Bone sarcomas, osteosarcoma (OS), Ewing sarcoma (ES)
and chondrosarcoma (CS), collectively account for 2–3% of all
childhood and adolescent types of cancer, yet they remain the third
leading cause of cancer-related mortality in patients aged 10–25
years (1). Globocan 2024 reports an
annual incidence of 0.3 for OS, 0.2 for ES and 0.4 for CS per
100,000, with no plateau in incidence over the past decade
(2). Distal extremity primaries
dominate OS and ES, whereas CS arises predominantly in axial
locations and is prone to local relapse (2). Genomic heterogeneity is extreme: OS is
characterized by TP53/RB1 disruption and chromothripsis, ES by
EWSR1-FLI1 translocations and CS by IDH1/2 or COL2A1 mutations,
which shape distinct immune landscapes (3,4). This
biological diversity translates into variable outcomes, 5-year
overall survival is 65–70% for localized OS but decreases to
<30% when metastatic (5); ES
achieves 75% survival with multimodal therapy but remains <25%
after recurrence (6) and high-grade
CS shows only 10–15% survival once unresectable (7). Despite their rarity, these tumors
impose a disproportionate clinical burden that calls for
mechanism-based therapeutic innovation.
Curative intent still hinges on surgery plus
multi-agent cytotoxic chemotherapy, with radiation reserved for
margin-positive or unresectable disease (8). Neoadjuvant response, defined by ≥90%
necrosis, associates with survival, yet 35–45% of patients with OS
achieve poor histologic response and carry a 3-fold higher risk of
metastatic relapse (8). High-grade
CS is intrinsically chemo- and radio-resistant, leaving wide
excision as the only curative option, which is often impossible in
pelvic or skull-base locations. Dose intensification has reached
ceiling toxicity without further survival benefit; therefore,
high-risk groups (metastatic presentation, axial primaries or
<90% necrosis) urgently require alternative strategies (9). Importantly, traditional regimens
neglect the tumor microenvironment (TME), which orchestrates
chemoresistance through hypoxia-induced quiescence, extracellular
matrix (ECM)-mediated drug trapping and immunosuppressive cytokines
such as TGF-β and IL-10. Recognizing these TME-centric escape
mechanisms is essential to explain why even aggressive multimodal
therapy fails in a substantial subset of patients (10,11).
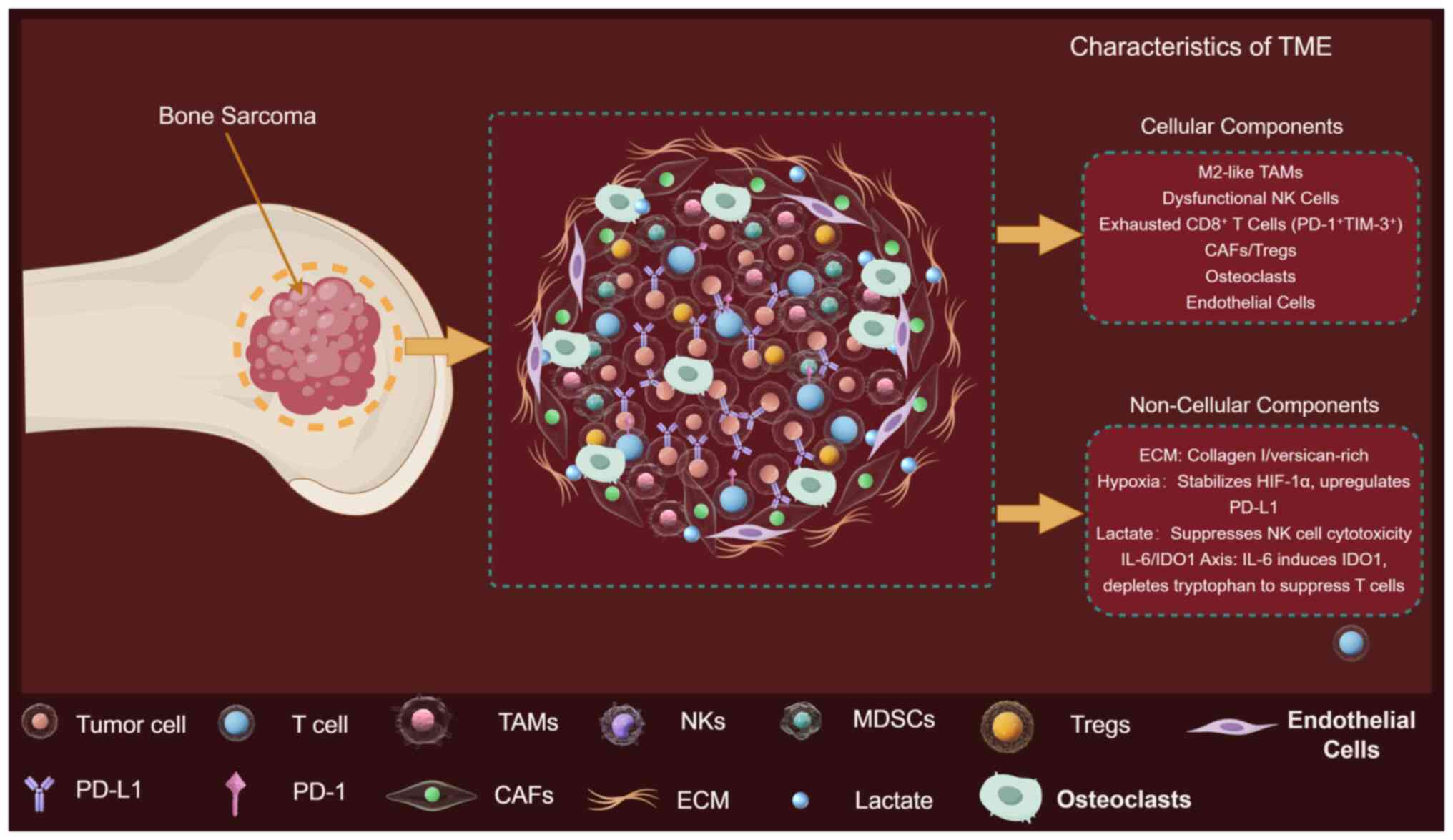
The bone sarcoma TME is an intricate ecosystem
composed of malignant cells interwoven with cancer-associated
fibroblasts (CAFs), bone-marrow-derived mesenchymal stromal cells,
osteoclasts, endothelial cells and a spectrum of innate and
adaptive immune cells (12).
Single-cell RNA-sequencing (scRNA-seq) of untreated OS resolved
nine major non-malignant populations, including C1Q+
tumor-associated macrophages (TAMs), exhausted CD8+ T cells,
regulatory T cells (Tregs) and dysfunctional dendritic cells (DCs)
(13). Non-cellular components are
equally important: A collagen- and versican-rich ECM increases
tissue stiffness, promotes integrin-mediated survival signaling and
impedes cytotoxic T-cell infiltration (14). Hypoxia and acidic pH gradients
generated by aerobic glycolysis stabilize hypoxia inducible factor
(HIF)-1α, upregulate programmed death-ligand 1 (PD-L1) and skew
macrophages toward an M2 immunosuppressive phenotype (15). Dynamic crosstalk between these
elements, for example, TAM-derived TGF-β induces OS stemness while
tumor-secreted CSF-1 reciprocally sustains TAMs survival, creates a
self-reinforcing niche that fosters progression and therapy
resistance (16,17).
Pre-clinical lineage-tracing studies demonstrate
that disseminated OS cells seed the lung as early as diagnosis, but
only those that have educated a pre-metastatic niche rich in
S100A8/A9+ myeloid cells and ECM crosslinking enzymes successfully
colonize (18,19). In ES, CD99-mediated reprogramming of
macrophages suppresses antigen presentation, facilitating immune
evasion during transit (20).
Locally, hypoxia-induced exosomes transfer micro (miR)-135b from
tumor cells to endothelial cells, promoting angiogenesis and
subsequent osteolysis (21).
Chemotherapy itself reshapes the TME: Cisplatin increases M2-TAMs
infiltration and PD-L1 expression, whereas doxorubicin enriches ECM
cross-linking enzymes, both contributing to acquired resistance
(22). Radiation upregulates
antigen presentation machinery yet simultaneously expands CD47+
macrophages that blunt T-cell cytotoxicity (23). Thus, the TME not only fuels primary
tumor growth but also orchestrates metastatic spread and shields
residual cells from cytotoxic, targeted and immune attack,
positioning it as the central bottleneck for durable cures.
OS is the most frequent primary malignant bone
neoplasm, with a bimodal age distribution encompassing adolescents
and the elderly (1,2). Despite aggressive multi-modal therapy,
~40% of patients with OS develop pulmonary metastases within 5
years, and the 5-year survival for metastatic disease remains
<30% (5). Single-cell RNA
sequencing coupled to intravital imaging has recently shown that
invasive osteosarcoma cells heighten focal adhesion kinase/SRC
signaling when confronted with extracellular-matrix stiffening
(Young's modulus >30 kPa), facilitating amoeboid migration and
trans-endothelial extravasation (24). Hypoxia-induced exosomal miR-135b
transferred from tumor to endothelial cells enhances vascular
endothelial growth factor (VEGF)-independent angiogenesis and
osteolysis, facilitating pre-metastatic niche formation in the lung
(21). Additionally, M2-polarized
TAMs secrete CCL18, which activates the PI3K/AKT pathway in OS
cells and increases MMP9 expression, further augmenting local
invasion and intravasation (25).
These observations position the TME not only as a barrier to
therapy but also as an active driver of metastatic cascade,
underscoring the urgency of targeting both tumor-intrinsic and
microenvironmental determinants of invasion.
Current regimens yield suboptimal survival in
patients with metastatic or relapsed bone sarcomas, partly because
cytotoxic protocols overlook the immunosuppressive, mechanically
rigid and metabolically hostile TME. Previous publications have
summarized individual TME components in OS pathogenesis and
discussed therapeutic targets (13–15).
This present review extends beyond these earlier reports by
integrating scRNA-seq, spatial proteomics and functional imaging
across OS, ES and CS to define subtype-specific TME archetypes. In
addition, a mechanistic framework that associates
mineralized-matrix mechanics, metabolic acidosis and myeloid
reprogramming to primary and acquired immunotherapy resistance is
presented and biomarker-guided combination strategies are proposed
for addressing these challenges.
Single-cell atlases and spatial proteomics now
resolve OS, Ewing and CS microenvironments into archetypes
dominated by M2 macrophages, exhausted CD8+ T cells and
stiff collagen-versican ECM. Fig. 1
provides the reference framework for dissecting subtype-specific
cellular and non-cellular determinants of immune privilege and drug
resistance.
scRNA-seq has revealed striking inter-tumoral
heterogeneity that directly conditions immunotherapy outcomes
(26). In OS, CD3+ T cells
constitute 8–15% of all viable cells; however, 60–80% of these
cells display an exhausted PD-1+ T-cell Immunoglobulin and
Mucin-domain containing-3 (TIM-3)+ Lymphocyte Activation
Gene 3 (LAG-3)+ phenotype and are spatially confined to
the tumor periphery by CXCL12-rich reticular networks (27). By contrast, ES lesions harbor an
even lower overall T-cell fraction (2–5%) but a higher
CD8+/ Tregs ratio, possibly explaining the 20% objective
response rate to pembrolizumab monotherapy observed in the
EURO-EWING-2012-NIS trial (NCT 02707557), whereas OS trials have
yet to >5% objective response rate (28). These divergent landscapes argue
against a ‘one-size-fits-all’ checkpoint blockade strategy and
suggest that CXCR4 or CXCL12 inhibition might preferentially
benefit OS by enhancing T-cell trafficking.
Myeloid populations dominate the sarcoma TME across
subtypes, yet their functional states are context-dependent
(29). In OS, two independent
scRNA-seq cohorts (n=11 and n=19) both identified a continuum of
TAMs skewed toward M2-like signatures
(CD163+CCL18+MMP12+), associating
with shorter metastasis-free survival (HR=2.3, P=0.007) (13). Conversely, CS TAMs express higher
inducible nitric oxide synthase (iNOS) and tumor necrosis factor- α
(TNF-α), resembling an M1 phenotype, but paradoxically co-express
PD-L1 and indoleamine 2,3-dioxygenase1 (IDO1), indicating a hybrid
state that may blunt anti-PD-1 efficacy despite apparent
‘inflammatory’ polarity (30).
These conflicting data underscore the need for multi-omic spatial
mapping to resolve whether M1/M2 classifications are sufficient
biomarkers in bone sarcomas.
Myeloid-derived suppressor cells (MDSCs) and
regulatory DCs further entrench immunosuppression. In canine OS,
PMN-MDSCs rise from <3% in healthy marrow to 18% within tumor
tissue and their depletion with PI3Kδ inhibitor GS-9820 restores
antigen-specific T-cell proliferation ex vivo (31). A separate study reported that
conventional DC1s are virtually absent in 70% of high-grade CS
specimens, and forced re-introduction of FLT3L-matured DC1s in
patient-derived organoids re-sensitized tumors to γδ T-cell killing
(32). Collectively, these findings
position combinatorial regimens that simultaneously deplete MDSCs
and replenish DC1s as a rational next step for clinical
testing.
Natural killer cells (NKs) abundance is high in ES
(<12%), but their cytotoxicity is crippled by transforming
growth factor-β1 (TGF-β1) concentrations >800 pg/ml, levels that
exceed those in OS by 3-fold (33).
Blockade of TGF-βRI with galunisertib restored NKs degranulation
against ES cell lines in vitro, providing a mechanistic
rationale for the ongoing phase I/II trial of galunisertib plus
dinutuximab β (NCT05461567) (34).
CAFs originate from resident bone-marrow mesenchymal
stem cells (MSCs) and adopt heterogeneous phenotypes. CAFs
constitute a dominant stromal component in bone sarcomas and are
increasingly recognized as co-architects of the immunosuppressive
niche (35). scRNA-seq of human OS
biopsies resolved two transcriptionally stable CAF
subsets-myofibroblastic CAFs (myCAFs, ACTA2high) and
inflammatory CAFs (iCAFs,
IL-6highCXCL12high), that together account
for 25–35% of all non-malignant cells (35). Lineage-tracing studies in
genetically engineered mouse models indicate that the majority of
CAFs arise from local bone-marrow-derived mesenchymal stromal/stem
cells (BM-MSCs) through TGF-β- and bone morphogenetic protein-2
(BMP-2)-driven reprogramming, a process that is molecularly
indistinguishable from the osteoblastic differentiation cascade
that gives rise to the malignant clone itself (35). Consequently, OS cells and CAFs share
an overlapping mesenchymal developmental program, including
expression of platelet-derived growth factor receptor (PDGFR)-β,
CD90 and the osteoblastic transcription factor RUNX2, suggesting
that the same oncogenic cues that initiate the tumor concurrently
instruct CAF fate. This ontological convergence explains why CAFs
are uniquely equipped to deposit a collagen- and versican-rich ECM
that matches the stiffness (Young's modulus 25–35 kPa) of the
surrounding mineralized bone, thereby shielding tumor cells from
immune attack and impeding chimeric antigen receptor T-cell (CAR-T)
cell penetration (36). Two
transcriptionally distinct CAF subpopulations have been delineated:
myCAFs (ACTA2high) and iCAFs (IL-6high
CXCL12high) (37). In
OS, myCAFs aligned along collagen bundles associate with increased
tissue stiffness (Young's modulus 25–35 kPa) and impaired CAR-T
penetration, whereas iCAFs secrete CXCL12 that recruits
CXCR4+ Tregs (38).
Targeting CXCL12 with plerixafor sensitized OS xenografts to
disialoganglioside 2 (GD2). CAR-T therapy, increasing intratumoral
CAR-T density 4-fold (39). These
data highlight CAFs subtyping as a prerequisite for effective
stromal reprogramming.
Osteoblast-lineage cells, often dismissed as
bystanders, actively shape immune privilege (40). Single-cell interactome analyses
revealed 21 ligand-receptor pairs between OS cells and osteoblasts,
including RANKL-RANK and Jagged1-Notch2 axes that polarize
macrophages toward an M2 phenotype and upregulate PD-L1 (41). Pharmacologic RANKL inhibition with
denosumab not only reduced osteoclastogenesis but also decreased
M2-TAMs abundance by 40% in a syngeneic OS model, suggesting dual
anti-resorptive and immunomodulatory benefits (42). Conversely, osteoclasts fuel a
vicious cycle of bone destruction and TGF-β release; TGF-β
concentrations >1 ng/ml were sufficient to convert 50% of CD8+ T
cells into FOXP3+ Tregs in Transwell assays (43). These observations position
osteoclast-directed therapy as a combinatorial partner for TGF-β
inhibition.
Bone sarcomas develop a chaotic vasculature
characterized by tortuous, CD31-high and NG2-low vessels with poor
pericyte coverage (44).
Microvessel density is highest in ES (mean 104 vessels
mm−2) and lowest in CS (42 vessels mm−2),
paralleling VEGF-A expression levels (45). Yet, bevacizumab achieved only modest
disease stabilization [6-month progression-free survival (PFS) 18%]
in a phase II OS trial, possibly because VEGF blockade
paradoxically increased hypoxia and HIF-1α-driven PD-L1 expression
on tumor cells (46). Alternative
strategies that normalize rather than prune vessels, such as
low-dose metronomic cyclophosphamide enhanced CART cells
infiltration in murine ES models without exacerbating hypoxia,
underscoring the need to balance anti-angiogenic and
immunotherapeutic dosing schedules (47).
MSCs are recruited to primary tumors via CCL5-CCR5
signaling and differentiate into either CAFs or osteoblast-like
cells depending on TGF-β and BMP-2 gradients (48). In OS, MSCs-secreted PGE2 induces
IDO1 in DCs, leading to a 60% reduction in T-cell proliferation in
mixed lymphocyte reactions (49).
Genetic ablation of cyclooxygenase-2 (COX-2) in MSCs restored DCs
function and improved anti-PD-1 efficacy in vivo (50). These findings suggest that
MSCs-educated immune suppression is reversible and that COX-2
inhibition could serve as an adjuvant to checkpoint blockade.
In summary, osteoblast-lineage cells, osteoclasts,
MSCs and endothelial cells each contribute distinct
immunosuppressive cues, including cytokine secretion, ECM
remodeling, and metabolic reprogramming. These interactions not
only reinforce tumor survival but also impede effective immune
surveillance. A summary of key bone-related cells, their ontogeny,
immunosuppressive mediators, and therapeutic implications is
presented in Table I (14,34,42,47,50),
highlighting potential targets for microenvironment-directed
interventions.
The bone sarcoma ECM is a composite of type-I
collagen, hydroxyapatite nanocrystals and oncofetal fibronectin
(51). Atomic-force microscopy
shows that OS ECM stiffness (10–40 kPa) is 3-fold higher than
adjacent marrow, a mechanical cue that activates yes-associated
protein (YAP)/transcriptional co-activator with PDZ-binding motif
(TAZ) and downregulates MHC-I in tumor cells (52). Lysyl oxidase-like 2 (LOXL2)-mediated
collagen cross-linking is elevated in metastatic bone sarcomas and
targeting LOXL2 improves both chemotherapeutic drug delivery and
the efficacy of adoptive T-cell therapies (14).
Multiplex cytokine profiling of OS biopsies
identified a high-TGF-β/low-interferon-γ (IFN-γ) signature that
independently predicted poor histologic response to neoadjuvant MAP
chemotherapy (53). Tumor-derived
IL-6 and CXCL12 establish an autocrine/paracrine loop that recruits
CXCR4+ MDSCs and polarizes macrophages to an M2
phenotype (54). These soluble
factors collectively sculpt an immunosuppressive milieu and
establish the rationale for co-targeting IL-6, CXCL12 and TGF-β in
combination regimens.
Lactate concentrations >15 mM in OS interstitial
fluid, suppressing NKs cytotoxicity by 50% via GPR81-dependent
mechanisms (57). Pharmacologic
buffering with oral sodium bicarbonate restored NK function and
improved anti-GD2 antibody efficacy in vivo, supporting a
readily translatable adjunct (58).
Pharmacologic buffering with oral sodium bicarbonate restored NK
cytotoxicity and enhanced anti-GD2 antibody efficacy in orthotopic
OS models, confirming rapid translatability of this metabolic
adjunct (58).
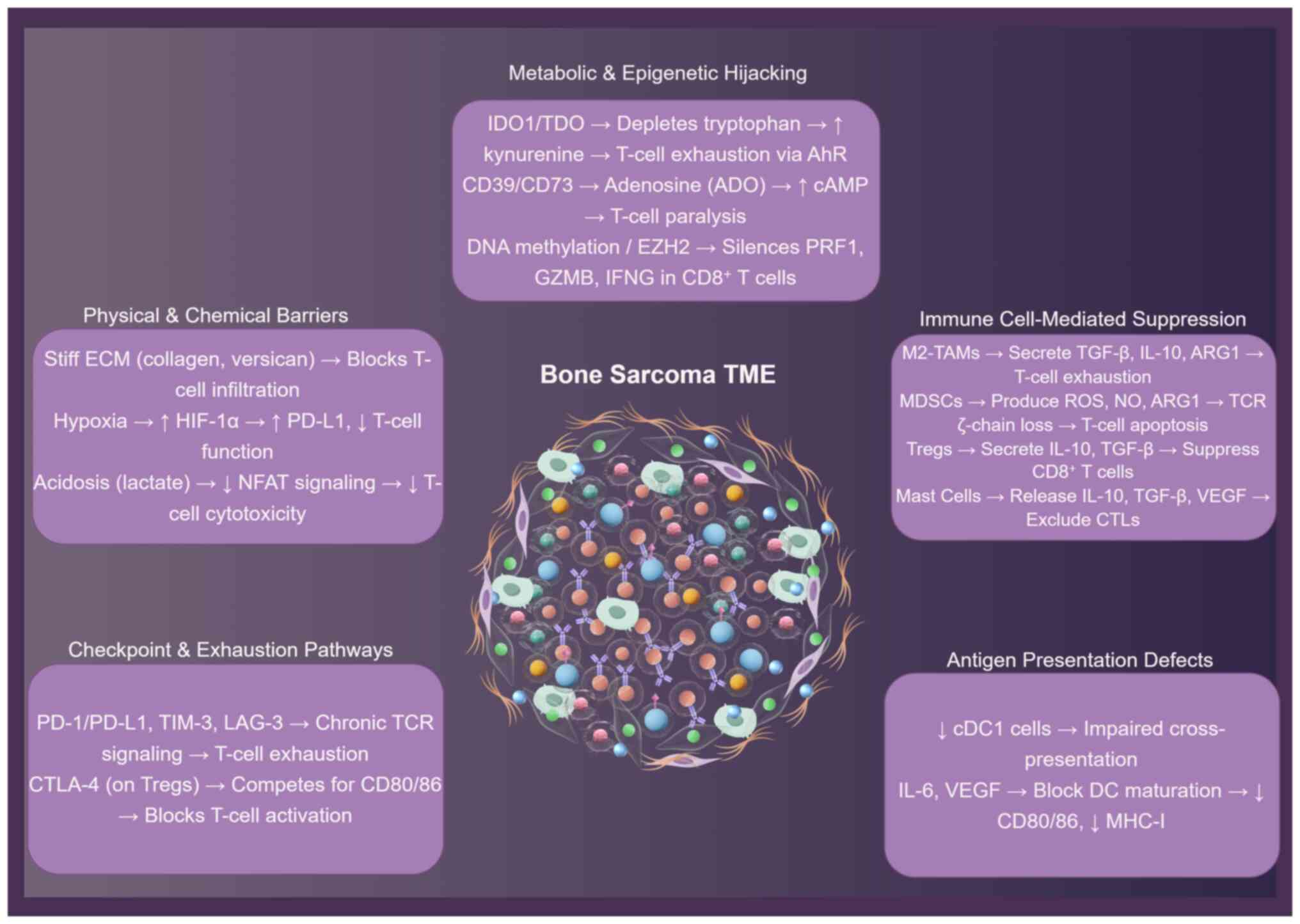
Dense collagen matrices and aberrant chemokines
exclude cytotoxic T cells, while M2 macrophages, MDSCs and Tregs
secrete arginase-1, TGF-β and adenosine to paralyze effector
function (Fig. 2) (13,14).
Exhaustion checkpoints, metabolic acidosis and IDO1-driven
tryptophan depletion reinforce this immunosuppressive circuit, and
immature dendritic cells fail to present antigen, forging an
immune-privileged sanctuary that defies therapy (53–56).
Physical barriers and aberrant chemokine gradients
cooperatively limit intratumoral T-cell accumulation (59). The ECM of OS is enriched in collagen
I/III, fibronectin and hyaluronan; high tissue stiffness reduces
T-cell motility and cytotoxicity in vitro and associates
with low CD8+ infiltration in vivo (60). Chemokine profiling of 22
treatment-naïve OSs revealed a marked paucity of CXCL9/10 and CCL5
concomitant with overexpression of CXCL12 and CCL18, a pattern that
preferentially recruits CXCR4+ Tregs and CCR8+ M2 macrophages while
excluding CXCR3+ CD8+ T cells (61). Consistently, spatial transcriptomics
showed an inverse association between CXCL9/10 levels and distance
of CD8+ T cells from tumor nests (Spearman ρ=−0.63, P<0.01)
(62). The clinical implication is
that forced intratumoral release of CXCL9 or CXCL10 via oncolytic
viruses or STING agonists could enhance ICI efficacy, an approach
currently being tested in early-phase trials (NCT05115319).
Tregs constitute 10–25% of CD4+ tumor-infiltrating
lymphocytes (TILs) in OS and exhibit an activated phenotype
(FOXP3high, CD25high and
CTLA-4high) (67).
Functionally, Tregs suppress CD8+ proliferation in vitro via
IL-10, TGF-β and IL-35 and physically deplete IL-2 through
high-affinity IL-2Rα (68,69). Notably, the Treg/CD8 ratio increases
from 0.2 in primary tumors to 0.7 in metastases (P<0.001),
paralleling reduced objective response rates to anti-PD-1 therapy
in metastatic cohorts (8 vs. 26% in localized disease) (70). Depletion models in murine OS (K7M2)
show that transient Treg reduction (anti-CD25) doubles CD8+
granzyme-B production and restores sensitivity to PD-1 blockade
(71).
Chronic antigen exposure in bone sarcomas drives a
progressive exhaustion program (78). sc TCR sequencing revealed
oligoclonal expansion (median 47 unique clonotypes per patient)
with over-representation of exhausted
TCF-1−TOX+ PD-1high
CD8+ subsets (79).
Continuous PD-1 signaling, reinforced by TIM-3 and LAG-3,
upregulates diacylglycerol kinase-α, dampening TCR signaling
(80). Metabolic stress further
exacerbates dysfunction: Lactate (15–20 mM) suppresses nuclear
factor of activated T-cells (NFAT) nuclear translocation, while
adenosine (via CD73/CD39 axis) elevates intracellular cyclic
adenosine monophosphate (cAMP), both impairing cytokine production
(81). Pre-clinical blockade of the
adenosine A2A receptor (A2AR) pathway restores T-cell effector
function in hypoxic OS models, providing a rationale for combining
adenosine-targeted agents with immune-checkpoint inhibitors in
future trials (82).
Metabolic reprogramming within the bone sarcoma TME
actively suppresses antitumor immunity (86). Tumor cells exhibit high glycolytic
flux, generating an acidic, lactate-rich milieu that impairs
CD8+ T-cell cytotoxicity and cytokine production,
notably via inhibition of NFAT signaling and histone acetylation
(87). Concurrently, tumor and
stromal cell upregulation of IDO1 depletes tryptophan and produces
immunosuppressive kynurenine metabolites, activating the aryl
hydrocarbon receptor in T cells and driving exhaustion (88). Furthermore, the CD39/CD73-A2AR
adenosine axis is a dominant immunosuppressive pathway; adenosine
generated extracellularly suppresses T-cell effector functions via
elevated intracellular cAMP. Elevated CD73 expression associates
with poor response to PD-1 blockade (88,89).
These pathways collectively establish a metabolically hostile
environment for effector immune cells.
DNA hypermethylation silences perforin 1 (PRF1),
granzyme B (GZMB) and IFNγ promoters in CD8+ TILs,
whereas H3K27me3 deposition represses T-box transcription factor 21
and eomesodermin loci, locking cells in an exhausted state
(90). OS-derived exosomal
miR-221-3p downregulates suppressor of cytokine signaling 3 in
macrophages, enhancing JAK2-STAT3 signaling and sustaining M2
polarization (91). Conversely,
pharmacologic inhibition of enhancer of zeste homolog 2 (EZH2;
tazemetostat) reactivates PRF1 and GZMB transcription in murine
models, improving response to PD-1 blockade (92). These data raise the prospect of
combining epigenetic modifiers with immune checkpoint inhibitors
(ICIs) to overcome adaptive resistance.
Building upon the aforementioned mechanistic
studies, the clinical record of immune checkpoint and adoptive cell
therapies in bone sarcomas is now systematically reviewed. Table II (93–100)
summarizes objective response rates from landmark ICI trials, while
Table III (35,36,101–124) collates pre-clinical and
early-phase adoptive cell therapies (ACT) data, together revealing
how the immunosuppressive TME continues to dictate therapeutic
outcome.
The advent of ICIs, particularly targeting the
PD-1/PD-L1 and CTLA-4 axes, revolutionized oncology. However, their
application in bone sarcomas, primarily OS, has yielded
predominantly modest clinical benefits, underscoring the profound
influence of the TME in shaping resistance (93,94).
Across the published pembrolizumab, nivolumab and
nivolumab-plus-ipilimumab series, objective responses in bone
sarcoma rarely exceed single digits. SARC028, the sentinel
multicenter phase II trial, enrolled 22 patients with OS or CS and
documented only one partial response lasting 32 weeks, yielding an
objective response rate of 5% (93). Two confirmatory studies have since
reproduced this figure: The PEMBROSARC cohort of 30 heavily
pre-treated (≥2 prior lines of systemic cytotoxic chemotherapy or
targeted therapy) patients with OS had no objective responses at
all (94), while the pediatric
ADVL1412 expansion treated 14 patients with OS with nivolumab plus
ipilimumab and observed just one durable partial response
(objective response rate, 7%) (95,96).
Single-arm studies using pembrolizumab monotherapy (Boye et
al (97), 28 patients) or the
nivolumab-sunitinib combination [Palmerini et al (98); 15 patients] produced identical null
response rates.
The mechanistic explanation for this uniformly low
activity lies in three interconnected features of the bone-sarcoma
TME detailed in ‘Mechanisms of immunosuppression within the bone
sarcoma TME’, immunologically ‘cold’ tumors: Spatial mapping shows
PD-L1-expressing cells physically separated from CD8+ CTLs,
creating an excluded infiltrate refractory to checkpoint
reactivation (99). Genomic
quietness: Low mutational burden and minimal neoantigen
presentation deprive T cells of the antigenic signal needed for
ICI-driven expansion (93).
Pronounced myeloid dominance: The SDF-1/CXCR4 axis recruits
monocytic MDSCs that outcompete CTLs for glucose and secrete
arginase-1, directly neutralizing anti-PD-1 efficacy in
pre-clinical models (100).
Notably, the lone responder in SARC028 had chondroblastic OS with
high PD-L1 expression (≥50% tumor cells) and brisk peritumoural CD8
infiltration, illustrating that exceptional responders may arise
when the tumor deviates from the archetypal ‘cold’ bone-sarcoma
microenvironment (93).
PD-L1 expression remains the most interrogated
biomarker, yet results are inconsistent. Discordant PD-L1 status
between primary and metastatic lesions occurs in 35% of patients
with OS, undermining archival tissue reliability (101). In PEMBROSARC, PD-L1 positivity
(≥1%) was present in 30% of cases but was unassociated with benefit
(94). Conversely, Le Cesne et
al (94) reported that high
PD-L1 combined with IDO1 expression enriched for a 10% long-term
disease-control subgroup, hinting at combinatorial biomarker
utility. Spatial transcriptomics now suggests that the spatial
proximity of PD-L1+ macrophages to CTLs, rather than
absolute PD-L1 levels, predicts non-progression (99). Beyond PD-L1, an ‘immune-hot’
signature (CD8+ CTL/Treg ratio >2 and low M-MDSC
density) emerged as an independent predictor of PFS in the
nivolumab-plus-ipilimumab ADVL1412 cohort (HR, 0.31; 95% CI,
0.12–0.78) (96). However, this
signature was present in only 6/38 (16%) sarcoma biopsies,
underscoring the rarity of immunologically favorable bone
sarcomas.
Emerging evidence suggests mast-cell stabilization
as a novel resistance mechanism. Mast cells are tissue-resident,
c-Kit+FcεRI+ innate immune cells that
differentiate in the bone marrow, circulate as progenitors and
mature locally under the influence of stem-cell factor, IL-3, IL-33
and CXCL12. In bone sarcomas, mast cells preferentially localize to
the hypoxic tumor margin and along neo-vessels, where they act as
central hubs integrating stromal and immune signals (102). Upon activation, mast cells rapidly
degranulate histamine, tryptase and chymase, and additionally
secrete IL-4, IL-10, VEGF-A, PDGF-B and TGF-β1. These mediators
collectively enhance vascular permeability, stimulate CAF
proliferation and promote M2-like macrophage polarization, thereby
reinforcing an immunosuppressive niche that blunts cytotoxic T-cell
influx. Pharmacological stabilization of mast cells with cromolyn
or targeted depletion via c-Kit inhibition restores CD8+
T-cell infiltration and sensitizes OS xenografts to anti-PD-1
therapy, underscoring their therapeutic relevance (44). Mast-cell-derived IL-10 and TGF-β
convert macrophages toward M2 and exclude CTLs; pharmacologic
stabilization restored PD-L1 blockade sensitivity pre-clinically
(44), validation in human studies
is required. Finally, host-related factors (human leukocyte antigen
loss of heterozygosity, low peripheral blood T-cell clonality)
associated with primary resistance in soft-tissue sarcomas warrant
investigation in bone sarcomas via ongoing multi-omics studies
(such as NCT 04339738) (34,35,103).
In summary, current ICI trials confirm modest
single-agent activity in bone sarcomas, driven by an
immunologically ‘cold’ and myeloid-dominated TME. Objective
responses cluster in rare cases with inflamed, PD-L1-high, CTL-rich
tumors, but robust predictive biomarkers are still lacking. Future
efforts must integrate spatially resolved TME profiling, systemic
immune monitoring and rational combination strategies to overcome
primary resistance.
CAR T cells therapy has shown notable preclinical
potential in bone sarcomas, targeting various tumor-associated
antigens. Early work demonstrated the efficacy of IGF1R- and
ROR1-specific CAR T cells against sarcoma cell lines and
xenografts, highlighting IGF1R as a particularly potent target
(104). Subsequent research
identified B7-H3 (CD276) as a highly expressed pan-cancer antigen
in pediatric solid tumors, including OS and ES. B7-H3-CAR T cells
exhibited robust antitumor activity in multiple preclinical OS
models, markedly reducing tumor burden and improving survival
(36,105,106). This efficacy was further enhanced
by strategies to overcome TME-driven homing limitations.
Engineering B7-H3-CAR T cells to express chemokine receptors such
as CXCR2 or CXCR1/CXCR2 ligands (for example, IL-8) or redirecting
them towards chemokines (CXCL9 and CXCL10) abundant in OS models,
considerably improved tumor infiltration and antitumor potency
compared with standard CAR T cells (35,107,108). Similarly, CAR T cells targeting
CD166/4-1BB showed efficacy against OS in vitro and in
vivo, inducing cytotoxicity and inhibiting tumor growth
(109). Other promising
preclinical targets include GD2 in ES [where combining GD2-CAR T
cells with hepatocyte growth factor-neutralizing antibody prevented
metastasis (110)], VEGFR2 in ES
vasculature (111), EphA2 [though
efficacy was variable (112)],
folate receptor α (FRα) via a bispecific adaptor approach (113), cancer stem cell antigens DnaJ Heat
Shock Protein Family Member B8 (DNAJB8) (114) and alkaline phosphatase,
biomineralization associated-1 (115) and oncofetal tenascin C using
IL-18R-supported CARs (116).
Membrane-anchored, tumor-targeted IL-12 expressed on CAR T cells or
PBMCs also demonstrated potent antitumor activity against
heterogeneous OS models by remodeling the TME (117,118). Recent strategies involve enhancing
homing via CXCR5/IL-7 co-expression (119), targeting immunosuppressive
pathways such as Regnase-1 to create a proinflammatory TME
(120) or utilizing switchable CAR
systems for improved safety (121).
Despite promising preclinical results, translating
CAR T-cells efficacy to the clinic for solid tumors such as bone
sarcomas faces major hurdles imposed by the TME. Trafficking to
tumor sites remains a key barrier. Poor homing has been observed,
partly due to inadequate chemokine receptor expression on CAR T
cells mismatched with the chemokine profile of the TME (108,111). Persistence of functional CAR T
cells in vivo is often limited. Wang et al (122) demonstrated the utility of PET
reporter gene imaging to monitor CAR T cells location and
persistence, revealing challenges in maintaining therapeutic cell
numbers within solid tumors. Factors contributing to poor
persistence include T-cell exhaustion within the immunosuppressive
TME and potential fratricide when targeting widely expressed
antigens. Suppression by the TME is a dominant challenge. The TME
fosters immunosuppressive cell populations (TAMs, MDSCs and Tregs)
and expresses immune checkpoint molecules. While Altvater et
al (123) found that HLA-G and
HLA-E, though expressed in ES, had limited functional impact on
GD2-CAR T cells in vitro, other immunosuppressive mechanisms
are potent. Tumor-derived soluble factors, particularly granulocyte
colony-stimulating factor, have been shown to create an
immunosuppressive myeloid-rich TME that markedly impairs the
efficacy of GD2-CAR T cells in OS models (124). The physical barriers of the tumor
stroma and hypoxia further impede CAR T cell function. Clinical
experience with CAR T cells in bone sarcomas remains limited
primarily to early-phase trials, often showing modest activity
compared with hematologic malignancies. Recent analyses, such as
the identification of immune determinants of CAR T cell expansion
in GD2-CAR T treated patients with solid tumor (including OS/ES),
underscore the complex interplay between patient factors, product
attributes and the TME that dictates clinical outcomes (125). Kaczanowska et al (125) found that early expansion kinetics
and T cell phenotypes notably influenced outcomes in GD2-CAR trials
for sarcomas and neuroblastoma, while Arnett and Heczey (126) emphasized that beyond T cell
fitness, the immunosuppressive TME is a major limiting factor
requiring combination approaches.
T cell receptor-engineered T (TCR-T) cell therapy
and TIL therapy represent alternative ACT strategies, but their
application in bone sarcomas is less advanced than CAR T therapy
and faces distinct challenges. TCR-T cells can target intracellular
antigens presented by MHC molecules, potentially broadening the
targetable antigen repertoire. Lu et al reported a clinical
trial using an MHC class II-restricted TCR targeting the cancer
germline antigen MAGE-A3. While the trial included patients with
various types of metastatic cancer, one patient with metastatic
sarcoma (unspecified subtype) achieved a complete response lasting
>24 months, demonstrating the potential therapeutic activity of
TCR-T cells in sarcoma (127).
Recently, Hamada et al (128) developed TCR-T cells targeting the
sarcoma-associated antigen papillomavirus binding factor (PBF).
These TCR-T cells demonstrated specific cytotoxicity against
PBF-positive sarcoma cell lines in vitro and inhibited tumor
growth in xenograft models. Watanabe et al (114) targeted the cancer stem cell
antigen DNAJB8 with TCR-T cells, showing efficacy against various
solid tumors in vitro and in vivo, though specific
bone sarcoma data were limited.
TCR-T therapy faces considerable hurdles in bone
sarcomas. Key challenges include identifying truly tumor-specific
intracellular antigens shared across subtypes, dependence on tumor
MHC expression, intratumoral heterogeneity enabling escape and the
critical risk of off-target toxicity due to TCR cross-reactivity
(114,128). Furthermore, as with CAR T cells,
TCR-T cells must overcome the profoundly immunosuppressive TME to
traffic, persist and function effectively, with specific bone
sarcoma TME interaction data currently limited.
Pre-clinical studies show that oncolytic viruses
can control OS growth, yet the immunocompetent microenvironment
rapidly blunts efficacy (129–131). Intratumoral Semliki Forest virus
encoding HSV-TK shrank orthotopic 143B-luc tumors by 74% in nude
mice, but complete regressions were absent and viral genomes
disappeared within 7 days, leaving the contribution of antitumor
immunity unaddressed (129).
Similarly, intravenous reovirus cleared Ras-activated xenografts
yet failed against A673 or SJSA-1 lesions, and its benefit was lost
in syngeneic K7M2 mice owing to neutralizing antibodies and
T-cell-mediated viral clearance (130).
Live-attenuated Listeria monocytogenes vaccines
offer a complementary strategy. Musser et al (133) administered Lm-LLO-E7 to 15
OS-bearing dogs; although only mild pyrexia and lymphopenia
occurred, median survival appeared prolonged vs. historical
controls (234 vs. 180 days). However, lack of randomization and
heterogeneous adjuvant chemotherapy preclude definitive efficacy
claims and anti-Listeria immunity may limit booster efficacy.
Pre-clinical reprogramming strategies, CSF-1R
inhibition, LOXL2 knockdown, hypoxia-activated prodrugs and EZH2
blockade, restore CTL infiltration and sensitize tumors to
checkpoint blockade (Table IV)
(55,61,135–144). Composite biomarker panels
integrating stiffness, hypoxia scores and immune infiltrate predict
2-3-fold increases in durable regressions across OS models.
Translation strategies: Systemic CSF-1R inhibition
(pexidartinib) eradicated >70% M2-TAMs in mice, tripled
CD8+ TILs, but caused grade-3 hepatotoxicity in 25% of
pediatric patients with only transient responses (145). While promising, repeated
intralesional injection is impractical for multifocal disease, and
long-term immunogenicity is unknown.
Collagen cross-linking enzyme LOXL2 is a central
regulator of ECM stiffness in OS. LOXL2 knockdown reduced stiffness
(35 to 15 kPa), doubled doxorubicin penetration and improved CAR-T
infiltration (145).
Paradoxically, partial ECM degradation increased metastasis by
releasing VEGF-sequestered matrices (138), suggesting indiscriminate
collagenolysis is counterproductive. This underscores the need for
spatiotemporally controlled strategies, such as photo-activatable
MMP-9 inhibitors.
CAFs fortify barriers via paracrine TGF-β and
CXCL12. CLTC-TFG signaling in CAFs upregulates TGF-β, excluding
CD8+ T cells (139).
The bifunctional anti-PD-L1/TGF-β antibody TQB2858 achieved a 33%
objective response rate in relapsed OS (140), yet CAFs heterogeneity predicted
variable CXCL12 levels and inconsistent T-cell recruitment. To
address plasticity, membrane-anchored IL-12 T cells converted
FAP+ CAFs to an inflammatory phenotype, reduced collagen
by 60% and improved CAR-T persistence (141). Unfortunately, sustained IL-12
induced cachexia in 20% of mice, necessitating inducible expression
systems.
Hypoxia-activated prodrugs such as TH-302 kill
hypoxic cells. Combined with anti-PD-1, it improved survival in
metastatic OS mice (55). However,
variable hypoxic thresholds and a lack of consensus imaging
biomarkers hinder application. Integrating 18F-MISO PET
with circulating hypoxia gene signatures may stratify responders,
but needs validation.
IDO1 is upregulated in 60% of OS and associated
with kynurenine-mediated T-cell suppression (143). Epacadostat plus pembrolizumab
produced a 33% ORR, yet IDO1 expression did not predict benefit,
possibly due to compensatory tryptophan 2,3-dioxygenase activation
(145). Dual IDO/TDO inhibitors
are under investigation, with off-target hepatic toxicity a
concern.
Translating the aforementioned mechanistic insights
and combination strategies into reliable clinical benefit demands
experimental platforms that faithfully replicate the mineralized,
immune-privileged bone sarcoma niche. Integrating stiffness-tunable
3D hydrogels with humanized mouse models now enables longitudinal
tracking of CAR T cells trafficking, myeloid reprogramming and ECM
modulation, thereby accelerating the validation of biomarker-driven
regimens before paediatric trial entry.
Conventional preclinical models, including cell
monolayers and immunodeficient xenografts, inadequately
recapitulate the spatial, mechanical and immunosuppressive features
of bone sarcoma TME. In vitro 2D cultures fail to model
mineralized bone matrices, which physically impede CAR-T cell
trafficking and alter metabolic crosstalk between tumor cells and
stromal components such as osteoblasts or marrow adipocytes
(149,150). Murine xenograft models (such as
NOD-SCID) lack functional human immune systems, rendering them
incapable of evaluating immunotherapies dependent on human-specific
antigen presentation or checkpoint interactions. For instance, such
models could not predict the clinical failure of HER2-targeted CAR
T cells in OS, which later was attributed to TME-driven exhaustion
markers (PD-1, TIM-3) and TGF-β-mediated suppression (149,151). Even syngeneic mouse models exhibit
interspecies discrepancies in cytokine networks; the
TGF-β/osteopontin (OPN) axis is key for osteoclast-mediated
immunosuppression in human bone metastases, poorly mirrored in
murine systems, leading to overstated efficacy of immune checkpoint
blockade in bone tumors (152).
These limitations underscore a key gap: Conventional models cannot
simulate the dynamic immunosuppressive reprogramming induced by
bone-specific niches, resulting in unreliable therapeutic
predictions.
To address these shortcomings, genetically
engineered and humanized murine platforms incorporating functional
human immunity have emerged (153,154). huHSC-NOG mice reconstituted with
human hematopoietic stem cells enable long-term (≥6 months) study
of human T-cell differentiation and memory responses, making them
ideal for evaluating sustained immunotherapy efficacy (153). For example, F. Hoffmann-La Roche
Ltd's CEA-TCB bispecific antibody combined with atezolizumab
(anti-PD-L1) showed synergistic tumor reduction in huHSC-NOG models
of colorectal cancer, a finding later validated clinically
(155,156). Conversely, huPBMC-NOG models,
which rapidly reconstitute mature T cells within 2–4 weeks, are
suited for short-term assessments of T-cell-engaging therapies such
as blinatumomab (157). However,
their utility is constrained by graft-vs.-host disease) and
deficient B-cell reconstitution, limiting studies on
antibody-dependent cellular cytotoxicity (158).
Advanced 3D engineered systems now integrate
biomechanical cues to mimic bone TME pathophysiology.
Stiffness-tunable hydrogels (such as poly-aspartate scaffolds)
revealed that matrix rigidity >40 kPa, mimicking mineralized
bone, induces YAP overexpression in OS cells, synergizing with
TNF-α from TAMs to drive STAT3-mediated chemoresistance (159). These platforms also demonstrate
context-dependent therapeutic vulnerabilities; STAT3 inhibition
reversed doxorubicin resistance in stiff 3D models but showed
minimal efficacy in 2D cultures (151). For giant cell tumor of bone
(GCTB), mass cytometry of patient-derived samples identified
denosumab-induced depletion of γδTCR+ osteoclast-like giant cells
and expansion of cytotoxic CD8+ T cells, a shift not reproducible
in non-humanized models (160).
Despite progress, discrepancies persist: while 3D models predict
enhanced CAR-T cytotoxicity under dynamic flow, clinical
translation remains hampered by poor infiltration into osteoid-rich
niches 5.
sc and spatial omics technologies are dissecting
the cellular heterogeneity and topographical drivers of
immunotherapy resistance in bone sarcomas. Mass cytometry (CyTOF)
of GCTB identified a spatially restricted PD-1hiTIM-3+CD69+ CD8+
T-cell population juxtaposed with SIRPα+ TAMs, suggesting localized
exhaustion within the TME (161).
In OS, multi-omics integration of transcriptomic and sc data
defined a palmitoylation-driven metabolic-immune subtype
characterized by ZDHHC3/21/23 upregulation, which induces ‘cold
tumor’ phenotypes via dual suppression of MAPK signaling and CD8+
T-cell infiltration. High-risk patients, as defined by a
palmitoylation-driven prognostic score (PPS) above the 75th
percentile, showed resistance to PD-L1 inhibitors in the IMvigor210
cohort, highlighting this subtype's clinical relevance (162).
Spatial transcriptomics further maps cytokine
gradients underpinning immunosuppressive niches. In bone
metastases, osteoclast-derived OPN creates CXCL12-rich ecological
niches that sequester Tregs and exclude cytotoxic T cells, a
mechanism validated through in situ hybridization in clinical
biopsies (152). Radiation
modeling in microphysiological systems revealed that proton therapy
amplifies localized TGF-β secretion from damaged osteoblasts,
spatially associating with PD-L1 upregulation in surviving tumor
cells (159). However,
technological constraints remain: Current spatial methods lack
resolution for bone's mineralized matrix and sc datasets are biased
toward non-mineralized zones due to tissue dissociation artifacts
(163). Discrepancies also arise
in immune subset classification; CyTOF-defined TAM subsets in GCTB
contradict scRNA-seq annotations due to antibody specificity issues
vs. dropout effects in sequencing (164,165).
The next translational leap hinges on replacing
PD-L1-centric paradigms with multidimensional TME biomarkers,
refining patient stratification through dynamic profiling and
engineering bone-selective delivery systems that respect skeletal
physiology. Integrating TME modulators demands rigorous
longitudinal toxicity surveillance, especially in pediatric
populations. Collaborative, biomarker-driven trials embedding
real-time immune-monitoring and skeletal health endpoints are
indispensable to convert mechanistic insights into durable clinical
benefit. Multiplex IHC and spatial transcriptomics of OS biopsies
reveal CD8+ T cells proximal to antigen-presenting
fibroblasts predict prolonged metastasis-free survival, while high
neutrophil-to-CD8+ distance associates with
pembrolizumab resistance (166).
scRNA-seq resolved a metabolically distinct arginine-depleting TAM
cluster inversely associating with post-chemotherapy T-cell
expansion (167). However, CyTOF
antibody cross-reactivity in mineralized tissue overestimates TAM
frequency by ~30% vs. transcriptomics, highlighting the need for
orthogonal validation (20).
Composite signatures integrating spatial proximity, metabolic flux
and cross-platform profiling likely outperform PD-L1 alone, but
standardization remains a challenge.
Unsupervised clustering of 84 OS transcriptomes
identified three TME subtypes: ‘immune-hot’
(CXCL9/PD-L1high), ‘myeloid-rich/cold’
(CSF1R/STAT3high) and ‘fibrotic-desert’
(COL1A1high/CD45low) (168). In SARC028, pembrolizumab response
was confined to immune-hot tumors (objective response rate, 38%),
while myeloid-rich tumors progressed rapidly despite PD-L1
(169). Conversely, neoadjuvant
axitinib plus pembrolizumab converted 5/9 myeloid-rich tumors to
immune-active and tripled CD8+ density, achieving 66%
3-month PFS (170). These findings
illustrate dynamic conversion is achievable, but baseline
stratification is insufficient; longitudinal TME monitoring is
required to guide adaptive combinations.
The mineralized matrix and high interstitial
pressure limit penetration of antibodies and cellular therapeutics
(171). Osteotropic peptide
(Asp-Ser-Ser)-modified liposomal alendronate achieved 7-fold higher
OS accumulation and reduced tumor burden when loaded with
doxorubicin (172).
Anti-CD117-conjugated mesoporous silica nanoparticles co-delivering
imatinib and STAT3 siRNA deplete MDSCs and sensitize to PD-1
blockade (173). Heterogeneity in
pediatric vs. adult bone density may impede nanoparticle
extravasation (174),
necessitating patient-specific models calibrated with PET perfusion
data.
Chronic CSF1R inhibition in adolescent mice
transiently decreased trabecular bone volume (BV/TV-22%) due to
impaired osteoclastogenesis (175). Combined with PD-1 blockade,
sustained Treg reduction led to persistent IFN-γ elevation and
prolonged suppression of bone formation markers (osteocalcin −35%
at 12 weeks), raising fracture risk concerns in growing patients
(176). Conversely, local low-dose
irradiation (8Gy) followed by GD2-CAR-T infusion increased bone
mineral density at metastatic sites via T-cell-mediated osteoblast
activation (177). These divergent
outcomes underscore the necessity of integrating serial skeletal
imaging and biomechanical testing into TME-targeting trials.
Pre-operative DNMT inhibitor (5-azacytidine) plus
HDAC inhibitor (entinostat) upregulated neoantigens and synergized
with doxorubicin, reducing lung micrometastases by 90% in mice
(178). Followed by GD2-CAR-T, the
triple combination extended median survival to 85 vs. 42 days for
chemo alone (179). However, a
phase I study combining pembrolizumab with standard MAP reported
unexpected early cardiotoxicity, possibly due to PD-1 blockade
enhancing doxorubicin-induced oxidative stress (180). Rational sequencing and dose
de-escalation informed by real-time TME profiling are key to
maximize synergy while limiting toxicities.
Despite compelling pre-clinical data, only two
registered trials (NCT04443235 and NCT05121269) prospectively
stratify patients with bone sarcoma by integrated TME signatures.
Early results from NCT04443235, a phase II study allocating
immune-hot OS to pembrolizumab plus stereotactic radiotherapy and
myeloid-rich tumors to CSF1R inhibitor pexidartinib plus
pembrolizumab, show an interim overall response rate of 45 vs. 11%
in historical controls. Cross-trial comparison reveals that trials
lacking biomarker selection consistently report objective response
rate below 15%, reinforcing the key impact of patient enrichment.
Standardized tissue acquisition protocols, centralized multiplex
imaging pipelines and open-access data repositories are proposed to
accelerate validation of next-generation biomarkers and ensure
reproducibility across centers.
Current regimens leave metastatic bone sarcomas
largely incurable because the immunosuppressive, mineralized and
metabolically hostile TME repels effector immunity. Integrating sc,
spatial and functional data across OS, ES and CS reveals
subtype-specific immune archetypes, tractable stromal targets and
delivery barriers. Translation now demands biomarker-driven,
bone-selective combinations with real-time monitoring to convert
mechanistic insight into durable cures.
Funding: No funding was received.
Not applicable.
WL contributed to conceptualization, literature
review, writing (original draft) and visualization. LL contributed
to literature review, writing (original draft) and retrieve
literature. YJ contributed to writing (review and editing) and
resources. XY contributed to supervision, project administration,
writing (review and editing, and funding acquisition). All authors
have read and approved the final manuscript. Data authentication
not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Strauss SJ, Frezza AM, Abecassis N, Bajpai
J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, Bonvalot I, et
al: Bone sarcomas: ESMO-EURACAN-GENTURIS-ERN PaedCan clinical
practice guideline for diagnosis, treatment and follow-up. Ann
Oncol. 32:1520–1536. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
3
|
Perry JA, Kiezun A, Tonzi P, Van Allen EM,
Carter SL, Baca SC, Cowley GS, Bhatt AS, Rheinbay E, Pedamallu CS,
et al: Complementary genomic approaches highlight the PI3K/mTOR
pathway as a common vulnerability in osteosarcoma. Proc Natl Acad
Sci USA. 111:E5564–E5573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Behjati S, Tarpey PS, Sheldon H,
Martincorena I, Van Loo P, Gundem G, Wedge DC, Ramakrishna M, Cooke
SL, Pillay N, et al: Recurrent PTPRB and PLCG1 mutations in
angiosarcoma. Nat Genet. 46:376–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marina NM, Smeland S, Bielack SS,
Bernstein M, Jovic G, Krailo MD, Hook JM, Arndt C, van den Berg H,
Brennan B, et al: Comparison of MAPIE versus MAP in patients with a
poor response to preoperative chemotherapy for newly diagnosed
high-grade osteosarcoma (EURAMOS-1): An open-label, international,
randomised controlled trial. Lancet Oncol. 17:1396–1408. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gaspar N, Hawkins DS, Dirksen U, Lewis IJ,
Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, et
al: Ewing sarcoma: Current management and future approaches through
collaboration. J Clin Oncol. 33:3036–3046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Maldegem AM, Gelderblom H, Palmerini
E, Dijkstra SD, Gambarotti M, Ruggieri P, Nout RA, van de Sande MA,
Ferrari C, Ferrari S, et al: Outcome of advanced, unresectable
conventional central chondrosarcoma. Cancer. 120:3159–3164. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakano K: Challenges of systemic therapy
investigations for bone sarcomas. Int J Mol Sci. 23:35402022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tlemsani C, Larousserie F, De Percin S,
Audard V, Hadjadj D, Chen J, Biau D, Anract P, Terris B, Goldwasser
F, et al: Biology and management of high-grade chondrosarcoma: An
Update on targets and treatment options. Int J Mol Sci.
24:13612023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan Z, Li Y, Zhang S, Wang X, Dou H, Yu
X, Zhang Z, Yang S and Xiao M: Extracellular matrix remodeling in
tumor progression and immune escape: From mechanisms to treatments.
Mol Cancer. 22:482023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Naser R, Fakhoury I, El-Fouani A,
Abi-Habib R and El-Sibai M: Role of the tumor microenvironment in
cancer hallmarks and targeted therapy (Review). Int J Oncol.
62:232023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alfranca A, Martinez-Cruzado L, Tornin J,
Abarrategi A, Amaral T, de Alava E, Menendez P, Garcia-Castro J and
Rodriguez R: Bone microenvironment signals in osteosarcoma
development. Cell Mol Life Sci. 72:3097–3113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Yang D, Yang Q, Lv X, Huang W,
Zhou Z, Wang Y, Zhang Z, Yuan T, Ding X, et al: Single-cell RNA
landscape of intratumoral heterogeneity and immunosuppressive
microenvironment in advanced osteosarcoma. Nat Commun. 11:63222020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nicolas-Boluda A, Vaquero J, Vimeux L,
Guilbert T, Barrin S, Kantari-Mimoun C, Ponzo M, Renault G, Deptula
P, Pogoda K, et al: Tumor stiffening reversion through collagen
crosslinking inhibition improves T cell migration and anti-PD-1
treatment. Elife. 10:e586882021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shurin MR and Umansky V: Cross-talk
between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy.
J Clin Invest. 132:e1594732022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jinushi M, Chiba S, Yoshiyama H, Masutomi
K, Kinoshita I, Dosaka-Akita H, Yagita H, Takaoka A and Tahara H:
Tumor-associated macrophages regulate tumorigenicity and anticancer
drug responses of cancer stem/initiating cells. Proc Natl Acad Sci
USA. 108:12425–12430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saito M, Ichikawa J, Ando T, Schoenecker
JG, Ohba T, Koyama K, Suzuki-Inoue K and Haro H: Platelet-derived
TGF-β induces tissue factor expression via the smad3 pathway in
osteosarcoma cells. J Bone Miner Res. 33:2048–2058. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiratsuka S, Watanabe A, Sakurai Y,
Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S,
Aburatani H and Maru Y: The S100A8-serum amyloid A3-TLR4 paracrine
cascade establishes a pre-metastatic phase. Nat Cell Biol.
10:1349–1355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Li J, Yang X, Li X, Kong J, Qi D,
Zhang F, Sun B, Liu Y and Liu T: Carcinoma-associated
fibroblast-derived lysyl oxidase-rich extracellular vesicles
mediate collagen crosslinking and promote epithelial-mesenchymal
transition via p-FAK/p-paxillin/YAP signaling. Int J Oral Sci.
15:322023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chevrier S, Crowell HL, Zanotelli VRT,
Engler S, Robinson MD and Bodenmiller B: Compensation of signal
spillover in suspension and imaging mass cytometry. Cell Syst.
6:612–620.e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Umezu T, Tadokoro H, Azuma K, Yoshizawa S,
Ohyashiki K and Ohyashiki JH: Exosomal miR-135b shed from hypoxic
multiple myeloma cells enhances angiogenesis by targeting
factor-inhibiting HIF-1. Blood. 124:3748–3757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Principe DR, Kamath SD, Korc M and Munshi
HG: The immune modifying effects of chemotherapy and advances in
chemo-immunotherapy. Pharmacol Ther. 236:1081112022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Han C, Liu Z, Zhang Y, Shen A, Dong C,
Zhang A, Moore C, Ren Z, Lu C, Cao X, et al: Tumor cells suppress
radiation-induced immunity by hijacking caspase 9 signaling. Nat
Immunol. 21:546–554. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Li K, Peng Y, Zhang Z, Pu F, Shao Z
and Wu W: Tumor microenvironment in osteosarcoma: From cellular
mechanism to clinical therapy. Genes Dis. 12:1015692025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dutour A, Pasello M, Farrow L, Amer MH,
Entz-Werlé N, Nathrath M, Scotlandi K, Mittnacht S and
Gomez-Mascard A: Microenvironment matters: Insights from the FOSTER
consortium on microenvironment-driven approaches to osteosarcoma
therapy. Cancer Metastasis Rev. 44:442025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azizi E, Carr AJ, Plitas G, Cornish AE,
Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V and Setty
M: Single-Cell map of diverse immune phenotypes in the breast tumor
microenvironment. Cell. 174:1293–1308.e36. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun CY, Zhang Z, Tao L, Xu FF, Li HY,
Zhang HY and Liu W: T cell exhaustion drives osteosarcoma
pathogenesis. Ann Transl Med. 9:14472021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Visser LL, Bleijs M, Margaritis T, van de
Wetering M, Holstege FCP and Clevers H: Ewing sarcoma single-cell
transcriptome analysis reveals functionally impaired
antigen-presenting cells. Cancer Res Commun. 3:2158–2169. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guimarães GR, Maklouf GR, Teixeira CE, de
Oliveira Santos L, Tessarollo NG, de Toledo NE, Serain AF, de Lanna
CA, Pretti MA, da Cruz JGV, et al: Single-cell resolution
characterization of myeloid-derived cell states with implication in
cancer outcome. Nat Commun. 15:56942024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kashfi K, Kannikal J and Nath N:
Macrophage reprogramming and cancer therapeutics: Role of
iNOS-Derived NO. Cells. 10:31942021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ka HI, Mun SH, Han S and Yang Y: Targeting
myeloid-derived suppressor cells in the tumor microenvironment:
Potential therapeutic approaches for osteosarcoma. Oncol Res.
33:519–531. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao L, Liu P, Mao M, Zhang S, Bigenwald
C, Dutertre CA, Lehmann CHK, Pan H, Paulhan N, Amon L, et al: BCL2
inhibition reveals a dendritic cell-specific immune checkpoint that
controls tumor immunosurveillance. Cancer Discov. 13:2448–2469.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shaim H, Shanley M, Basar R, Daher M,
Gumin J, Zamler DB, Uprety N, Wang F, Huang Y, Gabrusiewicz K, et
al: Targeting the αv integrin/TGF-β axis improves natural killer
cell function against glioblastoma stem cells. J Clin Invest.
131:e421162021. View Article : Google Scholar
|
|
34
|
Tran HC, Wan Z, Sheard MA, Sun J, Jackson
JR, Malvar J, Xu Y, Wang L, Sposto R, Kim ES, et al: TGFβR1
blockade with galunisertib (LY2157299) enhances anti-neuroblastoma
activity of the anti-GD2 antibody dinutuximab (ch14.18) with
natural killer cells. Clin Cancer Res. 23:804–813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao JW, Lake J, Impastato R, Chow L, Perez
L, Chubb L, Kurihara J, Verneris MR and Dow S: Targeting
osteosarcoma with canine B7-H3 CAR T cells and impact of CXCR2
Co-expression on functional activity. Cancer Immunol Immunother.
73:772024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Q, Zhang Z, Liu G, Li D, Gu Z, Zhang
L, Pan Y, Cui X, Wang L, Liu G, et al: B7-H3 targeted CAR-T cells
show highly efficient anti-tumor function against osteosarcoma both
in vitro and in vivo. BMC Cancer. 22:11242022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fotsitzoudis C, Koulouridi A, Messaritakis
I, Konstantinidis T, Gouvas N, Tsiaoussis J and Souglakos J:
Cancer-associated fibroblasts: The origin, biological
characteristics and role in cancer-a glance on colorectal cancer.
Cancers (Basel). 14:43942022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cao Z, Quazi S, Arora S, Osellame LD,
Burvenich IJ, Janes PW and Scott AM: Cancer-associated fibroblasts
as therapeutic targets for cancer: Advances, challenges, and future
prospects. J Biomed Sci. 32:72025. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Long AH, Highfill SL, Cui Y, Smith JP,
Walker AJ, Ramakrishna S, El-Etriby R, Galli S, Tsokos MG, Orentas
RJ and Mackall CL: Reduction of MDSCs with all-trans retinoic acid
improves CAR therapy efficacy for sarcomas. Cancer Immunol Res.
4:869–880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Calvi LM, Adams GB, Weibrecht KW, Weber
JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P,
Bringhurst FR, et al: Osteoblastic cells regulate the
haematopoietic stem cell niche. Nature. 425:841–846. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang S, Greenbaum J, Qiu C, Gong Y, Wang
Z, Lin X, Liu Y, He P, Meng X, Zhang Q, et al: Single-cell RNA
sequencing reveals in vivo osteoimmunology interactions between the
immune and skeletal systems. Front Endocrinol (Lausanne).
14:11075112023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Canon JR, Roudier M, Bryant R, Morony S,
Stolina M, Kostenuik PJ and Dougall WC: Inhibition of RANKL blocks
skeletal tumor progression and improves survival in a mouse model
of breast cancer bone metastasis. Clin Exp Metastasis. 25:119–129.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kiesel JR, Buchwald ZS and Aurora R:
Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J
Immunol. 182:5477–5487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Panagi M, Mpekris F, Voutouri C,
Hadjigeorgiou AG, Symeonidou C, Porfyriou E, Michael C, Stylianou
A, Martin JD, Cabral H, et al: Stabilizing tumor-resident mast
cells restores T-cell infiltration and sensitizes sarcomas to PD-L1
inhibition. Clin Cancer Res. 30:2582–2597. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Białas M, Dyduch G, Dudała J,
Bereza-Buziak M, Hubalewska-Dydejczyk A, Budzyński A and Okoń K:
Study of microvessel density and the expression of vascular
endothelial growth factors in adrenal gland pheochromocytomas. Int
J Endocrinol. 2014:1041292014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kreisl TN, Kim L, Moore K, Duic P, Royce
C, Stroud I, Garren N, Mackey M, Butman JA and Camphausen K: Phase
II trial of single-agent bevacizumab followed by bevacizumab plus
irinotecan at tumor progression in recurrent glioblastoma. J Clin
Oncol. 27:740–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dong X, Ren J, Amoozgar Z, Lee S, Datta M,
Roberge S, Duquette M, Fukumura D and Jain RK: Anti-VEGF therapy
improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic
glioblastoma models in mice. J Immunother Cancer. 11:e0055832023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Karnoub AE, Dash AB, Vo AP, Sullivan A,
Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R and Weinberg
RA: Mesenchymal stem cells within tumour stroma promote breast
cancer metastasis. Nature. 449:557–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Trabanelli S, Lecciso M, Salvestrini V,
Cavo M, Očadlíková D, Lemoli RM and Curti A: PGE2-induced IDO1
inhibits the capacity of fully mature DCs to elicit an in vitro
antileukemic immune response. J Immunol Res. 2015:2531912015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zelenay S, van der Veen AG, Böttcher JP,
Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais
R, Quezada SA, et al: Cyclooxygenase-dependent tumor growth through
evasion of immunity. Cell. 162:1257–1270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kolb AD and Bussard KM: The bone
extracellular matrix as an ideal milieu for cancer cell metastases.
Cancers (Basel). 11:10202019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Molina ER, Chim LK, Salazar MC, Mehta SM,
Menegaz BA, Lamhamedi-Cherradi SE, Satish T, Mohiuddin S, McCall D,
Zaske AM, et al: Mechanically tunable coaxial electrospun models of
YAP/TAZ mechanoresponse and IGF-1R activation in osteosarcoma. Acta
Biomater. 100:38–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu D, Peng Y, Li X, Zhu Z, Mi Z, Zhang Z
and Fan H: Comprehensive landscape of TGFβ-related signature in
osteosarcoma for predicting prognosis, immune characteristics, and
therapeutic response. J Bone Oncol. 40:1004842023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Highfill SL, Cui Y, Giles AJ, Smith JP,
Zhang H, Morse E, Kaplan RN and Mackall CL: Disruption of
CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy.
Sci Transl Med. 6:237ra672014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhao TT, Zhou TJ, Zhang C, Liu YX, Wang
WJ, Li C, Xing L and Jiang HL: Hypoxia inhibitor combined with
chemotherapeutic agents for antitumor and antimetastatic efficacy
against osteosarcoma. Mol Pharm. 20:2612–2623. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Khojastehnezhad MA, Seyedi SMR, Raoufi F
and Asoodeh A: Association of hypoxia-inducible factor 1
expressions with prognosis role as a survival prognostic biomarker
in the patients with osteosarcoma: A meta-analysis. Expert Rev Mol
Diagn. 22:1099–1106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shi W, Cassmann TJ, Bhagwate AV, Hitosugi
T and Ip WKE: Lactic acid induces transcriptional repression of
macrophage inflammatory response via histone acetylation. Cell Rep.
43:1137462024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hashim AI, Cornnell HH, Mde LC, Abrahams
D, Cunningham J, Lloyd M, Martinez GV, Gatenby RA and Gillies RJ:
Reduction of metastasis using a non-volatile buffer. Clin Exp
Metastasis. 28:841–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Aquino A and Franzese O: Reciprocal
modulation of tumour and immune cell motility: Uncovering dynamic
interplays and therapeutic approaches. Cancers (Basel).
17:15472025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cui J, Dean D, Hornicek FJ, Chen Z and
Duan Z: The role of extracelluar matrix in osteosarcoma progression
and metastasis. J Exp Clin Cancer Res. 39:1782020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Liu W, Hu H, Shao Z, Lv X, Zhang Z, Deng
X, Song Q, Han Y, Guo T, Xiong L, et al: Characterizing the tumor
microenvironment at the single-cell level reveals a novel immune
evasion mechanism in osteosarcoma. Bone Res. 11:42023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gorchs L, Oosthoek M, Yucel-Lindberg T,
Moro CF and Kaipe H: Chemokine receptor expression on T cells is
modulated by CAFs and chemokines affect the spatial distribution of
T cells in pancreatic tumors. Cancers (Basel). 14:38262022.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Park HK, Kim M, Sung M, Lee SE, Kim YJ and
Choi YL: Status of programmed death-ligand 1 expression in
sarcomas. J Transl Med. 16:3032018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang R, Sun L, Li CF, Wang YH, Yao J, Li
H, Yan M, Chang WC, Hsu JM, Cha JH, et al: Galectin-9 interacts
with PD-1 and TIM-3 to regulate T cell death and is a target for
cancer immunotherapy. Nat Commun. 12:8322021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Selby MJ, Engelhardt JJ, Quigley M,
Henning KA, Chen T, Srinivasan M and Korman AJ: Anti-CTLA-4
antibodies of IgG2a isotype enhance antitumor activity through
reduction of intratumoral regulatory T cells. Cancer Immunol Res.
1:32–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jiang X, Li L, Li Y and Li Q: Molecular
mechanisms and countermeasures of immunotherapy resistance in
malignant tumor. J Cancer. 10:1764–1771. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tian H, Cao J, Li B, Nice EC, Mao H, Zhang
Y and Huang C: Managing the immune microenvironment of
osteosarcoma: The outlook for osteosarcoma treatment. Bone Res.
11:112023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Starska-Kowarska K: The role of different
immunocompetent cell populations in the pathogenesis of head and
neck cancer-regulatory mechanisms of pro- and anti-cancer activity
and their impact on immunotherapy. Cancers (Basel). 15:16422023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li C, Jiang P, Wei S, Xu X and Wang J:
Regulatory T cells in tumor microenvironment: New mechanisms,
potential therapeutic strategies and future prospects. Mol Cancer.
19:1162020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Denize T, Jegede OA, Matar S, El Ahmar N,
West DJ, Walton E, Bagheri AS, Savla V, Laimon YN, Gupta S, et al:
PD-1 expression on intratumoral regulatory T cells is associated
with lack of benefit from Anti-PD-1 therapy in metastatic
clear-cell renal cell carcinoma patients. Clin Cancer Res.
30:803–813. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Merchant MS, Melchionda F, Sinha M, Khanna
C, Helman L and Mackall CL: Immune reconstitution prevents
metastatic recurrence of murine osteosarcoma. Cancer Immunol
Immunother. 56:1037–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Han Q, Shi H and Liu F: CD163(+) M2-type
tumor-associated macrophage support the suppression of
tumor-infiltrating T cells in osteosarcoma. Int Immunopharmacol.
34:101–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gallina G, Dolcetti L, Serafini P, De
Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I,
Zanovello P, et al: Tumors induce a subset of inflammatory
monocytes with immunosuppressive activity on CD8+ T cells. J Clin
Invest. 116:2777–2790. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Munder M: Arginase: An emerging key player
in the mammalian immune system. Br J Pharmacol. 158:638–651. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang Y, Li C, Liu T, Dai X and Bazhin AV:
Myeloid-derived suppressor cells in tumors: from mechanisms to
antigen specificity and microenvironmental regulation. Front
Immunol. 11:13712020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhu Y, Knolhoff BL, Meyer MA, Nywening TM,
West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC and
DeNardo DG: CSF1/CSF1R blockade reprograms tumor-infiltrating
macrophages and improves response to T-cell checkpoint
immunotherapy in pancreatic cancer models. Cancer Res.
74:5057–5069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
DeNardo DG, Brennan DJ, Rexhepaj E,
Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD,
Junaid SA, et al: Leukocyte complexity predicts breast cancer
survival and functionally regulates response to chemotherapy.
Cancer Discov. 1:54–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hashimoto M, Kamphorst AO, Im SJ, Kissick
HT, Pillai RN, Ramalingam SS, Araki K and Ahmed R: CD8 T cell
exhaustion in chronic infection and cancer: Opportunities for
interventions. Annu Rev Med. 69:301–318. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Depuydt MAC, Schaftenaar FH, Prange KHM,
Boltjes A, Hemme E, Delfos L, de Mol J, de Jong MJM, Kleijn MNA,
Peeters JAHM, et al: Single-cell T cell receptor sequencing of
paired human atherosclerotic plaques and blood reveals
autoimmune-like features of expanded effector T cells. Nat
Cardiovasc Res. 2:112–125. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shin J, O'Brien TF, Grayson JM and Zhong
XP: Differential regulation of primary and memory CD8 T cell immune
responses by diacylglycerol kinases. J Immunol. 188:2111–2117.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Gu XY, Yang JL, Lai R, Zhou ZJ, Tang D, Hu
L and Zhao LJ: Impact of lactate on immune cell function in the
tumor microenvironment: Mechanisms and therapeutic perspectives.
Front Immunol. 16:15633032025. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Leone RD, Sun IM, Oh MH, Sun IH, Wen J,
Englert J and Powell JD: Inhibition of the adenosine A2a receptor
modulates expression of T cell coinhibitory receptors and improves
effector function for enhanced checkpoint blockade and ACT in
murine cancer models. Cancer Immunol Immunother. 67:1271–1284.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Heger L, Hatscher L, Liang C, Lehmann CHK,
Amon L, Lühr JJ, Kaszubowski T, Nzirorera R, Schaft N, Dörrie J, et
al: XCR1 expression distinguishes human conventional dendritic cell
type 1 with full effector functions from their immediate
precursors. Proc Natl Acad Sci USA. 120:e23003431202023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Ohno Y, Kitamura H, Takahashi N, Ohtake J,
Kaneumi S, Sumida K, Homma S, Kawamura H, Minagawa N, Shibasaki S
and Taketomi A: IL-6 down-regulates HLA class II expression and
IL-12 production of human dendritic cells to impair activation of
antigen-specific CD4(+) T cells. Cancer Immunol Immunother.
65:193–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Oba T, Long MD, Keler T, Marsh HC,
Minderman H, Abrams SI, Liu S and Ito F: Overcoming primary and
acquired resistance to anti-PD-L1 therapy by induction and
activation of tumor-residing cDC1s. Nat Commun. 11:54152020.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ying H, Li ZQ, Li MP and Liu WC:
Metabolism and senescence in the immune microenvironment of
osteosarcoma: Focus on new therapeutic strategies. Front Endocrinol
(Lausanne). 14:12176692023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bogdanov A, Bogdanov A, Chubenko V, Volkov
N, Moiseenko F and Moiseyenko V: Tumor acidity: From hallmark of
cancer to target of treatment. Front Oncol. 12:9791542022.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Muller AJ, DuHadaway JB, Donover PS,
Sutanto-Ward E and Prendergast GC: Inhibition of indoleamine
2,3-dioxygenase, an immunoregulatory target of the cancer
suppression gene Bin1, potentiates cancer chemotherapy. Nat Med.
11:312–319. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
89
|
Bopp T, Becker C, Klein M, Klein-Hessling
S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt
S, et al: Cyclic adenosine monophosphate is a key component of
regulatory T cell-mediated suppression. J Exp Med. 204:1303–1310.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ji Y, Xiao C, Fan T, Deng Z, Wang D, Cai
W, Li J, Liao T, Li C and He J: The epigenetic hallmarks of immune
cells in cancer. Mol Cancer. 24:662025. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu W, Long Q, Zhang W, Zeng D, Hu B, Liu
S and Chen L: miRNA-221-3p derived from M2-polarized
tumor-associated macrophage exosomes aggravates the growth and
metastasis of osteosarcoma through SOCS3/JAK2/STAT3 axis. Aging
(Albany NY). 13:19760–19775. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hou Y, Zak J, Shi Y, Pratumchai I, Dinner
B, Wang W, Qin K, Weber EW, Teijaro JR and Wu P: Transient EZH2
suppression by tazemetostat during in vitro expansion maintains
T-cell stemness and improves adoptive T-cell therapy. Cancer
Immunol Res. 13:47–65. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tawbi HA, Burgess M, Bolejack V, Van Tine
BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA,
et al: Pembrolizumab in advanced soft-tissue sarcoma and bone
sarcoma (SARC028): A multicentre, two-cohort, single-arm,
open-label, phase 2 trial. Lancet Oncol. 18:1493–1501. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Le Cesne A, Marec-Berard P, Blay JY,
Gaspar N, Bertucci F, Penel N, Bompas E, Cousin S, Toulmonde M,
Bessede A, et al: Programmed cell death 1 (PD-1) targeting in
patients with advanced osteosarcomas: Results from the PEMBROSARC
study. Eur J Cancer. 119:151–157. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Davis KL, Fox E, Merchant MS, Reid JM,
Kudgus RA, Liu X, Minard CG, Voss S, Berg SL, Weigel BJ and Mackall
CL: Nivolumab in children and young adults with relapsed or
refractory solid tumours or lymphoma (ADVL1412): A multicentre,
open-label, single-arm, phase 1–2 trial. Lancet Oncol. 21:541–550.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Davis KL, Fox E, Isikwei E, Reid JM, Liu
X, Minard CG, Voss S, Berg SL, Weigel BJ and Mackall CL: A phase
I/II trial of nivolumab plus ipilimumab in children and young
adults with relapsed/refractory solid tumors: A children's oncology
group study ADVL1412. Clin Cancer Res. 28:5088–5097. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Boye K, Longhi A, Guren T, Lorenz S, Næss
S, Pierini M, Taksdal I, Lobmaier I, Cesari M, Paioli A, et al:
Pembrolizumab in advanced osteosarcoma: Results of a single-arm,
open-label, phase 2 trial. Cancer Immunol Immunother. 70:2617–2624.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Palmerini E, Pousa AL, Grignani G, Redondo
A, Hindi N, Provenzano S, Sebio A, Martin JA, Valverde C, Trufero
JM, et al: Nivolumab and sunitinib in patients with advanced bone
sarcomas: A multicenter, single-arm, phase 2 trial. Cancer.
131:e356282025. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yang C, Lai Y, Wang J, Chen Q, Pan Q, Xu
C, Mo P, Guo G, Chen R, Liu N and Wu Y: Spatial heterogeneity of
PD-1/PD-L1 defined osteosarcoma microenvironments at single-cell
spatial resolution. Lab Invest. 104:1021432024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jiang K, Li J, Zhang J, Wang L, Zhang Q,
Ge J, Guo Y, Wang B, Huang Y, Yang T, et al: SDF-1/CXCR4 axis
facilitates myeloid-derived suppressor cells accumulation in
osteosarcoma microenvironment and blunts the response to anti-PD-1
therapy. Int Immunopharmacol. 75:1058182019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Toda Y, Kohashi K, Yamada Y, Yoshimoto M,
Ishihara S, Ito Y, Iwasaki T, Yamamoto H, Matsumoto Y and Nakashima
Y: PD-L1 and IDO1 expression and tumor-infiltrating lymphocytes in
osteosarcoma patients: Comparative study of primary and metastatic
lesions. J Cancer Res Clin Oncol. 146:2607–2620. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ribatti D: Mast cells and resistance to
immunotherapy in cancer. Arch Immunol Ther Exp (Warsz). 71:112023.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Lee AQ, Hao C, Pan M, Ganjoo KN and Bui
NQ: Histologic and immunologic factors associated with response to
immune checkpoint inhibitors in advanced sarcoma. Clin Cancer Res.
31:678–684. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Huang X, Park H, Greene J, Pao J, Mulvey
E, Zhou SX, Albert CM, Moy F, Sachdev D, Yee D, et al: IGF1R- and
ROR1-Specific CAR T cells as a potential therapy for high risk
sarcomas. PLoS One. 10:e01331522015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Majzner RG, Theruvath JL, Nellan A,
Heitzeneder S, Cui Y, Mount CW, Rietberg SP, Linde MH, Xu P, Rota
C, et al: CAR T cells targeting B7-H3, a pan-cancer antigen,
demonstrate potent preclinical activity against pediatric solid
tumors and brain tumors. Clin Cancer Res. 25:2560–2574. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Talbot LJ, Chabot A, Funk A, Nguyen P,
Wagner J, Ross A, Tillman H, Davidoff A, Gottschalk S and DeRenzo
C: A novel orthotopic implantation technique for osteosarcoma
produces spontaneous metastases and illustrates dose-dependent
efficacy of B7-H3-CAR T cells. Front Immunol. 12:6917412021.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lake JA, Woods E, Hoffmeyer E, Schaller
KL, Cruz-Cruz J, Fernandez J, Tufa D, Kooiman B, Hall SC, Jones D,
et al: Directing B7-H3 chimeric antigen receptor T cell homing
through IL-8 induces potent antitumor activity against pediatric
sarcoma. J Immunother Cancer. 12:e0092212024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Talbot LJ, Chabot A, Ross AB, Beckett A,
Nguyen P, Fleming A, Chockley PJ, Shepphard H, Wang J, Gottschalk S
and DeRenzo C: Redirecting B7-H3.CAR T cells to chemokines
expressed in osteosarcoma enhances homing and antitumor activity in
preclinical models. Clin Cancer Res. 30:4434–4449. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Wang Y, Yu W, Zhu J, Wang J, Xia K, Liang
C and Tao H: Anti-CD166/4-1BB chimeric antigen receptor T cell
therapy for the treatment of osteosarcoma. J Exp Clin Cancer Res.
38:1682019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Charan M, Dravid P, Cam M, Audino A, Gross
AC, Arnold MA, Roberts RD, Cripe TP, Pertsemlidis A, Houghton PJ
and Cam H: GD2-directed CAR-T cells in combination with
HGF-targeted neutralizing antibody (AMG102) prevent primary tumor
growth and metastasis in Ewing sarcoma. Int J Cancer.
146:3184–3195. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Englisch A, Altvater B, Kailayangiri S,
Hartmann W and Rossig C: VEGFR2 as a target for CAR T cell therapy
of Ewing sarcoma. Pediatr Blood Cancer. 67:e283132020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hsu K, Middlemiss S, Saletta F, Gottschalk
S, McCowage GB and Kramer B: Chimeric antigen receptor-modified T
cells targeting EphA2 for the immunotherapy of paediatric bone
tumours. Cancer Gene Ther. 28:321–334. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lu YJ, Chu H, Wheeler LW, Nelson M,
Westrick E, Matthaei JF, Cardle II, Johnson A, Gustafson J, Parker
N, et al: Preclinical evaluation of bispecific adaptor molecule
controlled folate receptor CAR-T cell therapy with special focus on
pediatric malignancies. Front Oncol. 9:1512019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Watanabe Y, Tsukahara T, Murata K, Hamada
S, Kubo T, Kanaseki T, Hirohashi Y, Emori M, Teramoto A,
Nakatsugawa M, et al: Development of CAR-T cells specifically
targeting cancer stem cell antigen DNAJB8 against solid tumours. Br
J Cancer. 128:886–895. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Mensali N, Köksal H, Joaquina S, Wernhoff
P, Casey NP, Romecin P, Panisello C, Rodriguez R, Vimeux L,
Juzeniene A, et al: ALPL-1 is a target for chimeric antigen
receptor therapy in osteosarcoma. Nat Commun. 14:33752023.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wickman E, Lange S, Wagner J, Ibanez J,
Tian L, Lu M, Sheppard H, Chiang J, Koo SC, Vogel P, et al: IL-18R
supported CAR T cells targeting oncofetal tenascin C for the
immunotherapy of pediatric sarcoma and brain tumors. J Immunother
Cancer. 12:e0097432024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hu J, Yang Q, Zhang W, Du H, Chen Y, Zhao
Q, Dao L, Xia X, Wall FN, Zhang Z, et al: Cell membrane-anchored
and tumor-targeted IL-12 (attIL12)-T cell therapy for eliminating
large and heterogeneous solid tumors. J Immunother Cancer.
10:e0036332022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Yang Q, Hu J, Jia Z, Wang Q, Wang J, Dao
LH, Zhang W, Zhang S, Xia X, Gorlick R and Li S: Membrane-anchored
and tumor-targeted IL12 (attIL12)-PBMC therapy for osteosarcoma.
Clin Cancer Res. 28:3862–3873. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Hui X, Farooq MA, Chen Y, Ajmal I, Ren Y,
Xue M, Ji Y, Du B, Wu S and Jiang W: A novel strategy of
co-expressing CXCR5 and IL-7 enhances CAR-T cell effectiveness in
osteosarcoma. Front Immunol. 15:14620762024. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Adeshakin AO, Shi H, Perry SS, Sheppard H,
Nguyen P, Sun X, Zhou P, Métais JY, Cunningham T, Anil KC, et al:
Targeting Regnase-1 unleashes CAR T cell antitumor activity for
osteosarcoma and creates a proinflammatory tumor microenvironment.
bioRxiv. 23:2025.05.20.650777. 2025.
|
|
121
|
Hidalgo L, Somovilla-Crespo B,
Garcia-Rodriguez P, Morales-Molina A, Rodriguez-Milla MA and
Garcia-Castro J: Switchable CAR T cell strategy against
osteosarcoma. Cancer Immunol Immunother. 72:2623–2633. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wang Z, Yan N, Sheng H, Xiao Y, Sun J and
Cao C: Single-cell transcriptomic analysis reveals an
immunosuppressive network between POSTN CAFs and ACKR1 ECs in
TKI-resistant lung cancer. Cancer Genomics Proteomics. 21:65–78.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Altvater B, Kailayangiri S, Lanuza LF,
Urban K, Greune L, Flügge M, Meltzer J, Farwick N, König S, Görlich
D, et al: HLA-G and HLA-E immune checkpoints are widely expressed
in ewing sarcoma but have limited functional impact on the effector
functions of antigen-specific CAR T cells. Cancers (Basel).
13:28572021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Pezzella M, Quintarelli C, Quadraccia MC,
Sarcinelli A, Manni S, Iaffaldano L, Ottaviani A, Ciccone R, Camera
A, D'Amore ML, et al: Tumor-derived G-CSF induces an
immunosuppressive microenvironment in an osteosarcoma model,
reducing response to CAR.GD2 T-cells. J Hematol Oncol. 17:1272024.
View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kaczanowska S, Murty T, Alimadadi A,
Contreras CF, Duault C, Subrahmanyam PB, Reynolds W, Gutierrez NA,
Baskar R, Wu CJ, et al: Immune determinants of CAR-T cell expansion
in solid tumor patients receiving GD2 CAR-T cell therapy. Cancer
Cell. 42:35–51.e8. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Arnett AB and Heczey A: GD2-CAR CAR T
cells in patients with osteosarcoma and neuroblastoma-it's not only
the T cells that matter. Cancer Cell. 42:8–10. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lu YC, Parker LL, Lu T, Zheng Z, Toomey
MA, White DE, Yao X, Li YF, Robbins PF, Feldman SA, et al:
Treatment of patients with metastatic cancer using a major
histocompatibility complex class II-restricted T-cell receptor
targeting the cancer germline antigen MAGE-A3. J Clin Oncol.
35:3322–3329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Hamada S, Tsukahara T, Watanabe Y, Murata
K, Mizue Y, Kubo T, Kanaseki T, Hirohashi Y, Emori M, Nakatsugawa
M, et al: Development of T cell receptor-engineered T cells
targeting the sarcoma-associated antigen papillomavirus binding
factor. Cancer Sci. 115:24–35. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ketola A, Hinkkanen A, Yongabi F, Furu P,
Määttä AM, Liimatainen T, Pirinen R, Björn M, Hakkarainen T,
Mäkinen K, et al: Oncolytic Semliki forest virus vector as a novel
candidate against unresectable osteosarcoma. Cancer Res.
68:8342–8350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Hingorani P, Zhang W, Lin J, Liu L, Guha C
and Kolb EA: Systemic administration of reovirus (Reolysin)
inhibits growth of human sarcoma xenografts. Cancer. 117:1764–1774.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Christie JD, Appel N, Zhang L, Lowe K,
Kilbourne J, Daggett-Vondras J, Elliott N, Lucas AR, Blattman JN,
Rahman MM and McFadden G: Systemic delivery of mLIGHT-armed myxoma
virus is therapeutic for later-stage syngeneic murine lung
metastatic osteosarcoma. Cancers (Basel). 14:3372022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Laborda E, Puig-Saus C, Rodriguez-García
A, Moreno R, Cascalló M, Pastor J and Alemany R: A pRb-responsive,
RGD-modified, and hyaluronidase-armed canine oncolytic adenovirus
for application in veterinary oncology. Mol Ther. 22:986–998. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Musser ML, Berger EP, Tripp CD, Clifford
CA, Bergman PJ and Johannes CM: Safety evaluation of the canine
osteosarcoma vaccine, live Listeria vector. Vet Comp Oncol.
19:92–98. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chen A, Qiu Y, Yen YT, Wang C, Wang X, Li
C, Wei Z, Li L, Yu L, Liu F and Li R: Expression of cancer-testis
antigens MAGE-A1, MAGE-A4, NY-ESO-1 and PRAME in bone and soft
tissue sarcomas: The experience from a single center in China.
Cancer Med. 14:e707502025. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Gu J, Ji Z, Li D and Dong Q: Proliferation
inhibition and apoptosis promotion by dual silencing of VEGF and
Survivin in human osteosarcoma. Acta Biochim Biophys Sin
(Shanghai). 51:59–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhang Y, Cheng H, Li W, Wu H and Yang Y:
Highly-expressed P2X7 receptor promotes growth and metastasis of
human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β-catenin and
mTOR/HIF1α/VEGF signaling. Int J Cancer. 145:1068–1082. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Dong J, Chai X, Xue Y, Shen S, Chen Z,
Wang Z, Yinwang E, Wang S, Chen L, Wu F, et al: ZIF-8-encapsulated
pexidartinib delivery via targeted peptide-modified M1 macrophages
attenuates MDSC-mediated immunosuppression in osteosarcoma. Small.
20:e23090382024. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Gutiérrez LM, Alvarez MV, Yang Y, Spinelli
F, Cantero MJ, Alaniz L, García MG, Kleinerman ES, Correa A and
Bolontrade MF: Up-regulation of pro-angiogenic molecules and events
does not relate with an angiogenic switch in metastatic
osteosarcoma cells but to cell survival features. Apoptosis.
26:447–459. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
LShijie L, Zhen P, Kang Q, Hua G,
Qingcheng Y and Dongdong C: Deregulation of CLTC interacts with
TFG, facilitating osteosarcoma via the TGF-beta and AKT/mTOR
signaling pathways. Clin Transl Med. 11:e3772021. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Xie L, Liang X, Xu J, Sun X, Liu K, Sun K,
Li Y, Tang X, Li X, Zhan X, et al: Exploratory study of an
anti-PD-L1/TGF-β antibody, TQB2858, in patients with refractory or
recurrent osteosarcoma and alveolar soft part sarcoma: A report
from Chinese sarcoma study group (TQB2858-Ib-02). BMC Cancer.
23:8682023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Hu J, Lazar AJ, Ingram D, Wang WL, Zhang
W, Jia Z, Ragoonanan D, Wang J, Xia X, Mahadeo K, et al: Cell
membrane-anchored and tumor-targeted IL-12 T-cell therapy destroys
cancer-associated fibroblasts and disrupts extracellular matrix in
heterogenous osteosarcoma xenograft models. J Immunother Cancer.
12:e0069912024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Kong D, Ying B, Zhang J and Ying H: The
anti-osteosarcoma property of ailanthone through regulation of
miR-126/VEGF-A axis. Artif Cells Nanomed Biotechnol. 47:3913–3919.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li B, Dang X, Duan J, Zhang G, Zhang J and
Song Q: SIX4 upregulates IDH1 and metabolic reprogramming to
promote osteosarcoma progression. J Cell Mol Med. 27:259–265. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Shan J, Lin Z, Rashid H, Huang P, Qiang L,
Liu Y, Shen G, Li Y, Cui J, Su Z, et al: A novel therapeutic
strategy for osteosarcoma using anti-GD2 ADC and EZH2 inhibitor.
Biomark Res. 13:872025. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Taylor AM, Sheng J, Ng PKS, Harder JM,
Kumar P, Ahn JY, Cao Y, Dzis AM, Jillette NL, Goodspeed A, et al:
Immunosuppressive tumor microenvironment of osteosarcoma. Cancers
(Basel). 17:21172025. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Gassmann H, Schneider K, Evdokimova V,
Ruzanov P, Schober SJ, Xue B, von Heyking K, Thiede M, Richter GHS,
Pfaffl MW, et al: Ewing sarcoma-derived extracellular vesicles
impair dendritic cell maturation and function. Cells. 10:20812021.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wang L, Dou X, Xie L, Zhou X, Liu Y, Liu J
and Liu X: Metabolic landscape of osteosarcoma: Reprogramming of
lactic acid metabolism and metabolic communication. Front Biosci
(Landmark Ed). 29:832024. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wang Y, Wang X, Liu Y, Xu J, Zhu J, Zheng
Y and Qi Q: A novel hypoxia- and lactate metabolism-related
prognostic signature to characterize the immune landscape and
predict immunotherapy response in osteosarcoma. Front Immunol.
15:14670522024. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhu J, Simayi N, Wan R and Huang W: CAR T
targets and microenvironmental barriers of osteosarcoma.
Cytotherapy. 24:567–576. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Nguyen DT, Ogando-Rivas E, Liu R, Wang T,
Rubin J, Jin L, Tao H, Sawyer WW, Mendez-Gomez HR, Cascio M, et al:
CAR T cell locomotion in solid tumor microenvironment. Cells.
11:19742022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Chim LK, Williams IL, Bashor CJ and Mikos
AG: Tumor-associated macrophages induce inflammation and drug
resistance in a mechanically tunable engineered model of
osteosarcoma. Biomaterials. 296:1220762023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Cheng JN, Jin Z, Su C, Jiang T, Zheng X,
Guo J, Li X, Chu H, Jia J, Zhou Q, et al: Bone metastases diminish
extraosseous response to checkpoint blockade immunotherapy through
osteopontin-producing osteoclasts. Cancer Cell. 43:1093–1107.e9.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Martinov T, McKenna KM, Tan WH, Collins
EJ, Kehret AR, Linton JD, Olsen TM, Shobaki N and Rongvaux A:
Building the next generation of humanized hemato-lymphoid system
mice. Front Immunol. 12:6438522021. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Yin L and Wang XJ, Chen DX, Liu XN and
Wang XJ: Humanized mouse model: A review on preclinical
applications for cancer immunotherapy. Am J Cancer Res.
10:4568–4584. 2020.PubMed/NCBI
|
|
155
|
Bacac M, Klein C and Umana P: CEA TCB: A
novel head-to-tail 2:1 T cell bispecific antibody for treatment of
CEA-positive solid tumors. Oncoimmunology. 5:e12034982016.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lehmann S, Perera R, Grimm HP, Sam J,
Colombetti S, Fauti T, Fahrni L, Schaller T, Freimoser-Grundschober
A, Zielonka J, et al: In vivo fluorescence imaging of the activity
of CEA TCB, a novel T-Cell bispecific antibody, reveals highly
specific tumor targeting and fast induction of T-Cell-mediated
tumor killing. Clin Cancer Res. 22:4417–4427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Sun Z, Gu M, Yang Z, Shi L, Zhao L, Zheng
M, Wang Y, Zhang W, Han K and Tang N: Application of humanized mice
in the safety experiments of antibody drugs. Animal Model Exp Med.
8:1023–1032. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Gail LM, Schell KJ, Łacina P, Strobl J,
Bolton SJ, Ulriksen ES, Bogunia-Kubik K, Greinix H, Crossland RE,
Inngjerdingen M and Stary G: Complex interactions of cellular
players in chronic Graft-versus-Host Disease. Front Immunol.
14:11994222023. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Jackett KN, DaPonte DL, Soman P and Horton
JA: Modeling the effects of radiation on the bone tumor
microenvironment: Opportunities for exploring combination therapies
in microphysiologic systems. Cell Mol Biol Lett. 30:972025.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zheng BW, Zheng BY, Niu HQ, Yang YF, Zhu
GQ, Li J, Zhang TL and Zou MX: Tumor growth rate in spinal giant
cell tumors of bone and association with the immune
microenvironment and denosumab treatment responsiveness: A
multicenter study. Neurosurgery. 92:524–537. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Cheng X, Peng T, Chu T, Yang Y, Liu J, Gao
Q, Cao C and Wei J: Application of single-cell and spatial omics in
deciphering cellular hallmarks of cancer drug response and
resistance. J Hematol Oncol. 18:702025. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Chen J, Chen F, Zhang H, Hu Y, Zhou X,
Weng X, Bao G and Ding X: Multi-omics-based construction of a
palmitoylation-driven prognostic model reveals tumor immune
phenotypes in osteosarcoma. Discov Oncol. 16:15442025. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Yu B, Pacureanu A, Olivier C, Cloetens P
and Peyrin F: Assessment of the human bone lacuno-canalicular
network at the nanoscale and impact of spatial resolution. Sci Rep.
10:45672020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lu T, Park S, Zhu J, Wang Y, Zhan X, Wang
X, Wang L, Zhu H and Wang T: Overcoming Expressional drop-outs in
lineage reconstruction from single-cell RNA-sequencing data. Cell
Rep. 34:1085892021. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Leipold MD, Obermoser G, Fenwick C,
Kleinstuber K, Rashidi N, McNevin JP, Nau AN, Wagar LE, Rozot V,
Davis MM, et al: Comparison of CyTOF assays across sites: Results
of a six-center pilot study. J Immunol Methods. 453:37–43. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Lacinski RA, Dziadowicz SA, Roth CA, Ma L,
Melemai VK, Fitzpatrick B, Chaharbakhshi E, Heim T, Lohse I,
Schoedel KE, et al: Spatial multiplexed immunofluorescence analysis
reveals coordinated cellular networks associated with overall
survival in metastatic osteosarcoma. Bone Res. 12:552024.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Krieg C, Nowicka M, Guglietta S, Schindler
S, Hartmann FJ, Weber LM, Dummer R, Robinson MD, Levesque MP,
Becher B, et al: High-dimensional single-cell analysis predicts
response to anti-PD-1 immunotherapy. Nat Med. 24:144–153. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Jiang Y, Wang J, Sun M, Zuo D, Wang H,
Shen J, Jiang W, Mu H, Ma X, Yin F, et al: Multi-omics analysis
identifies osteosarcoma subtypes with distinct prognosis indicating
stratified treatment. Nat Commun. 13:72072022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Keung EZ, Burgess M, Salazar R, Parra ER,
Rodrigues-Canales J, Bolejack V, Van Tine BA, Schuetze SM, Attia S,
Riedel RF, et al: Correlative analyses of the SARC028 trial reveal
an association between sarcoma-associated immune infiltrate and
response to pembrolizumab. Clin Cancer Res. 26:1258–1266. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Gu L, Peng C, Liang Q, Huang Q, Lv D, Zhao
H, Zhang Q, Zhang Y, Zhang P, Li S, et al: Neoadjuvant toripalimab
plus axitinib for clear cell renal cell carcinoma with inferior
vena cava tumor thrombus: NEOTAX, a phase 2 study. Signal Transduct
Target Ther. 9:2642024. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Brekken C, Bruland ØS and de Lange Davies
C: Interstitial fluid pressure in human osteosarcoma xenografts:
Significance of implantation site and the response to intratumoral
injection of hyaluronidase. Anticancer Res. 20:3503–3512.
2000.PubMed/NCBI
|
|
172
|
Wu H, Luo Y, Xu D, Ke X and Ci T: Low
molecular weight heparin modified bone targeting liposomes for
orthotopic osteosarcoma and breast cancer bone metastatic tumors.
Int J Biol Macromol. 164:2583–2597. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Meng H, Mai WX, Zhang H, Xue M, Xia T, Lin
S, Wang X, Zhao Y, Ji Z, Zink JI and Nel AE: Codelivery of an
optimal drug/siRNA combination using mesoporous silica
nanoparticles to overcome drug resistance in breast cancer in vitro
and in vivo. ACS Nano. 7:994–1005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Kontulainen SA, Macdonald HM and McKay HA:
Change in cortical bone density and its distribution differs
between boys and girls during puberty. J Clin Endocrinol Metab.
91:2555–2561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Dai XM, Ryan GR, Hapel AJ, Dominguez MG,
Russell RG, Kapp S, Sylvestre V and Stanley ER: Targeted disruption
of the mouse colony-stimulating factor 1 receptor gene results in
osteopetrosis, mononuclear phagocyte deficiency, increased
primitive progenitor cell frequencies, and reproductive defects.
Blood. 99:111–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Joseph GJ, Vecchi Iii LA, Uppuganti S,
Kane JF, Durdan M, Hill P, McAdoo AG, Tanaka H, Kell D, Searcy MB,
et al: Programmed cell death protein 1 (PD-1) blockade regulates
skeletal remodeling in a sex- and age-dependent manner. J Bone
Miner Res. 40:950–964. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
DeSelm C, Palomba ML, Yahalom J, Hamieh M,
Eyquem J, Rajasekhar VK and Sadelain M: Low-dose radiation
conditioning enables CAR T cells to mitigate antigen escape. Mol
Ther. 26:2542–2552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Yu D, Kahen E, Cubitt CL, McGuire J,
Kreahling J, Lee J, Altiok S, Lynch CC, Sullivan DM and Reed DR:
Identification of synergistic, clinically achievable, combination
therapies for osteosarcoma. Sci Rep. 5:169912015. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Gargett T, Ebert LM, Truong NTH, Kollis
PM, Sedivakova K, Yu W, Yeo ECF, Wittwer NL, Gliddon BL, Tea MN, et
al: GD2-targeting CAR-T cells enhanced by transgenic IL-15
expression are an effective and clinically feasible therapy for
glioblastoma. J Immunother Cancer. 10:e0051872022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Rahouma M, Karim NA, Baudo M, Yahia M,
Kamel M, Eldessouki I, Abouarab A, Saad I, Elmously A, Gray KD, et
al: Cardiotoxicity with immune system targeting drugs: A
meta-analysis of anti-PD/PD-L1 immunotherapy randomized clinical
trials. Immunotherapy. 11:725–735. 2019. View Article : Google Scholar : PubMed/NCBI
|