Introduction
Tryptophan (Trp) is an essential aromatic amino acid
in the human body that performs various key physiological functions
(1–3). Trp metabolism primarily involves three
pathways: the kynurenine (Kyn) pathway, catalyzed by indoleamine
2,3-dioxygenase (IDO) or Trp 2,3-dioxygenase (TDO); the serotonin
[or 5-hydroxytryptamine (5-HT)] pathway, mediated by the gut
microbiota; and the indole pathway, which is largely dependent on
the gut microbiota. A total of ~95% of Trp is metabolized through
the Kyn pathway, catalyzed by TDO in the liver and IDO in immune
cells such as macrophages and dendritic cells (DCs). The products
of this pathway include Kyn, nicotinic acid, and nicotinamide,
which ultimately enter the nicotinamide adenine dinucleotide
(NAD+) synthesis pathway to produce essential
redox-regulating molecules for cells (4). The 5-HT pathway consumes ~1% Trp to
produce 5-HT, which is an important neurotransmitter and vasoactive
substance. The gut microbiota metabolizes Trp to indole derivatives
via the indole pathway. These metabolites activate the host aryl
hydrocarbon receptor (AhR), thereby regulating gut barrier function
and immune responses (5).
Dysregulated Trp metabolism is associated with cancer stem cell
(CSC) self-renewal, tumor angiogenesis, and immune evasion. For
example, tumors can use the Trp-Kyn pathway to suppress local
immune responses and promote the formation of an immunosuppressive
tumor microenvironment (TME) that facilitates cancer progression
(6). IDO is overexpressed in
various cancers and associated with poor prognosis (7). Therefore, targeting the Trp metabolism
has emerged as a promising therapeutic strategy in oncology.
Previous studies have focused on the therapeutic potential of
inhibiting key enzymes in the Trp metabolic pathway, such as IDO
and TDO, to enhance antitumor immune responses and improve the
efficacy of cancer immunotherapy (8). New compounds targeting Trp metabolism
are being investigated to assess their ability to modulate immune
responses and inhibit tumor growth, highlighting significant
opportunities and challenges (9).
Given the central role of Trp metabolism in cancer biology, recent
advances have highlighted the potential of multi-targeted
approaches, such as combining Trp pathway modulators with immune
checkpoint inhibitors (ICIs), to overcome drug resistance and
enhance antitumor efficacy. Additionally, emerging insights into
the crosstalk between Trp metabolism and the gut microbiome have
opened new avenues for drug discovery with the development of novel
compounds aimed at modulating these interactions. The aim of the
present review was to provide a comprehensive overview of Trp
metabolism and its dysregulation in cancer, focusing on its
therapeutic implications. Herein, the key metabolic pathways of
Trp, their roles in cancer development and progression, and the
latest advances in targeting these pathways for cancer therapy are
discussed. By understanding the complex mechanisms underlying Trp
metabolism, effective therapeutic strategies can be developed to
treat cancer and improve patient outcomes.
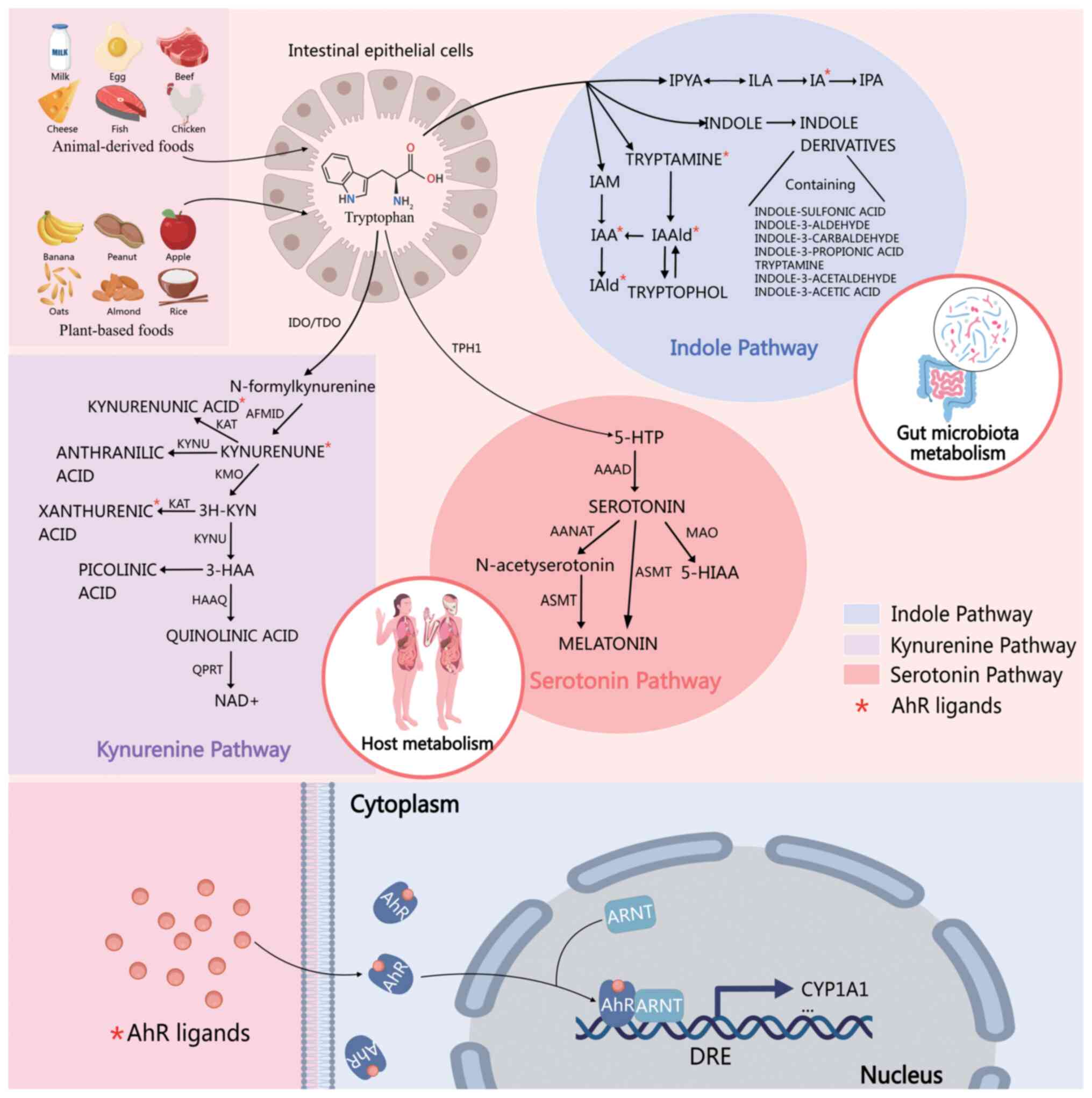
Trp metabolism pathways and their clinical
biomarker implications
Trp metabolism involves three key amino acid
pathways (Fig. 1). These
metabolites reflect the metabolic status of the body and can serve
as potential disease biomarkers with significant clinical
importance.
Kynurenine pathway
Trp is an essential substrate for protein synthesis
and is involved in various biological processes. In the human body,
the Kyn pathway is the predominant metabolic route accounting for
the majority of Trp metabolism, in which Trp is oxidized to
N-formyl-L-Kyn by TDO or IDO. Subsequently, N-formyl-L-kyn is
converted to L-kyn using formylase. L-Kyn is further metabolized to
3-hydroxy-L-Kyn in a reaction catalyzed by Kyn 3-monooxygenase
(KMO). 3-Hydroxy-L-Kyn is then transformed into
3-hydroxyanthranilic (3-HAA) acid via oxidation by 3-HAA oxidase,
which generates anthranilic acid. Kyn can be converted into
kynurenic acid (KYNA) by Kyn aminotransferases. KYNA exerts
neuroprotective effects by inhibiting glutamate receptors and
reducing neuroexcitatory toxicity (10–12).
The Kyn pathway is the predominant pathway for Trp metabolism,
accounting for over 95% of Trp catabolism. Various metabolites
generated in this metabolic pathway are closely associated with
immune regulation, inflammation and neurodegenerative diseases. For
instance, an elevated Kyn/Trp ratio often correlates with disease
progression and poor prognosis in patients with cancer (such as
lung cancer) (13–15). Studies have shown that Trp
metabolism is altered in the early adenoma stage and persists
throughout colorectal cancer (CRC) progression in patients with
CRC. Moreover, compared with the control group, the activities of
IDO1 and Trp hydroxylase (TPH) enzymes were significantly increased
in patients with CRC, suggesting that the Kyn and serotonin
pathways may play important roles in immune regulation. Further
analysis revealed that patients with colon cancer are more prone to
Trp catabolism than patients with rectal cancer. These findings
indicated that early abnormalities in Trp metabolism may help colon
cancer evade immune surveillance and resist immunosuppression
(16). Abnormalities in Trp
metabolism are significant in non-cancer-related diseases. For
example, elevated Kyn levels are considered to be closely related
to neuroinflammation and neurotoxicity in patients with depression
(17), and increased quinolinic
acid (QA) levels in the cerebrospinal fluid are significantly
associated with cognitive decline in patients with
neurodegenerative diseases (18).
These studies reveal the potential pathological mechanisms of Trp
metabolic abnormalities in various diseases and their importance as
biomarkers.
5-HT pathway
The 5-HT pathway is another important pathway
involved in Trp metabolism. This pathway converts Trp to the
neurotransmitter 5-HT, also known as serotonin, where Trp is
hydroxylated to form 5-hydroxyTrp (5-HTP) by TPH (19). The conversion of Trp to 5-HTP by TPH
is followed by decarboxylation of 5-HTP to 5-HT by aromatic L-amino
acid decarboxylase (AADC). TPH, the rate-limiting enzyme in this
pathway, has two isoforms: TPH1, which is predominantly expressed
in peripheral tissues, and TPH2, which is primarily expressed in
the central nervous system (CNS) (20). The decarboxylation of 5-HTP to 5-HT
by AADC occurs in the neuronal cytoplasm, and 5-HT is subsequently
stored in synaptic vesicles and released into the synaptic cleft to
exert its neurotransmitter effects (21). 5-HT binds various receptors in the
synaptic cleft to mediate its physiological effects. After the
action of 5-HT is terminated, primarily through reuptake by the
5-HT transporter (SERT), it is restored, metabolized, or degraded
(22). Within neurons, it is mainly
metabolized by monoamine oxidase to form
5-hydroxyindoleacetaldehyde, which is then converted to
5-hydroxyindoleacetic acid (5-HIAA) by aldehyde dehydrogenase and
ultimately excreted as a metabolic product (23). 5-HT is acetylated by
N-acetyltransferase to form N-acetyl-5-HT (NAc-5-HT), which is then
converted into melatonin via O-methylation by
hydroxy-indole-O-methyltransferase (24). The 5-HT pathway of Trp metabolism
and its metabolites have garnered widespread attention in clinical
studies as potential cancer biomarkers. Studies have demonstrated
that 5-HT plays a crucial role in the susceptibility to esophageal
cancer (EC) and may serve as a potential EC biomarker (25). Moreover, 5-HT metabolism is
implicated in the development and progression of various tumors,
and relevant research has revealed the therapeutic potential of
targeting key enzymes, metabolites and receptors within the 5-HT
metabolic pathway (26).
Specifically, Trp and its metabolites interact with 5-HT receptors
(5-HTR1A, 1B, 2A and 2B) to regulate cancer cell proliferation and
metastasis (27). Under normal
physiological conditions, Trp metabolism occurs through both 5-HT
and indole pathways, with these enzymes and their metabolites
widely distributed in various cells and tissues (28). In addition, the metabolic pathway of
the end product of Trp metabolism, 5-HIAA, in malignant melanoma is
an important direction for future studies (29).
Indole pathway
The indole-synthesis pathway is an essential
metabolic process. This pathway occurs primarily in the gut
microbiota, where Trp is converted into various bioactive molecules
via enzymatic reactions. These molecules play crucial roles in host
physiological and pathological processes (30). In the indole pathway, Trp is
catalytically degraded by tryptophanase (Trpase) secreted by gut
bacteria to produce tryptamine. Subsequently, in the presence of
gut bacteria, tryptamine undergoes a series of complex enzymatic
reactions to generate indoles. Tryptamine transaminase plays a key
role in this process by efficiently converting tryptamine into
indole (31,32). Indoles can be further oxidized to
indoxyl sulfate, an important intermediate in the indole pathway.
Additionally, indole can be metabolized into other derivatives,
such as indole-3-acetaldehyde and indole-3-pyruvic acid, via
alternative pathways (33,34). Indole-3-acetic acid (IAA) is an
auxin essential for plant growth regulation (35). Indole and its derivatives have
various biological functions in the cells that produce them,
primarily in gut health and immune regulation. Indole compounds
(for example, indole-3-propionic acid) activate AhR, modulate gut
barrier function, reduce the production of inflammatory cytokines,
and enhance the gut immune balance (30). Regarding the gut microbiota, indole
metabolites protect gut health by influencing gut barrier function
and inflammatory responses (31).
Trp metabolism plays a significant role in the interaction between
the host and pathogens and involves multiple metabolic pathways.
Indole and its derivatives are unique metabolites produced by human
gut microbiota. Trp metabolism undergoes significant changes in
CRC, and studies have shown that this is related to alterations in
the populations of indole-producing bacteria. Indole exhibits
anti-inflammatory effects and demonstrates potential therapeutic
value through specific anticancer mechanisms and may become part of
future anticancer adjuvant strategies (36). Inflammation can induce changes in
both the host and microbial Trp metabolism in colon cancer. For
example, the overexpression of IDO1 (indoleamine-2,3-dioxygenase 1)
shifts Trp metabolism towards the Kyn pathway, thereby promoting
tumor immune evasion. By contrast, Trp metabolites, such as indole,
can inhibit tumor occurrence and development. However, changes in
the gut microbiota often lead to decreased indole levels,
disrupting the symbiotic relationship between the host and
microbes, consequently enhancing inflammation and exacerbating the
vicious cycle (37). Therefore,
abnormal levels of indole metabolites are associated with diseases,
such as inflammatory bowel disease, metabolic syndrome and gut
microbiota dysbiosis, and thus have potential value as biomarkers.
In summary, Trp metabolism via the indole pathway is vital for
physiological and pathological processes in cells. Elucidating the
specific mechanisms of action of indole and its derivatives on cell
health is essential for exploring their potential applications in
disease prevention and treatment.
Disruption of Trp metabolism in cancer
Disruption of Trp metabolism can induce alterations
in various hallmark features of cancer across multiple systems,
including the digestive, nervous, respiratory and hematological
systems. Its role and underlying mechanisms in cancer are gradually
elucidated (Fig. 2). The effects of
Trp metabolism in cancer are summarized in Table I.
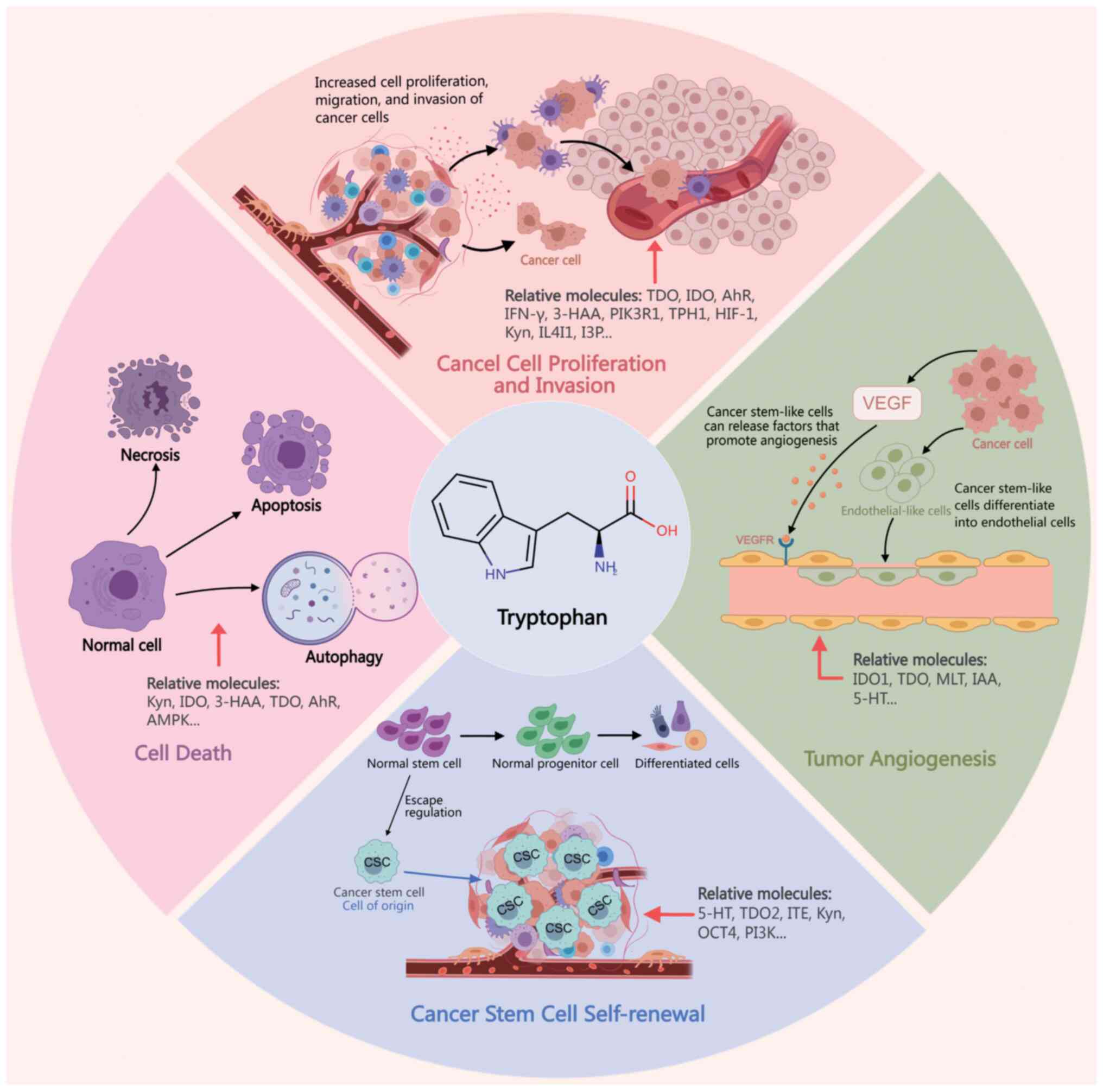 | Figure 2.Role of tryptophan metabolism and
related molecules in cancer-related processes such as cancer cell
proliferation and invasion, tumor angiogenesis, self-renewal of
cancer stem cells, and cell death. IDO, 2,3-dioxygenase; TDO, Trp
2,3-dioxygenase; 3-HAA, 3-hydroxyanthranilic acid; Kyn, kynurenine;
AhR, aryl hydrocarbon receptor; 5-HT, 5-hydroxytryptamine; HIF-1,
hypoxia-inducible factor 1. |
 | Table I.Role and mechanism of tryptophan in
tumors. |
Table I.
Role and mechanism of tryptophan in
tumors.
| First author,
year | Tumor type | Tryptophan
metabolism | Functions | Relative
molecules | (Refs.) |
|---|
| Chen et al,
2015; | CRC | Kyn, AhR | Self-renewal of
colon CSCs | PI3K/Akt, | (43,44,48) |
| Vermeulen et
al, 2010; |
|
|
| β-catenin |
|
| Pham et al,
2018 |
|
|
|
|
|
| Zhu et al,
2022 | CRC | 5-HT | Self-renewal of
colon CSCs and promotion of tumor angiogenesis | MMP-12 | (45) |
| Shi et al,
2022 | CRC | IDO1 | Cause of T-cell
dysfunction | - | (52) |
| Zheng et al,
2017; | Glioma | Melatonin | Inhibition of the
proliferation and | EZH2, Notch | (46,47) |
| Gürsel et
al, 2012 |
|
| tumorigenicity of
glioma stem cells |
|
|
| Guastella et
al, 2018 | Glioma | Kynurenic acid,
AhR | Promotion of
immunosuppression | - | (66) |
| Panitz et
al, 2021 | Glioma | TDO2, Kyn, AhR | Promotion of the
motility of tumor cells and inhibit immune cells | Akt | (67) |
| Sahm et al,
2013 | Glioma |
NAD+ | Enhancement of
resistance to oxidative stress | - | (71) |
| Sadik et al,
2020 | Glioma | I3P, Kyn, AhR | Promotion of the
motility and malignant phenotype of cancer cells | IL4I1 | (74) |
| Bosnyák et
al, 2015 | Glioblastoma | Kyn, AhR | Promotion of the
motility of tumor cells and inhibit immune cells | Trp catabolic
enzymes | (77) |
| Panitz et
al, 2021 | Glioblastoma | TDO2, Kyn,
3-HAA | Facilitation of the
preservation of tryptophan in tumor cells | HIF-1α | (67) |
| Zhang et al,
2022; | Esophageal | TDO2 | Participation in
the formation of | Oct4, EGFR | (39,48) |
| Pham et al,
2018 | cancer |
| tumor spheres of
esophageal CSCs |
|
|
| Zhao et al,
2012 | Liver cancer | IDO | Facilitation of the
immune escape of tumors | IFN-γ | (55) |
| Tummala et
al, 2014 | Liver cancer |
NAD+ | Prevention of DNA
damage and dysregulation of cell proliferation | URI | (56) |
| Yu et al,
2021 | Liver cancer | TDO2 | Inhibition of the
proliferation of HCC cells | p21, p27, CDK2,
CDK4 | (57) |
| Wu et al,
2021 | Liver cancer | TDO2, Kyn, AhR | Promotion of the
progression of HCC cells | IL-6, STAT3,
NF-kB | (58) |
| Li et al,
2021 | Liver cancer | TDO2 | Promotion of EMT of
cancer cells | - | (59) |
| Jin et al,
2015 | Liver cancer | KMO | Promotion of the
progression of HCC cells | - | (60) |
| Shi et al,
2021 | Liver cancer | 3-HAA | Induction of
apoptosis of HCC cells | YY1 | (61) |
| Zhang et al,
2017; | Pancreatic | IDO | Reflection of the
malignancy of | - | (63,65) |
| Koblish et
al, 2010 | cancer |
| pancreatic
adenocarcinoma |
|
|
| Hezaveh et
al, 2022 | Pancreatic
cancer | Indole, AhR | Promotion of tumor
growth and metastasis | - | (64) |
| Talari et
al, 2016; | Meningioma | IDO2, TDO2, | Promotion of tumor
growth and | - | (76,77) |
| Bosnyák et
al, 2015 |
| Kyn | metastasis |
|
|
| Ino et al,
2008; | Endometrial | IDO | Promotion of the
motility of tumor | - | (78–80) |
| Yoshida et
al, 2008; | cancer |
| cells and inhibit
immune cells |
|
|
| Ino et al,
2006 |
|
|
|
|
|
| Inaba et al,
2009; | Ovarian | IDO,
NAD+ | Promotion of the
motility of tumor | - | (81–83) |
| Odunsi et
al, 2022; | cancer |
| cells and inhibit
immune cells |
|
|
| Gostner et
al, 2018 |
|
|
|
|
|
| Liu et al,
2014 | Non-Hodgkin's
lymphoma | IDO1 | Facilitation of the
immune escape of tumors | Lactate
dehydrogenase | (87) |
| El Kholy et
al, 2011; | Acute | IDO, | Promotion of the
motility of tumor | - | (88,89) |
| Curti et al,
2007 | myeloid
leukemia | kynurenic acid | cells and inhibit
immune cells |
|
|
| Zhang et al,
2019 | Pulmonary
adenocarcinoma | IDO1 | Promotion of the
motility of tumor cells | EGFR | (90) |
| Tang et al,
2017; Hsu et al, 2016 | Lung cancer | TDO2, Kyn, AhR | Promotion of the
proliferation of CAFs and EMT in lung cancer cells | Akt/CREB,
Akt/WNK1 | (91,92) |
| Feng et al,
2022 | Lung cancer | TDO, Kyn, AhR | Enhancement of the
proliferation ability of NSCLC cells and their resistance to EGFR
tyrosine kinase inhibitors | Akt, ERK | (93) |
| Tang et al,
2017 | Lung cancer | IDO1 | Promotion of the
motility of tumor cells | P53 | (91) |
| Karayama et
al, 2021 | NSCLC | 3-HAA | Inhibition of the
therapeutic efficacy of immune checkpoint inhibitors | - | (94) |
| Levina et
al, 2012; | Breast cancer | IDO1, TDO2, | Promotion of tumor
growth and | - | (95,96) |
| D'Amato et
al, 2015 |
| AhR | metastasis |
|
|
| Li et al,
2021 | Prostate
cancer | AhR | Increase of the
apoptosis rate of prostate cancer cells treated with Abi or
Doc | c-Myc | (102) |
| Zhang et al,
2019 | Bladder cancer | IDO1 | Promotion of tumor
angiogenesis | microRNA-153 | (111) |
| Cecchi et
al, 2024 | Melanoma | TDO | Promotion of tumor
angiogenesis | - | (112) |
Correlation between Trp metabolism and
CSC self-renewal
Human pluripotent stem cells (hPSCs), which can
differentiate into various cell types, hold great promise as a
source of cells for regenerative therapies and drug discovery. Trp
metabolism is critical for promoting hPSC proliferation without
compromising their pluripotency (38). Several studies focused on the
relationship between Trp metabolism and CSCs. Modulation of the Trp
metabolic pathway can influence the self-renewal ability of CSCs.
For example, the Trp metabolite Kyn can activate AhR to regulate
self-renewal and differentiation of CSCs (39). In some diseases, colorectal CSCs
represent a small subpopulation of cells with self-renewal and
differentiation capabilities within CRC (40). These pathways are beneficial for the
self-renewal of CSCs. Abnormal hyperactivation of specific
signaling pathways in CSCs, such as the phosphoinositide
3-kinase/protein kinase B (PI3K/Akt) and β-catenin pathways, may
induce uncontrolled cell proliferation and abnormal
differentiation, leading to tissue-specific tumorigenesis (41–44).
The neurotransmitter 5-HT promotes self-renewal and tumorigenesis
of colorectal CSCs (45). The
Trp-derivative melatonin may inhibit the proliferation and
tumorigenicity of glioma stem-like cells by suppressing the EZH2
and Notch pathways, which are crucial for the survival of glioma
stem cells (46,47). Overexpression of TDO2 is associated
with tumor staging and recurrence status, as well as the CD44 CSC
marker in esophageal squamous cell carcinoma (ESCC) and is involved
in the formation of tumor spheres in esophageal CSCs. TDO2 may
promote the generation of esophageal CSCs by inducing the
expression of Oct4 and CD44, activating the epidermal growth factor
receptor (EGFR) pathway, stimulating epithelial-mesenchymal
transition (EMT) and invasion of esophageal CSCs, which are
associated with poor prognosis in patients with ESCC (39,48).
Additionally, indole-3-pyruvate, a Trp metabolite, reduces the
expression of Oct4 in CSCs by activating the AhR transcriptional
pathway, inducing CSC differentiation, and decreasing CSC
tumorigenicity (39). Moreover,
activating the Trp metabolic pathway helps CSCs evade attacks from
the host immune system through immune modulation and metabolic
reprogramming, thereby maintaining survival. For example, Trp
depletion and hypoxia preserve CSC phenotype by enhancing OCT4
transcription (49). Kyn inhibits
the activity of effector T-cells via the AhR pathway, enhances Treg
function, and suppresses antitumor immune responses. This
immunosuppressive effect provides a favorable environment for CSC
survival (7,39). In addition, Trp metabolites regulate
the metabolic state of CSCs to adapt to hypoxia and nutrient
deficiency in the TME (50). In
summary, Trp metabolism and its key enzymes are vital for the
maintenance and function of CSCs, promoting tumor progression and
drug resistance by directly affecting CSC behavior and indirectly
regulating immune responses in the TME.
Cancer cell proliferation, invasion
and immune evasion
Digestive system diseases
CRC
Kyn, a bioactive metabolite of the Trp metabolism,
acts as an ‘oncometabolite’ in CRC cells. It maintains the
proliferation of CRC cells by activating the transcription factor
AhR and regulating genes that promote cell proliferation (51). Additionally, Kyn accumulation
affects the TME, facilitating the proliferation and spread of CRC
cells. USP14 promotes Trp metabolism in CRC by stabilizing the
expression of IDO1, leading to T-cell dysfunction. This metabolic
alteration enables tumor cells to evade immune surveillance,
enhancing their proliferative capacity and metastatic potential
(52). High expression of IDO is
associated with the local depletion of Trp, which inhibits T-cell
proliferation and activity, allowing tumor cells to escape immune
attack and promote tumor proliferation and metastasis (53). The activity of IDO1 promotes immune
tolerance in the TME and directly enhances the proliferation of CRC
cells by activating the β-catenin signaling pathway (42). These studies indicate that
modulation of Trp metabolism influences the development and
progression of CRC. Therefore, in-depth studies on the relationship
between Trp metabolism and CRC may facilitate the development of
novel therapeutic strategies.
Hepatocellular carcinoma (HCC)
In HCC, IDO inhibits the proliferation of cytotoxic
T-cells by degrading Trp and promoting tumor immune evasion. High
IDO expression is significantly associated with the metastasis rate
and poor prognosis in patients with HCC, suggesting that IDO may
play a crucial role in tumor cell proliferation, invasion and
metastasis (54). CD69+
T-cells in the TME can induce high expression of IDO in
tumor-associated macrophages (TAMs) through the secretion of
interferon-γ (IFN-γ), which, in turn, inhibits T-cell proliferation
and function, promoting tumor immune evasion (55). The role of Trp metabolism in HCC is
reflected by its effect on NAD+ synthesis and DNA damage
mechanisms. Overexpression of URI (an unconventional prefoldin
RPB5-interacting factor) inhibits the Trp metabolism pathway,
leading to decreased NAD+ levels, DNA damage and
dysregulated cell proliferation, promoting the malignant
transformation of hepatocytes and tumorigenesis (56). Overexpression of TDO2 inhibits HCC
cell proliferation and induces cell cycle arrest by upregulating
p21 and p27 and downregulating CDK2 and CDK4. The absence of TDO2
may promote tumor proliferation and metastasis, indicating that
TDO2 is a potential biomarker and therapeutic target for HCC
(57). High TDO2 expression is
associated with poor prognosis in patients with HCC. TDO2 promotes
the conversion of Trp to Kyn, activates AhR, upregulates the
secretion of interleukin-6 (IL-6), activating signal transducer and
activator of transcription 3 (STAT3) and nuclear factor kappa B
(NF-Κb) signaling pathways, and promotes HCC cell proliferation and
metastasis (58). TDO2 promotes EMT
in cancer cells by activating the Kyn-AhR pathway, thereby
enhancing HCC cell proliferation, invasion and metastasis (59). High KMO expression in HCC tissues is
associated with poor prognosis. In vitro experiments have
shown that KMO overexpression promotes HCC cell proliferation,
migration and invasion, whereas KMO knockdown inhibits these
processes (60). 3-HAA, a
derivative of Trp, is present at low concentrations in tumor cells.
Exogenous 3-HAA induces apoptosis in HCC cells by binding to the
transcription factor YY1. The mechanism involves protein kinase C
Zeta (PKCζ)-mediated phosphorylation of YY1, enhancing its binding
to target genes, and regulating HCC cell proliferation, invasion
and metastasis through modulation of 3-HAA levels (61). 5-HT1D is significantly upregulated
in HCC tissues and cell lines, with its expression associated with
poor clinical pathological features. 5-HT1D stabilizes PIK3R1 to
activate the PI3K/Akt signaling pathway, enhances FoxO6 expression,
and promotes cancer cell proliferation, EMT and metastasis.
Additionally, 5-HT1D inhibits the expression of TPH1 via the
PI3K/Akt/CUX1 axis, thereby affecting 5-HT synthesis (62). In-depth research into the
relationship between Trp metabolism and HCC may facilitate the
development of novel therapeutic strategies against HCC.
Pancreatic cancer
High IDO expression is closely related to the
malignancy of pancreatic adenocarcinoma, particularly in patients
with poorly differentiated tumors, lymph node metastasis, and
advanced TNM staging, and is associated with a poor prognosis. This
suggests that IDO is involved in pancreatic cancer progression,
making it a potential therapeutic target (63). The gut microbiota metabolizes Trp to
produce indole compounds that activate the AhR, inhibit antitumor
immunity, and promote tumor growth and metastasis. Macrophages
lacking AhR function exhibit a stronger inflammatory phenotype,
increased CD8+ T-cell infiltration and tumor growth
inhibition (64). IDO catalyzes the
conversion of Trp to Kyn, depleting Trp levels, causing immune
suppression, and facilitating tumor cell evasion during immune
attacks. Inhibition of IDO activity can reduce Kyn levels in the
TME, enhance the function of tumor-infiltrating lymphocytes (TILs),
and inhibit tumor growth and metastasis (65). These results indicate that
modulation of Trp metabolism may be an effective strategy for
cancer therapy.
CNS diseases
Glioma
Dysregulation of the Kyn pathway in primary brain
tumors, such as gliomas, leads to local Trp depletion within cancer
cells and promotes an immunosuppressive TME, affecting tumor cell
proliferation and invasion capabilities. This pathway may also
affect tumor metastasis by modulating the AhR signaling pathway
(66). The upregulation of Trp
catabolic enzymes in glioblastoma (GBM) promotes Trp degradation to
produce metabolites, such as Kyn, which activate AhR and
subsequently enhance tumor cell motility while suppressing immune
cell function (67). TDO2
facilitates the generation of Kyn from Trp metabolism, which
activates the AhR and Akt signaling pathways, enhancing tumor cell
proliferation and invasion. Moreover, Kyn inhibits the
proliferation of functional T-cells, leading to immunosuppression
and the promotion of glioma cell proliferation and metastasis
(68). Under hypoxic conditions,
hypoxia-inducible factor-1α in GBM downregulates the expression of
TDO2, reducing Trp degradation and consequently decreasing the
production of Kyn and 3-HAA. This mechanism may help tumor cells
conserve Trp in the hypoxic microenvironment and maintain their
proliferative and survival properties (69). IDO expression in the brain increases
with age. This enzyme generates immunosuppressive metabolites from
Trp metabolism, weakening the efficacy of immunotherapy and
resulting in poor treatment outcomes for GBM in elderly patients
(70). Glioma cells generate QA via
microglia and utilize it to synthesize NAD+, enhancing
resistance to oxidative stress. Activation of this metabolic
pathway is closely related to the malignant phenotype of tumors,
and the accumulation of Quin endows glioma cells with a greater
survival capacity when subjected to radiotherapy and chemotherapy
(for example, temozolomide), promoting tumor invasion and
metastasis (71). Positron emission
tomography (PET) using α-11C-methyl-L-Trp (AMT) can be used to
assess 5-HT synthesis in the brain and track the upregulation of
the Kyn pathway in tumor tissues in patients with malignant
gliomas. Increased AMT uptake in the tumor tissues of patients with
high-grade gliomas (grades III–IV) suggests that alterations in Trp
metabolism may be closely related to the biological behavior of
tumors such as proliferation and metastasis (72). Increased Trp metabolism, mainly
through the Kyn pathway, observed using PET, positively correlates
with tumor proliferative activity (assessed using the Ki-67
labeling index) (73). Finally,
IL4I1 catalyzes Trp metabolism to generate indole-3-propionic acid
(I3P) and its derivatives (for example, Quin and KynA), which
activate AhR and promote cancer cell motility and a malignant
phenotype, thereby correlating with reduced survival rates in
patients with glioma (74). In
low-grade brain tumors, such as dysplastic neuroepithelial tumors,
widespread expression of the Trp-catabolic enzyme IDO may lead to
local Trp depletion and inhibition of cell proliferation, as
evidenced by a lower proliferative index (Ki-67). By contrast, IDO
expression is less frequent in high-grade tumors such as GBM. It is
mainly confined to endothelial cells and is possibly associated
with tumor invasiveness and metastatic capability (75). Trp metabolism is important for
immune evasion in gliomas and provides a survival advantage to
glioma cells; thus, it is emerging as a potential therapeutic
target.
Meningioma
Meningioma cells evade immune responses via the Trp
metabolic pathway, particularly the Kyn pathway, resulting in
elevated levels of Kyn and lower levels of Trp and its metabolites,
thereby promoting tumor progression. This process is accompanied by
the upregulation of enzymes such as IDO2 and INOS, highlighting the
significant role of Trp metabolism in cancer cell proliferation and
invasion (76). The grading of
meningiomas is positively correlated with PET imaging parameters of
Trp metabolism (for example, the k3′ ratio). These parameters can
be used to effectively distinguish meningiomas of different grades.
The key enzyme in Trp metabolism, TDO2, exhibits significant
immunostaining in meningiomas, suggesting its potential role in
tumor immune tolerance and proliferation (77). These findings indicate that Trp
metabolism is associated with the biological characteristics of
tumors and may provide new strategies for improving meningioma
treatment outcomes.
Reproductive system diseases
Endometrial cancer
IDO is highly expressed in endometrial cancer and is
significantly associated with tumor aggressiveness, lymph node
metastasis and involvement of vascular lymphatic spaces. IDO exerts
its effects by depleting Trp and generating toxic metabolites that
inhibit the function of TILs and natural killer (NK) cells and
promote tumor immune evasion (78).
However, in mouse xenograft models, tumors overexpressing IDO
exhibited faster growth rates, which correlated with a decrease in
the number and function of NK cells, suggesting that IDO inhibits
NK cell activity through Trp metabolism, thereby facilitating tumor
progression (79). High IDO
expression in endometrial cancer cells positively correlated with
the clinicopathological features of the tumor. Elevated IDO
expression is significantly associated with reduced overall and
progression-free survival (PFS) (80). IDO influences endometrial cancer
cell proliferation, invasion and metastasis by regulating Trp
metabolism, making it a potential therapeutic target.
Ovarian cancer
High IDO expression is associated with an
immunosuppressive state in tumor cells, reducing the number of TILs
and affecting patient survival. Although in vitro
experiments have shown no significant differences in the
proliferation, migration and invasion capabilities of
IDO-overexpressing ovarian cancer cells, mouse xenograft models
have demonstrated a substantial increase in peritoneal metastasis
in IDO-overexpressing cells (81).
IDO1 inhibition alters Trp metabolism and suppresses immune evasion
in tumor cells. It also induces metabolic adaptation by increasing
NAD+ synthesis and inhibiting T-cell proliferation and
function. This metabolic adaptation may limit the antitumor immune
response and promote the proliferation, invasion and metastasis of
ovarian cancer cells (82). An
elevated Kyn/Trp ratio is associated with disease progression in
patients with ovarian cancer. The presence of circulating tumor
cells (CTCs) correlates with alterations in Trp metabolism and
elevated levels of immune activation markers (such as neopterin),
suggesting that CTCs continuously stimulate the immune system by
releasing tumor antigens or cytokines, facilitating tumor immune
evasion and metastasis (83).
Hematological system diseases
Non-Hodgkin's lymphoma (NHL)
NHL is a hematological malignancy originating from
lymph nodes and other extranodal lymphoid tissues (84–86).
IDO1 expression is significantly upregulated in NHL tissues and is
associated with the clinical stage of the tumor, tumor size and
serum lactate dehydrogenase levels, indicating a poor prognosis.
IDO1 promotes local immune tolerance by depleting Trp and its
metabolites (for example L-kyn) and inhibiting the proliferation
and activation of antigen-specific T-cells. Upregulation of IDO1
enhances the generation and infiltration of Tregs, possibly
representing a mechanism by which NHL evades immune control
(87), making it a potential target
for treatment.
Acute myeloid leukemia (AML)
IDO is expressed in patients with AML. It suppresses
T-cell proliferation by reducing local Trp concentrations and
generating immunosuppressive metabolites (for example, Quin),
thereby promoting tumor immune tolerance. This mechanism may
enhance the proliferation and survival of tumor cells, facilitating
their invasion and metastasis (88). IDO expression is associated with
increased circulating
CD4+CD25+FOXP3+ regulatory T
cells. AML cells directly convert CD4+CD25− T
cells into CD4+CD25+ Treg cells via Trp
metabolism, inhibiting T-cell proliferation and activity and
promoting tumor immune evasion. This process affects the TME and
may contribute to cancer cell proliferation, invasion and
metastasis (89). The inhibition of
IDO may represent a novel therapeutic strategy against AML.
Other disease systems
The expression of IDO1 is significantly associated
with tumor aggressiveness, smoking history, and the abundance of
tumor-infiltrating CD8+ and T-bet+ cells; in
some cases, it is related to EGFR mutations. Although IDO1
expression is associated with tumor growth and metastasis, its
independent expression does not significantly affect patient
survival, suggesting that its immunosuppressive role in lung
adenocarcinoma may involve other mechanisms (90). High expression of IDO1 in lung
cancer tissues correlates with clinical stage and lymph node
metastasis. It enhances cancer cell migratory and invasive
capacities, which are partially attenuated by p53 through
suppression of the IDO signaling pathway (91). Lung cancer cells activate
cancer-associated fibroblasts (CAFs) to promote Trp metabolism and
generate Kyn via TDO2. Kyn inhibits DC function, promotes
proliferation and EMT in lung cancer cells, and activates Akt/CREB
and Akt/WNK1 signaling pathways to enhance cancer cell
proliferation and migration (92).
Kyn production is closely related to the activation in CAFs. It
promotes activation of the AhR signaling pathway, activates Akt and
ERK signaling, and enhances the proliferative capacity and
resistance to EGFR tyrosine kinase inhibitors in non-small cell
lung cancer (NSCLC) cells (93).
Changes in Trp metabolite 3-HAA in the plasma of patients with
NSCLC are associated with the efficacy of ICIs. Lower levels of
3-HAA correlate with improved treatment responses and longer PFS,
indicating that 3-HAA may influence tumor growth and metastasis by
modulating immune responses (94).
IDO1 plays a significant role in Trp metabolism in breast cancer
(BC), promoting tumor cell proliferation and metastasis through
both immune and non-immune pathways (95). Upregulation of TDO2 and AhR renders
triple-negative BC (TNBC) cells more resistant, allowing them to
survive in the absence of matrix attachment, which is crucial for
BC metastasis (96).
The complex relationship between
cancer cells and cell death: Evasion and manipulation
Some metabolites generated during Trp metabolism can
influence the immune system by regulating immune cell apoptosis,
potentially promoting tumor progression (97).
Apoptosis
Kyn, the first metabolite of Trp degradation via
IDO, also functions as an immunosuppressive molecule owing to the
generation of its derivatives 3-HAA and Quin, which induce
selective apoptosis in murine Th1 cells by activating caspase-8
in vitro (9). T-cell
apoptosis occurs at relatively low Kyn concentrations, independent
of Fas/Fas ligand interactions, and is associated with caspase-8
activation and mitochondrial release of cytochromes (97,98).
IDO and TDO deplete local Trp and accumulate Kyn in tumors and
antigen-presenting cells, leading to T-cell anergy and apoptosis
via the GCN2 pathway (99). Trp
metabolites further enhance their immunosuppressive effects by
inhibiting T-cell proliferation and inducing DC-mediated T-cell
apoptosis (97). In addition to
T-cell apoptosis, Trp metabolism also affects cancer cell apoptosis
to some extent. Trp deprivation significantly increases the
expression of ERRFI1 in sensitive HCC cells, activating the
apoptotic pathway and inducing cell death (100). Enhanced Kyn pathway activation
leads to a substantial accumulation of Quin in the CNS in several
inflammatory neurological diseases. By contrast, 3OH-Kyn and
3OH-anthranilic acids may induce neuronal apoptosis or necrosis.
Kyn hydroxylase inhibitors reduce neuronal death in both in
vitro and in vivo models of cerebral ischemia and
excitotoxicity (101). AhR can
cooperate with NF-κB to promote c-Myc activation in prostate cancer
cells, and overexpression of c-Myc upregulates the expression of
Trp transporters and ABC transporters, further increasing the
apoptosis rate of prostate cancer cells treated with Abi or Doc
(102).
Autophagy
In addition to apoptosis, Trp metabolism, which
involves various metabolic pathways involving Trp and its
metabolites, is associated with autophagy. Trp metabolites modulate
the autophagy pathway by activating AMPK and SIRT1, thereby
affecting intestinal inflammation (103). For instance, Trp metabolites, such
as IAA, regulate autophagy and ameliorate pulmonary fibrosis by
inhibiting the PI3K/Akt signaling pathway (104). Trp metabolites also modulate
autophagy via the mechanistic target of rapamycin (mTOR) signaling
pathway. L-Trp enhances susceptibility to nonalcoholic fatty liver
disease via the mTOR pathway (105). Trp metabolism is essential to the
immune system, particularly in T-cells, where Trp metabolites
modulate immune responses by affecting the autophagy pathway
(106). However, Trp metabolism
plays different roles in cancer cell autophagy. For example,
extracellular vesicles derived from IDO1-high ovarian cancer cells
upregulate SIRT3 expression in endothelial cells by increasing its
acetylation, which is essential for promoting endothelial
mitochondrial autophagy associated with tumor angiogenesis
(107).
Necrosis
Trp metabolism generates Kyn and its metabolites
(3-hydroxykynurenine and Quin), which may induce necrosis in tumor
cells through multiple mechanisms. Metabolites in the Kyn metabolic
pathway may induce oxidative stress, damaging the DNA and
organelles of tumor cells and ultimately leading to cell necrosis
(108). Although IDO/TDO primarily
promote tumor growth through immune suppression, certain Kyn
metabolites may activate immune cells under specific conditions,
exerting cytotoxic effects on tumor cells, which may lead to
alterations in the immune microenvironment. For example, Quin
activates specific immune cell subsets and promotes tumor necrosis
(109).
Ferroptosis
Ferroptosis is a newly discovered type of regulated
cell death that is distinct from apoptosis and autophagy. The Trp
metabolites, 5-HT and 3-hydroxykynurenine, are potent ferroptosis
inhibitors capable of suppressing ferroptosis in tumor cells and
promoting their proliferation (110). These effects jointly influence
cancer cell proliferation, survival and death and have significant
implications for cancer progression and treatment.
Induction of cancer angiogenesis by
Trp metabolites
Some metabolites formed during Trp metabolism
promote tumor angiogenesis, which is crucial for tumor growth and
metastasis. MicroRNA (miR)-153 inhibits tumor angiogenesis by
suppressing the expression of IDO1. Downregulation of miR-153 leads
to increased IDO1 expression in bladder cancer cells, promoting
tumor angiogenesis (111).
Angiogenesis is essential for the progression and metastasis of
melanoma and is based on the production and release of
pro-angiogenic molecules in the TME. The interaction between
melanoma cells and endothelial cells affects the molecular
signaling pathways involved in tumor growth and progression. TDO
affects different melanoma cell lines, and its expression
positively correlates with endothelial cell infiltration (112). The Trp metabolite 5-HT can
modulate the function of tumor-infiltrating macrophages by
regulating the expression of matrix metalloproteinase 12, which
influences tumor angiogenesis. This mechanism has been validated in
colorectal and lung cancers (113). Indole compounds related to Trp
metabolism, such as melatonin, IAA, 5-hydroxytryptophan and 5-HT,
significantly inhibit vascular endothelial growth factor
(VEGF)-induced VEGF receptor-2 activation and subsequent
angiogenesis in human umbilical vein endothelial cells, thereby
suppressing tumor angiogenesis. These compounds provide potential
molecular targets for developing anti-VEGF signaling pathway drugs
that may help control tumor growth and progression (114,115). In summary, Trp metabolites induce
tumor angiogenesis through significant tumor growth and
metastasis.
Discoveries in targeting Trp metabolism in
cancer
Recently, targeting Trp metabolism has led to
significant progress in cancer therapy. The Trp metabolic pathway
plays a crucial role in the TME, immune evasion and cancer
progression, providing a wealth of targets for developing novel
therapeutic strategies. It also offers theoretical support and
practical evidence for applying multi-target strategies,
combination therapies with ICIs, interactions with the microbiome,
and the use of AhR antagonists.
Progress in multi-target strategies
targeting Trp metabolism in cancer therapy
In recent years, therapeutic strategies targeting
Trp metabolism have garnered widespread attention, with the
potential for multi-target combination therapies gradually emerging
as a research hotspot. The small-molecule IDO1 inhibitor navoximod
(synonyms: GDC-0919, NLG-919) has demonstrated significant activity
in combination therapy in various tumor models. It has been
revealed that this inhibitor is well-tolerated and capable of
reducing plasma Kyn levels, which is consistent with its
pharmacokinetic (PK) half-life. In addition, a stable disease
response has been observed in some patients (116). Further research has revealed that
navoximod in combination with cisplatin can effectively reverse
immune resistance mediated by the Kyn-AhR-IL6 axis induced by
IDO1-positive CAFs and chemoresistance triggered by tumor lymphoid
structures by targeting IDO1. This mechanism has exhibited great
potential in evaluating chemoresistance and biosafety in oral
squamous cell carcinoma (117).
Moreover, when navoximod is used in combination with another IDO1
inhibitor, indoximod, it exhibits synergistic effects, prolonging
patient survival and enhancing therapeutic efficacy when combined
with chemotherapy. Currently, research is underway to optimize
prodrug formulations to improve their therapeutic effects (118). 1-Methyl-L-Trp (1-MT-L-Trp) is a
non-specific competitive IDO1 inhibitor that is widely used in
basic research (119). Notably,
even in the absence of IDO expression, 1-MT can induce a strong
inflammatory molecular genetic response by activating AhR. These
data provide important insights into the potential clinical
indications of 1-MT as a cancer immunotherapy and suggest that 1-MT
may exert therapeutic effects through AhR-related mechanisms, even
in IDO-deficient tumors (120).
This finding also suggests that 1-MT may have a multi-target
mechanism of action, thereby providing a new research direction for
further exploration of its potential value in cancer therapy.
Compared with 1-MT-L-Trp, 1-methyl-D-Trp (1-D-MT) is currently
undergoing clinical trials for patients with recurrent or
refractory solid tumors, with the aim of inhibiting tumor immune
evasion mediated by IDO. However, studies have reported that 1-D-MT
promotes immune evasion by upregulating the expression of IDO1 in
cancer cells. This off-target effect has raised concerns regarding
its safety and efficacy in clinical trials (121). Future research should combine
high-throughput screening technologies to develop more selective
IDO1 inhibitors and employ drug delivery systems (such as
nanoparticles or prodrugs) to reduce non-specific effects on normal
cells, which may help mitigate such off-target effects. TDO has
long been considered constitutively expressed only in the liver. It
has been identified that TDO is significantly expressed in various
cancers, including BC, ovarian cancer and gliomas. TDO is involved
in tumor progression and immune suppression through the
TDO-L-Kyn-AhR pathway, and its inhibition is considered to help
reverse immune evasion (29,36).
Although TDO inhibitors remain in the preclinical stage, research
is moving towards the development of dual-target inhibitors (IDO1
and TDO) that may enhance cancer immunotherapy efficacy by blocking
L-kyn synthesis. However, the clinical feasibility of multi-target
strategies faces challenges such as adverse reactions caused by
drug interactions and heterogeneous responses of tumors. In the
future, precise diagnostic tools need to be developed to identify
tumor metabolic phenotypes and optimize multi-target therapeutic
combination strategies.
Synergistic effects of Trp metabolism
and ICIs
Recently, the synergistic effects of Trp metabolism
and ICIs have become a popular research topic in the field of
cancer immunotherapy. The Trp metabolic pathway, particularly the
metabolic processes mediated by IDO and TDO, plays a crucial role
in tumor immune evasion (99). It
has been found that, in primary pulmonary squamous cell carcinoma,
the co-expression of IDO1 and programmed death-ligand 1 (PD-L1) has
significant prognostic implications and is closely related to TILs.
Co-expression of IDO1 and PD-L1 may be an important target for
immunotherapy in pulmonary squamous cell carcinoma (122). The IDO1 enzyme inhibitor,
BGB-5777, in combination with anti-PD-1 monoclonal antibodies and
radiotherapy, demonstrated a robust immune-enhancing effect in GBM
model studies. This mechanism may be related to an increase in
tumor-infiltrating T-cells, reduction in immunosuppressive IDO1
levels, restoration of immune cell function following PD-1
blockade, and the pro-inflammatory effects of radiation therapy
(123). Additionally, the novel
IDO1 inhibitor PF-06840003 reversed the T-cell non-responsive
state, reduced intra-tumoral Kyn levels, and inhibited tumor
growth. It has been shown that the antitumor effect of this drug in
combination with anti-PD-L1 antibody therapy is more pronounced,
and preclinical data have supported Phase I clinical trials
(27). Another IDO1 inhibitor,
LY3381916, demonstrated high selectivity and efficacy. LY3381916,
either as monotherapy or in combination with PD-L1 inhibitors,
significantly inhibited IDO1 activity in tumors and increased
CD8+ T-cell infiltration in studies on treating advanced
solid tumors. However, the clinical activity of combination therapy
is limited, and significant hepatotoxicity has been observed in
patients with TNBC, highlighting the importance of dose adjustment
and optimization (124). NLG-919,
in combination with indoximod and ICIs (such as inhibitors of the
PD-1/PD-L1/PD-L2 pathway), has shown synergistic antitumor effects
in multiple models. For example, in the B16F10 melanoma model,
NLG-919, in combination with indoximod, ICIs, chemotherapy and
hgp100 peptide vaccine therapy, significantly inhibited tumor
growth, further clarifying the mechanistic basis of such
combination strategies. Future studies of the synergistic effects
of Trp metabolism and ICIs should focus on several aspects. It has
been previously revealed that tumors induce an immunosuppressive
microenvironment by upregulating IDO1 expression. Specifically,
IDO1 promotes immune tolerance under inflammatory stimulation by
depleting Trp and generating metabolites, leading to the loss of
effector T-cell function and enhanced activity of Tregs, making it
an important target for immunotherapy (28). For example, epacadostat, a novel
IDO1 inhibitor, can significantly inhibit Trp catabolism and has
multifaceted effects on the maturation of DCs, activation of tumor
antigen-specific cytotoxic T lymphocytes, regulation of Tregs and
function of peripheral blood mononuclear cells, thereby enhancing
the efficacy of immunotherapy (37). Additionally, linifanib mesylate,
which occupies the heme cofactor-binding site, prevents the
activation of IDO1. In vitro studies have shown that it can
inhibit Kyn production and restore T-cell proliferation, while
significantly reducing Kyn levels in tumor xenograft models in
vivo, with favorable PK and pharmacodynamic properties,
providing a basis for clinical development (125). The synergistic effects of Trp
metabolism and ICIs offer new insights into cancer immunotherapy.
However, their efficacy is influenced by the differential
expression of IDO1 and PD-L1. Future studies should focus on
patient stratification by using these biomarkers. This combination
therapy has great potential to overcome the limitations of
monotherapy and provide more efficient antitumor strategies.
Further research is expected to drive the development of novel
combination therapies.
Interactions between Trp metabolism
and the microbiome
Tight interactions exist between Trp metabolism and
the gut microbiome, which has garnered widespread attention
recently because of its implications in disease pathogenesis and
treatment. The gut microbiota metabolizes Trp through various
pathways into indole and its derivatives, which exhibit significant
biological functions in immune regulation, metabolic homeostasis
and suppression of inflammation. For instance, it has been
identified that moderate dietary supplementation with Trp can
generate indole compounds via microbial metabolism, modulating
immune responses and effectively treating various immune-related
diseases (126). Additionally,
indole and its derivatives have shown considerable potential in
treating metabolic and hepato-intestinal diseases, with mechanisms
possibly involving multi-target regulation (127,128). Microbial indole metabolism is
considered a potential therapeutic strategy for CRC (129). Recently, the discovery of novel
microbial strains associated with Trp metabolism has increased the
depth of research in this area. For example, certain strains
isolated from the gut generate bioactive metabolites by modulating
the Trp metabolic pathway, thereby influencing disease onset and
progression. A representative example is the gut bacterium
Clostridium sporogenes, which captures energy via the
Stickland reaction. Its metabolic product, indolepropionic acid,
which is known for its antioxidant and anti-inflammatory
properties, is considered a probiotic with therapeutic potential
for the prevention and treatment of inflammatory bowel disease
(130,131). Similarly, Lactobacillus
plantarum DPUL-S164 (S164) and its Trp metabolite,
indole-lactic acid, demonstrated significant repair effects in
mouse models of intestinal barrier damage induced by antibiotic
mixtures and dextran sulfate sodium (132,133). The development of next-generation
probiotics offers new insights into Trp-metabolism-targeted
therapies. For example, by comparing the microbiota composition of
healthy and diseased individuals and designing recombinant microbes
that overexpress target genes, it is possible to achieve the local
delivery of metabolites and regulation of signaling pathways.
However, this strategy faces challenges such as regulatory
requirements, manufacturing difficulties, complexity of disease
models, and diversity of individual gut microbiota, which can lead
to variable therapeutic outcomes. Current research has primarily
focused on short-term efficacy, whereas long-term safety and
effectiveness require further validation. The interactions between
Trp metabolism and the gut microbiome provide new directions for
disease treatment. An in-depth exploration of their metabolic
pathways and microbial regulatory mechanisms can promote the
development of novel compounds and optimize therapeutic strategies.
However, future studies should address the challenges posed by
complex disease models and the need for personalized treatments to
achieve broader clinical applications and enhanced therapeutic
outcomes.
AhR antagonists targeting Trp
metabolism
Trp metabolites are closely related to AhR, and the
potential role of AhR antagonists in targeting Trp metabolism in
cancer therapy warrants further investigation. The AhR is a
ligand-activated transcription factor that plays a key role in
maintaining important physiological functions in the body. It
exhibits dual pro- and antitumorigenic activities during
tumorigenesis, with its expression and activity varying depending
on the tumor type and individual differences among patients
(134–136). It has been revealed that Trp
metabolites such as Kyn and indole, generated by endogenous enzymes
or microbial metabolism, can bind to and activate AhR, forming the
Trp-AhR pathway, which is closely related to cancer progression
(137). For example, in melanoma
and glioma, the continuous transcriptional activation of AhR is
driven by ligands produced by the TME and the tumor itself,
promoting tumor growth and suppressing immune defense functions.
Moreover, IL4I1, a metabolic pathway parallel to IDO1 and TDO, can
generate AhR ligands, further demonstrating the difficulty of
completely inhibiting AhR ligand production (138). Based on these mechanisms, the
potential applications of AhR antagonists in cancer therapy have
attracted widespread attention. Various AhR antagonists have shown
significant antitumor potential. For example, CH223191 is a
selective competitive AhR antagonist that lacks antagonistic
activity against non-aromatic hydrocarbon ligands. Although related
research is still mainly focused on the basic scientific field,
preliminary therapeutic effects have been demonstrated in
pancreatic cancer models (139,140). Additionally, StemRegenin-1, an
exogenously applied AhR antagonist, reversed the drug resistance of
MCF-7/ADR cells through the AhR/ABC transport and AhR/UGT pathways
(141). Furthermore, the
antagonistic properties of BAY 2416964 were confirmed using
transactivation assays. It effectively and selectively inhibits AhR
activation in human and mouse head, head and neck squamous cell
carcinoma, NSCLC and CRC cells in vitro, while restoring
immune cell function and enhancing antitumor responses. BAY 2416964
exhibited favorable drug tolerance and significant antitumor
efficacy in vivo by inducing a pro-inflammatory TME
(142). CB7993113 inhibited the
invasive capacity of human BC cells under three-dimensional culture
conditions and blocked tumor cell migration in two-dimensional
culture without affecting cell viability or proliferation.
Moreover, this compound effectively inhibits bone marrow ablation
induced by 7,12-dimethylbenz[a]anthracene in vivo, further
proving that its absorption and distribution can produce
pharmacological effects (143).
Current research has clarified the dual role of AhR in
tumorigenesis; however, the development of AhR antagonists remains
in its early stages, with numerous obstacles facing clinical
translation, such as balancing the pro-inflammatory and
anti-inflammatory effects of AhR and avoiding off-target effects.
Research targeting Trp metabolism is at a critical stage of
transitioning from basic science to clinical applications, and
there is still a need to address core issues such as optimizing
drug targeting and safety, dealing with tumor heterogeneity, and
integrating multi-target combination therapy strategies.
Concluding remarks
Trp metabolites are potential biomarkers for
clinical features, such as inflammation, mental state and cognitive
function, which can guide clinical decision-making. These
metabolites may facilitate the development of novel therapeutic
strategies for treating various diseases. Rate-limiting enzymes,
including IDO, TDO, KMO and TPH, are critical for Trp metabolism,
and research on their inhibitors has progressed. Although IDO/TDO
inhibitors have shown limited efficacy as monotherapies for cancer
treatment, they can significantly enhance the efficacy of
conventional therapeutic agents when used in combination with
traditional drugs. Moreover, KMO inhibitors can reduce the
neurological damage caused by acute pancreatitis, and the
combination of telotristat ethyl with somatostatin analogs has been
approved by the FDA for the treatment of carcinoid-associated
diarrhea. Thus, specific inhibitors targeting TPH1 or TPH2 may be
safer and more effective. Direct supplementation with indoles and
their derivatives is a promising therapeutic strategy.
Dysregulation of Trp metabolism in cancer is closely associated
with clinical features such as tumor stage, size and lymph node
metastasis, and the levels of Trp metabolites can thus serve as
predictive biomarkers. Therefore, targeting Trp metabolism has
emerged as a promising therapeutic approach for cancer treatment.
However, despite preclinical evidence demonstrating the anticancer
effects of Trp metabolism inhibition, its translation into clinical
success remains challenging, as exemplified by the failure of IDO1
inhibitors in clinical trials. Similarly, although niacin can
significantly increase HDL-C levels and has various
lipid-modulating effects, it has not shown the expected clinical
benefits of reducing the risk of cardiovascular events (144). Future research should delve into
the resistance mechanisms, utilize multi-omic approaches to
identify new biomarkers and therapeutic targets, and design
combination therapies to enhance therapeutic efficacy and minimize
resistance. Continued research on Trp metabolism combined with
advanced technologies and innovative strategies holds promise for
advancing cancer treatments and patient outcomes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of Guangxi (grant nos. 2023GXNSFAA026061,
2024GXNSFAA010335, 2023GXNSFBA026313 and 2025GXNSFAA069989), the
National Natural Science Foundation of China (grant nos. 32360170,
82160590, 81802884, 82460677, 82260602, 82460677 and 82204208) and
the Independent project of Guangxi Key Laboratory of Tumor Immunity
and Microenvironment Regulation (grant no. 203030302415).
Availability of data and materials
Not applicable.
Authors' contributions
JiZ, XB and JD contributed to conception and
manuscript writing. YC and XG contributed to the proofreading and
bioanalysis. JuZ and JG contributed to acquisition of data. PW, SC
and XZ contributed to table and figure production. JY, JJ and LG
contributed to manuscript editing. All authors read and approved
the final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barik S: The uniqueness of tryptophan in
biology: Properties, metabolism, interactions and localization in
proteins. Int J Mol Sci. 21:87762020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palego L, Betti L, Rossi A and Giannaccini
G: Tryptophan biochemistry: Structural, nutritional, metabolic, and
medical aspects in humans. J Amino Acids. 2016:89525202016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kałużna-Czaplińska J, Gątarek P,
Chirumbolo S, Chartrand MS and Bjørklund G: How important is
tryptophan in human health? Crit Rev Food Sci Nutr. 59:72–88. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schwarcz R, Bruno JP, Muchowski PJ and Wu
HQ: Kynurenines in the mammalian brain: When physiology meets
pathology. Nat Rev Neurosci. 13:465–477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma L and Riva A: Intestinal barrier
function in health and disease - Any role of SARS-CoV-2?
Microorganisms. 8:17442020.https://doi.org/10.3390/microorganisms8111744
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu XH and Zhai XY: Role of tryptophan
metabolism in cancers and therapeutic implications. Biochimie.
182:131–139. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan J, Chen D, Ye Z, Zhu X, Li X, Jiao H,
Duan M, Zhang C, Cheng J, Xu L, et al: Molecular mechanisms and
therapeutic significance of Tryptophan Metabolism and signaling in
cancer. Mol Cancer. 23:2412024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez-Castro L, Garcia R, Venkateswaran N,
Barnes S and Conacci-Sorrell M: Tryptophan and its metabolites in
normal physiology and cancer etiology. FEBS J. 290:7–27. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HL, Zhang AH, Miao JH, Sun H, Yan
GL, Wu FF and Wang XJ: Targeting regulation of tryptophan
metabolism for colorectal cancer therapy: A systematic review. RSC
Adv. 9:3072–3080. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badawy AA: Kynurenine pathway of
tryptophan metabolism: Regulatory and functional aspects. Int J
Tryptophan Res. 10:11786469176919382017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stone TW and Darlington LG: Endogenous
kynurenines as targets for drug discovery and development. Nat Rev
Drug Discov. 1:609–620. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lugo-Huitrón R, Ugalde Muñiz P, Pineda B,
Pedraza-Chaverrí J, Ríos C and Pérez-de la Cruz V: Quinolinic acid:
An endogenous neurotoxin with multiple targets. Oxid Med Cell
Longev. 2013:1040242013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandarano M, Orecchini E, Bellezza G,
Vannucci J, Ludovini V, Baglivo S, Tofanetti FR, Chiari R, Loreti
E, Puma F, et al: Kynurenine/tryptophan ratio as a potential
blood-based biomarker in non-small cell lung cancer. Int J Mol Sci.
22:44032021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwiatkowska I, Hermanowicz JM,
Przybyszewska-Podstawka A and Pawlak D: Not only immune escape-the
confusing role of the TRP metabolic pathway in carcinogenesis.
Cancers (Basel). 13:26672021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suzuki Y, Suda T, Furuhashi K, Suzuki M,
Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H and Chida K:
Increased serum kynurenine/tryptophan ratio correlates with disease
progression in lung cancer. Lung Cancer. 67:361–365. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crotti S, D'Angelo E, Bedin C, Fassan M,
Pucciarelli S, Nitti D, Bertazzo A and Agostini M: Tryptophan
metabolism along the kynurenine and serotonin pathways reveals
substantial differences in colon and rectal cancer. Metabolomics.
13:1482017. View Article : Google Scholar
|
|
17
|
Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa
S, Omura Y, Wada M, Tarumi R, Plitman E, Moriguchi S, et al:
Kynurenine pathway in depression: A systematic review and
meta-analysis. Neurosci Biobehav Rev. 90:16–25. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maddison DC and Giorgini F: The kynurenine
pathway and neurodegenerative disease. Semin Cell Dev Biol.
40:134–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwarcz R and Pellicciari R: Manipulation
of brain kynurenines: Glial targets, neuronal effects, and clinical
opportunities. J Pharmacol Exp Ther. 303:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Walther DJ and Bader M: A unique central
tryptophan hydroxylase isoform. Biochem Pharmacol. 66:1673–1680.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel PD, Pontrello C and Burke S: Robust
and tissue-specific expression of TPH2 versus TPH1 in rat raphe and
pineal gland. Biol Psychiatry. 55:428–433. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalinichenko LS, Kornhuber J, Sinning S,
Haase J and Müller CP: Serotonin signaling through lipid membranes.
ACS Chem Neurosci. 15:1298–1320. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Murphy DL and Lesch KP: Targeting the
murine serotonin transporter: Insights into human neurobiology. Nat
Rev Neurosci. 9:85–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi X, Zhao G, Li H, Zhao Z, Li W, Wu M
and Du YL: Hydroxytryptophan biosynthesis by a family of
heme-dependent enzymes in bacteria. Nat Chem Biol. 19:1415–1422.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung KH, LoRusso P, Burris H, Gordon M,
Bang YJ, Hellmann MD, Cervantes A, Ochoa de Olza M, Marabelle A,
Hodi FS, et al: Phase I study of the indoleamine 2,3-dioxygenase 1
(IDO1) inhibitor navoximod (GDC-0919) administered with PD-L1
inhibitor (Atezolizumab) in advanced solid tumors. Clin Cancer Res.
25:3220–3228. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ebata T, Shimizu T, Fujiwara Y, Tamura K,
Kondo S, Iwasa S, Yonemori K, Shimomura A, Kitano S, Koyama T, et
al: Phase I study of the indoleamine 2,3-dioxygenase 1 inhibitor
navoximod (GDC-0919) as monotherapy and in combination with the
PD-L1 inhibitor atezolizumab in Japanese patients with advanced
solid tumours. Invest New Drugs. 38:468–477. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gomes B, Driessens G, Bartlett D, Cai D,
Cauwenberghs S, Crosignani S, Dalvie D, Denies S, Dillon CP, Fantin
VR, et al: Characterization of the selective indoleamine
2,3-dioxygenase-1 (IDO1) catalytic inhibitor EOS200271/PF-06840003
Supports IDO1 as a critical resistance mechanism to PD-(L)1
blockade therapy. Mol Cancer Ther. 17:2530–2542. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crosignani S, Bingham P, Bottemanne P,
Cannelle H, Cauwenberghs S, Cordonnier M, Dalvie D, Deroose F, Feng
JL, Gomes B, et al: Discovery of a novel and selective indoleamine
2,3-dioxygenase (IDO-1) inhibitor
3-(5-Fluoro-1H-indol-3-yl)pyrrolidine-2,5-dione
(EOS200271/PF-06840003) and Its characterization as a potential
clinical candidate. J Med Chem. 60:9617–9629. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim M and Tomek P: Tryptophan: A rheostat
of cancer immune escape mediated by immunosuppressive enzymes IDO1
and TDO. Front Immunol. 12:6360812021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roager HM and Licht TR: Microbial
tryptophan catabolites in health and disease. Nat Commun.
9:32942018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Madella AM, Van Bergenhenegouwen J,
Garssen J, Masereeuw R and Overbeek SA: Microbial-derived
tryptophan catabolites, kidney disease and gut inflammation. Toxins
(Basel). 14:6452022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Medvedev A and Buneeva O: Tryptophan
metabolites as mediators of microbiota-gut-brain communication:
Focus on isatin. Front Behav Neurosci. 16:9222742022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghiboub M, Boneh RS, Sovran B, Wine E,
Lefèvre A, Emond P, Verburgt CM, Benninga MA, de Jonge WJ and Van
Limbergen JE: Sustained diet-induced remission in pediatric Crohn's
disease is associated with kynurenine and serotonin pathways.
Inflamm Bowel Dis. 29:684–694. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vanholder R, Nigam SK, Burtey S and
Glorieux G: What if not all metabolites from the uremic toxin
generating pathways are toxic? A hypothesis. Toxins (Basel).
14:2212022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Spaepen S and Vanderleyden J: Auxin and
plant-microbe interactions. Cold Spring Harb Perspect Biol.
3:a0014382011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ye Z, Yue L, Shi J, Shao M and Wu T: Role
of IDO and TDO in cancers and related diseases and the therapeutic
implications. J Cancer. 10:2771–2782. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jochems C, Fantini M, Fernando RI, Kwilas
AR, Donahue RN, Lepone LM, Grenga I, Kim YS, Brechbiel MW, Gulley
JL, et al: The IDO1 selective inhibitor epacadostat enhances
dendritic cell immunogenicity and lytic ability of tumor
antigen-specific T cells. Oncotarget. 7:37762–37772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Someya S, Tohyama S, Kameda K, Tanosaki S,
Morita Y, Sasaki K, Kang MI, Kishino Y, Okada M, Tani H, et al:
Tryptophan metabolism regulates proliferative capacity of human
pluripotent stem cells. iScience. 24:1020902021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang Q and Li W: Correlation between
amino acid metabolism and self-renewal of cancer stem cells:
Perspectives in cancer therapy. World J Stem Cells. 14:267–286.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Munro MJ, Wickremesekera SK, Peng L, Tan
ST and Itinteang T: Cancer stem cells in colorectal cancer: A
review. J Clin Pathol. 71:110–116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bishnupuri KS, Alvarado DM, Khouri AN,
Shabsovich M, Chen B, Dieckgraefe BK and Ciorba MA: IDO1 and
kynurenine pathway metabolites activate PI3K-Akt signaling in the
neoplastic colon epithelium to promote cancer cell proliferation
and inhibit apoptosis. Cancer Res. 79:1138–1150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thaker AI, Rao MS, Bishnupuri KS, Kerr TA,
Foster L, Marinshaw JM, Newberry RD, Stenson WF and Ciorba MA: IDO1
metabolites activate β-catenin signaling to promote cancer cell
proliferation and colon tumorigenesis in mice. Gastroenterology.
145:416–425.e1-e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen J, Shao R, Li F, Monteiro M, Liu JP,
Xu ZP and Gu W: PI 3K/Akt/mTOR pathway dual inhibitor BEZ 235
suppresses the stemness of colon cancer stem cells. Clin Exp
Pharmacol Physiol. 42:1317–1326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vermeulen L, De Sousa E, Melo F, Van Der
Heijden M, Cameron K, De Jong JH, Borovski T, Tuynman JB, Todaro M,
Merz C, et al: Wnt activity defines colon cancer stem cells and is
regulated by the microenvironment. Nat Cell Biol. 12:468–476. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhu P, Lu T, Chen Z, Liu B, Fan D, Li C,
Wu J, He L, Zhu X, Du Y, et al: 5-hydroxytryptamine produced by
enteric serotonergic neurons initiates colorectal cancer stem cell
self-renewal and tumorigenesis. Neuron. 110:2268–2282. e42022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng X, Pang B, Gu G, Gao T, Zhang R,
Pang Q and Liu Q: Melatonin inhibits glioblastoma stem-like cells
through suppression of EZH2-NOTCH1 signaling axis. Int J Biol Sci.
13:245–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gürsel DB, Berry N and Boockvar JA: The
contribution of Notch signaling to glioblastoma via activation of
cancer stem cell self-renewal: the role of the endothelial network.
Neurosurgery. 70:N19–N21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pham QT, Oue N, Sekino Y, Yamamoto Y,
Shigematsu Y, Sakamoto N, Sentani K, Uraoka N and Yasui W: TDO2
overexpression is associated with cancer stem cells and poor
prognosis in esophageal squamous cell carcinoma. Oncology.
95:297–308. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu J, Qin X, Pan D, Zhang B and Jin F:
Amino acid-mediated metabolism: A new power to influence properties
of stem cells. Stem Cells Int. 2019:69194632019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang R, Hu P, Zang Q, Yue X, Zhou Z, Xu
X, Xu J, Li S, Chen Y, Qiang B, et al: LC-MS-based metabolomics
reveals metabolic signatures related to glioma stem-like cell
self-renewal and differentiation. RSC Adv. 7:24221–24232. 2017.
View Article : Google Scholar
|
|
51
|
Venkateswaran N and Conacci-Sorrell M:
Kynurenine: An oncometabolite in colon cancer. Cell Stress.
4:24–26. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi D, Wu X, Jian Y, Wang J, Huang C, Mo
S, Li Y, Li F, Zhang C, Zhang D, et al: USP14 promotes tryptophan
metabolism and immune suppression by stabilizing IDO1 in colorectal
cancer. Nat Commun. 13:56442022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brandacher G, Perathoner A, Ladurner R,
Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G,
Weiss HG, Göbel G, et al: Prognostic value of indoleamine 2,
3-dioxygenase expression in colorectal cancer: Effect on
tumor-infiltrating T cells. Clin Cancer Res. 12:1144–1151. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pan K, Wang H, Chen MS, Zhang HK, Weng DS,
Zhou J, Huang W, Li JJ, Song HF and Xia JC: Expression and
prognosis role of indoleamine 2, 3-dioxygenase in hepatocellular
carcinoma. J Cancer Res Clin Oncol. 134:1247–1253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li
TJ and Zheng L: Activated CD69+ T cells foster immune privilege by
regulating IDO expression in tumor-associated macrophages. J
Immunol. 188:1117–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tummala KS, Gomes AL, Yilmaz M, Graña O,
Bakiri L, Ruppen I, Ximénez-Embún P, Sheshappanavar V,
Rodriguez-Justo M, Pisano DG, et al: Inhibition of de novo NAD+
synthesis by oncogenic URI causes liver tumorigenesis through DNA
damage. Cancer Cell. 26:826–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu C, Rao D, Zhu H, Liu Q, Huang W, Zhang
L, Liang H, Song J and Ding Z: TDO2 was downregulated in
hepatocellular carcinoma and inhibited cell proliferation by
upregulating the expression of p21 and p27. Biomed Res Int.
2021:47084392021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu Z, Yan L, Lin J, Ke K and Yang W:
Constitutive TDO2 expression promotes liver cancer progression by
an autocrine IL-6 signaling pathway. 2 Cancer Cell Int. 21:5382021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li L, Wang T, Li S, Chen Z, Wu J, Cao W,
Wo Q, Qin X and Xu J: TDO2 promotes the EMT of hepatocellular
carcinoma through Kyn-AhR pathway. Front Oncol. 10:5628232021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jin H, Zhang Y, You H, Tao X, Wang C, Jin
G, Wang N, Ruan H, Gu D, Huo X, et al: Prognostic significance of
kynurenine 3-monooxygenase and effects on proliferation, migration
and invasion of human hepatocellular carcinoma. Sci Rep.
5:104662015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shi Z, Gan G, Xu X, Zhang J, Yuan Y, Bi B,
Gao X, Xu P, Zeng W, Li J, et al: Kynurenine derivative 3-HAA is an
agonist ligand for transcription factor YY1. J Hematol Oncol.
14:1532021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zuo X, Chen Z, Cai J, Gao W, Zhang Y, Han
G, Pu L, Wu Z, You W, Qin J, et al: 5-Hydroxytryptamine receptor 1D
aggravates hepatocellular carcinoma progression through FoxO6 in
AKT-dependent and independent manners. Hepatology. 69:2031–2047.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang T, Tan XL, Xu Y, Wang ZZ, Xiao CH
and Liu R: Expression and prognostic value of indoleamine 2,
3-dioxygenase in pancreatic cancer. Chin Med J (Engl). 130:710–716.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hezaveh K, Shinde RS, Klötgen A, Halaby
MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, et
al: Tryptophan-derived microbial metabolites activate the aryl
hydrocarbon receptor in tumor-associated macrophages to suppress
anti-tumor immunity. Immunity. 55:324–40. e82022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Koblish HK, Hansbury MJ, Bowman KJ, Yang
G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, et
al: Hydroxyamidine inhibitors of indoleamine-2, 3-dioxygenase
potently suppress systemic tryptophan catabolism and the growth of
IDO-expressing tumors. Mol Cancer Ther. 9:489–498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Guastella AR, Michelhaugh SK, Klinger NV,
Fadel HA, Kiousis S, Ali-Fehmi R, Kupsky WJ, Juhász C and Mittal S:
Investigation of the aryl hydrocarbon receptor and the intrinsic
tumoral component of the kynurenine pathway of tryptophan
metabolism in primary brain tumors. J Neurooncol. 139:239–249.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Panitz V, Končarević S, Sadik A, Friedel
D, Bausbacher T, Trump S, Farztdinov V, Schulz S, Sievers P,
Schmidt S, et al: Tryptophan metabolism is inversely regulated in
the tumor and blood of patients with glioblastoma. Theranostics.
11:9217–9233. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhong C, Peng L, Tao B, Yin S, Lyu L, Ding
H, Yang X, Peng T, He H and Zhou P: TDO2 and tryptophan metabolites
promote kynurenine/AhR signals to facilitate glioma progression and
immunosuppression. Am J Cancer Res. 12:2558–2575. 2022.PubMed/NCBI
|
|
69
|
Mohapatra SR, Sadik A, Tykocinski LO,
Dietze J, Poschet G, Heiland I and Opitz CA: Hypoxia inducible
factor 1α inhibits the expression of immunosuppressive
tryptophan-2, 3-dioxygenase in glioblastoma. Front Immunol.
10:27622019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ladomersky E, Zhai L, Lauing KL, Bell A,
Xu J, Kocherginsky M, Zhang B, Wu JD, Podojil JR, Platanias LC, et
al: Advanced age increases immunosuppression in the brain and
decreases immunotherapeutic efficacy in subjects with glioblastoma.
Clin Cancer Res. 26:5232–5245. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sahm F, Oezen I, Opitz CA, Radlwimmer B,
Von Deimling A, Ahrendt T, Adams S, Bode HB, Guillemin GJ, Wick W
and Platten M: The endogenous tryptophan metabolite and NAD+
precursor quinolinic acid confers resistance of gliomas to
oxidative stress. Cancer Res. 73:3225–3234. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kamson DO, Lee TJ, Varadarajan K,
Robinette NL, Muzik O, Chakraborty PK, Snyder M, Barger GR, Mittal
S and Juhász C: Clinical significance of tryptophan metabolism in
the nontumoral hemisphere in patients with malignant glioma. J Nucl
Med. 55:1605–1610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Juhász C, Chugani DC, Barger GR, Kupsky
WJ, Chakraborty PK, Muzik O and Mittal S: Quantitative PET imaging
of tryptophan accumulation in gliomas and remote cortex:
Correlation with tumor proliferative activity. Clin Nucl Med.
37:838–842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Sadik A, Somarribas Patterson LF, Öztürk
S, Mohapatra SR, Panitz V, Secker PF, Pfänder P, Loth S, Salem H,
Prentzell MT, et al: IL4I1 is a metabolic immune checkpoint that
activates the AHR and promotes tumor progression. Cell.
182:1252–70. e342020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Batista CE, Juhász C, Muzik O, Kupsky WJ,
Barger G, Chugani HT, Mittal S, Sood S, Chakraborty PK and Chugani
DC: Imaging correlates of differential expression of indoleamine 2,
3-dioxygenase in human brain tumors. Mol Imaging Biol. 11:460–466.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Talari NK, Panigrahi M, Madigubba S,
Challa S and Phanithi PB: Altered tryptophan metabolism in human
meningioma. J Neurooncol. 130:69–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Bosnyák E, Kamson DO, Guastella AR,
Varadarajan K, Robinette NL, Kupsky WJ, Muzik O, Michelhaugh SK,
Mittal S and Juhász C: Molecular imaging correlates of tryptophan
metabolism via the kynurenine pathway in human meningiomas. Neuro
Oncol. 17:1284–1292. 2015.PubMed/NCBI
|
|
78
|
Ino K, Yamamoto E, Shibata K, Kajiyama H,
Yoshida N, Terauchi M, Nawa A, Nagasaka T, Takikawa O and Kikkawa
F: Inverse correlation between tumoral indoleamine 2, 3-dioxygenase
expression and tumor-infiltrating lymphocytes in endometrial
cancer: Its association with disease progression and survival. Clin
Cancer Res. 14:2310–2317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yoshida N, Ino K, Ishida Y, Kajiyama H,
Yamamoto E, Shibata K, Terauchi M, Nawa A, Akimoto H, Takikawa O,
et al: Overexpression of indoleamine 2, 3-dioxygenase in human
endometrial carcinoma cells induces rapid tumor growth in a mouse
xenograft model. Clin Cancer Res. 14:7251–7259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ino K, Yoshida N, Kajiyama H, Shibata K,
Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S,
et al: Indoleamine 2, 3-dioxygenase is a novel prognostic indicator
for endometrial cancer. Br J Cancer. 95:1555–1561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Inaba T, Ino K, Kajiyama H, Yamamoto E,
Shibata K, Nawa A, Nagasaka T, Akimoto H, Takikawa O and Kikkawa F:
Role of the immunosuppressive enzyme indoleamine 2, 3-dioxygenase
in the progression of ovarian carcinoma. Gynecol Oncol.
115:185–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Odunsi K, Qian F, Lugade AA, Yu H, Geller
MA, Fling SP, Kaiser JC, Lacroix AM, D'Amico L, Ramchurren N, et
al: Metabolic adaptation of ovarian tumors in patients treated with
an IDO1 inhibitor constrains antitumor immune responses. Sci Transl
Med. 14:eabg84022022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Gostner JM, Obermayr E, Braicu IE, Concin
N, Mahner S, Vanderstichele A, Sehouli J, Vergote I, Fuchs D and
Zeillinger R: Immunobiochemical pathways of neopterin formation and
tryptophan breakdown via indoleamine 2, 3-dioxygenase correlate
with circulating tumor cells in ovarian cancer patients-A study of
the OVCAD consortium. Gynecol Oncol. 149:371–380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Singh R, Shaik S, Negi BS, Rajguru JP,
Patil PB, Parihar AS and Sharma U: Non-Hodgkin's lymphoma: A
review. J Family Med Prim Care. 9:1834–1840. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shankland KR, Armitage JO and Hancock BW:
Non-hodgkin lymphoma. Lancet. 380:848–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Feller AC and Diebold J: Histopathology of
Nodal and Extranodal Non-Hodgkin's Lymphomas. Springer Science
& Business Media; Heidelberg: 2003
|
|
87
|
Liu XQ, Lu K, Feng LL, Ding M, Gao JM, Ge
XL and Wang X: Up-regulated expression of indoleamine 2,
3-dioxygenase 1 in non-Hodgkin lymphoma correlates with increased
regulatory T-cell infiltration. Leuk Lymphoma. 55:405–414. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
El Kholy NM, Sallam MM, Ahmed MB, Sallam
RM, Asfour IA, Hammouda JA, Habib HZ and Abu-Zahra F: Expression of
indoleamine 2, 3-dioxygenase in acute myeloid leukemia and the
effect of its inhibition on cultured leukemia blast cells. Med
Oncol. 28:270–278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Curti A, Pandolfi S, Valzasina B, Aluigi
M, Isidori A, Ferri E, Salvestrini V, Bonanno G, Rutella S, Durelli
I, et al: Modulation of tryptophan catabolism by human leukemic
cells results in the conversion of CD25− into CD25+ T regulatory
cells. Blood. 109:2871–2877. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhang ML, Kem M, Mooradian MJ, Eliane JP,
Huynh TG, Iafrate AJ, Gainor JF and Mino-Kenudson M: Differential
expression of PD-L1 and IDO1 in association with the immune
microenvironment in resected lung adenocarcinomas. Mod Pathol.
32:511–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tang D, Yue L, Yao R, Zhou L, Yang Y, Lu L
and Gao W: P53 prevent tumor invasion and metastasis by
down-regulating IDO in lung cancer. Oncotarget. 8:545482017.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hsu YL, Hung JY, Chiang SY, Jian SF, Wu
CY, Lin YS, Tsai YM, Chou SH, Tsai MJ and Kuo PL: Lung
cancer-derived galectin-1 contributes to cancer associated
fibroblast-mediated cancer progression and immune suppression
through TDO2/kynurenine axis. Oncotarget. 7:27584–27598. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Feng H, Cao B, Peng X and Wei Q:
Cancer-associated fibroblasts strengthen cell proliferation and
EGFR TKIs resistance through aryl hydrocarbon receptor dependent
signals in non-small cell lung cancer. BMC Cancer. 22:7642022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Karayama M, Masuda J, Mori K, Yasui H,
Hozumi H, Suzuki Y, Furuhashi K, Fujisawa T, Enomoto N, Nakamura Y,
et al: Comprehensive assessment of multiple tryptophan metabolites
as potential biomarkers for immune checkpoint inhibitors in
patients with non-small cell lung cancer. Clin Transl Oncol.
23:418–423. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Levina V, Su Y and Gorelik E:
Immunological and nonimmunological effects of indoleamine 2,
3-dioxygenase on breast tumor growth and spontaneous metastasis
formation. Clin Dev Immunol. 2012:1730292012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
D'Amato NC, Rogers TJ, Gordon MA, Greene
LI, Cochrane DR, Spoelstra NS, Nemkov TG, D'Alessandro A, Hansen KC
and Richer JK: A TDO2-AhR signaling axis facilitates anoikis
resistance and metastasis in triple-negative breast cancer. Cancer
Res. 75:4651–4664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Fallarino F, Grohmann U, Vacca C, Bianchi
R, Orabona C, Spreca A, Fioretti MC and Puccetti P: T cell
apoptosis by tryptophan catabolism. Cell Death Differ. 9:1069–1077.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Fallarino F, Grohmann U, Vacca C, Orabona
C, Spreca A, Fioretti MC and Puccetti P: T cell apoptosis by
kynurenines. Adv Exp Med Biol. 527:183–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Platten M, Wick W and Van den Eynde BJ:
Tryptophan catabolism in cancer: Beyond IDO and tryptophan
depletion. Cancer Res. 72:5435–5440. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Cui M, Liu D, Xiong W, Wang Y and Mi J:
ERRFI1 induces apoptosis of hepatocellular carcinoma cells in
response to tryptophan deficiency. Cell Death Discov. 7:2742021.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Moroni F: Tryptophan metabolism and brain
function: focus on kynurenine and other indole metabolites. Eur J
Pharmacol. 375:87–100. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li F, Zhao Z, Zhang Z, Zhang Y and Guan W:
Tryptophan metabolism induced by TDO2 promotes prostatic cancer
chemotherapy resistance in a AhR/c-Myc dependent manner. BMC
Cancer. 21:11122021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gao N, Yang Y, Liu S, Fang C, Dou X, Zhang
L and Shan A: Gut-derived metabolites from dietary tryptophan
supplementation quench intestinal inflammation through the
AMPK-SIRT1-autophagy pathway. J Agric Food Chem. 70:16080–16095.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhuo J, Chu L, Liu D, Cai S and Dong H:
Tryptophan metabolite indole-3-acetic acid ameliorates pulmonary
fibrosis by regulating lung microbiota and inducing autophagy via
inhibition of the PI3K/AKT/MTOR pathway. Am J Respir Crit Care Med.
A24592024.
|
|
105
|
Osawa Y, Kanamori H, Seki E, Hoshi M,
Ohtaki H, Yasuda Y, Ito H, Suetsugu A, Nagaki M, Moriwaki H, et al:
L-tryptophan-mediated enhancement of susceptibility to nonalcoholic
fatty liver disease is dependent on the mammalian target of
rapamycin. J Biol Chem. 286:34800–34808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Rathmell JC: Metabolism and autophagy in
the immune system: Immunometabolism comes of age. Immunol Rev.
249:52012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu X, Zhou W, Zhang X, Ding Y, Du Q and
Hu R: 1-L-MT, an IDO inhibitor, prevented colitis-associated cancer
by inducing CDC20 inhibition-mediated mitotic death of colon cancer
cells. Int J Cancer. 143:1516–1529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Prendergast GC, Malachowski WJ, Mondal A,
Scherle P and Muller AJ: Indoleamine 2, 3-dioxygenase and its
therapeutic inhibition in cancer. Int Rev Cell Mol Biol.
336:175–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Opitz CA, Litzenburger UM, Sahm F, Ott M,
Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller
M, et al: An endogenous tumour-promoting ligand of the human aryl
hydrocarbon receptor. Nature. 478:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Liu D, Liang CH, Huang B, Zhuang X, Cui W,
Yang L, Yang Y, Zhang Y, Fu X, Zhang X, et al: Tryptophan
metabolism acts as a new anti-ferroptotic pathway to mediate tumor
growth. Adv Sci (Weinh). 10:22040062023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zhang W, Mao S, Shi D, Zhang J, Zhang Z,
Guo Y, Wu Y, Wang R, Wang L, Huang Y and Yao X: MicroRNA-153
decreases tryptophan catabolism and inhibits angiogenesis in
bladder cancer by targeting indoleamine 2, 3-dioxygenase 1. Front
Oncol. 9:6192019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Cecchi M, Anceschi C, Silvano A, Coniglio
ML, Chinnici A, Magnelli L, Lapucci A, Laurenzana A and Parenti A:
Unveiling the role of tryptophan 2, 3-dioxygenase in the angiogenic
process. Pharmaceuticals (Basel). 17:5582024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Nocito A, Dahm F, Jochum W, Jang JH,
Georgiev P, Bader M, Graf R and Clavien PA: Serotonin regulates
macrophage-mediated angiogenesis in a mouse model of colon cancer
allografts. Cancer Res. 68:5152–5158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Cerezo AB, Hornedo-Ortega R,
Álvarez-Fernández MA, Troncoso AM and García-Parrilla MC:
Inhibition of VEGF-induced VEGFR-2 activation and HUVEC migration
by melatonin and other bioactive indolic compounds. Nutrients.
9:2492017. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Peters MA, Walenkamp AM, Kema IP, Meijer
C, de Vries EG and Oosting SF: Dopamine and serotonin regulate
tumor behavior by affecting angiogenesis. Drug Resist Updat.
17:96–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Nayak-Kapoor A, Hao Z, Sadek R, Dobbins R,
Marshall L, Vahanian NN, Jay Ramsey W, Kennedy E, Mautino MR, Link
CJ, et al: Phase Ia study of the indoleamine 2,3-dioxygenase 1
(IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent
advanced solid tumors. J Immunother Cancer. 6:612018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen F, Zhao D, Huang Y, Wen X and Feng S:
Synergetic impact of combined navoximod with cisplatin mitigates
chemo-immune resistance via blockading IDO1(+) CAFs-secreted
Kyn/AhR/IL-6 and pol ζ-prevented CIN in human oral squamous cell
carcinoma. Life Sci. 335:1222392023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang XX, Sun SY, Dong QQ, Wu XX, Tang W
and Xing YQ: Recent advances in the discovery of indoleamine
2,3-dioxygenase 1 (IDO1) inhibitors. Medchemcomm. 10:1740–1754.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Oh JE, Shim KY, Lee JI, Choi SI, Baik SK
and Eom YW: 1-Methyl-L-tryptophan promotes the apoptosis of hepatic
stellate cells arrested by interferon-γ by increasing the
expression of IFN-γRβ, IRF-1 and FAS. Int J Mol Med. 40:576–582.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lewis HC, Chinnadurai R, Bosinger SE and
Galipeau J: The IDO inhibitor 1-methyl tryptophan activates the
aryl hydrocarbon receptor response in mesenchymal stromal cells.
Oncotarget. 8:91914–91927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Opitz CA, Litzenburger UM, Opitz U, Sahm
F, Ochs K, Lutz C and Wick Wand Platten M: The
indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan
upregulates IDO1 in human cancer cells. PLoS One. 6:e198232011.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Takada K, Kohashi K, Shimokawa M, Haro A,
Osoegawa A, Tagawa T, Seto T, Oda Y and Maehara Y: Co-expression of
IDO1 and PD-L1 in lung squamous cell carcinoma: Potential targets
of novel combination therapy. Lung Cancer. 128:26–32. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ladomersky E, Zhai L, Lenzen A, Lauing KL,
Qian J, Scholtens DM, Gritsina G, Sun X, Liu Y, Yu F, et al: IDO1
inhibition synergizes with radiation and PD-1 blockade to durably
increase survival against advanced glioblastoma. Clin Cancer Res.
24:2559–2573. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Kotecki N, Vuagnat P, O'Neil BH, Jalal S,
Rottey S, Prenen H, Benhadji KA, Xia M, Szpurka AM, Saha A, et al:
A Phase I study of an IDO-1 inhibitor (LY3381916) as monotherapy
and in combination with an Anti-PD-L1 Antibody (LY3300054) in
patients with advanced cancer. J Immunother. 44:264–275. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Balog A, Lin TA, Maley D, Gullo-Brown J,
Kandoussi EH, Zeng J and Hunt JT: Preclinical characterization of
linrodostat mesylate, a novel, potent, and selective oral
indoleamine 2,3-dioxygenase 1 inhibitor. Mol Cancer Ther.
20:467–476. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lin J, Sun-Waterhouse D and Cui C: The
therapeutic potential of diet on immune-related diseases: Based on
the regulation on tryptophan metabolism. Crit Rev Food Sci Nutr.
62:8793–8811. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Hu W, Yan G, Ding Q, Cai J, Zhang Z, Zhao
Z, Lei H and Zhu YZ: Update of indoles: Promising molecules for
ameliorating metabolic diseases. Biomed Pharmacother.
150:1129572022. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Li X, Zhang B, Hu Y and Zhao Y: New
insights into gut-bacteria-derived indole and its derivatives in
intestinal and liver diseases. Front Pharmacol. 12:7695012021.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liu Y, Pei Z, Pan T, Wang H, Chen W and Lu
W: Indole metabolites and colorectal cancer: Gut microbial
tryptophan metabolism, host gut microbiome biomarkers, and
potential intervention mechanisms. Microbiol Res. 272:1273922023.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Krause FF, Mangold KI, Ruppert AL, Leister
H, Hellhund-Zingel A, Lopez Krol A, Pesek J, Watzer B, Winterberg
S, Raifer H, et al: Clostridium sporogenes-derived metabolites
protect mice against colonic inflammation. Gut Microbes.
16:24126692024. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liu Y, Chen H, Van Treuren W, Hou BH,
Higginbottom SK and Dodd D: Clostridium sporogenes uses reductive
Stickland metabolism in the gut to generate ATP and produce
circulating metabolites. Nat Microbiol. 7:695–706. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang A, Guan C, Wang T, Mu G and Tuo Y:
Lactiplantibacillus plantarum-derived indole-3-lactic acid
ameliorates intestinal barrier integrity through the AhR/Nrf2/NF-κB
Axis. J Agric Food Chem. April 10–2024.(Epub ahead of print).
|
|
133
|
Wang A, Guan C, Wang T, Mu G and Tuo Y:
Indole-3-lactic acid, a tryptophan metabolite of
lactiplantibacillus plantarum DPUL-S164, improved intestinal
barrier damage by activating AhR and Nrf2 signaling pathways. J
Agric Food Chem. 71:18792–18801. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Leclerc D, Staats Pires AC, Guillemin GJ
and Gilot D: Detrimental activation of AhR pathway in cancer: An
overview of therapeutic strategies. Curr Opin Immunol. 70:15–26.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Paris A, Tardif N, Galibert MD and Corre
S: AhR and cancer: From gene profiling to targeted therapy. Int J
Mol Sci. 22:7522022. View Article : Google Scholar
|
|
136
|
Wang Z, Monti S and Sherr DH: The diverse
and important contributions of the AHR to cancer and cancer
immunity. Current Opin Toxicol. 2:93–102. 2017. View Article : Google Scholar
|
|
137
|
Sun M, Ma N, He T, Johnston LJ and Ma X:
Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon
receptor (AhR). Crit Rev Food Sci Nutr. 60:1760–1768. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Griffith BD and Frankel TL: The aryl
hydrocarbon receptor: impact on the tumor immune microenvironment
and modulation as a potential therapy. Cancers (Basel). 16:4722024.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Leja-Szpak A, Góralska M, Link-Lenczowski
P, Czech U, Nawrot-Porąbka K, Bonior J and Jaworek J: The opposite
effect of L-kynurenine and Ahr inhibitor Ch223191 on apoptotic
protein expression in pancreatic carcinoma cells (Panc-1).
Anticancer Agents Med Chem. 19:2079–2090. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Choi EY, Lee H, Dingle RW, Kim KB and
Swanson HI: Development of novel CH223191-based antagonists of the
aryl hydrocarbon receptor. Mol Pharmacol. 81:3–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhang Y, Ma YC, Song J, Jin Y and Bao YN:
StemRegenin-1 reverses drug resistance of MCF-7/ADR Cells via
AhR/ABC transports and AhR/UGTs pathways. Current Proteomics.
21:113–128. 2024. View Article : Google Scholar
|
|
142
|
Kober C, Roewe J, Schmees N, Roese L,
Roehn U, Bader B, Stoeckigt D, Prinz F, Gorjánácz M, Roider HG, et
al: Targeting the aryl hydrocarbon receptor (AhR) with BAY 2416964:
A selective small molecule inhibitor for cancer immunotherapy. J
Immunother Cancer. 11:e0074952023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Parks AJ, Pollastri MP, Hahn ME, Stanford
EA, Novikov O, Franks DG, Haigh SE, Narasimhan S, Ashton TD, Hopper
TG, et al: In silico identification of an aryl hydrocarbon receptor
antagonist with biological activity in vitro and in vivo. Mol
Pharmacol. 86:593–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Garg A, Sharma A, Krishnamoorthy P, Garg
J, Virmani D, Sharma T, Stefanini G, Kostis JB, Mukherjee D and
Sikorskaya E: Role of niacin in current clinical practice: A
systematic review. Am J Med. 130:173–187. 2017. View Article : Google Scholar : PubMed/NCBI
|