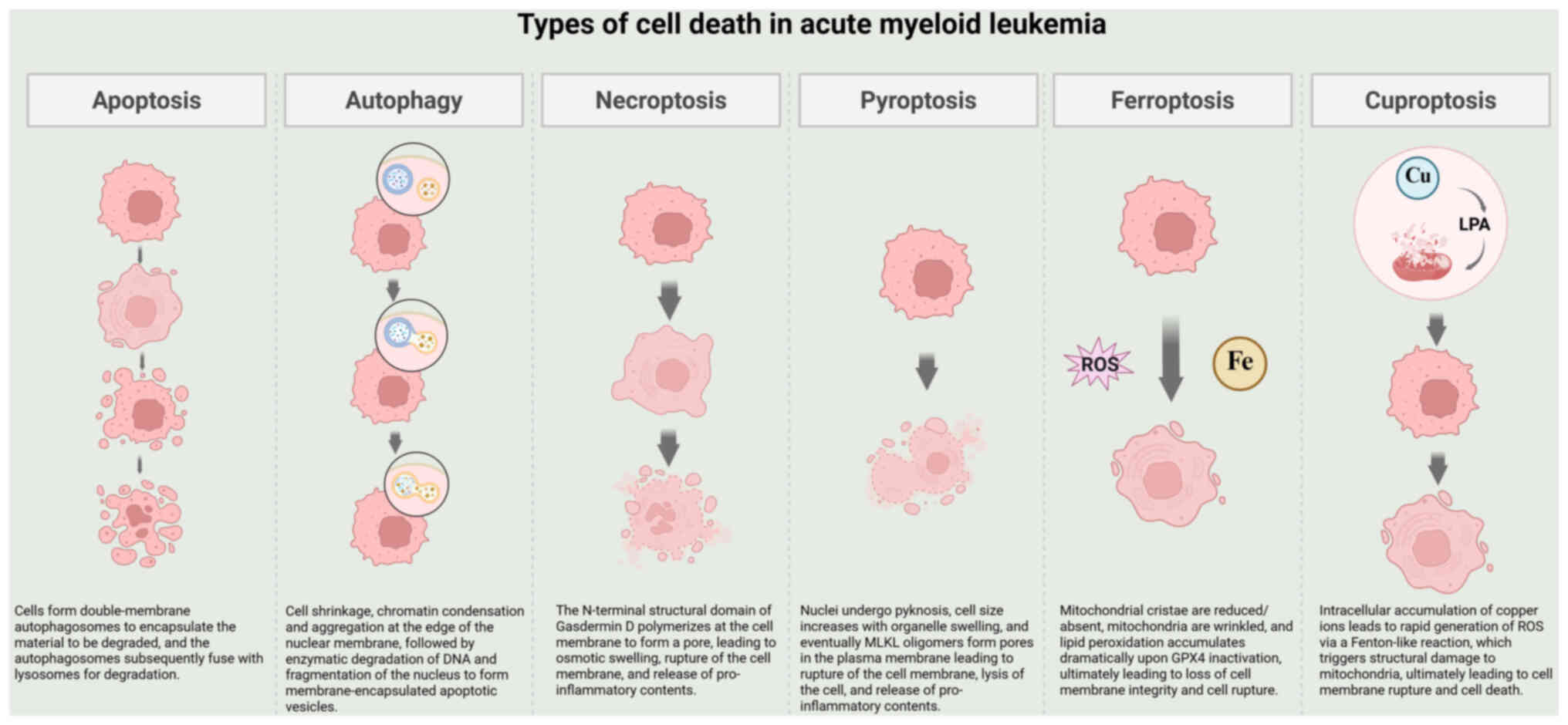
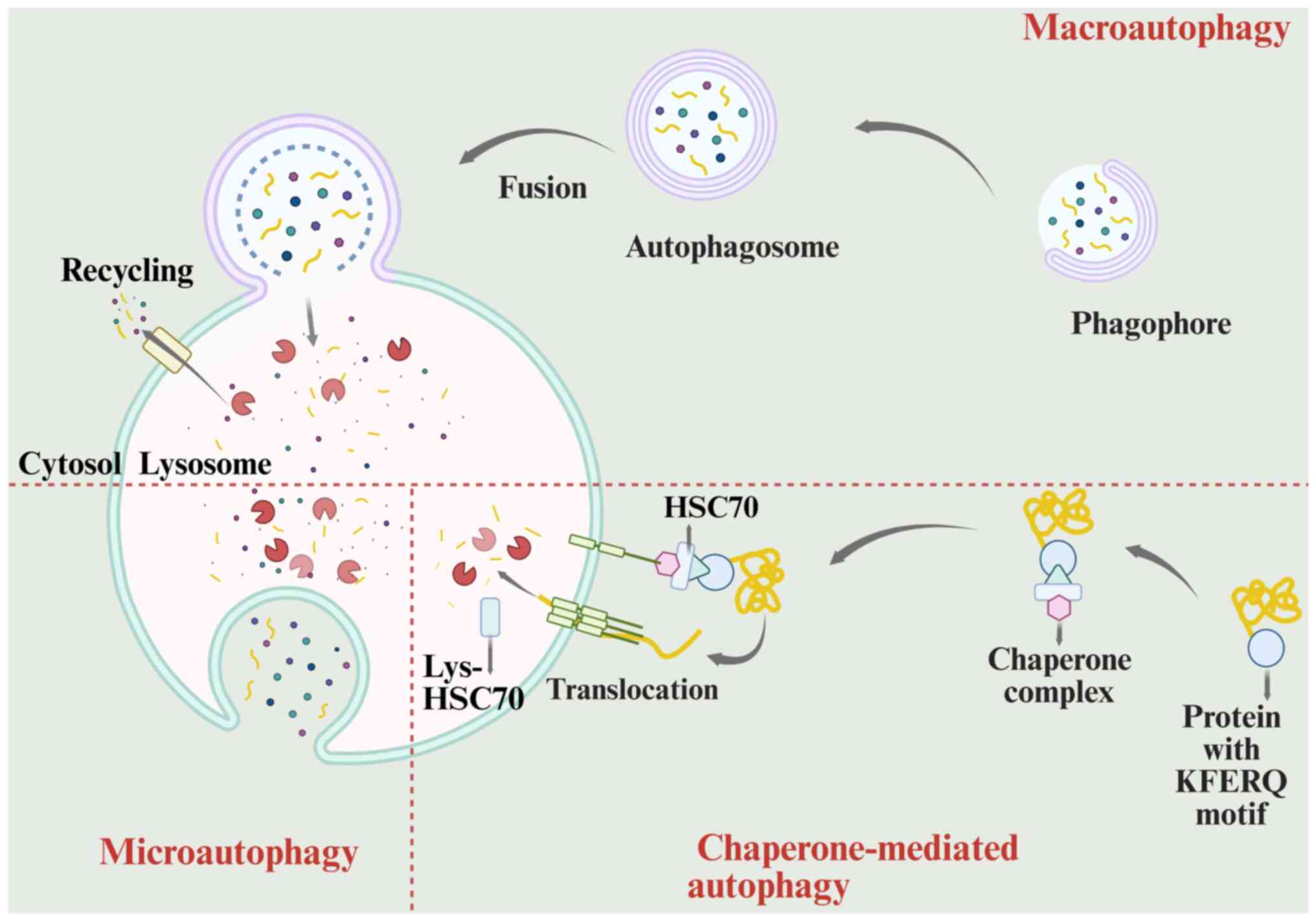
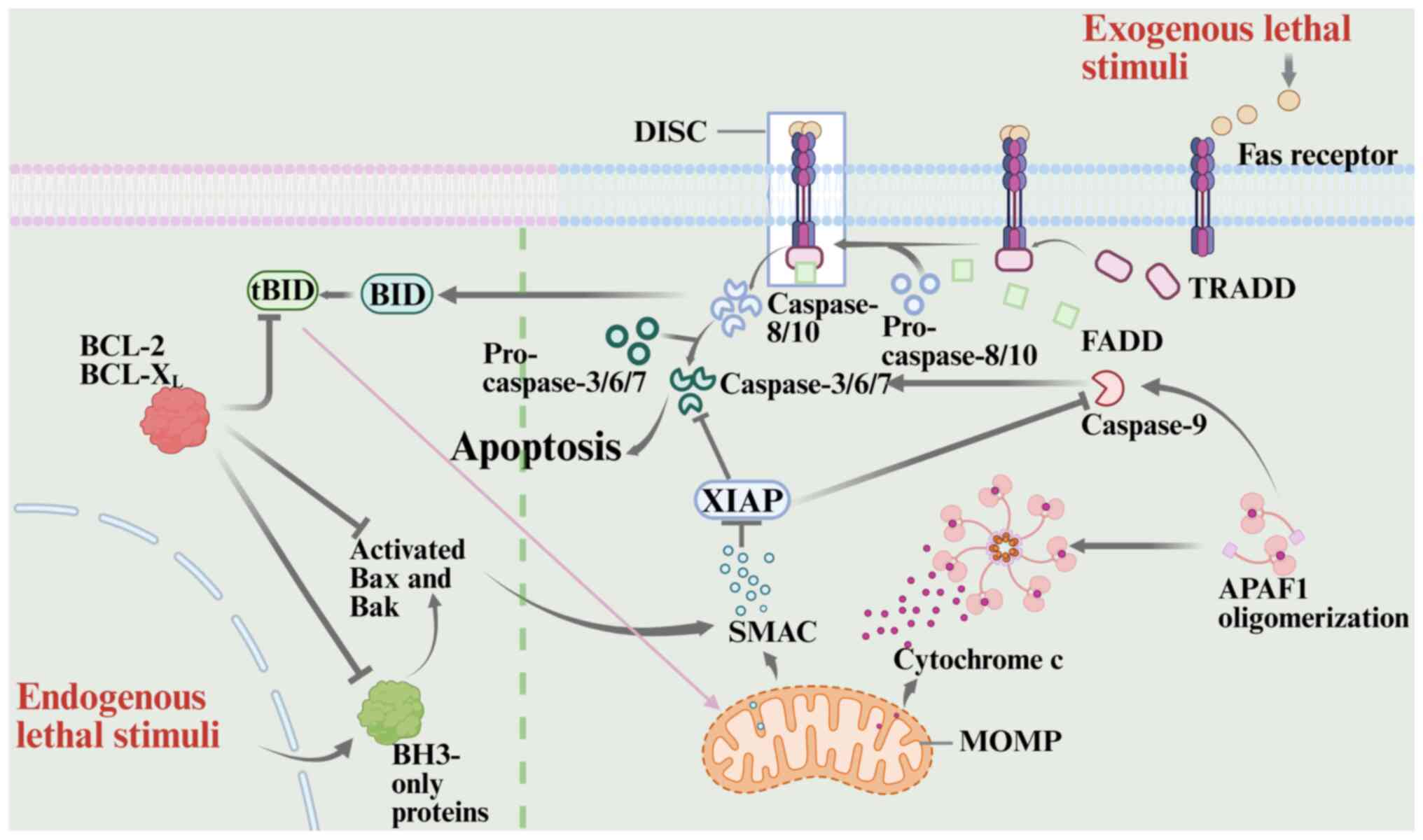
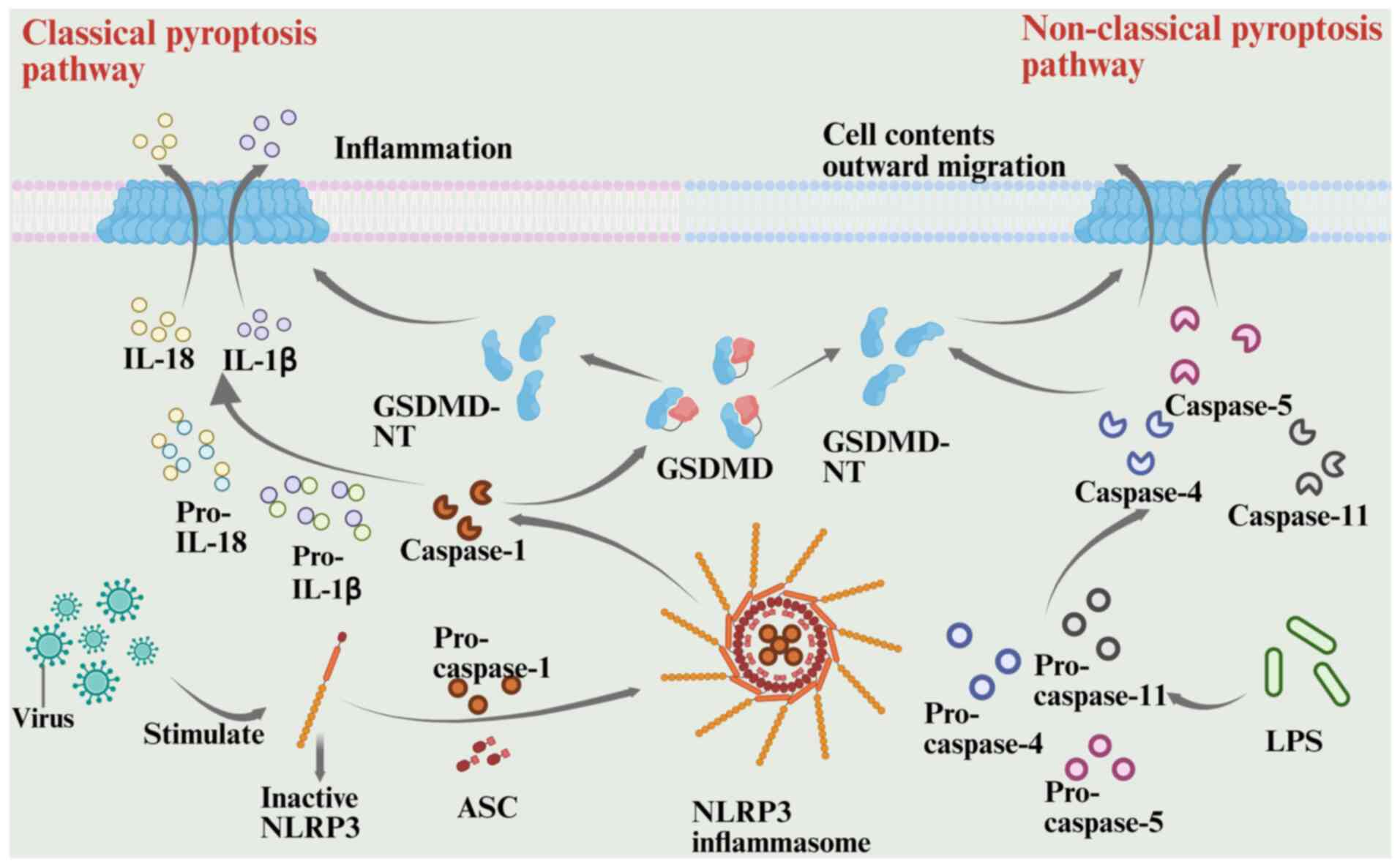
|
1
|
Pollyea DA, Altman JK, Assi R, Bixby D,
Fathi AT, Foran JM, Gojo I, Hall AC, Jonas BA, Kishtagari A, et al:
Acute myeloid leukemia, version 3.2023, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 21:503–513. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moore CG, Stein A, Fathi AT and Pullarkat
V: Treatment of Relapsed/Refractory AML-Novel treatment options
including immunotherapy. Am J Hematol. 100:23–37. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki K, Ravandi F, Kadia TM, DiNardo CD,
Short NJ, Borthakur G, Jabbour E and Kantarjian HM: De novo acute
myeloid leukemia: A population-based study of outcome in the United
States based on the Surveillance, Epidemiology, and End Results
(SEER) database, 1980 to 2017. Cancer. 127:2049–2061. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halik A, Tilgner M, Silva P, Estrada N,
Altwasser R, Jahn E, Heuser M, Hou HA, Pratcorona M, Hills RK, et
al: Genomic characterization of AML with aberrations of chromosome
7: A multinational cohort of 519 patients. J Hematol Oncol.
17:702024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kantarjian H, Borthakur G, Daver N,
DiNardo CD, Issa G, Jabbour E, Kadia T, Sasaki K, Short NJ, Yilmaz
M and Ravandi F: Current status and research directions in acute
myeloid leukemia. Blood Cancer J. 14:1632024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee E, Song CH, Bae SJ, Ha KT and Karki R:
Regulated cell death pathways and their roles in homeostasis,
infection, inflammation, and tumorigenesis. Exp Mol Med.
55:1632–1643. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng J, Zou M, Zhang Q, Liu D, Chen S,
Fang R, Gao Y, Yan X and Hao L: Symphony of regulated cell death:
Unveiling therapeutic horizons in sarcopenia. Metabolism.
172:1563592025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen C, Wang J, Zhang S, Zhu X, Hu J, Liu
C and Liu L: Epigenetic regulation of diverse regulated cell death
modalities in cardiovascular disease: Insights into necroptosis,
pyroptosis, ferroptosis, and cuproptosis. Redox Biol.
76:1033212024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sorokin O, Hause F, Wedler A, Alakhras T,
Bauchspiess T, Dietrich A, Günther WF, Guha C, Obika KB, Kraft J,
et al: Comprehensive analysis of regulated cell death pathways:
Intrinsic disorder, protein-protein interactions, and cross-pathway
communication. Apoptosis. 30:2110–2162. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu
L, Chen Y and Han B: Regulated cell death (RCD) in cancer: Key
pathways and targeted therapies. Signal Transduct Target Ther.
7:2862022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seo W, Silwal P, Song IC and Jo EK: The
dual role of autophagy in acute myeloid leukemia. J Hematol Oncol.
15:512022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren J, Tao Y, Peng M, Xiao Q, Jing Y,
Huang J, Yang J, Lin C, Sun M, Lei L, et al: Targeted activation of
GPER enhances the efficacy of venetoclax by boosting leukemic
pyroptosis and CD8+ T cell immune function in acute myeloid
leukemia. Cell Death Dis. 13:9152022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan FK: RIPK3 Slams the Brake on
Leukemogenesis. Cancer Cell. 30:7–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye F, Zhang W, Fan C, Dong J, Peng M, Deng
W, Zhang H and Yang L: Antileukemic effect of venetoclax and
hypomethylating agents via caspase-3/GSDME-mediated pyroptosis. J
Transl Med. 21:6062023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An Iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu B, Wang S, Li R, Chen K, He L, Deng M,
Kannappan V, Zha J, Dong H and Wang W: Disulfiram/copper
selectively eradicates AML leukemia stem cells in vitro and in vivo
by simultaneous induction of ROS-JNK and inhibition of NF-κB and
Nrf2. Cell Death Dis. 8:e27972017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo Z, Liu Y, Chen D, Sun Y, Li D, Meng Y,
Zhou Q, Zeng F, Deng G and Chen X: Targeting regulated cell death:
Apoptosis, necroptosis, pyroptosis, ferroptosis, and cuproptosis in
anticancer immunity. J Transl Int Med. 13:10–32. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garciaz S, Miller T, Collette Y and Vey N:
Targeting regulated cell death pathways in acute myeloid leukemia.
Cancer Drug Resist. 6:151–168. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He R, Liu Y, Fu W, He X, Liu S, Xiao D and
Tao Y: Mechanisms and Cross-talk of regulated cell death and their
epigenetic modifications in tumor progression. Mol Cancer.
23:2672024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dikic I and Elazar Z: Mechanism and
medical implications of mammalian autophagy. Nat Rev Mol Cell Biol.
19:349–364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaushik S and Cuervo AM: The coming of age
of Chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 19:365–381.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamamoto H and Matsui T: Molecular
mechanisms of macroautophagy, microautophagy, and
Chaperone-mediated autophagy. J Nippon Med Sch. 91:2–9. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niu X, You Q, Hou K, Tian Y, Wei P, Zhu Y,
Gao B, Ashrafizadeh M, Aref AR, Kalbasi A, et al: Autophagy in
cancer development, immune evasion, and drug resistance. Drug
Resist Updat. 78:1011702025. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sankar DS, Kaeser-Pebernard S, Vionnet C,
Favre S, de Oliveira Marchioro L, Pillet B, Zhou J, Stumpe M,
Kovacs WJ, Kressler D, et al: The ULK1 effector BAG2 regulates
autophagy initiation by modulating AMBRA1 localization. Cell Rep.
43:1146892024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xi H, Wang S, Wang B, Hong X, Liu X, Li M,
Shen R and Dong Q: The role of interaction between autophagy and
apoptosis in tumorigenesis (Review). Oncol Reps. 48:2082022.
View Article : Google Scholar
|
|
27
|
Cook ASI, Chen M, Nguyen TN, Cabezudo AC,
Khuu G, Rao S, Garcia SN, Yang M, Iavarone AT, Ren X, et al:
Structural pathway for PI3-kinase regulation by VPS15 in autophagy.
Science. 388:eadl37872025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nascimbeni AC, Codogno P and Morel E:
Local detection of PtdIns3P at autophagosome biogenesis membrane
platforms. Autophagy. 13:1602–1612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wani WY, Boyer-Guittaut M, Dodson M,
Chatham J, Darley-Usmar V and Zhang J: Regulation of autophagy by
protein post-translational modification. Lab Invest. 95:14–25.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang R, Xu Y, Wan W, Shou X, Qian J, You
Z, Liu B, Chang C, Zhou T, Lippincott-Schwartz J and Liu W:
Deacetylation of nuclear LC3 drives autophagy initiation under
starvation. Mol Cell. 57:456–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ichimura Y, Kirisako T, Takao T, Satomi Y,
Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi
M, et al: A ubiquitin-like system mediates protein lipidation.
Nature. 408:488–492. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rogov V, Dötsch V, Johansen T and Kirkin
V: Interactions between autophagy receptors and ubiquitin-like
proteins form the molecular basis for selective autophagy. Mol
Cell. 53:167–178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kimmelman AC and White E: Autophagy and
tumor metabolism. Cell Metab. 25:1037–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santos de Macedo BG, Albuquerque de Melo
M, Pereira-Martins DA, Machado-Neto JA and Traina F: An updated
outlook on autophagy mechanism and how it supports acute myeloid
leukemia maintenance. Biochim Biophys Acta Rev Cancer.
1879:1892142024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nomura N, Ito C, Ooshio T, Tadokoro Y,
Kohno S, Ueno M, Kobayashi M, Kasahara A, Takase Y, Kurayoshi K, et
al: Essential role of autophagy in protecting neonatal
haematopoietic stem cells from oxidative stress in a
p62-independent manner. Sci Rep. 11:16662021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y, Luo X, Zou Z and Liang Y: The role
of reactive oxygen species in tumor treatment and its impact on
bone marrow hematopoiesis. Curr Drug Targets. 21:477–498. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sharma P, Piya S, Ma H, Baran N, Anna Zal
M, Hindley CJ, Dao K, Sims M, Zal T, Ruvolo V, et al: ERK1/2
inhibition overcomes resistance in acute myeloid leukemia (AML) and
alters mitochondrial dynamics. Blood. 138:33382021. View Article : Google Scholar
|
|
38
|
Folkerts H, Wierenga AT, van den Heuvel
FA, Woldhuis RR, Kluit DS, Jaques J, Schuringa JJ and Vellenga E:
Elevated VMP1 expression in acute myeloid leukemia amplifies
autophagy and is protective against venetoclax-induced apoptosis.
Cell Death Dis. 10:4212019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khan A, Singh VK, Thakral D and Gupta R:
Autophagy in acute myeloid leukemia: A paradoxical role in
chemoresistance. Clin Transl Oncol. 24:1459–1469. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heydt Q, Larrue C, Saland E, Bertoli S,
Sarry JE, Besson A, Manenti S, Joffre C and Mansat-De Mas V:
Oncogenic FLT3-ITD supports autophagy via ATF4 in acute myeloid
leukemia. Oncogene. 37:787–797. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Omori I, Yamaguchi H, Miyake K, Miyake N,
Kitano T and Inokuchi K: D816V mutation in the KIT gene activation
loop has greater cell-proliferative and anti-apoptotic ability than
N822K mutation in core-binding factor acute myeloid leukemia. Exp
Hematol. 52:56–64.e54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Larrue C, Heydt Q, Saland E, Boutzen H,
Kaoma T, Sarry JE, Joffre C and Récher C: Oncogenic KIT mutations
induce STAT3-dependent autophagy to support cell proliferation in
acute myeloid leukemia. Oncogenesis. 8:392019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zou Q, Tan S, Yang Z, Zhan Q, Jin H, Xian
J, Zhang S, Yang L, Wang L and Zhang L: NPM1 mutant mediated PML
delocalization and stabilization enhances autophagy and cell
survival in leukemic cells. Theranostics. 7:2289–2304. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Folkerts H, Hilgendorf S, Wierenga ATJ,
Jaques J, Mulder AB, Coffer PJ, Schuringa JJ and Vellenga E:
Inhibition of autophagy as a treatment strategy for p53 wild-type
acute myeloid leukemia. Cell Death Dis. 8:e29272017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang Y, Pu J and Yang Y: Glycolysis and
chemoresistance in acute myeloid leukemia. Heliyon. 10:e357212024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang YH, Israelsen WJ, Lee D, Yu VWC,
Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG and Scadden DT:
Cell-state-specific metabolic dependency in hematopoiesis and
leukemogenesis. Cell. 158:1309–1323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen S, Tao Y, Wang Q, Ren J, Jing Y,
Huang J, Zhang L and Li R: Glucose Induced-AKT/mTOR activation
accelerates glycolysis and promotes cell survival in acute myeloid
leukemia. Leuk Res. 128:1070592023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stevens BM, Jones CL, Pollyea DA,
Culp-Hill R, D'Alessandro A, Winters A, Krug A, Abbott D, Goosman
M, Pei S, et al: Fatty acid metabolism underlies venetoclax
resistance in acute myeloid leukemia stem cells. Nat Cancer.
1:1176–1187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Carter JL, Su Y, Qiao X, Zhao J, Wang G,
Howard M, Edwards H, Bao X, Li J, Hüttemann M, et al: Acquired
resistance to venetoclax plus azacitidine in acute myeloid
leukemia: In vitro models and mechanisms. Biochem Pharmacol.
216:1157592023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang HB, Sun ZK, Zhong FM, Yao FY, Liu J,
Zhang J, Zhang N, Lin J, Li SQ, Li MY, et al: A novel fatty acid
metabolism-related signature identifies features of the tumor
microenvironment and predicts clinical outcome in acute myeloid
leukemia. Lipids Health Dis. 21:792022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tcheng M, Roma A, Ahmed N, Smith RW,
Jayanth P, Minden MD, Schimmer AD, Hess DA, Hope K, Rea KA, et al:
Very long chain fatty acid metabolism is required in acute myeloid
leukemia. Blood. 137:3518–3532. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhao Y, Guo H, Niu L and Zhao J: Metabolic
pathways and chemotherapy resistance in acute myeloid leukemia
(AML): Insights into Enoyl-CoA hydratase domain-containing protein
3 (ECHDC3) as a potential therapeutic target. Cancer Pathogenesis
and Therapy; 2025, View Article : Google Scholar
|
|
53
|
Du W, Xu A, Huang Y, Cao J, Zhu H, Yang B,
Shao X, He Q and Ying M: The role of autophagy in targeted therapy
for acute myeloid leukemia. Autophagy. 17:2665–2679. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li X, Qian Q, Li J, Zhang L, Wang L, Huang
D, Xu Q and Chen W: The RNA-binding protein CELF1 targets ATG5 to
regulate autophagy and promote drug resistance in acute myeloid
leukemia. Cell Death Dis. 16:5992025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Herschbein L and Liesveld JL: Dueling for
dual inhibition: Means to enhance effectiveness of PI3K/Akt/mTOR
inhibitors in AML. Blood Rev. 32:235–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Koschade SE, Klann K, Shaid S, Vick B,
Stratmann JA, Thölken M, Meyer LM, Nguyen TD, Campe J, Moser LM, et
al: Translatome proteomics identifies autophagy as a resistance
mechanism to on-target FLT3 inhibitors in acute myeloid leukemia.
Leukemia. 36:2396–2407. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cao Y, Wang Y, Chen Y, Zhang X, Zuo Y, Ge
X, Sun C, Ren B, Liu Y, Wang M and Lu J: Voacamine initiates the
PI3K/mTOR/Beclin1 pathway to induce autophagy and potentiate
apoptosis in acute myeloid leukemia. Phytomedicine. 143:1568592025.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nössing C and Ryan KM: 50 years on and
still very much alive: ‘Apoptosis: A basic biological phenomenon
with wide-ranging implications in tissue kinetics’. Br J Cancer.
128:426–431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yoon JH and Gores GJ: Death
receptor-mediated apoptosis and the liver. J Hepatol. 37:400–410.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
O'Neill KL, Huang K, Zhang J, Chen Y and
Luo X: Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak
through the outer mitochondrial membrane. Genes Dev. 30:973–988.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xiong S, Mu T, Wang G and Jiang X:
Mitochondria-mediated apoptosis in mammals. Protein Cell.
5:737–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Croce CM, Vaux D, Strasser A, Opferman JT,
Czabotar PE and Fesik SW: The BCL-2 protein family: from discovery
to drug development. Cell Death Differ. 32:1369–1381. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Reyna DE, Garner TP, Lopez A, Kopp F,
Choudhary GS, Sridharan A, Narayanagari SR, Mitchell K, Dong B,
Bartholdy BA, et al: Direct activation of BAX by BTSA1 overcomes
apoptosis resistance in acute myeloid leukemia. Cancer Cell.
32:490–505.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Singh G, Guibao CD, Seetharaman J,
Aggarwal A, Grace CR, McNamara DE, Vaithiyalingam S, Waddell MB and
Moldoveanu T: Structural basis of BAK activation in mitochondrial
apoptosis initiation. Nat Commun. 13:2502022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cain K, Bratton SB and Cohen GM: The
Apaf-1 apoptosome: A large caspase-activating complex. Biochimie.
84:203–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kashyap D, Garg VK and Goel N: Intrinsic
and extrinsic pathways of apoptosis: Role in cancer development and
prognosis. Adv Protein Chem Struct Biol. 125:73–120. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zheng L, Yao Y and Lenardo MJ: Death
receptor 5 rises to the occasion. Cell Res. 33:199–200. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Helmke C, Raab M, Rödel F, Matthess Y,
Oellerich T, Mandal R, Sanhaji M, Urlaub H, Rödel C, Becker S and
Strebhardt K: Ligand stimulation of CD95 induces activation of Plk3
followed by phosphorylation of caspase-8. Cell Res. 26:914–934.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang K, Zhang J, O'Neill KL, Gurumurthy
CB, Quadros RM, Tu Y and Luo X: Cleavage by Caspase 8 and
mitochondrial membrane association activate the BH3-only protein
bid during TRAIL-induced apoptosis. J Biol Chem. 291:11843–11851.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kantari C and Walczak H: Caspase-8 and
bid: Caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kim WS, Lee KS, Kim JH, Kim CK, Lee G,
Choe J, Won MH, Kim TH, Jeoung D, Lee H, et al: The
caspase-8/Bid/cytochrome c axis links signals from death receptors
to mitochondrial reactive oxygen species production. Free Radic
Biol Med. 112:567–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Moyer A, Tanaka K and Cheng EH: Apoptosis
in cancer biology and therapy. Annu Rev Pathol. 20:303–328. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pfeffer CM and Singh ATK: Apoptosis: A
target for anticancer therapy. Int J Mol Sci. 19:4482018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Campbell KJ and Tait SWG: Targeting BCL-2
regulated apoptosis in cancer. Open Biol. 8:1800022018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ashkenazi A, Fairbrother WJ, Leverson JD
and Souers AJ: From basic apoptosis discoveries to advanced
selective BCL-2 family inhibitors. Nat Rev Drug Discov. 16:273–284.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Konopleva M, Pollyea DA, Potluri J, Chyla
B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, et
al: Efficacy and biological correlates of response in a phase II
study of venetoclax monotherapy in patients with acute myelogenous
leukemia. Cancer Discov. 6:1106–1117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wei AH, Strickland SA Jr, Hou JZ, Fiedler
W, Lin TL, Walter RB, Enjeti A, Tiong IS, Savona M, Lee S, et al:
Venetoclax combined with Low-dose cytarabine for previously
untreated patients with acute myeloid leukemia: Results from a
Phase Ib/II study. J Clin Oncol. 37:1277–1284. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
DiNardo CD, Jonas BA, Pullarkat V, Thirman
MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et
al: Azacitidine and venetoclax in previously untreated acute
myeloid leukemia. N Engl J Med. 383:617–629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jiao CQ, Hu C, Sun MH, Li Y, Wu C, Xu F,
Zhang L, Huang FR, Zhou JJ, Dai JF, et al: Targeting METTL3
mitigates venetoclax resistance via proteasome-mediated modulation
of MCL1 in acute myeloid leukemia. Cell Death Dis. 16:2332025.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chai J, Du C, Wu JW, Kyin S, Wang X and
Shi Y: Structural and biochemical basis of apoptotic activation by
Smac/DIABLO. Nature. 406:855–862. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Carter BZ, Mak PY, Mak DH, Shi Y, Qiu Y,
Bogenberger JM, Mu H, Tibes R, Yao H, Coombes KR, et al:
Synergistic targeting of AML stem/progenitor cells with IAP
antagonist birinapant and demethylating agents. J Natl Cancer Inst.
106:djt4402014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Gencel-Augusto J and Lozano G: Targeted
degradation of mutant p53 reverses the Pro-oncogenic
Dominant-negative effect. Cancer Res. 85:1955–1956. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Tuval A, Strandgren C, Heldin A,
Palomar-Siles M and Wiman KG: Pharmacological reactivation of p53
in the era of precision anticancer medicine. Nat Rev Clin Oncol.
21:106–120. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Napolitano R, De Matteis S, Carloni S,
Bruno S, Abbati G, Capelli L, Ghetti M, Bochicchio MT, Liverani C,
Mercatali L, et al: Kevetrin induces apoptosis in TP53 wild-type
and mutant acute myeloid leukemia cells. Oncol Rep. 44:1561–1573.
2020.PubMed/NCBI
|
|
87
|
Fulda S: Targeting apoptosis for
anticancer therapy. Semin Cancer Biol. 31:84–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chow LQ, Eckhardt SG, Gustafson DL, Langer
CJ, Camidge DR, Padavic K, Gore L, Smith M, Chow LQ, von Mehren M,
et al: HGS-ETR1, an antibody targeting TRAIL-R1, in combination
with paclitaxel and carboplatin in patients with advanced solid
malignancies: Results of a phase 1 and PK study. J Clin Oncol.
24:25152006. View Article : Google Scholar
|
|
89
|
You R, Hou D, Wang B, Liu J, Wang X, Xiao
Q, Pan Z, Li D, Feng X, Kang L, et al: Bone marrow microenvironment
drives AML cell OXPHOS addiction and AMPK inhibition to resist
chemotherapy. J Leukoc Biol. 112:299–311. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cheung HL, Wong YH, Li YY, Yang X, Ko LH,
Tan Kabigting JE, Chan KC, Leung AYH and Chan BP: Microenvironment
matters: In vitro 3D bone marrow niches differentially modulate
survival, phenotype and drug responses of acute myeloid leukemia
(AML) cells. Biomaterials. 312:1227192025. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zou J, Zheng Y, Huang Y, Tang D, Kang R
and Chen R: The versatile gasdermin family: Their function and
roles in diseases. Front Immunol. 12:7515332021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Broz P, Pelegrín P and Shao F: The
gasdermins, a protein family executing cell death and inflammation.
Nat Rev Immunol. 20:143–157. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tamura M, Tanaka S, Fujii T, Aoki A,
Komiyama H, Ezawa K, Sumiyama K, Sagai T and Shiroishi T: Members
of a novel gene family, Gsdm, are expressed exclusively in the
epithelium of the skin and gastrointestinal tract in a highly
Tissue-specific manner. Genomics. 89:618–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang S, Yuan YH, Chen NH and Wang HB: The
mechanisms of NLRP3 inflammasome/pyroptosis activation and their
role in Parkinson's disease. Int Immunopharmacol. 67:458–464. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Miao EA, Rajan JV and Aderem A:
Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–214.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yu P, Zhang X, Liu N, Tang L, Peng C and
Chen X: Pyroptosis: Mechanisms and diseases. Signal Transduct
Target Ther. 6:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ding J, Wang K, Liu W, She Y, Sun Q, Shi
J, Sun H, Wang DC and Shao F: Pore-forming activity and structural
autoinhibition of the gasdermin family. Nature. 535:111–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Garlanda C, Dinarello CA and Mantovani A:
The interleukin-1 family: Back to the future. Immunity.
39:1003–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, Qiao
C, Liu W, Deng H, Li J, Ning P and Wang Z: Pyroptosis in
inflammatory diseases and cancer. Theranostics. 12:4310–4329. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yang J, Zhao Y and Shao F: Non-canonical
activation of inflammatory caspases by cytosolic LPS in innate
immunity. Curr Opin Immunol. 32:78–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li
P, Hu L and Shao F: Inflammatory caspases are innate immune
receptors for intracellular LPS. Nature. 514:187–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Hagar JA, Powell DA, Aachoui Y, Ernst RK
and Miao EA: Cytoplasmic LPS activates caspase-11: implications in
TLR4-independent endotoxic shock. Science. 341:1250–1253. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu Y, Pan R, Ouyang Y, Gu W, Xiao T, Yang
H, Tang L, Wang H, Xiang B and Chen P: Pyroptosis in health and
disease: Mechanisms, regulation and clinical perspective. Signal
Transduct Target Ther. 9:2452024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kayagaki N, Warming S, Lamkanfi M, Vande
Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, et al:
Non-canonical inflammasome activation targets caspase-11. Nature.
479:117–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Shi X, Sun Q, Hou Y, Zeng H, Cao Y, Dong
M, Ding J and Shao F: Recognition and maturation of IL-18 by
caspase-4 noncanonical inflammasome. Nature. 624:442–450. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Oltra SS, Colomo S, Sin L, Pérez-López M,
Lázaro S, Molina-Crespo A, Choi KH, Ros-Pardo D, Martínez L,
Morales S, et al: Distinct GSDMB protein isoforms and protease
cleavage processes differentially control pyroptotic cell death and
mitochondrial damage in cancer cells. Cell Death Differ.
30:1366–1381. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang S, Chang CW, Huang J, Zeng S, Zhang
X, Hung MC and Hou J: Gasdermin C sensitizes tumor cells to PARP
inhibitor therapy in cancer models. J Clin Invest. 134:e1668412024.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Alves-Hanna FS, Crespo-Neto JA, Nogueira
GM, Pereira DS, Lima AB, Ribeiro TLP, Santos VGR, Fonseca JRF,
Magalhães-Gama F, Sadahiro A and Costa AG: Insights regarding the
role of inflammasomes in leukemia: What Do We Know? J Immunol Res.
2023:55844922023. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Johnson DC, Taabazuing CY, Okondo MC, Chui
AJ, Rao SD, Brown FC, Reed C, Peguero E, de Stanchina E, Kentsis A
and Bachovchin DA: DPP8/DPP9 inhibitor-induced pyroptosis for
treatment of acute myeloid leukemia. Nat Med. 24:1151–1156. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Deng S, Pan Y, An N, Chen F, Chen H, Wang
H, Xu X, Liu R, Yang L, Wang X, et al: Downregulation of RCN1
promotes pyroptosis in acute myeloid leukemia cells. Mol Oncol.
17:2584–2602. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Askmyr M, Ågerstam H, Hansen N, Gordon S,
Arvanitakis A, Rissler M, Juliusson G, Richter J, Järås M and
Fioretos T: Selective killing of candidate AML stem cells by
antibody targeting of IL1RAP. Blood. 121:3709–3713. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Ågerstam H, Karlsson C, Hansen N, Sandén
C, Askmyr M, von Palffy S, Högberg C, Rissler M, Wunderlich M,
Juliusson G, et al: Antibodies targeting human IL1RAP (IL1R3) show
therapeutic effects in xenograft models of acute myeloid leukemia.
Proc Natl Acad Sci USA. 112:10786–10791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang Y, Sun X, Yuan S, Hou S, Guo T, Chu
Y, Pang T, Luo HR, Yuan W and Wang X: Interleukin-1β inhibits
normal hematopoietic expansion and promotes acute myeloid leukemia
progression via the bone marrow niche. Cytotherapy. 22:127–134.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wong J, Tran LT, Magun EA, Magun BE and
Wood LJ: Production of IL-1β by bone marrow-derived macrophages in
response to chemotherapeutic drugs: Synergistic effects of
doxorubicin and vincristine. Cancer Biol Ther. 15:1395–1403. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yan J, Wan P, Choksi S and Liu ZG:
Necroptosis and tumor progression. Trends Cancer. 8:21–27. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu X, Miao M, Sun J, Wu J and Qin X:
PANoptosis: A potential new target for programmed cell death in
breast cancer treatment and prognosis. Apoptosis. 29:277–288. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
He S, Wang L, Miao L, Wang T, Du F, Zhao L
and Wang X: Receptor interacting protein kinase-3 determines
cellular necrotic response to TNF-alpha. Cell. 137:1100–1111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Holler N, Zaru R, Micheau O, Thome M,
Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B and Tschopp
J: Fas triggers an alternative, caspase-8-independent cell death
pathway using the kinase RIP as effector molecule. Nat Immunol.
1:489–495. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mandal R, Barrón JC, Kostova I, Becker S
and Strebhardt K: Caspase-8: The double-edged sword. Biochim
Biophys Acta Rev Cancer. 1873:1883572020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kaiser WJ, Sridharan H, Huang C, Mandal P,
Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J and Mocarski ES:
Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J
Biol Chem. 288:31268–31279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Upton JW, Kaiser WJ and Mocarski ES:
DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced
programmed necrosis that is targeted by murine cytomegalovirus
vIRA. Cell Host Microbe. 11:290–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Pasparakis M and Vandenabeele P:
Necroptosis and its role in inflammation. Nature. 517:311–320.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Linkermann A and Green DR: Necroptosis. N
Engl J Med. 370:455–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Murphy JM, Czabotar PE, Hildebrand JM,
Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D,
Webb AI, et al: The pseudokinase MLKL mediates necroptosis via a
molecular switch mechanism. Immunity. 39:443–453. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gong YN, Guy C, Olauson H, Becker JU, Yang
M, Fitzgerald P, Linkermann A and Green DR: ESCRT-III acts
downstream of MLKL to regulate necroptotic cell death and its
consequences. Cell. 169:286–300.e16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Cai Z, Jitkaew S, Zhao J, Chiang HC,
Choksi S, Liu J, Ward Y, Wu LG and Liu ZG: Plasma membrane
translocation of trimerized MLKL protein is required for
TNF-induced necroptosis. Nat Cell Biol. 16:55–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Khan N, Downey J, Sanz J, Kaufmann E,
Blankenhaus B, Pacis A, Pernet E, Ahmed E, Cardoso S, Nijnik A, et
al: M. tuberculosis reprograms hematopoietic stem cells to limit
myelopoiesis and impair trained immunity. Cell. 183:752–770.e22.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan
K, Cheng H, Jin K, Ni Q, Yu X and Liu C: The role of necroptosis in
cancer biology and therapy. Mol Cancer. 18:1002019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhang T, Wang Y, Inuzuka H and Wei W:
Necroptosis pathways in tumorigenesis. Semin Cancer Biol. 86:32–40.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Höckendorf U, Yabal M, Herold T,
Munkhbaatar E, Rott S, Jilg S, Kauschinger J, Magnani G, Reisinger
F, Heuser M, et al: RIPK3 restricts myeloid leukemogenesis by
promoting cell death and differentiation of leukemia initiating
cells. Cancer Cell. 30:75–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Wang Y, Zhang TJ, Zhang LC, Xu ZJ, Chu MQ,
Zhao YJ, Lin J, Qian J and Zhou JD: Overexpression and oncogenic
role of RIPK3 in acute myeloid leukemia associated with specific
subtypes and treatment outcome. BMC Cancer. 25:2532025. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Xin J, You D, Breslin P, Li J, Zhang J,
Wei W, Cannova J, Volk A, Gutierrez R, Xiao Y, et al: Sensitizing
acute myeloid leukemia cells to induced differentiation by
inhibiting the RIP1/RIP3 pathway. Leukemia. 31:1154–1165. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhu S, Luo Y, Li K, Mei C, Wang Y, Jiang
L, Wang W, Zhang Q, Yang W, Lang W, et al: RIPK3 deficiency blocks
R-2-hydroxyglutarate-induced necroptosis in IDH-mutated AML cells.
Sci Adv. 10:eadi17822024. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hillert LK, Bettermann-Bethge K,
Nimmagadda SC, Fischer T, Naumann M and Lavrik IN: Targeting RIPK1
in AML cells carrying FLT3-ITD. Int J Cancer. 145:1558–1569. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li J, Liao D, Wang F, Wang Z, Li Y, Xiong
Y and Niu T: RIPK1 inhibition enhances the therapeutic efficacy of
chidamide in FLT3-ITD positive AML, both in vitro and in vivo. Leuk
Lymphoma. 63:1167–1179. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Yue X, Chen Q and He J: Combination
strategies to overcome resistance to the BCL2 inhibitor venetoclax
in hematologic malignancies. Leuk Lymphoma. 20:5242020.
|
|
140
|
Culver-Cochran AE, Hassan A, Hueneman K,
Choi K, Ma A, VanCauwenbergh B, O'Brien E, Wunderlich M, Perentesis
JP and Starczynowski DT: Chemotherapy resistance in acute myeloid
leukemia is mediated by A20 suppression of spontaneous necroptosis.
Nat Commun. 15:91892024. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Liang D, Minikes AM and Jiang X:
Ferroptosis at the intersection of lipid metabolism and cellular
signaling. Mol Cell. 82:2215–2227. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Chen F, Kang R, Tang D and Liu J:
Ferroptosis: principles and significance in health and disease. J
Hematol Oncol. 17:412024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Koleini N, Shapiro JS, Geier J and
Ardehali H: Ironing out mechanisms of iron homeostasis and
disorders of iron deficiency. J Clin Invest. 131:e1486712021.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Co HKC, Wu CC, Lee YC and Chen SH:
Emergence of large-scale cell death through ferroptotic trigger
waves. Nature. 631:654–662. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF and Clish CB: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Reyhani A, McKenzie TG, Fu Q and Qiao GG:
Fenton-Chemistry-Mediated Radical Polymerization. Macromol Rapid
Commun. 40:19002202019. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Dai E, Chen X, Linkermann A, Jiang X, Kang
R, Kagan VE, Bayir H, Yang WS, Garcia-Saez AJ, Ioannou MS, et al: A
guideline on the molecular ecosystem regulating ferroptosis. Nat
Cell Biol. 26:1447–1457. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Ma S, Henson ES, Chen Y and Gibson SB:
Ferroptosis is induced following siramesine and lapatinib treatment
of breast cancer cells. Cell Death Dis. 7:e23072016. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Bannai S: Exchange of cystine and
glutamate across plasma membrane of human fibroblasts. J Biol Chem.
261:2256–2263. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Koppula P, Zhang Y, Zhuang L and Gan B:
Amino acid transporter SLC7A11/xCT at the crossroads of regulating
redox homeostasis and nutrient dependency of cancer. Cancer Commun
(Lond). 38:122018.PubMed/NCBI
|
|
155
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Liu MR, Zhu WT and Pei DS: System Xc-: A
key regulatory target of ferroptosis in cancer. Invest New Drugs.
39:1123–1131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Tang D, Chen X, Kang R and Kroemer G:
Ferroptosis: Molecular mechanisms and health implications. Cell
Res. 31:107–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Scarpellini C, Klejborowska G, Lanthier C,
Hassannia B, Vanden Berghe T and Augustyns K: Beyond ferrostatin-1:
A comprehensive review of ferroptosis inhibitors. Trends Pharmacol
Sci. 44:902–916. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Brown CW, Amante JJ, Chhoy P, Elaimy AL,
Liu H, Zhu LJ, Baer CE, Dixon SJ and Mercurio AM: Prominin2 drives
ferroptosis resistance by stimulating iron export. Dev Cell.
51:575–586.e4. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Cao M, Li H, Sun D, He S, Yu Y, Li J, Chen
H, Shi J, Ren J, Li N and Chen W: Cancer screening in China: The
current status, challenges, and suggestions. Cancer Lett.
506:120–127. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhang H and Sun C, Sun Q, Li Y, Zhou C and
Sun C: Susceptibility of acute myeloid leukemia cells to
ferroptosis and evasion strategies. Front Mol Biosci.
10:12757742023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Akiyama H, Zhao R, Ostermann LB, Li Z,
Tcheng M, Yazdani SJ, Moayed A, Pryor ML II, Slngh S, Baran N, et
al: Mitochondrial regulation of GPX4 inhibition-mediated
ferroptosis in acute myeloid leukemia. Leukemia. 38:729–740. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Na X, Li L, Liu D, He J, Zhang L and Zhou
Y: Natural products targeting ferroptosis pathways in cancer
therapy (Review). Oncol Rep. 52:1232024. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Ma C, Wang J, Cui S and Xu R: Crotonoside
induces ferroptosis and mitochondrial dysfunction in AML. Eur J
Pharmacol. 1002:1778592025. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Birsen R, Larrue C, Decroocq J, Johnson N,
Guiraud N, Gotanegre M, Cantero-Aguilar L, Grignano E, Huynh T,
Fontenay M, et al: APR-246 induces early cell death by ferroptosis
in acute myeloid leukemia. Haematologica. 107:403–416. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lin AY, Rink JS, Yang E, Small S, Gerber
JJ, Zak TJ, Altman J, Abaza Y, Platanias LC, Gordon LI and Thaxton
CS: Targeting scavenger receptor class B type 1 with a bioinspired
ligand induces apoptosis or ferroptosis in AML. Blood Neoplasia.
2:1001222025. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Jiang F, Yu WJ, Wang XH, Tang YT, Guo L
and Jiao XY: Regulation of hepcidin through GDF-15 in
cancer-related anemia. Clin Chim Acta. 428:14–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Lu QW and Liao Y: GDF-15 upregulates the
SLC7A11/GPX4 signaling axis and promotes mitoxantrone resistance in
AML cells. Eur J Med Res. 30:5042025. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Yu Y, Meng Y, Xu X, Tong T, He C, Wang L,
Wang K, Zhao M, You X, Zhang W, et al: A Ferroptosis-inducing and
leukemic Cell-targeting drug nanocarrier formed by Redox-responsive
cysteine polymer for acute myeloid leukemia therapy. ACS Nano.
17:3334–3345. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Yu X, Wang Y, Tan J, Li Y, Yang P, Liu X,
Lai J, Zhang Y, Cai L, Gu Y, et al: Inhibition of NRF2 enhances the
acute myeloid leukemia cell death induced by venetoclax via the
ferroptosis pathway. Cell Death Discov. 10:352024. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Pardieu B, Pasanisi J, Ling F, Dal Bello
R, Penneroux J, Su A, Joudinaud R, Chat L, Wu HC, Duchmann M, et
al: Cystine uptake inhibition potentiates front-line therapies in
acute myeloid leukemia. Leukemia. 36:1585–1595. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Lyu T, Li X and Song Y: Ferroptosis in
acute leukemia. Chin Med J (Engl). 136:886–898. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Target Ther. 7:3782022. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Tian Z, Jiang S, Zhou J and Zhang W:
Copper homeostasis and cuproptosis in mitochondria. Life Sci.
334:1222232023. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Tsvetkov P, Detappe A, Cai K, Keys HR,
Brune Z, Ying W, Thiru P, Reidy M, Kugener G, Rossen J, et al:
Mitochondrial metabolism promotes adaptation to proteotoxic stress.
Nat Chem Biol. 15:681–689. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Zheng P, Zhou C, Lu L, Liu B and Ding Y:
Elesclomol: A copper ionophore targeting mitochondrial metabolism
for cancer therapy. J Exp Clin Cancer Res. 41:2712022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Vo TTT, Peng TY, Nguyen TH, Bui TNH, Wang
CS, Lee WJ, Chen YL, Wu YC and Lee IT: The crosstalk between
copper-induced oxidative stress and cuproptosis: A novel potential
anticancer paradigm. Cell Commun Signal. 22:3532024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zeng Y, Cao Y, Ren S, Zhang C, Liu J, Liu
K, Wang Y, Chen H, Zhou F, Yang X, et al: Responsive ROS-Augmented
prodrug hybridization nanoassemblies for multidimensionally
synergitic treatment of hepatocellular carcinoma in cascade
assaults. Adv Sci (Weinh). 12:e25014202025. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Cong Y, Li N, Zhang Z, Shang Y and Zhao H:
Cuproptosis: Molecular mechanisms, cancer prognosis, and
therapeutic applications. J Transl Med. 23:1042025. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Feng Y, Yang Z, Wang J and Zhao H:
Cuproptosis: Unveiling a new frontier in cancer biology and
therapeutics. Cell Commun Signal. 22:2492024. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Kahlson MA and Dixon SJ: Copper-induced
cell death. Science. 375:1231–1232. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Wang Y, Zhang L and Zhou F: Cuproptosis: A
new form of programmed cell death. Cell Mol Immunol. 19:867–868.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Dreishpoon MB, Bick NR, Petrova B, Warui
DM, Cameron A, Booker SJ, Kanarek N, Golub TR and Tsvetkov P: FDX1
regulates cellular protein lipoylation through direct binding to
LIAS. J Biol Chem. 299:1050462023. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Sun Z, Xu H, Lu G, Yang C, Gao X, Zhang J,
Liu X, Chen Y, Wang K, Guo J and Li J: AKT1 Phosphorylates FDX1 to
promote cuproptosis resistance in Triple-negative breast cancer.
Adv Sci (Weinh). 12:e24081062025. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Yip NC, Fombon IS, Liu P, Brown S,
Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL and Wang W:
Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast
cancer cells with cancer stem cell-like properties. Br J Cancer.
104:1564–1574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Valadbeigi S, Javadian S, Ebrahimi-Rad M,
Khatami S and Saghiri R: Assessment of trace elements in serum of
acute lymphoblastic and myeloid leukemia patients. Exp Oncol.
41:69–71. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Singh RP, Jeyaraju DV, Voisin V, Hurren R,
Xu C, Hawley JR, Barghout SH, Khan DH, Gronda M, Wang X, et al:
Disrupting mitochondrial copper distribution inhibits leukemic stem
cell Self-renewal. Cell Stem Cell. 26:926–937.e10. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
McMahon A, Chen W and Li F: Old wine in
new bottles: Advanced drug delivery systems for disulfiram-based
cancer therapy. J Control Release. 319:352–359. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Weiser Drozdkova D and Smesny Trtkova K:
Possible therapeutic potential of disulfiram for multiple myeloma.
Curr Oncol. 28:2087–2096. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Solak K, Mavi A and Yılmaz B:
Disulfiram-loaded functionalized magnetic nanoparticles combined
with copper and sodium nitroprusside in breast cancer cells. Mater
Sci Eng C Mater Biol Appl. 119:1114522021. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Solier S, Müller S, Cañeque T, Versini A,
Mansart A, Sindikubwabo F, Baron L, Emam L, Gestraud P, Pantoș GD,
et al: A druggable copper-signalling pathway that drives
inflammation. Nature. 617:386–394. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Karsa M, Xiao L, Ronca E, Bongers A,
Spurling D, Karsa A, Cantilena S, Mariana A, Failes TW, Arndt GM,
et al: FDA-approved disulfiram as a novel treatment for aggressive
leukemia. J Mol Med (Berl). 102:507–519. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Oliveri V: Selective targeting of cancer
cells by copper ionophores: An overview. Front Mol Biosci.
9:8418142022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Wang J, Luo C, Shan C, You Q, Lu J, Elf S,
Zhou Y, Wen Y, Vinkenborg JL, Fan J, et al: Inhibition of human
copper trafficking by a small molecule significantly attenuates
cancer cell proliferation. Nat Chem. 7:968–979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Cao C, Wang T, Luo Y, Zhang Y, Dai YY and
Shen Y: Comprehensive analysis of cuproptosis-associated LncRNAs
predictive value and related CeRNA network in acute myeloid
leukemia. Heliyon. 9:e225322023. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Moison C, Gracias D, Schmitt J, Girard S,
Spinella JF, Fortier S, Boivin I, Mendoza-Sanchez R, Thavonekham B,
MacRae T, et al: SF3B1 mutations provide genetic vulnerability to
copper ionophores in human acute myeloid leukemia. Sci Adv.
10:eadl40182024. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Zhang J, Zheng Y, Liu H and Liu B:
AC024896.1/miR-363-3p axis regulates the malignant progression of
acute myeloid leukemia by Cuproptosis-related gene MYO1B. Blood
Lymphat Cancer. 14:17–30. 2024.PubMed/NCBI
|
|
199
|
Huang X, Yan H, Xu Z, Yang B, Luo P and He
Q: The inducible role of autophagy in cell death: Emerging evidence
and future perspectives. Cell Commun Signal. 23:1512025. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Gao W, Wang X, Zhou Y, Wang X and Yu Y:
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor
immunotherapy. Signal Transduct Target Ther. 7:1962022. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Liu X, Tuerxun H and Zhao Y, Li Y, Wen S,
Li X and Zhao Y: Crosstalk between ferroptosis and autophagy:
Broaden horizons of cancer therapy. J Transl Med. 23:182025.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Newton K, Wickliffe KE, Maltzman A, Dugger
DL, Webster JD, Guo H and Dixit VM: Caspase cleavage of RIPK3 after
Asp333 is dispensable for mouse embryogenesis. Cell Death Differ.
31:254–262. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Fritsch M, Günther SD, Schwarzer R, Albert
MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H,
Seeger JM, et al: Caspase-8 is the molecular switch for apoptosis,
necroptosis and pyroptosis. Nature. 575:683–687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Qiu Y, Hüther JA, Wank B, Rath A, Tykwe R,
Aldrovandi M, Henkelmann B, Mergner J, Nakamura T and Laschat S:
Interplay of ferroptotic and apoptotic cell death and its
modulation by BH3-mimetics. Cell Death Differ. Apr 29–2025.doi:
10.1038/s41418-025-01514-7 (Epub ahead of print). View Article : Google Scholar
|
|
205
|
Xu R, Wang W and Zhang W: Ferroptosis and
the bidirectional regulatory factor p53. Cell Death Discov.
9:1972023. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Gao J, Xiong A, Liu J, Li X, Wang J, Zhang
L, Liu Y, Xiong Y, Li G and He X: PANoptosis: Bridging apoptosis,
pyroptosis, and necroptosis in cancer progression and treatment.
Cancer Gene Ther. 31:970–983. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Qu M, Wang Y, Qiu Z, Zhu S, Guo K, Chen W,
Miao C and Zhang H: Necroptosis, pyroptosis, ferroptosis in sepsis
and treatment. Shock. 57:161–171. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Zhang C and Liu N: Ferroptosis,
necroptosis, and pyroptosis in the occurrence and development of
ovarian cancer. Front Immunol. 13:9200592022. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Chen Y, Fang ZM, Yi X, Wei X and Jiang DS:
The interaction between ferroptosis and inflammatory signaling
pathways. Cell Death Dis. 14:2052023. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Li Y, Du Y, Zhou Y, Chen Q, Luo Z, Ren Y,
Chen X and Chen G: Iron and copper: Critical executioners of
ferroptosis, cuproptosis and other forms of cell death. Cell Commun
Signal. 21:3272023. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Hu X, Li L, Nkwocha J, Kmieciak M, Shang
S, Cowart LA, Yue Y, Horimoto K, Hawkridge A, Rijal A, et al: Src
inhibition potentiates MCL-1 antagonist activity in acute myeloid
leukemia. Signal Transduct Target Ther. 10:502025. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Ong F, Kim K and Konopleva MY: Venetoclax
resistance: Mechanistic insights and future strategies. Cancer Drug
Resist. 5:380–400. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Lim J and Murthy A: Targeting autophagy to
treat cancer: Challenges and opportunities. Front Pharmacol.
11:5903442020. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Hassani S, Ghaffari P, Chahardouli B,
Alimoghaddam K, Ghavamzadeh A, Alizadeh S and Ghaffari SH:
Disulfiram/copper causes ROS levels alteration, cell cycle
inhibition, and apoptosis in acute myeloid leukaemia cell lines
with modulation in the expression of related genes. Biomed
Pharmacother. 99:561–569. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Song F, Lin S, Xu T, Yang C, Sharavyn B,
Naranmandura H, Zhang Y and Huang P: Targeted therapy in acute
myeloid leukemia: Resistance and overcoming strategy. Drug Resist
Updat. 83:1012862025. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
D'Amico M and De Amicis F: Challenges of
regulated cell death: Implications for therapy resistance in
cancer. Cells. 13:10832024. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Tong X, Tang R, Xiao M, Xu J, Wang W,
Zhang B, Liu J, Yu X and Shi S: Targeting cell death pathways for
cancer therapy: Recent developments in necroptosis, pyroptosis,
ferroptosis, and cuproptosis research. J Hematol Oncol. 15:1742022.
View Article : Google Scholar : PubMed/NCBI
|