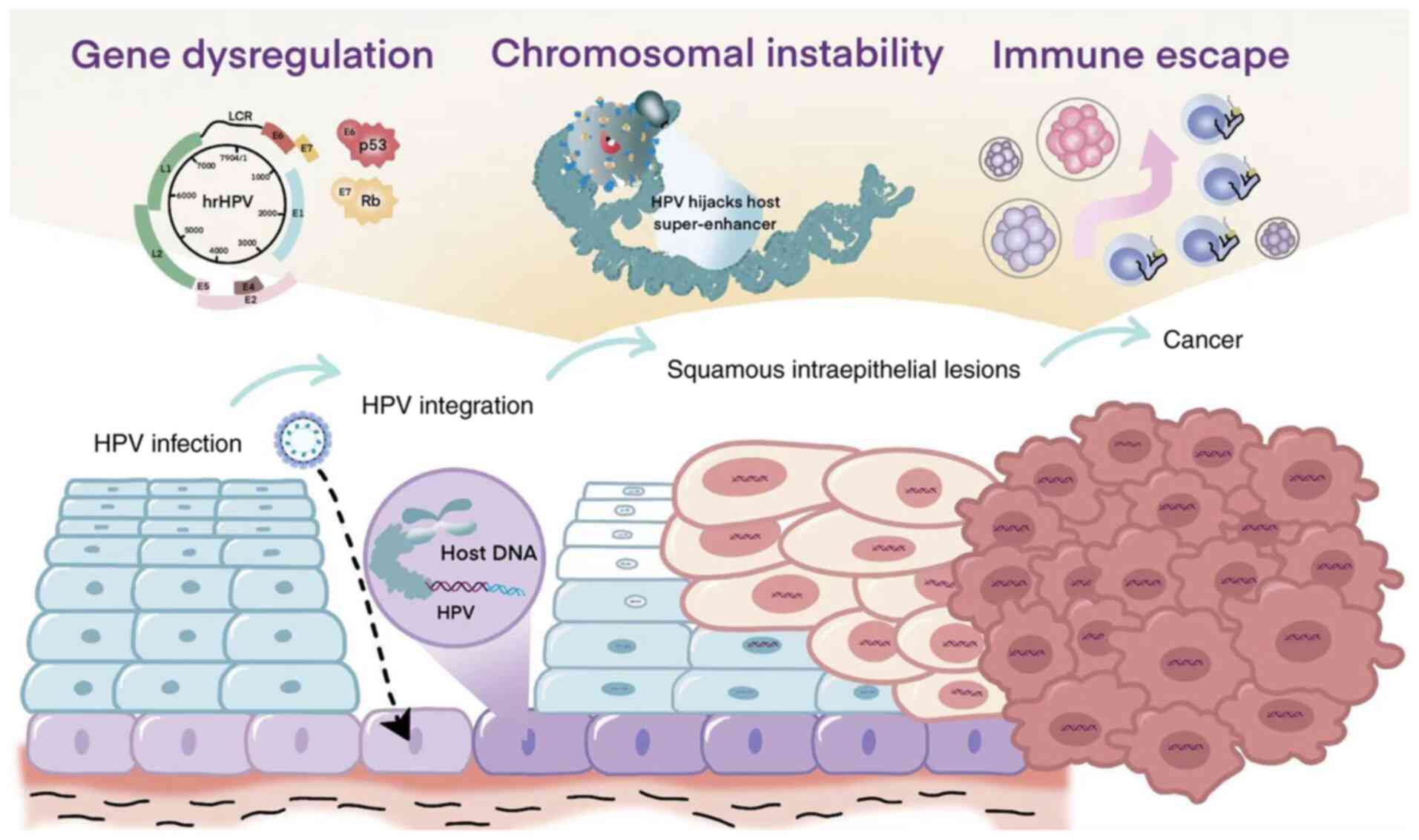
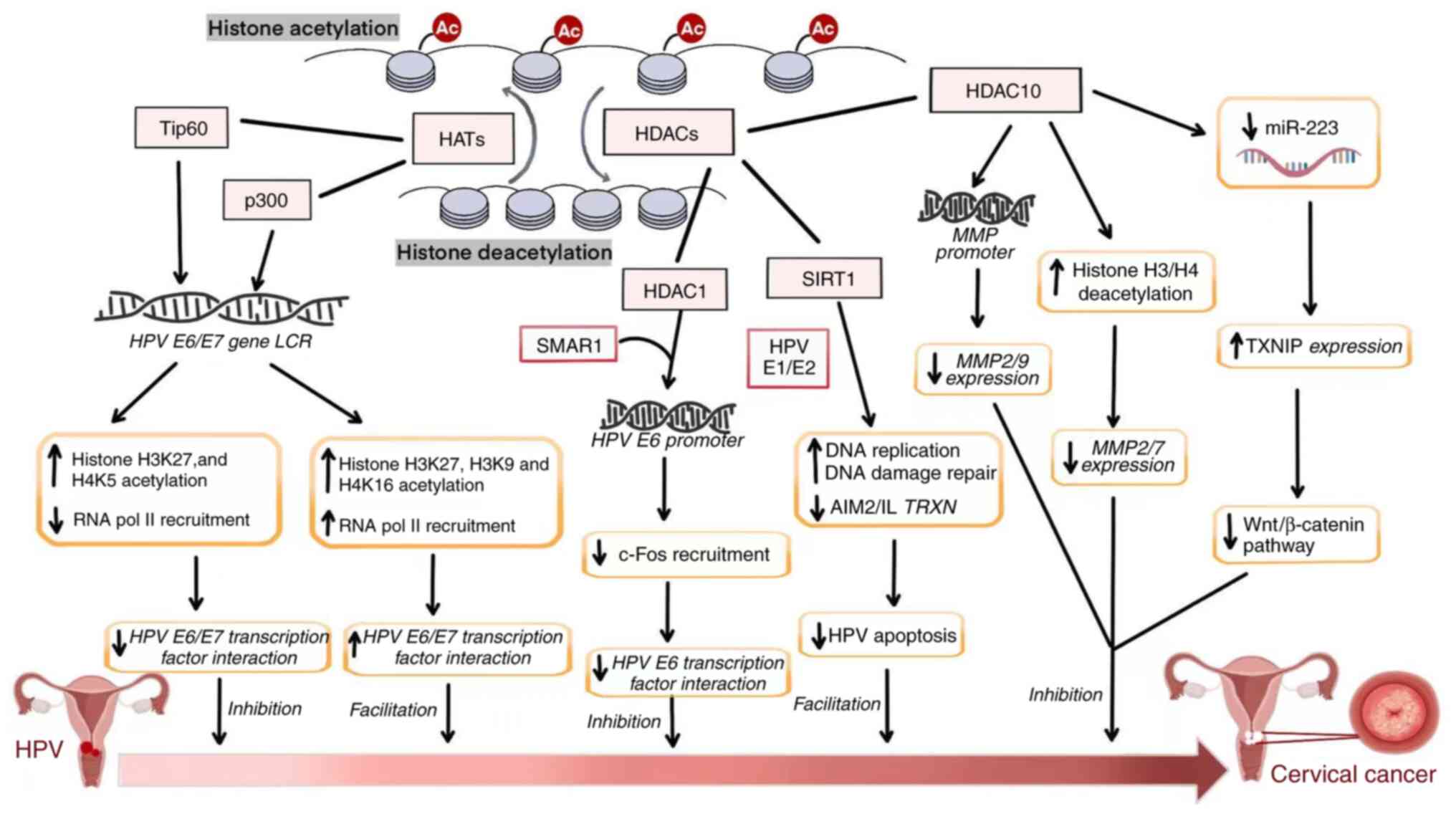
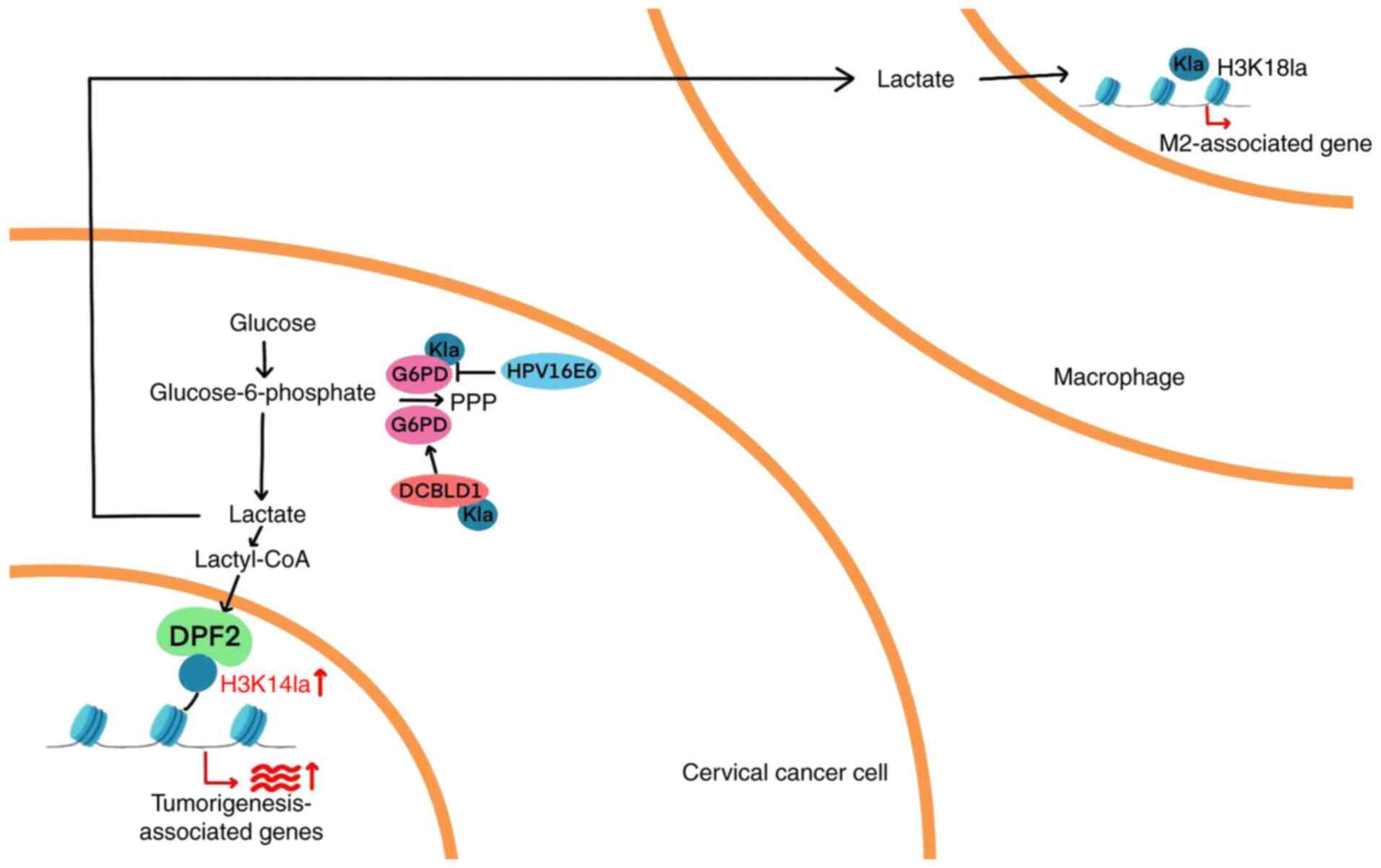
|
1
|
Vu M, Yu J, Awolude OA and Chuang L:
Cervical cancer worldwide. Curr Probl Cancer. 42:457–465. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao W, Qin K, Li F and Chen W:
Socioeconomic inequalities in cancer incidence and mortality: An
analysis of GLOBOCAN 2022. Chin Med J (Engl). 137:1407–1413. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma S, Deep A and Sharma AK: Current
treatment for cervical cancer: An update. Anticancer Agents Med
Chem. 20:1768–1779. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caird H, Simkin J, Smith L, Van Niekerk D
and Ogilvie G: The path to eliminating cervical cancer in canada:
Past, present and future directions. Curr Oncol. 29:1117–1122.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrall L, Lin KY, Roden RBS, Hung CF and
Wu TC: Cervical cancer immunotherapy: Facts and hopes. Clin Cancer
Res. 27:4953–4973. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu L, Lanqing G, Huang Z, Xin X, Minglin
L, Fa-Hui L, Zou H and Min J: T cell immunotherapy for cervical
cancer: Challenges and opportunities. Front Immunol.
14:11052652023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hake SB, Xiao A and Allis CD: Linking the
epigenetic ‘language’ of covalent histone modifications to cancer.
Br J Cancer. 90:761–769. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vinci MC, Polvani G and Pesce M:
Epigenetic programming and risk: The birthplace of cardiovascular
disease? Stem Cell Rev Rep. 9:241–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu D, Shi Y, Zhang H and Miao C:
Epigenetic mechanisms of Immune remodeling in sepsis: Targeting
histone modification. Cell Death Dis. 14:1122023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan X, Sun S, Yang H, Ma H, Zhao C, Niu W,
Fan J, Fang Z and Chen X: SETD2 palmitoylation mediated by ZDHHC16
in epidermal growth factor receptor-mutated glioblastoma promotes
ionizing radiation-induced DNA damage. Int J Radiat Oncol Biol
Phys. 113:648–660. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao X, Kuo CW, Main A, Brown E, Rios FJ,
Camargo LL, Mary S, Wypijewski K, Gök C, Touyz RM and Fuller W:
Palmitoylation regulates cellular distribution of and transmembrane
Ca flux through TrpM7. Cell Calcium. 106:1026392022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Yu T, Li X, He X, Zhang B and Yang
Y: Role of novel protein acylation modifications in immunity and
its related diseases. Immunology. 173:53–75. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, Shi Z and Bao L: An expanding
repertoire of protein acylations. Mol Cell Proteomics.
21:1001932022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zaib S, Rana N and Khan I: Histone
modifications and their role in epigenetics of cancer. Curr Med
Chem. 29:2399–2411. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park J, Lee K, Kim K and Yi SJ: The role
of histone modifications: From neurodevelopment to neurodiseases.
Signal Transduct Target Ther. 7:2172022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maksimovic I and David Y: Non-enzymatic
covalent modifications as a new chapter in the histone code. Trends
Biochem Sci. 46:718–730. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Srivastava R and Ahn SH: Modifications of
RNA polymerase II CTD: Connections to the histone code and cellular
function. Biotechnol Adv. 33:856–872. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin ML and Jeong KW: Histone modifications
in drug-resistant cancers: From a cancer stem cell and immune
evasion perspective. Exp Mol Med. 55:1333–1347. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Ren B, Ren J, Yang G, Fang Y, Wang
X, Zhou F, You L and Zhao Y: Epigenetic reprogramming-induced
guanidinoacetic acid synthesis promotes pancreatic cancer
metastasis and transcription-activating histone modifications. J
Exp Clin Cancer Res. 42:1552023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dueñas-González A, Lizano M, Candelaria M,
Cetina L, Arce C and Cervera E: Epigenetics of cervical cancer. An
overview and therapeutic perspectives. Mol Cancer. 4:382005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu M, Cao C, Wu P, Huang X and Ma D:
Advances in cervical cancer: Current insights and future
directions. Cancer Commun (Lond). 45:77–109. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
23
|
Gavinski K and DiNardo D: Cervical cancer
screening. Med Clin North Am. 107:259–269. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rahangdale L, Mungo C, O'Connor S,
Chibwesha CJ and Brewer NT: Human papillomavirus vaccination and
cervical cancer risk. BMJ. 379:e0701152022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sahasrabuddhe VV: Cervical cancer:
Precursors and prevention. Hematol Oncol Clin North Am. 38:771–781.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Viveros-Carreño D, Fernandes A and Pareja
R: Updates on cervical cancer prevention. Int J Gynecol Cancer.
33:394–402. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ang DJM and Chan JJ: Evolving standards
and future directions for systemic therapies in cervical cancer. J
Gynecol Oncol. 35:e652024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mayadev JS, Ke G, Mahantshetty U, Pereira
MD, Tarnawski R and Toita T: Global challenges of radiotherapy for
the treatment of locally advanced cervical cancer. Int J Gynecol
Cancer. 32:436–445. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Revathidevi S, Murugan AK, Nakaoka H,
Inoue I and Munirajan AK: APOBEC: A molecular driver in cervical
cancer pathogenesis. Cancer Lett. 496:104–116. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Willemsen A and Bravo IG: Origin and
evolution of papillomavirus (onco)genes and genomes. Philos Trans R
Soc Lond B Biol Sci. 374:201803032019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Olusola P, Banerjee HN, Philley JV and
Dasgupta S: Human papilloma virus-associated cervical cancer and
health disparities. Cells. 8:6222019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Doorbar J, Egawa N, Griffin H, Kranjec C
and Murakami I: Human papillomavirus molecular biology and disease
association. Rev Med Virol. 25 (Suppl 1):S2–S23. 2015. View Article : Google Scholar
|
|
34
|
Venuti A, Paolini F, Nasir L, Corteggio A,
Roperto S, Campo MS and Borzacchiello G: Papillomavirus E5: The
smallest oncoprotein with many functions. Mol Cancer. 10:1402011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Idres YM, McMillan NAJ and Idris A:
Hyperactivating p53 in human papillomavirus-driven cancers: A
potential therapeutic intervention. Mol Diagn Ther. 26:301–308.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoppe-Seyler K, Bossler F, Braun JA,
Herrmann AL and Hoppe-Seyler F: The HPV E6/E7 oncogenes: Key
factors for viral carcinogenesis and therapeutic targets. Trends
Microbiol. 26:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bhattacharjee R, Das SS, Biswal SS, Nath
A, Das D, Basu A, Malik S, Kumar L, Kar S, Singh SK, et al:
Mechanistic role of HPV-associated early proteins in cervical
cancer: Molecular pathways and targeted therapeutic strategies.
Crit Rev Oncol Hematol. 174:1036752022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao F, Yin J, Wang Y, Li H and Wang D:
miR-182 promotes cervical cancer progression via activating the
Wnt/β-catenin axis. Am J Cancer Res. 13:3591–3598. 2023.PubMed/NCBI
|
|
39
|
Maliekal TT, Bajaj J, Giri V, Subramanyam
D and Krishna S: The role of Notch signaling in human cervical
cancer: Implications for solid tumors. Oncogene. 27:5110–5114.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amboree TL, Paguio J and Sonawane K: HPV
vaccine: the key to eliminating cervical cancer inequities. BMJ.
385:q9962024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abu-Rustum NR, Yashar CM, Arend R, Barber
E, Bradley K, Brooks R, Campos SM, Chino J, Chon HS, Crispens MA,
et al: NCCN Guidelines® insights: Cervical cancer,
version 1.2024. J Natl Compr Canc Netw. 21:1224–1233. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kasius JC, van der Velden J, Denswil NP,
Tromp JM and Mom CH: Neo-adjuvant chemotherapy in fertility-sparing
cervical cancer treatment. Best Pract Res Clin Obstet Gynaecol.
75:82–100. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27:e432016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Turinetto M, Valsecchi AA, Tuninetti V,
Scotto G, Borella F and Valabrega G: Immunotherapy for cervical
cancer: Are we ready for prime time? Int J Mol Sci. 23:35592022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grau JF, Farinas-Madrid L, Garcia-Duran C,
Garcia-Illescas D and Oaknin A: Advances in immunotherapy in
cervical cancer. Int J Gynecol Cancer. 33:403–413. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang H, Nie CP, Liu XF, Song B, Yue JH,
Xu JX, He J, Li K, Feng YL, Wan T, et al: Phase I study of adjuvant
immunotherapy with autologous tumor-infiltrating lymphocytes in
locally advanced cervical cancer. J Clin Invest. 132:e1577262022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, Cao Y, Liu Y, Yu L, Zhang Z, Wang X,
Bai H, Zhang Y, Liu S, Gao M, et al: Multiomics profiling reveals
the benefits of gamma-delta (γδ) T lymphocytes for improving the
tumor microenvironment, immunotherapy efficacy and prognosis in
cervical cancer. J Immunother Cancer. 12:e0083552024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma Z, Zou X, Yan Z, Chen C, Chen Y and Fu
A: Preliminary analysis of cervical cancer immunotherapy. Am J Clin
Oncol. 45:486–490. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ogasawara A and Hasegawa K: Recent
advances in immunotherapy for cervical cancer. Int J Clin Oncol.
30:434–448. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ramanathan P, Dhandapani H, Jayakumar H,
Seetharaman A and Thangarajan R: Immunotherapy for cervical cancer:
Can it do another lung cancer? Curr Probl Cancer. 42:148–160. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Garzón-Porras AM, Chory E and Gryder BE:
Dynamic opposition of histone modifications. ACS Chem Biol.
18:1027–1036. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Santana DA, Smith MAC and Chen ES: Histone
modifications in Alzheimer's disease. Genes (Basel). 14:3472023.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yao W, Hu X and Wang X: Crossing
epigenetic frontiers: The intersection of novel histone
modifications and diseases. Signal Transduct Target Ther.
9:2322024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhao A, Xu W, Han R, Wei J, Yu Q, Wang M,
Li H, Li M and Chi G: Role of histone modifications in neurogenesis
and neurodegenerative disease development. Ageing Res Rev.
98:1023242024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Y: Modern epigenetics methods in
biological research. Methods. 187:104–113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sahu RK, Dhakshnamoorthy J, Jain S, Folco
HD, Wheeler D and Grewal SIS: Nucleosome remodeler exclusion by
histone deacetylation enforces heterochromatic silencing and
epigenetic inheritance. Mol Cell. 84:3175–3191.e8. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Perez MF and Sarkies P: Histone
methyltransferase activity affects metabolism in human cells
independently of transcriptional regulation. PLoS Biol.
21:e30023542023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Casciello F, Windloch K, Gannon F and Lee
JS: Functional role of G9a histone methyltransferase in cancer.
Front Immunol. 6:4872015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li S: Implication of posttranslational
histone modifications in nucleotide excision repair. Int J Mol Sci.
13:12461–12486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gao J, Liu R, Huang K, Li Z, Sheng X,
Chakraborty K, Han C, Zhang D, Becker L and Zhao Y: Dynamic
investigation of hypoxia-induced L-lactylation. Proc Natl Acad Sci
USA. 122:e24048991222025. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dong W, Lu J, Li Y, Zeng J, Du X, Yu A,
Zhao X, Chi F, Xi Z and Cao S: SIRT1: A novel regulator in
colorectal cancer. Biomed Pharmacother. 178:1171762024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yang Y, Liu Y, Wang Y, Chao Y, Zhang J,
Jia Y, Tie J and Hu D: Regulation of SIRT1 and its roles in
inflammation. Front Immunol. 13:8311682022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Fang Y, Yang C, Yu Z, Li X, Mu Q, Liao G
and Yu B: Natural products as LSD1 inhibitors for cancer therapy.
Acta Pharm Sin B. 11:621–631. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Marsolier J, Prompsy P, Durand A, Lyne AM,
Landragin C, Trouchet A, Bento ST, Eisele A, Foulon S, Baudre L, et
al: H3K27me3 conditions chemotolerance in triple-negative breast
cancer. Nat Genet. 54:459–468. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang K, Jiang X, Jiang Y, Liu J, Du Y,
Zhang Z, Li Y, Zhao X, Li J and Zhang R: EZH2-H3K27me3-mediated
silencing of mir-139-5p inhibits cellular senescence in
hepatocellular carcinoma by activating TOP2A. J Exp Clin Cancer
Res. 42:3202023. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Benard A, van de Velde CJ, Lessard L,
Putter H, Takeshima L, Kuppen PJ and Hoon DS: Epigenetic status of
LINE-1 predicts clinical outcome in early-stage rectal cancer. Br J
Cancer. 109:3073–3083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gerić M, Gajski G and Garaj-Vrhovac V:
γ-H2AX as a biomarker for DNA double-strand breaks in
ecotoxicology. Ecotoxicol Environ Saf. 105:13–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hinohara K, Wu HJ, Vigneau S, McDonald TO,
Igarashi KJ, Yamamoto KN, Madsen T, Fassl A, Egri SB, Papanastasiou
M, et al: KDM5 histone demethylase activity links cellular
transcriptomic heterogeneity to therapeutic resistance. Cancer
Cell. 34:939–953.e9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu H, Ma H, Li Y and Zhao H: Advances in
epigenetic modifications and cervical cancer research. Biochim
Biophys Acta Rev Cancer. 1878:1888942023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Yang X, Sun F, Gao Y, Li M, Liu M, Wei Y,
Jie Q, Wang Y, Mei J, Mei J, et al: Histone acetyltransferase
CSRP2BP promotes the epithelial-mesenchymal transition and
metastasis of cervical cancer cells by activating N-cadherin. J Exp
Clin Cancer Res. 42:2682023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xiang H, Tang H, He Q, Sun J, Yang Y, Kong
L and Wang Y: NDUFA8 is transcriptionally regulated by P300/H3K27ac
and promotes mitochondrial respiration to support proliferation and
inhibit apoptosis in cervical cancer. Biochem Biophys Res Commun.
693:1493742024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Pan B, Liu C, Su J and Xia C: Activation
of AMPK inhibits cervical cancer growth by hyperacetylation of H3K9
through PCAF. Cell Commun Signal. 22:3062024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Qiao L, Zhang Q, Zhang W and Chen JJ: The
lysine acetyltransferase GCN5 contributes to human papillomavirus
oncoprotein E7-induced cell proliferation via up-regulating E2F1. J
Cell Mol Med. 22:5333–5345. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Avvakumov N, Torchia J and Mymryk JS:
Interaction of the HPV E7 proteins with the pCAF acetyltransferase.
Oncogene. 22:3833–3841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Bernat A, Avvakumov N, Mymryk JS and Banks
L: Interaction between the HPV E7 oncoprotein and the
transcriptional coactivator p300. Oncogene. 22:7871–7881. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Groves IJ, Knight EL, Ang QY, Scarpini CG
and Coleman N: HPV16 oncogene expression levels during early
cervical carcinogenesis are determined by the balance of epigenetic
chromatin modifications at the integrated virus genome. Oncogene.
35:4773–4786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zimmermann H, Degenkolbe R, Bernard HU and
O'Connor MJ: The human papillomavirus type 16 E6 oncoprotein can
down-regulate p53 activity by targeting the transcriptional
coactivator CBP/p300. J Virol. 73:6209–6219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zhu J and Han S: Histone deacetylase 10
exerts anti-tumor effects on cervical cancer via a novel
microRNA-223/TXNIP/Wnt/β-catenin pathway. IUBMB Life. Jan
22–2021.(Epub ahead of print). View Article : Google Scholar
|
|
79
|
Lu X, Jin P, Tang Q, Zhou M, Xu H, Su C,
Wang L, Xu F, Zhao M, Yin Y, et al: NAD(+) metabolism reprogramming
drives SIRT1-dependent deacetylation inducing PD-L1 nuclear
localization in cervical cancer. Adv Sci (Weinh). 12:e24121092025.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sun X, Shu Y, Ye G, Wu C, Xu M, Gao R,
Huang D and Zhang J: Histone deacetylase inhibitors inhibit
cervical cancer growth through Parkin acetylation-mediated
mitophagy. Acta Pharm Sin B. 12:838–852. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
He H, Lai Y, Hao Y, Liu Y, Zhang Z, Liu X,
Guo C, Zhang M, Zhou H, Wang N, et al: Selective p300 inhibitor
C646 inhibited HPV E6-E7 genes, altered glucose metabolism and
induced apoptosis in cervical cancer cells. Eur J Pharmacol.
812:206–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lourenço de Freitas N, Deberaldini MG,
Gomes D, Pavan AR, Sousa Â, Dos Santos JL and Soares CP: Histone
deacetylase inhibitors as therapeutic interventions on cervical
cancer induced by human papillomavirus. Front Cell Dev Biol.
8:5928682021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhang T, Zhou C, Lv M, Yu J, Cheng S, Cui
X, Wan X, Ahmad M, X B, Qin J, et al: Trifluoromethyl quinoline
derivative targets inhibiting HDAC1 for promoting the acetylation
of histone in cervical cancer cells. Eur J Pharm Sci.
194:1067062024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu N, Zhao LJ, Li XP, Wang JL, Chai GL
and Wei LH: Histone deacetylase inhibitors inducing human cervical
cancer cell apoptosis by decreasing DNA-methyltransferase 3B. Chin
Med J (Engl). 125:3273–3278. 2012.PubMed/NCBI
|
|
85
|
Li H and Wu X: Histone deacetylase
inhibitor, Trichostatin A, activates p21WAF1/CIP1 expression
through downregulation of c-myc and release of the repression of
c-myc from the promoter in human cervical cancer cells. Biochem
Biophys Res Commun. 324:860–867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wagner W, Ciszewski WM and Kania KD: L-
and D-lactate enhance DNA repair and modulate the resistance of
cervical carcinoma cells to anticancer drugs via histone
deacetylase inhibition and hydroxycarboxylic acid receptor 1
activation. Cell Commun Signal. 13:362015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wasim L and Chopra M: Panobinostat induces
apoptosis via production of reactive oxygen species and synergizes
with topoisomerase inhibitors in cervical cancer cells. Biomed
Pharmacother. 84:1393–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Khanduja JS, Joh RI, Perez MM, Paulo JA,
Palmieri CM, Zhang J, Gulka AOD, Haas W, Gygi SP and Motamedi M:
RNA quality control factors nucleate Clr4/SUV39H and trigger
constitutive heterochromatin assembly. Cell. 187:3262–3283.e23.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Marmorstein R: Structure of SET domain
proteins: A new twist on histone methylation. Trends Biochem Sci.
28:59–62. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Yi Y and Ge S: Targeting the histone H3
lysine 79 methyltransferase DOT1L in MLL-rearranged leukemias. J
Hematol Oncol. 15:352022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang L, Tian S, Pei M, Zhao M, Wang L,
Jiang Y, Yang T, Zhao J, Song L and Yang X: Crosstalk between
histone modification and DNA methylation orchestrates the
epigenetic regulation of the costimulatory factors, Tim-3 and
galectin-9, in cervical cancer. Oncol Rep. 42:2655–2669.
2019.PubMed/NCBI
|
|
92
|
Beyer S, Zhu J, Mayr D, Kuhn C, Schulze S,
Hofmann S, Dannecker C, Jeschke U and Kost BP: Histone H3 acetyl K9
and histone H3 tri methyl K4 as prognostic markers for patients
with cervical cancer. Int J Mol Sci. 18:4772017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chen R, Chen Y, Zhao W, Fang C, Zhou W,
Yang X and Ji M: The role of methyltransferase NSD2 as a potential
oncogene in human solid tumors. Onco Targets Ther. 13:6837–6846.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ansari KI, Kasiri S and Mandal SS: Histone
methylase MLL1 has critical roles in tumor growth and angiogenesis
and its knockdown suppresses tumor growth in vivo. Oncogene.
32:3359–3370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhang L, Tian S, Zhao M, Yang T, Quan S,
Yang Q, Song L and Yang X: SUV39H1-DNMT3A-mediated epigenetic
regulation of Tim-3 and galectin-9 in the cervical cancer. Cancer
Cell Int. 20:3252020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Osawa T, Muramatsu M, Wang F, Tsuchida R,
Kodama T, Minami T and Shibuya M: Increased expression of histone
demethylase JHDM1D under nutrient starvation suppresses tumor
growth via down-regulating angiogenesis. Proc Natl Acad Sci USA.
108:20725–20729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gascoigne KE and Cheeseman IM:
CDK-dependent phosphorylation and nuclear exclusion coordinately
control kinetochore assembly state. J Cell Biol. 201:23–32. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yang D, He Y, Li R, Huang Z, Zhou Y, Shi
Y, Deng Z, Wu J and Gao Y: Histone H3K79 methylation by DOT1L
promotes Aurora B localization at centromeres in mitosis. Cell Rep.
42:1128852023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Banáth JP, Macphail SH and Olive PL:
Radiation sensitivity, H2AX phosphorylation, and kinetics of repair
of DNA strand breaks in irradiated cervical cancer cell lines.
Cancer Res. 64:7144–7149. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhao J, Wang Q, Li J, Si TB, Pei SY, Guo Z
and Jiang C: Comparative study of phosphorylated histone H2AX
expressions in the cervical cancer patients of pre- and
post-neoadjuvant chemotherapy. Eur J Gynaecol Oncol. 36:318–322.
2015.PubMed/NCBI
|
|
101
|
Bañuelos CA, Banáth JP, Kim JY,
Aquino-Parsons C and Olive PL: GammaH2AX expression in tumors
exposed to cisplatin and fractionated irradiation. Clin Cancer Res.
15:3344–3353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Brustmann H, Hinterholzer S and Brunner A:
Expression of phosphorylated histone H2AX (γ-H2AX) in normal and
neoplastic squamous epithelia of the uterine cervix: An
immunohistochemical study with epidermal growth factor receptor.
Int J Gynecol Pathol. 30:76–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Fuhrman CB, Kilgore J, LaCoursiere YD, Lee
CM, Milash BA, Soisson AP and Zempolich KA: Radiosensitization of
cervical cancer cells via double-strand DNA break repair
inhibition. Gynecol Oncol. 110:93–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Zhang L and Zhang S: ZM447439, the Aurora
kinase B inhibitor, suppresses the growth of cervical cancer SiHa
cells and enhances the chemosensitivity to cisplatin. J Obstet
Gynaecol Res. 37:591–600. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Cheung CH, Lin WH, Hsu JT, Hour TC, Yeh
TK, Ko S, Lien TW, Coumar MS, Liu JF, Lai WY, et al: BPR1K653, a
novel Aurora kinase inhibitor, exhibits potent anti-proliferative
activity in MDR1 (P-gp170)-mediated multidrug-resistant cancer
cells. PLoS One. 6:e234852011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhai G, Niu Z, Jiang Z, Zhao F, Wang S,
Chen C, Zheng W, Wang A, Zang Y, Han Y and Zhang K: DPF2 reads
histone lactylation to drive transcription and tumorigenesis. Proc
Natl Acad Sci USA. 121:e24214961212024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Huang C, Xue L, Lin X, Shen Y and Wang X:
Histone lactylation-driven GPD2 mediates M2 macrophage polarization
to promote malignant transformation of cervical cancer progression.
DNA Cell Biol. 43:605–618. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Han X, Xiang X, Yang H, Zhang H, Liang S,
Wei J and Yu J: p300-catalyzed lysine crotonylation promotes the
proliferation, invasion, and migration of HeLa cells via
heterogeneous nuclear ribonucleoprotein A1. Anal Cell Pathol
(Amst). 2020:56323422020.PubMed/NCBI
|
|
109
|
Chen D, Cai B, Zhu Y, Ma Y, Yu X, Xiong J,
Shen J, Tie W, Zhang Y and Guo F: Targeting histone demethylases
JMJD3 and UTX: Selenium as a potential therapeutic agent for
cervical cancer. Clin Epigenetics. 16:512024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kedhari Sundaram M, Hussain A, Haque S,
Raina R and Afroze N: Quercetin modifies 5′CpG promoter methylation
and reactivates various tumor suppressor genes by modulating
epigenetic marks in human cervical cancer cells. J Cell Biochem.
120:18357–18369. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mani E, Medina LA, Isaac-Olivé K and
Dueñas-González A: Radiosensitization of cervical cancer cells with
epigenetic drugs hydralazine and valproate. Eur J Gynaecol Oncol.
35:140–142. 2014.PubMed/NCBI
|
|
112
|
Saenglee S, Jogloy S, Patanothai A, Leid M
and Senawong T: Cytotoxic effects of peanut phenolics possessing
histone deacetylase inhibitory activity in breast and cervical
cancer cell lines. Pharmacol Rep. 68:1102–1110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bishop TR, Subramanian C, Bilotta EM,
Garnar-Wortzel L, Ramos AR, Zhang Y, Asiaban JN, Ott CJ, Rock CO
and Erb MA: Acetyl-CoA biosynthesis drives resistance to histone
acetyltransferase inhibition. Nat Chem Biol. 19:1215–1222. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chan HM and La Thangue NB: p300/CBP
proteins: HATs for transcriptional bridges and scaffolds. J Cell
Sci. 114:2363–2373. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lasko LM, Jakob CG, Edalji RP, Qiu W,
Montgomery D, Digiammarino EL, Hansen TM, Risi RM, Frey R, Manaves
V, et al: Discovery of a selective catalytic p300/CBP inhibitor
that targets lineage-specific tumours. Nature. 550:128–132. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhou Y and Shao C: Histone methylation can
either promote or reduce cellular radiosensitivity by regulating
DNA repair pathways. Mutat Res Rev Mutat Res. 787:1083622021.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mentch SJ and Locasale JW: One-carbon
metabolism and epigenetics: Understanding the specificity. Ann N Y
Acad Sci. 1363:91–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhao Y, Jiang B, Gu Z, Chen T, Yu W, Liu
S, Liu X, Chen D, Li F and Chen W: Discovery of cysteine-targeting
covalent histone methyltransferase inhibitors. Eur J Med Chem.
246:1150282023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lim Y, De Bellis D, Sandow JJ, Capalbo L,
D'Avino PP, Murphy JM, Webb AI, Dorstyn L and Kumar S:
Phosphorylation by Aurora B kinase regulates caspase-2 activity and
function. Cell Death Differ. 28:349–366. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang W, Zhang Z, Xiang Y, Gu DD, Chen J,
Chen Y, Zhai S, Liu Y, Jiang T, Liu C, et al: Aurora kinase
A-mediated phosphorylation triggers structural alteration of Rab1A
to enhance ER complexity during mitosis. Nat Struct Mol Biol.
31:219–231. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Mattiroli F and Penengo L: Histone
ubiquitination: An integrative signaling platform in genome
stability. Trends Genet. 37:566–581. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Oss-Ronen L, Sarusi T and Cohen I: Histone
mono-ubiquitination in transcriptional regulation and its mark on
life: Emerging roles in tissue development and disease. Cells.
11:24042022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yadav P, Subbarayalu P, Medina D, Nirzhor
S, Timilsina S, Rajamanickam S, Eedunuri VK, Gupta Y, Zheng S,
Abdelfattah N, et al: M6A RNA methylation regulates histone
ubiquitination to support cancer growth and progression. Cancer
Res. 82:1872–1889. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Bonfiglio JJ, Leidecker O, Dauben H,
Longarini EJ, Colby T, San Segundo-Acosta P, Perez KA and Matic I:
An HPF1/PARP1-Based chemical biology strategy for exploring
ADP-Ribosylation. Cell. 183:1086–1102.e23. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Messner S and Hottiger MO: Histone
ADP-ribosylation in DNA repair, replication and transcription.
Trends Cell Biol. 21:534–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Lv X, Lv Y and Dai X: Lactate, histone
lactylation and cancer hallmarks. Expert Rev Mol Med. 25:e72023.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhang D, Tang Z, Huang H, Zhou G, Cui C,
Weng Y, Liu W, Kim S, Lee S, Perez-Neut M, et al: Metabolic
regulation of gene expression by histone lactylation. Nature.
574:575–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Wu X, Li X, Wang L, Bi X, Zhong W, Yue J
and Chin YE: Lysine deacetylation is a key function of the lysyl
oxidase family of proteins in cancer. Cancer Res. 84:652–658. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Jambhekar A, Dhall A and Shi Y: Roles and
regulation of histone methylation in animal development. Nat Rev
Mol Cell Biol. 20:625–641. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Perillo B, Tramontano A, Pezone A and
Migliaccio A: LSD1: More than demethylation of histone lysine
residues. Exp Mol Med. 52:1936–1947. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liu R, Wu J, Guo H, Yao W, Li S, Lu Y, Jia
Y, Liang X, Tang J and Zhang H: Post-translational modifications of
histones: Mechanisms, biological functions, and therapeutic
targets. MedComm (2020). 4:e2922023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Roth SY and Allis CD: Chromatin
condensation: Does histone H1 dephosphorylation play a role? Trends
Biochem Sci. 17:93–98. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Clague MJ, Coulson JM and Urbé S:
Deciphering histone 2A deubiquitination. Genome Biol. 9:2022008.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
He X, Li Y, Li J, Li Y, Chen S, Yan X, Xie
Z, Du J, Chen G, Song J and Mei Q: HDAC2-Mediated METTL3
delactylation promotes DNA damage repair and chemotherapy
resistance in triple-negative breast cancer. Adv Sci (Weinh).
12:e24131212025. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wu N, Sun Q, Yang L, Sun H, Zhou Z, Hu Q,
Li C, Wang D, Zhang L, Hu Y and Cong X: HDAC3 and Snail2 complex
promotes melanoma metastasis by epigenetic repression of IGFBP3.
Int J Biol Macromol. 300:1403102025. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhu Y, Chen JC, Zhang JL, Wang FF and Liu
RP: A new mechanism of arterial calcification in diabetes:
interaction between H3K18 lactylation and CHI3L1. Clin Sci (Lond).
139:115–130. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Morschhauser F, Tilly H, Chaidos A, McKay
P, Phillips T, Assouline S, Batlevi CL, Campbell P, Ribrag V, Damaj
GL, et al: Tazemetostat for patients with relapsed or refractory
follicular lymphoma: An open-label, single-arm, multicentre, phase
2 trial. Lancet Oncol. 21:1433–1442. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zauderer MG, Szlosarek PW, Le Moulec S,
Popat S, Taylor P, Planchard D, Scherpereel A, Koczywas M, Forster
M, Cameron RB, et al: EZH2 inhibitor tazemetostat in patients with
relapsed or refractory, BAP1-inactivated malignant pleural
mesothelioma: a multicentre, open-label, phase 2 study. Lancet
Oncol. 23:758–767. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zinzani PL, Izutsu K, Mehta-Shah N, Barta
SK, Ishitsuka K, Córdoba R, Kusumoto S, Bachy E, Cwynarski K,
Gritti G, et al: Valemetostat for patients with relapsed or
refractory peripheral T-cell lymphoma (VALENTINE-PTCL01): A
multicentre, open-label, single-arm, phase 2 study. Lancet Oncol.
25:1602–1613. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Maruyama D, Jacobsen E, Porcu P, Allen P,
Ishitsuka K, Kusumoto S, Narita T, Tobinai K, Foss F, Tsukasaki K,
et al: Valemetostat monotherapy in patients with relapsed or
refractory non-Hodgkin lymphoma: A first-in-human, multicentre,
open-label, single-arm, phase 1 study. Lancet Oncol. 25:1589–1601.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yap TA, Winter JN, Giulino-Roth L, Longley
J, Lopez J, Michot JM, Leonard JP, Ribrag V, McCabe MT, Creasy CL,
et al: Phase I study of the novel enhancer of zeste homolog 2
(EZH2) inhibitor GSK2816126 in patients with advanced hematologic
and solid tumors. Clin Cancer Res. 25:7331–7339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Ribrag V, Iglesias L, De Braud F, Ma B,
Yokota T, Zander T, Spreafico A, Subbiah V, Illert AL, Tan D, et
al: A first-in-human phase 1/2 dose-escalation study of MAK683 (EED
inhibitor) in patients with advanced malignancies. Eur J Cancer.
216:1151222025. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Stein EM, Garcia-Manero G, Rizzieri DA,
Tibes R, Berdeja JG, Savona MR, Jongen-Lavrenic M, Altman JK,
Thomson B, Blakemore SJ, et al: The DOT1L inhibitor pinometostat
reduces H3K79 methylation and has modest clinical activity in adult
acute leukemia. Blood. 131:2661–2669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Issa GC, Aldoss I, DiPersio J, Cuglievan
B, Stone R, Arellano M, Thirman MJ, Patel MR, Dickens DS, Shenoy S,
et al: The menin inhibitor revumenib in KMT2A-rearranged or
NPM1-mutant leukaemia. Nature. 615:920–924. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Issa GC, Aldoss I, Thirman MJ, DiPersio J,
Arellano M, Blachly JS, Mannis GN, Perl A, Dickens DS, McMahon CM,
et al: Menin inhibition with revumenib for KMT2A-Rearranged
relapsed or refractory acute leukemia (AUGMENT-101). J Clin Oncol.
43:75–84. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Wang ES, Issa GC, Erba HP, Altman JK,
Montesinos P, DeBotton S, Walter RB, Pettit K, Savona MR, Shah MV,
et al: Ziftomenib in relapsed or refractory acute myeloid leukaemia
(KOMET-001): A multicentre, open-label, multi-cohort, phase 1
trial. Lancet Oncol. 25:1310–1324. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Gold S and Shilatifard A: Epigenetic
therapies targeting histone lysine methylation: Complex mechanisms
and clinical challenges. J Clin Invest. 134:e1833912024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Hollebecque A, Salvagni S, Plummer R,
Isambert N, Niccoli P, Capdevila J, Curigliano G, Moreno V,
Martin-Romano P, Baudin E, et al: Phase I study of lysine-specific
demethylase 1 inhibitor, CC-90011, in patients with advanced solid
tumors and relapsed/refractory non-hodgkin lymphoma. Clin Cancer
Res. 27:438–446. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wass M, Göllner S, Besenbeck B, Schlenk
RF, Mundmann P, Göthert JR, Noppeney R, Schliemann C, Mikesch JH,
Lenz G, et al: A proof of concept phase I/II pilot trial of LSD1
inhibition by tranylcypromine combined with ATRA in
refractory/relapsed AML patients not eligible for intensive
therapy. Leukemia. 35:701–711. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Tayari MM, Santos HGD, Kwon D, Bradley TJ,
Thomassen A, Chen C, Dinh Y, Perez A, Zelent A, Morey L, et al:
Clinical responsiveness to all-trans retinoic acid is potentiated
by LSD1 inhibition and associated with a quiescent transcriptome in
myeloid malignancies. Clin Cancer Res. 27:1893–1903. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang F, Jin Y, Wang M, Luo HY, Fang WJ,
Wang YN, Chen YX, Huang RJ, Guan WL, Li JB, et al: Combined
anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal
cancer: A randomized phase 2 trial. Nat Med. 30:1035–1043. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Younes A, Oki Y, Bociek RG, Kuruvilla J,
Fanale M, Neelapu S, Copeland A, Buglio D, Galal A, Besterman J, et
al: Mocetinostat for relapsed classical Hodgkin's lymphoma: An
open-label, single-arm, phase 2 trial. Lancet Oncol. 12:1222–1228.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Johnson ML, Strauss J, Patel MR, Garon EB,
Eaton KD, Neskorik T, Morin J, Chao R and Halmos B: Mocetinostat in
combination with durvalumab for patients with advanced NSCLC:
Results from a phase I/II study. Clin Lung Cancer. 24:218–227.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Awad MM, Le Bruchec Y, Lu B, Ye J, Miller
J, Lizotte PH, Cavanaugh ME, Rode AJ, Dumitru CD and Spira A:
Selective histone deacetylase inhibitor ACY-241 (Citarinostat) plus
nivolumab in advanced non-small cell lung cancer: Results from a
phase Ib study. Front Oncol. 11:6965122021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Jiang Z, Li W, Hu X, Zhang Q, Sun T, Cui
S, Wang S, Ouyang Q, Yin Y, Geng C, et al: Tucidinostat plus
exemestane for postmenopausal patients with advanced, hormone
receptor-positive breast cancer (ACE): A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 20:806–815. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kim YH, Bagot M, Pinter-Brown L, Rook AH,
Porcu P, Horwitz SM, Whittaker S, Tokura Y, Vermeer M, Zinzani PL,
et al: Mogamulizumab versus vorinostat in previously treated
cutaneous T-cell lymphoma (MAVORIC): An international, open-label,
randomised, controlled phase 3 trial. Lancet Oncol. 19:1192–1204.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Garcia-Manero G, Podoltsev NA, Othus M,
Pagel JM, Radich JP, Fang M, Rizzieri DA, Marcucci G, Strickland
SA, Litzow MR, et al: A randomized phase III study of standard
versus high-dose cytarabine with or without vorinostat for AML.
Leukemia. 38:58–66. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Monje M, Cooney T, Glod J, Huang J, Peer
CJ, Faury D, Baxter P, Kramer K, Lenzen A, Robison NJ, et al: Phase
I trial of panobinostat in children with diffuse intrinsic pontine
glioma: A report from the Pediatric Brain Tumor Consortium
(PBTC-047). Neuro Oncol. 25:2262–2272. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Horwitz SM, Nirmal AJ, Rahman J, Xu R,
Drill E, Galasso N, Ganesan N, Davey T, Hancock H, Perez L, et al:
Duvelisib plus romidepsin in relapsed/refractory T cell lymphomas:
A phase 1b/2a trial. Nat Med. 30:2517–2527. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Maher KR, Shafer D, Schaar D,
Bandyopadhyay D, Deng X, Wright J, Piekarz R, Rudek MA, Harvey RD
and Grant S: A phase I study of MLN4924 and belinostat in
relapsed/refractory acute myeloid leukemia or myelodysplastic
syndrome. Cancer Chemother Pharmacol. 95:242025. View Article : Google Scholar : PubMed/NCBI
|