Introduction
Mebendazole (Mbz), a broad-spectrum anthelmintic
drug, is widely used to treat various helminthic infections but has
also shown anticancer activity (1).
In preclinical models, Mbz induces dose-dependent direct cytotoxic
effects in adrenocortical carcinoma, lung cancer, melanoma, ovarian
cancer and glioblastoma (2–7). Furthermore, arrest of tumor growth has
been observed in cancer xenograft mouse models (2–5,8).
Clinically meaningful activity of Mbz has also been reported in two
case reports of patients with colorectal cancer (CRC) and
adrenocortical carcinoma (9,10). The
anticancer properties of Mbz have been proposed to be due to direct
cytotoxic actions such as tubulin polymerization inhibition
(6), protein kinase inhibition
(11), anti-angiogenesis (12) and inhibition of the Hedgehog pathway
(13). More recently, we observed
that Mbz also induces an antitumoral immune response ex vivo
by activation of M1 macrophages through the ERK I/II and Toll-like
receptor-8 dependent pathway (14,15)
along with induction of pro-inflammatory cytokines (16).
The concept of administering a combination of two or
more anticancer drugs has been a cornerstone in cancer therapy.
This approach generally enhances the treatment efficacy and reduces
drug resistance (17,18). Traditionally, anticancer drug
combinations have been identified through empirical screening for
non-overlapping toxicities, whereas contemporary strategies
increasingly employ mechanistic rationales for which drugs to
combine (19,20). Preclinical studies frequently report
synergistic interactions when combining drugs, whereas such
interactions hardly apply in the clinic (21). Additive or sub-additive interactions
are more realistic effects to expect and would still provide
clinical benefits (22).
Combinations of Mbz with established anticancer
drugs have been explored in cell line models. For example, in a
melanoma cell line, Mbz increases apoptosis when combined with
trametinib (23). Similarly,
synergetic effects have been observed when Mbz is combined with
temozolomide and vincristine or with gemcitabine in brain and
breast cancer cell lines, respectively (24,25).
We previously found single drug Mbz administration to be
ineffective in patients with advanced previously treated
gastrointestinal cancer (26).
However, in early clinical studies of glioblastoma and CRC,
combining Mbz with chemotherapy was found to be safe and appeared
to provide patient benefit (27–30).
With this background, we considered it relevant to
further characterize the anticancer properties of single agent Mbz
and Mbz in combination with irinotecan, gemcitabine and cisplatin
in a clinically relevant ex vivo model of primary cultures
of patient tumor cells as well as in vivo with a murine
colon cancer model. These cytotoxic drugs were selected based on
their well-characterized mechanisms of action (31), allowing for the assessment of
whether Mbz exhibits broad-spectrum synergy or if its interactions
are specific to certain drug classes. Moreover, all three agents
are standard-of-care therapies for several solid tumors (31). The study was designed to address
certain critical gaps in the research on Mbz as a potential
anticancer drug, mainly its cytotoxic activity as a single drug,
its interaction with standard cytotoxic anticancer drugs and its
modes of action. This motivated the use of a somewhat mixed study
design using clinically relevant ex vivo human tumor models
as well as an immune-competent in vivo colon cancer
model.
Materials and methods
Preparation of patient tumor cells for
ex vivo assessment of direct cytotoxicity
Tumor samples were obtained from adult patients aged
≥18 years with acute myeloid leukemia (AML) or colorectal, ovarian
or renal cancer at Akademiska Hospital (Uppsala University
Hospital) (Uppsala, Sweden) by bone marrow or peripheral blood
sampling, intraoperatively or by diagnostic biopsy in accordance
with the approval from the Regional Ethical Committee in Uppsala
(approval no. 2007/237). The samples were collected January
2008-December 2014, prepared as detailed below and then
cryopreserved until use in 2022 for experiments in the present
study. Written informed consent for sample collection and further
analysis was provided by patients before sampling. Inclusion
criteria for sampling were: i) Patients aged ≥18 years; ii)
localized or advanced untreated or previously treated disease; and
iii) the sampling procedure was considered to be safe and with a
prospect that drug sensitivity testing could be of potential
patient benefit. Sex distribution among the included patients was
70% (n=155) women and 30% (n=67) men.
The cell preparation protocol for solid and leukemic
tumors has been described in detail elsewhere (32). Briefly, for the solid tumors, the
samples were manually minced using sterile scissors and dispersed
in collagenase [collagenase type I, 1.5 mg/ml (Sigma-Aldrich; Merck
KGaA) and DNase type I, 100 µg/ml (Sigma-Aldrich; Merck KGaA) in
CO2 Independent Medium, pH 7.35–7.45 (Gibco; Thermo
Fisher Scientific, Inc.)] for 4 h at 37°C. The samples were then
purified by density gradient centrifugation [200 × g, 5 min, at
room temperature (RT)] (Histopaque®−1077; Sigma-Aldrich;
Merck KGaA) followed by the determination of cell viability and
count using a Bürker chamber. The cells were diluted in complete
culture medium [RPMI 1640 (Thermo Fisher Scientific, Inc.)
containing 10% fetal calf serum (Sigma-Aldrich; Merck KGaA) and 2%
glutamine/penicillin-streptomycin] in a 37°C humidified atmosphere
containing 5% CO2 before seeding in plates for further
analysis.
The leukemic cells were isolated by Ficoll-Paque
density gradient centrifugation (400 × g, 30 min, RT) and then
counted using a Bürker chamber after staining for viability in
Türks solution (33). The cells
were then diluted in complete culture medium until experimental
seeding.
Isolated cells were stained using the May-Grünwald
Giemsa staining method. Cells were placed on slides then first
stained with May-Grünwald solution for 5 min at RT, washed
thoroughly in deionized water and then stained in Giemsa solution
for 10 min at RT, followed by another wash. Slides were air-dried
and evaluated for tumor representativity, based on cell size,
nucleus to cytoplasm ratio, chromatin staining pattern, presence of
nucleoli and mitoses and cellular adhesion tendency, by a trained
cytopathologist. A representative image of May-grünwald Giemsa
stained isolated cells from a colorectal tumor is shown in Fig. 1. Only samples with ≥70% tumor cells
were included in the study.
Assessment of direct cellular toxicity
in patient tumor cells
The fluorometric microculture cytotoxicity assay
(FMCA) described in detail previously (34), was used for the measurement of cell
survival. In the FMCA, living cells with an intact membrane
hydrolyze fluorescein diacetate to fluorescein. By measuring
fluorescence, cell survival is expressed as the survival index %
(SI), calculated as:
SI=(Fsample-Fblank)/(Fcontrol-Fblank)
×100, where Fsample represents the fluorescence in wells
with the drug, Fcontrol the fluorescence in wells with
cells and medium and Fblank the fluorescence in wells
with medium alone. The FMCA is a total cell death assay, the
results of which correspond well with the much more laborious
clonogenic assay (35).
Briefly, 5,000 cells/well (solid tumor samples), or
20,000 cells/well (hematological tumor samples) were seeded in
384-well Nunc culture plates (cat. no. 164688; Thermo Fisher
Scientific, Inc.) using the pipetting robot, Biomek 4000 (Beckman
Coulter, Inc.). Drugs were added immediately after cell seeding
using an ECHO 550 system (Beckman Coulter, Inc.). The plates were
incubated for 72 h in a 37°C humidified atmosphere containing 5%
CO2 and then transferred to an integrated system for
automated fluorescence scanning and data calculation. A successful
assay was defined by a fluorescence signal in control wells of ≥5
times the mean blank and a coefficient of variation of cell
survival in control cultures of ≤30 and ≥70% tumor cells before
incubation and/or on the assay day.
Mbz was purchased from Sigma-Aldrich (Merck KGaA)
whereas cisplatin, irinotecan and gemcitabine were either from
commercial clinical preparations or obtained from Selleck Chemicals
or LC Laboratories. Mbz was tested at concentrations of 45, 15, 5,
1.67 and 0.56 µM, cisplatin at 30, 10, 3.3, 1.12 and 0.37 µM,
irinotecan at 180, 60, 20, 6.6 and 2.2 µM, and gemcitabine at 90,
30, 10, 3.34 and 1.12 µM. The procedure of drug dissolution and
storage has been described in detail by Blom et al (32).
Assessment of direct cellular toxicity
in the CT26 cell line
CT26 murine colon carcinoma cells (ATCC) were
diluted in complete culture medium (RPMI 1640 supplemented with 10%
fetal calf serum and 2% glutamine/penicillin-streptomycin) and
seeded at 500 cells per well into 384-well Corning plates (cat. no.
3764; Thermo Fisher Scientific, Inc.). Plates were incubated for 24
h at 37°C in a humidified atmosphere containing 5% CO2.
Mbz, cisplatin and gemcitabine were tested at the concentrations
detailed above and were dispensed using an Echo 650 (Backman
Coulter, Inc), followed by a 72 h incubation period in a 37°C
humidified atmosphere containing 5% CO2. After
incubation, the plates were centrifuged (200 × g, 5 min, RT) and
the supernatant was removed using an ELx405 plate washer.
CellTiter-Glo® 2.0 reagent (25 µl/well; cat. no. G9243;
Promega Corporation) was added using the Biomek 4000 liquid
handling system. Plates were sealed, wrapped in foil, shaken (600
rpm for 2 min) and incubated in the dark for 10 min. Luminescence
was then measured using a FLUOstar Omega microplate reader (BMG
Labtech GmbH). The ATP-based viability method was chosen over the
FMCA, as CT26 cells exhibit a pronounced fibroblast-like phenotype
that may interfere with fluorescence-based viability measurements
(36).
Drug interaction analysis ex vivo
The interaction between Mbz and cisplatin (0.37–30
µM), irinotecan (2.2–180 µM) or gemcitabine (1.1–90 µM) in solid
tumor samples was assessed by comparing the SI for each drug
separately and when combined with Mbz at 5 µM, a concentration that
induced modest cytotoxicity alone. The drug concentration ranges
used were previously shown to induce a suitable range of cytotoxic
effects in solid tumor samples.
For the assessment of drug interaction for each drug
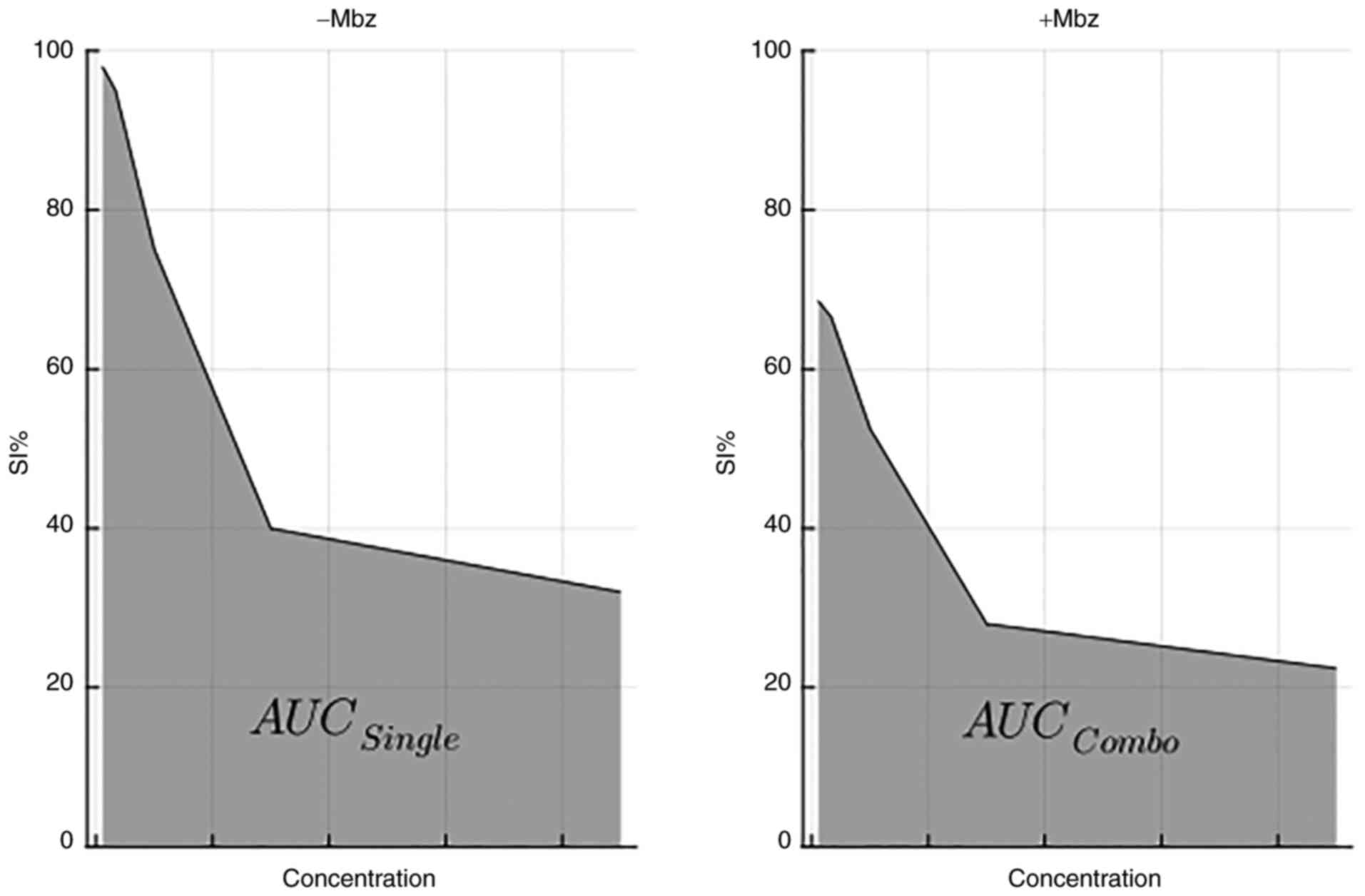
and sample SI, area under the curve (AUC) was calculated.
AUCi was defined as the
AUCCombo/AUCSingle ratio, where
AUCCombo denotes the area under the concentration-SI
curve for the drug combined with Mbz and AUCSingle
denotes the AUC for the cytotoxic drug alone, as illustrated in
Fig. 2. Thus, AUCi
ratios ≤1 indicate positive interactions (such as sub-additive or
synergistic), whereas ratios >1 indicate negative interactions
(such as antagonistic). The results are illustrated by heatmaps
plotted using the ‘pheatmap’ (version 1.0.12) package in R
(https://www.r-project.org; version
4.1.2), with rows and columns ordered by hierarchical clustering
(average linkage and Euclidean distance).
To further detail the interactions between Mbz and
irinotecan or cisplatin, FMCA experiments were performed using 4
CRC samples and analyzed in SynergyFinder 3.0 (https://synergyfinder.fimm.fi), an online
web-application that enables analysis and visualization of
multi-drug combination screening data (37,38).
The application offers interaction analysis with different models:
Loewe additivity (39), Bliss
excess (40), Zero Interaction
Potency (ZIP) (41) and Highest
Single Agent (HSA) (42). The
interactions are visualized as two-dimensional heatmaps and then
summarized over a dose-response matrix as an interaction score
calculated for each interaction model. The synergy experiments were
performed in triplicate.
Antitumor activity of Mbz and
irinotecan in vivo
The animal care and in vivo experimental
procedures were performed by Adlego Biomedical AB (Solna, Sweden)
according to Good Laboratory Practice standards and per ethical
approval by the regional animal ethics committee in Stockholm,
Sweden (approval no. 12201-2019). The study started late 2021 and
was finalized, including subsequent analyses in our laboratory,
late 2022.
In total, 54 female BALB/c mice aged 6–7 weeks at
arrival were obtained from Charles River Laboratories, Inc. The
animals were kept 4–5 animals/cage in individually ventilated cages
(type IVC 4). Bedding was provided by BeeKay Scanbur AB and all of
the cages were enriched with happy homes, play tunnels, sizzle nest
and soft paper wool (Scanbur AB). The mice were kept at 22±3°C with
50±20% humidity. Lighting was regulated to 12-h light and dark
cycles and the animals had free access to food and water (RM 3;
Special Diet Services). Animals weighed 17.0–18.4 g at arrival and
19.3–21.2 g on day 20 with no obvious differences between
groups.
CT26, a murine colorectal carcinoma cell line
derived from the BALB/c strain, was purchased from ATCC. Irinotecan
was purchased from Fresenius Kabi AG and Mbz was purchased from
Recipharm AB. On Day-11, animals were inoculated with
3×105 CT26 cells (diluted in complete RPMI 1640 medium
to a total volume of 100 µl), subcutaneously in the right rear
flank. Tumor volumes, body weights and the animal general health
status were measured 3 times weekly by designated animal house
technicians. In addition, the team conducting the experiments
monitored the animals on each day of treatment and during the
endpoint procedures. Tumor volumes (in mm3) were
measured using a caliper and calculated by the following formula:
Length × width × width ×0.44.
On Day-1, when the tumors had reached a mean volume
of 46.5±1.97 mm3, the animals were stratified into 6
experimental groups, with 9 animals per group. Treatment was then
started at day 0, as detailed in Table
I. The dosing schedule was aimed to be as clinically relevant
as possible with Mbz considered to be a drug continuously
administered orally and irinotecan intermittently intravenously,
each at doses giving effects allowing for analysis of interactions
(5,43). Treatment was to be continued for a
maximum of 41 days after which the animals were euthanized by
cervical dislocation. Animals with tumors >2,000 mm3
and/or tumor-related wounds, were to be euthanized pre-term in
accordance with the ethical permit governing these experiments. For
this reason, most animals were euthanized around day 20, thus tumor
growth data are presented up until that day. Survival was defined
as time from stratification to pre-term termination or day 41.
Following euthanasia, tumors were extracted and placed in medium
(CO2 Independent Medium) with 5% heat-inactivated fetal
calf serum (Sigma-Aldrich; Merck KGaA) and 1.1%
glutamine/penicillin-streptomycin consisting of equal parts 200 mM
L-glutamine (Sigma-Aldrich; Merck KGaA) and penicillin-streptomycin
(10,000 U/ml penicillin and 10 mg/ml streptomycin in 0.9% NaCl;
Sigma-Aldrich; Merck KGaA) and shipped directly to our laboratory
for preparation of the cells for flow cytometric evaluation.
 | Table I.Treatment schedules for the
experimental groups. |
Table I.
Treatment schedules for the
experimental groups.
| Compound | Administration
route | Dose (mg/kg) | Schedule |
|---|
| Saline | i.v. | - | Twice weekly |
| LD Mbz | p.o. | 20 | Five
times/week |
| HD Mbz | p.o. | 50 | Five
times/week |
| Irinotecan | i.p. | 15 | Once weekly |
| Irinotecan + LD
Mbz | i.p./p.o. | 15+20 | Once weekly + five
times/week |
| Irinotecan + HD
Mbz | i.p./p.o. | 15+50 | Once weekly + five
times/week |
Flow cytometry
Immune cell infiltration in the mouse tumors was
assessed by flow cytometry. Mouse tumor tissues were prepared by
mechanical disintegration and collagenase digestion as
aforementioned. Cells were then enriched for CD45+
expression using an AutoMACS Pro (Miltenyi Biotec GmbH).
Prior to flow cytometry analysis, the cells were
washed and diluted with PBS (Thermo Fisher Scientific, Inc.) and
human serum albumin (Sigma-Aldrich; Merck KGaA) (PBS-HSA) 0.1% for
5 min at 4°C. Gating antibodies were added to the cells according
to the following: i) Macrophage panel: Lymphocyte antigen 6
complex, locus G FITC (cat. no. 127605; Biolegend, Inc.), CD45
Pacific Orange (cat. no. MCD4530; Thermo Fisher Scientific, Inc.),
CD163 PE (cat. no. 156704; Biolegend, Inc.), CD38 Pacific blue
(cat. no. 102720; Biolegend, Inc.), F4/80 PE/Cy7 (cat. no. 123114;
Biolegend, Inc.) and CD11b Violet 711 (cat. no. 101241; Biolegend,
Inc.); and ii) T cell panel: CD8 FITC/CD4 PE/CD3 PC7 (cat. no.
558391; BD Biosciences) and CD45 Pacific Orange. All antibodies
were diluted in PBS-HSA 0.1%. The cell and antibody mixture was
incubated for 20 min in the dark, washed with cold 1% PBS-HSA and
centrifuged at 400 × g for 5 min at 4°C. Finally, flow cytometry
was performed using a Navios flow cytometer (Beckman Coulter, Inc.)
and data were analyzed using Navios software v1.2 (Beckman Coulter,
Inc.).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism™ 6 (Dotmatics). Between group differences were first analyzed
by one-way ANOVA and if statistically significant, comparisons
between groups were made using Dunnett's post hoc test. P<0.05
was considered to indicate a statistically significant difference.
The data are presented as mean ± SD throughout.
Results
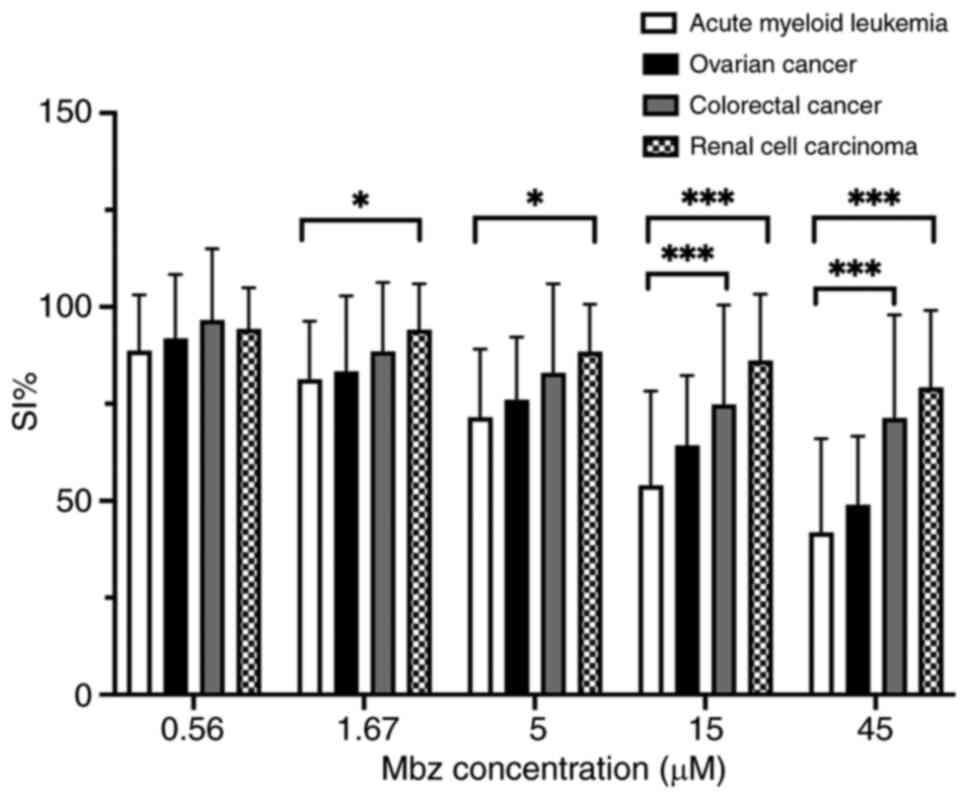
Mbz shows modest cytotoxicity in
patient tumor cells ex vivo
In total, 222 patient samples fulfilled the FMCA
quality criteria including AML (n=24) and ovarian (n=78),
colorectal (n=71) and renal (n=49) cancer samples. The single-drug
effect of Mbz was modest across all tumor types, with AML showing
the highest sensitivity, followed by ovarian, colorectal and renal
cancer (Fig. 3). Statistical
analysis confirmed a significant difference between AML and CRC at
the two highest concentrations (P<0.001). Moreover, a
significant difference was observed between AML and renal cell
carcinoma at all tested concentrations except the lowest
(P<0.001 for the two highest concentrations and P<0.05 for
the remaining concentrations). Mbz had a modest effect at 5 µM
(mean SI% 72, 76, 83 and 88 in AML, ovarian, colorectal and renal
cancer, respectively) and this concentration was selected for the
combination experiments.
Mbz enhances the effect of cytotoxic
drugs in patient tumor cells
Drug interactions notably differed between samples
and tumor types (Fig. 4). In all of
the samples (n=82), regardless of diagnosis, a positive interaction
following the addition of Mbz was observed in 68% of the samples
(50/74) for cisplatin, 77% (58/75) for gemcitabine and in 88%
(72/82) for irinotecan. The corresponding numbers for renal cancer
were 73% (14/19), 79% (15/19) and 85% (18/21), for ovarian cancer
64% (25/39), 68% (28/41) and 90% (38/42) and for CRC 84% (16/19),
69% (12/16) and 93% (14/15). Notably, some drug-sample combinations
were not tested in all experiments (indicated in grey in Fig. 4), which explains why the sum of
subgroup analyses does not equal the total number of samples.
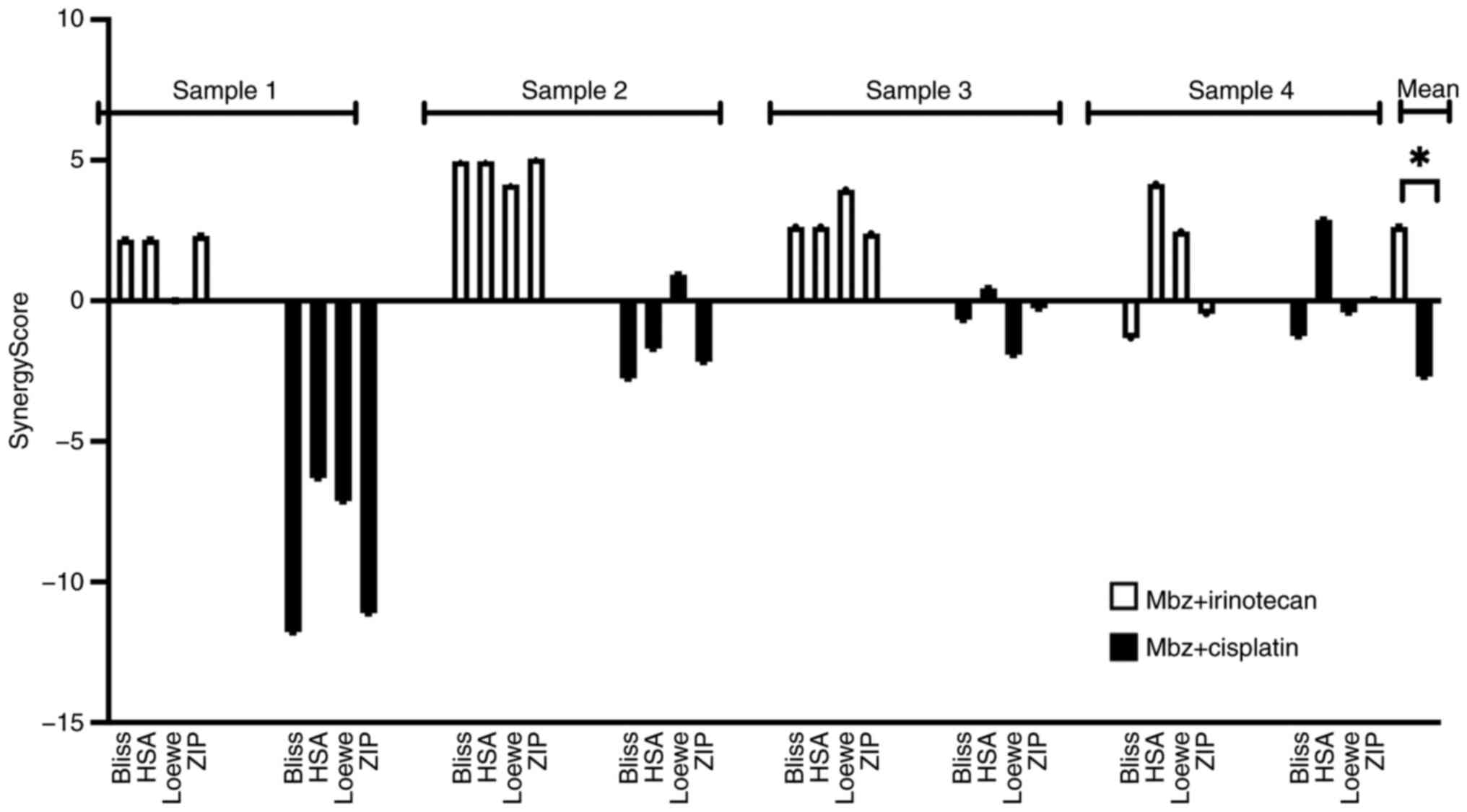
To investigate the observed interactions between Mbz
and irinotecan or cisplatin in more detail, a new set of FMCA
experiments with 4 CRC samples were performed using the
SynergyFinder application with four models for drug interactions.
According to the application, interaction scores in the −10 to +10
range suggests additive interactions, >+10 synergistic and
<-10 antagonistic interactions (36). The interaction scores for each
patient and each interaction model are shown in Fig. 5. Overall, the interaction scores for
the Mbz/irinotecan combination were higher across most interaction
models compared with the Mbz/cisplatin combination, suggesting the
Mbz/irinotecan combination to be the most favorable. Therefore,
this combination was selected for the in vivo study. The
majority of synergy scores for both combinations fell within the
range of −10 to +10, indicating additive effects. However, the
Mbz/irinotecan combinations occasionally exceeded +10 in certain
models and samples, suggesting potential synergistic
interactions.
Using Mann-Whitney test, a statistically
significantly higher overall mean score was observed for the
Mbz/irinotecan combination compared with the Mbz/cisplatin
combination [mean synergy score for Mbz/irinotecan, 2.63 (Bliss
excess, 2.11; HSA, 3.48; Loewe additivity, 2.63; ZIP, 2.32) vs.
mean synergy score for Mbz/cisplatin, −2.69 (Bliss excess, −4.11;
HSA, −1.16; Loewe additivity, −2.12; ZIP, −3.37); P<0.0001;
Fig. 5].
The low synergy scores for the Mbz/cisplatin
combination in sample 1 likely reflect the intrinsic drug
sensitivity and resistance of that particular tumor sample.
Individual tumor samples exhibit substantial heterogeneity in drug
response, which underscores the value of using a patient-derived
cell model to reflect drug activity in the clinic.
Cytotoxic effects in the CT26 murine
colon cancer cell line in vitro
As a basis for the assessment of drug interactions
in vivo, a corresponding set of experiments with in
vitro CT26 cells were conducted using Mbz alone and in
combination with cisplatin or irinotecan. Mbz alone showed a modest
and an almost concentration independent activity (Fig. S1A), whereas both cisplatin and
irinotecan induced a dose-dependent cytotoxicity (Fig. S1A and B). Furthermore, Mbz at 5 µM
enhanced the cytotoxic effect of cisplatin and irinotecan in an
additive/sub-additive pattern without notable differences between
these drugs (Fig. S1A-C).
Mbz inhibits tumor growth in the CT26
murine colon cancer model
Animal body weight and health status assessments
showed that drug administration was not associated with a decrease
in overall animal health status. Tumor growth was rapid and some
animals (1–3 animals in each group) developed tumor-related wounds
and were thus euthanized prior to the final study day.
Tumor-related wound development was evenly distributed throughout
groups and likely not correlated to drug administration. The
maximum measured tumor diameter and volume were 17.6 mm and 2,877
mm3, respectively.
The mean tumor volumes for the control and
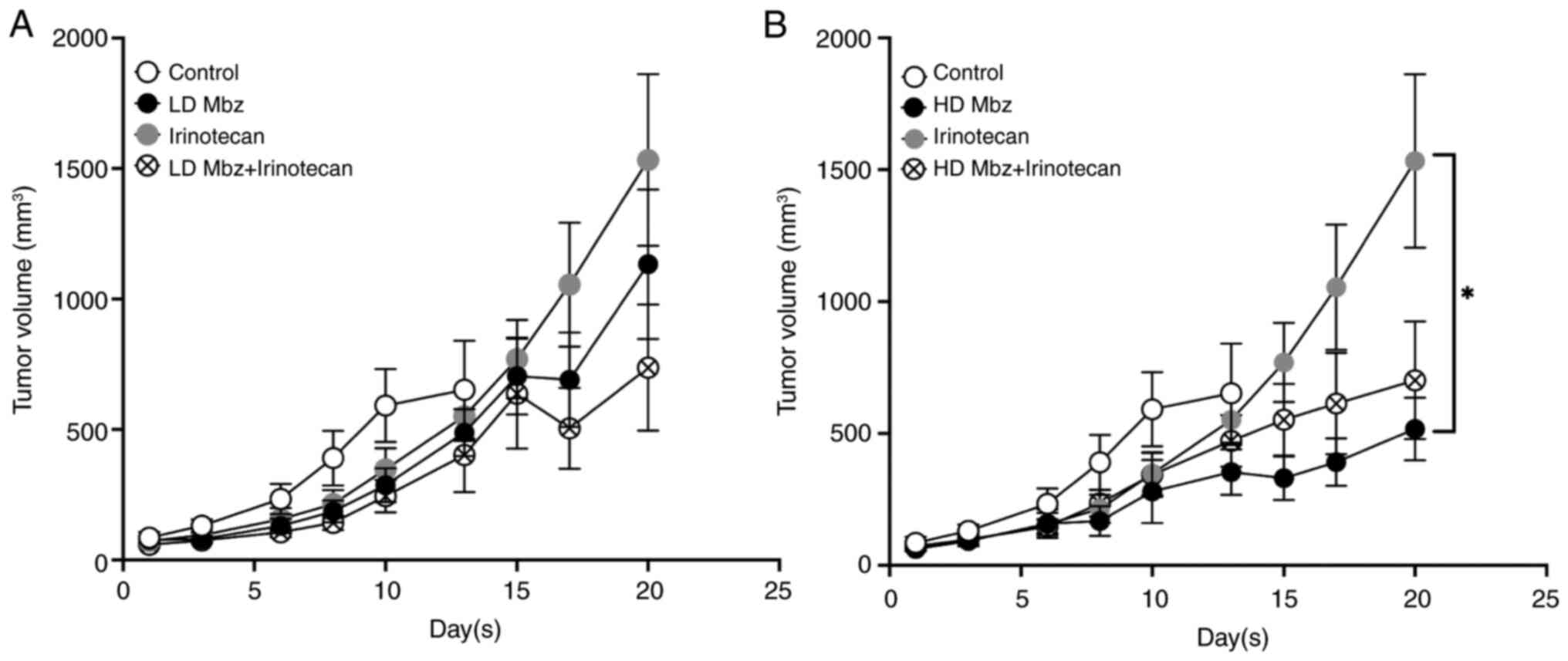
experimental groups are shown in Fig.
6, divided into a and b for clarity. The values are presented
up until day 13 for the control group as after this day most
animals in this group had been euthanized pre-term, in accordance
with the study protocol due to tumor growth, whereas most animals
in the experimental groups were continued to day 20. At day 13, the
control group had a mean tumor volume of 651 mm3
compared with 488 mm3 in the low dose Mbz group and 353
mm3 in the high dose Mbz group. The mean tumor volume at
day 13 in animals treated with irinotecan was 523 mm3
and when irinotecan was combined with low or high dose Mbz, the
volumes were 402 mm3 and 472 mm3,
respectively. Differences between the groups were not statically
significant. At day 20, the mean tumor volume in the irinotecan
group was 1,533 mm3, the low dose Mbz group was 1,133
mm3 and the high dose Mbz group was 517 mm3.
The tumor volume for irinotecan combined with low dose Mbz at day
20 was 737 mm3 and 699 mm3 for high dose Mbz.
Compared with irinotecan alone, the differences were not
statistically significant. At day 20, the difference between all
groups was statistically significant according to one-way ANOVA
(P=0.048), but only the difference between the mean tumor volumes
in the irinotecan and high dose Mbz groups was statistically
significant following post hoc test (P=0.04).
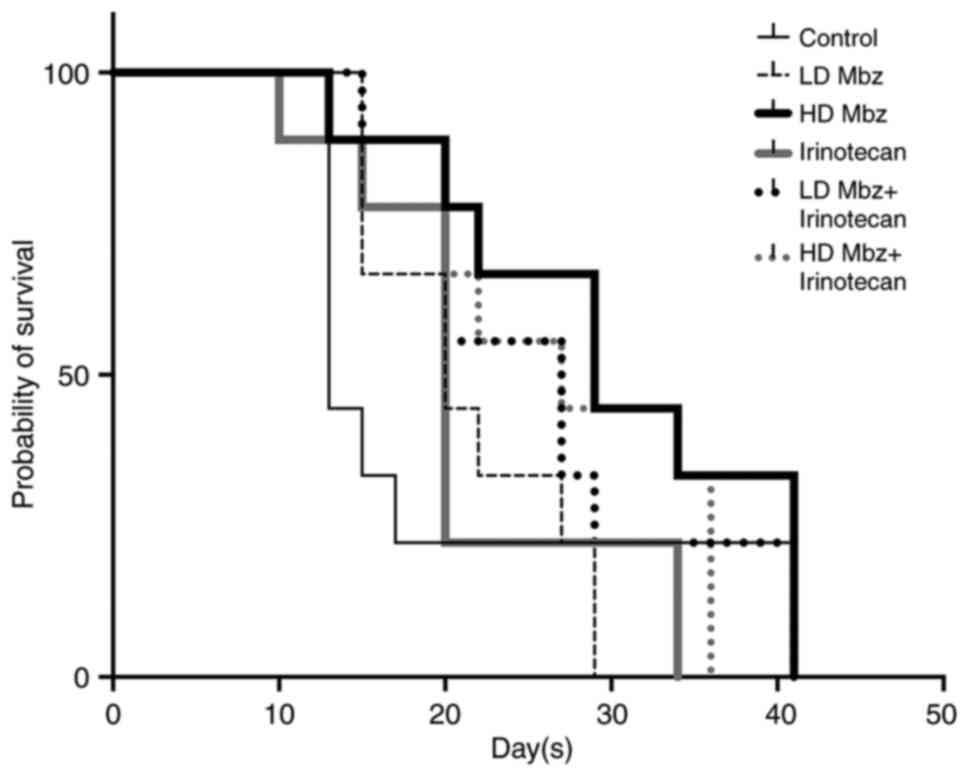
Kaplan Meier survival curves are presented in
Fig. 7. Drug exposed animals showed
a longer survival compared with the control animals. Control
animals had a median survival time of 13 days, compared with 20–29
days for those drugs exposed. In agreement with the effect on tumor
volumes, a dose-dependent effect of Mbz alone was indicated, with a
median survival time of 20 days in animals treated with low dose
Mbz compared with 29 days for high dose Mbz. Animals treated with
irinotecan combined with low or high dose Mbz had median survival
times of 27 days.
Mbz modulates tumor immune cell
infiltration in vivo
Based on our previous ex vivo findings of an
immunomodulatory effect of Mbz switching macrophages from the M2 to
M1 type (14,16), tumor tissue from the animals was
retrieved and analyzed by flow cytometry to detect the presence of
various subsets of immune cells. CD45+ cells were sorted
for CD4+/CD8+ T cells, and macrophages were
then classified further as type M1
(CD38+/CD80+) or type M2 (CD206+).
Fig. S2, Fig. S3, Fig.
S4, Fig. S5, Fig. S6, Fig.
S7 illustrate the gating strategies used to identify
macrophages and T cells, for each group separately.
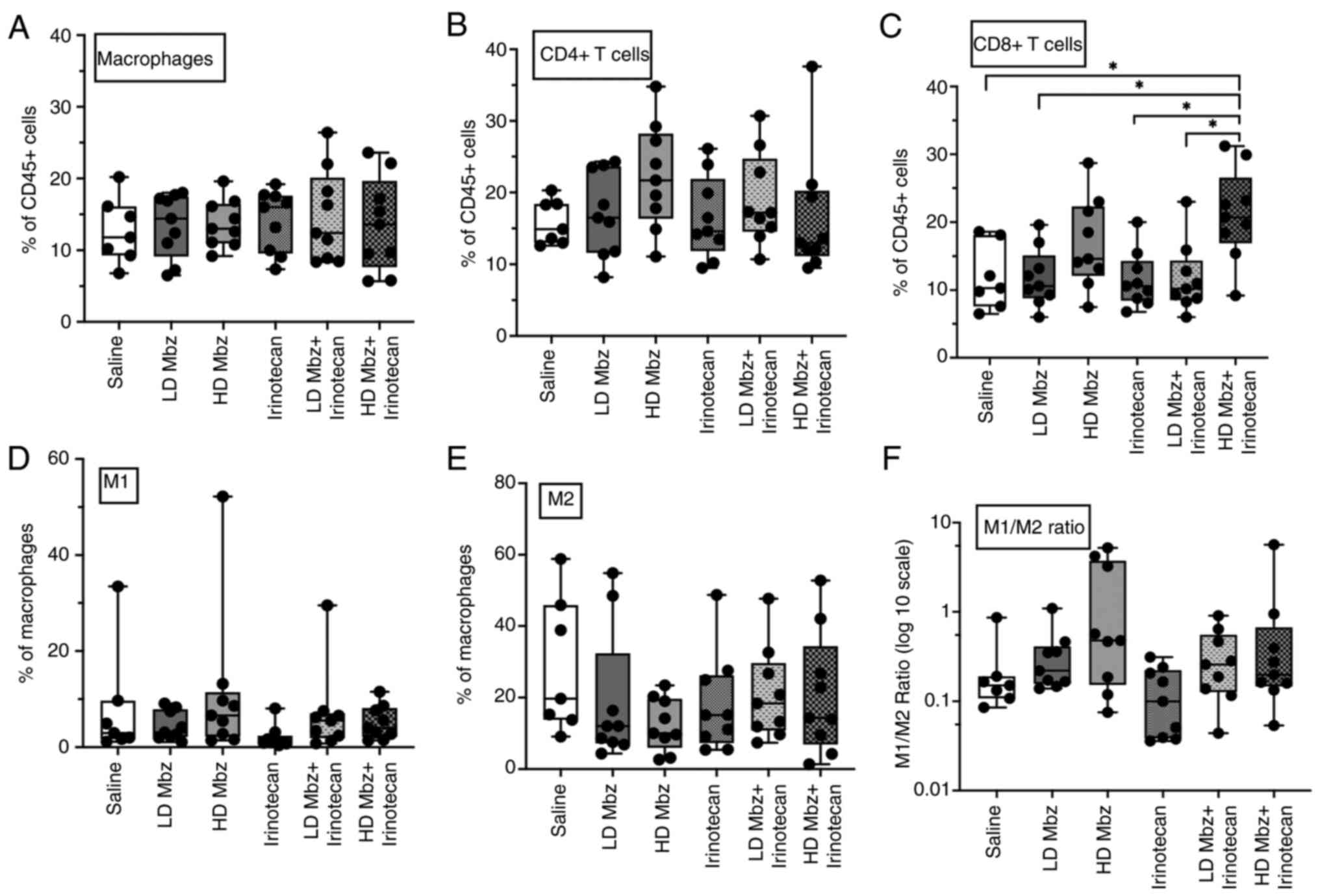
Overall, Mbz slightly increased the infiltration of
macrophages and CD4+ T cells (Fig. 8A and B). Mbz tended to
dose-dependently increase the levels of M1 macrophages and decrease
M2 macrophages (Fig. 8D-F). Thus,
the mean M1/M2 ratio for low and high dose Mbz were 0.26 (range,
0.15–1.09) and 1.35 (range, 0.12–5.22), respectively, compared with
0.25 (range, 0.08–0.78) for the control group.
Mbz in combination with irinotecan increased the
M1/M2 ratio, but not beyond the effect of Mbz alone. Irinotecan
alone seemingly increased the macrophage infiltration but otherwise
did not notably alter the immune cell infiltration. The tumor
immune cell data exhibited substantial variability and no
statistically significant differences were observed between groups,
except for CD8+ T cells. A significant increase in
CD8+ T cells was observed in the high dose Mbz +
irinotecan group compared with the control, low dose Mbz +
irinotecan, low dose Mbz and irinotecan alone groups (P=0.01;
Fig. 8C).
Discussion
Ex vivo drug sensitivity testing of primary
cultures of tumor cells from patients using FMCA has previously
shown a good correlation with clinical outcomes in several tumor
types (44–47). Using this model in the present
study, it was demonstrated that Mbz alone had a modest direct
cytotoxic activity, in agreement with the general chemotherapeutic
drug sensitivity known from our previous ex vivo
characterization and from the clinic (2,3,48,49).
This finding is in contrast to that observed in various cell line
models, in which Mbz alone exhibits strong cytotoxic activity
(2–7). However, cell lines have several
limitations as tumor models and generally do not adequately
reproduce drug sensitivity in the clinic (50,51),
in contrast to primary cultures of tumor cells from patients
(50). Nevertheless, it should be
acknowledged that single-cell culture models, including
patient-derived cells, lack the complex interactions with immune
cells and extracellular matrix components that are present in the
in vivo tumor microenvironment.
Several mechanisms for a direct tumor cell Mbz
cytotoxicity have been reported (52) such as tubulin polymerization
inhibition (6), protein kinase
inhibition (11), anti-angiogenesis
(12) and inhibition of the
Hedgehog pathway (13), and are
considered notable for an anticancer drug. Together with the very
advantageous safety profile of Mbz, these observations have fueled
an interest in trying single drug Mbz for cancer treatment in
patients. Mbz has been reported to induce tumor remission in case
reports of patients with neuroendocrine and colon cancer (9,10).
Furthermore, in early clinical trials with patients with brain
tumors, Mbz at higher dose (up to 200 mg/kg/day) seemingly stops
tumor progression (28). Such
observations are in line with the observed Mbz-induced tumor growth
inhibition in different mouse models (2,5) and
are corroborated by the dose-dependent inhibition of tumor growth
observed in the in vivo CT26 colon cancer model in the
present study.
However, the aforementioned findings contrast with
our phase II clinical trial of last line, individualized, single
drug Mbz in patients with advanced gastrointestinal cancer
(26). In the trial, no objective
tumor response to single-agent Mbz was observed, and a subset of
patients showed unusually rapid disease progression, suggestive of
hyperprogression, a phenomenon reported in patients treated with
immune checkpoint inhibitors (53).
Although it cannot be concluded that Mbz directly induced
hyperprogression, one possible explanation is that Mbz modulates
the immune system in a complex, context-dependent manner. While Mbz
can promote pro-inflammatory responses in controlled ex vivo
settings (16), the tumor
microenvironment in patients, particularly in heavily pre-treated
and immunosuppressed individuals, may respond differently.
The concept of combining several drugs remains a
cornerstone of cancer therapy. This approach generally improves the
benefit-risk balance compared with single drug treatment (17,18).
Traditionally, anticancer drug combinations were established
empirically, focusing on identifying regimens with non-overlapping
toxicities. More recently, however, combination strategies are
increasingly guided by mechanistic rationale based on tumor biology
(19,20). Preclinical studies frequently report
synergistic interactions when combining drugs whereas such
interactions hardly apply in the clinic (21). Instead, additive or sub-additive
interactions are more common and can still yield clinically
meaningful benefits (22). Given
the benefits of drug combinations and the negative results from our
aforementioned single-drug Mbz clinical trial, it is notable that a
recent randomized study reported improved outcomes in advanced CRC
when Mbz was combined with standard chemotherapy (FOLFOX) compared
with chemotherapy alone (27). This
finding prompted the investigation of the effect of Mbz combined
with mechanistically different standard cytotoxic drugs in the
present study. In patient tumor and CT26 cells, positive
interactions in the sub-additive to additive range were observed,
with the strongest effect noted for Mbz combined with irinotecan in
patient cells. This finding suggests that the Mbz/irinotecan
combination may be suitable for clinical testing, particularly in
tumor types where irinotecan is part of the standard treatment,
such as CRC.
In the present study, the observed positive
interaction between Mbz and irinotecan was partly corroborated in
the CT26 colon cancer mouse model. Low dose Mbz combined with
irinotecan was more active than each drug individually, although
this result was not statistically significant. By contrast, high
dose Mbz combined with irinotecan tended to be less active than Mbz
alone. The reason for this seemingly dose-dependent interaction
difference is unclear. However, it could be speculated that the
stronger tumor growth inhibition from high dose Mbz compared with
low dose Mbz was reduced by irinotecan-induced counteracting
changes in the tumor macrophage infiltration.
From a clinical point of view, the observations of
the present study make the selection of candidate Mbz combinations
for clinical testing complicated. If the anticancer effect of Mbz
in vivo is mainly due to changes in tumor immune cell
infiltration, screening of drugs suitable for combination with Mbz
using cell lines or patient-derived primary culture models will not
produce data applicable in vivo. Other models that also
reflect drug effects on immune cells would be necessary to select
drugs appropriate for combination with Mbz. Notably, Mbz has been
successfully combined with FOLFOX in treating CRC (27), but has also been combined with
bevazicumab, irinotecan, temozolomide or lomustine in early
clinical trials of patients with brain tumors (28–30).
The antitumor activity in these trials was seemingly very modest
and the trial design did not allow for any conclusions on whether
these combinations were justified from a mechanistic point of view.
In this context, combination of Mbz with other immunomodulatory
drugs is conceptually interesting. Indeed, preliminary results in
our laboratory from in vivo experiments of the CT26 model
treated with an immune checkpoint inhibiting antibody indicate a
positive interaction with Mbz. Another important avenue in
repurposing Mbz for use in cancer therapy is the development of
drug carriers or prodrugs to overcome the pharmacokinetic issues
associated with Mbz (54).
One strength of the present study is the use of
primary cultures of patient tumor cells, a model known to better
reflect clinical outcomes than tumor cell lines (55). While the findings of the present
study provide valuable insights into the anticancer properties of
Mbz, the limitations of the study should be acknowledged. The ex
vivo, in vivo and in vitro models utilized do not
recapitulate the complexity of cancer, necessitating further
validation in preclinical and clinical models.
In conclusion, the present study highlights the
multifaceted anticancer properties of Mbz. Thus, findings from this
and other published studies suggest that a key mechanism of the
anticancer effect of Mbz may be immune modulation in addition to
direct cytotoxicity. It would be of interest to explore whether
pharmacological agents known to promote M1 to M2 macrophage
polarization might antagonize the effects of Mbz. Such studies
could provide valuable insights into the immunomodulatory role of
Mbz and inform on combination strategies. However, selection of the
best drugs to combine with Mbz for efficacy optimization requires
careful consideration based on further research.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by grants from The Swedish Cancer
Society (grant nos. 14 445, 17 0661 and 20 844) and The Cancer
Research Fund at the Uppsala University hospital (grant no.
2022-PN).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
The study was designed and performed with
significant contributions from all authors. SM, CA, PN, MF and RL
conceptualized and designed the study. SM and KB performed the
experiments and collected the data. SM, KB, CA and PN analyzed the
data. SM and PN wrote the manuscript. KB, CA, MF and RL revised the
manuscript. SM and PN confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The collection of patient tumor samples was approved
from the Swedish Ethics Review Board (approval no. 2007/237).
Written informed consent for sample collection and further analysis
was provided by all patients before sample collection. The in
vivo experiments were approval by the regional animal ethics
committee in Stockholm (approval no. 12201-2019).
Patient consent for publication
Written patient consent for tumor sampling also
included the publication of results.
Competing interests
RL, PN and MF are co-founders and shareholders of
Repos Pharma AB, a Swedish research and development company
dedicated to investigations of drug repositioning. Repos Pharma AB
was not involved in the study design, execution, data analysis or
writing of the manuscript. The remaining authors declare that they
have no competing interests.
References
|
1
|
Pantziarka P, Bouche G, Meheus L, Sukhatme
V and Sukhatme VP: Repurposing drugs in oncology (ReDO)-mebendazole
as an anti-cancer agent. Ecancermedicalscience. 8:4432014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mukhopadhyay T, Sasaki J, Ramesh R and
Roth JA: Mebendazole elicits a potent antitumor effect on human
cancer cell lines both in vitro and in vivo. Clin Cancer Res.
8:2963–2969. 2002.PubMed/NCBI
|
|
3
|
Martarelli D, Pompei P, Baldi C and
Mazzoni G: Mebendazole inhibits growth of human adrenocortical
carcinoma cell lines implanted in nude mice. Cancer Chemother
Pharmacol. 61:809–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doudican NA, Byron SA, Pollock PM and
Orlow SJ: XIAP downregulation accompanies mebendazole growth
inhibition in melanoma xenografts. Anticancer Drugs. 24:181–188.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bai RY, Staedtke V, Aprhys CM, Gallia GL
and Riggins GJ: Antiparasitic mebendazole shows survival benefit in
2 preclinical models of glioblastoma multiforme. Neuro Oncol.
13:974–982. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sasaki J, Ramesh R, Chada S, Gomyo Y, Roth
JA and Mukhopadhyay T: The anthelmintic drug mebendazole induces
mitotic arrest and apoptosis by depolymerizing tubulin in non-small
cell lung cancer cells. Mol Cancer Ther. 1:1201–1209.
2002.PubMed/NCBI
|
|
7
|
Pourgholami MH, Cai ZY, Chu SW, Galettis P
and Morris DL: The influence of ovarian cancer induced peritoneal
carcinomatosis on the pharmacokinetics of albendazole in nude mice.
Anticancer Res. 30:423–428. 2010.PubMed/NCBI
|
|
8
|
Aliabadi A, Haghshenas MR, Kiani R,
Koohi-Hosseinabadi O, Purkhosrow A, Pirsalami F, Panjehshahin MR
and Erfani N: In vitro and in vivo anticancer activity of
mebendazole in colon cancer: A promising drug repositioning. Naunyn
Schmiedebergs Arch Pharmacol. 397:2379–2388. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nygren P and Larsson R: Drug repositioning
from bench to bedside: Tumour remission by the antihelmintic drug
mebendazole in refractory metastatic colon cancer. Acta Oncol.
53:427–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dobrosotskaya IY, Hammer GD, Schteingart
DE, Maturen KE and Worden FP: Mebendazole monotherapy and long-term
disease control in metastatic adrenocortical carcinoma. Endocr
Pract. 17:e59–e62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nygren P, Fryknäs M, Agerup B and Larsson
R: Repositioning of the anthelmintic drug mebendazole for the
treatment for colon cancer. J Cancer Res Clin Oncol. 139:2133–2140.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai RY, Staedtke V, Rudin CM, Bunz F and
Riggins GJ: Effective treatment of diverse medulloblastoma models
with mebendazole and its impact on tumor angiogenesis. Neuro Oncol.
17:545–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larsen AR, Bai RY, Chung JH, Borodovsky A,
Rudin CM, Riggins GJ and Bunz F: Repurposing the antihelmintic
mebendazole as a hedgehog inhibitor. Mol Cancer Ther. 14:3–13.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blom K, Rubin J, Berglund M, Jarvius M,
Lenhammar L, Parrow V, Andersson C, Loskog A, Fryknäs M, Nygren P
and Larsson R: Mebendazole-induced M1 polarisation of THP-1
macrophages may involve DYRK1B inhibition. BMC Res Notes.
12:2342019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blom K, Senkowski W, Jarvius M, Berglund
M, Rubin J, Lenhammar L, Parrow V, Andersson C, Loskog A, Fryknäs
M, et al: The anticancer effect of mebendazole may be due to M1
monocyte/macrophage activation via ERK1/2 and TLR8-dependent
inflammasome activation. Immunopharmacol Immunotoxicol. 39:199–210.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubin J, Mansoori S, Blom K, Berglund M,
Lenhammar L, Andersson C, Loskog A, Fryknäs M, Nygren P and Larsson
R: Mebendazole stimulates CD14+ myeloid cells to enhance T-cell
activation and tumour cell killing. Oncotarget. 9:30805–30813.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bayat Mokhtari R, Homayouni TS, Baluch N,
Morgatskaya E, Kumar S, Das B and Yeger H: Combination therapy in
combating cancer. Oncotarget. 8:38022–38043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Law MR, Wald NJ, Morris JK and Jordan RE:
Value of low dose combination treatment with blood pressure
lowering drugs: Analysis of 354 randomised trials. BMJ.
326:14272003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foucquier J and Guedj M: Analysis of drug
combinations: Current methodological landscape. Pharmacol Res
Perspect. 3:e001492015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boshuizen J and Peeper DS: Rational cancer
treatment combinations: An urgent clinical need. Mol Cell.
78:1002–1018. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ocana A, Amir E, Yeung C, Seruga B and
Tannock IF: How valid are claims for synergy in published clinical
studies? Ann Oncol. 23:2161–2166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwangbo H, Patterson SC, Dai A, Plana D
and Palmer AC: Additivity predicts the efficacy of most approved
combination therapies for advanced cancer. Nat Cancer. 4:1693–1704.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simbulan-Rosenthal CM, Dakshanamurthy S,
Gaur A, Chen YS, Fang HB, Abdussamad M, Zhou H, Zapas J, Calvert V,
Petricoin EF, et al: The repurposed anthelmintic mebendazole in
combination with trametinib suppresses refractory NRASQ61K
melanoma. Oncotarget. 8:12576–12595. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kipper FC, Silva AO, Marc AL, Confortin G,
Junqueira AV, Neto EP and Lenz G: Vinblastine and antihelmintic
mebendazole potentiate temozolomide in resistant gliomas. Invest
New Drugs. 36:323–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coyne CP, Jones T and Bear R:
Gemcitabine-(C4-amide)-[anti- HER2/neu] Anti-neoplastic
cytotoxicity in dual combination with mebendazole against
chemotherapeutic-resistant mammary adenocarcinoma. J Clin Exp
Oncol. 2:10001092013.PubMed/NCBI
|
|
26
|
Mansoori S, Fryknäs M, Alvfors C, Loskog
A, Larsson R and Nygren P: A phase 2a clinical study on the safety
and efficacy of individualized dosed mebendazole in patients with
advanced gastrointestinal cancer. Sci Rep. 11:89812021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hegazy SK, El-Azab GA, Zakaria F, Mostafa
MF and El-Ghoneimy RA: Mebendazole; from an anti-parasitic drug to
a promising candidate for drug repurposing in colorectal cancer.
Life Sci. 299:1205362022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gallia GL, Holdhoff M, Brem H, Joshi AD,
Hann CL, Bai RY, Staedtke V, Blakeley JO, Sengupta S, Jarrell TC,
et al: Mebendazole and temozolomide in patients with newly
diagnosed high-grade gliomas: Results of a phase 1 clinical trial.
Neurooncol Adv. 3:vdaa1542020.PubMed/NCBI
|
|
29
|
Krystal J, Hanson D, Donnelly D and Atlas
M: A phase 1 study of mebendazole with bevacizumab and irinotecan
in high-grade gliomas. Pediatr Blood Cancer. 71:e308742024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patil VM, Menon N, Chatterjee A, Tonse R,
Choudhari A, Mahajan A, Puranik AD, Epari S, Jadhav M, Pathak S, et
al: Mebendazole plus lomustine or temozolomide in patients with
recurrent glioblastoma: A randomised open-label phase II trial.
EClinicalMedicine. 49:1014492022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anand U, Dey A, Chandel AKS, Sanyal R,
Mishra A, Pandey DK, Falco VD, Upadhyay A, Kandimalla R, Chaudhary
A, et al: Cancer chemotherapy and beyond: Current status, drug
candidate, associated risks and progress in targeted therapeutics.
Genes Disease. 10:136–1401. 2022.
|
|
32
|
Blom K, Nygren P, Alvarsson J, Larsson R
and Andersson CR: Ex vivo assessment of drug activity in patient
tumor cells as a basis for tailored cancer therapy. J Lab Autom.
21:178–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Larsson R and Nygren P: Laboratory
prediction of clinical chemotherapeutic drug resistance: A working
model exemplified by acute leukaemia. Eur J Cancer. 29A:1208–1212.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lindhagen E, Nygren P and Larsson R: The
fluorometric microculture cytotoxicity assay. Nat Protoc.
3:1364–1369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Karlsson H, Fryknäs M, Senkowski W,
Larsson R and Nygren P: Selective radiosensitization by
nitazoxanice of quiescent clonogenic colon cancer tumour cells.
Oncol Lett. 23:1232022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miles FL, Lynch JE and Sikes RA:
Cell-based assays using calcein acetoxymethyl ester show variation
in fluorescence with treatment conditions. J Biol Method.
2:e292015. View Article : Google Scholar
|
|
37
|
Ianevski A, Giri AK and Aittokallio T:
SynergyFinder 2.0: Visual analytics of Multi-drug combination
synergies. Nucleic Acids Res. 48:W488–W493. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ianevski A, Giri AK and Aittokallio T:
SynergyFinder 3.0: An interactive analysis and consensus
interpretation of multi-drug synergies across multiple samples.
Nucleic Acids Res. 50:W739–W743. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Loewe S: The problem of synergism and
antagonism of combined drugs. Arzneimittelforschung. 3:285–290.
1953.PubMed/NCBI
|
|
40
|
Bliss CI: The toxicity of poisions applied
jointly. Ann Appl Biol. 26:585–615. 1939. View Article : Google Scholar
|
|
41
|
Yadav B, Wennerberg K, Aittokallio T and
Tang J: Searching for drug synergy in complex dose-Response
landscapes using an interaction potency model. Comput Struct
Biotechnol J. 13:504–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Berenbaum MC: What is synergy? Pharmacol
Rev. 41:93–141. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moon CH, Lee SJ, Lee HY, Lee JC, Cha H,
Cho WJ, Park JW, Park HJ, Seo J, Lee YH, et al: KML001 displays
vascular disrupting properties and irinotecan combined antitumor
activities in a murine tumor model. PLoS One. 8:e539002013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Csóka K, Tholander B, Gerdin E, de la
Torre M, Larsson R and Nygren P: In vitro determination of
cytotoxic drug response in ovarian carcinoma using the fluorometric
microculture cytotoxicity assay (FMCA). Int J Cancer. 72:1008–1012.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
von Heideman A, Tholander B, Grundmark B,
Cajander S, Gerdin E, Holm L, Axelsson A, Rosenberg P, Mahteme H,
Daniel E, et al: Chemotherapeutic drug sensitivity of primary
cultures of epithelial ovarian cancer cells from patients in
relation to tumour characteristics and therapeutic outcome. Acta
Oncol. 53:242–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cashin PH, Söderström M, Blom K, Artursson
S, Andersson C, Larsson R and Nygren P: Ex vivo assessment of
chemotherapy sensitivity of colorectal cancer peritoneal
metastases. Br J Surg. 110:1080–1083. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bjersand K, Blom K, Poromaa IS, Stålberg
K, Lejon AM, Bäckman F, Nyberg Å, Andersson C, Larsson R and Nygren
P: Ex vivo assessment of cancer drug sensitivity in epithelial
ovarian cancer and its association with histopathological type,
treatment history and clinical outcome. Int J Oncol. 61:1282022.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang X, Zhao J, Gao X, Pei D and Gao C:
Anthelmintic drug albendazole arrests human gastric cancer cells at
the mitotic phase and induces apoptosis. Exp Ther Med. 13:595–603.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang L, Zhao L, Zhang J, He F, Wang H,
Liu Q, Shi D, Ni N, Wagstaff W, Chen C, et al: Antiparasitic
mebendazole (MBZ) effectively overcomes cisplatin resistance in
human ovarian cancer cells by inhibiting multiple cancer-associated
signaling pathways. Aging (Albany NY). 13:17407–17427. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Salvadores M, Fuster-Tormo F and Supek F:
Matching cell lines with cancer type and subtype of origin via
mutational, epigenomic, and transcriptomic patterns. Sci Adv.
6:eaba18622020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mirabelli P, Coppola L and Salvatore M:
Cancer cell lines are useful model systems for medical research.
Cancers (Basel). 11:10982019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guerini AE, Triggiani L, Maddalo M, Bonù
ML, Frassine F, Baiguini A, Alghisi A, Tomasini D, Borghetti P,
Pasinetti N, et al: Mebendazole as a candidate for drug repurposing
in oncology: An extensive review of current literature. Cancers
(Basel). 11:12842019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Champiat S, Ferrara R, Massard C, Besse B,
Marabelle A, Soria JC and Ferté C: Hyperprogressive disease:
Recognizing a novel pattern to improve patient management. Nat Rev
Clin Oncol. 15:748–762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abu-Hdaib B, Nsairat H, El-Tanani M,
Al-Deeb I and Hasasna N: In vivo evaluation of mebendazole and Ran
GTPase inhibition in breast cancer model system. Nanomedicine
(Lond). 19:1087–1101. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Idrisova K, Simon H and Gomzikova M: Role
of patient-derived models of cancer in translational oncology.
Cancers. 15:1392022. View Article : Google Scholar : PubMed/NCBI
|