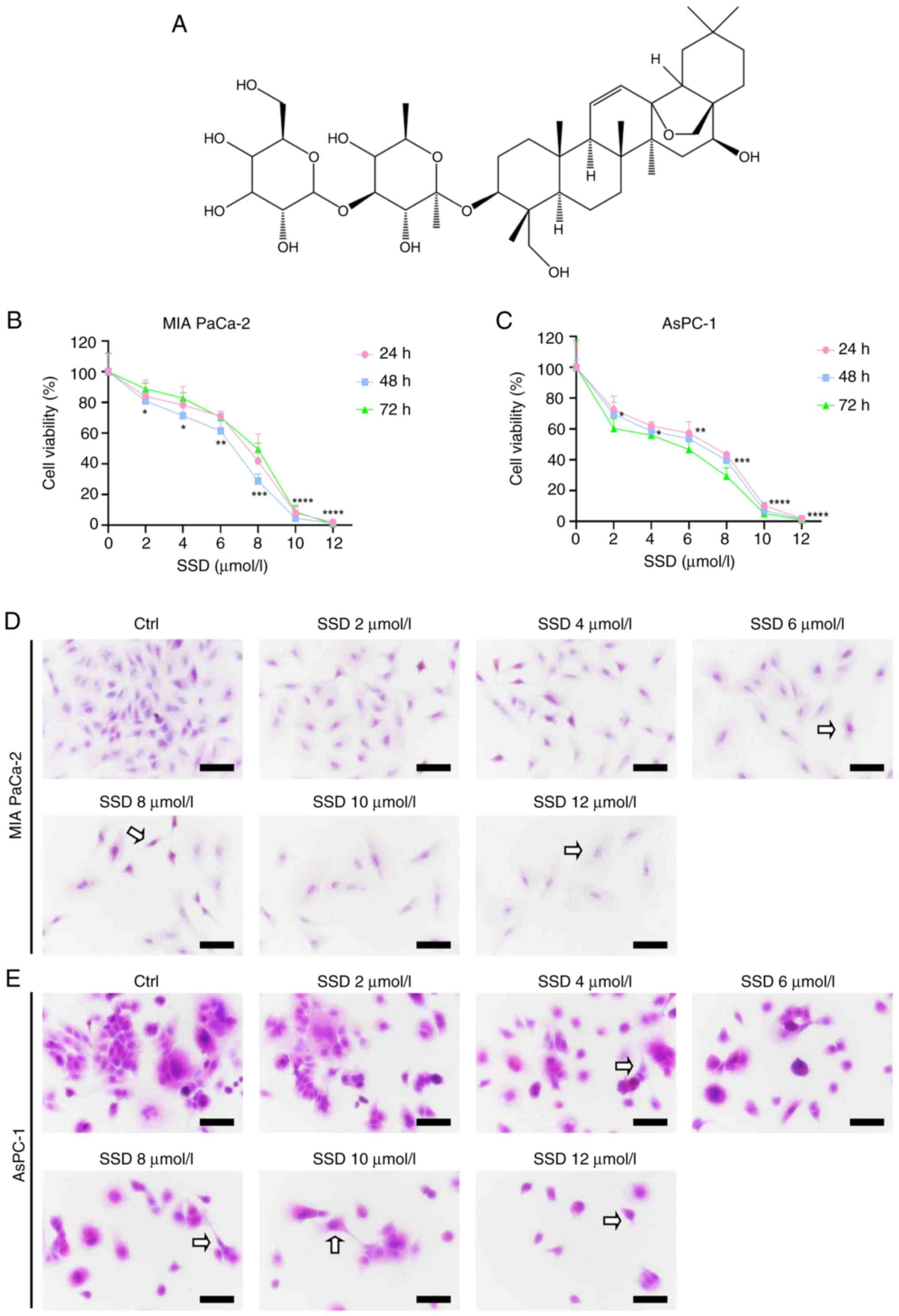
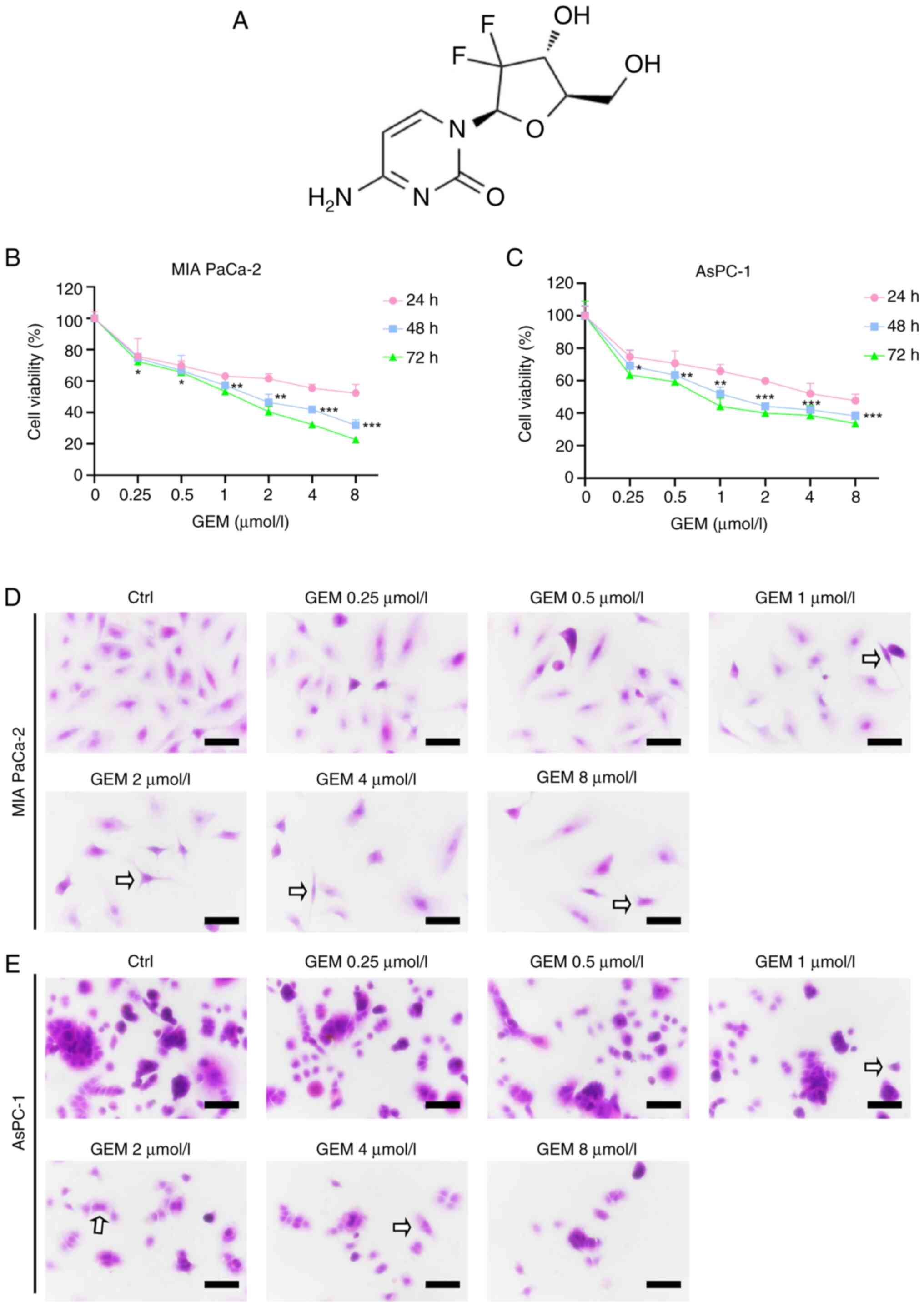
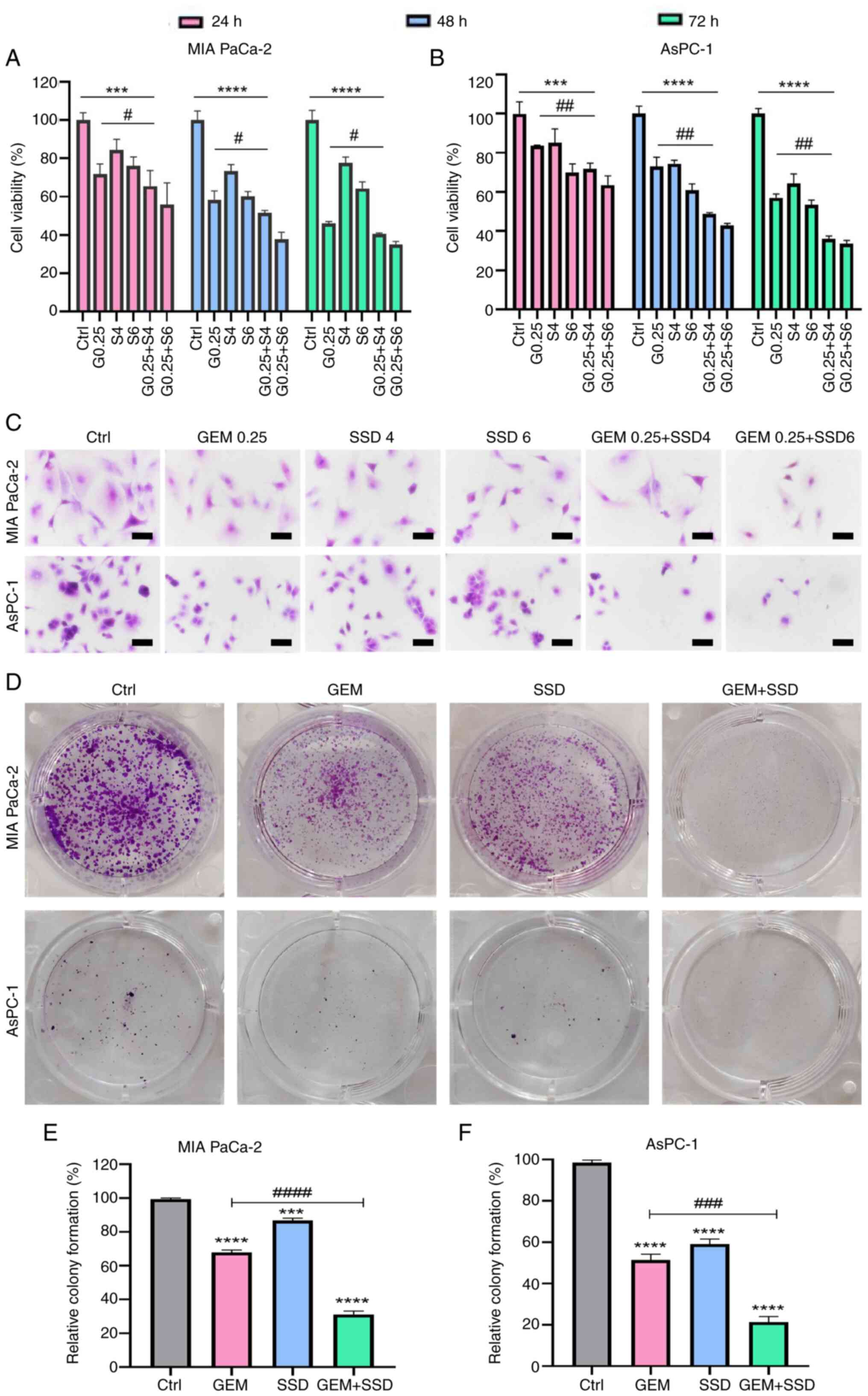
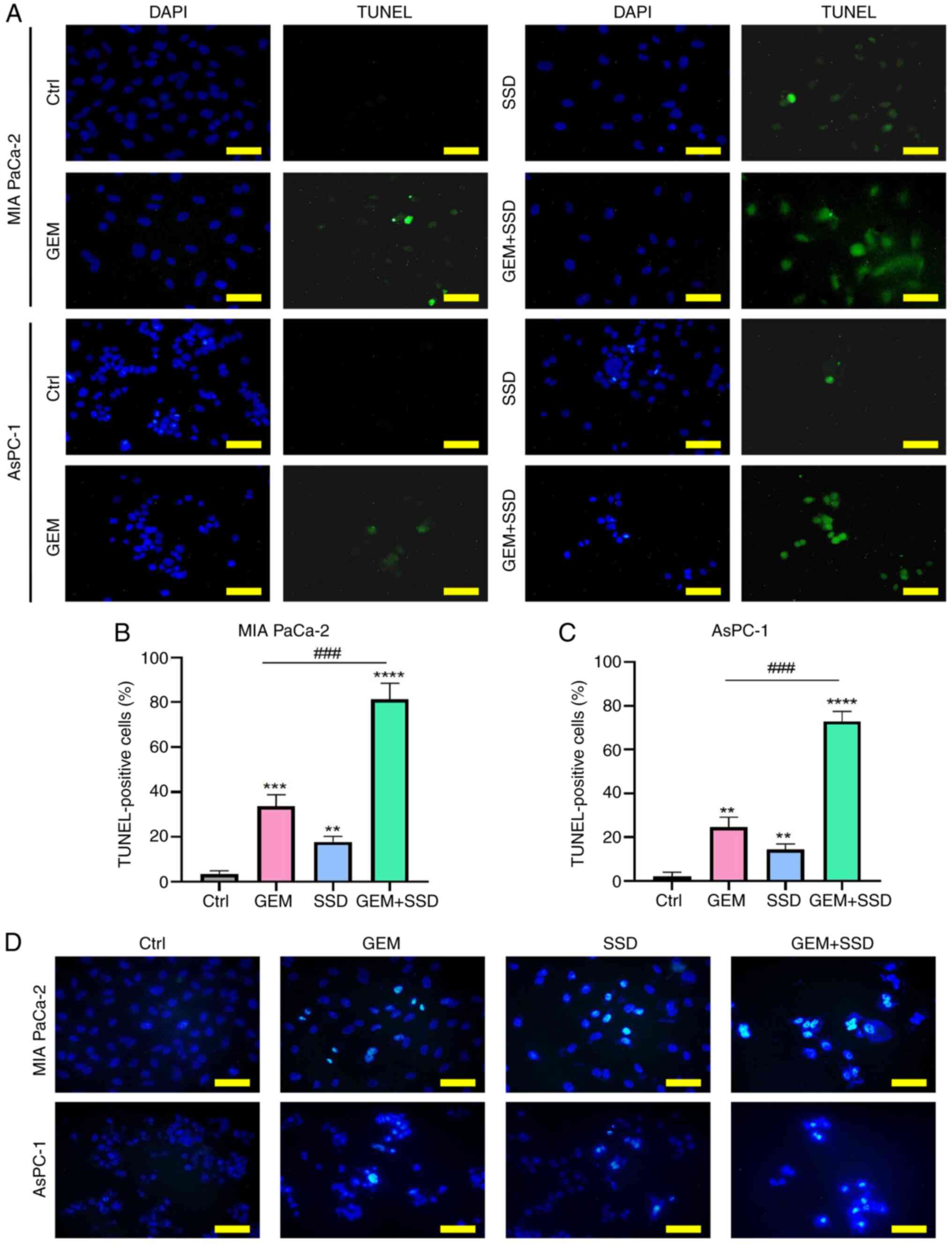
|
1
|
Cai J, Chen H, Lu M, Zhang Y, Lu B, You L,
Zhang T, Dai M and Zhao Y: Advances in the epidemiology of
pancreatic cancer: Trends, risk factors, screening, and prognosis.
Cancer Lett. 520:1–11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klatte DCF, Wallace MB, Löhr M, Bruno MJ
and van Leerdam ME: Hereditary pancreatic cancer. Best Pract Res
Clin Gastroenterol. 58-59:1017832022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Domagała-Haduch M, Gorzelak-Magiera A,
Michalecki Ł and Gisterek-Grocholska I: Radiochemotherapy in
pancreatic cancer. Curr Oncol. 31:3291–3300. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brozos-Vázquez E, Toledano-Fonseca M,
Costa-Fraga N, García-Ortiz MV, Díaz-Lagares Á, Rodríguez-Ariza A,
Aranda E and López-López R: Pancreatic cancer biomarkers: A pathway
to advance in personalized treatment selection. Cancer Treat Rev.
125:1027192024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miao H, Chen X and Luan Y: Small molecular
gemcitabine prodrugs for cancer therapy. Curr Med Chem.
27:5562–5582. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pandit B and Royzen M: Recent development
of prodrugs of gemcitabine. Genes (Basel). 13:4662022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cui J, Guo Y, Yin T, Gou S, Xiong J, Liang
X, Lu C and Peng T: USP8 promotes gemcitabine resistance of
pancreatic cancer via deubiquitinating and stabilizing Nrf2. Biomed
Pharmacother. 166:1153592023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shu X, Zhan PP, Sun LX, Yu L, Liu J, Sun
LC, Yang ZH, Ran YL and Sun YM: BCAT1 activates PI3K/AKT/mTOR
pathway and contributes to the angiogenesis and tumorigenicity of
gastric cancer. Front Cell Dev Biol. 9:6592602021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu L, Wei J and Liu P: Attacking the
PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment
in human cancer. Semin Cancer Biol. 85:69–94. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rahaman A, Chaudhuri A, Sarkar A,
Chakraborty S, Bhattacharjee S and Mandal DP: Eucalyptol targets
PI3K/Akt/mTOR pathway to inhibit skin cancer metastasis.
Carcinogenesis. 43:571–583. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou X, Zhao J, Yan T, Ye D, Wang Y, Zhou
B, Liu D, Wang X, Zheng W, Zheng B, et al: ANXA9 facilitates S100A4
and promotes breast cancer progression through modulating STAT3
pathway. Cell Death Dis. 15:2602024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Q, Guan Y, Zheng J and Lu H: TBK1
promotes thyroid cancer progress by activating the PI3K/Akt/mTOR
signaling pathway. Immun Inflamm Dis. 11:e7962023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang S, Cheng Z, Cui Y, Xu S, Luan Q, Jing
S, Du B, Li X and Li Y: PTPRH promotes the progression of non-small
cell lung cancer via glycolysis mediated by the PI3K/AKT/mTOR
signaling pathway. J Transl Med. 21:8192023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang S, Zhang C, Xu Z, Chen MH, Yu H, Wang
L and Liu R: Differential impact of PI3K/AKT/mTOR signaling on
tumor initiation and progression in animal models of prostate
cancer. Prostate. 83:97–108. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mortazavi M, Moosavi F, Martini M,
Giovannetti E and Firuzi O: Prospects of targeting PI3K/AKT/mTOR
pathway in pancreatic cancer. Crit Rev Oncol Hematol.
176:1037492022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang L, Sun J, Ma Y, Chen H, Tian C and
Dong M: MSI2 regulates NLK-mediated EMT and PI3K/AKT/mTOR pathway
to promote pancreatic cancer progression. Cancer Cell Int.
24:2732024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimia M, Amini M, Ravari AO, Tabnak P,
Valizadeh A, Ghaheri M and Yousefi B: Thymoquinone reversed
doxorubicin resistance in U87 glioblastoma cells via targeting
PI3K/Akt/mTOR signaling. Chem Biol Drug Des. 104:e145872024.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M, Xue W, Yuan H, Wang Z and Yu L:
Nano-drug delivery systems targeting CAFs: A promising treatment
for pancreatic cancer. Int J Nanomedicine. 19:2823–2849. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Xu H, Li Y, Sun Y and Peng X:
Advances in the treatment of pancreatic cancer with traditional
Chinese medicine. Front Pharmacol. 14:10892452023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manoharan S, Deivendran B and Perumal E:
Chemotherapeutic potential of saikosaponin D: Experimental
evidence. J Xenobiot. 12:378–405. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu G, Guan Y, Liu Y, Wang Y, Zhang J, Liu
Y and Liu X: Saikosaponin D inducing apoptosis and autophagy
through the activation of endoplasmic reticulum stress in
glioblastoma. Biomed Res Int. 2022:54895532022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang JC, Long F, Zhao J, Hang J, Ren YG,
Chen JY and Mu B: The effects and mechanisms by which
saikosaponin-D enhances the sensitivity of human non-small cell
lung cancer cells to gefitinib. J Cancer. 10:6666–6672. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu J, Li P, Shi B and Tie J: Effects and
mechanisms of saikosaponin d improving the sensitivity of human
gastric cancer cells to cisplatin. ACS Omega. 6:18745–18755. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang CY, Jiang ZM, Ma XF, Li Y, Liu XZ,
Li LL, Wu WH and Wang T: Saikosaponin-d inhibits the hepatoma cells
and enhances chemosensitivity through SENP5-dependent inhibition of
gli1 sumoylation under hypoxia. Front Pharmacol. 10:10392019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang J, Sun J, Liu A, Chen L, Ma X, Liu X
and Zhang C: Saikosaponin D improves chemosensitivity of
glioblastoma by reducing the its stemness maintenance. Biochem
Biophys Rep. 32:1013422022.PubMed/NCBI
|
|
26
|
Tang TT, Jiang L, Zhong Q, Ni ZJ, Thakur
K, Khan MR and Wei ZJ: Saikosaponin D exerts cytotoxicity on human
endometrial cancer ishikawa cells by inducing apoptosis and
inhibiting metastasis through MAPK pathways. Food Chem Toxicol.
177:1138152023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xi H, Wang S, Wang B, Hong X, Liu X, Li M,
Shen R and Dong Q: The role of interaction between autophagy and
apoptosis in tumorigenesis (Review). Oncol Rep. 48:2082022.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sorice M: Crosstalk of autophagy and
apoptosis. Cells. 11:14792022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu S, Yao S, Yang H, Liu S and Wang Y:
Autophagy: Regulator of cell death. Cell Death Dis. 14:6482023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Niu X, You Q, Hou K, Tian Y, Wei P, Zhu Y,
Gao B, Ashrafizadeh M, Aref AR, Kalbasi A, et al: Autophagy in
cancer development, immune evasion, and drug resistance. Drug
Resist Updat. 78:1011702025. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Wu Y, Meng S, Xu P, Li S, Li Y, Hu
X, Ouyang L and Wang G: Selective autophagy in cancer: mechanisms,
therapeutic implications, and future perspectives. Mol Cancer.
23:222024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao Y, Tang J, Jiang K, Liu SY, Aicher A
and Heeschen C: Liquid biopsy in pancreatic cancer-current
perspective and future outlook. Biochim Biophys Acta Rev Cancer.
1878:1888682023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhong BH, Ma YT, Sun J, Tang JT and Dong
M: Transcription factor FOXF2 promotes the development and
progression of pancreatic cancer by targeting MSI2. Oncol Rep.
52:932024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Hua Q, Wang H, Zhang D, Zhao L, Yu
D, Pi G, Zhang T and Lin Z: Meta-analysis and indirect treatment
comparison of modified FOLFIRINOX and gemcitabine plus
nab-paclitaxel as first-line chemotherapy in advanced pancreatic
cancer. BMC Cancer. 21:8532021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji J, Wen Q, Yu Y, Xiong F, Zheng X and
Ruan S: Personalized traditional Chinese medicine in oncology:
Bridging the macro state with micro targets. Am J Chin Med.
14:1–34. 2025.
|
|
37
|
Ke Y, Pan Y, Huang X, Bai X, Liu X, Zhang
M, Wei Y, Jiang T and Zhang G: Efficacy and safety of traditional
Chinese medicine (TCM) combined with immune checkpoint inhibitors
(ICIs) for the treatment of cancer: a systematic review and
meta-analysis. Front Pharmacol. 31:16615032025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okuno K, Xu C, Pascual-Sabater S, Tokunaga
M, Han H, Fillat C, Kinugas Y and Goel A: Berberine overcomes
gemcitabine-associated chemoresistance through regulation of
Rap1/PI3K-Akt signaling in pancreatic ductal adenocarcinoma.
Pharmaceuticals (Basel). 15:11992022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bhattacharyya S, Ghosh H,
Covarrubias-Zambrano O, Jain K, Swamy KV, Kasi A, Hamza A, Anant S,
Van Saun M, Weir SJ, et al: Anticancer activity of novel
difluorinated curcumin analog and its inclusion complex with
2-hydroxypropyl-β-cyclodextrin against pancreatic cancer. Int J Mol
Sci. 24:63362023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ashour ML and Wink M: Genus bupleurum: A
review of its phytochemistry, pharmacology and modes of action. J
Pharm Pharmacol. 63:305–321. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cheng Y, Liu G, Li Z, Zhou Y and Gao N:
Screening saikosaponin d (SSd)-producing endophytic fungi from
Bupleurum scorzonerifolium Willd. World J Microbiol Biotechnol.
38:2422022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lai M, Ge Y, Chen M, Sun S, Chen J and
Cheng R: Saikosaponin D inhibits proliferation and promotes
apoptosis through activation of MKK4-JNK signaling pathway in
pancreatic cancer cells. Onco Targets Ther. 13:9465–9479. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Conroy T, Castan F, Lopez A, Turpin A, Ben
Abdelghani M, Wei AC, Mitry E, Biagi JJ, Evesque L, Artru P, et al:
Five-year outcomes of FOLFIRINOX vs. gemcitabine as adjuvant
therapy for pancreatic cancer: A randomized clinical trial. JAMA
Oncol. 8:1571–1578. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun EJ, Wankell M, Palamuthusingam P,
McFarlane C and Hebbard L: Targeting the PI3K/Akt/mTOR pathway in
hepatocellular carcinoma. Biomedicines. 9:16392021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kale J, Kutuk O, Brito GC, Andrews TS,
Leber B, Letai A and Andrews DW: Phosphorylation switches Bax from
promoting to inhibiting apoptosis thereby increasing drug
resistance. EMBO Rep. 19:e452352018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li X, He S and Ma B: Autophagy and
autophagy-related proteins in cancer. Mol Cancer. 19:122020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mehra S, Deshpande N and Nagathihalli N:
Targeting PI3K pathway in pancreatic ductal adenocarcinoma:
Rationale and progress. Cancers (Basel). 13:44342021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sheng W, Shi X, Lin Y, Tang J, Jia C, Cao
R, Sun J, Wang G, Zhou L and Dong M: Musashi2 promotes EGF-induced
EMT in pancreatic cancer via ZEB1-ERK/MAPK signaling. J Exp Clin
Cancer Res. 39:162020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Deer EL, González-Hernández J, Coursen JD,
Shea JE, Ngatia J, Scaife CL, Firpo MA and Mulvihill SJ: Phenotype
and genotype of pancreatic cancer cell lines. Pancreas. 39:425–435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fianco G, Mongiardi MP, Levi A, De Luca T,
Desideri M, Trisciuoglio D, Del Bufalo D, Cinà I, Di Benedetto A,
Mottolese M, et al: Caspase-8 contributes to angiogenesis and
chemotherapy resistance in glioblastoma. Elife. 6:e225932017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wei S, Han C, Mo S, Huang H and Luo X:
Advancements in programmed cell death research in antitumor
therapy: A comprehensive overview. Apoptosis. 30:401–421. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Das S, Shukla N, Singh SS, Kushwaha S and
Shrivastava R: Mechanism of interaction between autophagy and
apoptosis in cancer. Apoptosis. 26:512–533. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hajdú B, Holczer M, Horváth G, Szederkényi
G and Kapuy O: Fine-tuning of mTORC1-ULK1-PP2A regulatory triangle
is crucial for robust autophagic response upon cellular stress.
Biomolecules. 12:15872022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fujiwara N, Usui T and Nagai H: Regulation
of the Beclin 1/VPS34 complex by post-translational modifications.
Biochem Biophys Res Commun. 566:155–161. 2021.PubMed/NCBI
|
|
56
|
Pareek G and Kundu M: Physiological
functions of ULK1/2. J Mol Biol. 436:1684722024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lu G, Wu Z, Shang J, Xie Z, Chen C and
Zhang C: The effects of metformin on autophagy. Biomed
Pharmacother. 137:1112862021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fujiwara N, Shibutani S, Ohama T and Sato
K: Protein phosphatase 6 dissociates the beclin 1/Vps34 complex and
inhibits autophagy. Biochem Biophys Res Commun. 552:191–195. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bekker M, Abrahams S, Loos B and Bardien
S: Can the interplay between autophagy and apoptosis be targeted as
a novel therapy for Parkinson's disease? Neurobiol Aging.
100:91–105. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vujić N, Bradić I, Goeritzer M, Kuentzel
KB, Rainer S, Kratky D and Radović B: ATG7 is dispensable for
LC3-PE conjugation in thioglycolate-elicited mouse peritoneal
macrophages. Autophagy. 17:3402–3407. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Foucquier J and Guedj M: Analysis of drug
combinations: Current methodological landscape. Pharmacol Res
Perspect. 3:e001492015. View Article : Google Scholar : PubMed/NCBI
|