. e42022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng X, Pang B, Gu G, Gao T, Zhang R,
Pang Q and Liu Q: Melatonin inhibits glioblastoma stem-like cells
through suppression of EZH2-NOTCH1 signaling axis. Int J Biol Sci.
13:245–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gürsel DB, Berry N and Boockvar JA: The
contribution of Notch signaling to glioblastoma via activation of
cancer stem cell self-renewal: the role of the endothelial network.
Neurosurgery. 70:N19–N21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pham QT, Oue N, Sekino Y, Yamamoto Y,
Shigematsu Y, Sakamoto N, Sentani K, Uraoka N and Yasui W: TDO2
overexpression is associated with cancer stem cells and poor
prognosis in esophageal squamous cell carcinoma. Oncology.
95:297–308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu J, Qin X, Pan D, Zhang B and Jin F:
Amino acid-mediated metabolism: A new power to influence properties
of stem cells. Stem Cells Int. 2019:69194632019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang R, Hu P, Zang Q, Yue X, Zhou Z, Xu
X, Xu J, Li S, Chen Y, Qiang B, et al: LC-MS-based metabolomics
reveals metabolic signatures related to glioma stem-like cell
self-renewal and differentiation. RSC Adv. 7:24221–24232. 2017.
View Article : Google Scholar
|
|
51
|
Venkateswaran N and Conacci-Sorrell M:
Kynurenine: An oncometabolite in colon cancer. Cell Stress.
4:24–26. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi D, Wu X, Jian Y, Wang J, Huang C, Mo
S, Li Y, Li F, Zhang C, Zhang D, et al: USP14 promotes tryptophan
metabolism and immune suppression by stabilizing IDO1 in colorectal
cancer. Nat Commun. 13:56442022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brandacher G, Perathoner A, Ladurner R,
Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G,
Weiss HG, Göbel G, et al: Prognostic value of indoleamine 2,
3-dioxygenase expression in colorectal cancer: Effect on
tumor-infiltrating T cells. Clin Cancer Res. 12:1144–1151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pan K, Wang H, Chen MS, Zhang HK, Weng DS,
Zhou J, Huang W, Li JJ, Song HF and Xia JC: Expression and
prognosis role of indoleamine 2, 3-dioxygenase in hepatocellular
carcinoma. J Cancer Res Clin Oncol. 134:1247–1253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li
TJ and Zheng L: Activated CD69+ T cells foster immune privilege by
regulating IDO expression in tumor-associated macrophages. J
Immunol. 188:1117–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tummala KS, Gomes AL, Yilmaz M, Graña O,
Bakiri L, Ruppen I, Ximénez-Embún P, Sheshappanavar V,
Rodriguez-Justo M, Pisano DG, et al: Inhibition of de novo NAD+
synthesis by oncogenic URI causes liver tumorigenesis through DNA
damage. Cancer Cell. 26:826–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu C, Rao D, Zhu H, Liu Q, Huang W, Zhang
L, Liang H, Song J and Ding Z: TDO2 was downregulated in
hepatocellular carcinoma and inhibited cell proliferation by
upregulating the expression of p21 and p27. Biomed Res Int.
2021:47084392021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu Z, Yan L, Lin J, Ke K and Yang W:
Constitutive TDO2 expression promotes liver cancer progression by
an autocrine IL-6 signaling pathway. 2 Cancer Cell Int. 21:5382021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li L, Wang T, Li S, Chen Z, Wu J, Cao W,
Wo Q, Qin X and Xu J: TDO2 promotes the EMT of hepatocellular
carcinoma through Kyn-AhR pathway. Front Oncol. 10:5628232021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jin H, Zhang Y, You H, Tao X, Wang C, Jin
G, Wang N, Ruan H, Gu D, Huo X, et al: Prognostic significance of
kynurenine 3-monooxygenase and effects on proliferation, migration
and invasion of human hepatocellular carcinoma. Sci Rep.
5:104662015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shi Z, Gan G, Xu X, Zhang J, Yuan Y, Bi B,
Gao X, Xu P, Zeng W, Li J, et al: Kynurenine derivative 3-HAA is an
agonist ligand for transcription factor YY1. J Hematol Oncol.
14:1532021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zuo X, Chen Z, Cai J, Gao W, Zhang Y, Han
G, Pu L, Wu Z, You W, Qin J, et al: 5-Hydroxytryptamine receptor 1D
aggravates hepatocellular carcinoma progression through FoxO6 in
AKT-dependent and independent manners. Hepatology. 69:2031–2047.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang T, Tan XL, Xu Y, Wang ZZ, Xiao CH
and Liu R: Expression and prognostic value of indoleamine 2,
3-dioxygenase in pancreatic cancer. Chin Med J (Engl). 130:710–716.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hezaveh K, Shinde RS, Klötgen A, Halaby
MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, et
al: Tryptophan-derived microbial metabolites activate the aryl
hydrocarbon receptor in tumor-associated macrophages to suppress
anti-tumor immunity. Immunity. 55:324–40. e82022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Koblish HK, Hansbury MJ, Bowman KJ, Yang
G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, et
al: Hydroxyamidine inhibitors of indoleamine-2, 3-dioxygenase
potently suppress systemic tryptophan catabolism and the growth of
IDO-expressing tumors. Mol Cancer Ther. 9:489–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Guastella AR, Michelhaugh SK, Klinger NV,
Fadel HA, Kiousis S, Ali-Fehmi R, Kupsky WJ, Juhász C and Mittal S:
Investigation of the aryl hydrocarbon receptor and the intrinsic
tumoral component of the kynurenine pathway of tryptophan
metabolism in primary brain tumors. J Neurooncol. 139:239–249.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Panitz V, Končarević S, Sadik A, Friedel
D, Bausbacher T, Trump S, Farztdinov V, Schulz S, Sievers P,
Schmidt S, et al: Tryptophan metabolism is inversely regulated in
the tumor and blood of patients with glioblastoma. Theranostics.
11:9217–9233. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhong C, Peng L, Tao B, Yin S, Lyu L, Ding
H, Yang X, Peng T, He H and Zhou P: TDO2 and tryptophan metabolites
promote kynurenine/AhR signals to facilitate glioma progression and
immunosuppression. Am J Cancer Res. 12:2558–2575. 2022.PubMed/NCBI
|
|
69
|
Mohapatra SR, Sadik A, Tykocinski LO,
Dietze J, Poschet G, Heiland I and Opitz CA: Hypoxia inducible
factor 1α inhibits the expression of immunosuppressive
tryptophan-2, 3-dioxygenase in glioblastoma. Front Immunol.
10:27622019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ladomersky E, Zhai L, Lauing KL, Bell A,
Xu J, Kocherginsky M, Zhang B, Wu JD, Podojil JR, Platanias LC, et
al: Advanced age increases immunosuppression in the brain and
decreases immunotherapeutic efficacy in subjects with glioblastoma.
Clin Cancer Res. 26:5232–5245. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sahm F, Oezen I, Opitz CA, Radlwimmer B,
Von Deimling A, Ahrendt T, Adams S, Bode HB, Guillemin GJ, Wick W
and Platten M: The endogenous tryptophan metabolite and NAD+
precursor quinolinic acid confers resistance of gliomas to
oxidative stress. Cancer Res. 73:3225–3234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kamson DO, Lee TJ, Varadarajan K,
Robinette NL, Muzik O, Chakraborty PK, Snyder M, Barger GR, Mittal
S and Juhász C: Clinical significance of tryptophan metabolism in
the nontumoral hemisphere in patients with malignant glioma. J Nucl
Med. 55:1605–1610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Juhász C, Chugani DC, Barger GR, Kupsky
WJ, Chakraborty PK, Muzik O and Mittal S: Quantitative PET imaging
of tryptophan accumulation in gliomas and remote cortex:
Correlation with tumor proliferative activity. Clin Nucl Med.
37:838–842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sadik A, Somarribas Patterson LF, Öztürk
S, Mohapatra SR, Panitz V, Secker PF, Pfänder P, Loth S, Salem H,
Prentzell MT, et al: IL4I1 is a metabolic immune checkpoint that
activates the AHR and promotes tumor progression. Cell.
182:1252–70. e342020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Batista CE, Juhász C, Muzik O, Kupsky WJ,
Barger G, Chugani HT, Mittal S, Sood S, Chakraborty PK and Chugani
DC: Imaging correlates of differential expression of indoleamine 2,
3-dioxygenase in human brain tumors. Mol Imaging Biol. 11:460–466.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Talari NK, Panigrahi M, Madigubba S,
Challa S and Phanithi PB: Altered tryptophan metabolism in human
meningioma. J Neurooncol. 130:69–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bosnyák E, Kamson DO, Guastella AR,
Varadarajan K, Robinette NL, Kupsky WJ, Muzik O, Michelhaugh SK,
Mittal S and Juhász C: Molecular imaging correlates of tryptophan
metabolism via the kynurenine pathway in human meningiomas. Neuro
Oncol. 17:1284–1292. 2015.PubMed/NCBI
|
|
78
|
Ino K, Yamamoto E, Shibata K, Kajiyama H,
Yoshida N, Terauchi M, Nawa A, Nagasaka T, Takikawa O and Kikkawa
F: Inverse correlation between tumoral indoleamine 2, 3-dioxygenase
expression and tumor-infiltrating lymphocytes in endometrial
cancer: Its association with disease progression and survival. Clin
Cancer Res. 14:2310–2317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yoshida N, Ino K, Ishida Y, Kajiyama H,
Yamamoto E, Shibata K, Terauchi M, Nawa A, Akimoto H, Takikawa O,
et al: Overexpression of indoleamine 2, 3-dioxygenase in human
endometrial carcinoma cells induces rapid tumor growth in a mouse
xenograft model. Clin Cancer Res. 14:7251–7259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ino K, Yoshida N, Kajiyama H, Shibata K,
Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S,
et al: Indoleamine 2, 3-dioxygenase is a novel prognostic indicator
for endometrial cancer. Br J Cancer. 95:1555–1561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Inaba T, Ino K, Kajiyama H, Yamamoto E,
Shibata K, Nawa A, Nagasaka T, Akimoto H, Takikawa O and Kikkawa F:
Role of the immunosuppressive enzyme indoleamine 2, 3-dioxygenase
in the progression of ovarian carcinoma. Gynecol Oncol.
115:185–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Odunsi K, Qian F, Lugade AA, Yu H, Geller
MA, Fling SP, Kaiser JC, Lacroix AM, D'Amico L, Ramchurren N, et
al: Metabolic adaptation of ovarian tumors in patients treated with
an IDO1 inhibitor constrains antitumor immune responses. Sci Transl
Med. 14:eabg84022022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gostner JM, Obermayr E, Braicu IE, Concin
N, Mahner S, Vanderstichele A, Sehouli J, Vergote I, Fuchs D and
Zeillinger R: Immunobiochemical pathways of neopterin formation and
tryptophan breakdown via indoleamine 2, 3-dioxygenase correlate
with circulating tumor cells in ovarian cancer patients-A study of
the OVCAD consortium. Gynecol Oncol. 149:371–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Singh R, Shaik S, Negi BS, Rajguru JP,
Patil PB, Parihar AS and Sharma U: Non-Hodgkin's lymphoma: A
review. J Family Med Prim Care. 9:1834–1840. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shankland KR, Armitage JO and Hancock BW:
Non-hodgkin lymphoma. Lancet. 380:848–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Feller AC and Diebold J: Histopathology of
Nodal and Extranodal Non-Hodgkin's Lymphomas. Springer Science
& Business Media; Heidelberg: 2003
|
|
87
|
Liu XQ, Lu K, Feng LL, Ding M, Gao JM, Ge
XL and Wang X: Up-regulated expression of indoleamine 2,
3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased
regulatory T-cell infiltration. Leuk Lymphoma. 55:405–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
El Kholy NM, Sallam MM, Ahmed MB, Sallam
RM, Asfour IA, Hammouda JA, Habib HZ and Abu-Zahra F: Expression of
indoleamine 2, 3-dioxygenase in acute myeloid leukemia and the
effect of its inhibition on cultured leukemia blast cells. Med
Oncol. 28:270–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Curti A, Pandolfi S, Valzasina B, Aluigi
M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli
I, et al: Modulation of tryptophan catabolism by human leukemic
cells results in the conversion of CD25− into CD25+ T regulatory
cells. Blood. 109:2871–2877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang ML, Kem M, Mooradian MJ, Eliane JP,
Huynh TG, Iafrate AJ, Gainor JF and Mino-Kenudson M: Differential
expression of PD-L1 and IDO1 in association with the immune
microenvironment in resected lung adenocarcinomas. Mod Pathol.
32:511–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tang D, Yue L, Yao R, Zhou L, Yang Y, Lu L
and Gao W: P53 prevent tumor invasion and metastasis by
down-regulating IDO in lung cancer. Oncotarget. 8:545482017.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hsu YL, Hung JY, Chiang SY, Jian SF, Wu
CY, Lin YS, Tsai YM, Chou SH, Tsai MJ and Kuo PL: Lung
cancer-derived galectin-1 contributes to cancer associated
fibroblast-mediated cancer progression and immune suppression
through TDO2/kynurenine axis. Oncotarget. 7:27584–27598. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Feng H, Cao B, Peng X and Wei Q:
Cancer-associated fibroblasts strengthen cell proliferation and
EGFR TKIs resistance through aryl hydrocarbon receptor dependent
signals in non-small cell lung cancer. BMC Cancer. 22:7642022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Karayama M, Masuda J, Mori K, Yasui H,
Hozumi H, Suzuki Y, Furuhashi K, Fujisawa T, Enomoto N, Nakamura Y,
et al: Comprehensive assessment of multiple tryptophan metabolites
as potential biomarkers for immune checkpoint inhibitors in
patients with non-small cell lung cancer. Clin Transl Oncol.
23:418–423. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Levina V, Su Y and Gorelik E:
Immunological and nonimmunological effects of indoleamine 2,
3-dioxygenase on breast tumor growth and spontaneous metastasis
formation. Clin Dev Immunol. 2012:1730292012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
D'Amato NC, Rogers TJ, Gordon MA, Greene
LI, Cochrane DR, Spoelstra NS, Nemkov TG, D'Alessandro A, Hansen KC
and Richer JK: A TDO2-AhR signaling axis facilitates anoikis
resistance and metastasis in triple-negative breast cancer. Cancer
Res. 75:4651–4664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fallarino F, Grohmann U, Vacca C, Bianchi
R, Orabona C, Spreca A, Fioretti MC and Puccetti P: T cell
apoptosis by tryptophan catabolism. Cell Death Differ. 9:1069–1077.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Fallarino F, Grohmann U, Vacca C, Orabona
C, Spreca A, Fioretti MC and Puccetti P: T cell apoptosis by
kynurenines. Adv Exp Med Biol. 527:183–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Platten M, Wick W and Van den Eynde BJ:
Tryptophan catabolism in cancer: Beyond IDO and tryptophan
depletion. Cancer Res. 72:5435–5440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cui M, Liu D, Xiong W, Wang Y and Mi J:
ERRFI1 induces apoptosis of hepatocellular carcinoma cells in
response to tryptophan deficiency. Cell Death Discov. 7:2742021.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Moroni F: Tryptophan metabolism and brain
function: focus on kynurenine and other indole metabolites. Eur J
Pharmacol. 375:87–100. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li F, Zhao Z, Zhang Z, Zhang Y and Guan W:
Tryptophan metabolism induced by TDO2 promotes prostatic cancer
chemotherapy resistance in a AhR/c-Myc dependent manner. BMC
Cancer. 21:11122021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gao N, Yang Y, Liu S, Fang C, Dou X, Zhang
L and Shan A: Gut-derived metabolites from dietary tryptophan
supplementation quench intestinal inflammation through the
AMPK-SIRT1-autophagy pathway. J Agric Food Chem. 70:16080–16095.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhuo J, Chu L, Liu D, Cai S and Dong H:
Tryptophan metabolite indole-3-acetic acid ameliorates pulmonary
fibrosis by regulating lung microbiota and inducing autophagy via
inhibition of the PI3K/AKT/MTOR pathway. Am J Respir Crit Care Med.
A24592024.
|
|
105
|
Osawa Y, Kanamori H, Seki E, Hoshi M,
Ohtaki H, Yasuda Y, Ito H, Suetsugu A, Nagaki M, Moriwaki H, et al:
L-tryptophan-mediated enhancement of susceptibility to nonalcoholic
fatty liver disease is dependent on the mammalian target of
rapamycin. J Biol Chem. 286:34800–34808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rathmell JC: Metabolism and autophagy in
the immune system: Immunometabolism comes of age. Immunol Rev.
249:52012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu X, Zhou W, Zhang X, Ding Y, Du Q and
Hu R: 1-L-MT, an IDO inhibitor, prevented colitis-associated cancer
by inducing CDC20 inhibition-mediated mitotic death of colon cancer
cells. Int J Cancer. 143:1516–1529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Prendergast GC, Malachowski WJ, Mondal A,
Scherle P and Muller AJ: Indoleamine 2, 3-dioxygenase and its
therapeutic inhibition in cancer. Int Rev Cell Mol Biol.
336:175–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Opitz CA, Litzenburger UM, Sahm F, Ott M,
Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller
M, et al: An endogenous tumour-promoting ligand of the human aryl
hydrocarbon receptor. Nature. 478:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu D, Liang CH, Huang B, Zhuang X, Cui W,
Yang L, Yang Y, Zhang Y, Fu X, Zhang X, et al: Tryptophan
metabolism acts as a new anti-ferroptotic pathway to mediate tumor
growth. Adv Sci (Weinh). 10:22040062023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang W, Mao S, Shi D, Zhang J, Zhang Z,
Guo Y, Wu Y, Wang R, Wang L, Huang Y and Yao X: MicroRNA-153
decreases tryptophan catabolism and inhibits angiogenesis in
bladder cancer by targeting indoleamine 2, 3-dioxygenase 1. Front
Oncol. 9:6192019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cecchi M, Anceschi C, Silvano A, Coniglio
ML, Chinnici A, Magnelli L, Lapucci A, Laurenzana A and Parenti A:
Unveiling the role of tryptophan 2, 3-dioxygenase in the angiogenic
process. Pharmaceuticals (Basel). 17:5582024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Nocito A, Dahm F, Jochum W, Jang JH,
Georgiev P, Bader M, Graf R and Clavien PA: Serotonin regulates
macrophage-mediated angiogenesis in a mouse model of colon cancer
allografts. Cancer Res. 68:5152–5158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Cerezo AB, Hornedo-Ortega R,
Álvarez-Fernández MA, Troncoso AM and García-Parrilla MC:
Inhibition of VEGF-induced VEGFR-2 activation and HUVEC migration
by melatonin and other bioactive indolic compounds. Nutrients.
9:2492017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Peters MA, Walenkamp AM, Kema IP, Meijer
C, de Vries EG and Oosting SF: Dopamine and serotonin regulate
tumor behavior by affecting angiogenesis. Drug Resist Updat.
17:96–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nayak-Kapoor A, Hao Z, Sadek R, Dobbins R,
Marshall L, Vahanian NN, Jay Ramsey W, Kennedy E, Mautino MR, Link
CJ, et al: Phase Ia study of the indoleamine 2,3-dioxygenase 1
(IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent
advanced solid tumors. J Immunother Cancer. 6:612018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen F, Zhao D, Huang Y, Wen X and Feng S:
Synergetic impact of combined navoximod with cisplatin mitigates
chemo-immune resistance via blockading IDO1(+) CAFs-secreted
Kyn/AhR/IL-6 and pol ζ-prevented CIN in human oral squamous cell
carcinoma. Life Sci. 335:1222392023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang XX, Sun SY, Dong QQ, Wu XX, Tang W
and Xing YQ: Recent advances in the discovery of indoleamine
2,3-dioxygenase 1 (IDO1) inhibitors. Medchemcomm. 10:1740–1754.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Oh JE, Shim KY, Lee JI, Choi SI, Baik SK
and Eom YW: 1-Methyl-L-tryptophan promotes the apoptosis of hepatic
stellate cells arrested by interferon-γ by increasing the
expression of IFN-γRβ, IRF-1 and FAS. Int J Mol Med. 40:576–582.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lewis HC, Chinnadurai R, Bosinger SE and
Galipeau J: The IDO inhibitor 1-methyl tryptophan activates the
aryl hydrocarbon receptor response in mesenchymal stromal cells.
Oncotarget. 8:91914–91927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Opitz CA, Litzenburger UM, Opitz U, Sahm
F, Ochs K, Lutz C and Wick Wand Platten M: The
indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan
upregulates IDO1 in human cancer cells. PLoS One. 6:e198232011.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Takada K, Kohashi K, Shimokawa M, Haro A,
Osoegawa A, Tagawa T, Seto T, Oda Y and Maehara Y: Co-expression of
IDO1 and PD-L1 in lung squamous cell carcinoma: Potential targets
of novel combination therapy. Lung Cancer. 128:26–32. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ladomersky E, Zhai L, Lenzen A, Lauing KL,
Qian J, Scholtens DM, Gritsina G, Sun X, Liu Y, Yu F, et al: IDO1
inhibition synergizes with radiation and PD-1 blockade to durably
increase survival against advanced glioblastoma. Clin Cancer Res.
24:2559–2573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kotecki N, Vuagnat P, O'Neil BH, Jalal S,
Rottey S, Prenen H, Benhadji KA, Xia M, Szpurka AM, Saha A, et al:
A Phase I study of an IDO-1 inhibitor (LY3381916) as monotherapy
and in combination with an Anti-PD-L1 Antibody (LY3300054) in
patients with advanced cancer. J Immunother. 44:264–275. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Balog A, Lin TA, Maley D, Gullo-Brown J,
Kandoussi EH, Zeng J and Hunt JT: Preclinical characterization of
linrodostat mesylate, a novel, potent, and selective oral
indoleamine 2,3-dioxygenase 1 inhibitor. Mol Cancer Ther.
20:467–476. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lin J, Sun-Waterhouse D and Cui C: The
therapeutic potential of diet on immune-related diseases: Based on
the regulation on tryptophan metabolism. Crit Rev Food Sci Nutr.
62:8793–8811. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hu W, Yan G, Ding Q, Cai J, Zhang Z, Zhao
Z, Lei H and Zhu YZ: Update of indoles: Promising molecules for
ameliorating metabolic diseases. Biomed Pharmacother.
150:1129572022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Li X, Zhang B, Hu Y and Zhao Y: New
insights into gut-bacteria-derived indole and its derivatives in
intestinal and liver diseases. Front Pharmacol. 12:7695012021.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu Y, Pei Z, Pan T, Wang H, Chen W and Lu
W: Indole metabolites and colorectal cancer: Gut microbial
tryptophan metabolism, host gut microbiome biomarkers, and
potential intervention mechanisms. Microbiol Res. 272:1273922023.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Krause FF, Mangold KI, Ruppert AL, Leister
H, Hellhund-Zingel A, Lopez Krol A, Pesek J, Watzer B, Winterberg
S, Raifer H, et al: Clostridium sporogenes-derived metabolites
protect mice against colonic inflammation. Gut Microbes.
16:24126692024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liu Y, Chen H, Van Treuren W, Hou BH,
Higginbottom SK and Dodd D: Clostridium sporogenes uses reductive
Stickland metabolism in the gut to generate ATP and produce
circulating metabolites. Nat Microbiol. 7:695–706. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang A, Guan C, Wang T, Mu G and Tuo Y:
Lactiplantibacillus plantarum-derived indole-3-lactic acid
ameliorates intestinal barrier integrity through the AhR/Nrf2/NF-κB
Axis. J Agric Food Chem. April 10–2024.(Epub ahead of print).
|
|
133
|
Wang A, Guan C, Wang T, Mu G and Tuo Y:
Indole-3-lactic acid, a tryptophan metabolite of
lactiplantibacillus plantarum DPUL-S164, improved intestinal
barrier damage by activating AhR and Nrf2 signaling pathways. J
Agric Food Chem. 71:18792–18801. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Leclerc D, Staats Pires AC, Guillemin GJ
and Gilot D: Detrimental activation of AhR pathway in cancer: An
overview of therapeutic strategies. Curr Opin Immunol. 70:15–26.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Paris A, Tardif N, Galibert MD and Corre
S: AhR and cancer: From gene profiling to targeted therapy. Int J
Mol Sci. 22:7522022. View Article : Google Scholar
|
|
136
|
Wang Z, Monti S and Sherr DH: The diverse
and important contributions of the AHR to cancer and cancer
immunity. Current Opin Toxicol. 2:93–102. 2017. View Article : Google Scholar
|
|
137
|
Sun M, Ma N, He T, Johnston LJ and Ma X:
Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon
receptor (AhR). Crit Rev Food Sci Nutr. 60:1760–1768. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Griffith BD and Frankel TL: The aryl
hydrocarbon receptor: impact on the tumor immune microenvironment
and modulation as a potential therapy. Cancers (Basel). 16:4722024.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Leja-Szpak A, Góralska M, Link-Lenczowski
P, Czech U, Nawrot-Porąbka K, Bonior J and Jaworek J: The opposite
effect of L-kynurenine and Ahr inhibitor Ch223191 on apoptotic
protein expression in pancreatic carcinoma cells (Panc-1).
Anticancer Agents Med Chem. 19:2079–2090. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Choi EY, Lee H, Dingle RW, Kim KB and
Swanson HI: Development of novel CH223191-based antagonists of the
aryl hydrocarbon receptor. Mol Pharmacol. 81:3–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhang Y, Ma YC, Song J, Jin Y and Bao YN:
StemRegenin-1 reverses drug resistance of MCF-7/ADR Cells via
AhR/ABC transports and AhR/UGTs pathways. Current Proteomics.
21:113–128. 2024. View Article : Google Scholar
|
|
142
|
Kober C, Roewe J, Schmees N, Roese L,
Roehn U, Bader B, Stoeckigt D, Prinz F, Gorjánácz M, Roider HG, et
al: Targeting the aryl hydrocarbon receptor (AhR) with BAY 2416964:
A selective small molecule inhibitor for cancer immunotherapy. J
Immunother Cancer. 11:e0074952023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Parks AJ, Pollastri MP, Hahn ME, Stanford
EA, Novikov O, Franks DG, Haigh SE, Narasimhan S, Ashton TD, Hopper
TG, et al: In silico identification of an aryl hydrocarbon receptor
antagonist with biological activity in vitro and in vivo. Mol
Pharmacol. 86:593–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Garg A, Sharma A, Krishnamoorthy P, Garg
J, Virmani D, Sharma T, Stefanini G, Kostis JB, Mukherjee D and
Sikorskaya E: Role of niacin in current clinical practice: A
systematic review. Am J Med. 130:173–187. 2017. View Article : Google Scholar : PubMed/NCBI
|