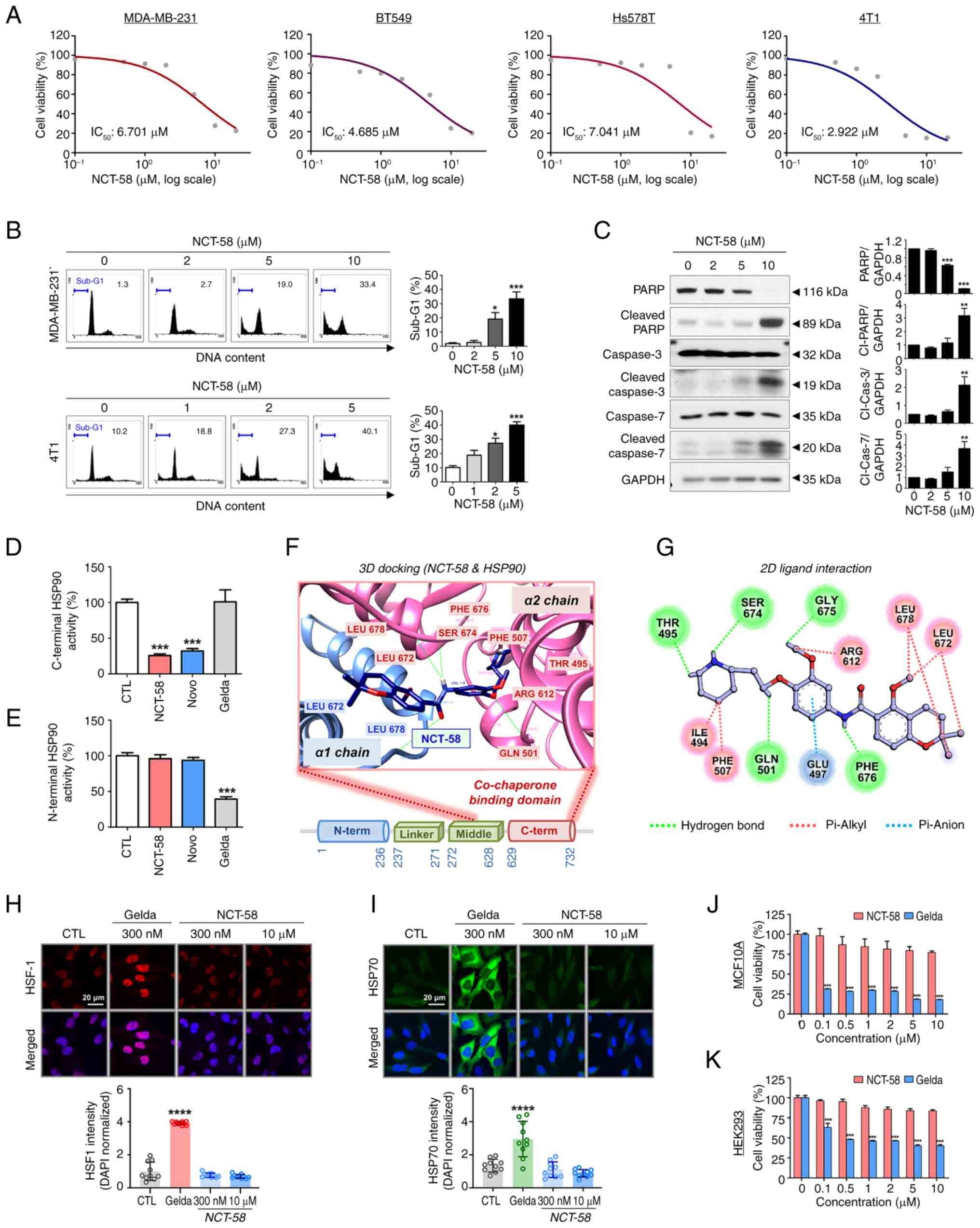
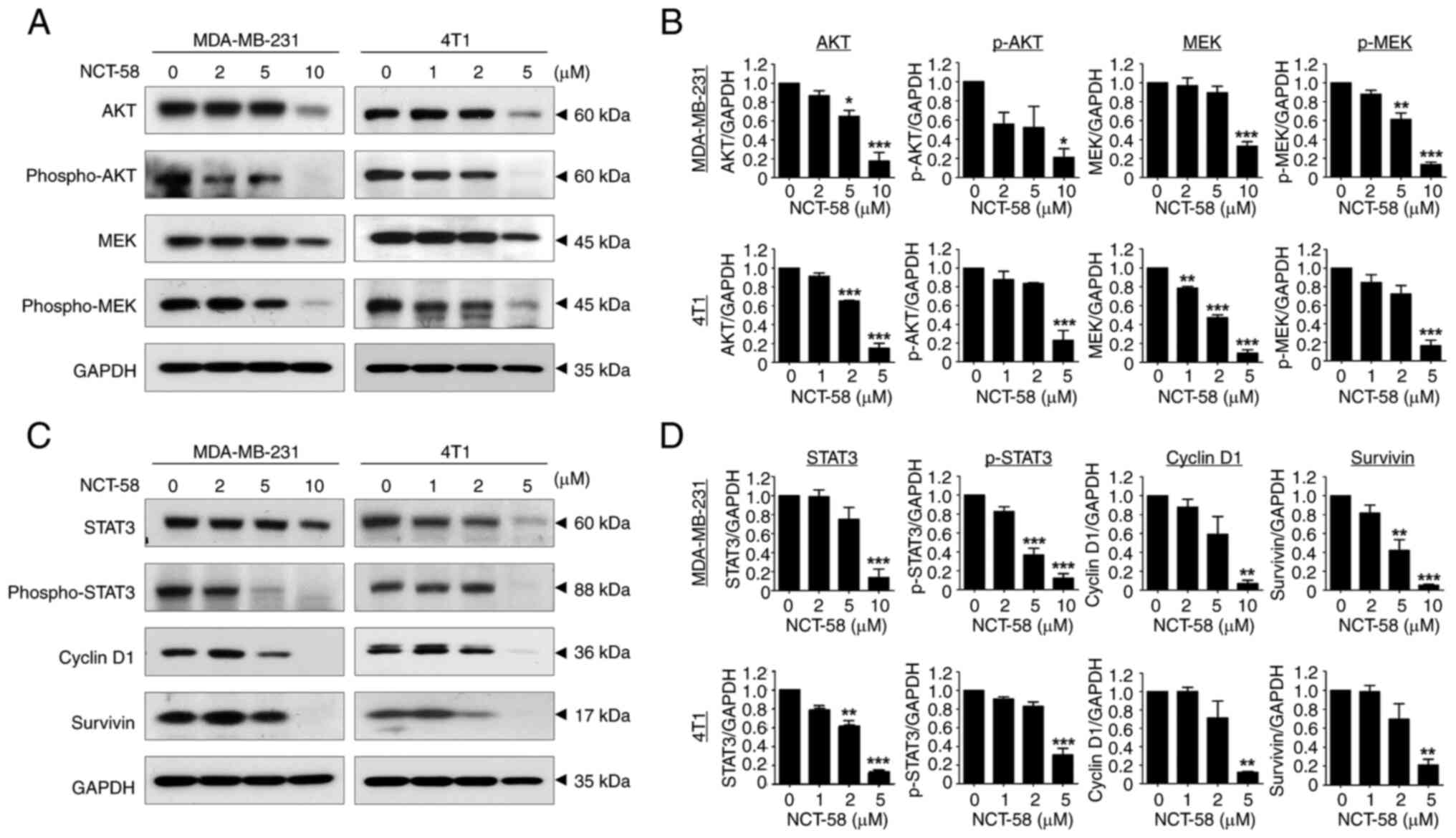
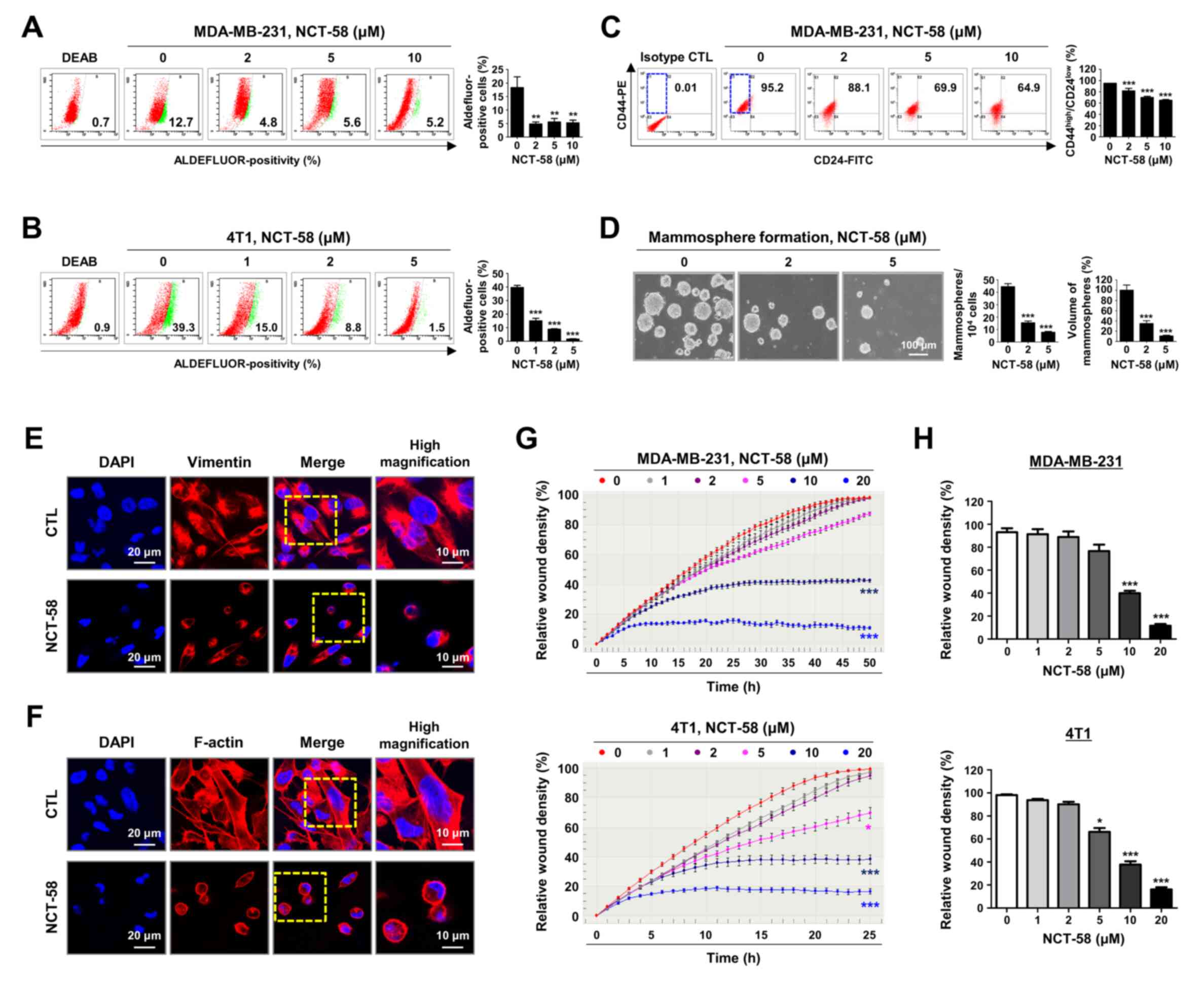
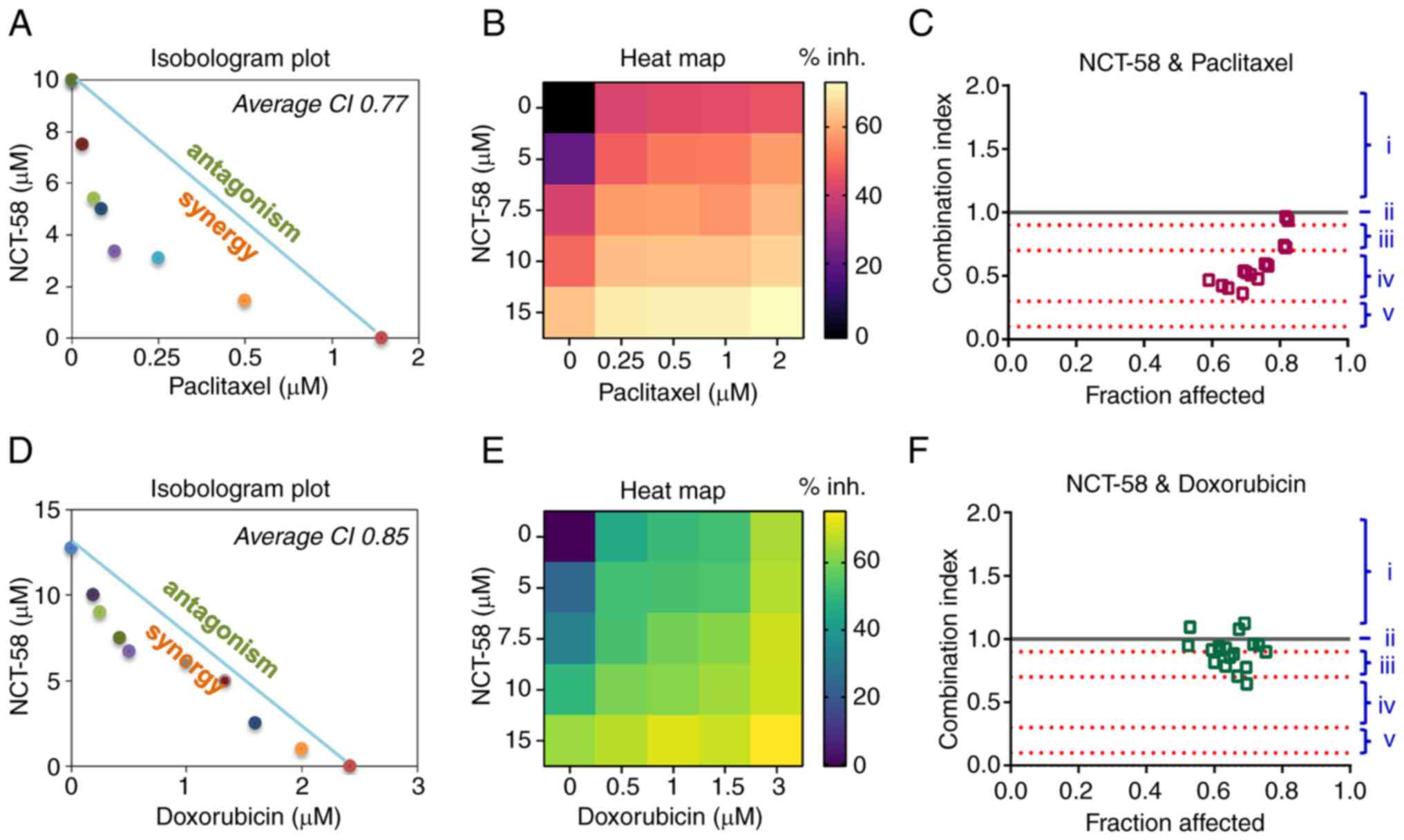
|
1
|
Zagami P and Carey LA: Triple negative
breast cancer: Pitfalls and progress. NPJ Breast Cancer. 8:952022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrarini M, Heltai S, Zocchi MR and
Rugarli C: Unusual expression and localization of heat-shock
proteins in human tumor cells. Int J Cancer. 51:613–619. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pick E, Kluger Y, Giltnane JM, Moeder C,
Camp RL, Rimm DL and Kluger HM: High HSP90 expression is associated
with decreased survival in breast cancer. Cancer Res. 67:2932–2937.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Isaacs JS, Xu W and Neckers L: Heat shock
protein 90 as a molecular target for cancer therapeutics. Cancer
Cell. 3:213–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bocchini CE, Kasembeli MM, Roh SH and
Tweardy DJ: Contribution of chaperones to STAT pathway signaling.
JAKSTAT. 3:e9704592014.PubMed/NCBI
|
|
6
|
Streicher JM: The role of heat shock
proteins in regulating receptor signal transduction. Mol Pharmacol.
95:468–474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edkins AL: Hsp90 co-chaperones as drug
targets in cancer: Current perspectives. Heat Shock Protein
Inhibitors. McAlpine S and Edkins A: Topics in Medicinal Chemistry,
19. Springer; Cham: pp. 21–54. 2016, View Article : Google Scholar
|
|
8
|
Collina F, Di Bonito M, Li Bergolis V, De
Laurentiis M, Vitagliano C, Cerrone M, Nuzzo F, Cantile M and Botti
G: Prognostic value of cancer stem cells markers in triple-negative
breast cancer. Biomed Res Int. 2015:1586822015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kabakov A, Yakimova A and Matchuk O:
Molecular chaperones in cancer stem cells: Determinants of stemness
and potential targets for antitumor therapy. Cells. 9:8922020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho TM, Kim JY, Kim YJ, Sung D, Oh E, Jang
S, Farrand L, Hoang VH, Nguyen CT, Ann J, et al: C-terminal HSP90
inhibitor L80 elicits anti-metastatic effects in triple-negative
breast cancer via STAT3 inhibition. Cancer Lett. 447:141–153. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JM, Kim YJ, Park S, Park M, Farrand
L, Nguyen CT, Ann J, Nam G, Park HJ, Lee J, et al: A novel HSP90
inhibitor targeting the C-terminal domain attenuates trastuzumab
resistance in HER2-positive breast cancer. Mol Cancer. 19:1612020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park M, Jung E, Park JM, Park S, Ko D, Seo
J, Kim S, Nam KD, Kang YK, Farrand L, et al: The HSP90 inhibitor
HVH-2930 exhibits potent efficacy against trastuzumab-resistant
HER2-positive breast cancer. Theranostics. 14:2442–2463. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoter A, El-Sabban ME and Naim HY: The
HSP90 family: Structure, regulation, function, and implications in
health and disease. Int J Mol Sci. 19:25602018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garcia-Carbonero R, Carnero A and Paz-Ares
L: Inhibition of HSP90 molecular chaperones: Moving into the
clinic. Lancet Oncol. 14:e358–e369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y and McAlpine SR: N-terminal and
C-terminal modulation of Hsp90 produce dissimilar phenotypes. Chem
Commun (Camb). 51:1410–1413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park S, Kim YJ, Park JM, Park M, Nam KD,
Farrand L, Nguyen CT, La MT, Ann J, Lee J, et al: The C-terminal
HSP90 inhibitor NCT-58 kills trastuzumab-resistant breast cancer
stem-like cells. Cell Death Discov. 7:3542021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nguyen CT, Ann J, Sahu R, Byun WS, Lee S,
Nam G, Park HJ, Park S, Kim YJ, Kim JY, et al: Discovery of novel
anti-breast cancer agents derived from deguelin as inhibitors of
heat shock protein 90 (HSP90). Bioorg Med Chem Lett. 30:1273742020.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim JY, Cho TM, Park JM, Park S, Park M,
Nam KD, Ko D, Seo J, Kim S, Jung E, et al: A novel HSP90 inhibitor
SL-145 suppresses metastatic triple-negative breast cancer without
triggering the heat shock response. Oncogene. 41:3289–3297. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Donnelly A and Blagg BSJ: Novobiocin and
additional inhibitors of the Hsp90 C-terminal nucleotide-binding
pocket. Curr Med Chem. 15:2702–2717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stebbins CE, Russo AA, Schneider C, Rosen
N, Hartl FU and Pavletich NP: Crystal structure of an
Hsp90-geldanamycin complex: Targeting of a protein chaperone by an
antitumor agent. Cell. 89:239–250. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gooljarsingh LT, Fernandes C, Yan K, Zhang
H, Grooms M, Johanson K, Sinnamon RH, Kirkpatrick RB, Kerrigan J,
Lewis T, et al: A biochemical rationale for the anticancer effects
of Hsp90 inhibitors: Slow, tight binding inhibition by geldanamycin
and its analogues. Proc Natl Acad Sci USA. 103:7625–7630. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu J, Liu T, Rios Z, Mei Q, Lin X and Cao
S: Heat shock proteins and cancer. Trends Pharmacol Sci.
38:226–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neckers L, Blagg B, Haystead T, Trepel JB,
Whitesell L and Picard D: Methods to validate Hsp90 inhibitor
specificity, to identify off-target effects, and to rethink
approaches for further clinical development. Cell Stress
Chaperones. 23:467–482. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin JJ, Yan L, Zhang J and Zhang WD: STAT3
as a potential therapeutic target in triple negative breast cancer:
A systematic review. J Exp Clin Cancer Res. 38:1952019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Wang L, Song Y, Wang S, Huang X,
Xuan Q, Kang X and Zhang Q: CD44+/CD24−
phenotype predicts a poor prognosis in triple-negative breast
cancer. Oncol Lett. 14:5890–5898. 2017.PubMed/NCBI
|
|
26
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh E, Kim YJ, An H, Sung D, Cho TM,
Farrand L, Jang S, Seo JH and Kim JY: Flubendazole elicits
anti-metastatic effects in triple-negative breast cancer via STAT3
inhibition. Int J Cancer. 143:1978–1993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang MH, Lee JS, Kim HJ, Jin DI, Kim JI,
Lee KJ and Seo JS: HSP90 protects apoptotic cleavage of vimentin in
geldanamycin-induced apoptosis. Mol Cell Biochem. 281:111–121.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamashita N, Tokunaga E, Kitao H,
Hisamatsu Y, Taketani K, Akiyoshi S, Okada S, Aishima S, Morita M
and Maehara Y: Vimentin as a poor prognostic factor for
triple-negative breast cancer. J Cancer Res Clin Oncol.
139:739–746. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sirkisoon SR, Carpenter RL, Rimkus T,
Anderson A, Harrison A, Lange AM, Jin G, Watabe K and Lo HW:
Interaction between STAT3 and GLI1/tGLI1 oncogenic transcription
factors promotes the aggressiveness of triple-negative breast
cancers and HER2-enriched breast cancer. Oncogene. 37:2502–2514.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei Y, Li Y, Chen Y, Liu P, Huang S, Zhang
Y, Sun Y, Wu Z, Hu M, Wu Q, et al: ALDH1: A potential therapeutic
target for cancer stem cells in solid tumors. Front Oncol.
12:10262782022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clark DW and Palle K: Aldehyde
dehydrogenases in cancer stem cells: Potential as therapeutic
targets. Ann Transl Med. 4:5182016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sládek NE: Aldehyde dehydrogenase-mediated
cellular relative insensitivity to the oxazaphosphorines. Curr
Pharm Des. 5:607–625. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sladek NE: Metabolism of
oxazaphosphorines. Pharmacol Ther. 37:301–355. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moreb JS: Aldehyde dehydrogenase as a
marker for stem cells. Curr Stem Cell Res Ther. 3:237–246. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schrörs B, Boegel S, Albrecht C, Bukur T,
Bukur V, Holtsträter C, Ritzel C, Manninen K, Tadmor AD, Vormehr M,
et al: Multi-omics characterization of the 4T1 murine mammary gland
tumor model. Front Oncol. 10:11952020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Herndon ME, Ayers M, Gibson-Corley KN,
Wendt MK, Wallrath LL, Henry MD and Stipp CS: The highly metastatic
4T1 breast carcinoma model possesses features of a hybrid
epithelial/mesenchymal phenotype. Dis Model Mech. 17:dmm0507712024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tao K, Fang M, Alroy J and Sahagian GG:
Imagable 4T1 model for the study of late stage breast cancer. BMC
Cancer. 8:2282008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu W and Kang Y: Epithelial-mesenchymal
plasticity in cancer progression and metastasis. Dev Cell.
49:361–374. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zagouri F, Bournakis E, Koutsoukos K and
Papadimitriou CA: Heat shock protein 90 (hsp90) expression and
breast cancer. Pharmaceuticals (Basel). 5:1008–1020. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei H, Zhang Y, Jia Y, Chen X, Niu T,
Chatterjee A, He P and Hou G: Heat shock protein 90: Biological
functions, diseases, and therapeutic targets. MedComm (2020).
5:e4702024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Burstein HJ,
Chew H, et al: NCCN guidelines® insights: Breast cancer,
version 4.2023. J Natl Compr Canc Netw. 21:594–608. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cortes J, Rugo HS, Cescon DW, Im SA, Yusof
MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et
al: Pembrolizumab plus chemotherapy in advanced triple-negative
breast cancer. N Engl J Med. 387:217–226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xie J, Li S, Li Y and Li J:
Cost-effectiveness of sacituzumab govitecan versus chemotherapy in
patients with relapsed or refractory metastatic triple-negative
breast cancer. BMC Health Serv Res. 23:7062023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Coates JT, Sun S, Leshchiner I, Thimmiah
N, Martin EE, McLoughlin D, Danysh BP, Slowik K, Jacobs RA,
Rhrissorrakrai K, et al: Parallel genomic alterations of antigen
and payload targets mediate polyclonal acquired clinical resistance
to sacituzumab govitecan in triple-negative breast cancer. Cancer
Discov. 11:2436–2445. 2021. View Article : Google Scholar : PubMed/NCBI
|