Introduction
Cancer is a disease that poses threats to human
health and life, and is characterized by aberrant cellular
proliferation and the development of malignant tumors. According to
data provided by the World Health Organization, millions of
individuals worldwide are diagnosed with cancer annually (1,2). The
incidence and mortality rates have exhibited an upward trend, and
are subject to temporal and regional variations (3). Although advancements in oncology
research have been made by scientists and clinicians in areas such
as drug discovery, immunotherapy and radiotherapy, effective
treatment of cancer is still facing great challenges, such as drug
resistance, immune tolerance and severe side-effects (4–6). It is
imperative to unravel the mechanisms underlying tumorigenesis and
to develop novel intervention strategies.
Osteopontin (OPN) is a multifunctional
glycophosphoprotein encoded by the secretory phosphoprotein 1
(SPP1) gene, which is expressed in various types of cells and
tissues, including in the bone, dentin, cementum, hypertrophic
cartilage, kidney, brain, bone-marrow-derived metrial gland cells,
vascular tissues, cytotrophoblasts of the chorionic villus in the
uterus and decidua, ganglia of the inner ear, brain cells,
specialized epithelia found in mammary, salivary and sweat glands,
bile and pancreatic ducts, distal renal tubules and the gut, as
well as in activated macrophages and lymphocytes (7,8). OPN
was first reported as a marker of transformation of epithelial
cells in 1979 (9). Subsequently,
more variants of OPN and their corresponding functions have been
identified. For example, OPN splicing isoforms in solid tumors have
been investigated in breast cancer, mesothelioma and lung cancer
(10,11). As a secreted protein, OPN binds with
its receptors, such as integrin or CD44, to exert functions,
including modulating cell proliferation, adhesion, inflammatory
responses, osteogenesis and wound healing (8). The role of OPN in tumorigenesis and
development has attracted increasing attention (12,13).
Abnormal OPN expression has been observed in different types of
cancer such as brain, lung, kidney, liver, bladder and breast
cancer (13,14). Diverse hypotheses have been proposed
regarding the association between OPN and tumorigenesis; however,
to the best of our knowledge, its precise molecular mechanisms
remain to be illustrated. Given the pivotal role of OPN in
tumorigenesis and progression, OPN-based interventions have been
developed and applied in both basic research and clinical settings
(15–17).
The present review, after briefly introducing the
molecular structure, receptors and physiological functions of OPN,
describes how OPN participates in cancer initiation, progression,
metastasis and drug resistance. Furthermore, the present review
summarizes current OPN-based tumor intervention strategies. The
present review also provides perspectives on how to deepen the
insights regarding the exact role of OPN in tumorigenesis in order
to help develop novel strategies for tumor therapeutics. The
present review broadens the knowledge regarding the
pathophysiological role of OPN, so that novel OPN-based cancer
treatment strategies may be proposed.
Profile of OPN
OPN was first reported as a transformation marker of
malignant cells by Senger et al (9) in 1979. With the deepening of research,
more definitions of OPN have been determined, including bone
sialoprotein, SPP1 and early T-lymphocyte activation 1 (Eta-1)
(8). Based on mRNA transcript
variants, OPN can be translated into five subtypes, including
OPN-a, OPN-b, OPN-c, OPN4 and OPN5, which can exist in secretory or
intracellular forms (10).
Therefore, OPN has multiple functions in pathophysiological
conditions such as inflammation, cell survival, adhesion,
migration, differentiation, apoptosis and bone matrix
mineralization (18,19). Comprehensive understanding of the
protein structure, receptors and functions of OPN is necessary and
crucial in order to further reveal its mechanism involved in tumor
genesis and development, and to develop therapeutic strategies.
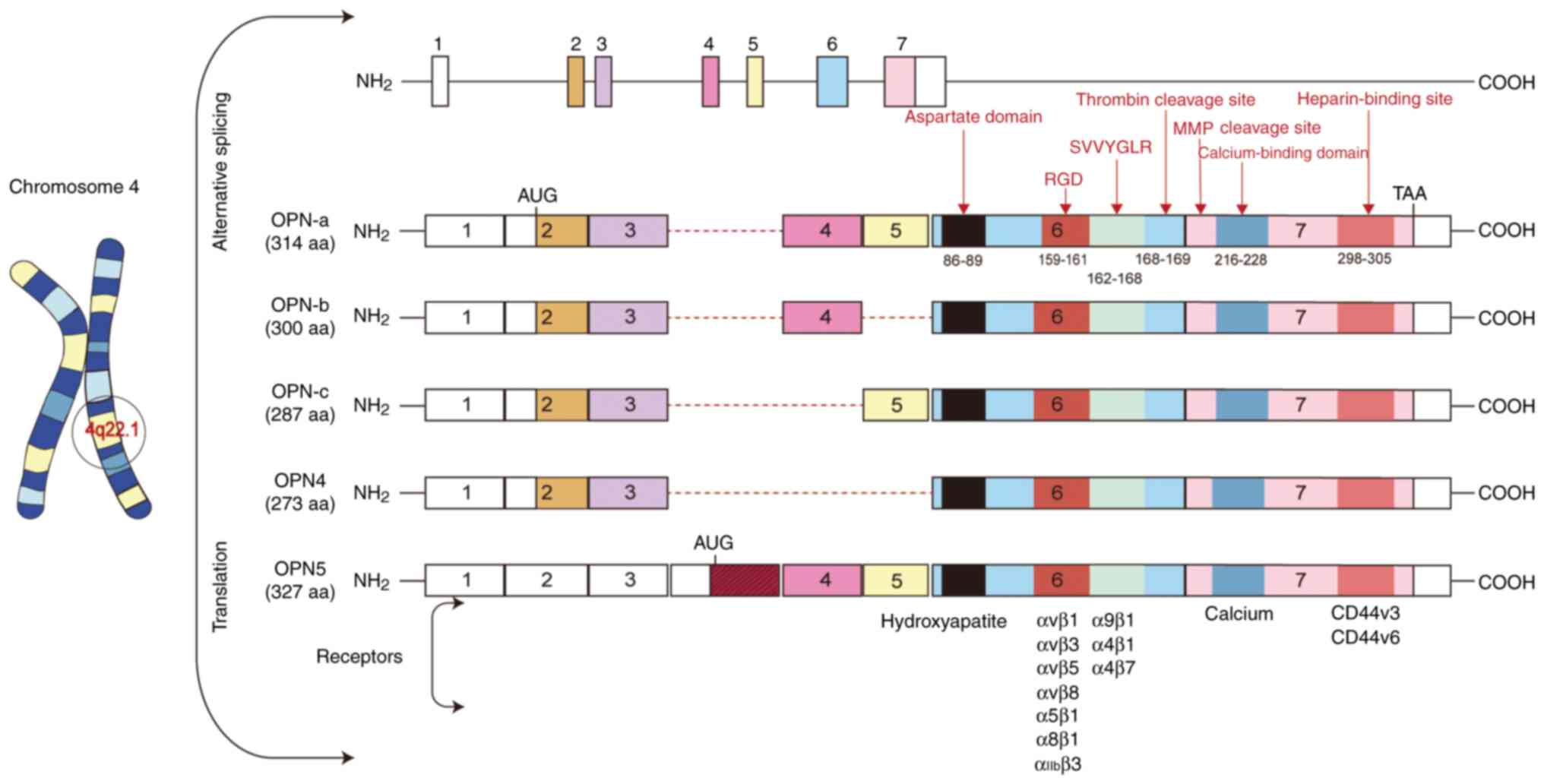
Genomic location and structure of
OPN
OPN is encoded by the SPP1 gene located on
chromosome 4q22.1 (19). The SPP1
gene contains seven exons, which undergo selective splicing to
generate distinct variants. At present, seven exons of SPP1 have
been reported. Exon 1 remains untranslated, whereas exons 2–7
contain coding sequences. Exon 2 encodes a signal peptide along
with two amino acids constituting the mature protein; exon 3
encodes a serine phosphorylation motif; exon 4 encodes a
proline-rich region and transglutaminase site; and exon 5 encodes
an additional protein phosphorylation site. Notably, exons 6 and 7
account for >80% of the expressed OPN protein (19). Five isoforms of OPN have been
reported, including OPN-a (comprising all exons and consisting of
314 amino acids), OPN-b (lacking exon 5 and comprising 300 amino
acids), OPN-c (lacking exon 4 and comprising 287 amino acids), OPN4
(lacking exons 4 and 5, and consisting of 273 amino acids) and OPN5
(containing an additional exon, with a length of 327 amino acids)
(10,20). Distinct splicing of the SPP1 gene
has been implicated in cancer occurrence, progression and prognosis
(19).
Protein structure of OPN
Human OPN contains ~314 amino acid residues, with a
molecular weight ranging between 41 and 75 kDa, depending on
different post-translational modifications (8,21). OPN
contains several highly conserved domains, including the aspartate
domain, arginine-glycine-aspartic acid (RGD) domain,
serine-valine-valine-tyrosine-glutamate-leucine-arginine (SVVYGLR)
domain, thrombin cleavage domain, calcium-binding domain and
C-terminal heparin-binding domain (22). Given that OPN is a secreted protein,
it is present not only in various tissues but also in biological
fluids. The secreted OPN is subjected to various post-translational
modifications, including glycosylation, phosphorylation and
sulfuration, which endows OPN with diversified biological functions
(8). Fig. 1 schematically illustrates the
structure and domains of OPN.
Receptors of OPN
OPN needs to bind with its receptors to exert its
function. The well-characterized receptors of OPN include integrins
and CD44 (23,24). Notably, under some conditions, OPN
is subjected to protease cleavage to expose its specific domains,
which exhibit binding affinity for potential receptors (25).
Integrins are transmembrane heterodimers that
recognize various ligands, including OPN and other extracellular
matrix proteins and cell surface proteins (26). The RGD sequence of OPN exhibits a
strong binding affinity for integrins, including αvβ1, αvβ3, αvβ5,
αvβ6, αvβ8, α8β1, α5β1 and αIIbβ3 (27,28).
Another integrin-binding domain (SVVYGLR) can only be exposed after
thrombin cleavage at Arg168-Ser169 (29). The exposed SVVYGLR interacts with
integrin subtypes such as α9β1, α4β1 and α4β7 (25,28,30).
Similarly, plasma proteases cleave OPN at
Lys154-Ser155, producing an N-terminal OPN
fragment that binds more strongly to α5β1 and αvβ3 integrins than
the full-length OPN (25). MMP
could cleave OPN at various sites such as
Gly166-Leu167,
Ala201-Tyr202 and
Asp210-Leu211, producing a fragment that
preferably binds with the α4β1 isoform of integrin (29).
CD44, also known as hyaluronic acid receptor, is
expressed in various cell types such as osteocytes, endothelial
cells, fibroblasts, epithelial cells and smooth muscle cells
(31). The C-terminal fragment
heparin-binding site of OPN can directly interact with CD44
variants CD44v3 and CD44v6 (30).
Therefore, OPN, with or without cleavage, interacts
with integrins or CD44 to regulate multiple cellular processes,
including cell attachment, migration, chemotaxis and immune
modulation in diverse types of cells (24).
Pathophysiological role of OPN
OPN is expressed in various types of cells and
tissues. With the deepening of research, diverse functions of OPN
in both physiological and pathological conditions have been
revealed (32,33).
Orchestrating the immune response
OPN is also known as Eta-1 and can trigger the
immune response of macrophages, T cells and B cells (34). For example, OPN binds with integrin
(αvβ3) of macrophages and reduces the production of
anti-inflammatory IL-10, while its binding with CD44 promotes the
secretion of proinflammatory IL-12 (23). OPN also influences the migration of
immune cells (27,35). Wang and Denhardt (27) reported that OPN served as a type 1 T
helper cell cytokine, contributing to mucosal defense against viral
pathogens. OPN may regulate inflammatory reactions by stimulating
the natural immune response of macrophages and neutrophils
(27). OPN could also be secreted
by activated T cells to aid in the recruitment and migration of
immune cells, particularly macrophages, toward the site of
inflammation, resulting in persistence and exacerbation of the
inflammatory response (36).
OPN exerts pro-inflammatory effects by activating
inflammatory signaling pathways, such as the NF-κB and STAT3
pathways, which leads to the secretion of cytokines, including
TNF-α and CD16 (37). Additionally,
OPN modulates immune cell functions by enhancing macrophage
chemotaxis and phagocytic activity, promoting their polarization
toward the pro-inflammatory M1 phenotype, and activating the immune
responses of T cells, natural killer cells and dendritic cells
(38,39). Furthermore, OPN stimulates tumor
cells to secrete IL-17, thereby activating the JNK/c-Jun signaling
pathway and contributing to immunosuppressive effects (40). OPN also serves a critical role in
mediating immune escape mechanisms, such as enabling tumor cells to
evade immune surveillance through the suppression of T cell
activity (41). Development of
single-cell RNA sequencing (scRNA-seq) has provided a better way to
define the context-dependent functions of OPN. Ding et al
(42) utilized scRNA-seq and
revealed that CD163+ macrophages were enriched in lung
tissues of patients with coronavirus disease 2019, and these cells
highly expressed OPN. Wang et al (43) used scRNA-seq analysis and revealed
that OPN expression was upregulated in menstrual blood cells from
patients with endometriosis, which in synergy with inflammatory
factors, such as IL-10 and IL-6, promoted local fibrotic
processes.
Aberrant OPN expression may induce dysregulation of
the immune response, thereby impairing the normal recognition and
response to self-antigens and foreign antigens, which contributes
to the initiation and progression of autoimmune disorders (44). Improper expression of OPN is
associated with the pathogenesis of various autoimmune diseases
(such as multiple sclerosis, systemic lupus erythematosus,
rheumatoid arthritis and atherosclerosis) and other inflammatory
diseases (including cardiovascular diseases, chronic obstructive
pulmonary disease, inflammatory bowel disease, liver diseases and
asthma) (27).
Regulating osteogenesis
OPN also participates in osteogenesis, regulating
bone cell adhesion and osteoclast function, and promoting matrix
mineralization. The strong affinity of OPN for hydroxyapatite leads
to its accumulation in the bone (45–47).
OPN exerts an effect on skeletal cell proliferation,
differentiation and migration, while facilitating calcium phosphate
mineralization (48). Given its
crucial role in bone metabolism, aberrant OPN expression is
associated with various types of bone diseases. In patient with
osteolysis, OPN expression is downregulated, whereas in patients
with fractures, OPN expression is increased (49).
Tissue repair
OPN exhibits an important role in tissue repair and
regeneration by facilitating cell migration, proliferation,
neoangiogenesis and the formation of fibrous connective tissue
(50). For example, OPN has been
found to promote fibroblast proliferation during wound healing
(50). Furthermore, Wen et
al (51) found that, in a model
of regenerating liver after partial hepatectomy, OPN promoted the
inflammatory response and activated the IL-6/STAT3 pathway to
facilitate the proliferation of hepatocytes and the repair of the
liver. A previous study has demonstrated that OPN expression could
induce vascular intimal thickening and promote neointimal formation
following injury, suggesting a role in vascular remodeling
(52). Upregulation of OPN leads to
tissue fibrosis, including liver and kidney fibrosis, by
facilitating the synthesis and deposition of extracellular matrix
proteins (53).
Cardiovascular diseases
OPN also participates in the regulation of
cardiovascular function. OPN is upregulated in atherosclerosis and
present in atherosclerotic plaques, suggesting its potential
relevance (54). Furthermore, it
has been demonstrated that OPN induces vascular calcification by
facilitating mineral uptake and formation of apatite crystals
(55). Speer et al (56) further revealed that phosphorylation
of OPN could induce vascular calcification.
In summary, OPN serves diverse and important
pathophysiological roles in a context-dependent manner. Elucidating
the intricate structure and multifaceted functionality of OPN will
consequently facilitate the development of OPN-based diagnostic and
therapeutic strategies.
Molecular mechanisms of OPN involved in
tumor genesis and development
The role and mechanisms of OPN in tumor genesis and
development have attracted increased attention (57–60).
Increased OPN expression is observed in various malignancies,
including breast cancer, leukemia, hepatocellular carcinoma (HCC),
bladder cancer, colorectal cancer, melanoma, lung cancer, squamous
cell carcinoma of the head and neck, and glioblastoma multiforme
(13,61). Numerous studies have demonstrated
that the upregulation of OPN is involved in tumorigenesis,
chemotherapy resistance, angiogenesis and immunosuppression, and
revealing how OPN participates in these processes is essential
(62–67).
OPN is involved in cancer pathogenesis. One study
has revealed high OPN expression in various types of cancer such as
brain tumors, lung cancer, kidney cancer, liver cancer, bladder
cancer, breast cancer, esophageal cancer, pancreatic cancer,
gastric cancer, colorectal cancer and prostate cancer (13). The precise role and mechanism of OPN
in cancer pathogenesis are not fully understood; however, the
following aspects have been widely implicated in these.
Promoting cell survival and inhibiting
apoptosis
Tumor cells exhibit anti-apoptosis capacities
(68). Sun et al (69) demonstrated that OPN regulated Bcl-2
and Bax expression, thus enhancing the viability of tumor cells.
Another group found that OPN could induce Bcl-2 expression and
attenuate apoptosis (70). In
addition, OPN facilitates tumor progression by regulating tumor
surveillance mechanisms and inhibiting tumor cell apoptosis
(71). Upregulated OPN interacts
with integrin αvβ3 or CD44, facilitating complement factor H cell
surface translocalization, and thus, complement-mediated cell death
is suppressed (72). Zhou et
al (73) found that OPN
knockdown enhanced apoptosis and caused cell cycle arrest in
leukemia stem cells.
Promoting cell migration and
invasion
Tumor metastasis is closely associated with the
capacity of cell migration and invasion (72,74).
Irby et al (75) reported
that overexpressed OPN in SW480, HT29 and HCT116 cells bound with
its receptors, including integrins (αvβ1, αvβ3 and αvβ5) and CD44,
and enhanced colon cancer invasion. OPN has the ability to mediate
the adhesion and interaction between tumor cells and the
surrounding matrix, thereby augmenting the metastatic potential of
tumor cells (76). Kale et
al (77) found that OPN, after
binding with integrin (α9β1), triggered cyclooxygenase-2 (COX-2)
and prostaglandin E2 expression, and thus, promoted tumor growth
and metastasis. OPN induces the expression and activation of MMP-2,
MMP-3 and urokinase-type plasminogen activator in an
integrin-dependent manner, which promotes extracellular matrix
degradation and consequent metastasis (78). The capacity of OPN to induce
cellular migration and extracellular matrix degradation contributes
to the invasive and metastatic potential of cancer (78).
Promoting angiogenesis
Angiogenesis is a feature of tumors, particularly
solid tumors. Neovascularization enhances the supply of nutrients
and oxygen, thus promoting tumor growth and metastasis (71,79).
OPN is involved in tumor angiogenesis by facilitating the migration
and proliferation of vascular endothelial cells, which promotes
neovascularization (79). Gupta
et al (80) reported that
OPN activated MMP-9, which resulted in increased VEGF secretion and
promoted the angiogenesis of prostate cancer, while OPN knockdown
or αvβ3 mutation reversed these effects. Fukusada et al
(81) reported that OPN induced
VEGF expression and angiogenesis, which promoted pancreatic ductal
adenocarcinoma progression.
Modulating the tumor microenvironment
(TME)
Tumor cells are surrounded by the TME, which
consists of extracellular matrix, stromal cells, blood vessels,
immune cells, fibroblasts and secretory factors (82). A recent study has demonstrated that
the interplay between tumor cells and the TME governs
tumorigenesis, invasion, metastasis, chemotherapy resistance and
the immune response, thereby driving tumor progression and invasion
(83). OPN, as a component of the
TME, could derive from tumor cells, endothelial cells, fibroblasts
and immune cells. OPN exerts an influence on the formation and
regulation of the TME (9,84,85).
Wei et al (36) found that
OPN promoted tumor progression by attracting macrophages and
activating T cells in the glioblastoma TME. Butti et al
(86) reported that OPN could
remodel the TME immune suppression and promoted tumor progression
by modulating the polarization of T helper cells, T-regulatory
cells and tumor-associated macrophages. Rogers et al
(87) reported that myofibroblastic
cancer-associated fibroblasts in the TME promoted cancer stem cell
proliferation and metastasis, whereas inhibition of OPN in a mouse
breast cancer model resulted in diminished tumor stemness.
Therefore, destruction of the TME by targeting OPN may effectively
inhibit tumor progression.
OPN exhibits distinct effects across various cancer
types. For example, OPN inhibits autophagy in colorectal cancer
cells, induces breast cancer cell migration and increases
angiogenesis in breast cancer, and leads to cell migration and
invasion in lung adenocarcinoma (88,89).
Further studies elucidating the precise involvement of OPN in
cancer pathogenesis are required.
OPN triggers signaling pathways in
tumor pathogenesis
The essential role of OPN in tumorigenesis and
development is well-established, and OPN exerts its effects by
activating multiple intertwined signaling pathways such as the JNK,
Ras/Raf/MEK/ERK, PI3K/Akt, Janus kinase (JAK)/STAT, NF-κB and TIAM
Rac1 associated GEF 1/Rac1 pathways (14,57).
These pathways regulate cell proliferation, adhesion, diffusion,
migration, invasion and epithelial-mesenchymal transition (EMT) of
tumor cells, as well as immunosuppression and drug resistance
(1). The main pathways regulated by
OPN that are involved in tumorigenesis and development are
summarized and illustrated in Fig.
2 (90–99).
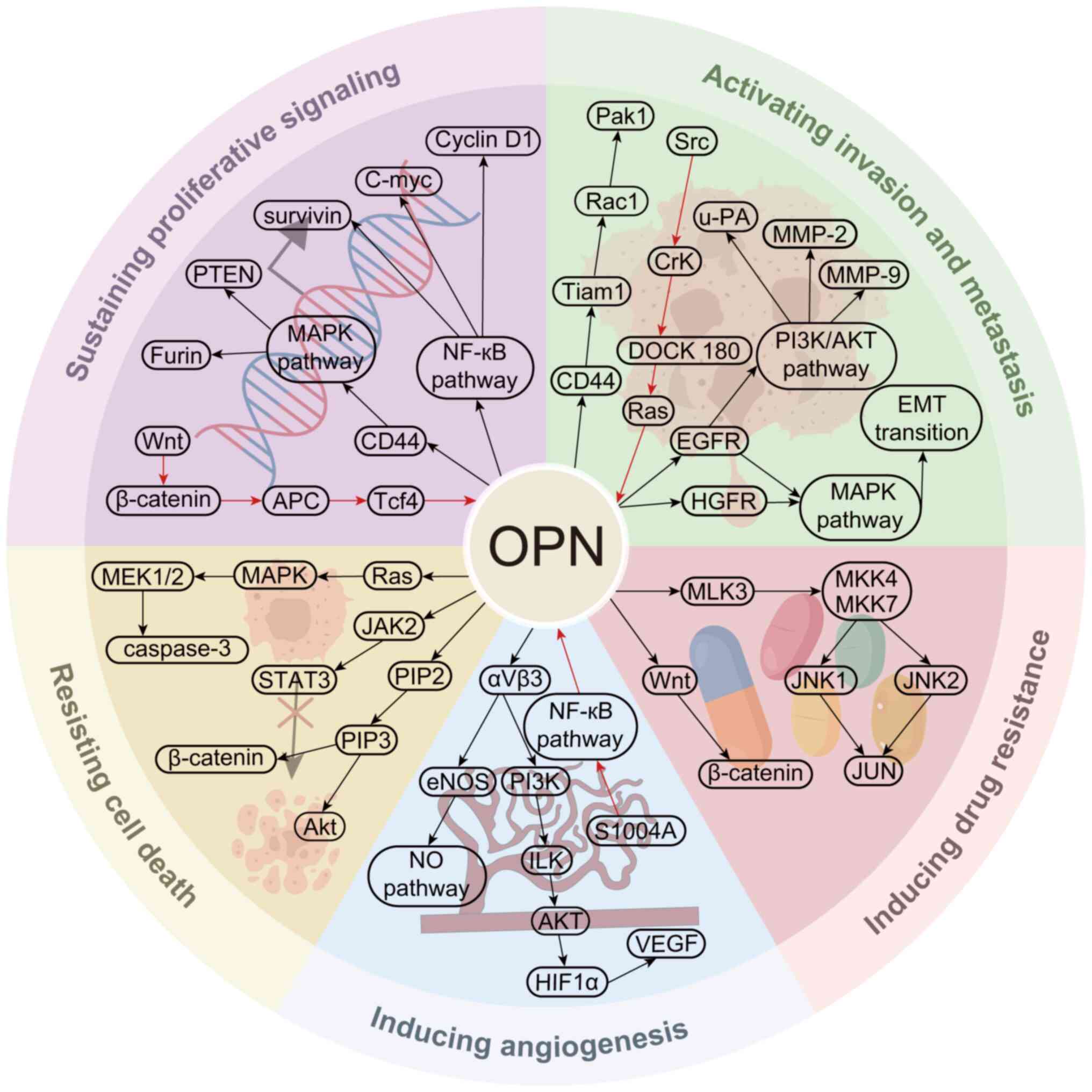 | Figure 2.Representative signaling pathways
involved in OPN participating in cancer genesis and development.
Black arrows indicates that OPN initiates the signaling pathway,
while red arrows indicate that the signaling pathway ends with OPN
modulation. APC, adenomatous polyposis coli; EMT,
epithelial-mesenchymal transition; eNOS, endothelial nitric oxide
synthase; HGFR, hepatocyte growth factor receptor; HIF1α,
hypoxia-inducible factor-1α; ILK, integrin linked kinase; JAK2,
Janus kinase 2; MKK, mitogen-activated protein kinase kinase; MLK3,
mixed lineage kinase 3; NO, nitric oxide; OPN, osteopontin; Pak1,
p21 (RAC1) activated kinase 1; PIP2, phosphatidylinositol (4,5)-bisphosphate; PIP3, phosphatidylinositol
(3,4,5)-trisphosphate; S1004A, S100 calcium
binding protein A4; Tcf4, transcription factor 4; Tiam1, TIAM Rac1
associated GEF 1; u-PA, urokinase-type plasminogen activator. |
JNK signaling pathway
The JNK signaling pathway has been demonstrated to
be activated in various types of cancer such as lymphoma,
pancreatic cancer, hepatocellular carcinoma and childhood sarcoma
(91). Messex et al
(100) found that extraneously
added OPN activated the JNK pathway, which accelerated cell
proliferation and prostate cancer development. Insua-Rodríguez
et al (101) demonstrated
that JNK was activated in breast cancer xenograft tumor mouse
models, which enhanced OPN expression, resulting in chemotherapy
resistance and metastasis. Li et al (102) demonstrated that OPN bound to MB231
breast cancer cells and activated the JNK signaling pathway, which
resulted in EMT initiation and cellular migration.
Ras/Raf/MEK/ERK pathway
The Ras/Raf/MEK/ERK signaling pathway serves a vital
role in promoting cell proliferation, survival and metastasis in
various cancer types (103). Yang
et al (104) reported that
knockdown of OPN in squalene synthase-overexpressing lung cancer
cells inhibited their migration and invasion. Chernaya et al
(105) found that, in thyroid
cancer, activation of the RAS-RAF-MEK pathway led to upregulation
of OPN and its receptors (integrin), which in turn increased the
metastatic potential of tumor cells. Sun et al (106) adopted RNA interference (RNAi) to
reduce OPN expression, which resulted in a decrease in the
expression levels of MMP-2 and inhibition of the MEK/ERK pathway,
eventually retarding hepatic carcinoma growth and metastasis.
PI3K/AKT signaling pathway
The PI3K/AKT signaling pathway is aberrantly
activated in various types of cancer (91). OPN has been found to activate the
PI3K/AKT pathway by binding with CD44, consequently promoting cell
proliferation and suppressing apoptosis (107). Zhang et al (108) reported that OPN bound with
integrin αvβ3 and activated the PI3K/AKT pathway in breast cancer,
while knockdown of OPN suppressed breast cancer metastasis.
Furthermore, Fu et al (109) revealed that upregulation of OPN
facilitated chemoresistance of non-small cell lung cancer (NSCLC),
while silencing of OPN inhibited the phosphorylation of AKT and
ERK, weakening gefitinib resistance in NSCLC cells.
JAK/STAT signaling pathway
The JAK/STAT signaling pathway is overactivated in
cancer (110). OPN interacts with
CD44 and triggers the activation of JAK protein kinase, which
induces STAT phosphorylation and consequent enhanced hepatic
carcinoma cell proliferation (111). Behera et al (112) demonstrated that OPN activated
JAK2/STAT3 signaling and stimulated breast tumor growth in mice.
JAK2 inhibitor (AG 490) administration could suppress OPN-induced
STAT3 phosphorylation and promote tumor cell apoptosis (112).
NF-κB signaling pathway
The NF-κB signaling pathway has been found to be
activated in cancer (104). OPN
binding to integrin αvβ3 results in the translocation of NF-κB from
the cytoplasm to the nucleus, and promotes tumorigenesis,
proliferation, invasion and angiogenesis of HCC (113). Chen et al (114) demonstrated that specific small
interfering RNA (siRNA) inhibition of OPN could effectively
attenuate NF-κB levels, thereby inhibiting proliferation of
esophageal squamous cell carcinoma cells and suppressing tumor
formation and metastasis. Saurav et al (115) reported that inhibition of OPN
and/or NF-κB signaling could enhance the efficacy of irinotecan,
and the combination of OPN and/or an NF-κB inhibitor with
irinotecan may enhance the therapeutic efficacy while reducing the
development of drug resistance.
p38 MAPK signaling pathway
The p38 MAPK signaling pathway is a key signal
transduction cascade that cells possess in response to
environmental stimuli, and has attracted much attention as a
promising target for cancer therapy (116,117). Yu et al (118) reported that OPN was highly
expressed in head and neck squamous cell carcinoma (HNSCC), and OPN
promoted the proliferation and invasion of HNSCC cells by
activating the p38-MAPK signaling pathway. Huang et al
(66) stated that OPN participated
in the activation of the p38 MAPK signaling pathway, thereby
promoting cell migration and invasion of colorectal cancer.
OPN-conferred chemoresistance is also associated with the p38 MAPK
signaling pathway. Pang et al (119) demonstrated that OPN mediated
cyclophosphamide resistance in MDA-MB-231 tumor cells by activating
p38 MAPK.
OPN-based cancer treatment
Cancer is threatening the life of millions of
individuals worldwide, and its therapeutics still face great
challenges (120). OPN possesses
various functions in the context of cancer, including the
enhancement of tumor progression and metastasis, inhibition of
tumor cell apoptosis, regulation of the TME, and induction of
chemotherapeutic drug resistance. OPN represents a promising
therapeutic target for the improved treatment of cancer. RNAi,
small-molecule inhibitors, aptamers targeting OPN, and blockade
binding of OPN and its receptor are expected to be alternative
methods for cancer therapy (14,89).
Table I outlines available
OPN-based therapeutic strategies.
 | Table I.OPN-based cancer interventions. |
Table I.
OPN-based cancer interventions.
| First author/s,
year | Targeting
strategy | Inhibitor | Target molecule(s)
and/or related signaling pathways | Application
model | Effect/outcome | (Refs.) |
|---|
| Kumar et al,
2010 | RNAi | siRNA | OPN, CD44, MKK3/6,
p38-dependent NF-κB activation | Human cervical
cancer cells (HeLa and SiHa), female NOD/SCID mice | OPN silencing
causes reduced growth, migration and invasion | (16) |
| Saleh et al,
2016 |
| siRNA | OPN | Murine mammary
tumor cells (RM11A, RJ348 and RJ345) | Inhibits cancer
cell proliferation, apoptosis, migration and invasion | (141) |
| Zhang et al,
2014 |
| siRNA | OPN, LC3, Beclin1,
PI3K/Akt/mTOR pathway | Human breast cancer
cells (MDA-MB-231) | Knockdown decreases
the activation of the PI3K/Akt/mTOR pathway, inhibits migration and
invasion, and induces autophagy in cancer cells | (108) |
| Cho et al,
2015 |
| siRNA | OPN, PSOT | Human lung
adenocarcinoma epithelial cell line (A549), large cell lung
carcinoma cancer cell line (H460), male nude mice (BALB/c-nu) | siOPN treatment
reduces tumor size and weight | (142) |
| Bhattacharya et
al, 2010 |
| miRNA | OPN, miRNA
181a | HCC cell lines (Hep
G2 and Hep 3B) | OPN expression is
regulated by miRNA 181a, and the presence of siOPN attenuates the
adhesion, migration and invasion of tumor cells | (143) |
| Zagani et
al, 2009 | Small-molecule
inhibitors | Parecoxib (COX-2
inhibitor) | OPN, Wnt/β-catenin
pathway | Mouse colon
adenocarcinoma (CT26) Apcmin/+ mice | Downregulation of
OPN expression by COX-2 inhibitors inhibits tumor growth | (17) |
| Matsuura et
al, 2010 |
| Simvastatin
(HMG-CoA inhibitors) | OPN,
3-hydroxy-3-methylgluaryl coenzyme A reductase | Human ovarian
carcinoma cell lines (RMG-1 and TOV-21G), human adenocarcinoma cell
lines (HTOA, MH and KFr), human mucinous adenocarcinoma cell line
(MCAS) | Apoptosis, growth
arrest and enhanced invasion were induced by reducing OPN
expression | (127) |
| Kumar et al,
2012 |
|
Andrographolide | OPN, PI3 kinase/Akt
signaling | Human breast
adenocarcinoma cell lines (MDA-MB-231 and MCF-7), mouse fibroblast
cell line (NIH-3T3) | Inhibition of
cancer cell proliferation, adhesion, apoptosis, migration and
invasion | (126) |
| Sharma et
al, 2010 |
| Trichostatin A
(histone deacetylase inhibitor) | HDAC1 inhibitor,
OPN, c-Jun, cyclin D1, u-PA | Human cervical
carcinoma cells (HeLa and SiHa), female NOD/SCID mice | Cancer cell cycle
arrest, stimulates differentiation and induces apoptosis | (125) |
| Lv et al,
2013 |
| Curcumin + BPS | OPN, CD44,
MMP-9 | Human ovarian
cancer cells (SKOV3) | Reduces invasion of
ovarian cancer and dendritic cells | (128) |
| Mason et al,
2008 |
| Agelastatin A | β-catenin, Tcf4
signaling | Mammary epithelial
cells (MDA-MB-231 and MDA-MB-435s) | Inhibits
OPN-mediated invasion, adhesion and colony formation of malignant
cells | (144) |
| Mi et al,
2009 | Aptamers | OPN-R3 | OPN, JNK1/2,
PI3K | Human breast cancer
cells (MDA-MB-231) | OPN-R3 effectively
inhibits adhesion, migration and invasion of cancer cells | (15) |
| Dai et al,
2010 | Blocking
antibodies | Anti-OPN
antibody | OPN, hu1A12 | Human breast cancer
cells (MDA-MB-435S) | Inhibition of
cancer cell proliferation, survival, adhesion, migration and
invasion potential | (145) |
| Ahmed and Kundu,
2010 |
| Anti-αvβ3 integrin
antibody | αvβ3-integrin | Breast cancer cells
(MCF-7 and MDA-MB-468) | Inhibits
OPN-induced tumor growth and angiogenesis | (132) |
| Teramoto et
al, 2005 |
| Anti-CD44
antibody | CD44 receptor | Mouse embryonic
fibroblasts (NIH3T3) | Reduces tumor
progression induced by OPN | (146) |
| Moorman et
al, 2020 |
| AOM1 | Blocking the αvβ3
integrin-binding site on OPN | NSCLC tumor-bearing
mice | Increases the
efficacy of PD-1-based ICB immunotherapy | (61) |
| Chiou et al,
2019 | OPN
inactivation | OPN | FSTL-1 | Lung cancer cell
lines (A549, H1299, PC13 and PC14) | OPN inactivation
prevents tumor progression | (134) |
OPN gene interference
siRNAs and short hairpin RNAs (shRNAs) can
efficiently downregulate the expression of specific genes.
Knockdown of OPN with siRNA represents a promising intervention for
various types of cancer such as liver tumors, chronic myeloid
leukemia, familial adenomatous polyposis and metastatic melanoma,
and it is currently undergoing clinical trial (121). Ben-David-Naim et al
(122) demonstrated that
administration of nanoparticle-encapsulated OPN siRNA effectively
reduced OPN expression and suppressed tumor growth in a mouse
breast cancer model. Yang et al (123) reported that knockdown of OPN with
specific siRNA or shRNA could inhibit breast tumor progression.
These findings suggest that downregulation of OPN expression could
inhibit tumor cell proliferation, migration, invasion, angiogenesis
and other processes. Using siRNA technology to silence OPN offers a
plethora of strategic opportunities to develop effective treatment
modalities against cancer.
Small-molecule inhibitors of OPN
Given their compact size and efficient cell membrane
permeability, small-molecule inhibitors targeting specific
signaling pathways have attracted increasing attention in disease
intervention (23,124). Small-molecule inhibitors toward
OPN have been applied for cancer treatment. Sharma et al
(125) demonstrated that
inhibition of OPN expression with trichostatin A effectively
prevented tumor growth and metastasis in a mouse xenograft model.
Androstenedione (Andro), a diterpenoid compound isolated from
androsacetum, inhibits OPN expression, resulting in cell cycle
arrest and apoptosis of breast cancer cells, as well as
tumor-endothelial cell interactions (126). Kumar et al (126) further demonstrated that Andro
suppressed breast cancer cell proliferation by downregulating c-jun
expression and inhibiting PI3K/Akt signaling activation. Parecoxib,
a traditional COX-2 inhibitor, has been reported to retard the
progression of colorectal cancer by suppressing OPN expression via
blockade of the nuclear receptor subfamily 4 group A member 2 and
Wnt signaling pathways (17).
Matsuura et al (127)
demonstrated that simvastatin, a high mobility group-CoA inhibitor,
downregulated the expression levels of OPN and αvβ1, αvβ3, α5β1 and
α5β3 integrin, accompanied by an enhancement in the invasion of
ovarian cancer. Lv et al (128) found that combined administration
of curcumin and basilic polysaccharides could effectively suppress
cell proliferation and invasion by downregulating OPN and its
receptor in ovarian cancer.
Aptamers of OPN
Aptamers, as short single-stranded deoxyribonucleic
acid or ribonucleic acid molecules, have unique biometric
capabilities. They are able to fold to form a three-dimensional
conformation and precisely bind to a protein target (129). This process enables the aptamers
to bind to specific targets with high selectivity, including
proteins, peptides, small carbohydrate molecules and even living
cells, showing great potential in biomedical research and
diagnostic applications (130).
Aptamers exhibit advantages such as high affinity for the target,
biochemical stability and low immunogenicity, and could potentially
be used as cancer therapeutics (130). Mi et al (15) demonstrated that the treatment of
MDA-MB-231 breast cancer cells with OPN aptamers, OPN-R3, resulted
in inhibition of cell adhesion, migration and invasion. The authors
further demonstrated that OPN-R3 inactivated OPN-associated
signaling pathways, including the PI3K, JNK1/2, Src and Akt
pathways (15). Furthermore, in
vivo, OPN-R3 administration prevented tumor growth in breast
cancer xenotransplantation mouse models (131).
Blocking binding of OPN with its
receptors
OPN needs to bind with its receptors to exert its
roles in tumor genesis and progression. Therefore, disrupting the
binding of OPN and its receptors represents an alternative strategy
for tumor treatment. Rangaswami et al (90) reported that blocking antibodies
against αvβ3-integrin effectively suppressed OPN-induced tumor
growth and angiogenesis. Furthermore, αvβ3-blocking antibodies have
been found to effectively inhibit OPN-mediated activator protein 1
activation in breast cancer cells (132). Ahmed et al (133) showed that administration of CD44
antibody disrupted the interaction between OPN and CD44,
consequently restricting OPN-CD44 mediated tumor invasion and
metastasis. Blocking the binding of OPN with its receptors offers a
novel therapeutic strategy.
OPN inactivation
OPN needs to be cleaved by specific proteases to
expose its receptor binding domain in order to exert its function.
Therefore, blocking enzyme cleavage could render OPN inactive.
Chiou et al (134) showed
that follistatin-like protein 1 directly bound to the uncleaved
form of OPN and inhibited the proteolytic activation of OPN, which
blocked OPN-integrin/CD44-related signaling pathways and consequent
lung cancer metastasis. Thrombin has been demonstrated to
participate in OPN hydrolysis, which is essential for effective
binding of OPN with its receptors (135). Peraramelli et al (136) reported that a thrombin inhibitor,
dabigatran etexilate, retarded B16 melanoma growth by blocking OPN
activation. Therefore, inhibiting OPN activation by blocking OPN
signaling and proteolytic processing may be a feasible therapeutic
approach to stop tumor progression. Current OPN-based interventions
are summarized in Table I.
Although OPN is a promising target for cancer
therapy, there are still some limitations in practical
applications. Gene interference technologies such as RNAi have poor
stability and low delivery efficiency, making it difficult to
continuously and specifically inhibit OPN expression in vivo
(137). Small-molecule inhibitors
have several drawbacks, including a short half-life, poor oral
bioavailability, drug resistance, multiple side effects and high
production costs. Off-target effects occur when a molecule in the
body binds non-specifically to molecules other than its intended
target, which may lead to unexpected biological effects or adverse
reactions (138). Blocking the
interaction between OPN and receptors is also challenging because
OPN can bind to multiple receptors, and single blocking has limited
effects, and may also trigger unknown biological effects. Complete
inactivation of OPN may affect its normal physiological functions
and cause side effects. Despite the limitations of current
strategies, each approach has its unique advantages and applicable
scenarios (23). In practical
applications, appropriate strategies should be selected based on
specific diseases and treatment requirements, and their limitations
should be overcome through technological innovation and
optimization. For example, the therapeutic efficacy and safety can
be effectively improved by optimizing aptamer design, improving
delivery systems and enhancing the bioavailability of
small-molecule inhibitors (89).
The combination of multiple therapeutic strategies
could provide more comprehensive and effective treatments, and a
representative combination is OPN inhibitors together with immune
checkpoint intervention. Lu et al (139) found that administration of the WD
repeat domain 5 protein (WDR5), an inhibitor of OPN, suppressed the
growth of orthotopic pancreatic tumors in mice. Furthermore, WDR5
enhanced the efficacy of anti-programmed cell death protein 1
immunotherapy in inhibiting pancreatic tumor growth in mice
(139). Zhu et al (140) reported that OPN expression in HCC
was positively associated with the programmed death-ligand 1
levels, whereas OPN knockout enhanced the antitumor function of
T-helper 1 cells. Future research should focus on mechanism
verification and optimization of multi-target combination
strategies to achieve more precise antitumor treatment.
Summary and perspectives
OPN, as a secretory protein, is aberrantly
expressed in various types of cancer and exhibits a crucial role in
both the pathogenesis and progression of cancer. OPN exerts an
oncogenesis-promoting effect by stimulating cell proliferation,
antagonizing apoptosis, promoting angiogenesis, suppressing
immunity and inducing drug resistance. Given its pivotal role, OPN
has been regarded as one of the targets for cancer treatment.
OPN-based cancer therapeutics based on gene interference,
inhibitors and immunoregulation have displayed great potential in
research and clinical settings. However, a few aspects deserve to
be considered to broaden OPN-based cancer interventions. Firstly,
the molecular pathways through which OPN participates in
tumorigenesis could be revealed by multiple-omics analysis.
Secondly, the dynamic changes of OPNs during cancer development and
treatment should be precisely monitored. Thirdly, OPN-based
therapeutics should be combined with other treatment strategies. In
summary, by elucidating the various roles of OPN in cancer genesis
and development, the present review could deepen the understanding
of cancer biology and help develop a more effective cancer
treatment strategy.
Acknowledgements
The authors would like to thank Mr. Peng Ye (Shanxi
Medical University, Taiyuan, Shanxi 030000, China) and Ms. Shangyu
Xie (Shanxi Medical University, Taiyuan, Shanxi 030000, China) for
their drafting guidance in preparing the manuscript.
Funding
The present study was supported by the Doctoral Fund of Shanxi
Medical University (grant no. XD1805), the Doctoral Provincial
Research Fund of Shanxi Medical University (grant no. SD1806), the
Research Fund of Shanxi Province Health Commission (grant no.
2021060), the Shanxi Basic Research Program for Young Scientists
(grant no. 202203021212366) and the Basic Research Plan Project of
Shanxi Province (grant no. 20210302123265).
Availability of data and materials
Not applicable.
Authors' contributions
CZ proposed the concept and edited the draft. WX
and ZB wrote original draft. FF, LL and LC reviewed and finalized
the draft. Data authentication is not applicable. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kariya Y and Kariya Y: Osteopontin in
cancer: Mechanisms and therapeutic targets. Int J Transl Med.
2:419–447. 2022.
|
|
2
|
Liu X, Fang J, Huang S, Wu X, Xie X, Wang
J, Liu F, Zhang M, Peng Z and Hu N: Tumor-on-a-chip: From
bioinspired design to biomedical application. Microsyst Nanoeng.
7:502021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
4
|
Yang M, Cui M, Sun Y, Liu S and Jiang W:
Mechanisms, combination therapy, and biomarkers in cancer
immunotherapy resistance. Cell Commun Signa. 22:3382024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toledo B, Deiana C, Scianò F, Brandi G,
Marchal JA, Perán M and Giovannetti E: Treatment resistance in
pancreatic and biliary tract cancer: Molecular and clinical
pharmacology perspectives. Expert Rev Clin Pharmacol. 17:323–347.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Z, Bao C, Jiang L, Wang S, Wang K,
Lu C and Fang H: When cancer drug resistance meets metabolomics
(bulk, single-cell and/or spatial): Progress, potential, and
perspective. Front Oncol. 12:10542332022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yim A, Smith C and Brown AM:
Osteopontin/secreted phosphoprotein-1 harnesses glial-, immune-,
and neuronal cell ligand-receptor interactions to sense and
regulate acute and chronic neuroinflammation. Immunol Rev.
311:224–233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sodek J, Ganss B and McKee MD:
Osteopontin. Crit Rev Oral Biol Med. 11:279–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Senger DR, Wirth DF and Hynes RO:
Transformed mammalian cells secrete specific proteins and
phosphoproteins. Cell. 16:885–893. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bastos ACSF, Blunck CB, Emerenciano M and
Gimba ERP: Osteopontin and their roles in hematological
malignancies: Splice variants on the new avenues. Cancer Lett.
408:138–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gimba ER and Tilli TM: Human osteopontin
splicing isoforms: Known roles, potential clinical applications and
activated signaling pathways. Cancer Lett. 331:11–17. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jia Q, Ouyang Y, Yang Y, Yao S, Chen X and
Hu Z: Osteopontin: A novel therapeutic target for respiratory
diseases. Lung. 202:25–39. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao H, Chen Q, Alam A, Cui J, Suen KC,
Soo AP, Eguchi S, Gu J and Ma D: The role of osteopontin in the
progression of solid organ tumour. Cell Death Dis. 9:3562018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panda VK, Mishra B, Nath AN, Butti R,
Yadav AS, Malhotra D, Khanra S, Mahapatra S, Mishra P, Swain B, et
al: Osteopontin: A key multifaceted regulator in tumor progression
and immunomodulation. Biomedicines. 12:15272024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mi Z, Guo H, Russell MB, Liu Y, Sullenger
BA and Kuo PC: RNA aptamer blockade of osteopontin inhibits growth
and metastasis of MDA-MB231 breast cancer cells. Mol Ther.
17:153–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kumar V, Behera R, Lohite K, Karnik S and
Kundu GC: p38 kinase is crucial for osteopontin-induced furin
expression that supports cervical cancer progression. Cancer Res.
70:10381–10391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zagani R, Hamzaoui N, Cacheux W, de
Reyniès A, Terris B, Chaussade S, Romagnolo B, Perret C and
Lamarque D: Cyclooxygenase-2 inhibitors down-regulate osteopontin
and Nr4A2-new therapeutic targets for colorectal cancers.
Gastroenterology. 137:1358–1366.e1-e3. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Standal T, Borset M and Sundan A: Role of
osteopontin in adhesion, migration, cell survival and bone
remodeling. Exp Oncol. 26:179–184. 2004.PubMed/NCBI
|
|
19
|
Briones-Orta MA, Avendaño-Vázquez SE,
Aparicio-Bautista DI, Coombes JD, Weber GF and Syn WK: Osteopontin
splice variants and polymorphisms in cancer progression and
prognosis. Biochim Biophys Acta Rev Cancer. 1868:93–108.A. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hao C, Cui Y, Owen S, Li W, Cheng S and
Jiang WG: Human osteopontin: Potential clinical applications in
cancer (review). Int J Mol Med. 39:1327–1337. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Regan A and Berman JS: Osteopontin: A
key cytokine in cell-mediated and granulomatous inflammation. Int J
Exp Pathol. 81:373–390. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mirzaei A, Mohammadi S, Ghaffari SH,
Yaghmaie M, Vaezi M, Alimoghaddam K and Ghavamzadeh A: Osteopontin
b and c splice isoforms in leukemias and solid tumors: Angiogenesis
alongside chemoresistance. Asian Pac J Cancer Prev. 19:615–623.
2018.PubMed/NCBI
|
|
23
|
Castello LM, Raineri D, Salmi L, Clemente
N, Vaschetto R, Quaglia M, Garzaro M, Gentilli S, Navalesi P,
Cantaluppi V, et al: Osteopontin at the crossroads of inflammation
and tumor progression. Mediators Inflamm. 2017:40490982017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamort AS, Giopanou I, Psallidas I and
Stathopoulos GT: Osteopontin as a link between inflammation and
cancer: The thorax in the spotlight. Cells. 8:8152019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schytte GN, Christensen B, Bregenov I,
Kjøge K, Scavenius C, Petersen SV, Enghild JJ and Sørensen ES:
FAM20C phosphorylation of the RGDSVVYGLR motif in osteopontin
inhibits interaction with the αvβ3 integrin. J Cell Biochem.
121:4809–4818. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clark EA and Brugge JS: Integrins and
signal transduction pathways: The road taken. Science. 268:233–239.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang KX and Denhardt DT: Osteopontin: Role
in immune regulation and stress responses. Cytokine Growth Factor
Rev. 19:333–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yokosaki Y, Matsuura N, Sasaki T, Murakami
I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y and
Sheppard D: The integrin alpha(9)beta(1) binds to a novel
recognition sequence (SVVYGLR) in the thrombin-cleaved
amino-terminal fragment of osteopontin. J Biol Chem.
274:36328–36334. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ito K, Kon S, Nakayama Y, Kurotaki D,
Saito Y, Kanayama M, Kimura C, Diao H, Morimoto J, Matsui Y and
Uede T: The differential amino acid requirement within osteopontin
in alpha4 and alpha9 integrin-mediated cell binding and migration.
Matrix Biol. 28:11–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maeda N and Maenaka K: The roles of
matricellular proteins in oncogenic virus-induced cancers and their
potential utilities as therapeutic targets. Int J Mol Sci.
17:21982017. View Article : Google Scholar
|
|
31
|
Denhardt DT and Guo X: Osteopontin: A
protein with diverse functions. FASEB J. 7:1475–1482. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kazanecki CC, Uzwiak DJ and Denhardt DT:
Control of osteopontin signaling and function by post-translational
phosphorylation and protein folding. J Cell Biochem. 102:912–924.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bellahcène A, Castronovo V, Ogbureke KUE,
Fisher LW and Fedarko NS: Small integrin-binding ligand N-linked
glycoproteins (SIBLINGs): Multifunctional proteins in cancer. Nat
Rev Cancer. 8:212–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dalal S, Zha Q, Daniels CR, Steagall RJ,
Joyner WL, Gadeau AP, Singh M and Singh K: Osteopontin stimulates
apoptosis in adult cardiac myocytes via the involvement of CD44
receptors, mitochondrial death pathway, and endoplasmic reticulum
stress. Am J Physiol Heart Circ Physiol. 306:H1182–H1191. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyazaki K, Okada Y, Yamanaka O, Kitano A,
Ikeda K, Kon S, Uede T, Rittling SR, Denhardt DT, Kao WW and Saika
S: Corneal wound healing in an osteopontin-deficient mouse. Invest
Ophth Vis Sci. 49:1367–1375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei J, Marisetty A, Schrand B,
Gabrusiewicz K, Hashimoto Y, Ott M, Grami Z, Kong LY, Ling X,
Caruso H, et al: Osteopontin mediates glioblastoma-associated
macrophage infiltration and is a potential therapeutic target. J
Clin Invest. 129:137–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun C, Rahman MSU, Enkhjargal B, Peng J,
Zhou K, Xie Z, Wu L, Zhang T, Zhu Q, Tang J, et al: Osteopontin
modulates microglial activation states and attenuates inflammatory
responses after subarachnoid hemorrhage in rats. Exp Neurol.
371:1145852024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y, Huang Z, Gao L, Ma H and Chang R:
Osteopontin/SPP1: A potential mediator between immune cells and
vascular calcification. Front Immunol. 15:13955962024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang S, Hu H, Li M, Zhang K, Wu Q, Liu X,
Wu L, Yu B and Chen X: OPN promotes pro-inflammatory cytokine
expression via ERK/JNK pathway and M1 macrophage polarization in
rosacea. Front Immunol. 14:12859512024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao L, Wang Z, Tan Y, Ma J, Huang W,
Zhang X, Jin C, Zhang T, Liu W and Yang YG: IL-17A/CEBPβ/OPN/LYVE-1
axis inhibits anti-tumor immunity by promoting tumor-associated
tissue-resident macrophages. Cell Rep. 43:1150392024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He Z, Shen S, Yi Y, Ren L, Tao H, Wang F
and Jia Y: CD31 promotes diffuse large B-cell lymphoma metastasis
by upregulating OPN through the AKT pathway and inhibiting CD8+ T
cells through the mTOR pathway. Am J Transl Res. 15:2656–2675.
2023.PubMed/NCBI
|
|
42
|
Ding W, Deng S, Wang Z, Chen X, Xu Z,
Zhong J, Wu X, Lin R, Ye S, Li C and Li H: CD163+ macrophages drive
rapid pulmonary fibrosis via osteopontin secretion. Int
Immunopharmacol. 161:1149762025. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Gan Z, Wang Y, Hu D, Zhang L, Ye F
and Duan P: A noninvasive menstrual Blood-based diagnostic platform
for endometriosis using digital droplet Enzyme-linked immunosorbent
assay and Single-cell RNA sequencing. Research (Wash DC).
8:06522025.PubMed/NCBI
|
|
44
|
Crawford HC, Matrisian LM and Liaw L:
Distinct roles of osteopontin in host defense activity and tumor
survival during squamous cell carcinoma progression in vivo. Cancer
Res. 58:5206–5215. 1998.PubMed/NCBI
|
|
45
|
Icer MA and Gezmen-Karadag M: The multiple
functions and mechanisms of osteopontin. Clin Biochem. 59:17–24.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Steitz SA, Speer MY, McKee MD, Liaw L,
Almeida M, Yang H and Giachelli CM: Osteopontin inhibits mineral
deposition and promotes regression of ectopic calcification. Am J
Pathol. 161:2035–2046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Giachelli CM and Steitz S: Osteopontin: A
versatile regulator of inflammation and biomineralization. Matrix
Biol. 19:615–622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
McKee MD, Pedraza CE and Kaartinen MT:
Osteopontin and wound healing in bone. Cells Tissues Organs.
194:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ishijima M, Rittling SR, Yamashita T,
Tsuji K, Kurosawa H, Nifuji A, Denhardt DT and Noda M: Enhancement
of osteoclastic bone resorption and suppression of osteoblastic
bone formation in response to reduced mechanical stress do not
occur in the absence of osteopontin. J Exp Med. 193:399–404. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Miragliotta V, Pirone A, Donadio E, Abramo
F, Ricciardi MP and Theoret CL: Osteopontin expression in healing
wounds of horses and in human keloids. Equine Vet J. 48:72–77.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wen Y, Feng D, Wu H, Liu W, Li H, Wang F,
Xia Q, Gao WQ and Kong X: Defective initiation of liver
regeneration in osteopontin-deficient mice after partial
hepatectomy due to insufficient activation of IL-6/Stat3 pathway.
Int J Biol Sci. 11:1236–1247. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ström A, Franzén A, Wängnerud C, Knutsson
AK, Heinegård D and Hultgårdh-Nilsson A: Altered vascular
remodeling in osteopontin-deficient atherosclerotic mice. J Vasc
Res. 41:314–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Khuri FR, Shin DM, Glisson BS, Lippman SM
and Hong WK: Treatment of patients with recurrent or metastatic
squamous cell carcinoma of the head and neck: Current status and
future directions. Semin Oncol. 27:25–33. 2000.PubMed/NCBI
|
|
54
|
Peng Y, Feng W, Huang H, Chen Y and Yang
SY: Macrophage-targeting antisenescence nanomedicine enables
in-situ NO induction for gaseous and antioxidative atherosclerosis
intervention. Bioact Mater. 48:294–312. 2025.PubMed/NCBI
|
|
55
|
Lomashvili KA, Cobbs S, Hennigar RA,
Hardcastle KI and O'Neill WC: Phosphate-induced vascular
calcification: Role of pyrophosphate and osteopontin. J Am Soc
Nephrol. 15:1392–1401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Speer MY, McKee MD, Guldberg RE, Liaw L,
Yang HY, Tung E, Karsenty G and Giachelli CM: Inactivation of the
osteopontin gene enhances vascular calcification of matrix Gla
Protein-deficient mice: Evidence for osteopontin as an inducible
inhibitor of vascular calcification in vivo. J Exp Med.
196:1047–1055. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bandopadhyay M, Bulbule A, Butti R,
Chakraborty G, Ghorpade P, Ghosh P, Gorain M, Kale S, Kumar D,
Kumar S, et al: Osteopontin as a therapeutic target for cancer.
Expert Opin Ther Targets. 18:883–895. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chambers AF, Wilson SM, Kerkvliet N,
O'Malley FP, Harris JF and Casson AG: Osteopontin expression in
lung cancer. Lung Cancer. 15:311–323. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Gotoh M, Sakamoto M, Kanetaka K, Chuuma M
and Hirohashi S: Overexpression of osteopontin in hepatocellular
carcinoma. Pathol Int. 52:19–24. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cook AC, Tuck AB, McCarthy S, Turner JG,
Irby RB, Bloom GC, Yeatman TJ and Chambers AF: Osteopontin induces
multiple changes in gene expression that reflect the six ‘hallmarks
of cancer’ in a model of breast cancer progression. Mol Carcinog.
43:225–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Moorman HR, Poschel D, Klement JD, Lu C,
Redd PS and Liu K: Osteopontin: A key regulator of tumor
progression and immunomodulation. Cancers (Basel). 12:33792020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kariya Y, Oyama M, Kariya Y and Hashimoto
Y: Phosphorylated osteopontin secreted from cancer cells induces
cancer cell motility. Biomolecules. 11:13232021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Klement JD, Paschall AV, Redd PS, Ibrahim
ML, Lu C, Yang D, Celis E, Abrams SI, Ozato K and Liu K: An
osteopontin/CD44 immune checkpoint controls CD8+ T cell activation
and tumor immune evasion. J Clin Invest. 128:5549–5560. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Cao J, Li J, Sun L, Qin T, Xiao Y, Chen K,
Qian W, Duan W, Lei J, Ma J, et al: Hypoxia-driven paracrine
osteopontin/integrin αvβ3 signaling promotes pancreatic cancer cell
epithelial-mesenchymal transition and cancer stem cell-like
properties by modulating forkhead box protein M1. Mol Oncol.
13:228–245. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Qian J, LeSavage BL, Hubka KM, Ma C,
Natarajan S, Eggold JT, Xiao Y, Fuh KC, Krishnan V, Enejder A, et
al: Cancer-associated mesothelial cells promote ovarian cancer
chemoresistance through paracrine osteopontin signaling. J Clin
Invest. 131:e1461862021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Huang R, Quan Y, Chen JH, Wang TF, Xu M,
Ye M, Yuan H, Zhang CJ, Liu XJ and Min ZJ: Osteopontin promotes
cell migration and invasion, and inhibits apoptosis and autophagy
in colorectal cancer by activating the p38 MAPK signaling pathway.
Cell Physiol Biochem. 41:1851–1864. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ahmed M, Sottnik JL, Dancik GM, Sahu D,
Hansel DE, Theodorescu D and Schwartz MA: An osteopontin/CD44 Axis
in RhoGDI2-Mediated metastasis suppression. Cancer Cell.
30:432–443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sun C, Enkhjargal B, Reis C, Zhou KR, Xie
ZY, Wu LY, Zhang TY, Zhu QQ, Tang JP, Jiang XD and Zhang JH:
Osteopontin attenuates early brain injury through regulating
autophagy-apoptosis interaction after subarachnoid hemorrhage in
rats. Cns Neurosci Ther. 25:1162–1172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wei R, Wong JPC and Kwok HF: Osteopontin-a
promising biomarker for cancer therapy. J Cancer. 8:2173–2183.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhao J, Dong L, Lu B, Wu G, Xu D, Chen J,
Li K, Tong X, Dai J, Yao S, et al: Down-regulation of osteopontin
suppresses growth and metastasis of hepatocellular carcinoma via
induction of apoptosis. Gastroenterology. 135:956–968. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fedarko NS, Fohr B, Robey PG, Young MF and
Fisher LW: Factor H binding to bone sialoprotein and osteopontin
enables tumor cell evasion of complement-mediated attack. J Biol
Chem. 275:16666–16672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhou J, Chen X, Zhou P, Sun X, Chen Y, Li
M, Chu Y, Zhou J, Hu X, Luo Y, et al: Osteopontin is required for
the maintenance of leukemia stem cells in acute myeloid leukemia.
Biochem Biophys Res Commun. 600:29–34. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chen Q, Shou P, Zhang L, Xu C, Zheng C,
Han Y, Li W, Huang Y, Zhang X, Shao C, et al: An
osteopontin-integrin interaction plays a critical role in directing
adipogenesis and osteogenesis by mesenchymal stem cells. Stem
Cells. 32:327–337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Irby RB, Mccarthy SM and Yeatman TJ:
Osteopontin regulates multiple functions contributing to human
colon cancer development and progression. Clin Exp Metastas.
21:515–523. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Denhardt DT, Mistretta D, Chambers AF,
Krishna S, Porter JF, Raghuram S and Rittling SR: Transcriptional
regulation of osteopontin and the metastatic phenotype: Evidence
for a Ras-activated enhancer in the human OPN promoter. Clin Exp
Metastas. 20:77–84. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kale S, Raja R, Thorat D, Soundararajan G,
Patil TV and Kundu GC: Osteopontin signaling upregulates
cyclooxygenase-2 expression in tumor-associated macrophages leading
to enhanced angiogenesis and melanoma growth via α9β1 integrin.
Oncogene. 33:2295–2306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Mi Z, Guo H, Wai PY, Gao C and Kuo PC:
Integrin-linked kinase regulates osteopontin-dependent MMP-2 and
uPA expression to convey metastatic function in murine mammary
epithelial cancer cells. Carcinogenesis. 27:1134–1145. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wai PY and Kuo PC: The role of osteopontin
in tumor metastasis. J Surg Res. 121:228–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gupta A, Zhou C and Chellaiah M:
Osteopontin and MMP9: Associations with VEGF expression/secretion
and angiogenesis in PC3 prostate cancer cells. Cancers (Basel).
5:617–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fukusada S, Shimura T, Natsume M,
Nishigaki R, Okuda Y, Iwasaki H, Sugimura N, Kitagawa M, Katano T,
Tanaka M, et al: Osteopontin secreted from obese adipocytes
enhances angiogenesis and promotes progression of pancreatic ductal
adenocarcinoma in obesity. Cell Oncol. 45:229–244. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wei R, Liu S, Zhang S, Min L and Zhu S:
Cellular and extracellular components in tumor microenvironment and
their application in early diagnosis of cancers. Anal Cell Pathol
(Amst). 2020:62837962020.PubMed/NCBI
|
|
83
|
Tan Y, Zhao L, Yang YG and Liu W: The role
of osteopontin in tumor progression through tumor-associated
macrophages. Front Oncol. 12:9532832022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shao L, Zhang B, Wang L, Wu L, Kan Q and
Fan K: MMP-9-cleaved osteopontin isoform mediates tumor immune
escape by inducing expansion of myeloid-derived suppressor cells.
Biochem Biophys Res Commun. 493:1478–1484. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kariya Y, Oyama M, Hashimoto Y, Gu J and
Kariya Y: β4-integrin/PI3K signaling promotes tumor progression
through the galectin-3-N-glycan complex. Mol Cancer Res.
16:1024–1034. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Butti R, Kumar TVS, Nimma R, Banerjee P,
Kundu IG and Kundu GC: Osteopontin signaling in shaping tumor
microenvironment conducive to malignant progression. Adv Exp Med
Biol. 1329:419–441. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rogers MP, Kothari A, Read M, Kuo PC and
Mi Z: Maintaining myofibroblastic-like cancer-associated
fibroblasts by cancer stemness signal transduction feedback loop.
Cureus. 14:e293542022.PubMed/NCBI
|
|
88
|
Leung LL, Myles T and Morser J: Thrombin
cleavage of osteopontin and the host anti-tumor immune response.
Cancers. 15:34802023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yan Z, Hu X, Tang B and Deng F: Role of
osteopontin in cancer development and treatment. Heliyon.
9:e210552023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: Role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Abdelrahman KS, Hassan HA, Abdel-Aziz SA,
Marzouk AA, Narumi A, Konno H and Abdel-Aziz M: JNK signaling as a
target for anticancer therapy. Pharmacol Rep. 73:405–434. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shi L and Wang X: Role of osteopontin in
lung cancer evolution and heterogeneity. Semin Cell Dev Biol.
64:40–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Iida T, Wagatsuma K, Hirayama D and Nakase
H: Is osteopontin a friend or foe of cell apoptosis in inflammatory
gastrointestinal and liver diseases? Int J Mol Sci. 19:72017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Katoh M: Multi-layered prevention and
treatment of chronic inflammation, organ fibrosis and cancer
associated with canonical WNT/β-catenin signaling activation
(review). Int J Mol Med. 42:713–725. 2018.PubMed/NCBI
|
|
95
|
Bastos ACSDF, Gomes AVP, Silva GR,
Emerenciano M, Ferreira LB and Gimba ERP: The intracellular and
secreted sides of osteopontin and their putative physiopathological
roles. Int J Mol Sci. 24:29422023. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Berge G, Pettersen S, Grotterød I, Bettum
IJ, Boye K and Mælandsmo GM: Osteopontin-an important downstream
effector of S100A4-mediated invasion and metastasis. Int J Cancer.
129:780–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Amilca-Seba K, Sabbah M, Larsen AK and
Denis JA: Osteopontin as a regulator of colorectal cancer
progression and its clinical applications. Cancers. 13:37932021.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hao C, Lane J and Jiang WG: Osteopontin
and cancer: Insights into its role in drug resistance.
Biomedicines. 11:1972023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Moldogazieva NT, Mokhosoev IM, Zavadskiy
SP and Terentiev AA: Proteomic profiling and artificial
intelligence for hepatocellular carcinoma translational medicine.
Biomedicines. 9:1592021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Messex JK, Byrd CJ, Thomas MU and Liou GY:
Macrophages cytokine Spp1 increases growth of prostate
intraepithelial neoplasia to promote prostate tumor progression.
Int J Mol Sci. 23:42472022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Insua-Rodríguez J, Pein M, Hongu T, Meier
J, Descot A, Lowy CM, De Braekeleer E, Sinn HP, Spaich S, Sütterlin
M, et al: Stress signaling in breast cancer cells induces matrix
components that promote chemoresistant metastasis. EMBO Mol Med.
10:e90032018. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li NY, Weber CE, Wai PY, Cuevas BD, Zhang
J, Kuo PC and Mi Z: An MAPK-dependent pathway induces
epithelial-mesenchymal transition via twist activation in human
breast cancer cell lines. Surgery. 154:404–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Montalto G, Cervello M, Nicoletti F, Fagone P, Malaponte
G, Mazzarino MC, et al: Mutations and deregulation of
ras/raf/MEK/ERK and PI3K/PTEN/akt/mTOR cascades which alter therapy
response. Oncotarget. 3:954–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Yang YF, Chang YC, Jan YH, Yang CJ, Huang
MS and Hsiao M: Squalene synthase promotes the invasion of lung
cancer cells via the osteopontin/ERK pathway. Oncogenesis.
9:782020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Chernaya G, Mikhno N, Khabalova T,
Svyatchenko S, Mostovich L, Shevchenko S and Gulyaeva L: The
expression profile of integrin receptors and osteopontin in thyroid
malignancies varies depending on the tumor progression rate and
presence of BRAF V600E mutation. Surg Oncol. 27:702–708. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sun BS, Dong QZ, Ye QH, Sun HJ, Jia HL,
Zhu XQ, Liu DY, Chen J, Xue Q, Zhou HJ, et al: Lentiviral-mediated
miRNA against osteopontin suppresses tumor growth and metastasis of
human hepatocellular carcinoma. Hepatology. 48:1834–1842. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lin YH and Yang-Yen HF: The
osteopontin-CD44 survival signal involves activation of the
phosphatidylinositol 3-kinase/akt signaling pathway. J Biol Chem.
276:46024–46030. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang H, Guo M, Chen JH, Wang Z, Du XF,
Liu PX and Li WH: Osteopontin knockdown inhibits αv,β3
integrin-induced cell migration and invasion and promotes apoptosis
of breast cancer cells by inducing autophagy and inactivating the
PI3K/akt/mTOR pathway. Cell Physiol Biochem. 33:991–1002. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Fu Y, Zhang Y, Lei Z, Liu T, Cai T, Wang
A, Du W, Zeng Y, Zhu J, Liu Z and Huang JA: Abnormally activated
OPN/integrin αVβ3/FAK signalling is responsible for EGFR-TKI
resistance in EGFR mutant Non-small-cell lung cancer. J Hematol
Oncol. 13:1692020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hui T, Sørensen ES and Rittling SR:
Osteopontin binding to the alpha 4 integrin requires highest
affinity integrin conformation, but is independent of
Post-translational modifications of osteopontin. Matrix Biol.
41:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wu Q, Li L, Miao C, Hasnat M, Sun L, Jiang
Z and Zhang L: Osteopontin promotes hepatocellular carcinoma
progression through inducing JAK2/STAT3/NOX1-mediated ROS
production. Cell Death Dis. 13:3412022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Behera R, Kumar V, Lohite K, Karnik S and
Kundu GC: Activation of JAK2/STAT3 signaling by osteopontin
promotes tumor growth in human breast cancer cells. Carcinogenesis.
31:192–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Cao L, Fan X, Jing W, Liang Y, Chen R, Liu
Y, Zhu M, Jia R, Wang H, Zhang X, et al: Osteopontin promotes a
cancer stem cell-like phenotype in hepatocellular carcinoma cells
via an integrin-NF-κB-HIF-1α pathway. Oncotarget. 6:6627–6640.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Chen B, Liang S, Guo H, Xu L, Li J and
Peng J: OPN promotes cell proliferation and invasion through NF-κB
in human esophageal squamous cell carcinoma. Genet Res (Camb).
2022:31548272022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Saurav S, Karfa S, Vu T, Liu Z, Datta A,
Manne U, Samuel T and Datta PK: Overcoming irinotecan resistance by
targeting its downstream signaling pathways in colon cancer.
Cancers (Basel). 16:34912024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Graessmann M, Berg B, Fuchs B, Klein A and
Graessmann A: Chemotherapy resistance of mouse WAP-SVT/t breast
cancer cells is mediated by osteopontin, inhibiting apoptosis
downstream of caspase-3. Oncogene. 26:2840–2850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yong HY, Koh MS and Moon A: The p38 MAPK
inhibitors for the treatment of inflammatory diseases and cancer.
Expert Opin Investig Drugs. 18:1893–1905. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yu X, Du Y, Liang S, Zhang N, Jing S, Sui
L, Kong Y, Dong M and Kong H: OPN up-regulated proliferation and
invasion of head and neck squamous cell carcinoma through the
p38MAPK signaling pathway. Oral Surg Oral Med Oral Pathol Oral
Radiol. 136:70–79. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Pang H, Cai L, Yang Y, Chen X, Sui G and
Zhao C: Knockdown of osteopontin chemosensitizes MDA-MB-231 cells
to cyclophosphamide by enhancing apoptosis through activating p38
MAPK pathway. Cancer Biother Radiopharm. 26:165–173.
2011.PubMed/NCBI
|
|
120
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
121
|
Davidson BL and McCray PB: Current
prospects for RNA interference-based therapies. Nat Rev Genet.
12:329–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ben-David-Naim M, Dagan A, Grad E, Aizik
G, Nordling-David MM, Morss Clyne A, Granot Z and Golomb G:
Targeted siRNA nanoparticles for mammary carcinoma therapy. Cancers
(Basel). 11:4422019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yang L, Zhao W, Zuo WS, Wei L, Song XR,
Wang XW, Zheng G and Zheng MZ: Silencing of osteopontin promotes
the radiosensitivity of breast cancer cells by reducing the
expression of hypoxia inducible factor 1 and vascular endothelial
growth factor. Chin Med J (Engl). 125:293–299. 2012.PubMed/NCBI
|
|
124
|
Wang F, Fu K, Wang Y, Pan C, Wang X, Liu
Z, Yang C, Zheng Y, Li X, Lu Y, et al: Small-molecule agents for
cancer immunotherapy. Acta Pharm Sin B. 14:905–952. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sharma P, Kumar S and Kundu GC:
Transcriptional regulation of human osteopontin promoter by histone
deacetylase inhibitor, trichostatin A in cervical cancer cells. Mol
Cancer. 9:1782010. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Kumar S, Patil HS, Sharma P, Kumar D,
Dasari S, Puranik VG, Thulasiram HV and Kundu GC: Andrographolide
inhibits osteopontin expression and breast tumor growth through
down regulation of PI3 kinase/akt signaling pathway. Curr Mol Med.
12:952–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Matsuura M, Suzuki T and Saito T:
Osteopontin is a new target molecule for ovarian clear cell
carcinoma therapy. Cancer Sci. 101:1828–1833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lv J, Shao Q, Wang H, Shi H, Wang T, Gao
W, Song B, Zheng G, Kong B and Qu X: Effects and mechanisms of
curcumin and basil polysaccharide on the invasion of SKOV3 cells
and dendritic cells. Mol Med Rep. 8:1580–1586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Nimjee SM, White RR, Becker RC and
Sullenger BA: Aptamers as therapeutics. Annu Rev Pharmacol Toxicol.
57:61–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
He S, Du Y, Tao H and Duan H: Advances in
aptamer-mediated targeted delivery system for cancer treatment. Int
J Biol Macromol. 238:1241732023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Talbot LJ, Mi Z, Bhattacharya SD, Kim V,
Guo H and Kuo PC: Pharmacokinetic characterization of an RNA
aptamer against osteopontin and demonstration of in vivo efficacy
in reversing growth of human breast cancer cells. Surgery.
150:224–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Ahmed M and Kundu GC: Osteopontin
selectively regulates p70S6K/mTOR phosphorylation leading to
NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast
cancer cells. Mol Cancer. 9:1012010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ahmed M, Behera R, Chakraborty G, Jain S,
Kumar V, Sharma P, Bulbule A, Kale S, Kumar S, Mishra R, et al:
Osteopontin: A potentially important therapeutic target in cancer.
Expert Opin Ther Targets. 15:1113–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chiou J, Chang YC, Tsai HF, Lin YF, Huang
MS, Yang CJ and Hsiao M: Follistatin-like protein 1 inhibits lung
cancer metastasis by preventing proteolytic activation of
osteopontin. Cancer Res. 79:6113–6125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Salih E, Ashkar S, Gerstenfeld LC and
Glimcher MJ: Identification of the phosphorylated sites of
metabolically 32P-labeled osteopontin from cultured chicken
osteoblasts. J Biol Chem. 272:13966–13973. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Peraramelli S, Zhou Q, Zhou Q, Wanko B,
Zhao L, Nishimura T, Leung TH, Mizuno S, Ito M, Myles T, et al:
Thrombin cleavage of osteopontin initiates osteopontin's
tumor-promoting activity. J Thromb Haemost. 20:1256–1270. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Zou Y, Sun X, Wang Y, Yan C, Liu Y, Li J,
Zhang D, Zheng M, Chung RS and Shi B: Single siRNA nanocapsules for
effective siRNA brain delivery and glioblastoma treatment. Adv
Mater. 32:e20004162020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bansal I, Pandey AK and Ruwali M:
Small-molecule inhibitors of kinases in breast cancer therapy:
Recent advances, opportunities, and challenges. Front Pharmacol.
14:12445972023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Lu C, Liu Z, Klement JD, Yang D, Merting
AD, Poschel D, Albers T, Waller JL, Shi H and Liu K: WDR5-H3K4me3
epigenetic axis regulates OPN expression to compensate PD-L1
function to promote pancreatic cancer immune escape. J Immunother
Cancer. 9:e0026242021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu
JL, Li CW, Lim SO, Sheng YY, Zhang Y, et al: Disruption of
tumour-associated macrophage trafficking by the osteopontin-induced
colony-stimulating factor-1 signalling sensitises hepatocellular
carcinoma to anti-PD-L1 blockade. Gut. 68:1653–1666. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Saleh S, Thompson DE, McConkey J, Murray P
and Moorehead RA: Osteopontin regulates proliferation, apoptosis,
and migration of murine Claudin-low mammary tumor cells. BMC
Cancer. 16:3592016. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Cho W-Y, Hong SH, Singh B, Islam MA, Lee
S, Lee AY, Gankhuyag N, Kim JE, Yu KN, Kim KH, et al: Suppression
of tumor growth in lung cancer xenograft model mice by
poly(sorbitol-co-PEI)-mediated delivery of osteopontin siRNA. Eur J
Pharm Biopharm. 94:450–462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Bhattacharya SD, Garrison J, Guo H, Mi Z,
Markovic J, Kim VM and Kuo PC: Micro-RNA-181a regulates
osteopontin-dependent metastatic function in hepatocellular cancer
cell lines. Surgery. 148:291–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Mason CK, McFarlane S, Johnston PG, Crowe
P, Erwin PJ, Domostoj MM, Campbell FC, Manaviazar S, Hale KJ,
El-Tanani M, et al: Agelastatin A: A novel inhibitor of
osteopontin-mediated adhesion, invasion, and colony formation. Mol
Cancer Ther. 7:548–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Dai J, Li B, Shi J, Peng L, Zhang D, Qian
W, Hou S, Zhao L, Gao J, Cao Z, et al: A humanized Anti-osteopontin
antibody inhibits breast cancer growth and metastasis in vivo.
Cancer Immunol Immunother. 59:355–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Teramoto H, Castellone MD, Malek RL,
Letwin N, Frank B, Gutkind JS and Lee NH: Autocrine activation of
an osteopontin-CD44-Rac pathway enhances invasion and
transformation by H-RasV12. Oncogene. 24:489–501. 2005. View Article : Google Scholar : PubMed/NCBI
|