In 2020, colorectal cancer (CRC) is the third most
prevalent malignancy globally and the second leading cause of
cancer-associated deaths (1)
Distant metastasis is a key driver of mortality in CRC, with the
liver being the most common site of metastasis (2). A total of 15–25% of patients with CRC
present with synchronous liver metastases at initial diagnosis,
while a further 15–25% develop metachronous liver metastases
following primary CRC resection (2). Currently, a limited number of these
liver metastases are amenable to surgical resection, and the 5-year
survival rate for these patients is 25–44%. Moreover, up to 60% of
patients experience rapid recurrence following resection (3).
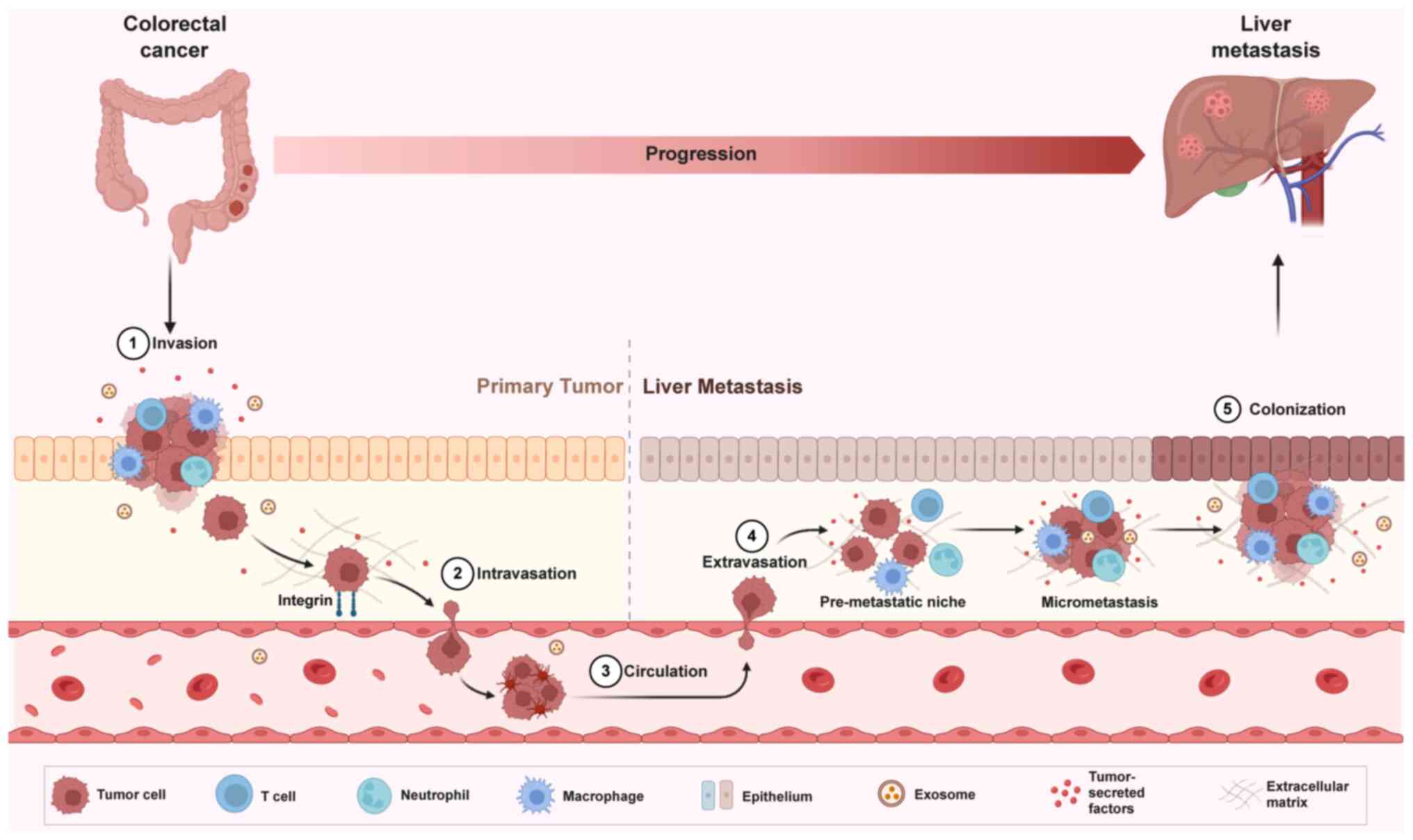
The exact mechanisms underlying colorectal liver
metastasis (CRLM) remain poorly understood. Tumor cell
dissemination from the primary site to distant organs and the
subsequent formation of metastatic tumors involve a dynamic process
regulated by numerous genes and signaling pathways. This process
includes tumor cell detachment from the primary site, entry into
the circulatory or lymphatic system, extravasation and colonization
at secondary sites. Tumor cell invasion, migration, adhesion,
extracellular matrix remodeling, neovascularization and immune
regulation are key in facilitating this metastatic cascade. The
rise of immunotherapy, with its notable clinical success, has
highlighted the critical role of the tumor immune microenvironment
(TIME) in CRLM. CRLM represents a complex interaction between tumor
and microenvironmental cells. Tumor cells, while acquiring invasive
phenotypes, maintain intercellular communication with
microenvironmental cells, regulating their functions through both
direct and indirect mechanisms. This regulation involves the
expression of immune checkpoint molecules and the secretion of
cytokines, establishing a microenvironment conducive to tumor cell
colonization and proliferation (4).
Moreover, suppressive immune cells enhance the invasiveness of
tumor cells by activating pro-metastatic signaling pathways. This
interaction between tumor cells and the microenvironment drives the
development of CRLM (Fig. 1).
The development, invasion and metastasis of CRC are
complex biological processes involving multiple genetic
alterations. Successive mutations and abnormal expression of genes
such as APC, KRAS, BRAF and PTEN activate signaling pathways
promoting CRC invasion and metastasis (5).
Epithelial-mesenchymal transition (EMT) is a
critical precursor to CRLM. EMT refers to the phenotypical
transformation of tumor epithelial cells into a mesenchymal
phenotype, which enhances their migratory capacity (6). This involves the loss of intercellular
junctions, increased secretion of intercellular plasma hydrolases
and disruption of apical polarity, resulting in a breakdown of
cell-cell adhesion. Concurrently, these changes induce the
expression of N-cadherin, vimentin and α-smooth muscle actin,
facilitating the transition from polarized epithelial cells to
multipolar mesenchymal cells. This transformation increases cell
motility, enabling tumor cells to detach from epithelial clusters
and migrate individually in a mesenchymal manner, further enhancing
the metastatic potential (7). The
E-cadherin-β-catenin complex serves a pivotal role in maintaining
epithelial integrity. Disruption of this complex leads to the
detachment of cells from the primary tumor, enabling invasion and
migration through the extracellular matrix and entry into the
circulatory or lymphatic system. This is an important initiating
step in the development of CRLM (8).
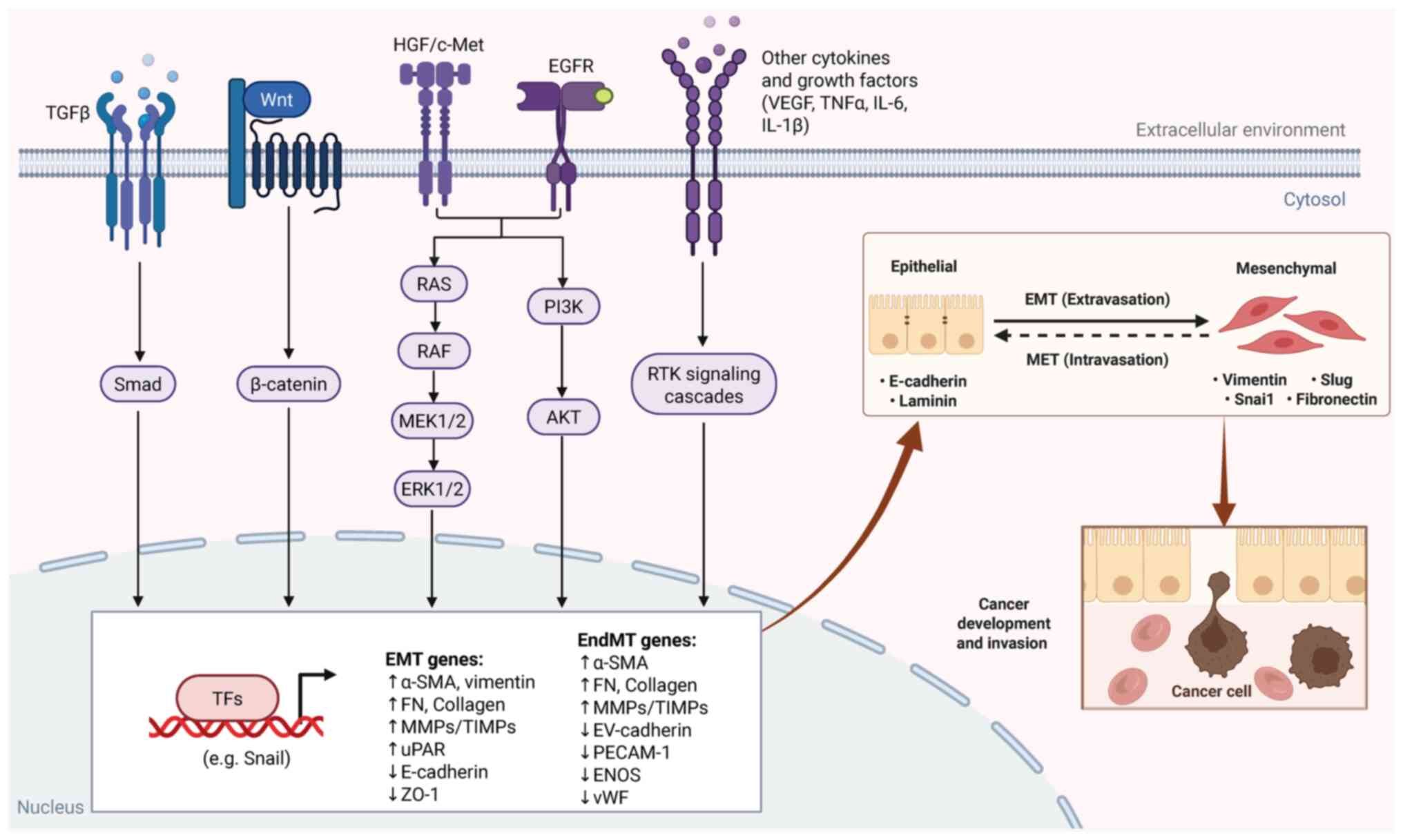
The acquisition of an invasive phenotype in CRC is
governed by signaling pathways, including Wnt/β-catenin (9,10),
TGF-β (11,12), PI3K/AKT (13–15),
MEK/ERK (16,17) and hepatocyte growth factor (HGF)/MET
(18). These pathways are regulated
by intracellular oncogenes and tumor suppressor genes, as well as
tumor microenvironmental signaling factors that influence tumor
cell invasion and metastasis (Fig.
2).
Hepatic susceptibility to colonization by
circulating tumor cells compared with other metastasis sites such
as the lungs and peritoneum is primarily attributed to its highly
permeable blood vessels, unique hemodynamic properties and distinct
immune microenvironment. Notably, the ability to tolerate immune
responses contributes to the creation of an immunosuppressive
microenvironment, which protects the organ from excessive immune
reactions to antigens. Additionally, liver homeostasis is
maintained by specialized resident cells and diverse immune cell
populations, which collectively regulate immune responses and
oncogenesis (19–21).
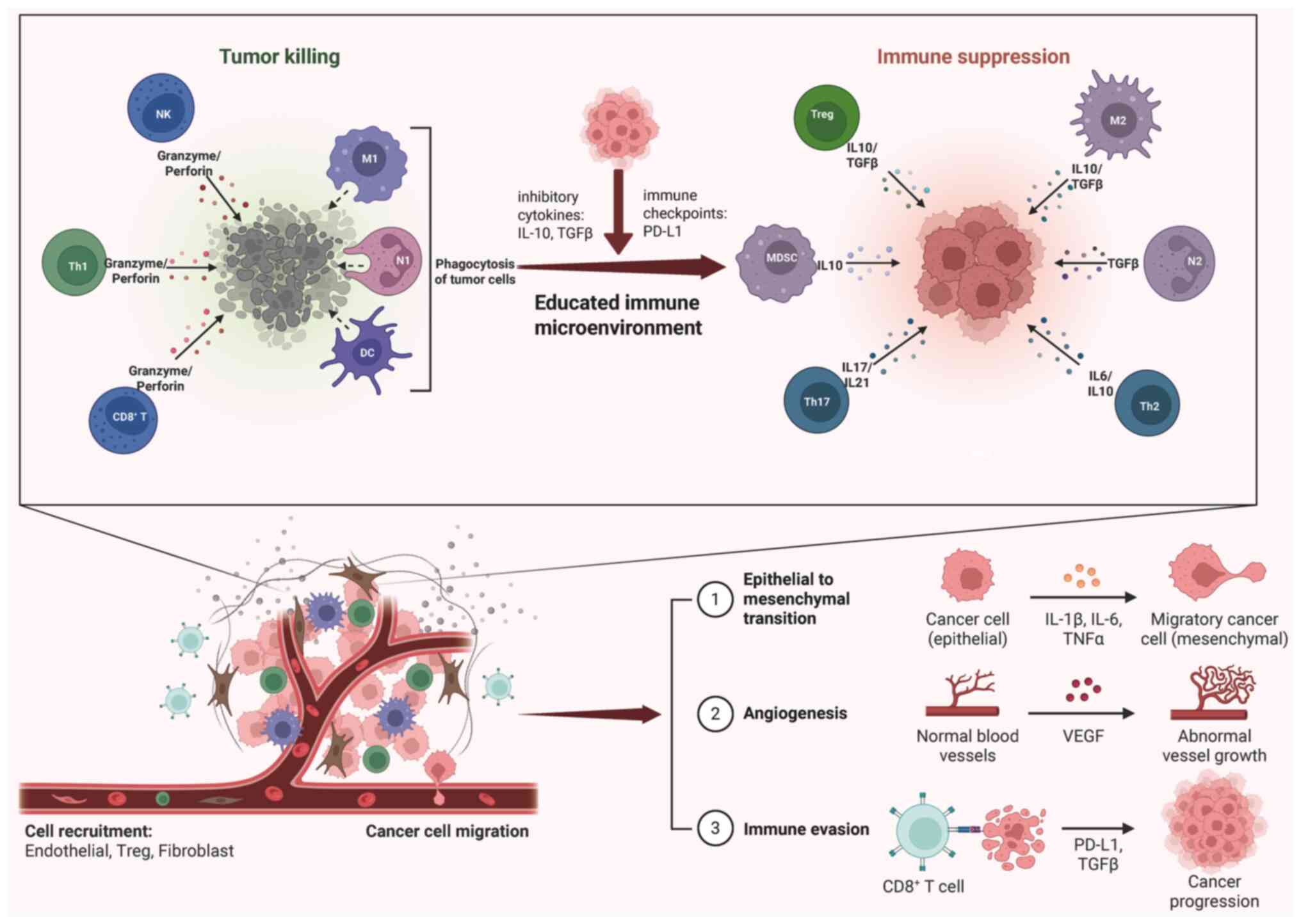
During liver metastasis, immune cells undergo
functional changes as a result of communication with tumor cells.
Typically, under the influence of tumor cells, immune cells shift
toward an immunosuppressive phenotype. Simultaneously, these immune
cells reverse their roles to promote the invasive behavior of tumor
cells by releasing pro-metastatic cytokines. This facilitates the
establishment of a pre-metastatic niche, supporting the
colonization of metastatic tumor cells and leading to the formation
of metastatic foci (Fig. 3)
(22).
Regulatory T cells (Tregs), identified by the
expression of CD25 and FoxP3, are classical immune suppressors
(38). In CRLM, Tregs are the
primary source of IL-10, which increases PD-L1 expression on
monocytes. This interaction diminishes CD8+ T cell
infiltration and impairs antitumor immunity in CRLM (39). Previous studies have shown a
significant increase in the proportion of Tregs in both mouse
models (39,40) and resected CRLM specimens from
patients (41). Furthermore,
Treg-mediated suppression of the antitumor immune response is
linked to clinical prognosis of patients with CRLM (42). Studies have revealed a paradox where
elevated infiltration of FOXP3+ T cells in CRC is
associated with improved relapse-free and disease-specific
survival, while low FOXP3+ T cell infiltration is
associated with poor prognosis (31,43).
This discrepancy may be due to the heterogeneity of Tregs within
the tumor microenvironment (TME). Saito et al (44) identified two distinct subpopulations
of Tregs in CRC terminally differentiated immunosuppressive
FoxP3high and pro-inflammatory FoxP3low
subtypes. Inflammatory Treg-infiltrating CRC showed significant
upregulation of genes associated with inflammation and immune
responses. Functionally distinct subpopulations of Tregs influence
CRC prognosis in opposing directions, with high FOXP3 expression in
immunosuppressive Treg-infiltrating tumors associated with poorer
outcomes. Pedroza-Gonzalez et al (45) demonstrated that, compared with Tregs
from primary hepatocellular carcinoma, Tregs in CRLM exhibit higher
expression of glucocorticoid-induced tumor necrosis factor receptor
and stronger immunosuppressive activity. These findings suggest
that a more precise characterization of Treg subpopulations in CRC
and its liver metastases may deepen the understanding of Treg
function in CRLM and help refine therapeutic strategies.
Macrophages within the TME exhibit notable
plasticity and heterogeneity, classified into M1-type macrophages
with pro-inflammatory, immune activating and antitumor properties,
and M2-type macrophages, which possess immunosuppressive and
pro-tumor functions. M2 macrophages are the dominant subtype in
liver metastases (46,47) and associated with poor prognosis
(48).
M2 macrophages facilitate tumor invasion and
metastasis through several mechanisms, such as remodeling the
extracellular matrix (49) and
inducing EMT (50,51). Additionally, M2 macrophages
contribute to tumor angiogenesis through the secretion of VEGF
(52) and support immune evasion by
releasing immunosuppressive cytokines such as IL-10 and TGF-β
(53). Aberrant expression and
activation of signaling molecules such as X-box binding protein 1
(XBP1) in M2 macrophages are further amplified by elevated cytokine
secretion of cytokines, including IL-6 and VEGFA, which accelerate
CRLM progression (54).
Cytokines and exosomes serve pivotal roles in CRC
cells by inducing macrophage polarization toward the M2 phenotype.
TGF-β, a classical cytokine, regulates macrophage polarization and,
in CRLM, is modulated by pro-oncogenic factors such as collagen
triple helix repeat containing 1, which further promotes CRLM by
remodeling infiltrating macrophages via TGF-β signaling (55). Additionally, CCL2 is a key regulator
of M2-type polarization, which fosters CRLM progression.
Pro-oncogenic factors such as STAT3, transcription factor 4 (TCF4)
and spondin 2 in CRC cells stimulate CCL2 secretion (56–58).
Targeting the CCL2/CCR2 chemokine pathway reduces
M2-typetumor-associated macrophages (TAMs) at metastatic sites,
disrupts the immunosuppressive TME and enhances the susceptibility
of mCRC to antitumor T cell responses (59). Tumor-derived factors such as CCL20,
IL-10, VEGF and IL-1β also serve key roles in promoting macrophage
infiltration and M2 polarization in CRLM (60,61).
Exosome-mediated release of pro-oncogenic factors also contributes
to the M2 polarization. CRC cells induce M2 polarization and
establish an immunosuppressive pre-metastatic niche via the
exosomal release of microRNAs (miRs; such as miR-25-3p, miR-130b-3p
and miR-425-5p, miR-21-5p, miR-203, miR-934, miR-135a-5p and
miR-106a-5p) and signaling molecules such as heat shock protein
90B1, and circ-0034880, which promote CRLM (62–69).
Neutrophils in the TME exhibit dual roles: In
early-stage tumors, they enhance T cell responses, while in
advanced tumors, they adopt an immunosuppressive function (72). Similarly to macrophages, neutrophils
in the TME can differentiate into distinct subsets, categorized as
antitumor N1- and pro-tumor N2-type (73). N1-type neutrophils enhance tumor
cell killing by expressing immune-activating cytokines and
chemokines while inhibiting arginase expression. TGF-β in the TME
induces the polarization of neutrophils from N1 to N2-type. N2-type
neutrophils suppress tumor-killing T cell activity (74) and promote tumor invasion and
metastasis by stimulating angiogenesis (75).
Tumor cells induce neutrophil infiltration via
multiple pathways. CRC cells secrete granulocyte colony stimulating
factor, which recruits neutrophils and upregulates Bv8/Prokineticin
2 expression, promoting immunosuppression and angiogenesis, thereby
contributing to CRC metastasis (75,79).
Seubert et al (80)
demonstrated that tumor-derived tissue inhibitor of
metalloproteinases 1(TIMP-1) upregulates stromal derived factor-1,
recruiting neutrophils to the liver, and promoting liver
metastasis. Aberrantly expressed molecules such as cell migration
inducing hyaluronidase 1 (81) and
DNA rrimase subunit 1 (82) in CRLM
ead to the production of CXCL1 and CXCL3, causing accumulation of
immunosuppressive neutrophils, ultimately enhancing CRLM
progression. These findings suggest potential therapeutic targets
for future CRLM research and treatment.
MDSCs, a heterogeneous group of immature myeloid
cells, serve a pivotal role in facilitating CRLM by inducing
immunosuppression, remodeling the extracellular matrix and
promoting angiogenesis (83).
Additionally, MDSCs are involved in the formation of NETs within
the pre-metastatic niche (84).
Abnormal expression and secretion of cytokines trigger MDSC
infiltration, further advancing CRLM. CCL2-CCR2 signaling has been
shown to induce MDSC infiltration in a STAT3-dependent manner,
enhancing their immunosuppressive functions and contributing to CRC
progression (85–87). Furthermore, CRC-derived CCL15
(88) and TME-derived CXCL1
(89,90) recruit MDSCs to establish a
pre-metastatic niche and promote CRLM. Cytokines such as CCL7
(91), IL-6 (92), IL-33 (93) and exosomes containing long
non-coding RNA MIR181A1HG (94)
also serve critical roles in inducing MDSC infiltration, enhancing
tumor invasiveness, supporting neovascularization and facilitating
the creation of a pre-metastatic ecological niche in the liver.
Targeted inhibition of these cytokines may offer an effective
therapeutic approach for CRLM.
DCs, as antigen-presenting cells, initiate immune
responses by capturing exogenous antigens and presenting them to T
cells. To evade immune surveillance, tumor cells suppress antigen
presentation by releasing inhibitory cytokines such as TGF-β
(95). Compared with DCs from
healthy individuals, those from patients with CRC exhibit impaired
antigen presentation, decreased expression of costimulatory
molecules, increased secretion of immunosuppressive IL-10 and
reduced levels of immunostimulatory IL-12 and TNF-α (96). Nagorsen et al (97) found that tumor-infiltrating
S100+ DCs in CRC are negatively associated with systemic
antigen-specific T cell responses and positively associated with
Tregs. Hsu et al (98)
discovered elevated CXCL1 expression in CRC patient-derived DCs,
which enhanced cell migration, increased matrix metalloproteinase 7
expression and promoted EMT, reflecting the altered functionality
of DCs within the CRC TME. Huang et al (99) revealed that tumor-associated
fibroblasts in CRC secrete WNT2, which suppresses DC function
through the JAK2/STAT3 pathway. Targeting WNT2 may restore
DC-mediated antitumor immunity. Further exploration of the
mechanisms regulating DC function in CRC may identify new
therapeutic targets for CRC treatment.
As a key component of innate immunity, NK cells
exert antitumor effects by releasing cytotoxic molecules such as
TRAIL and FasL. In a mouse model of CRLM, NK cells were shown to
inhibit liver metastasis of CRC (100). Increased NK cell infiltration is
associated with improved overall survival (OS) in patients with
CRLM (101).
However, NK cell function is impaired in both CRC
and liver metastases compared with NK cells in healthy livers. CRC
cells regulate the TME pH by producing lactic acid, which lowers
the pH within NK cells, leading to mitochondrial dysfunction and
apoptosis. This enables tumor cells to evade the cytotoxic effects
of NK cells (105). Metabolic
dysfunction in the metastatic niche notably impacts NK cell
functionality. Increased glutamine uptake by cancer cells depletes
glutamine availability for NK cells, decreasing their activity and
cytotoxicity, thereby promoting CRLM progression (106).
Upon entering the portal circulation, tumor cells
reach the hepatic sinusoidal capillaries through the portal vein.
These tumor cells trigger non-specific liver defense mechanisms,
leading to their phagocytosis by resident immune cells such as
Kupffer cells (KCs) and NK cells (107).
KCs, the resident macrophages of the liver, serve a
pivotal role in maintaining liver homeostasis and are key
contributors to the pathogenesis of liver disease. KCs are
essential in defending against liver metastasis due to their
phagocytic capability, cytokine production and promotion of
tertiary lymphoid structures (108). Dysfunction of phagocytosis in KCs
is a key driving force in CRLM. Some tumor cells can evade
phagocytosis by KCs, highlighting the need for further research
into this evasion mechanism to identify novel therapeutic targets
for liver metastasis (109). The
balance between pro-phagocytic ‘eat me’ signals, such as
tumor-associated antigens, calreticulin, SLAM Family Member 7 and
Erythroblast Membrane Associated Protein (ERMAP), and
anti-phagocytic ‘don't eat me’ signals, including CD47, PD-L1, CD24
and β2-microglobulin, is a key determinant in the phagocytosis
process (110). Additionally, the
functional reprogramming of KCs warrants attention. Following
metastatic colonization of the liver, KCs undergo transcriptional
reprogramming typical of TAMs, which facilitates tumor progression
(111). The phagocytosis of
exosomes released by the primary tumor into the circulation and
into the liver by KCs can initiate the formation of a
pre-metastatic niche in the liver (66).
Liver-resident specialized NK cells also serve a
significant role in the early stages of metastasis by contributing
to the establishment of pre-metastatic niches. Invariant NK T cells
in the liver promote metastasis by producing fibrogenic cytokines
such as IL-4 and IL-13, independent of T cell receptor activation,
thereby inducing a fibrotic niche in the liver. Targeted disruption
of IL-4 and IL-13 signaling pathways in hepatic stellate cells
inhibits their trans differentiation into extracellular
matrix-producing myofibroblasts, thereby impeding the metastatic
proliferation of disseminated cancer cells (112).
Tumor cells that evade innate immune surveillance
extravasate from blood vessels and form metastatic lesions. Tumor
cell adhesion to the vascular system is not only driven by
mechanical blockage of the vasculature, but also by specific
cellular adhesion processes that facilitate tumor cell attachment
and extravasation (113,114). Liver sinusoidal endothelial cells
express the cell adhesion molecule E-selectin, which promotes tumor
cell attachment to hepatic sinusoidal endothelial cells. Inhibition
of E-selectin effectively decreases the formation of liver
metastases (115). Moreover, tumor
cells can trigger the release of pro-inflammatory cytokines, such
as TNF-α, from KCs, which upregulates the expression of adhesion
molecules, such as E-selectin, vascular cell adhesion molecule 1
and intercellular adhesion molecule 1(ICAM1) on hepatic sinusoidal
endothelial cells. This increase in adhesion molecule expression
enhances tumor cell colonization in the liver (116).
Once tumor cells extravasate into the Disse
interstitium, hepatic stellate cells are activated by cytokines
such as TGF-β, which is secreted by KCs. This activation prompts
hepatic stellate cells to produce extracellular matrix proteins
such as collagen, laminin and fibronectin, creating a supportive
environment for tumor cell colonization. Additionally, KCs and
neutrophils secrete matrix metalloproteinases and elastases, which
degrade and remodel the extracellular matrix, facilitating tumor
cell invasion. Concurrently, hepatic stellate cells promote a
suppressive microenvironment by inducing the apoptosis of cytotoxic
T cells and expanding immune-regulatory T cells, creating a
favorable environment for tumor cell colonization in the liver
(117).
Research into liver metastasis in CRC has revealed
numerous potential therapeutic targets, with targeted therapy and
immunotherapy emerging as the leading strategies for managing CRLM
(121). In addition to traditional
neoadjuvant radiotherapy and chemotherapy and adjuvant therapy
following surgical resection, integrating targeted and
immunotherapies with radiotherapy/chemotherapy and surgery has
established a comprehensive treatment system for CRLM (122). This combination therapy offers
dual benefits: By initiating neoadjuvant treatment, previously
unresectable CRLM can become surgically resectable, increasing the
number of patients eligible for hepatic resection while decreasing
perioperative morbidity and mortality (123). Second, these combination therapies
show promise in enhancing long-term survival rates for patients
(124).
EGFR, a member of the receptor tyrosine kinase (TK)
family, serves a key role in CRC development and invasive
metastasis through downstream signaling pathways, including the
RAS/RAF/MEK/ERK and PI3K/AKT pathways (125). Cetuximab, the first monoclonal
antibody targeting EGFR, significantly improves OS and
progression-free survival (PFS) in patients with CRC who are
resistant to other treatment (126). When combined with chemotherapy,
cetuximab has demonstrated favorable therapeutic outcomes. The
combination of cetuximab with FOLFIRI (irinotecan, fluorouracil and
leucovorin) significantly decreases the risk of disease progression
in patients with mCRC compared with FOLFIRI alone (127). The combination of cetuximab with
FOLFOX4(oxaliplatin, leucovorin, and fluorouracil) as first-line
treatment for mCRC has also shown superior remission rates compared
with the FOLFOX4 regimen alone (128). However, mutations in the RAS gene
in CRC confer resistance to cetuximab, meaning cetuximab is
effective in patients with RAS wild-type mCRC (129) Another EGFR targeting drug,
panitumumab, is a fully humanized antibody that does not induce
antibody-dependent cell-mediated cytotoxicity (125). The PRIME trial, which examined the
efficacy of FOLFOX alone and in combination with panitumumab in
patients with mCRC, found that the combination therapy resulted in
higher OS and PFS compared with FOLFOX treatment alone (130,131).
Bevacizumab, a monoclonal antibody targeting the
angiogenesis inhibitor VEGF, has received US Food and Drug
Administration approval for the treatment of mCRC and demonstrated
favorable efficacy (132). A
meta-analysis by Cao et al (133), which included 1,838 patients with
mCRC, showed that chemotherapy combined with bevacizumab following
primary tumor resection significantly prolonged OS compared with
chemotherapy alone. The study also revealed improved OS in patients
who were initially unable to undergo resection of their primary
tumor when treated with bevacizumab. Additionally, the combination
of bevacizumab with chemotherapy may enhance the resectability of
CRLM. In a study by Tang et al (134), the combination of bevacizumab with
mFOLFOX6 as first-line treatment for patients with unresectable
CRLM harboring RAS mutations demonstrated significantly superior
efficacy compared with mFOLFOX6 monotherapy. This combination not
only markedly increased the R0 resection rate of liver metastases
but also improved the overall resectability of liver metastases,
leading to improved PFS and OS in patients. Ramucirumab, a VEGFR
antagonist that specifically binds VEGFR2 and blocks
ligand-receptor binding, has also shown promising results in mCRC
treatment (135). In a study by
Tabernero et al (136), the
combination of ramucirumab and FOLFIRI was evaluated against a
placebo in patients with mCRC. Ramucirumab and FOLFIRI combination
significantly improved OS in patients. Ramucirumab is currently
approved for use in the second-line treatment of mCRC.
Receptor TKs, located on the cell surface and
intracellularly, play a key role in intercellular signaling, which
influences cell function. TKIs block the activity of kinase
proteins that contribute to tumor cell proliferation and the
development of tumor vasculature. Regorafenib is an oral,
multi-targeted TKI that inhibits VEGFR1-3, PDGFR, FGFR, KIT, RET1
and BRAF and has been shown to improve survival in patients with
refractory mCRC (137).
Regorafenib is used to treat mCRC that progresses despite previous
chemotherapy, anti-VEGF or anti-EGFR therapy (138). In addition to regorafenib,
fruquintinib is an oral TKI that selectively inhibits different
subtypes of VEGFR, thereby inhibiting tumor angiogenesis and growth
(139). The FRESCO study evaluated
the effectiveness and safety of fruquintinib as a third-line or
subsequent treatment for patients with mCRC. The results
demonstrated that fruquintinib monotherapy significantly prolonged
survival in patients with mCRC who had failed second-line or higher
chemotherapy (140). FRESCO-2, an
international, multicentre, randomised, double-blind, phase 3
study, also demonstrated that fruquintinib significantly improved
overall survival in patients with refractory metastatic colorectal
cancer compared to placebo (141).
Fruquintinib is currently approved by FDA for use in patients with
mCRC who have previously undergone fluoropyrimidine-, oxaliplatin-,
and irinotecan-based chemotherapy, an anti-VEGF therapy, and if RAS
wild-type and medically appropriate, an anti-EGFR therapy (142).
In recent years, immune checkpoint inhibitors have
garnered attention due to their success in achieving long-lasting
responses in a range of previously difficult-to-treat solid tumors
(143). Overman et al
(144) demonstrated the
significant efficacy of the PD-1 inhibitor nivolumab alone in
individuals with deficient mismatch repair/microsatellite
instability(dMMR/MSI) CRC in a multicenter phase II clinical trial
(CheckMate142). Similarly, the KEYNOTE-177 (145) study showed a significant
improvement in PFS in patients with dMMR/MSI mCRC treated with the
PD-1 inhibitor pembrolizumab as first-line therapy, compared with
standard treatment. PD-1 inhibitors are currently approved by FDA
for patients with dMMR/MSI mCRC who experience disease progression
following standard chemotherapy (146). This approval highlights their
importance as a pivotal immunotherapy approach, particularly for
individuals with liver metastases from CRC. However, the efficacy
of PD-1 inhibitors varies among patients, and some do not benefit
from them. Patients with proficient mismatch repair/microsatellite
stability(pMMR/MSS) CRC, which constitutes the majority of the
patient population, derive limited benefits from PD-1 inhibitor
therapy. For individuals with dMMR/MSI CRC, who are currently
considered candidates for PD-1 inhibitor treatment, the observed
efficacy rate is suboptimal. In the KEYNOTE-016 trial,
pembrolizumab was administered to a cohort of 41 patients with CRC,
including both pMMR/MSS and dMMR/MSI subgroups, who experienced
disease progression following chemotherapy. In patients with
dMMR/MSI CRC, the immune-related objective response rate was 40%
and the 20-week PFS rate was 78%. By contrast, patients with
pMMR/MSS CRC exhibited an immune-related objective response rate of
0% and a 20-week PFS rate of 11% (147). Furthermore, liver metastases from
CRC induce systemic immune tolerance through a unique
immunosuppressive mechanism, which may further affect the efficacy
of PD-1 inhibitor therapy (148–150). Exploring effective combination
therapies may enhance the efficacy of PD-1 inhibitors. The
CheckMate-142 study demonstrated that combining PD-1 inhibitors
with CTLA4 inhibitors improves antitumor efficacy (151). In addition to immune checkpoint
inhibitors, drugs such as regorafenib have gained widespread
attention as potential combination therapies with PD-1 inhibitors
due to their promising role in modulating immunity and improving
the TME (152,153).
Cancer vaccines, adoptive cell transfer (ACT)
therapy and oncolytic viruses have emerged as prominent areas of
research in CRLM (154–156). As a form of active immunotherapy,
cancer vaccines present the immune system with tumor-specific or
-associated antigens, inducing antitumor cytotoxic responses that
help the immune system recognize and destroy cancer cells (157). ACT therapy enhances the natural
anti-cancer response by activating or genetically modifying
autologous or allogeneic immune cells in vitro to boost
tumor-fighting capabilities, followed by reinfusion into patients.
ACT includes therapies such as cytokine-induced killer cells and
chimeric antigen receptor T cell therapies (158). Oncolytic virus therapy uses
naturally occurring or genetically engineered viruses to
selectively target and lyse tumor cells. This strategy not only
modulates the TIME but also activates specific anti-tumor immune
responses (159). Additionally, it
is crucial to explore the role of cytokines, chemokines and
adjuvants in enhancing the precision and efficacy of
immunotherapies in CRLM.
Liver metastasis is a major factor influencing the
prognosis of patients with CRC. The process of liver metastasis in
CRC is complex and involves interconnected stages. Key pathways
that affect the invasive and metastatic potential of CRC cells have
been identified, along with prognostic and therapeutic molecules.
Additionally, factors within the TME influencing liver metastasis
in CRC have been preliminarily analyzed. Notably, the discovery of
immune-associated targets holds promise for treating liver
metastasis in CRC and improving prognosis. However, the clinical
application of these targets and associated drugs in individuals
with CRLM remains limited, and many patients do not benefit from
current targeted therapies and immunotherapies. Therapeutic
strategies aimed at modulating the immunosuppressive
microenvironment, such as depleting immunosuppressive cells,
inhibiting immune checkpoint pathways and stimulating cytotoxic
cells are critical approaches for enhancing the effectiveness of
immunotherapy.
Currently, novel immunotherapies for CRLM remain in
preclinical or clinical trials, and their successful integration
into clinical practice faces challenges. Firstly, CRLM creates a
highly immunosuppressive microenvironment within the liver. This
hostile TME actively inhibits the function of effector immune cells
(such as cytotoxic T cells and NK cells), rendering many
immunotherapies ineffective. Secondly, tumor heterogeneity and
evolution make it difficult to identify universal therapeutic
targets. Thirdly, lack of predictive biomarkers makes it difficult
to select patients most likely to benefit from expensive and
potentially toxic immunotherapies, leading to low response rates
and inefficient resource use. Lastly, overcoming the aforementioned
challenges above often requires combining immunotherapies (e.g.,
dual checkpoint blockade) with other modalities like chemotherapy,
targeted therapy (e.g., anti-VEGF, anti-EGFR), radiotherapy,
liver-directed therapies (ablation, embolization), or other
immunomodulators. Combinations significantly increase the risk of
severe immune-related adverse events (irAEs), including hepatitis,
colitis, pneumonitis, and endocrine toxicities. Managing
overlapping toxicities, especially in patients with liver
involvement, is challenging and can limit dosing or lead to
treatment discontinuation.
Overcoming these multifaceted challenges requires
further intensive studies to understand the CRLM biology and liver
immunology, elucidate the underlying mechanisms, discovering robust
biomarkers and identify novel therapeutic targets. Deeper
understanding of the key molecules and signaling pathways
influencing the invasive and metastatic potential of CRC is key,
achievable through the integration of high-throughput genomic
technology. Considering the complex and heterogeneous influence of
the TME on liver metastasis, it is necessary to investigate the
functional characteristics and dynamic changes of distinct cell
populations throughout the liver metastasis process, at a
single-cell level to identify key cells and molecules driving TME
remodeling. Moreover, as the understanding of the mechanisms
underlying CRLM advances, the diagnosis and treatment of this
condition may shift toward a multidisciplinary approach and enhance
patient care by facilitating a comprehensive and integrated
treatment strategy.
In conclusion, further research is required into the
mechanisms in CRLM, the exploration of novel targets for
personalized treatment and the development of innovative
intervention strategies to improve CRLM therapy efficacy.
Not applicable.
The present study was supported by the National Natural Science
Foundation of China (grant no. 82272841).
Not applicable.
CJY conceptualized the study and wrote the
manuscript. LZ performed the literature review. CHW and YJY
contributed to conception and design. ZLS revised and edited the
manuscript. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Horn SR, Stoltzfus KC, Lehrer EJ, Dawson
LA, Tchelebi L, Gusani NJ, Sharma NK, Chen H, Trifiletti DM and
Zaorsky NG: Epidemiology of liver metastases. Cancer Epidemiol.
67:1017602020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rees M, Tekkis PP, Welsh FK, O'Rourke T
and John TG: Evaluation of long-term survival after hepatic
resection for metastatic colorectal cancer: A multifactorial model
of 929 patients. Ann Surg. 247:125–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Medici B, Benatti S, Dominici M and
Gelsomino F: New frontiers of biomarkers in metastatic colorectal
cancer: Potential and critical issues. Int J Mol Sci. 26:52682025.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsubakihara Y and Moustakas A:
Epithelial-mesenchymal transition and metastasis under the control
of transforming growth factor β. Int J Mol Sci. 19:36722018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: Cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi J and Zhu YQ: Targeting the most
upstream site of Wnt signaling pathway provides a strategic
advantage for therapy in colorectal cancer. Curr Drug Targets.
9:548–557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3beta to the
APC-beta-catenin complex and regulation of complex assembly.
Science. 272:1023–1026. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Z, Gao Y, Qian Y, Wei B, Jiang K,
Sun Z, Zhang F, Yang M, Baldi S, Yu X, et al: The Lyn/RUVBL1
complex promotes colorectal cancer liver metastasis by regulating
arachidonic acid metabolism through chromatin remodeling. Adv Sci
(Weinh). 12:e24065622025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zubeldia IG, Bleau AM, Redrado M, Serrano
D, Agliano A, Gil-Puig C, Vidal-Vanaclocha F, Lecanda J and Calvo
A: Epithelial to mesenchymal transition and cancer stem cell
phenotypes leading to liver metastasis are abrogated by the novel
TGFβ1-targeting peptides P17 and P144. Exp Cell Res. 319:12–22.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Yang Y, Qi X, Cui P, Kang Y, Liu
H, Wei Z and Wang H: SLC14A1 and TGF-β signaling: A feedback loop
driving EMT and colorectal cancer metachronous liver metastasis. J
Exp Clin Cancer Res. 43:2082024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin AE, Sugiura K, Kariuki SW, Cohen DA,
Flashner SP, Klein-Szanto AJ, Nishiwaki N, De D, Vasan N, Gabre JT,
et al: LIN28B-mediated PI3K/AKT pathway activation promotes
metastasis in colorectal cancer models. J Clin Invest.
135:e1860352025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun X, Zhang J, Dong B, Xiong Q, Wang X,
Gu Y, Wang Z, Liu H, Zhang J, He X, et al: Targeting SLITRK4
restrains proliferation and liver metastasis in colorectal cancer
via regulating PI3K/AKT/NFκB pathway and tumor-associated
macrophage. Adv Sci (Weinh). 12:e24003672025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong Z, She X, Ma J, Chen Q, Gao Y, Chen
R, Qin H, Shen B and Gao H: The E3 Ligase NEDD4L prevents
colorectal cancer liver metastasis via degradation of PRMT5 to
inhibit the AKT/mTOR signaling pathway. Adv Sci (Weinh).
2025:e25047042025. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Urosevic J, Blasco MT, Llorente A,
Bellmunt A, Berenguer-Llergo A, Guiu M, Cañellas A, Fernandez E,
Burkov I, Clapés M, et al: ERK1/2 signaling induces upregulation of
ANGPT2 and CXCR4 to mediate liver metastasis in colon cancer.
Cancer Res. 80:4668–4680. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chu PC, Lin PC, Wu HY, Lin KT, Wu C,
Bekaii-Saab T, Lin YJ, Lee CT, Lee JC and Chen CS: Mutant KRAS
promotes liver metastasis of colorectal cancer, in part, by
upregulating the MEK-Sp1-DNMT1-miR-137-YB-1-IGF-IR signaling
pathway. Oncogene. 37:3440–3455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao JF, Li XJ, Yan LK, He S, Zheng JB,
Wang XR, Zhou PH, Zhang L, Wei GB and Sun XJ: Role of HGF/c-Met in
the treatment of colorectal cancer with liver metastasis. J Biochem
Mol Toxicol. 33:e223162019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu W, Xu J, Liu J, Wang N, Zhou L and Guo
J: Liver metastasis in cancer: Molecular mechanisms and management.
MedComm (2020). 6:e701192025. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dunbar KJ, Efe G, Cunningham K, Esquea E,
Navaridas R and Rustgi AK: Regulation of metastatic organotropism.
Trends Cancer. 11:216–231. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu C, Liao JY, Liu YY, Chen ZY, Chang RZ,
Chen XP, Zhang BX and Liang JN: Immune dynamics shaping
pre-metastatic and metastatic niches in liver metastases: From
molecular mechanisms to therapeutic strategies. Mol Cancer.
23:2542024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Liu F, Cai Q, Deng L, Ouyang Q,
Zhang XH and Zheng J: Invasion and metastasis in cancer: Molecular
insights and therapeutic targets. Signal Transduct Target Ther.
10:572025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Glaire MA, Domingo E, Sveen A, Bruun J,
Nesbakken A, Nicholson G, Novelli M, Lawson K, Oukrif D, Kildal W,
et al: Tumour-infiltrating CD8+ lymphocytes and
colorectal cancer recurrence by tumour and nodal stage. Br J
Cancer. 121:474–482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trailin A, Ali E, Ye W, Pavlov S,
Červenková L, Vyčítal O, Ambrozkiewicz F, Hošek P, Daum O, Liška V
and Hemminki K: Prognostic assessment of T-cells in primary
colorectal cancer and paired synchronous or metachronous liver
metastasis. Int J Cancer. 156:1282–1292. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang A, Zhou M, Gao Y and Zhang Y:
Mechanisms of CD8+ T cell exhaustion and its clinical
significance in prognosis of anti-tumor therapies: A review. Int
Immunopharmacol. 159:1148432025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shan T, Chen S, Wu T, Yang Y, Li S and
Chen X: PD-L1 expression in colon cancer and its relationship with
clinical prognosis. Int J Clin Exp Pathol. 12:1764–1769.
2019.PubMed/NCBI
|
|
27
|
Zhao T, Li Y, Zhang J and Zhang B: PD-L1
expression increased by IFN-γ via JAK2-STAT1 signaling and predicts
a poor survival in colorectal cancer. Oncol Lett. 20:1127–1134.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei XL, Luo X, Sheng H, Wang Y, Chen DL,
Li JN, Wang FH and Xu RH: PD-L1 expression in liver metastasis: Its
clinical significance and discordance with primary tumor in
colorectal cancer. J Transl Med. 18:4752020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rong D, Sun G, Zheng Z, Liu L, Chen X, Wu
F, Gu Y, Dai Y, Zhong W, Hao X, et al: MGP promotes CD8+
T cell exhaustion by activating the NF-κB pathway leading to liver
metastasis of colorectal cancer. Int J Biol Sci. 18:2345–2361.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun G, Zhao S, Fan Z, Wang Y, Liu H, Cao
H, Sun G, Huang T, Cai H, Pan H, et al: CHSY1 promotes
CD8+ T cell exhaustion through activation of succinate
metabolism pathway leading to colorectal cancer liver metastasis
based on CRISPR/Cas9 screening. J Exp Clin Cancer Res. 42:2482023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuwahara T, Hazama S, Suzuki N, Yoshida S,
Tomochika S, Nakagami Y, Matsui H, Shindo Y, Kanekiyo S, Tokumitsu
Y, et al: Intratumoural-infiltrating CD4+ and FOXP3 + T cells as
strong positive predictive markers for the prognosis of resectable
colorectal cancer. Br J Cancer. 121:659–665. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Katz SC, Pillarisetty V, Bamboat ZM, Shia
J, Hedvat C, Gonen M, Jarnagin W, Fong Y, Blumgart L, D'Angelica M
and DeMatteo RP: T cell infiltrate predicts long-term survival
following resection of colorectal cancer liver metastases. Ann Surg
Oncol. 16:2524–2530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Katz SC, Pillarisetty VG, Bleier JI,
Kingham TP, Chaudhry UI, Shah AB and DeMatteo RP: Conventional
liver CD4 T cells are functionally distinct and suppressed by
environmental factors. Hepatology. 42:293–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tosolini M, Kirilovsky A, Mlecnik B,
Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH,
Pagès F and Galon J: Clinical impact of different classes of
infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in
patients with colorectal cancer. Cancer Res. 71:1263–1271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu X, Wang X, Yang Q, Luo L, Liu Z, Ren
X, Lei K, Li S, Xie Z, Zheng G, et al: Th17 cells Secrete TWEAK to
trigger epithelial-mesenchymal transition and promote colorectal
cancer liver metastasis. Cancer Res. 84:1352–1371. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Simone V, Pallone F, Monteleone G and
Stolfi C: Role of T(H)17 cytokines in the control of colorectal
cancer. Oncoimmunology. 2:e266172013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kroemer M, Turco C, Spehner L, Viot J,
Idirène I, Bouard A, Renaude E, Deschamps M, Godet Y, Adotévi O, et
al: Investigation of the prognostic value of CD4 T cell subsets
expanded from tumor-infiltrating lymphocytes of colorectal cancer
liver metastases. J Immunother Cancer. 8:e0014782020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Olguín JE, Medina-Andrade I, Rodríguez T,
Rodríguez-Sosa M and Terrazas LI: Relevance of regulatory T cells
during colorectal cancer development. Cancers (Basel). 12:18882020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shiri AM, Zhang T, Bedke T, Zazara DE,
Zhao L, Lücke J, Sabihi M, Fazio A, Zhang S, Tauriello DVF, et al:
IL-10 dampens antitumor immunity and promotes liver metastasis via
PD-L1 induction. J Hepatol. 80:634–644. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang X, Chen Z, Zhang N, Zhu C, Lin X, Yu
J, Chen Z, Lan P and Wan Y: Increase in
CD4+FOXP3+ regulatory T cell number and
upregulation of the HGF/c-Met signaling pathway during the liver
metastasis of colorectal cancer. Oncol Lett. 20:2113–2118. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Katz SC, Bamboat ZM, Maker AV, Shia J,
Pillarisetty VG, Yopp AC, Hedvat CV, Gonen M, Jarnagin WR, Fong Y,
et al: Regulatory T cell infiltration predicts outcome following
resection of colorectal cancer liver metastases. Ann Surg Oncol.
20:946–955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brudvik KW, Henjum K, Aandahl EM,
Bjørnbeth BA and Taskén K: Regulatory T-cell-mediated inhibition of
antitumor immune responses is associated with clinical outcome in
patients with liver metastasis from colorectal cancer. Cancer
Immunol Immunother. 61:1045–1053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Salama P, Phillips M, Grieu F, Morris M,
Zeps N, Joseph D, Platell C and Iacopetta B: Tumor-infiltrating
FOXP3+ T regulatory cells show strong prognostic significance in
colorectal cancer. J Clin Oncol. 27:186–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Saito T, Nishikawa H, Wada H, Nagano Y,
Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, et
al: Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the
prognosis of colorectal cancers. Nat Med. 22:679–684. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pedroza-Gonzalez A, Verhoef C, Ijzermans
JN, Peppelenbosch MP, Kwekkeboom J, Verheij J, Janssen HL and
Sprengers D: Activated tumor-infiltrating CD4+ regulatory T cells
restrain antitumor immunity in patients with primary or metastatic
liver cancer. Hepatology. 57:183–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He Y, Han Y, Fan AH, Li D, Wang B, Ji K,
Wang X, Zhao X and Lu Y: Multi-perspective comparison of the immune
microenvironment of primary colorectal cancer and liver metastases.
J Transl Med. 20:4542022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang D, Wang X, Si M, Yang J, Sun S, Wu H,
Cui S, Qu X and Yu X: Exosome-encapsulated miRNAs contribute to
CXCL12/CXCR4-induced liver metastasis of colorectal cancer by
enhancing M2 polarization of macrophages. Cancer Lett. 474:36–52.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee YS, Song SJ, Hong HK, Oh BY, Lee WY
and Cho YB: The FBW7-MCL-1 axis is key in M1 and M2
macrophage-related colon cancer cell progression: Validating the
immunotherapeutic value of targeting PI3Kγ. Exp Mol Med.
52:815–831. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Afik R, Zigmond E, Vugman M, Klepfish M,
Shimshoni E, Pasmanik-Chor M, Shenoy A, Bassat E, Halpern Z, Geiger
T, et al: Tumor macrophages are pivotal constructors of tumor
collagenous matrix. J Exp Med. 213:2315–2331. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cai J, Xia L, Li J, Ni S, Song H and Wu X:
Tumor-associated macrophages derived TGF-β-induced epithelial to
mesenchymal transition in colorectal cancer cells through
Smad2,3-4/Snail signaling pathway. Cancer Res Treat. 51:252–266.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin
X, Liu Q, Dou R and Xiong B: Crosstalk between cancer cells and
tumor associated macrophages is required for mesenchymal
circulating tumor cell-mediated colorectal cancer metastasis. Mol
Cancer. 18:642019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Suarez-Lopez L, Sriram G, Kong YW,
Morandell S, Merrick KA, Hernandez Y, Haigis KM and Yaffe MB: MK2
contributes to tumor progression by promoting M2 macrophage
polarization and tumor angiogenesis. Proc Natl Acad Sci USA.
115:E4236–E4244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhong X, Chen B and Yang Z: The role of
Tumor-associated macrophages in colorectal carcinoma progression.
Cell Physiol Biochem. 45:356–365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao Y, Zhang W, Huo M, Wang P, Liu X,
Wang Y, Li Y, Zhou Z, Xu N and Zhu H: XBP1 regulates the protumoral
function of tumor-associated macrophages in human colorectal
cancer. Signal Transduct Target Ther. 6:3572021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang XL, Hu LP, Yang Q, Qin WT, Wang X,
Xu CJ, Tian GA, Yang XM, Yao LL, Zhu L, et al: CTHRC1 promotes
liver metastasis by reshaping infiltrated macrophages through
physical interactions with TGF-β receptors in colorectal cancer.
Oncogene. 40:3959–3973. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang C, Ou R, Chen X, Zhang Y, Li J,
Liang Y, Zhu X, Liu L, Li M, Lin D, et al: Tumor cell-derived SPON2
promotes M2-polarized tumor-associated macrophage infiltration and
cancer progression by activating PYK2 in CRC. J Exp Clin Cancer
Res. 40:3042021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang X, Wang J, Zhao J, Wang H, Chen J and
Wu J: HMGA2 facilitates colorectal cancer progression via
STAT3-mediated tumor-associated macrophage recruitment.
Theranostics. 12:963–975. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tu W, Gong J, Zhou Z, Tian D and Wang Z:
TCF4 enhances hepatic metastasis of colorectal cancer by regulating
tumor-associated macrophage via CCL2/CCR2 signaling. Cell Death
Dis. 12:8822021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Grossman JG, Nywening TM, Belt BA, Panni
RZ, Krasnick BA, DeNardo DG, Hawkins WG, Goedegebuure SP, Linehan
DC and Fields RC: Recruitment of CCR2+ tumor associated
macrophage to sites of liver metastasis confers a poor prognosis in
human colorectal cancer. Oncoimmunology. 7:e14707292018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu C, Fan L, Lin Y, Shen W, Qi Y, Zhang Y,
Chen Z, Wang L, Long Y, Hou T, et al: Fusobacterium
nucleatum promotes colorectal cancer metastasis through
miR-1322/CCL20 axis and M2 polarization. Gut Microbes.
13:19803472021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ohashi K, Wang Z, Yang YM, Billet S, Tu W,
Pimienta M, Cassel SL, Pandol SJ, Lu SC, Sutterwala FS, et al:
NOD-like receptor C4 inflammasome regulates the growth of colon
cancer liver metastasis in NAFLD. Hepatology. 70:1582–1599. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhou J, Song Q, Li H, Han Y, Pu Y, Li L,
Rong W, Liu X, Wang Z, Sun J, et al: Targeting
circ-0034880-enriched tumor extracellular vesicles to impede
SPP1highCD206+ pro-tumor macrophages mediated
pre-metastatic niche formation in colorectal cancer liver
metastasis. Mol Cancer. 23:1682024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shao Y, Chen T, Zheng X, Yang S, Xu K,
Chen X, Xu F, Wang L, Shen Y, Wang T, et al: Colorectal
cancer-derived small extracellular vesicles establish an
inflammatory premetastatic niche in liver metastasis.
Carcinogenesis. 39:1368–1379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Takano Y, Masuda T, Iinuma H, Yamaguchi R,
Sato K, Tobo T, Hirata H, Kuroda Y, Nambara S, Hayashi N, et al:
Circulating exosomal microRNA-203 is associated with metastasis
possibly via inducing tumor-associated macrophages in colorectal
cancer. Oncotarget. 8:78598–78613. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu
Y, Zhang Z, Cai S, Xu Y, Li X, et al: Tumor-derived exosomal
miR-934 induces macrophage M2 polarization to promote liver
metastasis of colorectal cancer. J Hematol Oncol. 13:1562020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sun H, Meng Q, Shi C, Yang H, Li X, Wu S,
Familiari G, Relucenti M, Aschner M, Wang X and Chen R:
Hypoxia-inducible exosomes facilitate liver-tropic premetastatic
niche in colorectal cancer. Hepatology. 74:2633–2651. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Li S, Fu X, Ning D, Liu Q, Zhao J, Cheng
Q, Chen X and Jiang L: Colon cancer exosome-associated HSP90B1
initiates pre-metastatic niche formation in the liver by polarizing
M1 macrophage into M2 phenotype. Biol Direct. 20:522025. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Liang Y, Li J, Yuan Y, Ju H, Liao H, Li M,
Liu Y, Yao Y, Yang L, Li T and Lei X: Exosomal miR-106a-5p from
highly metastatic colorectal cancer cells drives liver metastasis
by inducing macrophage M2 polarization in the tumor
microenvironment. J Exp Clin Cancer Res. 43:2812024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wei X, Ye J, Pei Y, Wang C, Yang H, Tian
J, Si G, Ma Y, Wang K and Liu G: Extracellular vesicles from
colorectal cancer cells promote metastasis via the NOD1 signalling
pathway. J Extracell Vesicles. 11:e122642022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu
K, Hu X, Bu Z, Peng J, Ren X and Zhang Z: Immune phenotypic linkage
between colorectal cancer and liver metastasis. Cancer Cell.
40:424–437.e5. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D,
Cheng Y, Huang S, Liu Y, Jiang S, et al: Spatiotemporal immune
landscape of colorectal cancer liver metastasis at Single-cell
level. Cancer Discov. 12:134–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Eruslanov EB, Bhojnagarwala PS, Quatromoni
JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A,
Litzky L, Hancock WW, et al: Tumor-associated neutrophils stimulate
T cell responses in early-stage human lung cancer. J Clin Invest.
124:5466–5480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Germann M, Zangger N, Sauvain MO, Sempoux
C, Bowler AD, Wirapati P, Kandalaft LE, Delorenzi M, Tejpar S,
Coukos G and Radtke F: Neutrophils suppress tumor-infiltrating T
cells in colon cancer via matrix metalloproteinase-mediated
activation of TGFβ. EMBO Mol Med. 12:e106812020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Itatani Y, Yamamoto T, Zhong C, Molinolo
AA, Ruppel J, Hegde P, Taketo MM and Ferrara N: Suppressing
neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF
antibody in a genetic model of colorectal cancer. Proc Natl Acad
Sci USA. 117:21598–21608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gordon-Weeks AN, Lim SY, Yuzhalin AE,
Jones K, Markelc B, Kim KJ, Buzzelli JN, Fokas E, Cao Y, Smart S
and Muschel R: Neutrophils promote hepatic metastasis growth
through fibroblast growth factor 2-dependent angiogenesis in mice.
Hepatology. 65:1920–1935. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yang L, Liu L, Zhang R, Hong J, Wang Y,
Wang J, Zuo J, Zhang J, Chen J and Hao H: IL-8 mediates a positive
loop connecting increased neutrophil extracellular traps (NETs) and
colorectal cancer liver metastasis. J Cancer. 11:4384–4396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tan H, Jiang Y, Shen L, Nuerhashi G, Wen
C, Gu L, Wang Y, Qi H, Cao F, Huang T, et al: Cryoablation-induced
neutrophil Ca2+ elevation and NET formation exacerbate
immune escape in colorectal cancer liver metastasis. J Exp Clin
Cancer Res. 43:3192024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jiang Y, Long G, Huang X, Wang W, Cheng B
and Pan W: Single-cell transcriptomic analysis reveals dynamic
changes in the liver microenvironment during colorectal cancer
metastatic progression. J Transl Med. 23:3362025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Seubert B, Grünwald B, Kobuch J, Cui H,
Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N,
et al: Tissue inhibitor of metalloproteinases (TIMP)-1 creates a
premetastatic niche in the liver through SDF-1/CXCR4-dependent
neutrophil recruitment in mice. Hepatology. 61:238–248. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang H, Zhang B, Li R, Chen J, Xu G, Zhu
Y, Li J, Liang Q, Hua Q, Wang L, et al: KIAA1199 drives immune
suppression to promote colorectal cancer liver metastasis by
modulating neutrophil infiltration. Hepatology. 76:967–981. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wu J, Song J, Ge Y, Hou S, Chang Y, Chen
X, Nie Z, Guo L and Yin J: PRIM1 enhances colorectal cancer liver
metastasis via promoting neutrophil recruitment and formation of
neutrophil extracellular trap. Cell Signal. 132:1118222025.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang QQ, Hu XW, Liu YL, Ye ZJ, Gui YH,
Zhou DL, Qi CL, He XD, Wang H and Wang LJ: CD11b deficiency
suppresses intestinal tumor growth by reducing myeloid cell
recruitment. Sci Rep. 5:159482015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cao X, Lan Q, Xu H, Liu W, Cheng H, Hu X,
He J, Yang Q, Lai W and Chu Z: Granulocyte-like myeloid-derived
suppressor cells: The culprits of neutrophil extracellular traps
formation in the pre-metastatic niche. Int Immunopharmacol.
143:1135002024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lim SY, Gordon-Weeks AN, Zhao L, Tapmeier
TT, Im JH, Cao Y, Beech J, Allen D, Smart S and Muschel RJ:
Recruitment of myeloid cells to the tumor microenvironment supports
liver metastasis. Oncoimmunology. 2:e231872013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhao L, Lim SY, Gordon-Weeks AN, Tapmeier
TT, Im JH, Cao Y, Beech J, Allen D, Smart S and Muschel RJ:
Recruitment of a myeloid cell subset (CD11b/Gr1 mid) via CCL2/CCR2
promotes the development of colorectal cancer liver metastasis.
Hepatology. 57:829–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chun E, Lavoie S, Michaud M, Gallini CA,
Kim J, Soucy G, Odze R, Glickman JN and Garrett WS: CCL2 promotes
colorectal carcinogenesis by enhancing polymorphonuclear
myeloid-derived suppressor cell population and function. Cell Rep.
12:244–257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Inamoto S, Itatani Y, Yamamoto T,
Minamiguchi S, Hirai H, Iwamoto M, Hasegawa S, Taketo MM, Sakai Y
and Kawada K: Loss of SMAD4 promotes colorectal cancer progression
by accumulation of myeloid-derived suppressor cells through the
CCL15-CCR1 chemokine axis. Clin Cancer Res. 22:492–501. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang D, Sun H, Wei J, Cen B and DuBois RN:
CXCL1 is critical for premetastatic niche formation and metastasis
in colorectal cancer. Cancer Res. 77:3655–3665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dang Y, Yu J, Zhao S, Cao X and Wang Q:
HOXA7 promotes the metastasis of KRAS mutant colorectal cancer by
regulating myeloid-derived suppressor cells. Cancer Cell Int.
22:882022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ren X, Xiao J, Zhang W, Wang F, Yan Y, Wu
X, Zeng Z, He Y, Yang W, Liao W, et al: Inhibition of CCL7 derived
from Mo-MDSCs prevents metastatic progression from latency in
colorectal cancer. Cell Death Dis. 12:4842021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lin Q, Ren L, Jian M, Xu P, Li J, Zheng P,
Feng Q, Yang L, Ji M, Wei Y and Xu J: The mechanism of the
premetastatic niche facilitating colorectal cancer liver metastasis
generated from myeloid-derived suppressor cells induced by the
S1PR1-STAT3 signaling pathway. Cell Death Dis. 10:6932019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang Y, Davis C, Shah S, Hughes D, Ryan
JC, Altomare D and Peña MM: IL-33 promotes growth and liver
metastasis of colorectal cancer in mice by remodeling the tumor
microenvironment and inducing angiogenesis. Mol Carcinog.
56:272–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Gu Y, Mi Y, Cao Y, Yu K, Zhang Z, Lian P,
Li D, Qin J and Zhao S: The lncRNA MIR181A1HG in extracellular
vesicles derived from highly metastatic colorectal cancer cells
promotes liver metastasis by remodeling the extracellular matrix
and recruiting myeloid-derived suppressor cells. Cell Biosci.
15:232025. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kobie JJ, Wu RS, Kurt RA, Lou S, Adelman
MK, Whitesell LJ, Ramanathapuram LV, Arteaga CL and Akporiaye ET:
Transforming growth factor beta inhibits the antigen-presenting
functions and antitumor activity of dendritic cell vaccines. Cancer
Res. 63:1860–1864. 2003.PubMed/NCBI
|
|
96
|
Orsini G, Legitimo A, Failli A, Ferrari P,
Nicolini A, Spisni R, Miccoli P and Consolini R: Defective
generation and maturation of dendritic cells from monocytes in
colorectal cancer patients during the course of disease. Int J Mol
Sci. 14:22022–22041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nagorsen D, Voigt S, Berg E, Stein H,
Thiel E and Loddenkemper C: Tumor-infiltrating macrophages and
dendritic cells in human colorectal cancer: Relation to local
regulatory T cells, systemic T-cell response against
tumor-associated antigens and survival. J Transl Med. 5:622007.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hsu YL, Chen YJ, Chang WA, Jian SF, Fan
HL, Wang JY and Kuo PL: Interaction between tumor-associated
dendritic cells and colon cancer cells contributes to tumor
progression via CXCL1. Int J Mol Sci. 19:24272018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Huang TX, Tan XY, Huang HS, Li YT, Liu BL,
Liu KS, Chen X, Chen Z, Guan XY, Zou C and Fu L: Targeting
cancer-associated fibroblast-secreted WNT2 restores dendritic
cell-mediated antitumour immunity. Gut. 71:333–344. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Sun Y, Hu H, Liu Z, Xu J, Gao Y, Zhan X,
Zhou S, Zhong W, Wu D, Wang P, et al: Macrophage STING signaling
promotes NK cell to suppress colorectal cancer liver metastasis via
4-1BBL/4-1BB co-stimulation. J Immunother Cancer. 11:e0064812023.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Donadon M, Hudspeth K, Cimino M, Di
Tommaso L, Preti M, Tentorio P, Roncalli M, Mavilio D and Torzilli
G: Increased infiltration of natural killer and T cells in
colorectal liver metastases improves patient overall survival. J
Gastrointest Surg. 21:1226–1236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Dupaul-Chicoine J, Arabzadeh A, Dagenais
M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton
V, Colpitts SL, Beauchemin N and Saleh M: The Nlrp3 inflammasome
suppresses colorectal cancer metastatic growth in the liver by
promoting natural killer cell tumoricidal activity. Immunity.
43:751–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Takeda K, Hayakawa Y, Smyth MJ, Kayagaki
N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H and Okumura K:
Involvement of tumor necrosis factor-related apoptosis-inducing
ligand in surveillance of tumor metastasis by liver natural killer
cells. Nat Med. 7:94–100. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
104
|
Russo E, D'Aquino C, Di Censo C,
Laffranchi M, Tomaipitinca L, Licursi V, Garofalo S, Promeuschel J,
Peruzzi G, Sozio F, et al: Cxcr3 promotes protection from
colorectal cancer liver metastasis by driving NK cell infiltration
and plasticity. J Clin Invest. 135:e1840362025. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Harmon C, Robinson MW, Hand F, Almuaili D,
Mentor K, Houlihan DD, Hoti E, Lynch L, Geoghegan J and O'Farrelly
C: Lactate-mediated acidification of tumor microenvironment induces
apoptosis of liver-resident NK cells in colorectal liver
metastasis. Cancer Immunol Res. 7:335–346. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Fang H, Dai W, Gu R, Zhang Y, Li J, Luo W,
Tong S, Han L, Wang Y, Jiang C, et al: myCAF-derived exosomal PWAR6
accelerates CRC liver metastasis via altering glutamine
availability and NK cell function in the tumor microenvironment. J
Hematol Oncol. 17:1262024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Matsumura H, Kondo T, Ogawa K, Tamura T,
Fukunaga K, Murata S and Ohkohchi N: Kupffer cells decrease
metastasis of colon cancer cells to the liver in the early stage.
Int J Oncol. 45:2303–2310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wortzel I, Seo Y, Akano I, Shaashua L,
Tobias GC, Hebert J, Kim KA, Kim D, Dror S, Liu Y, et al: Unique
structural configuration of EV-DNA primes Kupffer cell-mediated
antitumor immunity to prevent metastatic progression. Nat Cancer.
5:1815–1833. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lu WP, Liu YD, Zhang ZF, Liu J, Ye JW,
Wang SY, Lin XY, Lai YR, Li J, Liu SY, et al:
m6A-modified MIR670HG suppresses tumor liver metastasis
through enhancing Kupffer cell phagocytosis. Cell Mol Life Sci.
82:1852025. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li J, Liu XG, Ge RL, Yin YP, Liu YD, Lu
WP, Huang M, He XY, Wang J, Cai G, et al: The ligation between
ERMAP, galectin-9 and dectin-2 promotes Kupffer cell phagocytosis
and antitumor immunity. Nat Immunol. 24:1813–1824. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bresesti C, Carito E, Notaro M, Giacca G,
Breggion S, Kerzel T, Mercado CM, Beretta S, Monti M, Merelli I, et
al: Reprogramming liver metastasis-associated macrophages toward an
anti-tumoral phenotype through enforced miR-342 expression. Cell
Rep. 44:1155922025. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nater M, Brügger M, Cecconi V, Pereira P,
Forni G, Köksal H, Dimakou D, Herbst M, Calvanese AL, Lucchiari G,
et al: Hepatic iNKT cells facilitate colorectal cancer metastasis
by inducing a fibrotic niche in the liver. iScience. 28:1123642025.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Gassmann P, Hemping-Bovenkerk A, Mees ST
and Haier J: Metastatic tumor cell arrest in the liver-lumen
occlusion and specific adhesion are not exclusive. Int J Colorectal
Dis. 24:851–858. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Haier J, Korb T, Hotz B, Spiegel HU and
Senninger N: An intravital model to monitor steps of metastatic
tumor cell adhesion within the hepatic microcirculation. J
Gastrointest Surg. 7:507–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Khatib AM, Fallavollita L, Wancewicz EV,
Monia BP and Brodt P: Inhibition of hepatic endothelial E-selectin
expression by C-raf antisense oligonucleotides blocks colorectal
carcinoma liver metastasis. Cancer Res. 62:5393–5398.
2002.PubMed/NCBI
|
|
116
|
Khatib AM, Auguste P, Fallavollita L, Wang
N, Samani A, Kontogiannea M, Meterissian S and Brodt P:
Characterization of the host proinflammatory response to tumor
cells during the initial stages of liver metastasis. Am J Pathol.
167:749–759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Huang WH, Zhou MW, Zhu YF, Xiang JB, Li
ZY, Wang ZH, Zhou YM, Yang Y, Chen ZY and Gu XD: The role of
hepatic stellate cells in promoting liver metastasis of colorectal
carcinoma. Onco Targets Ther. 12:7573–7580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zeng X, Zhou J, Xiong Z, Sun H, Yang W,
Mok MTS, Wang J, Li J, Liu M, Tang W, et al: Cell cycle-related
kinase reprograms the liver immune microenvironment to promote
cancer metastasis. Cell Mol Immunol. 18:1005–1015. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yang Y, Chen Y, Liu Z, Chang Z, Sun Z and
Zhao L: Concomitant NAFLD facilitates liver metastases and
PD-1-refractory by recruiting MDSCs via CXCL5/CXCR2 in Colorectal
Cancer. Cell Mol Gastroenterol Hepatol. 18:1013512024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang Z, Kim SY, Tu W, Kim J, Xu A, Yang
YM, Matsuda M, Reolizo L, Tsuchiya T, Billet S, et al:
Extracellular vesicles in fatty liver promote a metastatic tumor
microenvironment. Cell Metab. 35:1209–1226.e13. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ruff SM, Brown ZJ and Pawlik TM: A review
of targeted therapy and immune checkpoint inhibitors for metastatic
colorectal cancer. Surg Oncol. 51:1019932023. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hernandez Dominguez O, Yilmaz S and Steele
SR: Stage IV colorectal cancer management and treatment. J Clin
Med. 12:20722023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Cheng XF, Zhao F, Chen D and Liu FL:
Current landscape of preoperative neoadjuvant therapies for initial
resectable colorectal cancer liver metastasis. World J
Gastroenterol. 30:663–672. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Tatsuta K, Sakata M, Kojima T, Booka E,
Kurachi K and Takeuchi H: Updated insights into the impact of
adjuvant chemotherapy on recurrence and survival after curative
resection of liver or lung metastases in colorectal cancer: A rapid
review and meta-analysis. World J Surg Oncol. 23:562025. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Yarom N and Jonker DJ: The role of the
epidermal growth factor receptor in the mechanism and treatment of
colorectal cancer. Discov Med. 11:95–105. 2011.PubMed/NCBI
|
|
126
|
Jonker DJ, O'Callaghan CJ, Karapetis CS,
Zalcberg JR, Tu D, Au HJ, Au HJ, Berry SR, Krahn M, Price T, et al:
Cetuximab for the treatment of colorectal cancer. N Engl J Med.
357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin, and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Misale S, Yaeger R, Hobor S, Scala E,
Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M,
Siravegna G, et al: Emergence of KRAS mutations and acquired
resistance to anti-EGFR therapy in colorectal cancer. Nature.
486:532–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Randomized, phase III trial of panitumumab with
infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4)
versus FOLFOX4 alone as first-line treatment in patients with
previously untreated metastatic colorectal cancer: The PRIME study.
J Clin Oncol. 28:4697–4705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Douillard JY, Siena S, Cassidy J,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Final results from PRIME: Randomized phase III
study of panitumumab with FOLFOX4 for first-line treatment of
metastatic colorectal cancer. Ann Oncol. 25:1346–1355. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Choi HY and Chang JE: Targeted therapy for
cancers: From ongoing clinical trials to FDA-Approved drugs. Int J
Mol Sci. 24:136182023. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Cao D, Zheng Y, Xu H, Ge W and Xu X:
Bevacizumab improves survival in metastatic colorectal cancer
patients with primary tumor resection: A meta-analysis. Sci Rep.
9:203262019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Tang W, Ren L, Liu T, Ye Q, Wei Y, He G,
Lin Q, Wang X, Wang M, Liang F, et al: Bevacizumab Plus mFOLFOX6
versus mFOLFOX6 Alone as First-line treatment for RAS mutant
unresectable colorectal Liver-limited metastases: The BECOME
randomized controlled trial. J Clin Oncol. 38:3175–3184. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Debeuckelaere C, Murgioni S, Lonardi S,
Girardi N, Alberti G, Fano C, Gallimberti S, Magro C,
Ahcene-Djaballah S, Daniel F, et al: Ramucirumab: The long and
winding road toward being an option for mCRC treatment. Expert Opin
Biol Ther. 19:399–409. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): A randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: Regorafenib monotherapy for previously treated metastatic
colorectal cancer (CORRECT): An international, multicentre,
randomised, placebo-controlled, phase 3 trial. Lancet. 381:303–312.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Goel G: Evolution of regorafenib from
bench to bedside in colorectal cancer: Is it an attractive option
or merely a ‘me too’ drug? Cancer Manag Res. 10:425–437. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhang Y, Zou JY, Wang Z and Wang Y:
Fruquintinib: A novel antivascular endothelial growth factor
receptor tyrosine kinase inhibitor for the treatment of metastatic
colorectal cancer. Cancer Manag Res. 11:7787–7803. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y,
Yang L, Deng Y, Chen ZD, Zhong H, et al: Effect of fruquintinib vs
placebo on overall survival in patients with previously treated
metastatic colorectal cancer: The FRESCO randomized clinical trial.
JAMA. 319:2486–2496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Dasari A, Lonardi S, Garcia-Carbonero R,
Elez E, Yoshino T, Sobrero A, Yao J, García-Alfonso P, Kocsis J,
Cubillo Gracian A, et al: Fruquintinib versus placebo in patients
with refractory metastatic colorectal cancer (FRESCO-2): An
international, multicentre, randomised, double-blind, phase 3
study. Lancet. 402:41–53. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Fusco MJ, Casak SJ, Mushti SL, Cheng J,
Christmas BJ, Thompson MD, Fu W, Wang H, Yoon M, Yang Y, et al: FDA
approval summary: Fruquintinib for the treatment of refractory
metastatic colorectal cancer. Clin Cancer Res. 30:3100–3104. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Mc Neil V and Lee SW: Advancing cancer
treatment: A review of immune checkpoint inhibitors and combination
STrategies. Cancers (Basel). 17:14082025. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Overman MJ, McDermott R, Leach JL, Lonardi
S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, et al:
Nivolumab in patients with metastatic DNA mismatch Repair-deficient
or microsatellite instability-high colorectal cancer (CheckMate
142): An open-label, multicentre, phase 2 study. Lancet Oncol.
18:1182–1191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in Microsatellite-Instability-High advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Weng J, Li S, Zhu Z, Liu Q, Zhang R, Yang
Y and Li X: Exploring immunotherapy in colorectal cancer. J Hematol
Oncol. 15:952022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 Blockade in tumors with Mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Yu J, Green MD, Li S, Sun Y, Journey SN,
Choi JE, Rizvi SM, Qin A, Waninger JJ, Lang X, et al: Liver
metastasis restrains immunotherapy efficacy via macrophage-mediated
T cell elimination. Nat Med. 27:152–164. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Saberzadeh-Ardestani B, Jones JC,
McWilliams RR, Tougeron D, Halfdanarson TR, Guimbaud R, Hubbard JM,
Flecchia C, Shi Q, Alouani E, et al: Metastatic site and clinical
outcome of patients with deficient mismatch repair metastatic
colorectal cancer treated with an immune checkpoint inhibitor in
the first-line setting. Eur J Cancer. 196:1134332024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wang C, Sandhu J, Ouyang C, Ye J, Lee PP
and Fakih M: Clinical response to immunotherapy targeting
programmed cell death Receptor 1/Programmed cell death Ligand 1 in
patients with treatment-resistant microsatellite stable colorectal
cancer with and without liver metastases. JAMA Netw Open.
4:e21184162021. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Overman MJ, Lonardi S, Wong KYM, Lenz HJ,
Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill
A, et al: Durable clinical benefit with nivolumab plus ipilimumab
in DNA mismatch Repair-deficient/microsatellite instability-high
metastatic colorectal cancer. J Clin Oncol. 36:773–779. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Fukuoka S, Hara H, Takahashi N, Kojima T,
Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et
al: Regorafenib plus nivolumab in patients with advanced gastric or
colorectal cancer: An open-label, dose-escalation, and
dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol.
38:2053–2061. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Yang C, Zhao L, Lin Y, Wang S, Ye Y and
Shen Z: Improving the efficiency of immune checkpoint inhibitors
for metastatic pMMR/MSS colorectal cancer: Options and strategies.
Crit Rev Oncol Hematol. 200:1042042024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Gutiu AG, Zhao L, Marrah AJ, Maher AJ,
Voss BB, Mayberry TG, Cowan BC, Wakefield MR and Fang Y: Promising
immunotherapeutic treatments for colon cancer. Med Oncol.
42:1752025. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Kaviyarasan V, Das A, Deka D, Saha B,
Banerjee A, Sharma NR, Duttaroy AK and Pathak S: Advancements in
immunotherapy for colorectal cancer treatment: a comprehensive
review of strategies, challenges, and future prospective. Int J
Colorectal Dis. 40:12024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Fatemi N, Mirbahari SN, Tierling S,
Sanjabi F, Shahrivari S, AmeliMojarad M, Amelimojarad M, Mirzaei
Rezaei M, Nobaveh P, Totonchi M and Nazemalhosseini Mojarad E:
Emerging frontiers in colorectal cancer therapy: From targeted
molecules to immunomodulatory breakthroughs and cell-based
approaches. Dig Dis Sci. 70:919–942. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Liu N, Xiao X, Zhang Z, Mao C, Wan M and
Shen J: Advances in cancer vaccine research. ACS Biomater Sci Eng.
9:5999–6023. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Liu C, Liu N, Zhang T and Tu Y: Adoptive
immune cell therapy for colorectal cancer. Front Immunol.
16:15579062025. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pérez-Domínguez F, Quezada-Monrás C,
Cárcamo L, Muñoz JP and Carrillo-Beltrán D: Oncolytic viruses as a
novel therapeutic approach for colorectal cancer: Mechanisms,
current advances, and future directions. Cancers (Basel).
17:18542025. View Article : Google Scholar : PubMed/NCBI
|