Introduction
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) induces apoptosis in various cancer cells and
xenograft models by activating TRAIL-receptor 1 and 2 (TRAIL-R1 and
TRAIL-R2, respectively) through trimerization (1). Although a number of TRAIL-R2 agonistic
antibodies and recombinant TRAILs have entered clinical trials
(2) and exhibit potential cytotoxic
activity against tumors, no approved therapeutics targeting
TRAIL-R2 are currently available. Early TRAIL-R2 antibodies, such
as tigatuzumab (3) and conatumumab
(4), require secondary crosslinking
mechanisms involving FcγR to exhibit antitumor activity, but they
have not shown adequate efficacy in clinical trials (2). Therefore, novel antibodies that are
directly activated without secondary crosslinking strategies are
needed. For example, KMTR2 and TAS266 have shown good efficacy
in vitro and in vivo without secondary crosslinking,
but they were toxic to human hepatocytes, PXB mice and humans in
clinical trials (5–7). Although several TRAIL-R2 agonistic
proteins fused with tumor-targeting elements have been developed to
achieve both apoptosis induction and tumor selectivity (8,9), the
risk of inducing liver damage persists. This is because these
proteins are capable of inducing apoptosis without relying on
secondary crosslinking, including the induction of apoptosis in
nontarget tissues expressing TRAIL-R2. Bispecific antibodies, such
as RG7386 (FAP/DR5) (10), BaCa
(FOLR1/DR5) (11), BI 905711
(CDH17/DR5) (12) and IMV-M
(MUC16/DR5) (13), which combine an
antibody for tumor targeting with an antibody for TRAIL-R2,
reportedly induce tumor cell apoptosis through trans or
cis interactions in a FAP, FOLR1, CDH17 and MUC16
binding-dependent manner, respectively. However, information on
whether these tetravalent antibodies can potentially avoid
hepatotoxicity in vitro and in vivo is scarce.
Prostate-specific membrane antigen (PSMA), a
transmembrane glycoprotein present on the cell surface, is highly
expressed in prostate cancer (14)
as well as other cancers, including non-small cell lung cancer
(NSCLC) (15) and breast cancers
(16,17) and in the neovascular endothelium
around various tumors (18), but
not in the vasculature of normal tissues. This unique PSMA
expression profile makes it an attractive therapeutic target and
candidate for imaging (19–21) and PSMA antibody-drug conjugates and
radioisotope-labeled antibodies have been used in clinical trials
for relapsed/refractory prostate cancer (22–25).
The present study developed REGULGENT™, a novel
tetravalent bispecific antibody based on human IgG4, with mutations
to reduce FcγR binding. It comprises a TRAIL-R2 antibody, E11,
which does not trigger apoptosis independently and a PSMA antibody
as the targeting element for treating PSMA-positive cancer and the
neovasculature. The aim was to develop a therapeutic agent that
effectively induces tumor cell death in PSMA/TRAIL-R2
double-positive cells while avoiding toxicity in normal tissues,
particularly the liver.
Materials and methods
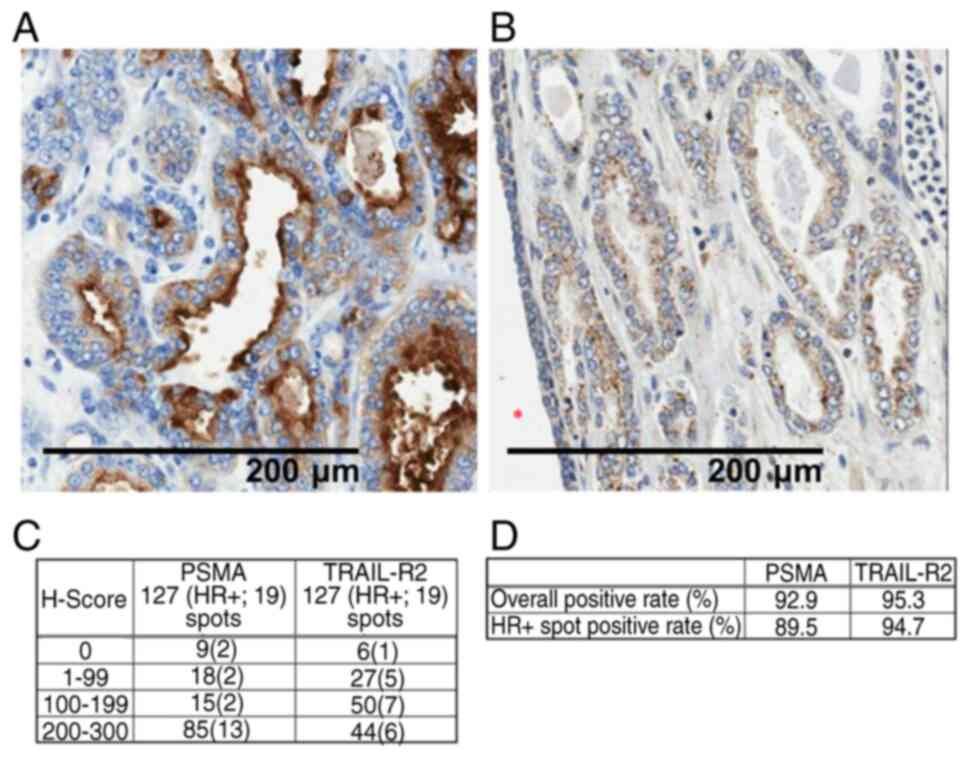
Immunohistochemistry
PSMA and TRAIL-R2 expression was analyzed using a
prostate cancer tissue microarray (cat. no. 79562483; Tristar
Technology Group LLC). The spots were stained using an anti-PSMA
antibody (cat. no. EPR6253; Abcam) and an anti-DR5 antibody (cat.
no. ab47179; Abcam). The primary antibodies were incubated for 60
min at 25°C. For secondary detection, Histofine Simple Stain Mouse
MAX-PO (R) (cat. no. 414341; Nichirei Biosciences, Inc.) was
applied and incubated for 30 min at 25°C. The stained sections were
observed using a light microscope at a magnification of ×200. The
H-score was calculated as follows: i) The intensity score was
determined based on the staining intensity of positive cells among
cancer cells (0; no staining, 1; low, 2; middle, 3: high). ii) The
positive occupancy rates (%) of cancer cells for each score were
calculated in increments of 5%. iii) Each score was multiplied by
the positive occupancy rate and the result was used as the
H-score=Σ (positive occupancy rates (%) × intensity score).
Construction and expression of
antibodies
The variable domains of the heavy chain (VH) and
light chain (VL) regions of E11 (26), a non-agonistic antibody TRAIL-R2
clone, were used in the present study. The novel PSMA antibody
clones with a VL in common with E11 were generated using phage
display with an E11 VL fixed library (27). cDNA extracted from the spleen of
PSMA-immunized human antibody transgenic mice (28) was used as the VH gene source in the
library by isolating total RNA using ISOGEN (cat. no. 315-02504;
Nippon Gene Co., Ltd.), synthesizing cDNA by reverse transcription,
and amplifying VH fragments by PCR. M13 phage panning was then
performed on PSMAcoated tubes to obtain clones that bound to PSMA.
The PSMA-binding clones were in-frame cloned into the tetravalent
bispecific antibody expression vector REGULGENT™ that included a
signal peptide and human IgG4 (S228P/L235E/R409K) Fc region
designed to reduce Fcγ receptor binding, thereby eliminating
effector functions (29). The
designed gene sequences were synthesized by Azenta Life Sciences
and inserted into the expression vector using restriction enzyme
digestion and ligation. The tetravalent bispecific antibody
PSMA/TRAIL-R2 REGULGENT™ plasmid was generated by fusing
anti-TRAIL-R2 Fab to the N-terminus of the anti-PSMA heavy chain.
The bivalent bispecific antibody plasmid was constructed based on
IgG1 with knobs-into-holes and Fc-silencing (L234A/L235A/G237A)
mutations (10,30,31).
The two plasmids were transfected into 293F or Expi293F™ (Thermo
Fisher Scientific, Inc.) cells using the FreeStyle™ 293 or Expi293™
Expression Systems (cat. no. A14635; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol; briefly, cells were
cultured in suspension, transfected with the supplied transfection
reagent, and cultured for 5 days before harvesting. Antibodies were
purified using Protein A (cat. no. 29048576; Cytiva). After eluting
the purified antibodies, the buffer used to dissolve the sample was
replaced with phosphate-buffered saline (PBS, cat. no. 14249-24;
Nacalai Tesque, Inc.). Subsequently, the monomer fraction was
separated from the antibody solution using an NGC™ Chromatography
System (Bio-Rad Laboratories, Inc.) with Superdex™ High-Performance
Columns (28990944; Cytiva).
Analysis of antibody purity
The purity of the prepared antibodies was determined
using microfluidic chip electrophoresis (LabChip GXII Touch;
PerkinElmer, Inc.) and ultra-high-pressure size exclusion
chromatography (UHP-SEC). The LabChip analysis was performed using
a Protein Express Assay Reagent Kit (cat. no. 760499; PerkinElmer,
Inc.); all microfluidic chips were primed and prepared according to
the manufacturer's protocol, the antibody samples and ladder were
loaded and the run was performed on the LabChip GXII Touch system
under standard settings. The non-reducing sample buffer contained
N-ethylmaleimide at a final concentration of 6.36 mM and the heat
treatment conditions were 70°C for 15 min. The denatured samples
were diluted with deionized water and loaded onto a primed chip and
the analysis was conducted according to the manufacturer's
instructions. Finally, the LabChip® GX Reviewer program
(PerkinElmer, Inc.) was used to automatically calculate the size
and concentration of each separated peak. The proportions of
monomers, high-molecular-weight species (HMWS) and
low-molecular-weight species (LMWS) were determined using UHP-SEC
on an ACQUITY UPLC Protein BEH SEC Column [200 À 1.7 µm, 4.6×150 mm
(Waters Corporation)]. The mobile phase contained 20 mM sodium
phosphate (pH 6.8), 500 mM NaCl and 5% ethanol. The flow rate and
detection wavelength were 0.4 ml/min and 215 nm, respectively.
Peaks indicative of antibody protein monomers, HMWS and LMWS were
analyzed by integrating the area under each eluting peak using
LabSolutions Software (Shimadzu Corporation).
Preparation of PSMA-expressing
cells
Human lung adenocarcinoma (NCI-H2122; ATCC no.
CRL-5985), human prostate cancer (PC-3; ATCC no. CRL-1435) and
lymph node carcinoma of the prostate (LNCaP) clone fast-growing
colony (FGC; ATCC no. CRL-1740) cells were seeded in RPMI 1640
medium (cat. no. 11875093; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (cat. no. 10099141; Thermo Fisher
Scientific, Inc.). Human PSMA cDNA was transfected into the cell
lines via lipofection using HilyMax (cat. no. H357; Dojindo
Laboratories, Inc.) according to the manufacturer's instructions.
The cells were incubated with the transfection mixture at 37°C and
the medium was replaced with fresh growth medium 2 days after
transfection. The transfected cells were then cultured under
blasticidin selection to establish stable clones, and subsequent
experiments were conducted approximately 1 month after
transfection. Nontransfected cells were included as a negative
control. The transduced cells were detached by adding 0.25%
trypsin/1 mM EDTA to the adhered cells and then counted.
Thereafter, the cell suspension was diluted and seeded at a density
of one cell per well containing 200 µl of drug selection medium in
96-well plates. Wells in which the cells formed a single colony
were expanded. Subsequently, PSMA expression was confirmed using a
FACSCanto II Flow Cytometer (BD Biosciences).
Western blot analysis
PSMA expression was confirmed using the Wes™
capillary-based automated western system (ProteinSimple) system.
Cells were lysed in RIPA buffer (MilliporeSigma) at 100 µl per
106 cells, and the lysates were directly analyzed by
loading 0.2 µl of each sample into the capillaries. The analysis
was performed with the 12-230 kDa Separation Module (cat. no.
SM-W001; ProteinSimple) according to the manufacturer's standard
protocol. Blocking, separation, immunodetection, and signal
development were carried out automatically within the system. For
primary detection, an anti PSMA/GCPII (cat. no. 13163-1-AP;
Proteintech Group, Inc.) was applied at a concentration of 20 µg/ml
and incubated at 25°C under instrument default settings. The
anti-Rabbit Detection Module for Wes, Peggy Sue or Sally Sue (cat.
no. DM-001; ProteinSimple) were used for secondary detection and
for blocking and chemiluminescent visualization. An anti-β-actin
antibody provided in the detection module was used as a loading
control. Signal acquisition and quantification were performed using
Compass software version 6.1.0 (ProteinSimple).
Biacore™ urface plasmon resonance
The antigen-binding affinities of the antibodies
were measured using a Biacore™ T200 surface plasmon resonance (SPR)
system (Cytiva) at 25°C with HBS-EP+ pH 7.4 (Cytiva) as the buffer.
Briefly, a Human Antibody Capture Kit (cat. no. BR100839; Cytiva)
was used to immobilize the anti-human IgG onto a CM5 sensor chip
(Cytiva). Next, the antibodies were injected, followed by TRAIL-R2
(cat. no. 310-19; PeproTech) and PSMA (PSA-H82Qb-25 µg;
ACROBiosystems) solution in a single cycle. A dilution series of
the antigens was injected for 4 min at a flow rate of 30 µl/min.
The dissociation time was 400 sec. After each binding event, the
surface was regenerated using 3 M MgCl2. The sensorgrams
were analyzed using Biacore Evaluation software (Cytiva), fitting a
1:1 binding model to determine the following binding kinetics: ka,
kd and KD.
Flow cytometric analysis
Cells were suspended in PBS (cat. no. 14249-24;
Nacalai Tesque, Inc.) supplemented with 5% fetal bovine serum (cat.
no. 10099141; Thermo Fisher Scientific, Inc.), 1 mM EDTA and 0.1%
sodium azide (staining buffer) and added to a 96-well plate. After
centrifugation at 340 × g for 3 min at 4°C, the supernatant was
removed and each antibody was added to the pellet and incubated for
30 min on ice. After washing, a goat F(ab')2 anti-human IgG (γ
chain-specific) R-phycoerythrin conjugate (cat. no. 2043-09;
SouthernBiotech) was added and the cells were incubated for 30 min
at 4°C. After washing, the cells were suspended in a staining
buffer containing 7-aminoactinomycin D staining solution (cat. no.
559925; BD Biosciences) and incubated for 10 min at 25°C. The
fluorescence intensity of each cell was measured using a FACSCanto
II Flow Cytometer (BD Biosciences). Data were analyzed using FlowJo
software version 9.6.4 (BD Biosciences).
Cell proliferation assay
Cells were seeded in 96-well plates and cultured
overnight at 37°C under 5% CO2. Anti-2,4-dinitrophenol
(DNP), anti-TRAIL-R2 agonistic KMTR2, anti-TRAIL-R2 E11, anti-PSMA
(PN7 or PI101) and PSMA/TRAIL-R2 bispecific antibodies diluted with
culture medium were added at 50 µl per well and incubated at 37°C
for 2 days. Cell Counting Kit-8 solution (cat. no. CK04; Dojindo
Laboratories, Inc.) was added (10 µl per well) to the cells and the
mixture was incubated for 4 h at 37°C under 5% CO2.
Next, 0.1 M HCl (10 µl per well) was added to stop the reaction.
The percent cell viability was calculated as follows:
(1-([absorbance at each antibody concentration]-[absorbance of
medium only])/([absorbance at an antibody concentration of 0
µg/ml]-[absorbance of medium only])) ×100. Data were expressed as
mean ± SE of quadruplicate experiments.
Flow cytometry apoptosis assay
Antibodies were added to PC-3 and PSMA/PC-3 cells,
after which activated caspases in the cells were detected using a
FAM-FLICA Poly Caspase Assay kit (cat. no. 92; ImmunoChemistry
Technologies, LLC). Thereafter, the cells were suspended in a
medium containing antibodies and TRAIL/Apo2 ligand (1 µg/ml) and
incubated at 37°C for 6 h. After centrifugation at 340 × g for 3
min at 4°C, the supernatant was removed, 50 µl/ml of FLICA supplied
with the kit (diluted in medium) were added and the mixture was
incubated at 37°C for 30 min at 4°C. After incubation, the cells
were washed four times with the apoptosis wash buffer provided with
the kit and suspended in 100 µl (per well) of apoptosis wash
buffer, after which the fluorescence intensity of each cell was
measured using a FACSCanto II Flow Cytometer (BD Biosciences). Data
were analyzed using FlowJo software version 9.6.4 (BD Biosciences).
The apoptotic rate was calculated as the percentage of
FAMFLICA-positive cells (early and late apoptotic cells combined)
among the total cell population.
Effect of crosslinker and intravenous
immunoglobulin (IVIG) on tumor cell death
PSMA/PC-3 cells were seeded in 96-well plates and
incubated overnight at 37°C to attach to the well. The
anti-TRAIL-R2 antibody, apomab (32,33)
and PSMA/TRAIL-R2 REGULGENT™ were added at final concentrations of
1 µg/ml each. Antibody crosslinkers [anti-human IgG
(γ-chain-specific) antibody; cat. no. I3382; MilliporeSigma] were
added to the wells and the cells were cultured at 37°C for 3 days.
Thereafter, Cell Counting Kit-8 solution was added and the mixture
was incubated for 4 h to determine cell viability. To examine the
effect of IVIG on cell death, it was applied before apomab and
PSMA/TRAIL-R2 REGULGENT™ were added to the wells.
Mouse xenograft model
SCID mice were purchased from CLEA Japan, Inc. A
total of 80 mice were used in the present study. The mice were
housed under specific pathogenfree conditions at 23±3°C with a 12-h
light/dark cycle and 50±20% relative humidity. The body weight of
the mice at the start of the experiment ranged from 19-24 g. A
xenograft mouse model was developed by injecting NCI-H2122 and
PSMA/NCI-H2122 transfectant cells (5×106) subcutaneously
into the dorsal flank of 6-week-old male SCID mice. Tumor volume
was measured twice a week using the following formula: (length ×
width2)/2. When the mean tumor volume reached 100
mm3 after 7 days, the mice were randomly assigned to
treatment groups using a computer-generated randomization method to
ensure unbiased distribution. Each group consisted of five mice.
Drug administration was initiated with the following treatments:
anti-DNP, anti-TRAIL-R2 agonistic antibody KMTR2 and PSMA/TRAIL-R2
REGULGENT™, diluted in saline containing 0.05 mg/ml Polysorbate 80,
administered intravenously through the tail vein at doses of 5
ml/kg once a week. After treatment, tumor volume was measured twice
a week to verify efficacy. Data are expressed as mean ± SE. Tumor
volumes and animal body weights were measured bi-weekly. The
experiments were terminated 2 weeks after the start of treatment.
No animal reached the predetermined humane endpoint. Mice were
anesthetized by inhalation of 3% isoflurane for ~10 min. The depth
of anesthesia was confirmed by assessing reflex responses and
respiratory patterns to ensure adequate anesthetic depth.
Subsequently, the mice were euthanized by cervical dislocation. The
mortality of the experimental animals was verified by the cessation
of respiration and heartbeat.
Pharmacokinetic profile analysis
The pharmacokinetic profile of REGULGENT™ was
assessed in 6-week-old male SCID mice after a single tail vein
injection of 1 or 10 mg/kg of anti-TRAIL-R2 antibody E11, anti-PSMA
antibody PI101, or PSMA/TRAIL-R2 REGULGENT™ (PI101/E11). Blood
samples were collected at 1, 6, 24, 48, 120 and 168 h post-dose.
Serum antibody concentrations were measured by
electrochemiluminescence immunoassay using streptavidin-coated
plates blocked with PBS containing 1% casein. Biotinylated
anti-human antibodies, calibration standards, quality control
samples and test samples were incubated in duplicate, followed by
HRP-labeled anti-human IgG and ruthenium-labeled anti-HRP detection
reagents. Luminescence was read on a SECTOR Imager 2400 (Meso Scale
Diagnostics, LLC). Between steps, plates were washed with PBS
containing 1% Tween-20.
In vitro hepatocyte toxicity
assay
Human hepatocytes from six donors (cat. no.
M00995-P; BioIVT) were seeded in collagen-coated 96-well plates and
cultured overnight at 37°C under 5% CO2. The anti-DNP
antibody, anti-TRAIL-R2 agonistic antibody KMTR2 and PSMA/TRAIL-R2
REGULGENT™, diluted in culture medium, were added at 50 µl per well
and incubated at 37°C for 4 days. Cell Counting Kit-8 solution was
added at 10 µl per well and the plates were incubated for 4 h at
37°C under 5% CO2. Next, 0.1 M HCl (10 µl per well) was
added to stop the reaction. The percent cell death was calculated
as follows: (1-([absorbance at each antibody
concentration]-[absorbance of medium only])/([absorbance at an
antibody concentration of 0 µg/ml]-[absorbance of medium only]))
×100. Data were expressed as mean ± SE of triplicate
experiments.
In vivo liver toxicity
Chimeric PXB mice with a humanized liver were
purchased from PhoenixBio Co., Ltd. Chimeric PXB mice with a
humanized liver were purchased from PhoenixBio Co., Ltd. A total of
16 mice were used in this study. The mice were housed under
specific pathogenfree conditions at 23±5°C with a 12-h light/dark
cycle and 55±25% relative humidity. All animals were male, 12-18
weeks old at the start of the study and weighed 18-23 g. The mice
were produced by xeno-transplanting human hepatocytes into
immunodeficient recipient cDNA-uPA+/-/SCID mice. The PXB mice were
assigned randomly to four groups (n=4 per group) and injected
intravenously with vehicle, anti-TRAIL-R2 agonistic antibody KMTR2
(1 mg/kg), or PSMA/TRAIL-R2 REGULGENT™ (1 or 10 mg/kg) and
monitored for 1 week. Individual body weights were recorded once
daily before blood sampling on Day 1, before dosing on Day 1 and
before blood sampling on Days 3 and 8. After the completion of
serial blood sampling for Day 8, mice were anesthetized by
inhalation of 3% isoflurane for ~10 min for induction, followed by
maintenance with 2.5% isoflurane. The depth of anesthesia was
confirmed by assessing reflex responses and respiratory patterns to
ensure adequate anesthetic depth. Subsequently, both the abdominal
cavity and the thoracic cavity were opened to access the heart for
the terminal blood sampling. The blood was collected from each
animal via the heart using disposable needles after which the
animals were euthanized by exsanguination. The serum was diluted
with saline by a factor of 2.5. The serum human alanine
aminotransferase (ALT) concentration was determined using a Human
ALT ELISA Kit (cat. no. ab234578; Abcam) (34). Serum ALT/ Aspartate aminotransferase
(AST) activities were determined by measuring diaryl imidazole type
of leucopigment. A DRI CHEM 7000 (Fujifilm) analyzer was used for
these measurements.
Negative stain electron microscopy
(NS-EM)
For preparation of the antigen–antibody complexes,
PSMA/TRAILR2 REGULGENT™, TRAIL/Apo2 ligand, and soluble PSMA were
mixed at equal protein amounts to achieve a final concentration of
1 mg/ml and incubated at 4°C for 16 ho to allow complex formation.
Antigenantibody complex fractions were then separated using
Superdex™ High-Performance Columns (Cytiva). The antigen-antibody
complexes were diluted to 0.1 µg/ml in phosphate-buffered saline
and crosslinked with 0.1% glutaraldehyde at 4°C for 4 h.
Thereafter, sample particles were adsorbed on to a carbon foil and
stained with Nano-W® containing methylamine tungstate
(Nanoprobes) and the sample grids were imaged using a TF20
microscope (FEI; Thermo Fisher Scientific, Inc.). The particles
were selected semi-automatically using COW (https://www.cow-em.de/) and were used for 2D
classification using RELION (https://www2.mrc-lmb.cam.ac.uk/relion/index.php/Main_Page).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism version 8 (Dotmatics). To evaluate the effect of cross-linker
on cytotoxic activity, data were analyzed using one-way ANOVA
followed by Dunnett's post hoc test. To assess the combined effects
of cross-linker and IVIG on cytotoxic activity, two-way ANOVA with
Bonferroni's post hoc correction was applied. For all other
analyses in which statistical significance is indicated,
repeated-measures one-way ANOVA followed by Tukey's multiple
comparisons test was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
PSMA and TRAIL-R2 expression in
patients with prostate cancer
The present study first investigated the expression
of PSMA and TRAIL-R2 in human prostate cancer, including
hormone-resistant cancer, using a tissue microarray. The H-score
was calculated as described in the Materials and methods.
The results showed that >90% of prostate cancers that were
tested were positive for both PSMA and TRAIL-R2 (Fig. 1A and B). In addition, ~90% of the
samples from hormone-resistant cancers were positive for both PSMA
and TRAIL-R2. Collectively, double positivity for PSMA and TRAIL-R2
was present in almost all human prostate cancers, including
hormone-resistant cancers ((Fig. 1C and
D).
Constructing PSMA/TRAIL-R2 bispecific
antibodies
Agonistic TRAIL-R2 antibodies are known to induce
apoptotic cell death through peripheral blood mononuclear cells
expressing FcγR. However, this poses some adverse risks to normal
cells, such as hepatocytes, which also express TRAIL-R2 (26,35).
Therefore, a non-agonistic TRAIL-R2 antibody, E11, was selected to
construct bispecific antibodies. Additionally, mispairing of
antibody light chains can occur if bispecific antibodies are
generated using two different antibody clones. The present study
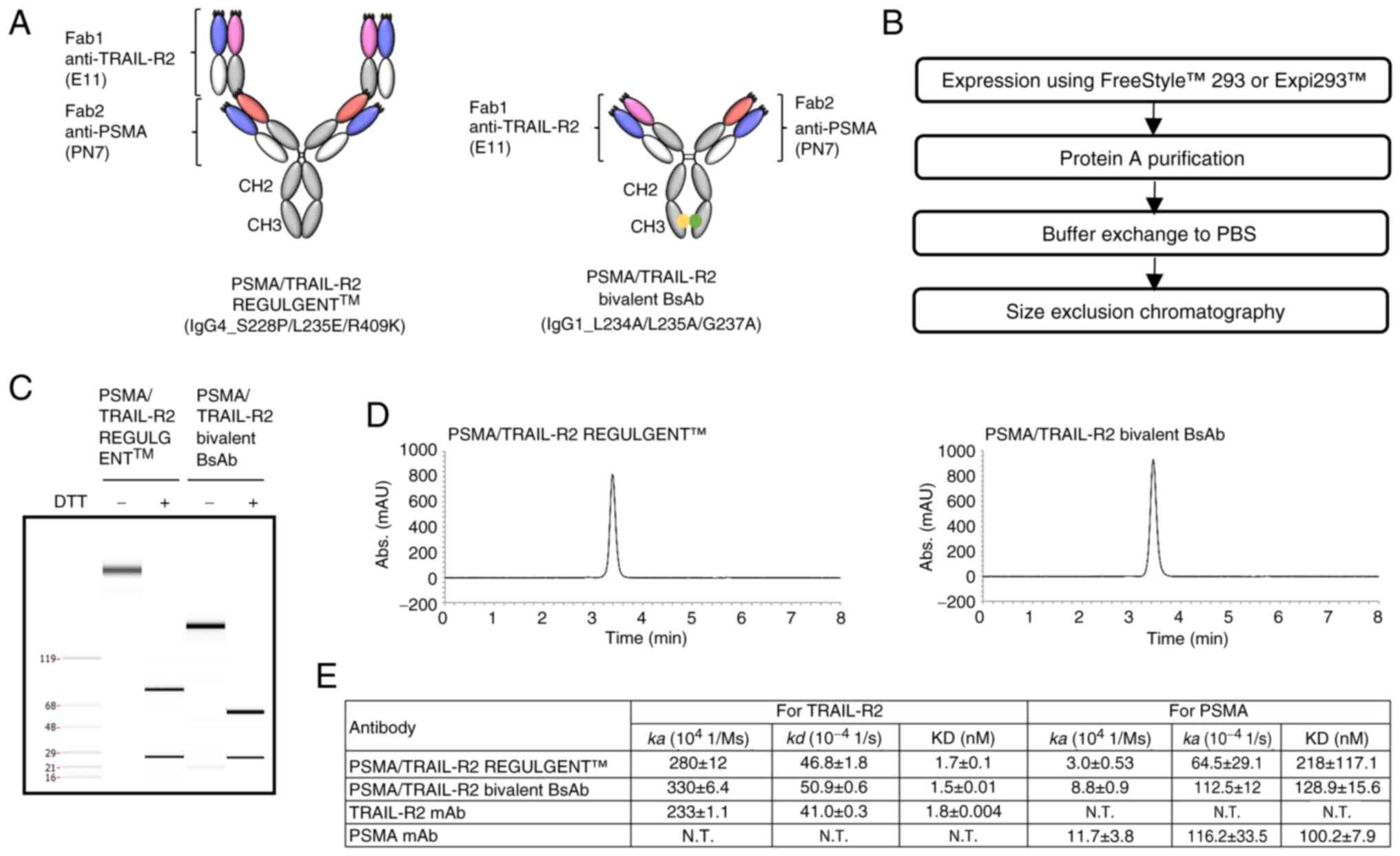
used a novel tetravalent and symmetric bispecific antibody, namely
REGULGENT™, comprised of a fused Fab in the N-terminus of the IgG4
heavy chain and a common light chain (Fig. 2A, left) (36). The present study successfully
constructed some PSMA antibody clones that shared a light chain
with the TRAIL-R2 antibody E11 and they were generated via phage
display using an E11 VL fixed library. For comparison with
PSMA/TRAIL-R2 REGULGENT™, a bivalent bispecific antibody was
constructed with a common light chain and IgG1 Fc mutations lacking
effector function (Fig. 2A, right)
(30). Based on previous reports
(10), the present study selected
effector function-null IgG variants of IgG4 and IgG1 Fc regions to
eliminate nonspecific TRAIL-R2 signaling mediated by Fc-dependent
mechanisms. This approach allowed the present study to specifically
evaluate activity independent of ADCC against PSMA-expressing
cells. Both bispecific antibodies were produced using Expi293F™ or
HEK293 cells (Fig. 2B) and their
purity was analyzed using LabChip and SEC (Fig. 2C and D). The purity was 97.4% for
PSMA/TRAIL-R2 REGULGENT™ and 99.3% for the bivalent bispecific
antibody. Subsequently, the PSMA/TRAIL-R2 binding of these
bispecific antibodies was evaluated using BIAcore SPR (Fig. 2E). The results indicated that both
bispecific antibodies bound to both PSMA and TRAIL-R2, with no
marked difference in the binding levels.
Superior tumor cell death-inducing
effect of PSMA/TRAIL-R2 REGULGENT™ over the monovalent
heterodimeric bispecific antibody
To investigate the biological activities of the
bispecific antibodies, the present study evaluated their abilities
to induce cell death in some tumor cell lines. First,
PSMA-overexpressing variants of the prostate cancer cell line PC-3
and the human lung adenocarcinoma cell line NCI-H2122 were
generated, termed PSMA/PC-3 and PSMA/NCI-H2122, respectively. PSMA
expression was confirmed by western blot analysis (Fig. S1). Next, PSMA/TRAIL-R2 expression
in PC-3 and PSMA/PC-3 cells was verified using flow cytometry
(Fig. 3A). The super-agonistic
TRAIL-R2 antibody, KMTR2, induced death in both PC-3 and PSMA/PC-3
cells (Fig. 3B and C), whereas the
non-agonistic TRAIL-R2 antibody, E11 and PSMA antibody, PN7, did
not elicit any cell death. By contrast, PSMA/TRAIL-R2 REGULGENT™
selectively induced death in PSMA/PC-3 but not in PC-3 cells, with
a potency superior to that of the KMTR2 antibody. The efficacy of
tumor cell death was the same even if another PSMA antibody, clone
PI101, was used in REGULGENT™ instead of PN7 (Fig. S2). These findings implied
PSMA-dependent biological activity of PSMA/TRAIL-R2 REGULGENT™.
Notably, the bivalent bispecific antibody did not induce death in
PSMA/PC-3 cells. Comparable results were obtained for NCI-H2122
cells, PSMA/NCI-H2122 and the LNCaP clone FGC (Fig. 3D-H).
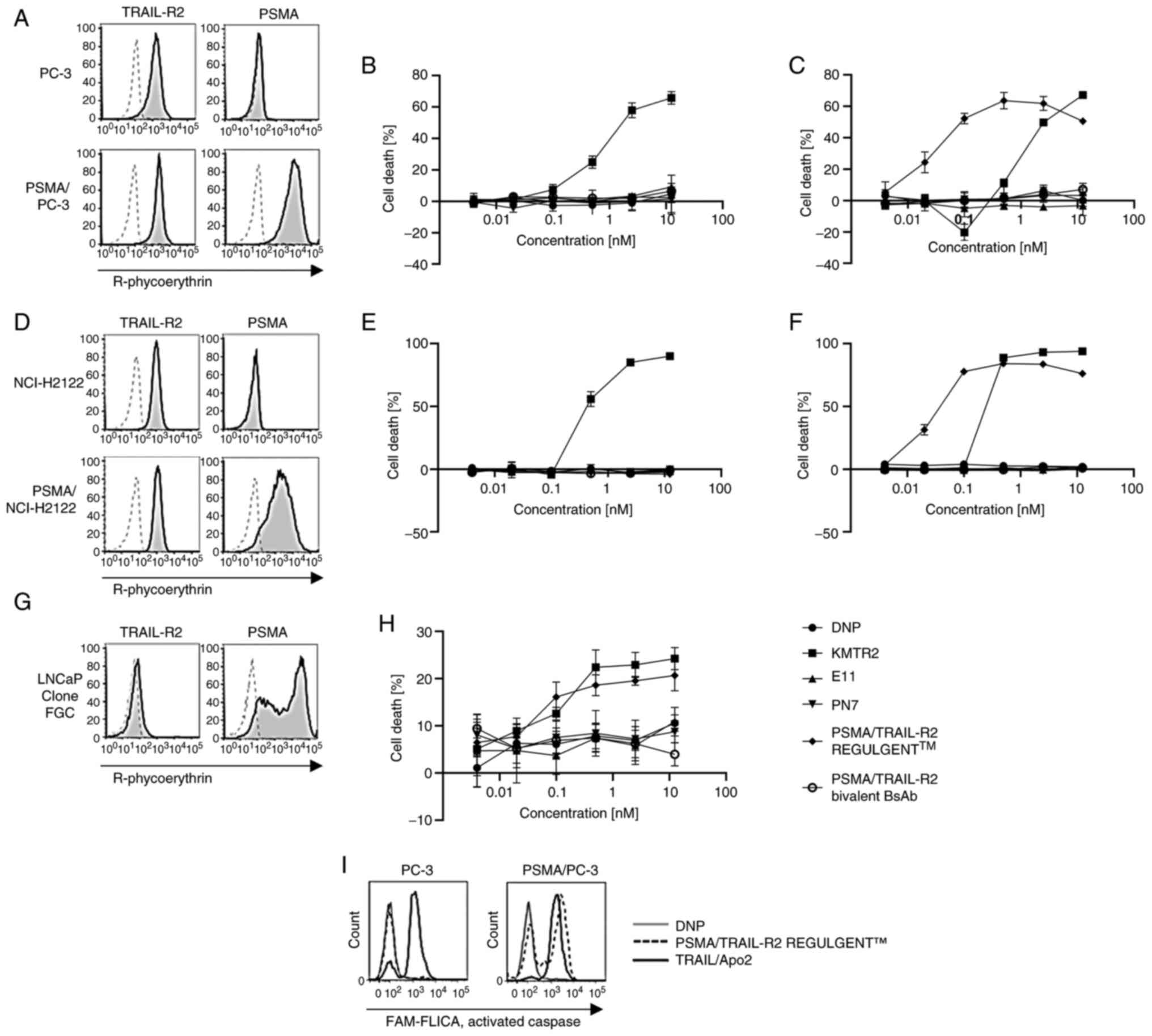 | Figure 3.Cancer cell death induced by
prostate-specific membrane antigen and tumor necrosis
factor-related apoptosis-inducing ligand-receptor 2 (PSMA/TRAIL-R2)
bispecific antibodies. (A, D and G) Evaluation of PSMA and TRAIL-R2
expression in various cell lines using flow cytometry. (B, C, E, F
and H) Viability of human tumor cell lines in response to treatment
with PSMA/TRAIL-R2 bispecific antibodies (REGULGENT™ and bivalent
bispecific antibody) in a 96-well cell proliferation assay using
the (B) PC-3 and (E) NCI-H2122 cell lines
(PSMA−TRAIL-R+), (C) PSMA/PC-3 and (F) a
PSMA/NCI-H2122 transfectants and (H) the LNCaP clone FGC cell line
(PSMA+TRAIL-R+). Antibodies were tested in
fivefold dilutions starting at 12.5 nM to 0.004 nM. Data represent
quadruplicate experiments and are shown as means ± SE. The vertical
axes represent cell death (%) and the horizontal axes represent the
antibody concentration (nM). An anti-DNP antibody was used as the
negative control. (I) Caspase activation in PC-3 or PSMA/PC-3 cells
by the TRAIL/Apo2 ligand and PSMA/TRAIL-R2 REGULGENT™ using the
FAM-FLICA Poly Caspase Assay. The solid line shows caspase
activation by the TRAIL/Apo2 ligand, the dotted line shows caspase
activation by TRAIL-R2/PSMA REGULGENT™ and the gray line shows
caspase activation by the anti-DNP antibody used as the negative
control. PSMA, prostate-specific membrane antigen; TRAIL-R2, tumor
necrosis factor-related apoptosis-inducing ligand-receptor 2; DNP,
2,4-dinitrophenol. |
To confirm that the cell death induced by
PSMA/TRAIL-R2 REGULGENT™ was apoptotic, activated caspase levels
were evaluated using flow cytometry (Fig. 3I). The results showed that
PSMA/TRAIL-R2 REGULGENT™ activated caspases only in PSMA/PC-3
cells, suggesting PSMA-dependent apoptotic induction.
Effect of IVIG and crosslinkers on
PSMA/TRAIL-R2 REGULGENT™ activity
The first-generation anti-TRAIL-R2 antibody, apomab,
requires a crosslinker to induce apoptosis. Apomab markedly
decreased cell viability in a crosslinker dose-dependent manner,
whereas PSMA/TRAIL-R2 REGULGENTTM slightly increased cell viability
at high crosslinker concentrations (Fig. 4A). To examine the effects of these
antibodies on cell death, IVIG was used to mimic the in vivo
environment. The efficacy of Apomab was markedly inhibited by IVIG
in a dose-dependent manner. On the other hand, PSMA/TRAIL-R2
REGULGENTTM did not markedly affect its activity in the presence of
IVIG (Fig. 4B).
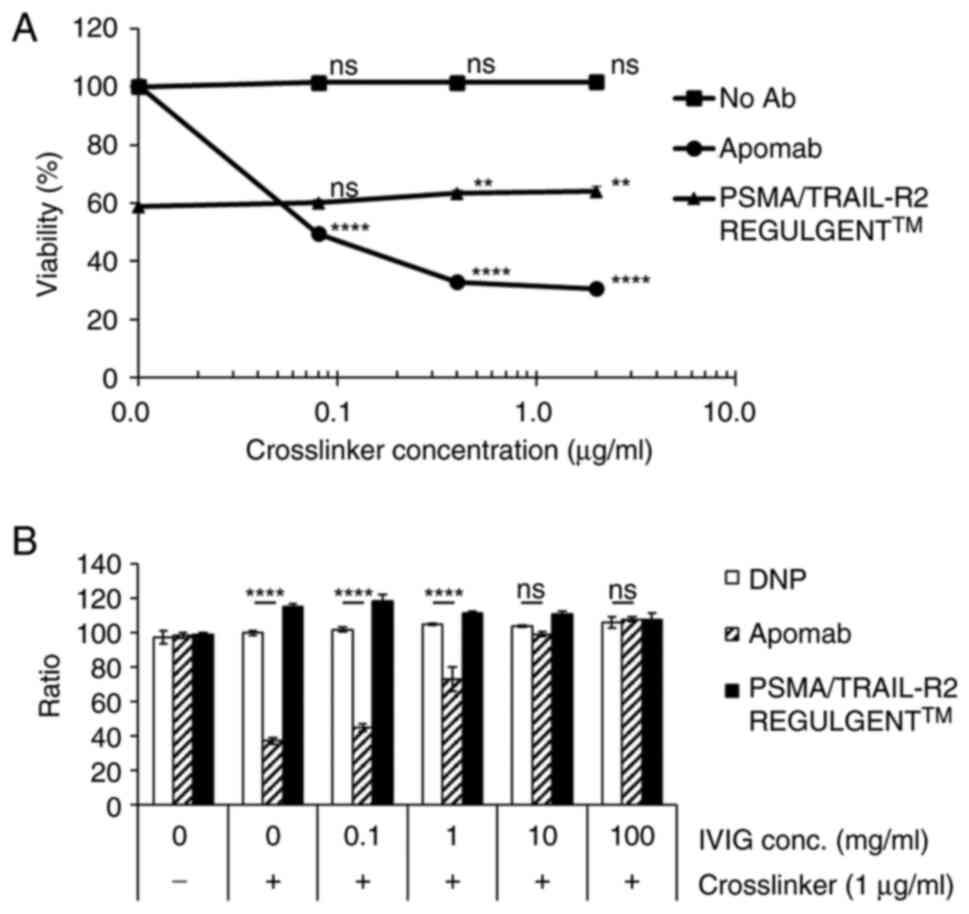 | Figure 4.Effect of crosslinker and IVIG on
tumor cell death induced by the prostate-specific membrane antigen
and the tumor necrosis factor-related apoptosis-inducing
ligand-receptor 2 (PSMA/TRAIL-R2) bispecific antibody (REGULGENT™).
(A) PSMA/PC-3 cells were seeded onto 96 well plates. Then apomab
and PSMA/TRAIL-R2 REGULGENT™ were added and cultured with
crosslinkers for 3 days. Statistical analysis was performed using
one-way ANOVA with Dunnett's post hoc test to compare each
treatment group at a given concentration with the corresponding
no-antibody (no Ab) control group. **P<0.01, ****P<0.0001,
ns, no significance. (B) IVIG affected the efficacy of crosslinked
apomab and PSMA/TRAIL-R2 REGULGENT™ in a concentration-dependent
manner. Statistical analysis was performed using twowayANOVA with
Bonferroni post hoc test. ****P<0.0001, ns, no significance.
IVIG, intravenous immunoglobulin; PSMA, prostate-specific membrane
antigen; TRAIL-R2, tumor necrosis factor-related apoptosis-inducing
ligand-receptor 2; DNP, 2,4-dinitrophenol. |
PSMA-dependent in vivo antitumor
activity of PSMA/TRAIL-R2 REGULGENT™
To assess whether PSMA/TRAIL-R2 REGULGENT™ exhibited
a selective antitumor effect on PSMA-expressing tumors in
vivo, NCI-H2122 and PSMA/NCI-H2122 cells were implanted
subcutaneously into SCID mice (Fig.
5A). Although images of the excised xenograft tumor were not
captured, the tumor was visually confirmed to be normal in volume
and size. The maximum diameter of the tumor in all mice was 17.9 mm
and the maximum volume was 1,195 mm3. KMTR2 showed a
strong antitumor effect in both NCI-H2122- and
PSMA/NCI-H2122-bearing mice (Fig.
5B, C and Table SI, Table SII, Table SIII, Table SIV, Table SV, Table SVI). By contrast, PSMA/TRAIL-R2
REGULGENT™ showed a specific and significant antitumor effect in
PSMA/NCI H2122- but not in NCI-H2122-bearing mice. The
pharmacokinetic profile of PSMA/TRAIL-R2 REGULGENT™ in wild-type
mice was favorable compared with that of monoclonal antibodies
(Fig. S3). These results indicated
that PSMA/TRAIL-R2 REGULGENT™ exerted a potent antitumor effect in
a PSMA-dependent manner in vivo.
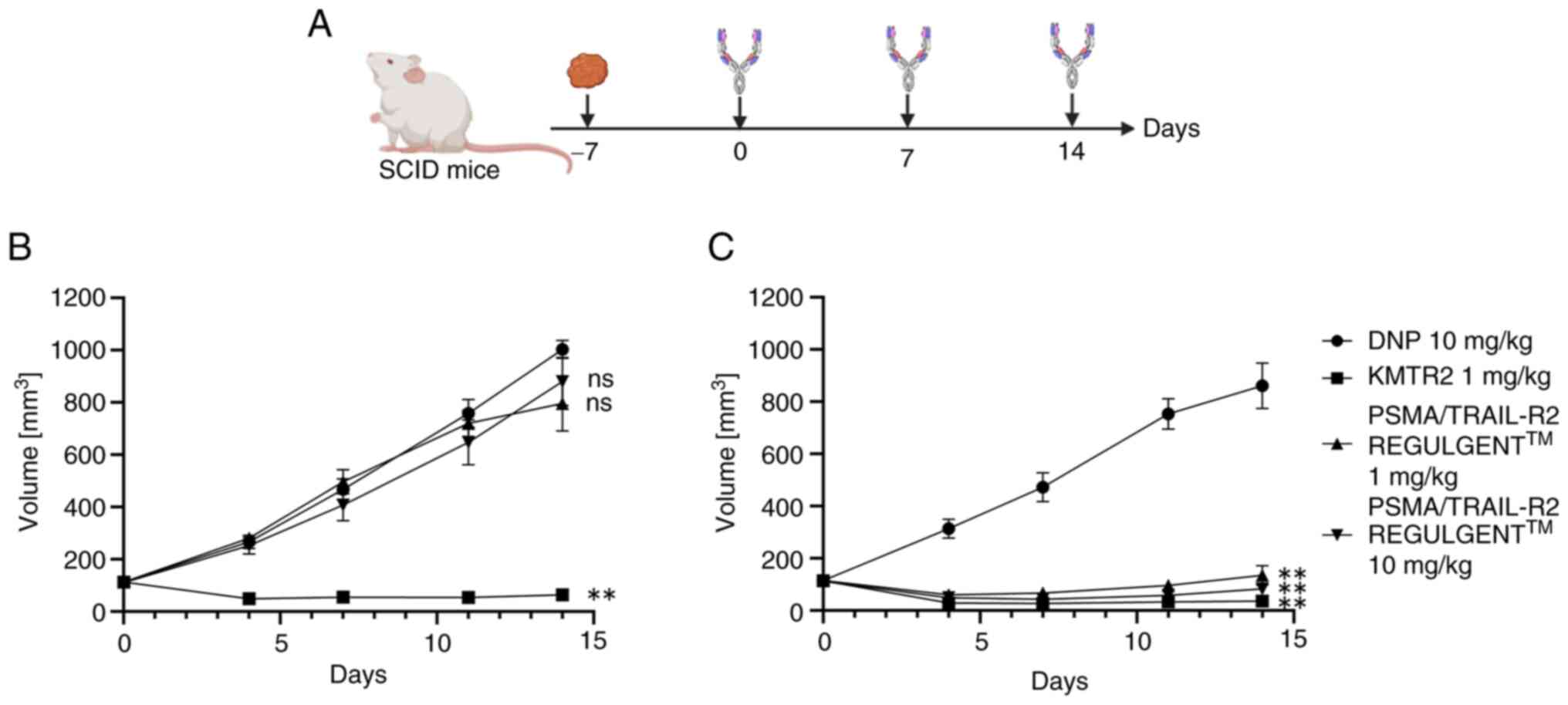 | Figure 5.In vivo PSMA-dependent
antitumor activity of the PSMA/TRAIL-R2 bispecific antibody
(REGULGENT™). (A) Schedule of antibody administration and tumor
measurement in a xenograft model. (B) NCI-H2122 and (C)
PSMA/NCI-H2122 transfectant cells were transplanted subcutaneously
into SCID mice. The mice were grouped 7 days after transplantation
to start receiving the anti-2,4-dinitrophenol (DNP) antibody,
anti-TRAIL-R2 agonistic antibody KMTR2, or PSMA/TRAIL-R2
REGULGENT™. The vertical axes indicate tumor size (mm3)
and the horizontal axes indicate the time (days) after the start of
antibody administration. Plotted values are the means (SEM) of
duplicates of four independent experiments. To determine the
statistical significance, an RM one-way ANOVA with Tukey's multiple
comparisons was performed (**P<0.01, ns, no significance). PSMA,
prostate-specific membrane antigen; TRAIL-R2, tumor necrosis
factor-related apoptosis-inducing ligand-receptor 2; DNP,
2,4-dinitrophenol. |
PSMA/TRAIL-R2 REGULGENT™ does not
cause injury to human hepatocytes in vitro or liver toxicity to PXB
mice in vivo
Following previous reports, the present study
evaluated the toxicity in human hepatocytes and chimeric livers
derived from PBX mice, which exhibit higher sensitivity to
TRAIL-mediated apoptosis compared to hepatocytes from rodents and
non-human primates (6). Therefore,
human hepatocytes and PXB mice were used to evaluate whether
PSMA/TRAIL-R2 REGULGENT™ exerts liver toxicity. Human hepatocytes
did not express PSMA but slightly expressed TRAIL-R2 on their cell
surface (Fig. 6A). KMTR2 induced
cell death in human hepatocytes whereas PSMA/TRAIL-R2 REGULGENT™
did not (Fig. 6B). In vitro
toxicity evaluations were performed using hepatocytes from multiple
donors and PSMA/TRAIL-R2 REGULGENT™ did not induce cell death in
any of the hepatocytes tested (Fig.
6C).
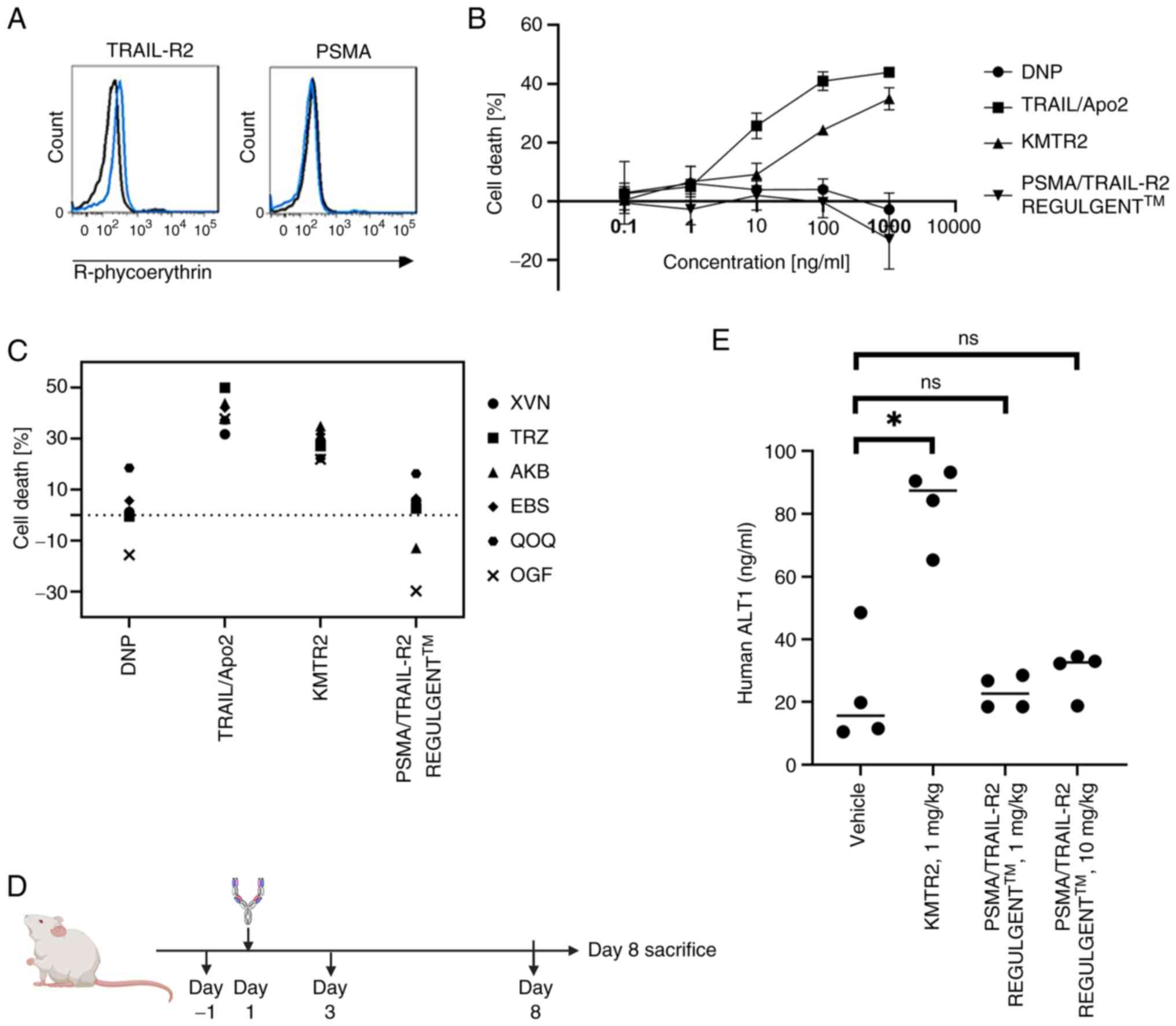 | Figure 6.In vitro/in vivo liver
toxicity of prostate-specific membrane antigen and tumor necrosis
factor-related apoptosis-inducing ligand-receptor 2 (PSMA/TRAIL-R2)
bispecific antibodies. (A) PSMA and TRAIL-R2 expression in
hepatocytes was determined using flow cytometry. (B) Hepatocytes
were treated with serially diluted antibodies anti-DNP,
anti-TRAIL-R2 agonistic KMTR2, anti-TRAIL-R2 non-agonistic E11, or
PSMA/TRAIL-R2 REGULGENT™) and incubated for 96 h. Cell viability
was determined using Cell Counting Kit-8. (C) Antibody-induced
toxicity in human primary hepatocytes from six donors (lot numbers:
XVN, TRZ, AKB, EBS, QOQ and OGF). (D) Schedule of in vivo
safety assessment using PXB mice. (E) Serum human ALT1
concentrations in response to anti-TRAIL-R2 agonistic antibody
KMTR2 and PSMA/TRAIL-R2 REGULGENT™ treatment. *P<0.05, ns, no
significance. PSMA, prostate-specific membrane antigen; TRAIL-R2,
tumor necrosis factor-related apoptosis-inducing ligand-receptor 2;
DNP, 2,4-dinitrophenol; ALT, alanine aminotransferase. |
Subsequently, PXB mice were administered a single
dose of KMTR2 or PSMA/TRAIL-R2 REGULGENT™ intravenously to assess
liver toxicity (Fig. 6D). Compared
with vehicle-treated mice, KMTR2-treated mice displayed
statistically significant increase in serum human ALT1 level, an
indicator of liver toxicity (Fig.
6E). By contrast, no significant changes in the serum human
ALT1 levels were observed in mice administered 1 or 10 mg/kg of
TRAIL-R2/PSMA REGULGENT™ (Fig. 6E).
Previous studies have reported that human ALT is the optimal
indicator of hepatotoxicity in PXB mice (34). In addition to human ALT1, the
present study also evaluated the activities of AST and ALT.
Significant increases in these values were observed in mice
administered KMTR2, whereas no elevations were detected in mice
treated with PSMA/TRAIL-R2 REGULGENT™ (Fig. S4). Moreover, the body weights of
mice did not differ markedly among the treatment groups (data not
shown). These results suggested that PSMA/TRAIL-R2 REGULGENT™ does
not exhibit hepatotoxic effects in PXB mice when administered
intravenously as a single dose of up to 10 mg/kg. By contrast,
KMTR2 showed hepatotoxic effects following a single intravenous
administration at 1 mg/kg.
Discussion
The present study reported the preclinical antitumor
activity of PSMA/TRAIL-R2 REGULGENT™, a novel tetravalent
bispecific antibody with common light chains. PSMA/TRAIL-R2
REGULGENT™ binds to PSMA as an anchor to induce TRAIL-R2
activation, leading to tumor cell apoptosis in a PSMA-dependent
manner.
Consistent with the findings of a previous study
(14), the present study confirmed
that PSMA and TRAIL-R2 are expressed in patients with prostate
cancer. This finding suggested that PSMA/TRAIL-R2 REGULGENT™ could
serve as a therapeutic agent for PSMA/TRAIL-R2 double-positive
cancer. Furthermore, PSMA is expressed in NSCLC and breast cancers,
suggesting that PSMA/TRAIL-R2 REGULGENT™ could be effective against
cancers other than prostate cancer. The expression of PSMA in
breast cancers is related to tumor subtype and malignancy,
exhibiting heterogeneity (37).
Therefore, stratifying patients by types or subtypes of cancer with
high PSMA positivity could yield more effective targets. In
addition, PSMA/TRAIL-R2 REGULGENT™ may have applications across a
broad spectrum of tumors owing to the unique expression of PSMA in
the tumor neovasculature.
The present study demonstrated that PSMA/TRAIL-R2
REGULGENT™ exhibited high tumor cell death activity in a PSMA
binding-dependent manner without any secondary crosslinkers both
in vitro and in vivo. Additionally, the apoptotic
activity remained unaffected in the presence of high IgG levels,
suggesting that this bispecific antibody effectively induced tumor
cell death in vivo. BI 905711, a tetravalent bispecific
CDH17/TRAIL-R2 antibody, induces apoptosis specifically in
CDH17-positive tumor cells, similar to PSMA/TRAIL-R2 REGULGENT™
(12). However, information on its
hepatocytotoxicity is limited, although no cytotoxicity was
observed in HepG2 human hepatocellular carcinoma cells (12). The present study detected human ALT1
in the serum of KMTR2-treated PXB mice as reported previously
(6), implying that cytotoxic
activity against human hepatocytes can be observed directly.
Nevertheless, no elevation in the serum human ALT1 level was
detected in PSMA/TRAIL-R2 REGULGENT™-treated PXB mice. Thus, it was
demonstrated, for the first time to the best of the authors'
knowledge, that PSMA/TRAIL-R2 REGULGENT™ lacks hepatotoxicity,
based on studies with primary hepatocytes and PXB chimeric mice
with humanized livers.
Currently, some biologics (IGM-8444, ABBV-621,
INBRX-109 and BI 905711) are being investigated in clinical trials
(NCT04553692, NCT04570631 and NCT04137289/NCT05087992,
respectively). The first three are TRAIL-R2 antibodies without
cancer-targeting molecules, making it difficult to avoid
hepatotoxicity. Therefore, their doses should be reduced to lower
adverse effects. Cancer-targeting molecules are designed to
mitigate hepatotoxicity, as mentioned previously. Recently, Phase 1
data of BI 905711 were released in ASCO2023 (NCT04137289) (38). No dose-limiting toxicities were
observed and the maximum tolerated dose was not reached. In heavily
pretreated patients, it showed a tolerable safety profile. AST
level increased in only 2 of 43 patients, suggesting that the
expected mechanism of the tetravalent bispecific antibody, using a
TRAIL-R2 non-agonistic antibody clone, might effectively suppress
hepatotoxicity when targeted to tumor cells.
Although apoptosis induction by TRAIL-R2 effectively
kills tumor cells, not all tumors are sensitive to the TRAIL-R
signaling pathway. Molecules that inhibit TRAIL-R signaling have
been reported, including cellular FLICE-like inhibitory protein,
which competes with caspase-8 for FADD binding, as well as XIAP,
cIAp-1 and cIAP-2, which block active caspases (39). In addition, combining TRAIL with
small molecules targeting inhibitory molecules against TRAIL-R
signaling reportedly induces TRAIL sensitization (40). The present study observed a tendency
for c-FLIP to be inversely associated with TRAIL-R2 signaling (data
not shown), but no information is available on whether c-FLIP
inhibitors synergistically affect PSMA/TRAIL-R2 REGULGENT™-induced
tumor cell death. Further analyses will be required to determine
which cancers are sensitive to TRAIL-R2 signaling.
Several agents and chemotherapeutic drugs, including
paclitaxel, doxorubicin and camptothecin, enhance TRAIL-induced
apoptosis in prostate cancer cell lines through TRAIL-R2
upregulation. Neferine treatment also enhances the TRAIL-induced
apoptosis of human prostate cancer cells via autophagic flux and
the c-Jun N-terminal kinase pathway (41). Bortezomib-mediated TRAIL
sensitization was facilitated by enhanced formation of the TRAIL
death-inducing signaling complex (DISC) and c-FLIP downregulation
in the DISC (42–44). The combination of TRAIL with SMAC or
BH3 mimetics has been evaluated in various cancers (45,46).
CDK9 inhibitors induce tumor cell death in TRAIL-resistant NSCLC
cell lines through downregulation of Mcl-1 and c-FLIP expression
(47,48). Additionally, elastin and RSL3 might
influence TRAIL-R2 sensitivity owing to the accumulation of ROS
produced by mitochondria during elastin- and RSL3-induced
ferroptosis (49), which
purportedly regulates ROS production and affects TRAIL-R2
sensitivity (50). Collectively,
these findings suggest that chemotherapeutic drugs might enhance
tumor cell death induced by PSMA/TRAIL-R2 REGULGENT™. In the
future, it is necessary to screen for agents that exhibit a strong
synergistic effect with PSMA/TRAIL-R2 REGULGENT™, tailored to the
specific type of cancer being targeted and consider their combined
use.
Previous studies have shown that diverse clones
within bivalent bispecific antibodies can effectively minimize
off-target toxicity (51). By
contrast, the current findings indicate that the bivalent
bispecific antibody did not demonstrate any tumor cell
death-inducing activity, with only the tetravalent format of
PSMA/TRAIL-R2 REGULGENT™ exhibiting such activity. For an improved
understanding of this difference, the structure of PSMA/TRAIL-R2
REGULGENT™, TRAIL-R2 and the PSMA trimer complex was observed using
NS-EM. When the trimeric complex structures of PSMA/TRAIL-R2
REGULGENT™, TRAIL-R2 and PSMA were observed using NS-EM, a
structure showing three TRAIL-R2 molecules in proximity was
detected (Fig. S5). This may
explain why REGULGENT™ induces apoptotic signals more effectively
than bivalent bispecific antibodies. In this structure, three
PSMA/TRAIL-R2 REGULGENT™ molecules bind through a dimeric PSMA,
leading to the aggregation of TRAIL-R2. However, in the bivalent
format, aggregation beyond the trimerization of REGULGENT and
TRAIL-R2 is not feasible. However, regarding TRAIL-R2, the bivalent
format may not allow for the aggregation of REGULGENT and TRAIL-R2
beyond trimerization, potentially impairing signal induction.
PSMA/TRAIL-R2 REGULGENT™ slightly decreased cell death at high
crosslinker concentrations, assuming that the decrease occurred as
a result that PSMA/TRAIL-R2 REGULGENT™ was randomly associated by
crossliner. As the trimerization/multimerization of TRAIL-R2 is
critical for the induction of apoptosis signaling pathways, it is
plausible that the tetravalent format of PSMA/TRAIL-R2 REGULGENT™
was able to efficiently transmit cell death-inducing signals.
Moreover, the present study demonstrated that a tetravalent
bispecific antibody using a TRAIL-R2 non-agonistic antibody (or a
simple binder) was not hepatotoxic. The authors are currently
conducting clinical trials to apply this format to different
tetravalent bispecific antibodies (NCT06248411, NCT06266299).
In summary, the present study demonstrated that
PSMA/TRAIL-R2 REGULGENT™ induced tumor cell apoptosis in a PSMA
expression-dependent manner in vitro and in vivo.
Additionally, PSMA/TRAIL-R2 REGULGENT™ did not exert toxicity
against human hepatocytes or PXB mice, representing an improvement
of the therapeutic window. Taken together, novel non-hepatotoxic
tetravalent bispecific antibodies could serve as effective
therapeutic agents for cancer treatment. Future studies involving
more advanced preclinical evaluations and clinical trials are
warranted to further elucidate the therapeutic value of
PSMA/TRAIL-R2 REGULGENT™ in patients.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors thank Dr Shinya Miyamoto and Dr Seiji
Saito (Core Research Laboratories, BioPharmaceutical Center,
Research Division, Kyowa Kirin Co., Ltd.) for providing
experimental information and valuable advice. The authors also
thank Mr. Masao Asada, Mr. Ryusei Ogura, and Ms. Kaori Yamazaki
(Core Research Laboratories, BioPharmaceutical Center, Research
Division, Kyowa Kirin Co., Ltd.) for their cooperation in
preliminary studies and experimental work. In addition, Dr Yohei
Inai, Dr Junko Iwano, and Dr Kenichirou Nanya (Translational
Research Laboratories, BioPharmaceutical Center, Research Division,
Kyowa Kirin Co., Ltd.) are acknowledged for their support in
experiments, provision of experimental information, and helpful
advice. The authors would also like to thank Dr Masakazu Kakuni
(Study Service Department, PhoenixBio Co., Ltd.) for his
contribution to the PXB mouse experiments.
Funding
The present study was funded by Kyowa Kirin Co., Ltd.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MN, STM, YM, KN and MI designed and performed the
experiments. MN and KU confirm the authenticity of all the raw
data. MN, STM, YM, KN and MI analyzed the data. MN, NT and KU
conceived and supervised the study. MN, STM and KU wrote the
manuscript. KU revised the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
All animal studies were performed in accordance with
Standards for Proper Conduct of Animal Experiments at Kyowa Kirin
Co., Ltd. under the approval of the company's Institutional Animal
Care and Use Committee (approval nos. 15J0057 and 16J1063). Tokyo
Research Park of Kyowa Kirin Co., Ltd. is fully accredited by the
AAALAC international. PXB mouse experiments were performed in
accordance with ethical approval of the PhoenixBio Ethics Board
(approval no. 1701).
Patient consent for publication
Not applicable.
Competing interests
All authors are employees of the company Kyowa Kirin
Co., Ltd., who were responsible for manufacturing the novel
tetravalent bispecific antibody, PSMA/TRAIL-R2 REGULGENT™, as
investigated in the present study.
Glossary
Abbreviations
Abbreviations:
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
DISC
|
death-inducing signaling complex
|
|
FGC
|
fast-growing colony
|
|
HMWS
|
high-molecular-weight species
|
|
LMWS
|
low-molecular-weight species
|
|
NS-EM
|
negative stain electron microscopy
|
|
NSCLC
|
non-small cell lung cancer
|
|
PSMA
|
prostate-specific membrane antigen
|
|
VH
|
variable domain of heavy chain
|
|
VL
|
variable domain of light chain
|
|
TRAIL
|
tumor necrosis factor-related
apoptosis-inducing ligand
|
|
PBS
|
phosphate-buffered saline
|
|
SPR
|
surface plasmon resonance
|
|
IVIG
|
intravenous immunoglobulin
|
|
UHP-SEC
|
ultra-high-pressure size exclusion
chromatography
|
References
|
1
|
Ashkenazi A, Pai RC, Fong S, Leung S,
Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert
A, et al: Safety and antitumor activity of recombinant soluble Apo2
ligand. J Clin Invest. 104:155–162. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Snajdauf M, Havlova K, Vachtenheim J Jr,
Ozaniak A, Lischke R, Bartunkova J, Smrz D and Strizova Z: The
TRAIL in the treatment of human cancer: An update on clinical
trials. Front Mol Biosci. 8:6283322021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yada A, Yazawa M, Ishida S, Yoshida H,
Ichikawa K, Kurakata S and Fujiwara K: A novel humanized anti-human
death receptor 5 antibody CS-1008 induces apoptosis in tumor cells
without toxicity in hepatocytes. Ann Oncol. 19:1060–1067. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuchs CS, Fakih M, Schwartzberg L, Cohn
AL, Yee L, Dreisbach L, Kozloff MF, Hei YJ, Galimi F, Pan Y, et al:
TRAIL receptor agonist conatumumab with modified FOLFOX6 plus
bevacizumab for first-line treatment of metastatic colorectal
cancer: A randomized phase 1b/2 trial. Cancer. 119:4290–4298. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motoki K, Mori E, Matsumoto A, Thomas M,
Tomura T, Humphreys R, Albert V, Muto M, Yoshida H Aoki, et al:
Enhanced apoptosis and tumor regression induced by a direct agonist
antibody to tumor necrosis factor-related apoptosis-inducing ligand
receptor 2. Clin Cancer Res. 11:3126–3135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nihira K, Nan-Ya KI, Kakuni M, Ono Y,
Yoshikawa Y, Ota T, Hiura M and Yoshinari K: Chimeric mice with
humanized livers demonstrate human-specific hepatotoxicity caused
by a therapeutic antibody against TRAIL-receptor 2/death receptor
5. Toxicol Sci. 167:190–201. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Papadopoulos KP, Isaacs R, Bilic S,
Kentsch K, Huet HA, Hofmann M, Rasco D, Kundamal N, Tang Z, Cooksey
J and Mahipal A: Unexpected hepatotoxicity in a phase I study of
TAS266, a novel tetravalent agonistic Nanobody®
targeting the DR5 receptor. Cancer Chemother Pharmacol. 75:887–895.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutt M, Fellermeier-Kopf S, Seifert O,
Schmitt LC, Pfizenmaier K and Kontermann RE: Targeting
scFv-Fc-scTRAIL fusion proteins to tumor cells. Oncotarget.
9:11322–11335. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Y, Bassoff N, Reinshagen C, Bhere D,
Nowicki MO, Lawler SE, Roux J and Shah K: Bi-specific molecule
against EGFR and death receptors simultaneously targets
proliferation and death pathways in tumors. Sci Rep. 7:26022017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brünker P, Wartha K, Friess T,
Grau-Richards S, Waldhauer I, Koller CF, Weiser B, Majety M, Runza
V, Niu H, et al: RG7386, a novel tetravalent FAP-DR5 antibody,
effectively triggers FAP-dependent, avidity-driven DR5
hyperclustering and tumor cell apoptosis. Mol Cancer Ther.
15:946–957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shivange G, Urbanek K, Przanowski P, Perry
JSA, Jones J, Haggart R, Kostka C, Patki T, Stelow E, Petrova Y, et
al: A single-agent dual-specificity targeting of FOLR1 and DR5 as
an effective strategy for ovarian cancer. Cancer Cell.
34:331–345.e311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
García-Martínez JM, Wang S, Weishaeupl C,
Wernitznig A, Chetta P, Pinto C, Ho J, Dutcher D, Gorman PN,
Kroe-Barrett R, et al: Selective tumor cell apoptosis and tumor
regression in CDH17-positive colorectal cancer models using BI
905711, a novel liver-sparing TRAILR2 agonist. Mol Cancer Ther.
20:96–108. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldmacher VS, Gershteyn I, Chari R and
Kovtun Y: A bispecific anti-MUC16/anti-death receptor 5 antibody
achieves effective and tumor-selective death receptor 5-mediated
tumor regression. Sci Rep. 15:99092025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minner S, Wittmer C, Graefen M, Salomon G,
Steuber T, Haese A, Huland H, Bokemeyer C, Yekebas E, Dierlamm J,
et al: High level PSMA expression is associated with early PSA
recurrence in surgically treated prostate cancer. Prostate.
71:281–288. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang HL, Wang SS, Song WH, Pan Y, Yu HP,
Si TG, Liu Y, Cui XN and Guo Z: Expression of prostate-specific
membrane antigen in lung cancer cells and tumor neovasculature
endothelial cells and its clinical significance. PLoS One.
10:e01259242015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tolkach Y, Gevensleben H, Bundschuh R,
Koyun A, Huber D, Kehrer C, Hecking T, Keyver-Paik MD, Kaiser C,
Ahmadzadehfar H, et al: Prostate-specific membrane antigen in
breast cancer: A comprehensive evaluation of expression and a case
report of radionuclide therapy. Breast Cancer Res Treat.
169:447–455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andryszak N, Kurzawa P, Krzyżaniak M,
Nowicki M, Ruchała M, Iżycki D and Czepczyński R: Evaluation of
prostate-specific membrane antigen (PSMA) immunohistochemical
expression in early-stage breast cancer ubtypes. Int J Mol Sci.
25:65192024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Milowsky MI, Nanus DM, Kostakoglu L,
Sheehan CE, Vallabhajosula S, Goldsmith SJ, Ross JS and Bander NH:
Vascular targeted therapy with anti-prostate-specific membrane
antigen monoclonal antibody J591 in advanced solid tumors. J Clin
Oncol. 25:540–547. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tolmachev V, Malmberg J, Estrada S,
Eriksson O and Orlova A: Development of a 124I-labeled version of
the anti-PSMA monoclonal antibody capromab for immunoPET staging of
prostate cancer: Aspects of labeling chemistry and biodistribution.
Int J Oncol. 44:1998–2008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang Z, Guo J, Hu L, Yang S, Meng B and
Tang Q; Diagnostic performance of (18)F-DCFPyL PET vs. (68)Ga-PSMA
PET/CT in patients with suspected prostate cancer, : A systemic
review and meta-analysis. Oncol Lett. 2024.27:188 View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simon H, Henkel D, Chiron P and Helissey
C: New perspectives on metabolic imaging in the management of
prostate cancer in 2022: A focus on radiolabeled PSMA-PET/CT
(Review). Mol Clin Oncol. 19:512023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petrylak DP, Vogelzang NJ, Chatta K,
Fleming MT, Smith DC, Appleman LJ, Hussain A, Modiano M, Singh P,
Tagawa ST, et al: PSMA ADC monotherapy in patients with progressive
metastatic castration-resistant prostate cancer following
abiraterone and/or enzalutamide: efficacy and safety in open-label
single-arm phase 2 study. Prostate. 80:99–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saga T, Nakamoto Y, Ishimori T, Inoue T,
Shimizu Y, Kimura H, Akamatsu S, Goto T, Watanabe H, Kitaguchi K,
et al: Initial evaluation of PET/CT with (18) F-FSU-880 targeting
prostate-specific membrane antigen in prostate cancer patients.
Cancer Sci. 110:742–750. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou H, Lin Y, Pan Y, Ma Y, Hou G, Sun X
and Gao F: Synthesis and preclinical evaluation of (68)Ga-labeled
PSMA tracers with improved pharmacological properties. Eur J Med
Chem. 274:1165452024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Droghetti M, Bianchi L, Presutti M,
Vetrone L, Farolfi A, Mei R, Giunchi F, Degiovanni A, Mottaran A,
Piazza P, et al: Immunohistochemistry analysis of PSMA expression
at prostatic biopsy in high-risk prostate cancer: Potential
implications for PSMA-PET patient selection. Front Oncol.
14:13246312024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mori E, Thomas M, Motoki K, Nakazawa K,
Tahara T, Tomizuka K, Ishida I and Kataoka S: Human normal
hepatocytes are susceptible to apoptosis signal mediated by both
TRAIL-R1 and TRAIL-R2. Cell Death Differ. 11:203–207. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Krah S, Schröter C, Eller C, Rhiel L,
Rasche N, Beck J, Sellmann C, Günther R, Toleikis L, Hock B, et al:
Generation of human bispecific common light chain antibodies by
combining animal immunization and yeast display. Protein Eng Des
Sel. 30:291–301. 2017.PubMed/NCBI
|
|
28
|
Ishida I, Tomizuka K, Yoshida H, Tahara T,
Takahashi N, Ohguma A, Tanaka S, Umehashi M, Maeda H, Nozaki C, et
al: Production of human monoclonal and polyclonal antibodies in
TransChromo animals. Cloning Stem Cells. 4:91–102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Namisaki H, Saito S, Hiraishi K, Haba T,
Tanaka Y, Yoshida H, Iida S and Takahashi N: R409K mutation
prevents acid-induced aggregation of human IgG4. PLoS One.
15:e02290272020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Merchant AM, Zhu Z, Yuan JQ, Goddard A,
Adams CW, Presta LG and Carter P: An efficient route to human
bispecific IgG. Nat Biotechnol. 16:677–681. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hezareh M, Hessell AJ, Jensen RC, van de
Winkel JG and Parren PW: Effector function activities of a panel of
mutants of a broadly neutralizing antibody against human
immunodeficiency virus type 1. J Virol. 75:12161–12168. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Camidge DR: Apomab: An agonist monoclonal
antibody directed against death receptor 5/TRAIL-receptor 2 for use
in the treatment of solid tumors. Expert Opin Biol Ther.
8:1167–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zinonos I, Labrinidis A, Lee M, Liapis V,
Hay S, Ponomarev V, Diamond P, Zannettino AC, Findlay DM and
Evdokiou A: Apomab, a fully human agonistic antibody to DR5,
exhibits potent antitumor activity against primary and metastatic
breast cancer. Mol Cancer Ther. 8:2969–2980. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tateno C, Iwanari H, Shimada T, Kimura T,
Iwasaki Y, Yamasaki C, Kakuni M and Ishida Y: Detection of human
hepatic toxicity in chimeric mice with humanized liver by human
ALT1 ELISA system. The 53rd Annual Meeting of the Society of
Toxicology Phoenix, AZ: 2014
|
|
35
|
Mori E, Thomas M, Motoki K and Kataika S:
Distinct function of monoclonal antibody to TRAIL-R2 as potentiator
or inhibitor of the ligand TRAIL-induced apoptosis. FEBS Lett.
579:5379–5384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saito S, Nakayama M, Yamazaki K, Miyamoto
Y, Hiraishi K, Tomioka D, Takagi-Maeda S, Usami K, Takahashi N,
Nara S and Imai E: Engineering and physicochemical characterization
of a novel, stable, symmetric bispecific antibody with dual
target-binding using a common light chain. Protein Sci.
33:e51212024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cieślewicz M, Andryszak N, Pełka K,
Szczepanek-Parulska E, Ruchała M, Kunikowska J and Czepczyński R:
Evaluation of prostate-specific membrane antigen (PSMA)
immunohistochemical expression in early-stage breast cancer
subtype. Int J Mol Sci. 25:65192024. View Article : Google Scholar
|
|
38
|
Harding JJ, Hofheinz RD, Elez E, Kuboki Y,
Rasco DW, Cecchini M, Shen L, He M, Archuadze S, Chhaya N and Pant
S: A phase Ia/b first-in-human, open-label, multicenter study of BI
905711, a bispecific TRAILR2 agonist, in patients with advanced
gastrointestinal cancers. J Clin Oncol. 41:115. 2023. View Article : Google Scholar
|
|
39
|
Irmler M, Thome M, Hahne M, Schneider P,
Hofmann K, Steiner V, Bodmer JL, Schröter M, Burns K, Mattmann C,
et al: Inhibition of death receptor signals by cellular FLIP.
Nature. 388:190–195. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shankar S, Chen X and Srivastava RK:
Effects of sequential treatments with chemotherapeutic drugs
followed by TRAIL on prostate cancer in vitro and in vivo.
Prostate. 62:165–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nazim UM, Yin H and Park SY: Neferine
treatment enhances the TRAIL-induced apoptosis of human prostate
cancer cells via autophagic flux and the JNK pathway. Int J Oncol.
56:1152–1161. 2020.PubMed/NCBI
|
|
42
|
Koschny R, Ganten TM, Sykora J, Haas TL,
Sprick MR, Kolb A, Stremmel W and Walczak H: TRAIL/bortezomib
cotreatment is potentially hepatotoxic but induces cancer-specific
apoptosis within a therapeutic window. Hepatology. 45:649–658.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang J, Xiu M, Wang J, Gao Y and Li Y:
Proteasome inhibition sensitizes hepatocellular carcinoma cells,
but not human hepatocytes, to TRAIL. Hepatology. 42:588–597. 2005.
View Article : Google Scholar
|
|
44
|
Koschny R, Holland H, Sykora J, Erdal H,
Krupp W, Bauer M, Bockmuehl U, Ahnert P, Meixensberger J, Stremmel
W, et al: Bortezomib sensitizes primary human esthesioneuroblastoma
cells to TRAIL-induced apoptosis. J Neurooncol. 97:171–185. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang FJ, Steeg PS, Price JE, Chiu WT,
Chou PC, Xie K, Sawaya R and Huang S: Molecular basis for the
critical role of suppressor of cytokine signaling-1 in melanoma
brain metastasis. Cancer Res. 68:9634–9642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cristofanon S and Fulda S: ABT-737
promotes tBid mitochondrial accumulation to enhance TRAIL-induced
apoptosis in glioblastoma cells. Cell Death Dis. 3:e4322012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lemke J, von Karstedt S, Zinngrebe J and
Walczak H: Getting TRAIL back on track for cancer therapy. Cell
Death Differ. 21:1350–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Montinaro A, Areso Zubiaur I, Saggau J,
Kretz AL, Ferreira RMM, Hassan O, Kitzig E, Müller I, El-Bahrawy
MA, von Karstedt S, et al: Potent pro-apoptotic combination therapy
is highly effective in a broad range of cancers. Cell Death Differ.
29:492–503. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiuhan S, Xiangyu H and Bao TZ: Role of
mitochondrial reactive oxygen species in chemically-induced
ferroptosis. Free Radic Biol Med. 223:473–492. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Suzuki-Karasaki M, Ochiai T and
Suzuki-Karasaki Y: Crosstalk between mitochondrial ROS and
depolarization in the potentiation of TRAIL-induced apoptosis in
human tumor cells. Int J Oncol. 44:616–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kaur M, Rüger K, Chen EC, Rangaswamy US,
Davison LM, Arteaga SM, Smith I, Chu R, Chattopadhyay S, Rickert M,
et al: Potency-optimized CD28-activating bispecific antibody for
the targeted treatment of Nectin-4 positive cancers. J Immunother
Cancer. 13:e0113232025. View Article : Google Scholar : PubMed/NCBI
|