Introduction
Drug repositioning involves identifying new
therapeutic applications for existing drugs beyond their original
indications by uncovering novel effects or targets. This approach
offers considerable advantages over traditional drug development,
including shorter development timelines and lower costs, as the
safety profile of the drug is already known (1). A well-known example is aspirin,
initially developed as an analgesic, which has since been
repurposed for its anti-platelet properties, reducing the risk of
cardiovascular thrombosis, and preventing colon cancer (2). Likewise, the demand for drug
repurposing in oncology has surged in recent decades (3).
STAT3 transcriptionally regulates a wide range of
genes that drive key cancer hallmarks, including sustained
proliferation and resistance to apoptosis and chemotherapy, such as
survivin and myeloid cell leukemia-1 (Mcl-1) (4,5).
Increased nuclear localization of phosphorylated (p)-STAT3 has been
shown to play a critical role in tumor initiation and development,
including breast and prostate cancer (6,7).
Hyperactivation of STAT3 is frequently observed in advanced
clinical stages of oral squamous cell carcinoma (OSCC) and is
associated with poor overall survival (8,9). Given
this evidence, STAT3 represents a promising target for cancer
therapy. However, despite efforts to develop new drugs targeting
STAT3 over the past decades, important advancements have been
elusive due to various challenges, including inadequate drug
delivery and unwanted side effects (5). Therefore, developing novel
STAT3-targeted therapies with established safety and efficacy
profiles remains a promising strategy for cancer treatment.
BBI608, an orphan drug approved by the Food and Drug
Administration for treating patients with advanced gastric cancer,
is a first-in-class cancer stemness inhibitor that targets STAT3
signaling (10,11). BBI608 has demonstrated tolerability
and potential efficacy, such as cancer stemness inhibition and
chemosensitization, in patients with advanced solid tumors, as a
monotherapy and in combination with conventional chemotherapies in
early-phase clinical trials (12,13).
In line with clinical trials results, BBI608 exhibited strong
antitumor activity and markedly improved survival outcomes in
various preclinical tumor models, including nude mouse tumor
xenograft models and orthotopic tumor animal models (14). However, the anticancer effects and
underlying mechanisms of BBI608 in human OSCC remain
unexplored.
The present study, for the first time to the best of
our knowledge, aimed to investigate the anticancer effects of
BBI608 in both conventional two-dimensional (2D) and spheroid OSCC
cultures, and to discover the regulatory mechanisms through which
BBI608 influences its downstream target genes, survivin and Mcl-1,
examining the potential of BBI608 as a therapeutic option for
patients with OSCC exhibiting high STAT3 expression.
Materials and methods
Cell culture and pharmacological
chemicals
The iHOK cell line (accession no. CVCL_C191) was
gifted by Kyung Hee University (Seoul, South Korea). The HSC-2
(accession no. CVCL_1287), HSC-3 (accession no. CVCL_1288) and
HSC-4 (accession no. CVCL_1289) cell lines were kindly provided by
Hokkaido University (Sapporo, Japan). The HN22 cell line (accession
no. CVCL_5522) was provided by Dankook University (Cheonan, South
Korea). For 2D culture, OSCC cell lines were cultured in DMEM/F-12
medium (Welgene, Inc.) and the iHOK cell line was cultured in KBM™
Gold Keratinocyte Growth Basal Medium (Lonza Group, Ltd),
supplemented with 10% fetal bovine serum (Welgene, Inc.) and 1%
penicillin/streptomycin (Welgene, Inc.), in a humidified atmosphere
at 37°C with 5% CO2. For 3D culture, OSCC spheroids were
generated using a hanging drop assay (15). Briefly, 5,000 cells in 20 µl
complete medium containing 0.24% methylcellulose (cat. no. M0387;
Sigma-Aldrich; Merck KGaA) were plated on the lid of 100-mm dishes,
which were then inverted over dishes containing 6 ml of PBS. After
a 3-day incubation at 37°C, spheroids were transferred to
poly(2-hydroxyethyl methacrylate) (HEMA)-coated 96-well or 6-well
plates for further experiments. To prepare poly-HEMA-coated plates,
each well of uncoated 96-well or 6-well plates was filled in with
0.5% poly-HEMA in 95% ethanol and then incubated for 2 days at
37°C. BBI608 was purchased from MedChemExpress (cat. no. HY-13919),
dissolved in DMSO, and stored at −20°C. Z-VAD-FMK was obtained from
R&D Systems, Inc., while cycloheximide (CHX) and MG132 were
sourced from Sigma-Aldrich (Merck KGaA) and Santa Cruz
Biotechnology, Inc., respectively. HSC-3 and HSC-4 cells were
pretreated with 10 and 5 µM Z-VAD-FMK, 50 and 100 ng/ml CHX and 300
and 500 nM MG132 for 1 h at 37°C. DMSO was used as control and its
concentration did not exceed 0.1%.
Western blot analysis
Cells (iHOK, HSC-2, HSC-3, HSC-4 and HN22) were
lysed using RIPA lysis buffer (MilliporeSigma) supplemented with
protease inhibitors (Roche Applied Science) and phosphatase
inhibitors (Thermo Fisher Scientific, Inc.). For 3D culture,
spheroids were sonicated on ice for 5 cycles (frequency, 20 kHz;5
sec of sonication and rest) with a Vibra-Cell VCX500 sonicator
(Sonics & Materials, Inc.). Total protein concentrations were
measured with the DC Protein Assay kit (Bio-Rad Laboratories,
Inc.), followed by separation on 10–15% SDS-PAGE gels depending on
protein size and transfer to PVDF membranes. A total of 30–50 µg
protein was loaded per lane. After blocking with 5% skimmed milk
(cat. no. 232100; Becton, Dickinson and Company) for 2 h at room
temperature (RT), the membranes were incubated overnight at 4°C
with the specified primary antibodies. The membranes were then
treated with HRP-conjugated goat anti-rabbit IgG (1:2,000; cat. no.
GTX213110-01; GeneTex, Inc.) and goat anti-mouse IgG (1:2,000, cat.
no. GTX213111-01, GeneTex, Inc.) for 2 h at RT. Immunoreactive
signals were visualized using the WestGlow™ PICO PLUS ECL
Chemiluminescent Substrate (BIOMAX) on X-ray film. Densitometric
analyses were performed with ImageJ software version 1.54f
(National Institutes of Health). The primary antibodies used were
as follows: STAT3 (1:5,000; cat. no. 4904),
p-STAT3Tyr705 (1:2,000; cat. no. 9145),
p-STAT3Ser727 (1:2,000; cat. no. 9134), poly(ADP-ribose)
polymerase (PARP; 1:2,000; cat. no. 9542), cleaved (c)-PARP
(1:2,000; cat. no. 9541), caspase 3 (1:3,000; cat. no. 14220),
c-caspase 3 (1:1,000; cat. no. 9664), survivin (1:1,000; cat. no.
2802), Mcl-1 (1:2,000; cat. no. 5453), Bcl-xL (1:5,000; cat. no.
2764), p-Mcl-1Ser159/Thr163 (1:1,000; cat. no. 4579),
p-ERK1/2Thr202/Tyr204 (1:5,000; cat. no. 9101) and
ERK1/2 (1:5,000; cat. no. 9102) were purchased from Cell Signaling
Technology, Inc. Bcl-2 (1:1,000; cat. no. sc-7382), β-actin
(1:5,000; cat. no. sc-47778), α-tubulin (1:5,000; cat. no.
sc-5286), p-GSK3βSer9 (1:1,000; cat. no. sc-373800) and
GSK3β (1:1,000; cat. no. sc-377213) were purchased from Santa Cruz
Biotechnology, Inc. Histone H3 (1:5,000; cat. no. ab1791; Abcam)
was kindly provided by Kangwon National University (Chuncheon,
South Korea).
Cell Counting Kit-8 (CCK-8) assay
Cell viability was assessed using the CCK-8 assay
(Dojindo Laboratories, Inc.) following the manufacturer's
instructions. For 2D culture, cells (5×104 cells/well)
were seeded into 96-well plates overnight and then treated with the
specified concentrations (0.1–10 µM) of BBI608 for 22 h at 37°C.
Subsequently, a 10-µl CCK-8 solution was added to each well and
incubated at 37°C with 5% CO2 for 2 h. For 3D culture,
spheroids (5×105 cells/well) were transferred to
poly-HEMA-coated U-bottom 96-well plates (16) and treated with the indicated
concentrations (2.5 µM for HSC-3 and 20 µM for HSC-4) of BBI608 for
6 h, followed by CCK-8 treatment for an additional 18 h. The
mixture of CCK-8 and media was then transferred to a new 96-well
plate, and absorbance at 450 nm was measured using a microplate
reader (Hidex Oy).
Crystal violet staining
Crystal violet staining was used to visually assess
the number of viable OSCC cells (HSC-3 and HSC-4) following BBI608
treatment. Briefly, cells (2.5×105 cells/well) were
plated into 6-well plates overnight and then treated with various
concentrations (0.125–10 µM) of BBI608 at 37°C. After 24 h, the
media containing BBI608 was removed, and the cells were washed
twice with PBS. Next, the cells were fixed with 100% methanol for 2
min and stained with a 1% crystal violet solution diluted in 20%
methanol for 30 min at RT. The stained cells were then photographed
using an inverted light microscope (Nikon Corporation).
Trypan blue exclusion assay
OSCC cell lines (HSC-3 and HSC-4) seeded onto 6-well
plate (2.5×105 cells/well) were treated with DMSO (0.1%)
or BBI608 (0.2–3.2 µM) for 24 h at 37°C. Trypan blue solution in a
concentration of 0.4% (Corning, Inc.) was used to stain the cells
for 3 min at a 1:1 v/v ratio at RT. Subsequently, cell viability
was examined using a CytoSMART automatic cell counter (Corning,
Inc.).
Colony formation assay
A total of 3 ml 1.25% agar solution was poured into
6-well plates and allowed to solidify at RT for 1–2 h. Cells
(2.4×104 cells/well) were suspended in 10% Basal Medium
Eagle (Sigma-Aldrich; Merck KGaA) and mixed with agar before 1 ml
of this cell mixture was layered on top of the solidified agar in
the plates. After the top layer solidified at RT for 1–2 h, the
plates were incubated in a humidified incubator at 37°C with 5%
CO2 for ~2 weeks. Specific concentrations of BBI608 (0.4
µM for HSC-3 and 3.2 µM for HSC-4) were added to both the bottom
and top agar layers before agar solidified. The colonies
(>3-pixel size; cells were not fixed or stained) that formed
were visualized using a CKX53 microscope (Olympus Corporation). To
prevent potential bias, each well was divided into four quadrants,
and non-overlapping images were captured separately from each
quadrant. These images were merged and quantified with ImageJ
software version 1.54f.
DAPI staining
Following a 24-h treatment with specific
concentrations (0.4 µM for HSC-3 and 3.2 µM for HSC-4) of BBI608 in
OSCC cell lines seeded onto 6-well plates (2.5×105
cells/well) at 37°C, adherent and detached cells were collected and
fixed in 70% ethanol at −20°C overnight. The fixed cells were then
resuspended in 100% methanol at RT for 10 min. The cells were
transferred onto glass slides and stained with a 2 µg/ml DAPI
solution at RT for 1–5 min. Images of the stained cells were
captured using a fluorescence microscope (Leica DMi8; Leica
Microsystems GmbH).
Measurement of cell cycle
distribution
HSC-3 and HSC-4 cells were seeded onto a 6-well
plate (2.5×105 cells/well) and fixed overnight at −20°C
with 70% ethanol. After removing the supernatant, the cells were
stained with a PI solution containing 20 µg/ml RNase A for 15 min
at 37°C. Cell cycle distribution was assessed using 10,000
cells/sample with an LSRFortessa X-20 flow cytometer (BD
Biosciences) and the sub-G1, G1, S, and
G2/M populations were analyzed using FlowJo software
(FlowJo LLC; BD Biosciences). The gating strategy of cell cycle
distribution is presented in Fig.
S1.
Annexin V-FITC/PI double staining
Apoptotic cells were examined using the FITC-Annexin
V Apoptosis Detection Kit (cat. no. 556547; BD Biosciences). The
cells collected from a 60 mm2 dish (seeding
concentration, 2.5×105 cells/ml) were resuspended in 400
µl Annexin V binding buffer, which included 5 µl FITC-conjugated
Annexin V and 1 µl PI solution, at RT in the dark. The stained
cells were analyzed with the LSRFortessa X-20 flow cytometer, and
the percentage of the apoptotic cell population was calculated
using FlowJo software.
Cross-linking assay
The cross-linking assay was conducted as previously
described (17). Briefly, cells
(HSC-3 and HSC-4) were suspended in a conjugation buffer containing
10 mM EDTA (dissolved in PBS). The cell lysates were then incubated
with 0.2 mM bismaleimidohexane (cat. no. 22330; Thermo Fisher
Scientific, Inc.) at RT for 1 h, followed by extraction with lysis
buffer for western blotting, as aforementioned.
Subcellular fractionation of the
nucleus and cytoplasm
Nuclear and cytoplasmic proteins were extracted
using the NE-PER™ Nuclear and Cytoplasmic Extraction Reagents kit
(cat. no. 78833; Thermo Fisher Scientific, Inc.). Briefly,
harvested cells (HSC-3 and HSC-4) were vortexed with Cytoplasmic
Extraction Reagent I and incubated on ice for 10 min. The cells
were then resuspended in Cytoplasmic Extraction Reagent II for 1
min and centrifuged at 13,000 g for 5 min at 4°C. The supernatant,
containing cytoplasmic proteins, was transferred to a pre-chilled
Eppendorf tube (the cytoplasmic fraction). The pellets were
resuspended in Nuclear Extraction Reagent and incubated for 40 min
at 4°C, with vortexing every 10 min and thorough sonication on ice
for 5 cycles (frequency, 20 kHz; 2 sec of sonication and rest). The
solution was then centrifuged at 13,000 g for 10 min at 4°C. The
supernatant containing nuclear proteins (the nuclear fraction) was
used for western blot analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (cat. no. 15596026; Thermo Fisher Scientific, Inc.) and the
quantity of RNA was measured with NanoPhotometer N50 (IMPLEN).
Reverse transcription of 1 µg total RNA was performed using the
AMPIGENE cDNA Synthesis Kit (Enzo Life Sciences, Inc.). The
resulting cDNA was then analyzed by qPCR with the AMPIGENE qPCR
Green Mix Hi-Rox (Enzo Life Sciences, Inc.) using the StepOnePlus™
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR conditions for all genes were as follows: An initial
step at 95°C for 2 min, followed by 40 cycles of 95°C for 10 sec
and 60°C for 30 sec. The relative expression of each gene was
determined using the 2−ΔΔCq method (18) and normalized to GAPDH as an internal
control. All primers were synthesized by Cosmo Genetech Co., Ltd.,
with the following sequences: Mcl-1 (accession no. NG_029069.1)
sense, 5′-GTATCACAGACGTTCTCGTAAGG-3′ and antisense,
5′-CCACCTTCTAGGTCCTCTACAT-3′; survivin (accession no. NG_029146.2)
sense, 5′-ACTTGGCCCAGTGTTTCTT-3′ and antisense,
5′-GACAGAAAGGAAAGCGCAAC-3′; GAPDH (accession no. NG_007073.2)
sense, 5′-GTGGTCTCCTCTGACTTCAAC-3′ and antisense,
5′-CCTGTTGCTGTAGCCAAATTC-3′.
Chromatin immunoprecipitation (ChIP)
assay
A ChIP kit from Abcam (cat. no. ab500) was used
according to the manufacturer's instructions. Briefly, cells (HSC-3
and HSC-4) were harvested and cross-linked with 1.1% formaldehyde
for 10 min at RT, and the reaction was then quenched with 1.25M
glycine for an additional 10 min at RT. Chromatin was sheared using
a Vibra-Cell VCX500 sonicator on ice for 20 cycles, alternating
between 15 sec of sonication and rest (frequency, 20 kHz). In each
of the three independent experiments, DNA fragments ranging from
200 to 500 base pairs were verified by electrophoresis on a 1.5%
agarose gel. The sheared chromatin was incubated overnight at 4°C
with either a rabbit isotype IgG antibody (0.24 µg, cat. no. 3900;
Cell Signaling Technology, Inc.) or a rabbit STAT3 antibody (0.24
µg, cat. no. 4904; cell Signaling Technology, Inc.). After
immunoprecipitation, cross-linking was reversed by adding DNA
purification slurry and proteinase K (both contained within the
ChIP kit used). The purified DNA was then amplified via RT-qPCR
using primers targeting the known STAT3 binding site on the
survivin promoter region (19). The
primer sequences were as follows: Survivin sense,
5′-CAGTGAGCTGAGATCATGCC-3′ and antisense,
5′-TATTAGCCCTCCAGCCCCAC-3′.
Live/dead assay
The presence of live and dead cells in the spheroids
was assessed using the LIVE/DEAD™ Viability/Cytotoxicity Kit (cat.
no. L3224; Thermo Fisher Scientific, Inc.), following the
manufacturer's instructions. Briefly, spheroids (5×105
cells/well) were transferred into poly-HEMA-coated 96-well plates
and treated with the specified concentration (2.5 µM for HSC-3 and
20 µM for HSC-4) of BBI608 for 24 h at 37°C. Calcein AM (4 mM) and
ethidium homodimer-1 (2 mM) (Themo Fisher Scientific, Inc.) were
added to each well in PBS at final concentrations of 4 and 2 µM,
respectively, and the spheroids were incubated for 1 h at RT. The
spheroids were then visualized using a digital inverted
fluorescence microscope (Nikon Corporation).
Statistical analysis
All graphs were generated using GraphPad Prism
version 8.4.2 (GraphPad; Dotmatics), and statistical analyses were
performed using SPSS version 25.0 (IBM Corp.). Data are presented
as the mean ± SD from at least three independent experiments. A
two-tailed unpaired Student's t-test was used for comparisons
between two groups, while one-way ANOVA followed by Tukey's post
hoc test was applied for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
BBI608 inhibits growth and induces
apoptosis in OSCC cell lines
To select OSCC cell lines with high STAT3 activity
for subsequent experiments, the protein levels of p-STAT3 at the
tyrosine 705 residue were screened across a panel consisting of one
immortalized human oral keratinocyte and four OSCC cell lines.
Among them, HSC-3 and HSC-4 cells showed the highest levels of
p-STAT3 (Fig. 1A). To evaluate the
cytotoxic effects of BBI608 on OSCC cell lines, a CCK-8 assay and
crystal violet staining were performed. The results indicated that
BBI608 inhibited the growth of both OSCC cell lines and normal oral
keratinocytes in a concentration-dependent manner (Fig. S2). The IC50 values for
iHOK, HSC-3, HSC-4 and HN22 were 3.56, 0.57, 2.16 and 3.58 µM,
respectively. The optimal concentrations of BBI608 (0.6 µM for
HSC-3 and 3.2 µM for HSC-4) that inhibit STAT3 activation and
simultaneously inhibit cell growth and induce the expression of
c-PARP were examined using trypan blue exclusion assay (Fig. S3A) and western blot analysis
(Fig. S3B). It was also observed
that BBI608 treatment significantly reduced colony forming
efficiency in HSC-3 and HSC-4 cell lines (Fig. 1B). To determine whether the
cytotoxicity of BBI608 was mediated by apoptosis, which is
characterized by nuclear condensation and DNA fragmentation, DAPI
staining and cell cycle analysis were performed (20). The results revealed that BBI608
significantly increased the presence of condensed nuclei and the
sub-G1 population compared with those in DMSO-treated
cells (Figs. 1C and D and S5A). Additionally, an increase in Annexin
V-positive cells and enhanced cleavage of PARP and caspase 3
following BBI608 treatment was observed in HSC-3 and HSC-4 cells
(Fig. 1E and F). To investigate
whether BBI608-mediated apoptosis was dependent on caspase
activation, OSCC cell lines were treated with Z-VAD-FMK, a
pan-caspase inhibitor, at the concentrations used in our previous
studies (21–23). Based on preliminary results, the
minimum concentrations (10 µM for HSC-3 and 5 µM for HSC-4) of
Z-VAD-FMK required to hinder PARP cleavage were selected (Fig. S4), and it was observed that
BBI608-mediated apoptosis was significantly rescued by Z-VAD-FMK
(Figs. 1G and S5B). Collectively, these findings
demonstrated that BBI608 exhibited strong growth-inhibitory effects
and induced apoptosis in OSCC cell lines.
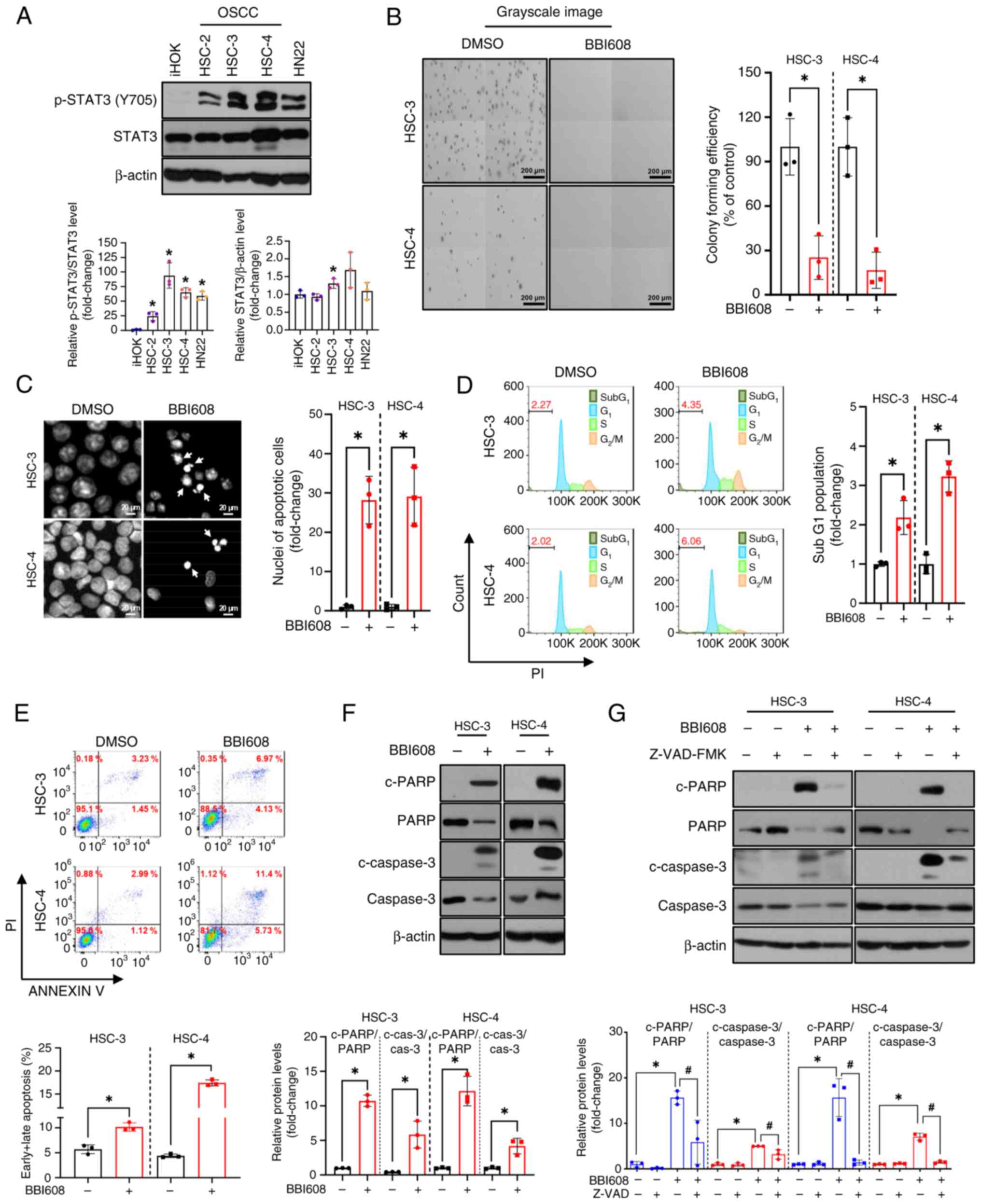 | Figure 1.Growth inhibitory activity and
apoptotic effects of BBI608 in OSCC cell lines. (A) Protein levels
of p-STAT3 in OSCC cell lines were analyzed by western blotting.
The expression levels of total STAT3 were normalized to those of
β-actin, while p-STAT3(Y705) levels were normalized to those of
total STAT3. (B) HSC-3 and HSC-4 cells were treated with BBI608 at
0.4 and 3.2 µM, respectively, for ~2 weeks. Colony formation assays
were performed to assess non-adherent, anchorage-independent
growth. (C) HSC-3 and HSC-4 cells were treated with BBI608 at 0.4
and 3.2 µM for 24 h, respectively. Representative images of DAPI
staining are shown, with condensed nuclei indicated by white
arrows. (D) Cell cycle evaluation of OSCC cell lines was performed
using flow cytometry analysis with PI staining. The horizontal axis
represents PI staining intensity, and the vertical axis represents
cell counts. (E) Apoptotic cell death was evaluated by Annexin V/PI
staining. The horizontal axis shows Annexin V intensity, and the
vertical axis shows PI intensity. (F) HSC-3 and HSC-4 cells were
treated with BBI608 at 0.6 and 3.2 µM for 24 h, respectively. The
expression levels of c-PARP and c-caspase 3, total PARP, total
caspase 3 and β-actin (loading control) were analyzed. The
expression levels of c-PARP and c-caspase 3 levels were normalized
to those of total PARP and caspase 3, respectively. (G) HSC-3 and
HSC-4 cells were pre-treated with Z-VAD-FMK at 10 and 5 µM for 1 h,
followed by treatment with BBI608 at 0.6 and 3.2 µM for 24 h,
respectively. All experiments were conducted in triplicate and the
results are presented as the mean ± SD. *P<0.05 vs. iHOK cells
(panel A) or as indicated (panels B-G); #P<0.05.
OSCC, oral squamous cell carcinoma; p, phosphorylated; c, cleaved;
PARP, poly(ADP-ribose) polymerase; cas, caspase. |
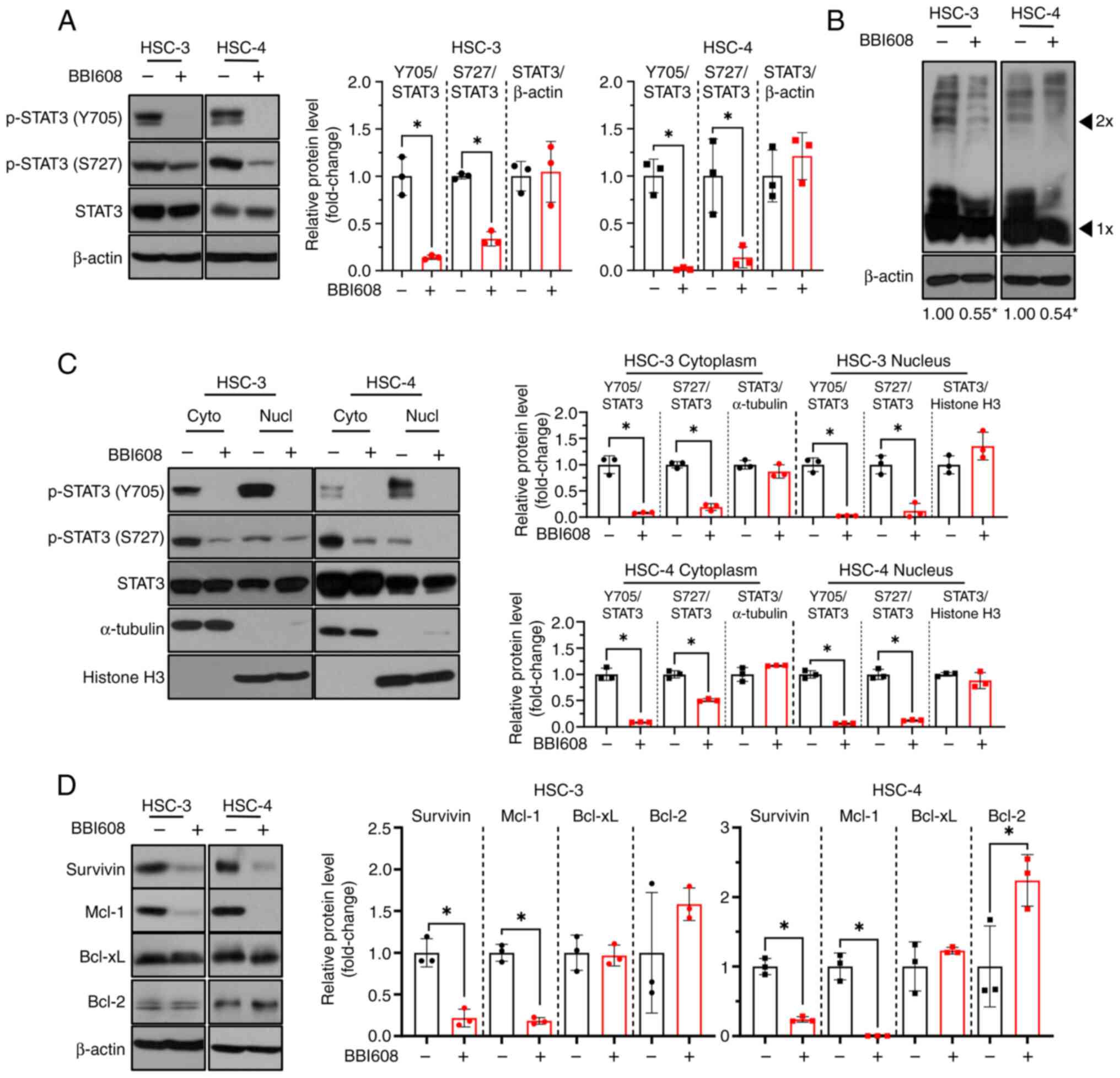
BBI608 inhibits STAT3 activity and the
expression of STAT3 target genes in OSCC cell lines
To determine whether BBI608 could inhibit STAT3
translocation into the nucleus in OSCC cell lines, western blot
analysis after BBI608 treatment was performed. The results showed
that BBI608 effectively suppressed STAT3 activation at both
tyrosine 705 and serine 727 residues (Fig. 2A). Additionally, BBI608 effectively
inhibited STAT3 dimerization compared with that in DMSO-treated
cells (Fig. 2B), indicating that
BBI608 may inhibit p-STAT3 nuclear translocation (24). Interestingly, BBI608 significantly
reduced nuclear p-STAT3 levels at both tyrosine 705 and serine 727
residues (Fig. 2C). Next, it was
investigated whether BBI608 could inhibit the expression of
STAT3-regulated genes that are related to apoptosis (25,26).
The results demonstrated that BBI608 significantly downregulated
survivin and Mcl-1 expression, while Bcl-2 expression was increased
only in HSC-4 cells and Bcl-xL was not changed in either cell line
(Fig. 2D). Furthermore, BBI608
reduced the level of p-STAT3 and those of its downstream target
genes, accompanied by an increase in c-PARP expression, in a
time-dependent manner (Fig. S6).
Overall, these findings indicated that BBI608 effectively inhibited
STAT3 activation and induced apoptosis in OSCC cell lines, at least
in part by inhibiting the STAT3 signaling pathway.
BBI608 regulates the expression of
survivin at the transcriptional level
To evaluate how BBI608 regulates survivin and Mcl-1
in OSCC cell lines, their mRNA levels were measured over time after
treatment with BBI608 in HSC-3 and HSC-4 cells. Interestingly, the
results showed that BBI608 induced a significant decrease in
survivin mRNA levels at all time points, while significantly
increasing Mcl-1 mRNA levels in both cell lines (Fig. 3A). To further investigate whether
the reduction in survivin expression was transcriptionally
regulated by BBI608 treatment in response to decreased STAT3
activation, a ChIP assay was performed. The results revealed that
BBI608 significantly impaired STAT3 binding to the survivin
promoter region in HSC-3 and HSC-4 cells, which was larger than the
binding impairment in the IgG control group (Fig. 3B). These findings suggested that
BBI608 may regulate survivin expression through a transcriptional
mechanism in OSCC cell lines.
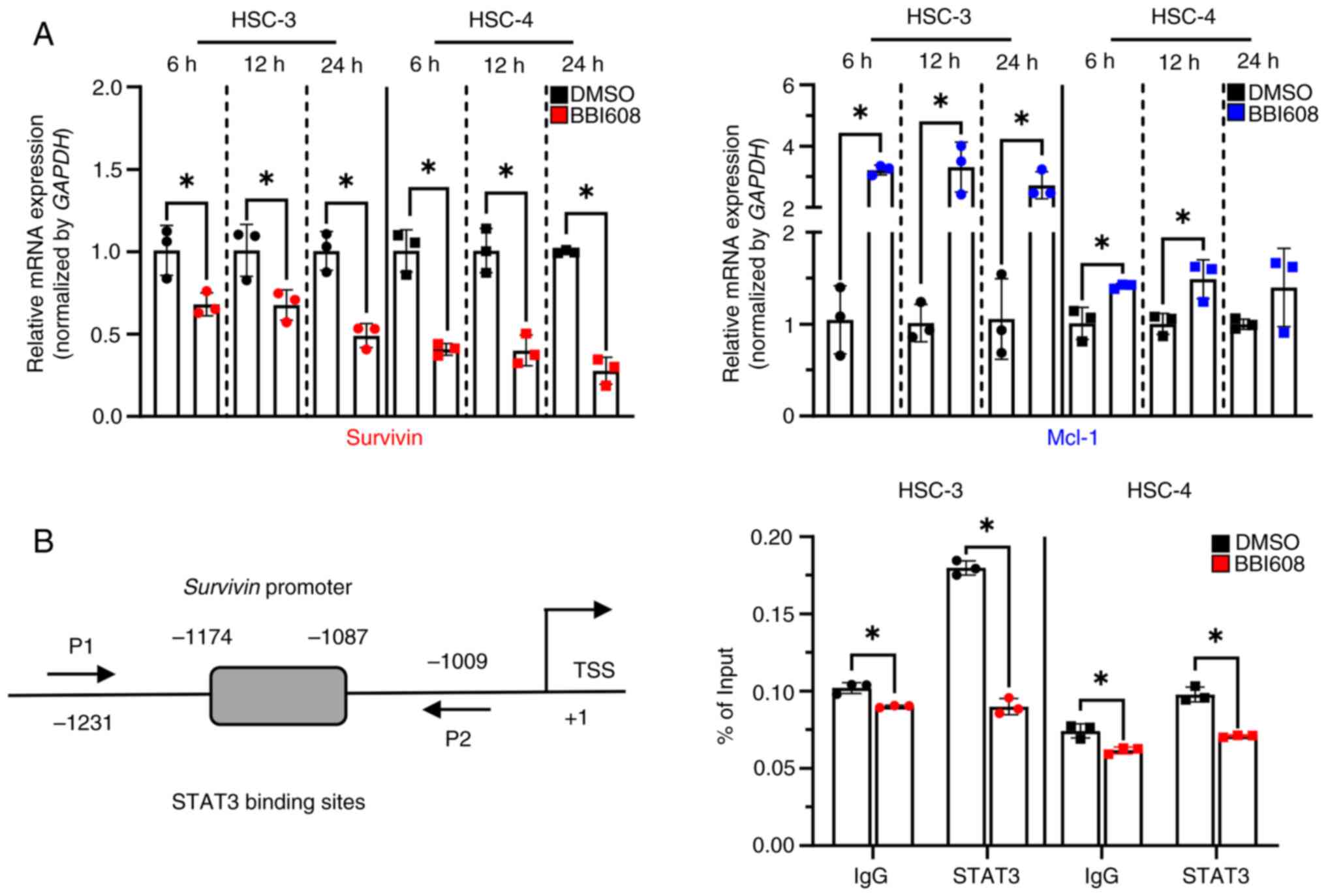 | Figure 3.Effect of BBI608 on survivin
expression in oral squamous cell carcinoma cell lines. To
investigate changes in mRNA levels of survivin and Mcl-1 in
response to BBI608, HSC-3, and HSC-4 cells were treated with BBI608
at concentrations of 0.6 and 3.2 µM for 6 to 24 h. (A) RT-qPCR
analysis showed a decrease in survivin mRNA levels and an increase
in Mcl-1 mRNA levels following BBI608 treatment. The data were
normalized to the expression of the housekeeping gene GAPDH. (B)
Schematic diagram of STAT3-binding sites and ChIP primer locations
on the survivin promoter. HSC-3 and HSC-4 cells were treated with
BBI608 at 0.6 and 3.2 µM, respectively, for 24 h. To confirm the
presence of STAT3 at the survivin promoter region, a ChIP assay
coupled with RT-qPCR on the survivin promoter region was performed.
Data are presented as % of input. All experiments were performed in
triplicate, and the results are presented as the mean ± SD.
*P<0.05. TSS, transcription start site; RT-qPCR, reverse
transcription-quantitative PCR; Mcl-1, myeloid cell leukemia-1;
ChIP, chromatin immunoprecipitation; P1, survivin ChIP sense; P2,
survivin ChIP antisense. |
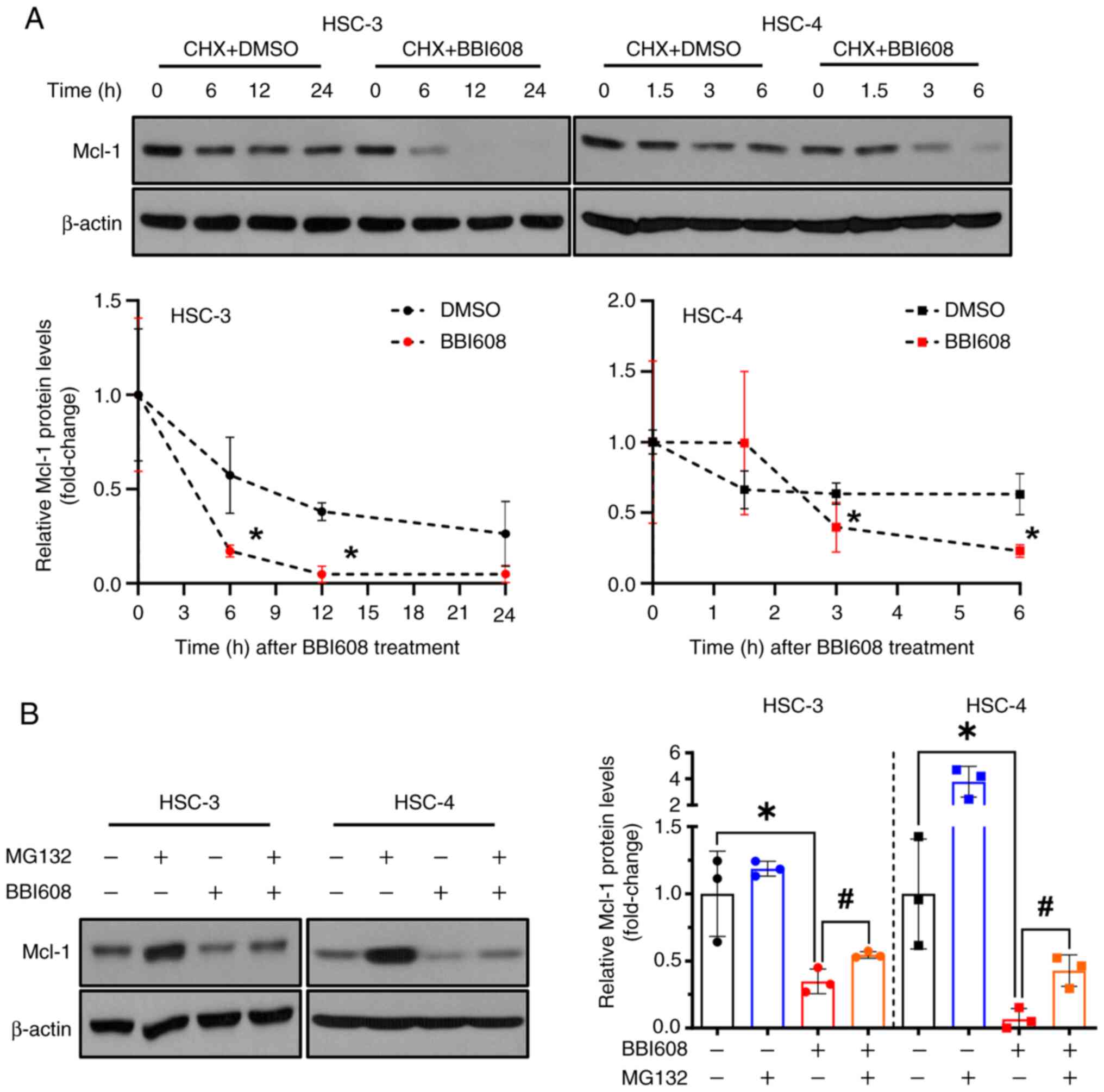
BBI608 modulates the expression of
Mcl-1 at the post-translational level
The stability of the Mcl-1 protein can be influenced
by proteasomal degradation (27).
Although BBI608 increased Mcl-1 mRNA levels, it was further
investigated whether Mcl-1 expression is regulated
post-translationally by BBI608 treatment. To examine this, HSC-3
and HSC-4 cells were co-treated with the protein synthesis
inhibitor CHX at time points (6 h for HSC-3 and 3 h for HSC-4) when
Mcl-1 protein levels begin to decline following BBI608 treatment
(Fig. S6). The results showed that
Mcl-1 protein levels decreased more rapidly with CHX plus BBI608
treatment compared with those observed with CHX plus DMSO treatment
(Fig. 4A). To further confirm
whether BBI608 affects Mcl-1 protein levels at the
post-translational level, cells were co-treated with the
proteasomal inhibitor MG132. The results indicated that combining
MG132 with BBI608 partially reversed the reduction in Mcl-1
expression caused by BBI608 alone (Fig.
4B). To elucidate how BBI608 induces Mcl-1 protein degradation,
the levels of p-Mcl-1, p-GSK3β and p-ERK were evaluated. However,
BBI608 decreased the level of p-Mcl-1, increased the level of
p-GSK3β, and did not alter the level of p-ERK (Fig. S7). These findings suggested that
BBI608 may regulate Mcl-1 expression through a post-translational
mechanism in OSCC cell lines.
BBI608 effectively triggers apoptosis
and impairs STAT3 signaling in OSCC spheroids
Since spheroid cultures closely mimic the hypoxic
and proliferative characteristics of in vivo tumors, they
serve as a valuable model for screening potential drug candidates
(15,28). Therefore, it was investigated
whether BBI608 exhibits anticancer effects in 3D cultures of OSCC
cell lines. To achieve this, a hanging drop assay was used to
establish 3D cultures of OSCC cell lines. Consistent with the
results from 2D cultures, BBI608 demonstrated robust growth
inhibitory activity in both OSCC spheroids (Fig. 5A), which was further confirmed by
live/dead assay showing that BBI608 increased the number of dead
cells (red color) in both cell lines (Fig. 5B). Additionally, BBI608 effectively
induced apoptosis in OSCC spheroids, as evidenced by the increased
cleavage of PARP and caspase 3, as well as the higher number of
apoptotic cells following BBI608 treatment (Fig. 5C and D). Subsequently, western
blotting and RT-qPCR were performed to determine whether and/or how
BBI608 inhibits STAT3 signaling in OSCC spheroids. The results
showed that BBI608 had an inhibitory effect on STAT3 signaling in
OSCC 3D spheroids (Fig. 5E).
Interestingly, BBI608 reduced survivin mRNA levels in both OSCC
spheroids and significantly decreased Mcl-1 mRNA levels only in
HSC-4 spheroids (Fig. 5F). Overall,
these results indicated that BBI608 induced apoptosis and
significantly inhibited STAT3 signaling in OSCC spheroids.
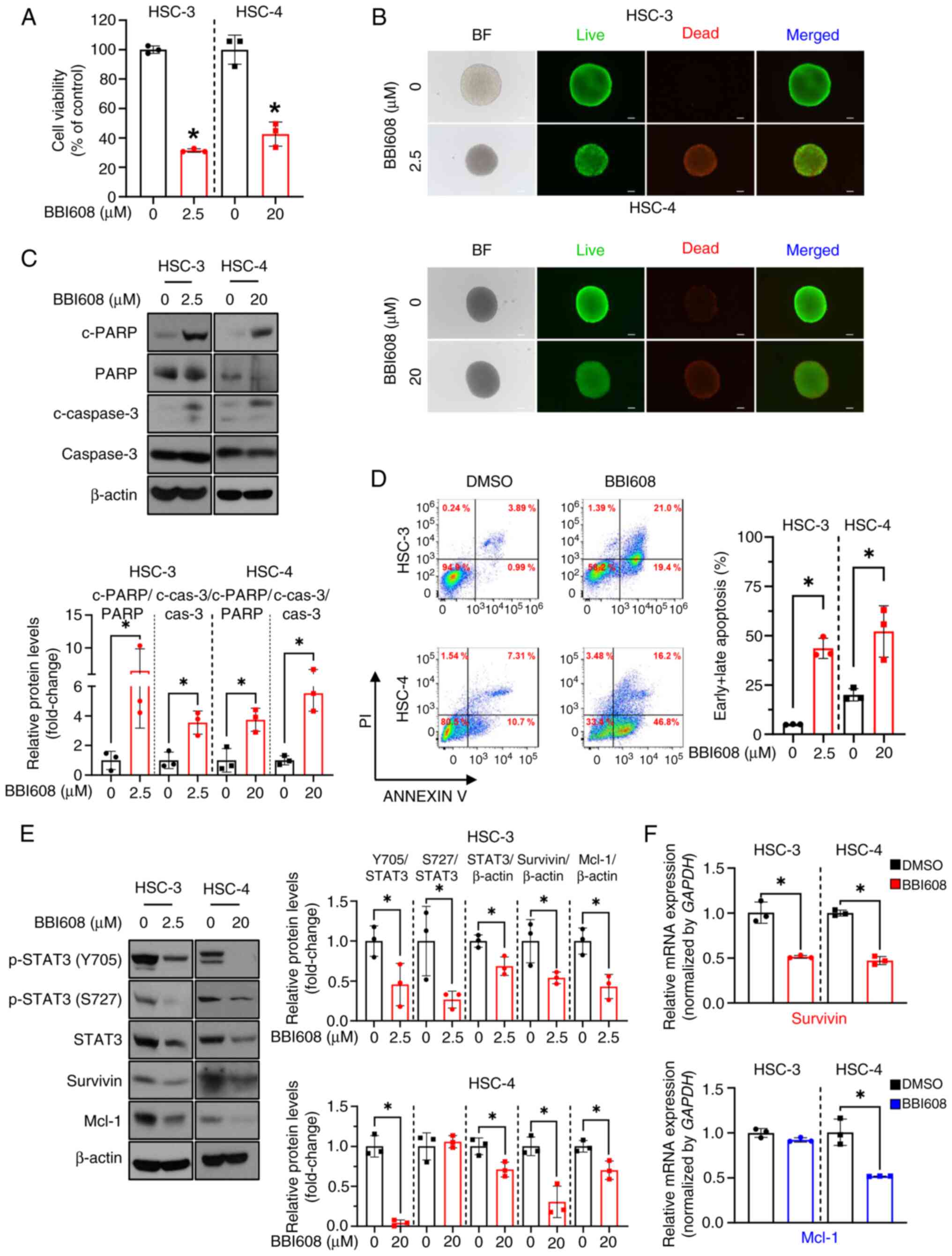 | Figure 5.Inhibitory effects of BBI608 on the
growth of OSCC spheroids and STAT3 signaling. HSC-3 and HSC-4 cells
were treated with BBI608 at concentrations of 2.5 and 20 µM for 24
h, respectively. Cell viability of OSCC spheroids was assessed to
determine the cytotoxic effects of BBI608 using (A) Cell Counting
Kit-8 and (B) live/dead assays (magnification, ×100; scale bar, 100
µm). Apoptotic cell death in OSCC spheroids was evaluated by (C)
the expression of c-PARP and c-caspase 3 and (D) Annexin V/PI
staining. The horizontal axis represents Annexin V staining, while
the vertical axis represents PI staining. (E) Western blot analysis
indicated the expression levels of STAT3-regulated proteins along
with β-actin. Protein expression levels were normalized to β-actin,
except for p-STAT3 levels, which were normalized to those of total
STAT3. (F) The mRNA levels of survivin and Mcl-1 in OSCC spheroids
were analyzed using reverse-transcription-quantitative PCR. The
data were normalized to the expression of the housekeeping gene
GAPDH. All experiments were performed in triplicate, and the
results are presented as the mean ± SD. *P<0.05. OSCC, oral
squamous cell carcinoma; Mcl-1, myeloid cell leukemia-1; p,
phosphorylated; c, cleaved; PARP, poly(ADP-ribose) polymerase; cas,
caspase; BF, bright field. |
Discussion
Aberrant upregulation of STAT3, which is commonly
observed in various cancers, including hepatocellular carcinoma and
non-Hodgkin's lymphoma, is associated with numerous malignant
activities, including tumorigenesis and enhanced cell survival
(24,29). This is consistent with our previous
study showing that p-STAT3 levels are significantly upregulated in
tissues from patients with OSCC compared with normal oral mucosa
(9). Mounting evidence indicates
that STAT3 is essential for early embryonic development, whereas
conditional deletion of the STAT3 gene in adult mouse tissues
results in only minor phenotypes, including defects during the
second hair cycle and thymic hypoplasia (5,30).
This suggests that STAT3 represents an attractive therapeutic
target for cancer therapy. In the present study, BBI608 exerted a
marked inhibitory effect on STAT3 activity in OSCC cell lines,
leading to pronounced apoptotic cell death. Given the potent
anticancer effects of BBI608 observed in multiple studies and its
approval for the treatment of advanced gastric and gastroesophageal
junction adenocarcinoma (10,11,31),
the present results raise the possibility that BBI608 may be
applied to clinical trials in patients with OSCC.
The proteasomal degradation of Mcl-1, which involves
its phosphorylation and subsequent ubiquitination, is regulated by
several signaling pathways, including ERK and GSK3β (27). Especially, GSK3β-mediated
hyper-phosphorylation of Mcl-1 at Ser159, together with Thr163
phosphorylation, promotes its proteasomal degradation (32). However, the present results showed
that BBI608 decreased the level of p-Mcl-1, indicating that the
post-translational modulation of Mcl-1 by BBI608 was not associated
with its phosphorylation. To rule out the involvement of GSK3β and
ERK pathways in Mcl-1 degradation, the levels of these kinases were
also examined, but the result was not consistent with this
hypothesis. Previous research demonstrated that the activation of
MAPK by reactive oxygen species (ROS) influences Mcl-1 stability in
response to BBI608 and increases Noxa protein expression in a
concentration-dependent manner (33). Since Noxa can bind to the
hydrophobic groove of Mcl-1 through its BH3 domain, making Mcl-1
accessible to ubiquitin ligases and leading to its ubiquitination
and proteasomal degradation (32),
it is possible that BBI608 modulates Mcl-1 protein stability, at
least in part, through Noxa-mediated ubiquitination in OSCC cell
lines. However, this needs to be examined in future studies.
Interestingly, it was observed that Mcl-1 mRNA expression increased
following BBI608 treatment. Considering that Mcl-1 stability was
significantly decreased by proteasomal degradation, it may be
hypothesized that complementary feedback mechanisms, intrinsic to
cellular homeostasis, take place against the downregulation of
Mcl-1 protein by BBI608 treatment.
The present study revealed that total STAT3
expression remained unchanged in 2D culture, while a significant
reduction was observed in 3D culture following BBI608 treatment.
Similarly, Mcl-1 mRNA expression decreased in HSC-4 spheroids but
not in 2D culture. These findings contrast those of a previous
study, which reported that BBI608 eliminated total STAT3 expression
in glioma stem cells in both 2D and 3D culture systems (34). The differences between 2D and 3D
cultures, including variations in cell polarity, as well as
cell-cell and cell-extracellular environment interactions, result
in changes in gene expression and signaling pathways (35). It must also be noted that 3D
spheroids exhibit lower proliferation rates and more hypoxic
conditions compared with 2D cultures (36). These factors may lead to
discrepancies in drug responses, as altered cellular properties can
affect the efficacy of treatments that rely on active cell division
or oxygen availability. For example, a previous study observed that
the anti-proliferative effect of 5-fluorouracil was more pronounced
in 2D culture than in 3D culture, whereas the hypoxia-activated
agent tirapazamine was more effective in 3D culture (37). Consistent with this notion, the
present results showed that a higher concentration of BBI608 was
required to achieve cytotoxic effects of BBI608 on OSCC 3D
spheroids compared with that required for the 2D culture.
Furthermore, it has been reported that BBI608 can affect STAT3
expression in osteosarcoma cells by regulating protein synthesis in
2D culture (38). However, given
the notion that BBI608 is a potent ROS inducer (11), the possibility that STAT3 stability
could be regulated by ROS-mediated proteasomal degradation cannot
be ruled out (39). Therefore,
further investigation is necessary to determine if the mechanism by
which BBI608 exerts differential regulation of STAT3 protein in 2D
and 3D culture is context dependent.
In a retrospective study, patients with advanced
colorectal cancer treated with BBI608 in cases of elevated p-STAT3
levels in tumor cells and in the tumor microenvironment (TME)
exhibited notably prolonged overall survival in comparison with the
placebo group (40). Furthermore,
BBI608 sensitivity in tumor cells has been demonstrated to result
in an increase in STAT3 activity in both the tumor cells and TME
(41), suggesting that BBI608 may
be more efficient in OSCC cells with high p-STAT3 levels. However,
in the present study, BBI608 reduced cell viability in OSCC cells
irrespectively of p-STAT3 levels, implying that BBI608-mediated
apoptotic cell death may involve other pathways as well as STAT3.
According to certain studies, BBI608 may also exhibit off-target
effects on other molecules, including AKT/mTOR or MAPK pathways
(33,42). Notably, it has been reported that
BBI608 could be a substrate for NAD(P)H dehydrogenase [quinone] 1,
thereby resulting in ROS-mediated STAT3 inhibition (11), which suggests that these pathways
could also contribute to BBI608-mediated antitumor effects,
particularly in OSCC cell line with low p-STAT3 levels, where the
mechanism of BBI608 is unclear. It is possible that BBI608 may
directly bind to STAT3 (although this has not been demonstrated in
the literature to the best of our knowledge) or modulate STAT3
indirectly by altering cellular stress responses. Therefore,
additional studies are needed to further clarify these off-target
effects of BBI608.
The present study demonstrated that BBI608
significantly inhibited STAT3 activation in OSCC cell lines
regardless of the culture conditions. Given the important role of
STAT3 in OSCC progression and the absence of effective therapeutic
options for STAT3 inhibition (7,9), the
present findings suggest the potential repurposing of BBI608 for
treating patients with OSCC exhibiting high STAT3 activity.
However, a limitation of the current study is the absence of
preclinical validation in animal models; therefore, future research
integrating animal studies may help to further elucidate the
therapeutic potential of BBI608 in OSCC. In conclusion, the present
study validated the feasibility and efficacy of repurposing BBI608
in 2D and 3D OSCC models. BBI608 demonstrated a potent anticancer
effect by inhibiting STAT3 activation and its downstream target
genes, survivin and Mcl-1, leading to the induction of
apoptosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea grant funded by the Korean government (grant
nos. 2022R1A2C1091608 and 2023-00247502).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
DGP conceived and designed the study, acquired and
analyzed the data, and drafted the manuscript. HJK, SL, HMJ and SDH
analyzed and interpreted the data. SJC and SDC conceived and
designed the study, and reviewed and edited the manuscript. DGP and
SDC confirmed the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pushpakom S, Iorio F, Eyers PA, Escott KJ,
Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, et
al: Drug repurposing: Progress, challenges and recommendations. Nat
Rev Drug Discov. 18:41–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abdelsayed M, Kort EJ, Jovinge S and
Mercola M: Repurposing drugs to treat cardiovascular disease in the
era of precision medicine. Nat Rev Cardiol. 19:751–764. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia Y, Sun M, Huang H and Jin WL: Drug
repurposing for cancer therapy. Signal Transduct Tar. 9:922024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D: Hallmarks of Cancer: New
dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu YM, Dong ZG and Liu KD: Unraveling the
complexity of STAT3 in cancer: Molecular understanding and drug
discovery. J Exp Clin Canc Res. 43:232024. View Article : Google Scholar
|
|
6
|
Yu H and Jove R: The stats of cancer - New
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Macha MA, Matta A, Kaur J, Chauhan SS,
Thakar A, Shukla NK, Gupta SD and Ralhan R: Prognostic significance
of nuclear pSTAT3 in oral cancer. Head Neck-J Sci Spec. 33:482–489.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei LY, Lin HC, Tsai FC, Ko JY, Kok SH,
Cheng SJ, Lee JJ and Chia JS: Effects of interleukin-6 on
STAT3-regulated signaling in oral cancer and as a prognosticator of
patient survival. Oral Oncol. 124:1056652022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim LH, Khadka S, Shin JA, Jung JY, Ryu
MH, Yu HJ, Lee HN, Jang B, Yang IH, Won DH, et al: Nitidine
chloride acts as an apoptosis inducer in human oral cancer cells
and a nude mouse xenograft model via inhibition of STAT3.
Oncotarget. 8:91306–91315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Rogoff HA, Keates S, Gao Y,
Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB and Li CJ:
Suppression of cancer relapse and metastasis by inhibiting cancer
stemness. Proc Natl Acad Sci USA. 112:1839–1844. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Froeling FEM, Swamynathan MM, Deschenes A,
Chio IIC, Brosnan E, Yao MA, Alagesan P, Lucito M, Li J, Chang AY,
et al: Bioactivation of napabucasin triggers reactive oxygen
species-mediated cancer cell death. Clin Cancer Res. 25:7162–7174.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bendell JC, Hubbard JM, O'Neil BH, Jonker
DJ, Starodub A, Peyton JD, Pitot HC, Halfdanarson TR, Nadeau BR,
Zubkus JD, et al: Phase 1b/II study of cancer stemness inhibitor
napabucasin (BBI-608) in combination with FOLFIRI+/-bevacizumab
(bev) in metastatic colorectal cancer (mCRC) patients (pts). J Clin
Oncol. 2017. View Article : Google Scholar
|
|
13
|
Bekaii-Saab TS, Starodub A, El-Rayes BF,
Shahda S, O'Neil BH, Noonan AM, Shaib WL, Hanna WT, Mikhail S, Neki
AS, et al: Phase 1b/2 trial of cancer stemness inhibitor
napabucasin (NAPA)+ nab-paclitaxel (nPTX) and gemcitabine (Gem) in
metastatic pancreatic adenocarcinoma (mPDAC). J Clin Oncol. 36
(Suppl 15):41102018. View Article : Google Scholar
|
|
14
|
Shao Z, Wang H, Ren H, Sun Y and Chen X:
The anticancer effect of napabucasin (BBI608), a natural
naphthoquinone. Molecules. 28:56782023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ware MJ, Colbert K, Keshishian V, Ho J,
Corr SJ, Curley SA and Godin B: Generation of homogenous
three-dimensional pancreatic cancer cell spheroids using an
improved hanging drop technique. Tissue Eng Part C-Me. 22:312–321.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oner E, Gray SG and Finn SP: Cell
viability assay with 3d prostate tumor spheroids. Methods Mol Biol.
2645:263–275. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu HJ, Park C, Kim SJ, Cho NP and Cho SD:
Signal transducer and activators of transcription 3 regulates
cryptotanshinone-induced apoptosis in human mucoepidermoid
carcinoma cells. Pharmacogn Mag. 10:622–629. 2014. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of STAT3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Riccardi C and Nicoletti I: Analysis of
apoptosis by propidium iodide staining and flow cytometry. Nat
Protoc. 1:1458–1461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HJ, Shin JA, Lee YG, Jin B, Lee WW,
Lee Y, Choi SJ, Han JM, Ahn MH, Kim JH, et al: Zingiber officinale
promotes autophagy and apoptosis in human oral cancer through the
C/EBP homologous protein. Cancer Sci. 115:2701–2717. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Won DH, Kim LH, Jang B, Yang IH, Kwon HJ,
Jin B, Oh SH, Kang JH, Hong SD, Shin JA and Cho SD: In vitro and in
vivo anti-cancer activity of silymarin on oral cancer. Tumour Biol.
40:10104283187761702018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang IH, Hong SH, Jung M, Ahn CH, Yoon HJ,
Hong SD, Cho SD and Shin JA: Cryptotanshinone chemosensitivity
potentiation by TW-37 in human oral cancer cell lines by targeting
STAT3-Mcl-1 signaling. Cancer Cell Int. 20:4052020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hsieh FC, Cheng G and Lin J: Evaluation of
potential Stat3-regulated genes in human breast cancer. Biochem
Bioph Res Co. 335:292–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zou SL, Tong QY, Liu BW, Huang W, Tian Y
and Fu XH: Targeting STAT3 in cancer immunotherapy. Mol Cancer.
19:1452020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Guo M, Wei H and Chen Y: Targeting
MCL-1 in cancer: Current status and perspectives. J Hematol Oncol.
14:672021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Breslin S and O'Driscoll L:
Three-dimensional cell culture: The missing link in drug discovery.
Drug Discov Today. 18:240–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geiger JL, Grandis JR and Bauman JE: The
STAT3 pathway as a therapeutic target in head and neck cancer:
Barriers and innovations. Oral Oncol. 56:84–92. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Levy DE and Lee CK: What does Stat3 do? J
Clin Invest. 109:1143–1148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Becerra C, Stephenson J, Jonker DJ, Cohn
AL, Asmis TR, Bekaii-Saab TS, Conkling PR, Garbo LE, Lenz HJ, et
al: Phase Ib/II study of cancer stem cell (CSC) inhibitor BBI608
combined with paclitaxel in advanced gastric and gastroesophageal
junction (GEJ) adenocarcinoma. J Clin Oncol. 33 (Suppl
15):40692015. View Article : Google Scholar
|
|
32
|
Senichkin VV, Streletskaia AY, Gorbunova
AS, Zhivotovsky B and Kopeina GS: Saga of Mcl-1: Regulation from
transcription to degradation. Cell Death Differ. 27:405–419. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Wei YQ and Wei XW: Napabucasin, a
novel inhibitor of STAT3, inhibits growth and synergises with
doxorubicin in diffuse large B-cell lymphoma. Cancer Lett.
491:146–161. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han D, Yu T, Dong N, Wang B, Sun F and
Jiang D: Napabucasin, a novel STAT3 inhibitor suppresses
proliferation, invasion and stemness of glioblastoma cells. J Exp
Clin Cancer Res. 38:2892019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zajączkowska M, Teresiak A, Filas V, Ibbs
M, Bliźniak R, Łuczewski Ł and Lamperska K: 2D and 3D cell cultures
- A comparison of different types of cancer cell cultures. Arch Med
Sci. 14:910–919. 2018.PubMed/NCBI
|
|
36
|
Edmondson R, Broglie JJ, Adcock AF and
Yang LJ: Three-dimensional cell culture systems and their
applications in drug discovery and cell-based biosensors. Assay
Drug Dev Techn. 12:207–218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tung YC, Hsiao AY, Allen SG, Torisawa YS,
Ho M and Takayama S: High-throughput 3D spheroid culture and drug
testing using a 384 hanging drop array. Analyst. 136:473–478. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zuo D, Shogren KL, Zang J, Jewison DE,
Waletzki BE, Miller AL II, Okuno SH, Cai Z and Yaszemski MJ:
Inhibition of STAT3 blocks protein synthesis and tumor metastasis
in osteosarcoma cells. J Exp Clin Cancer Res. 37:2442018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim J, Park A, Hwang J, Zhao X, Kwak J,
Kim HW, Ku M, Yang J, Kim TI, Jeong KS, et al: KS10076, a chelator
for redox-active metal ions, induces ROS-mediated STAT3 degradation
in autophagic cell death and eliminates ALDH1 stem cells. Cell Rep.
40:1110772022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jonker DJ, Nott L, Yoshino T, Gill S,
Shapiro J, Ohtsu A, Zalcberg J, Vickers MM, Wei AC, Gao Y, et al:
Napabucasin versus placebo in refractory advanced colorectal
cancer: A randomised phase 3 trial. Lancet Gastroenterol.
3:263–270. 2018.
|
|
41
|
Chang AY, Hsu E, Patel J, Li Y, Zhang M,
Iguchi H and Rogoff HA: Evaluation of tumor cell-tumor
microenvironment component interactions as potential predictors of
patient response to napabucasin. Mol Cancer Res. 17:1429–1434.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Petsri K, Thongsom S, Racha S, Chamni S,
Jindapol S, Kaekratoke N, Zou H and Chanvorachote P: Novel
mechanism of napabucasin, a naturally derived furanonaphthoquinone:
Apoptosis and autophagy induction in lung cancer cells through
direct targeting on Akt/mTOR proteins. Bmc Complement Med.
22:2502022. View Article : Google Scholar : PubMed/NCBI
|