Breast cancer (BC) is among the most common cancers
in female patients globally. It has seen rising prevalence and
mortality rates, representing a challenge to both the medical
ERcommunity and society (1). The
etiology of BC involves a complex interplay of modifiable and
non-modifiable factors, which include genetic susceptibility,
environmental exposure, hormone levels, nutritional status and
lifestyle choices (2). Notable risk
factors for BC include personal or positive family history of BC,
obesity, tall height, smoking, alcohol consumption, early menarche,
late menopause, sedentary lifestyle, nulliparity and hormone
replacement therapy (3). Factors
associated with a decreased risk of BC include high parity,
breastfeeding, regular physical activity and weight management.
Increases in BC incidence are attributed to rising obesity rates
and declining fertility (4,5). BC in female patients is the second
most common cancer globally according to the Global Cancer
Observatory Database 2022 (6), with
an estimated 2.3 million new cases accounting for 11.6% of all
cancer cases. It is the fourth leading cause of cancer-associated
death worldwide, resulting in ~666,000 deaths, which constitute
6.9% of all cancer deaths in 2022 (6). This presents significant challenges to
public health and healthcare systems.
The development and homeostasis of multicellular
organisms rely on regulated cell proliferation and proper disposal
of unnecessary or potentially harmful cells. Inflammation is a
biological response of the immune system triggered by viruses,
toxic compounds and other factors (12). Cell damage and infection activate
the immune system, resulting in inflammation, regulated cell death
(RCD) and regeneration of inflammatory cells (13).
Programmed cell death (PCD) was first used to
describe a form of cell death actively driven by endogenous genetic
programs during individual development and tissue homeostasis
maintenance, such as classical apoptosis (14). PCD typically does not trigger an
inflammatory response and is morphologically characterized by cell
shrinkage, chromatin condensation and preservation of membrane
integrity (15). Subsequent studies
have found that, in addition to PCD, numerous cell death events do
not result from external mechanical damage, but occur depending on
the activation or inhibition of specific signaling pathways
(16,17). Therefore, the broader concept of RCD
was proposed. RCD not only includes apoptosis, but also involves
necroptosis, pyroptosis and ferroptosis. These death modes differ
in terms of morphology, immunological effects and inflammatory
responses, but collectively reflect the regulation of cell death.
PCD can be regarded as a physiological and evolutionarily conserved
type of RCD, while RCD reveals the dynamic characteristics of cells
regulated through molecular mechanisms to affect immune responses,
inflammation and disease progression under pathological stress and
microenvironmental changes (18).
Membrane-bound and cytoplasmic proteins participate
in RCD, forming a complex cascade through transcriptional changes
and post-translational protein modifications that induce cell death
(19).
Necrotic material activates the immune system and is
promptly phagocytosed and degraded by both macrophages and
neutrophils, efficiently eliminating necrotic cell debris (20,21).
The dissolution of infected or damaged cells constitutes the
primary strategy for eliminating pathogens and preserving health.
This process ensures the replenishment of normal cells in the body
and sustains tissue homeostasis (22). Inflammation becomes problematic when
harmful stimuli cannot be eliminated through RCD (23). Certain scholars believe that RCD
compromises host defense mechanisms against intracellular
pathogens, while others propose that cell bodies resulting from RCD
modulate the innate immune response to facilitate infection
clearance under appropriate immunological and microenvironmental
conditions (24,25).
The present review aimed to explore the interplay
among the immune system, inflammatory response and RCD in BC,
seeking novel therapeutic targets and approaches at the molecular
level (Fig. 1).
Microbial and viral infections represent threats to
human health. The immune system has evolved complex mechanisms to
recognize and eliminate pathogens, thereby safeguarding the host
from disease. Human immune response comprises innate and adaptive
immunity (26).
The innate immune system serves as the primary
defense mechanism and initiates the rapid response (27). This system includes physical
barriers such as the skin and mucous membranes, as well as cellular
components, such as phagocytes and natural killer (NK) and
dendritic cells (DCs) (28). Innate
immune cells undergo phenotypical and functional maturation upon
encountering pathogens, leading to the production of highly
efficient antigen-presenting cells that bridge innate and adaptive
immunity (29).
The innate immune system serves a key role in
eradicating nascent tumor cells during the clearance phase of
cancer immunoediting. NK cells recognize stress ligands, such as
major histocompatibility complex (MHC) class I polypeptide-related
sequence A/B and poliovirus receptor, on the surface of tumor cells
through their activating receptors natural killer group 2 member D
(NKG2D) and DNAX accessory molecule-1 (DNAM-1), and induce tumor
cell apoptosis by releasing perforin and granzyme B (30–33).
IFN-γ secreted by NK cells inhibits angiogenesis and activates
macrophages (34). However, the TME
reprograms myeloid cells during the equilibrium and escape stages,
leading to notable immunosuppressive cell infiltration.
Tumor-associated macrophages (TAMs) are one of the most abundant
types of immune cell with high plasticity in the TME. They exhibit
an M1-like phenotype, secreting pro-inflammatory factors and
exerting antitumor effects. However, they are more commonly
polarized into an M2-like phenotype in most solid tumors, releasing
factors such as IL-10, transforming growth factor (TGF)-β and VEGF,
that promote angiogenesis, tissue remodeling and tumor metastasis,
while suppressing T cell-mediated immune responses. Therefore, TAMs
serve a key role in tumor progression and immune escape and are
important targets for current immunotherapy interventions (35).
The innate immune system responds promptly via
macrophages and neutrophils phagocytosing pathogens and sending
inflammatory signals that initiate DC recruitment (36,37).
Pattern recognition receptors (PRRs) enable innate immune cells to
detect two types of danger signals: Pathogen-associated molecular
patterns from microbial infection and danger-associated molecular
patterns (DAMPs) from dying or damaged cells. PRRs include
toll-like receptors (TLRs), C-type lectin receptors, retinoic
acid-inducible gene-I (RIG-1)-like receptors and nucleotide-binding
oligomerization domain-like receptors (NLRs), which recognize these
signals and activate immune responses (38).
Immune cells eliminate pathogens upon activation via
cytokine and chemokine secretion, as well as phagocytosis.
Cytokines, such as IL-1, IL-6, tumor necrosis factor (TNF-α) and
IFN, initiate signaling pathways. For example, IL-1 and TNF-α are
involved in the activation of the NF-κB pathway, while IFN
stimulates the JAK/STAT pathway to induce an antiviral state
mediated by IFN-stimulated genes (39,40).
Cytokines in the TME construct a complex signaling network with a
context-dependent function. Type I IFN is a core coordinating
factor in antitumor immunity. It directly inhibits tumor cell
proliferation via the JAK1/tyrosine kinase 2-mediated
STAT1/STAT2/interferon regulatory factor 9 (IRF9) signaling complex
(41), while upregulating MHC-I
molecule expression, promoting DC maturation (42) and enhancing the survival and
effector functions of CD8+ T cells (43).
The inflammasome is a molecular platform of innate
immunity that responds to cell stress and infection. The NLRP3
inflammasome senses multiple upstream signals [ion flux, reactive
oxygen species (ROS) and lysosomal damage] and forms a complex that
activates caspase-1 (44).
Activated caspase-1 serves two key functions: Processing pro-IL-1β
and pro-IL-18 into their mature cytokine forms (45) and cleaving gasdermin D (GSDMD) to
release its N-terminal fragment, which oligomerizes in the plasma
membrane to form pores and induce pyroptosis (46).
Inflammasome signaling in BC has context-dependent
effects. NLRP3 activation within tumor cells in estrogen
receptor-negative tumors, such as triple-negative BC (TNBC),
promotes pre-metastatic niche formation and distant metastasis
through IL-1β autocrine/paracrine loops (47). By contrast, inflammasome activation
in hematopoietic cells enhances antitumor immunity, where
pyroptosis releases tumor antigens, which facilitate DC priming and
T cell activation (48). Histone
deacetylase inhibitors in TNBC can switch caspase-3 activity from
apoptosis toward pyroptosis by cleaving GSDME (49). This causes tumor cell lysis and DAMP
release, which recruit CD11b+ myeloid cells, enhance
antigen presentation and increase CD8+ T cell
infiltration and granzyme B secretion, restoring antitumor immunity
(50). Conversely, silencing or
loss of pyroptotic effectors, such as GSDME, during tumor
progression allows BC cells to maintain a ‘cold tumor’ state,
characterized by low immune cell infiltration and weak immune
activation, and escape immune surveillance (51).
The adaptive immune system mediates antigen-specific
responses through specific T and B cell receptors, forming
long-lasting immune memory. It serves as the core force for
eliminating tumor cells (52–54).
The efficacy of the adaptive immune response in BC directly
determines patient prognosis and immunotherapy response (55,56).
Numerous types of conventional anticancer therapy, such as
anthracyclines, oxaliplatin and radiotherapy, can induce
immunogenic cell death, exposing tumor cells to calreticulin and
releasing DAMPs such as ATP and high mobility group box 1 (HMGB1).
These signals enhance the phagocytic, maturation and
cross-presentation capabilities of DCs (57).
The core of the adaptive immune system antitumor
effect lies in cellular immunity, particularly the recognition and
killing of tumor neoantigens presented by tumor cells via MHC-I
molecules by CD8+ cytotoxic T lymphocytes (CTLs)
(58). CD4+ T helper
(Th) cells regulate the function of CTLs and NK and
antigen-presenting cells by secreting cytokines, such as IFN-γ and
IL-2. They also provide assistance to B cells, coordinating the
humoral immune response (59).
However, the TME of BC typically secretes factors,
such as VEGF, IL-10 and TGF-β, to suppress DC maturation, thereby
impeding T cell priming (60).
Simultaneously, activated T cells are prone to exhaustion under
sustained antigenic stimulation, manifesting as high expression of
inhibitory receptors, such as PD-1, T-cell immunoglobulin and mucin
domain-containing protein 3 (TIM-3) and lymphocyte activation gene
3 (LAG-3), and gradually losing their effector function (61). BC cells actively induce T cell
exhaustion by binding PD-1 through high programmed death-ligand 1
(PD-L1) expression, representing one of the primary adaptive immune
escape mechanisms. Immune checkpoint inhibitors (ICIs) restore T
cell function and enhance antitumor immune responses by blocking
the PD-1/PD-L1 pathway (62).
Beyond antibody production, B cells participate in
antigen presentation and cytokine secretion, forming tertiary
lymphoid structures (TLSs) within tumor tissue. TLSs comprise T and
B cells and DCs and sustain local antitumor immune responses. Their
presence is typically associated with higher T cell infiltration,
more active immune response and improved patient prognosis
(63).
Adaptive immune pathways serve tumor-suppressing
roles and drive immune escape in BC development and progression.
The efficacy of cancer vaccines, adoptive cell therapy and ICIs
depends on the integrity of antigen presentation, T cell activation
and effector function (64–66). CTLs become exhausted due to
persistent antigen stimulation when DCs are blocked. Alternatively,
tumor cells evade immune recognition by downregulating MHC-I and
upregulating PD-L1. The adaptive immune response becomes difficult
to sustain, allowing tumors to progress (67). The presence of B cells and TLSs
enhances local immune responses, but their antitumor functions are
typically weakened under the regulation of immunosuppressive
cytokines, such as TGF-β and IL-10, and may shift toward pro-tumor
effects (68). Therefore, immune
escape in BC is the outcome of a prolonged ‘arms race’ between the
adaptive immune system and the tumor. On one hand, the immune
system shapes tumor evolution by recognizing and eliminating it
(69). On the other hand, the tumor
reciprocally reprograms the immune response through multiple
mechanisms, such as secreting immunosuppressive cytokines (TGF-β
and IL-10), inducing regulatory T cells, and upregulating immune
checkpoint molecules such as PD-L1 (70,71).
Future therapeutic strategies may simultaneously enhance adaptive
immune effects and prevent TME suppression to disrupt this
malignant equilibrium and achieve durable antitumor control.
Continuous signal release during apoptosis attracts
phagocytes and facilitates cell clearance (72). Granzyme family molecules released
from activated T and NK cells induce apoptosis. These protein
hydrolases target cysteine aspartyl proteases or BH3-interacting
domain death agonist, activating them and bypassing upstream
signaling, thus initiating target cell apoptosis (73). Tanzer et al (74) identified a specific secretome
signature distinguishing apoptosis from other forms of cell death,
where apoptotic cells predominantly release nucleosomal components.
Saxena et al (64)
discovered that apoptotic lymphocytes and macrophages release
anti-inflammatory metabolites, maintaining plasma membrane
integrity and inducing genetic programs promoting inflammation
suppression, cell proliferation and wound healing.
Apoptosis is key for tissue development and renewal
as it regulates cell population balance. Additionally, CTLs
maintain tissue health by inducing apoptosis in target or
virus-infected cells (75). Reports
indicate that apoptosis is key in the adaptive immune system,
contributing to the loss of auto- or non-reactive T cell receptor
expression by thymocytes and the absence of auto-reactive immature
B cells (76,77). Inflammation resolution involves the
emergence of apoptotic neutrophils (78).
Apoptosis defects result in autoimmune
abnormalities. Lymphoproliferation (LPR) and generalized
lymphoproliferative disease (GLD) are natural mouse mutants
associated with lymph node and spleen enlargement and the
development of systemic lupus erythematosus-like autoimmune
disorder (79). Functional defects
in Fas and FasL genes result in LPR and GLD phenotypes (80). Patients with autoimmune
lymphoproliferative syndrome carry mutations in somatic or germ
cells encoding Fas or FasL genes. The Fas-mediated extrinsic
apoptotic pathway clears peripheral T cells and eliminates
self-reactive B cells. Studies have shown that Bcl-2-interacting
mediator of cell death (Bim)-mediated apoptosis regulates
short-lived myeloid cells, including eosinophils, neutrophils and
monocytes (81,82). These findings highlight the key role
of the Fas-FasL system and Bim-mediated endogenous and exogenous
apoptotic pathways in autoimmune disease development (83).
In addition to exogenous stimuli, apoptosis is
regulated or activated by internal stimuli, such as DNA damage and
oxidative stress (84). The
intrinsic pathway for this process is characterized by
mitochondrial regulation and non-receptor-mediated initiation.
Stimuli such as oxidative stress cause mitochondrial membrane
disruption, leading to the formation of mitochondrial permeability
transition pores, thereby allowing pro-apoptotic factors to enter
the cytosol. This intrinsic apoptosis pathway involves key
mediators, such as Bcl-2 family proteins, including pro-apoptotic
Bax and anti-apoptotic Bcl-2 (85).
These proteins regulate the release of cytochrome c from the
mitochondria into the cytosol, where it forms a complex with
apoptotic protease-activating factor 1 and procaspase-9, known as
the apoptosome (86). This complex
activates mTOR, which triggers executioner caspases such as
caspase-3, −6 and −7, leading to apoptosis (87). Additionally, proteins, such as
second mitochondria-derived activator of caspase/direct IAP-binding
protein with low pI (SMAC/DIABLO) and apoptosis-inducing factor,
participate in caspase-dependent and -independent pathways,
respectively. SMAC/DIABLO inactivates apoptosis protein inhibitors,
thus facilitating apoptosis, while apoptosis-inducing factor
translocates to the nucleus once released from the mitochondria to
induce DNA fragmentation and chromatin condensation (88).
Research indicates that the endoplasmic reticulum
(ER) is also involved in apoptosis (89). Excess accumulation of proteins
within the ER and disruption of calcium homeostasis trigger ER
stress, leading to apoptosis (90).
Caspase-12 is located on the ER membrane and is key for ER-mediated
apoptosis (91). The ER response
triggers caspase-12 expression while translocating cytosolic
caspase-7 to the ER membrane, where caspase-12 is activated,
leading to apoptosis (87).
Autophagy and apoptosis exhibit a complex and
dynamic interplay in BC, jointly determining cell fate. Autophagy
is a survival mechanism that can suppress apoptosis by clearing
damaged organelles, such as dysfunctional mitochondria, and
decreasing ROS accumulation, promoting cancer cell survival under
chemotherapy stress and leading to therapeutic resistance (92,93).
On the other hand, excessive or sustained autophagic activity can
result in type II PCD, also known as autophagic cell death,
characterized by massive autophagosome formation and degradation of
cellular components. Key proteins, such as glucose-regulated
protein 78 (GRP78), serve an important role during ER stress. GRP78
activates protective autophagy by inhibiting the mTOR pathway while
suppressing apoptosis, thus supporting cell survival under estrogen
deprivation or tamoxifen treatment and contributing to endocrine
therapy resistance (94,95).
Autophagy serves as a key adaptive mechanism for BC
cell survival in the nutrient-deficient TME, such as that of
hypoxia or glucose deprivation (96,97).
Autophagy is activated under stress conditions to degrade redundant
or damaged intracellular proteins and organelles, recycling key
metabolites, such as amino and fatty acids, for energy production
and biosynthesis, maintaining cellular energy homeostasis (98). Moreover, the hypoxia signaling
pathway mediated by hypoxia-inducible factor-1α (HIF-1α)
upregulates autophagy (99),
facilitating the clearance of toxic substances generated under
hypoxic conditions and sustaining the stemness and self-renewal
capacity of BC stem cell populations (100,101). This enables residual cancer cells
to enter a dormant state and contribute to disease recurrence
(102).
Disruptions in lipid metabolism promote autophagy.
Lipophagy is a key autophagy function, in which lipid droplets are
selectively degraded to release free fatty acids for β-oxidation
and energy production, which is key under glucose-deprived
conditions. BC cells typically undergo metabolic reprogramming,
while autophagy influences intracellular metabolite levels by
regulating amino acid transporters, such as solute carrier family 6
member 14 (SLC6A14). Decreased activity of these transporters
disrupts amino acid availability and stimulates autophagy,
contributing to endocrine therapy resistance (95). Thus, metabolic dysregulation may
modulate autophagic activity by affecting key energy-sensing
pathways, such as mTOR and AMP-activated protein kinase (AMPK),
forming a metabolic adaptive feedback loop that influences cancer
cell survival, proliferation and therapeutic response (103,104).
Cell pyroptosis is a type of RCD with lytic and
inflammatory characteristics. It is mediated by the cleavage of
GSDM family proteins. GSDMD is cleaved by inflammatory caspases
(caspase-1, −4, −5, and −11) at a specific site to release its
N-terminal domain, which forms pores in the plasma membrane,
whereas GSDME is cleaved by caspase-3 at a distinct site, linking
apoptosis and pyroptosis (105).
This results in cell swelling, lysis and release of
pro-inflammatory factors, including IL-1β, IL-18, ATP and HMGB1.
Acute pyroptosis activation may induce immune cell infiltration,
potentially inhibiting tumor growth (106). GSDM proteins are activated by
distinct signaling pathways to execute pyroptotic functions in
specific contexts. GSDMD serves as the primary executor of
classical inflammatory pyroptosis. It is primarily cleaved by
caspase-1 or −4/5/11 activated by inflammasomes, commonly observed
during immune cell defense against pathogenic infection (46,107).
However, GSDME is typically cleaved by apoptosis-associated
caspase-3. When caspase-3 is activated, the cell fate shifts from
non-inflammatory apoptosis to inflammatory pyroptosis (108). In addition, granzyme B secreted by
CTLs directly cleaves GSDME (109). Overall, inducing cellular
pyroptosis within tumors may represent a potential strategy for
treating numerous types of cancers. Tumor cells undergoing
pyroptosis recruit tumor-suppressing immune cells. Wang et
al (110) employed a
bioorthogonal system to demonstrate that pyroptosis in <15% of
tumor cells eliminates entire tumor grafts in live mice. This
orthogonal system is capable of controlled drug release by
combining nanoparticle-mediated delivery with Phe-boron
trifluoride-catalyzed desilylation to selectively release client
proteins, including active GSDM, into tumor cells in mice. Zhang
et al (109) demonstrated
that CD8+ T and NK cells induce pyroptosis in tumor
cells through granzyme B in a pyroptosis-activated immune ME,
establishing a positive feedback loop. However, tumor suppression
is abolished in perforin-deficient mice and mice lacking killer
lymphocytes. A total of 20/22 tested cancer-associated GSDME
mutations decrease its function, indicating that GSDME inactivation
may be employed by cancer cells to evade immune attacks. This is
particularly relevant in BC. On one hand, genetic mutations
directly impair the pore-forming ability of GSDME and decrease the
occurrence of pyroptosis. On the other hand, promoter
hypermethylation of GSDME is frequently reported in BC, and
demethylating treatment can restore its expression and enhance
chemotherapy- or immune-induced pyroptosis (108,111). The loss or dysfunction of GSDME in
BC allows tumor cells to evade immune surveillance by decreasing
pyroptosis and DAMP release, which decreases the infiltration and
activation of CD8+ T and NK cells. This loss can result
from gene mutations or promoter methylation, suggesting that
restoring GSDME expression or function may be a potential strategy
to enhance antitumor immune responses.
Phagocytosis removes cell debris generated during
RCD and breaks it into macronutrients for use by other cells
(112). This maintains
intracellular environment stability and recycles cell components,
providing the necessary material for normal cell function.
Specialized cells such as macrophages, neutrophils, monocytes, DCs
and osteoclasts are capable of phagocytosis. Phagocytosis is not an
isolated cell reaction. It typically occurs concurrently with other
cellular processes, such as ROS production, proinflammatory
mediator secretion, antimicrobial molecule degranulation and
cytokine production (113).
Phagocytosis eliminates pathogens and host cells undergoing
apoptosis during infection (114).
Phagocytosis is key for antitumor immunity. Specific
macrophages enhance the antitumor response through distinct
phenotypes and functions. For example, TAMs hinder the efficacy of
glioblastoma immunotherapy (115).
CD169 macrophages enhance tumor-specific T cell responses by
promoting apoptotic glioma cell phagocytosis (116). Inflammatory macrophages regulate
macrophage phagocytosis and enhance tumor cell phagocytosis via
non-traditional pro-phagocytic integrins such as the CD47/signal
regulatory protein α) signaling pathway (117). Additionally, CTL-associated
protein 4 (CTLA-4) blockade stimulates microglial cells and
enhances tumor cell phagocytosis, promoting antitumor effects
(118). Immunotherapeutic
strategies may offer novel approaches for glioblastoma treatment by
activating synergistic interactions between macrophages and Th1 and
microglial cells and by boosting immune cell activation and
infiltration.
Tumor cells employ diverse mechanisms within the TME
to escape or inhibit phagocytosis, promoting tumor progression and
immune evasion. Tumor cells hinder phagocytosis and evade immune
clearance by expressing innate immune checkpoints, such as the
CD47/SIRPα signaling pathway. Blocking phagocytosis checkpoints
enhances phagocytic activity against tumor cells and encourages
their clearance (119). PD-L1
expression may hinder T cell cytotoxicity and macrophage-mediated
phagocytosis. Disrupting this pathway may trigger antitumor
immunity (120). Overall,
phagocytosis serves a pivotal role in pathogen clearance, cellular
waste disposal and antitumor defense mechanisms.
Optimal nutritional status is key for regulating
inflammatory and oxidative stress processes, which are connected
with the immune system (121).
Inflammation is recognized as a primary driver of malnutrition in
disease (122). It triggers
various physiological responses, such as loss of appetite,
decreased food intake, muscle breakdown, and insulin resistance,
leading to a catabolic state (122). Patients with colorectal cancer are
prone to chronic inflammation, malnutrition and complications
arising from chronic nutritional and energy depletion, insufficient
dietary intake, stress and metabolic disruptions due to surgeries,
chemotherapy or radiation therapy (123). Though most commonly attributed to
systemic inflammatory responses, hypoalbuminemia can result from
various other causes. For example, liver cirrhosis causes
hepatocyte damage and reduced synthetic capacity, while kidney
disease leads to increased albumin excretion in urine.
Additionally, dietary deficiencies in key amino acids cause
decreased albumin levels (124).
Albumin serves a key role in maintaining oncotic pressure,
neutralizing reactive species and preserving microvascular
integrity, thus protecting against inflammatory tissue damage
(125). Albumin is the most
abundant plasma protein and reflects both nutritional status and
inflammatory responses of the host. Recent evidence has
demonstrated its independent prognostic value in BC (126). In a large cohort study of nearly
3,000 patients with BC, a low albumin-to-globulin ratio and
decreased prealbumin levels were significantly associated with
inferior overall survival (OS) and disease-free survival.
Importantly, these markers retained independent prognostic
significance in multivariate models following adjustment for
conventional factors, such as TNM stage and molecular subtype
(127). Similarly, serum albumin
levels <43 g/l are predictive of shorter OS in an independent
cohort of patients with metastatic BC, with low albumin identified
as an independent adverse prognostic factor [hazard ratio
(HR)=0.47, P<0.01] (128).
Low levels of long-chain n-3 polyunsaturated fatty
acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid in
Western diets may contribute to chronic inflammation (129), as these fatty acids exert
anti-inflammatory effects by inhibiting pro-inflammatory cytokine
production and modulating eicosanoid synthesis (130). Various dietary and nutrient
components, such as omega-3 fatty acids, vitamin A and C and
phytochemicals such as polyphenols and carotenoids found in plant
foods, possess anti-inflammatory and antioxidant properties.
Moreover, dietary fiber in plant foods that is fermented by gut
microbiota to produce short-chain fatty acids is associated with
health benefits, including anti-inflammatory properties (131).
Lipids, including fatty acids, cholesterol and
phospholipids, form a diverse group of metabolites crucial for
constructing cell and organelle membranes, generating cell energy
and engaging in intracellular and hormonal signaling (132). In a healthy organism, free fatty
acids undergo β-oxidation to produce ATP and maintain nutrient flux
balance. Excess fatty acids are packaged into inert triglycerides
using a glycerol backbone and stored in intracellular lipid
droplets (133). Excessive
nutrition can lead to the accumulation of lipid intermediates in
new non-adipose tissue, resulting in lipotoxicity and further
tissue damage (134).
Large lipid loads in organ systems impair the
efficient fat catabolism and conversion of fatty acids to
triglycerides in cells, leading to increased lipogenesis or
triglyceride storage in adipose tissue. Glucose oxidation is
enhanced in muscle cells, while lipolysis in adipose tissue and
fatty acid oxidation in muscle cells increase to provide energy
during fasting conditions. These metabolic changes primarily occur
in the brain, heart, liver, pancreas, kidney and adipose tissue
(135).
In human clinical studies, lipid accumulation is
associated with renal dysfunction, while dyslipidemia directly or
indirectly impacts the kidney via systemic inflammation, oxidative
stress, vascular injury and alterations in signaling molecules such
as hormones (136,137). Glomeruli and tubules, especially
proximal tubules, are prone to lipid accumulation, contributing to
renal injury and dysfunction, which are key factors in diabetic
nephropathy (138). Non-alcoholic
fatty liver disease is associated with dysregulated lipid synthesis
and lipolysis pathways, with studies suggesting decreased lysosomal
acid lipase activity in such patients (139,140).
Moreover, endogenous lipid palmitic acid hydroxy
stearic acids positively impact blood glucose levels and insulin
sensitivity by regulating fat deposition and adipose tissue
lipolysis. Long-chain fatty acid oxidation influences postnatal
skeletal development. EPA is a long-chain polyunsaturated n-3 fatty
acid that regulates fatty acid re-esterification, impacting
substrate cycling in human skeletal muscle cells (141). The heart demonstrates the highest
energy demand and the most extensive fatty acid oxidation in the
body that efficiently accesses circulating lipids. However,
increased lipid availability exacerbates ischemia-induced cardiac
dysfunction (142). Lipid droplet
accumulation in glial cells is hypothesized to offer a protective
mechanism against the detrimental effects of neuronal activity by
detoxifying toxic fatty acids (143).
Abnormal lipid metabolism and inflammatory responses
form a cycle in certain pathological conditions, such as obesity,
type 2 diabetes, atherosclerosis and non-alcoholic fatty liver
disease, exacerbating disease progression. Lipid accumulation
triggers oxidative stress and inflammatory responses, releasing
oxidized lipid intermediates that stimulate cytokine production by
inflammatory cells, leading to abnormal lipid metabolism (134). The lipid intermediates invade
healthy non-adipose tissue, causing lipotoxicity and additional
damage. Damaged cells undergo PCD to release more nutrients. During
this process, the levels of the primary products of the
inflammatory response (lipid intermediates) are amplified,
initiating a cycle of deleterious stimuli to the tissues/cells.
Free fatty acids can act as endogenous danger signals by binding
the TLR4/myeloid differentiation factor 2 (MD2) complex, activating
the downstream MyD88/interleukin-1 receptor-associated kinase
(IRAK)/TNF receptor-associated factor 6 (TRAF6) signaling pathway.
This leads to the activation of the IκB kinase complex and IκB
degradation, releasing NF-κB dimers that translocate into the
nucleus to induce the transcription and secretion of
pro-inflammatory cytokines, such as IL-6 and TNF-α. This mechanism
serves a key role in chronic low-grade inflammation associated with
obesity and insulin resistance and contributes to the initiation
and progression of cancer such as BC (144,145).
The cycle between abnormal lipid metabolism and
inflammation impairs normal cellular and tissue metabolic functions
and accelerates the progression of conditions such as
atherosclerosis (146), obesity
and fatty liver disease (147). In
the context of BC, this lipid-inflammation feedback loop
contributes to a tumor-promoting microenvironment by enhancing
cytokine production, sustaining NF-κB activation and supporting
cancer cell survival. It triggers a cytokine storm with the release
of large amounts of cytokines, and the dysregulated inflammatory
response forms a self-reinforcing feedback loop that may endanger
the host (148). The levels of
pro-inflammatory cytokines (IL-1, IL-6, TNF and IFN-γ), especially
TNF and IFN-γ, are elevated during cytokine storms (149). These factors induce cell death in
numerous types of cell, leading to diseases, such as neurological
disorder, liver injury, chronic obstructive pulmonary disease,
fibrosis and osteoporosis (150).
TNF and IFN-γ activation also induces cell death pathways,
including pyroptosis, apoptosis and necrosis, further stimulating
cytokine release and triggering a cytokine storm (151).
The link between BC and viral infection is debated
in terms of its causes and may interact with other environmental
factors to promote tumorigenesis (152,153). DNA from viruses, including human
papillomavirus (HPV), Epstein-Barr virus (EBV), human
cytomegalovirus (HCMV), herpes simplex virus and Kaposi's
sarcoma-associated herpesvirus/human γ herpesvirus 8, have been
detected in BC samples (152).
However, these viral DNA particles are not present in all BC
subtypes and do not control apoptosis, autophagy and pyroptosis in
the same way across all subtypes (152). The impact of viral infections can
vary depending on the specific BC subtype and the viral mechanisms
involved.
High-risk HPV subtypes, particularly HPV16 and
HPV18, are most commonly associated with carcinogenic effects and
employ unique mechanisms to suppress apoptosis (154). HPV inhibits cancer cell apoptosis
by upregulating the tumor necrosis factor receptor superfamily
death receptor (155).
Furthermore, autophagy inhibition, which is typically mediated by
the E7 oncoprotein, exhibits subtype specificity. A study in
oropharyngeal squamous cell carcinoma indicated that HPV16 uses
E7-mediated degradation of the autophagy and beclin 1 regulator 1
protein to suppress autophagy, rendering cells more sensitive to
cisplatin-induced apoptosis (156). By contrast, EBV encodes an
anti-apoptotic product that enhances infected cell viability and
resistance to chemotherapy, promoting the development of
EBV-associated disease (157).
HCMV is a slow-replicating virus that has evolved and acquired
anti-apoptotic genes, including pUL38 and UL138, which encode
apoptosis inhibitors, ensuring HCMV replication by inhibiting
apoptosis (158).
Mounting evidence suggests the BC microenvironment
is diverse and dynamic, with cell pyroptosis playing a crucial role
in its control (159,160). GSDMD and GSDME are key pyroptosis
substrates that play key roles in BC etiology and pathogenesis
(161). GSDM protein family plays
a pivotal role in cellular pyroptosis (162). MicroRNA-200b activates GSDMD by
targeting the NF-κB, maternally expressed gene 3 (MEG3), juxtaposed
with another zinc finger 1 and JAK2/STAT3 pathways, whereas
uncoupling protein 1, dopamine receptor D2, the
AMPK/SIRT1/NF-κB/BAK and the STAT3/ROS/JNK pathway activate GSDME
(163). Additionally, some PRRs,
such as absent in melanoma 2, melanoma differentiation-associated
gene 5 and RIG-I, can activate GSDME (164). Furthermore, complexes containing
PD-L1 and STAT3 upregulate GSDMC expression under hypoxic
conditions (165).
Multiple mechanisms prevent apoptosis in BC TME.
Cancer-associated fibroblasts (CAFs) secrete factors such as IL-6
and CXCL12 that activate survival pathways in cancer cells,
enhancing their resistance to apoptosis (166). TAMs produce cytokines, such as
IL-10 and TGF-β, which promote tumor cell survival (70). Additionally, the hypoxic conditions
within tumors induce HIF-1α expression, leading to the upregulation
of anti-apoptotic proteins such as Bcl-2 and survivin (167). These factors create an environment
that supports cancer cell survival and proliferation by inhibiting
apoptotic pathways (168).
The inflammatory microenvironment in BC continuously
shapes RCD pathways through persistent signaling, consequently
influencing tumor progression (169). Pro-inflammatory cytokines,
including IL-1, IL-6, and TNF-α, activate key signaling cascades,
such as NF-κB, JAK/STAT3, and PI3K/AKT. These drive the
transcription of anti-apoptotic molecules such as Bcl-2, Bcl-xL and
X-linked inhibitor of apoptosis protein, while simultaneously
suppressing Bax/Bak-mediated mitochondrial outer membrane
permeabilization and blocking cytochrome c release (170). This weakens the mitochondrial
apoptotic response and allows tumor cells to maintain survival
advantages under adverse conditions. At the same time, these
signals alter inflammatory cell death modalities, for example, by
activating the NLRP3/caspase-1/GSDMD or GSDME pathways to promote
pyroptosis, inducing immune activation and T cell infiltration
(171). Pyroptosis may be
attenuated in the absence of effector molecules or when signals are
reprogrammed, enabling tumors to evade immune surveillance
(172). The inflammatory
microenvironment in BC exhibits both pro-tumor and antitumor
potential through this dynamic regulation of both the threshold and
mode of cell death, ultimately determining the course of disease
progression and therapeutic responses.
The innate immune system is key for BC immunity. It
comprises innate lymphocytes (ILCs), whose differentiation and
function are associated with the immune response (173,174). ILCs are a newly discovered class
of innate immune cell derived from pluripotent hematopoietic stem
cells (175). These cells are
predominantly found in tissue and are common on the mucosal
surfaces of the lung and intestines (176). ILCs are divided into three groups.
The first group includes ILC1s and NK cells, the second group
comprises ILC2s and regulatory ILCs (ILCregs) and the third group
consists of ILC3s and lymphoid tissue-inducing cells (177). ILC1s are characterized by their
ability to produce IFN-γ and are key in the early BC stages due to
their role in activating cytotoxic immune responses. In advanced
tumors, ILC1s are typically induced by factors such as TGF-β in the
TME to drive the conversion of NK cells into an ILC1-like phenotype
(178–180). These cells lose their potent
cytotoxic activity and promote angiogenesis and immune tolerance
via secretion of factors such as VEGF. At the same time, ILC1s
within tumor tissue commonly upregulate multiple immune checkpoint
receptors, including NKG2A, killer cell lectin-like receptor
subfamily G member 1, CTLA-4, CD96 and LAG3, leading to marked
functional suppression. Overall, they exhibit immunosuppressive and
tumor-promoting effects (181).
ILC2s produce type 2 cytokines, including IL-4, IL-5 and IL-13, and
are involved in tissue repair and immune regulation. However, their
role in cancer is either promotive or inhibitory depending on the
context. ILC3s produce IL-17 and IL-22, contributing to both
pro-inflammatory and tissue-regenerative processes. Their role in
tumor development can also vary (182).
Myeloid cells, notably granulocytes, macrophages and
DCs, are crucial for maintaining immune system homeostasis and
exert key effects within the TME (183). Myeloid-derived suppressor cells
(MDSCs) are immune-suppressive cells that serve a key role in BC
development and progression. Their regulatory mechanisms involve
multiple cytokines, such as granulocyte-macrophage
colony-stimulating factor (GM-CSF), granulocyte colony-stimulating
factor (G-CSF), VEGF and interleukins (IL-1, IL-6, IL-13, IL-17,
IL-20, IL-33 and IL-34), as well as signaling pathways, including
the STAT family (STAT3, STAT6), NF-κB, Notch, ER stress response
and JAK/STAT and PI3K/AKT/mTOR cascades (184). The complement system activity is
crucial in BC immunity, with components such as C1q, C3a, and C5a
associated with tumor growth, metastasis and immune responses
(185). The complement system also
promotes tumor progression through various mechanisms by enhancing
angiogenesis, suppressing antitumor immune responses and aiding in
the creation of a tumor-friendly microenvironment (186). Additionally, host defense peptides
and antimicrobial agents have antitumor activity against cancer
cells (187).
B and T cells from the adaptive immune system are
key for the antitumor response. B cells secrete antibodies and
regulate immune responses. Activated B cells infiltrating tumors
have antitumor effects in BC (188). Regulatory B cells may influence
the tumor immune microenvironment by secreting inhibitory factors
such as IL-10 and TGF-β (189). T
cells, notably CD4+ and CD8+ T cells, are key
in BC. CD8+ T cells directly eliminate cancer cells via
CTL production, while the differentiation status and secreted
cytokines of CD4+ T cells impact the immune response
direction (190). Th1 cell
activity contributes to tumor growth inhibition and spread
(191). Moreover, T regulatory
cells (Tregs), a subset of CD4+ T cells characterized by
transcription factor FOXP3 expression, play a critical role in
maintaining immune homeostasis and preventing autoimmune responses.
Tregs also contribute to immunosuppression in cancer by inhibiting
the function of effector T cells, including CD8+ CTLs,
which can facilitate tumor progression (192). Additionally, T cell exhaustion,
which is marked by the upregulation of inhibitory receptors, such
as PD-1 and CTLA-4, leads to a dysfunctional state in which T cells
lose their effector functions, contributing to the
immunosuppressive TME (193).
Additionally, specific immunomodulatory proteins such as
prolactin-inducible protein are crucial in BC immunomodulation, and
their absence may result in tumor development (194).
BC cells typically downregulate MHC-I molecules via
epigenetic mechanisms to evade immune surveillance, with DNA
methylation and histone modification serving key roles. DNA
methyltransferase-mediated promoter hypermethylation can silence
genes, such as human leukocyte antigen (HLA)-A/B/C and
β2-microglobulin, while also suppressing the expression of
antigen-processing components, including transporter associated
with antigen processing (TAP)1/2, thereby weakening antigen
presentation. At the same time, the EZH2/PRC2 complex suppresses
transcriptional regulators such as CIITA via H3K27me3
modifications, further reducing MHC-I transcription. As a result,
the surface expression of MHC-I on BC cells is markedly diminished,
leading to impaired recognition by CD8+ T cells,
resulting in immune evasion (195,196).
Glycolytic reprogramming (the Warburg effect) in
the TME provides energy and metabolic intermediates for tumor cells
and impairs the antitumor functions of the immune system via
multiple mechanisms (204).
Excessive lactate accumulation leads to local acidification, which
directly suppresses the effector activity of cytotoxic T and NK
cells, while decreasing the production of key cytokines, such as
IFN-γ and TNF-α (205). At the
same time, the high glucose demand of tumors creates energy
competition, placing T cells and DCs in a metabolically restricted
state, thus diminishing their proliferative capacity and antitumor
responses (206). Lactate also
drives TAMs toward an immunosuppressive M2 polarization and hinders
DC maturation, further promoting MDSC accumulation and shaping an
immunosuppressive microenvironment (207). In addition, glycolytic metabolites
activate signaling pathways, such as HIF-1α, and induce PD-L1
upregulation, inhibiting T cell function through immune checkpoint
mechanisms (196,208,209).
In summary, the interplay between the innate and
adaptive immune systems, coupled with the regulatory roles of
diverse cells and molecules, is key in the onset, progression and
treatment of BC. Gaining a deeper understanding of these mechanisms
may aid in devising more effective immunotherapy strategies,
enhancing the survival and quality of life for patients with
BC.
BC development is associated with inflammation,
which is marked by the presence of immune system and other
inflammation-associated cells in its tissues. Infiltration of
inflammatory cells, including lymphocytes, macrophages, DCs,
monocytes and neutrophils, is a common characteristic of BC
(210). It can result in the
release of inflammatory factors, such as IL-17A and IL-6, which
activate pathways associated with cancer and facilitate BC
development (211). Studies have
demonstrated an association between the degree of inflammatory cell
infiltration in BC tissue and cancer survival (212,213). Specifically, the presence of
CD8+ T cells within the tumor is linked to patient
survival (214). Notably, IL-6
serves an important role in the inflammatory microenvironment of
BC, particularly in aggressive subtypes, including TNBC and
HER2+ BC. IL-6 promotes tumor progression and metastasis
via the JAK/STAT3 signaling pathway in TNBC, and high IL-6 serum
levels are associated with poor prognosis (215,216). IL-6 contributes to trastuzumab
resistance in HER2+ BC by expanding cancer stem cell
populations via an autocrine inflammatory loop (217). These findings highlight the
potential of targeting IL-6 signaling as a therapeutic strategy in
specific BC subtypes.
Inflammatory cytokines in BC regulate the balance
between apoptosis and autophagy through multiple signaling
pathways, influencing cell fate. Studies have shown that TNF-α and
IL-1β can upregulate the expression of the anti-apoptotic Bcl-2 via
the NF-κB pathway, inhibiting mitochondrial-mediated apoptosis
(218,219). However, TNF-α also activates the
JNK pathway under strong inflammatory stimulation to promote
Bax/Bak-mediated mitochondrial membrane permeabilization,
ultimately inducing caspase cascades and triggering apoptosis
(220). At the same time, IFN-γ
enhances the expression of effector molecules, such as caspase-3,
through the JAK/STAT1 pathway, amplifying apoptotic effects.
Furthermore, IL-6 suppresses autophagic activity via the
JAK/STAT3/mTOR signaling pathway, helping BC cells to maintain
survival and acquire drug resistance under adverse conditions
(221). The IL-6/STAT3 pathway,
persistently activated in TNBC and some HER2+ BC, not
only suppresses autophagy but also induces PD-L1 expression,
driving immune evasion and serving as a key target for combined
immunotherapy (222). The NLRP3
inflammasome and its downstream caspase-1/GSDMD pyroptotic pathway
can both promote metastasis via IL-1β secretion and trigger
immunogenic cell death, thus offering bidirectional therapeutic
value. Hypermethylation-mediated silencing of GSDME dampens
pyroptosis-associated immunogenic signals, whereas demethylating
agents restore GSDME expression and enhance the efficacy of
immunotherapy (223). In addition,
the IL-17A/STAT3/VEGF axis promotes angiogenesis and
immunosuppression, making it a promising target to block metastasis
(11). Thus, inflammatory cytokines
are not only inducers of cell death but also remodel the dynamic
balance between autophagy and apoptosis through key pathways, such
as NF-κB, JAK/STAT and mTOR, impacting BC progression and
therapeutic responses.
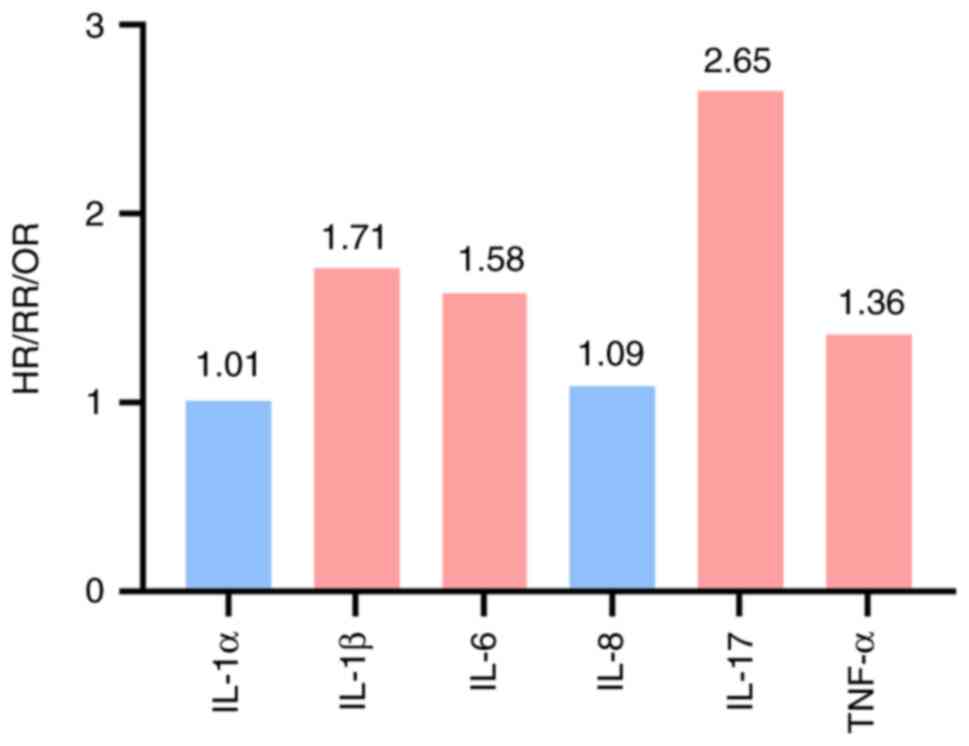
The association between inflammatory factors and BC
risk has been reported in multiple studies (224,225). The strength of the role served by
different inflammatory factors in BC pathogenesis varies. IL-17
confers the hazard risk (HR=2.65), with IL-1β [odds ratio
(OR)=1.71] and IL-6 [relative risk (RR)=1.58] also associated with
increased risk (226,227). The risks associated with TNF-α
(RR=1.36) and IL-8 (OR=1.09) are slightly increased, while IL-1α
(OR=1.01) is not associated with increased risk (Fig. 2) (226–228).
Numerous types of inflammatory cell play distinct
roles in BC. For example, macrophages serve key roles in chronic
inflammation associated with BC and can adopt M1 or M2 phenotype
(229). M1 macrophages release
pro-inflammatory cytokines, including TNF-α, IL-1 and IL-6, as well
as reactive oxygen and nitrogen species, which influence tumor
proliferation, invasion and metastasis. By contrast, M2 macrophages
release anti-inflammatory cytokines, such as IL-10, CCL5 and TGF-β,
which contribute to tissue repair and tumor progression. IL-6
exerts both pro- and anti-inflammatory effects (230). However, the concept that
pro-inflammatory M1 and anti-inflammatory M2 macrophages play
opposing roles in inflammation is oversimplified due to the high
plasticity of these cells in response to microenvironmental stimuli
(231). Moreover, the abundance of
macrophages is associated with clinical characteristics and
prognosis of BC (232).
Conversely, the presence of cancer-associated
adipocytes and corpuscular structures within BC tissue also
significantly influences tumor development (233). Cancer-associated adipocytes
secrete diverse inflammatory factors that alter BC cell behavior,
thereby promoting tumor invasion and metastasis (233). On the other hand, corpuscular
structures comprise macrophages, which serve as histological
indicators of pro-inflammatory processes and are present in adipose
tissue adjacent to BC (234).
These cells release pro-inflammatory factors that may induce a
chronic inflammatory state, influencing tumor progression and
prognosis.
Mesenchymal stem cells (MSCs) and CAFs serve key
roles in inflammatory processes associated with BC. MSCs secrete
chemokines and cytokines that influence tumor growth and
metastasis. MSCs have been shown to promote tumorigenesis by
differentiating into CAFs and enhancing the immunosuppressive
environment, leading to increased tumor cell proliferation and
invasion (235). CAFs represent
one of the predominant stromal cell populations in BC and
participate in inflammation-mediated TME regulation. CAFs
contribute to tumorigenesis by remodeling the extracellular matrix,
promoting angiogenesis and secreting factors, such as TGF-β, VEGF
and IL-6, which enhance the metastatic potential of tumor cells
(236). CAFs are involved in
resistance to chemotherapy and targeted therapies by creating a
protective niche for cancer cells and modulating immune cell
infiltration (237). This dual
role of MSCs and CAFs underscores their importance in BC
progression and treatment resistance.
Chronic inflammation is associated with BC
development and may also accelerate metastatic progression. Immune
cells and inflammatory mediators in the inflammatory
microenvironment enhance tumor cell migration and invasion, thus
elevating metastatic risk. Similarly, inflammation exerts
beneficial antitumor effects in the early stages of tumor
development. IFN-γ is a key factor in this process, as it
upregulates MHC-I expression in tumor cells, enhances antigen
presentation and promotes DC-mediated CD8+ T cell
activation, enabling them to directly kill tumor cells (238,239). Inflammation strengthens the
functions of NK cells and macrophages, further eliminating abnormal
cells and providing long-term surveillance via the formation of
immune memory, protecting the host during the initial tumorigenesis
stages (240). Thus, deeper
comprehension of chronic inflammation in BC is key for devising
more efficacious therapeutic approaches, potentially improving
patient prognosis and guiding future research.
Overnutrition is a notable risk factor for various
human diseases, especially those associated with obesity, such as
neurodegenerative diseases, metabolic disorder and cancer (241). Therefore, adopting a reasonable
fasting regimen is key. Fasting has notable lasting positive
effects on multiple health indicators by improving insulin
sensitivity, lowering blood pressure, decreasing body fat content
and stabilizing blood glucose levels and lipid metabolism (242). The relationship between obesity
and BC has garnered notable attention (243,244). The biological effects of obesity
extend beyond weight gain to include inflammatory responses,
endocrine alterations and metabolic dysregulation, which are
factors that collectively promote BC initiation and progression
(245,246).
Obesity increases systemic inflammatory responses
and drives metabolic disorder, including insulin resistance,
hyperinsulinemia and dysregulated lipid metabolism. These metabolic
abnormalities impair immune surveillance through multiple
mechanisms. For example, hyperinsulinemia and elevated insulin-like
growth factor 1 (IGF-1) signaling enhances tumor cell proliferation
and survival by activating the PI3K/AKT/mTOR pathway (247,248). Obesity-associated lipid
accumulation skews macrophages toward an M2-like immunosuppressive
phenotype and impairs DC maturation, weakening antigen presentation
(249). In addition, chronic
low-grade inflammation leads to persistent IL-6, TNF-α and leptin
secretion, which promotes Treg expansion and MDSC recruitment and
decreases CD8+ T cell cytotoxicity (250,251). Collectively, these
metabolic-immune alterations create a permissive TME that
facilitates BC cell proliferation, immune evasion and therapeutic
resistance.
The association between obesity and BC is a complex
and multifactorial research area that has attracted attention
(252,253). Epidemiological evidence indicates
that each 1 kg/m2 increase in body mass index (BMI) is
associated with a ~3.4% increase in BC risk in postmenopausal
patients [RR=1.034; 95% confidence interval (CI): 1.020–1.048].
Higher BMI shows a neutral or inverse association with BC risk in
premenopausal patients (RR=0.79; 95% CI: 0.70–0.88) (254). Severe obesity (BMI ≥35
kg/m2) further increases the risk, with HR of ~1.58 (95%
CI: 1.40–1.79) for invasive BC and ~1.86 (95% CI: 1.60–2.17) for
the ER+/PR+ subtype (255). Overweight and obese patients are
at a higher risk for lymph node-positive disease (HR=1.64; 95% CI:
1.09–2.48) and ER+ tumors (HR: 1.20–1.40) (256). Weight gain after adulthood is also
a key factor, with each 5 kg increase in body weight associated
with ~12% higher risk of postmenopausal BC (257).
Adipokines are bioactive hormones originating from
adipose tissue that regulate metabolism, caloric intake,
angiogenesis and cell proliferation. Adipokine levels typically
become dysregulated in obesity and are associated with cancer
development and metastasis (258).
Lipid metabolism influences BC proliferation, migration and
apoptosis (259). Lipids are key
to cellular structure and play pivotal roles in intercellular
signaling and metabolism (260).
Research indicates that cancer cells require substantial lipid
amounts for synthesizing biofilms, organelles and signaling
molecules that drive tumor progression (261). Chronic inflammation of white
adipose tissue is a crucial mechanistic link between obesity and
elevated BC risk (262). Activated
macrophages and elevated inflammatory mediators (IL-6, TNF-α and
IL-1β) amplify both local and systemic inflammation in obesity,
reshaping the TME and promoting angiogenesis, proliferation and
metastasis (263).
Cholesterol is a notable lipid component that plays
a vital role in BC pathogenesis. High cholesterol levels are
associated with increased BC risk and progression (264). Studies have shown that BC cells
exhibit altered cholesterol metabolism, which promotes tumor growth
and metastasis (265,266). Cholesterol influences BC cell
signaling pathways, such as the PI3K/AKT and ERK pathways,
contributing to cancer cell survival and proliferation. Moreover,
cholesterol-rich lipid rafts in the cell membrane serve as
platforms for oncogenic signaling. For instance, cholesterol
involvement in the PI3K/AKT pathway leads to the activation of a
key regulator of cell proliferation and survival, mTOR, thereby
promoting tumor development (267). The ERK pathway is modulated by
cholesterol and serves a crucial role in cell division and
differentiation, further aiding cancer progression (268).
Cholesterol also affects the composition and
fluidity of cell membranes, influencing the function of
membrane-bound proteins involved in signal transduction (269). This modulation enhances the
ability of cancer cells to invade surrounding tissue and
metastasize. Furthermore, the interaction between cholesterol and
caveolin-1, a structural protein in caveolae (specialized lipid
rafts), is implicated in tumorigenesis regulation (270).
Elevated circulating estrogen levels are associated
with a higher risk of hormone-sensitive BC (271). Peripheral adipose tissue becomes
the primary site of estrogen production during the postmenopausal
period, where aromatase converts androgens into estrogens.
Excessive adipose tissue leads to higher circulating estrogen
levels with an increasing BMI, contributing to an increase in BC
incidence (272). However, this
appears to contradict the decrease in circulating estrogen levels
following menopause, which may demonstrate the complex association
between obesity and BC (273).
Metabolic syndrome and insulin resistance are
associated with BC development and prognosis. Hyperinsulinemia is
associated with heightened synthesis of IGF-1, activating signaling
pathways that enhance cancer cell proliferation, migration and
survival (274). Insulin also
serves a key role in lipid metabolism (275). Elevated insulin levels,
characteristic of insulin resistance, trigger lipolysis and the
release of substantial amounts of free fatty acids, impacting
metabolic health status and establishing a detrimental cycle
(276,277).
In summary, the association between obesity and BC
involves a complex, multifactorial process that involves the
interaction of various biological mechanisms. A thorough
understanding of these mechanisms may aid in more effective
prevention and treatment of BC, especially in obese patients.
Traditional treatments for BC, such as surgical
excision, radiotherapy, chemotherapy and endocrine therapy, are not
universally applicable and have a range of side effects. Thus,
identifying novel treatment strategies is key for enhancing the
prognosis of patients with BC. Immunotherapy, which comprises both
passive and active approaches, has potential in treating various
cancers (278,279). Passive immunotherapy involves the
administration of immune components, such as monoclonal antibodies
(trastuzumab), to directly target tumor cells. Active immunotherapy
aims to stimulate the host immune system to attack tumors. These
strategies include ICIs, cancer vaccines and cellular therapy
(280).
Subtype-specific immunotherapeutic strategies are
key given the heterogeneity of BC. For example, combining
trastuzumab with IL-6R inhibitors (tocilizumab) in HER2+
BC has shown promise in overcoming resistance mediated by
IL-6-driven cancer stem cell expansion (217). Dual blockade of IL-6 and CCL5
signaling has been demonstrated to synergistically inhibit tumor
growth and metastasis in TNBC (216). Ongoing clinical trials are
evaluating the efficacy of IL-6 pathway inhibitors in combination
with standard therapies for patients with metastatic
HER2+ BC and TNBC, underscoring the translational
potential of targeting inflammatory pathways in BC immunotherapy
(281–283).
Immunotherapeutic approaches for BC involve
therapies using humanized monoclonal antibodies that target altered
molecules expressed by cancer cells (284). Trastuzumab is a humanized
monoclonal antibody approved in 1998 for HER2+ BC that
represents a key therapeutic advancement (285). Trastuzumab is traditionally
classified as a molecularly targeted drug because it inhibits
HER2-mediated signaling and blocks downstream proliferation
pathways (286). However, growing
evidence indicates that its therapeutic effects also depend on
immune-mediated mechanisms, particularly antibody-dependent
cellular cytotoxicity (ADCC) (287,288). In this process, the Fc fragment of
trastuzumab binds FcγRIIIa (CD16) on the surface of NK cells,
prompting them to release perforin, granzyme and pro-inflammatory
cytokines, such as IFN-γ and TNF-α (287). This leads to tumor cell lysis and
recruitment of other immune cells to participate in the response.
The dual action links HER2 signaling inhibition with immune
activation, enabling trastuzumab and other HER2-targeted antibodies
to be incorporated into cancer immunotherapy (289). HER2-targeted drugs suppress
oncogene signaling and mobilize the immune response, highlighting
their dual attributes as both targeted therapy and immunotherapy
(288,290). Other anti-HER2 agents, including
the monoclonal antibody pertuzumab and small molecule tyrosine
kinase inhibitors (lapatinib, neratinib, gefitinib and afatinib),
have subsequently been used (291). Monoclonal antibodies primarily
exert immune-mediated effects such as ADCC, while tyrosine kinase
inhibitors function via intracellular signal blockade (292). Nevertheless, monoclonal antibody
therapy encounters challenges, including moderate remission rates
and drug resistance development (293). Another strategy involves the use
of antibody-drug conjugates and T cell bispecific antibodies
(294). The IMpassion130 trial
demonstrated that atezolizumab combined with nab-paclitaxel
improves progression-free survival in patients with TNBC, but the
clinical benefit is confined to the PD-L1-positive subgroup
(295). The aforementioned trial
did not show a significant OS advantage in the intention-to-treat
population and was discontinued due to the lack of efficacy in the
overall cohort (295).
Notable progress has been made in immunotherapy
based on BC subtypes. Clinical trial, such as KEYNOTE-355, have
confirmed that PD-1 inhibitors (pembrolizumab) combined with
chemotherapy significantly improve progression-free survival and OS
in TNBC, and this regimen has been approved by the US Food and Drug
Administration as a first-line standard treatment (296). Antibody-drug conjugates, such as
trastuzumab deruxtecan, have demonstrated efficacy in later-line
treatments for HER2+ BC and are being investigated in
combination with ICIs (297). In
addition, cancer vaccines, bispecific antibodies and cell therapies
are also increasingly explored in subtype-stratified BC treatments
(298,299). There are notable differences in
the immune response rate to PD-L1 inhibitors between patients,
which may be associated with biomarkers, such as tumor-infiltrating
lymphocyte (TIL) density, PD-L1 expression heterogeneity and tumor
mutational burden (TMB) (300).
Integrating molecular biomarkers, including PD-L1 expression, TIL
density and TMB, with the immune microenvironment status to guide
personalized treatment represents a key direction for improving
therapeutic efficacy (178,301).
Immune evasion mechanisms in BC involve alterations
in both the TME and tumor cells. Tumor immunogenicity depends on BC
subtype. For example, HER2-positive BC and TNBC exhibit distinct
immunogenic characteristics (297). Tumor cell recognition is key for
the success of immunotherapy, and elevated estrogen levels may
interfere with immune system activity (302). Therefore, combining anti-estrogen
therapy with immunotherapy may represent a reasonable strategy.
Furthermore, the anti-apoptotic tumor cell mechanisms and HLA-I
expression also impact the efficacy of immunotherapy (303). Targeting alterations in tumor
cells, such as via combination therapy with inhibitors, may enhance
clinical benefits for patients and alleviate treatment resistance.
Overall, the success of BC immunotherapy depends on overcoming the
tumor immune evasion mechanisms, optimizing the TME and enhancing
tumor cell recognition, which require consideration of both tumor
characteristics and individual patient differences.
The primary mechanism of BC vaccines is to activate
antigen-specific T cells, which target and eliminate cancer cells
(304). BC vaccines are classified
into those targeting HER2 or associated antigens and those
targeting non-HER2-associated antigens. The E75 vaccine is a safe
and effective immunotherapeutic agent that induces a peptide-based
immune response. A clinical trial demonstrated that the E75 vaccine
significantly decreases cancer recurrence in 95.2% of patients with
high-risk BC expressing HER2 when combined with booster
immunization (305). GP2 exhibits
lower HLA-A2 affinity compared with E75, but it may have higher
immunogenicity levels. GP2 induces T cell responses and
delayed-type hypersensitivity when administered concurrently with
GM-CSF in patients with high-risk BC (306). The AE37 vaccine is typically used
as adjuvant immunotherapy for BC (307).
As an emerging field in BC treatment, immunotherapy
faces challenges, such as low complete remission rates and
increased adverse events. Successful treatment involves overcoming
tumor immune evasion mechanisms and optimizing the TME to enhance
tumor cell recognition. Integrating individual patient differences
may help develop more effective treatment regimens.
The immune system, inflammatory response and RCD
are interconnected in BC, collectively influencing tumor
progression and treatment outcomes. The immune system is typically
suppressed in patients with BC, leading to immune evasion by the
tumor. Prolonged chronic inflammation promotes tumor development
and is associated with the malignant features of BC. Cytokines and
inflammatory mediators produced during inflammation affect the
proliferation, invasion and metastatic potential of tumor cells.
Abnormal regulation of apoptosis and autophagy in BC cells leads to
tumor proliferation and survival. Aberrant regulatory mechanisms of
RCD include apoptosis evasion and autophagy inhibition. These
abnormalities are associated with drug resistance and malignant
tumor characteristics. Studying the mechanisms and regulation of
these interactions may deepen understanding of BC development and
progression, offering novel strategies for its prevention and
treatment. In recent years, immunotherapy has achieved progress in
BC treatment, particularly that of TNBC (328). Pembrolizumab
combined with neoadjuvant chemotherapy significantly improves
pathological complete response rates in high-risk patients with
early TNBC and demonstrates favorable efficacy and safety in
clinical practice (KEYNOTE-522 and KEYNOTE-756 Phase III trials and
real-world studies) (309–311). These data support immunotherapy as
a standard treatment approach in high-risk early TNBC and highlight
the importance of individualized treatment strategies. Future
research directions should focus on BC subtypes and the role of
cytokines in the TME, while also adopting advanced technical
strategies. Single-cell RNA sequencing is used to map the
transcriptional profiles of immune cells across BC subtypes in
order to delineate subtype-specific immune regulatory networks.
Gene-editing approaches, such as clustered regularly interspaced
short palindromic repeats/CRISPR-associated protein 9
(CRISPR/Cas9), may be employed to investigate the functional roles
of key cytokines in tumor progression and therapeutic resistance to
establish causal mechanisms. Moreover, integrating these approaches
with spatial transcriptomics or proteomics may further uncover
cell-cell interactions and microenvironmental heterogeneity.
Together, these strategies may provide more precise insight and
promote the development of personalized immunotherapeutic
approaches.
Future research should focus on the roles of
inflammatory factors and cell death-associated pathways in
different molecular subtypes of BC, with particular attention to
the key functions of IL-6, IL-1β, TNF-α and their associated
signaling pathways in driving immune evasion and therapy resistance
(312). It is also important to
address the loss of immunogenicity caused by the silencing of GSDME
through promoter methylation, which impairs pyroptosis, as well as
the decrease in antigen presentation resulting from the
downregulation of PD-L1 and HLA-I (313,314). In addition, attention should be
given to obesity-associated inflammatory factors and abnormal lipid
metabolism, which contribute to an immunosuppressive TME.
Approaches such as single-cell RNA sequencing and spatial
transcriptomics can be employed to map the distribution and
interactions of immune cells and these key factors across BC
subtypes (315). Functional
validation of molecules such as IL-6, GSDME and TAP1/2 can be
performed using CRISPR/Cas9 technology in patient-derived organoids
and humanized mouse models. Furthermore, the effects of modulating
epigenetic states and metabolic pathways on immune cell
infiltration and treatment responses can be explored. Integrating
these findings with large-scale clinical data to analyze the
associations between these key molecules and patient prognosis and
treatment outcomes may provide novel targets and evidence-based
support for the development of more precise, subtype-specific
immunotherapy combinations for BC, such as ICIs combined with IL-6
inhibitors or epigenetic drugs.
Not applicable.
The present study was supported by the Science and Technology
Innovation Fund Project of Dalian (grant no. 2023JJ13SN050) and
Natural Science Foundation of Liaoning Province (grant no.
2024-MS-281), Liaoning Province ‘Xingliao Talent Program’ Medical
Experts Project, Young Medical Experts Special Project
(XLYC2412097).
Not applicable.
GL, BJ and JZ wrote the manuscript. ZF and SF
revised the manuscript. Data authentication is not applicable. All
authors have read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Britt KL, Cuzick J and Phillips KA: Key
steps for effective breast cancer prevention. Nat Rev Cancer.
20:417–436. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Admoun C and Mayrovitz HN: The etiology of
breast cancer. Mayrovitz HN: Breast Cancer [Internet] Brisbane
(AU): Exon Publications; 2022, View Article : Google Scholar
|
|
3
|
Youn HJ and Han W: A review of the
epidemiology of breast cancer in Asia: Focus on risk factors. Asian
Pac J Cancer Prev. 21:867–880. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ligibel JA, Ballman KV, McCall L, Goodwin
PJ, Alfano CM, Bernstein V, Crane TE, Delahanty LM, Frank E, Hahn
O, et al: Impact of a weight loss intervention on 1-year weight
change in women with stage II/III breast cancer: Secondary analysis
of the breast cancer weight loss (BWEL) trial. JAMA Oncol.
11:1194–1203. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bellanger M, Lima SM, Cowppli-Bony A,
Molinié F and Terry MB: Effects of fertility on breast cancer
incidence trends: Comparing France and US. Cancer Causes Control.
32:903–910. 2021.PubMed/NCBI
|
|
6
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
7
|
Herdiana Y, Sriwidodo S, Sofian FF, Wilar
G and Diantini A: Nanoparticle-based antioxidants in stress
signaling and programmed cell death in breast cancer treatment.
Molecules. 28:53052023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Propper DJ and Balkwill FR: Harnessing
cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol.
19:237–253. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruggieri L, Moretti A, Berardi R, Cona MS,
Dalu D, Villa C, Chizzoniti D, Piva S, Gambaro A and La Verde N:
Host-related factors in the interplay among inflammation, immunity
and dormancy in breast cancer recurrence and prognosis: An overview
for clinicians. Int J Mol Sci. 24:49742023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Yu R, Cai T, Chen Z, Lan M, Zou T,
Wang B, Wang Q, Zhao Y and Cai Y: Effects of immune cells and
cytokines on inflammation and immunosuppression in the tumor
microenvironment. Int Immunopharmacol. 88:1069392020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Habanjar O, Bingula R, Decombat C,
Diab-Assaf M, Caldefie-Chezet F and Delort L: Crosstalk of
inflammatory cytokines within the breast tumor microenvironment.
Int J Mol Sci. 24:40022023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu L, Abd Ghani MK, Aghemo A, Barh D,
Bassetti M, Catena F, Gallo G, Gholamrezanezhad A, Kamal MA, Lal A,
et al: SARS-CoV-2 infection, inflammation, immunonutrition, and
pathogenesis of COVID-19. Curr Med Chem. 30:4390–4408. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Li X, Yang M and Liu SB: Research
progress on morphology and mechanism of programmed cell death. Cell
Death Dis. 15:3272024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Della Torre L, Beato A, Capone V,
Carannante D, Verrilli G, Favale G, Del Gaudio N, Megchelenbrink
WL, Benedetti R, Altucci L and Carafa V: Involvement of regulated
cell deaths in aging and age-related pathologies. Ageing Res Rev.
95:1022512024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fernández-Lázaro D, Sanz B and Seco-Calvo
J: The mechanisms of regulated cell death: Structural and
functional proteomic pathways induced or inhibited by a specific
protein-A narrative review. Proteomes. 12:32024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian S, Long Y, Tan G, Li X, Xiang B, Tao
Y, Xie Z and Zhang X: Programmed cell death: Molecular mechanisms,
biological functions, diseases, and therapeutic targets. MedComm
(2020). 5:e700242024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong X, Tang R, Xiao M, Xu J, Wang W,
Zhang B, Liu J, Yu X and Shi S: Targeting cell death pathways for
cancer therapy: Recent developments in necroptosis, pyroptosis,
ferroptosis, and cuproptosis research. J Hematol Oncol. 15:1742022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He R, Liu Y, Fu W, He X, Liu S, Xiao D and
Tao Y: Mechanisms and cross-talk of regulated cell death and their
epigenetic modifications in tumor progression. Mol Cancer.
23:2672024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagata S: Apoptosis and clearance of
apoptotic cells. Annu Rev Immunol. 36:489–517. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel AA, Ginhoux F and Yona S: Monocytes,
macrophages, dendritic cells and neutrophils: An update on lifespan
kinetics in health and disease. Immunology. 163:250–261. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Upton JW, Shubina M and Balachandran S:
RIPK3-driven cell death during virus infections. Immunol Rev.
277:90–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu L: Cell self-destruction (programmed
cell death), immunonutrition and metabolism. Biology (Basel).
12:9492023.PubMed/NCBI
|
|
24
|
Santagostino SF, Assenmacher CA, Tarrant
JC, Adedeji AO and Radaelli E: Mechanisms of regulated cell death:
Current perspectives. Vet Pathol. 58:596–623. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jorgensen I, Rayamajhi M and Miao EA:
Programmed cell death as a defence against infection. Nat Rev
Immunol. 17:151–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bekkering S, Domínguez-Andrés J, Joosten
LAB, Riksen NP and Netea MG: Trained immunity: Reprogramming innate
immunity in health and disease. Annu Rev Immunol. 39:667–693. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Liu S, Goraya MU, Maarouf M, Huang
S and Chen JL: Host immune response to influenza A virus infection.
Front Immunol. 9:3202018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Purnamasari S and Hidayat R: The role of
natural physical, mechanical, and biochemical barriers as innate
immunity: A narrative literature review. Open Access Indones J Med
Rev. 3:361–364. 2023.
|
|
29
|
Place DE and Kanneganti TD: The innate
immune system and cell death in autoinflammatory and autoimmune
disease. Curr Opin Immunol. 67:95–105. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Molfetta R, Quatrini L, Santoni A and
Paolini R: Regulation of NKG2D-dependent NK cell functions: The Yin
and the Yang of receptor endocytosis. Int J Mol Sci. 18:16772017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren X, Peng M, Xing P, Wei Y, Galbo PM Jr,
Corrigan D, Wang H, Su Y, Dong X, Sun Q, et al: Blockade of the
immunosuppressive KIR2DL5/PVR pathway elicits potent human NK
cell-mediated antitumor immunity. J Clin Invest. 132:e1636202022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paolini R and Molfetta R: Dysregulation of
DNAM-1-mediated NK cell anti-cancer responses in the tumor
microenvironment. Cancers (Basel). 15:46162023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Voskoboinik I, Whisstock JC and Trapani
JA: Perforin and granzymes: Function, dysfunction and human
pathology. Nat Rev Immunol. 15:388–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin W, Luo Y, Wu J, Zhang H, Jin G, Guo C,
Zhou H, Liang H and Xu X: Loss of ADAR1 in macrophages in
combination with interferon gamma suppresses tumor growth by
remodeling the tumor microenvironment. J Immunother Cancer.
11:e0074022023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang S, Liu G, Li Y and Pan Y: Metabolic
reprogramming induces macrophage polarization in the tumor
microenvironment. Front Immunol. 13:8400292022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boada-Romero E, Martinez J, Heckmann BL
and Green DR: The clearance of dead cells by efferocytosis. Nat Rev
Mol Cell Biol. 21:398–414. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Telser AG: Molecular biology of the cell,
4th edition. Shock. 18:2892002. View Article : Google Scholar
|
|
38
|
Li D and Wu M: Pattern recognition
receptors in health and diseases. Signal Transduct Target Ther.
6:2912021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koike A, Tsujinaka K and Fujimori K:
Statins attenuate antiviral IFN-β and ISG expression via inhibition
of IRF3 and JAK/STAT signaling in poly(I:C)-treated hyperlipidemic
mice and macrophages. FEBS J. 288:4249–4266. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Turner MD, Nedjai B, Hurst T and
Pennington DJ: Cytokines and chemokines: At the crossroads of cell
signalling and inflammatory disease. Biochim Biophys Acta.
1843:2563–2582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ivashkiv LB and Donlin LT: Regulation of
type I interferon responses. Nat Rev Immunol. 14:36–49. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li G, Zhao X, Zheng Z, Zhang H, Wu Y, Shen
Y and Chen Q: cGAS-STING pathway mediates activation of dendritic
cell sensing of immunogenic tumors. Cell Mol Life Sci. 81:1492024.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong H, Franklin NA, Ritchea SB, Yagita H,
Glennie MJ and Bullock TN: CD70 and IFN-1 selectively induce
eomesodermin or T-bet and synergize to promote CD8+ T-cell
responses. Eur J Immunol. 45:3289–3301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jo EK, Kim JK, Shin DM and Sasakawa C:
Molecular mechanisms regulating NLRP3 inflammasome activation. Cell
Mol Immunol. 13:148–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng M and Kanneganti TD: The regulation
of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis,
apoptosis, and necroptosis (PANoptosis). Immunol Rev. 297:26–38.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shi J, Zhao Y, Wang K, Shi X, Wang Y,
Huang H, Zhuang Y, Cai T, Wang F and Shao F: Cleavage of GSDMD by
inflammatory caspases determines pyroptotic cell death. Nature.
526:660–665. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tengesdal IW, Li S, Powers NE, May M, Neff
CP, Joosten LAB, Marchetti C and Dinarello CA: Activation of
host-NLRP3 inflammasome in myeloid cells dictates response to
anti-PD-1 therapy in metastatic breast cancers. Pharmaceuticals
(Basel). 15:5742022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jing L, An Y, Cai T, Xiang J, Li B, Guo J,
Ma X, Wei L, Tian Y, Cheng X, et al: A subpopulation of
CD146+ macrophages enhances antitumor immunity by
activating the NLRP3 inflammasome. Cell Mol Immunol. 20:908–923.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yan L, Liu Y, Ma XF, Hou D, Zhang YH, Sun
Y, Shi SS, Forouzanfar T, Lin HY, Fan J and Wu G: Triclabendazole
induces pyroptosis by activating caspase-3 to cleave GSDME in
breast cancer cells. Front Pharmacol. 12:6700812021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Z, Zhang H, Li D, Zhou X, Qin Q and
Zhang Q: Caspase-3-mediated GSDME induced Pyroptosis in breast
cancer cells through the ROS/JNK signalling pathway. J Cell Mol
Med. 25:8159–8168. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang X, Cui X, Wang G, Zhou M, Wu Y, Du Y,
Li X and Xu T: HDAC inhibitor regulates the tumor immune
microenvironment via pyroptosis in triple negative breast cancer.
Mol Carcinog. 63:1800–1813. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Adams NM, Grassmann S and Sun JC: Clonal
expansion of innate and adaptive lymphocytes. Nat Rev Immunol.
20:694–707. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Boraschi D, Toepfer E and Italiani P:
Innate and germline immune memory: Specificity and heritability of
the ancient immune mechanisms for adaptation and survival. Front
Immunol. 15:13865782024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sette A and Crotty S: Adaptive immunity to
SARS-CoV-2 and COVID-19. Cell. 184:861–880. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Loi S, Drubay D, Adams S, Pruneri G,
Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria
S, et al: Tumor-infiltrating lymphocytes and prognosis: A pooled
individual patient analysis of early-stage triple-negative breast
cancers. J Clin Oncol. 37:559–569. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Herbert JA and Panagiotou S: Immune
response to viruses. Encycl Infect Immun. 1:429–444. 2022.
|
|
58
|
Schumacher TN and Schreiber RD:
Neoantigens in cancer immunotherapy. Science. 348:69–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Borst J, Ahrends T, Bąbała N, Melief CJM
and Kastenmüller W: CD4+ T cell help in cancer
immunology and immunotherapy. Nat Rev Immunol. 18:635–647. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Qian D, Li J, Huang M, Cui Q, Liu X and
Sun K: Dendritic cell vaccines in breast cancer: Immune modulation
and immunotherapy. Biomed Pharmacother. 162:1146852023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cai L and Li Y, Tan J, Xu L and Li Y:
Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J
Hematol Oncol. 16:1012023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jin M, Fang J, Peng J, Wang X, Xing P, Jia
K, Hu J, Wang D, Ding Y, Wang X, et al: PD-1/PD-L1 immune
checkpoint blockade in breast cancer: Research insights and
sensitization strategies. Mol Cancer. 23:2662024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schumacher TN and Thommen DS: Tertiary
lymphoid structures in cancer. Science. 375:eabf94192022.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Saxena M, van der Burg SH, Melief CJM and
Bhardwaj N: Therapeutic cancer vaccines. Nat Rev Cancer.
21:360–378. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chamorro DF, Somes LK and Hoyos V:
Engineered adoptive T-cell therapies for breast cancer: Current
progress, challenges, and potential. Cancers (Basel). 16:1242023.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cejuela M, Vethencourt A and Pernas S:
Immune checkpoint inhibitors and novel immunotherapy approaches for
breast cancer. Curr Oncol Rep. 24:1801–1819. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Duong E, Fessenden TB, Lutz E, Dinter T,
Yim L, Blatt S, Bhutkar A, Wittrup KD and Spranger S: Type I
interferon activates MHC class I-dressed CD11b+
conventional dendritic cells to promote protective anti-tumor
CD8+ T cell immunity. Immunity. 55:308–323.e9. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wu X, Huang Q, Chen X and Zhang B, Liang J
and Zhang B: B cells and tertiary lymphoid structures in tumors:
immunity cycle, clinical impact, and therapeutic applications.
Theranostics. 15:605–631. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Thol K, Pawlik P and McGranahan N: Therapy
sculpts the complex interplay between cancer and the immune system
during tumour evolution. Genome Med. 14:1372022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mirlekar B: Tumor promoting roles of
IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer
immunotherapy. SAGE Open Med. 10:205031212110690122022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li C, Jiang P, Wei S, Xu X and Wang J:
Regulatory T cells in tumor microenvironment: New mechanisms,
potential therapeutic strategies and future prospects. Mol Cancer.
19:1162020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ravichandran KS: Find-me and eat-me
signals in apoptotic cell clearance: Progress and conundrums. J Exp
Med. 207:1807–1817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bertheloot D, Latz E and Franklin BS:
Necroptosis, pyroptosis and apoptosis: An intricate game of cell
death. Cell Mol Immunol. 18:1106–1121. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tanzer MC, Frauenstein A, Stafford CA,
Phulphagar K, Mann M and Meissner F: Quantitative and dynamic
catalogs of proteins released during apoptotic and necroptotic cell
death. Cell Rep. 30:1260–1270.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nössing C and Ryan KM: 50 Years on and
still very much alive: ‘Apoptosis: A basic biological phenomenon
with wide-ranging implications in tissue kinetics’. Br J Cancer.
128:426–431. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang R, Lan C, Benlagha K, Camara NOS,
Miller H, Kubo M, Heegaard S, Lee P, Yang L, Forsman H, et al: The
interaction of innate immune and adaptive immune system. MedComm
(2020). 5:e7142024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Moyer A, Tanaka K and Cheng EH: Apoptosis
in cancer biology and therapy. Annu Rev Pathol. 20:303–328. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nagata S and Tanaka M: Programmed cell
death and the immune system. Nat Rev Immunol. 17:333–340. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Halkom A, Wu H and Lu Q: Contribution of
mouse models in our understanding of lupus. Int Rev Immunol.
39:174–187. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Rieux-Laucat F, Magérus-Chatinet A and
Neven B: The autoimmune lymphoproliferative syndrome with defective
FAS or FAS-ligand functions. J Clin Immunol. 38:558–568. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Leis K, Gałązka P, Kazik J, Jamrożek T,
Bereźnicka W and Czajkowski R: Resveratrol in the treatment of
asthma based on an animal model. Postepy Dermatol Alergol.
39:433–438. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Juric V, Hudson L, Fay J, Richards CE,
Jahns H, Verreault M, Bielle F, Idbaih A, Lamfers MLM, Hopkins AM,
et al: Transcriptional CDK inhibitors, CYC065 and THZ1 promote
Bim-dependent apoptosis in primary and recurrent GBM through cell
cycle arrest and Mcl-1 downregulation. Cell Death Dis. 12:7632021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kotzin JJ, Spencer SP, McCright SJ, Kumar
DBU, Collet MA, Mowel WK, Elliott EN, Uyar A, Makiya MA, Dunagin
MC, et al: The long non-coding RNA Morrbid regulates Bim and
short-lived myeloid cell lifespan. Nature. 537:239–243. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Guo H, Yang Y, Lou Y, Zuo Z, Cui H, Deng
H, Zhu Y and Fang J: Apoptosis and DNA damage mediated by ROS
involved in male reproductive toxicity in mice induced by Nickel.
Ecotoxicol Environ Saf. 268:1156792023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Green DR: The mitochondrial pathway of
apoptosis part II: The BCL-2 protein family. Cold Spring Harb
Perspect Biol. 14:a0410462022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yadav N, Gogada R, O'Malley J, Gundampati
RK, Jayanthi S, Hashmi S, Lella R, Zhang D, Wang J, Kumar R, et al:
Molecular insights on cytochrome c and nucleotide regulation of
apoptosome function and its implication in cancer. Biochim Biophys
Acta Mol Cell Res. 1867:1185732020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Sahoo G, Samal D, Khandayataray P and
Murthy MK: A review on caspases: Key regulators of biological
activities and apoptosis. Mol Neurobiol. 60:5805–5837. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shoshan-Barmatz V, Arif T and
Shteinfer-Kuzmine A: Apoptotic proteins with non-apoptotic
activity: Expression and function in cancer. Apoptosis. 28:730–753.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang Y, Li Y, Wu Y, Wu A, Xiao B, Liu X,
Zhang Q, Feng Y, Yuan Z, Yi J, et al: Endoplasmic reticulum stress
promotes oxidative stress, inflammation, and apoptosis: A novel
mechanism of citrinin-induced renal injury and dysfunction.
Ecotoxicol Environ Saf. 284:1169462024. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Fu X, Cui J, Meng X, Jiang P, Zheng Q,
Zhao W and Chen X: Endoplasmic reticulum stress, cell death and
tumor: Association between endoplasmic reticulum stress and the
apoptosis pathway in tumors (Review). Oncol Rep. 45:801–808. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kara M and Oztas E: Endoplasmic reticulum
stress-mediated cell death. Program Cell Death. 1:1–14. 2020.
|
|
92
|
Wen N, Lv Q and Du ZG: MicroRNAs involved
in drug resistance of breast cancer by regulating autophagy. J
Zhejiang Univ Sci B. 21:690–702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liang DH, Choi DS, Ensor JE, Kaipparettu
BA, Bass BL and Chang JC: The autophagy inhibitor chloroquine
targets cancer stem cells in triple negative breast cancer by
inducing mitochondrial damage and impairing DNA break repair.
Cancer Lett. 376:249–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Cook KL, Shajahan AN, Wärri A, Jin L,
Hilakivi-Clarke LA and Clarke R: Glucose-regulated protein 78
controls cross-talk between apoptosis and autophagy to determine
antiestrogen responsiveness. Cancer Res. 72:3337–3349. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Crawford AC, Riggins RB, Shajahan AN,
Zwart A and Clarke R: Co-inhibition of BCL-W and BCL2 restores
antiestrogen sensitivity through BECN1 and promotes an
autophagy-associated necrosis. PLoS One. 5:e86042010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Thomas M, Davis T, Loos B, Sishi B,
Huisamen B, Strijdom H and Engelbrecht AM: Autophagy is essential
for the maintenance of amino acids and ATP levels during acute
amino acid starvation in MDAMB231 cells. Cell Biochem Funct.
36:65–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Martin S, Dudek-Peric AM, Garg AD, Roose
H, Demirsoy S, Van Eygen S, Mertens F, Vangheluwe P, Vankelecom H
and Agostinis P: An autophagy-driven pathway of ATP secretion
supports the aggressive phenotype of BRAFV600E
inhibitor-resistant metastatic melanoma cells. Autophagy.
13:1512–1527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bednarczyk M, Zmarzły N, Grabarek B,
Mazurek U and Muc-Wierzgoń M: Genes involved in the regulation of
different types of autophagy and their participation in cancer
pathogenesis. Oncotarget. 9:34413–34428. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Daskalaki I, Gkikas I and Tavernarakis N:
Hypoxia and selective autophagy in cancer development and therapy.
Front Cell Dev Biol. 6:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Bousquet G, El Bouchtaoui M, Sophie T,
Leboeuf C, de Bazelaire C, Ratajczak P, Giacchetti S, de
Roquancourt A, Bertheau P, Verneuil L, et al: Targeting autophagic
cancer stem-cells to reverse chemoresistance in human triple
negative breast cancer. Oncotarget. 8:35205–35221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yeo SK, Wen J, Chen S and Guan JL:
Autophagy differentially regulates distinct breast cancer stem-like
cells in murine models via EGFR/Stat3 and Tgfβ/Smad signaling.
Cancer Res. 76:3397–3410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Vera-Ramirez L, Vodnala SK, Nini R, Hunter
KW and Green JE: Autophagy promotes the survival of dormant breast
cancer cells and metastatic tumour recurrence. Nat Commun.
9:19442018. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Gwinn DM, Shackelford DB, Egan DF,
Mihaylova MM, Mery A, Vasquez DS, Turk BE and Shaw RJ: AMPK
phosphorylation of raptor mediates a metabolic checkpoint. Mol
Cell. 30:214–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Casimiro MC, Di Sante G, Di Rocco A, Loro
E, Pupo C, Pestell TG, Bisetto S, Velasco-Velázquez MA, Jiao X, Li
Z, et al: Cyclin D1 restrains oncogene-induced autophagy by
regulating the AMPK-LKB1 signaling axis. Cancer Res. 77:3391–3405.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Galluzzi L, Buqué A, Kepp O, Zitvogel L
and Kroemer G: Immunogenic cell death in cancer and infectious
disease. Nat Rev Immunol. 17:97–111. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kayagaki N, Stowe IB, Lee BL, O'Rourke K,
Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT,
et al: Caspase-11 cleaves gasdermin D for non-canonical
inflammasome signalling. Nature. 526:666–671. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang Y, Gao W, Shi X, Ding J, Liu W, He H,
Wang K and Shao F: Chemotherapy drugs induce pyroptosis through
caspase-3 cleavage of a gasdermin. Nature. 547:99–103. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu
X, Junqueira C, Meza-Sosa KF, Mok TMY, Ansara J, et al: Gasdermin E
suppresses tumour growth by activating anti-tumour immunity.
Nature. 579:415–420. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wang Q, Wang Y, Ding J, Wang C, Zhou X,
Gao W, Huang H, Shao F and Liu Z: A bioorthogonal system reveals
antitumour immune function of pyroptosis. Nature. 579:421–426.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wang Y, Peng J, Mi X and Yang M: p53-GSDME
elevation: A path for CDK7 inhibition to suppress breast cancer
cell survival. Front Mol Biosci. 8:6974572021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Broderick NA: A common origin for immunity
and digestion. Front Immunol. 6:722015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Uribe-Querol E and Rosales C:
Phagocytosis: Our current understanding of a universal biological
process. Front Immunol. 11:10662020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kourtzelis I, Hajishengallis G and
Chavakis T: Phagocytosis of apoptotic cells in resolution of
inflammation. Front Immunol. 11:5532020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Sprooten J, Vanmeerbeek I, Datsi A,
Govaerts J, Naulaerts S, Laureano RS, Borràs DM, Calvet A, Malviya
V, Kuballa M, et al: Lymph node and tumor-associated
PD-L1+ macrophages antagonize dendritic cell vaccines by
suppressing CD8+ T cells. Cell Rep Med. 5:1013772024.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Kim HJ, Park JH, Kim HC, Kim CW, Kang I
and Lee HK: Blood monocyte-derived CD169+ macrophages
contribute to antitumor immunity against glioblastoma. Nat Commun.
13:62112022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Tang Z, Davidson D, Li R, Zhong MC, Qian
J, Chen J and Veillette A: Inflammatory macrophages exploit
unconventional pro-phagocytic integrins for phagocytosis and
anti-tumor immunity. Cell Rep. 37:1101112021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chen D, Varanasi SK, Hara T, Traina K, Sun
M, McDonald B, Farsakoglu Y, Clanton J, Xu S, Garcia-Rivera L, et
al: CTLA-4 blockade induces a microglia-Th1 cell partnership that
stimulates microglia phagocytosis and anti-tumor function in
glioblastoma. Immunity. 56:2086–2104.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Liu Y, Wang Y, Yang Y, Weng L, Wu Q, Zhang
J, Zhao P, Fang L, Shi Y and Wang P: Emerging phagocytosis
checkpoints in cancer immunotherapy. Signal Transduct Target Ther.
8:1042023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mantovani A, Allavena P, Marchesi F and
Garlanda C: Macrophages as tools and targets in cancer therapy. Nat
Rev Drug Discov. 21:799–820. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Gabriele M and Pucci L: Diet bioactive
compounds: implications for oxidative stress and inflammation in
the vascular system. Endocr Metab Immune Disord Drug Targets.
17:264–275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Stumpf F, Keller B, Gressies C and Schuetz
P: Inflammation and nutrition: Friend or foe? Nutrients.
15:11592023. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ye J, Hu Y, Chen X, Chang C and Li K:
Comparative effects of different nutritional supplements on
inflammation, nutritional status, and clinical outcomes in
colorectal cancer patients: A systematic review and network
meta-analysis. Nutrients. 15:27722023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Soeters PB, Wolfe RR and Shenkin A:
Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J
Parenter Enteral Nutr. 43:181–193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wiedermann CJ: Hypoalbuminemia as
surrogate and culprit of infections. Int J Mol Sci. 22:44962021.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Pan C, Gu Y and Ni Q: The prognostic value
of serum albumin to globulin ratio in patients with breast cancer:
A retrospective study. Breast Cancer (Dove Med Press). 16:403–411.
2024.PubMed/NCBI
|
|
127
|
Wei C, Ai H, Mo D, Wang P, Wei L, Liu Z,
Li P, Huang T and Liu M: A nomogram based on inflammation and
nutritional biomarkers for predicting the survival of breast cancer
patients. Front Endocrinol (Lausanne). 15:13888612024. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Xiang M, Zhang H, Tian J, Yuan Y, Xu Z and
Chen J: Low serum albumin levels and high neutrophil counts are
predictive of a poorer prognosis in patients with metastatic breast
cancer. Oncol Lett. 24:4322022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Al-Shaer AE, Buddenbaum N and Shaikh SR:
Polyunsaturated fatty acids, specialized pro-resolving mediators,
and targeting inflammation resolution in the age of precision
nutrition. Biochim Biophys Acta Mol Cell Biol Lipids.
1866:1589362021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wautier JL and Wautier MP: Pro- and
anti-inflammatory prostaglandins and cytokines in humans: A mini
review. Int J Mol Sci. 24:96472023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Iddir M, Brito A, Dingeo G, Fernandez Del
Campo SS, Samouda H, La Frano MR and Bohn T: Strengthening the
immune system and reducing inflammation and oxidative stress
through diet and nutrition: Considerations during the COVID-19
crisis. Nutrients. 12:15622020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Flores J, White BM, Brea RJ, Baskin JM and
Devaraj NK: Lipids: Chemical tools for their synthesis,
modification, and analysis. Chem Soc Rev. 49:4602–4614. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Choi RH, Tatum SM, Symons JD, Summers SA
and Holland WL: Ceramides and other sphingolipids as drivers of
cardiovascular disease. Nat Rev Cardiol. 18:701–711. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Yu B, Yu L and Klionsky DJ: Nutrition
acquisition by human immunity, transient overnutrition and the
cytokine storm in severe cases of COVID-19. Med Hypotheses.
155:1106682021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yoon H, Shaw JL, Haigis MC and Greka A:
Lipid metabolism in sickness and in health: Emerging regulators of
lipotoxicity. Mol Cell. 81:3708–3730. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ruan XZ, Varghese Z and Moorhead JF: An
update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol.
5:713–721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Mitrofanova A, Merscher S and Fornoni A:
Kidney lipid dysmetabolism and lipid droplet accumulation in
chronic kidney disease. Nat Rev Nephrol. 19:629–645. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
D'Agati VD, Chagnac A, de Vries AP, Levi
M, Porrini E, Herman-Edelstein M and Praga M: Obesity-related
glomerulopathy: Clinical and pathologic characteristics and
pathogenesis. Nat Rev Nephrol. 12:453–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhou P, Santoro A, Peroni OD, Nelson AT,
Saghatelian A, Siegel D and Kahn BB: PAHSAs enhance hepatic and
systemic insulin sensitivity through direct and indirect
mechanisms. J Clin Invest. 129:4138–4150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Bhat N and Mani A: Dysregulation of lipid
and glucose metabolism in nonalcoholic fatty liver disease.
Nutrients. 15:23232023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Løvsletten NG, Bakke SS, Kase ET, Ouwens
DM, Thoresen GH and Rustan AC: Increased triacylglycerol-fatty acid
substrate cycling in human skeletal muscle cells exposed to
eicosapentaenoic acid. PLoS One. 13:e02080482018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Jelenik T, Flögel U, Álvarez-Hernández E,
Scheiber D, Zweck E, Ding Z, Rothe M, Mastrototaro L, Kohlhaas V,
Kotzka J, et al: Insulin resistance and vulnerability to cardiac
ischemia. Diabetes. 67:2695–2702. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Ioannou MS, Jackson J, Sheu SH, Chang CL,
Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Matthies D, et al:
Neuron-astrocyte metabolic coupling protects against
activity-induced fatty acid toxicity. Cell. 177:1522–1535.e14.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wang Y, Qian Y, Fang Q, Zhong P, Li W,
Wang L, Fu W, Zhang Y, Xu Z, Li X and Liang G: Saturated palmitic
acid induces myocardial inflammatory injuries through direct
binding to TLR4 accessory protein MD2. Nat Commun. 8:139972017.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Nicholas DA, Zhang K, Hung C, Glasgow S,
Aruni AW, Unternaehrer J, Payne KJ, Langridge WHR and De Leon M:
Palmitic acid is a toll-like receptor 4 ligand that induces human
dendritic cell secretion of IL-1β. PLoS One. 12:e01767932017.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Tabas I: Consequences and therapeutic
implications of macrophage apoptosis in atherosclerosis: The
importance of lesion stage and phagocytic efficiency. Arterioscler
Thromb Vasc Biol. 25:2255–2264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Neri CR, Scapaticci S, Chiarelli F and
Giannini C: Liver steatosis: A marker of metabolic risk in
children. Int J Mol Sci. 23:48222022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Jarczak D and Nierhaus A: Cytokine
storm-definition, causes, and implications. Int J Mol Sci.
23:117402022. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Dinarello CA: Proinflammatory cytokines.
Chest. 118:503–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Nie J, Zhou L, Tian W, Liu X, Yang L, Yang
X, Zhang Y, Wei S, Wang DW and Wei J: Deep insight into cytokine
storm: From pathogenesis to treatment. Signal Transduct Target
Ther. 10:1122025. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Man SM, Karki R and Kanneganti TD:
Molecular mechanisms and functions of pyroptosis, inflammatory
caspases and inflammasomes in infectious diseases. Immunol Rev.
277:61–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Afzal S, Fiaz K, Noor A, Sindhu AS, Hanif
A, Bibi A, Asad M, Nawaz S, Zafar S, Ayub S, et al: Interrelated
oncogenic viruses and breast cancer. Front Mol Biosci.
9:7811112022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wu Q, Nie DY, Ba-Alawi W, Ji Y, Zhang Z,
Cruickshank J, Haight J, Ciamponi FE, Chen J, Duan S, et al: PRMT
inhibition induces a viral mimicry response in triple-negative
breast cancer. Nat Chem Biol. 18:821–830. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zu Y, Ou Z, Wu D, Liu W, Liu L, Wu D, Zhao
Y, Ren P, Zhang Y, Li W, et al: Genetic characteristics of human
papillomavirus type 16, 18, 52 and 58 in southern China. Genomics.
113:3895–3906. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Khalil MI, Yang C, Vu L, Chadha S, Nabors
H, Welbon C, James CD, Morgan IM, Spanos WC and Pyeon D: HPV
upregulates MARCHF8 ubiquitin ligase and inhibits apoptosis by
degrading the death receptors in head and neck cancer. PLoS Pathog.
19:e10111712023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Antonioli M, Pagni B, Vescovo T, Ellis R,
Cosway B, Rollo F, Bordoni V, Agrati C, Labus M, Covello R, et al:
HPV sensitizes OPSCC cells to cisplatin-induced apoptosis by
inhibiting autophagy through E7-mediated degradation of AMBRA1.
Autophagy. 17:2842–2855. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wyżewski Z, Mielcarska MB,
Gregorczyk-Zboroch KP and Myszka A: Virus-mediated inhibition of
apoptosis in the context of EBV-associated diseases: Molecular
mechanisms and therapeutic perspectives. Int J Mol Sci.
23:72652022. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Yu Z, Wang Y, Liu L, Zhang X, Jiang S and
Wang B: Apoptosis disorder, a key pathogenesis of HCMV-related
diseases. Int J Mol Sci. 22:41062021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Gatti-Mays ME, Balko JM, Gameiro SR, Bear
HD, Prabhakaran S, Fukui J, Disis ML, Nanda R, Gulley JL, Kalinsky
K, et al: If we build it they will come: Targeting the immune
response to breast cancer. NPJ Breast Cancer. 5:372019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Huang G, Zhou J, Chen J and Liu G:
Identification of pyroptosis related subtypes and tumor
microenvironment infiltration characteristics in breast cancer. Sci
Rep. 12:106402022. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Berkel C and Cacan E: Differential
expression and copy number variation of gasdermin (GSDM) family
members, pore-forming proteins in pyroptosis, in normal and
malignant serous ovarian tissue. Inflammation. 44:2203–2216. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zhu C, Xu S, Jiang R, Yu Y, Bian J and Zou
Z: The gasdermin family: Emerging therapeutic targets in diseases.
Signal Transduct Target Ther. 9:872024. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wu H, Qian D, Bai X and Sun S: Targeted
pyroptosis is a potential therapeutic strategy for cancer. J Oncol.
2022:25155252022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Hu H, Yang H, Liu Y and Yan B:
Pathogenesis of anti-melanoma differentiation-associated gene 5
antibody-positive dermatomyositis: A concise review with an
emphasis on Type I interferon system. Front Med (Lausanne).
8:8331142022. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Wu L, Lu H, Pan Y, Liu C, Wang J, Chen B
and Wang Y: The role of pyroptosis and its crosstalk with immune
therapy in breast cancer. Front Immunol. 13:9739352022. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Zhao Q, Huang L, Qin G, Qiao Y, Ren F,
Shen C, Wang S, Liu S, Lian J, Wang D, et al: Cancer-associated
fibroblasts induce monocytic myeloid-derived suppressor cell
generation via IL-6/exosomal miR-21-activated STAT3 signaling to
promote cisplatin resistance in esophageal squamous cell carcinoma.
Cancer Lett. 518:35–48. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Zaarour RF, Ribeiro M, Azzarone B, Kapoor
S and Chouaib S: Tumor microenvironment-induced tumor cell
plasticity: Relationship with hypoxic stress and impact on tumor
resistance. Front Oncol. 13:12225752023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Igney FH and Krammer PH: Death and
anti-death: Tumour resistance to apoptosis. Nat Rev Cancer.
2:277–288. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
169
|
Liao M, Qin R, Huang W, Zhu HP, Peng F,
Han B and Liu B: Targeting regulated cell death (RCD) with
small-molecule compounds in triple-negative breast cancer: A
revisited perspective from molecular mechanisms to targeted
therapies. J Hematol Oncol. 15:442022. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Attiq A and Afzal S: Trinity of
inflammation, innate immune cells and cross-talk of signalling
pathways in tumour microenvironment. Front Pharmacol.
14:12557272023. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Wang JL, Hua SN, Bao HJ, Yuan J, Zhao Y
and Chen S: Pyroptosis and inflammasomes in cancer and
inflammation. MedComm (2020). 4:e3742023. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ji X, Huang X, Li C, Guan N, Pan T, Dong J
and Li L: Effect of tumor-associated macrophages on the pyroptosis
of breast cancer tumor cells. Cell Commun Signal. 21:1972023.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
van Beek JJP, Martens AWJ, Bakdash G and
de Vries IJM: Innate lymphoid cells in tumor immunity.
Biomedicines. 4:72016. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Spits H, Artis D, Colonna M, Diefenbach A,
Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius
RE, et al: Innate lymphoid cells-a proposal for uniform
nomenclature. Nat Rev Immunol. 13:145–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Verma D, Verma M and Mishra R: Stem cell
therapy and innate lymphoid cells. Stem Cells Int.
2022:35305202022. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Zhao M, Shao F, Yu D, Zhang J, Liu Z, Ma
J, Xia P and Wang S: Maturation and specialization of group 2
innate lymphoid cells through the lung-gut axis. Nat Commun.
13:76002022. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Srivastava RK, Sapra L, Bhardwaj A, Mishra
PK, Verma B and Baig Z: Unravelling the immunobiology of innate
lymphoid cells (ILCs): Implications in health and disease. Cytokine
Growth Factor Rev. 74:56–75. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Danziger N, Sokol ES, Graf RP, Hiemenz MC,
Maule J, Parimi V, Palmieri C, Pusztai L, Ross JS and Huang RSP:
Variable landscape of PD-L1 expression in breast carcinoma as
detected by the DAKO 22C3 immunohistochemistry assay. Oncologist.
28:319–326. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Levi I, Amsalem H, Nissan A, Darash-Yahana
M, Peretz T, Mandelboim O and Rachmilewitz J: Characterization of
tumor infiltrating natural killer cell subset. Oncotarget.
6:13835–13843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Montaldo E, Vacca P, Chiossone L, Croxatto
D, Loiacono F, Martini S, Ferrero S, Walzer T, Moretta L and
Mingari MC: Unique eomes(+) NK cell subsets are present in uterus
and decidua during early pregnancy. Front Immunol. 6:6462016.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Salimi M, Wang R, Yao X, Li X, Wang X, Hu
Y, Chang X, Fan P, Dong T and Ogg G: Activated innate lymphoid cell
populations accumulate in human tumour tissues. BMC Cancer.
18:3412018. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Sivori S, Pende D, Quatrini L, Pietra G,
Della Chiesa M, Vacca P, Tumino N, Moretta F, Mingari MC, Locatelli
F and Moretta L: NK cells and ILCs in tumor immunotherapy. Mol
Aspects Med. 80:1008702021. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Fenis A, Demaria O, Gauthier L, Vivier E
and Narni-Mancinelli E: New immune cell engagers for cancer
immunotherapy. Nat Rev Immunol. 24:471–486. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Liu H, Wang Z, Zhou Y and Yang Y: MDSCs in
breast cancer: An important enabler of tumor progression and an
emerging therapeutic target. Front Immunol. 14:11992732023.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Nakasone ES, Hurvitz SA and McCann KE:
Harnessing the immune system in the battle against breast cancer.
Drugs Context. 7:2125202018. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Revel M, Daugan MV, Sautés-Fridman C,
Fridman WH and Roumenina LT: Complement system: Promoter or
suppressor of cancer progression? Antibodies (Basel). 9:572020.
View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Deslouches B and Di YP: Antimicrobial
peptides with selective antitumor mechanisms: Prospect for
anticancer applications. Oncotarget. 8:46635–46651. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Angelico G, Broggi G, Tinnirello G, Puzzo
L, Vecchio GM, Salvatorelli L, Memeo L, Santoro A, Farina J, Mulé
A, et al: Tumor infiltrating lymphocytes (TILS) and PD-L1
expression in breast cancer: A review of current evidence and
prognostic implications from Pathologist's perspective. Cancers
(Basel). 15:44792023. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Tang Y, Jiang Q, Ou Y, Zhang F, Qing K,
Sun Y, Lu W, Zhu H, Gong F, Lei P and Shen G: BIP induces mice
CD19(hi) regulatory B cells producing IL-10 and highly expressing
PD-L1, FasL. Mol Immunol. 69:44–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Burugu S, Asleh-Aburaya K and Nielsen TO:
Immune infiltrates in the breast cancer microenvironment:
Detection, characterization and clinical implication. Breast
Cancer. 24:3–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Shang Q, Yu X, Sun Q, Li H, Sun C and Liu
L: Polysaccharides regulate Th1/Th2 balance: A new strategy for
tumor immunotherapy. Biomed Pharmacother. 170:1159762024.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Togashi Y, Shitara K and Nishikawa H:
Regulatory T cells in cancer immunosuppression-implications for
anticancer therapy. Nat Rev Clin Oncol. 16:356–371. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Dolina JS, Van Braeckel-Budimir N, Thomas
GD and Salek-Ardakani S: CD8+ T cell exhaustion in
cancer. Front Immunol. 12:7152342021. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Sauer N, Matkowski I, Bodalska G, Murawski
M, Dzięgiel P and Calik J: Prognostic role of prolactin-induced
protein (PIP) in breast cancer. Cells. 12:22522023. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Lehmann BD, Colaprico A, Silva TC, Chen J,
An H, Ban Y, Huang H, Wang L, James JL, Balko JM, et al:
Multi-omics analysis identifies therapeutic vulnerabilities in
triple-negative breast cancer subtypes. Nat Commun. 12:62762021.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Truax AD, Thakkar M and Greer SF:
Dysregulated recruitment of the histone methyltransferase EZH2 to
the class II transactivator (CIITA) promoter IV in breast cancer
cells. PLoS One. 7:e360132012. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kubaev A, Faez Sead F, Pirouzbakht M,
Nazari M, Riyahi H, Sargazi Aval O, Hasanvand A, Mousavi F and
Soleimani Samarkhazan H: Platelet-derived extracellular vesicles:
Emerging players in hemostasis and thrombosis. J Liposome Res.
35:334–344. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Han X, Song X, Xiao Z, Zhu G, Gao R, Ni B
and Li J: Study on the mechanism of MDSC-platelets and their role
in the breast cancer microenvironment. Front Cell Dev Biol.
12:13104422024. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Wang L, Zhang K, Feng J, Wang D and Liu J:
The progress of platelets in breast cancer. Cancer Manag Res.
15:811–821. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Mendoza-Almanza G, Burciaga-Hernández L,
Maldonado V, Melendez-Zajgla J and Olmos J: Role of platelets and
breast cancer stem cells in metastasis. World J Stem Cells.
12:1237–1254. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Catani MV, Savini I, Tullio V and Gasperi
V: The ‘Janus Face’ of platelets in cancer. Int J Mol Sci.
21:7882020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Zielińska KA and Katanaev VL: The
signaling duo CXCL12 and CXCR4: Chemokine fuel for breast cancer
tumorigenesis. Cancers (Basel). 12:30712020. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Wang X, Zhao S, Wang Z and Gao T:
Platelets involved tumor cell EMT during circulation:
Communications and interventions. Cell Commun Signal. 20:822022.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Wang J, He Y, Hu F, Hu C, Sun Y, Yang K
and Yang S: Metabolic reprogramming of immune cells in the tumor
microenvironment. International Int J Mol Sci. 25:122232024.
View Article : Google Scholar
|
|
205
|
Singh L, Nair L, Kumar D, Arora MK, Bajaj
S, Gadewar M, Mishra SS, Rath SK, Dubey AK, Kaithwas G, et al:
Hypoxia induced lactate acidosis modulates tumor microenvironment
and lipid reprogramming to sustain the cancer cell survival. Front
Oncol. 13:10342052023. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Yan Y, Huang L, Liu Y, Yi M, Chu Q, Jiao D
and Wu K: Metabolic profiles of regulatory T cells and their
adaptations to the tumor microenvironment: Implications for
antitumor immunity. J Hematol Oncol. 15:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Guo R, Wang R, Zhang W, Li Y, Wang Y, Wang
H, Li X and Song J: Macrophage polarisation in the tumour
microenvironment: recent research advances and therapeutic
potential of different macrophage reprogramming. Cancer Control.
32:107327482513166042025. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Chang CH, Qiu J, O'Sullivan D, Buck MD,
Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ,
et al: Metabolic competition in the tumor microenvironment is a
driver of cancer progression. Cell. 162:1229–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Xie J, Guo Z, Zhu Y, Ma M and Jia G:
Peripheral blood inflammatory indexes in breast cancer: A review.
Medicine (Baltimore). 102:e363152023. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Shibabaw T, Teferi B and Ayelign B: The
role of Th-17 cells and IL-17 in the metastatic spread of breast
cancer: As a means of prognosis and therapeutic target. Front
Immunol. 14:10948232023. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Danforth DN: The Role of chronic
inflammation in the development of breast cancer. (Basel).
13:39182021. View Article : Google Scholar
|
|
213
|
Ruan GT, Xie HL, Hu CL, Liu CA, Zhang HY,
Zhang Q, Wang ZW, Zhang X, Ge YZ, Lin SQ, et al: Comprehensive
prognostic effects of systemic inflammation and Insulin resistance
in women with breast cancer with different BMI: A prospective
multicenter cohort. Sci Rep. 13:43032023. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Egelston CA, Avalos C, Tu TY, Simons DL,
Jimenez G, Jung JY, Melstrom L, Margolin K, Yim JH, Kruper L, et
al: Human breast tumor-infiltrating CD8+ T cells retain
polyfunctionality despite PD-1 expression. Nat Commun. 9:42972018.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Hartman ZC, Poage GM, den Hollander P,
Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck
SG, Mills GB and Brown PH: Growth of triple-negative breast cancer
cells relies upon coordinate autocrine expression of the
proinflammatory cytokines IL-6 and IL-8. Cancer Res. 73:3470–3480.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Jin K, Pandey NB and Popel AS:
Simultaneous blockade of IL-6 and CCL5 signaling for synergistic
inhibition of triple-negative breast cancer growth and metastasis.
Breast Cancer Res. 20:542018. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Korkaya H, Kim GI, Davis A, Malik F, Henry
NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK,
et al: Activation of an IL6 inflammatory loop mediates trastuzumab
resistance in HER2+ breast cancer by expanding the cancer stem cell
population. Mol Cell. 47:570–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H:
Targeting NF-κB pathway for the therapy of diseases: Mechanism and
clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Li Z, Liu M, Li J, Yan G and Xu X:
Diosmetin alleviates AFB1-induced endoplasmic reticulum stress,
autophagy, and apoptosis via PI3K/AKT pathway in mice. Ecotoxicol
Environ Saf. 292:1179972025. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Diep S, Maddukuri M, Yamauchi S, Geshow G
and Delk NA: Interleukin-1 and nuclear factor kappa B signaling
promote breast cancer progression and treatment resistance. Cells.
11:16732022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Xu J, Zhang J, Mao QF, Wu J and Wang Y:
The interaction between autophagy and JAK/STAT3 signaling pathway
in tumors. Front Genet. 13:8803592022. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Dai Z, Liu WC, Chen XY, Wang X, Li JL and
Zhang X: Gasdermin D-mediated pyroptosis: Mechanisms, diseases, and
inhibitors. Front Immunol. 14:11786622023. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Liu X, Xu X, Ye P, Jiang Z, Tian L, Yin Y
and Feng L: Genetic evidence for causal effects of inflammatory
protein factors on breast cancer. Discov Oncol. 16:14902025.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Liu F, Li L, Lan M, Zou T, Kong Z, Cai T,
Wu XY and Cai Y: Key factor regulating inflammatory
microenvironment, metastasis, and resistance in breast cancer:
Interleukin-1 signaling. Mediators Inflamm. 2021:77858902021.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Dong W, Gu X, Li J and Zhuang Z:
Characterization of immune landscape and prognostic value of
IL-17-related signature in invasive breast cancer. Transl Cancer
Res. 14:907–929. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Cui Y, Cui S, Lu W, Wang Y, Zhuo Z, Wang
R, Zhang D, Wu X, Chang L, Zuo X, et al: CRP, IL-1α, IL-1β, and
IL-6 levels and the risk of breast cancer: A two-sample Mendelian
randomization study. Sci Rep. 14:19822024. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Kehm RD, McDonald JA, Fenton SE,
Kavanaugh-Lynch M, Leung KA, McKenzie KE, Mandelblatt JS and Terry
MB: Inflammatory biomarkers and breast cancer risk: A systematic
review of the evidence and future potential for intervention
research. Int J Environ Res Public Health. 17:54452020. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Liang Y, He J, Chen X, Yin L, Yuan Q, Zeng
Q, Zu X and Shen Y: The emerging roles of metabolism in the
crosstalk between breast cancer cells and tumor-associated
macrophages. Int J Biol Sci. 19:4915–4930. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Liu J, Geng X, Hou J and Wu G: New
insights into M1/M2 macrophages: Key modulators in cancer
progression. Cancer Cell Int. 21:3892021. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Strizova Z, Benesova I, Bartolini R,
Novysedlak R, Cecrdlova E, Foley LK and Striz I: M1/M2 macrophages
and their overlaps-myth or reality? Clin Sci (Lond). 137:1067–1093.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Wang C, Lin Y, Zhu H, Zhou Y, Mao F, Huang
X, Sun Q and Li C: The prognostic and clinical value of
tumor-associated macrophages in patients with breast cancer: A
systematic review and meta-analysis. Front Oncol. 12:9058462022.
View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Wu C, Dong S, Huang R and Chen X:
Cancer-associated adipocytes and breast cancer: Intertwining in the
tumor microenvironment and challenges for cancer therapy. Cancers
(Basel). 15:7262023. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Maliniak ML, Miller-Kleinhenz J,
Cronin-Fenton DP, Lash TL, Gogineni K, Janssen EAM and McCullough
LE: Crown-like structures in breast adipose tissue: Early evidence
and current issues in breast cancer. Cancers (Basel). 13:22222021.
View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Zhang L: The role of mesenchymal stem
cells in modulating the breast cancer microenvironment. Cell
Transplant. 32:96368972312200732023. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Zheng J and Hao H: The importance of
cancer-associated fibroblasts in targeted therapies and drug
resistance in breast cancer. Front Oncol. 13:13338392023.
View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Rubinstein-Achiasaf L, Morein D,
Ben-Yaakov H, Liubomirski Y, Meshel T, Elbaz E, Dorot O, Pichinuk
E, Gershovits M, Weil M and Ben-Baruch A: Persistent inflammatory
stimulation drives the conversion of MSCs to inflammatory CAFs that
promote pro-metastatic characteristics in breast cancer cells.
Cancers (Basel). 13:14722021. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Chen Y, Yu D, Qian H, Shi Y and Tao Z:
CD8+ T cell-based cancer immunotherapy. J Transl Med.
22:3942024. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Bhandarkar V, Dinter T and Spranger S:
Architects of immunity: How dendritic cells shape CD8+ T
cell fate in cancer. Sci Immunol. 10:eadf47262025. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Giles JR, Globig AM, Kaech SM and Wherry
EJ: CD8+ T cells in the cancer-immunity cycle. Immunity.
56:2231–2253. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Tang D, Tang Q, Huang W, Zhang Y, Tian Y
and Fu X: Fasting: From physiology to pathology. Adv Sci (Weinh).
10:e22044872023. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Wilkinson MJ, Manoogian ENC, Zadourian A,
Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda
S and Taub PR: Ten-hour time-restricted eating reduces weight,
blood pressure, and atherogenic lipids in patients with metabolic
syndrome. Cell Metab. 31:92–104.e5. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Yelek C, Mignion L, Paquot A, Bouzin C,
Corbet C, Muccioli GG, Cani PD and Jordan BF: Tumor metabolism is
affected by obesity in preclinical models of triple-negative breast
cancer. Cancers (Basel). 14:5622022. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
James FR, Wootton S, Jackson A, Wiseman M,
Copson ER and Cutress RI: Obesity in breast cancer-what is the risk
factor? Eur J Cancer. 51:705–720. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Ng WH, Abu Zaid Z, Mohd Yusof BN, Amin
Nordin S and Lim PY: Association between dietary inflammatory index
and body fat percentage among newly diagnosed breast cancer
patients. Ann Med. 55:23033992023. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Savva C, Copson E, Johnson PWM, Cutress RI
and Beers SA: Obesity is associated with immunometabolic changes in
adipose tissue that may drive treatment resistance in breast
cancer: immune-metabolic reprogramming and novel therapeutic
strategies. Cancers (Basel). 15:24402023. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: Epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Gallagher EJ and LeRoith D: Obesity and
diabetes: The increased risk of cancer and cancer-related
mortality. Physiol Rev. 95:727–748. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Ringel AE, Drijvers JM, Baker GJ, Catozzi
A, García-Cañaveras JC, Gassaway BM, Miller BC, Juneja VR, Nguyen
TH, Joshi S, et al: Obesity shapes metabolism in the tumor
microenvironment to suppress anti-tumor immunity. Cell.
183:1848–1866.e26. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Quail DF and Dannenberg AJ: The obese
adipose tissue microenvironment in cancer development and
progression. Nat Rev Endocrinol. 15:139–154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Iyengar NM, Gucalp A, Dannenberg AJ and
Hudis CA: Obesity and cancer mechanisms: Tumor microenvironment and
inflammation. J Clin Oncol. 34:4270–4276. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Fortner RT, Katzke V, Kühn T and Kaaks R:
Obesity and breast cancer. Recent Results Cancer Res. 208:43–65.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Jiralerspong S and Goodwin PJ: Obesity and
breast cancer prognosis: Evidence, challenges, and opportunities. J
Clin Oncol. 34:4203–4216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Suzuki R, Orsini N, Saji S, Key TJ and
Wolk A: Body weight and incidence of breast cancer defined by
estrogen and progesterone receptor status-a meta-analysis. Int J
Cancer. 124:698–712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Cecchini RS, Costantino JP, Cauley JA,
Cronin WM, Wickerham DL, Land SR, Weissfeld JL and Wolmark N: Body
mass index and the risk for developing invasive breast cancer among
high-risk women in NSABP P-1 and STAR breast cancer prevention
trials. Cancer Prev Res (Phila). 5:583–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Chan DSM, Vieira AR, Aune D, Bandera EV,
Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R
and Norat T: Body mass index and survival in women with breast
cancer-systematic literature review and meta-analysis of 82
follow-up studies. Ann Oncol. 25:1901–1914. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Key TJ, Appleby PN, Reeves GK, Roddam A,
Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE Jr, Falk RT,
Miller R, et al: Body mass index, serum sex hormones, and breast
cancer risk in postmenopausal women. J Natl Cancer Inst.
95:1218–1226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
He M, Xu S, Yan F, Ruan J and Zhang X:
Fatty acid metabolism: A new perspective in breast cancer precision
therapy. Front Biosci (Landmark Ed). 28:3482023. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Guo R, Chen Y, Borgard H, Jijiwa M, Nasu
M, He M and Deng Y: The function and mechanism of lipid molecules
and their roles in the diagnosis and prognosis of breast cancer.
Molecules. 25:48682020. View Article : Google Scholar
|
|
260
|
Lu S and Archer MC: Sp1 coordinately
regulates de novo lipogenesis and proliferation in cancer cells.
Int J Cancer. 126:416–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Martin-Perez M, Urdiroz-Urricelqui U,
Bigas C and Benitah SA: The role of lipids in cancer progression
and metastasis. Cell Metab. 34:1675–1699. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Solsona-Vilarrasa E and Vousden KH:
Obesity, white adipose tissue and cancer. FEBS J. 292:2189–2207.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Kolb R, Kluz P, Tan ZW, Borcherding N,
Bormann N, Vishwakarma A, Balcziak L, Zhu P, Davies BS, Gourronc F,
et al: Obesity-associated inflammation promotes angiogenesis and
breast cancer via angiopoietin-like 4. Oncogene. 38:2351–2363.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Magalhães A, Cesário V, Coutinho D, Matias
I, Domingues G, Pinheiro C, Serafim T and Dias S: A
high-cholesterol diet promotes the intravasation of breast tumor
cells through an LDL-LDLR axis. Sci Rep. 14:94712024. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Zipinotti Dos Santos D, de Souza JC,
Pimenta TM, da Silva Martins B, Junior RSR, Butzene SMS, Tessarolo
NG, Cilas PML Jr, Silva IV and Rangel LBA: The impact of lipid
metabolism on breast cancer: A review about its role in
tumorigenesis and immune escape. Cell Commun Signal. 21:1612023.
View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Centonze G, Natalini D, Piccolantonio A,
Salemme V, Morellato A, Arina P, Riganti C and Defilippi P:
Cholesterol and its derivatives: Multifaceted players in breast
cancer progression. Front Oncol. 12:9066702022. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Li P, Zhang Z, Lv H and Sun P: Inhibiting
the expression of STARD3 induced apoptosis via the inactivation of
PI3K/AKT/mTOR pathway on ER+ breast cancer. Tissue Cell.
79:1019712022. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Bandyopadhayaya S, Akimov MG, Verma R,
Sharma A, Sharma D, Kundu GC, Gretskaya NM, Bezuglov VV and Mandal
CC: N-arachidonoyl dopamine inhibits epithelial-mesenchymal
transition of breast cancer cells through ERK signaling and
decreasing the cellular cholesterol. J Biochem Mol Toxicol.
35:e226932021. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Baek AE, Krawczynska N, Das Gupta A,
Dvoretskiy SV, You S, Park J, Deng YH, Sorrells JE, Smith BP, Ma L,
et al: The cholesterol metabolite 27HC increases secretion of
extracellular vesicles which promote breast cancer progression.
Endocrinology. 162:bqab0952021. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Godina C, Indira Chandran V, Barbachowska
M, Tryggvadottir H, Nodin B, Visse E, Borgquist S, Jirström K,
Isaksson K, Bosch A, et al: Interplay between caveolin-1 and body
and tumor size affects clinical outcomes in breast cancer. Transl
Oncol. 22:1014642022. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Trabert B, Bauer DC, Buist DSM, Cauley JA,
Falk RT, Geczik AM, Gierach GL, Hada M, Hue TF, Lacey JV Jr, et al:
Association of circulating progesterone with breast cancer risk
among postmenopausal women. JAMA Netw Open. 3:e2036452020.
View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Mohanty SS and Mohanty PK: Obesity as
potential breast cancer risk factor for postmenopausal women. Genes
Dis. 8:117–123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Glassman I, Le N, Asif A, Goulding A,
Alcantara CA, Vu A, Chorbajian A, Mirhosseini M, Singh M and
Venketaraman V: The role of obesity in breast cancer pathogenesis.
Cells. 12:20612023. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Dong S, Wang Z, Shen K and Chen X:
Metabolic syndrome and breast cancer: Prevalence, treatment
response, and prognosis. Front Oncol. 11:6296662021. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Zhao X, An X, Yang C, Sun W, Ji H and Lian
F: The crucial role and mechanism of insulin resistance in
metabolic disease. Front Endocrinol (Lausanne). 14:11492392023.
View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Dieli-Conwright CM, Wong L, Waliany S and
Mortimer JE: Metabolic syndrome and breast cancer survivors: A
follow-up analysis after completion of chemotherapy. Diabetol Metab
Syndr. 14:362022. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Viedma-Rodríguez R, Martínez-Hernández MG,
Martínez-Torres DI and Baiza-Gutman LA: Epithelial mesenchymal
transition and progression of breast cancer promoted by diabetes
mellitus in mice are associated with increased expression of
glycolytic and proteolytic enzymes. Horm Cancer. 11:170–181. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Rahman MM, Behl T, Islam MR, Alam MN,
Islam MM, Albarrati A, Albratty M, Meraya AM and Bungau SG:
Emerging management approach for the adverse events of
immunotherapy of cancer. Molecules. 27:37982022. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Mehanna J, Haddad FG, Eid R, Lambertini M
and Kourie HR: Triple-negative breast cancer: Current perspective
on the evolving therapeutic landscape. Int J Womens Health.
11:431–437. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Ye F, Dewanjee S, Li Y, Jha NK, Chen ZS,
Kumar A, Vishakha Behl T, Jha SK and Tang H: Advancements in
clinical aspects of targeted therapy and immunotherapy in breast
cancer. Mol Cancer. 22:1052023. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Morrow RJ, Allam AH, Yeo B, Deb S, Murone
C, Lim E, Johnstone CN and Ernst M: Paracrine IL-6 signaling
confers proliferation between heterogeneous inflammatory breast
cancer sub-clones. Cancers (Basel). 14:22922022. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Manore SG, Doheny DL, Wong GL and Lo HW:
IL-6/JAK/STAT3 signaling in breast cancer metastasis: Biology and
treatment. Front Oncol. 12:8660142022. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Ding R, Kan Q, Wang T, Xiao R, Song Y and
Li D: Ginsenoside Rh2 regulates triple-negative breast cancer
proliferation and apoptosis via the IL-6/JAK2/STAT3 pathway. Front
Pharmacol. 15:14838962025. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Sun X, Liu K, Lu S, He W and Du Z:
Targeted therapy and immunotherapy for heterogeneous breast cancer.
Cancers (Basel). 14:54562022. View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Swain SM, Shastry M and Hamilton E:
Targeting HER2-positive breast cancer: Advances and future
directions. Nat Rev Drug Discov. 22:101–126. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Maadi H, Soheilifar MH, Choi WS,
Moshtaghian A and Wang Z: Trastuzumab mechanism of action; 20 years
of research to unravel a dilemma. Cancers (Basel). 13:35402021.
View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Li F and Liu S: Focusing on NK cells and
ADCC: A promising immunotherapy approach in targeted therapy for
HER2-positive breast cancer. Front Immunol. 13:10834622022.
View Article : Google Scholar : PubMed/NCBI
|
|
288
|
Mandó P, Rivero SG, Rizzo MM, Pinkasz M
and Levy EM: Targeting ADCC: A different approach to HER2 breast
cancer in the immunotherapy era. Breast. 60:15–25. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
289
|
Waks AG, Martínez-Sáez O, Tarantino P,
Braso-Maristany F, Pascual T, Cortés J, Tolaney SM and Prat A: Dual
HER2 inhibition: Mechanisms of synergy, patient selection, and
resistance. Nat Rev Clin Oncol. 21:818–832. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Yeh R, O'Donoghue JA, Jayaprakasam VS,
Mauguen A, Min R, Park S, Brockway JP, Bromberg JF, Zhi WI, Robson
ME, et al: First-in-human evaluation of site-specifically labeled
89Zr-pertuzumab in patients with HER2-positive breast
cancer. J Nucl Med. 65:386–393. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
291
|
García-Aranda M and Redondo M: Protein
kinase targets in breast cancer. Int J Mol Sci. 18:25432017.
View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Zhang S, Chen W, Zhou J, Liang Q, Zhang Y,
Su M, Zhang Z and Qu J: The benefits and safety of monoclonal
antibodies: implications for cancer immunotherapy. J Inflamm Res.
18:4335–4357. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
293
|
Wang R, Hu B, Pan Z, Mo C, Zhao X, Liu G,
Hou P, Cui Q, Xu Z, Wang W, et al: Antibody-drug conjugates (ADCs):
Current and future biopharmaceuticals. J Hematol Oncol. 18:512025.
View Article : Google Scholar : PubMed/NCBI
|
|
294
|
Torres ETR and Emens LA: Emerging
combination immunotherapy strategies for breast cancer: Dual immune
checkpoint modulation, antibody-drug conjugates and bispecific
antibodies. Breast Cancer Res Treat. 191:291–302. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Cortes J, Cescon DW, Rugo HS, Nowecki Z,
Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, et
al: Pembrolizumab plus chemotherapy versus placebo plus
chemotherapy for previously untreated locally recurrent inoperable
or metastatic triple-negative breast cancer (KEYNOTE-355): A
randomised, placebo-controlled, double-blind, phase 3 clinical
trial. Lancet. 396:1817–1828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Ayoub NM, Al-Shami KM and Yaghan RJ:
Immunotherapy for HER2-positive breast cancer: Recent advances and
combination therapeutic approaches. Breast Cancer (Dove Med Press).
11:53–69. 2019.PubMed/NCBI
|
|
298
|
Mittendorf EA, Zhang H, Barrios CH, Saji
S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, et al:
Neoadjuvant atezolizumab in combination with sequential
nab-paclitaxel and anthracycline-based chemotherapy versus placebo
and chemotherapy in patients with early-stage triple-negative
breast cancer (IMpassion031): A randomised, double-blind, phase 3
trial. Lancet. 396:1090–1100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
299
|
Pusztai L, Yau C, Wolf DM, Han HS, Du L,
Wallace AM, String-Reasor E, Boughey JC, Chien AJ, Elias AD, et al:
Durvalumab with olaparib and paclitaxel for high-risk HER2-negative
stage II/III breast cancer: Results from the adaptively randomized
I-SPY2 trial. Cancer Cell. 39:989–998.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
El Bairi K, Haynes HR, Blackley E,
Fineberg S, Shear J, Turner S, de Freitas JR, Sur D, Amendola LC,
Gharib M, et al: The tale of TILs in breast cancer: A report from
the international immuno-oncology biomarker working group. NPJ
Breast Cancer. 7:1502021. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Loi S, Salgado R, Adams S, Pruneri G,
Francis PA, Lacroix-Triki M, Joensuu H, Dieci MV, Badve S, Demaria
S, et al: Tumor infiltrating lymphocyte stratification of
prognostic staging of early-stage triple negative breast cancer.
NPJ Breast Cancer. 8:32022. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Moisand A, Madéry M, Boyer T, Domblides C,
Blaye C and Larmonier N: Hormone receptor signaling and breast
cancer resistance to anti-tumor immunity. Int J Mol Sci.
24:150482023. View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Axelrod ML, Cook RS, Johnson DB and Balko
JM: Biological consequences of MHC-II expression by tumor cells in
cancer. Clin Cancer Res. 25:2392–2402. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Smith PL, Piadel K and Dalgleish AG:
Directing T-cell immune responses for cancer vaccination and
immunotherapy. Vaccines (Basel). 9:13922021. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Mittendorf EA, Clifton GT, Holmes JP,
Schneble E, van Echo D, Ponniah S and Peoples GE: Final report of
the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine
with booster inoculations to prevent disease recurrence in
high-risk breast cancer patients. Ann Oncol. 25:1735–1742. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
306
|
Carmichael MG, Benavides LC, Holmes JP,
Gates JD, Mittendorf EA, Ponniah S and Peoples GE: Results of the
first phase 1 clinical trial of the HER-2/neu peptide (GP2) vaccine
in disease-free breast cancer patients: United States military
cancer institute clinical trials group study I-04. Cancer.
116:292–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
307
|
McCarthy PM, Clifton GT, Vreeland TJ,
Adams AM, O'Shea AE and Peoples GE: AE37: A HER2-targeted vaccine
for the prevention of breast cancer recurrence. Expert Opin
Investig Drugs. 30:5–11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
308
|
Liu Y, Hu Y, Xue J, Li J, Yi J, Bu J,
Zhang Z, Qiu P and Gu X: Advances in immunotherapy for
triple-negative breast cancer. Mol Cancer. 22:1452023. View Article : Google Scholar : PubMed/NCBI
|
|
309
|
Yuan Y, Lee JS, Yost SE, Frankel PH, Ruel
C, Egelston CA, Guo W, Gillece JD, Folkerts M, Reining L, et al: A
phase II clinical trial of pembrolizumab and enobosarm in patients
with androgen receptor-positive metastatic triple-negative breast
cancer. Oncologist. 26:99–e217. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
310
|
Wood SJ, Gao Y, Lee JH, Chen J, Wang Q,
Meisel JL and Li X: High tumor infiltrating lymphocytes are
significantly associated with pathological complete response in
triple negative breast cancer treated with neoadjuvant KEYNOTE-522
chemoimmunotherapy. Breast Cancer Res Treat. 205:193–199. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
311
|
Cardoso F, O'Shaughnessy J, Liu Z,
McArthur H, Schmid P, Cortes J, Harbeck N, Telli ML, Cescon DW,
Fasching PA, et al: Pembrolizumab and chemotherapy in high-risk,
early-stage, ER+/HER2− breast cancer: A
randomized phase 3 trial. Nat Med. 31:442–448. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
312
|
Thuya WL, Cao Y, Ho PC, Wong AL, Wang L,
Zhou J, Nicot C and Goh BC: Insights into IL-6/JAK/STAT3 signaling
in the tumor microenvironment: Implications for cancer therapy.
Cytokine Growth Factor Rev. 85:26–42. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
313
|
Kahaer G, Pan S, Yang C, Xie W and Lu Y:
Dual function of Gasdermin E: Pyroptosis-mediated pan-cancer
suppression versus HCC-specific oncogenic activity. Front Immunol.
16:16263112025. View Article : Google Scholar : PubMed/NCBI
|
|
314
|
Yang J, Xu J, Wang W, Zhang B, Yu X and
Shi S: Epigenetic regulation in the tumor microenvironment:
Molecular mechanisms and therapeutic targets. Signal Transduct
Target Ther. 8:2102023. View Article : Google Scholar : PubMed/NCBI
|
|
315
|
Kwon YY and Hui S: IL-6 promotes tumor
growth through immune evasion but is dispensable for cachexia. EMBO
Rep. 25:2592–2609. 2024. View Article : Google Scholar : PubMed/NCBI
|