Introduction
Breast cancer (BC) remains the leading cause of
cancer-related incidence and mortality among women worldwide and is
characterized by substantial biological heterogeneity (1). This heterogeneity significantly
influences therapeutic responses and clinical outcomes, posing a
major challenge to the implementation of personalized medicine
(2,3). The progression of BC, particularly
invasion, metastasis and therapeutic resistance, results not only
from genetic mutations within cancer cells but also from complex
interactions with the tumor microenvironment (TME) (4,5).
In clinical oncology, tumor-associated elevations of
serum Krebs von den Lungen-6 (KL-6) levels are commonly observed
(6). KL-6, a high-molecular-weight
mucin glycoprotein linked to mucin 1 (MUC1), is primarily used as a
biomarker for interstitial pneumonia (7). Although KL-6 is considered to be
co-expressed with membrane-bound MUC1 in tumor tissues, a previous
study indicated its potential role in facilitating invasion and
metastasis in pancreatic cancer; however, its functional
significance in other malignancies remains unclear (8).
Tumor hypoxia and the subsequent activation of
hypoxia-inducible factor (HIF) are key central drivers of cancer
cell invasion, metastasis and TME remodeling (9–11).
Notably, MUC1 expression is upregulated by HIF-1α under hypoxic
conditions in models of lung adenocarcinoma and renal cell
carcinoma (12–14). Although HIF-1α-mediated regulation
of MUC1 has been established, the impact of hypoxia on KL-6
expression has not yet been reported.
The present study aimed to investigate the effects
of hypoxia on KL-6 expression and its association with invasive
behavior in BC cells and spheroid models. Hypoxia-induced changes
in KL-6 expression were examined in triple-negative BC cell lines,
with particular attention to membrane localization and association
with invasion-associated phenotypes, using immunostaining, western
blot analysis and electron microscopy. Additionally, the interplay
between KL-6 expression and HIF-1α activity was investigated to
elucidate the role of KL-6 in the hypoxic response. These findings
provide novel insights into the regulatory mechanisms underlying
KL-6 expression and its contribution to BC invasion and metastasis,
suggesting its potential as a prognostic biomarker.
Materials and methods
Clinical data of patients with BC
The clinical data of 30 patients with early-stage BC
who underwent treatment at Kochi Medical School Hospital (Nankoku,
Japan) from January 2015 to December 2019 and completed at least 1
year of follow-up were retrospectively reviewed. All 30 patients
were women, with a median age of 64 years (range, 40–91 years). The
study was reviewed and approved by the Ethical Review Board of
Kochi Medical School (approval no. 2023-146; April 19, 2024), which
waived the requirement for obtaining individual written informed
consent and approved the use of an opt-out procedure as an
alternative. All patients underwent surgical treatment, and the
resected tissues were formalin-fixed at room temperature (about
20–25°C) in 20% neutral buffered formalin for 12–24 h and then
embedded in paraffin blocks. Immunohistochemical (IHC) evaluations
of the estrogen receptor (ER), progesterone receptor (PR), and
human epidermal growth factor receptor type 2 (HER2) status were
obtained from clinicopathological reports at the time of initial
diagnosis. ER and PR positivity was defined as positive staining in
≥1% of tumor cells. HER2 expression was evaluated using a
four-tiered scale (0, 1+, 2+, or 3+) in accordance with the 2018
American Society of Clinical Oncology/College of American
Pathologists guidelines. Patients with a score of 2+ were
re-evaluated by in situ hybridization, and HER2 positivity
was defined as a score of 3+ or a positive in situ
hybridization result.
KL-6 IHC evaluation
A total of 9 tissue samples obtained from patients
who developed metastases or recurrence within the 1-year follow-up
period were sectioned into 4-µm-thick slices and subjected to
heat-induced antigen retrieval using ULTRA Cell Conditioning 1
Solution (Roche Tissue Diagnostics) at 95°C for 30 min. IHC
staining was performed with an anti-KL-6 mouse monoclonal antibody
(cat. no. LNM-KR-067; Cosmo Bio Co., Ltd.) using the Ventana
automated system at a dilution of 1:2,000, with incubation at 4°C
overnight (18 h). A total of 21 consecutive tissue samples from
patients without metastasis or recurrence were processed
identically and served as controls. KL-6 expression was evaluated
using the Allred scoring system (13,14).
Briefly, the proportion of positively stained cells was scored as
follows: 0 (none), 1 (<1%), 2 (1–10%), 3 (11–33%), 4 (34–66%)
and 5 (≥66%). Staining intensity in the most predominant areas was
rated as 0 (none), 1 (weak), 2 (intermediate), or 3 (strong). The
Allred score (range: 0–8 points) was calculated by summing the
proportion and intensity scores. Immunostaining assessments were
independently performed by two experienced pathologists (Mitsuko
Iguchi and Makoto Toi).
Fluorescent immunocytochemical
staining
For immunocytochemical staining, MCF-7 and
MDA-MB-231 cells (Health Science Research Resources Bank), each at
a density of 4×104 cells/ml, were cultured in slide
chambers, fixed with 4% paraformaldehyde (PFA; Nacalai Tesque,
Inc.) at 4°C for 30 min, and washed with phosphate-buffered saline
(PBS). The cells were then incubated overnight at 4°C with an
anti-KL-6 mouse monoclonal antibody diluted at 1:2,000, followed by
incubation with fluorescein isothiocyanate-conjugated anti-mouse
immunoglobulin (IgG) antibodies (cat. no. F-2761; 1:200; Molecular
Probes; Thermo Fisher Scientific, Inc.) for 1 h at room
temperature. Nuclei were counterstained with
4′,6-diamidino-2-phenylindole (DAPI; 0.001 mg/ml; MilliporeSigma).
Fluorescence images were acquired using a BX53 fluorescence
microscope (Olympus Corporation).
Western blot analysis
Total protein was extracted from cells using a
radioimmunoprecipitation assay buffer (FUJIFILM Wako Pure Chemical
Corporation). Protein concentrations were measured using the Pierce
BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.). Equal
amounts of protein (20 µg per lane) were separated by 10% sodium
dodecyl-sulfate polyacrylamide gel electrophoresis (Bio-Rad
Laboratories, Inc.) and transferred to polyvinylidene difluoride
membranes using the Trans-Blot Turbo Transfer System (Bio-Rad
Laboratories, Inc.). Membranes were blocked at room temperature for
1 h with Blocking One (cat. no. 03953-93; Nacalai Tesque, Inc.) and
subsequently incubated overnight incubation at 4°C with an
anti-KL-6 mouse monoclonal antibody (1:2,000) and a glyceraldehyde
3-phosphate dehydrogenase mouse monoclonal antibody (1:2,000; cat.
no. 20035, ProMab Biotechnologies, Inc.). Subsequently, membranes
were incubated for 1 h at room temperature with a horseradish
peroxidase (HRP)-conjugated anti-mouse polyclonal antibody
(1:2,000; cat. no. P0447; Dako, Agilent Technologies, Inc.).
Protein bands were visualized using Enhanced Chemiluminescence
Prime Western Blotting Detection Reagents (Amersham; Cytiva) and
detected with an LAS-4000 Lumino-Image Analyzer (FUJIFILM Wako Pure
Chemicals Corporation).
Wound healing assay
Wound healing assays were performed as previously
described (15). Trypsinized MCF-7
cells were counted using a C-Chip™ disposable hemocytometer (SKC,
Inc.; Thermo Fisher Scientific, Inc.), seeded at a density of
2×104 cells/ml into 35-mm diameter dishes, and incubated
at 37°C for 48 h. Upon reaching ~90% confluency, three linear
scratches were created in the cell monolayer of each dish using a
20-µl pipette tip. Phase-contrast images were captured at 0, 12, 24
and 36 h post-scratch at 50 randomly marked locations per 35-mm
dish using an Olympus CKX41 microscope (original magnification,
×200; Olympus Corporation). The scratch areas devoid of cell
migration were quantified using the ImageJ software version 1.53
(National Institutes of Health). The average migration across the
50 sites per dish was calculated. The relative cell migration rates
at each time point were normalized to the 0-h measurement, which
was set as 1.0.
Hematoxylin-eosin and IHC staining of
MDA-MB-231 cells cultured in Cell Matrix®
MDA-MB-231 cells (1×104 cells/ml) were
prepared as described in Fluorescent immunocytochemical
staining. Cellmatrix (Nitta Gelatin Inc.) was prepared
according to the manufacturer's instructions. Each cell group was
mixed with 500 µl of Cellmatrix® and plated into 35-mm
dishes pre-coated with Cellmatrix, followed by incubation at 37°C
for 30 min. The gels were overlaid with 200 µl of Dulbecco's
Modified Eagle Medium (DMEM; MilliporeSigma) and cultured at 37°C
for 10 days. After incubation, the gels were fixed overnight in 20%
buffered formaldehyde at room temperature and embedded in paraffin.
Hematoxylin-eosin staining was performed using Mayer's hematoxylin
(10 min) and 1% eosin (5 min) at room temperature. IHC staining for
KL-6 and HIF-1α was subsequently performed. Paraffin-embedded
tissue sections (4-µm thick) were subjected to antigen retrieval by
immersion in Immunosaver (Nisshin EM Co., Ltd.) at 98°C for 30 min.
Endogenous peroxidase activity was quenched by incubation in 0.3%
hydrogen peroxide in methanol for 10 min at room temperature. Then,
sections were blocked with Blocking One for 1 h at room temperature
and incubated overnight at 4°C with the following antibodies:
anti-KL-6 mouse monoclonal antibody (1:2,000) and anti-HIF-1α mouse
monoclonal antibody (1:100; cat. no. AP2054a, Abgent, Inc.). After
washing with PBS, the sections were incubated for 1 h at room
temperature with Universal ImmPRESS HRP Reagent (MP-7500; Vector
Laboratories, Inc.), followed by additional PBS washes. Color
development was achieved using the DAB Peroxidase Substrate Kit
(SK-4100; Vector Laboratories, Inc.), and the nuclei were
counterstained with Mayer's hematoxylin for 1 min at room
temperature. Optical microscopic images were captured using an
Olympus BX53 microscope equipped with the cellSens imaging system
(Olympus Corporation).
Spheroids of MCF-7 and MDA-MB-231
cells
A total of 1×104 parental, MCF-7 and
MDA-MB-231 cells per well were counted as aforementioned and seeded
into PrimeSurface® 96-well ultra-low attachment
round-bottom plates (Sumitomo Bakelite Co., Ltd.). The cells were
cultured at 37°C in a 5% CO2 atmosphere for 6 days to
form multicellular spheroids. Following culture, spheroids were
fixed and subjected to IHC staining for KL-6 and HIF-1α, as
aforementioned and observed under an Olympus BX53 microscope.
Hypoxia induction experiment using
MDA-MB-231 cells
Hypoxia-induced changes in KL-6 protein expression
were evaluated in MDA-MB-231 cells using immunocytochemistry. Cells
(1×104) were cultured for 2 days in three 2-well slide
chambers (cat. no. 192-002; Watson Co., Ltd.). In each chamber, one
well was treated with cobalt chloride (CoCl2) to induce
hypoxia, whereas the other well served as an untreated control. The
cells were incubated in DMEM supplemented with CoCl2 (2
µmol/l; cat. no. 15862-1ML-F; MilliporeSigma) at 37°C for 8 h.
Following incubation, the cells were washed twice with PBS and
fixed in 4% PFA at 4°C for 30 min. Each of the three slides was
then assigned to a specific assay: One for KL-6 immunocytochemical
staining, one for HIF-1α immunocytochemical staining, and one for
hypoxia detection. Hypoxia was visualized using the LOX-1 hypoxia
probe (cat. no. NC-LOX-1S; MBL Co., Ltd.). Briefly, after the 8-h
incubation, LOX-1 was added to the DMEM at a concentration of 10
µl/ml. The cells were subsequently fixed again in 4% PFA (4°C for
30 min), washed twice with PBS, counterstained with DAPI, and
examined under a fluorescence microscope.
Immunoelectron microscopy
Immunoelectron microscopy was performed as
previously described (16).
MDA-MB-231 cells and spheroids were cultured on chamber slides or
PrimeSurface 96-well ultra-low attachment plates and fixed with 4%
PFA containing 0.01% glutaraldehyde (Wako Pure Chemical Industries)
at room temperature for 10 min. Endogenous peroxidase activity was
blocked by incubating the samples in methanol containing 0.3%
H2O2 for 10 min. After washing with PBS,
non-specific binding was blocked by incubation in PBS supplemented
with 10% normal goat serum for 10 min. The samples were then
incubated overnight at 4°C with a mouse monoclonal antibody against
KL-6. After washing with PBS, the slides were incubated at room
temperature for 60 min with the ImmPRESS detection reagent (Vector
Laboratories, Inc.). The peroxidase reaction was visualized using
DAB substrate solution (Vector Laboratories, Inc.) for 10 min.
After several rinses with distilled water, the slides were treated
with 0.01% aqueous hydrogen tetrachloroaurate(III) tetrahydrate
(HAuCl4·3H2O; MilliporeSigma) for 10 min,
rinsed again in distilled water, and dried using an air blower. The
slides were then incubated in a humid chamber at 37°C for 15 h,
followed by air-drying. As a control, parallel slides were
incubated under dry air conditions. All samples were examined under
a JSM-6010LV microscope (JEOL, Ltd.).
Statistical analyses
All quantitative data were obtained from at least
three independent experiments and are expressed as the mean ±
standard deviation. To compare KL-6-positive cell rates between the
MCF-7 and MDA-MB-231 cell lines, Levene's test was initially used
to assess the homogeneity of variances. As unequal variances were
observed, Welch's t-test was applied for statistical comparison.
Western blotting was performed to evaluate the KL-6 expression in
MCF-7 cells treated with or without CoCl2, a chemical
hypoxia-inducing agent. The Shapiro-Wilk test confirmed normal
distribution in both groups, whereas Levene's test revealed unequal
variances; therefore, Welch's t-test was used for statistical
comparison.
In the wound healing assay, the relative area
covered by cells was measured at 0 and 36 h post-treatment. As the
data from both treatment groups (KL-6 neutralizing antibody and
non-immune IgG control) were not normally distributed, according to
the Shapiro-Wilk test, comparisons were performed using the
non-parametric Mann-Whitney U test. All statistical analyses were
performed using the R software (version 4.4.3, http://www.R-project.org/) with a significance level
set at α=0.05. P<0.05 was considered to indicate a statistically
significant difference.
Results
Association between KL-6 and HIF-1α
expressions and BC recurrence and metastasis
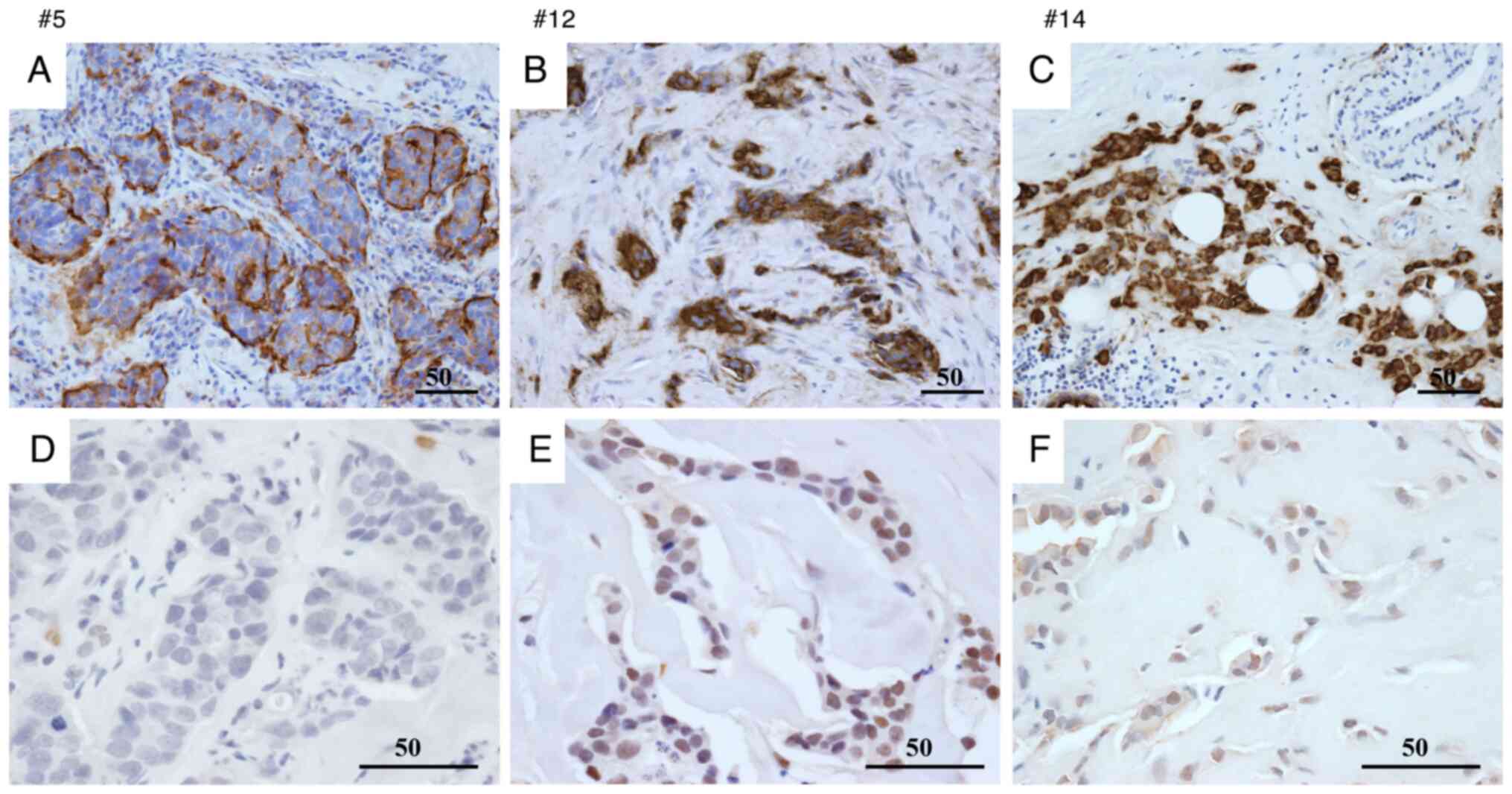
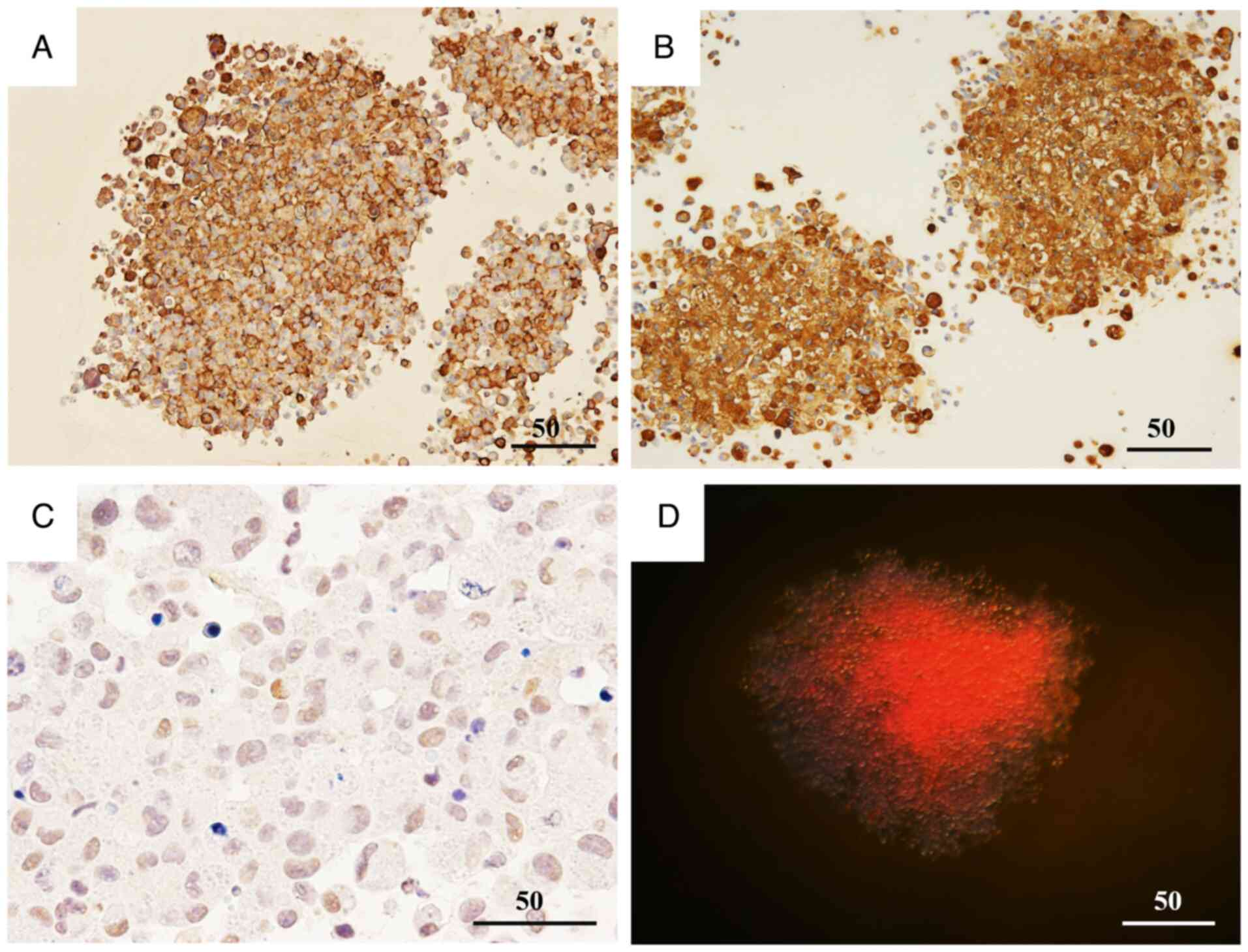
The association between BC recurrence or metastasis
and the IHC expression of KL-6 and HIF-1α in surgical pathology
specimens was investigated. Among the 30 patients, eight
experienced recurrence or metastasis, whereas 20 showed no evidence
of disease progression (Table
SI).
Expression levels were quantified using the Allred
scoring system (range, 0–8). Based on the observed score
distribution, the top three score categories were defined as ‘high’
expression, and the remaining categories were classified as ‘low’
expression.
For KL-6, 7/9 patients in the recurrence/metastasis
group were classified as having high expression, whereas 2/9 were
classified as having low expression. This pattern is suggestive but
should be interpreted with caution given the small sample (Fig. 1A-C).
For HIF-1α, 4/9 patients in the
recurrence/metastasis group were classified as having high
expression, whereas 5/9 were classified as having low expression.
In the non-recurrence/metastasis group, 1/21 patients were
classified as having high expression, whereas 20/21 were classified
as having low expression. These counts indicate a numerical
enrichment of higher HIF-1α expression in patients with recurrence
or metastasis (Fig. 1D-F).
A single-center analysis was conducted with a
limited number of events (n=9), yielding an events-per-variable
(EPV) below commonly accepted thresholds for stable multivariable
modeling. To prevent overfitting and unstable estimates,
statistical analyses, including multivariable modeling and
hypothesis testing, were not performed; accordingly, inferential
statistics (for example, P-values or confidence intervals) were not
reported in this subsection. The findings are therefore descriptive
and hypothesis-generating and require validation in larger,
multicenter cohorts with adequate EPV.
KL-6 expression in BC cell lines
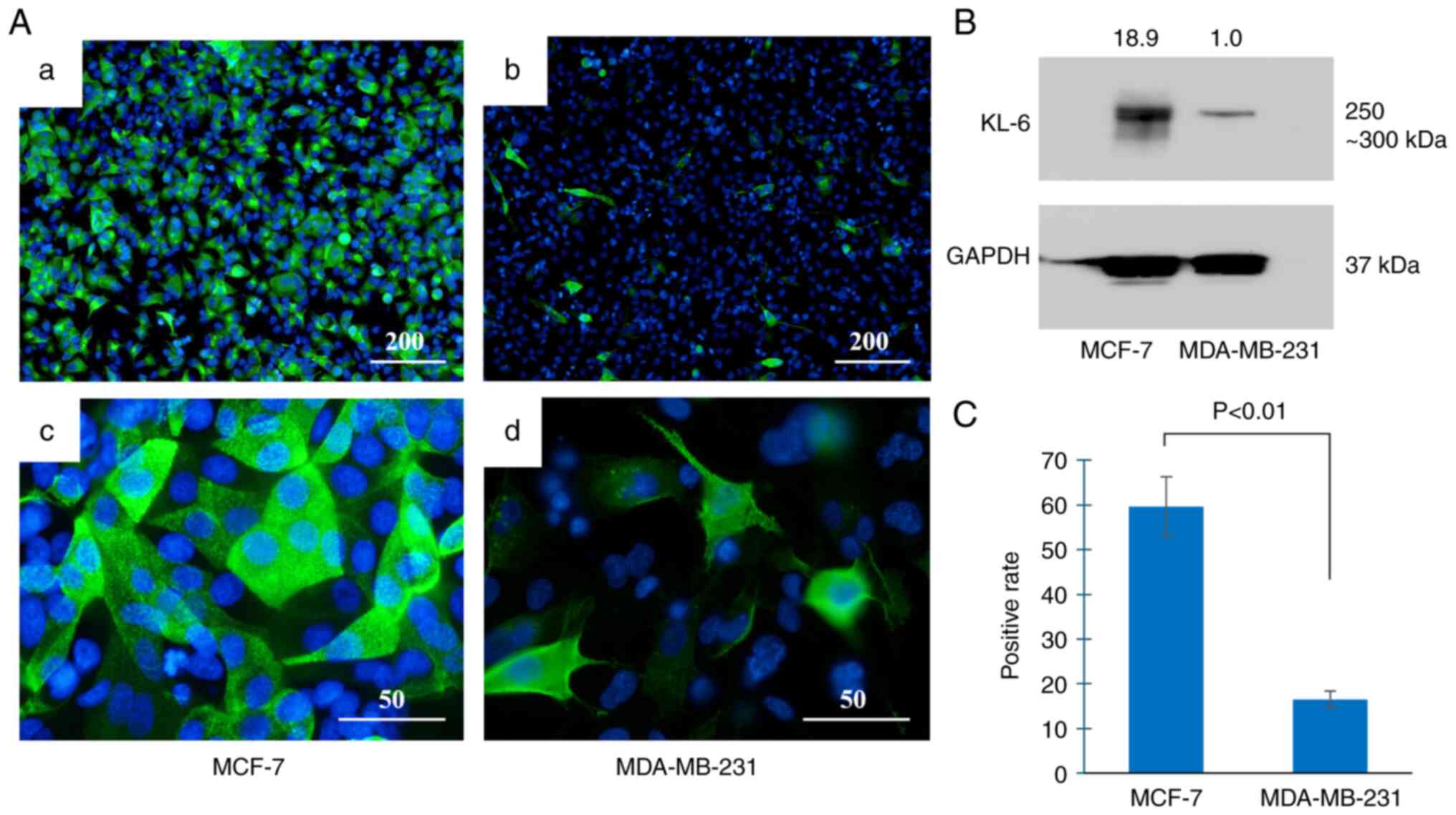
KL-6 protein expression in the BC cell lines was
evaluated using immunofluorescence staining and western blot
analysis (Fig. 2).
Immunofluorescence staining (Fig.
2A) demonstrated that the majority of MCF-7 cells exhibited
KL-6-positive signals (Fig. 2Aa),
whereas only a small proportion of MDA-MB-231 cells were positive
for KL-6 expression (Fig. 2Ab).
Subcellular localization analysis revealed that KL-6 in MCF-7 cells
was present as punctate staining along the cell membrane (Fig. 2Ac). By contrast, KL-6 localization
in MDA-MB-231 cells was primarily observed at the membrane edges
and cellular protrusions (Fig.
2Ad).
Western blot analysis (Fig. 2B) demonstrated markedly lower KL-6
expression in MDA-MB-231 cells compared with MCF-7 cells.
Quantitative analysis (Fig. 2C)
confirmed a higher proportion of KL-6-positive cells in MCF-7 than
in MDA-MB-231 cells.
Prior to group comparison, data distribution was
assessed using the Shapiro-Wilk test, which confirmed normality in
both cell lines. Levene's test indicated unequal variances;
therefore, Welch's t-test was applied. A significantly higher
percentage of KL-6-positive cells was observed in MCF-7 cells
compared with MDA-MB-231 cells (P<0.01).
Changes in cell migration capacity
following KL-6 functional inhibition: a wound healing assay
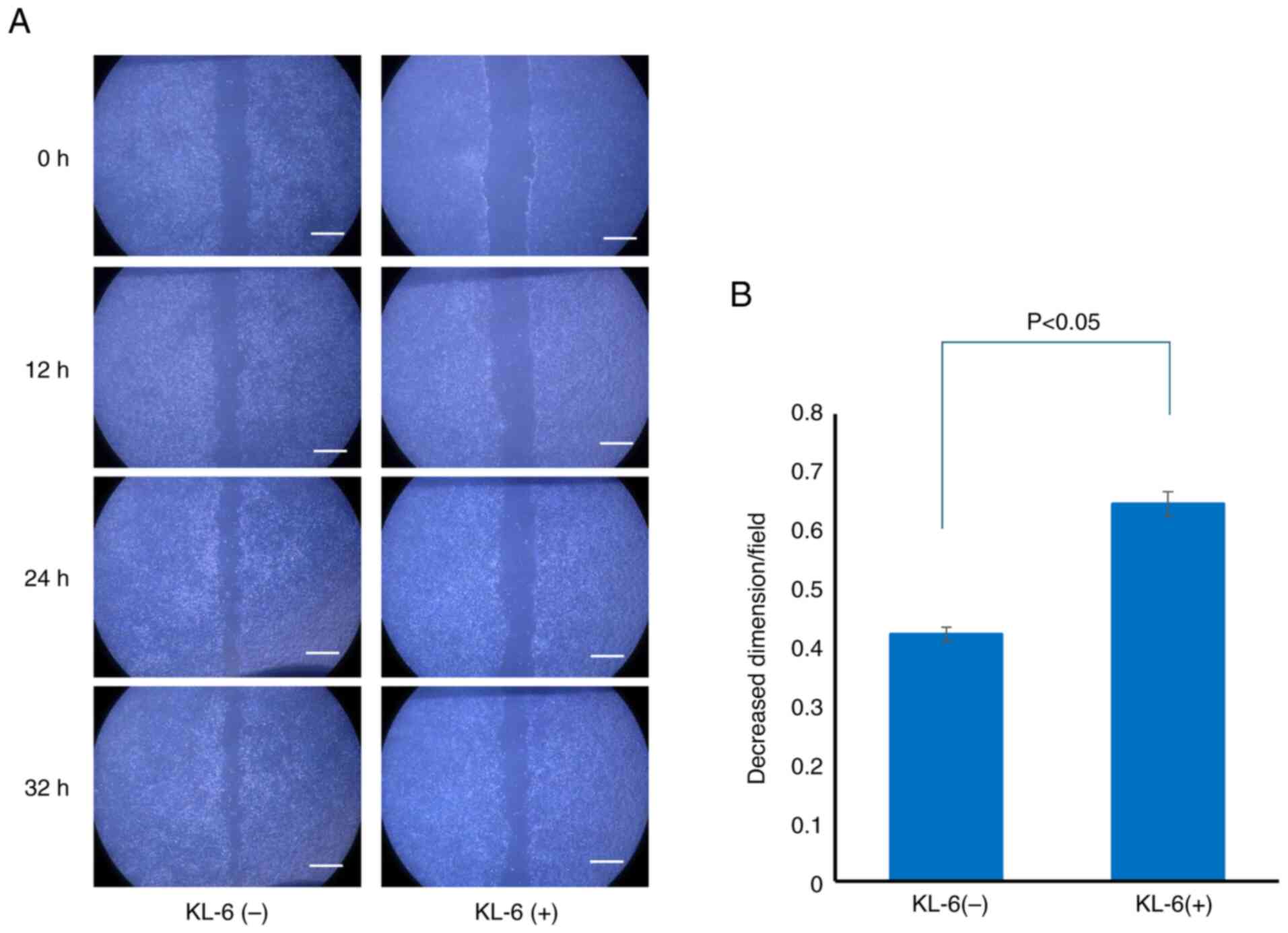
To elucidate the role of KL-6 in cell migration, a
wound healing assay was conducted. MCF-7 cells were selected for
this analysis due to their markedly higher KL-6 expression compared
with MDA-MB-231 cells, as demonstrated using western blot
analysis.
Following the creation of a linear wound in the cell
monolayer, two treatment groups were established: cells treated
with a KL-6 neutralizing antibody and control cells treated with
non-immune IgG. Wound closure was assessed 36 h after treatment.
Substantial wound closure was observed in the control group,
whereas closure was notably inhibited in the KL-6 antibody-treated
group (Fig. 3A).
For quantitative analysis, the relative wound area
covered at 0 and 36 h post-treatment was calculated and compared
between the groups. The Shapiro-Wilk test indicated that data from
both groups were not normally distributed; therefore, the
non-parametric Mann-Whitney U test was employed. The analysis
demonstrated that the KL-6 neutralizing antibody-treated group
exhibited a significantly smaller relative covered area compared
with the control group, indicating reduced migratory capacity
(P<0.05; Fig. 3B).
These findings suggested that KL-6 positively
regulates MCF-7 cell migration, supporting its role as a functional
contributor to BC cell invasiveness.
Expression of KL-6 and HIF-1α in the
spheroid model
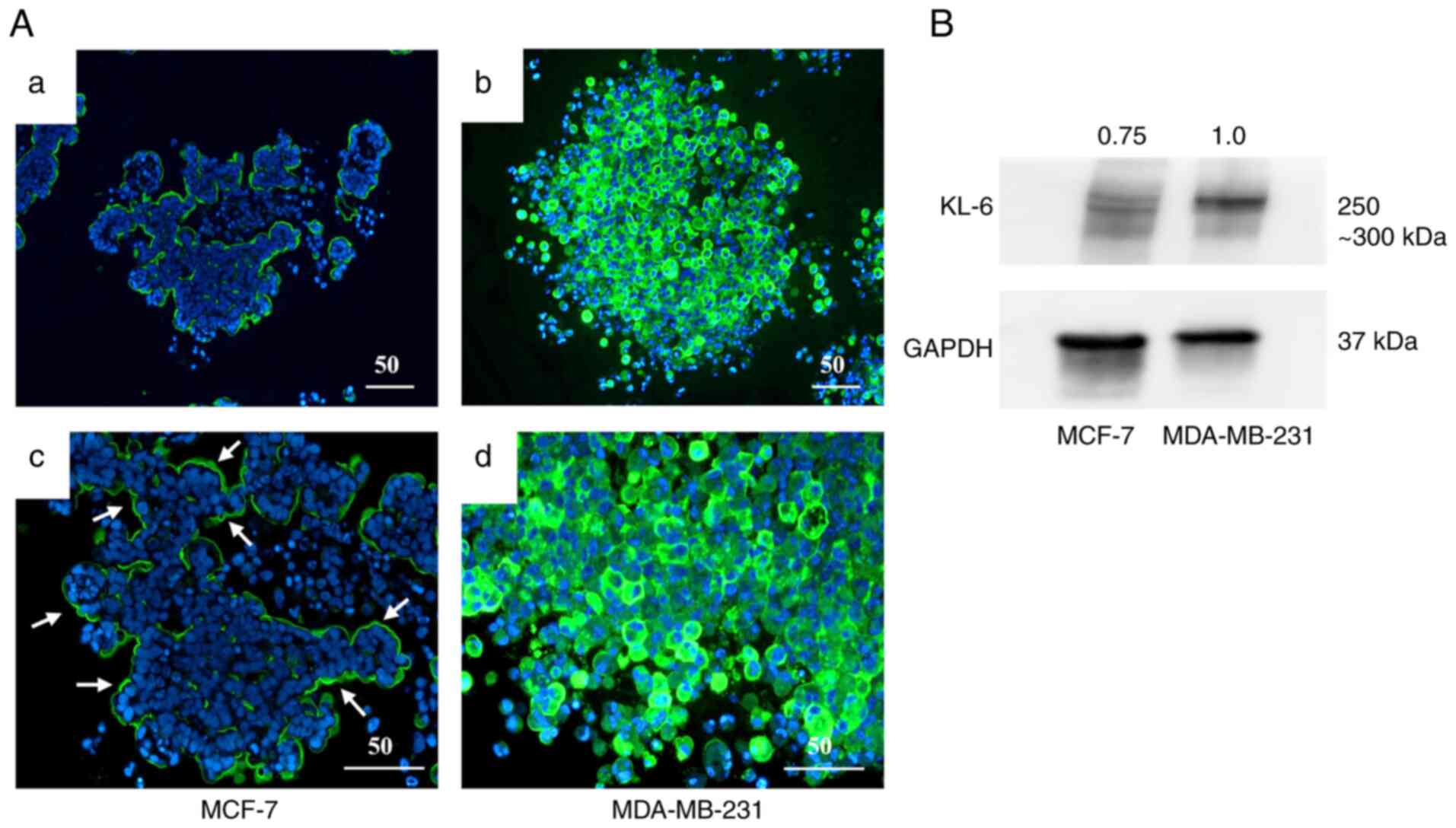
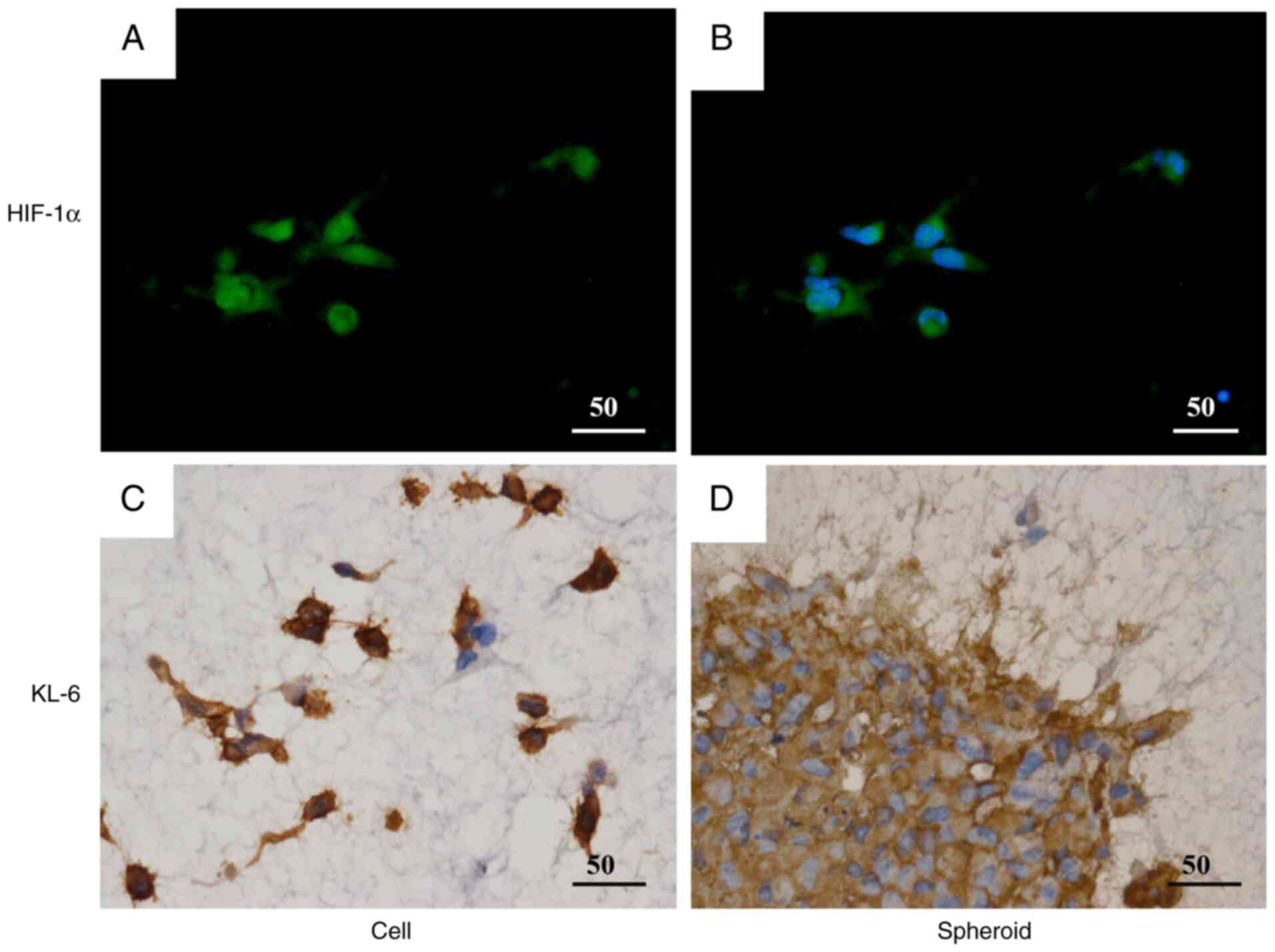
A three-dimensional spheroid culture model was used
to evaluate KL-6 expression in the BC cell lines MCF-7 and
MDA-MB-231 through IHC and western blot analysis (Fig. 4).
IHC analysis (Fig.
4A) demonstrated that KL-6 positivity in MCF-7 spheroids was
predominantly localized to the peripheral regions of the spheroid
structure (Fig. 4Aa and c). By
contrast, strong KL-6 positivity in MDA-MB-231 spheroids was
observed throughout the entire spheroid, including the central
regions (Fig. 4Ab and d).
Quantitative assessment by western blotting
(Fig. 4B) demonstrated higher KL-6
protein levels (Fig. 4B) in
MDA-MB-231 spheroids compared with MCF-7 spheroids. These findings
indicated that KL-6 expression was markedly upregulated in
MDA-MB-231 cells under three-dimensional culture conditions.
Temporal changes in hypoxia induction
and KL-6 expression in spheroid cultures and their involvement in
cell invasion
To further examine the relationship between KL-6
expression and hypoxia, the MDA-MB-231 spheroids were analyzed at
various time points (Fig. 5). IHC
analysis demonstrated a mosaic-like pattern of KL-6 expression 2
days after seeding (Fig. 5A); by
day 6, the majority of cells exhibited KL-6 positivity (Fig. 5B). Nuclear expression of HIF-1α, a
marker of hypoxia, was detected on day 6 (Fig. 5C). Furthermore, hypoxia probe-based
detection demonstrated strong fluorescence signals within the
spheroid core at this time point (Fig.
5D). These findings suggested that hypoxia develops
progressively during spheroid culture and is associated with
increased KL-6 expression in MDA-MB-231 cells.
KL-6 expression was subsequently assessed in
MDA-MB-231 cells and spheroids embedded within a three-dimensional
extracellular matrix (Fig. 6).
Immunofluorescence analysis demonstrated that most embedded cells
exhibited nuclear localization of HIF-1α, as evidenced by
co-localization with DAPI staining (Fig. 6A and B). IHC analysis further
revealed prominent KL-6 expression in cellular protrusions
extending from the spheroids into the surrounding matrix (Fig. 6C and D). These results suggested
that KL-6 contributes to the invasive behavior of MDA-MB-231 cells
under hypoxic conditions within a three-dimensional matrix
environment.
Induction of KL-6, HIF-1α and LOX-1 by
CoCl2 treatment
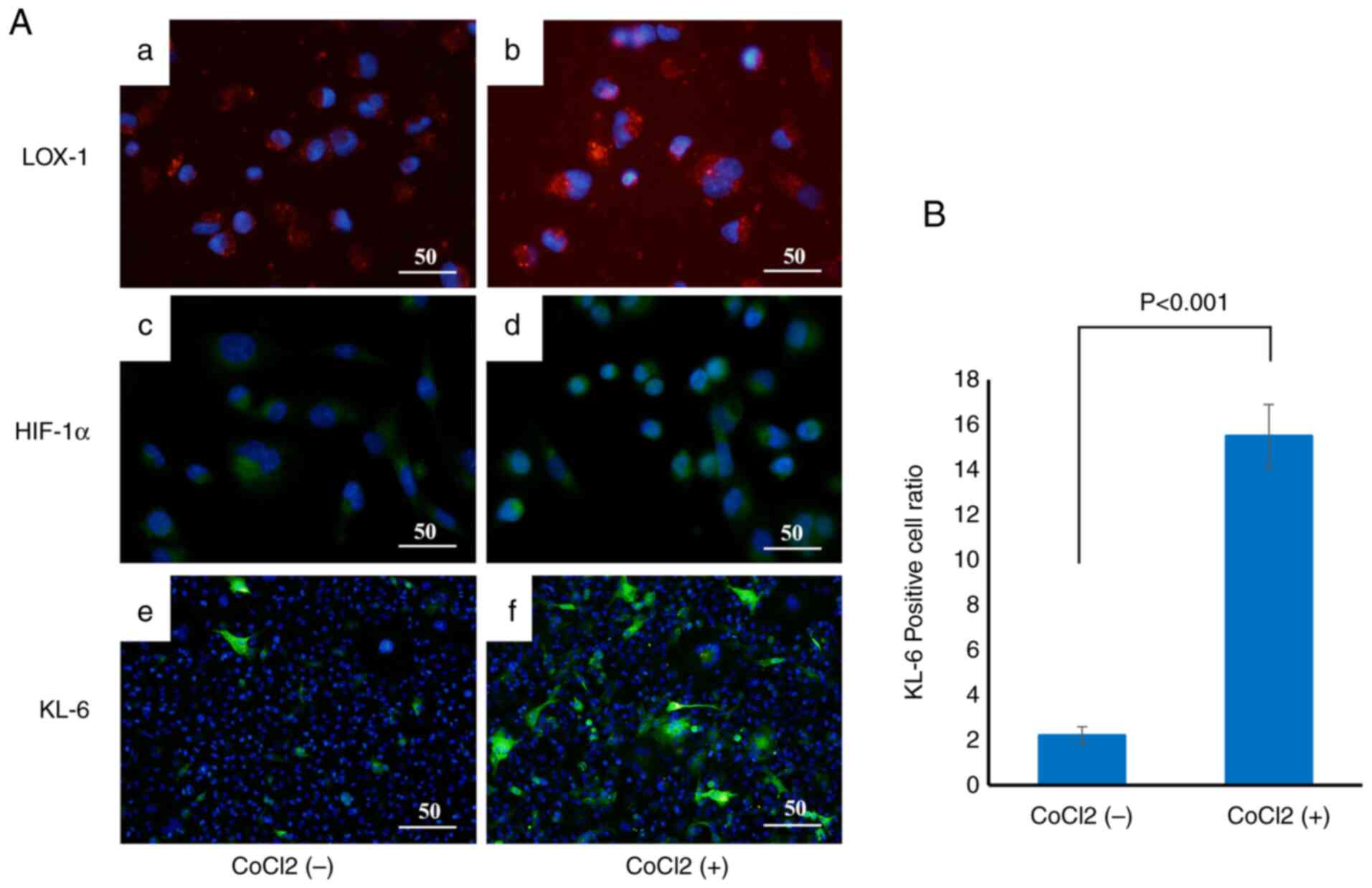
To examine the effect of hypoxic conditions on KL-6
expression in BC cells, CoCl2, a chemical hypoxia
inducer, was used to treat the cells, and changes in the expression
of the hypoxia markers LOX-1 and HIF-1α, as well as KL-6, were
evaluated by immunofluorescence staining (Fig. 7A).
MCF-7 cells were incubated with CoCl2 at
concentrations of 2 and 4 µmol/l for 6 h at 37°C.
Immunofluorescence analysis demonstrated that the fluorescence
intensities of LOX-1 (Fig. 7Aa and
b) and HIF-1α (Fig. 7Ac and d)
were markedly increased in the CoCl2-treated groups,
indicating effective induction of hypoxic conditions. KL-6
fluorescence was also significantly enhanced in
CoCl2-treated cells (Fig.
7Af) compared with the untreated controls (Fig. 7Ae).
Quantitative analysis of the proportion of
KL-6-positive cells relative to the total cell population revealed
a significant increase in the CoCl2-treated group
compared with the untreated group (P<0.001), as determined by
Welch's t-test (Fig. 7B).
Morphological observation of KL-6
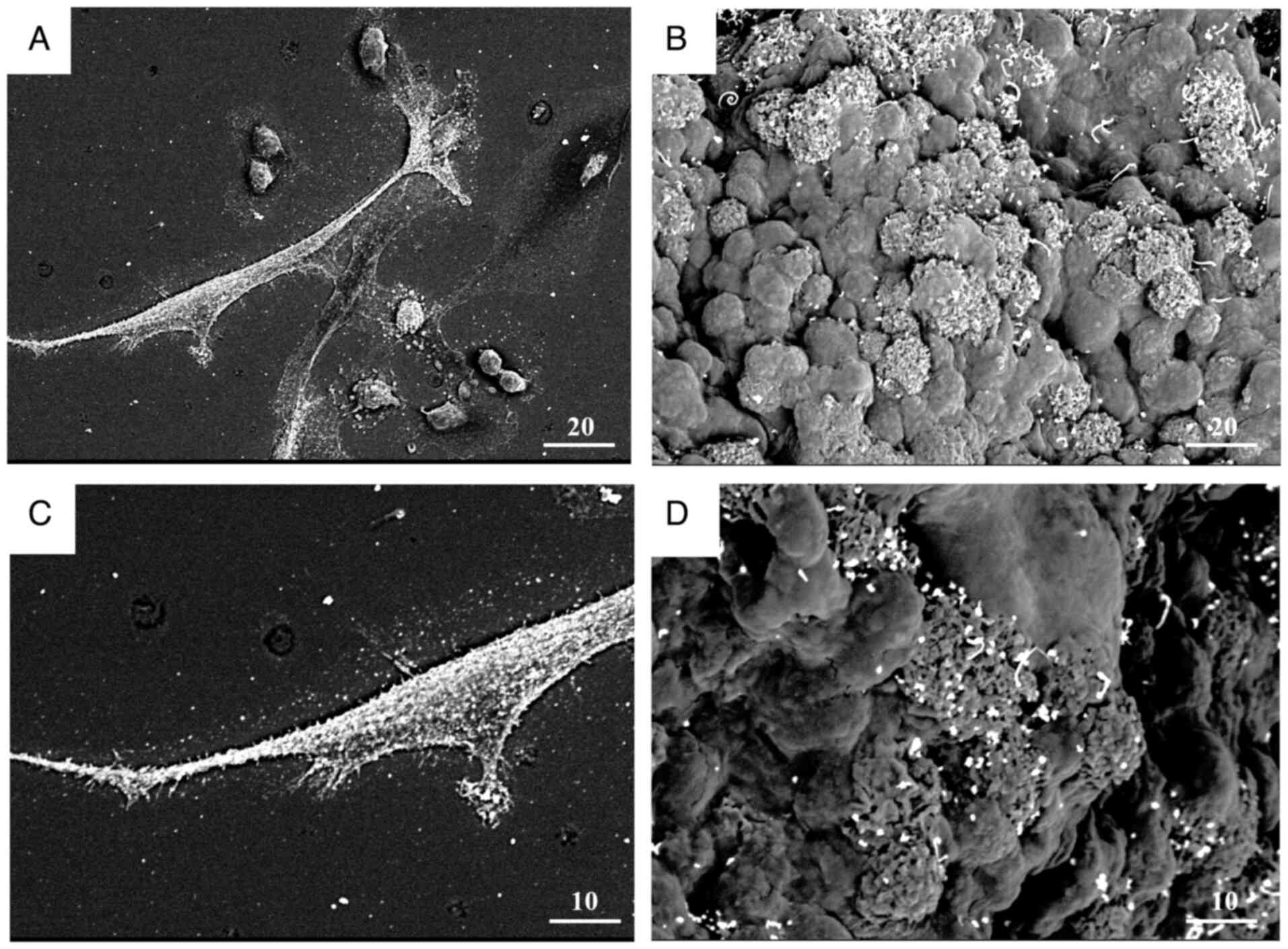
localization via immunoelectron microscopy
To elucidate the ultrastructural localization of
KL-6, immunogold labeling combined with low-vacuum scanning
electron microscopy was performed on MDA-MB-231 cells and spheroids
(Fig. 8).
Immunoelectron micrographs of MDA-MB-231 monolayer
cells and spheroids (Fig. 8A and C)
demonstrated KL-6-positive gold particles prominently localized on
the cell membrane, within cellular protrusions, and at sites of
cell-cell contact. Surface imaging of spheroids (Fig. 8B and D) further demonstrated the
accumulation of KL-6-associated gold particles in regions enriched
with protrusive structures.
Discussion
The present study demonstrated that hypoxia
increases KL-6/MUC1 expression in BC, at least in part via HIF-1α,
and that KL-6 contributes to invasive behavior. Supporting evidence
includes KL-6 enrichment on invasive protrusions, its upregulation
in hypoxic spheroids and CoCl2-treated models, and the
reduction of cell migration following KL-6 blockade.
In the clinical cohort, the KL-6 and HIF-1α
expression levels were numerically higher in patients who
experienced recurrence or metastasis, suggesting a potential
association. However, this analysis was conducted in a
single-center cohort with a limited number of events (n=9),
resulting in an EPV below commonly accepted thresholds for stable
multivariable modeling. To avoid overfitting and unstable
estimates, multivariable modeling and formal hypothesis testing
were not performed, and only descriptive associations were
reported. Consequently, these findings are hypothesis-generating
and should be interpreted with caution, as potential confounding
factors such as tumor stage, treatment, and subtype could not be
adjusted for in this analysis.
In the experiments of the present study (Fig. 2), the proportion of KL-6-positive
cells was significantly higher in MCF-7 than in MDA-MB-231 cells
(P<0.01), indicating that basal KL-6 expression is enriched in
MCF-7 under the assay conditions employed. Consistent with
established biology, MCF-7 cells display a luminal A epithelial
phenotype characterized by strong E-cadherin and low vimentin
expression. Conversely, MDA-MB-231 cells exhibit a mesenchymal
program, including loss of E-cadherin, vimentin and N-cadherin
expression; activation of the Ras-related C3 botulinum toxin
substrate-extracellular signal-regulated kinase-nuclear factor
kappa-light-chain-enhancer of activated B cells signaling pathway;
and elevated matrix metalloproteinase activity. These traits
support extracellular matrix degradation, stromal adhesion and
motility, thereby conferring greater invasive potential (17–21).
Collectively, these observations indicate that higher KL-6
expression in MCF-7 cells does not, by itself, determine invasive
capacity; rather, invasion likely reflects the integrated effects
of KL-6-dependent and KL-6-independent mechanisms, including
epithelial-mesenchymal transition (EMT) status, NF-κB/Rac
signaling, protease activity, and tumor-stroma interactions.
The structural characteristics of KL-6, particularly
its sialic acid modifications, have been implicated in promoting
cancer cell invasiveness and metastatic potential (22). Sialylation represents a key
molecular alteration that facilitates tumor cell migration,
adhesion and immune evasion (22).
Consistent with other sialylated glycans, KL-6 was observed at the
cell membrane in the current electron microscopy analyses (23). This localization suggests a role in
mediating cell-cell adhesion and interactions with the
extracellular matrix. Given the established involvement of
sialylated glycans in promoting cancer cell invasiveness and
dissemination, KL-6 may similarly enhance invasion and metastasis
by modulating cell-cell and cell-matrix interactions within the TME
(22). These findings suggest that
KL-6 may function beyond its utility as a biomarker and actively
contribute to malignant BC phenotypes through mechanisms
characteristic of sialylated glycoconjugates (24).
IHC and western blot analyses demonstrated elevated
KL-6 expression in MDA-MB-231 cell spheroids compared with
conventional monolayer cultures. This observation led to the
hypothesis that the increased KL-6 expression observed in the
spheroid models may be attributable to the hypoxic conditions
characteristic of three-dimensional cultures (25). To test this hypothesis, a chemical
hypoxia model was established using CoCl2. Following
CoCl2 treatment, the upregulation of HIF-1α expression
was observed, confirming the successful induction of a hypoxic
environment. Concurrently, a significant increase in the proportion
of KL-6-positive cells was detected, indicating that KL-6
expression is inducible under hypoxic conditions. Hypoxia modulates
the expression of adhesion molecules and glycosylation-related
proteins, enhancing the invasive and metastatic potential of cancer
cells (26,27). HIF-1α, a central regulator of the
cellular hypoxic response, governs the expression of numerous
tumor-associated genes (10). The
present findings suggest that KL-6 may act downstream of the HIF-1α
signaling, contributing to the malignant phenotype of BC cells.
Additionally, sialic acid modifications, which are promoted under
hypoxic conditions, have been shown to facilitate tumor invasion
and metastasis; KL-6 may similarly participate in these
sialylation-mediated mechanisms (22).
KL-6 is a glycan-dependent epitope on MUC1 (cluster
9). The minimal epitope recognized by the KL-6 antibody is located
within the MUC1 tandem repeat bearing an α2,3-sialyl-T structure
(7). Consequently, increased MUC1
expression and sialylation are expected to enhance KL-6 detection
in tumor cells. Under chronic hypoxia, HIF-1α and NF-κB p65
cooperatively induce MUC1/MUC1-C, and direct genetic perturbation
abolishes this response. Using a hypoxia fate-mapping/CRISPR
system, knockout of HIF-1α or NF-κB p65 prevented hypoxia-induced
MUC1-C accumulation (14).
Functionally, either deletion of MUC1 under hypoxic conditions or
pharmacologic inhibition of MUC1-C with the cell-penetrant
inhibitor GO-203 increased mitochondrial reactive oxygen species in
circulating tumor cells and reduced the contribution of
hypoxia-experienced cells to lung metastasis in vivo
(14).
Multiple independent lines of evidence indicate that
MUC1 is a direct HIF-1 target gene (12,13,28).
In clear-cell renal cell carcinoma models, stabilization of HIF-1α
by CoCl2 led to increased MUC1 mRNA levels, whereas
HIF-1α knockdown via small interfering RNA and the HIF-1 inhibitor
YC-1 reduced hypoxia-induced MUC1 expression. Chromatin
immunoprecipitation and electrophoretic mobility shift assay
identified HIF-1α binding at two hypoxia-responsive elements within
the MUC1 promoter, establishing direct transcriptional control
(13). In conjunction with studies
that MUC1 stabilizes HIF-1α and co-activates hypoxia-metabolic
targets, these findings support the existence of a feed-forward
HIF-MUC1 axis that reinforces hypoxic adaptation. Functionally,
hypoxia increased invasion and migration by ~4.7-fold and 3.9-fold,
respectively, whereas MUC1 knockdown significantly reversed these
hypoxia-enhanced phenotypes (13).
Building on these findings, a coherent model is proposed in which
KL-6/MUC1 promotes invasion and metastasis through HIF-1α-dependent
transcriptional control.
Sialic acid-modified mucin family molecules have
been reported to alter the physical and biological properties of
cell surfaces, thereby facilitating the acquisition of invasive and
metastatic capabilities (29).
α2,3-linked sialylation has been consistently associated with
metastatic behavior across multiple tumor types. In gastric cancer,
elevated α2,3-linked sialic acids correlate with increased
metastasis and poorer prognosis, consistent with the present
observation that the α2,3-sialylated KL-6 epitope is elevated in
motile states (30). These findings
support a model in which α2,3 sialylation marks an invasive
phenotype. ST3GAL1 catalyzes the installation of terminal
α2,3-sialic acid on O-glycans, the same linkage that defines the
KL-6 (sialyl-T) motif on MUC1. Functionally, ST3GAL1 promotes
migration, invasion, and TGF-β1-induced EMT in ovarian cancer
(31). In melanoma, ST3GAL1
enhances dimerization and activation of the receptor tyrosine
kinase AXL, increasing invasion and metastatic seeding (32). Collectively, these findings support
a coherent mechanism in which a KL-6-high state reflects
ST3GAL1-driven α2,3 sialylation on MUC1 and other surface proteins,
thereby potentiating receptor tyrosine kinase (RTK)-centered
signaling (for example, AXL) that promotes invasion (31,32).
This delineates a plausible downstream pathway by which KL-6 may
facilitate invasion through ST3GAL1-dependent α2,3 sialylation and
subsequent activation of RTK signaling.
The present study has certain limitations. First,
the relatively small sample size necessitates cautious
interpretation when generalizing the observed association between
KL-6 expression and recurrence or metastasis risk (29). Future studies with larger sample
sizes and multi-institutional collaborations are required to
validate these findings. Second, although a chemical hypoxia model
(CoCl2 treatment) was employed, it may not fully
replicate the sustained and multifactorial hypoxic conditions
present within TMEs. Future studies should evaluate KL-6 expression
dynamics using physical hypoxia culture systems and in vivo
models.
Furthermore, the downstream signaling pathways and
functional roles of KL-6 in cell adhesion and invasion require
further elucidation. In particular, the identification of specific
receptor molecules and extracellular matrix components that
interact with KL-6 remains a critical challenge and is directly
relevant to the development of novel therapeutic strategies for
BC.
In conclusion, the present study provides novel
insights into the biological significance of KL-6 in BC. Further
investigations are warranted to explore its potential clinical
applications.
Supplementary Material
Supporting Data
Acknowledgements
We acknowledge the Division of Functional Analysis
for Life Science, Science Research Center and Kochi University for
their valuable support in immunohistochemical analysis and for
providing technical expertise and access to electron microscopy,
which greatly contributed to this study.
Funding
The present study was supported by the Kochi Medical School
Hospital President's Discretionary Grant for the procurement of
essential research materials and additional support was provided by
the Department of Pathology, Kochi Medical School.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TI conceived and designed the study, drafted the
manuscript, and performed data analysis and interpretation. YH
performed the experimental procedures. TS contributed to the
study's conceptualization and assisted in data analysis and
interpretation. YH and TS served as primary contributors to the
research. MI and MT performed the immunohistochemical staining of
pathological tissues. HS and IM confirm the authenticity of all the
raw data and contributed to the interpretation of the experimental
findings and to the intellectual development of the manuscript,
including the conception and logical organization of the
background, methods, results, and discussion sections. All authors
critically reviewed the manuscript for intellectual content, read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Ethical Review Board of Kochi Medical School (approval no.
2023-146; April 19, 2024). The study was conducted using an opt-out
approach, whereby information about the research was publicly
disclosed, and participants were provided the opportunity to
decline participation to the fullest extent possible.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript
Glossary
Abbreviations
Abbreviations:
|
KL-6
|
Krebs von den Lungen-6
|
|
MUC1
|
mucin 1
|
|
TME
|
tumor microenvironment
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
type2
|
|
PFA
|
paraformaldehyde
|
|
PBS
|
phosphate-buffered saline
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
HRP
|
horseradish peroxidase
|
|
CoCl2
|
cobalt chloride
|
|
EPV
|
events-per-variable
|
|
Rac-ERK-NF-κB
|
signaling pathway
|
|
EMT
|
epithelial-mesenchymal transition
|
|
RTK
|
receptor tyrosine kinase
|
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Salemme V, Centonze G, Avalle L, Natalini
D, Piccolantonio A, Arina P, Morellato A, Ala U, Taverna D, Turco E
and Defilippi P: The role of tumor microenvironment in drug
resistance: Emerging technologies to unravel breast cancer
heterogeneity. Front Oncol. 13:11702642023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer Genome Atlas Network, .
Comprehensive molecular portraits of human breast tumours. Nature.
490:61–70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mittal S, Brown NJ and Holen I: The breast
tumor microenvironment: role in cancer development, progression and
response to therapy. Expert Rev Mol Diagn. 18:227–243. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogawa Y, Ishikawa T, Ikeda K, Nakata B,
Sawada T, Ogisawa K, Kato Y and Hirakawa K: Evaluation of serum
KL-6, a mucin-like glycoprotein, as a tumor marker for breast
cancer. Clin Cancer Res. 6:4069–4072. 2000.PubMed/NCBI
|
|
7
|
Ohyabu N, Hinou H, Matsushita T, Izumi R,
Shimizu H, Kawamoto K, Numata Y, Togame H, Takemoto H, Kondo H and
Nishimura S: An essential epitope of anti-MUC1 monoclonal antibody
KL-6 revealed by focused glycopeptide library. J Am Chem Soc.
131:17102–17109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu HL, Zhao X, Zhang KM, Tang W and Kokudo
N: Inhibition of KL-6/MUC1 glycosylation limits aggressive
progression of pancreatic cancer. World J Gastroenterol.
20:1217–12181. 2014. View Article : Google Scholar
|
|
9
|
Wong CC, Gilkes DM, Zhang H, Chen J, Wei
H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, et al:
Hypoxia-inducible factor 1 is a master regulator of breast cancer
metastatic niche formation. Proc Natl Acad Sci USA.
108:16369–16374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basheeruddin M and Qausain S:
Hypoxia-inducible factor 1-alpha (HIF-1alpha) and cancer:
Mechanisms of tumor hypoxia and therapeutic targeting. Cureus.
16:e707002024.PubMed/NCBI
|
|
11
|
Rankin EB, Nam JM and Giaccia AJ: Hypoxia:
Signaling the metastatic cascade. Trends Cancer. 2:295–304. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mikami Y, Hisatsune A, Tashiro T, Isohama
Y and Katsuki H: Hypoxia enhances MUC1 expression in a lung
adenocarcinoma cell line. Biochem Biophys Res Commun.
379:1060–1065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aubert S, Fauquette V, Hémon B, Lepoivre
R, Briez N, Bernard D, Van Seuningen I, Leroy X and Perrais M:
MUC1, a new hypoxia inducible factor target gene, is an actor in
clear renal cell carcinoma tumor progression. Cancer Res.
69:5707–5715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Godet I, Oza HH, Shi Y, Joe NS, Weinstein
AG, Johnson J, Considine M, Talluri S, Zhang J, Xu R, et al:
Hypoxia induces ROS-resistant memory upon reoxygenation in vivo
promoting metastasis in part via MUC1-C. Nat Commun. 15:84162024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto Y, Hayashi Y, Sakaki H and
Murakami I: Downregulation of fascin induces collective cell
migration in triple-negative breast cancer. Oncol Rep. 50:1502023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayashi Y, Yamamoto Y and Murakami I:
Micromorphological observation of HLE cells under knockdown of
fascin using LV-SEM. Med Mol Morphol. 56:257–265. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Isert L, Mehta A, Loiudice G, Oliva A,
Roidl A and Merkel OM: An in vitro approach to model emt in breast
cancer. Int J Mol Sci. 24:77572023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu CY, Lin HH, Tang MJ and Wang YK:
Vimentin contributes to epithelial-mesenchymal transition cancer
cell mechanics by mediating cytoskeletal organization and focal
adhesion maturation. Oncotarget. 6:15966–15983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gest C, Joimel U, Huang L, Pritchard LL,
Petit A, Dulong C, Buquet C, Hu CQ, Mirshahi P, Laurent M, et al:
Rac3 induces a molecular pathway triggering breast cancer cell
aggressiveness: differences in MDA-MB-231 and MCF-7 breast cancer
cell lines. BMC Cancer. 13:632013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen CH, Senfter D, Basilio J, Holzner
S, Stadler S, Krieger S, Huttary N, Milovanovic D, Viola K,
Simonitsch-Klupp I, et al: NF-κB contributes to MMP1 expression in
breast cancer spheroids causing paracrine PAR1 activation and
disintegrations in the lymph endothelial barrier in vitro.
Oncotarget. 6:39262–39275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H, Qiu Z, Li F and Wang C: The
relationship between MMP-2 and MMP-9 expression levels with breast
cancer incidence and prognosis. Oncol Lett. 14:5865–5870.
2017.PubMed/NCBI
|
|
22
|
Dobie C and Skropeta D: Insights into the
role of sialylation in cancer progression and metastasis. Br J
Cancer. 124:76–90. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Julien S, Lagadec C, Krzewinski-Recchi MA,
Courtand G, Le Bourhis X and Delannoy P: Stable expression of
sialyl-Tn antigen in T47-D cells induces a decrease of cell
adhesion and an increase of cell migration. Breast Cancer Res
Treat. 90:77–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou X, Chi K, Zhang C, Liu Q and Yang G:
Sialylation: A cloak for tumors to trick the immune system in the
microenvironment. Biology (Basel). 12:8322023.PubMed/NCBI
|
|
25
|
Tian X, Wang W, Zhang Q, Zhao L, Wei J,
Xing H, Song Y, Wang S, Ma D, Meng L and Chen G: Hypoxia-inducible
factor-1α enhances the malignant phenotype of multicellular
spheroid HeLa cells in vitro. Oncol Lett. 1:893–897. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koike T, Kimura N, Miyazaki K, Yabuta T,
Kumamoto K, Takenoshita S, Chen J, Kobayashi M, Hosokawa M,
Taniguchi A, et al: Hypoxia induces adhesion molecules on cancer
cells: a missing link between Warburg effect and induction of
selectin-ligand carbohydrates. Proc Natl Acad Sci USA.
101:8132–8137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arriagada C, Silva P and Torres VA: Role
of glycosylation in hypoxia-driven cell migration and invasion.
Cell Adh Migr. 13:13–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goudarzi H, Iizasa H, Furuhashi M,
Nakazawa S, Nakane R, Liang S, Hida Y, Yanagihara K, Kubo T,
Nakagawa K, et al: Enhancement of in vitro cell motility and
invasiveness of human malignant pleural mesothelioma cells through
the HIF-1α-MUC1 pathway. Cancer Lett. 339:82–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Habeeb IF, Alao TE, Delgado D and Buffone
A Jr: When a negative (charge) is not a positive: Sialylation and
its role in cancer mechanics and progression. Front Oncol.
14:14873062024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen L, Luo Z, Wu J, Qiu L, Luo M, Ke Q
and Dong X: Enhanced expression of α2,3-linked sialic acids
promotes gastric cancer cell metastasis and correlates with poor
prognosis. Int J Oncol. 50:1201–1210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu X, Zhao J, Ruan Y, Sun L, Xu C and
Jiang H: Sialyltransferase ST3GAL1 promotes cell migration,
invasion, and TGF-β1-induced EMT and confers paclitaxel resistance
in ovarian cancer. Cell Death Dis. 9:11022018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pietrobono S, Anichini G, Sala C, Manetti
F, Almada LL, Pepe S, Carr RM, Paradise BD, Sarkaria JN, Davila JI,
et al: ST3GAL1 is a target of the SOX2-GLI1 transcriptional complex
and promotes melanoma metastasis through AXL. Nat Commun.
11:58652020. View Article : Google Scholar : PubMed/NCBI
|