Colorectal cancer (CRC) is one of the most prevalent
malignancies of the digestive system. According to global cancer
statistics, >1.9 million new CRC cases and ~900,000 mortalities
due to CRC were recorded in 2022, making it the third most commonly
diagnosed cancer and the second leading cause of cancer-related
mortality worldwide (1). The
incidence and progression of CRC are influenced by various factors,
including age, genetic predisposition, lifestyle choices and
environmental exposures. Because early symptoms are subtle, several
patients present with advanced disease or distant spread, leading
to a poor outcome (2). Currently,
the mainstay of CRC treatment is comprehensive therapy centered
around surgical resection, complemented by radiotherapy,
chemotherapy, targeted therapy and immunotherapy. Preoperative
neoadjuvant therapies and postoperative adjuvant chemotherapy or
radiotherapy have improved the chances of curative outcomes.
Standard adjuvant chemotherapy regimens include oxaliplatin based
xeloda and oxaliplatin and folinic acid and fluorouracil and
oxaliplatin regimens (3,4). Additionally, targeted therapies
focusing on EGFR and vascular endothelial growth factor (VEGF)
pathways have demonstrated clinical benefit in select patient
populations (5,6). Nevertheless, response rates are modest
and adverse effects substantial (7–9).
Immunotherapy has transformed outcomes in several
malignancies and is now a key focus in CRC. Immune checkpoint
inhibitors (ICIs), in particular, have shown favorable efficacy in
patients with microsatellite instability-high (MSI-H) CRC.
Nonetheless, ~85% of CRC cases exhibit microsatellite stability
(MSS) or proficient mismatch repair (pMMR) status, and these
patients typically exhibit limited responses to ICIs (10). Thus, elucidating the immune
microenvironment of CRC and developing novel immunotherapeutic
strategies have emerged as key areas of current research.
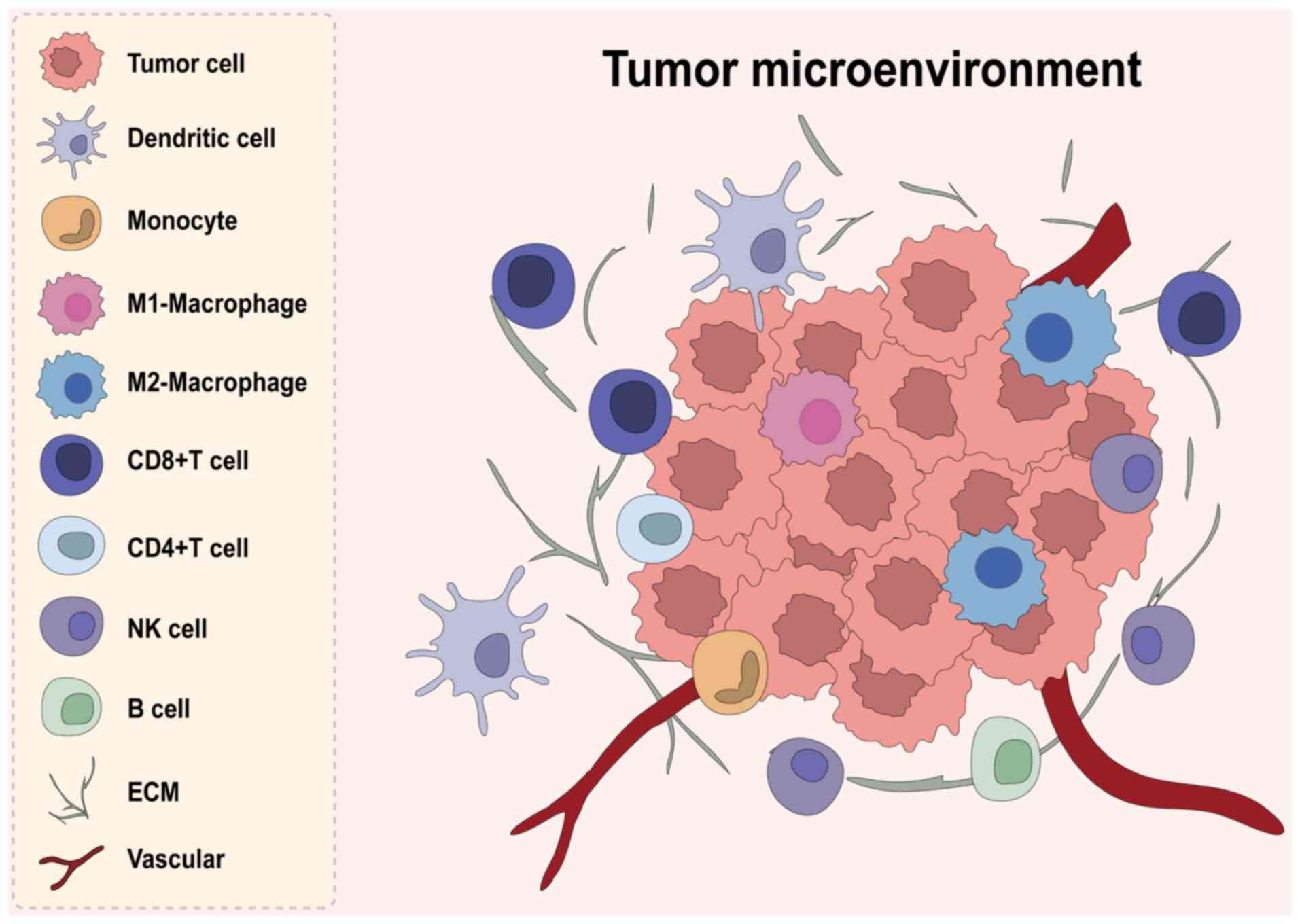
The tumor microenvironment (TME) comprises various
components associated with tumor tissues, carrying out key roles in
tumor initiation and progression. Multiple immune cells, including
but not limited to macrophages, T cells, B cells, natural killer
(NK) cells, dendritic cells (DCs) and neutrophils, are recruited
into the vicinity of tumor cells, interacting with extracellular
matrix (ECM) components and cytokines to collectively form the
tumor immune microenvironment (TIME) (11) (Fig.
1). These cells can mount potent antitumor responses yet also
enable immune escape. Their relative abundance and functional state
help determine tumor immunogenicity, therapy response and patient
survival. Intensive research has explored the roles and regulatory
mechanisms of various immune cells within the CRC TIME.
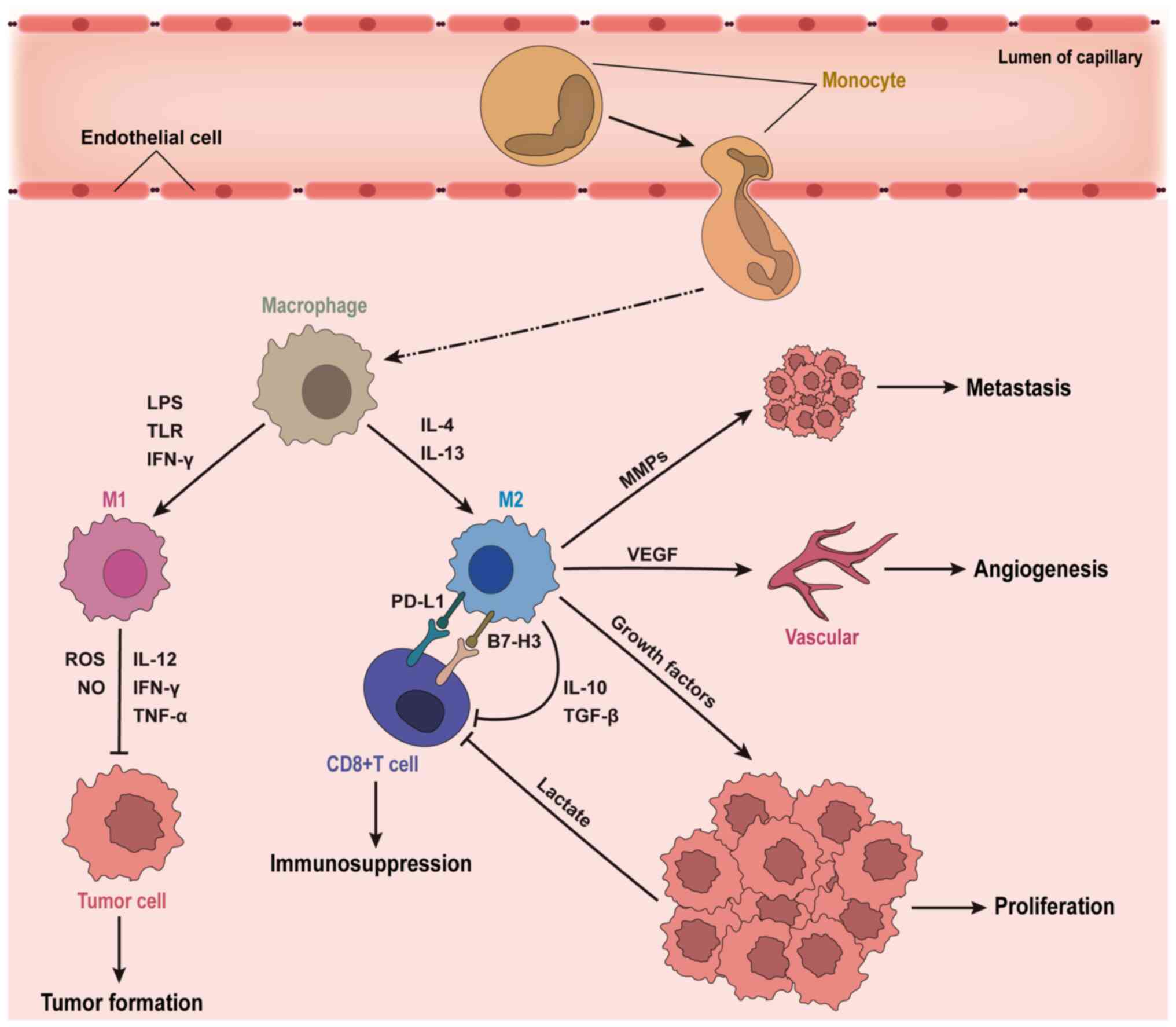
Based on functional characteristics, TAMs are
typically classified into pro-inflammatory M1 and anti-inflammatory
M2 phenotypes. M1 macrophages, activated by IFN-γ,
lipopolysaccharide or Toll-like receptor ligands, express markers
such as major histocompatibility complex (MHC)-II, CD68, CD80 and
CD86. They enhance anti-tumor immune responses through secretion of
cytokines including IL-12, TNF-α and IFN-γ, and directly exert
cytotoxic effects on tumor cells via reactive oxygen species and
nitric oxide (NO) production (15–19).
Conversely, M2 macrophages exhibit immunosuppressive roles,
expressing inhibitory receptors such as programmed death-ligand 1
(PD-L1) and B7-H3 (20–22). Activated by Th2 cytokines IL-4 and
IL-13, M2 macrophages express CD163, CD206 and ARG1, and secrete
cytokines such as IL-10, TGF-β and VEGF, suppressing T cell
activity, promoting angiogenesis and facilitating immune escape
(Fig. 2) (23–25).
High infiltration of M1 macrophages associates with
improved prognosis in CRC. Studies have shown an inverse
relationship between the proportion of M1 macrophages and lymph
node and distant metastases (26–28).
Wang et al (29) reported
that ANKRD22 expression in M1 macrophages positively associates
with survival and macrophage infiltration levels in patients with
colon cancer. Nussbaum et al (30)Q analyzing single-cell RNA sequencing
(scRNA-seq) data from 23 patients with CRC, identified high
SPP1-CD44 interactions within inflammatory macrophages. Targeting
SPP1+ macrophages exhibiting an anti-inflammatory phenotype could
disrupt these interactions, potentially reducing tumor progression
and immunosuppression.
Conversely, M2 macrophage infiltration in CRC is
associated with tumor progression and immune evasion. Their
presence associates notably with increased tumor aggressiveness,
metastatic potential and immunosuppressive capabilities (31,32).
Tumor-derived exosomes serve as key mediators of intercellular
communication, facilitating tumor-macrophage interactions. After
internalization by macrophages, these exosomes activate
polarization pathways, driving M2 macrophage polarization, creating
pre-metastatic niches and subsequently promoting CRC metastasis
(33–36). M2 macrophages further enhance tumor
invasion and metastasis by secreting pro-tumor factors such as
VEGF, MMPs, IL-4 and IL-13, contributing to angiogenesis and ECM
degradation (37–39). Additionally, M2 macrophages
facilitate immune escape through expression of PD-L1, engaging with
PD-1 and cytotoxic T-lymphocyte associated protein 4 (CTLA-4),
impairing T-cell receptor (TCR) signaling and inhibiting cytotoxic
T cell function (40). They also
expand Tregs via TGF-β secretion, further suppressing CD8+ T cell
activities and promoting immune evasion (41).
Studies have demonstrated the dual influence of TAMs
on the effectiveness of radiotherapy and chemotherapy, either
antagonizing therapeutic efficacy by facilitating tissue repair and
immunosuppression or enhancing overall antitumor effects (42,43).
De Palma et al (44) found
that TAMs also drive repair mechanisms following vascular-targeted
therapy. Furthermore, low-dose γ irradiation of macrophages during
neoadjuvant therapy can induce vascular normalization and
counteract immunosuppression, diminishing pro-tumor effects of TAMs
(45). Studies indicate that ICIs,
such as PD-1/PD-L1 inhibitors and agents such as selicrelumab, can
enhance macrophage phagocytosis, reducing tumor burden (46,47).
Current clinical trials targeting TAMs primarily follow three major
strategies, with differential therapeutic efficacy revealing
distinct underlying mechanisms of action. First, strategy targeting
the C-C motif chemokine receptor 2/C-C motif chemokine ligand 2 or
colony-stimulating factor 1 receptor (CSF1R) axis represent a
common therapeutic approach. This strategy inhibits TAM
recruitment, effectively reducing their overall density within the
TIME. Published results from clinical studies largely support the
feasibility of this approach (48–51).
However, likely due to limited anti-tumor clinical
activity, the majority of ongoing trials in solid tumors are Phase
I/II studies focusing on safety and preliminary efficacy (50,52,53).
This constrained efficacy may be partly attributed to the
concurrent suppression of potentially anti-tumor M1-type TAM
recruitment, thereby weakening anti-tumor immunity. Furthermore,
the high plasticity of TAMs may trigger compensatory activation of
other pro-tumor signaling pathways upon CSF1R inhibition,
potentially restoring the pro-tumor functions of TAMs and
diminishing therapeutic effectiveness (54). Second, blocking the ‘don't eat me’
signal using anti-CD47/SIRPα antibodies to activate macrophage
phagocytosis constitutes another common strategy. This approach
bypasses the dependency on TAM quantity and directly enhances their
intrinsic tumor-killing capacity (55). Results from the NCT02953782 trial
demonstrated that combined anti-CD47 and anti-EGFR therapy was
tolerable and showed potential anti-tumor activity in heavily
pre-treated patients with CRC, despite no notable changes in immune
cell infiltration levels being observed (56). Additionally, since CD47 is involved
in maintaining erythrocyte homeostasis, off-target toxicity of CD47
antibodies often limits the efficacy of monotherapy (57). Third, Chimeric Antigen Receptor
Macrophage (CAR-M) therapy, which utilizes genetically engineered
macrophages, represents a precision empowerment strategy. This
approach not only enables specific tumor antigen recognition but
may also promote macrophage polarization towards the M1 phenotype
upon activation. The subsequent secretion of pro-inflammatory
cytokines such as IL-12 could potentially convert ‘cold’ tumors
into ‘hot’ tumors. Clinical trial results (NCT04660929) indicated
that CAR-M therapy is safe and feasible in patients with
HER2-overexpressing solid tumors and can induce TME remodeling and
anti-tumor immune responses (58),
offering a novel strategy to address prevalent immunosuppression in
CRC (Table I).
Substantial advancements have been realized in the
field of cancer immunotherapy over recent years (59–61).
Although numerous early-phase clinical trials have explored
targeting TAMs, these studies (62–64)
have revealed several key and unresolved challenges: i) The lack of
predictive biomarkers to prospectively identify patients who are
likely to benefit; ii) difficulties in managing toxicities
associated with combination therapies, as well as uncertainties
regarding the optimal sequencing of treatments and their underlying
mechanistic basis; iii) obstacles in genetically engineering
macrophages, including their poor expansion in vitro and
limited self-renewal capacity following adoptive transfer.
Therefore, future efforts should focus on integrating specific
biomarkers to identify patients most likely to benefit, and
exploring combination regimens with chemotherapy, radiotherapy,
immunotherapy or therapies targeting other immune cells. This
strategy is essential to overcome treatment resistance and maximize
therapeutic efficacy.
T cells are prominent immune cell populations within
the CRC TIME, playing essential roles in tumor defense. They are
categorized primarily as CD8+ cytotoxic T cells and
CD4+ helper T cells based on functional differentiation.
CD8+ T cells serve as key effectors in adaptive
immunity, recognizing tumor antigens presented via MHC class I
molecules and directly killing tumor cells through secretion of
perforin and granzyme B (65). They
also release IFN-γ and TNF-α, strengthening local immune responses
and initiating apoptosis via the Fas/FasL signaling pathway
(66). Increased infiltration of
activated CD8+ T cells is associated with improved
patient prognosis (67).
B cells represent a key component of adaptive
immunity, mediating anti-tumor responses primarily through antibody
production, antigen presentation and the promotion of T cell
activation (101). The antibodies
secreted by B cells can bind to tumor cells, facilitating cytotoxic
activities mediated by NK cells, macrophages and the complement
system. Additionally, B cells function as antigen-presenting cells,
stimulating CD4+ T cells via MHC class II molecules, thereby
enhancing antitumor immune responses (102). However, the role of B cells within
the TME is not exclusively protective.
Certain subsets of B cells, particularly regulatory
B cells (Bregs), may promote tumor progression (103). Bregs secrete immunosuppressive
cytokines such as IL-10 and TGF-β, inhibiting CD8+ T
cell and NK cell functions, thus facilitating tumor immune escape
(104). Moreover, B cells can
exacerbate tumor-associated inflammation by producing
pro-inflammatory cytokines, including IL-6, thereby enhancing
cancer cell proliferation and metastasis (105). Clinical studies demonstrate that
increased infiltration of Bregs in CRC tissues associates
positively with tumor progression and predicts poor patient
prognosis (106,107).
Targeting B cells for immunomodulation has emerged
as a novel direction for CRC treatment. On one hand, enhancing
antigen-specific B cell responses, such as developing B-cell-based
vaccines, might potentiate antitumor immunity (108). On the other hand, inhibiting Breg
functions to reduce their immunosuppressive effects could improve
responses to immunotherapy. Monoclonal antibodies targeting
Breg-specific markers, such as CD19 or CD38, have been proposed as
promising therapeutic approaches (109,110). Additionally, interactions between
B cells and T cells are key within the CRC immune microenvironment
(111). Thus, future therapeutic
strategies should comprehensively consider dynamic interactions
among various immune cell subsets to optimize immunotherapy
outcomes.
NK cells constitute essential components of innate
immunity, capable of directly recognizing and killing tumor cells
independently of antigen presentation. NK cells induce apoptosis in
target cells through the secretion of perforin and granzyme B, and
enhance antitumor immune responses via IFN-γ production (112). Additionally, NK cells contribute
markedly to antibody-dependent cellular cytotoxicity, enhancing the
efficacy of antibody-based therapies (113). NK cells are principally stratified
into two distinct functional subsets contingent upon the surface
density of CD56 expression: The CD56dim and CD56bright
subpopulations. The former constitutes the numerically predominant
fraction, characterized by potent cytotoxicity and serving as the
primary effector killer cells; in contradistinction, the latter
subpopulation is specialized chiefly in cytokine secretion and the
orchestration of immune responses (114).
NK cell activity is notably impaired. CRC tissues
often exhibit reduced NK cell infiltration and functionality due to
immunosuppressive factors present within the TME (115). For example, cytokines such as
TGF-β and IL-10, abundant in the CRC microenvironment, directly
inhibit NK cell cytotoxicity, inducing a state of functional
exhaustion (116,117). Furthermore, increased expression
of inhibitory receptors, such as PD-1 on NK cells within CRC
tumors, reduces their capacity to recognize and eliminate tumor
cells, thereby allowing tumor cells to evade immune surveillance
(118). The modalities
underpinning this immune escape are multifaceted. Initially, CRC
neoplastic cells elude immunosurveillance mediated by the NK
activating receptor NKG2D via the specific downregulation of MHC-I
related molecular expression (119). Concomitantly, NK cells exhibit
elevated surface expression patterns of novel immune checkpoint
receptors beyond PD-1, namely NKG2A/CD94, TIGIT and TIM-3. The
cognate ligands for these inhibitory receptors are abundantly
expressed by tumor cells or residing within the TME,
collaboratively engendering a state of profound NK exhaustion
phenotype (120). Moreover, the
deleterious metabolic milieu within the TME constitutes another key
determinant: Overexpressed CD73 enzymatically catabolizes
extracellular ATP into adenosine, which subsequently engages the
A2AR receptor on the NK cell surface, thereby abrogating their
proliferative capacity and cytotoxic effector functions (121).
Strategies to enhance NK cell antitumor activities
include CAR-NK cell therapy, NK cell stimulants, such as IL-15 and
combination treatments involving PD-1/PD-L1 blockade (122). Preclinical studies indicate CAR-NK
cells exhibit potent antitumor activity and possess superior safety
profiles compared with CAR-T cell therapies (123–125). However, due to challenges
associated with NK cell proliferation and survival in vitro,
further optimization of NK cell-based immunotherapies remains
essential to improve their clinical utility in CRC treatment.
DCs, originating from hematopoietic stem cells in
the bone marrow, are the most potent professional
antigen-presenting cells. DCs capture exogenous antigens, process
them into antigenic peptides and present them in complex with MHC
molecules on their surface, initiating naïve T cell activation. DCs
are a highly heterogeneous group, primarily categorized into
conventional DCs (cDCs) and plasmacytoid DCs (pDCs). Functionally,
cDCs specialize in antigen processing and presentation, while pDCs
are less proficient in antigen presentation but produce substantial
amounts of type I interferons (126).
Within the TME, DCs serve as pivotal
antigen-presenting cells, capturing, processing and presenting
tumor antigens to activate T cell-mediated antitumor immune
responses. DCs stimulate CD4+ T cells via MHC-II
molecules and also facilitate CD8+ T cell activation
through cross-presentation (127).
cDCs constitute the predominant DC population within the TME and
are further delineated into distinct cDC1 and cDC2 subsets.
Notably, the cDC1 subpopulation, whose development is dependent
upon the transcription factor BATF3, assumes an indispensable role
in orchestrating anti-tumor T cell immune responses (128), whereas cDC2s are principally
tasked with initiating Th2-type immunity. However, DC functionality
in the CRC microenvironment is frequently compromised, impairing
effective antitumor immunity. Immunosuppressive factors, such as
prostaglandin E2 and IL-6, inhibit DC maturation, thereby limiting
their capacity to activate T cells effectively. This specific
functional deficit is particularly salient within the context of
CRC. Investigations have elucidated that in MSS CRC phenotypes,
aberrant intrinsic activation of the Wnt/β-catenin signaling axis
constitutes a pivotal mechanism underpinning immunosuppression
(129). Mechanistically, this
pathway acts to repress the expression of chemokines CCL4 and CCL5,
thereby specifically impeding the efficacious intratumoral
infiltration of the key cDC1 subset; this cascade consequently
renders the TME an immunologically ‘cold’ landscape, ultimately
conferring primary resistance to ICIs (130). Additionally, some DC populations
may acquire tolerogenic properties, failing to effectively present
antigens and instead promoting Treg expansion, further suppressing
antitumor responses (131).
Current strategies aiming to restore DC-mediated
antitumor immunity include DC vaccines, autologous DC infusion and
combination therapies with ICIs (132,133). DC vaccines hold promise for CRC
treatment, with several clinical trials currently evaluating their
efficacy in combination with ICIs. Furthermore, prospective
therapeutic avenues could involve targeted intervention against the
Wnt/β-catenin signaling axis to ameliorate the established
impediments to cDC1 recruitment within the TME, thereby
orchestrating the conversion of immunologically ‘cold’ tumors into
a ‘hot’ phenotype (134–136). This strategy could subsequently be
leveraged in synergistic combinations with ICIs to augment overall
antitumor therapeutic efficacy.
Neutrophils represent a primary barrier of innate
immunity, carrying out key roles in inflammation and immune
responses. Within the TME, neutrophils can be further categorized
into N1 phenotypes, which exert anti-tumor effects and N2
phenotypes, which facilitate tumor progression. Growth factors,
cytokines and chemokines present in the TME recruit neutrophils
into the tumor tissue, where they participate in the regulation of
diverse and complex biological processes (137). Prevailingly, elevated
concentrations of TGF-β function as a cardinal impetus driving the
phenotypic skewing of neutrophils from an N1 state towards the
pro-tumorigenic N2 phenotype (138). N2-polarized neutrophils
predominantly manifest immunosuppressive functionalities, exerting
these effects via the regulated secretion of a complex array of
chemokines, pro-angiogenic factors and immunosuppressive moieties.
Consequently, this bioactive secretome actively recruits and
activates collateral immunosuppressive cell populations,
facilitates neovascularization and concurrently subverts host
immune system recognition and cytotoxic clearance of tumors
(139).
Neutrophils carry out a key role in the metastatic
process of CRC. Under specific signaling stimuli, neutrophils
undergo a unique form of cell death known as neutrophil
extracellular trap (NET) formation. The released NETs not only
physically capture circulating tumor cells (140), but studies have also revealed that
NETs interact via histones on DNA with specific receptors on tumor
cell surfaces, activating downstream signaling pathways that
promote the colonization and survival of tumor cells in distant
organs (141,142). Furthermore, NETs facilitate immune
evasion by degrading anti-tumor molecules within the TME (143). Clinical evidence further confirms
that an elevated peripheral blood neutrophil-to-lymphocyte ratio
serves as an independent predictor of poor prognosis and resistance
to immunotherapy in patients with CRC (144).
Therapeutic approaches targeting neutrophils include
inhibition of NET formation and blockade of the CXCR2 signaling
pathway (145,146), with the objective of diminishing
neutrophil recruitment within the TME and augmenting the efficacy
of immunotherapeutic interventions. Consequently, the strategic
modulation of neutrophil polarization states, the suppression of
NET generation and the targeted disruption of their recruitment
signals represent viable avenues for enhancing therapeutic outcomes
in CRC management.
Multi-omics approaches integrate multiple molecular
analyses to elucidate interactions among various biological
components. Recently, notable advancements in scRNA-seq and spatial
transcriptomics have profoundly enhanced the understanding of the
CRC TIME (77,147,148), illuminating immune cell
heterogeneity, functional states and interactions with tumor cells,
thereby offering novel insights into CRC pathophysiology.
ScRNA-seq provides comprehensive information at the
transcriptomic, genomic and epigenomic levels for individual cells,
enabling researchers to identify distinct immune cell subsets
within the TME, characterize their functional states and reveal
potential intercellular communication pathways. Zhang et al
(149) conducted a detailed
scRNA-seq analysis of tumor-infiltrating myeloid cells in patients
with CRC, elucidating the characteristics and lineage trajectories
of TAM and DC subpopulations, as well as their interactions with T
cells and other cell types. Their study revealed a specific
enrichment of secreted phosphoprotein 1+ (SPP1+) TAMs in CRC
tissues and demonstrated the similarity between CSF1R
inhibitor-resistant TAM subsets and SPP1+ TAM populations,
providing a mechanistic explanation for the limited efficacy of
anti-CSF1R monotherapy and highlighting the role of SPP1+
macrophages in promoting tumor metastasis (149,150). Importantly, the identification of
this SPP1+ TAM subset provides the molecular evidence for the
therapeutic resistance mentioned in the TAM section, validating
that the compensatory activation limiting CSF1R inhibitor efficacy
is driven by distinct, intrinsically resistant macrophage
subpopulations. The investigation by Zhang et al (149) had elucidated, at single-cell
resolution, a landscape of TAM heterogeneity that was markedly more
intricate when compared with the canonical M1/M2 stratification
paradigm. This had substantially augmented the comprehension of
intratumoral TAM diversity by precisely pinpointing an SPP1+ TAM
subset exhibiting inherent resistance to CSF1R inhibitors. This key
discovery not only offers a mechanistic rationale for the
aforementioned therapeutic predicaments but further delineated that
prospective targeting avenues must focus on the precise therapeutic
interrogation of such distinct pro-tumorigenic subpopulations.
Additionally, Wu et al (151) applied combined scRNA-seq and
spatial transcriptomics to CRC liver metastasis samples,
systematically illustrating spatial expression patterns of SPP1+
macrophages and mannose receptor C-type 1+, CCL18+ M2-like
macrophages, suggesting a key immunosuppressive role of the TAM in
CRC liver metastasis (151).
The advancement and integrated application of
multi-omics technologies have shown considerable potential in
dissecting immune cell composition, functional dynamics and
tumor-immune cell interactions within the CRC microenvironment.
Such comprehensive insights provide key foundations for developing
novel therapeutic strategies and advancing personalized
medicine.
Notwithstanding the remarkable preclinical strides
in TIME-targeted immunotherapies, translating these findings into
tangible clinical benefits for patients with CRC is thwarted by
multifaceted impediments, principally stemming from the profound
heterogeneity of the CRC microenvironment (77). While ICIs demonstrate efficacy in
MSI-H subsets, the vast majority of patients with MSS exhibit an
‘immune desert’ or ‘immune exclusion’ phenotype. This intrinsic
immunological dormancy renders single-agent TIME-directed
strategies insufficient to revert their immune-silent status.
Moreover, the dynamic adaptability of the TIME is another key
determinant of therapeutic failure. The TME possesses a high degree
of compensatory capacity. This mechanism of compensatory immune
evasion severely curtails the durable efficacy achievable with
monotherapies.
The safety profile and the predictive value of
preclinical models also urgently warrant resolution. Several TIME
targets, such as CD47 and TGF-β, are ubiquitously expressed in
healthy tissues, meaning that off-target systemic toxicity severely
constrains the therapeutic window for these agents (154,155). While combinatorial regimens are
deemed the principal strategy to circumvent resistance, this
approach invariably precipitates compounding toxicities and an
escalated risk of immune-related adverse events. Concomitantly,
clear clinical guidance remains lacking for the precise
determination of the optimal sequencing for distinct therapeutic
agents. Furthermore, the widely employed subcutaneous xenograft
models exhibit rapid growth dynamics and lack the complex human CRC
stromal architecture and the long-term co-evolutionary interplay
between the immune system and the tumor (156). The disparity between these models
and clinical pathological features often results in a failure to
recapitulate efficacy in human trials, despite promising results
observed in mice. Therefore, future research should focus on
developing more clinically relevant models and exploring
combination therapy strategies to overcome the limitations of
targeting single pathways.
Accumulating evidence indicates that tumor
progression involves not only the intrinsic characteristics of
tumor cells but also key interactions within the TIME (157,158). Immune cells within the TIME carry
out pivotal roles in CRC initiation, progression and immune evasion
processes. Specifically, these immune cells shape the host immune
responses, considerably influencing tumor development, thereby
positioning the TIME as a promising therapeutic target for cancer
treatment. Immunotherapy has evolved from simply enhancing immune
effector functions to specifically targeting immunosuppressive
factors within the TIME. Consequently, strategies aimed at
effectively modulating the TIME to amplify the therapeutic effects
of immunotherapy are emerging as a key area of ongoing and future
research.
To advance the transition from targeting single
cells toward systematically reshaping the TIME through combination
therapies, future research should focus on multidisciplinary
integration and overcoming challenges such as drug resistance and
the limitations of immunotherapy, thereby enabling the development
of personalized treatment regimens. Although patients with
dMMR/MSI-H CRC benefit from ICIs, the majority of CRC cases are
pMMR/MSS and remain unresponsive to ICI-based immunotherapy
(10). Therefore, a primary
objective is to overcome the immunosuppressive state in pMMR/MSS
CRC by enhancing immunogenicity to render these tumors susceptible
to immunotherapy. For instance, immunosuppressive M2-type TAMs
express inhibitory ligands such as PD-L1 and B7H3, which contribute
to immune suppression and diminish the efficacy of T cell-directed
ICIs. Combining therapies that target these immunosuppressive TAMs
with PD-1/PD-L1 blockade is thus a rational strategy. Clinical
evidence supports this approach: The combination of the CSF1R
inhibitor emactuzumab with the anti-PD-L1 agent atezolizumab has
demonstrated a notable objective response rate in ICI-naïve
patients (159). This provides a
promising avenue for improving outcomes in pMMR/MSS patients with
CRC. Furthermore, breakthroughs in drug development technologies
and insights from spatial transcriptomics into TIME-mediated
immunosuppression and resistance mechanisms will be instrumental in
achieving precision medicine and addressing treatment insensitivity
in a subset of patients with CRC. For example, a previous study
showed that deep learning can predict MSI status directly from
routine histology slides with high concordance with standard
molecular testing (160),
underscoring the potential of multidisciplinary integration to
inform individualized precision therapy.
Comprehensive understanding of the composition and
functional dynamics of the TIME, along with its interplay in CRC
development and immune escape mechanisms, can provide key insights
into the underlying pathogenesis of CRC. Such knowledge offers a
robust theoretical framework for developing novel immunotherapeutic
approaches. Future research dedicated to unraveling the regulatory
mechanisms and interactive networks within the TIME will facilitate
individualized, precision-based therapeutic strategies, ultimately
improving both survival outcomes and quality of life for patients
with CRC.
Not applicable.
This work was supported by grants from the Guangxi Natural
Science Foundation (grant no. 2025GXNSFAA069131) and First-class
discipline innovation-driven talent program of Guangxi Medical
University.
Not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: Globocan estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Sargent D, Sobrero A, Grothey A, O'Connell
MJ, Buyse M, Andre T, Zheng Y, Green E, Labianca R, O'Callaghan C,
et al: Evidence for cure by adjuvant therapy in colon cancer:
Observations based on individual patient data from 20,898 patients
on 18 randomized trials. J Clin Oncol. 27:872–877. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maughan TS, Adams RA, Smith CG, Meade AM,
Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL,
et al: Addition of cetuximab to oxaliplatin-based first-line
combination chemotherapy for treatment of advanced colorectal
cancer: Results of the randomised phase 3 mrc coin trial. Lancet.
377:2103–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin, and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Larsen AK, Poindessous V, Ouaret D, El
Ouadrani K, Megalophonos VF, Batistella A, Petitprez A, Escargueil
AE, Tournigand C and De Gramont A: Feasibility of combining EGFR-
and VEGF(R)-targeted agents in colorectal cancer. J Clin Oncol. 29
(4_suppl):S4432011. View Article : Google Scholar
|
|
6
|
Chibaudel B, Henriques J, Rakez M, Brenner
B, Kim TW, Martinez-Villacampa M, Gallego-Plazas J, Cervantes A,
Shim K, Jonker D, et al: Association of bevacizumab plus
oxaliplatin-based chemotherapy with disease-free survival and
overall survival in patients with stage II colon cancer: A
secondary analysis of the avant trial. JAMA Netw Open.
3:e20204252020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cutroneo PM, Giardina C, Ientile V,
Potenza S, Sottosanti L, Ferrajolo C, Trombetta CJ and Trifirò G:
Overview of the safety of anti-VEGF drugs: Analysis of the Italian
spontaneous reporting system. Drug Saf. 40:1131–1140. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Passaro A, Jänne PA, Mok T and Peters S:
Overcoming therapy resistance in EGFR-mutant lung cancer. Nat
Cancer. 2:377–391. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oxnard GR, Yang JC, Yu H, Kim SW, Saka H,
Horn L, Goto K, Ohe Y, Mann H, Thress KS, et al: TATTON: A
multi-arm, phase Ib trial of osimertinib combined with selumetinib,
savolitinib, or durvalumab in egfr-mutant lung cancer. Ann Oncol.
31:507–516. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ganesh K, Stadler ZK, Cercek A, Mendelsohn
RB, Shia J, Segal NH and Diaz LA Jr: Immunotherapy in colorectal
cancer: Rationale, challenges and potential. Nat Rev Gastroenterol
Hepatol. 16:361–375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang T, Huang X, Zhang G, Hong Z, Bai X
and Liang T: Advantages of targeting the tumor immune
microenvironment over blocking immune checkpoint in cancer
immunotherapy. Signal Transduct Target Ther. 6:722021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sedighzadeh SS, Khoshbin AP, Razi S,
Keshavarz-Fathi M and Rezaei N: A narrative review of
tumor-associated macrophages in lung cancer: Regulation of
macrophage polarization and therapeutic implications. Transl Lung
Cancer Res. 10:1889–1916. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized m2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Müller E, Christopoulos PF, Halder S,
Lunde A, Beraki K, Speth M, Øynebråten I and Corthay A: Toll-like
receptor ligands and interferon-γ synergize for induction of
antitumor m1 macrophages. Front Immunol. 8:13832017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jing M, Ma M, Zhang M, Mei Y, Wang L,
Jiang Y, Li J, Song R, Yang Z, Pu Y, et al: The photothermal effect
induces m1 macrophage-derived tnf-α-type exosomes to inhibit
bladder tumor growth. Chem Eng J. 498:1550232024. View Article : Google Scholar
|
|
17
|
Aslam M, Ashfaq-Khan M, Qureshi A, Celik
S, Werle J-T, Senkowski M, Kim YO, KAPS L and Schuppan D:
Fri-453-rapamycin promotes tumoricidal immunity in murine HCC by
inducing M1-type macrophages and dentritic cells polarization and
enhancement of cytotoxic T-cells. J Hepatol. 70:e5952019.
View Article : Google Scholar
|
|
18
|
Han Y, Sun J, Yang Y, Liu Y, Lou J, Pan H,
Yao J and Han W: TMP195 exerts antitumor effects on colorectal
cancer by promoting M1 macrophages polarization. Int J Biol Sci.
18:5653–5666. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Griess B, Mir S, Datta K and
Teoh-Fitzgerald M: Scavenging reactive oxygen species selectively
inhibits M2 macrophage polarization and their pro-tumorigenic
function in part, via Stat3 suppression. Free Radic Biol Med.
147:48–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cassetta L and Pollard JW:
Tumor-associated macrophages. Curr Biol. 30:R246–R248. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng Y, Ren S, Zhang Y, Liu S, Meng L,
Liu F, Gu L, Ai N and Sang M: Circular RNA circWWC3 augments breast
cancer progression through promoting M2 macrophage polarization and
tumor immune escape via regulating the expression and secretion of
IL-4. Cancer Cell Int. 22:2642022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee YH, Martin-Orozco N, Zheng P, Li J,
Zhang P, Tan H, Park HJ, Jeong M, Chang SH, Kim BS, et al:
Inhibition of the B7-H3 immune checkpoint limits tumor growth by
enhancing cytotoxic lymphocyte function. Cell Res. 27:1034–1045.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kee JY, Ito A, Hojo S, Hashimoto I,
Igarashi Y, Tsuneyama K, Tsukada K, Irimura T, Shibahara N,
Takasaki I, et al: CXCL16 suppresses liver metastasis of colorectal
cancer by promoting TNF-α-induced apoptosis by tumor-associated
macrophages. BMC Cancer. 14:9492014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brigati C, Noonan DM, Albini A and Benelli
R: Tumors and inflammatory infiltrates: Friends or foes? Clin Exp
Metastasis. 19:247–258. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Locati M, Curtale G and Mantovani A:
Diversity, mechanisms, and significance of macrophage plasticity.
Annu Rev Pathol. 15:123–147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inagaki K, Kunisho S, Takigawa H, Yuge R,
Oka S, Tanaka S, Shimamoto F, Chayama K and Kitadai Y: Role of
tumor-associated macrophages at the invasive front in human
colorectal cancer progression. Cancer Sci. 112:2692–2704. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma J, Liu L, Che G, Yu N, Dai F and You Z:
The M1 form of tumor-associated macrophages in non-small cell lung
cancer is positively associated with survival time. BMC Cancer.
10:1122010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edin S, Wikberg ML, Dahlin AM, Rutegård J,
Öberg Å, Oldenborg PA and Palmqvist R: The distribution of
macrophages with a M1 or M2 phenotype in relation to prognosis and
the molecular characteristics of colorectal cancer. PLoS One.
7:e470452012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Yang K, Yang B, Wang R, Zhu Y and
Pan T: ANKRD22 participates in the proinflammatory activities of
macrophages in the colon cancer tumor microenvironment. Cancer
Immunol Immunother. 74:862025. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nussbaum YI, Manjunath Y, Kaifi JT, Warren
W and Mitchem J: Analysis of tumor-associated macrophages'
heterogeneity in colorectal cancer patients using single-cell
RNA-seq data. J Clin Oncol. 40 (4_suppl):S1462025. View Article : Google Scholar
|
|
31
|
Liang Y, Li J, Yuan Y, Ju H, Liao H, Li M,
Liu Y, Yao Y, Yang L, Li T and Lei X: Exosomal miR-106a-5p from
highly metastatic colorectal cancer cells drives liver metastasis
by inducing macrophage M2 polarization in the tumor
microenvironment. J Exp Clin Cancer Res. 43:2812024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Q, Yang C, Wang S, Shi D, Wei C, Song
J, Lin X, Dou R, Bai J, Xiang Z, et al: Wnt5a-induced M2
polarization of tumor-associated macrophages via IL-10 promotes
colorectal cancer progression. Cell Commun Signal. 18:512020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu
Y, Zhang Z, Cai S, Xu Y, Li X, et al: Tumor-derived exosomal
miR-934 induces macrophage M2 polarization to promote liver
metastasis of colorectal cancer. J Hematol Oncol. 13:1562020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun Z, Xu Y, Shao B, Dang P, Hu S, Sun H,
Chen C, Wang C, Liu J, Liu Y and Hu J: Exosomal circPOLQ promotes
macrophage M2 polarization via activating Il-10/STAT3 axis in a
colorectal cancer model. J Immunother Cancer. 12:e0084912024.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang C, Dou R, Wei C, Liu K, Shi D, Zhang
C, Liu Q, Wang S and Xiong B: Tumor-derived exosomal
microRNA-106b-5p activates EMT-cancer cell and M2-subtype tam
interaction to facilitate CRC metastasis. Mol Ther. 29:2088–2107.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu Y, Xiao Y, Ding Y, Ran R, Wei K, Tao S,
Mao H, Wang J, Pang S, Shi J, et al: Colorectal cancer cell-derived
exosomal miRNA-372-5p induces immune escape from colorectal cancer
via PTEN/AKT/NF-κB/PD-L1 pathway. Int Immunopharmacol. 143((Pt 1)):
1132612024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu X, Liang R, Lan T, Ding D, Huang S,
Shao J, Zheng Z, Chen T, Huang Y, Liu J, et al: Tumor-associated
macrophage-specific CD155 contributes to M2-phenotype transition,
immunosuppression, and tumor progression in colorectal cancer. J
Immunother Cancer. 10:e0042192022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang D, Wang X, Si M, Yang J, Sun S, Wu H,
Cui S, Qu X and Yu X: Exosome-encapsulated miRNAS contribute to
CXCL12/CXCR4-induced liver metastasis of colorectal cancer by
enhancing M2 polarization of macrophages. Cancer Lett. 474:36–52.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hardbower DM, Coburn LA, Asim M, Singh K,
Sierra JC, Barry DP, Gobert AP, Piazuelo MB, Washington MK and
Wilson KT: EGFR-mediated macrophage activation promotes
colitis-associated tumorigenesis. Oncogene. 36:3807–3819. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP,
Wu C and Zheng L: Activated monocytes in peritumoral stroma of
hepatocellular carcinoma foster immune privilege and disease
progression through PD-L1. J Exp Med. 206:1327–1337. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu X, Qian J, Ding L, Pan C, Liu X, Wu Q,
Wang S, Liu J, Shang M, Su R, et al: Galectin-1-induced tumor
associated macrophages repress antitumor immunity in hepatocellular
carcinoma through recruitment of tregs. Adv Sci (Weinh).
12:e24087882025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Palma M and Lewis CE: Macrophage
regulation of tumor responses to anticancer therapies. Cancer Cell.
23:277–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Klug F, Prakash H, Huber PE, Seibel T,
Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et
al: Low-dose irradiation programs macrophage differentiation to an
iNOS+/M1 phenotype that orchestrates effective T cell
immunotherapy. Cancer Cell. 24:589–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gordon SR, Maute RL, Dulken BW, Hutter G,
George BM, McCracken MN, Gupta R, Tsai JM, Sinha R, Corey D, et al:
PD-1 expression by tumour-associated macrophages inhibits
phagocytosis and tumour immunity. Nature. 545:495–499. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Byrne KT, Betts CB, Mick R, Sivagnanam S,
Bajor DL, Laheru DA, Chiorean EG, O'Hara MH, Liudahl SM, Newcomb C,
et al: Neoadjuvant selicrelumab, an agonist CD40 antibody, induces
changes in the tumor microenvironment in patients with resectable
pancreatic cancer. Clin Cancer Res. 27:4574–4586. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dowlati A, Harvey RD, Carvajal RD, Hamid
O, Klempner SJ, Kauh JSW, Peterson DA, Yu D, Chapman SC, Szpurka
AM, et al: LY3022855, an anti-colony stimulating factor-1 receptor
(CSF-1R) monoclonal antibody, in patients with advanced solid
tumors refractory to standard therapy: Phase 1 dose-escalation
trial. Invest New Drugs. 39:1057–1071. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Voissière A, Gomez-Roca C, Chabaud S,
Rodriguez C, Nkodia A, Berthet J, Montane L, Bidaux AS, Treilleux
I, Eberst L, et al: The CSF-1R inhibitor pexidartinib affects
FLT3-dependent DC differentiation and may antagonize durvalumab
effect in patients with advanced cancers. Sci Transl Med.
16:eadd18342024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Brana I, Calles A, LoRusso PM, Yee LK,
Puchalski TA, Seetharam S, Zhong B, de Boer CJ, Tabernero J and
Calvo E: Carlumab, an anti-C-C chemokine ligand 2 monoclonal
antibody, in combination with four chemotherapy regimens for the
treatment of patients with solid tumors: An open-label, multicenter
phase 1b study. Target Oncol. 10:111–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gomez-Roca CA, Italiano A, Le Tourneau C,
Cassier PA, Toulmonde M, D'Angelo SP, Campone M, Weber KL, Loirat
D, Cannarile MA, et al: Phase I study of emactuzumab single agent
or in combination with paclitaxel in patients with
advanced/metastatic solid tumors reveals depletion of
immunosuppressive M2-like macrophages. Ann Oncol. 30:1381–1392.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Falchook GS, Peeters M, Rottey S, Dirix
LY, Obermannova R, Cohen JE, Perets R, Frommer RS, Bauer TM, Wang
JS, et al: A phase 1a/1b trial of CSF-1R inhibitor ly3022855 in
combination with durvalumab or tremelimumab in patients with
advanced solid tumors. Invest New Drugs. 39:1284–1297. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nywening TM, Wang-Gillam A, Sanford DE,
Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA,
Yano M, et al: Targeting tumour-associated macrophages with CCR2
inhibition in combination with FOLFIRINOX in patients with
borderline resectable and locally advanced pancreatic cancer: A
single-centre, open-label, dose-finding, non-randomised, phase 1b
trial. Lancet Oncol. 17:651–662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cannarile MA, Weisser M, Jacob W, Jegg AM,
Ries CH and Rüttinger D: Colony-stimulating factor 1 receptor
(CSF1R) inhibitors in cancer therapy. J Immunother Cancer.
5:532017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qu T, Li B and Wang Y: Targeting
CD47/SIRPα as a therapeutic strategy, where we are and where we are
headed. Biomark Res. 10:202022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Eng C, Lakhani NJ, Philip PA, Schneider C,
Johnson B, Kardosh A, Chao MP, Patnaik A, Shihadeh F, Lee Y, et al:
A phase 1b/2 study of the anti-CD47 antibody magrolimab with
cetuximab in patients with colorectal cancer and other solid
tumors. Target Oncol. 20:519–530. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yamada-Hunter SA, Theruvath J, McIntosh
BJ, Freitas KA, Lin F, Radosevich MT, Leruste A, Dhingra S,
Martinez-Velez N, Xu P, et al: Engineered CD47 protects T cells for
enhanced antitumour immunity. Nature. 630:457–465. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Reiss KA, Angelos MG, Dees EC, Yuan Y,
Ueno NT, Pohlmann PR, Johnson ML, Chao J, Shestova O, Serody JS, et
al: Car-macrophage therapy for HER2-overexpressing advanced solid
tumors: A phase 1 trial. Nat Med. 31:1171–1182. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tan PB, Verschoor YL, van den Berg JG,
Balduzzi S, Kok NFM, Ijsselsteijn ME, Moore K, Jurdi A, Tin A,
Kaptein P, et al: Neoadjuvant immunotherapy in
mismatch-repair-proficient colon cancers. Nature. 648:726–735.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wang F, Chen G, Qiu M, Ma J, Mo X, Liu H,
Li Y, Ding P, Wan X, Hu Y, et al: Neoadjuvant treatment of IBI310
plus sintilimab in locally advanced MSI-H/DMMR colon cancer: A
randomized phase 1b study. Cancer Cell. 43:1958–1967.e2. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang X, Wang J, Wang G, Zhang Y, Fan Q,
Lu C, Hu C, Sun M, Wan Y, Sun S, et al: First-line sugemalimab plus
chemotherapy for advanced gastric cancer: The gemstone-303
randomized clinical trial. JAMA. 333:1305–1314. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ohtani Y, Ross K, Dandekar A, Gabbasov R
and Klichinsky M: 128 development of an M1-polarized, non-viral
chimeric antigen receptor macrophage (car-m) platform for cancer
immunotherapy. Journal for ImmunoTherapy of Cancer. 8 (Suppl
3):doi.org/10.1136/jitc-2020-SITC2020.0128. 2020.
|
|
63
|
Razak AR, Cleary JM, Moreno V, Boyer M,
Aller EC, Edenfield W, Tie J, Harvey RD, Rutten A, Shah MA, et al:
Safety and efficacy of AMG 820, an anti-colony-stimulating factor 1
receptor antibody, in combination with pembrolizumab in adults with
advanced solid tumors. J Immunother Cancer. 8:e0010062020.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Spierenburg G, van der Heijden L, van
Langevelde K, Szuhai K, Bovée JVGM, van de Sande MAJ and Gelderblom
H: Tenosynovial giant cell tumors (TGCT): Molecular biology, drug
targets and non-surgical pharmacological approaches. Expert Opin
Ther Targets. 26:333–345. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Golstein P and Griffiths GM: An early
history of T cell-mediated cytotoxicity. Nat Rev Immunol.
18:527–535. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pei S, Pollyea DA, Gustafson A,
Minhajuddin M, Stevens BM, Ye HY, Inguva A, Amaya ML, Krug A, Jones
CL, et al: Developmental plasticity of acute myeloid leukemia
mediates resistance to venetoclax-based therapy. Blood. 134
(Suppl_1):S1852019. View Article : Google Scholar
|
|
67
|
Li X, Gruosso T, Zuo D, Omeroglu A,
Meterissian S, Guiot MC, Salazar A, Park M and Levine H:
Infiltration of CD8(+) T cells into tumor cell clusters in
triple-negative breast cancer. Proc Natl Acad Sci USA.
116:3678–3687. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Gajewski TF, Schreiber H and Fu YX: Innate
and adaptive immune cells in the tumor microenvironment. Nat
Immunol. 14:1014–1022. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang L, Shi YC, Yang YX, Wang ZG, Wang SS
and Zhang H: Association of T lymphocyte subset counts with the
clinical features of colorectal cancer. J Nutr Oncol. 8:178–185.
2023. View Article : Google Scholar
|
|
70
|
Doering TA, Crawford A, Angelosanto JM,
Paley MA, Ziegler CG and Wherry EJ: Network analysis reveals
centrally connected genes and pathways involved in CD8+ T cell
exhaustion versus memory. Immunity. 37:1130–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wherry EJ and Kurachi M: Molecular and
cellular insights into T cell exhaustion. Nat Rev Immunol.
15:486–499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Angelosanto JM, Blackburn SD, Crawford A
and Wherry EJ: Progressive loss of memory T cell potential and
commitment to exhaustion during chronic viral infection. J Virol.
86:8161–8170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Im SJ, Hashimoto M, Gerner MY, Lee J,
Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al:
Defining CD8+ T cells that provide the proliferative burst after
PD-1 therapy. Nature. 537:417–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Alfei F and Zehn D: T cell exhaustion: An
epigenetically imprinted phenotypic and functional makeover. Trends
Mol Med. 23:769–771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Baitsch L, Baumgaertner P, Devêvre E,
Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke
B, Romero P, et al: Exhaustion of tumor-specific CD8+ T
cells in metastases from melanoma patients. J Clin Invest.
121:2350–2360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Odorizzi PM, Pauken KE, Paley MA, Sharpe A
and Wherry EJ: Genetic absence of PD-1 promotes accumulation of
terminally differentiated exhausted Cd8+ T cells. J Exp Med.
212:1125–1137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen Y, Wang D, Li Y, Qi L, Si W, Bo Y,
Chen X, Ye Z, Fan H, Liu B, et al: Spatiotemporal single-cell
analysis decodes cellular dynamics underlying different responses
to immunotherapy in colorectal cancer. Cancer Cell.
42:1268–1285.e7. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Liu Z, Zhang Y, Ma N, Yang Y, Ma Y, Wang
F, Wang Y, Wei J, Chen H, Tartarone A, et al: Progenitor-like
exhausted SPRY1(+)CD8(+) t cells potentiate responsiveness to
neoadjuvant PD-1 blockade in esophageal squamous cell carcinoma.
Cancer Cell. 41:1852–1870.e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Liu Z, Yang Z, Wu J, Zhang W, Sun Y, Zhang
C, Bai G, Yang L, Fan H, Chen Y, et al: A single-cell atlas reveals
immune heterogeneity in anti-PD-1-treated non-small cell lung
cancer. Cell. 188:3081–3096.e19. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kim YJ, Park SJ and Broxmeyer HE:
Phagocytosis, a potential mechanism for myeloid-derived suppressor
cell regulation of CD8+ T cell function mediated through programmed
cell death-1 and programmed cell death-1 ligand interaction. J
Immunol. 187:2291–2301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tanaka A and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Cell Res. 27:109–118. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ma S, Dahabieh MS, Mann TH, Zhao S,
McDonald B, Song WS, Chung HK, Farsakoglu Y, Garcia-Rivera L,
Hoffmann FA, et al: Nutrient-driven histone code determines
exhausted CD8(+) T cell fates. Science. 387:eadj30202025.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li J, Wu C, Hu H, Qin G, Wu X, Bai F,
Zhang J, Cai Y, Huang Y, Wang C, et al: Remodeling of the immune
and stromal cell compartment by pd-1 blockade in mismatch
repair-deficient colorectal cancer. Cancer Cell. 41:1152–1169.e7.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Borràs DM, Verbandt S, Ausserhofer M,
Sturm G, Lim J, Verge GA, Vanmeerbeek I, Laureano RS, Govaerts J,
Sprooten J, et al: Single cell dynamics of tumor specificity vs
bystander activity in CD8(+) T cells define the diverse immune
landscapes in colorectal cancer. Cell Discov. 9:1142023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Donia M, Andersen R, Kjeldsen JW, Fagone
P, Munir S, Nicoletti F, Andersen MH, Straten PT and Svane IM:
Aberrant expression of MHC class II in melanoma attracts
inflammatory tumor-specific CD4+ T- cells, which dampen CD8+ T-cell
antitumor reactivity. Cancer Res. 75:3747–3759. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Borst J, Ahrends T, Bąbała N, Melief CJM
and Kastenmüller W: CD4(+) T cell help in cancer immunology and
immunotherapy. Nat Rev Immunol. 18:635–647. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ahrends T, Spanjaard A, Pilzecker B,
Bąbała N, Bovens A, Xiao Y, Jacobs H and Borst J: CD4(+) T cell
help confers a cytotoxic T cell effector program including
coinhibitory receptor downregulation and increased tissue
invasiveness. Immunity. 47:848–861.e5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shamoun L, Skarstedt M, Andersson RE,
Wågsäter D and Dimberg J: Association study on IL-4, IL-4Rα and
Il-13 genetic polymorphisms in swedish patients with colorectal
cancer. Clin Chim Acta. 487:101–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li W, Chen F, Gao H, Xu Z, Zhou Y, Wang S,
Lv Z, Zhang Y, Xu Z, Huo J, et al: Cytokine concentration in
peripheral blood of patients with colorectal cancer. Front Immunol.
14:11755132023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor rorgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Huber S, Gagliani N, Zenewicz LA, Huber
FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W Jr, Murphy AJ, et
al: IL-22BP is regulated by the inflammasome and modulates
tumorigenesis in the intestine. Nature. 491:259–263. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kryczek I, Wei S, Szeliga W, Vatan L and
Zou W: Endogenous IL-17 contributes to reduced tumor growth and
metastasis. Blood. 114:357–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Bai Y, Li T, Wang Q, You W, Yang H, Xu X,
Li Z, Zhang Y, Yan C, Yang L, et al: Shaping immune landscape of
colorectal cancer by cholesterol metabolites. EMBO Mol Med.
16:334–360. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
De Simone V, Pallone F, Monteleone G and
Stolfi C: Role of TH17 cytokines in the control of
colorectal cancer. Oncoimmunology. 2:e266172013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sayour EJ, McLendon P, McLendon R, De Leon
G, Reynolds R, Kresak J, Sampson JH and Mitchell DA: Increased
proportion of FoxP3+ regulatory T cells in tumor infiltrating
lymphocytes is associated with tumor recurrence and reduced
survival in patients with glioblastoma. Cancer Immunol Immunother.
64:419–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Merlo A, Casalini P, Carcangiu ML,
Malventano C, Triulzi T, Mènard S, Tagliabue E and Balsari A: Foxp3
expression and overall survival in breast cancer. J Clin Oncol.
27:1746–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zi R, Zhao X, Liu L, Wang Y, Zhang R, Bian
Z, Jiang H, Liu T, Sun Y, Peng H, et al: Metabolic-immune
suppression mediated by the SIRT1-CX3CL1 axis induces functional
enhancement of regulatory T cells in colorectal carcinoma. Adv Sci
(Weinh). 12:e24047342025. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Gu J, Zhou J, Chen Q, Xu X, Gao J, Li X,
Shao Q, Zhou B, Zhou H, Wei S, et al: Tumor metabolite lactate
promotes tumorigenesis by modulating MOESIN lactylation and
enhancing TGF-beta signaling in regulatory T cells. Cell Rep.
39:1109862022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Adamczyk A, Pastille E, Kehrmann J, Vu VP,
Geffers R, Wasmer MH, Kasper S, Schuler M, Lange CM, Muggli B, et
al: GPR15 facilitates recruitment of regulatory T cells to promote
colorectal cancer. Cancer Res. 81:2970–2982. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Akkaya M, Kwak K and Pierce SK: B cell
memory: Building two walls of protection against pathogens. Nat Rev
Immunol. 20:229–238. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Choi IK, Wang Z, Ke Q, Hong M, Paul DW Jr,
Fernandes SM, Hu Z, Stevens J, Guleria I, Kim HJ, et al: Mechanism
of EBV inducing anti-tumour immunity and its therapeutic use.
Nature. 590:157–162. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Manfroi B and Fillatreau S: Regulatory B
cells gain muscles with a leucine-rich diet. Immunity. 55:970–972.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Murakami Y, Saito H, Shimizu S, Kono Y,
Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Ashida K, Sakabe
T, et al: Increased regulatory B cells are involved in immune
evasion in patients with gastric cancer. Sci Rep. 9:130832019.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Griss J, Bauer W, Wagner C, Simon M, Chen
M, Grabmeier-Pfistershammer K, Maurer-Granofszky M, Roka F, Penz T,
Bock C, et al: B cells sustain inflammation and predict response to
immune checkpoint blockade in human melanoma. Nat Commun.
10:41862019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Mao H, Pan F, Wu Z, Wang Z, Zhou Y, Zhang
P, Gou M and Dai G: Colorectal tumors are enriched with regulatory
plasmablasts with capacity in suppressing T cell inflammation. Int
Immunopharmacol. 49:95–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liao Y, Zhuo X, Huang Y, Xu H, Hao Z,
Huang L, Zheng H and Zhou J: A SENP7-SIRT1-IL-10 axis driven by
DeSUMOylation promotes breg differentiation and immune evasion in
colorectal cancer. Int J Biol Sci. 22:111–125. 2026. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hou D, Castro B, Dapash M, Rashidi A,
Zhang P, Han Y, Lopez-Rosas A, Lesniak M, Miska J and Chang C:
Immu-36. B cell-vaccine elicits long term immunity against
glioblastoma via activation and differentiation of tumor-specific
CD8+ memory T cells. Neuro Oncol. 23 (Suppl_6):vi100.
2021. View Article : Google Scholar
|
|
109
|
Irimia RM, Gerke MB, Thakar M, Ren Z,
Helmenstine E, Imus PH, Ghiaur G, Leone R and Gocke GB: CD38 is a
key regulator of tumor growth by modulating the metabolic signature
of malignant plasma cells. Blood. 138 (Suppl 1):S26522021.
View Article : Google Scholar
|
|
110
|
Malik H, Buelow B, Rangaswamy U,
Balasubramani A, Boudreau A, Dang K, Davison L, Aldred SF, Harris
KM, Iyer S, Jorgensen B, Pham D, Prabhakar K, Schellenberger U,
Ugamraj H, Trinklein N and Van Schooten W: Tnb-486, a novel fully
human bispecific CD19 × CD3 antibody that kills CD19-positive tumor
cells with minimal cytokine secretion. Blood. 134 (Suppl_1):S4070.
2019. View Article : Google Scholar
|
|
111
|
Wang W, Zhong Y, Zhuang Z, Xie J, Lu Y,
Huang C, Sun Y, Wu L, Yin J, Yu H, et al: Multiregion single-cell
sequencing reveals the transcriptional landscape of the immune
microenvironment of colorectal cancer. Clin Transl Med.
11:e2532021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Nicolai CJ and Raulet DH: Killer cells add
fire to fuel immunotherapy. Science. 368:943–944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Dixon K, Hullsiek R, Snyder K, Davis Z,
Khaw M, Lee T, Chu HY, Abujarour R, Dinella J, Rogers P, et al:
Engineered iPSC-derived NK cells expressing recombinant CD64 for
enhanced ADCC. Blood. 136 (Suppl 1):S10–S11. 2020. View Article : Google Scholar
|
|
114
|
Fauriat C, Long EO, Ljunggren HG and
Bryceson YT: Regulation of human NK-cell cytokine and chemokine
production by target cell recognition. Blood. 115:2167–2176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Liu YJ, Han M, Li JP, Zeng SH, Ye QW, Yin
ZH, Liu SL and Zou X: An analysis regarding the association between
connexins and colorectal cancer (CRC) tumor microenvironment. J
Inflamm Res. 15:2461–2476. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Yang L, Yi J, He W, Kong P, Xie Q, Jin Y,
Xiong Z and Xia L: Death receptors 4/5 mediate tumour sensitivity
to natural killer cell-mediated cytotoxicity in mismatch repair
deficient colorectal cancer. Br J Cancer. 131:334–346. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Otegbeye F, Ojo E, Moreton S, Mackowski N,
Lee DA, de Lima M and Wald DN: Inhibiting TGF-beta signaling
preserves the function of highly activated, in vitro expanded
natural killer cells in AML and colon cancer models. PLoS One.
13:e01913582018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Vuletić A, Martinović KM, Miletić NT,
Zoidakis J, Castellvi-Bel S and Čavić M: Cross-talk between tumor
cells undergoing epithelial to mesenchymal transition and natural
killer cells in tumor microenvironment in colorectal cancer. Front
Cell Dev Biol. 9:7500222021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Tang S, Fu H, Xu Q and Zhou Y: Mir-20a
regulates sensitivity of colorectal cancer cells to NK cells by
targeting mica. Biosci Rep. 39:BSR201806952019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lin A, Ye P, Li Z, Jiang A, Liu Z, Cheng
Q, Zhang J and Luo P: Natural killer cell immune checkpoints and
their therapeutic targeting in cancer treatment. Research (Wash
DC). 8:07232025.PubMed/NCBI
|
|
121
|
Lupo K and Matosevic S: 123 natural killer
cells engineered with an inducible, responsive genetic construct
targeting tigit and CD73 to relieve immunosuppression within the
gbm microenvironment. Journal for ImmunoTherapy of Cancer. 8 (Suppl
3):doi.org/10.1136/jitc-2020-SITC2020.0123. 2020.PubMed/NCBI
|
|
122
|
Strassheimer F, Strecker MI, Alekseeva T,
Macas J, Demes MC, Mildenberger IC, Tonn T, Wild PJ, Sevenich L,
Reiss Y, et al: Os12.6.A combination therapy of CAR-NK-cells and
anti-pd-1 results in high efficacy against advanced-stage
glioblastoma in a syngeneic mouse model and induces protective
anti-tumor immunity in vivo. Neuro Oncol. 23 (Suppl_2):ii152021.
View Article : Google Scholar
|
|
123
|
Kong R, Liu B, Wang H, Lu T and Zhou X:
CAR-NK cell therapy: Latest updates from the 2024 ASH annual
meeting. J Hematol Oncol. 18:222025. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Duan J, Zhao S, Duan Y, Sun D, Zhang G, Yu
D, Lou Y, Liu H, Yang S, Liang X, et al: Mnox nanoenzyme
armed CAR-NK cells enhance solid tumor immunotherapy by alleviating
the immunosuppressive microenvironment. Adv Healthc Mater.
13:e23039632024. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Wang W, Liu Y, He Z, Li L, Liu S, Jiang M,
Zhao B, Deng M, Wang W, Mi X, et al: Breakthrough of solid tumor
treatment: CAR-NK immunotherapy. Cell Death Discov. 10:402024.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Reizis B: Plasmacytoid dendritic cells:
Development, regulation, and function. Immunity. 50:37–50. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wculek SK, Cueto FJ, Mujal AM, Melero I,
Krummel MF and Sancho D: Dendritic cells in cancer immunology and
immunotherapy. Nat Rev Immunol. 20:7–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Jhunjhunwala S, Hammer C and Delamarre L:
Antigen presentation in cancer: Insights into tumour immunogenicity
and immune evasion. Nat Rev Cancer. 21:298–312. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Klempner SJ, Cecchini M, Khushman M,
Kummar S, Pelster M, Rodon J, Sharma S, Yaeger R, Rojo J, Wells AL,
et al: Abstract B031: A phase 1/2 trial of fog-001, a
first-in-class direct β-catenin:Tcf4 inhibitor, preliminary safety
and efficacy in patients with solid tumors bearing wnt
pathway-activating mutations (WPAM+). Mol Cancer Ther. 24
(10_Suppl):B0312025. View Article : Google Scholar
|
|
130
|
Suryawanshi A, Hussein MS, Prasad PD and
Manicassamy S: Wnt signaling cascade in dendritic cells and
regulation of anti-tumor immunity. Front Immunol. 11:1222020.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Dhodapkar MV, Dhodapkar KM and Palucka AK:
Interactions of tumor cells with dendritic cells: Balancing
immunity and tolerance. Cell Death Differ. 15:39–50. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Itai YS, Barboy O, Salomon R, Bercovich A,
Xie K, Winter E, Shami T, Porat Z, Erez N, Tanay A, et al:
Bispecific dendritic-T cell engager potentiates anti-tumor
immunity. Cell. 187:375–389.e18. 2024. View Article : Google Scholar
|
|
133
|
Aznar MA, Planelles L, Perez-Olivares M,
Molina C, Garasa S, Etxeberría I, Perez G, Rodriguez I, Bolaños E,
Lopez-Casas P, et al: Immunotherapeutic effects of intratumoral
nanoplexed poly I:C. J Immunother Cancer. 7:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
He F, Wu Z, Liu C, Zhu Y, Zhou Y, Tian E,
Rosin-Arbesfeld R, Yang D, Wang MW and Zhu D: Targeting BCL9/BCL9L
enhances antigen presentation by promoting conventional type 1
dendritic cell (cDC1) activation and tumor infiltration. Signal
Transduct Target Ther. 9:1392024. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Singer M, Valerin J, Zhang Z, Zhang Z,
Dayyani F, Yaghmai V, Choi A, Imagawa D and Abi-Jaoudeh N:
Promising cellular immunotherapy for colorectal cancer using
classical dendritic cells and natural killer T cells. Cells.
14:1662025. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Passardi A, Sullo FG, Bittoni A, Matteucci
L, De Rosa F, Bulgarelli J, Tazzari M, Petrini M, Scarpi E, Testoni
S, et al: CombiCoR-Vax trial: Study protocol for a phase II,
single-arm, multicenter trial of sequential pembrolizumab plus
dendritic cell vaccine followed by trifluridine/tipiracil and
bevacizumab in refractory microsatellite-stable metastatic
colorectal cancer. BMC Cancer. 25:19212025. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Guo FF and Cui JW: The role of neutrophils
in cancer development. Journal of Nutritional Oncology. 4:85–90.
2019.https://journals.lww.com/jno/Fulltext/2019/05150/The_Role_of_Neutrophils_in_Cancer_Development.5.aspx
View Article : Google Scholar
|
|
138
|
Peng H, Shen J, Long X, Zhou X, Zhang J,
Xu X, Huang T, Xu H, Sun S, Li C, et al: Local release of TGF-β
inhibitor modulates tumor-associated neutrophils and enhances
pancreatic cancer response to combined irreversible electroporation
and immunotherapy. Adv Sci (Weinh). 9:e21052402022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Giese MA, Hind LE and Huttenlocher A:
Neutrophil plasticity in the tumor microenvironment. Blood.
133:2159–2167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Yang L, Liu L, Zhang R, Hong J, Wang Y,
Wang J, Zuo J, Zhang J, Chen J and Hao H: IL-8 mediates a positive
loop connecting increased neutrophil extracellular traps (NETs) and
colorectal cancer liver metastasis. J Cancer. 11:4384–4396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yang L, Liu Q, Zhang X, Liu X, Zhou B,
Chen J, Huang D, Li J, Li H, Chen F, et al: DNA of neutrophil
extracellular traps promotes cancer metastasis via CCDC25. Nature.
583:133–138. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi
AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H and Tsung A:
Neutrophil extracellular traps promote the development and
progression of liver metastases after surgical stress. Cancer Res.
76:1367–1380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Wang X, He S, Gong X, Lei S, Zhang Q,
Xiong J and Liu Y: Neutrophils in colorectal cancer: Mechanisms,
prognostic value, and therapeutic implications. Front Immunol.
16:15386352025. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Yuan Y, Chen YF, Liu XM, Hu Y, Hao S and
Dai XY: Analysis of risk factors and prognostic prediction in
advanced colorectal cancer undergoing immunotherapy combined with
targeted therapy. Front Med (Lausanne). 12:16404692025. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhu T, Zou X, Yang C, Li L, Wang B, Li R,
Li H, Xu Z, Huang D and Wu Q: Neutrophil extracellular traps
promote gastric cancer metastasis by inducing
epithelial-mesenchymal transition. Int J Med. 48:1272021.
|
|
146
|
Cheng Y, Mo F, Li Q, Han X, Shi H, Chen S,
Wei Y and Wei X: Targeting CXCR2 inhibits the progression of lung
cancer and promotes therapeutic effect of cisplatin. Mol Cancer.
20:622021. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Chu X, Li X, Zhang Y, Dang G, Miao Y, Xu
W, Wang J, Zhang Z and Cheng S: Integrative single-cell analysis of
human colorectal cancer reveals patient stratification with
distinct immune evasion mechanisms. Nat Cancer. 5:1409–1426. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Oliveira MF, Romero JP, Chung M, Williams
SR, Gottscho AD, Gupta A, Pilipauskas SE, Mohabbat S, Raman N,
Sukovich DJ, et al: High-definition spatial transcriptomic
profiling of immune cell populations in colorectal cancer. Nat
Genet. 57:1512–1523. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhang L, Li Z, Skrzypczynska KM, Fang Q,
Zhang W, O'Brien SA, He Y, Wang L, Zhang Q, Kim A, et al:
Single-cell analyses inform mechanisms of myeloid-targeted
therapies in colon cancer. Cell. 181:442–459.e29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu
K, Hu X, Bu Z, Peng J, Ren X and Zhang Z: Immune phenotypic linkage
between colorectal cancer and liver metastasis. Cancer Cell.
40:424–437.e5. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D,
Cheng Y, Huang S, Liu Y, Jiang S, et al: Spatiotemporal immune
landscape of colorectal cancer liver metastasis at single-cell
level. Cancer Discov. 12:134–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhang L, Yu X, Zheng L, Zhang Y, Li Y,
Fang Q, Gao R, Kang B, Zhang Q, Huang JY, et al: Lineage tracking
reveals dynamic relationships of T cells in colorectal cancer.
Nature. 564:268–272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Feng Y, Ma W, Zang Y, Guo Y, Li Y, Zhang
Y, Dong X, Liu Y, Zhan X, Pan Z, et al: Spatially organized
tumor-stroma boundary determines the efficacy of immunotherapy in
colorectal cancer patients. Nat Commun. 15:102592024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Massagué J and Sheppard D: TGF-β signaling
in health and disease. Cell. 186:4007–4037. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Sikic BI, Lakhani N, Patnaik A, Shah SA,
Chandana SR, Rasco D, Colevas AD, O'Rourke T, Narayanan S,
Papadopoulos K, et al: First-in-human, first-in-class phase I trial
of the anti-CD47 antibody Hu5f9-G4 in patients with advanced
cancers. J Clin Oncol. 37:946–953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Pyo DH, Hong HK, Lee WY and Cho YB:
Patient-derived cancer modeling for precision medicine in
colorectal cancer: Beyond the cancer cell line. Cancer Biol Ther.
21:495–502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Balaban E and Cohen M: Decoding
multicellular interaction networks-a new horizon in tumor
microenvironment research. Mol Oncol. 19:957–960. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Gomez-Roca C, Cassier P, Zamarin D,
Machiels JP, Gracia JL, Hodi FS, Taus A, Garcia M, Boni V, Eder JP,
et al: Anti-CSF-1R emactuzumab in combination with anti-PD-L1
atezolizumab in advanced solid tumor patients naïve or experienced
for immune checkpoint blockade. J Immunother Cancer.
10:e0040762022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wagner SJ, Reisenbüchler D, West NP,
Niehues JM, Zhu J, Foersch S, Veldhuizen GP, Quirke P, Grabsch HI,
van den Brandt PA, et al: Transformer-based biomarker prediction
from colorectal cancer histology: A large-scale multicentric study.
Cancer Cell. 41:1650–1661.e4. 2023. View Article : Google Scholar : PubMed/NCBI
|