Introduction
MicroRNAs (miRNAs) are highly conserved non-coding
RNAs consisting of 21–24 nucleotides that target specific 3′
untranslated regions of mRNAs through the RNA-induced silencing
complex to regulate the expression of target genes (1,2). In
recent years, a large number of studies have confirmed the
important role of miRNAs in cancer and non-cancer diseases
(3,4).
One of the most important members of the miR-99
family, miR-100 regulates a wide variety of biological processes,
including migration, cell death, metabolism and response to drugs.
For instance, Liu et al (5)
demonstrated that miR-100-5p can target and reduce the expression
of myotubularin related protein 3 (MTMR3), thereby activating the
PIP3/AKT and ERK signaling pathways and promoting the proliferation
of epidermal stem cells, which in turn is beneficial to the healing
of skin wounds. Wang et al (6) found that miR-100-5p promotes
proliferation and inhibits differentiation of myofibroblasts by
downregulating tribbles pseudokinase 2. Numerous studies have shown
that miR-100 has atypical expression patterns in different forms of
cancer, where it can either restrict or promote tumor growth,
depending on the tumor setting. Of note, in recent years, an
increasing number of studies have focused on the exosome-mediated
miR-100 delivery system, exploring its application in regulating
tumor progression and providing new strategies for the clinical
translation of miR-100. In addition, miR-100 plays an important
role in the pathogenesis of noncancerous diseases, such as
osteoporosis, cerebral infarction, Parkinson's disease,
atherosclerosis, rheumatoid arthritis and autoimmune
dacryoadenitis. The present study was the first systematic review
of the dual regulatory roles of miR-100 in cancers of different
systems and comprehensively summarizes the application of
exosome-delivered miR-100 in the regulation of tumor progression,
as well as the research progress of miR-100 in non-cancerous
diseases, with the aim of elucidating its molecular mechanism and
biological function, and providing new insights for disease
diagnosis, prognosis assessment and treatment.
miR-100-overview
miR-100, belonging to the miR-99 family, is composed
of three distinct members: miR-99a, miR-99b and miR-100, all of
which exhibit a shared seed region sequence (ACCCGUA) (7). This molecule originates from the
miR-100/let-7/miR-125 miRNA cluster and is transcribed from the
third intron of the multi-exonic MIR-100HG gene, which is situated
on human chromosome 11. As one of the oldest miRNAs, tracing its
origins back to bilaterian ancestors, miR-100 is highly conserved
and functionally diverse. This miRNA exists in two mature forms:
miR-100-5p (mature sequence: AACCCGUAGAUCCGAACUUGUG) and miR-100-3p
(mature sequence: CAAGCUUGUAUCUAUAGGUAUG) (https://www.miRbase.org/) (8–10).
These forms exhibit distinct sequences, implying they target
different mRNA sequences and fulfill separate roles. For instance,
in gastric cancer (GC), miR-100-3p targets bone morphogenetic
protein receptor type 2 (BMPR2), whereas miR-100-5p targets mTOR
(11,12).
miR-100 and non-cancerous diseases
Diseases of the skeletal system
Osteoporosis
The growth and osteogenic differentiation of human
bone marrow mesenchymal stem cells (hBMSCs) play a crucial role in
mitigating bone loss in individuals with osteoporosis. Wang et
al (13) found that miR-100-5p
is upregulated in hBMSCs from patients with osteoporosis, where it
directly targets and suppresses transmembrane protein 135
expression. This suppression negatively regulates the proliferation
and osteogenic differentiation of hBMSCs, thereby disrupting the
bone formation process in osteoporosis (13). In a comparable study, Ai et
al (14) noted an increase in
miR-100-5p expression within the knee joint tissues of individuals
with osteoporosis, indicating that miR-100-5p plays a role in the
downregulation of lysine demethylase 6B (KDM6B) expression. This
reduction in KDM6B's capacity to eliminate histone H3K27 (H3K27me3)
methylation from the RUNX family transcription factor 2 (RUNX2)
promoter led to diminished RUNX2 expression and hindered osteoblast
differentiation, ultimately affecting the bone formation ability in
these patients (14). Of note,
several clinical studies have confirmed the potential diagnostic
value of miR-100 for osteoporosis. The miR-100 expression levels
were compared in 120 plasma samples taken from patients with
osteoporosis and 120 samples taken from healthy controls using
reverse transcription-quantitative PCR (RT-qPCR), in a study by
Ding et al (15). The
researchers found that the osteoporosis group had significantly
higher levels of miR-100 expression. An area under the curve (AUC)
of 0.8916 was determined by the receiver operating characteristic
(ROC) curve analysis to indicate that miR-100 has diagnostic
potential for osteoporosis (15).
This result can be corroborated with the study by Chen et al
(16), who also observed an
upregulation of miR-100 expression in the serum of patients with
osteoporosis and obtained a similar diagnostic efficacy
(AUC=0.8875). These studies not only revealed the key regulatory
role of miR-100-5p in the pathogenesis of osteoporosis, but also
provided an important theoretical basis for the development of
miRNA-based early diagnostic methods and targeted therapeutic
strategies.
Osteoarthritis (OA)
Wu et al (17) demonstrated that infrapatellar fat
pad MSC-derived exosomes could deliver miR-100-5p into
chondrocytes, reduce mTOR expression and promote autophagy
activation, thereby inhibiting apoptosis and maintaining cartilage
homeostasis to protect articular cartilage from damage and
alleviate the condition of OA. Lai et al (18) used RT-qPCR to detect the expression
level of miR-100-5p in the serum of 150 patients with knee OA (KOA)
and 150 normal controls, and found that its expression was
downregulated, with an AUC of 0.845, which suggests that miR-100-5p
is closely related to KOA, and it also has a high diagnostic
value.
Non-traumatic osteonecrosis of the femoral head
(NONFH)
Yang et al (19) identified an upregulation of
miR-100-5p in exosomes derived from bone tissue of patients with
NONFH. The research found that miR-100-5p inhibits osteogenic
differentiation of hBMSCs and angiogenesis of human umbilical vein
endothelial cells by inactivating the BMPR2/Smad1/5/9 signaling
pathway. The results suggest that miR-100-5p could be a promising
target for NONFH therapy (19).
Diseases of the nervous system
Cerebral infarction
In the acute phase after stroke, neuronal
abnormalities are one of the key factors promoting the formation
and expansion of infarct foci, whereas microglia activation plays
an important role in the progression of neuroinflammation (20,21).
The study by Xin et al (22)
found that ischemia induced hyperactivation of M1 neurons, which in
turn upregulated miR-100-5p expression in neurons and promoted its
enrichment in extracellular vesicles (EVs). These
miR-100-5p-carrying EVs can be taken up by neighboring microglia
and neurons, and subsequently, miR-100-5p specifically binds to and
activates the Toll-like receptor (TLR)7 through its
U18U19G20 motif, which in turn
activates the NF-κB signaling pathway. This process not only leads
to neuronal overexcitation and apoptosis but also exacerbates the
neuroinflammatory response, ultimately exacerbating the pathology
of ischemic brain injury (22).
However, Cao et al (23)
found that miR-100-5p can target to reduce the expression level of
mTOR, activate autophagy response, inhibit apoptosis and thus
alleviate the condition of cerebral infarction. This suggests a
possible dual regulatory role for miR-100 in cerebral
infarction.
Parkinson's disease (PD)
It has been shown that MSC-derived exosomes
(MSC-Exo) are effective in attenuating dopaminergic (DA) neuronal
damage and reducing oxidative stress levels in PD models. The
molecular mechanism is that miR-100-5p, delivered by MSCs-Exo,
inhibits the expression of its target gene NADPH oxidase 4 (NOX4)
and upregulates the expression levels of the antioxidant factors
Keap-1, nuclear factor erythroid 2-related factor 2, heme oxygenase
1, superoxide dismutase (SOD)-1 and SOD-2, which reduces the
accumulation of reactive oxygen species and attenuates the damage
of DA neurons, and improves the motor deficit in PD (24). Loss of DA neurons caused by
microglia activation is considered an important pathological factor
in PD. Adipose-derived stem cells with small EVs reduce microglia
activation by delivering miR-100-5p, targeting downregulation of
deltex E3 ubiquitin ligase 3L expression, which in turn reduces the
expression level of STAT1 and attenuates microglial cell
activation, thereby decreasing the loss of DA neurons and
ameliorating motor deficits (25).
Spinal cord injury (SCI)
It was found that the regulation of inflammation and
microenvironment after SCI was beneficial to the recovery of neural
tissues. miR-100 could attenuate the inflammatory response induced
by microglia by inhibiting the activation of the NF-κB pathway by
downregulating the expression level of TLR4. miR-100 also inhibited
neuronal apoptosis by decreasing the expression of
apoptosis-related proteins. These anti-inflammatory and
anti-apoptotic effects together promoted the repair of neural
tissues, which ultimately significantly improved motor function
after SCI (26).
Heart diseases
Coronary atherosclerosis
As key effector cells of allergic inflammation,
eosinophils and the cytotoxic granule proteins they release have
been shown to promote atherosclerotic plaque development (27). Gao et al (28) found that miR-100-5p in human
umbilical cord MSC exosomes (hUCMSC-Exo) could target and
downregulate frizzled class receptor (FZD)5, inhibit the
Wnt/β-catenin pathway, significantly reduce the migration ability
of eosinophils, promote apoptosis and reduce the release of
eosinophil cationic protein and inflammatory factors, thus
improving the atherosclerotic lesions in mice. Similarly, Ji et
al (29) found that knockdown
of circ-0004104 in vascular endothelial cells (VECs) with
atherosclerosis-induced injury resulted in upregulation of miR-100
expression, which targeted and downregulated of TNF-α-induced
protein 8 expression levels, and attenuation of VEC injury, thereby
inhibiting the progression of atherosclerosis.
Cardiac hypertrophy
Zeng et al (30) observed upregulation of miR-100-5p
expression in tissues of a rat model of cardiac hypertrophy induced
by abdominal aortic constriction and in a model of cardiac
hypertrophic cells generated by angiotensin II stimulation. Their
molecular pathogenic mechanism is that miR-100-5p promotes the
activation of autophagy by decreasing the expression of mTOR,
leading to an increase in the surface area of cardiomyocytes, a
decrease in cardiac function and the progression of cardiac
hypertrophy (30).
Heart failure
Zhong et al (31) constructed a heart failure cell model
by adriamycin induction. It was found that hUCMSC-EVs could inhibit
oxidative stress and apoptosis by delivering miR-100-5p and
targeting to reduce the expression level of NOX4, thus alleviating
the condition of heart failure (31).
Autoimmune diseases
Liu et al (32) demonstrated that miR-100-5p
expression was downregulated in EVs derived from macrophages in the
rheumatoid arthritis microenvironment. Overexpression of miR-100-5p
can target and reduce the expression level of mTOR, inhibit the
proliferation of synovial cells and the exacerbation of
inflammation and attenuate the disease progression of rheumatoid
arthritis. Li et al (33)
found that hUCMSC-sEVs deliver miR-100-5p, promote macrophage
polarization toward an anti-inflammatory M2 phenotype and increase
the proportion of regulatory T cells, thus playing an important
role in the treatment of autoimmune dacryoadenitis.
Other diseases
The incidence of acute kidney injury (AKI) caused by
ischemia/reperfusion (IR) injury has been increasing year by year.
Chen et al (34) found that
hUCMSC-sEVs could deliver miR-100-5p into HK-2 cells exposed to IR
injury, which could inhibit apoptosis by decreasing the expression
of FKBP5 and activating the AKT pathway. The hUCMSC-sEVs were
injected intravenously into mice with IR injury and found to
significantly inhibit apoptosis and protect the kidneys from
damage. This provides a new approach for the treatment of AKI
(34). Wu et al (35) found that miR-100-5p can treat atopic
dermatitis. miR-100-5p exerts anti-inflammatory effects by
downregulating the expression of forkhead box (FOX)O3, thereby
inhibiting the activation of the downstream NLR family pyrin domain
containing 3 signaling pathway. Zhang et al (36) demonstrated that miR-100 from
hUCMSC-EVs promotes endometriosis development by inhibiting HS3ST2
expression and promoting endometrial stromal cell proliferation,
invasion and migration. Furthermore, in the context of a high-fat
diet, mice that overexpress miR-100 exhibited a reduction in weight
gain, a decrease in both visceral and subcutaneous fat, lower
levels of serum low-density lipoprotein cholesterol, as well as
enhanced glucose tolerance and insulin sensitivity. The results
indicate that miR-100 could provide protective advantages in the
context of metabolic syndrome and hepatic steatosis induced by a
high-fat diet (37).
miR-100 function and molecular mechanisms in
different systemic cancers
A multitude of research findings has illustrated
that miR-100 is crucial in diverse systemic cancers, influencing
the proliferation, invasion, migration and apoptosis of malignant
tumor cells. As illustrated in Table
I and Fig. 1 and Fig. 2, the mechanisms by which miR-100
influences tumor development can be summarized as follows: i)
miR-100 directly targets and regulates its downstream genes,
impacting tumor progression (12,38–50);
ii) interactions between miR-100 and long non-coding RNAs (lncRNAs)
(11,51–55),
circular RNAs (circRNAs) (56,57)
and cytokines (58–61) modulate its expression, indirectly
affecting the expression of downstream target genes; and iii)
miR-100 regulates the expression of target genes and further
modulates tumor progression by affecting key signaling pathways
(53,58,62–66).
Furthermore, increasing attention has been given to the use of
miRNAs in clinical treatments. Exosomes are membrane-bound vesicles
released by diverse cells found in mammalian tissues or body
fluids, and they are crucial for facilitating communication between
cells (67–69). Research indicates that the
administration of miR-100 through exosomes into neoplastic cells
can modulate tumor advancement, highlighting a potentially
beneficial pathway for oncological therapy (62,63,70–72).
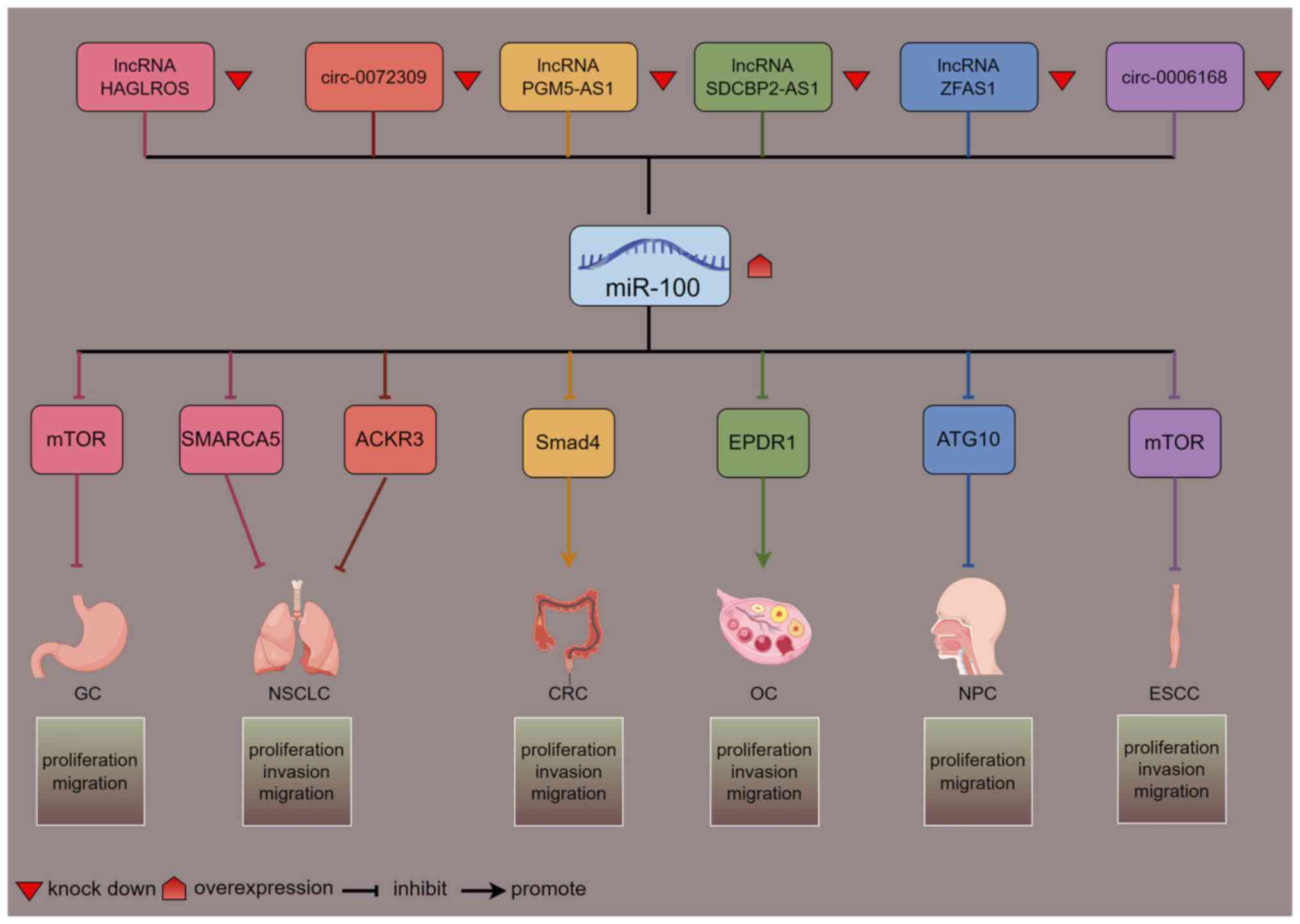 | Figure 1.Network diagram of lncRNAs/circRNAs
regulating miR-100: Down-regulation of lncRNAs/circRNAs results in
upregulation of miR-100, which in turn targets downstream target
genes to regulate cancer progression (figure generated with
figdraw). mTOR, mechanistic target of rapamycin; SMARCA5, SWI/SNF
related, matrix associated, actin dependent regulator of chromatin,
subfamily A, member 5; ACKR3, atypical chemokine receptor 3; Smad4,
SMAD family member 4; EPDR1, epithelial-derived protein 1; ATG10,
autophagy related 10; GC, gastric cancer; NSCLC, non-small cell
lung cancer; CRC, colorectal cancer; OC, ovarian cancer; NPC,
nasopharyngeal carcinoma; ESCC, esophageal squamous cell carcinoma;
lncRNA, long non-coding RNA; circRNA, circular RNA; miR,
microRNA. |
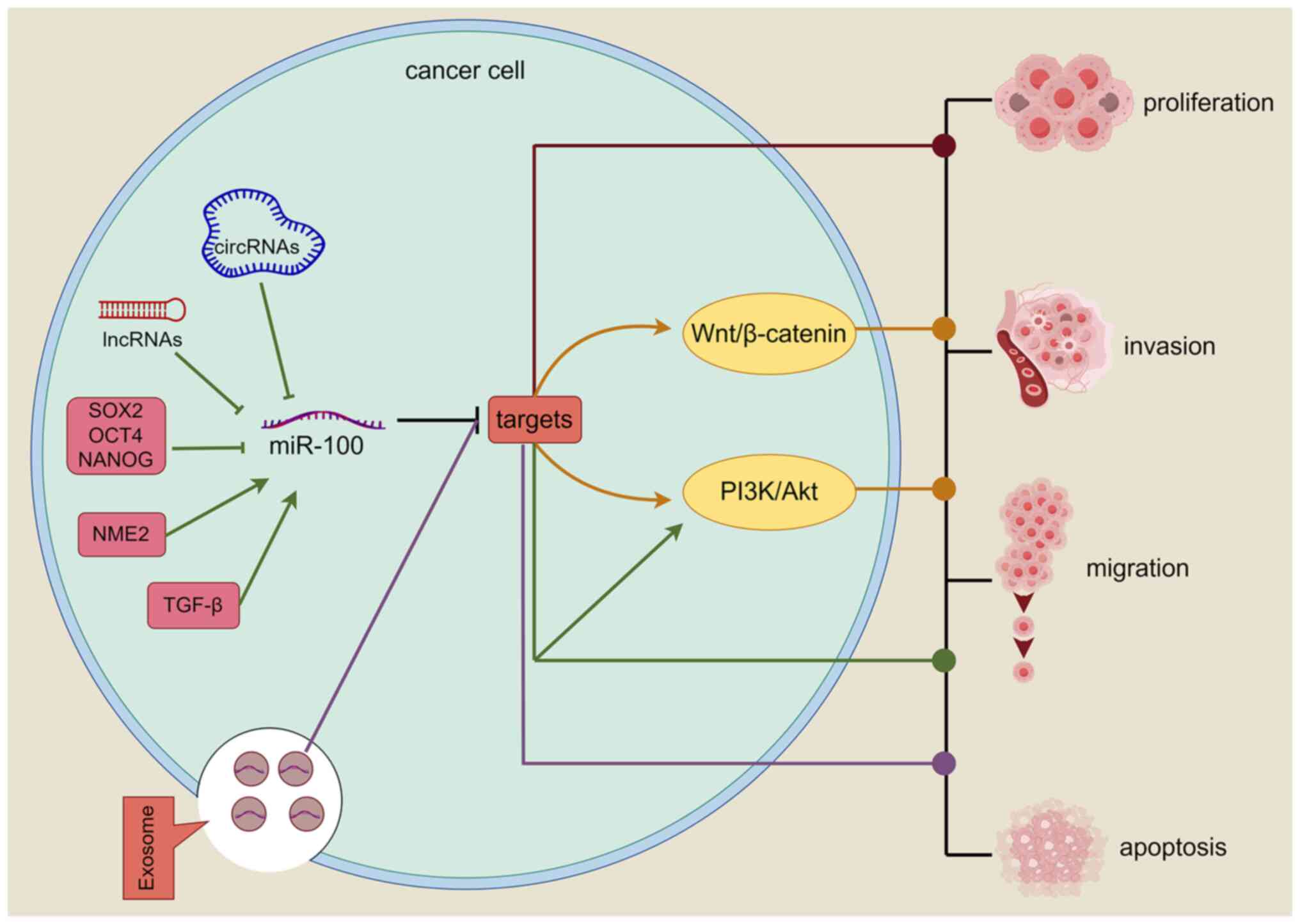 | Figure 2.Mechanism of miR-100. Red pathway:
miR-100 directly inhibits relevant target genes regulating tumor
proliferation, invasion, migration and apoptosis. Green pathway:
LncRNAs, circRNAs, cytokines (SOX2, OCT4, NANOG, NME2, TGF-β)
upregulate/downregulate the level of miR-100 and inhibit the level
of target genes, and the relevant target genes regulate tumor
progression by activating the relevant signaling pathways or
directly. Yellow pathway: miR-100 activates relevant signaling
pathways by inhibiting target genes and plays corresponding roles
in tumors. Purple pathway: miR-100 is delivered into tumor cells
via exosomes, inhibiting the expression of target genes and
regulating tumor progression (figure generated with figdraw).
LncRNA, long non-coding RNA; circRNA, circular RNA; miR, microRNA;
SOX2, sex determining region Y-box 2; OCT4, octamer binding
transcription factor 4; NANOG, recombinant NANOG homeobox protein;
NME2, NME/NM23 nucleoside diphosphate kinase 2; TGF-β, transforming
growth factor-β; ⊣, inhibition; →, promotion. |
 | Table I.microRNA-100 target genes and their
roles in different cancers. |
Table I.
microRNA-100 target genes and their
roles in different cancers.
| Type of cancer | Targets | Roles | (Refs.) |
|---|
| Liver cancer | CXCR7 | Direct inhibition
of cell proliferation, migration and invasion | (38) |
|
| IGF2 | Inhibition of the
PI3K/AKT/mTOR pathway reduces tumor stem cell stemness | (58) |
|
| CLDN11 | Activation of
PI3K/AKT pathway to enhance cell migration and invasion | (62) |
|
| mTOR | Inhibition of
cell-dependent VETC migration | (73) |
|
| LDHA | Inhibits glycolysis
and thus cell proliferation and invasion | (75) |
| Gastric cancer | mTOR | Promotes autophagy,
which in turn inhibits cell proliferation and migration | (11) |
|
| BMPR2 | Direct inhibition
of cell proliferation and promotion of apoptosis | (12) |
|
| CXCR7 | Direct inhibition
of cell proliferation | (39) |
| Esophageal
cancer | CXCR7 | Direct inhibition
of cell proliferation, migration and invasion | (40) |
|
| mTOR | Direct inhibition
of cell proliferation and invasion | (56) |
|
| IGF1R | Promotes
lymphangiogenesis and enhances metastasis | (63) |
| Colorectal
cancer | Smad4 | Promotes cell
proliferation, migration and invasion | (51) |
|
| mTOR | Direct inhibition
of cell proliferation, migration and invasion | (70) |
| Breast cancer | FOXA1 | Direct inhibition
of cell proliferation, migration and invasion | (41) |
|
| CDC25A | Direct inhibition
of cell proliferation, migration and invasion | (42) |
|
| FZD8 | Inhibition of the
Wnt/β-catenin pathway, which in turn inhibits cell migration and
invasion | (64) |
|
| mTOR | Inhibition of
HIF-1α expression, which in turn inhibits cell proliferation,
migration and invasion | (72) |
|
| mTOR | Maintenance of
tumor-associated macrophage phenotype for tumor progression | (83) |
| Endometrial
cancer | mTOR | Promotes autophagy
and induces apoptosis | (87) |
| Cervical
cancer | SATB1 | Inhibits the
AKT/mTOR signaling pathway and suppresses cell proliferation,
migration and invasion | (65) |
| Ovarian cancer | EPDR1 | Promotes tumor
progression | (52) |
| Nasopharyngeal | HOXA1 | Direct inhibition
of tumor progression | (43) |
| carcinoma | IGF1R | Direct inhibition
of cell motility and invasion | (44) |
|
| ATG10 | Activates PI3K/AKT
pathway, inhibits autophagy, inhibits cell proliferation and
migration | (53) |
|
| RASGRP3 | Promotes cell
proliferation, migration and invasion | (61) |
| Chordoma | IGF1R | Direct inhibition
of tumor progression | (45) |
| Thyroid cancer | FZD8 | Inhibits the
Wnt/β-catenin signaling pathway, inhibits cell proliferation and
promotes apoptosis | (66) |
| Non-small cell
lung | HOXA1 | Direct inhibition
of cell proliferation, motility and invasion | (46) |
| cancer | SOAT1 | Direct inhibition
of cell proliferation and migration | (47) |
|
| SMARCA5 | Direct inhibition
of cell proliferation, migration, and invasion | (54) |
|
| ACKR3 | Direct inhibition
of tumor progression | (57) |
| Prostate
cancer | mTOR | Inhibits NOX4,
inhibits cell proliferation, migration and invasion | (94) |
| Renal cell
carcinoma | NOX4 | Inhibits mTOR,
promotes autophagy and inhibits cell migration and invasion | (95) |
| Mantle cell
lymphoma | mTOR | Inhibition of cell
proliferation | (48) |
| Acute myeloid
leukemia in children | ATM | Promotes cell
proliferation and inhibits apoptosis | (49) |
| Multiple
myeloma | MTMR3 | Promotes cell
migration and inhibits apoptosis | (50) |
Digestive system tumors
Liver cancer
Ge et al (38) showed that miR-100 downregulates
C-X-C motif chemokine receptor 7 (CXCR7) expression in
hepatocellular carcinoma (HCC) LM3 cells, which decreases
proliferation, migration and invasion. The cancer stem cells of HCC
showed a marked downregulation of miR-100 and miR-125, according to
another study (58). In addition,
the study demonstrated that stemness regulators, including SOX2,
OCT4 and NANOG, reduced miR-100 and miR-125 expression, which in
turn increased insulin-like growth factor (IGF)2 expression,
activated the PI3K/AKT/mTOR pathway and preserved tumor stem cell
characteristics (58). Vessels
encapsulating tumor clusters (VETC) are a typical vascular
architecture in HCC that allows complete tumor clusters to enter
the bloodstream non-invasively. Elevated levels of angiopoietin 2
(Angpt2) in HCC cells are critical for the formation of VETCs.
miR-100 targets and reduces mTOR expression, which in turn
diminishes p70S6K phosphorylation, leading to a decrease in Angpt2
levels. This action inhibits VETC-dependent metastasis of HCC
cells, preventing their migration into the bloodstream in a
non-invasive manner (73). The
‘Warburg effect’ is a characteristic of cancer metabolism; it
occurs when cancer cells generate energy primarily through
glycolysis (74). Tumor cell
metabolism and survival are greatly impacted by lactate
dehydrogenase A (LDHA), an essential glycolysis enzyme. By focusing
on and reducing LDHA expression, miR-100-5p blocks glycolysis in
cancer cells when oxygen levels are low. This inhibits HCC cell
proliferation and invasion by reducing lactate generation and
glucose uptake (75). The results
of these investigations provide credence to the idea that miR-100
can slow the development of HCC. Nevertheless, there is evidence
that miR-100 may contribute to the aggressive development of HCC,
according to certain research. Wang et al (62) found that MHCC-97H, a highly
metastatic HCC cell line, which has high expression of
β-galactoside α2,6 sialyltransferase I (ST6Gal-I), was better able
to invade and migrate than its ST6Gal-I-knockdown counterpart. The
stimulation of α-2,6 sialylation by ST6Gal-I was thought to be
responsible for this action. It led to an increase in the activity
of nerve sheath phospholipase-2 and caused miR-100-5p to be sorted
into exosomes. When these exosomes were co-cultured with
low-invasive HCC cells (HepG2), miR-100-5p was transferred into the
HepG2 cells, resulting in reduced claudin 11 expression, increased
PI3K expression and AKT phosphorylation. These changes led to the
activation of the PI3K/AKT signaling pathway and enhanced the
migratory and invasive potential of HCC cells (62).
GC
Peng et al (12) demonstrated that BMPR2 expression
could be enhanced by removing miR-100-3p, and that BMPR2 expression
could be suppressed by increasing the levels of miR-100-3p.
Subsequently, this inhibition slowed GC cell proliferation and set
off cell death. Cao et al (39) found that miR-100 could target and
reduce CXCR7 expression, which in turn suppressed GC-cell
proliferation. The ability of lncRNAs to operate as competing
endogenous RNAs allows for the regulation of miRNA activity
(76). To inhibit miR-100-5p
expression, Chen et al (11)
found the lncRNA HAGLROS. After HAGLROS knockdown increased
miR-100-5p and decreased mTOR expression, autophagy was improved
and GC-cell proliferation and migration were suppressed (11). Evidence indicates, on the other
hand, demonstrated that miR-100 expression is elevated in GC
tissues and cells, and that levels show marked increases in
relation to tumor aggressiveness. The transcription factor NME/NM23
nucleoside diphosphate kinase 2 (NME2) plays a critical role in
miR-100 transcription. To achieve this, it acts with RNA polymerase
II at its C-terminal domain, specifically targeting serine 5 for
phosphorylation. This leads to an increase in miR-100 expression,
which prevents GC cells from terminating their lives (59).
Esophageal cancer (EC)
Through its direct targeting of CXCR7, miR-100
inhibits EC cell proliferation, migration and invasion (40). Additionally, circ-0006168 serves as
an oncogenic circRNA, with its expression being markedly elevated
in esophageal squamous cell carcinoma (ESCC) tissues and cell
lines. Reducing circ-0006168 expression increased miR-100
expression and decreased mTOR expression, which suppressed ESCC
cell motility, invasion and proliferation (56). Patients with ESCC have a poor
prognosis due to lymphangiogenesis, which is a critical component
of metastasis (77). There are
multiple routes by which cancer-associated fibroblasts (CAF), an
important part of the tumor microenvironment (TME), can promote
tumorigenesis and progression (78). The study demonstrated that in ESCC,
overexpression of IGF1R was caused by the deletion of miR-100-5p in
CAF-derived exosomes. This overexpression activated the PI3K/AKT
pathway, which in turn promoted the creation of lymphatic vessels
and enhanced the metastasis of ESCC to lymph nodes. Based on these
results, miR-100-5p may be able to target the lymphatic metastases
of ESCC via exosome-mediated transport and suppress
lymphangiogenesis (63).
Colorectal cancer (CRC)
Relative to non-metastatic CRC tissues, miR-100
expression is substantially higher in lymph node metastatic CRC
tissues, according to various studies. By reducing the expression
of targets such as mTOR, IGF1R, Fas and X-linked inhibitor of
apoptosis, overexpression of miR-100-5p can prevent CRC metastasis
(79). Furthermore, Jahangiri et
al (70) discovered that
miR-100, which was delivered via MSCs-Exo, reduced mTOR expression
and indirectly increased miR-143. The expression of hexokinase 2
and KRAS was subsequently downregulated as a result of this,
thereby inhibiting CRC cellular activities (70). Of note, Zhou et al (51) found that lncRNA PGM5-AS1 could
target and inhibit miR-100-5p. The elevation of miR-100-5p
expression and the subsequent downregulation of Smad4 promoted the
proliferation, migration and invasion of CRC cells when PGM5-AS1
was knocked down (51).
Pancreatic ductal adenocarcinoma (PDA)
Ottaviani et al (60) discovered that the SMAD2/3 signaling
pathway is activated by TGF-β, leading to an increase in miR-100
transcription and the progression of PDA. However, miR-100-5p was
found in significant levels in exosomes from hUCMSCs, according to
Ding et al (71). Pancreatic
cancer cells sped up the disease's development after absorbing
these exosomes, which allowed miR-100-5p to enter the cells and
stimulate cell proliferation and invasion.
Cancer of the reproductive system
Breast cancer (BC)
Through downregulating FOXA1 expression, Xie et
al (41) discovered that
miR-100 impeded BC-cell proliferation, migration and invasion. In a
similar study, Li et al (42) showed that miR-100-5p may reduce cell
division cycle 25A expression, which in turn delayed BC cell
migration, invasion and proliferation while speeding up apoptosis.
The Wnt/β-catenin system is crucial in the genesis of cancer and
regulates numerous key biological processes. It is also a highly
conserved pathway. After being engaged, the Wnt pathway makes
β-catenin more stable, which encourages it to go to the nucleus and
take part in cellular activities (80,81).
To enhance Wnt/β-catenin signaling, FZD8, a receptor for Wnt
proteins, activates signaling pathways that are dependent on
β-catenin, as well as those that are independent of it (82). According to Jiang et al
(64), miR-100 suppresses the
migration and invasion of BC cells by downregulating FZD8, which in
turn reduces the expression of β-catenin, MMP-7, transcription
factor 4 and lymphoid enhancer binding factor 1. Ultimately, this
leads to inactivation of the Wnt/β-catenin pathway (64). Separately, Pakravan et al
(72) transported miR-100 into BC
cells using exosomes produced by MSCs. Once inside, miR-100 reduced
mTOR expression, which in turn reduced hypoxia-inducible factor 1α
expression, leading to less VEFG transcription and a reduction in
BC cell proliferation, migration and invasion (72). Remarkably, a different study
proposed that miR-100 could enhance the tumor-associated macrophage
phenotype, which in turn promotes BC metastasis. Angiogenesis,
tumor migration and anti-tumor immunity are all promoted by
tumor-associated macrophages (TAM), an important part of the TME
immune cell population. In BC, TAM express a high level of miR-100,
which helps to preserve their phenotype by reducing the production
of mTOR, an enzyme that promotes tumor growth. Furthermore, the
Hedgehog pathway can be activated to improve the stemness and
migration of BC cells, as miR-100-induced reductions in mTOR
expression result in an increase in STAT5A-mediated IL-1R secretion
(83).
Endometrial, cervical and ovarian cancer
(OC)
Cancer cells may die when autophagy, a mechanism of
cellular breakdown, is stimulated (84). There is a strong correlation between
the amount of autophagosomes and the expression of light chain
(LC)3; therefore, an increase in LC3 often correlates with an
increase in autophagosome numbers. Beclin1 is involved in
autophagosome formation during the early stages of autophagy
(85,86). By reducing mTOR expression, Cai
et al (87) demonstrated
that miR-100-5p accelerates autophagy and promotes autophagosome
formation. Endometrial cancer cells die and the disease advances
more slowly as a result of this upregulation of Beclin1 and LC3
expression (87). By reducing SATB
homeobox 1 expression, miR-100 suppressed cervical cancer cell
proliferation, migration and invasion, as well as epithelial to
mesenchymal transition (EMT) and the AKT/mTOR pathway, according to
research by Huang et al (65). In OC, the lncRNA SDCBP2-AS1 was
shown by Liu et al (52) to
modulate miR-100-5p expression. Through inhibition of SDCBP2-AS1,
miR-100-5p was upregulated, leading to the suppression of
epithelial-derived protein 1 expression. This, in turn, enhanced
migration, invasion and proliferation of OC cells while preventing
their apoptosis (52).
Head and neck tumors
Nasopharyngeal carcinoma (NPC)
Through its direct targeting and suppression of
homeobox (HOX)A1 expression, He et al (43) showed that miR-100 suppresses the
growth of NPC cells. A different team of researchers discovered
that miR-100 can decrease IGF1R expression, which in turn decreases
NPC cell motility and invasion (44). In RNA, the reversible methylation of
the sixth position of adenine, called N6-methyladenosine (m6A), is
dynamically regulated by methyltransferases and demethylases. The
methyltransferases that play a role include methyltransferase 3,
N6-adenosine-methyltransferase complex catalytic subunit (METTL3),
METTL14, RNA binding motif protein 15B and zinc finger CCCH-type
containing 13, with METTL3 serving as the primary catalytic enzyme.
Research has demonstrated that m6A alteration modulates RNA
function through controlling RNA expression, splicing,
translocation, stabilization of lncRNAs and miRNA processing
(88–91). Peng et al (53) discovered a variety of differentially
expressed m6A-associated genes in NPC, including METTL3 and alkB
homolog 5, RNA demethylase. The expression of METTL3 was observed
to be markedly elevated in tumor tissues. METTL3 promotes the
expression of the lncRNA ZFAS1 by decelerating RNA degradation
processes and providing stability to the methylated ZFAS1
transcripts. The increased levels of ZFAS1 expression are
significantly associated with unfavorable outcomes in NPC. The
depletion of ZFAS1 led to an increase in miR-100-3p levels, which
subsequently reduced autophagy-related 10 expression, stimulated
the PI3K/AKT pathway and suppressed autophagy in tumor cells. The
increased autophagy within the TME supplies tumor cells with
additional energy, leading to the conclusion that the inhibition of
autophagy by miR-100-3p diminishes the proliferation and migration
of NPC cells (53,92). However, additional research
indicates that miR-100-5p could also be involved in the advancement
of NPC. The downregulation of FOXA1, a pioneer factor implicated in
multiple tumors (93), resulted in
heightened expression of miR-100-5p. This increase subsequently
diminished RAS guanyl releasing protein 3 expression, thereby
facilitating cell proliferation, migration and invasion in NPC
(61).
Chordoma and thyroid cancer
Zhang et al (45) discovered that miR-100-5p has the
capacity to suppress the proliferation of chordoma cells while
enhancing apoptosis through the downregulation of IGF1R expression.
Furthermore, it notably reduced the levels of N-calmodulin and
waveform protein, while simultaneously enhancing the expression of
E-calmodulin. This modulation effectively hinders the migration and
invasion of chordoma cells by disrupting the EMT process (45). In a distinct investigation, Ma and
Han (66) demonstrated that
miR-100-5p has the capacity to inactivate the Wnt/β-catenin pathway
through the suppression of FZD8 expression, subsequently leading to
the inhibition of thyroid cancer cell proliferation and the
induction of apoptosis.
Tumors of the respiratory system
The two main histological subtypes of lung cancer
(LC) are small cell LC (SCLC) and non-SCLC (NSCLC), the former of
which is more frequent. The development and progression of NSCLC
are regulated by miR-100, according to multiple studies. Based on
what we know about its upstream regulators, miR-100 is frequently
downregulated in NSCLC. For instance, in NSCLC, brain metastasis is
reduced when circ-0072309 is downregulated and miR-100 is
upregulated. This, in turn, decreases atypical chemokine receptor 3
expression (57). A similar pattern
was observed when the lncRNA HAGLROS was knocked down: miR-100 was
upregulated, SWI/SNF related, matrix associated, actin dependent
regulator of chromatin, subfamily A, member 5 was downregulated and
NSCLC cell proliferation, migration, and invasion were all reduced
(54). Furthermore, it has been
demonstrated that miR-100 can lower HOXA1 expression, which in turn
inhibits NSCLC cell proliferation, motility and invasion (46). Sevoflurane inhibited cell
proliferation and migration by re-establishing miR-100-3p
expression, which in turn decreased sterol O-acyltransferase 1
expression, as discovered by Fu et al (47), who noted that miR-100-3p was
downregulated in A549 NSCLC cells.
Other tumors
The expression of miR-100 is negatively impacted by
a number of cancers and is strongly linked to the advancement of
tumors. By reducing the expression of NOX4, for instance,
miR-100-5p blocks the proliferation, colony formation, migration
and invasion of prostate cancer (PC) cells. This is achieved by
targeting and suppressing the expression of mTOR (94). In renal cell carcinoma (RCC), Liu
et al (95) discovered that
miR-100 inhibits mTOR pathway expression, which in turn induces
autophagy and downregulates NOX4 expression. As a result, the
migration and invasion of RCC cells are suppressed (95). In mantle cell lymphoma, miR-100
inhibits cell growth via targeting mTOR (48). On the other hand, miR-100 is
overexpressed and targets ATM in child acute myeloid leukemia,
which promotes the proliferation of leukemia cells while preventing
their death (49). In their study,
Wei et al (50) discovered
that miR-100-5p inhibits apoptosis and increases the survival and
metastatic capacity of multiple myeloma cells by targeting and
downregulating MTMR3 expression. Diffuse large B-cell lymphoma
cells are unable to proliferate, migrate or invade when the lncRNA
HAGLROS is silenced; this is because miR-100 is upregulated in this
tumor type (55).
Value in cancer diagnosis and prognostic
assessment
A significant contributor to high cancer mortality
is the failure to diagnose tumors early, which leads to missed
treatment opportunities. Furthermore, inadequate or ineffective
methods for assessing prognosis can result in suboptimal treatment
for patients. These issues, including missed early diagnoses and
improper prognostic assessments, contribute to increased mortality
rates in cancer patients (96).
Early diagnosis and accurate prognostic evaluation are thus
critical. While most tumor markers currently used in clinical
settings are protein-based, only ~2% of human genome genes are
translated into proteins, meaning that relying solely on protein
markers may not provide a comprehensive view of the tumor. The
non-coding regions of the genome contain a wealth of information
beyond that found in the protein-coding regions. Therefore, a
deeper exploration of the role of these non-coding regions is
essential for improving early cancer diagnosis and prognostic
assessments (97). miRNA expression
is generally tissue-specific, with changes in expression levels
corresponding to the growth or regression of tumor tissue (98). Additionally, miRNAs are highly
stable in body fluids, making them detectable and valuable for
diagnostic purposes (99).
Consequently, miRNAs, including miR-100, are increasingly
recognized for their potential as biomarkers in clinical cancer
diagnosis and prognosis.
Diagnostic value
There is strong evidence that miR-100 could be used
as a diagnostic tool in a number of cancer types, such as PC
(100), multiple myeloma (50), BC (101), nephroblastoma (102) and bladder cancer (103), based on studies that measured
miR-100 levels in cancer patients' tissues or sera and compared
those results to other relevant factors (Table II). One study looked at 100 men
with PC and 100 men with benign prostatic hyperplasia to see how
miR-100-5p was expressed in their tissues. They discovered that
miR-100-5p expression was lower in PC and that this decrease was
increasingly pronounced as the tumor grade rose. With an AUC of
0.72, miR-100-5p may be useful as a PC biomarker, according to an
ROC curve analysis (100).
Similarly, miRNA sequencing and RT-qPCR both indicated that
patients with multiple myeloma had significantly higher miR-100-5p
expression levels than those with iron deficiency anemia. With an
AUC of 0.983, miR-100-5p is clearly a highly useful biomarker for
the diagnosis of multiple myeloma (50). Wang et al (101) found that miR-100-5p, miR-191-5p
and miR-342-3p were all considerably higher in the plasma of 108
patients with BC compared to 103 healthy controls. These levels
were particularly high in stages I and II of the disease. Together
and separately, these three miRNAs successfully differentiated
patients with BC from healthy controls; however, miR-191-5p and
miR-100-5p demonstrated superior diagnostic performance in the
early detection of BC. In comparison, more conventional biomarkers
like CEA and CA153 showed less diagnostic efficacy (101). Similarly, Ludwig et al
(102) found that the serum
expression level of miR-100-5p was significantly higher in 32
patients with nephroblastoma (or Wilms' tumor) compared to normal
controls, with an AUC value of 0.90. When Motawi et al
(103) compared miR-92a, miR-100
and miR-143 levels in the blood of 62 healthy controls with those
of 70 patients with bladder cancer, they discovered that the cancer
patients' levels were substantially lower. miR-100 showed a 90%
sensitivity and 66.7% specificity with an AUC of 0.823. When
miR-143 and miR-92a were added to the mix, the assay's sensitivity
and specificity went up to 94.3 and 83.3%, respectively, with an
AUC of 0.926. Therefore, miR-92a, miR-100 and miR-143 in plasma
show promise as circulating biomarkers for the clinical
identification of bladder cancer (103). Alongside the previously discussed
malignancies, miR-100 demonstrates promise as a diagnostic
biomarker in conditions such as cervical cancer (AUC 0.879)
(104) and leukemia (AUC 0.642)
(105). Overall, the unusual
expression of miR-100-5p in the context of cancer development
suggests its potential as a valuable candidate for cancer
diagnosis. However, to improve its reliability, additional
validation is necessary across a wider spectrum of cancer types and
more extensive patient groups.
 | Table II.Potential utility of miR-100 in
cancer diagnosis. |
Table II.
Potential utility of miR-100 in
cancer diagnosis.
| Diagnostic | Cases | Sample type | Testing
technology | Expression | AUC | Sensitivity,
specificity, % | (Refs.) |
|---|
| MM | 5 MM vs. 5 IDA | Plasma cells | MiRNA-seq | Upward | 0.983 | – | (50) |
| PC | 100 PC vs. 100
BPH | Tissue | RT-qPCR | Downward | 0.720 | – | (100) |
| BC | 108 BC vs. 103
HC | Plasma | RT-qPCR | Upward | 0.961 | 93.5, 93.2 | (101) |
| Nephroblastoma | 32 WT vs. 12
HC | Serum | RT-qPCR | Upward | 0.900 | – | (102) |
| BlC | 70 BlC vs. 62
HC | Plasma | RT-qPCR | Downward | 0.823 | 90.0, 66.7 | (103) |
| CC | 46 CC vs. 34
HC | Serum | RT-qPCR | Downward | 0.879 | 91.2, 80.4 | (104) |
| Leukemia | 85 ALL vs. 12
HC | Bone marrow | RT-qPCR | Downward | 0.642 | 64.7, 62.5 | (105) |
Prognostic assessment value
Several studies have shown that miR-100-5p is
important for predicting cancer outcomes. Studying the link between
miR-100-5p expression levels and patient survival, overall survival
(OS), recurrence-free survival (RFS) and event-free survival (EFS)
allowed to determine miR-100-5p's prognostic importance (Table III). In a survival analysis, Liao
et al (106) found that
patients whose miR-100-5p expression was lower had worse survival
results. A subsequent study revealed that the overexpression of
polo-like kinase 1 (PLK1), an oncogene associated with adverse
outcomes in HCC, was caused by miR-100-5p's insufficient targeting
and repression of PLK1 (106). A
related study by He et al (107) found that low-expression patients
with HCC had a much lower OS rate compared to high-expression
individuals. Furthermore, tumor grade, metastasis and tumor stage
were significantly correlated with miR-100-5p levels, which are
important clinicopathological indicators (107). Additionally, Song et al
(108) found that miR-100-5p was
downregulated in HCC cases with major vascular invasion and that
its low expression was significantly associated with poorer RFS and
OS. A potential prognostic factor in HCC is overexpression of
miR-100-5p, which was associated with improved clinical outcomes.
Overexpression of miR-100-5p in HER2-positive non-luminal subtype
BC cells improved EFS and OS, according to Fuso et al
(109). Overexpression of
miR-100-5p in combination with let-7a-5p, miR-101-3p and
miR-199a-3p improved EFS and OS (109). Patients had significantly better
3- and 5-year survival rates when miR-100-5p expression was
downregulated in EC tissues, as reported by Zhang and Tang
(110). Higher levels of
miR-100-5p were associated with improved survival rates in
patients. According to Wang et al (111), greater expression of miR-100-5p is
strongly related to cutaneous melanoma patient survival, suggesting
improved clinical prognosis. Conversely, Jakob et al
(112) found that patients with
oral squamous cell carcinoma with high miR-100-5p expression had
poorer OS and progression-free survival. A research team has
proposed using the miR-182/miR-100 ratio as a predictive biomarker
for patients with bladder cancer after finding an association
between this ratio and the pT stage, histologic grade, recurrence
and carcinoma in situ. Multifactorial Cox regression
analysis demonstrated that the miR-182/miR-100 ratio is an
independent predictor for OS. Kaplan-Meier curve analysis showed
that individuals with bladder cancer had a much shorter survival
time when the miR-182/miR-100 ratio was high. Accordingly, this
ratio shows promise as a novel biomarker for survival prediction
(113). In addition, OC (114), glioblastoma (115) and gastric adenocarcinoma (116) are just a few of the cancers where
miR-100 has demonstrated prognostic value. The importance of
miR-100 as a predictive biomarker for various cancer types is
underscored by these findings.
 | Table III.Potential utility of miR-100 in
cancer prognostic assessment. |
Table III.
Potential utility of miR-100 in
cancer prognostic assessment.
| Cancer type |
Characteristics | (Refs.) |
|---|
| Liver cancer | Low expression is
associated with shorter OS | (106) |
| Liver cancer | Decreased OS with
low expression | (107) |
| Liver cancer | Low expression
associated with poorer RFS and OS | (108) |
| Breast cancer | Low expression
associated with poorer EFS and OS | (109) |
| Esophageal
cancer | Reduced 3- and
5-year survival with low expression | (110) |
| Skin melanoma | Low expression
associated with shorter survival time | (111) |
| Oral squamous cell
carcinoma | High expression
associated with poorer OS and PFS | (112) |
| Bladder cancer | High levels of
miR-182/miR-100 ratio significantly associated with shorter
survival | (113) |
Impact on cancer drug resistance
Although multidrug resistance is still a major
problem in cancer treatment, researchers have made great strides in
understanding its molecular processes and regulatory pathways, with
miRNAs being named as key intracellular regulators (117). It has been acknowledged that
miR-100 plays a major role in the development of treatment
resistance in several cancer types. To illustrate the point,
tyrosine kinase inhibitor (TKI) resistance is substantially related
to elevated miR-100-5p expression in NSCLC cell lines. A drop in
cell viability rates is observed when miR-100-5p expression is
suppressed with lock nucleic acid, which greatly increases the
sensitivity of cancer cells to TKI therapy (118). These results highlight the
critical role of miR-100-5p in promoting NSCLC resistance to TKIs.
Reduced miR-100-5p expression causes mTOR levels to rise in LC,
which in turn makes LC cells resistant to cisplatin therapy
(119). In addition, treatment
resistance and metastasis in malignant cells, commonly called
dormant cancer cells, are often associated with the presence of
residual tumor cells and disseminated tumor cells. Malignant cells
in PC can evade conventional treatments by entering a dormant
phase, which they can then progress through to castration-resistant
prostate cancer (CRPC) and transdifferentiated neuroendocrine
prostate cancer (NEPC). These latent cells consistently showed an
increase of miR-100-5p, which is involved in the development of
CRPC and NEPC. Knockdown of miR-100-5p promotes apoptosis in
dormant prostate cancer cells and thus inhibits CRPC and NEPC
progression (120). A possible
involvement for miR-100-5p in the development of paclitaxel
resistance in this cancer was suggested by the significantly higher
levels of miR-100-5p in paclitaxel-resistant PC cell lines compared
to non-resistant ones (121).
Notably, in the setting of cervical cancer, hypoxia-induced
overexpression of miR-100 slowed the pace of cell viability
reduction following paclitaxel treatment. On the other hand,
paclitaxel sensitivity was enhanced in cells lacking miR-100,
suggesting that overexpression of miR-100 may promote paclitaxel
resistance in cervical cancer cells (122). These studies highlight the various
roles of miR-100 in the development of resistance to drugs in
various cancer types. Although further research is needed to
determine the exact mechanisms of action, miR-100 is a potential
option for future oncology therapeutic treatments due to its
evident involvement in cancer drug resistance.
Conclusion
The exploration of diagnostic markers and
therapeutic strategies for cancer remains a pivotal area of
investigation, as numerous previously daunting challenges are
progressively being resolved. In recent years, miRNAs have been
acknowledged for their crucial functions in tumor development and
the advancement of cancer. Of note, miR-100 has been identified as
a significant factor that can either facilitate or suppress cancer
progression, contingent upon the specific tumor type. For instance,
in various studies, miR-100 has demonstrated tumor-suppressive
effects in esophageal cancer (40,56,63),
endometrial cancer (87), cervical
cancer (65), chordoma (45), thyroid cancer (66), NSCLC (46,47,54,57),
PC (94), RCC (95), mantle cell lymphoma (48) and diffuse large B-cell lymphoma
(55). In the context of PDA
(60,71), OC (52), acute myeloid leukemia in children
(49) and multiple myeloma
(50), miR-100 exhibits a role that
promotes tumorigenesis. In various malignancies, including liver
cancer (38,58,62,73,75),
GC (11,12,39,59),
CRC (51,70,79),
BC (41,42,64,72,83)
and NPC (43,44,53,61),
the function of miR-100 is still a subject of debate, as it may
either facilitate or suppress tumor development. The analysis of
molecular mechanisms has demonstrated that miR-100 plays a
significant role in regulating essential processes in cancer cells,
primarily through the targeting of various downstream genes.
Furthermore, the expression of miR-100 is regulated by upstream
signaling factors that influence tumor progression through the
modulation of target genes. miR-100 plays a role in modulating
cancer-associated signaling pathways, thereby impacting the
behavior of tumor cells. Furthermore, the application of exosomes
for the delivery of miR-100 has demonstrated potential in
effectively modulating tumor progression. Consequently, a more
profound comprehension of these molecular mechanisms aids in
clarifying the processes that contribute to cancer development and
provides fresh insights for therapeutic approaches to cancer. The
expression patterns specific to certain tissues and the notable
dysregulation of miR-100 across different cancer types underscore
its potential utility as a biomarker for the early detection of
cancer. Furthermore, the relationship between miR-100 expression
levels and patient survival following treatment highlights its
importance as a prognostic indicator. In addition, the varying
levels of miR-100 expression observed in both drug-sensitive and
drug-resistant cell lines indicate its potential role in the
mechanisms underlying cancer drug resistance. Subsequent research
could yield novel approaches to address chemoresistance in clinical
applications. It is worth noting that miR-100 also has an important
role in the disease development of numerous non-cancer diseases,
and in-depth exploration of its molecular mechanism and study of
the clinical translational approach may provide new ideas for the
treatment of diseases. Despite its promising potential, there are
still several limitations in current research: i) The specific
behavior and mechanisms of miR-100 in the complex cancer
microenvironment remain to be further explored; ii) efficient
utilization of miR-100 for early diagnosis and accurate prognostic
assessment remains an unresolved challenge; iii) much of the
current research on miR-100 is primarily at the basic experimental
level, with insufficient integration into clinical applications.
Consequently, future research should focus on advancing the
molecular mechanisms of miR-100, facilitating its clinical
translation, and improving its diagnostic and therapeutic
applications. At the basic research level, deeper exploration is
needed to better understand miR-100's dual role in cancer and to
analyze its dynamic mechanisms in the TME. Regarding therapeutic
development, efforts should focus on optimizing targeted delivery
systems using exosomes or nanocarriers, and exploring the combined
effects of miR-100 mimetics or inhibitors with conventional
therapies. In diagnostic applications, establishing body
fluid-based miR-100 detection systems and developing precise tools
for early diagnosis and prognosis assessment, possibly integrating
artificial intelligence, should be prioritized. By adopting a
‘basic-translational-clinical’ research model, miR-100 can be
accelerated from a molecular marker to a clinical diagnostic and
treatment strategy, ultimately offering new hope and possibilities
for patients.
Acknowledgements
Not applicable.
Funding
The author(s) declare financial support was received for the
research, authorship and/or publication of this article. This work
was supported by the Inner Mongolia Science and Technology Research
Project (grant no. 2021MS08093), the Key Technologies Research and
Development Program of Inner Mongolia (grant no. 2021GG0170), the
General Program of Inner Mongolia Medical University (grant no.
YKD2021006), the 14th Five-Year Plan of Education Science in Inner
Mongolia Autonomous Region (grant no. NGJGH2021307), the 14th
Five-Year Plan of Science and Technology Innovation in Inner
Mongolia Autonomous Region (grant no. 2022YFSH0078), Zhiyuan Talent
Program of Inner Mongolia Medical University (grant no. ZY0202020),
Key Project of Inner Mongolia Medical University (grant no.
YKD2021ZD007), Inner Mongolia Natural Science Foundation (grant no.
2024MS08069), Science and Technology Program of the Joint Fund of
Scientific Research for the Public Hospitals of Inner Mongolia
Academy of Medical Sciences (grant no. 2024GLLH0323), Zhiyuan
Talent Program of Inner Mongolia Medical University (grant no.
ZY20242107), Doctoral Start-up Foundation Project of Inner Mongolia
Medical University (grant no. YKD2024BSQD026) and the Undergraduate
Teaching Reform Research and Practice Project of Inner Mongolia
Medical University in 2024 (grant no. NYJXGGSJ20244046).
Availability of data and materials
Not applicable.
Authors' contributions
JL and HD conceived and designed the study and were
responsible for manuscript writing. YS and JL were responsible for
the collection and assembly of data. YS and HD were responsible for
data analysis and interpretation. GH participated in the revision
of the paper. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hussen BM, Hidayat HJ, Salihi A, Sabir DK,
Taheri M and Ghafouri-Fard S: MicroRNA: A signature for cancer
progression. Biomed Pharmacother. 138:1115282021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soifer HS, Rossi JJ and Saetrom P:
MicroRNAs in disease and potential therapeutic applications. Mol
Ther. 15:2070–2079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Budakoti M, Panwar AS, Molpa D, Singh RK,
Büsselberg D, Mishra AP, Coutinho HDM and Nigam M: Micro-RNA: The
darkhorse of cancer. Cell Signal. 83:1099952021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douvris A, Viñas J and Burns KD:
miRNA-486-5p: Signaling targets and role in non-malignant disease.
Cell Mol Life Sci. 79:3762022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Z, Yang Y, Ju J, Zhang G, Zhang P, Ji
P, Jin Q, Cao G, Zuo R, Wang H, et al: miR-100-5p promotes
epidermal stem cell proliferation through targeting MTMR3 to
activate PIP3/AKT and ERK signaling pathways. Stem Cells Int.
2022:14742732022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang K, Liufu S, Yu Z, Xu X, Ai N, Li X,
Liu X, Chen B, Zhang Y, Ma H and Yin Y: miR-100-5p regulates
skeletal muscle myogenesis through the Trib2/mTOR/S6K signaling
pathway. Int J Mol Sci. 24:89062023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eniafe J and Jiang S: MicroRNA-99 family
in cancer and immunity. Wiley Interdiscip Rev RNA. 12:e16352021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Belles X: MicroRNAs and the evolution of
insect metamorphosis. Annu Rev Entomol. 62:111–125. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heimberg AM, Sempere LF, Moy VN, Donoghue
PC and Peterson KJ: MicroRNAs and the advent of vertebrate
morphological complexity. Proc Natl Acad Sci USA. 105:2946–2950.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu Y, Wang Z, Yu S, Liu D and Sun L:
LncmiRHG-MIR100HG: A new budding star in cancer. Front Oncol.
12:9975322022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen JF, Wu P, Xia R, Yang J, Huo XY, Gu
DY, Tang CJ, De W and Yang F: STAT3-induced lncRNA HAGLROS
overexpression contributes to the malignant progression of gastric
cancer cells via mTOR signal-mediated inhibition of autophagy. Mol
Cancer. 17:62018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peng CW, Yue LX, Zhou YQ, Tang S, Kan C,
Xia LM, Yang F and Wang SY: miR-100-3p inhibits cell proliferation
and induces apoptosis in human gastric cancer through targeting to
BMPR2. Cancer Cell Int. 19:3542019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang R, Zhang M, Hu Y, He J, Lin Q and
Peng N: MiR-100-5p inhibits osteogenic differentiation of human
bone mesenchymal stromal cells by targeting TMEM135. Hum Cell.
35:1671–1683. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ai L, Yi W, Chen L, Wang H and Huang Q:
Xian-Ling-Gu-Bao protects osteoporosis through promoting osteoblast
differentiation by targeting miR-100-5p/KDM6B/RUNX2 axis. In Vitro
Cell Dev Biol Anim. 57:3–9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding W, Ding S, Li J, Peng Z, Hu P, Zhang
T and Pan L: Aberrant expression of miR-100 in plasma of patients
with osteoporosis and its potential diagnostic value. Clin Lab.
65:1903272019. View Article : Google Scholar
|
|
16
|
Chen R, Liao X, Chen F, Wang B, Huang J,
Jian G, Huang Z, Yin G, Liu H and Jin D: Circulating microRNAs,
miR-10b-5p, miR-328-3p, miR-100 and let-7, are associated with
osteoblast differentiation in osteoporosis. Int J Clin Exp Pathol.
11:1383–1390. 2018.PubMed/NCBI
|
|
17
|
Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li
T, Chen H, Huang S, Fu Z, Li J, et al: miR-100-5p-abundant exosomes
derived from infrapatellar fat pad MSCs protect articular cartilage
and ameliorate gait abnormalities via inhibition of mTOR in
osteoarthritis. Biomaterials. 206:87–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai Z and Cao Y: Plasma miR-200c-3p,
miR-100-5p, and miR-1826 serve as potential diagnostic biomarkers
for knee osteoarthritis: Randomized controlled trials. Medicine
(Baltimore). 98:e181102019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang W, Zhu W, Yang Y, Guo M, Qian H,
Jiang W, Chen Y, Lian C, Xu Z, Bai H, et al: Exosomal miR-100-5p
inhibits osteogenesis of hBMSCs and angiogenesis of HUVECs by
suppressing the BMPR2/Smad1/5/9 signalling pathway. Stem Cell Res
Ther. 12:3902021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chamorro Á, Dirnagl U, Urra X and Planas
AM: Neuroprotection in acute stroke: Targeting excitotoxicity,
oxidative and nitrosative stress, and inflammation. Lancet Neurol.
15:869–881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia X, Chen J, Ren H, Zhou C, Zhang Q,
Cheng H and Wang X: Gypenoside pretreatment alleviates the cerebral
ischemia injury via inhibiting the microglia-mediated
neuroinflammation. Mol Neurobiol. 61:1140–1156. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xin D, Li T, Zhao Y, Guo X, Gai C, Jiang
Z, Yu S, Cheng J, Song Y, Cheng Y, et al: MiR-100-5p-rich small
extracellular vesicles from activated neuron to aggravate
microglial activation and neuronal activity after stroke. J
Nanobiotechnology. 22:5342024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao X, Zhang X, Chen J, Sun Q, Sun Y, Lin
N and Liu X: miR-100-5p activation of the autophagy response
through inhibiting the mTOR pathway and suppression of cerebral
infarction progression in mice. Aging (Albany NY). 15:8315–8324.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He S, Wang Q, Chen L, He YJ, Wang X and Qu
S: miR-100a-5p-enriched exosomes derived from mesenchymal stem
cells enhance the anti-oxidant effect in a Parkinson's disease
model via regulation of Nox4/ROS/Nrf2 signaling. J Transl Med.
21:7472023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng N, Huang X and Jia Y: Small
extracellular vesicles from adipose derived stem cells alleviate
microglia activation and improve motor deficit of Parkinson's
disease via miR-100-5p/DTX3L/STAT1 signaling axis. Exp Neurol.
389:1152502025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li XH, Fu NS and Xing ZM: MiR-100
suppresses inflammatory activation of microglia and neuronal
apoptosis following spinal cord injury via TLR4/NF-κB pathway. Eur
Rev Med Pharmacol Sci. 23:8713–8720. 2019.PubMed/NCBI
|
|
27
|
Marx C, Novotny J, Salbeck D, Zellner KR,
Nicolai L, Pekayvaz K, Kilani B, Stockhausen S, Bürgener N, Kupka
D, et al: Eosinophil-platelet interactions promote atherosclerosis
and stabilize thrombosis with eosinophil extracellular traps.
Blood. 134:1859–1872. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao H, Yu Z, Li Y and Wang X: miR-100-5p
in human umbilical cord mesenchymal stem cell-derived exosomes
mediates eosinophilic inflammation to alleviate atherosclerosis via
the FZD5/Wnt/β-catenin pathway. Acta Biochim Biophys Sin
(Shanghai). 53:1166–1176. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji P, Song X and Lv Z: Knockdown of
circ_0004104 alleviates oxidized low-density lipoprotein-induced
vascular endothelial cell injury by regulating miR-100/TNFAIP8
axis. J Cardiovasc Pharmacol. 78:269–279. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng J, Wang L, Zhao J, Zheng Z, Peng J,
Zhang W, Wen T, Nie J, Ding L and Yi D: MiR-100-5p regulates
cardiac hypertrophy through activation of autophagy by targeting
mTOR. Hum Cell. 34:1388–1397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhong Z, Tian Y, Luo X, Zou J, Wu L and
Tia J: Extracellular vesicles derived from human umbilical cord
mesenchymal stem cells protect against DOX-induced heart failure
through the miR-100-5p/NOX4 pathway. Front Bioeng Biotechnol.
9:7032412021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu H, Chen Y, Huang Y, Wei L, Ran J, Li
Q, Tian Y, Luo Z, Yang L, Liu H, et al: Macrophage-derived
mir-100-5p orchestrates synovial proliferation and inflammation in
rheumatoid arthritis through mTOR signaling. J Nanobiotechnology.
22:1972024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li N, Gao Z, Zhao L, Du B, Ma B, Nian H
and Wei R: MSC-derived small extracellular vesicles attenuate
autoimmune dacryoadenitis by promoting M2 macrophage polarization
and inducing tregs via miR-100-5p. Front Immunol. 13:8889492022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen G, Li X, Zhou X, Li Y, Yu H, Peng X,
Bai X, Zhang C, Feng Z, Mei Y, et al: Extracellular vesicles
secreted from mesenchymal stem cells ameliorate renal ischemia
reperfusion injury by delivering miR-100-5p targeting FKBP5/AKT
axis. Sci Rep. 14:67202024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu Z, He L, Yan L, Tan B, Ma L, He G, Dai
Z, Sun R and Li C: Hydrogels treat atopic dermatitis by
transporting marine-derived miR-100-5p-abundant extracellular
vesicles. ACS Biomater Sci Eng. 10:7667–7682. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang F, Li F and Lu J: microRNA-100
shuttled by human umbilical cord MSC-secreted extracellular
vesicles induces endometriosis by inhibiting HS3ST2. Cell Signal.
102:1105322023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smolka C, Schlosser D, Hohnloser C,
Bemtgen X, Jänich C, Schneider L, Martin J, Pfeifer D, Moser M,
Hasselblatt P, et al: MiR-100 overexpression attenuates high fat
diet induced weight gain, liver steatosis, hypertriglyceridemia and
development of metabolic syndrome in mice. Mol Med. 27:1012021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ge Y, Shu J, Shi G, Yan F, Li Y and Ding
H: miR-100 suppresses the proliferation, invasion, and migration of
hepatocellular carcinoma cells via targeting CXCR7. J Immunol Res.
2021:99207862021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao Y, Song J, Ge J, Song Z, Chen J and Wu
C: MicroRNA-100 suppresses human gastric cancer cell proliferation
by targeting CXCR7. Oncol Lett. 15:453–458. 2018.PubMed/NCBI
|
|
40
|
Zhou SM, Zhang F, Chen XB, Jun CM, Jing X,
Wei DX, Xia Y, Zhou YB, Xiao XQ, Jia RQ, et al: miR-100 suppresses
the proliferation and tumor growth of esophageal squamous cancer
cells via targeting CXCR7. Oncol Rep. 35:3453–3459. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie H, Xiao R, He Y, He L, Xie C, Chen J
and Hong Y: MicroRNA-100 inhibits breast cancer cell proliferation,
invasion and migration by targeting FOXA1. Oncol Lett. 22:8162021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Ren Y, Liu D, Yu X and Chen K: Role
of miR-100-5p and CDC25A in breast carcinoma cells. PeerJ.
9:e122632022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He W, Huang Y, Jiang CC, Zhu Y, Wang L,
Zhang W, Huang W, Zhou T and Tang S: miR-100 inhibits cell growth
and proliferation by targeting HOXA1 in nasopharyngeal carcinoma.
Onco Targets Ther. 13:593–602. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun X, Liu X, Wang Y, Yang S, Chen Y and
Yuan T: miR-100 inhibits the migration and invasion of
nasopharyngeal carcinoma by targeting IGF1R. Oncol Lett.
15:8333–8338. 2018.PubMed/NCBI
|
|
45
|
Zhang H, Yang K, Ren T, Huang Y, Liang X,
Yu Y, Wang W, Niu J, Lou J, Tang X and Guo W: miR-100-5p inhibits
malignant behavior of chordoma cells by targeting IGF1R. Cancer
Manag Res. 12:4129–4137. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Han W, Ren X, Yang Y, Li H, Zhao L and Lin
Z: microRNA-100 functions as a tumor suppressor in non-small cell
lung cancer via regulating epithelial-mesenchymal transition and
Wnt/β-catenin by targeting HOXA1. Thorac Cancer. 11:1679–1688.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fu B, Zhou F, Zhang J, Kong X, Ni B, Bu J,
Xu S and He C: Sevoflurane attenuates proliferative and migratory
activity of lung cancer cells via mediating the
microRNA-100-3p/sterol O-Acyltransferase 1 axis. Chin J Physiol.
66:456–465. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin L, Huang Y, Zhuang W, Lin P and Ma X:
miR-100 inhibits cell proliferation in mantle cell lymphoma by
targeting mTOR. Exp Hematol Oncol. 9:252020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun Y, Wang H and Luo C: MiR-100 regulates
cell viability and apoptosis by targeting ATM in pediatric acute
myeloid leukemia. Biochem Biophys Res Commun. 522:855–861. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wei X, Feng Y, Fu Y, Liu F, Chen Q, Zhang
W, Zhao Y, Huang X, Chen Y, Li Q and Zhang Q: miR-100-5p is
upregulated in multiple myeloma and involves in the pathogenesis of
multiple myeloma through targeting MTMR3. Hematology.
28:21968572023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou B, Yi F, Chen Y, Li CH, Cheng YS and
Yang K: Reduced long noncoding RNA PGM5-AS1 facilitated
proliferation and invasion of colorectal cancer through sponging
miR-100-5p. Eur Rev Med Pharmacol Sci. 24:7972–7981.
2020.PubMed/NCBI
|
|
52
|
Liu X, Liu C, Zhang A, Wang Q, Ge J, Li Q
and Xiao J: Long non-coding RNA SDCBP2-AS1 delays the progression
of ovarian cancer via microRNA-100-5p-targeted EPDR1. World J Surg
Oncol. 19:1992021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Peng J, Zheng H, Liu F, Wu Q and Liu S:
The m6A methyltransferase METTL3 affects autophagy and progression
of nasopharyngeal carcinoma by regulating the stability of lncRNA
ZFAS1. Infect Agent Cancer. 17:12022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li L, Zhu H, Li X, Ke Y, Yang S and Cheng
Q: Long non-coding RNA HAGLROS facilitates the malignant phenotypes
of NSCLC cells via repressing miR-100 and up-regulating SMARCA5.
Biomed J. 44 (6 Suppl 2):S305–S315. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shu L, Guo K, Lin ZH and Liu H: Long
non-coding RNA HAGLROS promotes the development of diffuse large
B-cell lymphoma via suppressing miR-100. J Clin Lab Anal.
36:e241682022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shi Y, Guo Z, Fang N and Liu H:
hsa_circ_0006168 sponges miR-100 and regulates mTOR to promote the
proliferation, migration and invasion of esophageal squamous cell
carcinoma. Biomed Pharmacother. 117:1091512019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang XQ, Song Q and Zeng LX: Circulating
hsa_circ_0072309, acting via the miR-100/ACKR3 pathway, maybe a
potential biomarker for the diagnosis, prognosis, and treatment of
brain metastasis from non-small-cell lung cancer. Cancer Med.
12:18005–18019. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Seol HS, Akiyama Y, Lee SE, Shimada S and
Jang SJ: Loss of miR-100 and miR-125b results in cancer stem cell
properties through IGF2 upregulation in hepatocellular carcinoma.
Sci Rep. 10:214122020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gong Y, Yang G, Wang Q, Wang Y and Zhang
X: NME2 is a master suppressor of apoptosis in gastric cancer cells
via transcriptional regulation of miR-100 and other survival
factors. Mol Cancer Res. 18:287–299. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ottaviani S, Stebbing J, Frampton AE,
Zagorac S, Krell J, de Giorgio A, Trabulo SM, Nguyen VTM, Magnani
L, Feng H, et al: TGF-β induces miR-100 and miR-125b but blocks
let-7a through LIN28B controlling PDAC progression. Nat Commun.
9:18452018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Peng Q, Zhang L, Li J, Wang W, Cai J, Ban
Y, Zhou Y, Hu M, Mei Y, Zeng Z, et al: FOXA1 suppresses the growth,
migration, and invasion of nasopharyngeal carcinoma cells through
repressing miR-100-5p and miR-125b-5p. J Cancer. 11:2485–2495.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang L, Chen X, Meng F, Huang T, Wang S,
Zheng Z, Zheng G, Li W, Zhang J and Liu Y: α2,6-Sialylation
promotes hepatocellular carcinoma cells migration and invasion via
enhancement of nSmase2-mediated exosomal miRNA sorting. J Physiol
Biochem. 79:19–34. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen C, Yang C, Tian X, Liang Y, Wang S,
Wang X, Shou Y, Li H, Xiao Q, Shu J, et al: Downregulation of
miR-100-5p in cancer-associated fibroblast-derived exosomes
facilitates lymphangiogenesis in esophageal squamous cell
carcinoma. Cancer Med. 12:14468–14483. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jiang Q, He M, Guan S, Ma M, Wu H, Yu Z,
Jiang L, Wang Y, Zong X, Jin F and Wei M: MicroRNA-100 suppresses
the migration and invasion of breast cancer cells by targeting
FZD-8 and inhibiting Wnt/β-catenin signaling pathway. Tumour Biol.
37:5001–5011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Huang C, Qin X, Zhao N, Jin H, Zhang S and
Yang H: MicroRNA-100 functions as a tumor suppressor in cervical
cancer via downregulating the SATB1 expression and regulating
AKT/mTOR signaling pathway and epithelial-to-mesenchymal
transition. Oncol Lett. 20:1336–1344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ma P and Han J: Overexpression of
miR-100-5p inhibits papillary thyroid cancer progression via
targeting FZD8. Open Med (Wars). 17:1172–1182. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Adamo A, Dal Collo G, Bazzoni R and
Krampera M: Role of mesenchymal stromal cell-derived extracellular
vesicles in tumour microenvironment. Biochim Biophys Acta Rev
Cancer. 1871:192–198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Mathieu M, Martin-Jaular L, Lavieu G and
Théry C: Specificities of secretion and uptake of exosomes and
other extracellular vesicles for cell-to-cell communication. Nat
Cell Biol. 21:9–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shojaei S, Hashemi SM, Ghanbarian H,
Salehi M and Mohammadi-Yeganeh S: Effect of mesenchymal stem
cells-derived exosomes on tumor microenvironment: Tumor progression
versus tumor suppression. J Cell Physiol. 234:3394–3409. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jahangiri B, Khalaj-Kondori M, Asadollahi
E, Dizaj LP and Sadeghizadeh M: MSC-Derived exosomes suppress
colorectal cancer cell proliferation and metastasis via
miR-100/mTOR/miR-143 pathway. Int J Pharm. 627:1222142022.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ding Y, Mei W, Zheng Z, Cao F, Liang K,
Jia Y, Wang Y, Liu D, Li J and Li F: Exosomes secreted from human
umbilical cord mesenchymal stem cells promote pancreatic ductal
adenocarcinoma growth by transferring miR-100-5p. Tissue Cell.
73:1016232021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pakravan K, Babashah S, Sadeghizadeh M,
Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N and Javan M:
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes
suppresses in vitro angiogenesis through modulating the
mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol
(Dordr). 40:457–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou HC, Fang JH, Shang LR, Zhang ZJ, Sang
Y, Xu L, Yuan Y, Chen MS, Zheng L, Zhang Y and Zhuang SM: MicroRNAs
miR-125b and miR-100 suppress metastasis of hepatocellular
carcinoma by disrupting the formation of vessels that encapsulate
tumour clusters. J Pathol. 240:450–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chelakkot C, Chelakkot VS, Shin Y and Song
K: Modulating glycolysis to improve cancer therapy. Int J Mol Sci.
24:26062023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou Y, Huang Y, Hu K, Zhang Z, Yang J and
Wang Z: HIF1A activates the transcription of lncRNA RAET1K to
modulate hypoxia-induced glycolysis in hepatocellular carcinoma
cells via miR-100-5p. Cell Death Dis. 11:1762020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang Y, Zhang W, Liu W, Huang L, Wang Y,
Li D, Wang G, Zhao Z, Chi X, Xue Y, et al: Long noncoding RNA
VESTAR regulates lymphangiogenesis and lymph node metastasis of
esophageal squamous cell carcinoma by enhancing VEGFC mRNA
stability. Cancer Res. 81:3187–3199. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ishii G, Ochiai A and Neri S: Phenotypic
and functional heterogeneity of cancer-associated fibroblast within
the tumor microenvironment. Adv Drug Deliv Rev. 99:186–196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Fujino Y, Takeishi S, Nishida K, Okamoto
K, Muguruma N, Kimura T, Kitamura S, Miyamoto H, Fujimoto A,
Higashijima J, et al: Downregulation of microRNA-100/microRNA-125b
is associated with lymph node metastasis in early colorectal cancer
with submucosal invasion. Cancer Sci. 108:390–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu
C, Wang C and Ye L: Wnt/β-catenin signaling in cancers and targeted
therapies. Signal Transduct Target Ther. 6:3072021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen W, Liu Z, Mai W, Xiao Y, You X and
Qin L: FZD8 indicates a poor prognosis and promotes gastric cancer
invasion and metastasis via B-catenin signaling pathway. Ann Clin
Lab Sci. 50:13–23. 2020.PubMed/NCBI
|
|
83
|
Wang W, Liu Y, Guo J, He H, Mi X, Chen C,
Xie J, Wang S, Wu P, Cao F, et al: miR-100 maintains phenotype of
tumor-associated macrophages by targeting mTOR to promote tumor
metastasis via Stat5a/IL-1ra pathway in mouse breast cancer.
Oncogenesis. 7:972018. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
248:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Pattingre S, Espert L, Biard-Piechaczyk M
and Codogno P: Regulation of macroautophagy by mTOR and Beclin 1
complexes. Biochimie. 90:313–323. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cai J, Zhang Y, Huang S, Yan M, Li J, Jin
T and Bao S: MiR-100-5p, miR-199a-3p and miR-199b-5p induce
autophagic death of endometrial carcinoma cell through targeting
mTOR. Int J Clin Exp Pathol. 10:9262–9272. 2017.PubMed/NCBI
|
|
88
|
Deng X, Su R, Weng H, Li Z and Chen J: RNA
N(6)-methyladenosine modification in cancers: Current status and
perspectives. Cell Res. 28:507–517. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yang Y, Hsu PJ, Chen YS and Yang YG:
Dynamic transcriptomic m(6)A decoration: Writers, erasers, readers
and functions in RNA metabolism. Cell Res. 28:616–624. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zaccara S, Ries RJ and Jaffrey SR:
Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell
Biol. 20:608–624. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL,
Lv JW, Huang XD, Liu RQ, Chen F, He XJ, et al: Long noncoding RNA
FAM225A promotes nasopharyngeal carcinoma tumorigenesis and
metastasis by acting as ceRNA to sponge miR-590-3p/miR-1275 and
upregulate ITGB3. Cancer Res. 79:4612–4626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Katheder NS, Khezri R, O'farrell F,
Schultz SW, Jain A, Rahman MM, Schink KO, Theodossiou TA, Johansen
T, Juhász G, et al: Microenvironmental autophagy promotes tumour
growth. Nature. 541:417–420. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Cirillo LA, Lin FR, Cuesta I, Friedman D,
Jarnik M and Zaret KS: Opening of compacted chromatin by early
developmental transcription factors HNF3 (FoxA) and GATA-4. Mol
Cell. 9:279–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ye Y, Li SL and Wang JJ: miR-100-5p
downregulates mTOR to suppress the proliferation, migration, and
invasion of prostate cancer cells. Front Oncol. 10:5789482020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Liu X, Zhong L, Li P and Zhao P:
MicroRNA-100 enhances autophagy and suppresses migration and
invasion of renal cell carcinoma cells via disruption of
NOX4-dependent mTOR pathway. Clin Transl Sci. 15:567–575. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Raji S, Sahranavard M, Mottaghi M and
Sahebkar A: MiR-212 value in prognosis and diagnosis of cancer and
its association with patient characteristics: A systematic review
and meta-analysis. Cancer Cell Int. 22:1632022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Qian Y, Shi L and Luo Z: Long non-coding
RNAs in cancer: Implications for diagnosis, prognosis, and therapy.
Front Med (Lausanne). 7:6123932020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Cheng G: Circulating miRNAs: Roles in
cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev.
81:75–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wnuk J, Strzelczyk JK and Gisterek I:
Clinical value of circulating miRNA in diagnosis, prognosis,
screening and monitoring therapy of pancreatic ductal
adenocarcinoma-a review of the literature. Int J Mol Sci.
24:51132023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Damodaran M, Dandapani MC, Simondurairaj,
SandhyaSundaram, VenkatRamanan S, Ramachandran I and Venkatesan V:
Differentially expressed miR-20, miR-21, miR-100, miR-125a and
miR-146a as a potential biomarker for prostate cancer. Mol Biol
Rep. 48:3349–3356. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang S, Li L, Yang M, Wang X, Zhang H, Wu
N, Jia K, Wang J, Li M, Wei L and Liu J: Identification of three
circulating MicroRNAs in plasma as clinical biomarkers for breast
cancer detection. J Clin Med. 12:3222022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ludwig N, Nourkami-Tutdibi N, Backes C,
Lenhof HP, Graf N, Keller A and Meese E: Circulating serum miRNAs
as potential biomarkers for nephroblastoma. Pediatr Blood Cancer.
62:1360–1367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Motawi TK, Rizk SM, Ibrahim TM and Ibrahim
IA: Circulating microRNAs, miR-92a, miR-100 and miR-143, as
non-invasive biomarkers for bladder cancer diagnosis. Cell Biochem
Funct. 34:142–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yamanaka Z, Sasaki T, Yamanaka A, Kato K
and Nishi H: Circulating and tissue miR-100 acts as a potential
diagnostic biomarker for cervical cancer. Cancer Biomark.
32:551–558. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hassan NM, Refaat LA, Ismail GN,
Abdellateif M, Fadel SA and AbdelAziz RS: Diagnostic, prognostic
and predictive values of miR-100 and miR-210 in pediatric acute
lymphoblastic leukemia. Hematology. 25:405–413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Liao Z, Zhang Q, Yang L, Li H, Mo W, Song
Z, Huang X, Wen S, Cheng X and He M: Increased hsa-miR-100-5p
expression improves hepatocellular carcinoma prognosis in the asian
population with PLK1 variant rs27770A>G. Cancers (Basel).
16:1292023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
He QL, Qin SY, Tao L, Ning HJ and Jiang
HX: Prognostic value and prospective molecular mechanism of
miR-100-5p in hepatocellular carcinoma: A comprehensive study based
on 1,258 samples. Oncol Lett. 18:6126–6142. 2019.PubMed/NCBI
|
|
108
|
Song SK, Jung WY, Park SK, Chung CW and
Park Y: Significantly different expression levels of microRNAs
associated with vascular invasion in hepatocellular carcinoma and
their prognostic significance after surgical resection. PLoS One.
14:e02168472019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fuso P, Di Salvatore M, Santonocito C,
Guarino D, Autilio C, Mulè A, Arciuolo D, Rinninella A, Mignone F,
Ramundo M, et al: Let-7a-5p, miR-100-5p, miR-101-3p, and
miR-199a-3p hyperexpression as potential predictive biomarkers in
early breast cancer patients. J Pers Med. 11:8162021. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zhang HC and Tang KF: Clinical value of
integrated-signature miRNAs in esophageal cancer. Cancer Med.
6:1893–1903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang J, Tao Y and Bian Q: miRNA and mRNA
expression profiling reveals potential biomarkers for metastatic
cutaneous melanoma. Expert Rev Anticancer Ther. 21:557–567. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Jakob M, Mattes LM, Küffer S, Unger K,
Hess J, Bertlich M, Haubner F, Ihler F, Canis M, Weiss BG and Kitz
J: MicroRNA expression patterns in oral squamous cell carcinoma:
Hsa-mir-99b-3p and hsa-mir-100-5p as novel prognostic markers for
oral cancer. Head Neck. 41:3499–3515. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Chen Z, Wu L, Lin Q, Shi J, Lin X and Shi
L: Evaluation of miR-182/miR-100 ratio for diagnosis and survival
prediction in bladder cancer. Arch Iran Med. 19:645–651.
2016.PubMed/NCBI
|
|
114
|
Azizmohammadi S, Azizmohammadi S, Safari
A, Kosari N, Kaghazian M, Yahaghi E and Seifoleslami M: The role
and expression of miR-100 and miR-203 profile as prognostic markers
in epithelial ovarian cancer. Am J Transl Res. 8:2403–2410.
2016.PubMed/NCBI
|
|
115
|
Zhang H, Wang J, Wang Z, Ruan C, Wang L
and Guo H: Serum miR-100 is a potential biomarker for detection and
outcome prediction of glioblastoma patients. Cancer Biomark.
24:43–49. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang G, Yang L, Hu M, Hu R, Wang Y, Chen
B, Jiang X and Cui R: Comprehensive analysis of the prognostic
significance of Hsa-miR-100-5p and its related gene signature in
stomach adenocarcinoma. Front Cell Dev Biol. 9:7362742021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hu XY, Song Z, Yang ZW, Li JJ, Liu J and
Wang HS: Cancer drug resistance related microRNAs: recent advances
in detection methods. Analyst. 147:2615–2632. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Lai Y, Kacal M, Kanony M, Stukan I, Jatta
K, Kis L, Norberg E, Vakifahmetoglu-Norberg H, Lewensohn R,
Hydbring P and Ekman S: miR-100-5p confers resistance to ALK
tyrosine kinase inhibitors Crizotinib and Lorlatinib in EML4-ALK
positive NSCLC. Biochem Biophys Res Commun. 511:260–265. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Qin X, Yu S, Zhou L, Shi M, Hu Y, Xu X,
Shen B, Liu S, Yan D and Feng J: Cisplatin-resistant lung cancer
cell-derived exosomes increase cisplatin resistance of recipient
cells in exosomal miR-100-5p-dependent manner. Int J Nanomedicine.
12:3721–3733. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Nabavi N, Saidy NRN, Venalainen E, Haegert
A, Parolia A, Xue H, Wang Y, Wu R, Dong X, Collins C, et al:
miR-100-5p inhibition induces apoptosis in dormant prostate cancer
cells and prevents the emergence of castration-resistant prostate
cancer. Sci Rep. 7:40792017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Samli H, Samli M, Vatansever B, Ardicli S,
Aztopal N, Dincel D, Sahin A and Balci F: Paclitaxel resistance and
the role of miRNAs in prostate cancer cell lines. World J Urol.
37:1117–1126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Nishi H, Ono M, Ohno S, Yamanaka Z, Sasaki
T, Ohyashiki K, Ohyashiki JH and Kuroda M: Hypoxia-induced
paclitaxel resistance in cervical cancer modulated by miR-100
targeting of USP15. Gynecol Oncol Rep. 45:1011382023. View Article : Google Scholar : PubMed/NCBI
|
















