Introduction
Senescent cells are characterized by a permanent
cell cycle arrest, resistance to apoptosis and the bioactive
secretion phenotype known as the senescence-associated secretory
phenotype (SASP) (1–4). While cellular senescence has
beneficial effects in early carcinogenesis and tumor therapy,
studies have shown that the accumulation of senescent cells in
age-related diseases, including cancer, has detrimental
consequences. The persistence of these cells promotes cancer cell
growth, aggressiveness and metastasis while contributing to an
immunosuppressive tumor microenvironment (TME) (5,6).
Previous research has highlighted the potential prognostic
importance of senescent cells in HNSCC patients (7). The expression of SASP components has
been linked to certain clinicopathological features, suggesting
that senescence-related mechanisms may impact tumor progression and
patient outcomes (8). Specific
senescence-related genes hold prognostic value in HNSCC. For
instance, a prognostic risk model incorporating these genes has
been developed, offering potential as a biomarker for patient
stratification and personalized treatment approaches (9). Cellular senescence can be effected by
DNA damage caused by radiation therapy, which is one of the main
treatment pillars in HNSCC. This therapy-induced senescence (TIS)
contributes to tumor control by arresting the growth of cancer
cells. However, the accumulation of senescent cells
post-irradiation can also promote radioresistance. Early onset of
senescence and the subsequent production of SASP factors have been
identified as key determinants of this resistance in HNSCC
(9).
The expression of radiation-induced
senescence-associated genes has been suggested to have prognostic
implications in HNSCC (10).
Exposure to IR causes compensatory activation of intracellular
signaling cascades securing tumor cell survival (11–13)
such as signaling pathways MEK/ERK and PI3K/AKT in HNSCC (14,15).
Our own previous studies verify the IR-dependent induction of these
cascades in vitro as well as in a 3D HNSCC ex vivo
model most likely as a mechanism of resistance against radiotherapy
(16–19). The response to therapeutic
strategies in HNSCC is generally determined by a pronounced
intratumorigenic heterogeneity (20) followed by a high variety in
treatment response.
Immunotherapy has revolutionized treatment for a
number of cancers including HNSCC over the past decade. In
recurrent or metastatic HNSCC PD-1 inhibitors play an essential
role (21,22). However, the majority of patients do
not exhibit stable responses, suggesting the presence of mechanisms
underlying de novo or acquired resistance to checkpoint
inhibitors (CPI). Despite advances in the field, there remains a
lack of robust biomarkers to predict clinical response and/or
resistance to CPI (23).
Senescent cells have been shown to heterogeneously
express the immune checkpoint molecule PD-L1 and that PD-L1+
senescent cells accumulate with age in vivo. In the context
of potential anti-ageing therapy, the elimination of PD-L1+
senescent cells by immune checkpoint blockade has been described as
a promising approach (24).
Senescent cells upregulate PD-L1, the ligand for PD-1 on immune
cells, leading to immune cell inactivation. Consequently, targeting
PD-L1 has been proposed as a novel strategy for addressing
senescence (25). Additionally,
PD-L1 plays a role in the senescence of malignant cells via
interferon-dependent cell cycle regulatory cascades or through its
destabilization by cyclin D-CDK4 kinase (26–28).
Notably, silencing PD-L1 in cancer cells has been shown to induce
senescence (29). A potential link
between checkpoint regulation and senescence is further supported
by an investigation by Moreira et al (30), which investigated whether senescence
markers could predict response to CPI in melanoma patients.
Additionally, aging-related genes have been associated with
chemotherapy resistance and linked to CPI response in HNSCC
(7). Early senescence and the
production of senescence-associated cytokines have also been
identified as major determinants of radioresistance in HNSCC
(9). Moreover, there is a notable
connection between mitogen-activated protein kinase (MAPK) pathway
activation and senescence. This relationship is exemplified by the
association between MAPK ERK1/2 and p21, a key marker of senescence
activation (31). Recently, we
demonstrated a potential co-regulation of pERK1/2 and PD-L1
expression as a response to standard HNSCC treatment revealing a
direct link between oncogenic drivers and PD-L1 expression
(32).
Building on these findings, the present study used a
2D in vitro and a 3D ex vivo HNSCC model to map the
effect of fractionated IR on the induction of senescence as well as
its influence on checkpoint expression and survival signaling as
part of a potential tumoral bailout mechanism.
Materials and methods
Cell culture
The cell lines UM-SCC-11B, UM-SCC-14C and UM-SCC-22B
were obtained from Dr TE Carey (University of Michigan, Ann Arbor,
MI, USA). Origins of the cell lines were larynx, oral cavity and
hypopharynx, respectively (Table I)
(33). Original tumors were not
human papilloma virus-driven. The cells were cultivated in
Dulbecco´s modified Eagle´s medium (DMEM; Thermo Fisher Scientific
Inc.) supplemented with 10% fetal calf serum (FCS) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific Inc.). The
authentication of all cell lines was confirmed by a recent Short
Tandem Repeat (STR) profiling which is a DNA-based method used for
authenticating and identifying cell lines (CLS Cell Lines Service
GmbH; latest update Nov 2022).
 | Table I.Characteristics of the head and neck
squamous cell carcinoma cell lines (33). |
Table I.
Characteristics of the head and neck
squamous cell carcinoma cell lines (33).
| Characteristic | UM-SCC-11B | UM-SCC-14C | UM-SCC-22B |
|---|
| Specimen | Primary | Local
recurrence | Lymph node
metastasis |
| Tumor site | Larynx | Floor of mouth | Hypopharynx |
| TNM status | T2N2a | T2N0 | T2N1 |
| Previous
therapy | CT | SU + CRT | None |
Irradiation experiments
For each cell line, 200,000 cells/well were seeded
in six-well plates and irradiated day 4 post-seeding on four
consecutive days with a daily dose of 2 Gy using a linear
accelerator (a device used in radiation therapy to deliver
high-energy X-rays or electrons to tumors) with 6 megavolts (MV)
photon energy (Elekta Synergy AB) and polymethylmethacrylate (PMMA)
plates as water and tissue equivalents, respectively. Cells were
left to recover again on days 8 and 9 and were harvested on day 10.
Controls were mock-treated. Each experiment was performed three
times (n=3).
Senescence-associated ß-galactosidase
(SA-ß-Gal) staining
The cells were cultured in 9-well plates. After 48 h
in culture, cells were irradiated with 2 Gy on four consecutive as
aforementioned. Mock-treated samples served as controls. At 4 h
after the last irradiation session, SA-ß-Gal staining was performed
according to the manufacturer's instructions (Senescence
β-galactosidase Staining Kit; cat. no. 9860; Cell Signaling
Technology, Inc.). First, the growth medium was removed and the
cells were washed once with 1X PBS. Then, 1 ml of 1X fixative
solution was added to each 35 mm well and the cells were fixed for
10–15 min at room temperature (RT). After rinsing off the fixative
solution with 1X PBS, 1 ml of the prepared β-galactosidase staining
solution (comprising 930 µl of 1X Staining Solution, 10 µl of 100X
Solution A, 10 µl of 100X Solution B and 50 µl of 20 mg/ml X-Gal)
was added to each 35 mm well. The plate was sealed to prevent
evaporation and incubated overnight at 37°C in a dry incubator. The
cells were then examined under the microscope (magnification, ×200)
for the development of blue staining. For long-term storage, the
β-galactosidase staining solution was removed and the cells were
overlaid with 70% glycerol. The plates were stored at 4°C. The
experiment was repeated three times.
Senescence induction was quantified by ImageJ
software (National Institutes of Health, version 1.54p) and is
given as relative amount of positive cells. Due to the small sample
size normal distribution could not be assumed hence statistical
assessment was performed using Mann Whitney U Test.
Immunohistochemical (IHC)
staining
Cells were treated as described for SA
ß-galactosidase staining and fixed by ice-cold ethanol/acetone
(2:1) for 15 min on ice followed by treatment with antigen
retrieval with Tris-EDTA buffer or Citrate buffer for antigen
retrieval and subsequently endogenous peroxidase blockage with DAKO
Peroxidase blocking solution (Agilent Technologies, Inc.). After
preincubation with sheep normal serum 1:10 (BIOZOL Diagnostica
Vertrieb GmbH) for 30 min at RT to avoid unspecific binding,
primary antibodies [phosphorylated-p44/42 MAPK (Erk1/2;
Thr202/Tyr204); cat. no. 9101 Cell Signaling Technology, Inc.;
1:400; PD-L1; cat. no. 13684, Cell Signaling Technology, Inc.;
1:400; p21 Waf1/Cip1 (12D1) Rabbit mAb cat. no. 2947, Cell
Signaling Technology, Inc.; 1:500, Phospho-Histone H2A.X (Ser139;
20E3) Rabbit mAb cat. no. 9718, Cell Signaling Technology, Inc.;
1:1,000)] were incubated overnight. A biotinylated secondary
antibody, diluted 1:200 in streptavidin biotinylated horseradish
peroxidase for 45 min at RT (Cytiva), was added. Afterwards,
3-amino-9-ethylcarbazole (AEC; ScyTek Laboratories, Inc.) was used
as a substrate. All washing procedures were performed in PBS.
Slides were counterstained with hematoxylin for 5 min at RT.
Sections incubated without the primary antibody served as negative
controls and additional controls were performed with the secondary
antibody only (data not shown). Samples were imaged using Zeiss
Axio Observer Z1 with AxioCam 505 color and analysed with
Zen-software version 2.3 (Carl Zeiss AG).
Ex vivo treatment of HNSCC vital
tissue cultures
Fresh tissue HNSCC samples (n=4; 1 from the
oropharynx, 1 from the oral cavity, 1 from the hypopharynx and 1
from the larynx; Table II) were
procured immediately after surgical resection at the Department of
Otorhinolaryngology, Head and Neck Surgery, Mannheim University
Hospital, Germany. All four donors were male. The mean age was 56.5
years (±1.73 years). Recruitment took place between February 2025
and June 2025. Informed consent was obtained from all subjects
involved in the study after the review of the local ethics board
(ethic vote 2020-558N). The consent forms provided clear
information on the study's purpose, procedures, risks, benefits and
the right to withdraw at any time and were presented tailored to
the participants' comprehension level. Patients were informed by
the principal investigator of the study and consented orally and in
written after having the opportunity to ask questions and address
concerns. Patients were to be included in the study if they had
been diagnosed with HNSCC and have undergone treatment at the
Department of Otorhinolaryngology, Head and Neck Surgery,
University Hospital Mannheim. Sufficient formalin-fixed
paraffin-embedded (FFPE) tumor material had to be available for the
planned analyses. Patients were to be excluded from the study if
they have a pre-existing mental illness that might interfere with
study participation, a history of a previous HNSCC diagnosis, or
another active malignant disease at the time of inclusion. Patients
with medical conditions associated with an increased risk of
bleeding or impaired wound healing were also excluded. Further
exclusion criteria include being under the age of 18, not being
fully capable of giving informed consent, or testing positive for
HIV, HBV, or HCV. Table II gives
characteristics of ex vivo specimens.
 | Table II.Characteristics of ex vivo
specimens. |
Table II.
Characteristics of ex vivo
specimens.
| Fresh tissue
sample | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|
| Patient age,
sex | 59, male | 56, male | 56, male | 55, male |
| Tumor site | Oropharynx (Left
Tonsil) | Oral cavity (Lower
lip) | Larynx | Hypopharynx |
| TNM status | pT1 N0 M0 | pT3 pN0 M0 | rcT4a cN0 M0 | pT4a pN2b M0 |
| Histological risk
factors | V0 L0 Pn0 R0
G2 | V0 L0 Pn1 R0
G2 | L0 V0 Pn0 G2 | L1 V0 Pn1 R0
G3 |
| Date (month/year)
of sample collection | 02/2024 | 05/2024 | 05/2024 | 06/2024 |
Specimens were transported to the laboratory in
transport media (Dulbecco's modified Eagle's medium, supplemented
with Primocin) within 30 min after resection. Fresh tissue was
sectioned with a scalpel to fragments of 2–3 mm2 under
sterile conditions. For ex vivo analysis of tumor response
to fractionated IR, tumor sections were maintained in twelve-well
plates with inserts (ThinCert; Greiner Bio One Ltd.) in Dulbecco's
modified Eagle's medium, supplemented with 10% fetal bovine serum
and antibiotics (penicillin 100 U/ml and streptomycin 100 µg/ml).
After five days in culture, samples were irradiated on three
consecutive days with a daily dose of 2 Gy. Mock-treated samples
served as controls. According to the cell line experiments, a
linear accelerator with 6 megavolts (MV) photon energy (Elekta
Synergy AB) and PMMA plates as water and tissue equivalents,
respectively, were employed.
Previously, the total dose and time scheme of 3×2 Gy
applied on days 6–8 had been evaluated by dose and time row
experiments to achieve an optimal IR effect without overdosing the
tissue samples. Fractionated IR instead of a single dose was chosen
to adapt the experimental setting to the norm fractionation scheme
applied in HNSCC patients as closely as possible (32,34).
The medium was changed every second day. The samples were harvested
on day 10 to be evaluated for histopathological and
immunohistochemical features (34,35).
Morphological and immunohistochemical
evaluation of ex vivo HNSCC cultures
Ex vivo cultivated tissue was FFPE. From
these FFPE tissue blocks, 0.5 µM sections were cut and
deparaffinized. Hematoxylin and eosin staining, a routine
histological technique to visualize tissue morphology was performed
(Eosin 10 min, hematoxylin 5 min, at RT). According to the
immunohistochemistry protocol used in cell lines, staining was
performed analogously with adaptation of the concentrations of
antibodies as follows: [Phospho-p44/42 MAPK (Erk1/2;
Thr202/Tyr204); cat. no. 9101; Cell Signaling Technology, Inc.;
1:100; t(total) ERK1/2 p44/42 MAPK (ERK1/2); cat. no. 9102; Cell
Signaling Technology, Inc.; 1:100; PD-L1; cat. no. 13684; Cell
Signaling Technology, Inc.; 1:200, p21 Waf1/Cip1 (12D1) Rabbit mAb
cat. no. 2947 Cell Signaling Technology, Inc.; 1:100,
phospho-Histone H2A.X (Ser139; 20E3) Rabbit mAb cat. no. 9718, Cell
Signaling Technology, Inc.; 1:200]. Incubation with the primary
antibodies was at 4°C overnight.
Quantification of IHC results in cell
and tissue cultures
The intensity of IHC staining was determined using
the immunoreactive score (IRS) proposed by Remmele and Stegner
(36) with slight modifications as
follows.
Staining intensity × percentage of positive
cells=IRS.
The percentage of immunoreactive tumor cells was
rated as follows: No staining=0; less than 10% positive cells=1;
10–50% positive cells=2; 51–80% positive cells=3; greater than 80%
positive cells=4. The intensity of staining was evaluated as
follows: no color reaction=0; weak/mild color reaction=1; moderate
=2; strong/intense color reaction=3. The expression score was
obtained by multiplying the percentage of immunoreactive tumor
cells by the staining intensity. The score was determined by three
independent observers and the mean value was calculated.
Shapiro-Wilk-Test was performed with the null hypothesis of normal
distribution of the data. Since most p-values were significant a
Gaussian distribution was not presumed and statistical significance
was calculated using Mann Whitney U test.
Statistical analysis
Results were graphed and analyzed using GraphPad
Prism software (version 9.4.1; Dotmatics). Data are presented as
means (bars) with standard error of the mean (whiskers) from three
independent experiments.
A Shapiro-Wilk test was conducted to assess the
normality of the data. Since most P-values were significant, the
present study did not assume a Gaussian distribution.
For SA-ß-Gal staining, a Mann-Whitney U test was
performed. The null hypothesis stated that there was no difference
in positive SA-ß-Gal staining between IR cells and controls.
The results of both ex vivo and in
vitro experiments were also analyzed statistically.
Immunoreactivity scores assigned by three independent observers
were compared using the Mann-Whitney U test. The null hypothesis
stated that there was no difference in scoring between treated
cells and mock-treated controls. P<0.05 was considered to
indicate a statistically significant difference.
Results
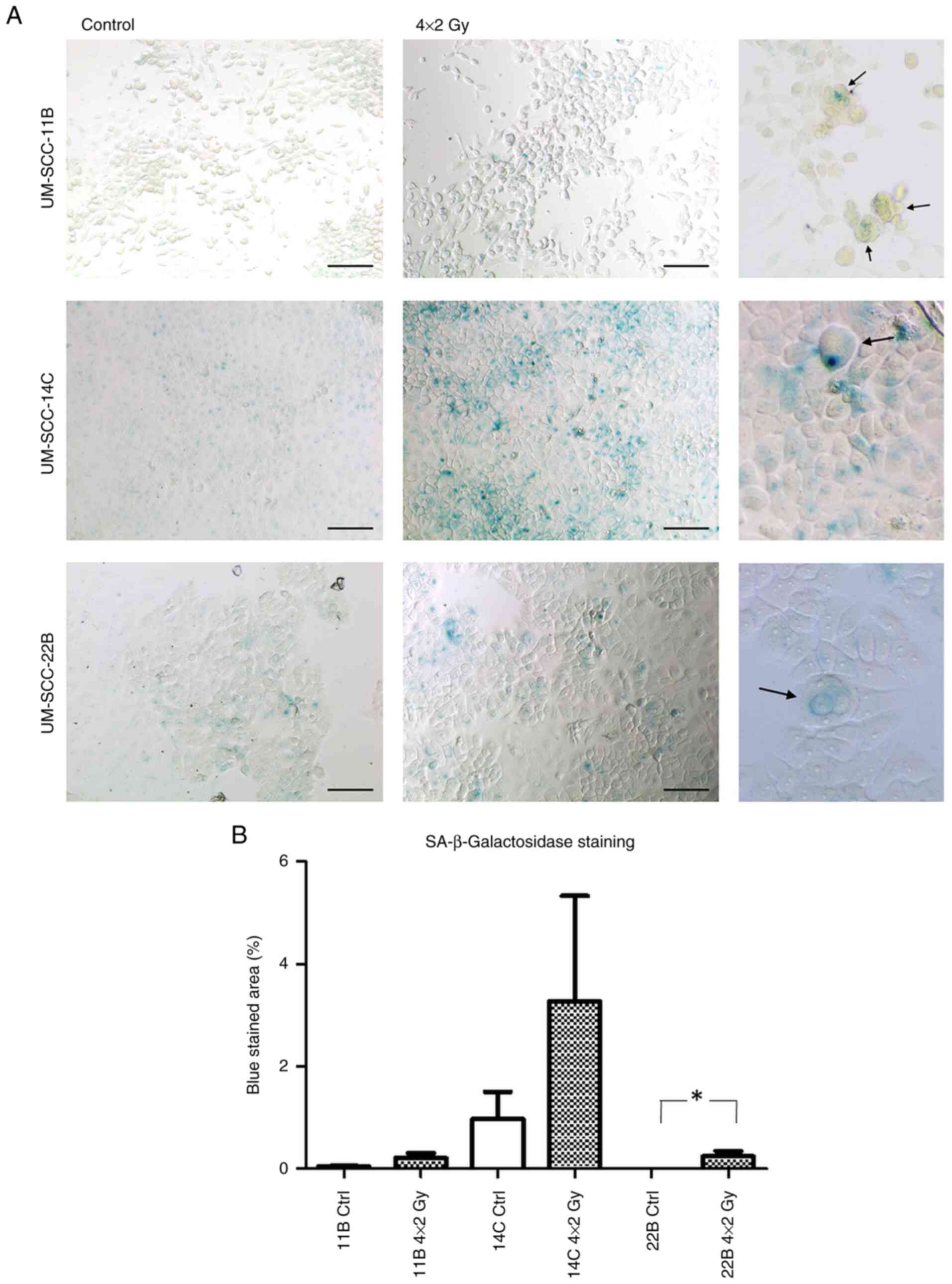
Morphologic alterations of the
nuclei
Multiple publications describe morphologic
alterations, such as enlarged cell volume and nuclei, irregularly
shaped nuclei and cytoplasmic vacuoles as characteristic features
of senescent cells (37–39). After fractionated IR, the present
study found a subpopulation of tumor cells with enlarged nuclei in
all three HNSCC cell lines (Fig.
1A). Other morphologic alterations, such as enlarged cell
volume, irregularly shaped nuclei and cytoplasmic vacuoles as
characteristic features of senescence, were also visible. The
altered morphology was clearly recognizable across all cell lines.
There was a small number of cells with this phenotype in untreated
cells, which distinctly increased in response to fractionated
IR.
Evidence for senescence induction by
SA-ß-Gal staining
To confirm a potential induction of senescence by
fractionated IR, SA-ß-Gal staining was performed.
SA-ß-Gal staining is a widely used histochemical
assay to detect cellular senescence. It identifies senescent cells
by measuring ß-galactosidase enzyme activity at pH 6.0, a
characteristic feature of senescent cells. The assay involves
staining cells or tissue samples with X-gal, a substrate that
produces a blue color when cleaved by SA-ß-Gal.
An increase in SA-ß-Gal was observed after
irradiation in all cell lines, which was most distinct in UMSCC-14C
cells. In UM-SCC 22B the changes where statistically significant
(P=0.0286; Fig. 1A; for
quantification see Fig. 1B).
Effect of fractioned IR on
proliferation of senescence marker p21
To further confirm senescence induction, IHC
staining of the senescence marker p21 was performed. As seen in
Fig. 2, there was a slight increase
in p21 protein expression in UM-SCC-11B and UM-SCC-22B as well as a
marked increase in UM-SCC-14C compared with mock-treated controls
(Fig. 2A and B).
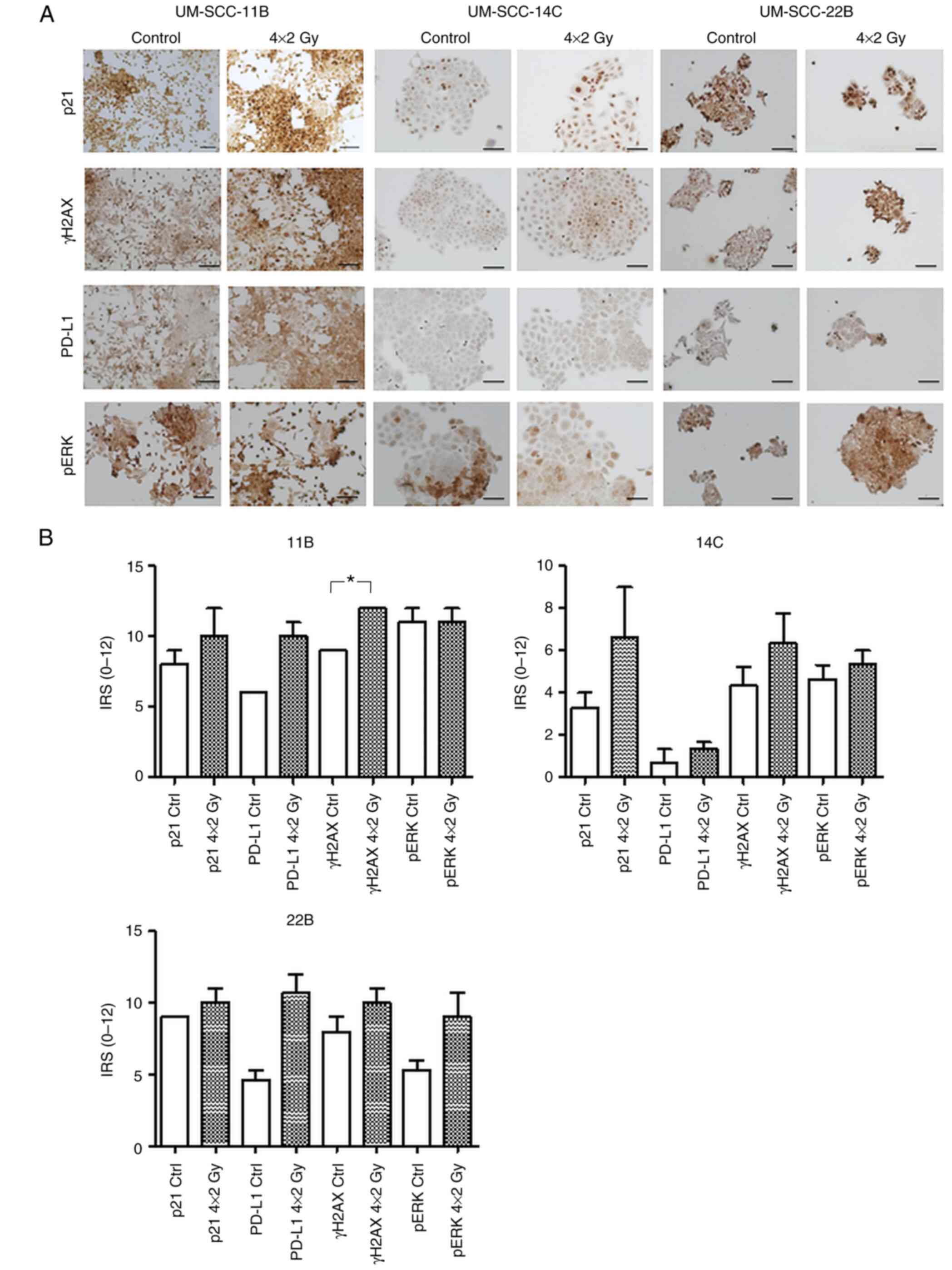 | Figure 2.Differential expression and
quantification of p21, pERK1/2, PD-L1 and γH2AX in HNSCC cell lines
following fractionated IR. (A) Expression of p21, pERK1/2, PD-L1
and γH2AX in HNSCC cell lines UM-SCC-11B, UM-SCC-14C and UM-SCC-22B
post IR. IHC staining of p21, γH2AX, PD-L1 and pERK1/2 after
fractionated IR (4×2 Gy) compared with mock-treated controls
displayed differential modulations of target proteins
(representative images shown). (B) Quantification of IHC results.
The IRS was obtained by visual examination. Results were quantified
using the IRS modified according to Remmele and Stegner (36). The mean ± SD value was calculated.
Statistical significance (*P<0.05) was assessed using Mann
Whitney U test. p, phosphorylated; PD-L1, programmed death-ligand
1; γH2AX, histone H2AX; HNSCC, head and neck squamous cell
carcinoma; IR, ionizing radiation; IHC, immunohistochemistry; IRS,
immunoreactive score. Scale bar=100 µm. |
Effect of fractioned IR on
proliferation of γH2AX
There was a visible increase in positive staining of
γH2AX indicating postradiogenic DNA double strand breaks in all
three cell lines, but due to the small sample size the results of
the statistical evaluation were non-significant (11B P=0.059; 14C
P=0.48; 22B P=0.3; Fig. 2A and
B).
Fractionated IR modulates PD-L1
surface expression
Unlike in previous studies where cells and tissue
samples were treated with a single irradiation dose and immediately
analyzed post IR, the present study was interested in examining
also mid- and long-term adjustment mechanisms to a fractionated
protocol (4×2 Gy) according to the norm fractionation scheme
applied to HNSCC patients. All three cell lines displayed a strong
induction of PD-L1 which was most distinct in cells from the
UM-SCC-22B cell line (Fig. 2A and
B).
Effect of fractionated IR on MAPK ERK
signaling
UM-SCC-22B cells showed a strong induction of
activated ERK1/2, similar to the PD-L1 expression pattern. There
was a minor induction in pERK1/2 in UM-SCC-14C, while no
modifications in pERK1/2 protein expression were detected in
UM-SCC-11B compared with mock-treated controls (Fig. 2A and B).
Validation of senescence-associated
markers in a 3D ex vivo HNSCC tissue culture model
In order to verify the findings of cellular response
to fractionated IR observed in the cell lines, the expression of
senescence-associated markers was validated in human HNSCC tumor
tissue. This explant model offers the advantage of taking the
composition of the individual patient's tumor and a potential
impact of the TME into account, which is not depicted in 2D cell
culture model.
One specimen each from the typical anatomical HNSCC
sites (oral cavity, oropharynx, hypopharynx, larynx) was
included.
A statistically significant increase in p21
expression post IR was observed in all four specimens indicating
that radiotherapy affects senescence regulation (EV1 P=0.0001, EV2
P=0.003, EV 3 P=0.004, EV 4 P=0.0005). Accordingly, there was a
parallel raise in γH2AX as additional senescence marker in all
samples. The difference reached significance in oral cavity, larynx
and hypopharynx samples (EV2 P=0.0002, EV3 P=0.0137, EV 4
P=0.0004).
As expected, the 3D model enabled confirmation of
the 2D results regarding a postradiogenic upregulation of PD-L1 and
pERK1/2. Except the oropharyngeal specimen, which did not express
any PD-L1 at baseline and after treatment, PD-L1 levels were
significantly enhanced after IR in the hypopharyngeal tissue
(P=0.0066) while there was a minor increase in ex vivo
specimens derived from the larynx and the oral cavity. pERK1/2 was
activated in all four ex vivo samples reaching significance
in all cases (EV1: P=0.011; EV2: 0.0039; EV3: 0.0052; EV4: 0,0004).
tERK1/2 expression appeared stable after fractionated IR in all
specimens (Fig. 3A and B).
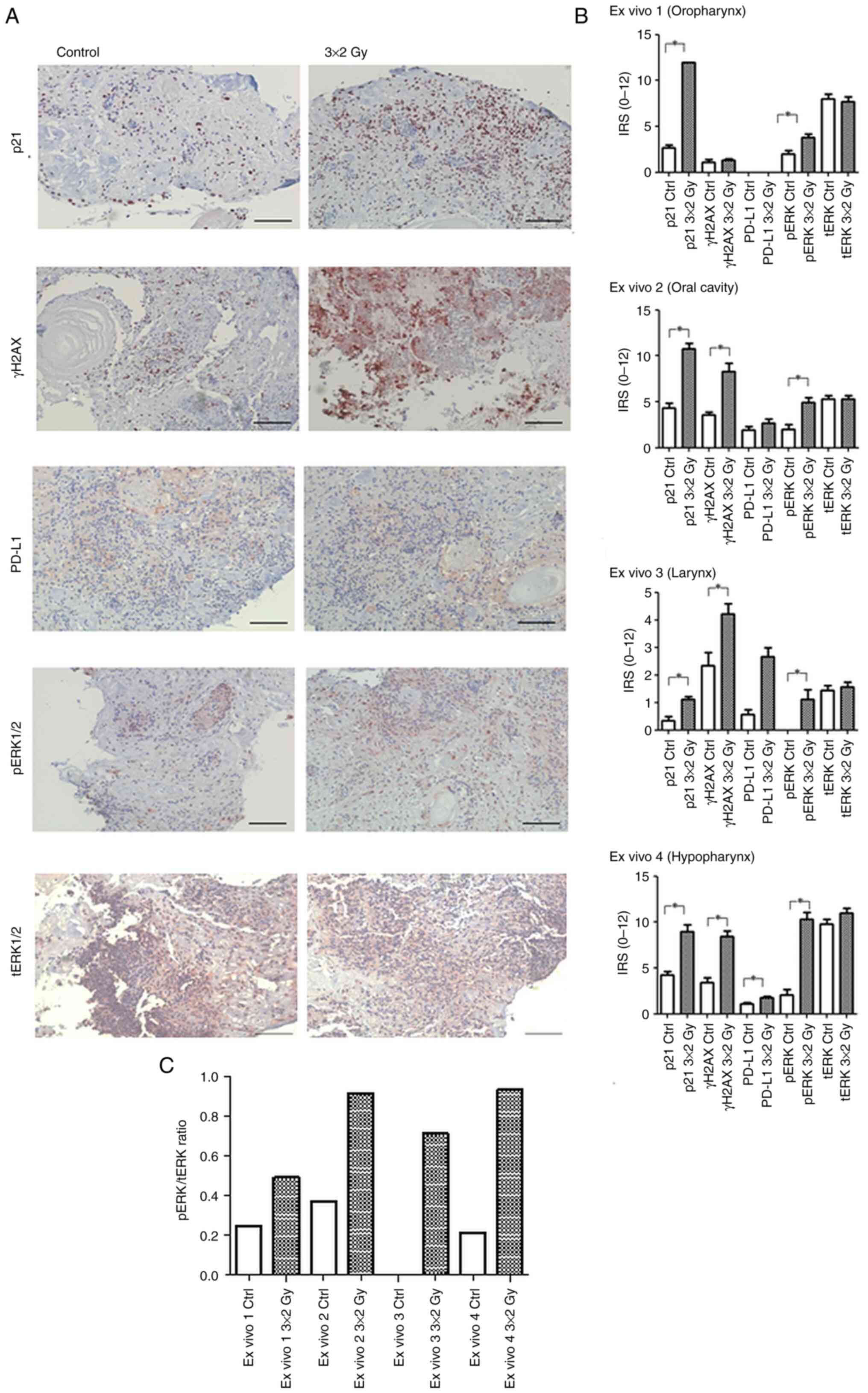 | Figure 3.Expression and quantification of p21,
γH2AX, PD-L1, and ERK1/2 in irradiated 3D ex vivo HNSCC
tissue. (A) IHC staining of p21, γH2AX, PD-L1, pERK1/2 and tERK1/2
in irradiated 3D ex vivo HNSCC tissue cultures. Expression
patterns in an ex vivo human HNSCC tissue culture model.
Simultaneous increase of PD-L1 and p21 after fractionated IR in the
IHC, compared with mock-treated controls (representative images
shown; scale bar, 100 µm). (B) Quantification of IHC results. The
IRS was obtained by visual examination. Results were quantified
using the IRS modified according to Remmele and Stegner (36) as performed for the in vitro
data. The mean ± SD was calculated. Statistical significance
(*P<0.05) was assessed using Mann Whitney U tests. (C) pERK1/2
to tERK1/2 ratio in ex vivo samples following radiation
treatment. Bar graph showing the pERK1/2/ERK1/2 ratio in ex
vivo 1–4. IHC, immunohistochemistry; γH2AX, histone H2AX;
PD-L1, programmed death-ligand 1; p, phosphorylated; HNSCC, head
and neck squamous cell carcinoma; IR, ionizing radiation; IHC,
immunohistochemistry; IRS, immunoreactive score. Scale bar=100
µm. |
Discussion
The present study described the expression of
senescence-associated molecules γH2AX, SA-β-Gal and p21, alongside
morphological criteria for detecting senescence in HNSCC cell lines
and viable HNSCC human tissue samples. The findings demonstrated
that these features are associated with irradiation-induced
induction of PD-L1 along with activation of the MAPK ERK survival
pathway.
In HNSCC patients, norm-fractionated radiotherapy
alone is most commonly administered at a dose of 1.6–2.0 Gy per
fraction per day, five days a week, over a period of 6–7 weeks
(40). Therefore, the present study
established an experimental regimen using single daily fractions of
2 Gy, based on our previous demonstration that these doses are
sufficient to produce an effect (32,34).
It is well established that standard oncological treatments, such
as radiochemotherapy (RCT), induce senescence in solid tumors
(40–42). Additionally, senescent cells are
known to contribute to certain chemotherapy-related side effects
(43,44). The senescent phenotype is
characterized by irreversible growth arrest while maintaining
viability and metabolic activity. Key features of this phenotype
include distinct cellular morphology (enlarged and flattened
cells), increased expression of p53 and p21, markers of DNA
double-strand breaks (DSBs) and the presence of SA-ß-Gal, along
with an elevated number of γH2AX foci, one of the most sensitive
markers of DSBs (45–48).
Standard oncological treatment such as RCT induces
senescence in solid tumors (41–43)
Additionally, senescent cells are known to cause certain
chemotherapy-related side effects (38,44).
The senescent phenotype is featured by irreversible growth arrest
while maintaining viability and metabolic activity. This phenotype
includes a distinct cellular morphology (enlarged and flattened
cells), increased expression of p53, p21, markers of DNA DSBs and
the presence of SA-ß-Gal as well as an increased number of γH2AX
foci, the most sensitive marker of DSBs (39,45–47).
Senescence has been described as a double-edged sword exhibiting
both tumor suppressive and promoting properties (48–51).
While therapy-induced senescence can contribute to metastasis,
recurrence and adverse reactions to cancer treatment (52), current anti-cancer strategies
provide an ideal foundation for TME-associated damage responses,
which result in the promotion of senescence as a resistance
mechanism (53): It is well
documented that senescent cells within the TME contribute to tumor
progression, metastasis and therapy resistance (54). Cellular senescence is increasingly
recognized as a patients' response to various anticancer therapies
(55) and is probably associated
with poor treatment response in different cancer entities (56–59).
As one of the most prominent markers of senescence,
p21 has been described to harbor either tumor suppressor activity
or tumor-promoting properties (60). In the p21 analyses, the present
study detected an increased postradiogenic nuclear p21 expression
in HNSCC tumor cell lines and ex vivo cultures, accompanied
by an increase in ß Gal positive cells and γH2AX protein levels,
compared with control, indicating that IR might trigger senescence
in HNSCC cells which could induce resistance to this fundamental
treatment pillar.
In parallel, the MAPK signaling pathway plays an
important role in oncogene-induced senescence (61,62).
MEK and subsequently ERK1/2, so-called gerogens, are capable of
arresting the cell cycle via p53, p21 and p16 (63). Patel et al (64) recently showed that continued
MAPK/ERK activation in oncogene-induced senescent cells offers a
potential escape route after ~1 month leading to c-MYC dependent
reactivation of telomerase. This reinforces the role of MAPK/ERK in
the formation of resistance based on senescent cells. While there
are various 3D models of ERK-induced senescence, so far none have
been detected specifically for head and neck cancer (65).
Woo et al (66) describe that that inhibition of MEK
and PI3K signaling pathways increases the sensitivity of HNSCC
cells to radiotherapy by enhancing therapy-induced senescence.
However, this study was only conducted in a 2D cell culture
approach, which does not reflect tumor-TME interactions. Indeed, a
rationale for including the tumor-TME-interrelations when
investigating senescence is provided by studies showing that the
epithelial to mesenchymal transition phenotype is resistant to
radiochemotherapy (67) and that
interactions with the TME can influence the tumor's sensitivity
against treatment (68). A improved
understanding of this mechanism is of great interest as there is
evidence for radioresistance already in quiescent cells in HNSCC
cell lines (69). This aggressive
phenotype could be further promoted by coexisting senescent cells
(70,71) leading to an undesirable outcome that
must not be underestimated.
Similarly, the immune checkpoint molecule PD-L1, the
ligand for PD-1 on immune cells, which drives immune cell
inactivation, is associated with cellular senescence. In line with
the present data, Onorati et al (25) report that senescent cells upregulate
PD-L1. This induction of PD-L1 in senescence is shown to depend on
the proinflammatory program. The clinical benefit of PD-1
inhibitors is restricted by immunosuppressive properties of the TME
(72–74) and the insufficient reactivation of
antitumor immunity by the drug alone (75). There have been considerations as to
whether responses to CPI are linked to cellular senescence as CPI
treatment has been observed to increase the presence of senescent T
cells (76). These findings on a
potential link between senescence and CPI response justify the
employment of 2D and 3D HNSCC models as performed in the present
study as only <20% of HNSCC patients respond to CPI monotherapy
(77). So more elucidation of
factors contributing to CPI sensitivity are clearly needed. For
other tumor entities such as breast cancer this issue has already
been addressed. Si et al (4)
observed that blockade of PD-L1 and STAT3 could prevent
tumor-associated regulatory T cell-induced senescence in dendritic
cells. Although that study focuses on breast cancer, the results
may also be relevant for head and neck cancer, as similar
mechanisms of immunomodulation may be involved.
The present study included cell lines and tissue
samples from different localities in the head and neck region to
illustrate diversity in tumoral behavior, which is known to be
associated with varying response to cancer treatment and found
comparable results. This observation is particularly notable
because it has variously been shown that differences in survival
and molecular biology exist between HNSCC anatomical sites and TME
of these sites may guide immune and radiation therapies (78). However, this observation is
provisional, as the present sample size was small, which must be
considered as a limitation of the study. The results can be
considered more of a pilot project but are worth testing in a
larger cohort. In terms of clinical applicability, it could be of
relevance that recent data show senolytic agents such as B-cell
lymphoma-x large inhibitor ABT-263 (Navitoclax) in combination with
cisplatin probably being useful for eliminating residual senescent
tumor cells after chemotherapy and thereby potentially delay
disease recurrence in head and neck cancer patients (79).
Hence, it is expected, based on these and previous
data, that the ex vivo model is a useful tool to provide a
platform for sensitivity testing of novel combination therapies,
such as cisplatin and senolytics, as part of a personalized
treatment approach.
The prognostic effect of therapy-induced senescence
remains incomplete. Previously we demonstrated tumor cell-mediated
regulation of PD-L1 upon platinum-based CRT in HNSCC and their
exosomes (32). The present study
aimed to define whether there is a correlation between
postradiogenic induction of senescence, ERK1/2 signaling and PD-L1
expression. The data provided evidence that fractionated IR
generated a subpopulation of HNSCC tumor cells exhibiting
senescence-associated cellular changes and increased expression of
pERK1/2 and PD-L1. The results suggested a link between survival
signaling, radiation-induced checkpoint activation and the
emergence of senescence in HNSCC. Additionally, the ex vivo
3D tissue model has proved to be a valuable tool for validation and
complementing in vitro data, offering insights into
tumor-TME correlations. In the future, further analyses,
particularly in larger cohorts, are required to better elucidate
the clinical relevance and significance of therapy-induced
senescence in the treatment of cancer patients.
Acknowledgements
The authors gratefully acknowledge the excellent
technical support of Ms. Petra Prohaska, Scientific Laboratory at
Department of Otorhinolaryngology, Head and Neck Surgery,
University Hospital Mannheim, Medical Faculty Mannheim of
Heidelberg University, Mannheim, Germany.
Funding
The present study was substantially supported by the Program for
the Promotion of Equality and Careers of Female Physicians and
Scientists at the University Medical Center Mannheim (to AA).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
LB and AA were responsible for data curation and
formal analysis. Funding acquisition was by AA and ES. FJ, JF and
AL made substantial contributions to the acquisition, analysis, and
interpretation of data for the work. Supervision was by JK, NR and
CS. Visualization was by AA, LB and ES. Writing the original draft
was by LB and AA. Writing, reviewing and editing was by LB, AA, CS,
JK and NR. LB, AA, and JK confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki and approved by the Institutional
Review Board (or Ethics Committee) of Heidelberg University,
Medical Faculty Mannheim (protocol code 2020-558N, date of approval
4 Jun 2020). Informed consent was obtained from all subjects
involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Herranz N and Gil J: Mechanisms and
functions of cellular senescence. J Clin Invest. 128:1238–1246.
2018. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HS, Song MC, Kwak IH, Park TJ and Lim
IK: Constitutive induction of p-Erk1/2 accompanied by reduced
activities of protein phosphatases 1 and 2A and MKP3 due to
reactive oxygen species during cellular senescence. J Biol Chem.
278:37497–37510. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White RR and Vijg J: Do DNA double-strand
breaks drive aging? Mol Cell. 63:729–738. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Si F, Liu X, Tao Y, Zhang Y, Ma F, Hsueh
EC, Puram SV and Peng G: Blocking senescence and tolerogenic
function of dendritic cells induced by γδ Treg cells enhances
tumor-specific immunity for cancer immunotherapy. J Immunother
Cancer. 12:e0082192024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruhland MK and Alspach E: Senescence and
immunoregulation in the tumor microenvironment. Front Cell Dev
Biol. 9:7540692021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toso A, Revandkar A, Di Mitri D, Guccini
I, Proietti M, Sarti M, Pinton S, Zhang J, Kalathur M, Civenni G,
et al: Enhancing chemotherapy efficacy in Pten-deficient prostate
tumors by activating the senescence-associated antitumor immunity.
Cell Rep. 9:75–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Zhou CC, Sun HC, Li Q, Hu JD,
Jiang T and Zhou S: Identification of several senescence-associated
genes signature in head and neck squamous cell carcinoma. J Clin
Lab Anal. 36:e245552022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ostrowska K, Niewinski P, Piotrowski I,
Ostapowicz J, Koczot S, Suchorska WM, Golusiński P, Masternak MM
and Golusiński W: Senescence in head and neck squamous cell
carcinoma: relationship between senescence-associated secretory
phenotype (SASP) mRNA expression level and clinicopathological
features. Clin Transl Oncol. 26:1022–1032. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schoetz U, Klein D, Hess J, Shnayien S,
Spoerl S, Orth M, Mutlu S, Hennel R, Sieber A, Ganswindt U, et al:
Early senescence and production of senescence-associated cytokines
are major determinants of radioresistance in head-and-neck squamous
cell carcinoma. Cell Death Dis. 12:11622021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee YC, Nam Y, Kim M, Kim SI, Lee JW, Eun
YG and Kim D: Prognostic significance of senescence-related tumor
microenvironment genes in head and neck squamous cell carcinoma.
Aging (Albany NY). 16:985–1001. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Golding SE, Morgan RN, Adams BR, Hawkins
AJ, Povirk LF and Valerie K: Pro-survival AKT and ERK signaling
from EGFR and mutant EGFRvIII enhances DNA double-strand break
repair in human glioma cells. Cancer Biol Ther. 8:730–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hein AL, Ouellette MM and Yan Y:
Radiation-induced signaling pathways that promote cancer cell
survival (review). Int J Oncol. 45:1813–1819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chung EJ, Brown AP, Asano H, Mandler M,
Burgan WE, Carter D, Camphausen K and Citrin D: In vitro and in
vivo radiosensitization with AZD6244 (ARRY-142886), an inhibitor of
mitogen-activated protein kinase/extracellular signal-regulated
kinase 1/2 kinase. Clin Cancer Res. 15:3050–3057. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leiker AJ, DeGraff W, Choudhuri R, Sowers
AL, Thetford A, Cook JA, Van Waes C and Mitchell JB: Radiation
enhancement of head and neck squamous cell carcinoma by the Dual
PI3K/mTOR Inhibitor PF-05212384. Clin Cancer Res. 21:2792–2801.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Affolter A, Drigotas M, Fruth K,
Schmidtmann I, Brochhausen C, Mann WJ and Brieger J: Increased
radioresistance via G12S K-Ras by compensatory upregulation of MAPK
and PI3K pathways in epithelial cancer. Head Neck. 35:220–228.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Affolter A, Fruth K, Brochhausen C,
Schmidtmann I, Mann WJ and Brieger J: Activation of
mitogen-activated protein kinase extracellular signal-related
kinase in head and neck squamous cell carcinomas after irradiation
as part of a rescue mechanism. Head Neck. 33:1448–1457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Affolter A and Hess J: Preclinical models
in head and neck tumors: Evaluation of cellular and molecular
resistance mechanisms in the tumor microenvironment. HNO.
64:860–869. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Affolter A, Samosny G, Heimes AS,
Schneider J, Weichert W, Stenzinger A, Sommer K, Jensen A, Mayer A,
Brenner W, et al: Multikinase inhibitors sorafenib and sunitinib as
radiosensitizers in head and neck cancer cell lines. Head Neck.
39:623–632. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perri F, Pacelli R, Della Vittoria
Scarpati G, Cella L, Giuliano M, Caponigro F and Pepe S:
Radioresistance in head and neck squamous cell carcinoma:
Biological bases and therapeutic implications. Head Neck.
37:763–770. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharon S and Bell RB: Immunotherapy in
head and neck squamous cell carcinoma: A narrative review. Front
Oral Maxillofac Med. 4:282022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Z, Wong PY, Ng CWK, Lan L, Fung S, Li
JW, Cai L, Lei P, Mou Q, Wong SH, et al: The intersection between
oral microbiota, host gene methylation and patient outcomes in head
and neck squamous cell carcinoma. Cancers (Basel). 12:34252020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Affolter A, Kern J, Bieback K, Scherl C,
Rotter N and Lammert A: Biomarkers and 3D models predicting
response to immune checkpoint blockade in head and neck cancer
(Review). Int J Oncol. 61:882022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang TW, Johmura Y, Suzuki N, Omori S,
Migita T, Yamaguchi K, Hatakeyama S, Yamazaki S, Shimizu E, Imoto
S, et al: Blocking PD-L1-PD-1 improves senescence surveillance and
ageing phenotypes. Nature. 611:358–364. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onorati A, Havas AP, Lin B, Rajagopal J,
Sen P, Adams PD and Dou Z: Upregulation of PD-L1 in Senescence and
Aging. Mol Cell Biol. 42:e00171222022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brenner E, Schörg BF, Ahmetlić F, Wieder
T, Hilke FJ, Simon N, Schroeder C, Demidov G, Riedel T,
Fehrenbacher B, et al: Cancer immune control needs senescence
induction by interferon-dependent cell cycle regulator pathways in
tumours. Nat Commun. 11:13352020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Z, Hu K, Feng L, Su R, Lai N, Yang Z
and Kang S: Senescent cells re-engineered to express soluble
programmed death receptor-1 for inhibiting programmed death
receptor-1/programmed death ligand-1 as a vaccination approach
against breast cancer. Cancer Sci. 109:1753–1763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Bu X, Wang H, Zhu Y, Geng Y,
Nihira NT, Tan Y, Ci Y, Wu F, Dai X, et al: Cyclin D-CDK4 kinase
destabilizes PD-L1 via cullin 3-SPOP to control cancer immune
surveillance. Nature. 553:91–95. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee JJ, Kim SY, Kim SH, Choi S, Lee B and
Shin JS: STING mediates nuclear PD-L1 targeting-induced senescence
in cancer cells. Cell Death Dis. 13:7912022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moreira A, Gross S, Kirchberger MC,
Erdmann M, Schuler G and Heinzerling L: Senescence markers:
Predictive for response to checkpoint inhibitors. Int J Cancer.
144:1147–1150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Abdel-Naby R, Wang K, Song D, Bozentka J,
LaFonte M, Ou P, Stanek A, Mueller C, Alfonso A and Huan C:
Extracellular Signal-Regulated Kinase (ERK)-dependent p21
(WAF1/Cip1) Expression Is Associated with Gemcitabine Resistance in
Pancreatic Cancer Cells. J Am Coll Surg. 219:S272014. View Article : Google Scholar
|
|
32
|
Affolter A, Liebel K, Tengler L, Seiz E,
Tiedtke M, Azhakesan A, Schütz J, Theodoraki MN, Kern J, Ruder AM,
et al: Modulation of PD-L1 expression by standard therapy in head
and neck cancer cell lines and exosomes. Int J Oncol. 63:1022023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Welters MJ, Fichtinger-Schepman AM, Baan
RA, Hermsen MA, van der Vijgh WJ, Cloos J and Braakhuis BJ:
Relationship between the parameters cellular differentiation,
doubling time and platinum accumulation and cisplatin sensitivity
in a panel of head and neck cancer cell lines. Int J Cancer.
71:410–415. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Engelmann L, Thierauf J, Koerich Laureano
N, Stark HJ, Prigge ES, Horn D, Freier K, Grabe N, Rong C,
Federspil P, et al: Organotypic co-cultures as a novel 3D model for
head and neck squamous cell carcinoma. Cancers (Basel).
12:23302020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Affolter A, Muller MF, Sommer K,
Stenzinger A, Zaoui K, Lorenz K, Wolf T, Sharma S, Wolf J, Perner
S, et al: Targeting irradiation-induced mitogen-activated protein
kinase activation in vitro and in an ex vivo model for human head
and neck cancer. Head Neck. 38 (Suppl 1):E2049–E2061. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
37
|
Muñoz-Espín D and Serrano M: Cellular
senescence: From physiology to pathology. Nat Rev Mol Cell Biol.
15:482–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Demaria M: Senescent cells: New target for
an old treatment? Mol Cell Oncol. 4:e12996662017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bernadotte A, Mikhelson VM and Spivak IM:
Markers of cellular senescence. Telomere shortening as a marker of
cellular senescence. Aging (Albany NY). 8:3–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matuschek C, Haussmann J, Bölke E, Gripp
S, Schuler PJ, Tamaskovics B, Gerber PA, Djiepmo-Njanang FJ,
Kammers K, Plettenberg C, et al: Accelerated vs. conventionally
fractionated adjuvant radiotherapy in high-risk head and neck
cancer: A meta-analysis. Radiat Oncol. 13:1952018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qin S, Schulte BA and Wang GY: Role of
senescence induction in cancer treatment. World J Clin Oncol.
9:180–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Milanovic M, Fan DNY, Belenki D, Däbritz
JHM, Zhao Z, Yu Y, Dörr JR, Dimitrova L, Lenze D, Monteiro Barbosa
IA, et al: Senescence-associated reprogramming promotes cancer
stemness. Nature. 553:96–100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nicolas AM, Pesic M, Engel E, Ziegler PK,
Diefenhardt M, Kennel KB, Buettner F, Conche C, Petrocelli V,
Elwakeel E, et al: Inflammatory fibroblasts mediate resistance to
neoadjuvant therapy in rectal cancer. Cancer Cell. 40:168–184.e13.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Demaria M, O'Leary MN, Chang J, Shao L,
Liu S, Alimirah F, Koenig K, Le C, Mitin N, Deal AM, et al:
Cellular senescence promotes adverse effects of chemotherapy and
cancer relapse. Cancer Discov. 7:165–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gu L and Kitamura M: Sensitive detection
and monitoring of senescence-associated secretory phenotype by
SASP-RAP assay. PLoS One. 7:e523052012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Redon CE, Nakamura AJ, Sordet O, Dickey
JS, Gouliaeva K, Tabb B, Lawrence S, Kinders RJ, Bonner WM and
Sedelnikova OA: γ-H2AX detection in peripheral blood lymphocytes,
splenocytes, bone marrow, xenografts, and skin. Methods Mol Biol.
682:249–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sedelnikova OA, Horikawa I, Zimonjic DB,
Popescu NC, Bonner WM and Barrett JC: Senescing human cells and
ageing mice accumulate DNA lesions with unrepairable double-strand
breaks. Nat Cell Biol. 6:168–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Golomb L, Sagiv A, Pateras IS, Maly A,
Krizhanovsky V, Gorgoulis VG, Oren M and Ben-Yehuda A:
Age-associated inflammation connects RAS-induced senescence to stem
cell dysfunction and epidermal malignancy. Cell Death Differ.
22:1764–1774. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Krtolica A, Parrinello S, Lockett S,
Desprez PY and Campisi J: Senescent fibroblasts promote epithelial
cell growth and tumorigenesis: A link between cancer and aging.
Proc Natl Acad Sci USA. 98:12072–12077. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Halazonetis TD, Gorgoulis VG and Bartek J:
An oncogene-induced DNA damage model for cancer development.
Science. 319:1352–1355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Serrano M, Lin AW, McCurrach ME, Beach D
and Lowe SW: Oncogenic ras provokes premature cell senescence
associated with accumulation of p53 and p16INK4a. Cell. 88:593–602.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang B, Kohli J and Demaria M: Senescent
cells in cancer therapy: Friends or Foes? Trends Cancer. 6:838–857.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gordon RR and Nelson PS: Cellular
senescence and cancer chemotherapy resistance. Drug Resist Updat.
15:123–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
D'Ambrosio M and Gil J: Reshaping of the
tumor microenvironment by cellular senescence: An opportunity for
senotherapies. Dev Cell. 58:1007–1021. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Domen A, Deben C, Verswyvel J, Flieswasser
T, Prenen H, Peeters M, Lardon F and Wouters A: Cellular senescence
in cancer: Clinical detection and prognostic implications. J Exp
Clin Cancer Res. 41:3602022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
George B, Horn D, Bayo P, Zaoui K,
Flechtenmacher C, Grabe N, Plinkert P, Krizhanovsky V and Hess J:
Regulation and function of Myb-binding protein 1A (MYBBP1A) in
cellular senescence and pathogenesis of head and neck cancer.
Cancer Lett. 358:191–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mosieniak G, Sliwinska M, Alster O,
Strzeszewska A, Sunderland P, Piechota M, Was H and Sikora E:
Polyploidy formation in doxorubicin-treated cancer cells can favor
escape from senescence. Neoplasia. 17:882–893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang Q, Wu PC, Dong DZ, Ivanova I, Chu E,
Zeliadt S, Vesselle H and Wu DY: Polyploidy road to therapy-induced
cellular senescence and escape. Int J Cancer. 132:1505–1515. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhai J, Han J, Li C, Lv D, Ma F and Xu B:
Tumor senescence leads to poor survival and therapeutic resistance
in human breast cancer. Front Oncol. 13:10975132023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Stivala LA, Cazzalini O and Prosperi E:
The cyclin-dependent kinase inhibitor p21CDKN1A as a target of
anti-cancer drugs. Curr Cancer Drug Targets. 12:85–96. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu Y, Li N, Xiang R and Sun P: Emerging
roles of the p38 MAPK and PI3K/AKT/mTOR pathways in
oncogene-induced senescence. Trends Biochem Sci. 39:268–276. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Anerillas C, Abdelmohsen K and Gorospe M:
Regulation of senescence traits by MAPKs. Geroscience. 42:397–408.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chambard JC, Lefloch R, Pouysségur J and
Lenormand P: ERK implication in cell cycle regulation. Biochim
Biophys Acta. 1773:1299–1310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Patel PL, Suram A, Mirani N, Bischof O and
Herbig U: Derepression of hTERT gene expression promotes escape
from oncogene-induced cellular senescence. Proc Natl Acad Sci USA.
113:E5024–E5033. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Schmitt CA, Wang B and Demaria M:
Senescence and cancer-role and therapeutic opportunities. Nat Rev
Clin Oncol. 19:619–636. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Woo SH, Yang LP, Chuang HC, Fitzgerald A,
Lee HY, Pickering C, Myers JN and Skinner HD: Down-regulation of
malic enzyme 1 and 2: Sensitizing head and neck squamous cell
carcinoma cells to therapy-induced senescence. Head Neck. 38 (Suppl
1):E934–E940. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Khalaf K, Hana D, Chou JT, Singh C,
Mackiewicz A and Kaczmarek M: Aspects of the tumor microenvironment
involved in immune resistance and drug resistance. Front Immunol.
12:6563642021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Eckers JC, Kalen AL, Sarsour EH, Tompkins
VS, Janz S, Son JM, Doskey CM, Buettner GR and Goswami PC: Forkhead
box M1 regulates quiescence-associated radioresistance of human
head and neck squamous carcinoma cells. Radiat Res. 182:420–429.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cahu J, Bustany S and Sola B:
Senescence-associated secretory phenotype favors the emergence of
cancer stem-like cells. Cell Death Dis. 3:e4462012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Özcan S, Alessio N, Acar MB, Mert E,
Omerli F, Peluso G and Galderisi U: Unbiased analysis of senescence
associated secretory phenotype (SASP) to identify common components
following different genotoxic stresses. Aging (Albany NY).
8:1316–1329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fares CM, Van Allen EM, Drake CG, Allison
JP and Hu-Lieskovan S: Mechanisms of resistance to immune
checkpoint blockade: Why does checkpoint inhibitor immunotherapy
not work for all patients? Am Soc Clin Oncol Educ Book. 39:147–164.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nowicki TS, Hu-Lieskovan S and Ribas A:
Mechanisms of resistance to PD-1 and PD-L1 blockade. Cancer J.
24:47–53. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Saleh R and Elkord E: Acquired resistance
to cancer immunotherapy: Role of tumor-mediated immunosuppression.
Semin Cancer Biol. 65:13–27. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Haratani K, Yonesaka K, Takamura S,
Maenishi O, Kato R, Takegawa N, Kawakami H, Tanaka K, Hayashi H,
Takeda M, et al: U3-1402 sensitizes HER3-expressing tumors to PD-1
blockade by immune activation. J Clin Invest. 130:374–388. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Arasanz H, Zuazo M, Bocanegra A, Gato M,
Martínez-Aguillo M, Morilla I, Fernández G, Hernández B, López P,
Alberdi N, et al: Early detection of hyperprogressive disease in
non-small cell lung cancer by monitoring of systemic T cell
dynamics. Cancers (Basel). 12:3442020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Hirsch L, Zitvogel L, Eggermont A and
Marabelle A: PD-Loma: A cancer entity with a shared sensitivity to
the PD-1/PD-L1 pathway blockade. Br J Cancer. 120:3–5. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Kim HAJ, Zeng PYF, Shaikh MH, Mundi N,
Ghasemi F, Di Gravio E, Khan H, MacNeil D, Khan MI, Patel K, et al:
All HPV-negative head and neck cancers are not the same: Analysis
of the TCGA dataset reveals that anatomical sites have distinct
mutation, transcriptome, hypoxia, and tumor microenvironment
profiles. Oral Oncol. 116:1052602021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ahmadinejad F, Bos T, Hu B, Britt E,
Koblinski J, Souers AJ, Leverson JD, Faber AC, Gewirtz DA and
Harada H: Senolytic-mediated elimination of head and neck tumor
cells induced into senescence by cisplatin. Mol Pharmacol.
101:168–180. 2022. View Article : Google Scholar : PubMed/NCBI
|