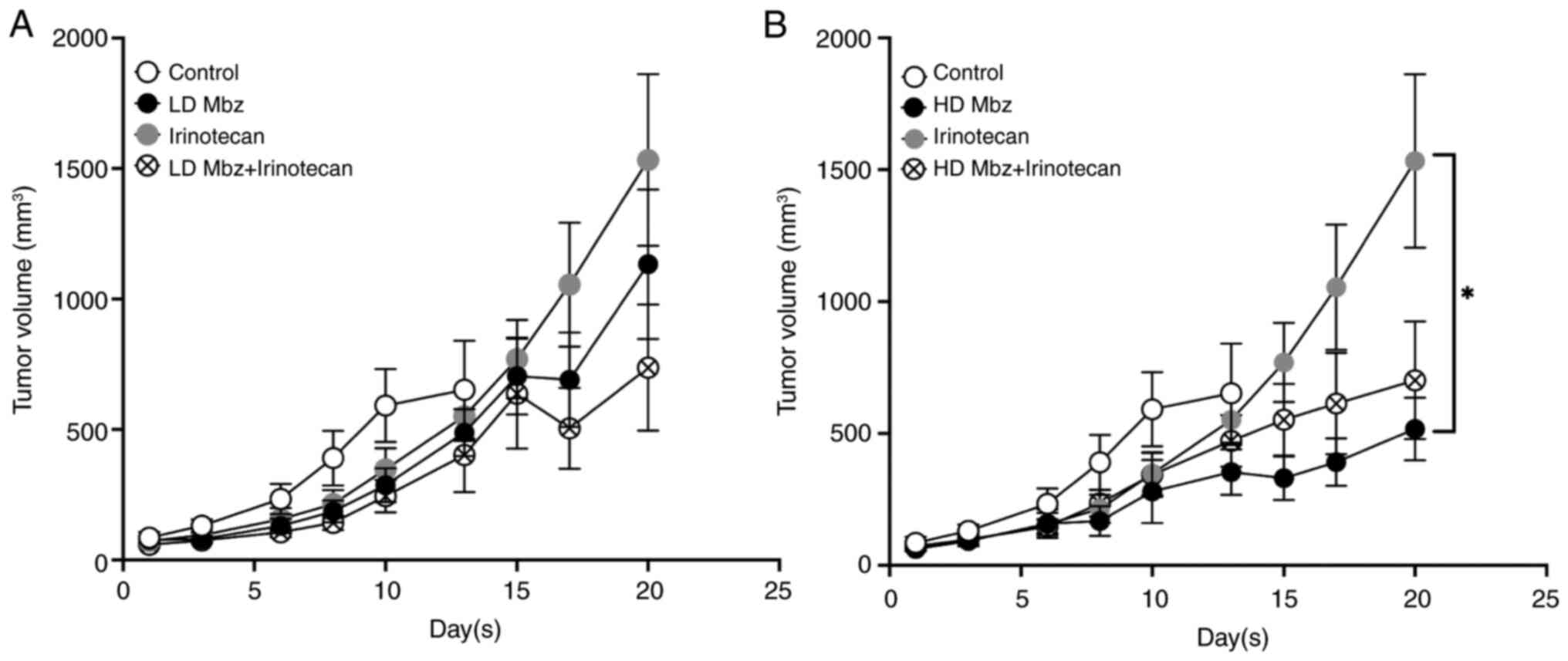
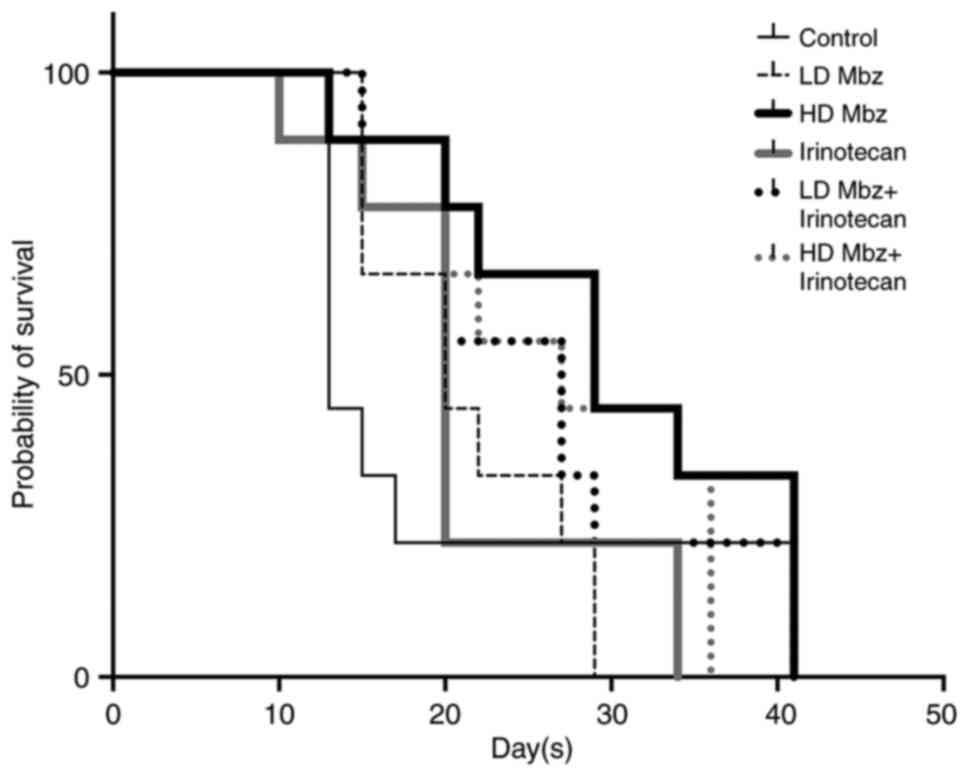
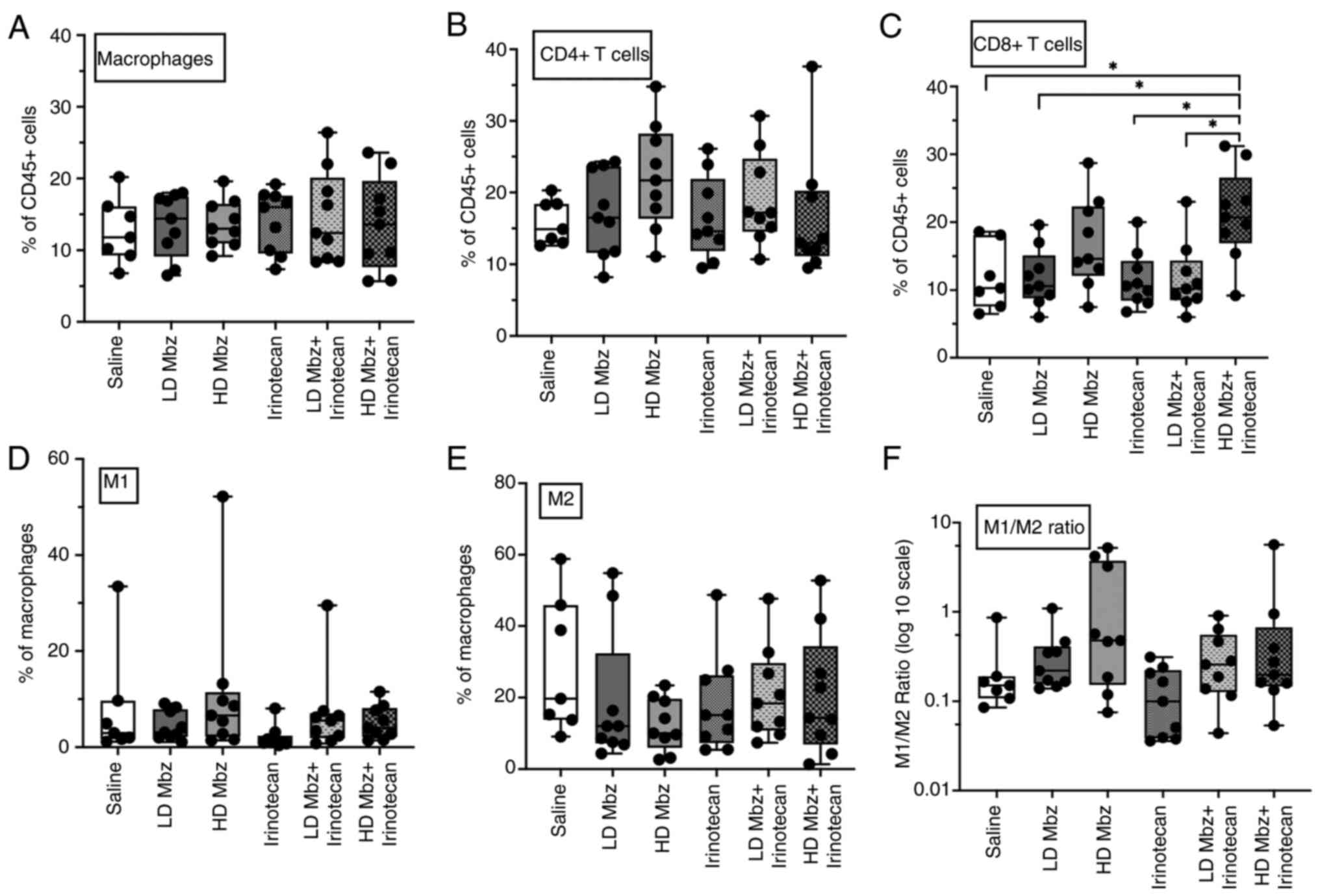
|
1
|
Pantziarka P, Bouche G, Meheus L, Sukhatme
V and Sukhatme VP: Repurposing drugs in oncology (ReDO)-mebendazole
as an anti-cancer agent. Ecancermedicalscience. 8:4432014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mukhopadhyay T, Sasaki J, Ramesh R and
Roth JA: Mebendazole elicits a potent antitumor effect on human
cancer cell lines both in vitro and in vivo. Clin Cancer Res.
8:2963–2969. 2002.PubMed/NCBI
|
|
3
|
Martarelli D, Pompei P, Baldi C and
Mazzoni G: Mebendazole inhibits growth of human adrenocortical
carcinoma cell lines implanted in nude mice. Cancer Chemother
Pharmacol. 61:809–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doudican NA, Byron SA, Pollock PM and
Orlow SJ: XIAP downregulation accompanies mebendazole growth
inhibition in melanoma xenografts. Anticancer Drugs. 24:181–188.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai RY, Staedtke V, Aprhys CM, Gallia GL
and Riggins GJ: Antiparasitic mebendazole shows survival benefit in
2 preclinical models of glioblastoma multiforme. Neuro Oncol.
13:974–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaki J, Ramesh R, Chada S, Gomyo Y, Roth
JA and Mukhopadhyay T: The anthelmintic drug mebendazole induces
mitotic arrest and apoptosis by depolymerizing tubulin in non-small
cell lung cancer cells. Mol Cancer Ther. 1:1201–1209.
2002.PubMed/NCBI
|
|
7
|
Pourgholami MH, Cai ZY, Chu SW, Galettis P
and Morris DL: The influence of ovarian cancer induced peritoneal
carcinomatosis on the pharmacokinetics of albendazole in nude mice.
Anticancer Res. 30:423–428. 2010.PubMed/NCBI
|
|
8
|
Aliabadi A, Haghshenas MR, Kiani R,
Koohi-Hosseinabadi O, Purkhosrow A, Pirsalami F, Panjehshahin MR
and Erfani N: In vitro and in vivo anticancer activity of
mebendazole in colon cancer: A promising drug repositioning. Naunyn
Schmiedebergs Arch Pharmacol. 397:2379–2388. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nygren P and Larsson R: Drug repositioning
from bench to bedside: Tumour remission by the antihelmintic drug
mebendazole in refractory metastatic colon cancer. Acta Oncol.
53:427–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dobrosotskaya IY, Hammer GD, Schteingart
DE, Maturen KE and Worden FP: Mebendazole monotherapy and long-term
disease control in metastatic adrenocortical carcinoma. Endocr
Pract. 17:e59–e62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nygren P, Fryknäs M, Agerup B and Larsson
R: Repositioning of the anthelmintic drug mebendazole for the
treatment for colon cancer. J Cancer Res Clin Oncol. 139:2133–2140.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai RY, Staedtke V, Rudin CM, Bunz F and
Riggins GJ: Effective treatment of diverse medulloblastoma models
with mebendazole and its impact on tumor angiogenesis. Neuro Oncol.
17:545–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larsen AR, Bai RY, Chung JH, Borodovsky A,
Rudin CM, Riggins GJ and Bunz F: Repurposing the antihelmintic
mebendazole as a hedgehog inhibitor. Mol Cancer Ther. 14:3–13.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blom K, Rubin J, Berglund M, Jarvius M,
Lenhammar L, Parrow V, Andersson C, Loskog A, Fryknäs M, Nygren P
and Larsson R: Mebendazole-induced M1 polarisation of THP-1
macrophages may involve DYRK1B inhibition. BMC Res Notes.
12:2342019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blom K, Senkowski W, Jarvius M, Berglund
M, Rubin J, Lenhammar L, Parrow V, Andersson C, Loskog A, Fryknäs
M, et al: The anticancer effect of mebendazole may be due to M1
monocyte/macrophage activation via ERK1/2 and TLR8-dependent
inflammasome activation. Immunopharmacol Immunotoxicol. 39:199–210.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubin J, Mansoori S, Blom K, Berglund M,
Lenhammar L, Andersson C, Loskog A, Fryknäs M, Nygren P and Larsson
R: Mebendazole stimulates CD14+ myeloid cells to enhance T-cell
activation and tumour cell killing. Oncotarget. 9:30805–30813.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bayat Mokhtari R, Homayouni TS, Baluch N,
Morgatskaya E, Kumar S, Das B and Yeger H: Combination therapy in
combating cancer. Oncotarget. 8:38022–38043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Law MR, Wald NJ, Morris JK and Jordan RE:
Value of low dose combination treatment with blood pressure
lowering drugs: Analysis of 354 randomised trials. BMJ.
326:14272003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foucquier J and Guedj M: Analysis of drug
combinations: Current methodological landscape. Pharmacol Res
Perspect. 3:e001492015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boshuizen J and Peeper DS: Rational cancer
treatment combinations: An urgent clinical need. Mol Cell.
78:1002–1018. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ocana A, Amir E, Yeung C, Seruga B and
Tannock IF: How valid are claims for synergy in published clinical
studies? Ann Oncol. 23:2161–2166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwangbo H, Patterson SC, Dai A, Plana D
and Palmer AC: Additivity predicts the efficacy of most approved
combination therapies for advanced cancer. Nat Cancer. 4:1693–1704.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simbulan-Rosenthal CM, Dakshanamurthy S,
Gaur A, Chen YS, Fang HB, Abdussamad M, Zhou H, Zapas J, Calvert V,
Petricoin EF, et al: The repurposed anthelmintic mebendazole in
combination with trametinib suppresses refractory NRASQ61K
melanoma. Oncotarget. 8:12576–12595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kipper FC, Silva AO, Marc AL, Confortin G,
Junqueira AV, Neto EP and Lenz G: Vinblastine and antihelmintic
mebendazole potentiate temozolomide in resistant gliomas. Invest
New Drugs. 36:323–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coyne CP, Jones T and Bear R:
Gemcitabine-(C4-amide)-[anti- HER2/neu] Anti-neoplastic
cytotoxicity in dual combination with mebendazole against
chemotherapeutic-resistant mammary adenocarcinoma. J Clin Exp
Oncol. 2:10001092013.PubMed/NCBI
|
|
26
|
Mansoori S, Fryknäs M, Alvfors C, Loskog
A, Larsson R and Nygren P: A phase 2a clinical study on the safety
and efficacy of individualized dosed mebendazole in patients with
advanced gastrointestinal cancer. Sci Rep. 11:89812021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hegazy SK, El-Azab GA, Zakaria F, Mostafa
MF and El-Ghoneimy RA: Mebendazole; from an anti-parasitic drug to
a promising candidate for drug repurposing in colorectal cancer.
Life Sci. 299:1205362022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gallia GL, Holdhoff M, Brem H, Joshi AD,
Hann CL, Bai RY, Staedtke V, Blakeley JO, Sengupta S, Jarrell TC,
et al: Mebendazole and temozolomide in patients with newly
diagnosed high-grade gliomas: Results of a phase 1 clinical trial.
Neurooncol Adv. 3:vdaa1542020.PubMed/NCBI
|
|
29
|
Krystal J, Hanson D, Donnelly D and Atlas
M: A phase 1 study of mebendazole with bevacizumab and irinotecan
in high-grade gliomas. Pediatr Blood Cancer. 71:e308742024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patil VM, Menon N, Chatterjee A, Tonse R,
Choudhari A, Mahajan A, Puranik AD, Epari S, Jadhav M, Pathak S, et
al: Mebendazole plus lomustine or temozolomide in patients with
recurrent glioblastoma: A randomised open-label phase II trial.
EClinicalMedicine. 49:1014492022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anand U, Dey A, Chandel AKS, Sanyal R,
Mishra A, Pandey DK, Falco VD, Upadhyay A, Kandimalla R, Chaudhary
A, et al: Cancer chemotherapy and beyond: Current status, drug
candidate, associated risks and progress in targeted therapeutics.
Genes Disease. 10:136–1401. 2022.
|
|
32
|
Blom K, Nygren P, Alvarsson J, Larsson R
and Andersson CR: Ex vivo assessment of drug activity in patient
tumor cells as a basis for tailored cancer therapy. J Lab Autom.
21:178–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Larsson R and Nygren P: Laboratory
prediction of clinical chemotherapeutic drug resistance: A working
model exemplified by acute leukaemia. Eur J Cancer. 29A:1208–1212.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lindhagen E, Nygren P and Larsson R: The
fluorometric microculture cytotoxicity assay. Nat Protoc.
3:1364–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karlsson H, Fryknäs M, Senkowski W,
Larsson R and Nygren P: Selective radiosensitization by
nitazoxanice of quiescent clonogenic colon cancer tumour cells.
Oncol Lett. 23:1232022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miles FL, Lynch JE and Sikes RA:
Cell-based assays using calcein acetoxymethyl ester show variation
in fluorescence with treatment conditions. J Biol Method.
2:e292015. View Article : Google Scholar
|
|
37
|
Ianevski A, Giri AK and Aittokallio T:
SynergyFinder 2.0: Visual analytics of Multi-drug combination
synergies. Nucleic Acids Res. 48:W488–W493. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ianevski A, Giri AK and Aittokallio T:
SynergyFinder 3.0: An interactive analysis and consensus
interpretation of multi-drug synergies across multiple samples.
Nucleic Acids Res. 50:W739–W743. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Loewe S: The problem of synergism and
antagonism of combined drugs. Arzneimittelforschung. 3:285–290.
1953.PubMed/NCBI
|
|
40
|
Bliss CI: The toxicity of poisions applied
jointly. Ann Appl Biol. 26:585–615. 1939. View Article : Google Scholar
|
|
41
|
Yadav B, Wennerberg K, Aittokallio T and
Tang J: Searching for drug synergy in complex dose-Response
landscapes using an interaction potency model. Comput Struct
Biotechnol J. 13:504–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Berenbaum MC: What is synergy? Pharmacol
Rev. 41:93–141. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moon CH, Lee SJ, Lee HY, Lee JC, Cha H,
Cho WJ, Park JW, Park HJ, Seo J, Lee YH, et al: KML001 displays
vascular disrupting properties and irinotecan combined antitumor
activities in a murine tumor model. PLoS One. 8:e539002013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Csóka K, Tholander B, Gerdin E, de la
Torre M, Larsson R and Nygren P: In vitro determination of
cytotoxic drug response in ovarian carcinoma using the fluorometric
microculture cytotoxicity assay (FMCA). Int J Cancer. 72:1008–1012.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
von Heideman A, Tholander B, Grundmark B,
Cajander S, Gerdin E, Holm L, Axelsson A, Rosenberg P, Mahteme H,
Daniel E, et al: Chemotherapeutic drug sensitivity of primary
cultures of epithelial ovarian cancer cells from patients in
relation to tumour characteristics and therapeutic outcome. Acta
Oncol. 53:242–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cashin PH, Söderström M, Blom K, Artursson
S, Andersson C, Larsson R and Nygren P: Ex vivo assessment of
chemotherapy sensitivity of colorectal cancer peritoneal
metastases. Br J Surg. 110:1080–1083. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bjersand K, Blom K, Poromaa IS, Stålberg
K, Lejon AM, Bäckman F, Nyberg Å, Andersson C, Larsson R and Nygren
P: Ex vivo assessment of cancer drug sensitivity in epithelial
ovarian cancer and its association with histopathological type,
treatment history and clinical outcome. Int J Oncol. 61:1282022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang X, Zhao J, Gao X, Pei D and Gao C:
Anthelmintic drug albendazole arrests human gastric cancer cells at
the mitotic phase and induces apoptosis. Exp Ther Med. 13:595–603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang L, Zhao L, Zhang J, He F, Wang H,
Liu Q, Shi D, Ni N, Wagstaff W, Chen C, et al: Antiparasitic
mebendazole (MBZ) effectively overcomes cisplatin resistance in
human ovarian cancer cells by inhibiting multiple cancer-associated
signaling pathways. Aging (Albany NY). 13:17407–17427. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Salvadores M, Fuster-Tormo F and Supek F:
Matching cell lines with cancer type and subtype of origin via
mutational, epigenomic, and transcriptomic patterns. Sci Adv.
6:eaba18622020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mirabelli P, Coppola L and Salvatore M:
Cancer cell lines are useful model systems for medical research.
Cancers (Basel). 11:10982019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guerini AE, Triggiani L, Maddalo M, Bonù
ML, Frassine F, Baiguini A, Alghisi A, Tomasini D, Borghetti P,
Pasinetti N, et al: Mebendazole as a candidate for drug repurposing
in oncology: An extensive review of current literature. Cancers
(Basel). 11:12842019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Champiat S, Ferrara R, Massard C, Besse B,
Marabelle A, Soria JC and Ferté C: Hyperprogressive disease:
Recognizing a novel pattern to improve patient management. Nat Rev
Clin Oncol. 15:748–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abu-Hdaib B, Nsairat H, El-Tanani M,
Al-Deeb I and Hasasna N: In vivo evaluation of mebendazole and Ran
GTPase inhibition in breast cancer model system. Nanomedicine
(Lond). 19:1087–1101. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Idrisova K, Simon H and Gomzikova M: Role
of patient-derived models of cancer in translational oncology.
Cancers. 15:1392022. View Article : Google Scholar : PubMed/NCBI
|