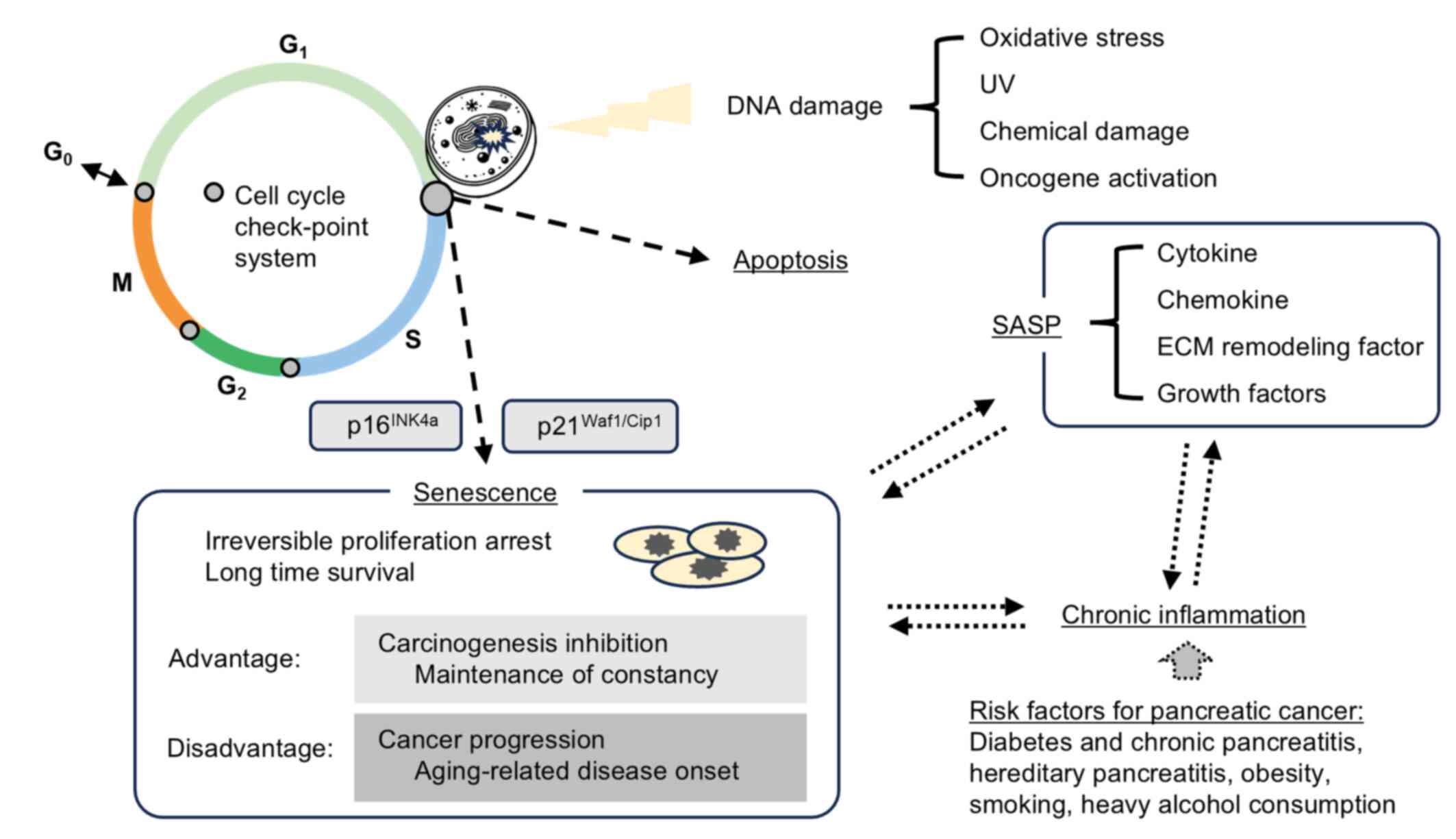
Introduction
In healthy proliferative cells, irreversible growth
arrest is induced when irreparable DNA damage occurs as a result of
telomere shortening, oncogene activation, excessive oxidative
stress; this state is referred to as cellular senescence (1–3).
Senescent cells have been observed in vivo and are
recognized as a physiological phenomenon (4–7). Key
features of senescent cells include permanent cell cycle arrest,
morphological changes such as cellular enlargement and flattening,
formation of senescence-associated heterochromatic foci, activation
of senescence-associated β-galactosidase (SA-β-gal) and
upregulation of the cell cycle inhibitors p21 and p16 (3). Similar to apoptosis, cellular
senescence is considered an important tumor-suppressive mechanism
that prevents abnormal proliferation and cancer development
(8,9). However, studies have shown that
senescent cells not only cease proliferating but can also persist
for extended periods, contributing to a phenomenon known as the
senescence-associated secretory phenotype (SASP) (5,8,9). The
SASP involves the elevated secretion of various bioactive molecules
with pro-tumorigenic effects, including inflammatory cytokines,
chemokines, extracellular matrix-remodeling enzymes and growth
factors (8). Although SASP factors
serve physiological roles in processes such as tissue development
and wound healing, excessive accumulation of senescent cells and
SASP factors may have detrimental effects. These include the
development of age-related diseases such as atherosclerosis,
induction of chronic inflammation and promotion and progression of
cancer (10,11). Thus, although cellular senescence
serves as a short-term cancer-suppressive mechanism, its long-term
presence and associated SASP may contribute to carcinogenesis and
tumor progression. These findings suggest that cellular senescence
has a dual nature: Protective in the short term, yet potentially
harmful over time (Fig. 1)
(8,9,11).
Senescent cells exhibit morphological changes,
appearing larger and more flattened than proliferating cells. They
accumulate dysfunctional mitochondria and display elevated levels
of reactive oxygen species (ROS). Lysosomal content is increased,
reflected by enhanced β-galactosidase activity in acidic
environments (pH 6.0). Cells exhibiting the SASP secrete a wide
array of factors that promote angiogenesis, extracellular matrix
remodeling, epithelial-mesenchymal transition and tumor-promoting
activities (12). Chronic
inflammation induced by senescent cells may also lead to
immunosuppression, contributing to cancer development and
age-related diseases (10,11).
The tissue microenvironment is shaped by the
physical and chemical influences of resident and surrounding cells,
along with local factors such as growth factors, cytokines,
temperature and oxygen levels (8).
In cancerous tissues, the tumor microenvironment is composed of
various cells and signaling molecules that surround cancer cells
and promote tumor progression and invasion. Among these, certain
fibroblasts function as cancer-associated fibroblasts (CAFs)
(13–16). Studies suggest that a CAF-rich tumor
microenvironment contributes to cancer invasion, metastasis, immune
evasion and drug resistance (17,18).
Stromal depletion through chemotherapy has been shown to improve
drug resistance in cancer (19,20).
Furthermore, senescence and SASP expression have been observed in
fibroblasts within the stroma of several solid tumors (such as
hepatocellular carcinoma, ovarian cancer, and prostate cancer) and
are thought to influence tumor progression, suggesting these are
common features across tumor types (21–23).
Notably, pancreatic ductal adenocarcinoma (PDAC) and diffuse type
gastric cancer are characterized by abundant fibrous stroma and
particularly poor prognoses (24,25).
However, the current understanding of stromal senescence and SASP
expression in PDAC or their prognostic relevance is limited.
The present study focused on PDAC, which is
particularly characterized by a dense fibrotic stroma among types
of digestive system cancer. Using resected specimens from patients
with PDAC, the present study aimed to investigate the presence of
senescent fibroblasts and SASP factor expression in the tumor
stroma, and their association with cancer progression and patient
prognosis. Furthermore, senescent human pancreatic fibroblasts were
established and co-cultured with pancreatic cancer cells with the
aim of evaluating the effects of SASP on tumor cell behavior.
Elucidating the impact of stromal senescence and SASP expression on
patient prognosis in PDAC may help identify novel prognostic
biomarkers and therapeutic targets, potentially leading to the
development of stroma-targeted treatments for this aggressive
cancer.
Materials and methods
Patients and pancreatic tissue
The present study utilized 90 resected specimens
from patients who underwent pancreaticoduodenectomy for PDAC
involving the pancreatic head at Kanazawa University Hospital
between 2006 and 2017; retrospective study was conducted on
patients treated between 2006 and 2015. The study protocol was
approved by the Ethics Committee of Kanazawa University Hospital
(approval no. 2016-318; Kanazawa, Japan), and complied with the
Declaration of Helsinki (1975) and the Ethical Guidelines for
Medical and Biological Research Involving Human Subjects by the
Japanese Government (26). Written
informed consent was obtained from all participants for the
anonymous use of resected specimens and clinical data. The clinical
characteristics of the patients were as follows: Median age, 66
years (age range, 35–81); sex distribution, male (62%) and female
(38%); pathological stage of PDAC based on the Union for
International Cancer Control (8th edition) TNM classification
(27): Stage I, n=34; stage II,
n=31; and stage III, n=25; and preoperative chemotherapy in 59
cases (66%). Of the total cohort, 1 patient received preoperative
radiation therapy. All patients were followed up after surgery for
up to 8 years, and survival was defined as the time from
pancreaticoduodenectomy to death or the last visit to the
hospital.
Immunohistochemistry and
immunofluorescence
Paraffin-embedded sections of 4-µm thickness (10%
neutral buffered formalin-fixed for 24 h at room temperature) were
prepared for immunohistochemistry and immunofluorescence. The Dako
Envision system (Dako; Agilent Technologies, Inc.), which uses
dextran polymers conjugated to horseradish peroxidase, was employed
for immunohistochemical and immunofluorescent staining to avoid any
endogenous biotin contamination. Sections were deparaffinized in
xylene and rehydrated in a graded ethanol series. Endogenous
peroxidase was blocked by immersing sections in 3%
H2O2 in 100% methanol for 20 min at room
temperature (~26°C). Antigen retrieval was achieved by microwaving
sections at 95°C for 10 min in 0.001 M citrate buffer (pH 6.7).
After blocking endogenous peroxidase, sections were incubated with
Protein Block Serum-Free (Dako; Agilent Technologies, Inc.) at room
temperature (~26°C) for 10 min to block non-specific staining.
Fibroblasts in the pancreatic cancer stroma were
identified via immunohistochemistry and immunofluorescence staining
with a mouse monoclonal anti-a smooth muscle actin (αSMA; cat. no.
ab7817; Abcam) antibody. A rabbit polyclonal anti-CDKN2A/p16INK4a
(cat. no. ab189034; Abcam) antibody and a mouse monoclonal
anti-interleukin-6 (IL-6; cat. no. ab9324; Abcam) antibody were
used to identify p16- and IL-6-positive fibroblasts within the PDAC
stroma. Primary antibodies were applied for 2 h at room temperature
(~26°C). The fluorescent dyes used were goat anti-mouse IgG H&L
(Alexa Fluor 488; cat. no. ab150113; Abcam), donkey anti-rabbit IgG
H&L (Alexa Fluor 647; cat. no. ab150075; Abcam) and DAPI (cat.
no. ab104139; Abcam). Secondary antibodies were applied for 1 h at
room temperature (~26°C).
When staining culture plates, cells were fixed with
4% paraformaldehyde for 15 min, permeabilized with 0.05% Triton
X-100 in 1X PBS for 15 min and blocked with 1% fetal bovine serum
(FBS; Nichirei Biosciences, Inc.) in 1X PBS for 30 min at room
temperature (~26°C). A mouse monoclonal anti-AE1/AE3 (cat. no.
sc-81714; Santa Cruz Biotechnology) antibody and a mouse monoclonal
anti-Vimentin (cat. no. sc-6260; Santa Cruz Biotechnology) antibody
were used to identify established human pancreatic fibroblasts.
Primary antibodies were applied for 2 h at room temperature
(~26°C). Peroxidase activity was detected using the enzyme
substrate DAB. Samples were counterstained with hematoxylin for 1
min or DAPI for 30 min at room temperature (~26°C).
The mean expression intensities of p16- and
IL-6-positive fibroblasts were quantified by counting three fields
per specimen (magnification, ×100). To evaluate expression levels
quantitively, H-score analysis was employed (28,29).
Immunohistochemical images were captured using an optical
microscope (BX-50; Olympus Corporation), and fluorescent images
were captured using a BZ-X710 microscope (Keyence Corporation).
Establishment of human pancreatic
fibroblasts
A human pancreatic fibroblast cell line was
established using a resected pancreatic specimen of the
non-tumorous area from a patient with pancreatic cancer, performed
as previously described (30,31).
To maintain sterility, pancreatic tissue from the non-tumorous area
(~5 µm3) with adequate margin was collected under clean
conditions. The tissue was minced using scissors and cultured in
Dulbecco's Modified Eagle Medium (DMEM; Nacalai Tesque, Inc.)
supplemented with 10% FBS in a 10-cm culture dish. After 3–4 days
of incubation at 37°C, proliferation of bipolar or multipolar
spindle-shaped cells was observed, whereas no proliferation was
seen in cell populations with other morphologies. Pancreatic
fibroblasts were successfully established based on cell morphology
and marker expression; spindle-shaped cells tested positive for
αSMA and vimentin by immunostaining. Fibroblasts between passages 4
and 8 were used.
Cell lines and culture conditions
The Panc-1 cell line, derived from human pancreatic
cancer cells (cat. no. CRL-1469; American Type Culture Collection)
was used in the present study. Pancreatic cancer cell lines and
pancreatic fibroblasts were cultured in DMEM supplemented with 10%
FBS at 37°C in a humidified atmosphere with 5% CO2. The
culture medium was replaced 2–3 times per week.
Induction of cellular senescence in
human pancreatic fibroblasts
Confluent monolayers of human pancreatic fibroblasts
cultured in 6-well plates were irradiated with X-rays at a dose of
10 Gy. The fibroblasts were maintained using the culture conditions
described above, with medium changes every 3 days. On days 0, 3, 6
and 9 after irradiation, SA-β-gal staining was performed using a
Cellular Senescence Kit (OZ Biosciences) according to the
manufacturer's instructions to evaluate cellular senescence. To
assess the dose-dependent induction of senescence, fibroblasts were
irradiated at 3, 6 and 10 Gy, and SA-β-gal staining was performed
on day 9 after irradiation. Fibroblasts irradiated with 10 Gy in
6-cm culture dishes were collected on day 9 post-irradiation for
further analysis.
Real-time PCR
Total mRNA was extracted using TRIzol reagent
(Thermo Fisher Scientific, Inc.) and reverse transcription was
performed using the AffinityScript QPCR cDNA Synthesis Kit (Agilent
Technologies, Inc.) according to the manufacturer's protocol.
Reverse transcription-quantitative PCR (RT-qPCR) was conducted
using an Mx3000P system (Agilent Technologies, Inc.) with a SYBR
Green PCR Kit (Qiagen GmbH) in triplicate, using specific primers.
The primer sequences used to assess target gene expression were as
follows: IL-6, forward (F), 5′-ATGAACTCCTTCTCCACAAGC-3′ and reverse
(R), 5′-GTTTTCTGCCAGTGCCTCTTTG-3′; IL-1, F,
5′-ATCAGTACCTCACGGCTGCT-3′ R, 5′-TGGGTATCTCAGGCATCTCC-3′; IL-8 F,
5′-AACATGACTTCCAAGCTGGC-3′ R, 5′-AAACTTCTCCACAACCCTCTGC-3′; MMP-1
F, 5′-AGCTAGCTCAGGATGACATTGATG-3′ R, 5′-GCCGATGGGCTGGACAG-3′; VEGF
F, 5′-ATGAACTTTCTGCTGTCTTGGGT-3′ R, 5′-TGGCCTTGGTGAGGTTTGATCC-3′;
p16 F, 5′-CCCAACGCACCGAATAGTTA-3′ R, 5′-ACCAGCGTGTCCAGGAAG-3′; and
GAPDH F, 5′-GACAGTCAGCCGCATCTTCT-3′ R, 5′-TTAAAAGCAGCCCTGGTGAC-3′
The following thermocycling conditions were used for qPCR: Initial
denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C
for 30 sec; annealing at 55°C for 1 min; and extension at 72°C for
1 min. Real-time PCR was conducted according to the
2−ΔΔCq method (32),
with GAPDH as the internal control.
Western blotting
After collection, cells were lysed on ice in RIPA
lysis buffer (Wako Pure Chemical Industries, Ltd.) containing a
protease inhibitor. Protein concentrations were measured using the
Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.).
Total cellular proteins (20 µg) from each sample were separated by
12.5% gradient polyacrylamide gel electrophoresis (e-PAGEL; ATTO
Corporation) and transferred onto a nitrocellulose membrane.
Membranes were blocked with Odyssey Blocking Buffer (LI-COR
Biosciences) for 2 h at 26°C and incubated overnight at 4°C with
the primary antibodies. The following primary antibodies were used:
p16 (1:1,000; cat. no. ab189034; Abcam), IL-6 (1:1,000; cat. no.
ab9324; Abcam) and β-actin (1:10,000; cat. no. A5441; Sigma-Adrich;
Merck KGaA), which served as the internal control. After three
10-min washes with Tris-buffered saline containing 0.05% of Tween
20, membranes were incubated for 1 h at 26°C with either IRDye
680RD-conjugated goat anti-rabbit IgG (1:10,000; cat. no.
925-68071; LI-COR Biosciences) or IRDye 800CW-conjugated goat
anti-mouse IgG (1:10,000; cat. no. 925-32210; LI-COR Biosciences).
Western blot images were analyzed using an Odyssey infrared imaging
system software (version 4.0; LI-COR Biosciences).
Matrigel invasion assay
A Corning Matrigel Invasion Chamber (cat. no.
354480; Corning, Inc.) in a 24-well plate format (cat. no. 353504;
Corning, Inc.) was used and two groups of fibroblasts were
prepared: Pancreatic fibroblasts collected 9 days after irradiation
and non-irradiated pancreatic fibroblasts maintained in culture for
the same duration (9 days). Matrigel precoating was performed at
37°C for 1 h for the invasion assay. First, 1×105
fibroblasts in 1 ml DMEM were seeded into the lower chamber. After
2 h, 5×104 Panc-1 cells in 500 µl DMEM were added to the
upper chamber to initiate co-culture (n=6). The culture medium used
was DMEM supplemented with 10% FBS. After 24 h of incubation at
37°C, the chambers were collected. Following gel removal, membranes
were stained with hematoxylin for 2 min at 26°C. The number of
pancreatic cancer cells that had invaded through the membrane was
counted under an optical microscope (BX-50, Olympus Corporation).
As a control, Panc-1 cells were cultured in the upper chamber
without fibroblasts in the lower chamber.
Scratch assay and application of
culture supernatant
Pancreatic fibroblasts were cultured into confluent
in 10-cm dishes containing (either 9 days post-irradiation or
non-irradiated). After culturing the fibroblasts in DMEM
supplemented with 1% FBS for 48 h at 37°C, the culture supernatant
was collected for use in subsequent experiments. Simultaneously,
6-well plates containing confluent monolayers of Panc-1 cells
cultured in serum-free DMEM for 24 h at 37°C were scratched using a
pipette tip. The collected culture supernatants from irradiated and
non-irradiated fibroblasts were added to the wells (2 ml/well), and
the plates were incubated at 37°C. After 24 h, the area into which
Panc-1 cells had migrated was measured. As a control, scratched
confluent monolayers of Panc-1 cells were cultured in serum-free
DMEM not containing fibroblast culture supernatant. A microscope
(BX-50, Olympus Corporation) was used for cell observation.
Migration was quantified by comparing the final images with
baseline images captured immediately after scratching (n=6), using
the ImageJ software (version 1.51k; National Institutes of Health)
(33). For each group, the cell
migration area was calculated by subtracting the area of the
cell-free region (scratch line) at 24 h from the area at 0 h. The
cell migration area for the control group was set as 1, and the
cell migration area for each group was calculated as a ratio.
Cell proliferation assay
Cell proliferation was measured using the MTT assay
(n=8). Confluent monolayers of irradiated pancreatic fibroblasts in
10-cm dishes were cultured in DMEM supplemented with 1% FBS for 48
h, and the resulting culture supernatant was collected. Panc-1
cells were seeded at a density of 6,000 cells per well in 96-well
plates, and 200 µl of the fibroblast supernatant, adjusted to
contain 10% FBS, was added to each well. Cells were incubated for
24 and 48 h at 37°C. After incubation, the medium was removed, and
the crystallized formazan was solubilized by addition of dimethyl
sulfoxide. Absorbance was measured at 535 nm using a microplate
reader (Bio-Rad 550; Bio-Rad Laboratories, Inc.). Panc-1 cells
cultured without the fibroblast supernatant were used as
controls.
Statistical analysis
Statistical analyses were performed using EZR
software (version 1.63; Saitama Medical Center; Jichi Medical
University). Demographic and clinicopathological characteristics
were summarized using descriptive statistics. To compare
characteristics between groups, the χ2 test was used for
categorical variables, and Student's t-test or one-way ANOVA
followed by Turkey's post hoc test was used for unpaired continuous
variables. Pearson's correlation coefficient (r) was used to assess
correlations between the expression levels of p16- and
IL-6-positive fibroblasts in the pancreatic cancer stroma, and
between IL-6-positive fibroblast expression and postoperative
survival time. Receiver operating characteristic (ROC) curve
analysis was used to determine the IL-6 cut-off value for
prognosis. Survival curves were generated using the Kaplan-Meier
method and compared using the log-rank test. Multivariate analysis
was performed using the Cox proportional hazards model on variables
that showed significance in univariate analysis. Results are
presented as mean ± standard deviation. All statistical tests were
two-tailed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Assessment of PDAC stromal senescence
and expression of SASP factors in PDAC specimens
Using resected pancreatic cancer specimens, the
presence of senescent fibroblasts in the pancreatic cancer stroma
and the expression of SASP factors were evaluated. αSMA was used as
a marker for pancreatic fibroblasts (13,19).
Although multiple proteins are known markers of cellular senescence
(8,13,16),
p16 expression levels were evaluated as its expression is extremely
low in proliferating cells but increases markedly when cells reach
their mitotic limit or are exposed to carcinogenic stress, inducing
cellular senescence (34,35). Simultaneous immunofluorescence
staining for αSMA and p16 revealed double-positive cells within the
pancreatic cancer stroma, which confirmed the presence of
fibroblasts exhibiting features of cellular senescence (Fig. 2A). Although various cytokines,
chemokines, growth factors and extracellular matrix-degrading
factors are recognized as SASP components, IL-6 was assessed as a
representative and pleiotropic proinflammatory cytokine (8,9,13). The
fibroblasts were positive for both p16 and IL-6 in the tumor stroma
(Fig. 2B), indicating that
senescent fibroblasts in the PDAC stroma express the SASP factor
IL-6. Using resected pancreatic cancer specimens,
immunohistochemistry with p16 and IL-6 targeting the pancreatic
cancer stroma was then performed. A significant positive
correlation was found between p16 and IL-6 expression (r=0.585,
P<0.0001; Fig. 2C), which
suggested that IL-6 expression increased proportionally with the
number of senescent fibroblasts in the tumor stroma. Based on these
findings, subsequent analyses on IL-6 expression in the PDAC stroma
was conducted.
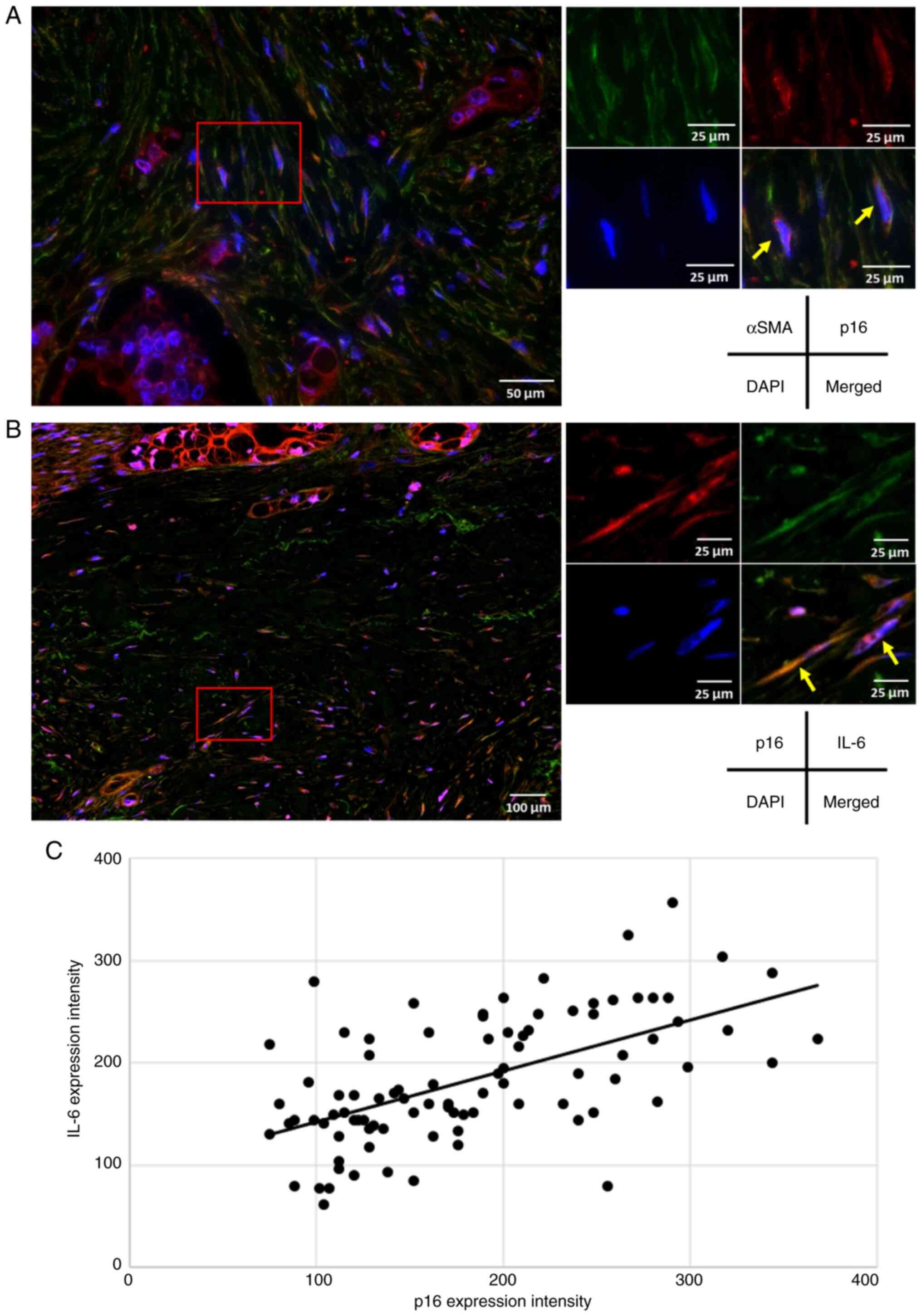 | Figure 2.Evaluation of p16- and IL-6-positive
cells in the stroma of pancreatic ductal adenocarcinoma. (A)
Representative immunofluorescence staining of a pancreatic cancer
resection specimen (αSMA, green; p16, red; DAPI, blue; scale bar,
50 and 25 µm). In the pancreatic cancer stroma, pancreatic
fibroblasts (αSMA-positive) expressing the senescence marker p16
were identified (yellow arrows). (B) Representative
immunofluorescence staining of a pancreatic cancer resection
specimen (p16, red; IL-6, green; DAPI, blue; scale bar, 100 and 25
µm). Fibroblasts positive for p16 and the senescence-associated
secretory phenotype factor IL-6 were observed in the pancreatic
cancer stroma (yellow arrows). (C) A significant positive
correlation between the expression intensity of p16- and
IL-6-positive fibroblasts in the pancreatic cancer stroma
(Pearson's correlation coefficient, r=0.585; P<0.0001). |
Postoperative prognosis according to
IL-6 expression in the PDAC stroma
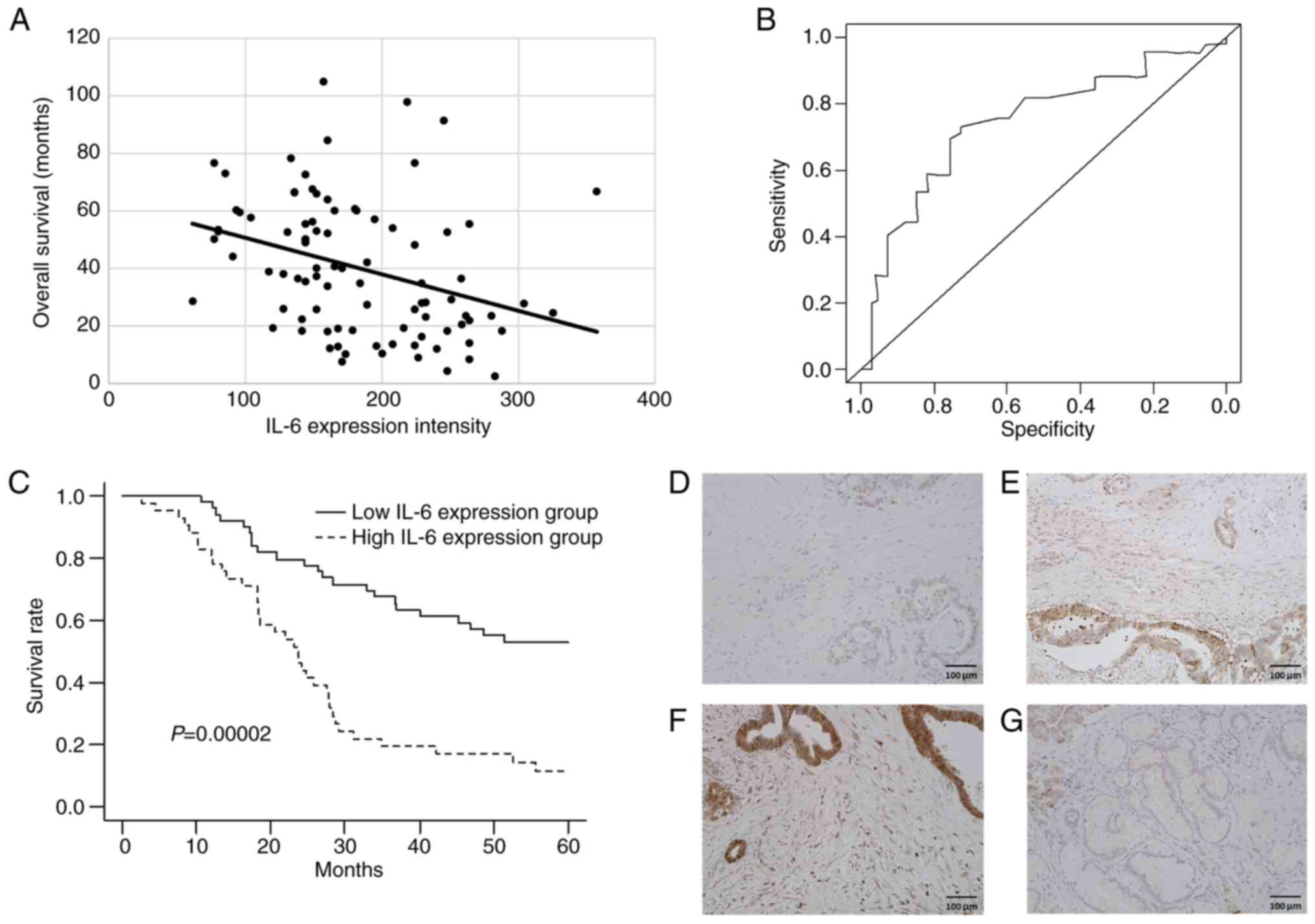
The association between IL-6 expression in stromal
fibroblasts and overall survival (OS) following
pancreaticoduodenectomy was investigated. A significant negative
correlation was found between IL-6-positive fibroblast expression
and postoperative survival (r=−0.336, P=0.0012; Fig. 3A). Therefore, it was considered that
IL-6 expression in the tumor stroma may potentially serve as a
prognostic factor after surgery. Using an ROC curve, the H-score
cut-off value of 179 of IL-6 expression was determined to
distinguish prognostic groups [area under the curve (AUC)=0.754,
sensitivity=0.736 and specificity=0.734; Fig. 3B]. The H-score cut-off was used to
patients divide into high- (≥179) and low-IL-6 (<179) expression
groups and Kaplan-Meier analysis of OS of the two groups was
performed. The high-IL-6 expression group demonstrated a
significantly poorer OS compared with that of the low-IL-6 group
(log-rank test, P=0.00002; Fig.
3C). IHC was performed on representative PDAC specimens with
weak (Fig. 3D), moderate (Fig. 3E) and strong (Fig. 3F) IL-6 positivity, and a
non-tumorous pancreatic specimen (Fig.
3G). Univariate analysis identified curability, T factor (T3 or
T4), lymph node metastasis, neuroplexus invasion and high-IL-6
expression as significant prognostic factors. Multivariate analysis
further confirmed lymph node metastasis and high IL-6 expression as
independent prognostic indicators (Table I). Patient demographics and
clinicopathological characteristics of high- and low-IL-6
expression groups are summarized in Table II. There were no significant
differences between groups in terms of baseline characteristics,
including preoperative chemotherapy, combined portal vein or
arterial resection and curative resection. The high IL-6 expression
group demonstrated a notably higher proportion of patients with
advanced T and N classifications compared with that of the
low-expression group, although this not statistically significant.
To evaluate whether patient characteristics or tumoral factors
influenced IL-6 expression in stromal fibroblasts of PDAC, a
subgroup analysis was conducted. Stromal IL-6 expression was not
associated with any patient characteristics or tumoral factors
(Table III). In this cohort,
neither patient age nor preoperative chemotherapy were associated
with IL-6 expression in senescent stromal fibroblasts.
 | Table I.Factors associated with overall
survival in operated patients with pancreatic ductal
adenocarcinoma. |
Table I.
Factors associated with overall
survival in operated patients with pancreatic ductal
adenocarcinoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, ≥65 years |
|
| 0.401 |
|
|
|
| Sex, male |
|
| 0.748 |
|
|
|
| Neoadjuvant
chemotherapy |
|
| 0.838 |
|
|
|
| Curability (R1 or
2) | 2.173 | 1.372–3.441 | 0.001 | 1.214 | 0.670–2.107 | 0.490 |
| Histological
differentiationa |
|
| 0.119 |
|
|
|
| Tumor
factora |
|
|
|
|
|
|
| T3 or
4 | 2.671 | 1.445–4.938 | 0.002 | 2.101 | 0.860–5.135 | 0.104 |
| N1 or
2 | 4.607 | 2.411–8.806 | 0.00001 | 3.594 | 1.636–7.893 | 0.001 |
| Histopathological
factor |
|
|
|
|
|
|
|
Lymphatic invasion |
|
| 0.516 |
|
|
|
|
Vascular invasion |
|
| 0.102 |
|
|
|
|
Neuroplexus invasion | 2.025 | 1.181–3.473 | 0.010 | 1.576 | 0.835–2.972 | 0.160 |
| High IL-6
expression | 4.486 | 2.564–7.846 | 0.00001 | 3.483 | 1.519–6.852 | 0.001 |
 | Table II.Relationship between stromal IL-6
expression and clinicopathological factors. |
Table II.
Relationship between stromal IL-6
expression and clinicopathological factors.
| Characteristic | Low IL-6
expression, n | High IL-6
expression, n | P-value |
|---|
| Total patients | 49 | 41 |
|
| Age, years |
|
| 0.623 |
|
<65 | 30 | 23 |
|
|
≥65 | 19 | 18 |
|
| Sex |
|
| 0.823 |
|
Male | 31 | 25 |
|
|
Female | 18 | 16 |
|
| Neoadjuvant
chemotherapy |
|
| 0.957 |
|
Present | 32 | 27 |
|
|
Absent | 17 | 14 |
|
| Portal vein
resection |
|
| 0.317 |
|
Present | 31 | 30 |
|
|
Absent | 18 | 11 |
|
| Artery
resection |
|
| 0.525 |
|
Present | 4 | 5 |
|
|
Absent | 45 | 36 |
|
| Curability |
|
| 0.828 |
| R0 | 38 | 31 |
|
|
R1/2 | 11 | 10 |
|
| T
categorya |
|
| 0.189 |
|
T1/2 | 41 | 33 |
|
|
T3/4 | 8 | 8 |
|
| N
categorya |
|
| 0.156 |
| N0 | 24 | 12 |
|
| N1 | 14 | 15 |
|
| N2 | 11 | 14 |
|
| UICC
stagea |
|
| 0.267 |
| I | 22 | 12 |
|
| II | 16 | 15 |
|
|
III | 11 | 14 |
|
| Histological
differentiationa |
|
| 0.449 |
|
Well | 10 | 6 |
|
|
Moderate | 34 | 32 |
|
|
Poor | 7 | 3 |
|
| Lymphatic
invasion |
|
| 0.881 |
|
Present | 45 | 38 |
|
|
Absent | 4 | 3 |
|
| Vascular
invasion |
|
| 0.361 |
|
Present | 41 | 37 |
|
|
Absent | 8 | 4 |
|
| Neuroplexus
invasion |
|
| 0.365 |
|
Present | 24 | 24 |
|
|
Absent | 25 | 17 |
|
 | Table III.Subgroup analysis of the association
with IL-6 expression in stromal fibroblasts was performed. |
Table III.
Subgroup analysis of the association
with IL-6 expression in stromal fibroblasts was performed.
| Factor | Hazard ratio | 95% CI | P-value |
|---|
| Age (≥65
years) | 0.749 | 0.293–1.910 | 0.546 |
| Sex (male) | 0.800 | 0.312–2.050 | 0.643 |
| Neoadjuvant
chemotherapy | 0.963 | 0.364–2.540 | 0.939 |
| Curability (R1 or
2) | 0.731 | 0.301–1.780 | 0.491 |
| Histological
differentiationa
(moderate or poor) | 0.947 | 0.232–3.860 | 0.939 |
| Tumor factor
a |
|
|
|
| T3 or
4 | 0.904 | 0.278–2.930 | 0.867 |
| N1 or
2 | 2.680 | 0.901–7.960 | 0.076 |
| Histopathological
factor |
|
|
|
|
Lymphatic invasion | 0.440 | 0.047–4.110 | 0.471 |
|
Vascular invasion | 2.370 | 0.387–14.600 | 0.350 |
|
Neuroplexus invasion | 1.190 | 0.433–3.300 | 0.731 |
Induction of cellular senescence in
established human pancreatic fibroblasts and co-cultures with PDAC
cell lines
Hematoxylin and eosin staining of cells established
from resected pancreatic specimens was used to confirm the
proliferation of bipolar or multipolar spindle-shaped cells with
basophilic nuclei and cytoplasm. AE1/3 staining was negative,
whereas αSMA and vimentin were diffusely positive (Fig. 4A), which demonstrated the successful
isolation of pancreatic fibroblasts from pancreatic specimens.
Next, cellular senescence in the established pancreatic fibroblasts
was induced. Cells were exposed to 10 Gy X-ray irradiation to
induce DNA damage, and changes were observed over time by every 3
days (up to 9 days) post irradiation. The irradiated fibroblasts
gradually ceased proliferation and exhibited cell and nuclear
enlargement, which are hallmarks of senescent cells, and exhibited
positive SA-β-gal staining (Fig.
4B), which indicated that radiation-induced DNA damage
successfully induced cellular senescence in pancreatic fibroblasts.
To assess the degree of senescence, a dose-gradient approach (3, 6
and 10 Gy irradiation) was applied. SA-β-gal staining was performed
9 days after irradiation. With increasing radiation doses,
fibroblasts appeared flattened and exhibited enhanced SA-β-gal
staining (Fig. 4C). Based on these
temporal and dose-dependent findings, fibroblasts that were
irradiated with 10 Gy and cultured for 9 days were used in
subsequent experiments. The expression levels of p16INK4a, as a
marker of senescence in the fibroblasts, were then evaluated.
Western blotting demonstrated no p16 expression in non-irradiated
cells; however, irradiated fibroblasts showed consistently
increased p16 expression from day 3 onward (Fig. 4D). Regarding the SASP factor IL-6,
western blotting demonstrated a gradual increase in IL-6 protein
expression beginning on day 3 (Fig.
4E), paralleled by a similar significant increase in IL-6 mRNA
levels as confirmed by RT-qPCR (Fig.
4F). Immunofluorescence analysis on day 9 post-irradiation
demonstrated cytoplasmic IL-6 staining in pancreatic fibroblasts
(Fig. 4G). Additionally, the mRNA
expression of other SASP components, including cytokines (IL-1),
chemokines (IL-8), extracellular matrix remodeling factors (MMP-1
and PAI-1) and a growth factor (VEGF) were assessed; these SASP
components were significantly upregulated in irradiated fibroblasts
(Fig. S1). These results suggested
that radiation-induced DNA damage triggered cellular senescence and
promoted SASP factor expression in pancreatic fibroblasts.
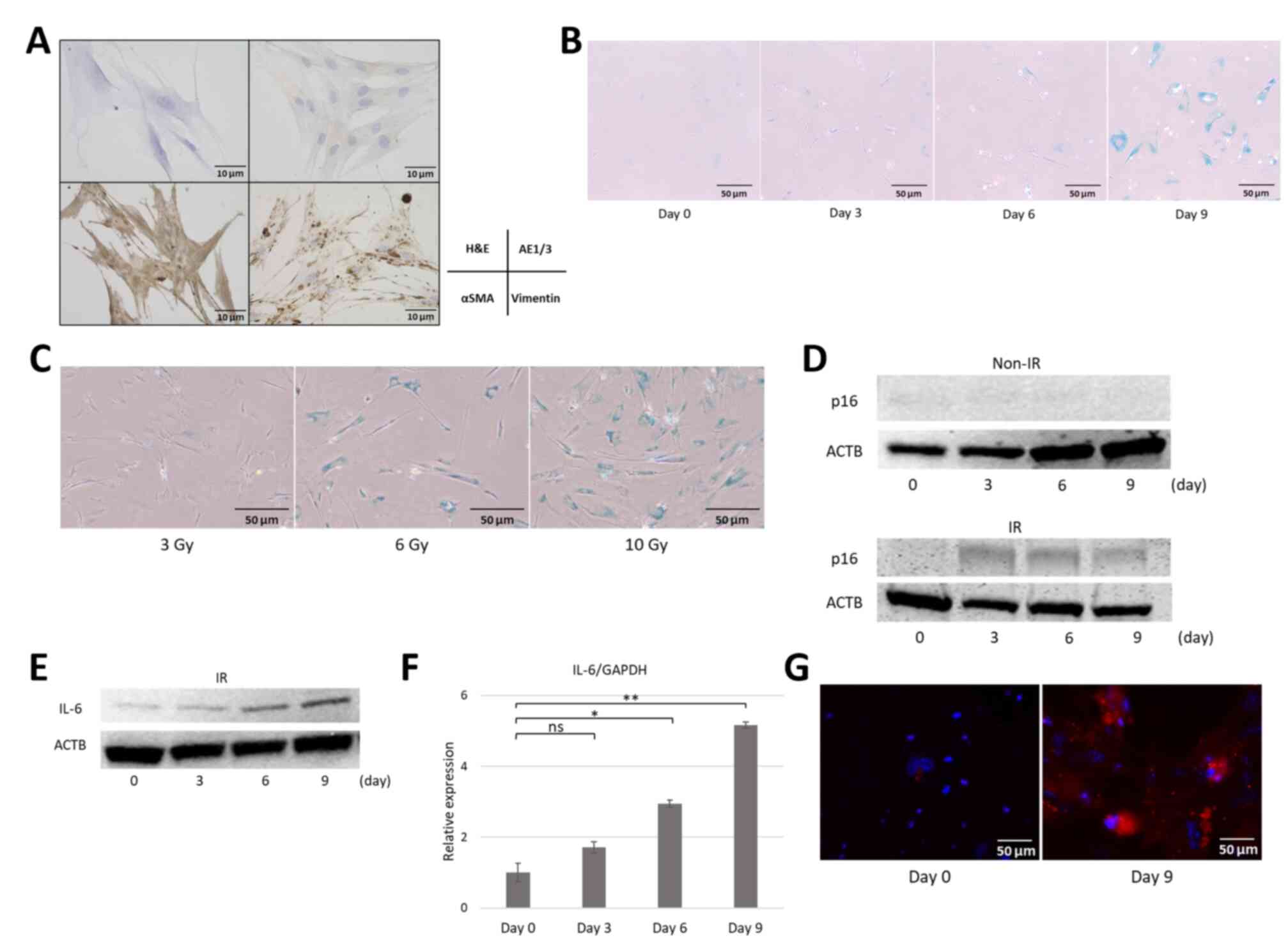 | Figure 4.Establishment of human pancreatic
fibroblast cell lines and induction of cellular senescence and the
senescence-associated secretory phenotype. (A) Pancreatic
fibroblasts established from resected pancreatic specimens. Cells
showed a spindle-shaped morphology with cytoplasmic protrusions
(AE1/3-negative, αSMA- and vimentin-positive; scale bar, 10 µm).
(B) SA-β-gal staining of IR pancreatic fibroblasts (10 Gy)
increased over time (scale bar, 50 µm). (C) SA-β-gal staining of
pancreatic fibroblasts 9 days increased with gradient irradiation
(3, 6 and 10 Gy; scale bar, 50 µm). (D) Western blot analysis of
p16 protein expression in IR pancreatic fibroblasts (10 Gy X-rays
on days 0, 3, 6 and 9) showed increased p16 protein expression from
day 3 onward compared with the non-IR group. (E) Western blot
analysis showed a gradual increase of IL-6 protein expression from
day 3 onwards to day 9 post-irradiation. (F) Reverse
transcription-quantitative PCR confirmed increased IL-6 mRNA
expression after irradiation over 9 days of culture. Data are
presented as mean ± SD (n=6). (G) Immunofluorescence of pancreatic
fibroblasts. IL-6 expression was increased on day 9 (IL-6, red;
DAPI, blue; scale bar, 50 µm). *P<0.01 and **P<0.001. ns, not
significant; H&E, hemoxylin and eosin; SA-β-gal,
senescence-associated β-galactosidase; aSMA, a smooth muscle actin;
ACTB, β-actin; IR, irradiated. |
Invasive, migratory and proliferative
capacities of Panc-1 cells with irradiated human pancreatic
fibroblasts or culture supernatant
The invasive potential of Panc-1 cells co-cultured
with fibroblasts 9 days post-irradiation was evaluated. Panc-1
cells were co-cultured with fibroblasts, and the number of cancer
cells that migrated through the Matrigel and membrane was
quantified. Co-culture with pancreatic fibroblasts significantly
enhanced Panc-1 invasion compared with that of control cells, and
this effect was further amplified by co-culture with irradiated,
SASP-expressing fibroblasts (Fig. 5A
and B).
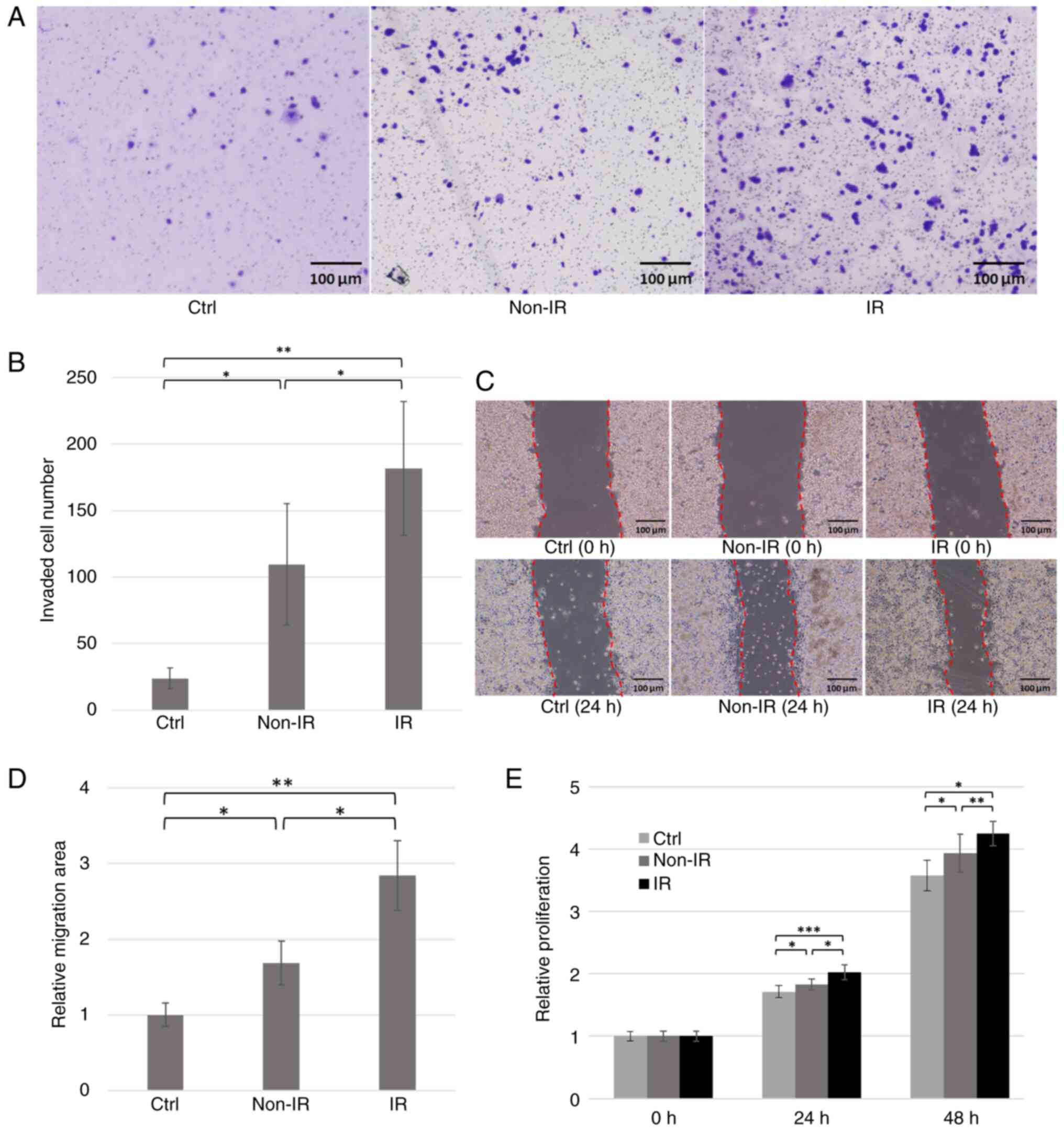 | Figure 5.Co-culture experiments of Panc-1
cells with IR human pancreatic fibroblasts or their culture
supernatants. (A) Representative images of pancreatic fibroblasts
(10 Gy IR or non-IR) that were co-cultured with Panc-1 cells (scale
bar, 100 µm), and (B) the number of cells that invaded through the
gel and membrane was counted (n=6). (C) A confluent monolayer of
Panc-1 cells was scratched with a pipette, and the culture
supernatant of IR or non-IR pancreatic fibroblasts was added (scale
bar, 100 µm). (D) After 12 h, the area of cell migration was
measured (n=6). *P<0.05 and **P<0.01. (E) Panc-1 cells were
cultured with culture supernatants of IR or non-IR pancreatic
fibroblasts, and proliferation was assessed after 24 and 48 h using
the MTT assay (n=8). Data are presented as mean ± SD. *P<0.05,
**P<0.01 and ***P<0.001. aSMA, a smooth muscle actin; Ctrl,
control; IR, irradiated. |
Panc-1 cells were cultured in the conditioned medium
from irradiated or non-irradiated fibroblasts, and their migration
capacity was evaluated. The groups treated with conditioned medium
showed significantly larger migration areas compared with control.
Furthermore, the group treated with conditioned medium from
irradiated, SASP-expressing fibroblasts showed a significantly
larger migration area compared with that of cells treated with
medium from non-irradiated fibroblasts (Fig. 5C and D).
Cell proliferation of Panc-1 cells was assessed
using the MTT assay (n=8). Panc-1 cells cultured in
fibroblast-conditioned medium exhibited significantly higher
proliferation compared with those cultured in standard
serum-containing medium. Furthermore, conditioned medium from
irradiated fibroblasts demonstrated significantly increased Panc-1
proliferation compared with cells treated with either
serum-containing medium or medium from non-irradiated fibroblasts
(Fig. 5E). These findings indicated
that pancreatic fibroblasts expressing the SASP significantly
enhanced the proliferative capacity of pancreatic cancer cells.
Discussion
Research has shown that cells and molecules within
or around the cancer stroma form the cancer microenvironment in
various carcinomas. Furthermore, the fibrous stroma serves an
important role in shaping the cancer microenvironment (13–16).
In particular, cellular senescence of the fibrotic stroma and the
expression of SASP factors associated with cellular senescence have
been reported to significantly impact cancer progression in several
carcinomas (such as hepatocellular carcinoma, ovarian and prostate
cancer) (21–23). In gastrointestinal carcinomas,
pancreatic ductal and diffuse-type gastric cancer have a more
abundant fibrotic stroma than other malignant tumors of the
digestive tract and are known to have poor prognoses (24,25).
Certain underlying health conditions and lifestyle
factors are risk factors for pancreatic cancer, including diabetes,
chronic pancreatitis, hereditary pancreatitis, obesity, smoking and
heavy drinking (36–39). These are also risk factors for
chronic inflammation, whereas obesity, smoking and alcohol
consumption are known risk factors for other types of malignancies
(40–43). This suggests that systemic and/or
local chronic inflammation in the pancreas caused by underlying
diseases and/or lifestyle factors induces cellular senescence and
SASP in the pancreatic stroma, and that oxidative stress, DNA
damage and oncogene activation occur in the pancreatic stroma
before pancreatic cancer develops. After carcinogenesis, various
cytokines, chemokines and reactive oxygen species produced by
cancer cells interact with the surrounding stroma, inducing further
fibroblast production and forming a desmoplastic microenvironment
(13,17).
The status of lymph node metastasis is widely
recognized as an important prognostic factor for pancreatic cancer
and is reflected in the TNM classification of cancer staging
(27,44). In the present study, multivariate
analysis using the Cox proportional hazards model indicated that
lymph node metastasis and high IL-6 expression were significant
prognostic factors. The presence of fibroblasts positive for p16
and IL-6, representative SASP factors, was confirmed in the stroma
of resected PDAC specimens. These results suggested that senescent
fibroblasts exist in the pancreatic cancer stroma and express SASP
factors. The expression intensity of IL-6-positive cells in the
pancreatic cancer stroma was calculated using H-score analysis, and
a cut-off value of 179 was defined using an ROC curve. Based on
this cut-off value, it was demonstrated that the OS of patients in
the high IL-6 expression group was significantly shorter compared
with that in the low IL-6 expression group. In the present study,
consecutive patients with PDAC who underwent surgery were included.
As multidisciplinary treatment is the standard for pancreatic
cancer, it could be considered that the inclusion of numerous
patients who received preoperative chemotherapy (66%) may reflect
the current status of pancreatic cancer treatment. Although it was
considered that advanced age and preoperative chemotherapy may
directly contribute to pancreatic stromal senescence, subgroup
analysis demonstrated that IL-6 expression in PDAC stromal
fibroblasts was not associated with any patient demographics and
clinicopathological characteristics. Since pancreatic
carcinogenesis is suggested to occur against a background of
chronic inflammation (36–39), it was considered that cellular
senescence of the pancreatic stroma begins at the
pre-carcinogenesis stage. Although stromal depletion after
preoperative chemotherapy has been reported (19), the present study found no evidence
that preoperative chemotherapy directly induces further senescence
in the cancer stroma. In summary, high expression intensity of IL-6
in the pancreatic cancer stromal fibroblasts was significantly
associated with shorter survival after pancreatic cancer surgery.
The presence of senescent fibroblasts and expression of SASP
factors in the pancreatic cancer stroma may potentially serve as
prognostic factors for pancreatic cancer.
The present in vitro studies demonstrated the
successful establishment of pancreatic fibroblasts and confirmed
the induction of cellular senescence and the expression of SASP
factors in these fibroblasts following DNA damage by irradiation.
Co-culture of pancreatic fibroblasts expressing SASP factors with
pancreatic cancer cells revealed that the invasive, migratory and
proliferative capacities of the cancer cells were significantly
enhanced. The results of the in vitro analyses suggest that
a microenvironment promoting cancer growth and spread is formed in
pancreatic tissues subjected to X-ray irradiation, mimicking stress
or DNA damage caused by chronic inflammation. Most forms of
cellular senescence, such as replicative exhaustion,
therapy-induced senescence or expression of oncogenic RAS, are
associated with unrepaired DNA damage, similar to that caused by
X-rays, and the choice of senescence inducer has been shown to have
little effect on the senescent phenotype (8). Based on this finding, X ray
irradiation was employed in the present study. To the best of our
knowledge, no basic research on pancreatic cancer, a typical
refractory solid tumor, has focused on cellular senescence and SASP
in cancer stromal fibroblasts to clarify their effects on the tumor
microenvironment and the promotion of cancer progression.
Numerous reports have demonstrated the role of CAFs
in the tumor microenvironment. Several CAF markers have been
documented, with the most commonly used including fibroblast
activation protein α (FAP), αSMA and podoplanin (13–16).
In the present study, increased FAP expression levels were observed
in irradiated pancreatic fibroblasts (data not shown), which
exhibited cellular senescence and the SASP. However, the expression
of CAF markers is highly heterogeneous and varies significantly
among CAF subpopulations (45).
Furthermore, identifying CAFs remains challenging, and further
investigation is needed to standardize CAF identification across
studies. A limitation of the present study is that the overlap
between senescent fibroblasts and CAFs was not assessed; therefore,
it could not be determined whether they represent the same or
distinct cell populations. Nevertheless, it remains possible that
some senescent fibroblasts exhibiting SASP may function as CAFs
within the tumor microenvironment to promote invasion and
metastasis.
Cellular senescence is characterized by irreversible
cell cycle arrest. Representative protein markers include p16INK4a,
p21 (Waf1/Clip1/Sdi1), p53 and Rb (3). Activation of p53 directly induces
apoptosis and enhances the expression of p21, a cyclin-dependent
kinase (CDK) inhibitor; by inhibiting CDKs, p21 activates Rb and
suppresses the function of E2F, which regulates the transcription
of genes that promote cell proliferation (3). E2F is responsible for the
G1-to-S phase transition of the cell cycle; its
inhibition arrests cells in the G1 phase (3). In parallel, p16INK4a inhibits CDK4/6,
which also serves a role in G1 progression. During
cellular senescence, Rb remains constantly activated through these
two CDK-inhibitory pathways. Although p21 and p16INK4a alone weakly
activate Rb, their combined action robustly maintains Rb activation
and arrests cell cycle progression (46). p16INK4a expression is extremely low
in cells with normal proliferative potential and is nearly
undetectable. However, its expression markedly increases when
normal cells reach their mitotic limit or experience oncogenic
stress, thereby inducing senescence (34,35).
Therefore, p16INK4a is a key marker of cellular senescence.
The SASP comprises multiple factors, including IL-6,
which may act synergistically within the cancer microenvironment.
IL-6, a pleiotropic proinflammatory cytokine, has been shown to
promote senescence and influence tumor formation (47,48).
IL-6 is associated with DNA damage (and tumorigenic stress) induced
senescence in keratinocytes, melanocytes, monocytes, fibroblasts
and epithelial cells (47,49–51).
According to previous reports, IL-6 affects tumor cells and the
surrounding microenvironment by upregulating various genes (such as
granulocyte-macrophage colony-stimulating factor, ILs, matrix
metalloproteinase-2, nuclear factor erythroid 2-related factor 2,
receptor for advanced glycation end-products and VEGF) and
activating multiple tumor-promoting processes, such as angiogenesis
and metastasis (52). The IL-6
signaling pathway is considered a key contributor to pancreatic
cancer development. Through IL-6 expression, senescent fibroblasts
can influence nearby cells expressing the IL-6 receptor (gp80) and
gp130 signaling complex, such as epithelial and endothelial cells
(8). IL-6 binding to its receptor
activates Janus kinase 2 (JAK2), which transmits signals to the
downstream STAT3 protein. STAT3 promotes pancreatic cancer
progression by inducing genes such as cyclin D1 and Bcl-2,
upregulating VEGF, promoting epithelial-mesenchymal transition and
inhibiting dendritic cell-mediated immune responses (53). STAT3 is considered an oncogene owing
to its widespread activation in cancer. In addition to JAK-STAT3,
IL-6 also activates the Ras-mitogen-activated protein kinase (MAPK)
and phosphoinositide-3-kinase (PI3K)-Akt pathways, which contribute
to anti-apoptotic and tumorigenic effects (54). In various malignancies, including
colorectal, renal, prostate, breast and ovarian cancer, as well as
multiple myeloma and non-small cell lung cancer, increased serum
IL-6 concentrations have been associated with advanced tumor stage
and poor survival (52). In the
present study, it was found that senescent pancreatic fibroblasts
induced by X-ray irradiation exhibited simultaneous upregulation of
multiple mRNA transcripts, including IL-6. A key limitation of the
present study is the lack of a systematic evaluation of IL-6 in
relation to other critical factors. This limitation arises from the
inherent constraints of in vitro systems in recapitulating
the complex tumor microenvironment, which is shaped by the dynamic
interplay of various cell types and molecular signals. Furthermore,
the SASP encompasses a broad array of factors, making it difficult
to comprehensively assess SASP function. However, given that IL-6
signaling is widely recognized as a major contributor to pancreatic
cancer progression, the present study focused on IL-6 as the most
representative SASP factor. Future research on cellular senescence
within the tumor microenvironment will enable a more complete
evaluation of SASP function. Specifically, these include spatial
gene expression analysis to visualize gene expression in specific
regions of biological tissue and investigate the localization of
SASP factors and the microenvironment in which SASP factors are
released, as well as biological approaches such as creating animal
models with specific SASP factors deleted to identify their
functions and examine their effects on senescence and disease
within the organism.
A previous report established a mouse model
(p16-CreERT2-DTR-tdTomato) that enables the removal of p16-positive
cells, showing positive results (55). In that study, non-alcoholic
steatohepatitis (NASH) was induced by feeding mice a
choline-deficient L-amino acid high-fat diet for 6 weeks. Removal
of p16-positive cells during the final 3 weeks improved hepatic
steatosis and inflammation. These findings indicate that NASH
progression may be suppressed by eliminating p16-positive cells.
Senescent cells have an apoptosis resistance mechanism similar to
that of cancer cells, including high expression of the
anti-apoptotic Bcl-2 family proteins. Based on the concept that
senescent cells can be selectively eliminated by chemotherapy,
various drugs have been screened, and the development of senolytic
agents that specifically target senescent cells is advancing.
Dasatinib plus quercetin, the first senolytic drug, has progressed
to clinical trials for multiple diseases and has shown efficacy
(56,57). In addition, studies have reported
that the removal of senescent cells from atherosclerotic plaques
(58) and the central nervous
system (59) in mice suppresses the
progression of age-related diseases. Other reports demonstrate the
effectiveness of senolytic drugs via p53 activation, which promotes
apoptosis in senescent cells (60,61).
Most of these studies have targeted age-related diseases and
excluded malignant tumors. Presently, no senolytic drugs have shown
statistically significant effects in clinical trials involving
living organisms. However, in the future, new therapies targeting
malignant tumors by removing senescent cells may be developed.
Senescent fibroblasts in the cancer stroma produce
various substances, such as SASP factors, that influence cancer
progression. The present study demonstrated the presence of
senescent fibroblasts in the pancreatic cancer stroma, with a
positive correlation between p16 and IL-6 expression in
fibroblasts. Furthermore, IL-6 expression in pancreatic cancer
stromal fibroblasts was identified as a significant prognostic
factor, along with lymph node metastasis, following pancreatic
cancer surgery. Therefore, evaluating the presence of senescent
cells and the expression of SASP factors in the pancreatic cancer
stroma may be useful for predicting postoperative prognosis in
patients with pancreatic cancer in the future.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Yasuyo Futakuchi
and Ms. Kyoko Yoshida, the Medical Technologist of Gastrointestinal
Surgery, Kanazawa University, who provided technical support for
the experiments and made significant contribution.
Funding
The present study was supported by the JSPS KAKENHI (grant no.
JP23K08163).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YK, TM, YY, NI and SY designed and conceived the
study. YK, TM and TS analyzed and interpreted the results and
drafted the manuscript. TM and YY supervised this study. YK and IM
collected the data. YE contributed to the establishment of
pancreatic fibroblasts. TS, YY, NI and SY edited and revised the
manuscript critically for intellectual content. All the authors
have read and approved the final version of the manuscript. YK and
TM confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study followed the provisions of the
Declaration of Helsinki and was approved by the Ethical Review
Committee of Kanazawa University Hospital (approval no. 2016-318).
Written informed consent was obtained from all participants for the
anonymous use of resected specimens and clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
YK, 0000-0003-1941-9074; TM, 0000-0002-3771-0773;
TS, 0009-0001-5490-8271; IM, 0000-0001-6023-7163; YE,
0000-0003-0785-2006; YY, 0000-0003-1326-4649; NI,
0000-0002-4241-5015; SY, 0000-0001-7465-5761.
References
|
1
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Campisi J and d'Adda di Fagagna F:
Cellular senescence: When bad things happen to good cells. Nat Rev
Mol Cell Biol. 8:729–740. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohtani N, Mann DJ and Hara E: Cellular
senescence: Its role in tumor suppression and aging. Cancer Sci.
100:792–797. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:9363–9367.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harper JW and Elledge SJ: The DNA damage
response: Ten years after. Mol Cell. 28:739–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mallette FA and Ferbeyre G: The DNA damage
signaling pathway connects oncogenic stress to cellular senescence.
Cell Cycle. 6:1831–1836. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tchkonia T, Zhu Y, van Deursen J, Campisi
J and Kirkland JL: Cellular senescence and the senescent secretory
phenotype: Therapeutic opportunities. J Clin Invest. 123:966–972.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muñoz-Espín D, Cañamero M, Maraver A,
Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A,
Varela-Nieto I, Ruberte J, Collado M and Serrano M: Programmed cell
senescence during mammalian embryonic development. Cell.
155:1104–1118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demaria M, Ohtani N, Youssef SA, Rodier F,
Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé
ME, et al: An essential role for senescent cells in optimal wound
healing through secretion of PDGF-AA. Dev Cell. 31:722–733. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laberge RM, Awad P, Campisi J and Desprez
PY: Epithelial-mesenchymal transition induced by senescent
fibroblasts. Cancer Microenviron. 5:39–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Von Ahrens D, Bhagat TD, Nagrath D, Maitra
A and Verma A: The role of stromal cancer-associated fibroblasts in
pancreatic cancer. J Hematol Oncol. 10:762017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawase T, Yasui Y, Nishina S, Hara Y,
Yanatori I, Tomiyama Y, Nakashima Y, Yoshida K, Kishi F, Nakamura M
and Hino K: Fibroblast activation protein-α-expressing fibroblasts
promote the progression of pancreatic ductal adenocarcinoma. BMC
Gastroenterol. 15:1092015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabola R, Zaremba-Czogalla M, Baczynska D,
Cirocchi R, Stach K, Grabowski K and Augoff K: Fibroblast
activating protein-α expression in squamous cell carcinoma of the
esophagus in primary and irradiated tumors: The use of archival
FFPE material for molecular techniques. Eur J Histochem.
61:27932017.PubMed/NCBI
|
|
16
|
Shindo K, Aishima S, Ohuchida K, Fujiwara
K, Fujino M, Mizuuchi Y, Hattori M, Mizumoto K, Tanaka M and Oda Y:
Podoplanin expression in cancer-associated fibroblasts enhances
tumor progression of invasive ductal carcinoma of the pancreas. Mol
Cancer. 12:1682013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su S, Chen J, Yao H, Liu J, Yu S, Lao L,
Wang M, Luo M, Xing Y, Chen F, et al: CD10+GPR77+ Cancer-associated
fibroblasts promote cancer formation and chemoresistance by
sustaining cancer stemness. Cell. 172:841–856.e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paulsson J, Rydén L, Strell C, Frings O,
Tobin NP, Fornander T, Bergh J, Landberg G, Stål O and Östman A:
High expression of stromal PDGFRβ is associated with reduced
benefit of tamoxifen in breast cancer. J Pathol Clin Res. 3:38–43.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyashita T, Tajima H, Makino I, Okazaki
M, Yamaguchi T, Ohbatake Y, Nakanuma S, Hayashi H, Takamura H,
Ninomiya I, et al: Neoadjuvant chemotherapy with gemcitabine plus
nab-paclitaxel reduces the number of cancer-associated fibroblasts
through depletion of pancreatic stroma. Anticancer Res. 38:337–343.
2018.PubMed/NCBI
|
|
20
|
Miyashita T, Tajima H, Gabata R, Okazaki
M, Shimbashi H, Ohbatake Y, Okamoto K, Nakanuma S, Sakai S, Makino
I, et al: Impact of extravasated platelet activation and
podoplanin-positive cancer-associated fibroblasts in pancreatic
cancer stroma. Anticancer Res. 39:5565–5572. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JS, Yoo JE, Kim H, Rhee H, Koh MJ,
Nahm JH, Choi JS, Lee KH and Park YN: Tumor stroma with
senescence-associated secretory phenotype in steatohepatitic
hepatocellular carcinoma. PLoS One. 12:e01719222017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harper EI, Sheedy EF and Stack MS: With
great age comes great metastatic ability: Ovarian cancer and the
appeal of the aging peritoneal microenvironment. Cancers (Basel).
10:2302018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mori JO, Elhussin I, Brennen WN, Graham
MK, Lotan TL, Yates CC, De Marzo AM, Denmeade SR, Yegnasubramanian
S, Nelson WG, et al: Prognostic and therapeutic potential of
senescent stromal fibroblasts in prostate cancer. Nat Rev Urol.
21:258–273. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakayama I: Therapeutic strategy for
scirrhous type gastric cancer. Jpn J Clin Oncol. 55:860–870. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto Y, Kasashima H, Fukui Y, Tsujio
G, Yashiro M and Maeda K: The heterogeneity of cancer-associated
fibroblast subpopulations: Their origins, biomarkers, and roles in
the tumor microenvironment. Cancer Sci. 114:16–24. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ministry of Education Culture Sports
Science Technology Ministry of Health Labour Welfare, Ministry of
Economy, TradeIndustry, . Ethical guidelines for medical and
biological research involving human subjects (In Japanese).
https://www.mhlw.go.jp/content/000757566.pdf2021.
|
|
27
|
Brierley JD, Gospodarowicz MK and
Wittekind C: Union for International Cancer Control (UICC). TNM
Classification of Malignant Tumors. 8th Edition. Wiley-Blackwell;
2017
|
|
28
|
Tsuta K, Wistuba II and Moran CA:
Differential expression of somatostatin receptors 1–5 in
neuroendocrine carcinoma of the lung. Pathol Res Pract.
208:470–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmid HA, Lambertini C, van Vugt HH,
Barzaghi-Rinaudo P, Schäfer J, Hillenbrand R, Sailer AW, Kaufmann M
and Nuciforo P: Monoclonal antibodies against the human
somatostatin receptor subtypes 1–5: Development and
immunohistochemical application in neuroendocrine tumors.
Neuroendocrinology. 95:232–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roncoroni L, Elli L, Doneda L, Piodi L,
Ciulla MM, Paliotti R and Bardella MT: Isolation and culture of
fibroblasts from endoscopic duodenal biopsies of celiac patients. J
Transl Med. 7:402009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seluanov A, Vaidya A and Gorbunova V:
Establishing primary adult fibroblast cultures from rodents. J Vis
Exp. 5:20332010.
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rasband WS: ImageJ. U.S. National
Institutes of Health. (Bethesda, MD). 2011.http://imagej.nih.gov/ij
|
|
34
|
Hara E, Smith R, Parry D, Tahara H, Stone
S and Peters G: Regulation of p16CDKN2 expression and its
implications for cell immortalization and senescence. Mol Cell
Biol. 16:859–867. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Serrano M, Lin AW, McCurrach ME, Beach D
and Lowe SW: Oncogenic ras provokes premature cell senescence
associated with accumulation of p53 and p16INK4a. Cell. 88:593–602.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Raimondi S, Lowenfels AB, Morselli-Labate
AM, Maisonneuve P and Pezzilli R: Pancreatic cancer in chronic
pancreatitis; aetiology, incidence, and early detection. Best Pract
Res Clin Gastroenterol. 24:349–358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiu D, Kurosawa M, Lin Y, Inaba Y, Matsuba
T, Kikuchi S, Yagyu K, Motohashi Y and Tamakoshi A; JACC Study
Group, : Overview of the epidemiology of pancreatic cancer focusing
on the JACC study. J Epidemiol. 15 (Suppl II):S157–S167. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iodice S, Gandini S, Maisonneuve P and
Lowenfels AB: Tobacco and the risk of pancreatic cancer: A review
and meta-analysis. Langenbecks Arch Surg. 393:535–545. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tramacere I, Scotti L, Jenab M, Bagnardi
V, Bellocco R, Rota M, Corrao G, Bravi F, Boffetta P and La Vecchia
C: Alcohol drinking and pancreatic cancer risk: A meta-analysis of
the dose-risk relation. Int J Cancer. 126:1474–1486. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gosavi R, Jaya J, Yap R, Narasimhan V and
Ooi G: Obesity and gastrointestinal cancer: A converging epidemic
with surgical consequence. Eur J Surg Oncol. 51:1103582025.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cuomo RE: Distinct somatic mutation
profiles in colon cancer by behavioral comorbidity. Future Sci OA.
11:25615012025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Qiu K, Zhou S, Gan Y, Jiang K,
Wang D and Wang H: Risk factors for hepatocellular carcinoma: An
umbrella review of systematic review and meta-analysis. Ann Med.
57:24555392025. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ni L, Liu Z, Xiang L, Li Y and Zhang Y:
Comprehensive evaluation of risk factors for LNM and distant
metastasis in patients with NSCLC. Sci Rep. 15:308092025.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Min SK, You Y, Choi DW, Han IW, Shin SH,
Yoon S, Jung JH, Yoon SJ and Heo JS: Prognosis of pancreatic head
cancer with different patterns of lymph node metastasis. J
Hepatobiliary Pancreat Sci. 29:1004–1013. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nurmik M, Ullmann P, Rodriguez F, Haan S
and Letellier E: In search of definitions: Cancer-associated
fibroblasts and their markers. Int J Cancer. 146:895–905. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ohtani N, Imamura Y, Yamakoshi K, Hirota
F, Nakayama R, Kubo Y, Ishimaru N, Takahashi A, Hirao A, Shimizu T,
et al: Visualizing the dynamics of p21(Waf1/Cip1) cyclin-dependent
kinase inhibitor expression in living animals. Proc Natl Acad Sci
USA. 104:15034–15039. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kuilman T, Michaloglou C, Vredeveld LC,
Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ and Peeper DS:
Oncogene-induced senescence relayed by an interleukin-dependent
inflammatory network. Cell. 133:1019–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ancrile B, Lim KH and Counter CM:
Oncogenic Ras-induced secretion of IL6 is required for
tumorigenesis. Genes Dev. 21:1714–1719. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Coppé JP, Patil CK, Rodier F, Sun Y, Munoz
DP, Goldstein J, Nelson PS, Desprez PY and Campisi J:
Senescence-associated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS Biol. 6:2853–2868. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lu SY, Chang KW, Liu CJ, Tseng YH, Lu HH,
Lee SY and Lin SC: Ripe areca nut extract induces G1 phase arrests
and senescence-associated phenotypes in normal human oral
keratinocyte. Carcinogenesis. 27:1273–1284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sarkar D, Lebedeva IV, Emdad L, Kang DC,
Baldwin AS Jr and Fisher PB: Human polynucleotide phosphorylase
(hPNPaseold-35): A potential link between aging and inflammation.
Cancer Res. 64:7473–7478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Holmer R, Goumas FA, Waetzig GH, Rose-John
S and Kalthoff H: Interleukin-6: A villain in the drama of
pancreatic cancer development and progression. Hepatobiliary
Pancreat Dis Int. 13:371–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Song M, Tang Y, Cao K, Qi L and Xie K:
Unveiling the role of interleukin-6 in pancreatic cancer occurrence
and progression. Front Endocrinol (Lausanne). 15:14083122024.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ara T and Declerck YA: Interleukin-6 in
bone metastasis and cancer progression. Eur J Cancer. 46:1223–1231.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Omori S, Wang TW, Johmura Y, Kanai T,
Nakano Y, Kido T, Susaki EA, Nakajima T, Shichino S, Ueha S, et al:
Generation of a p16 reporter mouse and its use to characterize and
target p16high cells in vivo. Cell Metab. 32:814–828.e6.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hickson LJ, Langhi Prata LGP, Bobart SA,
Evans TK, Giorgadze N, Hashmi SK, Herrmann SM, Jensen MD, Jia Q,
Jordan KL, et al: Senolytics decrease senescent cells in humans:
Preliminary report from a clinical trial of dasatinib plus
quercetin in individuals with diabetic kidney disease.
EBiomedicine. 47:446–456. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Farr JN, Atkinson EJ, Achenbach SJ,
Volkman TL, Tweed AJ, Vos SJ, Ruan M, Sfeir J, Drake MT, Saul D, et
al: Effects of intermittent senolytic therapy on bone metabolism in
postmenopausal women: A phase 2 randomized controlled trial. Nat
Med. 30:2605–2612. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bussian TJ, Aziz A, Meyer CF, Swenson BL,
van Deursen JM and Baker DJ: Clearance of senescent glial cells
prevents tau-dependent pathology and cognitive decline. Nature.
562:578–582. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Childs BG, Baker DJ, Wijshake T, Conover
CA, Campisi J and van Deursen JM: Senescent intimal foam cells are
deleterious at all stages of atherosclerosis. Science. 354:472–477.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Paez-Ribes M, González-Gualda E, Doherty
GJ and Muñoz-Espín D: Targeting senescent cells in translational
medicine. EMBO Mol Med. 11:e102342019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Baar MP, Brandt RMC, Putavet DA, Klein
JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van
Willigenburg H, Feijtel DA, et al: Targeted apoptosis of senescent
cells restores tissue homeostasis in response to chemotoxicity and
aging. Cell. 169:132–147.e16. 2017. View Article : Google Scholar : PubMed/NCBI
|