Nasopharyngeal carcinoma (NPC) is a malignant tumor
originating from the mucosal epithelium of the nasopharynx,
exhibiting distinct geographical distribution patterns. It is
highly prevalent in regions such as South China (for example,
Guangdong, Guangxi, Fujian and Hunan), Southeast Asia and North
Africa (1). The pathogenesis of NPC
involves genetic susceptibility, environmental factors and
Epstein-Barr virus (EBV) infection, with EBV being a
well-established oncogenic driver (1). Currently, the standard treatment for
NPC primarily consists of radiotherapy combined with
cisplatin-based concurrent chemoradiotherapy. However, chemotherapy
resistance and severe side effects notably limit clinical efficacy
(2). Consequently, there is a need
to develop novel therapeutic strategies that are more effective,
less toxic and capable of overcoming drug resistance.
Ferroptosis is a novel form of iron-dependent
programmed cell death that is distinct from apoptosis and autophagy
(15,16). Its core mechanism involves the
catalysis of lipid peroxidation (LPO) in membrane polyunsaturated
fatty acids (PUFAs) by ferrous iron (Fe2+) or
lipoxygenases (LOXs), leading to membrane damage and subsequent
cell death. Characteristic morphological features include
mitochondrial shrinkage and increased membrane density, while
nuclear structure typically remains intact (17–19).
Currently identified regulatory pathways of ferroptosis include:
The system cystine/glutamate transporter (Xc)-/glutathione
(GSH)/GSH peroxidase 4 (GPX4) axis; guanosine triphosphate (GTP)
cyclohydrolase 1 (GCH1)/tetrahydrobiopterin (BH4) pathway;
dihydroorotate dehydrogenase (DHODH)-mediated pathway;
membrane-bound O-acyltransferase 1/2 (MBOAT1/2)-monounsaturated
fatty acid (MUFA) regulation; Nrf2 signaling; LPO mechanisms and
apoptosis-inducing factor mitochondria-associated 2 (FSP1 otherwise
known as AIFM2) pathway. The present review systematically examines
the molecular mechanisms and key signaling pathways of ferroptosis.
By integrating the epidemiological characteristics and risk factors
of NPC, the present review elucidates the relationship between
ferroptosis and NPC pathogenesis. Furthermore, the present review
discusses the potential therapeutic value of targeting ferroptosis
in NPC treatment, providing a theoretical foundation for developing
novel anti-tumor strategies.
Ferroptosis has emerged as a prominent research
focus in cell biology as a distinct form of programmed cell death.
A precise understanding of its definition and characteristics forms
the essential foundation for in-depth investigation of this cell
death mechanism. Defined as an iron-overload and reactive oxygen
species (ROS)-dependent cell death process driven by lipid peroxide
accumulation, ferroptosis reveals two key pathogenic factors: Iron
ions and ROS (20–24). Morphologically, ferroptosis exhibits
unique ultrastructural features: Markedly shrunken mitochondria
with increased membrane density, reduced or vanished mitochondrial
cristae, outer mitochondrial membrane rupture and loss of plasma
membrane integrity (25–27). These characteristics distinctly
differentiate ferroptosis from other cell death modalities such as
apoptosis and necroptosis. Notably, nuclear morphology typically
remains intact during ferroptosis, contrasting with classical
apoptotic nuclear fragmentation.
These distinctive features suggest that ferroptosis
is regulated through specific molecular mechanisms. In the
following sections, the present study systematically elaborates on
the key metabolic pathways and molecular mechanisms governing
ferroptosis regulation.
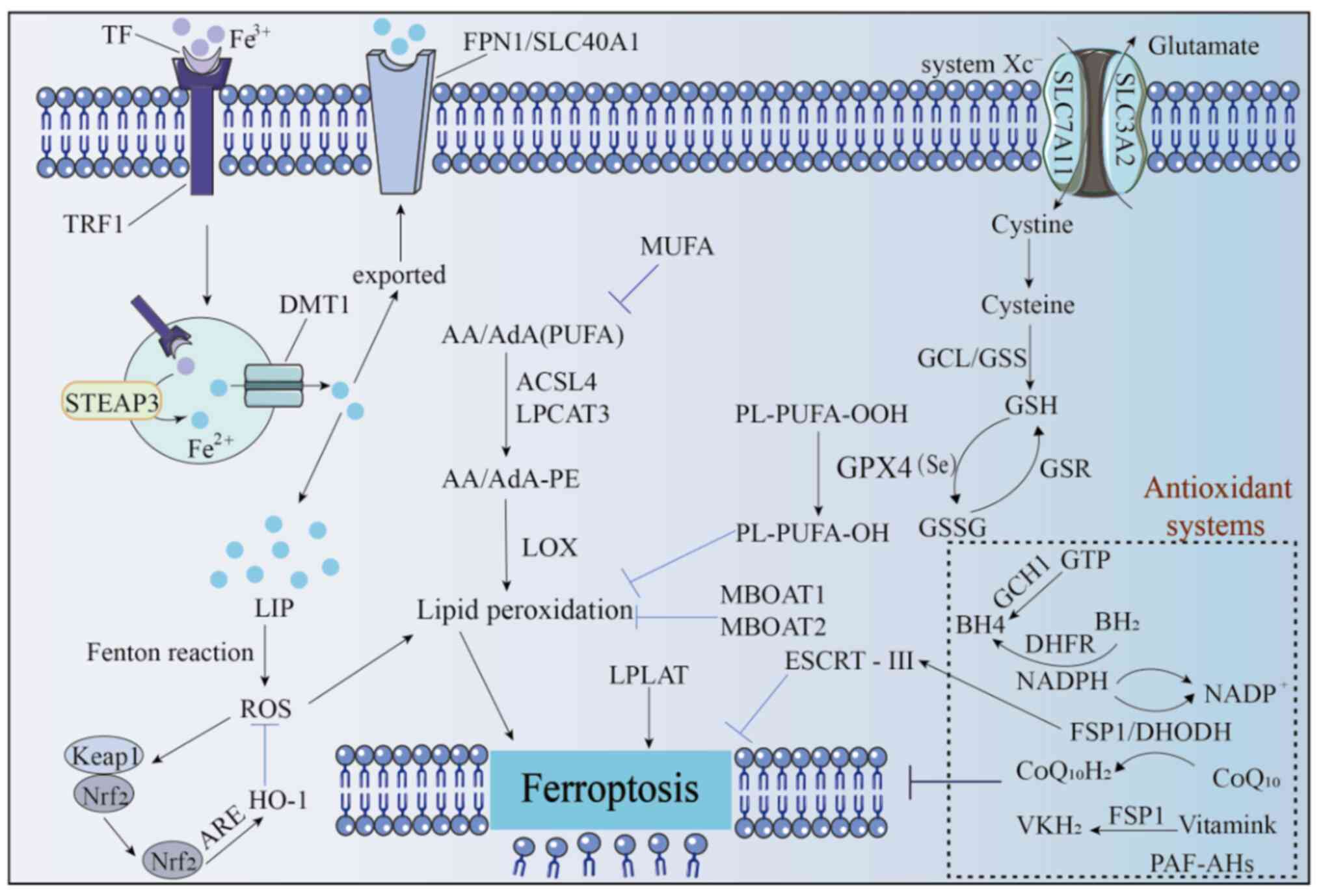
Iron is important for human survival, participating
in physiological activities in the forms of Fe3+ and
Fe2+: It is not only a key participant in the electron
transport chain during oxidative phosphorylation but also the core
of heme in hemoglobin, responsible for oxygen transport in the
blood. The majority of iron in the body is bound to proteins or
stored by ferritin, with only a small amount of free iron forming
the labile iron pool (LIP) (28).
The transport, metabolism and storage of iron are tightly regulated
because free Fe2+, with its redox activity, can promote
the generation of ROS through the Fenton reaction, exacerbating LPO
(29–32). Extracellular Fe3+ first
binds to transferrin and enters cells via endocytosis mediated by
transferrin receptor 1. Within the endosome-lysosome system, as the
pH decreases, Fe3+ dissociates and is reduced to
Fe2+ by the metal reductase STEAP3, then transported to
the cytoplasm by the divalent metal transporter 1 (33,34).
In the cytoplasm, Fe2+ can be reduced and stored as
Fe3+ by ferritin, exported out of the cell via
ferroportin (FPN1/SLC40A1), involved in the synthesis of
iron-containing proteins or exist as part of the transient LIP
(35). Iron homeostasis is vital
for cell survival and iron overload disrupts this balance, thereby
inducing ferroptosis (Fig. 1).
Lipids are the core structural components of cell
membranes and organelle membranes. Under normal physiological
conditions, lipid oxidation and reduction maintain a dynamic
balance, but cellular carcinogenesis or external stimuli can
disrupt this equilibrium (36).
Ferroptosis, a novel form of cell death, is characterized by
oxidative damage to PUFA-containing phospholipids in cell membranes
(37,38). PUFAs are the primary substrates of
LPO and their oxidation severely disrupts membrane structure and
function. By contrast, MUFAs can antagonize ferroptosis by
inhibiting LPO (39–41). Thus, PUFA levels regulate both LPO
and susceptibility to ferroptosis.
At the molecular level, acyl-CoA synthetase
long-chain family member 4 (ACSL4) and lysophosphatidylcholine
acyltransferase 3 (LPCAT3) are key enzymes regulating PUFA
incorporation into phospholipids. Inhibition or loss of their
activity confers resistance to ferroptosis (42–44).
Specifically, ACSL4 and LPCAT3 work synergistically to esterify
arachidonic acid (AA) or adrenic acid (AdA) into
phosphatidylethanolamine (PE). Subsequently, LOXs catalyze the
formation of lipid hydroperoxides. The breakdown products of these
peroxides attack proteins, induce plasma membrane rupture and
ultimately drive ferroptosis (45–47)
(Fig. 1).
As a key precursor, cysteine, together with
glutamate and glycine, is catalyzed by glutamate-cysteine ligase
and GSH synthetase in sequence to synthesize GSH. GSH is an
essential cofactor for GPX4, a selenium-dependent enzyme that can
reduce toxic lipid peroxides (PL-PUFA-OOH) to harmless lipid
alcohols (PL-PUFA-OH), maintaining redox homeostasis (49,50,55).
In addition, GSH reductase can regenerate oxidized
GSH into reduced GSH, thereby maintaining the activity of GPX4 and
blocking ferroptosis triggered by LPO (50,51,56).
Therefore, the targeted regulation of the three-level defense
network of cystine uptake, GSH synthesis and GPX4 function is an
effective strategy for intervening in ferroptosis (Fig. 1).
BH4 and BH2 form a redox cycle that synergistically
scavenges oxygen-free radicals to inhibit ferroptosis (61). This cycle is regulated by
dihydrofolate reductase (DHFR), which uses NAD(P)H as a cofactor to
reduce BH2 to BH4. Elevated BH4 levels can induce cellular lipid
remodeling, reducing the proportion of phospholipids containing
di-polyunsaturated fatty acyl groups (diPUFA) and thereby blocking
ferroptosis (62).
Furthermore, BH4 enhances the biosynthesis of
coenzyme Q10 (CoQ10) by promoting the synthesis of 4-hydroxybenzoic
acid, associating the GCH1-BH4-DHFR pathway to the FSP1-CoQ10 axis
and forming a synergistic anti-ferroptosis network (Fig. 1).
In the biosynthetic pathway of pyrimidine
nucleotides, DHODH occupies a key position and carries out an
indispensable role in the synthesis of DNA and RNA. Once DHODH is
inhibited, this biosynthetic pathway will be disrupted, resulting
in abnormal or even terminated synthesis of pyrimidine nucleotides
(66). This further leads to a
decrease in the availability of pyrimidine nucleotides, making the
purine-pyrimidine base pairing process unable to proceed normally
and ultimately severely hindering the synthesis of RNA (67). Since RNA is a cofactor of GSH, a
decrease in the amount of RNA will lead to an increase in the
amount of GSH. At this time, GPX4 can reduce the peroxidized lipids
back to their original state. As the level of LPO decreases, the
process of ferroptosis is inhibited and the incidence of
ferroptosis also decreases accordingly (68,69)
(Fig. 1).
PE-PUFAs are the preferred substrates for LPO, and
their content directly affects the degree of LPO and cellular
sensitivity to ferroptosis. Thus, MBOAT1 and MBOAT2 regulate the
composition of unsaturated fatty acids in membrane phospholipids,
reducing oxidizable PE-PUFAs and increasing stable PE-MUFAs, to
form a defense against ferroptosis, effectively inhibiting its
occurrence (71). Further studies
showed that their expression and function are specifically
regulated by sex hormone receptors: MBOAT1 is regulated by estrogen
receptors, with activity related to estrogen signaling; MBOAT2 is
regulated by androgen receptors, with function dependent on
androgen signal activation. This makes their ferroptosis-inhibiting
effect sex hormone-dependent, providing a new perspective for
analyzing the differential regulation of ferroptosis under
different sex or hormonal microenvironments (70,72,73).
This mechanism does not rely on classical pathways (70) such as GPX4 or AIFM2, but acts by
directly remodeling the fatty acid composition of membrane
phospholipids, adding new insights into the complexity and
diversity of the ferroptosis regulatory network (Fig. 1).
FSP1, a type II nicotinamide adenine dinucleotide-H
(NADH): quinone oxidoreductase (NDH-2) with N-terminal
hydrophobic/membrane (aa 1–27), NADH oxidoreductase (aa 81–285) and
FAD (aa 286–308) domains (74), is
a key anti-ferroptosis factor. Its GSH-independent antioxidant
pathway, parallel to GPX4, regulates iron metabolism and protects
against iron-dependent death (75).
Vitamin K, a lipophilic molecule with
2-methyl-1,4-naphthoquinone and polyisoprene side chains
(plant-derived phylloquinone K1; animal/bacterial menaquinone K2),
converts to hydroquinone (VKH2) for the vitamin K cycle (79,80–82).
FSP1 acts as a vitamin K reductase, consuming NAD(P)H to produce
VKH2, which inhibits ferroptosis by blocking LPO (83,84)
(Fig. 1).
Additionally, FSP1 enhances membrane repair via the
ESCRT-III-dependent pathway (CoQ-independent) to inhibit
ferroptosis (85). Ferroptosis
inducers trigger FSP1 to suppress tumor cell ferroptosis; FSP1
knockout blocks RSL3-induced plasma membrane expression of CHMP5/6,
while CHMP5 overexpression reverses inducer- and FSP1
knockout-induced cell death (86–89)
(Fig. 1).
Nrf2, the core of cellular antioxidant responses,
binds to antioxidant response elements (AREs) to promote downstream
gene transcription (90). It
carries out a key role in regulating ferroptosis as an important
transcription factor against it, involved in iron, lipid and amino
acid metabolism (91). Its
regulated antioxidant effectors [such as heme oxygenase-1 (HO-1)
and GSH] which contain AREs (92).
Elevated ROS activate the p62-Keap1-Nrf2 pathway: Nrf2 dissociates
from Keap1, translocates to the nucleus, binds to AREs, initiates
transcriptional cascades and upregulates downstream antioxidant
genes, which is important for maintaining redox balance and
inhibiting ferroptosis (77)
(Fig. 1).
Additionally, cytoplasmic platelet-activating factor
(PAF) acetylhydrolase (II) specifically inhibits short-chain fatty
acid oxidation, blocks oxidized phospholipid (such as PAF)
accumulation by interfering with cellular redox capacity, thus
inhibiting ferroptosis (78).
whereas LPLAT disrupts lipid bilayers, increases membrane
permeability and triggers ferroptosis (93) (Fig.
1).
NPC is a malignant tumor originating from the
mucosal epithelium of the nasopharynx. Its incidence shows obvious
regional characteristics and is particularly high in Southeast Asia
and North Africa (79). The
pathogenesis of this disease is multifactorial, involving the
complex interaction of various risk factors such as genetic
susceptibility, environmental exposure, EBV infection and lifestyle
(79).
Genetic factors carry out an important role in the
pathogenesis of NPC. Epidemiological studies have shown that NPC
exhibits notable familial aggregation. The risk of disease in
first-degree relatives is markedly compared with that in the
general population, indicating the key role of genetic
susceptibility in the occurrence of this disease (80–82).
Currently, it is considered that specific gene polymorphisms (such
as the HLA gene cluster), mutations in tumor susceptibility genes
(such as TP53) and epigenetic changes may jointly affect the
susceptibility of an individual to NPC. In addition, genetic
factors may interact with environmental factors (such as EBV
infection), further increasing the risk of developing the disease
(Table I).
Environmental exposure is one of the important risk
factors for NPC. Long-term exposure to air pollutants (such as
PM2.5, sulfur dioxide and nitrogen oxides) can lead to chronic
inflammation of the nasopharyngeal mucosa and DNA damage, thus
promoting malignant transformation (94). In addition, occupational exposure to
certain chemical carcinogens (such as formaldehyde or
benzo(a)pyrene) may also increase the risk of NPC. Viral infection
carries out a central role in the development of NPC, especially
EBV infection. EBV can infect nasopharyngeal epithelial cells. By
expressing latent membrane proteins (LMP) 1 and 2 and oncogenic
proteins such as EBV nuclear antigen 1 (EBNA1), it can interfere
with the regulation of the cell cycle and induce genomic
instability (86,95) (Table
I).
Unhealthy lifestyles are modifiable risk factors for
NPC. Smoking can notably increase the risk of developing the
disease. Carcinogens in tobacco, such as nitrosamines and
polycyclic aromatic hydrocarbons, can directly damage the
nasopharyngeal mucosa and promote the oncogenic effect of EBV
(87,88). Excessive alcohol consumption may
induce the formation of DNA adducts through metabolites (such as
acetaldehyde), exacerbating mucosal damage (89). Dietary factors also play a key role.
Long-term consumption of pickled foods (such as salted fish and
cured meat) may increase the risk of NPC because they are rich in
nitrites, which can be converted into the potent carcinogen
N-nitroso compounds in the body (85). In addition, heterocyclic amines and
polycyclic aromatic hydrocarbons produced by high-temperature
cooking (such as grilling and smoking) may also promote
tumorigenesis (Table I).
EBV belongs to the gammaherpesvirus family and is
widely prevalent in the human population, with >90% of adults
having been infected. It can establish a lifelong latent state in
epithelial cells and B cells. EBV is closely associated with a
variety of lymphoid malignancies, such as Burkitt's lymphoma,
Hodgkin's lymphoma, B-cell lymphoma, NK/T-cell lymphoma, as well as
two types of epithelial cancer: NPC and gastric cancer (96). EBV exhibits two distinctly different
life cycle states: lytic (productive) and latent (persistent).
During primary infection, EBV first replicates in nasopharyngeal
epithelial cells, then crosses the epithelial layer of the
nasopharyngeal lining, infects naive B cells and continues to
replicate, and finally establishes a stable latent state in host
cells in the form of histone-associated episomes. To maintain
latent viruses and serve as a stable reservoir for EBV-induced
tumorigenesis, latent viruses need to be periodically reactivated.
In NPC, the prevalence of type 2 and type 3 EBV reaches 100%, and
high-level viral reactivation is a risk factor for EBV-associated
NPC (97). The interaction between
EBV infection, environmental factors, and genetic factors is the
core of the pathogenesis of NPC. Recent studies have shown that
abortive lytic infection promotes the establishment of the latent
state during primary infection and the development of
EBV-associated tumors. In-depth exploration of the mechanisms by
which EBV viral products drive the development of NPC will help
design more effective EBV-targeted therapies (98,99).
During the latent period, the virus only expresses a
small number of genes key for genome maintenance and regulation.
Based on late gene expression profiles, EBV latency can be divided
into four types (100,101). In NPC, EBV infection is largely in
type 2 latency, during which EBNA1, LMP1 and LMP2 protein are
expressed. At the same time, some non-coding RNAs are also
expressed, such as EBV-encoded RNA (EBER), BamHI A rightward
transcript (BART), and BART miRNA. These expressed viral genes
provide signals necessary for the maintenance of replication and
survival of both EBV and host cells. However, EBV lytic phase
proteins, such as BGLF5 (DNase) and BALF3, cause host genome
instability and play an important role in the tumorigenesis of NPC
(100).
Ferroptosis is a novel form of programmed cell
death, characterized by iron-dependent accumulation of lipid
peroxides, which ultimately leads to cell death (102,103). The occurrence of ferroptosis is
associated with the balance of intracellular iron metabolism, lipid
metabolism and antioxidant systems. In the field of tumor research,
abnormal regulation of ferroptosis is associated with the
occurrence, development and therapeutic sensitivity of tumors
(104–106). The regulatory role of EBV
infection on ferroptosis in NPC is an important content that
urgently needs to be supplemented and improved in current research.
In the type 2 latency of EBV infection in NPC, the expressed EBNA1,
LMP1, LMP2 proteins and non-coding RNAs such as EBER, BART and BART
miRNA may carry out key roles in regulating the ferroptosis process
(107–109).
EBNA1, as an important nuclear antigen during EBV
latency, not only participates in the replication and maintenance
of the viral genome but also affects the physiological functions of
host cells by regulating intracellular signaling pathways. Studies
have shown that EBNA1 can affect the expression of intracellular
antioxidant-related genes by activating the NF-κB signaling
pathway. The activation of the NF-κB signaling pathway can promote
the expression of GPX4 (110–113). As a key inhibitor of ferroptosis,
GPX4 can inhibit the occurrence of ferroptosis by reducing LPOs to
non-toxic alcohols. Therefore, EBNA1 may upregulate the expression
of GPX4 by activating the NF-κB signaling pathway, thereby
inhibiting ferroptosis in NPC cells and providing favorable
conditions for the survival of cancer cells (114).
As a transmembrane protein, LMP1 can mimic the
signal transduction function of members of the tumor necrosis
factor receptor family and activate a variety of intracellular
signaling pathways, such as MAPK and PI3K/Akt (115,116). Among them, the activation of the
PI3K/Akt signaling pathway can promote the degradation of
intracellular iron regulatory protein 2 (IRP2). IRP2 can bind to
the mRNAs of ferritin heavy chain (FTH1) and ferritin light chain
(FTL), inhibiting their translation and thus reducing the synthesis
of intracellular ferritin (117,118). Ferritin is an important protein
for intracellular iron storage; a decrease in ferritin content
leads to an increase in intracellular free iron levels, which in
turn promotes the occurrence of ferroptosis. However, after LMP1
activates the PI3K/Akt signaling pathway, it can increase the
expression of FTH1 and FTL by promoting the degradation of IRP2,
thereby increasing the intracellular ferritin content and reducing
free iron levels, which inhibits ferroptosis in NPC cells (119,120). In addition, LMP1 can also
upregulate the expression of SLC7A11 by activating the MAPK
signaling pathway. SLC7A11 is an important component of the Xc-,
which can promote the entry of cystine into cells and provide raw
materials for the synthesis of GSH. GSH is an important coenzyme
for GPX4 to exert its antioxidant effect; an increase in GSH
content can enhance the activity of GPX4 and further inhibit the
occurrence of ferroptosis (121–124).
LMP2 protein mainly includes two subtypes: LMP2A and
LMP2B, among which LMP2A carries out an important role in the
occurrence and development of NPC. LMP2A can activate signaling
pathways such as PI3K/Akt and MAPK by mimicking the signal
transduction of the B-cell receptor and its regulation of
ferroptosis may be similar to that of LMP1. In addition, studies
have found that LMP2A can affect the expression of intracellular
iron metabolism-related genes, such as downregulating the
expression of TFR1 (125–128). TFR1 is an important receptor for
iron uptake by cells; a decrease in TFR1 expression reduces iron
uptake by cells, lowers intracellular iron levels and thereby
inhibits the occurrence of ferroptosis.
EBER is a small non-coding RNA encoded by EBV,
mainly including two types: EBER1 and EBER2. Although EBER does not
encode proteins, it can regulate the physiological functions and
signaling pathways of cells by interacting with a variety of host
cell proteins. Studies have shown that EBER can induce the
production of IFNs by activating the toll-like receptor 3 signaling
pathway and IFN can affect the balance of intracellular iron
metabolism and antioxidant systems. In addition, EBER can also
interact with RNA-activated protein (RIG-I) to activate downstream
signaling pathways and regulate the expression of associated genes,
which may further affect ferroptosis. For example, after EBER
activates the RIG-I signaling pathway, it can promote the
production of pro-inflammatory cytokines and some pro-inflammatory
cytokines can upregulate the expression of SLC7A11 and inhibit the
occurrence of ferroptosis.
EBV lytic phase proteins, such as BGLF5 and BALF3,
not only cause host genome instability but also may regulate
ferroptosis in NPC cells (103).
BGLF5 is a DNase expressed during the EBV lytic phase, which can
degrade the DNA of host cells and viruses, leading to genome
instability. Genome instability causes an increase in intracellular
oxidative stress levels and oxidative stress is one of the
important inducers of ferroptosis (129–131). By degrading DNA, BGLF5 may
increase intracellular ROS levels; ROS can attack intracellular
lipid molecules, trigger LPO reactions and thereby promote the
occurrence of ferroptosis. In addition, BGLF5 may also regulate the
cellular stress response by affecting the expression of
intracellular DNA damage repair-related genes, further influencing
the process of ferroptosis. For example, BGLF5 can inhibit the
expression of DNA damage repair proteins, preventing cells from
effectively repairing DNA damage, leading cells to be in a
continuous stress state and increasing their sensitivity to
ferroptosis (132,133).
BALF3 is a DNA helicase expressed during the EBV
lytic phase and is involved in the replication and packaging of
viral DNA. In the process of exerting its biological functions,
BALF3 may affect the intracellular redox balance and iron
metabolism. Studies have shown that BALF3 can interact with certain
intracellular antioxidant proteins, inhibiting their activity,
reducing the antioxidant capacity of cells and thereby increasing
the sensitivity of cells to ferroptosis (134–136). In addition, BALF3 may also
regulate the expression of iron metabolism-related genes, such as
upregulating the expression of TFR1, increasing iron uptake by
cells, raising intracellular iron levels and promoting the
occurrence of ferroptosis (137,138).
In addition to BGLF5 and BALF3, the EBV lytic phase
also expresses a variety of other proteins, such as ZEBRA (BZLF1)
and RTA (BRLF1). These proteins carry out key roles in initiating
EBV lytic infection and may also regulate ferroptosis. For example,
ZEBRA can regulate the expression of intracellular oxidative stress
and iron metabolism-related genes by activating a variety of
signaling pathways, thereby influencing the occurrence of
ferroptosis (139,140). RTA can also interact with host
cell proteins, affecting the physiological functions and signaling
pathways of cells, which may indirectly affect ferroptosis
(141,142).
In summary, EBV can regulate the ferroptosis process
of NPC cells from multiple aspects, including iron metabolism,
lipid metabolism and antioxidant systems, through its
latency-related molecules and lytic phase-related proteins.
In-depth study of the mechanism by which EBV regulates ferroptosis
in NPC can not only improve the research on the pathogenesis of EBV
and NPC but also provide new targets and strategies for the
treatment of NPC (102,143,144). For example, designing
corresponding inhibitors or antagonists targeting key molecules or
signaling pathways involved in EBV-regulated ferroptosis may
enhance the sensitivity of NPC cells to ferroptosis and improve the
therapeutic effect of NPC (145,146) (Table
I).
Genomic instability refers to an increased tendency
for errors in DNA replication or repair, leading to changes such as
mutations and deletions in the genome. Causes for genomic
instability include endogenous abnormal cellular processes and
exogenous environmental factors (such as radiation, chemicals and
viruses) (147). There is evidence
indicating that genomic instability is key in the development of
NPC and several factors can promote the genomic instability of NPC.
For example, EBV can cause DNA damage, inhibit the DNA repair
mechanism of infected cells and increase the risk of mutations and
chromosomal abnormalities. Exposure to environmental factors such
as tobacco smoke, alcohol, formaldehyde and nitrosamines is
associated with DNA damage and genomic instability, which can
trigger NPC (148–150). Genetic factors (such as mutations
in tumor suppressor genes or DNA repair genes) can also increase
the susceptibility to NPC by impairing the ability of a cell to
maintain genomic stability.
As a malignant tumor, NPC has complex and diverse
clinical manifestations and early diagnosis is difficult. The
clinical manifestations of NPC vary with the progression of the
disease course. In the early stage, NPC may have no obvious
symptoms. Some patients with NPC may have mild symptoms such as
blood in nasal discharge or nasal bleeding. These symptoms are
often ignored by patients, thus delaying the best treatment
opportunity. As the disease progresses, the symptoms of NPC
gradually become more obvious, including nasal bleeding, ulcers or
cauliflower-like masses, tinnitus, hearing loss, nasal congestion,
persistent unilateral headache or eye symptoms (151,152). Currently, radiotherapy alone or in
combination with chemotherapy is the main treatment method for NPC;
however, a large number of patients succumb to NPC due to
recurrence and tumor metastasis. Distant metastasis is an important
cause of treatment failure and mortality in patients with NPC.
Previous studies have shown the key role of ferroptosis in tumor
metastasis, emphasizing the importance of ferroptosis in tumor
growth and metastasis (13,153–155). Determining new anti-cancer
strategies and discovering new drugs that induce ferroptosis will
be beneficial for improving the cure rate of advanced patients with
NPC (Table I).
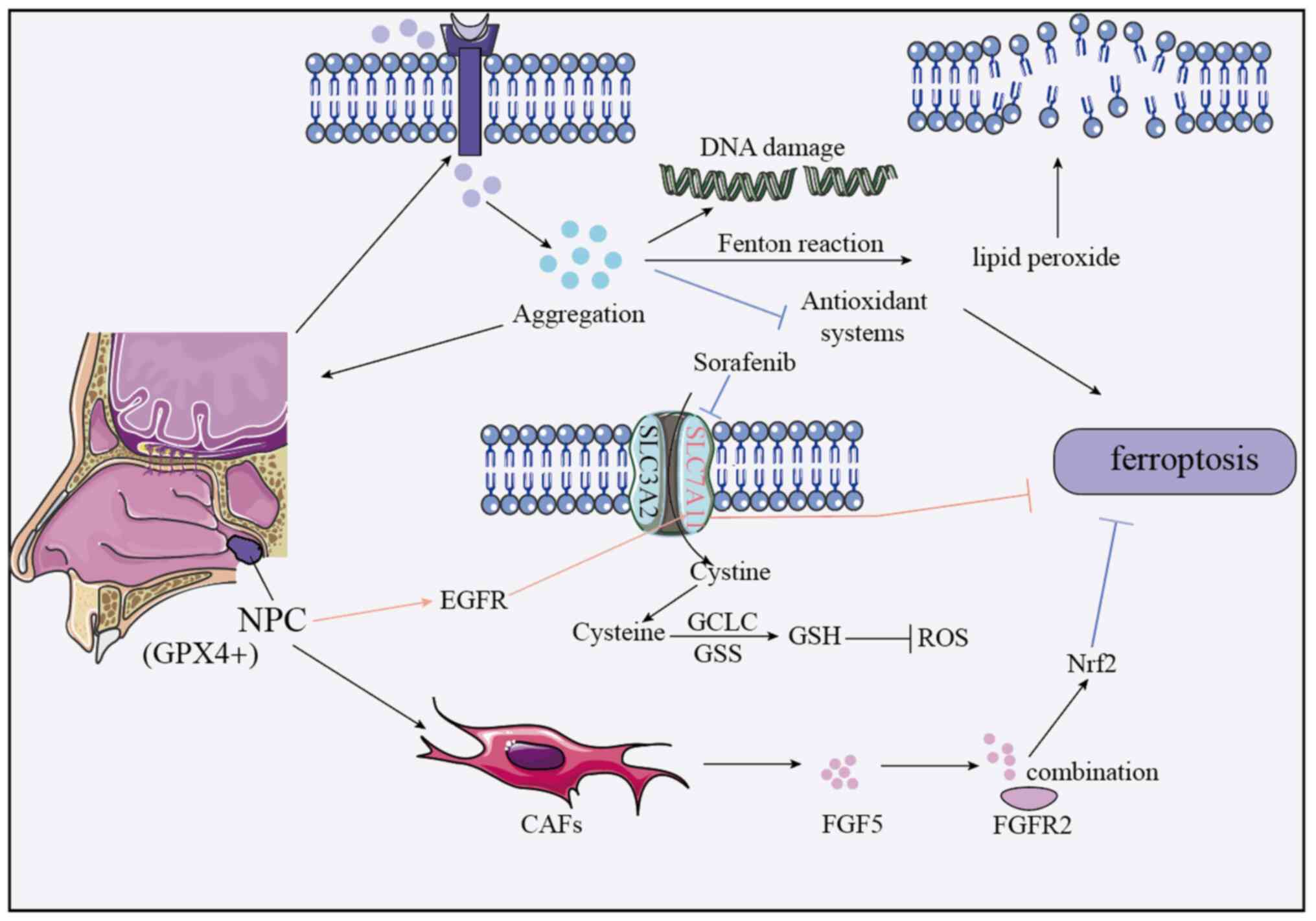
NPC development is associated with abnormal iron
metabolism. Cancer cells, with higher iron demand, accumulate
intracellular iron by increasing Trf for enhanced uptake and
regulating genes to reduce iron transport/storage (156). High iron levels trigger Fenton
reactions, leading to LPO accumulation, membrane damage and
exacerbated ferroptosis (possibly via antioxidant system
inhibition). Iron imbalance also fuels proliferation, oxidative
stress and DNA damage, accelerating progression (Fig. 2).
Ferroptosis interacts with other pathways in
regulating NPC, such as the FGF5/FGFR2/Nrf2 pathway inhibits
ferroptosis and reduces cisplatin sensitivity. Cancer-associated
fibroblasts (CAFs)-secreted FGF5 binds FGFR2, activating Nrf2 to
inhibit ferroptosis (159),
indicating a role for CAFs and potential targets (Fig. 2).
GPX4, a key antioxidant enzyme converting lipid
peroxides to inactive forms, has higher positivity in
nasopharyngeal carcinoma (NPC) tissues than normal nasopharyngeal
tissues (102). Its expression
associates with clinical features of NPC: Higher in stage III–IV
vs. I–II NPC, poorly vs. well-differentiated tumors, patients with
vs. without lymph node metastasis, and those with poor vs. good
radiotherapy response. Moreover, GPX4-positive NPC cases have lower
5-year survival rates than GPX4-negative cases (160), indicating high GPX4 as a poor
prognosis marker for NPC (Fig.
2).
The protein arginine methyltransferase (Prmt) family
catalyzes the methylation of arginine residues in both histones and
non-histone proteins. As a key post-translational modification,
arginine methylation is widely involved in regulating various
cellular processes. Prmt4, also known as coactivator-associated
arginine methyltransferase 1, is the first identified member of the
PRMT family. It can catalyze the asymmetric dimethylation of
arginine residues in protein substrates, carries out a key role in
the regulation of gene transcription and participates in the
modulation of multiple cellular processes (161).
Studies have shown that Prmt4 is overexpressed in a
variety of tumors, including breast cancer, prostate cancer and
colorectal cancer (162–164). Its overexpression activates key
factors of multiple oncogenic signaling pathways, such as FOS,
E2F1, Wnt/β-catenin and nuclear receptor coactivator 3
(NCOA3/AIB1), thereby creating a favorable microenvironment for
tumor growth, invasion and metastasis. A study by Pu et al
(161) further revealed that the
upregulation of PRMT4 reduces the sensitivity of
cisplatin-resistant NPC cells to erastin-induced ferroptosis
through mitochondrial damage; additionally, the interaction between
PRMT4 and Nrf2 promotes the enzymatic methylation activity of
PRMT4. These findings indicate that m6A methylation enhances the
stability of PRMT4 in cisplatin-resistant NPC cells, thereby
affecting the erastin-induced ferroptosis process.
Currently, research on PRMT4 is mainly in the
preclinical stage. Although notable achievements have been made in
cellular and animal experiments, progress in clinical trials is
relatively slow. The application of PRMT4 as a therapeutic target
for tumors faces numerous challenges in clinical practice. Firstly,
due to the complexity of the human physiological environment,
PRMT4-targeted drugs are difficult to act precisely and tend to
interfere with the normal physiological functions of healthy cells,
leading to adverse reactions such as myelosuppression and liver
injury. Secondly, tumor cells exhibit high heterogeneity, and there
are key differences in PRMT4-related characteristics (including
expression level, activity and associated signaling pathways) among
tumor cells from patients with different types of cancer or even
within the same cancer type, making it difficult to develop a
unified and effective treatment regimen. Thirdly, long-term use of
PRMT4 inhibitors may lead to tumor drug resistance; tumor cells can
evade the effects of drugs through alternative pathways or gene
mutations, thereby impairing therapeutic efficacy (161). Therefore, to promote the
translational application of PRMT4-targeted tumor therapy in the
future, it is necessary to optimize the specificity of drugs and
reduce their toxic and side effects by leveraging structural
biology and targeted delivery technologies; establish personalized
diagnosis and treatment regimens through multi-omics stratification
and predictive models; and clarify the mechanisms of drug
resistance while exploring the combined use of PRMT4 inhibitors
with ferroptosis inducers (such as erastin) and chemotherapeutic
drugs (such as cisplatin) to reverse drug resistance (Table II).
GSH S-transferase mu 3 (GSTM3) is a member of the
GSH S-transferase family and has multiple effects on the
progression of various malignant tumors (160). To investigate the effect of GSTM3
on ionizing radiation (IR; induced ferroptosis in vivo,
researchers subcutaneously injected GSTM3-overexpressing stable
5–8F cells into nude mice, leading to the formation of palpable
tumors (174,175). Subsequently, the mice bearing
xenograft tumors received conventional IR treatment. The results
showed that the body weight of the mice remained stable throughout
the treatment period (176,177).
Compared with the control group, in the xenograft model, GSTM3
overexpression alone did not exhibit any effect on tumor growth,
while IR effectively inhibited tumor growth. Notably, compared with
the group treated with IR alone, the combination of GSTM3
overexpression and IR treatment led to a notable reduction in tumor
size and weight. 4-Hydroxy-2-nonenal (4-HNE) serves as a
ferroptosis marker reflecting the level of LPO. Immunohistochemical
staining showed that IR moderately increased the contents of GSTM3
and 4-HNE (178–180). In addition, GSTM3 overexpression
combined with IR treatment resulted in a marked increase in the
signal level of 4-HNE (181,182). Overall, these results indicate
that GSTM3 enhances IR-mediated ferroptosis and improves the
radiosensitivity of NPC.
To the best of our knowledge, currently, clinical
research on GSTM3 is relatively limited. Expression of GSTM3 is
associated with the inhibition of breast cancer stem cell
phenotypes and favorable clinical prognosis (183,184). Additionally, chemotherapeutic
drugs can inhibit GSTM3 expression, which promotes the enrichment
of breast cancer stem cells and ultimately leads to tumor
recurrence and metastasis (182,185). In clinical application, due to the
functional redundancy of the GSTM3 family and high heterogeneity of
tumor cells, single-target intervention on GSTM3 shows poor
efficacy, and it is difficult to develop a unified targeted
treatment plan. In the future, it is necessary to analyze the
functional cooperation mechanism between GSTM3 and its family
members, and construct a patient stratification model using
multi-omics technology to promote the translation of GSTM3-related
targeted strategies from basic research to clinical practice,
thereby providing a new direction for improving tumor treatment
efficacy (186) (Table II).
Ferroptosis is triggered by LPO and is strictly
regulated by SLC7A11 and SLC3A2, which are key components of the
cystine-glutamate antiporter (187). Due to the inhibition of LPO, the
downregulation of GPX4 can directly or indirectly trigger
ferroptosis. To determine the specific molecular mechanism of
BBR-induced ferroptosis, Wu et al (187) treated NPC cells with different
concentrations of BBR and detected the mRNA and protein levels of
GPX4, SLC7A11 and SLC3A2. The results showed that the protein
levels of GPX4, SLC7A11 and SLC3A2 in S18 and 5–8F NPC cells
decreased in a dose-dependent manner. Meanwhile, the mRNA levels of
GPX4, SLC7A11 and SLC3A2 also decreased (188–190). Moreover, the use of deferoxamine
(DFO) and Fer-1 reversed the expression levels of proteins and
mRNAs associated with BBR-induced ferroptosis. In addition, the
results of the study on the in vivo anti-metastatic effect
of berberine (BBR) on nasopharyngeal carcinoma (NPC) cells (using a
nude mouse xenograft model) showed that the number of metastatic
lesions in the BBR treatment group was reduced compared with that
in the control group (191).
Moreover, compared with the control group, the proteins of GPX4,
SLC7A11 and SLC3A2 were elevated, which was consistent with the
in vitro results of the downregulation of the expression of
GPX4, SLC7A11 and SLC3A2 in the BBR treatment group (190,192). These findings indicate that BBR
considerably inhibits NPC metastasis both in vitro and in
vivo. In conclusion, GPX4 is a major molecule and the Xc-/GPX4
axis system carries out an important role in BBR-induced
ferroptosis of NPC cells (189,193).
In terms of clinical trial progress, studies have
shown that BBR can inhibit high glucose-induced ferroptosis in
cells associated with diabetic retinopathy by activating the
Nrf2/HO-1/GPX4 pathway (194–196), providing a theoretical basis for
its application in the treatment of associated diseases. Although
in vitro and animal experiments have confirmed that BBR can
inhibit tumor cell growth and induce ferroptosis (197–200), its clinical application in NPC
still faces challenges. Specifically, the complex human
physiological environment leads to differences in the
pharmacokinetics of BBR between in vivo and in vitro
settings, making it difficult to ensure that BBR acts precisely on
NPC cells to induce ferroptosis while avoiding damage to normal
cells. Additionally, NPC cells exhibit high heterogeneity, patients
vary in their sensitivity to BBR and the expression of molecules
related to the Xc−/GPX4 axis, which hinders the
development of a unified and effective clinical treatment regimen
(201–203). In the future, more large-scale,
multi-center clinical trials are needed to investigate the safety
and efficacy of BBR in humans, explore personalized treatment
strategies and promote the translation of BBR from basic research
to clinical application in NPC treatment (Table II).
Numerous studies have shown that the NF-κB pathway
carries out an important role in the regulation of oxidative stress
and ferroptosis (204–208). Based on this, Luo et al
(209) speculated that
isoquercitrin might play a promoting role in the oxidative stress
and ferroptosis processes of NPC cells by regulating the NF-κB
pathway (210,211). By detecting the expression levels
of proteins associated with the NF-κB pathway, it was found that
isoquercitrin markedly reduced the ratios of phosphorylated
(p)-p65/p65 and p-IκB/IκB and simultaneously inhibited the
expression of IL-1β. These results indicate that isoquercitrin can
inhibit the activation of the NF-κB pathway (212,213).
In addition, as a key molecule regulating various
metabolic processes (including oxidative stress), the activity of
AMPK is also affected by isoquercitrin. Zhang et al
(214) showed that isoquercitrin
markedly reduced the ratio of p-AMPK/AMPK, indicating that it has
an inhibitory effect on the activity of AMPK. These results suggest
that isoquercitrin may carry out a role in NPC by inhibiting the
AMPK/NF-κB p65 signaling axis. To further verify this mechanism,
Luo et al (209)
established a xenograft tumor model. Analysis revealed that
isoquercitrin markedly reduced tumor weight, however, had no
obvious effect on the body weight of the mice. At the same time,
the level of LPO in the isoquercitrin treatment group was notably
increased, while the expressions of ferroptosis-related markers
(such as ATF4, xCT, GPX4 and HO-1) were considerably decreased,
indicating that isoquercitrin can induce ferroptosis in vivo
(215,216). In conclusion, isoquercitrin may
inhibit tumorigenesis of NPC in vivo, enhance oxidative
stress and promote ferroptosis by inhibiting the AMPK/NF-κB p65
signaling pathway (209,217).
To the best of our knowledge, to date, there are no
publicly available dedicated clinical studies on isoquercitrin for
NPC. However, research has explored its potential in other diseases
and tumor types: for instance, in early clinical observations of
colorectal cancer, isoquercitrin combined with chemotherapy showed
a synergistic effect in inhibiting tumor growth without notably
increasing adverse reactions, initially demonstrating its safety
and potential for combined therapy (218). In small-sample trials for
ulcerative colitis (a chronic inflammation-related disease),
isoquercitrin alleviated intestinal inflammation by regulating the
NF-κB pathway (219,220), this mechanism shares commonality
with the ‘NF-κB pathway inhibition’ observed in NPC research
(221), providing an indirect
reference value. Nevertheless, to promote the application of
isoquercitrin in NPC treatment, targeted Phase I and II clinical
trials are still needed to verify its pharmacokinetic
characteristics, the efficacy of monotherapy or combined therapy
and long-term safety. Additionally, it is necessary to clarify the
differences in efficacy across patients with NPC with different
molecular subtypes, so as to provide a basis for formulating
subsequent clinical protocols (222–226) (Table
II).
As a key element in the mitochondrial respiratory
chain, iron carries out an important role in the process of
ferroptosis. Lipid hydroperoxides are considered to be the main
driving force and marker of ferroptosis. To explore the mechanism
of action of CuB, Huang et al (227) detected the concentrations of
intracellular iron and lipid peroxides. The results showed that
after treatment with CuB, the contents of intracellular iron and
lipid peroxides increased considerably in a dose-dependent manner,
and this effect could be effectively reversed by inhibitors such as
DFO, CPX and Fer-1. In addition, GPX4, as a key regulatory factor
of ferroptosis, its expression level decreased markedly after CuB
treatment, further supporting the role of CuB in inducing
ferroptosis.
To the best of our knowledge, there are currently
no publicly available clinical trial data on CuB for NPC, and it
has not yet entered the formal clinical trial phase, with only some
preliminary explorations conducted. To promote the clinical
translation of CuB in the future, targeted Phase II and III
clinical trials are still needed to evaluate the objective response
rate, progression-free survival, and long-term safety (especially
the effects on the hematopoietic system and liver/kidney function)
of its monotherapy or combined therapy regimens, thereby providing
sufficient clinical evidence (Table
II).
DSF is a drug clinically used for the treatment of
alcoholism, which exerts its effect by inhibiting the activity of
aldehyde dehydrogenase (228).
Studies have shown that DSF has potential application value in
cancer treatment. The combined action of DSF and Cu can induce the
aggregation of NPL4, leading to complex cellular phenotypes and
ultimately triggering cell death (229–231). Experiments have shown that DSF/Cu
treatment upregulates the expression of p53 protein and its
downstream targets p21 and BAX, and this effect can be reversed by
the p53 inhibitor Pifithrin-α (232). In addition, DSF/Cu markedly
increases the level of lipid ROS in 5–8F cells, and the ROS
scavenger N-acetylcysteine can partially reverse this phenomenon.
In the 5–8F xenograft model, DSF/Cu markedly inhibits tumor growth
without causing changes in the body weight of the mice (233–235). These results indicate that DSF/Cu
carries out an important role in the treatment of NPC by inducing
ROS-mediated ferroptosis and has the potential to be used as an
adjuvant therapeutic drug in clinical practice (236,237).
Although DSF/Cu shows promising prospects in NPC
treatment research, its clinical application faces challenges: DSF
has a short half-life and is easily metabolized into inactive
substances after oral administration, making it difficult to
maintain effective concentrations (238,239); the complex human physiological
environment and pronounced individual differences in
pharmacokinetics complicate the determination of precise dosages;
additionally, tumor cell heterogeneity increases the difficulty of
personalized treatment (240,241). In the future, it is necessary to
deepen basic research to clarify the molecular mechanism of
DSF/Cu-induced ferroptosis and differences across NPC subtypes,
conduct Phase I–III clinical trials to verify its pharmacokinetics,
efficacy and safety, screen patients sensitive to DSF/Cu with
precision medicine technologies (such as genetic testing and liquid
biopsy), and explore innovative drug delivery systems to improve
pharmacokinetic properties of DSF, all to promote its development
as a clinical adjuvant treatment for NPC (242,243) (Table
II).
P4H is a heterotetramer composed of P4HA subtypes
(P4HA1, P4HA2 and P4HA3) and P4HB, forming P4H1, P4H2 and P4H3
holoenzymes respectively. It carries out a key role in the proline
hydroxylation of procollagen, catalyzing the formation of
hydroxyproline from the proline residues in the Xaa-Pro-Gly
triplet, which is essential for the folding of procollagen into a
stable triple-helix structure and its secretion out of the cell
(244,245). In addition, P4H also regulates the
hydroxylation modification of proteins containing collagen-like
sequences (246,247) (such as AGO2).
Previous studies have found that P4HA1 is a novel
regulator of ferroptosis. Its overexpression enhances the
ferroptosis resistance of NPC cells by activating HMGCS1 (248,249). Meanwhile, the P4HA1/HMGCS1
regulatory axis also promotes the proliferation of NPC cells, as
well as the survival and ferroptosis resistance of NPC cells that
are separated from the extracellular matrix (248,250,251).
Currently, relevant research on P4HA1 is mostly in
the stages of basic research and model validation, but preliminary
attempts have been made in other cancer fields: A retrospective
study on non-small cell lung cancer revealed that high expression
of P4HA1 is associated with poor prognosis in patients, providing
indirect evidence for its potential as a prognostic marker and
therapeutic target (252,253); In early preclinical trials for
pancreatic cancer, small-molecule inhibitors of P4HA1 were able to
inhibit tumor growth without obvious toxicity, laying a foundation
for research on P4HA1 in NPC (254). In the future, it is first
necessary to conduct large-scale retrospective studies to clarify
the association between P4HA1 expression and the prognosis, staging
and treatment response of patients with NPC. On this basis, Phase
I/II clinical trials of P4HA1 inhibitors should be advanced to
evaluate their safety, pharmacokinetic characteristics and
efficacy, thereby providing evidence for clinical translation
(Table II).
Icaritin has a variety of pharmacological effects,
including antioxidant effects, prevention and treatment of
osteoporosis, improvement of cardiovascular function and protection
against neurodegenerative damage (255,256). In terms of anti-tumor effects,
icaritin has been proven to inhibit the proliferation and induce
apoptosis of a variety of tumor cells. These effects make icaritin
a potential anti-tumor drug.
Studies have shown that icaritin can regulate the
expression of proteins associated with ferroptosis. For example, In
NPC cells, the expression of ACSL4, a marker protein of
ferroptosis, is upregulated, while the expression of GPX4 is
downregulated. Similarly, when icaritin is combined with
radiotherapy, it can markedly promote the accumulation of ROS in
NPC cells (255,257,258). The accumulation of ROS will
further lead to DNA damage, such as the upregulation of the
expression of γ-H2AX (259,260).
These damages will exacerbate the death of NPC cells, thereby
improving the effect of radiotherapy. In addition, icaritin can
also cause NPC cells to arrest in the G2 phase. This arrest will
further affect the proliferation and division ability of cells,
thus enhancing the killing effect of radiotherapy on NPC cells. The
aforementioned studies indicate that icaritin may enhance the
radiosensitivity of NPC cells by promoting the occurrence of
ferroptosis (261,262).
However, there are still challenges in applying
icariin to the clinical treatment of NPC: It remains unclear
whether the mechanism by which icariin regulates ferroptosis and
enhances radiosensitivity in experiments is fully applicable in the
complex human environment (263,264). Additionally, there is a lack of
clinical data to support balancing efficacy and adverse reactions
(such as mucosal damage and myelosuppression) when combined with
radiotherapy (256,266). To address these issues in the
future, breakthroughs can be made in three aspects: Conducting
clinical sample studies on NPC to verify the effect of icariin on
ferroptosis-related pathways and the impact of molecular subtypes;
developing local formulations for the nasopharynx (such as
nano-sprays) to increase drug concentration in tumor tissues; and
advancing small-sample Phase I/II clinical trials to explore the
safe dosage and administration timing of icariin combined with
radiotherapy (267) (Table II).
Nasopharyngeal carcinoma (NPC) is a head and neck
malignant tumor, with >70% of new cases occurring in East Asia
and Southeast Asia (268–270), and its treatment has been a major
focus of clinical and research attention (271). Traditional treatment methods, such
as radiotherapy and chemotherapy, have achieved curative effects,
but present side effects and drugs that need to be addressed. In
recent years, with the in-depth study of the mechanism of cell
death, ferroptosis, as a new type of programmed cell death, has
provided a new perspective and strategy for the treatment of NPC
(13,272–274).
Ferroptosis, also referred to as iron-dependent
LPO-mediated cell death, is a unique form of cell death driven by
iron ions and LPO. Its core characteristic is the intracellular
accumulation of iron ions coupled with an uncontrolled LPO
reaction, which ultimately results in cell membrane rupture and
subsequent cell death. Compared with traditional cell death methods
such as apoptosis and necrosis, ferroptosis has its own uniqueness
in morphological, biochemical and genetic characteristics.
Numerous studies have shown that ferroptosis
carries out an important role in the occurrence, development and
treatment of NPC (10,275–278). Conversely, the abnormal iron
metabolism and increased LPO levels in NPC cells make them more
sensitive to ferroptosis. On the other hand, some chemotherapeutic
drugs, such as cisplatin, have been shown to be able to induce
ferroptosis in NPCnuo cells (279–281), thereby exerting an anti-tumor
effect. Based on the important role of ferroptosis in NPC,
researchers have begun to explore the application of ferroptosis
inducers in the treatment of NPC. For example, Erastin, as a small
molecule compound that can induce ferroptosis in a variety of
cancer cells, has been proven to enhance the sensitivity of
traditional anti-cancer drugs to NPC cells (161,282). In addition, some new
chemotherapeutic drugs and targeted therapeutic drugs are also
being developed. They induce ferroptosis in NPC cells by regulating
ferroptosis-related signaling pathways, such as GPX4 and SLC7A11.
Although ferroptosis inducers have shown great potential in the
treatment of NPC, ferroptosis inhibitors also carry out a role that
cannot be ignored. A number of studies have shown that ferroptosis
inhibitors can protect normal cells from ferroptosis, thereby
reducing the side effects of chemotherapeutic drugs (283–285). In addition, ferroptosis inhibitors
can also be used in combination with ferroptosis inducers. By
regulating the degree and speed of ferroptosis, a more precise
therapeutic effect can be achieved.
Although the application of ferroptosis in the
treatment of NPC has made progress, there are still several issues
that remain to be solved. For example, how to precisely regulate
the degree and speed of ferroptosis to achieve the best therapeutic
effect; how to overcome the problem of drug resistance and improve
the sensitivity of ferroptosis inducers; how to develop safer and
more effective ferroptosis inducers and inhibitors and new
treatment methods all need to go through clinical trials and
evaluations to ensure their safety and effectiveness. In the
future, with the in-depth study of the mechanism of ferroptosis and
the development of new drugs, it is considered that ferroptosis
will carry out a greater role in the treatment of NPC, and it is
expected to achieve more precise and effective therapeutic
effects.
Not applicable.
The present review was supported by the grants from Yan'an
science and technology bureau (grant no. 2024SF-YBXM-037
Y.Y.L).
Not applicable.
SB was responsible for writing and revising the
manuscript. YG was responsible for the preparation of figures and
revisions of the present review. QQ was responsible for the
revisions of the present review. JQ was responsible for the
revisions of the present review. YY was responsible for revising
the article. All authors contributed to the article and all authors
read and approved the final manuscript. Data authentication not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Lim CY, Ng GWY, Goh CK, Lee MKC, Cheong I,
Ooi EE, Liu J, West RB, Loh KS and Tay JK: Impact of high-risk EBV
strains on nasopharyngeal carcinoma gene expression. Oral Oncol.
157:1069412024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yip PL, Lee AWM and Chua MLK: Adjuvant
chemotherapy in nasopharyngeal carcinoma. Lancet Oncol. 24:713–715.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee S, Hwang N, Seok BG, Lee S, Lee SJ and
Chung SW: Autophagy mediates an amplification loop during
ferroptosis. Cell Death Dis. 14:4642023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kinowaki Y, Taguchi T, Onishi I, Kirimura
S, Kitagawa M and Yamamoto K: Overview of ferroptosis and synthetic
lethality strategies. Int J Mol Sci. 22:92712021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alborzinia H, Chen Z, Yildiz U, Freitas
FP, Vogel FCE, Varga JP, Batani J, Bartenhagen C, Schmitz W, Büchel
G, et al: LRP8-mediated selenocysteine uptake is a targetable
vulnerability in MYCN-amplified neuroblastoma. EMBO Mol Med.
15:e180142023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Floros KV, Cai J, Jacob S, Kurupi R,
Fairchild CK, Shende M, Coon CM, Powell KM, Belvin BR, Hu B, et al:
MYCN-amplified neuroblastoma is addicted to iron and vulnerable to
inhibition of the system Xc-/glutathione axis. Cancer Res.
81:1896–1908. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Yang Q, Su Y, Ji Y, Li G, Yang X, Xu
L, Lu Z, Dong J, Wu Y, et al: MYCN mediates TFRC-dependent
ferroptosis and reveals vulnerabilities in neuroblastoma. Cell
Death Dis. 12:5112021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou J, Guo T, Zhou L, Bao M, Wang L, Zhou
W, Tan S, Li G, He B and Guo Z: The ferroptosis signature predicts
the prognosis and immune microenvironment of nasopharyngeal
carcinoma. Sci Rep. 13:18612023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teng Y, Gao L, Mäkitie AA, Florek E,
Czarnywojtek A, Saba NF and Ferlito A: Iron, ferroptosis, and head
and neck cancer. Int J Mol Sci. 24:151272023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mao C, Wang M, Zhuang L and Gan B:
Metabolic cell death in cancer: Ferroptosis, cuproptosis,
disulfidptosis, and beyond. Protein Cell. 15:642–660. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo T, Wang Y and Wang J: Ferroptosis
assassinates tumor. J Nanobiotechnology. 20:4672022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu Y, Wang Q, Li X, Chen Y and Xu G:
Itraconazole attenuates the stemness of nasopharyngeal carcinoma
cells via triggering ferroptosis. Environ Toxicol. 36:257–266.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Du Y, Zhou Y, Chen Q, Luo Z, Ren Y,
Chen X and Chen G: Iron and copper: critical executioners of
ferroptosis, cuproptosis and other forms of cell death. Cell Commun
Signal. 21:3272023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hadian K and Stockwell BR: SnapShot:
Ferroptosis. Cell. 181:1188–1188.e1. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma TL, Chen JX, Zhu P, Zhang CB, Zhou Y
and Duan JX: Focus on ferroptosis regulation: Exploring novel
mechanisms and applications of ferroptosis regulator. Life Sci.
307:1208682022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gan B: Mitochondrial regulation of
ferroptosis. J Cell Biol. 220:e2021050432021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Guo X, Zeng Y, Mo X, Hong S, He H,
Li J, Fatima S and Liu Q: Oxidative stress induces mitochondrial
iron overload and ferroptotic cell death. Sci Rep. 13:155152023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wahida A and Conrad M: Ferroptosis: Under
pressure! Curr Biol. 33:R269–R272. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lai L, Tan M, Hu M, Yue X, Tao L, Zhai Y
and Li Y: Important molecular mechanisms in ferroptosis. Mol Cell
Biochem. 480:639–658. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XD, Liu ZY, Wang MS, Guo YX, Wang
XK, Luo K, Huang S and Li RF: Mechanisms and regulations of
ferroptosis. Front Immunol. 14:12694512023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Z, Lu M, Chen C, Tong X, Li Y, Yang
K, Lv H, Xu J and Qin L: Holo-lactoferrin: the link between
ferroptosis and radiotherapy in triple-negative breast cancer.
Theranostics. 11:3167–3182. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Kang R and Tang D: Signaling
pathways and defense mechanisms of ferroptosis. FEBS J.
289:7038–7050. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 17:2054–2081.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Seibt T, Wahida A, Hoeft K, Kemmner S,
Linkermann A, Mishima E and Conrad M: The biology of ferroptosis in
kidney disease. Nephrol Dial Transplant. 39:1754–1761. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dutt S, Hamza I and Bartnikas TB:
Molecular mechanisms of iron and heme metabolism. Annu Rev Nutr.
42:311–335. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao Y, Yang M and Liang X: The role of
mitochondria in iron overload-induced damage. J Transl Med.
22:10572024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Zhang Z, Ruan S, Yan Q, Chen Y,
Cui J, Wang X, Huang S and Hou B: Regulation of iron metabolism and
ferroptosis in cancer stem cells. Front Oncol. 13:12515612023.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li S, Zhang G, Hu J, Tian Y and Fu X:
Ferroptosis at the nexus of metabolism and metabolic diseases.
Theranostics. 14:5826–5852. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Venkataramani V: Iron homeostasis and
metabolism: Two sides of a coin. Adv Exp Med Biol. 1301:25–40.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang L, Bian M, Zhang J and Jiang L: Iron
metabolism and ferroptosis in peripheral nerve injury. Oxid Med
Cell Longev. 2022:59182182022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Capelletti MM, Manceau H, Puy H and Peoc'h
K: Ferroptosis in liver diseases: An Overview. Int J Mol Sci.
21:49082020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu L, Xian X, Tan Z, Dong F, Xu G, Zhang M
and Zhang F: The role of iron metabolism, lipid metabolism, and
redox homeostasis in Alzheimer's disease: From the perspective of
ferroptosis. Mol Neurobiol. 60:2832–2850. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lyamzaev KG, Panteleeva AA, Simonyan RA,
Avetisyan AV and Chernyak BV: Mitochondrial lipid peroxidation is
responsible for ferroptosis. Cells. 12:6112023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Do Q and Xu L: How do different lipid
peroxidation mechanisms contribute to ferroptosis? Cell Rep Phys
Sci. 4:1016832023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Naowarojna N, Wu TW, Pan Z, Li M, Han JR
and Zou Y: Dynamic regulation of ferroptosis by lipid metabolism.
Antioxid Redox Signal. 39:59–78. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xin S and Schick JA: PUFAs dictate the
balance of power in ferroptosis. Cell Calcium. 110:1027032023.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cui S, Simmons G Jr, Vale G, Deng Y, Kim
J, Kim H, Zhang R, McDonald JG and Ye J: FAF1 blocks ferroptosis by
inhibiting peroxidation of polyunsaturated fatty acids. Proc Natl
Acad Sci USA. 119:e21071891192022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qiu B, Zandkarimi F, Bezjian CT, Reznik E,
Soni RK, Gu W, Jiang X and Stockwell BR: Phospholipids with two
polyunsaturated fatty acyl tails promote ferroptosis. Cell.
187:1177–1190.e18. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma XH, Liu JH, Liu CY, Sun WY, Duan WJ,
Wang G, Kurihara H, He RR, Li YF, Chen Y and Shang H:
ALOX15-launched PUFA-phospholipids peroxidation increases the
susceptibility of ferroptosis in ischemia-induced myocardial
damage. Signal Transduct Target Ther. 7:2882022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Do Q, Zhang R, Hooper G and Xu L:
Differential contributions of distinct free radical peroxidation
mechanisms to the induction of ferroptosis. JACS Au. 3:1100–1117.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Li Z, Ran Q and Wang P: Sterols in
ferroptosis: From molecular mechanisms to therapeutic strategies.
Trends Mol Med. 31:36–49. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gan B: ACSL4, PUFA, and ferroptosis: New
arsenal in anti-tumor immunity. Signal Transduct Target Ther.
7:1282022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sokol KH, Lee CJ, Rogers TJ, Waldhart A,
Ellis AE, Madireddy S, Daniels SR, House RRJ, Ye X, Olesnavich M,
et al: Lipid availability influences ferroptosis sensitivity in
cancer cells by regulating polyunsaturated fatty acid trafficking.
Cell Chem Biol. 32:408–422.e6. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hirata Y, Ferreri C, Yamada Y, Inoue A,
Sansone A, Vetica F, Suzuki W, Takano S, Noguchi T, Matsuzawa A and
Chatgilialoglu C: Geometrical isomerization of arachidonic acid
during lipid peroxidation interferes with ferroptosis. Free Radic
Biol Med. 204:374–384. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu S, Zhang HL, Li J, Ye ZP, Du T, Li LC,
Guo YQ, Yang D, Li ZL, Cao JH, et al: Tubastatin A potently
inhibits GPX4 activity to potentiate cancer radiotherapy through
boosting ferroptosis. Redox Biol. 62:1026772023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nishida Xavier da Silva T, Friedmann
Angeli JP and Ingold I: GPX4: Old lessons, new features. Biochem
Soc Trans. 50:1205–1213. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen T, Leng J, Tan J, Zhao Y, Xie S, Zhao
S, Yan X, Zhu L, Luo J, Kong L and Yin Y: Discovery of novel potent
covalent glutathione peroxidase 4 inhibitors as highly selective
ferroptosis inducers for the treatment of triple-negative breast
cancer. J Med Chem. 66:10036–10059. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rochette L, Dogon G, Rigal E, Zeller M,
Cottin Y and Vergely C: Lipid peroxidation and iron metabolism: Two
corner stones in the homeostasis control of ferroptosis. Int J Mol
Sci. 24:4492022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guo W, Li K, Sun B, Xu D, Tong L, Yin H,
Liao Y, Song H, Wang T, Jing B, et al: Dysregulated glutamate
transporter SLC1A1 propels cystine uptake via Xc− for
glutathione synthesis in lung cancer. Cancer Res. 81:552–566. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Z, Li Y, Yang Y, Gong Z, Zhu H and Qian
Y: In vivo tracking cystine/glutamate antiporter-mediated
cysteine/cystine pool under ferroptosis. Anal Chim Acta.
1125:66–75. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Koppula P, Zhuang L and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang Y, Zheng L, Shang W, Yang Z, Li T,
Liu F, Shao W, Lv L, Chai L, Qu L, et al: Wnt/beta-catenin
signaling confers ferroptosis resistance by targeting GPX4 in
gastric cancer. Cell Death Differ. 29:2190–2202. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xia C, Xing X, Zhang W, Wang Y, Jin X,
Wang Y, Tian M, Ba X and Hao F: Cysteine and homocysteine can be
exploited by GPX4 in ferroptosis inhibition independent of GSH
synthesis. Redox Biol. 69:1029992024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang F and Min J: DHODH tangoing with GPX4
on the ferroptotic stage. Signal Transduct Target Ther. 6:2442021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hu Q, Wei W, Wu D, Huang F, Li M, Li W,
Yin J, Peng Y, Lu Y, Zhao Q and Liu L: Blockade of GCH1/BH4 axis
activates ferritinophagy to mitigate the resistance of colorectal
cancer to erastin-induced ferroptosis. Front Cell Dev Biol.
10:8103272022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Feng Y, Feng Y, Gu L, Mo W, Wang X, Song
B, Hong M, Geng F, Huang P, Yang H, et al: Tetrahydrobiopterin
metabolism attenuates ROS generation and radiosensitivity through
LDHA S-nitrosylation: Novel insight into radiogenic lung injury.
Exp Mol Med. 56:1107–1122. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Soula M, Weber RA, Zilka O, Alwaseem H, La
K, Yen F, Molina H, Garcia-Bermudez J, Pratt DA and Birsoy K:
Metabolic determinants of cancer cell sensitivity to canonical
ferroptosis inducers. Nat Chem Biol. 16:1351–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lv Y, Wu M, Wang Z and Wang J:
Ferroptosis: From regulation of lipid peroxidation to the treatment
of diseases. Cell Biol Toxicol. 39:827–851. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang S, Kang L, Dai X, Chen J, Chen Z,
Wang M, Jiang H, Wang X, Bu S, Liu X, et al: Manganese induces
tumor cell ferroptosis through type-I IFN dependent inhibition of
mitochondrial dihydroorotate dehydrogenase. Free Radic Biol Med.
193:202–212. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Amos A, Amos A, Wu L and Xia H: The
Warburg effect modulates DHODH role in ferroptosis: A review. Cell
Commun Signal. 21:1002023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Desler C, Durhuus JA, Hansen TLL, Anugula
S, Zelander NT, Bøggild S and Rasmussen LJ: Partial inhibition of
mitochondrial-linked pyrimidine synthesis increases tumorigenic
potential and lysosome accumulation. Mitochondrion. 64:73–81. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tarangelo A, Rodencal J, Kim JT, Magtanong
L, Long JZ and Dixon SJ: Nucleotide biosynthesis links glutathione
metabolism to ferroptosis sensitivity. Life Sci Alliance.
5:e2021011572022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yang C, Zhao Y, Wang L, Guo Z, Ma L, Yang
R, Wu Y, Li X, Niu J, Chu Q, et al: De novo pyrimidine biosynthetic
complexes support cancer cell proliferation and ferroptosis
defence. Nat Cell Biol. 25:836–847. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu Y, Lu S, Wu LL, Yang L, Yang L and
Wang J: The diversified role of mitochondria in ferroptosis in
cancer. Cell Death Dis. 14:5192023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liang D, Feng Y, Zandkarimi F, Wang H,
Zhang Z, Kim J, Cai Y, Gu W, Stockwell BR and Jiang X: Ferroptosis
surveillance independent of GPX4 and differentially regulated by
sex hormones. Cell. 186:2748–64.e22. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun S, Shen J, Jiang J, Wang F and Min J:
Targeting ferroptosis opens new avenues for the development of
novel therapeutics. Signal Transduct Target Ther. 8:3722023.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li P, Xu J, Xu B, Hu X, Xiong Y, Wang Y
and Liu P: NR5A2 (located on chromosome 1q32) inhibits ferroptosis
and promotes drug resistance by regulating phospholipid remodeling
in multiple myeloma. Int J Biol Sci. 21:5789–5801. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Phat NK, Tien NTN, Anh NK, Yen NTH, Lee
YA, Trinh HKT, Le KM, Ahn S, Cho YS, Park S, et al: Alterations of
lipid-related genes during anti-tuberculosis treatment: insights
into host immune responses and potential transcriptional
biomarkers. Front Immunol. 14:12103722023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nguyen HP, Yi D, Lin F, Viscarra JA,
Tabuchi C, Ngo K, Shin G, Lee AY, Wang Y and Sul HS: Aifm2, a NADH
oxidase, supports robust glycolysis and is required for cold- and
diet-induced thermogenesis. Mol Cell. 77:600–617.e4. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lv Y, Liang C, Sun Q, Zhu J, Xu H, Li X,
Li YY, Wang Q, Yuan H, Chu B and Zhu D: Structural insights into
FSP1 catalysis and ferroptosis inhibition. Nat Commun. 14:59332023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Guo J, Chen L and Ma M: Ginsenoside Rg1
suppresses ferroptosis of renal tubular epithelial cells in
sepsis-induced acute kidney injury via the FSP1-CoQ10-
NAD(P)H pathway. Curr Med Chem. 31:2119–2132. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mikac S, Dziadosz A, Padariya M, Kalathiya
U, Fahraeus R, Marek-Trzonkowska N, Chruściel E, Urban-Wójciuk Z,
Papak I, Arcimowicz Ł, et al: Keap1-resistant ΔN-Nrf2 isoform does
not translocate to the nucleus upon electrophilic stress. bioRxiv.
2022.06. 10.495609. 2022.
|
|
78
|
Zhang Q, Sun T, Yu F, Liu W, Gao J, Chen
J, Zheng H, Liu J, Miao C, Guo H, et al: PAFAH2 suppresses
synchronized ferroptosis to ameliorate acute kidney injury. Nat
Chem Biol. 20:835–846. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Su ZY, Siak PY, Lwin YY and Cheah SC:
Epidemiology of nasopharyngeal carcinoma: Current insights and
future outlook. Cancer Metastasis Rev. 43:919–939. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Siak PY, Khoo AS, Leong CO, Hoh BP and
Cheah SC: Current status and future perspectives about molecular
biomarkers of nasopharyngeal carcinoma. Cancers (Basel).
13:34902021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zheng MQ, Wang TM, Liao Y, Xue WQ, He YQ,
Wu ZY, Yang DW, Li DH, Deng CM, Jia YJ, et al: Nasopharyngeal
Epstein-Barr virus DNA loads in high-risk nasopharyngeal carcinoma
families: Familial aggregation and host heritability. J Med Virol.
92:3717–3725. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liao Y, Tong XT, Zhou T, Xue WQ, Wang TM,
He YQ, Zheng MQ, Jia YJ, Yang DW, Wu YX, et al: Unveiling familial
aggregation of nasopharyngeal carcinoma: Insights from oral
microbiome dysbiosis. Cell Rep Med. 6:1019792025. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li W, Liang L, Liu S, Yi H and Zhou Y:
FSP1: A key regulator of ferroptosis. Trends Mol Med. 29:753–764.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ward NP and DeNicola GM: Long-sought
mediator of vitamin K recycling discovered. Nature. 608:673–674.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Feng H, Zhou Y, Wang L, Wang Y, Zhou S and
Tian F: Consumption of processed food and risk of nasopharyngeal
carcinoma: A systematic review and meta-analysis. Transl Cancer
Res. 11:872–879. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhong L, Krummenacher C, Zhang W, Hong J,
Feng Q, Chen Y, Zhao Q, Zeng MS, Zeng YX, Xu M, Zhang X, et al:
Urgency and necessity of Epstein-Barr virus prophylactic vaccines.
NPJ Vaccines. 7:1592022. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lin JH, Wen CP, Jiang CQ, Yuan JM, Chen
CJ, Ho SY, Gao W, Zhang W, Wang R, Chien YC, et al: Smoking and
nasopharyngeal cancer: Individual data meta-analysis of six
prospective studies on 334 935 men. Int J Epidemiol. 50:975–986.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Arfaeinia H, Ghaemi M, Jahantigh A,
Soleimani F and Hashemi H: Secondhand and thirdhand smoke: A review
on chemical contents, exposure routes, and protective strategies.
Environ Sci Pollut Res Int. 30:78017–78029. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Feng R, Chang ET, Liu Q, Cai Y, Zhang Z,
Chen G, Huang QH, Xie SH, Cao SM, Zhang Y, et al: Intake of alcohol
and tea and risk of nasopharyngeal carcinoma: A population-based
case-control study in Southern China. Cancer Epidemiol Biomarkers
Prev. 30:545–553. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
George M, Reddy AP, Reddy PH and
Kshirsagar S: Unraveling the NRF2 confusion: Distinguishing nuclear
respiratory factor 2 from nuclear erythroid factor 2. Ageing Res
Rev. 98:1023532024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yan R, Lin B, Jin W, Tang L, Hu S and Cai
R: NRF2, a superstar of ferroptosis. Antioxidants (Basel).
12:17392023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Yuan L, Wang Y, Li N, Yang X, Sun X, Tian
He and Zhang Y: Mechanism of action and therapeutic implications of
Nrf2/HO-1 in inflammatory bowel disease. Antioxidants (Basel).
13:10122024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang D, Tang L, Zhang Y, Ge G, Jiang X, Mo
Y, Wu P, Deng X, Li L, Zuo S, et al: Regulatory pathways and drugs
associated with ferroptosis in tumors. Cell Death Dis. 13:5442022.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Huang HC, Tantoh DM, Hsu SY, Nfor ON,
Frank CL, Lung CC, Ho CC, Chen CY and Liaw YP: Association between
coarse particulate matter (PM10-2.5) and nasopharyngeal
carcinoma among Taiwanese men. J Investig Med. 68:419–424. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Xue WQ, Wang TM, Huang JW, Zhang JB, He
YQ, Wu ZY, Liao Y, Yuan LL, Mu J and Jia WH: A comprehensive
analysis of genetic diversity of EBV reveals potential high-risk
subtypes associated with nasopharyngeal carcinoma in China. Virus
Evol. 7:veab0102021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Damania B, Kenney SC and Raab-Traub N:
Epstein-Barr virus: Biology and clinical disease. Cell.
185:3652–3670. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Manners O, Murphy JC, Coleman A, Hughes DJ
and Whitehouse A: Contribution of the KSHV and EBV lytic cycles to
tumourigenesis. Curr Opin Virol. 32:60–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Phan TG: Epstein-Barr virus and multiple
sclerosis: The dawn of a new age. Clin Transl Immunology.
12:e14572023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tyler KL: The enigmatic links between
Epstein-Barr virus infection and multiple sclerosis. J Clin Invest.
132:e1604682022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lyu L, Li Q and Wang C: EBV latency
programs: Molecular and epigenetic regulation and its role in
disease pathogenesis. J Med Virol. 97:e705012025. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Murray-Nerger LA, Lozano C, Burton EM,
Liao Y, Ungerleider NA, Guo R and Gewurz BE: The nucleic acid
binding protein SFPQ represses EBV lytic reactivation by promoting
histone H1 expression. Nat Commun. 15:41562024. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Yuan L, Li S, Chen Q, Xia T, Luo D, Li L,
Liu S, Guo S, Liu L, Du C, et al: EBV infection-induced GPX4
promotes chemoresistance and tumor progression in nasopharyngeal
carcinoma. Cell Death Differ. 29:1513–1527. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
He S, Luo C, Shi F, Zhou J and Shang L:
The emerging role of ferroptosis in EBV-associated cancer:
Implications for cancer therapy. Biology (Basel).
13:5432024.PubMed/NCBI
|
|
104
|
Ge A, Xiang W, Li Y, Zhao D, Chen J, Daga
P, Dai CC, Yang K, Yan Y, Hao M, et al: Broadening horizons: The
multifaceted role of ferroptosis in breast cancer. Front Immunol.
15:14557412024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li X, Zhang R, Zhao X and Zhuang X:
Advances in the role of FOXM1 and ferroptosis in the diagnosis,
treatment, and prognosis of hepatocellular carcinoma. Curr Protein
Pept Sci. Oct 28–2025.(Epub ahead of print). View Article : Google Scholar
|
|
106
|
Wu J, Zhu S, Wang P, Wang J, Huang J, Wang
T, Guo L, Liang D, Meng Q and Pan H: Regulators of epigenetic
change in ferroptosis-associated cancer (Review). Oncol Rep.
48:2152022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Li J, Zhang XS, Xie D, Deng HX, Gao YF,
Chen QY, Huang WL, Masucci MG and Zeng YX: Expression of
immune-related molecules in primary EBV-positive Chinese
nasopharyngeal carcinoma: Associated with latent membrane protein 1
(LMP1) expression. Cancer Biol Ther. 6:1997–2004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Abstracts from the 22nd International
Symposium on Signal Transduction at the Blood-Brain Barriers.
Würzburg, Germany. September 11–13, 2019. Fluids Barriers CNS. 16
(Suppl 2):292019.PubMed/NCBI
|
|
109
|
Verhoeven RJA, Tong S, Mok BWY, Liu J, He
S, Zong J, Chen Y, Tsao SW, Lung ML and Chen H: Epstein-Barr virus
BART long non-coding RNAs function as epigenetic modulators in
nasopharyngeal carcinoma. Front Oncol. 9:11202019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Xu H, Koganti S, Li C, McIntosh MT and
Bhaduri-McIntosh S: STAT3, MYC, and EBNA1 cooperate through a
ZC3H18 transcriptional network to regulate survival and
proliferation of EBV-positive lymphomas. PLoS Pathog.
21:e10131662025. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Ji HZ, Chen L, Ren M, Li S, Liu TY, Chen
HJ, Yu HH and Sun Y: CXCL8 promotes endothelial-to-mesenchymal
transition of endothelial cells and protects cells from
erastin-induced ferroptosis via CXCR2-mediated activation of the
NF-κB signaling pathway. Pharmaceuticals (Basel). 16:12102023.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Verhoeven RJA, Tong S, Zong J, Chen Y,
Tsao SW, Pan J and Chen H: NF-κB signaling regulates epstein-barr
virus BamHI-Q-driven EBNA1 expression. Cancers (Basel). 10:1192018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yuan L, Zhang L, Yao N, Wu L, Liu J, Liu
F, Zhang H, Hu X, Xiong Y and Xia C: Upregulation of UGT1A1
expression by ursolic acid and oleanolic acid via the inhibition of
the PKC/NF-κB signaling pathway. Phytomedicine. 92:1537262021.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Iglesias-Matesanz P, Lacalle-Gonzalez C,
Lopez-Blazquez C, Ochieng' Otieno M, Garcia-Foncillas J and
Martinez-Useros J: Glutathione peroxidases: An emerging and
promising therapeutic target for pancreatic cancer treatment.
Antioxidants (Basel). 13:14052024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Morgos DT, Stefani C, Miricescu D, Greabu
M, Stanciu S, Nica S, Stanescu-Spinu II, Balan DG,
Balcangiu-Stroescu AE, Coculescu EC, et al: Targeting PI3K/AKT/mTOR
and MAPK signaling pathways in gastric cancer. Int J Mol Sci.
25:18482024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Tóthová Z, Šemeláková M, Solárová Z, Tomc
J, Debeljak N and Solár P: The role of PI3K/AKT and MAPK signaling
pathways in erythropoietin signalization. Int J Mol Sci.
22:76822021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chen K, Wang J, Guo L, Wang J, Yang L, Hu
T, Zhao Y, Wang X and Zhu Y: Lactobacillus johnsonii L531
ameliorates salmonella enterica serovar typhimurium diarrhea by
modulating iron homeostasis and oxidative stress via the IRP2
pathway. Nutrients. 15:11272023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jia F, Li H, Jiao Q, Li C, Fu L, Cui C,
Jiang H and Zhang L: Deubiquitylase OTUD3 prevents Parkinson's
disease through stabilizing iron regulatory protein 2. Cell Death
Dis. 13:4182022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Jiao Q, Du X, Wei J, Li Y and Jiang H:
Oxidative stress regulated iron regulatory protein IRP2 through
FBXL5-mediated ubiquitination-proteasome way in SH-SY5Y cells.
Front Neurosci. 13:202019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Terzi EM, Sviderskiy VO, Alvarez SW,
Whiten GC and Possemato R: Iron-sulfur cluster deficiency can be
sensed by IRP2 and regulates iron homeostasis and sensitivity to
ferroptosis independent of IRP1 and FBXL5. Sci Adv. 7:eabg43022021.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Dasgupta S and Gan B: Ferroptosis
vulnerability in MYCN-driven neuroblastomas. Clin Transl Med.
12:e9632022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Hill RA and Liu YY:
N6-methyladenosine-RNA methylation promotes expression
of solute carrier family 7 member 11, an uptake transporter of
cystine for lipid reactive oxygen species scavenger glutathione
synthesis, leading to hepatoblastoma ferroptosis resistance. Clin
Transl Med. 12:e8892022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li X, Qi H, Zhang X, Liang H and Zeng N:
Jing-Fang n-butanol extract and its isolated JFNE-C inhibit
ferroptosis and inflammation in LPS induced RAW264.7 macrophages
via STAT3/p53/SLC7A11 signaling pathway. J Ethnopharmacol.
316:1166892023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li X, Zou Y, Fu YY, Xing J, Wang KY, Wan
PZ and Zhai XY: A-Lipoic acid alleviates folic acid-induced renal
damage through inhibition of ferroptosis. Front Physiol.
12:6805442021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhao X, Shi D, Sun L, Gong Z, Liu W, Zhang
Y and Luo B: Epstein-Barr virus modulates iron metabolism and
ferritin expression to promote tumorigenesis in gastric cancer. J
Mol Histol. 56:2312025. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Altamura S, Colucci S, Schmidt J, Muedder
K, Neves J, Hentze M and Muckenthaler M: Hepatocyte iron content
controls BMP6-dependent hepcidin regulation. Blood. 132 (Suppl
1):S3626. 2018. View Article : Google Scholar
|
|
127
|
Cabrera C, Frisk C, Löfström U, Lyngå P,
Linde C, Hage C, Persson H, Eriksson MJ, Wallén H, Persson B and
Ekström M: Relationship between iron deficiency and expression of
genes involved in iron metabolism in human myocardium and skeletal
muscle. Int J Cardiol. 379:82–88. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Huang H, Mei L, Wang L, Bai Y, Gao K, Song
J, Jiang M, Chen Y, Zhang S, Pang B, et al: Ferroptosis contributes
to lead-induced cochlear spiral ganglion neurons injury.
Toxicology. 509:1539382024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ameziane El Hassani R, Buffet C,
Leboulleux S and Dupuy C: Oxidative stress in thyroid carcinomas:
Biological and clinical significance. Endocr Relat Cancer.
26:R131–R143. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Backert S, Linz B and Tegtmeyer N:
Helicobacter pylori-induced host cell DNA damage and genetics of
gastric cancer development. Curr Top Microbiol Immunol.
444:185–206. 2023.PubMed/NCBI
|
|
131
|
da Silva MS, Segatto M, Pavani RS,
Gutierrez-Rodrigues F, Bispo VD, de Medeiros MH, Calado RT, Elias
MC and Cano MI: Consequences of acute oxidative stress in
Leishmania amazonensis: From telomere shortening to the selection
of the fittest parasites. Biochim Biophys Acta Mol Cell Res.
1864:138–150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Lee J, Lim JW and Kim H: Astaxanthin
inhibits oxidative stress-induced Ku protein degradation and
apoptosis in gastric epithelial cells. Nutrients. 14:39392022.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Sekhar KR, Hanna DN, Cyr S, Baechle JJ,
Kuravi S, Balusu R, Rathmell K and Baregamian N: Glutathione
peroxidase 4 inhibition induces ferroptosis and mTOR pathway
suppression in thyroid cancer. Sci Rep. 12:193962022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Dong Q, Shen X, Fang G, Shi J, Zhu X, Du
J, Zhang H and Ge C: Theory-screened Prussian blue analogues-based
nanozymes for promoting diabetic wound healing via ferroptosis
inhibition. Mater Today Bio. 32:1018392025. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yang R, Zhou Y, Zhang T, Wang S, Wang J,
Cheng Y, Li H, Jiang W, Yang Z and Zhang X: The transcription
factor HBP1 promotes ferroptosis in tumor cells by regulating the
UHRF1-CDO1 axis. PLoS Biol. 21:e30018622023. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhu JF, Liu Y, Li WT, Li MH, Zhen CH, Sun
PW, Chen JX, Wu WH and Zeng W: Ibrutinib facilitates the
sensitivity of colorectal cancer cells to ferroptosis through
BTK/NRF2 pathway. Cell Death Dis. 14:1512023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hitt MM, Allday MJ, Hara T, Karran L,
Jones MD, Busson P, Tursz T, Ernberg I and Griffin BE: EBV gene
expression in an NPC-related tumour. EMBO J. 8:2639–2651. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Chiu SH, Wu MC, Wu CC, Chen YC, Lin SF,
Hsu JTA, ang CS, Tsai CH, Takada K, Chen MR and Chen JY:
Epstein-Barr virus BALF3 has nuclease activity and mediates mature
virion production during the lytic cycle. J Virol. 88:4962–4975.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Ragoczy T, Heston L and Miller G: The
Epstein-Barr virus Rta protein activates lytic cycle genes and can
disrupt latency in B lymphocytes. J Virol. 72:7978–7984. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Germini D, Sall FB, Shmakova A, Wiels J,
Dokudovskaya S, Drouet E and Vassetzky Y: Oncogenic properties of
the EBV ZEBRA protein. Cancers (Basel). 12:14792020. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Meng C, Dai X, Sun L, Huang D, Xu X and
Cheng Y: Meeting abstracts from the 10th international conference
on cGMP: Generators, effectors and therapeutic implications. J
Transl Med. Jan 31–2023.(Epub ahead of print).
|
|
142
|
Meng C, Dai X, Sun L, Huang D, Xu X, Cheng
Y and Zhang W: ZDHHC21-driven S-palmitoylation of Themis regulates
the function of T cells and maintains homeostatic balance. Cell
Commun Signal. 23:4012025. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Png YT, Yang AZY, Lee MY, Chua MJM and Lim
CM: The role of NK cells in EBV infection and EBV-associated NPC.
Viruses. 13:3002021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hsu WL, Tao J, Fu S, Yu KJ, Simon J, Chen
TC, Chen CJ, Goldstein AM, Yu K, Hildesheim A, et al: Kinetics of
EBV antibody-based NPC risk scores in Taiwan NPC multiplex
families. Int J Cancer. 155:1400–1408. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang J and Cao X: Dietary long-chain fatty
acid metabolism boosts antitumor immune response. Cancer Commun
(Lond). 44:580–583. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Peng WX and Mo YY: Connecting
N6-methyladenosine modification to ferroptosis resistance in
hepatoblastoma. Clin Transl Med. 12:e8202022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chiu SH, Wu CC, Fang CY, Yu SL, Hsu HY,
Chow YH and Chen JY: Epstein-Barr virus BALF3 mediates genomic
instability and progressive malignancy in nasopharyngeal carcinoma.
Oncotarget. 5:8583–8601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Benedetti F, Curreli S, Gallo RC and Zella
D: Tampering of viruses and bacteria with host DNA repair:
Implications for cellular transformation. Cancers (Basel).
13:2412021. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Dylawerska A, Barczak W, Wegner A,
Golusinski W and Suchorska WM: Association of DNA repair genes
polymorphisms and mutations with increased risk of head and neck
cancer: A review. Med Oncol. 34:1972017. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Mekonnen N, Yang H and Shin YK: Homologous
recombination deficiency in ovarian, breast, colorectal,
pancreatic, non-small cell lung and prostate cancers, and the
mechanisms of resistance to PARP inhibitors. Front Oncol.
12:8806432022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
No authors listed. The international
headache congress-IHS and EHF joint congress 2021: Late breaking
abstracts: Virtual. 8–12 September 2021. J Headache Pain. 22 (Suppl
2):S1302021. View Article : Google Scholar
|
|
152
|
Abstracts from the 17th European headache
congress (EHC). J Headache Pain. 25 (Suppl 1):1232024. View Article : Google Scholar
|
|
153
|
Li F, Xu T, Chen P, Sun R, Li C, Zhao X,
Ou J, Li J, Liu T, Zeng M, et al: Platelet-derived extracellular
vesicles inhibit ferroptosis and promote distant metastasis of
nasopharyngeal carcinoma by upregulating ITGB3. Int J Biol Sci.
18:5858–5872. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Jin N, Qian YY, Jiao XF, Wang Z, Li X, Pan
W, Jiang JK, Huang P, Wang SY, Jin P, et al: Niraparib restricts
intraperitoneal metastases of ovarian cancer by eliciting
CD36-dependent ferroptosis. Redox Biol. 80:1035282025. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhang B, Hou Q, Zhang X, Ma Y, Yuan J, Li
S, Zhao X, Sun L, Wang H and Zheng H: Anesthetic propofol inhibits
ferroptosis and aggravates distant cancer metastasis via Nrf2
upregulation. Free Radic Biol Med. 195:298–308. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhang R, Shen Y, Zhou X, Li J, Zhao H,
Zhang Z, Zhao J, Jin H, Guo S, Ding H, et al: Hypoxia-tropic
delivery of nanozymes targeting transferrin receptor 1 for
nasopharyngeal carcinoma radiotherapy sensitization. Nat Commun.
16:8902025. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Wang HH, Fan SQ, Zhan YT, Peng SP and Wang
WY: Suppression of the SLC7A11/glutathione axis causes ferroptosis
and apoptosis and alters the mitogen-activated protein kinase
pathway in nasopharyngeal carcinoma. Int J Biol Macromol.
254:1279762024. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Nhung DT, Yousif OEA and Kwon B: Sorafenib
induces ferroptosis in human renal cell carcinoma cells through
CCAT/enhancer-binding protein homologous protein. Biochem Biophys
Rep. 43:1021432025.PubMed/NCBI
|
|
159
|
Liu F, Tang L, Liu H, Chen Y, Xiao T, Gu
W, Yang H, Wang H and Chen P: Cancer-associated fibroblasts secrete
FGF5 to inhibit ferroptosis to decrease cisplatin sensitivity in
nasopharyngeal carcinoma through binding to FGFR2. Cell Death Dis.
15:2792024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Chen Y, Feng Y, Lin Y, Zhou X, Wang L,
Zhou Y, Lin K and Cai L: GSTM3 enhances radiosensitivity of
nasopharyngeal carcinoma by promoting radiation-induced ferroptosis
through USP14/FASN axis and GPX4. Br J Cancer. 130:755–768. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Pu X, Wu H, Liu X and Yang F: PRMT4
reduced erastin-induced ferroptosis in nasopharyngeal carcinoma
cisplatin-resistant cells by Nrf2/GPX4 pathway. J Environ Pathol
Toxicol Oncol. 44:57–71. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Du P, Luo K, Li G, Zhu J, Xiao Q, Li Y and
Zhang X: PRMT4 promotes hepatocellular carcinoma progression by
activating AKT/mTOR signaling and indicates poor prognosis. Int J
Med Sci. 18:3588–3598. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Berman AY, Manna S, Schwartz NS, Katz YE,
Sun Y, Behrmann CA, Yu JJ, Plas DR, Alayev A and Holz MK: ERRα
regulates the growth of triple-negative breast cancer cells via
S6K1-dependent mechanism. Signal Transduct Target Ther.
2:e170352017. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Xiao H, Zhang F, Zou Y, Li J, Liu Y and
Huang W: The function and mechanism of long non-coding RNA-ATB in
cancers. Front Physiol. 9:3212018. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Cui X, Gong Y, Ge J, Feng X, Xiong X, Shi
Z, Zheng Q, Li D and Bi S: α-Solanine induces ferroptosis in
nasopharyngeal carcinoma via targeting HSP90α/p53 axis. J Funct
Foods. 104:1055172023. View Article : Google Scholar
|
|
166
|
Yap TA, Bessudo A, Hamilton E, Sachdev J,
Patel MR, Rodon J, Evilevitch L, Duncan M, Guo W, Kumar S, et al:
IOLite: Phase 1b trial of doublet/triplet combinations of
dostarlimab with niraparib, carboplatin-paclitaxel, with or without
bevacizumab in patients with advanced cancer. J Immunother Cancer.
10:e0039242022. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wang Y, Xu H, Sa Q, Zhou Y, Cheng H, Gao
R, Xu B and Wang J: Efficacy and safety of vascular-targeting
agents in advanced soft tissue sarcoma: A systematic review and
network meta-analysis. Ther Adv Med Oncol.
17:175883592513789342025. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Ochsenreither S, Fiedler WM, Conte GD,
Macchini M, Matos I, Habel B, Ahrens-Fath I, Raspagliesi F, Lorusso
D, Keilholz U, et al: Safety and preliminary activity results of
the GATTO study, a phase Ib study combining the anti-TA-MUC1
antibody gatipotuzumab with the anti-EGFR tomuzotuximab in patients
with refractory solid tumors. ESMO Open. 7:1004472022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Zargarani N, Kavousi M and Aliasgari E: A
potential new strategy for BC treatment: NPs containing solanine
and evaluation of its anticancer and antimetastatic properties. BMC
Cancer. 25:8602025. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Chowański S, Winkiel M, Szymczak-Cendlak
M, Marciniak P, Mańczak D, Walkowiak-Nowicka K, Spochacz M, Bufo
SA, Scrano L and Adamski Z: Solanaceae glycoalkaloids: α-solanine
and α-chaconine modify the cardioinhibitory activity of verapamil.
Pharm Biol. 60:1317–1330. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Li W, Liu Y, Wei M, Yang Z, Tang H and
Huang W: Chondrocyte-targeted α-Solanine through HIF-1α regulating
glycolysis to reduce the ferroptosis of chondrocyte in
osteoarthritis. Int Immunopharmacol. 159:1148412025. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Wei Z, Wang H, Zhong R, Chen L, Vigors S
and Zhang H: Biodegradation of α-solanine and α-chaconine: Insights
into microbial detoxification and enzymatic deglycosylation
pathways. Food Chem X. 31:1029682025. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Zhou J, Wu J, Fu F, Yao S, Zheng W, Du W,
Luo H, Jin H, Tong P, Wu C and Ruan H: α-Solanine attenuates
chondrocyte pyroptosis to improve osteoarthritis via suppressing
NF-κB pathway. J Cell Mol Med. 28:e181322024. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Seven D, Dalan AB and Bayrak ÖF: Targeting
GSTM3 for therapeutic potential in advanced prostate cancer. BMC
Cancer. 25:14932025. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Llavanera M, Ribas-Maynou J,
Delgado-Bermúdez A, Recuero S, Salas-Huetos A, Benet J and Yeste M:
P-049 sperm GSTM3: A potential molecular biomarker for sperm
quality and male (in)fertility. Hum Reprod. 37 (Suppl
1):deac104.111. 2022. View Article : Google Scholar
|
|
176
|
Wang B, Gu Q and Li J: DOC-2/DAB2
interactive protein regulates proliferation and mobility of
nasopharyngeal carcinoma cells by targeting PI3K/Akt pathway. Oncol
Rep. 38:317–324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Niu Z, Liu H, Zhou M, Wang H, Liu Y, Li X,
Xiong W, Ma J, Li X and Li G: Knockdown of c-Myc inhibits cell
proliferation by negatively regulating the Cdk/Rb/E2F pathway in
nasopharyngeal carcinoma cells. Acta Biochim Biophys Sin
(Shanghai). 47:183–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Soulage CO, Pelletier CC, Florens N,
Lemoine S, Dubourg L, Juillard L and Guebre-Egziabher F: Two toxic
lipid aldehydes, 4-hydroxy-2-hexenal (4-HHE) and
4-hydroxy-2-nonenal (4-HNE), accumulate in patients with chronic
kidney disease. Toxins (Basel). 12:5672020. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Li Y, Zhao T, Li J, Xia M, Li Y, Wang X,
Liu C, Zheng T, Chen R, Kan D, et al: Oxidative stress and
4-hydroxy-2-nonenal (4-HNE): Implications in the pathogenesis and
treatment of aging-related diseases. J Immunol Res.
2022:22339062022.PubMed/NCBI
|
|
180
|
Globisch M, Kaden D and Henle T:
4-Hydroxy-2-nonenal (4-HNE) and Its lipation product
2-pentylpyrrole lysine (2-PPL) in peanuts. J Agric Food Chem.
63:5273–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Llavanera M, Delgado-Bermúdez A,
Fernandez-Fuertes B, Recuero S, Mateo Y, Bonet S, Barranco I and
Yeste M: GSTM3, but not IZUMO1, is a cryotolerance marker of boar
sperm. J Anim Sci Biotechnol. 10:612019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Li B, Zhou J, Zhang G, Wang Y, Kang L, Wu
J, Chen J and Guan H: Relationship between the altered expression
and epigenetics of GSTM3 and age-related cataract. Invest
Ophthalmol Vis Sci. 57:4721–4732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Mandic-Maravic V, Mitkovic-Voncina M,
Pljesa-Ercegovac M, Savic-Radojevic A, Djordjevic M, Ercegovac M,
Pekmezovic T, Simic T and Pejovic-Milovancevic M: Glutathione
S-transferase polymorphisms and clinical characteristics in autism
spectrum disorders. Front Psychiatry. 12:6723892021. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Li X, Gou J, Li H and Yang X:
Bioinformatic analysis of the expression and prognostic value of
chromobox family proteins in human breast cancer. Sci Rep.
10:177392020. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Li G, Cai Y, Wang C, Huang M and Chen J:
LncRNA GAS5 regulates the proliferation, migration, invasion and
apoptosis of brain glioma cells through targeting GSTM3 expression.
The effect of LncRNA GAS5 on glioma cells. J Neurooncol.
143:525–536. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Chen T, Jinlin D, Wang F, Yuan Z, Xue J,
Lu T, Huang W, Liu Y and Zhang Y: GSTM3 deficiency impedes DNA
mismatch repair to promote gastric tumorigenesis via
CAND1/NRF2-KEAP1 signaling. Cancer Lett. 538:2156922022. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Wu Y, Jia Q, Tang Q, Deng H, He Y and Tang
F: Berberine-mediated ferroptosis through system
Xc−/GSH/GPX4 axis inhibits metastasis of nasopharyngeal
carcinoma. J Cancer. 15:685–698. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Hou J, Zi L, Shi M, Wang Y, Gao F and Chen
W: The contribution of SLC7A11-mediated ferroptosis to cardiac
injury in iron overload cardiomyopathy: An in vitro study. Eur
Heart J. 45 (Suppl 1):ehae666.3648. 2024. View Article : Google Scholar
|
|
189
|
Wang F, Sun Z, Zhang Q, Yang H, Yang G,
Yang Q, Zhu Y, Wu W, Xu W and Wu X: Curdione induces ferroptosis
mediated by m6A methylation via METTL14 and YTHDF2 in colorectal
cancer. Chin Med. 18:1222023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Zhao YY, Yang YQ, Sheng HH, Tang Q, Han L,
Wang SM and Wu WY: GPX4 plays a crucial role in Fuzheng Kang'ai
decoction-induced non-small cell lung cancer cell ferroptosis.
Front Pharmacol. 13:8516802022. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Gao X, Guo N, Xu H, Pan T, lei H, Yan A,
Mi Y and Xu L: Ibuprofen induces ferroptosis of glioblastoma cells
via downregulation of nuclear factor erythroid 2-related factor 2
signaling pathway. Anticancer Drugs. 31:27–34. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Zheng J, Fang Y, Zhang M, Gao Q, Li J,
Yuan H, Jin W, Lin Z and Lin W: Mechanisms of ferroptosis in
hypoxic-ischemic brain damage in neonatal rats. Exp Neurol.
372:1146412024. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Yujiao C, Meng Z, Shanshan L, Wei W,
Yipeng W and Chenghong Y: Exposure to bisphenol A induces abnormal
fetal heart development by promoting ferroptosis. Ecotoxicol
Environ Saf. 255:1147532023. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Wang S, Xu Z, Cai B and Chen Q: Berberine
as a potential multi-target agent for metabolic diseases: A review
of investigations for berberine. Endocr Metab Immune Disord Drug
Targets. 21:971–979. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Chen J, Peng W, Yang W, You Z and Zou Y:
Berberine inhibits high glucose-induced ferroptosis in retinal
vascular endothelial cells: Mechanism and implications. Exp Eye
Res. 259:1105312025. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Bao L, Jin Y, Han J, Wang W, Qian L and Wu
W: Berberine regulates GPX4 to inhibit ferroptosis of islet β
cells. Planta Med. 89:254–261. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Shah D, Challagundla N, Dave V, Patidar A,
Saha B, Nivsarkar M, Trivedi VB and Agrawal-Rajput R: Berberine
mediates tumor cell death by skewing tumor-associated
immunosuppressive macrophages to inflammatory macrophages.
Phytomedicine. 99:1539042022. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Sun Q, Tu K, Xu Q, Yan L, Yang S, Wang J,
Lv L, Liu H and Cai L: Berberine suppresses colorectal cancer
progression by inducing ferroptosis-mediated energy metabolism
disorders. J Adv Res. Oct 24–2025.(Epub ahead of print). View Article : Google Scholar
|
|
199
|
Xie Z, Zhou Y, Lin M and Huang C: Binding
of berberine to PEBP1 synergizes with sorafenib to induce the
ferroptosis of hepatic stellate cells. Amino Acids. 55:1867–1878.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Zhang Y, Liu X, Yu M, Xu M, Xiao Y, Ma W,
Huang L, Li X and Ye X: Berberine inhibits proliferation and
induces G0/G1 phase arrest in colorectal cancer cells by
downregulating IGF2BP3. Life Sci. 260:1184132020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Eke I, Makinde AY, Aryankalayil MJ,
Sandfort V, Palayoor ST, Rath BH, Liotta L, Pierobon M, Petricoin
EF, Brown MF, et al: Exploiting radiation-induced signaling to
increase the susceptibility of resistant cancer cells to targeted
drugs: AKT and mTOR inhibitors as an example. Mol Cancer Ther.
17:355–367. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Bagnulo M and García-Martínez A: When less
is more: BBR versus LEDBAT++. Comput Netw. 219:1094602022.
View Article : Google Scholar
|
|
203
|
Atxutegi E, Liberal F, Haile HK, Grinnemo
KJ, Brunstrom A and Arvidsson Å: On the use of TCP BBR in cellular
networks. IEEE Commun Mag. 56:172–179. 2018. View Article : Google Scholar
|
|
204
|
Abdelmawgood IA, Kotb MA, Hassan HS,
Mahana NA, Rochdi AM, Sayed NH, Elsafoury RH, Saber AM, Youssef MN,
Waheeb NG, et al: Gentisic acid attenuates ovalbumin-induced airway
inflammation, oxidative stress, and ferroptosis through the
modulation of Nrf2/HO-1 and NF-κB signaling pathways. Int
Immunopharmacol. 146:1137642025. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Zhong X, Zhang Z, Shen H, Xiong Y, Shah
YM, Liu Y, Liu Y, Fan XG and Rui L: Hepatic NF-κB-inducing kinase
and inhibitor of NF-κB kinase subunit α promote liver oxidative
stress, ferroptosis, and liver injury. Hepatol Commun. 5:1704–1720.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Zhou JC, Wu B, Zhang JJ and Zhang W:
Lupeol triggers oxidative stress, ferroptosis, apoptosis and
restrains inflammation in nasopharyngeal carcinoma via AMPK/NF-κB
pathway. Immunopharmacol Immunotoxicol. 44:621–631. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Wang Z, Di Y, Ye L, Fang W, Wen X, Zhang
X, Qin J, Wang Y, Hu K, Zhu Z, et al: NANS suppresses NF-κB
signaling to promote ferroptosis by perturbing iron homeostasis.
Cell Rep. 44:1157012025. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Wu S, Zhou Y, Liang J, Ying P, Situ Q, Tan
X and Zhu J: Upregulation of NF-κB by USP24 aggravates ferroptosis
in diabetic cardiomyopathy. Free Radic Biol Med. 210:352–366. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Luo X, Gong Y, Jiang Q, Wang Q, Li S and
Liu L: Isoquercitrin promotes ferroptosis and oxidative stress in
nasopharyngeal carcinoma via the AMPK/NF-κB pathway. J Biochem Mol
Toxicol. 38:e235422024. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Song X, Zhu H, Chen Z, Wang Y, Zhang J,
Wang Y, Rong P and Wang J: Transcutaneous auricular vagus nerve
stimulation alleviates inflammation-induced depression by
modulating peripheral-central inflammatory cytokines and the NF-κB
pathway in rats. Front Immunol. 16:15360562025. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Gong X, Yang Y, Huang L, Zhang Q, Wan RZ,
Zhang P and Zhang X: Antioxidation, anti-inflammation and
anti-apoptosis by paeonol in LPS/d-GalN-induced acute liver failure
in mice. Int Immunopharmacol. 46:124–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Zeng MY and Tong QY: Anti-inflammation
effects of sinomenine on macrophages through suppressing activated
TLR4/NF-κB signaling pathway. Curr Med Sci. 40:130–137. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Zhao S, Zuo W, Chen H, Bao T, Liu X, Sun T
and Wang S: Effects of pilose antler peptide on bleomycin-induced
pulmonary fibrosis in mice. Biomed Pharmacother. 109:2078–2083.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Zhang J, Qi S, Du Y, Dai H and Lu N:
Effect of quercetin on inhibiting gefitinib-activated non-small
cell lung cancer-induced cell pyroptosis in cardiomyocytes via
modulating mitochondrial autophagy mediated by the
SHP2/ROS/AMPK/XBP-1/DJ-1 signaling pathway. Oncol Rep. 53:572025.
View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Wang N, Chen HQ, Zeng Y, Shi Y, Zhang Z,
Li JY, Zhou SM, Li YW, Deng SW, Han X, et al: Benzo(a)pyrene
promotes the malignant progression of malignant-transformed BEAS-2B
cells by regulating YTH N6-methyladenosine RNA binding protein 1 to
inhibit ferroptosis. Toxicology. 507:1538862024. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Shao C, Luo T, Wang S, Li Z, Yu X, Wu Y,
Jiang S, Zhou B, Song Q, Song S, et al: Selenium nanoparticles
alleviates cadmium induced hepatotoxicity by inhibiting ferroptosis
and oxidative stress in vivo and in vitro. Chemosphere.
364:1430042024. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Meng J, Hu C, Qian Z, Yue J, Zhang S,
Jiang W, Su R, Jiang G and Huang G: Isoquercitrin inhibits
ferroptosis and ameliorates insulin resistance: Evidence from
network pharmacology and in vitro studies. Biochem Biophys Res
Commun. 781:1525002025. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Lee J, Jang CH, Kim Y, Oh J and Kim JS:
Quercetin-Induced glutathione depletion sensitizes colorectal
cancer cells to oxaliplatin. Foods. 12:17332023. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Kottakis G, Kambouri K, Giatromanolaki A,
Valsami G, Kostomitsopoulos N, Tsaroucha A and Pitiakoudis M:
Effects of the antioxidant quercetin in an experimental model of
ulcerative colitis in mice. Medicina (Kaunas). 59:872022.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Li Y, Xu Z, Zhao S, Huang T, Xu J, Wang S,
Sang Y, Yu W and Wang X: Oral administration of curcumin and
quercetin nanoparticles can improve ulcerative colitis by
regulating intestinal microorganisms. Front Nutr. 12:16966992025.
View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Wei Q, Jiang H, Zeng J, Xu J, Zhang H,
Xiao E, Lu Q and Huang G: Quercetin protected the gut barrier in
ulcerative colitis by activating aryl hydrocarbon receptor.
Phytomedicine. 140:1566332025. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Li J, Yan Y and Chen F: Clinical trial
landscape for histone deacetylation inhibitors in breast cancer: A
dawn in the darkness? J Transl Med. 22:10812024. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Tan LLY, Le QT, Lee NYY and Chua MLK:
JUPITER-02 trial: Advancing survival for recurrent metastatic
nasopharyngeal carcinoma and next steps. Cancer Commun (Lond).
42:56–59. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Zhu D, Wang Z, Zhang G, Ma C, Qiu X, Wang
Y, Liu M, Guo X, Chen H, Deng Q and Kang X: Periostin promotes
nucleus pulposus cells apoptosis by activating the Wnt/β-catenin
signaling pathway. FASEB J. 36:e223692022. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Takashima K, Okano H, Ojiro R, Tang Q,
Takahashi Y, Ozawa S, Zou X, Koyanagi M, Maronpot RR, Yoshida T and
Shibutani M: Continuous exposure to alpha-glycosyl isoquercitrin
from gestation ameliorates disrupted hippocampal neurogenesis in
rats induced by gestational injection of valproic acid. Neurotox
Res. 40:2278–2296. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Takashima K, Okano H, Ojiro R, Tang Q,
Takahashi Y, Ozawa S, Zou X, Koyanagi M, Maronpot RR, Yoshida T and
Shibutani M: Continuous exposure to alpha-glycosyl isoquercitrin
from mid-gestation ameliorates polyinosinic-polycytidylic
acid-disrupted hippocampal neurogenesis in rats. J Chem Neuroanat.
128:1022192023. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Huang S, Cao B, Zhang J, Feng Y, Wang L,
Chen X, Su H, Liao S, Liu J, Yan J and Liang B: Induction of
ferroptosis in human nasopharyngeal cancer cells by cucurbitacin B:
Molecular mechanism and therapeutic potential. Cell Death Dis.
12:2372021. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Li Y, Chen F, Chen J, Chan S, He Y, Liu W
and Zhang G: Disulfiram/copper induces antitumor activity against
both nasopharyngeal cancer cells and cancer-associated fibroblasts
through ROS/MAPK and ferroptosis pathways. Cancers (Basel).
12:1382020. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Chen C, Nie D, Huang Y, Yu X, Chen Z,
Zhong M, Liu X, Wang X, Sui S, Liu Z, et al: Anticancer effects of
disulfiram in T-cell malignancies through NPL4-mediated
ubiquitin-proteasome pathway. J Leukoc Biol. 112:919–929. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Serra R, Zhao T, Huq S, Gorelick NL,
Casaos J, Cecia A, Mangraviti A, Bai R, Olivi A, Brem H, et al:
Disulfiram and copper combination therapy targets NPL4, cancer stem
cells and prolongs survival in group 3 medulloblastoma.
Neurosurgery. 87 (Suppl 1):S9082020.
|
|
231
|
Serra R, Zhao T, Huq S, Gorelick NL,
Casaos J, Cecia A, Mangraviti A, Eberhart C, Bai R, Olivi A, et al:
Disulfiram and copper combination therapy targets NPL4, cancer stem
cells and extends survival in a medulloblastoma model. PLoS One.
16:e02519572021. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Lian Q, Chen F, Sha Z, Zhao H, Li J, Chen
T, Liu C, Wang B, Wang Z and Qiao S: Disulfiram upgrades the
radiosensitivity of osteosarcoma by enhancing apoptosis and
P53-induced cell cycle arrest. Radiat Res. 202:752–764. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Wu X, Xue X, Wang L, Wang W, Han J, Sun X,
Zhang H, Liu Y, Che X, Yang J and Wu C: Suppressing autophagy
enhances disulfiram/copper-induced apoptosis in non-small cell lung
cancer. Eur J Pharmacol. 827:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Wang K, Michelakos T, Wang B, Shang Z,
DeLeo AB, Duan Z, Hornicek FJ, Schwab JH and Wang X: Targeting
cancer stem cells by disulfiram and copper sensitizes
radioresistant chondrosarcoma to radiation. Cancer Lett. 505:37–48.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Park YM, Kim DH, Kang MS, Koh YW, Choi EC
and Kim SH: Abstract B25: Anticancer effects of disulfiram in head
and neck squamous cell carcinoma via autophagic cell death. Clin
Cancer Res. 26 (12 Suppl 2):B252020. View Article : Google Scholar
|
|
236
|
Li Z, Cao S, Sun Y, Niu Z, Liu X, Niu J
and Zhou Y: TIPE3 is a candidate prognostic biomarker promoting
tumor progression via elevating RAC1 in pancreatic cancer. Mol
Cancer. 21:1602022. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Li Z, Cao S, Sun Y, Niu Z, Liu X, Niu J,
PavelM A, Sridhar A, Maienschein-Cline M, Ong SG, et al:
Late-breaking basic science abstracts from the american heart
association's scientific sessions 2021. Circ Res. Dec 2–2021.(Epub
ahead of print).
|
|
238
|
Guo J, Ma Y, Tang T, Bian Z, Li Q, Tang L,
Li Z, Li M, Wang L, Zeng A, et al: Modulation of immune-responses
by DSF/Cu enhances the anti-tumor effects of DTX for metastasis
breast cancer. J Cancer. 15:1523–1535. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Ghaffarianhoseini A, Ghaffarianhoseini A,
Berardi U, Tookey J, Li DHW and Kariminia S: Exploring the
advantages and challenges of double-skin façades (DSFs). Renew
Sustain Energy Rev. 60:1052–1065. 2016. View Article : Google Scholar
|
|
240
|
Zhu Y, Lei C, Jiang Q, Yu Q and Qiu L:
DSF/Cu induces antitumor effect against diffuse large B-cell
lymphoma through suppressing NF-κB/BCL6 pathways. Cancer Cell Int.
22:2362022. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Waldron EJ, Snyder D, Fernandez NL, Sileo
E, Inoyama D, Freundlich JS, Waters CM, Cooper VS and Neiditch MB:
Structural basis of DSF recognition by its receptor RpfR and its
regulatory interaction with the DSF synthase RpfF. PLoS Biol.
17:e30001232019. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Gennaro G, Catto Lucchino E, Goia F and
Favoino F: Modelling double skin façades (DSFs) in whole-building
energy simulation tools: Validation and inter-software comparison
of naturally ventilated single-story DSFs. Build Environ.
231:1100022023. View Article : Google Scholar
|
|
243
|
Catto Lucchino E, Gelesz A, Skeie K,
Gennaro G, Reith A, Serra V and Goia F: Modelling double skin
façades (DSFs) in whole-building energy simulation tools:
Validation and inter-software comparison of a mechanically
ventilated single-story DSF. Build Environ. 199:1079062021.
View Article : Google Scholar
|
|
244
|
Rempfer C, Hoernstein SNW, van Gessel N,
Graf AW, Spiegelhalder RP, Bertolini A, Bohlender LL, Parsons J,
Decker EL and Reski R: Differential prolyl hydroxylation by six
Physcomitrella prolyl-4 hydroxylases. bioRxiv. 2024.
|
|
245
|
Rempfer C, Hoernstein SNW, van Gessel N,
Graf AW, Spiegelhalder RP, Bertolini A, Bohlender LL, Parsons J,
Decker EL and Reski R: Differential prolyl hydroxylation by six
Physcomitrella prolyl-4 hydroxylases. Comput Struct Biotechnol J.
23:2580–2594. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
McKenzie AJ, Hoshino D, Hong NH, Cha DJ,
Franklin JL, Coffey RJ, Patton JG and Weaver AM: KRAS-MEK signaling
controls Ago2 sorting into exosomes. Cell Rep. 15:978–987. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Hu P, Zhao H, Zhu P, Xiao Y, Miao W, Wang
Y and Jin H: Dual regulation of Arabidopsis AGO2 by arginine
methylation. Nat Commun. 10:8442019. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Zhou R, Qiu L, Zhou L, Geng R, Yang S and
Wu J: P4HA1 activates HMGCS1 to promote nasopharyngeal carcinoma
ferroptosis resistance and progression. Cell Signal.
105:1106092023. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Meng Y, Sun HY, He Y, Zhou Q, Liu YH, Su
H, Yin MZ, Zeng FR, Chen X and Deng GT: BET inhibitors potentiate
melanoma ferroptosis and immunotherapy through AKR1C2 inhibition.
Mil Med Res. 10:612023.PubMed/NCBI
|
|
250
|
Li Q and Gan B: Uncovering the
IL-1β-PCAF-NNT axis: A new player in ferroptosis and tumor immune
evasion. Cancer Commun (Lond). 43:1048–1050. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Qiu L, Zhou R, Zhou L, Yang S and Wu J:
CAPRIN2 upregulation by LINC00941 promotes nasopharyngeal carcinoma
ferroptosis resistance and metastatic colonization through HMGCR.
Front Oncol 6. 12:9317492022. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Ning Y, Zheng H, Zhan Y, Liu S, Yang Y,
Zang H, Wen Q, Zhang Y and Fan S: Overexpression of P4HA1
associates with poor prognosis and promotes cell proliferation and
metastasis of lung adenocarcinoma. J Cancer. 12:6685–6694. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Robinson AD, Chakravarthi BVSK, Agarwal S,
Chandrashekar DS, Davenport ML, Chen G, Manne U, Beer DG, Edmonds
MD and Varambally S: Collagen modifying enzyme P4HA1 is
overexpressed and plays a role in lung adenocarcinoma. Transl
Oncol. 14:1011282021. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Navarro-Serer B, Wissler MF, Glover BK,
Lerner MG, Oza HH, Wang V, Knutsdottir H, Shojaeian F, Noller K,
Baskaran SG, et al: P4HA1 mediates hypoxia-induced invasion in
human pancreatic cancer organoids. Cancer Res Commun. 5:881–895.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Hu T, Gou W, Ren Z, Liu G, Li Y, Zuo D and
Hou W: Icaritin increases radiosensitivity of nasopharyngeal
carcinoma cells by regulating iron death. Nan Fang Yi Ke Da Xue Xue
Bao. 43:1665–1673. 2023.(In Chinese). PubMed/NCBI
|
|
256
|
Zhang D, Wu X, Xue X, Li W, Zhou P, Lv Z,
Zhao K and Zhu F: Ancient dormant virus remnant ERVW-1 drives
ferroptosis via degradation of GPX4 and SLC3A2 in schizophrenia.
Virol Sin. 39:31–43. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Chen H, Li Y, Deng C, Liang X and Liu G:
O-254 ACTRT2 deficiency increases spermatogonia vulnerability to
ferroptosis. Hum Reprod. 38:dead093.308. 2023. View Article : Google Scholar
|
|
258
|
Qin J, Chen Z, Ye M, Liang L and Ding X:
High glucose promotes O-GlcNAcylation of ACSL4 to induce
ferroptosis of renal tubular epithelial cell. Autoimmunity.
58:25768812025. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Zhou R, Wang X, Jin Y, Chen B, Liu H, Zhao
X, Jing D and Zhao B: Mechanism of lidocaine-induced ROS generation
triggering DNA double-strand breaks and promoting intervertebral
disc cell senescence via the MYC-DUSP1-P53 axis. Free Radic Biol
Med. 240:457–471. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Yue C, Qian Y, Wang C, Chen J, Wang J,
Wang Z, Wan X, Cao S, Zhu J, Tao Q, et al: TRIM29 acts as a
potential senescence suppressor with epigenetic activation in
nasopharyngeal carcinoma. Cancer Sci. 114:3176–3189. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25:1010842019. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Ding Y, Xiu H, Zhang Y, Ke M, Lin L, Yan
H, Hu P, Xiao M, He X and Zhang T: Learning and investigation of
the role of angiotensin-converting enzyme in radiotherapy for
nasopharyngeal carcinoma. Biomedicines. 11:15812023. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Wang SY, Xu XW, Yao JJ, Peng PJ, Zhou B,
Liu QD, Huang XP and Lin Z: Dose escalation of lobaplatin
concurrent with IMRT for the treatment of stage III–IVb NPC: A
phase I clinical trial. Transl Oncol. 11:1007–1011. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Tang Q, Mei C, Huang B, Huang R, Kang L,
Chen A, Lei N, Deng P, Ying S, Zhang P and Qin Y: Risk
stratification of LA-NPC during chemoradiotherapy based on clinical
classification and TVRR. Cancer Med. 13:e70292024. View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Kim KY, Le QT, Yom SS, Ng RHW, Chan KCA,
Bratman SV, Welch JJ, Divi RL, Petryshyn RA and Conley BA: Clinical
utility of Epstein-Barr virus DNA testing in the treatment of
nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys.
98:996–1001. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
He Q, Tuo Y, Zhou Y, Yan Y, Liu X, Zhao D,
Wang Q, Luo H, Zhang Z, Meng F, et al: MB based RT-qPCR increase
the clinical application of cfEBV DNA for NPC in non-endemic area
of China. Sci Rep. 15:91862025. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Burton BK, Ellis AG, Orr B, Chatlani S,
Yoon K, Shoaff JR and Gallo D: Estimating the prevalence of
Niemann-Pick disease type C (NPC) in the United States. Mol Genet
Metab. 134:182–187. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Wang MD, Li HT, Peng LX, Mei Y, Zheng LS,
Li CZ, Meng DF, Lang YH, Xu L, Peng XS, et al: TSPAN1 inhibits
metastasis of nasopharyngeal carcinoma via suppressing NF-kB
signaling. Cancer Gene Ther. 31:454–463. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Zhang X, Qian S, Wu P, Yu B, Yin D, Peng
X, Li S, Xiao Z and Xie Z: Tumor-associated macrophage-derived
itaconic acid contributes to nasopharyngeal carcinoma progression
by promoting immune escape via TET2. Cell Commun Signal.
22:4132024. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
Zhang X, Song X, Lai Y, Zhu B, Luo J, Yu H
and Yu Y: Identification of key pseudogenes in nasopharyngeal
carcinoma based on RNA-Seq analysis. BMC Cancer. 21:4832021.
View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Renaud S, Lefebvre A, Mordon S, Moralès O
and Delhem N: Novel therapies boosting T cell immunity in epstein
barr virus-associated nasopharyngeal carcinoma. Int J Mol Sci.
21:42922020. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Cheu JWS, Lee D, Li Q, Goh CC, Bao MHR,
Yuen VWH, Zhang MS, Yang C, Chan CY, Tse AP, et al: Ferroptosis
suppressor protein 1 inhibition promotes tumor ferroptosis and
anti-tumor immune responses in liver cancer. Cell Mol Gastroenterol
Hepatol. 16:133–159. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Cui K, Wang K and Huang Z: Ferroptosis and
the tumor microenvironment. J Exp Clin Cancer Res. 43:3152024.
View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Kojima Y, Tanaka M, Sasaki M, Ozeki K,
Shimura T, Kubota E and Kataoka H: Induction of ferroptosis by
photodynamic therapy and enhancement of antitumor effect with
ferroptosis inducers. J Gastroenterol. 59:81–94. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Cai W, Wu S, Lin Z, Ming X, Yang X, Yang M
and Chen X: Hypoxia-induced BAP1 enhances erastin-induced
ferroptosis in nasopharyngeal carcinoma by stabilizing H2A. Cancer
Cell Int. 24:3072024. View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Chen W, Zuo F, Zhang K, Xia T, Lei W,
Zhang Z, Bao L and You Y: Exosomal MIF derived from nasopharyngeal
carcinoma promotes metastasis by repressing ferroptosis of
macrophages. Front Cell Dev Biol. 9:7911872021. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Chen X, Wang X, Zou Y, Wang Y, Duan T,
Zhou Z, Huang Y and Ye Q: EMC2 suppresses ferroptosis via
regulating TFRC in nasopharyngeal carcinoma. Transl Oncol.
52:1022512025. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Zheng D, Chu T, Yang D, Liang S, Yang L,
Yang Y, Zhang K and Ma W: Targeting ferroptosis in nasopharyngeal
carcinoma: Mechanisms of therapy resistance and therapeutic
opportunities. Adv Biol (Weinh). Oct 30–2025.(Epub ahead of print).
View Article : Google Scholar
|
|
279
|
Liang Z, Zhao W, Li X, Wang L, Meng L and
Yu R: Cisplatin synergizes with PRLX93936 to induce ferroptosis in
non-small cell lung cancer cells. Biochem Biophys Res Commun.
569:79–85. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Roh JL, Kim EH, Jang H and Shin D: Nrf2
inhibition reverses the resistance of cisplatin-resistant head and
neck cancer cells to artesunate-induced ferroptosis. Redox Biol.
11:254–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
281
|
Takahashi R, Kamizaki K, Yamanaka K, Terai
Y and Minami Y: Expression of Ferredoxin1 in cisplatin-resistant
ovarian cancer cells confers their resistance against ferroptosis
induced by cisplatin. Oncol Rep. 49:1242023. View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Perera L, Brown SM, Silver BB, Tokar EJ
and Sinha BK: Ferroptosis inducers erastin and RSL3 enhance
adriamycin and topotecan sensitivity in ABCB1/ABCG2-expressing
tumor cells. Int J Mol Sci. 26:6352025. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Bao C, Liu C, Liu Q, Hua L, Hu J, Li Z and
Xu S: Liproxstatin-1 alleviates LPS/IL-13-induced bronchial
epithelial cell injury and neutrophilic asthma in mice by
inhibiting ferroptosis. Int Immunopharmacol. 109:1087702022.
View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Zhang B, Chen X, Ru F, Gan Y, Li B, Xia W,
Dai G, He Y and Chen Z: Liproxstatin-1 attenuates unilateral
ureteral obstruction-induced renal fibrosis by inhibiting renal
tubular epithelial cells ferroptosis. Cell Death Dis. 12:8432021.
View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Cui J, Chen Y, Yang Q, Zhao P, Yang M,
Wang X, Mang G, Yan X, Wang D, Tong Z, et al: Protosappanin A
protects DOX-induced myocardial injury and cardiac dysfunction by
targeting ACSL4/FTH1 axis-dependent ferroptosis. Adv Sci (Weinh).
11:e23102272024. View Article : Google Scholar : PubMed/NCBI
|