Platelets are universally recognized for pivotal
role in hemostasis. However, emerging evidence has revealed their
substantial involvement in cancer progression (1). This seminal study revealed how
platelets become indispensable accomplices of circulating tumor
cells (CTCs). The molecular crosstalk between platelets and CTCs
plays a vital role in tumor metastasis, the primary cause of
cancer-related mortality. CTCs, identified as malignant cells
shedding from primary tumors into the vasculature, serve as the
precursors to metastasis by disseminating via the bloodstream to
distant organs (2). Understanding
CTCs helps further explore platelet roles in CTC generation and
metastasis. Platelets confer multifaceted protection to CTCs
through direct or indirect interactions: i) shielding from immune
surveillance and hemodynamic shear forces, and ii) facilitating
endothelial adhesion and extravasation (3,4).
Mechanistically, platelet-derived bioactive molecules (for example,
TGF-β) induce epithelial-mesenchymal transition (EMT), enabling
tumor cell detachment and intravasation (3). Furthermore, platelet-tumor aggregates
formed via surface receptor interactions enhance CTC survival and
hematogenous spread (5,6).
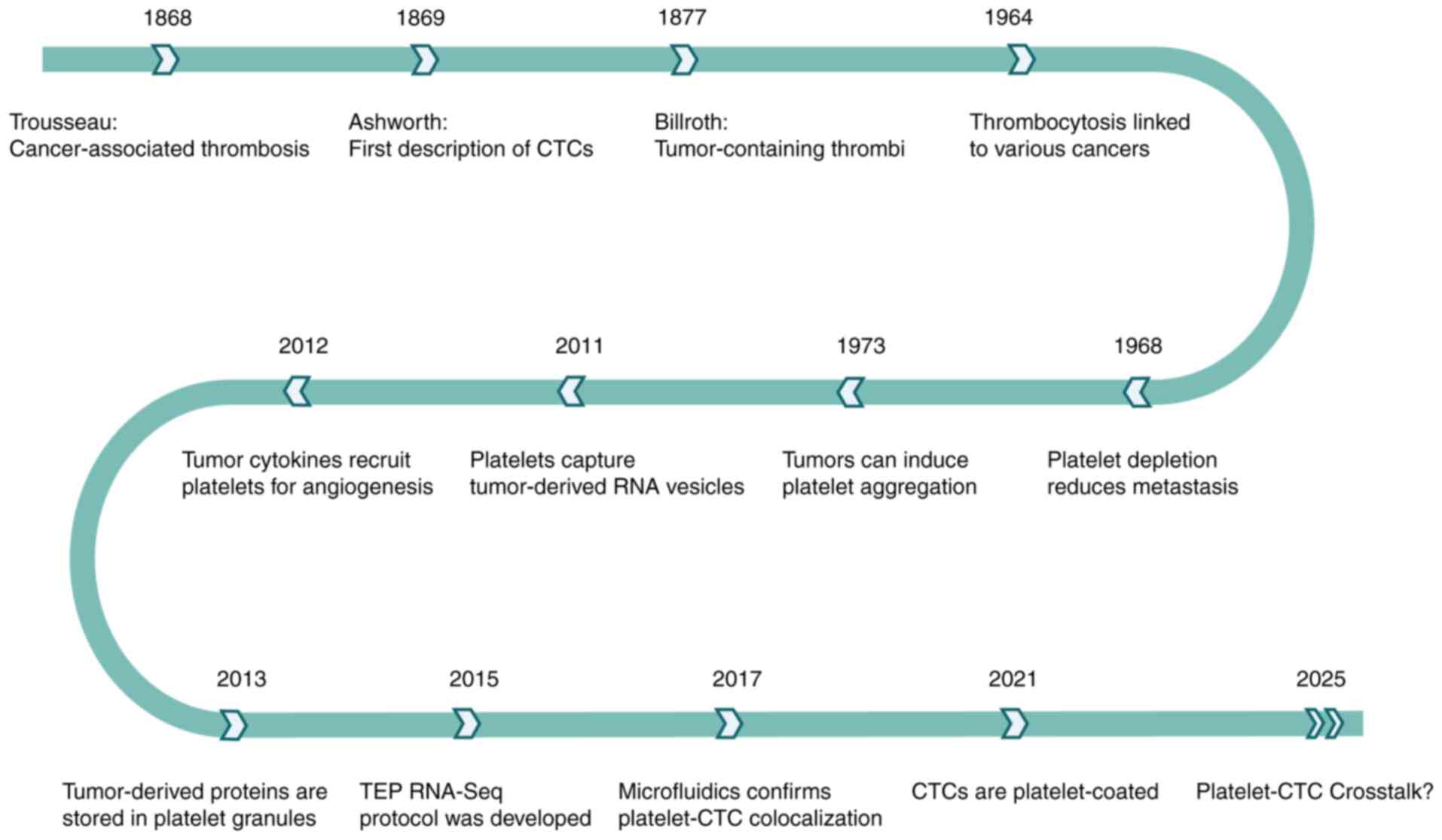
As time goes by, our comprehension of the
platelet-CTC crosstalk has been continuously deepened, gradually
revealing its significant relationships in multiple aspects such as
promoting the survival of tumor cells, immune escape and metastatic
dissemination (7). Extending the
study of Wang et al (7),
these mechanisms underlying platelet-CTC crosstalk were further
elucidated and their diagnostic and therapeutic potential were
explored. The present review synthesizes contemporary insights into
the following aspects: i) the historical context and progression of
platelet-CTC crosstalk; ii) the molecular mechanisms underlying
platelet-CTC interactions; iii) the bidirectional interactions
between platelets and CTCs; iv) the diagnostic utility of
platelet-CTC-derived biomarkers in liquid biopsies; and v) emerging
therapeutic strategies targeting the platelet-CTC interface.
Platelets are primarily recognized for their
critical function in hemostasis; however, their multifaceted roles
in oncogenesis have garnered increasing scientific interest.
Seminal insights into this relationship emerged in the late 19th
century. In 1868, Trousseau (8)
reported an association between malignancy and spontaneous
coagulation, implicating platelet involvement. Subsequently in
1869, Ashworth documented the presence of aberrant neoplastic cells
within the circulatory system, now acknowledged as the inaugural
identification of CTCs (9). In
1877, Billroth (10) observed
‘thrombi with tumor components’ within the tumor metastasis,
further establishing a direct connection between cancer cells and
blood platelets. Advancing to the mid-20th century, a comprehensive
analysis of blood smears from 14,000 patients revealed a
significant correlation between increased platelet levels and
cancer incidence (11). The
findings indicated that thrombocythemia was notably more prevalent
in malignancies of the lung, stomach, colorectal tract, breast and
ovary. In 1968, Gasic et al (12) observed that thrombocytopenia was
associated with cancer metastasis in murine models. Injecting mice
with antiplatelet serum significantly reduced the number of
metastatic foci; this effect was reversible through platelet-rich
plasma infusion. These findings underscore platelets' facilitatory
role in metastatic dissemination.
In the late 20th century, research elucidated
complex bidirectional interactions between tumors and platelets.
Numerous malignancies induce platelet aggregation both in
vitro and in vivo, resulting in thrombocytopenia,
frequently associated with pulmonary platelet sequestration
(13). By 2011, mechanistic studies
revealed that platelets contribute to cancer progression via
internalization of RNA from tumor-derived extracellular vesicles
(EVs), establishing an RNA transfer network within the bloodstream
by releasing pro-tumorigenic vesicles (14). Concomitantly, tumor-secreted
cytokines are internalized by platelets, recruiting them to the
neoplastic site to support angiogenesis (15). Platelets sequester these proteins
via α-granules, thereby modulating specific cellular pathways
through regulated exocytosis (16).
Clinical investigations concurrently identified thrombocytosis as
an independent prognostic factor in primary lung carcinoma,
suggesting platelet count as a straightforward biomarker for risk
stratification (17,18). Collectively, these advances
highlight platelets' integral roles beyond hemostasis, encompassing
tumor proliferation, metastasis and vascular remodeling.
The advent of microfluidic technology in the 21st
century has revolutionized the research of platelet-CTC crosstalk.
In 2017, microfluidic-based isolation leveraged platelet-derived
surface markers on CTCs, confirming platelet-CTC co-localization
(19). In 2021, Lim et al
(20) discovered using centrifugal
microfluidic technology that 90.7% of CTCs were platelet-coated,
and platelet-encased CTC clusters correlated with rapid disease
progression. Recent investigations (21) have elucidated the molecular
mechanisms underlying the crosstalk between platelets and CTCs. The
interplay provides a critical scientific foundation for advancing
metastasis research and harbors significant potential for clinical
applications. Since the initial recognition of platelet-tumor
interactions in the 19th century, the field has undergone
substantial evolution, with contemporary studies comprehensively
exploring the platelet-CTC crosstalk to uncover pro-tumorigenic
effects (Fig. 1).
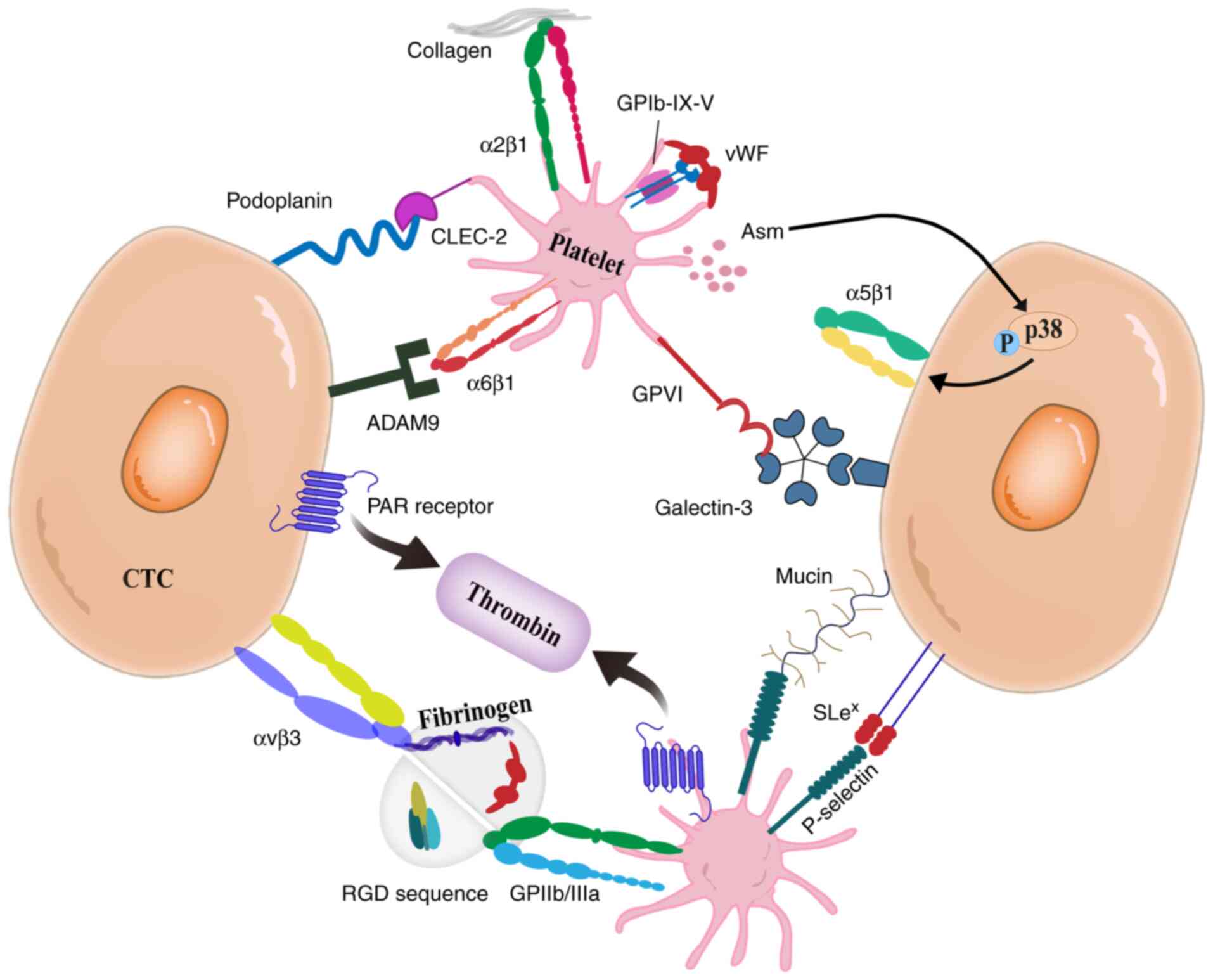
The interplay between platelets and CTCs is
primarily mediated by direct surface receptor binding and by
extracellular proteins that facilitate receptor bridging (Fig. 2). Building on the previous study of
Erpenbeck and Schön (22), the
adhesive mechanisms underlying platelet-CTC crosstalk were refined,
clearly revealing the physical basis of their interaction. Key
mechanisms include: i) the engagement of platelet C-type
lectin-like receptor 2 (CLEC-2) with Podoplanin expressed on tumor
cells, which induces platelet activation and facilitates tumor cell
metastasis (6); ii) the interaction
of the platelet glycoprotein (GP) Ib-IX–V complex with tumor cells
via von Willebrand factor (vWF), mediating adhesion (22); iii) platelet GPIIb/IIIa (αIIbβ3
integrin) binding to ligands such as fibrinogen and vWF, enhancing
platelet-tumor cell aggregation (23–25);
iv) metastatic potential is augmented by platelet GPVI receptor
interactions with galectin-3, collagen, or fibrin on tumor cells
(26,27); v) integrin αvβ3 on tumor cells
binding to the Arg-Gly-Asp (RGD) sequence, promoting adhesion
(28); vi) the activation of
protease-activated receptors (PAR) on tumor cells by thrombin,
enhancing their interaction with platelets (22); vii) integrin α6β1 binding to ADAM9
on tumor cells, promoting metastasis (29); viii) platelet-derived acidic
sphingomyelinase (Asm) inducing p38K phosphorylation in tumor
cells, thereby activating integrin α5β1 and facilitating adhesion
and metastasis (30,31); and ix) P-selectin on the surface of
platelets binds to sialyl Lewisx (SLex) on
the surface of CTCs (22,32). This complex subsequently interacts
with endothelial P-selectin, resulting in CTCs rolling and vascular
retention (22,32). Collectively, these molecular
interactions represent potential therapeutic targets for cancer
treatment.
Secondly, platelet adhesion to CTCs is facilitated
through surface adhesion molecules, including P-selectin, CLEC-2
and GPIIb/IIIa. This interaction promotes CTC-endothelial adhesion
and vascular retention, establishing critical prerequisites for
metastatic colonization in distant organs (38–41). A
previous study by Schlesinger (41)
summarized the role of platelet receptors in tumor metastasis,
which advanced our comprehension of platelet-CTC adhesion. Notably,
surgical stress induces TLR4-dependent ERK5 phosphorylation,
resulting in GPIIb/IIIa integrin activation and subsequent
platelet-CTC aggregation (42).
These aggregates exhibit enhanced entrapment by neutrophil
extracellular traps, thereby promoting their retention within the
pulmonary microvasculature (42).
Heparanase released from α-granules cooperates with P-selectin to
enhance platelet adhesion and promote thrombogenicity, facilitating
metastasis (43). Furthermore,
tissue factor (TF)-induced coagulation facilitates the coating of
CTCs by platelets, leading to the formation of circulating tumor
microemboli (CTM) (44).
Additionally, platelets secrete multiple bioactive
mediators that collectively facilitate metastatic progression. The
platelet release contains TGFβ, ATP, and serotonin, which, in
combination with matrix metalloproteinases (MMPs) and histamine,
synergistically enhance tumor cell invasiveness, increase vascular
permeability, and promote extravasation and metastatic niche
formation (45–49). For example, TGFβ released via
platelet-CTC crosstalk can activate the metastatic capacity of CTCs
by triggering metabolic reprogramming and bioenergetic adjustment
(50). On the other hand, TGFβ
activates the TGFβ/Smad pathway, inducing SERPINE1/PAI-1 expression
to activate PI3K/AKT signaling, thereby enhancing CTC metastatic
competence (51). Molecular
analyses reveal that platelet-CTC crosstalk upregulates key
oncogenic pathways in CTCs, including MYC, IL33, VEGFB, PTGER2,
PTGS2 and TGFβ2 expression profiles (52). Regulation of angiogenesis is a key
mechanism in tumor progression, with extensive evidence indicating
that platelets directly contribute to this process, likely through
factors released upon their activation (53). Ghosh et al (54) developed an angiogenesis-enabled
tumor microenvironment (TME) chip that recapitulates multicellular
interactions among tumor cells, endothelial cells and platelets in
ovarian cancer, providing direct evidence of platelet-mediated
angiogenesis enhancement. Furthermore, platelets orchestrate
immune-modulatory functions through chemokine release (particularly
CXCL7 and CXCL5 from α-granules), facilitating immune cells'
recruitment and pre-metastatic niche establishment (55,56).
Platelets significantly facilitate EMT during the
dissemination of CTCs via diverse molecular mechanisms (57). TGFβ secreted by platelets triggers
EMT by activating both TGFβ/Smad and NF-κB signaling pathways
(46). Furthermore,
platelet-derived growth factor-D (PDGF-D) promotes EMT by
upregulating transcriptional regulators such as Twist1 and Notch1
in colorectal cancer cells, as well as interacting with PDGFRβ in
tongue squamous cell carcinoma cells, thereby inducing
phosphorylation of key kinases including p38, AKT and ERK (58,59).
Platelets also secrete lysophosphatidic acid (LPA), which binds to
LPA receptors on tumor cells to enhance their invasive and
migratory capacities (57,60). Additionally, platelet-derived
microvesicles modulate EMT-associated gene expression in tumor
cells through the transfer of regulatory RNAs, including mRNA and
microRNA (miRNA) (61). Building on
Wang et al's (57) detailed
delineation of platelet-induced EMT signaling pathways, the present
review integrates recent advances to provide a more comprehensive
mechanistic overview. For instance, platelets contribute to CTC
plasticity by maintaining a mesenchymal phenotype during
circulation, enabling more efficient transition to an epithelial
state upon reaching distant metastatic sites. This
epithelial-mesenchymal plasticity augments tumor cell invasiveness
and metastatic potential (52).
These multifaceted roles establish platelets as critical mediators
of cancer metastasis and provide a mechanistic basis for
therapeutic strategies targeting platelet-CTC crosstalk. However,
while current research predominantly focuses on platelet-mediated
EMT in CTC generation, few investigations address how platelets
support CTC colonization via mesenchymal-epithelial transition
(MET) in distant organs.
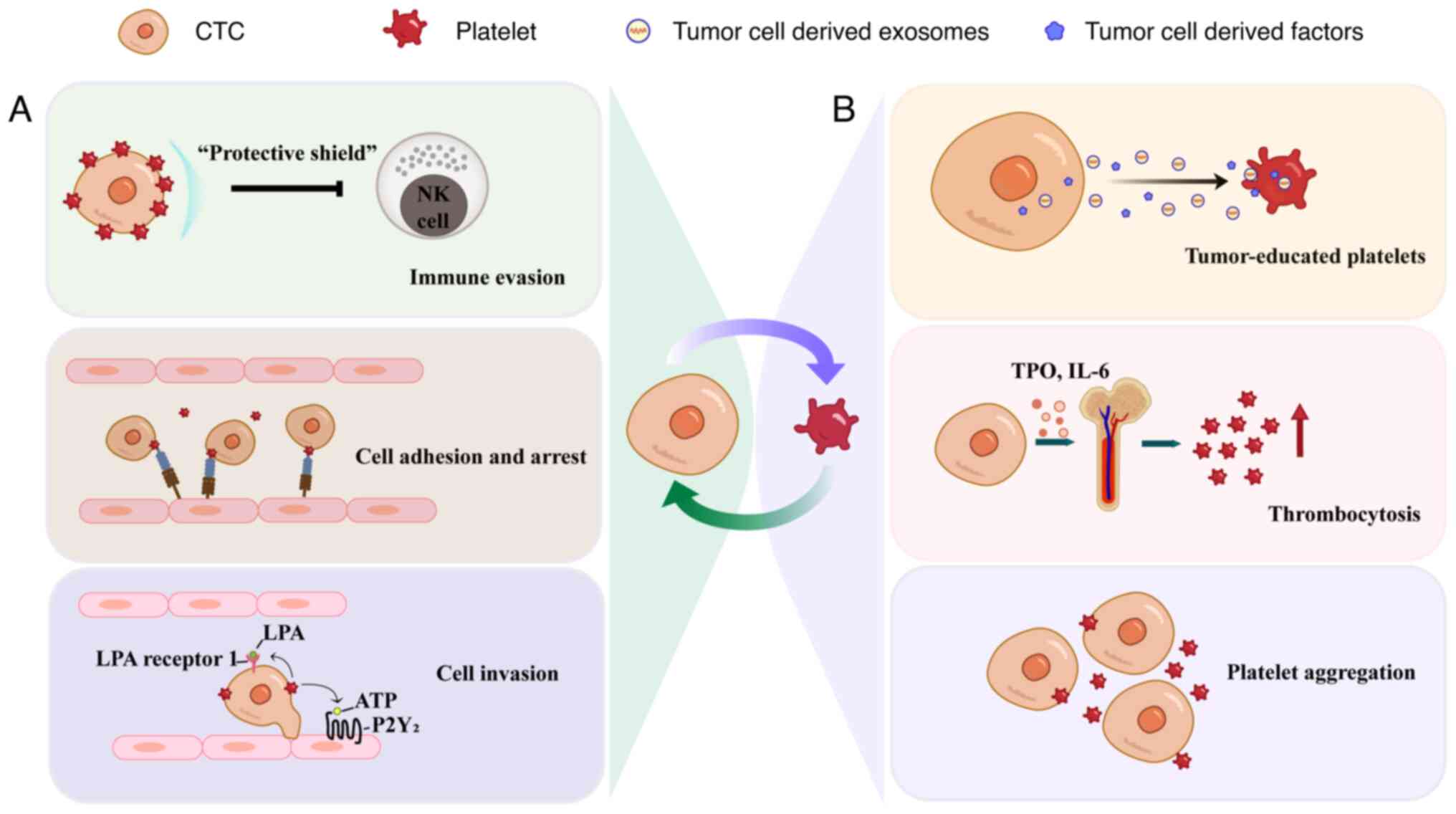
Emerging evidence demonstrates a complex
bidirectional interplay between platelets and tumor cells (7) (Fig.
3B). This interaction induces platelet activation, phenotypic
transformation and transcriptomic reprogramming, culminating in the
formation of tumor-educated platelets (TEPs). TEPs are
characterized by their capacity to sequester and enrich
tumor-specific substances (including proteins, nucleic acids, EVs)
during their interaction with tumors, leading to distinct
alterations in DNA, RNA and protein expression profiles (62). A recent study has demonstrated that
platelets actively internalize DNA-containing EVs and membrane-free
DNA fragments, thereby sequestering tumor-derived DNA (63). These platelets play pivotal roles in
tumor growth, angiogenesis and metastasis (41). Through interaction with cancer
cells, platelets are ‘educated’ and carry tumor-related biological
information (64). Due to the short
lifespan and enclosed membrane structure, TEPs provide real-time
molecular snapshots of tumor activity; therefore, they are regarded
as promising indicators for cancer detection and disease monitoring
(65).
The tumor-induced platelet education process
operates through several critical aspects. Firstly, tumor cells
initiate platelet aggregation via direct binding to platelet
surface receptors or through extracellular protein-mediated
bridging, which is known as ‘tumor cell-induced platelet
aggregation’ (TCIPA) (33). In
metastatic models, TCIPA formation occurs within 1 min of tumor
cell intravasation, shielding tumor cells from immune attacks and
facilitating tumor growth and metastasis (33,56,66).
Beyond inducing TCIPA, Strasenburg et al (66) elucidated multiple mechanisms of
tumor-mediated platelet activation, substantially expanding our
understanding of this process. Secondly, tumor-derived factors,
including thrombopoietin and interleukin-6, stimulate
megakaryopoiesis and platelet generation in bone marrow, leading to
paraneoplastic thrombocytosis, a well-established poor prognostic
indicator across multiple malignancies (67). Furthermore, tumor cells trigger
platelet degranulation, releasing immunosuppressive (for example,
CD40L) and procoagulant (for example, TF) mediators that reshape
the TME (68,69). Elevated tumor TF expression
initiates the coagulation cascade, inducing platelet activation and
fibrin production. The resulting fibrin mediates TCIPA and enhances
adhesion of CTM to the mesothelium, ultimately promoting polyclonal
metastasis (70). Fibrinogen, upon
conversion to fibrin, may form a matrix scaffold that influences
the recruitment of immune cells, mediates inflammatory responses,
stimulates angiogenesis, and increases vascular permeability,
collectively facilitating the development of a pre-metastatic niche
that supports tumor progression (71). Collectively, tumor cells orchestrate
the function and quantity of platelets through multiple mechanisms,
establishing a microenvironment conducive to tumor development and
metastasis (72).
Cancer diagnosis has historically relied on biopsy,
a method offering high specificity but constrained by invasiveness,
spatial resolution limitations, and incomplete capture of tumor
heterogeneity. To address the increasing need for early cancer
detection and monitoring, non-invasive approaches have been
investigated, including circulating tumor DNA/RNA (ctDNA/RNA) and
CTCs (73–76). ctDNA/RNA analysis enables
plasma-based sequencing. In addition, CTCs can be enriched by size,
density, or surface markers, followed by quantification using
fluorescence immunostaining or morphological examination (77). Moreover, CTCs can be captured intact
or through secreted exosomes for nucleic acid analysis, enhancing
their utility as tumor biomarkers (78).
Platelets, as abundant and readily isolatable blood
components, interact with CTCs to form platelet-CTC complexes,
thereby presenting novel diagnostic opportunities. Platelet-related
indices, including platelet count, mean platelet volume, and
platelet-to-lymphocyte ratio, are associated with tumor progression
(79,80). However, these parameters may be
confounded by inflammation or chemotherapy-induced bone marrow
suppression (79–81). Technological advancements now
facilitate precise analysis of platelet-CTC complexes, enhancing
diagnostic accuracy and specificity. For instance, a microfluidic
platform was developed to isolate platelet-covered CTCs through a
two-stage strategy: Initial removal of unbound platelets, followed
by antibody-mediated CTC capture targeting platelet-specific
markers (19). The shielding of CTC
surface markers (for example, EpCAM) by platelet encapsulation
impairs antibody-dependent capture and can also alter cellular
morphology and molecular profiles, complicating subsequent
analysis. To address this, bifunctional monomers or zwitterionic 3D
network structures can be employed to suppress platelet adhesion
and preserve CTC integrity (82,83).
This technology represents a promising tool for cancer metastasis
research and non-invasive cancer diagnosis, though further
mechanistic and clinical validation remains essential.
Although anucleate, platelets harbor diverse
biomolecules, including RNA, DNA and proteins derived from
megakaryocytes or the TME. Their 7-to-10-day circulatory lifespan
confers superior stability compared with ctDNA/RNA or exosomes,
making platelets ideal for longitudinal monitoring. During
circulation, platelets continuously assimilate and enrich
tumor-related substances, culminating in the formation of TEPs
(84). TEPs exhibit diagnostic
utility across multiple cancer types, including pan-cancer
detection and companion diagnostics (85). The RNA profiles of TEPs, including
miRNA, lncRNA, mRNA, and small nuclear RNA (snRNA), exhibit
significant potential for tumor diagnosis (Table I) (67,75,86–92).
Specifically, dysregulation of snRNAs (for example, U1, U2 and U5),
which mediate RNA splicing, is mechanistically linked to tumor
progression and displays diagnostic potential in malignancies such
as lung cancer (67,93). Previous studies have detailed the
diagnostic utility of snRNA, mRNA and proteins from TEPs,
highlighting the great potential of TEP RNA profiles in cancer
diagnosis and prognosis monitoring (67,84).
Particularly, platelet RNA-seq emerges as a highly promising
screening methodology (94). This
approach offers several advantages, including minimal invasiveness,
relatively low cost, and absence of radiation exposure. Moreover,
it has the potential to identify primary tumor origins (95). Protocols, such as thromboSeq, were
proposed based on TEP RNA analysis for cancer diagnosis and
therapeutic monitoring. Notably, TEP-derived RNA signatures also
enable dynamic monitoring of tumor progression. Comparative
efficacy of TEP RNA sequencing across tumor types is systematically
summarized in Table I (96).
Platelets accumulate tumor-derived DNA across a wide
spectrum of neoplastic conditions, from advanced malignancies with
high ctDNA load to low-burden diseases such as early-stage cancers
and precancerous colorectal polyps (63). Compared with other blood components,
including red blood cells and mononuclear cells, platelets exhibit
superior efficiency in the uptake of tumor-derived DNA, suggesting
their role as a reservoir for genetic material derived from tumors
(63). An important advantage of
this platelet-based capture is that the DNA is shielded from
nuclease activity, thereby preserving low-frequency mutations that
might otherwise be degraded (63).
Consequently, platelet DNA analysis emerges as a highly promising
tool for liquid biopsy. However, the extraction of platelet DNA
necessitates an initial platelet isolation step to avoid
contamination from other cellular components, rendering this method
more technically complex than ctDNA analysis. Furthermore, the
field currently lacks standardized protocols, large-scale clinical
validation, and robust evidence supporting its diagnostic utility.
Future research in large-scale clinical cohorts is warranted to
translate this discovery into improved strategies for cancer
diagnosis and monitoring.
In addition to RNA and DNA, platelets contain a
diverse array of biologically active proteins that represent a
promising source for cancer biomarkers. Utilizing advanced
proteomics approaches, researchers have identified protein panels
exhibiting differential expression patterns between ovarian cancer
(FIGO stages III–IV) and benign adnexal lesions, with diagnostic
accuracies showing high sensitivity (96%) and specificity (88%)
(97). Similarly, targeted
methodologies involving nano liquid chromatography-tandem mass
spectrometry have revealed seven potential platelet protein markers
associated with early-stage malignancies (98). Nevertheless, the underlying
molecular mechanisms remain insufficiently characterized.
Therefore, further research is essential to elucidate the
mechanisms of these proteins.
Extensive preclinical and clinical evidence
demonstrates that common antiplatelet agents, including aspirin,
clopidogrel and c7E3/ReoPro, exert significant antitumor effects
(99). The multifaceted mechanisms
of aspirin include: Direct suppression of tumor cell proliferation,
inhibition of platelet-CTC crosstalk, and attenuation of
platelet-derived pro-angiogenic and growth factors, cytokines and
chemokines (100). Notably,
aspirin may exert anticancer properties through non-COX-dependent
pathways, including the inhibition of inflammatory processes and
the induction of apoptosis, as well as the suppression of signal
transduction mediated by IκB kinase β and ERK (99,101).
In 2016, the U.S. Preventive Services Task Force endorsed low-dose
aspirin for cancer prevention in selected populations (aged 50–69
years), albeit with the caveat that its benefits may be offset by
the risk of major bleeding (102,103).
In addition to aspirin, other antiplatelet agents
also exhibit significant roles in cancer therapy. Preclinical
studies demonstrated the therapeutic potential of P2Y12
inhibitors (for example, clopidogrel and ticagrelor) in disrupting
platelet-tumor interactions, thereby inhibiting cancer cell
migration and metastasis (104–106). Experimental models demonstrate
that ticagrelor and EP3 antagonist DG-041 inhibit platelet-induced
phenotypic changes in colon cancer cells (106). Such changes include the
downregulation of E-cadherin, upregulation of Twist1, enhanced cell
motility and increased platelet aggregation (106).
Some antiplatelet agents inhibit platelet-CTC
crosstalk by targeting specific platelet surface receptors.
Notably, multiple surface proteins, including GPVI, CLEC-2,
GPIIb/IIIa, etc., represent validated therapeutic targets for
attenuating tumor progression and metastasis (107). For instance, GPIIb/IIIa
antagonists (for example, eptifibatide) have demonstrated efficacy
in inhibiting cancer cell metastasis both in vitro and in
animal models (108,109). Furthermore, activated GPIIb/IIIa
receptors serve as dual-function targets for molecular imaging
probes, enabling tumor visualization, and chemotherapeutic agents,
facilitating precise therapeutic delivery (110,111). GPVI blockers (for example,
revacept) reduce thrombosis by disrupting platelet-collagen
interactions and concurrently block platelet-induced EMT and COX-2
expression in cancer cells (112).
Targeting podoplanin on CTCs selectively inhibits the binding of
CLEC-2 receptors on platelets to podoplanin, thereby preventing
platelet-mediated tumor protection (113). Additionally, inhibitors of GPIbα
and P-selectin exhibit potential in preclinical studies for
inhibiting the interaction between platelets and cancer cells
(39,114). Several published reviews have
summarized various antiplatelet agents for cancer therapy,
synthesizing fragmented findings in the field and further advancing
the understanding of targeting platelets for cancer treatment
(78,107). Based on current advances,
integrating antiplatelet agents into comprehensive therapeutic
strategies, such as in combination with chemotherapy or targeted
therapy, may enhance efficacy through complementary mechanisms.
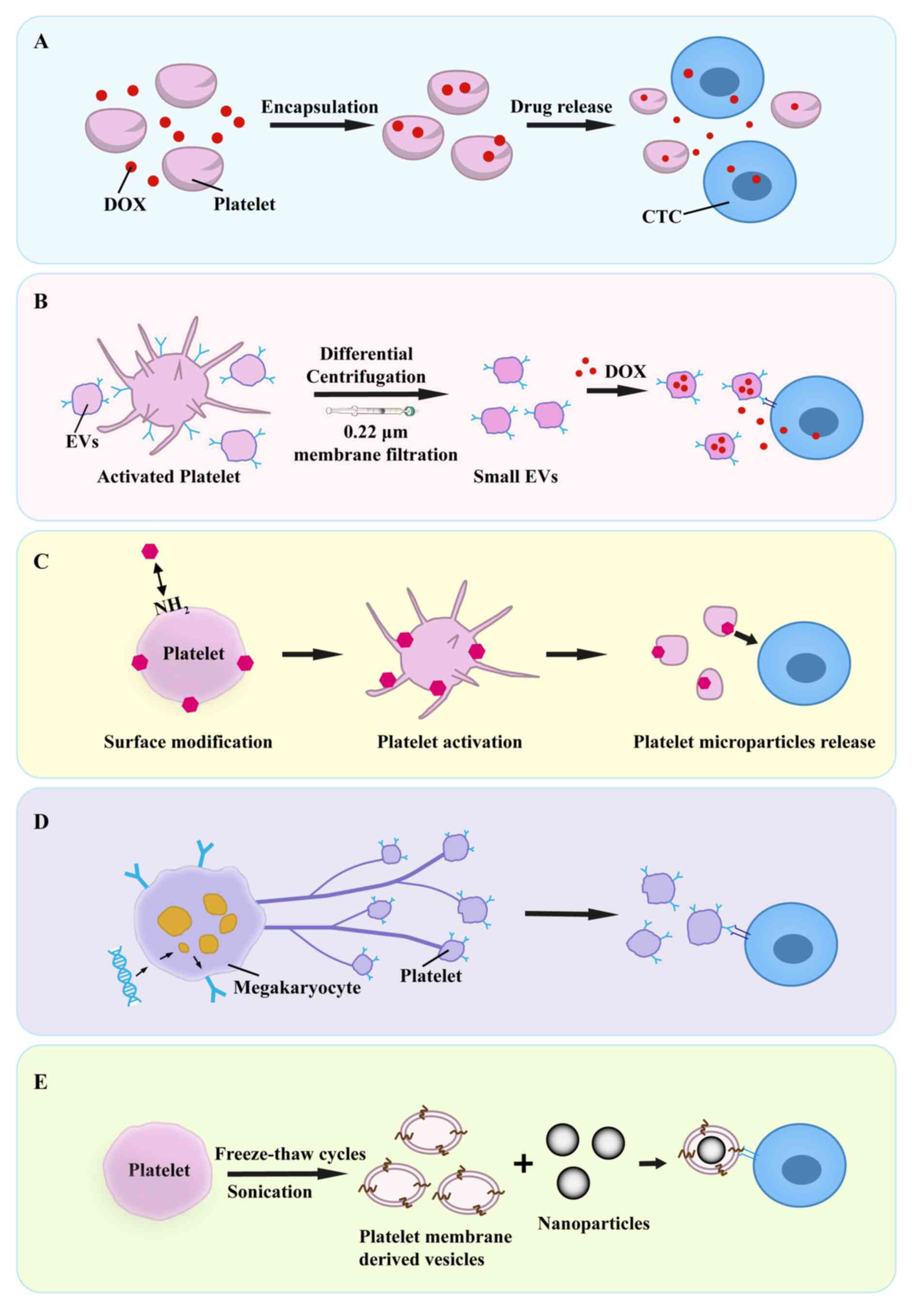
Engineered platelet technologies represent a
promising paradigm for cancer therapy, leveraging fabrication
techniques such as drug loading, genetic engineering and membrane
modification to create intelligent drug delivery systems.
Concerning the combination therapy with chemotherapeutic agents,
two principal strategies have been established. Firstly, the
incorporation of doxorubicin (DOX) into platelets significantly
enhances therapeutic efficacy against lymphoma (Fig. 4A) (115). Secondly, small EVs derived from
platelets can be exploited to encapsulate DOX, subsequently
facilitating targeted delivery to CTCs (Fig. 4B) (116). An innovative approach involves
disrupting CTC clusters through platelet-CTC crosstalk; loading
platelet decoys or lyophilized platelets with tissue-type
plasminogen activator facilitates dissociation of these clusters,
thereby inhibiting metastatic potential (117). Within the domain of genetic
engineering, a study by Li et al (118) utilized lentiviral vector-mediated
transduction of hematopoietic stem cells to generate platelets
expressing tumor necrosis factor-related apoptosis-inducing ligand
(TRAIL) (Fig. 4D). These engineered
platelets substantially reduced hepatic metastasis in a prostate
cancer mouse model while preserving native hemostatic function
(118). Collectively, these
advancements not only enhance treatment specificity but also
minimize off-target effects.
Furthermore, engineered platelets represent a
promising platform for integration with tumor immunotherapy,
enabling novel therapeutic strategies. One approach utilizes
engineered platelets as carriers for immune checkpoint blockade.
Surface conjugation of anti-PD-L1 antibodies onto platelets
leverages their inherent tumor tropism, facilitating targeted
accumulation within post-resection inflammatory niches (119). Upon activation, platelets release
microparticles encapsulating anti-PD-L1 antibodies, which block the
PD-L1/PD-1 interaction between tumor cells and T cells,
consequently reversing immunosuppression and potentiating T
cell-mediated antitumor responses (Fig.
4C) (119). Engineered
platelets can also augment chimeric antigen receptor T (CAR-T) cell
therapy. Surface-anchored anti-PD-L1 antibodies obstruct the
PD-1/PD-L1 pathway, preventing CAR-T cell exhaustion, while
activated platelets secrete cytokines such as IL-15 to enhance
CAR-T cell proliferation and activity (120). Additionally, platelet-derived
microparticles and chemokines enhance CAR-T cell infiltration into
tumors (120). Beyond immune
modulation, a distinct strategy involves loading engineered
platelets with cytotoxic complexes, such as granzyme B and
perforin, enabling immune-independent cytotoxicity against CTCs
through mechanisms mimicking T cell effector functions, thereby
inhibiting metastasis (121).
These strategies enhance therapeutic efficacy synergistically when
combined with chemotherapy or immunotherapy.
However, research on engineered platelets as
carriers for radiosensitizers remains limited but promising;
targeted tumor delivery improves radiotherapy efficacy while
sparing normal tissues (122,123). In vitro megakaryocyte
cultures provide platelets for engineering, yet scaling
clinical-grade, donation-independent production requires overcoming
yield and quality barriers (124).
Nanotechnology demonstrates significant potential in
modulating platelet-CTC crosstalk. For instance, perfluorocarbon
nanoparticles inhibit platelet activation, thereby enhancing T cell
infiltration into tumors and improving immunotherapy efficacy
(125). Similarly, chlorogenic
acid nanoparticles also suppress platelet activation, disrupting
the tumor vascular barrier to augment chemotherapeutic drug
permeability (126). Additionally,
nanoparticles functionalized with activated platelet membranes
target CTC-associated microthrombi, significantly reducing lung
metastasis in a breast cancer model (Fig. 4E) (127). Photodynamic nanostrategies enable
the release of nitric oxide, which suppresses platelet activation
by downregulating the expression of P-selectin and blocking the
activated configuration of GPIIb/IIIa (128). This intervention not only reduces
tumor-derived procoagulant factors secretion but also avoids
hemorrhagic risks associated with systemic anticoagulation.
Collectively, these approaches provide a novel therapeutic paradigm
for inhibiting tumor metastasis through precise platelet function
modulation.
Several nanostrategies disrupt platelet-CTC
crosstalk without direct targeting. For example, MMP2-responsive
polymer-lipid-peptide nanoparticles encapsulate DOX and an
anti-platelet antibody R300 (129). Upon MMP2 activation in the TME,
the release of R300 induces platelet micro-aggregation and
depletion, which facilitates vascular permeabilization and DOX
delivery for enhanced anticancer efficacy (129). Furthermore, strategies focusing on
adhesion disruption include CD44-targeted DOX-loaded liposomes that
incorporate the Lys-Leu-Val-Phe-Phe (KLVFF) peptide motifs that
self-assemble into nanofiber networks on CTC surfaces, physically
impeding platelet aggregation (130). By blocking platelet-CTC crosstalk,
such approaches effectively suppress tumor invasion and metastasis.
These nanotechnological innovations illustrate promising avenues
for cancer therapy, offering novel insights for developing precise
and efficient treatment strategies.
Beyond the aforementioned strategies, substantial
advancements have been achieved in direct approaches specifically
targeting CTCs. An intelligent nano-diagnostic system was developed
for targeting CTCs of metastatic breast cancer (131). This nano-diagnostic system is
composed of targeted multi-responsive nanomicelles encapsulating
near-infrared fluorescent superparamagnetic iron oxide
nanoparticles, featuring dual-mode imaging and dual toxicity. It
tracks and eliminates CTCs before colonization at distant sites,
thereby impeding metastatic progression (131). Furthermore, CTCs exhibit unique
functional properties that render them ideal targets for
personalized drug sensitivity testing. In vitro isolation
and culture of CTCs, followed by pharmacological screening, can
predict patient-specific therapeutic responses, mitigating the risk
of ineffective or adverse treatments (132).
The present review systematically examines the
mechanisms, diagnostic applications and therapeutic implications of
platelet-CTC crosstalk. Beginning with historical observations such
as cancer-related thrombocytosis, the evolution of research toward
molecular-level insights is traced. A systematic overview of
adhesion molecules between CTCs and platelets is provided,
clarifying the physical basis of their interactions, a perspective
that distinguishes the present study from Yang et al
(21), who focused primarily on
platelets as CTC ‘shelters’. The review further offers a broader
examination of CTC effects on platelets, covering platelet
education (leading to TEP formation), TCIPA, enhanced
thrombocytosis and platelet activation. Beyond existing concepts,
particular emphasis is placed on the novel capacity of platelets to
capture tumor-derived DNA, adding a molecular dimension to TEP
research.
A key focus lies in the clinical translational
potential of TEPs. In addition to systematically summarizing the
diagnostic performance of TEP RNA-seq across multiple cancer types
in tabular form, the review highlights the promising applications
of RNA, DNA and protein profiles in TEPs as emerging biomarkers, an
aspect not sufficiently explored in the study by Yang et al
(21). Moreover, given the
extensive reviews on CTC detection technologies elsewhere, only a
brief overview was provided. Therapeutically, antiplatelet agents
and strategies targeting platelet-CTC crosstalk were reviewed to
highlight promising avenues for future therapeutic development.
Furthermore, advances in smart delivery systems enable exploration
of engineered platelets, leveraging their natural homing properties
for drug loading, and their combination with chemotherapy or
immunotherapy, thereby revealing potential therapeutic avenues.
Progress in nanotechnology targeting this interaction was also
discussed, broadening research perspectives in the field.
The present review advances the field by
synthesizing fundamental research on platelet-CTC crosstalk,
organized around several integrative themes. Its uniqueness lies in
several key integrative aspects: i) a dedicated focus on the
bidirectional crosstalk in the vascular niche; ii) an expansion of
the TEP concept to include the novel dimension of tumor-DNA capture
for diagnostics, complemented by a systematic consolidation of the
current landscape of TEP RNA-seq for cancer diagnosis and its
clinical translational potential; and iii) a comprehensive,
cross-disciplinary synthesis of next-generation therapeutic
platforms (for example, engineered platelets and nanotherapeutics),
moving beyond traditional antiplatelet agents. By bridging
molecular mechanisms with translational applications, the present
review offers a forward-looking perspective and a strategic
resource for ongoing research and therapeutic development.
Looking forward, critical research directions
warrant further investigation: Elucidating the precise mechanisms
through which platelets facilitate CTC extravasation across vessel
walls and influence MET during metastatic colonization;
investigating variations in platelet-CTC crosstalk across diverse
cancer types to enable precise therapeutic targeting; advancing the
clinical translation of TEP-based diagnostics, particularly
validating the efficacy of integrated platelet DNA analysis in
large-scale clinical cohorts; and overcoming challenges in the
large-scale ex vivo culture of functional platelets to
realize the potential of engineered platelet therapies. Future
efforts include preclinical evaluation of translationally promising
engineered platelet strategies, leveraging their inherent
tumor-homing properties for drug delivery, and exploring
synergistic combinations with multimodal treatments. Progress in
these areas may inform novel therapeutic strategies to inhibit
metastatic progression and enhance patient survival outcomes.
Not applicable.
The present study was supported by the Opening Project of Hubei
Key Laboratory of Regenerative Medicine and Multi-disciplinary
Translational Research (grant no. 2023zsyx007).
Not applicable.
LZ wrote the original draft and created the
visualizations. YY acquired funding, conceived the study, performed
critical revision, and provided substantial improvement of the
manuscript. YD reviewed the manuscript, contributed to
visualizations, provided constructive feedback, and polished the
language. FC and LW jointly supervised the study. FC oversaw the
research design and project administration. LW led the
investigation, provided resources, and performed technical
validation. All authors read and approved the final version of the
manuscript and take full responsibility for the integrity and
accuracy of all aspects of the work. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lou XL, Sun J, Gong SQ, Yu XF, Gong R and
Deng H: Interaction between circulating cancer cells and platelets:
Clinical implication. Chin J Cancer Res. 27:450–460.
2015.PubMed/NCBI
|
|
2
|
Lawrence R, Watters M, Davies CR, Pantel K
and Lu YJ: Circulating tumour cells for early detection of
clinically relevant cancer. Nat Rev Clin Oncol. 20:487–500. 2023.
View Article : Google Scholar
|
|
3
|
Morris K, Schnoor B and Papa AL: Platelet
cancer cell interplay as a new therapeutic target. Biochim Biophys
Acta Rev Cancer. 1877:1887702022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Li T, Ding L, Wang J, Chen C, Liu
T, Liu Y, Li Q, Wang C, Huo R, et al: Platelet-mediated circulating
tumor cell evasion from natural killer cell killing through immune
checkpoint CD155-TIGIT. Hepatology. 81:791–807. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi Y, Chen W, Liang X, Xu K, Gu X, Wu F,
Fan X, Ren S, Liu J, Zhang J, et al: Novel antibodies against GPIbα
inhibit pulmonary metastasis by affecting vWF-GPIbα interaction. J
Hematol Oncol. 11:1172018. View Article : Google Scholar
|
|
6
|
Suzuki-Inoue K: Platelets and
cancer-associated thrombosis: Focusing on the platelet activation
receptor CLEC-2 and podoplanin. Blood. 134:1912–1918. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S, Li Z and Xu R: Human cancer and
platelet interaction, a potential therapeutic target. Int J Mol
Sci. 19:12462018. View Article : Google Scholar
|
|
8
|
Trousseau A: Lectures on clinical
medicine, delivered at the Hotel-Dieu, Paris, New Sydenham Society.
London: 1868
|
|
9
|
Ashworth TR: A case of cancer in which
cells similar to those in the tumours were seen in the blood after
death. Australas Med J. 14:146–147. 1869.
|
|
10
|
Billroth T: Metastatic tumours. Lectures
on surgical pathology and therapeutics. A handbook for students and
practitioners. The New Sydenham society; London: pp. 352–368.
1877
|
|
11
|
Levin J and Conley CL: Thrombocytosis
associated with malignant disease. Arch Intern Med. 114:497–500.
1964. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gasic GJ, Gasic TB and Stewart CC:
Antimetastatic effects associated with platelet reduction. Proc
Natl Acad Sci USA. 61:46–52. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gasic GJ, Gasic TB, Galanti N, Johnson T
and Murphy S: Platelet-tumor-cell interactions in mice. The role of
platelets in the spread of malignant disease. Int J Cancer.
11:704–718. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nilsson RJA, Balaj L, Hulleman E, van Rijn
S, Pegtel DM, Walraven M, Widmark A, Gerritsen WR, Verheul HM,
Vandertop WP, et al: Blood platelets contain tumor-derived RNA
biomarkers. Blood. 118:3680–3683. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuznetsov HS, Marsh T, Markens BA, Castaño
Z, Greene-Colozzi A, Hay SA, Brown VE, Richardson AL, Signoretti S,
Battinelli EM and McAllister SS: Identification of luminal breast
cancers that establish a tumor-supportive macroenvironment defined
by proangiogenic platelets and bone marrow-derived cells. Cancer
Discov. 2:1150–1165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kerr BA, McCabe NP, Feng W and Byzova TV:
Platelets govern pre-metastatic tumor communication to bone.
Oncogene. 32:4319–4324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Costantini V, Zacharski LR, Moritz TE and
Edwards RL: The platelet count in carcinoma of the lung and colon.
Thromb Haemost. 64:501–505. 1990. View Article : Google Scholar
|
|
18
|
Pedersen LM and Milman N: Prognostic
significance of thrombocytosis in patients with primary lung
cancer. Eur Respir J. 9:1826–1830. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang X, Wong KHK, Khankhel AH, Zeinali M,
Reategui E, Phillips MJ, Luo X, Aceto N, Fachin F, Hoang AN, et al:
Microfluidic isolation of platelet-covered circulating tumor cells.
Lab Chip. 17:3498–3503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lim M, Park S, Jeong H, Park SH, Kumar S,
Jang A, Lee S, Kim DU and Cho Y: Circulating tumor cell clusters
are cloaked with platelets and correlate with poor prognosis in
unresectable pancreatic cancer. Cancers (Basel). 13:52722021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Xu P, Zhang G, Wang D, Ye B and Wu
L: Advances and potentials in platelet-circulating tumor cell
crosstalk. Am J Cancer Res. 15:407–425. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Erpenbeck L and Schön MP: Deadly allies:
The fatal interplay between platelets and metastasizing cancer
cells. Blood. 115:3427–3436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goh CY, Patmore S, Smolenski A, Howard J,
Evans S, O'Sullivan J and McCann A: The role of von Willebrand
factor in breast cancer metastasis. Transl Oncol. 14:1010332021.
View Article : Google Scholar
|
|
24
|
Kitagawa H, Yamamoto N, Yamamoto K, Tanoue
K, Kosaki G and Yamazaki H: Involvement of platelet membrane
glycoprotein Ib and glycoprotein IIb/IIIa complex in
thrombin-dependent and -independent platelet aggregations induced
by tumor cells. Cancer Res. 49:537–541. 1989.PubMed/NCBI
|
|
25
|
Lonsdorf AS, Krämer BF, Fahrleitner M,
Schönberger T, Gnerlich S, Ring S, Gehring S, Schneider SW, Kruhlak
MJ, Meuth SG, et al: Engagement of αIIbβ3 (GPIIb/IIIa) with ανβ3
integrin mediates interaction of melanoma cells with platelets: A
connection to hematogenous metastasis. J Biol Chem. 287:2168–2178.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mammadova-Bach E, Gil-Pulido J,
Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, Stegner D,
Remer K, Nurden P, Nurden AT, et al: Platelet glycoprotein VI
promotes metastasis through interaction with cancer cell-derived
galectin-3. Blood. 135:1146–1160. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saha B, Mathur T, Tronolone JJ, Chokshi M,
Lokhande GK, Selahi A, Gaharwar AK, Afshar-Kharghan V, Sood AK, Bao
G and Jain A: Human tumor microenvironment chip evaluates the
consequences of platelet extravasation and combinatorial
antitumor-antiplatelet therapy in ovarian cancer. Sci Adv.
7:eabg52832021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eliceiri BP and Cheresh DA: The role of
alphav integrins during angiogenesis: Insights into potential
mechanisms of action and clinical development. J Clin Invest.
103:1227–1230. 1999. View Article : Google Scholar
|
|
29
|
Mammadova-Bach E, Zigrino P, Brucker C,
Bourdon C, Freund M, De Arcangelis A, Abrams SI, Orend G, Gachet C
and Mangin PH: Platelet integrin α 6 β 1 controls lung metastasis
through direct binding to cancer cell-derived ADAM9. JCI Insight.
1:e882452016. View Article : Google Scholar
|
|
30
|
Carpinteiro A, Becker KA, Japtok L,
Hessler G, Keitsch S, Požgajovà M, Schmid KW, Adams C, Müller S,
Kleuser B, et al: Regulation of hematogenous tumor metastasis by
acid sphingomyelinase. EMBO Mol Med. 7:714–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carpinteiro A, Beckmann N, Seitz A,
Hessler G, Wilker B, Soddemann M, Helfrich I, Edelmann B, Gulbins E
and Becker KA: Role of acid sphingomyelinase-induced signaling in
melanoma cells for hematogenous tumor metastasis. Cell Physiol
Biochem. 38:1–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McCarty OJ, Mousa SA, Bray PF and
Konstantopoulos K: Immobilized platelets support human colon
carcinoma cell tethering, rolling, and firm adhesion under dynamic
flow conditions. Blood. 96:1789–1797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shu L, Lin S, Zhou S and Yuan T:
Glycan-Lectin interactions between platelets and tumor cells drive
hematogenous metastasis. Platelets. 35:23150372024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Egan K, Cooke N and Kenny D: Living in
shear: Platelets protect cancer cells from shear induced damage.
Clin Exp Metastasis. 31:697–704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nieswandt B, Hafner M, Echtenacher B and
Männel DN: Lysis of tumor cells by natural killer cells in mice is
impeded by platelets. Cancer Res. 59:1295–1300. 1999.PubMed/NCBI
|
|
36
|
Placke T, Kopp H and Salih HR: Modulation
of natural killer cell anti-tumor reactivity by platelets. J Innate
Immun. 3:374–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anvari S, Osei E and Maftoon N:
Interactions of platelets with circulating tumor cells contribute
to cancer metastasis. Sci Rep. 11:154772021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fabricius HÅ, Starzonek S and Lange T: The
role of platelet cell surface P-selectin for the direct
platelet-tumor cell contact during metastasis formation in human
tumors. Front Oncol. 11:6427612021. View Article : Google Scholar
|
|
39
|
Ling T and Liu J, Dong L and Liu J: The
roles of P-selectin in cancer cachexia. Med Oncol. 40:3382023.
View Article : Google Scholar
|
|
40
|
Liu Y, Zhao F, Gu W, Yang H, Meng Q, Zhang
Y, Yang H and Duan Q: The roles of platelet GPIIb/IIIa and
alphavbeta3 integrins during HeLa cells adhesion, migration, and
invasion to monolayer endothelium under static and dynamic shear
flow. J Biomed Biotechnol. 2009:8292432009. View Article : Google Scholar
|
|
41
|
Schlesinger M: Role of platelets and
platelet receptors in cancer metastasis. J Hematol Oncol.
11:1252018. View Article : Google Scholar
|
|
42
|
Ren J, He J, Zhang H, Xia Y, Hu Z,
Loughran P, Billiar T, Huang H and Tsung A: Platelet TLR4-ERK5 axis
facilitates NET-mediated capturing of circulating tumor cells and
distant metastasis after surgical stress. Cancer Res. 81:2373–2385.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cui H, Tan YX, Österholm C, Zhang X, Hedin
U, Vlodavsky I and Li JP: Heparanase expression upregulates
platelet adhesion activity and thrombogenicity. Oncotarget.
7:39486–39496. 2016. View Article : Google Scholar
|
|
44
|
Li H, Yu Y, Gao L, Zheng P, Liu X and Chen
H: Tissue factor: A neglected role in cancer biology. J Thromb
Thrombolysis. 54:97–108. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Labelle M, Begum S and Hynes RO: Direct
signaling between platelets and cancer cells induces an
epithelial-mesenchymal-like transition and promotes metastasis.
Cancer Cell. 20:576–590. 2011. View Article : Google Scholar
|
|
47
|
Medina VA and Rivera ES: Histamine
receptors and cancer pharmacology. Br J Pharmacol. 161:755–767.
2010. View Article : Google Scholar
|
|
48
|
Schumacher D, Strilic B, Sivaraj KK,
Wettschureck N and Offermanns S: Platelet-derived nucleotides
promote tumor-cell transendothelial migration and metastasis via
P2Y2 receptor. Cancer Cell. 24:130–137. 2013. View Article : Google Scholar
|
|
49
|
Skolnik G, Bagge U, Blomqvist G, Djärv L
and Ahlman H: The role of calcium channels and serotonin (5-HT2)
receptors for tumour cell lodgement in the liver. Clin Exp
Metastasis. 7:169–174. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhong C, Wang W, Yao Y, Lian S, Xie X, Xu
J, He S, Luo L, Ye Z, Zhang J, et al: TGF-β secreted by cancer
cells-platelets interaction activates cancer metastasis potential
by inducing metabolic reprogramming and bioenergetic adaptation. J
Cancer. 16:1310–1323. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tong H, Li K, Zhou M, Wu R, Yang H, Peng
Z, Zhao Q and Luo KQ: Coculture of cancer cells with platelets
increases their survival and metastasis by activating the
TGFβ/Smad/PAI-1 and PI3K/AKT pathways. Int J Biol Sci.
19:4259–4277. 2023. View Article : Google Scholar
|
|
52
|
Eslami-S Z, Cortés-Hernández LE,
Glogovitis I, Antunes-Ferreira M, D Ambrosi S, Kurma K, Garima F,
Cayrefourcq L, Best MG, Koppers-Lalic D, et al: In vitro cross-talk
between metastasis-competent circulating tumor cells and platelets
in colon cancer: A malicious association during the harsh journey
in the blood. Front Cell Dev Biol. 11:12098462023. View Article : Google Scholar
|
|
53
|
Filippelli A, Del Gaudio C, Simonis V,
Ciccone V, Spini A and Donnini S: Scoping review on platelets and
tumor angiogenesis: Do we need more evidence or better analysis?
Int J Mol Sci. 23:134012022. View Article : Google Scholar
|
|
54
|
Ghosh LD, Mathur T, Tronolone JJ, Chuong
A, Rangel K, Corvigno S, Sood AK and Jain A: Angiogenesis-enabled
human ovarian tumor microenvironment-chip evaluates pathophysiology
of platelets in microcirculation. Adv Healthc Mater.
13:e23042632024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gleissner CA: Platelet-derived chemokines
in atherogenesis: What's new? Curr Vasc Pharmacol. 10:563–569.
2012. View Article : Google Scholar
|
|
56
|
Labelle M, Begum S and Hynes RO: Platelets
guide the formation of early metastatic niches. Proc Natl Acad Sci
USA. 111:E3053–E3061. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang X, Zhao S, Wang Z and Gao T:
Platelets involved tumor cell EMT during circulation:
Communications and interventions. Cell Commun Signal. 20:822022.
View Article : Google Scholar
|
|
58
|
Chen J, Yuan W, Wu L, Tang Q, Xia Q, Ji J,
Liu Z, Ma Z, Zhou Z, Cheng Y and Shu X: PDGF-D promotes cell
growth, aggressiveness, angiogenesis and EMT transformation of
colorectal cancer by activation of Notch1/Twist1 pathway.
Oncotarget. 8:9961–9973. 2017. View Article : Google Scholar
|
|
59
|
Zhang H, Sun JD, Yan LJ and Zhao XP:
PDGF-D/PDGFRβ promotes tongue squamous carcinoma cell (TSCC)
progression via activating p38/AKT/ERK/EMT signal pathway. Biochem
Biophys Res Commun. 478:845–851. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Takagi S, Sasaki Y, Koike S, Takemoto A,
Seto Y, Haraguchi M, Ukaji T, Kawaguchi T, Sugawara M, Saito M, et
al: Platelet-derived lysophosphatidic acid mediated LPAR1
activation as a therapeutic target for osteosarcoma metastasis.
Oncogene. 40:5548–5558. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Plantureux L, Mège D, Crescence L,
Carminita E, Robert S, Cointe S, Brouilly N, Ezzedine W,
Dignat-George F, Dubois C and Panicot-Dubois L: The interaction of
platelets with colorectal cancer cells inhibits tumor growth but
promotes metastasis. Cancer Res. 80:291–303. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li W, Liu JB, Hou LK, Yu F, Zhang J, Wu W,
Tang XM, Sun F, Lu HM, Deng J, et al: Liquid biopsy in lung cancer:
Significance in diagnostics, prediction, and treatment monitoring.
Mol Cancer. 21:252022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Murphy L, Inchauspé J, Valenzano G,
Holland P, Sousos N, Belnoue-Davis HL, Li R, Jooss NJ, Benlabiod C,
Murphy E, et al: Platelets sequester extracellular DNA, capturing
tumor-derived and free fetal DNA. Science. 389:eadp39712025.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Roweth HG and Battinelli EM: Lessons to
learn from tumor-educated platelets. Blood. 137:3174–3180. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Best MG, Wesseling P and Wurdinger T:
Tumor-educated platelets as a noninvasive biomarker source for
cancer detection and progression monitoring. Cancer Res.
78:3407–3412. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Strasenburg W, Jóźwicki J, Durślewicz J,
Kuffel B, Kulczyk MP, Kowalewski A, Grzanka D, Drewa T and
Adamowicz J: Tumor cell-induced platelet aggregation as an emerging
therapeutic target for cancer therapy. Front Oncol. 12:9097672022.
View Article : Google Scholar
|
|
67
|
Ding S, Dong X and Song X: Tumor educated
platelet: The novel biosource for cancer detection. Cancer Cell
Int. 23:912023. View Article : Google Scholar
|
|
68
|
Amirkhosravi A, Amaya M, Desai H and
Francis JL: Platelet-CD40 ligand interaction with melanoma cell and
monocyte CD40 enhances cellular procoagulant activity. Blood Coagul
Fibrinolysis. 13:505–512. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mezouar S, Darbousset R, Dignat-George F,
Panicot-Dubois L and Dubois C: Inhibition of platelet activation
prevents the P-selectin and integrin-dependent accumulation of
cancer cell microparticles and reduces tumor growth and metastasis
in vivo. Int J Cancer. 136:462–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Miyazaki M, Nakabo A, Nagano Y, Nagamura
Y, Yanagihara K, Ohki R, Nakamura Y, Fukami K, Kawamoto J,
Umayahara K, et al: Tissue factor-induced fibrinogenesis mediates
cancer cell clustering and multiclonal peritoneal metastasis.
Cancer Lett. 553:2159832023. View Article : Google Scholar
|
|
71
|
Zhang Y, Li Z, Zhang J, Mafa T, Zhang J,
Zhu H, Chen L, Zong Z and Yang L: Fibrinogen: A new player and
target on the formation of pre-metastatic niche in tumor
metastasis. Crit Rev Oncol Hematol. 207:1046252025. View Article : Google Scholar
|
|
72
|
Catani MV, Savini I, Tullio V and Gasperi
V: The ‘janus face’ of platelets in cancer. Int J Mol Sci.
21:7882020. View Article : Google Scholar
|
|
73
|
Allegra A, Cancemi G, Mirabile G, Tonacci
A, Musolino C and Gangemi S: Circulating tumour cells, cell free
DNA and tumour-educated platelets as reliable prognostic and
management biomarkers for the liquid biopsy in multiple myeloma.
Cancers (Basel). 14:41362022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Feng W, Jia N, Jiao H, Chen J, Chen Y,
Zhang Y, Zhu M, Zhu C, Shen L and Long W: Circulating tumor DNA as
a prognostic marker in high-risk endometrial cancer. J Transl Med.
19:512021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hu H, Song H, Han B, Zhao H and He J:
Tumor-educated platelet RNA and circulating free RNA: Emerging
liquid biopsy markers for different tumor types. Front Biosci
(Landmark Ed). 29:802024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pereira-Veiga T, Martínez-Fernández M,
Abuin C, Piñeiro R, Cebey V, Cueva J, Palacios P, Blanco C,
Muinelo-Romay L, Abalo A, et al: CTCs expression profiling for
advanced breast cancer monitoring. Cancers (Basel). 11:19412019.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li TT, Liu H, Yu J, Shi GY, Zhao LY and Li
GX: Prognostic and predictive blood biomarkers in gastric cancer
and the potential application of circulating tumor cells. World J
Gastroenterol. 24:2236–2246. 2018. View Article : Google Scholar
|
|
78
|
Haemmerle M, Stone RL, Menter DG,
Afshar-Kharghan V and Sood AK: The platelet lifeline to cancer:
Challenges and opportunities. Cancer Cell. 33:965–983. 2018.
View Article : Google Scholar
|
|
79
|
Gao L, Zhang H, Zhang B, Zhang L and Wang
C: Prognostic value of combination of preoperative platelet count
and mean platelet volume in patients with resectable non-small cell
lung cancer. Oncotarget. 8:15632–15641. 2017. View Article : Google Scholar
|
|
80
|
Mandaliya H, Jones M, Oldmeadow C and
Nordman IIC: Prognostic biomarkers in stage IV non-small cell lung
cancer (NSCLC): Neutrophil to lymphocyte ratio (NLR), lymphocyte to
monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and
advanced lung cancer inflammation index (ALI). Transl Lung Cancer
Res. 8:886–894. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Palacios-Acedo AL, Mège D, Crescence L,
Dignat-George F, Dubois C and Panicot-Dubois L: Platelets,
thrombo-inflammation, and cancer: Collaborating with the enemy.
Front Immunol. 10:18052019. View Article : Google Scholar
|
|
82
|
Kobayashi S, Sugasaki A, Yamamoto Y,
Shigenoi Y, Udaka A, Yamamoto A and Tanaka M: Enrichment of cancer
cells based on antibody-free selective cell adhesion. ACS Biomater
Sci Eng. 8:4547–4556. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ma Y, Zhang J, Tian Y, Fu Y, Tian S, Li Q,
Yang J and Zhang L: Zwitterionic microgel preservation platform for
circulating tumor cells in whole blood specimen. Nat Commun.
14:49582023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Plantureux L, Mège D, Crescence L,
Dignat-George F, Dubois C and Panicot-Dubois L: Impacts of cancer
on platelet production, activation and education and mechanisms of
cancer-associated thrombosis. Cancers (Basel). 10:4412018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Best MG, Sol N, Kooi I, Tannous J,
Westerman BA, Rustenburg F, Schellen P, Verschueren H, Post E,
Koster J, et al: RNA-seq of tumor-educated platelets enables
blood-based pan-cancer, multiclass, and molecular pathway cancer
diagnostics. Cancer Cell. 28:666–676. 2015. View Article : Google Scholar
|
|
86
|
D'Ambrosi S, Nilsson RJ and Wurdinger T:
Platelets and tumor-associated RNA transfer. Blood. 137:3181–3191.
2021. View Article : Google Scholar
|
|
87
|
Heinhuis KM, In't Veld SGJG, Dwarshuis G,
van den Broek D, Sol N, Best MG, Coevorden FV, Haas RL, Beijnen JH,
van Houdt WJ, et al: RNA-sequencing of tumor-educated platelets, a
novel biomarker for blood-based sarcoma diagnostics. Cancers
(Basel). 12:13722020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Antunes-Ferreira M, D Ambrosi S, Arkani M,
Post E, In't Veld SGJG, Ramaker J, Zwaan K, Kucukguzel ED, Wedekind
LE, Griffioen AW, et al: Tumor-educated platelet blood tests for
non-small cell lung cancer detection and management. Sci Rep.
13:93592023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gao Y, Liu CJ, Li HY, Xiong XM, Li GL,
In't Veld SGJG, Cai GY, Xie GY, Zeng SQ, Wu Y, et al: Platelet RNA
enables accurate detection of ovarian cancer: An intercontinental,
biomarker identification study. Protein Cell. 14:579–590. 2023.
|
|
90
|
Sol N, In't Veld SGJG, Vancura A,
Tjerkstra M, Leurs C, Rustenburg F, Schellen P, Verschueren H, Post
E, Zwaan K, et al: Tumor-educated platelet RNA for the detection
and (pseudo)progression monitoring of glioblastoma. Cell Rep Med.
1:1001012020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Mantini G, Meijer LL, Glogovitis I, In't
Veld SGJG, Paleckyte R, Capula M, Le Large TYS, Morelli L, Pham TV,
Piersma SR, et al: Omics analysis of educated platelets in cancer
and benign disease of the pancreas. Cancers (Basel). 13:662020.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Łukasiewicz M, Pastuszak K,
Łapińska-Szumczyk S, Różański R, Veld SGJG, Bieńkowski M, Stokowy
T, Ratajska M, Best MG, Würdinger T, et al: Diagnostic accuracy of
liquid biopsy in endometrial cancer. Cancers (Basel).
13:57312021.
|
|
93
|
Dong X, Ding S, Yu M, Niu L, Xue L, Zhao
Y, Xie L and Song X and Song X: Small nuclear RNAs (U1, U2, U5) in
tumor-educated platelets are downregulated and act as promising
biomarkers in lung cancer. Front Oncol. 10:16272020. View Article : Google Scholar
|
|
94
|
Xu L, Li X, Li X, Wang X, Ma Q, She D, Lu
X, Zhang J, Yang Q, Lei S, et al: RNA profiling of blood platelets
noninvasively differentiates colorectal cancer from healthy donors
and noncancerous intestinal diseases: A retrospective cohort study.
Genome Med. 14:262022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
In't Veld SGJG, Arkani M, Post E,
Antunes-Ferreira M, D'Ambrosi S, Vessies DCL, Vermunt L, Vancura A,
Muller M, Niemeijer AN, et al: Detection and localization of early-
and late-stage cancers using platelet RNA. Cancer Cell.
40:999–1009.e6. 2022. View Article : Google Scholar
|
|
96
|
Xiao R, Liu C, Zhang B and Ma L:
Tumor-educated platelets as a promising biomarker for blood-based
detection of renal cell carcinoma. Front Oncol. 12:8445202022.
View Article : Google Scholar
|
|
97
|
Lomnytska M, Pinto R, Becker S, Engström
U, Gustafsson S, Björklund C, Templin M, Bergstrand J, Xu L,
Widengren J, et al: Platelet protein biomarker panel for ovarian
cancer diagnosis. Biomark Res. 6:22018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Sabrkhany S, Kuijpers MJE, Knol JC, Olde
Damink SWM, Dingemans AC, Verheul HM, Piersma SR, Pham TV,
Griffioen AW, Oude Egbrink MGA and Jimenez CR: Exploration of the
platelet proteome in patients with early-stage cancer. J
Proteomics. 177:65–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tao DL, Tassi Yunga S, Williams CD and
McCarty OJT: Aspirin and antiplatelet treatments in cancer. Blood.
137:3201–3211. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Johnson KE, Ceglowski JR, Roweth HG,
Forward JA, Tippy MD, El-Husayni S, Kulenthirarajan R, Malloy MW,
Machlus KR, Chen WY, et al: Aspirin inhibits platelets from
reprogramming breast tumor cells and promoting metastasis. Blood
Adv. 3:198–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
McCarty MF and Block KI: Preadministration
of high-dose salicylates, suppressors of NF-kappaB activation, may
increase the chemosensitivity of many cancers: An example of
proapoptotic signal modulation therapy. Integr Cancer Ther.
5:252–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chan AT and Ladabaum U: Where do we stand
with aspirin for the prevention of colorectal cancer? The USPSTF
recommendations. Gastroenterology. 150:14–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Chubak J, Whitlock EP, Williams SB,
Kamineni A, Burda BU, Buist DSM and Anderson ML: Aspirin for the
prevention of cancer incidence and mortality: Systematic evidence
reviews for the U.S. Preventive services task force. Ann Intern
Med. 164:814–825. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bruno A, Dovizio M, Tacconelli S, Contursi
A, Ballerini P and Patrignani P: Antithrombotic agents and cancer.
Cancers (Basel). 10:2532018. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Cho MS, Noh K, Haemmerle M, Li D, Park H,
Hu Q, Hisamatsu T, Mitamura T, Mak SLC, Kunapuli S, et al: Role of
ADP receptors on platelets in the growth of ovarian cancer. Blood.
130:1235–1242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guillem-Llobat P, Dovizio M, Bruno A,
Ricciotti E, Cufino V, Sacco A, Grande R, Alberti S, Arena V,
Cirillo M, et al: Aspirin prevents colorectal cancer metastasis in
mice by splitting the crosstalk between platelets and tumor cells.
Oncotarget. 7:32462–32477. 2016. View Article : Google Scholar
|
|
107
|
Xu XR, Yousef GM and Ni H: Cancer and
platelet crosstalk: Opportunities and challenges for aspirin and
other antiplatelet agents. Blood. 131:1777–1789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang C, Liu Y, Gao Y, Shen J, Zheng S,
Wei M and Zeng X: Modified heparins inhibit integrin
alpha(IIb)beta(3) mediated adhesion of melanoma cells to platelets
in vitro and in vivo. Int J Cancer. 125:2058–2065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kononczuk J, Surazynski A, Czyzewska U,
Prokop I, Tomczyk M, Palka J and Miltyk W: αIIbβ3-integrin ligands:
Abciximab and eptifibatide as proapoptotic factors in MCF-7 human
breast cancer cells. Curr Drug Targets. 16:1429–1437. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yap ML, McFadyen JD, Wang X, Zia NA,
Hohmann JD, Ziegler M, Yao Y, Pham A, Harris M, Donnelly PS, et al:
Targeting activated platelets: A unique and potentially universal
approach for cancer imaging. Theranostics. 7:2565–2574. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Yap ML, McFadyen JD, Wang X, Ziegler M,
Chen YC, Willcox A, Nowell CJ, Scott AM, Sloan EK, Hogarth PM, et
al: Activated platelets in the tumor microenvironment for targeting
of antibody-drug conjugates to tumors and metastases. Theranostics.
9:1154–1169. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Dovizio M, Maier TJ, Alberti S, Di
Francesco L, Marcantoni E, Münch G, John CM, Suess B, Sgambato A,
Steinhilber D, et al: Pharmacological inhibition of platelet-tumor
cell cross-talk prevents platelet-induced overexpression of
cyclooxygenase-2 in HT29 human colon carcinoma cells. Mol
Pharmacol. 84:25–40. 2013. View Article : Google Scholar
|
|
113
|
Tsai HJ, Cheng KW, Li JC, Ruan TX, Chang
TH, Wang JR and Tseng CP: Identification of podoplanin aptamers by
SELEX for protein detection and inhibition of platelet aggregation
stimulated by C-type lectin-like receptor 2. Biosensors (Basel).
14:4642024. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Erpenbeck L, Nieswandt B, Schön M,
Pozgajova M and Schön MP: Inhibition of platelet GPIb alpha and
promotion of melanoma metastasis. J Invest Dermatol. 130:576–586.
2010. View Article : Google Scholar
|
|
115
|
Xu P, Zuo H, Chen B, Wang R, Ahmed A, Hu Y
and Ouyang J: Doxorubicin-loaded platelets as a smart drug delivery
system: An improved therapy for lymphoma. Sci Rep. 7:426322017.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Zhao J, Ye H, Lu Q, Wang K, Chen X, Song
J, Wang H, Lu Y, Cheng M, He Z, et al: Inhibition of post-surgery
tumour recurrence via a sprayable chemo-immunotherapy gel releasing
PD-L1 antibody and platelet-derived small EVs. J Nanobiotechnology.
20:622022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Schnoor B, Morris K, Kottana RK, Muldoon
R, Barron J and Papa AL: Fibrinolytic platelet decoys reduce cancer
metastasis by dissociating circulating tumor cell clusters. Adv
Healthc Mater. 13:e23043742024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Li J, Sharkey CC, Wun B, Liesveld JL and
King MR: Genetic engineering of platelets to neutralize circulating
tumor cells. J Control Release. 228:38–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang C, Sun W, Ye Y, Hu Q, Bomba HN and Gu
Z: In situ activation of platelets with checkpoint inhibitors for
post-surgical cancer immunotherapy. Nat Biomed Eng. 1:00112017.
View Article : Google Scholar
|
|
120
|
Hu Q, Li H, Archibong E, Chen Q, Ruan H,
Ahn S, Dukhovlinova E, Kang Y, Wen D, Dotti G and Gu Z: Inhibition
of post-surgery tumour recurrence via a hydrogel releasing CAR-T
cells and anti-PDL1-conjugated platelets. Nat Biomed Eng.
5:1038–1047. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Yang Y, Wang Y, Yao Y, Wang S, Zhang Y,
Dotti G, Yu J and Gu Z: T cell-mimicking platelet-drug conjugates.
Matter. 6:2340–2355. 2023. View Article : Google Scholar
|
|
122
|
Chen M, Wang P, Jiang D, Bao Z and Quan H:
Platelet membranes coated gold nanocages for tumor targeted drug
delivery and amplificated low-dose radiotherapy. Front Oncol.
11:7930062021. View Article : Google Scholar
|
|
123
|
Xia D, Hang D, Li Y, Jiang W, Zhu J, Ding
Y, Gu H and Hu Y: Au-hemoglobin loaded platelet alleviating tumor
hypoxia and enhancing the radiotherapy effect with low-dose X-ray.
ACS Nano. 14:15654–15668. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Sim X, Poncz M, Gadue P and French DL:
Understanding platelet generation from megakaryocytes: Implications
for in vitro-derived platelets. Blood. 127:1227–1233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhou Z, Zhang B, Zai W, Kang L, Yuan A, Hu
Y and Wu J: Perfluorocarbon nanoparticle-mediated platelet
inhibition promotes intratumoral infiltration of T cells and boosts
immunotherapy. Proc Natl Acad Sci USA. 116:11972–11977. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ke Y, Ma Z, Ye H, Guan X, Xiang Z, Xia Y
and Shi Q: Chlorogenic acid-conjugated nanoparticles suppression of
platelet activation and disruption to tumor vascular barriers for
enhancing drug penetration in tumor. Adv Healthc Mater.
12:22022052023. View Article : Google Scholar
|
|
127
|
Li J, Ai Y, Wang L, Bu P, Sharkey CC, Wu
Q, Wun B, Roy S, Shen X and King MR: Targeted drug delivery to
circulating tumor cells via platelet membrane-functionalized
particles. Biomaterials. 76:52–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu R, Xu B, Ma Z, Ye H, Guan X, Ke Y,
Xiang Z and Shi Q: Controlled release of nitric oxide for enhanced
tumor drug delivery and reduction of thrombosis risk. RSC Adv.
12:32355–32364. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Li S, Zhang Y, Wang J, Zhao Y, Ji T, Zhao
X, Ding Y, Zhao X, Zhao R, Li F, et al: Nanoparticle-mediated local
depletion of tumour-associated platelets disrupts vascular barriers
and augments drug accumulation in tumours. Nat Biomed Eng.
1:667–679. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Luo S, Feng J, Xiao L, Guo L, Deng L, Du
Z, Xue Y, Song X, Sun X, Zhang Z, et al: Targeting self-assembly
peptide for inhibiting breast tumor progression and metastasis.
Biomaterials. 249:1200552020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Dhandapani R, Sethuraman S, Krishnan UM
and Subramanian A: Self-assembled multifunctional nanotheranostics
against circulating tumor clusters in metastatic breast cancer.
Acta Pharm Sin B. 13:1711–1725. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Smit DJ, Pantel K and Jücker M:
Circulating tumor cells as a promising target for individualized
drug susceptibility tests in cancer therapy. Biochem Pharmacol.
188:1145892021. View Article : Google Scholar
|