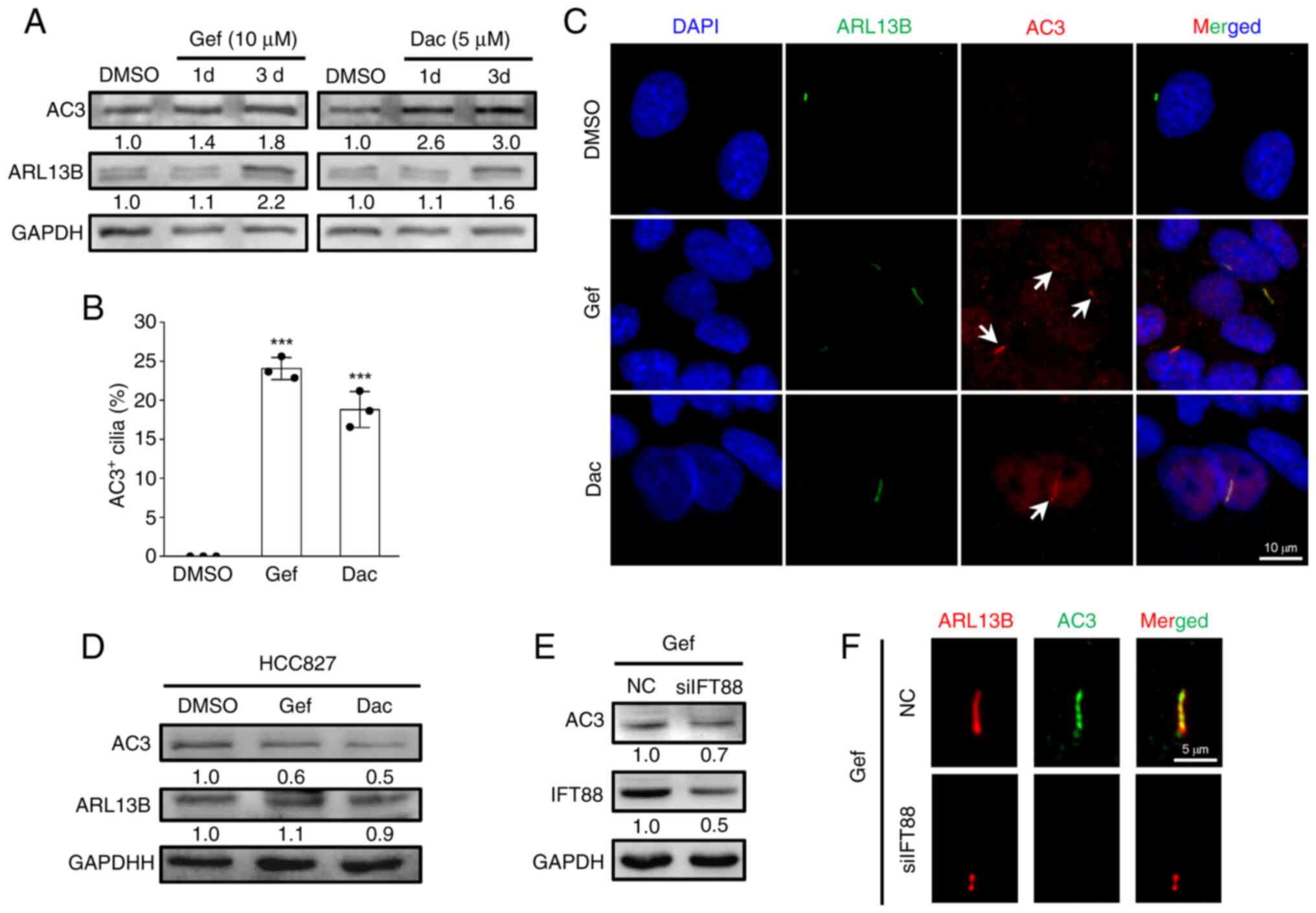
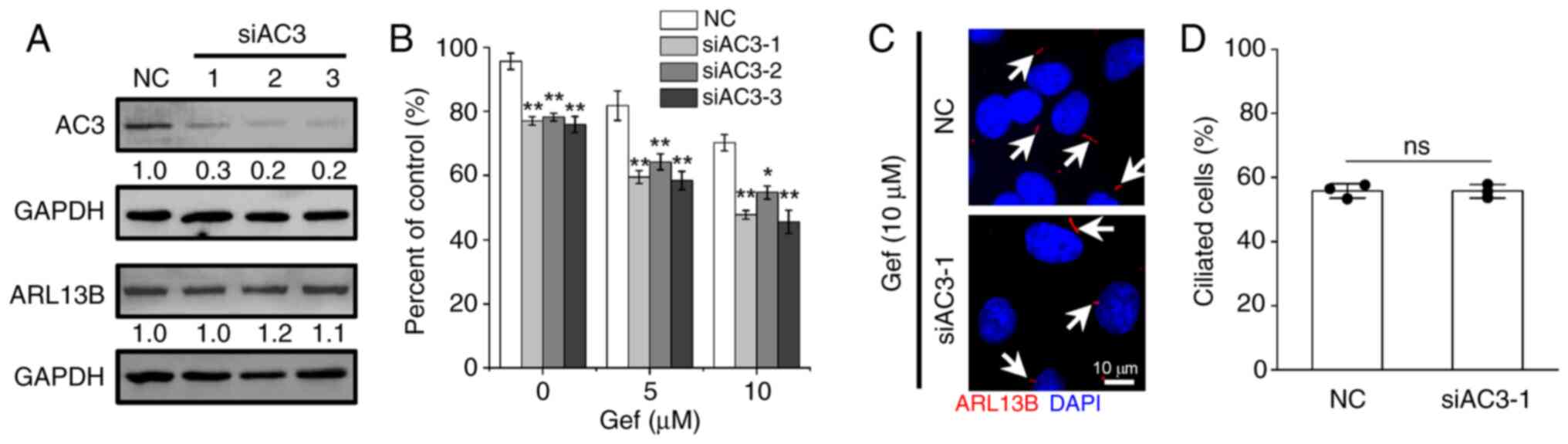
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Hendriks LEL, Remon J, Faivre-Finn C,
Garassino MC, Heymach JV, Kerr KM, Tan DSW, Veronesi G and Reck M:
Non-small-cell lung cancer. Nat Rev Dis Primers. 10:712024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pao W and Chmielecki J: Rational,
biologically based treatment of EGFR-mutant non-small-cell lung
cancer. Nat Rev Cancer. 10:760–774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoue A, Suzuki T, Fukuhara T, Maemondo M,
Kimura Y, Morikawa N, Watanabe H, Saijo Y and Nukiwa T: Prospective
phase II study of gefitinib for chemotherapy-naive patients with
advanced non-small-cell lung cancer with epidermal growth factor
receptor gene mutations. J Clin Oncol. 24:3340–3346. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cappuzzo F, Ciuleanu T, Stelmakh L,
Cicenas S, Szczésna A, Juhász E, Esteban E, Molinier O, Brugger W,
Melezínek I, et al: Erlotinib as maintenance treatment in advanced
non-small-cell lung cancer: A multicentre, randomised,
placebo-controlled phase 3 study. Lancet Oncol. 11:521–529. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du Z, Kan H, Sun J, Liu Y, Gu J,
Akemujiang S, Zou Y, Jiang L, Wang Q, Li C, et al: Molecular
mechanisms of acquired resistance to EGFR tyrosine kinase
inhibitors in non-small cell lung cancer. Drug Resist Updat.
82:1012662025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Satir P, Pedersen LB and Christensen ST:
The primary cilium at a glance. J Cell Sci. 123:499–503. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anvarian Z, Mykytyn K, Mukhopadhyay S,
Pedersen LB and Christensen ST: Cellular signalling by primary
cilia in development, organ function and disease. Nat Rev Nephrol.
15:199–219. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiseleva AA, Nikonova AS and Golemis EA:
Patterns of ciliation and ciliary signaling in cancer. Rev Physiol
Biochem Pharmacol. 185:87–105. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shireman JM, Atashi F, Lee G, Ali ES,
Saathoff MR, Park CH, Savchuk S, Baisiwala S, Miska J, Lesniak MS,
et al: De novo purine biosynthesis is a major driver of
chemoresistance in glioblastoma. Brain. 144:1230–1246. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chao YY, Huang BM, Peng IC, Lee PR, Lai
YS, Chiu WT, Lin YS, Lin SC, Chang JH, Chen PS, et al: ATM- and
ATR-induced primary ciliogenesis promotes cisplatin resistance in
pancreatic ductal adenocarcinoma. J Cell Physiol. 237:4487–4503.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei L, Ma W, Cai H, Peng SP, Tian HB, Wang
JF, Gao L and He JP: Inhibition of ciliogenesis enhances the
cellular sensitivity to temozolomide and ionizing radiation in
human glioblastoma cells. Biomed Environ Sci. 35:419–436.
2022.PubMed/NCBI
|
|
13
|
Ma W, Wei L, Jin L, Ma Q, Zhang T, Zhao Y,
Hua J, Zhang Y, Wei W, Ding N, et al: YAP/Aurora A-mediated
ciliogenesis regulates ionizing radiation-induced senescence via
Hedgehog pathway in tumor cells. Biochim Biophys Acta Mol Basis
Dis. 1870:1670622024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jenks AD, Vyse S, Wong JP, Kostaras E,
Keller D, Burgoyne T, Shoemark A, Tsalikis A, de la Roche M,
Michaelis M, et al: Primary cilia mediate diverse kinase inhibitor
resistance mechanisms in cancer. Cell Rep. 23:3042–3055. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee C, Yi J, Park J, Ahn B, Won YW, Jeon
J, Lee BJ, Cho WJ and Park JW: Hedgehog signalling is involved in
acquired resistance to KRASG12C inhibitors in lung
cancer cells. Cell Death Dis. 15:562024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan NA, Willemarck N, Talebi A, Marchand
A, Binda MM, Dehairs J, Rueda-Rincon N, Daniels VW, Bagadi M,
Thimiri Govinda Raj DB, et al: Identification of drugs that restore
primary cilium expression in cancer cells. Oncotarget. 7:9975–9992.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo R, Liu T, Shasaltaneh MD, Wang X,
Imani S and Wen Q: Targeting adenylate cyclase family: New concept
of targeted cancer therapy. Front Oncol. 12:8292122022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson JLF and Leroux MR: cAMP and cGMP
signaling: Sensory systems with prokaryotic roots adopted by
eukaryotic cilia. Trends Cell Biol. 20:435–444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brewer KK, Brewer KM, Terry TT, Caspary T,
Vaisse C and Berbari NF: Postnatal dynamic ciliary ARL13B and ADCY3
localization in the mouse brain. Cells. 13:2592024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner
JB and van der Hoorn FA: Adenylate cyclase regulates elongation of
mammalian primary cilia. Exp Cell Res. 315:2802–2817. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hong SH, Goh SH, Lee SJ, Hwang JA, Lee J,
Choi IJ, Seo H, Park JH, Suzuki H, Yamamoto E, et al: Upregulation
of adenylate cyclase 3 (ADCY3) increases the tumorigenic potential
of cells by activating the CREB pathway. Oncotarget. 4:1791–1803.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Quinn SN, Graves SH, Dains-McGahee C,
Friedman EM, Hassan H, Witkowski P and Sabbatini ME: Adenylyl
cyclase 3/adenylyl cyclase-associated protein 1 (CAP1) complex
mediates the anti-migratory effect of forskolin in pancreatic
cancer cells. Mol Carcinog. 56:1344–1360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu L, Dong C, Wang Z, He S, Yang Y, Zi M,
Li H, Zhang Y, Chen C, Zheng R, et al: A rationally designed
fluorescence probe achieves highly specific and long-term detection
of senescence in vitro and in vivo. Aging Cell. 22:e138962023.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Li S, Wang Y, Zhao Y and Li Q:
Protein tyrosine kinase inhibitor resistance in malignant tumors:
Molecular mechanisms and future perspective. Signal Transduct
Target Ther. 7:3292022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SO, Kim BY and Lee KH: Synergistic
effect of anticancer drug resistance and Wnt3a on primary
ciliogenesis in A549 cell-derived anticancer drug-resistant subcell
lines. Biochem Biophys Res Commun. 635:1–11. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Duffy MP, Sup ME and Guo XE: Adenylyl
cyclase 3 regulates osteocyte mechanotransduction and primary
cilium. Biochem Biophys Res Commun. 573:145–150. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sterpka A and Chen X: Neuronal and
astrocytic primary cilia in the mature brain. Pharmacol Res.
137:114–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hilgendorf KI, Myers BR and Reiter JF:
Emerging mechanistic understanding of cilia function in cellular
signalling. Nat Rev Mol Cell Biol. 25:555–573. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kiseleva AA, Korobeynikov VA, Nikonova AS,
Zhang P, Makhov P, Deneka AY, Einarson MB, Serebriiskii IG, Liu H,
Peterson JR and Golemis EA: Unexpected activities in regulating
ciliation contribute to off-target effects of targeted drugs. Clin
Cancer Res. 25:4179–4193. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guen VJ and Prigent C: Targeting Primary
ciliogenesis with small-molecule inhibitors. Cell Chem Biol.
27:1224–1228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Collinson R and Tanos B: Primary cilia and
cancer: A tale of many faces. Oncogene. 44:1551–1566. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saito M, Otsu W, Miyadera K and Nishimura
Y: Recent advances in the understanding of cilia mechanisms and
their applications as therapeutic targets. Front Mol Biosci.
10:12321882023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pant K, Richard S, Peixoto E, Baral S,
Yang R, Ren Y, Masyuk TV, LaRusso NF and Gradilone SA:
Cholangiocyte ciliary defects induce sustained epidermal growth
factor receptor signaling. Hepatology. 81:1132–1145. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin F, Chen Q, Shi Y, Xu H, Huang J, Qing
M, Zhong L, Li J, Xie L and Zeng X: Activation of EGFR-Aurora A
induces loss of primary cilia in oral squamous cell carcinoma. Oral
Dis. 28:621–630. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kasahara K, Aoki H, Kiyono T, Wang S,
Kagiwada H, Yuge M, Tanaka T, Nishimura Y, Mizoguchi A, Goshima N
and Inagaki M: EGF receptor kinase suppresses ciliogenesis through
activation of USP8 deubiquitinase. Nat Commun. 9:7582018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsai YC, Kuo TN, Chao YY, Lee PR, Lin RC,
Xiao XY, Huang BM and Wang CY: PDGF-AA activates AKT and ERK
signaling for testicular interstitial Leydig cell growth via
primary cilia. J Cell Biochem. 124:89–102. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee JE, Park HS, Lee D, Yoo G, Kim T, Jeon
H, Yeo MK, Lee CS, Moon JY, Jung SS, et al: Hippo pathway effector
YAP inhibition restores the sensitivity of EGFR-TKI in lung
adenocarcinoma having primary or acquired EGFR-TKI resistance.
Biochem Biophys Res Commun. 474:154–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guo Y, Dupart M, Irondelle M, Peraldi P,
Bost F and Mazure NM: YAP1 modulation of primary cilia-mediated
ciliogenesis in 2D and 3D prostate cancer models. FEBS Lett.
598:3071–3086. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee J, Park KC, Sul HJ, Hong HJ, Kim KH,
Kero J and Shong M: Loss of primary cilia promotes
mitochondria-dependent apoptosis in thyroid cancer. Sci Rep.
11:41812021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jeffries EP, Di Filippo M and Galbiati F:
Failure to reabsorb the primary cilium induces cellular senescence.
FASEB J. 33:4866–4882. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma X, Zhang Y, Zhang Y, Zhang X, Huang Y,
He K, Chen C, Hao J, Zhao D, LeBrasseur NK, et al: A stress-induced
cilium-to-PML-NB route drives senescence initiation. Nat Commun.
14:18402023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Breslin L, Prosser SL, Cuffe S and
Morrison CG: Ciliary abnormalities in senescent human fibroblasts
impair proliferative capacity. Cell Cycle. 13:2773–2779. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Teng YN, Chang HC, Chao YY, Cheng HL, Lien
WC and Wang CY: Etoposide triggers cellular senescence by inducing
multiple centrosomes and primary cilia in adrenocortical tumor
cells. Cells. 10:14662021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu X, Wei L, Zhang R, Chen J, Zhang T,
Hua J, Wang J, He J and Xie X: The DNA-PKcs-primary cilia axis
maintains ionizing radiation-induced senescence in tumor cells.
Acta Biochim Biophys Sin. 2025.Doi: 10.3724/abbs.2025168.
View Article : Google Scholar
|
|
45
|
Zhang H, Liu Y, Liu J, Chen J, Wang J, Hua
H and Jiang Y: cAMP-PKA/EPAC signaling and cancer: The interplay in
tumor microenvironment. J Hematol Oncol. 17:52024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ginsberg G, Angle K, Guyton K and Sonawane
B: Polymorphism in the DNA repair enzyme XRCC1: Utility of current
database and implications for human health risk assessment. Mutat
Res. 727:1–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zou T, Liu J, She L, Chen J, Zhu T, Yin J,
Li X, Li X, Zhou H and Liu Z: A perspective profile of ADCY1 in
cAMP signaling with drug-resistance in lung cancer. J Cancer.
10:6848–6857. 2019. View Article : Google Scholar : PubMed/NCBI
|