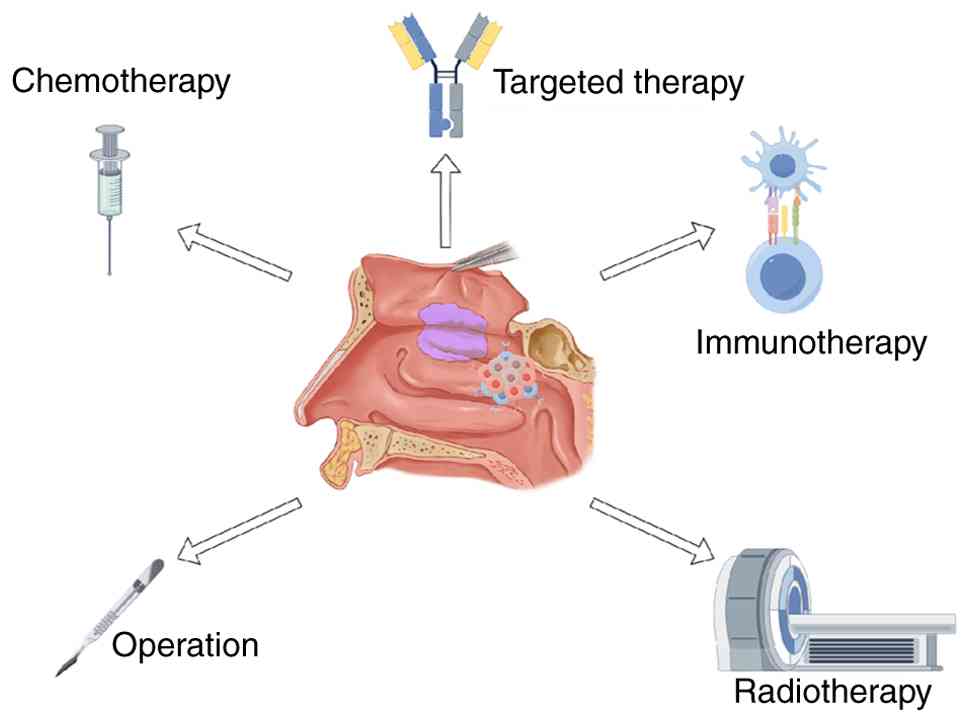
Introduction
Epidemiology and unmet clinical
needs
Nasopharyngeal carcinoma (NPC) is a malignant
epithelial tumor of the nasopharynx with an uneven global
distribution. East and Southeast Asia account for ~80% of cases,
with the highest incidence concentrated in southern China,
particularly Guangdong, Guangxi and Fujian (1), making NPC both regionally clustered
and globally consequential. The disease primarily affects
individuals aged 40–70 years and shows a pronounced sex disparity,
with men affected 2- to 3-fold more often than women (1). Despite advances in cross-sectional
imaging and conformal radiotherapy, early detection remains elusive
and long-term quality of life is still shaped by late toxicities,
such as xerostomia, dysphagia and otologic injury.
Clinical presentation in the early stages is
frequently silent or non-specific and includes blood-tinged sputum,
unilateral nasal obstruction or painless cervical lymphadenopathy,
so that 70–80% of patients are diagnosed at advanced stages
(III/IV) (2) This late-stage
predominance at diagnosis largely explains the pronounced survival
gradient across stages and highlights the clinical value of
shifting detection toward earlier disease: The 5-year survival rate
is >90% when NPC is detected early, but falls to >40% in
late-stage disease. Distant metastasis and local recurrence remain
the principal modes of treatment failure (3). Collectively, these realities define
three persistent unmet needs: i) Scalable, non-invasive screening
deployable at the population level; ii) risk-stratified care
pathways that minimise overtreatment without compromising oncologic
safety; and iii) implementation models that preserve quality and
outcomes in resource-limited settings (4). Addressing these gaps will require
earlier, more reliable detection and exact therapies capable of
improving durable survival while mitigating treatment-related
morbidity.
Current diagnostic bottlenecks and
emerging precision solutions
Legacy serology and conventional imaging lack the
resolution to capture preclinical or very-early NPC across diverse
populations (5). Epstein-Barr virus
(EBV)-centric assays have advanced non-invasive detection but
remain vulnerable to EBV-negative disease and cross-platform
variability; T-cell receptor (TCR) repertoire profiling and
radiomics/radiogenomics add discrimination yet require standardized
pipelines and prospective external validation (6). In practice, the present review
advocates a molecular-imaging co-screening approach that pairs
liquid-biopsy signals with advanced endoscopy to address historical
blind spots in very-early disease (4).
The emergence of molecular and
surgical precision paradigms
Converging advances now support a dual-front
precision strategy for NPC. On the molecular/imaging side,
CRISPR-enabled assays, antibody-augmented platforms and integrated
multi-omics/radiomics enable non-invasive, risk-stratified
co-screening suitable for population deployment (2). On the interventional side, endoscopic
nasopharyngectomy (ENPG) has re-emerged as an organ-preserving
option restricted to carefully selected T1-T2 disease in
experienced centers; it does not constitute a universal substitute
for radiotherapy. Bringing these strands together, a pragmatic
tri-axis framework was adopted: Molecular detection, precision
surgery (indication-bounded ENPG) and immune modulation,
coordinated through analytics spanning screening, diagnosis/triage,
treatment and surveillance (long-term endpoints and PROs have been
synthesised in Table SI) (4).
Looking ahead, the field will hinge on harmonising
precision detection with targeted intervention to shift care
upstream. Artificial intelligence-assisted models that fuse
multi-omics [including circulating tumor DNA (ctDNA)],
immune-repertoire signatures and radiomic biomarkers can refine
dynamic risk stratification and flag pre-clinical disease. In
parallel, rational sequencing, for example, protocol-guided
programmed cell death protein 1 (PD-1)/programmed death ligand 1
(PD-L1) strategies alongside indication-bounded ENPG, may sustain
oncologic control while preserving function in selected patients.
Progress will depend on multi-center prospective programs with
harmonized endpoints [5/10-year OS; late ≥G3 toxicities and PROs
such as M.D. Anderson dysphagia inventory (MDADI)/quality of life
questionnaire-head and neck module (QLQ-H&N35)], and on
equitable implementation via portable diagnostics and tele-enabled
expertise in endemic regions where the burden is greatest.
Early screening for NPC: Current status and
recent advances
EBV and its associated biomarkers
The EBV, a central oncogenic driver of NPC,
establishes its pathogenicity through persistent latent infection
and immune evasion (1,2), making EBV-specific biomarkers
important for early detection. Current serological screening
strategies targeting EBV antigens, such as viral capsid antigen IgA
(VCA-IgA) and early antigen IgA, exhibit limited diagnostic
utility, with single-antibody assays achieving only a sensitivity
of ~25% (6). Dual-antibody
approaches (such as VCA-IgA with EBNA1-IgA) have been shown to
improve sensitivity to ~75%, yet their clinical translation is
hampered by persistently low positive predictive values (<20%)
and high false-positive rates, underscoring the need for
complementary biomarkers (7,8).
Legacy serology and conventional imaging lack the
granularity to consistently detect preclinical or very-early NPC
across heterogeneous populations. EBV-centric assays, including
plasma EBV DNA and methylome (cfDNA), exhibit advanced non-invasive
detection but remain vulnerable to EBV-negative disease,
pre-analytical variability and cross-platform threshold drift
(9–11). TCR repertoire profiling and
radiomics/radiogenomics add discriminatory power, yet require
standardised pipelines and rigorous prospective external validation
(7,12). The net effect is a patchwork
evidence base marked by heterogeneous cut-offs and inconsistent
reporting windows, which impedes guideline adoption and real-world
scale-up (13,14).
Recent advances in NPC screening have revolutionized
EBV biomarker detection through multi-dimensional technological
innovations. Next-generation sequencing (NGS)-enabled composite
models now decode both quantitative and fragmentomic profiles of
plasma EBV DNA via real-time PCR, achieving unprecedented precision
(15). A landmark study by Lam
et al (15) demonstrated
that algorithmic optimization of NGS data elevates screening
specificity from 98.6 to 99.3% and boosts positive predictive value
by 78% (from 11.0 to 19.6%), markedly enhancing early-stage
detection. Parallel breakthroughs in nucleic acid testing include
the CRISPR-Cas12a-based non-amplification digital detection system
developed at Sun Yat-sen University Cancer Center, Guangzhou, China
(15,16). By targeting EBV genomic repetitive
sequences, this platform outperforms conventional quantitative PCR
(qPCR), achieving superior sensitivity (98.5 vs. 95.2%) and
specificity (99.1 vs. 97.8%), while reducing assay time by 60% and
enabling real-time monitoring of tumor load dynamics. These
innovations not only address historical limitations in EBV-driven
screening but are also aligned with global efforts to reduce
mortality due to NPC in endemic regions, where >80% of the cases
occur (17).
Serological testing for NPC has undergone
transformative innovation, addressing long-standing gaps in early
detection. The VCA-IgA/EBNA1-IgA dual-antibody ELISA platform
increases screening sensitivity threefold (from 25 to 75%) while
maintaining 98.5% specificity, earning endorsement as the National
Health Commission's preferred protocol for high-incidence regions
(15). Complementing this, the P85
antibody (P85-Ab) assay, co-developed by Xiamen University (Xiamen,
China) and Wantai Biotech marks a paradigm shift in serological
screening. As a standalone test, it achieves 97.9% sensitivity and
98.3% specificity, surpassing conventional biomarkers. When
integrated with dual-antibody screening (18), P85-Ab elevated the positive
predictive value from 10 to 44.6%, quadrupling diagnostic
efficiency while reducing per-test costs by 30% in pilot
implementations (19). Approved for
clinical use in late 2024, this combinatorial strategy redefines
cost-effective mass screening, particularly in endemic areas where
NPC accounts for >80% of global cases (6). By harmonizing high-throughput serology
with precision biomarkers, these advances align with World Health
Organization targets to reduce NPC-caused mortality through early
interception, exemplifying how translational innovation can bridge
diagnostic accuracy and scalability in resource-variable settings
(20).
The current landscape of EBV-related biomarker
detection is characterized by three key developments. First, NGS
and CRISPR-based technologies are enhancing diagnostic precision
through multi-indicator nucleic acid testing strategies. Secondly,
the identification of novel biomarkers, such as P85-Ab, has opened
new avenues for early screening. In combination, these advances
provide key technical support for establishing a tiered prevention
and control framework encompassing initial screening, refined
screening and definitive diagnosis. Of note, the advent of high
positive predictive value assays contributes to reducing the risk
of overdiagnosis in clinical settings. Despite these promising
developments, challenges remain. The high costs associated with NGS
and CRISPR limit their accessibility at the primary care level,
while the long-term stability and clinical utility of emerging
biomarkers such as P85-Ab require validation in large-scale
cohorts. Future research directions include the development of
portable, low-cost diagnostic platforms, the integration of
multi-omics data to construct dynamic early warning systems and the
application of artificial intelligence to optimize screening
algorithms. These innovations aim to enable standardized, accurate
and widely accessible early detection of NPC.
Plasma free DNA cfDNA and ctDNA
Recent advances in circulating biomarkers and
protein-based assays have transformed the non-invasive diagnosis
and prognostic stratification of NPC. cfDNA, comprising short DNA
fragments shed into the bloodstream, includes a tumor-derived
fraction, ctDNA, that harbors somatic alterations such as TP53 and
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(PIK3CA) mutations (21,22). Leveraging ultra-sensitive platforms
such as digital PCR and NGS, trace levels of ctDNA can now be
reliably detected, enabling earlier diagnosis and real-time
monitoring of tumor burden in patients with NPC. Complementing
these molecular assays, the immunohistochemical analysis of the p53
tumor suppressor protein provides key prognostic insights:
Loss-of-function TP53 mutations often result in aberrant p53
accumulation and elevated p53 expression within NPC tissues has
been consistently associated with worse overall and disease-free
survival (23). Together, ctDNA
profiling and p53 immunostaining offer a powerful, minimally
invasive toolkit for guiding individualized treatment decisions,
facilitating both the early detection of NPC and the tailoring of
therapeutic intensity based on tumor biology (Fig. 1).
Biomarker detection
Progress in microRNA (miRNAs) research
of related biomarkers
miRNAs, a class of ~22-nucleotide non-coding RNAs,
modulate gene expression post-transcriptionally by binding to the
3′ untranslated regions of target messenger RNAs, thereby playing
key roles in oncogenesis. In NPC, a distinct and consistent pattern
of miRNA dysregulation has been widely observed, underscoring their
potential as diagnostic and prognostic biomarkers (24–26).
This aberrant miRNA expression landscape not only reflects
underlying tumor biology but also offers a promising avenue for
non-invasive molecular diagnostics. However, the clinical
translation of miRNA-based biomarkers in NPC remains contingent on
the development of robust, standardized detection platforms and
optimized analytical strategies that ensure sensitivity,
specificity and reproducibility across diverse patient
populations.
Early studies on miRNAs focused on identifying
miRNAs specific to NPC. Zhang et al (27) found that miR-93 was markedly
upregulated in NPC tissues and Zhou et al (28) further confirmed its serum levels
were positively associated with tumour stage. However, the
sensitivity and specificity of miR-93 alone for diagnosis are
insufficient, suggesting the limited clinical value of a single
miRNA. Zhou et al (28),
Duan et al (29), and Zhang
et al (30) demonstrated
that the combined detection of miR-17-5p and miR-20a can increase
the sensitivity of early NPC to 80% and the specificity to 87%,
notably outperforming traditional serum antibodies (such as
VCA-IgA). Of note, the specificity of miR-17-5p in distinguishing
patients with early-stage NPC from healthy individuals is
relatively low (73%), indicating the need to combine other markers
to improve accuracy.
The inherent limitations of single-miRNA diagnostic
approaches have catalyzed the development of multi-analyte
biomarker integration strategies, with recent advancements
demonstrating marked improvements in clinical detection accuracy.
Of note, Zhu et al (31)
pioneered a serum triplex miRNA panel (miR-140-3p, miR-192-5p and
miR-223-3p) achieving 93.2% sensitivity and 93.5% specificity
through the synergistic regulation of Wnt/β-catenin signaling and
epithelial-mesenchymal transition (EMT) pathways. This paradigm
shift toward biomarker combination was further validated by Jiang
et al (32), who reported
that incorporating circulating EBV microRNA BART2-5p (miR-BART2-5p)
into screening/triage markedly improved diagnostic performance (AUC
~0.96–0.97 in validation cohorts)., outperforming conventional
plasma EBV DNA assays (50% detection rate), while maintaining 94.2%
sensitivity. Considerably advancing the field, Li et al
(24,25) and Allaya et al (22) established a multi-omics diagnostic
framework combining miR-10b with EBV Latent Membrane Protein 1
(LMP1). EBV-related transcripts (for example, LMP1) and host
regulators such as TWIST1 have been associated with NPC
pathobiology, supporting their potential as components of
multi-marker panels (24). These
collective findings underscore the potential of cross-modal
biomarker integration in overcoming biological heterogeneity and
pathway redundancy limitations inherent to single-marker
approaches, establishing a new standard for precision diagnostics
in complex disease states.
Although miRNA biomarkers hold considerable promise
for the clinical management of NPC, their translation into routine
practice is hindered by three principal challenges. First, tumor
heterogeneity poses a notable obstacle: Molecular subtypes of NPC,
such as variations in EBV latency patterns and the degree of
stromal infiltration, result in marked differences in miRNA
expression profiles. For instance, miR-93 is consistently
upregulated in EBV type III latency tumors, yet exhibits variable
expression in type I, limiting its utility as a broadly applicable
biomarker (33). Secondly, the lack
of technical standardization undermines reproducibility and
comparability across studies. While qPCR and microarray platforms
are widely used for miRNA detection, there remains no consensus on
key methodological parameters, including sample preprocessing
protocols (such as exosome isolation) and reference gene selection
(such as U6 small nuclear RNA vs. cel-miR-39). Finally, the
clinical utility of miRNAs has been predominantly explored in the
context of diagnosis, whereas their potential roles in prognosis
and therapy remain underdeveloped. Of note, miR-205 has been
implicated in radiotherapy resistance, highlighting the need to
further investigate miRNAs as prognostic indicators and therapeutic
targets (34). Addressing these
challenges will be essential for harnessing the full clinical
potential of miRNA-based tools in NPC.
Genetic markers
Recent large-scale genomic sequencing efforts have
substantially advanced understanding of the molecular landscape of
NPC, revealing recurrent alterations in tumor suppressor genes and
oncogenic drivers with marked clinical implications. Among these,
TP53 mutations, one of the most common genetic events across
different types of human cancer, display distinct patterns in NPC,
being predominantly enriched in EBV-negative subtypes and recurrent
tumors. These mutations, which include missense variants, point
mutations and splice site alterations, result in the functional
inactivation of the p53 protein, thereby impairing key pathways
involved in DNA damage response, cell cycle regulation and
apoptosis. The consequence is enhanced tumor resistance to
radiotherapy and chemotherapy, often associated with poor clinical
outcomes (35–38).
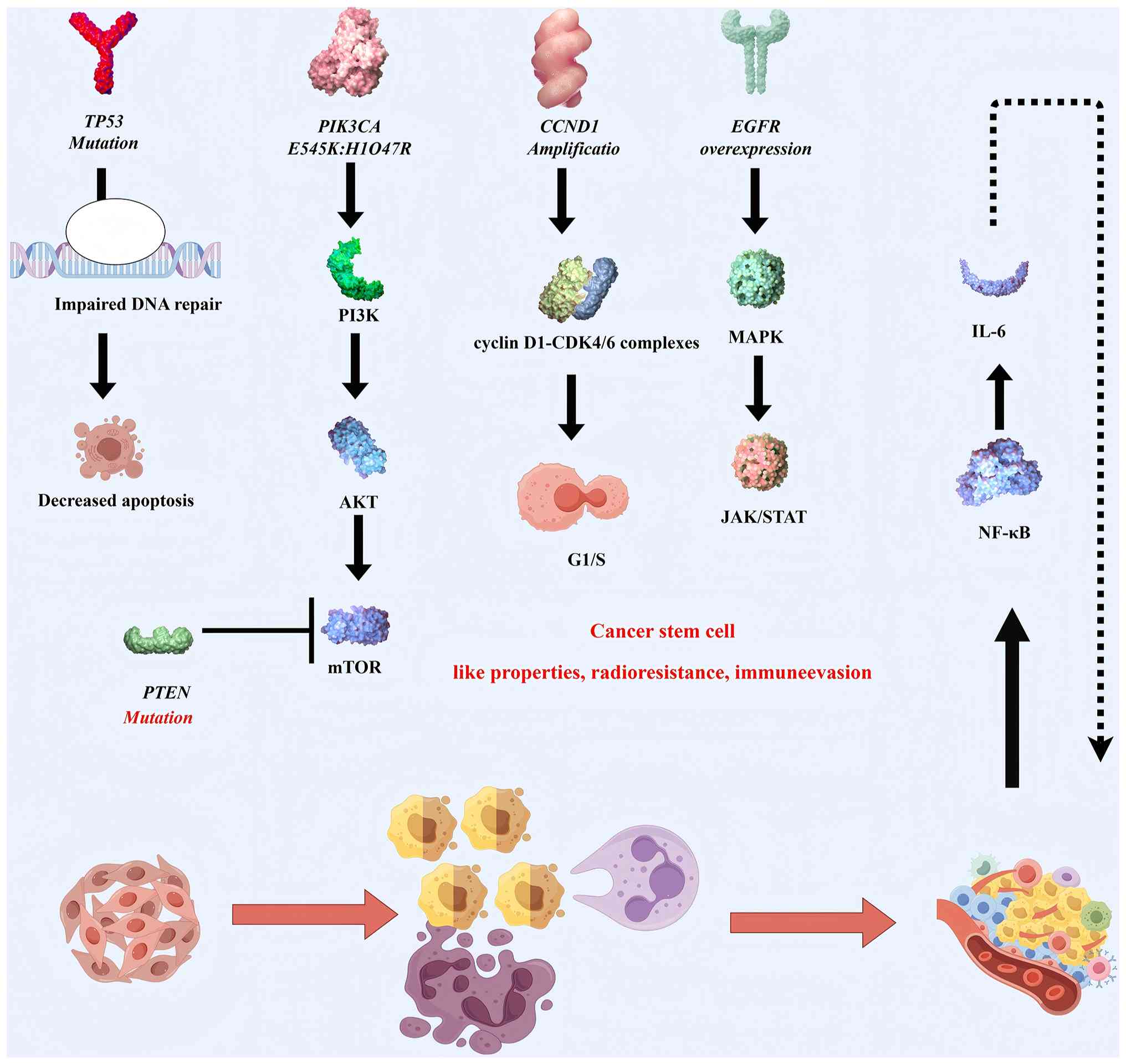
In parallel, the dysregulation of the PI3K/AKT
signaling pathway carries out a complementary oncogenic role.
Activating mutations in PIK3CA, notably E545K and H1047R, directly
enhance AKT phosphorylation, promoting cell proliferation and
survival (39). Meanwhile, the
tumor-suppressive function of PTEN, a key negative regulator of
this pathway, is frequently compromised by inactivating mutations
or copy number loss, resulting in a sustained activation of the
PI3K/AKT/mechanistic target of rapamycin axis. These molecular
aberrations are further amplified by the dysregulation of EGFR
signaling (39,40). In EBV-positive NPCs, EGFR
amplification or overexpression concurrently activates multiple
downstream pathways, including MAPK, PI3K/AKT, and Janus
kinase/signal transducer and activator of transcription signaling
pathway, which collectively contribute to radioresistance and the
acquisition of cancer stem cell-like properties. This mechanistic
convergence provides a compelling rationale for the clinical
evaluation of EGFR-targeted therapies, such as erlotinib, in
selected subsets of patients with NPC (41–43).
Given the clinical relevance of EGFR, the use of targeted
therapies, including anti-EGFR monoclonal antibodies such as
cetuximab (44,45), are increasing. Detecting EGFR
expression levels allows for the selection of more appropriate
treatment strategies and facilitates the prediction of patient
response and overall prognosis.
The dysregulation of cell cycle checkpoints in NPC
exemplifies a paradigm shift in understanding tumorigenic
mechanisms. The amplification of cyclin D1 (CCND1) drives the
aberrant G1/S transition through the enhanced assembly
of cyclin D1-Cyclin-dependent kinase 4 and 6 (CDK4/6) complexes,
while the concomitant epigenetic silencing of cyclin-dependent
kinase inhibitor 2A (CDKN2A) via promoter hypermethylation induces
the functional inactivation of cyclin-dependent kinase inhibitor
2A, isoform p16, thereby relieving its restraint on CDK4/6 kinase
activity. This dual disruption of cell cycle surveillance
constitutes a hallmark of NPC pathogenesis, providing a robust
biological rationale for the clinical evaluation of CDK4/6
inhibitors that have shown a therapeutic promise in other head and
neck malignancies (46–49). Paralleling these proliferation
anomalies, the tumor microenvironment (TME) exhibits constitutive
NF-κB activation, a pathognomonic feature mediated by convergent
genetic lesions including nuclear factor of κ light polypeptide
gene enhancer in B-cells inhibitor, α truncating mutations
impairing IκBα function and heterozygous deletions affecting TNF
receptor-associated factor 3/cylindromatosis-mediated negative
regulation. These molecular defects sustain NF-κB transcriptional
activity, resulting in the paracrine secretion of immunosuppressive
cytokines (such as IL-6 and TNF-α) that create a pro-tumorigenic
niche while conferring radioresistance through EMT programming
(50–52).
Of note, the immunogenetic predisposition of NPC
reveals an evolutionary arms race between viral persistence and
host immunity: Genome-wide association studies implicate human
leukocyte antigen A/B (HLA-A/B) class I allele variants in
defective EBV antigen presentation, compromising cytotoxic T-cell
surveillance and enabling viral oncogene-mediated transformation
(53–55). The synergistic interplay between
these cell-intrinsic (CCND1/CDKN2A axis) and cell-extrinsic
(NF-κB-inflammatory loop) drivers, superimposed upon
(HLA)-restricted immune evasion mechanisms, defines the landscape
of the molecular heterogeneity of NPC. Such integrative molecular
cartography not only elucidates the biological basis of the
distinct clinical behavior of NPC but also informs precision
therapeutics, exemplified by the combinatorial targeting of
PI3K/AKT and EGFR axes that converge on dysregulated growth factor
signaling. This multi-layered mechanistic dissection positions NPC
as a paradigm for viro-immunogenetic tumor models requiring
context-specific therapeutic interventions (Fig. 2).
Epigenetic markers
The epigenetic and transcriptional dysregulation of
key genetic elements has emerged as a key mechanism in the
pathogenesis of NPC. One notable example is Septin 9 (SEPT9_v2), a
tumor suppressor gene whose promoter hypermethylation leads to
transcriptional silencing and is closely associated with NPC
progression. Functional studies have demonstrated that SEPT9_v2
suppresses tumor cell proliferation, migration and invasion by
inhibiting the Wnt/β-catenin signaling cascade through the
miR-92b-3p/frizzled class receptor 10 axis (55,56).
Conversely, the kinesin family member kinesin family member 23
(KIF23), an established oncogene, is notably overexpressed in NPC
tissues and promotes tumor aggressiveness through the activation of
the same Wnt/β-catenin pathway. Of note, KIF23 expression is
transcriptionally regulated by the androgen receptor, providing a
mechanistic association with the observed sex-specific differences
in NPC incidence and outcomes. Collectively, these findings
underscore the dual role of the Wnt/β-catenin pathway as both a
downstream effector and an integrative hub for epigenetic and
hormonal regulation in NPC, positioning SEPT9_v2 and KIF23 as
promising biomarkers for prognostic evaluation and as candidate
targets for therapeutic intervention.
Emerging evidence highlights the key role of
non-coding RNA signatures and epigenetic alterations in shaping the
molecular landscape and clinical trajectory of NPC. Specific miRNA
expression profiles, particularly hsa-miR-142-3p, hsa-miR-29c and
hsa-miR-30e, have shown robust associations with overall survival
and when integrated with clinical parameters, substantially improve
the accuracy of prognostic models. In addition to their
intracellular regulatory functions, miRNAs packaged into exosomes,
such as miR-34a-5p, exert paracrine effects that modulate the TME
and intercellular signaling networks (57,58).
Epigenetic dysregulation, especially DNA
methylation, is another hallmark of NPC. The promoter
hypermethylation of protocadherin 17 (PCDH17) leads to its complete
silencing across NPC cell lines and the functional restoration of
PCDH17 inhibits angiogenesis and suppresses vascular endothelial
growth factor secretion (59). In
addition, differentially methylated regions in genes such as
calcitonin-related polypeptide α, ALX homeobox 4 and homeobox D9
are detectable in cfDNA, laying the foundation for non-invasive
liquid biopsy-based diagnostics (60,61).
Of particular note, the methylation status of SEPT9_v2 and
protocadherin 17 not only holds diagnostic potential but may also
serve as predictive biomarkers for treatment responsiveness,
underscoring the multifaceted utility of epigenetic markers in
early detection, prognosis stratification and therapy
optimization.
Metabolomics markers
Metabolic reprogramming represents a hallmark of
NPC, contributing to tumor progression, invasion and metastasis.
One notable feature of NPC is the abnormal accumulation of
metabolites, such as lactic acid and glutamine, within the TME,
reflecting a shift in energy metabolism and biosynthetic demands
(62). By integrating single-cell
Raman spectroscopy with mass spectrometry, recent studies (63–65)
have revealed markedly elevated levels of unsaturated fatty acids
in highly metastatic NPC cell lines (such as 5-8F and CNE2),
implicating dysregulated lipid metabolism as a driver of metastatic
potential. Complementary NMR-based metabolomics analyses have
identified specific lipoprotein subfractions, very low-density
lipoprotein and low-density lipoprotein, as closely associated with
NPC onset. A metabolite-derived risk score incorporating these
lipoproteins demonstrated strong discriminative power in
multicentre cohorts (AUC=0.841), supporting their potential as
early diagnostic and stratification biomarkers (66).
Furthermore, disturbances in amino acid metabolism,
particularly elevated levels of 4-aminobutyric acid, have been
shown to facilitate tumor invasion through the activation of the
ubiquitin-proteasome pathway, specifically through
ubiquitin-conjugating enzyme E2C (67). These findings not only enhance
understanding of the metabolic underpinnings of NPC but also
provide a framework for the development of targeted metabolic
interventions. The integration of multi-omics platforms with
artificial intelligence-aided analysis is expected to further
refine metabolic phenotyping and identify actionable
vulnerabilities in NPC (Fig.
3).
Application and cutting-edge progress of
immunotherapy in NPC
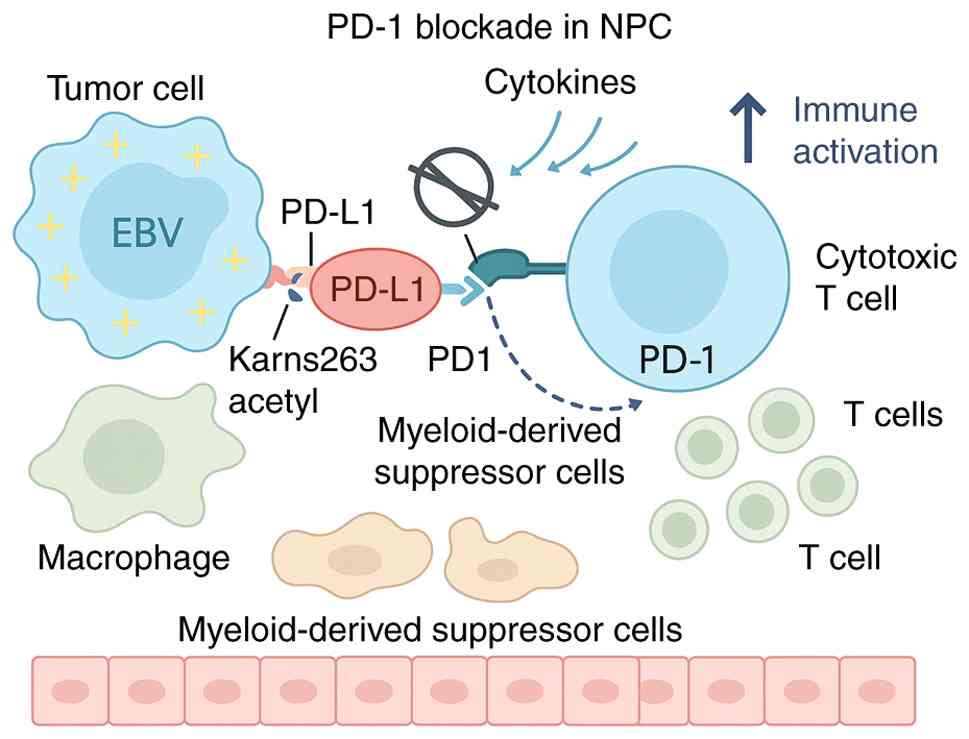
In NPC, an EBV-associated malignancy with pronounced
immune infiltration and viral antigenicity, immune checkpoint
blockade has emerged as a transformative modality. The PD-1/PD-L1
axis plays a central role in immune evasion by impairing cytotoxic
T-cell activation within the TME. High PD-L1 expression, driven in
part by EBV-related inflammation and epigenetic regulation (such as
acetylation-mediated nuclear translocation), suppresses anti-tumor
immunity, enabling tumor persistence (68–70).
PD-1/PD-L1 inhibitors restore T-cell function and have demonstrated
meaningful clinical efficacy in recurrent/metastatic NPC (R/M
NPC).
Multiple early-phase trials have established the
monotherapeutic activity of PD-1 blockade in pretreated R/M NPC.
The KEYNOTE-028 study reported an objective response rate (ORR) of
25.9% and median overall survival (OS) of 16.5 months with
pembrolizumab (71), while the
NCI-9742 trial showed similar outcomes for nivolumab (ORR, 20.5%;
OS 17.1, months) (72).
Camrelizumab also achieved an ORR of 28.2% in the CAPTAIN trial
(73). However, despite encouraging
activity, monotherapy responses were limited by modest durability
and heterogeneous patient benefit, prompting combination
strategies.
The paradigm shifted with the emergence of
immune-chemotherapy combinations. In the landmark JUPITER-02 trial,
toripalimab plus gemcitabine-cisplatin prolonged progression-free
survival (PFS) to 11.7 vs. 8.0 months with chemotherapy alone,
reducing the risk of mortality by 40% (73). Similar benefits were observed in
NCT03707509 with camrelizumab, further confirming the synergy
between chemotherapy-induced immunogenic cell death and PD-1
blockade (74). Of note, PD-L1
positivity and EBV DNA clearance were predictive of superior
responses (75), underscoring the
importance of biomarker-driven treatment selection.
Yet, immune resistance remains a key challenge.
Mechanisms include loss of PD-L1 expression, T-cell exhaustion,
immune desertification and neoantigen depletion (75–78).
Tumor-intrinsic alterations, such as NF-κB pathway activation and
cytokine-driven immunosuppression, further impair durable response
(79). To overcome these barriers,
neoadjuvant immunotherapy (such as PD-1 inhibitors preceding ENPG),
immune-antiangiogenic combinations and AI-assisted immune landscape
modeling are being investigated.
In conclusion, PD-1/PD-L1 inhibitors have redefined
the therapeutic landscape of NPC, particularly in the metastatic
setting and are increasingly being integrated into frontline
regimens. Future directions focus on optimizing combinatorial
immunotherapy, identifying predictive biomarkers and tailoring
interventions based on EBV load, TME and host immune features.
These efforts aim to achieve durable functional remission in a
historically therapy-resistant malignancy (Fig. 4).
Examination and treatment of NPC
In the early screening system for NPC, endoscopic
technology has progressed from traditional white light observation
to advanced image enhancement stages. Optical enhancement
platforms, represented by narrow band imaging (NBI) and I-scan
virtual chromoendoscopy, have markedly transformed the mucosal
lesion identification system (80–82).
NBI enhances microvascular patterns using narrow-band blue-green
light, excelling in detecting microinvasive lesions with diameters
>5 mm, achieving a sensitivity of 92.3% and specificity of
93.1%, far surpassing white light endoscopy. I-scan, through
digital image post-processing, enhances vascular morphology and
glandular structure details, identifying potential vascular
abnormalities in white-light negative regions with a detection rate
of 23%, thus making it a powerful secondary screening tool for
high-risk populations. However, challenges in clinical application
remain, such as high false-positive rates, operator dependence and
high equipment costs (83). Future
breakthroughs will likely focus on building a multimodal fusion
intelligent screening system, integrating signals such as EBV DNA
load, TCR subtype typing and AI image recognition, to establish a
new ‘visual + molecular diagnosis’ paradigm for grassroots
applications.
Radiotherapy has long been regarded as the
first-line treatment for early-stage NPC. However, the long-term
survival benefit of re-irradiation is limited (5-year OS <45%),
with a high incidence of complications (such as NP necrosis and
temporal lobe damage), leading to the return of surgery as the core
treatment approach. Traditional open surgical methods, such as
those involving the hard palate or maxillary bone, achieve high
local control rates (10-year OS ≤73.8%) (84–87).
However, their high margin positivity rates (≤29%), postoperative
functional impairment (such as swallowing and facial issues) and
aesthetic damage limit their applicability. The rise of endoscopic
technology (such as ENPG) marks a key turning point in NPC surgical
treatment paradigms. By offering high-definition magnification
views, ENPG allows for precise exposure of the skull base
structures while preserving key anatomy (such as the eustachian
tube and internal carotid artery), achieving a postoperative 5-year
OS improvement to 48–52%, while markedly reducing the incidence of
≥3-grade toxicity events (88,89).
More importantly, the anatomical classification system developed
based on the endoscopic platform, such as the four-segment internal
carotid artery classification for the parapharyngeal segment and
four-level recurring NPC surgical classification, provides key
support for enhancing surgical safety and expanding
indications.
Building on the demonstrated efficacy of ENPG in
treating recurrent NPC, clinical studies have progressively
extended its application to patients with early-stage NPC (90–92).
Several prospective studies indicate that for patients at T1-T2
stage, the combination of ENPG with chemotherapy or low-dose
radiotherapy achieves survival outcomes comparable with those of
standard radiotherapy [3-year OS of 100% and disease-free survival
(DFS) of 95.8–100%]. Of note, ENPG also enhances quality of life
metrics, particularly in preserving salivary gland function,
reducing dry mouth and improving speech function. More importantly,
ENPG successfully meets the dual objectives of precise lesion
resection and functional preservation by shortening the irradiation
path, circumventing the need for repeated radiation and minimizing
the trauma to the adhesion zones (Table
I).
 | Table I.Summary of key advances in early
detection technologies for NPC, focusing on innovations in
diagnostic platforms and their clinical applicability. The table
provides an overview of sensitivity, specificity, advantages and
limitations of each method discussed in the manuscript. |
Table I.
Summary of key advances in early
detection technologies for NPC, focusing on innovations in
diagnostic platforms and their clinical applicability. The table
provides an overview of sensitivity, specificity, advantages and
limitations of each method discussed in the manuscript.
| Detection
method | Key technology | Sensitivity
(%) | Specificity
(%) | Advantages | Limitations |
|---|
| CRISPR-Cas12a | CRISPR-based | 98.5 | 99.1 | High sensitivity
and | Requires
advanced |
|
(Amplification-free) |
non-amplification |
|
| specificity;
rapid | technology and
trained |
|
| digital
detection |
|
| and
cost-effective | personnel |
| P85 antibody
profiling | Antibody
detection | 97.9 | 98.3 | High positive | Limited
validation |
|
| (P85) |
|
| predictive
value; | across
different |
|
|
|
|
| cost-efficient
for | populations |
|
|
|
|
| mass screening |
|
| T-Cell
receptor | TCR sequencing
of | 98.6 | - | Ultra-early | Not widely
accessible; |
| sequencing | EBV-related
clonal |
|
| prediction of
NPC | requires high- |
|
| expansions |
|
| risk (6–12
months | throughput
sequencing |
|
|
|
|
| prior to
clinical | platforms |
|
|
|
|
| diagnosis) |
|
| Narrow-band
imaging | Optical | 92.3 | 93.1 | Can detect | High false-positive
rate; |
|
| enhancement
with |
|
| micro-invasive |
operator-dependent |
|
| blue-green
light |
|
| lesions <5
mm; |
|
|
|
|
|
| superior to
white |
|
|
|
|
|
| light
endoscopy |
|
| I-Scan virtual | Image
enhancement | 87.5 | - | Enhances
vascular | Limited to
higher-end |
|
chromoendoscopy | for detailed
mucosal | (Stage II) |
| morphology and | equipment;
operator- |
|
| patterns |
|
| glandular
structure | dependent |
|
|
|
|
| detail |
|
Although ENPG is currently used as part of a
multimodal treatment regimen, emerging evidence is gradually
confirming its potential as a standalone therapy for stage INPC. A
10-year retrospective study from the Cancer Center of Sun Yat-sen
University revealed that patients with stage T1N0M0 NPC who
underwent ENPG alone achieved a 100% three-year PFS, markedly
surpassing intensity-modulated radiotherapy (IMRT) in terms of
cost-effectiveness and quality of life (93,94).
This finding suggested that minimally invasive surgery could
challenge the established standards of radiotherapy for specific
NPC subtypes. However, this approach remains in its infancy and
current evidence is constrained by small sample sizes and limited
follow-up durations. Future research should prioritize multicenter
randomized controlled trials that incorporate molecular
characteristics, such as EBV DNA load, PD-L1 expression and TME
features. This would enable the development of a more refined
treatment model, emphasizing surgery complemented by
immunoradiotherapy. The focus should be on targeting low-risk
molecular subgroups, with the aim of expanding the role of ENPG as
a curative therapy. Accordingly, ENPG is not recommended beyond
T1-T2 indications and should not be generalized outside centers
with the requisite skull-base expertise; radiotherapy remains the
reproducible standard elsewhere.
Looking ahead, three key strategies are poised to
establish ENPG as a primary treatment modality: i) Preoperative
neoadjuvant therapy, such as PD-1 inhibitors combined with
gemcitabine-platinum regimens, can markedly reduce tumor volume and
broaden surgical indications; ii) intraoperative integration of
AI-assisted navigation, enhanced vascular imaging and robotic
manipulation will lower technical barriers and iii) the
incorporation of multidisciplinary collaboration (involving ENT,
radiotherapy, oncology and immunology) alongside the dynamic
monitoring of EBV load will enable the development of an
individualized treatment decision-making system, ultimately
targeting ‘functional cure’ rather than mere ‘lesion elimination’
(Table II).
 | Table II.A comparative analysis of ENPG and
traditional radiotherapy in treating early-stage NPC. This table
highlights key clinical outcomes, functional preservation and the
challenges associated with each treatment modality. |
Table II.
A comparative analysis of ENPG and
traditional radiotherapy in treating early-stage NPC. This table
highlights key clinical outcomes, functional preservation and the
challenges associated with each treatment modality.
| Parameter | ENPG | Traditional
radiotherapy |
|---|
| 5-Year survival
rate | 92.1% | ~90% |
| Negative resection
margins | ≥90% | N/A |
| Functional
preservation | Swallowing, hearing
and cervical mobility preserved | Notable loss in
swallowing, hearing and neck mobility |
|
Radiotherapy-related morbidity | Reduced incidence
of mucosal necrosis, fibrosis and other side effects | High incidence of
mucosal necrosis, neck fibrosis and functional impairments |
| Eligibility | Early-stage (I/II)
and recurrent NPC; precision-based patient selection | Broader
eligibility, including advanced stages |
| Technical
requirements | High precision
surgery; AI-assisted navigation; specialized equipment | High-dose
radiotherapy; established protocols |
| Patient
recovery | Faster recovery;
fewer long-term side effects | Slower recovery;
long-term functional impairments |
| Cost and
accessibility | Expensive; limited
access in low-resource regions | Widely available;
lower initial treatment cost |
Long-term outcomes and functional
assessment
Across available comparative studies, endoscopic
nasopharyngectomy has been explored as an organ-preserving option
in highly selected early-stage disease, with oncologic outcomes
that appear broadly comparable to radiotherapy in some cohorts,
while potentially offering advantages in selected functional
domains; however, the evidence remains limited by small sample
size, centre expertise, and heterogeneity in endpoints and
follow-up (90,93,94).
Beyond oncologic parity, convergent patient-reported data indicate
functional advantages, with higher MDADI scores for
swallowing-related quality of life and lower European Organisation
for Research and Treatment of Cancer QLQ-H and N35 symptom burdens
in xerostomia-linked domains (such as dry mouth and sticky saliva),
consistent with the organ-preserving intent of ENPG (1,3,95).
Accordingly, radiotherapy remains the reproducible standard,
whereas ENPG should be reserved for carefully selected T1-T2
patients in experienced centres, ideally within registries or
prospective trials (90,93). To provide a transparent evidence map
for clinicians and health systems, 5-/10-year survival and
functional readouts were synthesized and summarized in
Supplementary Table SI, annotated
by assessment windows (baseline, 12–24 months, and last follow-up)
and highlighting the heterogeneity of instruments that currently
limits cross-study pooling (93,96–100).
Conclusion
In recent years, notable advances have been made in
the early screening and treatment strategies for NPC, culminating
in the development of a new paradigm of ‘precise
screening-minimally invasive intervention-functional preservation’
(9). In the realm of screening,
continuous innovations in detection technologies for EBV-related
markers have substantially improved diagnostic performance
(18,101). The introduction of
CRISPR/Cas12a-based non-amplification detection and the novel
P85-Ab has enhanced the sensitivity and specificity of NPC
screening to 97.9 and 98.3%, respectively (18,102).
Furthermore, the integration of mRNA multi-marker combined
detection (such as the miR-140-3p triad model) with imaging
techniques, such as NBI and I-scan, marks a notable shift from a
‘single indicator’ approach to a more robust ‘molecular-imaging’
collaborative model (30,82).
In the treatment domain, ENPG has raised the 5-year
survival rate for patients with early-stage NPC to 92.1% through
precise resection and functional preservation. In addition,
quality-of-life metrics, such as swallowing and hearing functions,
are markedly superior when compared with those achieved with
traditional radiotherapy, signifying a shift in treatment goals
from ‘survival first’ to a balanced emphasis on both ‘survival and
quality of life’ (90,93). However, several key challenges
persist, tumor heterogeneity results in limited universality of
markers (such as fluctuations in miR-93 expression) (103); the low penetration of NBI/I-scan
technologies at the grassroots level and the lack of operational
standards hinder their widespread use, and the indications of ENPG
remain confined to early-stage NPC, with long-term efficacy
requiring further large-scale validation (104). EBV-based detection anchors current
non-invasive screening, yet gaps persist in EBV-negative disease
and cross-population generalizability; ENPG remains
indication-bounded (selected T1-T2; experienced centers), with
radiotherapy as the reproducible standard elsewhere (105,106).
Future research should prioritize multi-omics
integration to build AI-driven risk stratification models combining
EBV-related signals with immune and metabolic features.
Surgery-immunotherapy synergy also warrants exploration, including
neoadjuvant PD-1 blockade with ENPG in trial settings and adjuvant
strategies guided by dynamic EBV monitoring. Meanwhile,
standardization and access should be improved through portable
assays and consensus endoscopic grading systems. Multidisciplinary
collaboration across ENT/head-and-neck surgery, oncology and
radiotherapy is essential to implement stage-appropriate precision
care. Finally, multicenter prospective studies with harmonized
endpoints and standardized PROs are required to validate long-term
efficacy and functional benefit.
The diagnosis and treatment of NPC are rapidly
evolving from empirical approaches to precision medicine. By
fostering interdisciplinary collaboration and embracing
technological innovation, the goal of achieving an ‘early diagnosis
rate of >80% and functional preservation rate >90%’ is within
reach. Ultimately, these advancements will help overcome the
persistent challenges in the prevention and control of NPC.
Going forward, the present review will adopt an
indication-bounded pathway anchored by three coordinated axes.
Molecular detection (EBV DNA/methylome cfDNA, TCR and selected
multi-omics) will be deployed as co-screening with predefined
cut-offs and external controls (107,108). Treatment selection remains
stage-appropriate: Radiotherapy as the reproducible standard, and
ENPG reserved for carefully selected T1-T2 in experienced centers
(with optional dose-optimized low-dose RT). Immune modulation
(PD-1/PD-L1) is integrated for high-risk biology and in trial
settings. Implementation will prioritize registries and embedded
pragmatic designs with harmonized endpoints, 5/10-year OS, late ≥G3
toxicities and standardized PROs (MDADI/QLQ-H&N35 at baseline,
12 months, 24 months and long-term), and will explicitly address
equity and access (such as, portable assays, tele-navigation and
center-stratified analytics) (109,110). This program is intended to confirm
survival parity where suggested and to quantify any durable
functional advantages under real-world constraints (Fig. 5).
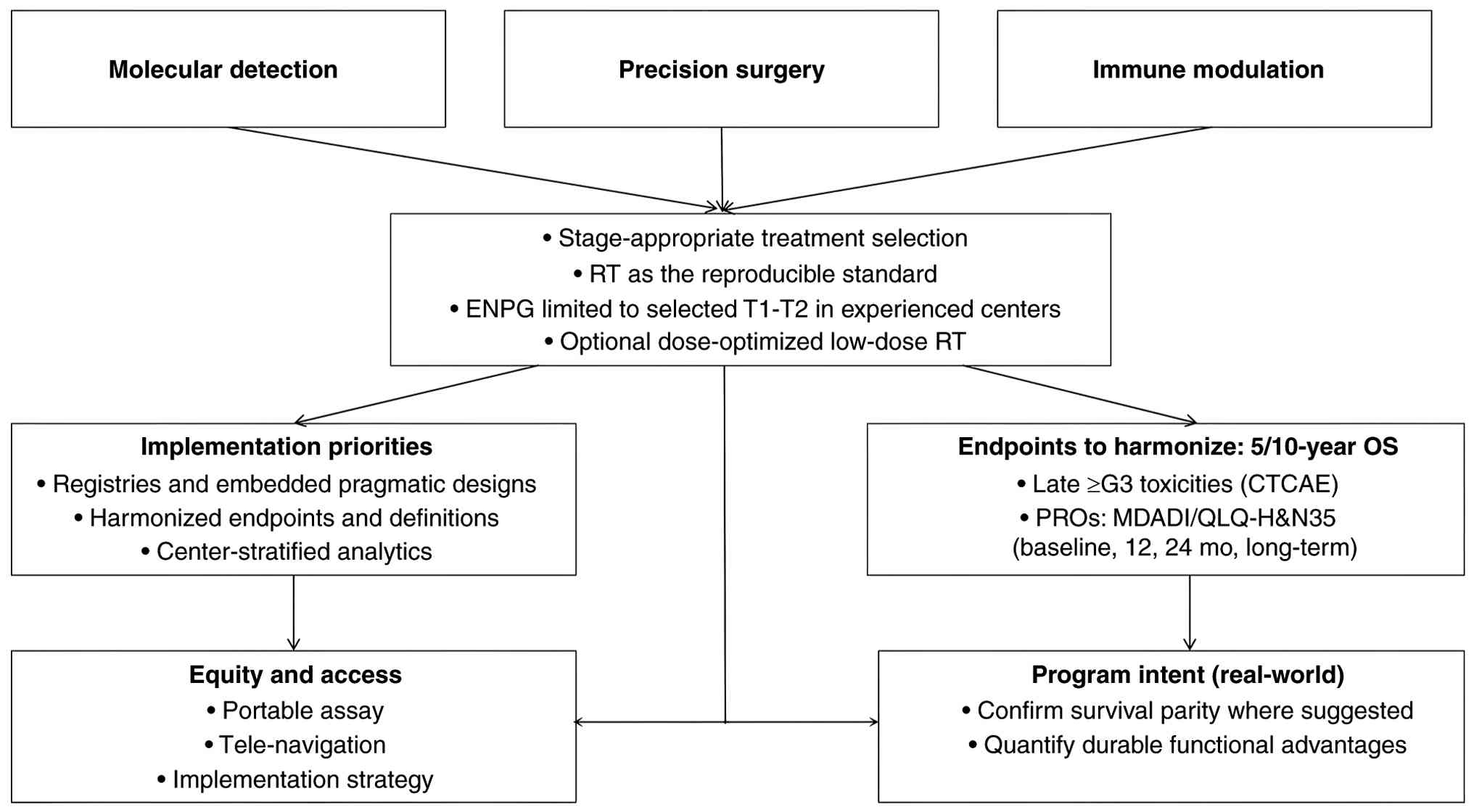 | Figure 5.Practice-facing outlook for NPC
precision care. NPC, nasopharyngeal carcinoma. RT, radiotherapy;
ENPG, endoscopic nasopharyngectomy; OS, overall survival; CTCAE,
Common Terminology Criteria for Adverse Events; PROs,
patient-reported outcomes; MDADI, MD Anderson Dysphagia Inventory;
QLQ-H&N35, EORTC Quality of Life Questionnaire-Head and Neck
35; mo, months; T1-T2, tumor stage T1-T2; G3, grade 3. |
Limitations
Several constraints temper inference from the
present synthesis. i) Biology and case-mix: EBV-centric algorithms
underperform in EBV-negative NPC and may vary across ethnicities
and prevalence settings; current evidence lacks adequately powered,
externally validated cohorts for these subgroups. ii) Assay and
pipeline heterogeneity: Platforms (qPCR/ddPCR/NGS/CRISPR),
thresholds and pre-analytics (plasma handling, batching) differ
across studies, introducing spectrum and incorporation biases that
limit cross-study pooling. iii) Functional outcomes and toxicity:
PROs (MDADI, QLQ-H&N35) are inconsistently timed and analyzed
(directionality, responder thresholds) and acute vs. late (≥G3)
toxicities are not uniformly separated. iv) Surgical
generalizability: ENPG results largely reflect stringent selection
(T1-T2) and center experience/learning curves; requirements for
navigation, imaging and rescuability restrict applicability in
low-resource settings. v) Long-term endpoints: 10-year OS/DFS and
durable functional stability remain sparse and heterogeneous, with
non-randomized designs susceptible to residual confounding
(adjuvant and salvage policies). vi) Implementation and equity: Few
studies report cost-effectiveness, access or workforce
implications, impeding translation at scale.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
EJ contributed to the study design, literature
search and selection and analysis of the literature/information. WL
and XZ were involved in the writing process, including manuscript
drafting, editing and reviewing, as well as the creation of figures
and tables. All authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tang LL, Chen YP, Chen CB, Chen MY, Chen
NY, Chen XZ, Du XJ, Fang WF, Feng M, Gao J, et al: The Chinese
society of clinical oncology (CSCO) clinical guidelines for the
diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun
(Lond). 41:1195–1227. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bossi P, Chan AT, Licitra L, Trama A,
Orlandi E, Hui EP, Halámková J, Mattheis S, Baujat B, Hardillo J,
et al: Nasopharyngeal carcinoma: ESMO-EURACAN clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
32:452–465. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen YP, Ismaila N, Chua ML, Colevas AD,
Haddad R, Huang SH, Wee JT, Whitley AC, Yi JL, Yom SS, et al:
Chemotherapy in combination with radiotherapy for definitive-intent
treatment of stage II–IVA nasopharyngeal carcinoma: CSCO and ASCO
guideline. J Clin Oncol. 39:840–859. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lam WJ, King AD, Miller JA, Liu Z, Yu KJ,
Chua ML, Ma BB, Chen MY, Pinsky BA, Lou PJ, et al: Recommendations
for Epstein-Barr virus-based screening for nasopharyngeal cancer in
high-and intermediate-risk regions. J Natl Cancer Inst.
115:355–364. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao SM, Liu Z, Jia WH, Huang QH, Liu Q,
Guo X, Huang TB, Ye W and Hong MH: Fluctuations of epstein-barr
virus serological antibodies and risk for nasopharyngeal carcinoma:
A prospective screening study with a 20-year follow-up. PLoS One.
6:e191002011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Huang Q, Liu W, Liu Q, Jia W, Chang
E, Chen F, Liu Z, Guo X, Mo H, et al: Establishment of VCA and
EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as
preferred screening method for nasopharyngeal carcinoma: A
two-stage design with a preliminary performance study and a mass
screening in southern China. Int J Cancer. 131:406–416. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Wang K, Yin SK, Zheng HL and Min DL:
Expression of Epstein-Barr virus antibodies EA-IgG, Rta-IgG, and
VCA-IgA in nasopharyngeal carcinoma and their use in a combined
diagnostic assay. Genet Mol Res. Mar 18–2016.(Epub ahead of
print).
|
|
8
|
Ai P, Wang T, Zhang H, Wang Y, Song C,
Zhang L, Li Z and Hu H: Determination of antibodies directed at EBV
proteins expressed in both latent and lytic cycles in
nasopharyngeal carcinoma. Oral Oncol. 49:326–331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang W, Zheng B and Wei H: Recent
advances in early detection of nasopharyngeal carcinoma. Discov
Oncol. 15:3652024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo KW, To KF and Huang DP: Focus on
nasopharyngeal carcinoma. Cancer Cell. 5:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong KC, Hui EP, Lo KW, Lam WKJ, Johnson
D, Li L, Tao Q, Chan KCA, To KF, King AD, et al: Nasopharyngeal
carcinoma: An evolving paradigm. Nat Rev Clin Oncol. 18:679–695.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S, Zhou Y, Liu Z, Wang Y, Zhou X,
Chen H, Zhang X, Chen Y, Feng Q, Ye X, et al: Immunosequencing
identifies signatures of T cell responses for early detection of
nasopharyngeal carcinoma. Cancer Cell. 43:1423–1441. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ni XG and Wang GQ: The role of narrow band
imaging in head and neck cancers. Curr Oncol Rep. 18:102016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan JL, Lin L, Feiler M, Wolf AI, Cardona
DM and Gellad ZF: Comparative effectiveness of i-SCAN™ and
high-definition white light characterizing small colonic polyps.
World J Gastroenterol. 18:5905–5911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lam WKJ, Jiang P, Chan KCA, Cheng SH,
Zhang H, Peng W, Tse OYO, Tong YK, Gai W, Zee BCY, et al:
Sequencing-based counting and size profiling of plasma Epstein-Barr
virus DNA enhance population screening of nasopharyngeal carcinoma.
Proc Natl Acad Sci USA. 115:E5115–E5124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X, Tay JK, Goh CK, Chan C, Lee YH,
Springs SL, Wang Y, Loh KS, Lu TK and Yu H: Digital CRISPR-based
method for the rapid detection and absolute quantification of
nucleic acids. Biomaterials. 274:1208762021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang C, Zheng X, Lin L, Li X, Li X, Liao
Y, Jia W and Shu B: CRISPR Cas12a-mediated amplification-free
digital DNA assay improves the diagnosis and surveillance of
Nasopharyngeal carcinoma. Biosens Bioelectron. 237:1155462023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li T, Li F, Guo X, Hong C, Yu X, Wu B,
Lian S, Song L, Tang J, Wen S, et al: Anti-Epstein-Barr virus
BNLF2b for mass screening for nasopharyngeal cancer. N Engl J Med.
389:808–819. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan YX, Huang Q, Xing S and Zhu QY: A
novel serum protein biomarker for the late-stage diagnosis of
nasopharyngeal carcinoma. BMC Cancer. 25:5852025. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji MF, He YQ, Tang MZ, Xue WQ, Yu X, Diao
H, Yang DW, Mai ZM, Cheong IH and Zhao ZY: Epstein Barr virus
antibody and cancer risk in two prospective cohorts in Southern
China. Nat Commun. 16:59402025. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Y, Zhao W, Mo Y, Ma N, Midorikawa K,
Kobayashi H, Hiraku Y, Oikawa S, Zhang Z and Huang G: Combination
of RERG and ZNF671 methylation rates in circulating cell-free DNA:
A novel biomarker for screening of nasopharyngeal carcinoma. Cancer
Sci. 111:2536–2545. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu CL, Chang YS and Li HP: Molecular
diagnosis of nasopharyngeal carcinoma: Past and future. Biomed J.
48:1007482025. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Islam KA, Chow LKY, Kam NW, Wang Y, Chiang
CL, Choi HCW, Xia YF, Lee AWM, Ng WT and Dai W: Prognostic
biomarkers for survival in nasopharyngeal carcinoma: A systematic
review of the literature. Cancers. 14:20222122.
|
|
24
|
Li G, Wu Z, Peng Y, Liu X, Lu J, Wang L,
Pan Q, He ML and Li XP: MicroRNA-10b induced by Epstein-Barr
virus-encoded latent membrane protein-1 promotes the metastasis of
human nasopharyngeal carcinoma cells. Cancer Lett. 299:29–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allaya N, Khabir A, Sallemi-Boudawara T,
Sellami N, Daoud J, Ghorbel A, Frikha M, Gargouri A,
Mokdad-Gargouri R and Ayadi W: Over-expression of miR-10b in NPC
patients: Correlation with LMP1 and Twist1. Tumor Biol.
36:3807–3814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Lin T, Tang L, He L and He Y:
MiRNA signatures in nasopharyngeal carcinoma: Molecular mechanisms
and therapeutic perspectives. Am J Cancer Res. 13:5805–5824.
2023.PubMed/NCBI
|
|
27
|
Zhang Y and Xu Z: miR-93 enhances cell
proliferation by directly targeting CDKN1A in nasopharyngeal
carcinoma. Oncol Lett. 15:1723–1727. 2018.PubMed/NCBI
|
|
28
|
Zhou W, Chang A, Zhao H, Ye H, Li D and
Zhuo X: Identification of a novel microRNA profile including
miR-106b, miR-17, miR-20b, miR-18a and miR-93 in the metastasis of
nasopharyngeal carcinoma. Cancer Biomark. 27:533–539. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duan B, Shi S, Yue H, You B, Shan Y, Zhu
Z, Bao L and You Y: Exosomal miR-17-5p promotes angiogenesis in
nasopharyngeal carcinoma via targeting BAMBI. J Cancer.
10:6681–6692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Zou X, Wu L, Zhang S, Wang T, Liu
P, Zhu W and Zhu J: Identification of a 7-microRNA signature in
plasma as promising biomarker for nasopharyngeal carcinoma
detection. Cancer Med. 9:1230–1241. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou X, Zhu D, Zhang H, Zhang S, Zhou X, He
X, Zhu J and Zhu W: MicroRNA expression profiling analysis in serum
for nasopharyngeal carcinoma diagnosis. Gene. 727:1442432020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang C, Chen J, Xie S, Zhang L, Xiang Y,
Lung M, Kam NW, Kwong DL, Cao S and Guan XY: Evaluation of
circulating EBV microRNA BART2-5p in facilitating early detection
and screening of nasopharyngeal carcinoma. Int J Cancer.
143:3209–3217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lyu X, Fang W, Cai L, Zheng H, Ye Y, Zhang
L, Li J, Peng H, Cho WC, Wang E, et al: TGFβR2 is a major target of
miR-93 in nasopharyngeal carcinoma aggressiveness. Mol Cancer.
13:512014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang W, Tan S, Yang L, Chen X, Yang R,
Oyang L, Lin J, Xia L, Wu N, Han Y, et al: Exosomal miR-205-5p
enhances angiogenesis and nasopharyngeal carcinoma metastasis by
targeting desmocollin-2. Mol Ther Oncolytics. 24:612–623. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsia B, Sure A, Dongre R, Jo N, Kuzniar J,
Bitar G, Alshaka SA, Kim JD, Valencia-Sanchez BA, Brandel MG, et
al: Molecular profiling of nasopharyngeal carcinoma using the AACR
project GENIE repository. Cancers (Basel). 17:15442025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan X, Liu Y, Peng Y, Wang J, Yan SM,
Zhang L, Wu W, Zhao L, Chen X, Ren K, et al: Primary and orthotopic
murine models of nasopharyngeal carcinoma reveal molecular
mechanisms underlying its malignant progression. Adv Sci (Weinh).
11:e24031612024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen W, Yang KB, Zhang YZ, Lin ZS, Chen
JW, Qi SF, Wu CF, Feng GK, Yang DJ, Chen M, et al: Synthetic
lethality of combined ULK1 defection and p53 restoration induce
pyroptosis by directly upregulating GSDME transcription and
cleavage activation through ROS/NLRP3 signaling. J Exp Clin Cancer
Res. 43:2482024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Agaoglu FY, Dizdar Y, Dogan O, Alatli C,
Ayan I, Savci N, Tas S, Dalay N and Altun M: P53 overexpression in
nasopharyngeal carcinoma. In Vivo. 18:555–560. 2004.PubMed/NCBI
|
|
39
|
Li YY, Chung GT, Lui VW, To KF, Ma BB,
Chow C, Woo JKS, Yip KY, Seo J, Hui EP, et al: Exome and genome
sequencing of nasopharynx cancer identifies NF-κB pathway
activating mutations. Nat Commun. 8:141212017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Papa A and Pandolfi PP: The PTEN-PI3K axis
in cancer. Biomolecules. 9:1532019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang S, Hong HI, Mak VCY, Zhou Y, Lu Y,
Zhuang G and Cheung LWT: Vertical inhibition of p110α/AKT and
N-cadherin enhances treatment efficacy in PIK3CA-aberrated ovarian
cancer cells. Mol Oncol. 19:1132–1154. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shamsuri AS and Sim EU: In silico
prediction of the action of bromelain on PI3K/Akt signalling
pathway to arrest nasopharyngeal cancer oncogenesis by targeting
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha protein. BMC Res Notes. 17:3462024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou Z, Li P, Zhang X, Xu J, Xu J, Yu S,
Wang D, Dong W, Cao X, Yan H, et al: Mutational landscape of
nasopharyngeal carcinoma based on targeted next-generation
sequencing: Implications for predicting clinical outcomes. Mol Med.
28:552022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu Q, Duan XB, Hu H, You R, Xia TL, Yu T,
Xiang T and Chen MY: EBV-induced upregulation of CD55 reduces the
efficacy of cetuximab treatment in nasopharyngeal carcinoma. J
Transl Med. 22:11112024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Piao Y, Yang Y, Wu S and Han L:
Toripalimab plus cetuximab combined with radiotherapy in a locally
advanced platinum-based chemotherapy-insensitive nasopharyngeal
carcinoma patient: A case report. Front Oncol. 14:13832502024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang Y, Lv X, Li G, Wang P, Zhang L, Wang
R, Jia L and Liang S: Schisandrin B targets CDK4/6 to suppress
proliferation and enhance radiosensitivity in nasopharyngeal
carcinoma by inducing cell cycle arrest. Sci Rep. 15:84522025.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li HP, Huang CY, Lui KW, Chao YK, Yeh CN,
Lee LY, Huang Y, Lin TL, Kuo YC, Huang MY, et al: Nasopharyngeal
carcinoma patient-derived xenograft mouse models reveal potential
drugs targeting cell cycle, mTOR, and autophagy pathways. Transl
Oncol. 38:1017852023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li HP, Huang CY, Lui KW, Chao YK, Yeh CN,
Lee LY, Huang Y, Lin TL, Kuo YC, Huang MY, et al: Combination of
epithelial growth factor receptor blockers and CDK4/6 inhibitor for
nasopharyngeal carcinoma treatment. Cancers (Basel). 13:29542021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liao LJ, Hsu WL, Chen CJ and Chiu YL:
Feature reviews of the molecular mechanisms of nasopharyngeal
carcinoma. Biomedicines. 11:15282023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tao W, Jiang C, Velu P, Lv C and Niu Y:
Rosmanol suppresses nasopharyngeal carcinoma cell proliferation and
enhances apoptosis, the regulation of MAPK/NF-κB signaling pathway.
Biotechnol Appl Biochem. 72:e27502025. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liang Y, Xiong XY, Lin GW, Bai X, Li F, Ko
JM, Zhou YH, Xu AY, Liu SQ, He S, et al: Integrative
transcriptome-wide association study with expression quantitative
trait loci colocalization identifies a causal VAMP8 variant for
nasopharyngeal carcinoma susceptibility. Adv Sci (Weinh).
12:e24125802025. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Y, Chen Y, Liu Y, Zhao J, Wang G,
Chen H, Tang Y, Ouyang D, Xie S, You J, et al: Tumor vascular
endothelial cells promote immune escape by upregulating PD-L1
expression via crosstalk between NF-κB and STAT3 signaling pathways
in nasopharyngeal carcinoma. Cell Death Dis. 16:1292025. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang R, Hu Y, Yindom LM, Huang L, Wu R,
Wang D, Chang C, Rostron T, Dong T and Wang X: Association analysis
between HLA-A, -B, -C, -DRB1, and -DQB1 with nasopharyngeal
carcinoma among a Han population in Northwestern China. Hum
Immunol. 75:197–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Barber LD, Percival L, Valiante NM, Chen
L, Lee C, Gumperz JE, Phillips JH, Lanier LL, Bigge JC, Parekh RB
and Parham P: The inter-locus recombinant HLA-B*4601 has high
selectivity in peptide binding and functions characteristic of
HLA-C. J Exp Med. 184:735–740. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Su WH, Chiu CC and Shugart YY:
Heterogeneity revealed through meta-analysis might link
geographical differences with nasopharyngeal carcinoma incidence in
Han Chinese populations. BMC Cancer. 15:5982015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xu H, Liu J, Zhang Y, Zhou Y, Zhang L,
Kang J, Ning C, He Z and Song S: KIF23, under regulation by
androgen receptor, contributes to nasopharyngeal carcinoma
deterioration by activating the Wnt/β-catenin signaling pathway.
Funct Integr Genomics. 23:1162023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ji Y, Wang M, Li X and Cui F: The long
noncoding RNA NEAT1 targets miR-34a-5p and drives nasopharyngeal
carcinoma progression via Wnt/β-catenin signaling. Yonsei Med J.
60:336–345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Song P, Ye LF, Zhang C, Peng T and Zhou
XH: Long non-coding RNA XIST exerts oncogenic functions in human
nasopharyngeal carcinoma by targeting miR-34a-5p. Gene. 592:8–14.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang S, Dai Z, Li W, Wang R and Huang D:
Aberrant promoter methylation reduced the expression of
protocadherin 17 in nasopharyngeal cancer. Biochem Cell Biol.
97:364–368. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Loyo M, Brait M, Kim MS, Ostrow KL, Jie
CC, Chuang AY, Califano JA, Liégeois NJ, Begum S, Westra WH, et al:
A survey of methylated candidate tumor suppressor genes in
nasopharyngeal carcinoma. Int J Cancer. 128:1393–1403. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li T, Patel KB, Yu X, Yao S, Wang L, Chung
CH and Wang X: Unveiling targeted cell-free DNA methylation regions
through paired methylome analysis of tumor and normal tissues.
bioRxiv. 29:10.1101/2023.06.27.546654. 2023.
|
|
62
|
Xu J, Chen D, Wu W, Ji X, Dou X, Gao X, Li
J, Zhang X, Huang WE and Xiong D: A metabolic map and artificial
intelligence-aided identification of nasopharyngeal carcinoma via a
single-cell Raman platform. Br J Cancer. 130:1635–1646. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yi L, Dong N, Shi S, Deng B, Yun Y, Yi Z
and Zhang Y: Metabolomic identification of novel biomarkers of
nasopharyngeal carcinoma. RSC Advances. 4:59094–59101. 2014.
View Article : Google Scholar
|
|
64
|
Liao Z, Zhao L, Zhong F, Zhou Y, Lu T, Liu
L, Gong X, Li J and Rao J: Serum and urine metabolomics analyses
reveal metabolic pathways and biomarkers in relation to
nasopharyngeal carcinoma. Rapid Commun Mass Spectrom. 37:e94692023.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhou J, Deng Y, Huang Y, Wang Z, Zhan Z,
Cao X, Cai Z, Deng Y, Zhang L, Huang H, et al: An individualized
prognostic model in patients with locoregionally advanced
nasopharyngeal carcinoma based on serum metabolomic profiling. Life
(Basel). 13:11672023.PubMed/NCBI
|
|
66
|
Zhou Z, Tang T, Li N, Zheng Q, Xiao T,
Tian Y, Sun J, Zhang L, Wang X, Wang Y, et al: VLDL and LDL
subfractions enhance the risk stratification of individuals who
underwent epstein-barr virus-based screening for nasopharyngeal
carcinoma: A multicenter cohort study. Adv Sci (Weinh).
11:e23087652024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu H, Wang J, Wang L, Tang W, Hou X, Zhu
YZ and Chen X: Multi-omics exploration of the mechanism of curcumol
to reduce invasion and metastasis of nasopharyngeal carcinoma by
inhibiting NCL/EBNA1-mediated UBE2C upregulation. Biomolecules.
14:11422024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nie RC, Zhao CB, Xia XW, Luo YS, Wu T,
Zhou ZW, Yuan SQ, Wang Y and Li YF: The efficacy and safety of
PD-1/PD-L1 inhibitors in combination with conventional therapies
for advanced solid tumors: A meta-analysis. Biomed Res Int.
2020:50590792020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhou S, Zhu J, Xu J, Gu B, Zhao Q, Luo C,
Gao Z, Chin YE and Cheng X: Anti-tumour potential of PD-L1/PD-1
post-translational modifications. Immunology. 167:471–481. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lu MM and Yang Y: Exosomal PD-L1 in cancer
and other fields: Recent advances and perspectives. Front Immunol.
15:13953322024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hsu C, Lee SH, Ejadi S, Even C, Cohen RB,
Le Tourneau C, Mehnert JM, Algazi A, van Brummelen EMJ, Saraf S, et
al: Safety and antitumor activity of pembrolizumab in patients with
programmed death-ligand 1-positive nasopharyngeal carcinoma:
Results of the KEYNOTE-028 study. J Clin Oncol. 35:4050–4056. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW,
Pettinger A, Foster NR, Riess JW, Agulnik M, Chang AYC, et al:
Antitumor activity of nivolumab in recurrent and metastatic
nasopharyngeal carcinoma: An international, multicenter study of
the mayo clinic phase 2 consortium (NCI-9742). J Clin Oncol.
36:1412–1418. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yang Y, Zhou T, Chen X, Li J, Pan J, He X,
Lin L, Shi YR, Feng W, Xiong J, et al: Efficacy, safety, and
biomarker analysis of camrelizumab in previously treated recurrent
or metastatic nasopharyngeal carcinoma (CAPTAIN study). J
Immunother Cancer. 9:e0037902021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou
T, Shen L, Wu H, Lang J, et al: Camrelizumab versus placebo in
combination with gemcitabine and cisplatin as first-line treatment
for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st):
A multicentre, randomised, double-blind, phase 3 trial. Lancet
Oncol. 22:1162–1174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shi Y, Han G, Zhou J, Shi X, Jia W, Cheng
Y, Jin Y, Hua X, Wen T, Wu J, et al: Toripalimab plus bevacizumab
versus sorafenib as first-line treatment for advanced
hepatocellular carcinoma (HEPATORCH): A randomised, open-label,
phase 3 trial. Lancet Gastroenterol Hepatol. 10:658–670. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xue J, Ma T and Zhang X: TRA2: The
dominant power of alternative splicing in tumors. Heliyon.
9:e155162023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xiao M, Xue J and Jin E: SPOCK: Master
regulator of malignant tumors (Review). Mol Med Rep. 30:2312024.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Briante R, Zhai Q, Mohanty S, Zhang P,
O'Connor A, Misker H, Wang W, Tan C, Abuhay M, Morgan J, et al:
Successful targeting of multidrug-resistant tumors with bispecific
antibodies. Mabs. 17:24922382025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Li D, Wang J, Li X, Wang Z, Yu Q, Koh SB,
Wu R, Ye L, Guo Y, Okoli U, et al: Interactions between
radiotherapy resistance mechanisms and the tumor microenvironment.
Crit Rev Oncol Hematol. 210:1047052025. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kumazawa Y, Ikenoyama Y, Takamatsu M, Kido
K, Namikawa K, Tokai Y, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio
T, et al: ]Differences in clinical characteristics between missed
and detected laryngopharyngeal cancers. J Gastroenterol Hepatol.
40:2037–2045. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
He R, Jie P, Hou W, Long Y, Zhou G, Wu S,
Liu W, Lei W, Wen W and Wen Y: Real-time artificial
intelligence-assisted detection and segmentation of nasopharyngeal
carcinoma using multimodal endoscopic data: A multi-center,
prospective study. EClinicalMedicine. 81:1031202025. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Si YF, Deng ZX, Weng JJ, Si JY, Lan GP,
Zhang BJ, Yang Y, Huang B, Han X, Qin Y, et al: A study on the
value of narrow-band imaging (NBI) for the general investigation of
a high-risk population of nasopharyngeal carcinoma (NPC). World J
Surg Oncol. 16:1262018. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Filauro M, Vallin A, Fragale M, Sampieri
C, Guastini L, Mora F and Peretti G: Office-based procedures in
laryngology. Acta Otorhinolaryngol Ital. 41:243–247. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wu KP, Li QQ, Luo XQ, Wang XX, Lai YZ,
Tian D, Yang HC, Wei XL, Wang LY, Li QM, et al: Chemoimmunotherapy
as induction treatment in concurrent chemoradiotherapy for patients
with nasopharyngeal carcinoma stage IVa. Ann Med. 57:24530912025.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lan K, Liu S, Li S, Sun X, Xie S, Jia G,
Sun R and Mai H: Comparing outcomes and toxicities among patients
with nasopharyngeal carcinoma treated with daytime versus evening
radiotherapy: A retrospective analysis with propensity score
matching. Int J Cancer. 157:345–354. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Liang YJ, Luo MJ, Wen DX, Wang P, Tang LQ,
Mai HQ and Liu LT: Optimal induction chemotherapy courses in
nasopharyngeal carcinoma in the IMRT era: A recursive partitioning
risk stratification analysis based on EBV DNA and AJCC staging.
Oral Oncol. 167:1074042025. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lin SJ, Guo QJ, Liu Q, Ng WT, Ahn YC,
AlHussain H, Chan AW, Chow J, Chua M, Corry J, et al: International
consensus guideline on delineation of the clinical target volumes
(CTV) at different dose levels for nasopharyngeal carcinoma (2024
Version). Int J Radiat Oncol Biol Phys. 123:415–421. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Qiu QH, Li N, Zhang QH, Chen Z, Huang Y,
Jiang Y and Yang XT: Clinical efficacy of endoscopic
nasopharyngectomy for initially diagnosed advanced nasopharyngeal
carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
52:365–371. 2017.(In Chinese). PubMed/NCBI
|
|
89
|
Emanuelli E, Albu S, Cazzador D, Pedruzzi
B, Babighian G and Martini A: Endoscopic surgery for recurrent
undifferentiated nasopharyngeal carcinoma. J Craniofac Surg.
25:1003–1008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Liu YP, Wen YH, Tang J, Wei Y, You R, Zhu
XL, Li J, Chen L, Ling L, Zhang N, et al: Endoscopic surgery
compared with intensity-modulated radiotherapy in resectable
locally recurrent nasopharyngeal carcinoma: A multicentre,
open-label, randomised, controlled, phase 3 trial. Lancet Oncol.
22:381–390. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
You R, Liu YP, Xie YL, Lin C, Duan CY,
Chen DP, Pan Y, Qi B, Zou X, Guo L, et al: Hyperfractionation
compared with standard fractionation in intensity-modulated
radiotherapy for patients with locally advanced recurrent
nasopharyngeal carcinoma: A multicentre, randomised, open-label,
phase 3 trial. Lancet. 401:917–927. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Weng J, Gao J, Li M, Wei J, Zhang S, Lan
G, Li B, Qin D, Huang B, Zhu Z, et al: Effect of endoscopic surgery
combined with chemotherapy and radiotherapy on prognosis of early
nasopharyngeal carcinoma patients in high incidence area. Lin
Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 38:472–476.
2024.PubMed/NCBI
|
|
93
|
Liu YP, Lv X, Zou X, Hua YJ, You R, Yang
Q, Xia L, Guo SY, Hu W, Zhang MX, et al: Minimally invasive surgery
alone compared with intensity-modulated radiotherapy for primary
stage I nasopharyngeal carcinoma. Cancer Commun (Lond). 39:752019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Liu J, Zeng Z, Wang D and Qin G: Minimally
invasive surgery for early-stage nasopharyngeal carcinoma. J
Craniofac Surg. 33:e834–e837. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen YP, Wang YQ, Li WF, Chen L, Xu C, Lu
TX, Lin AH, Yao JJ, Li YC, Sun Y, et al: Critical evaluation of the
quality and recommendations of clinical practice guidelines for
nasopharyngeal carcinoma. J Natl Compr Canc Netw. 15:336–344. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhang B, Li Y, Weng J, Huang B, Ban M, Lan
G, Lu Y, Luo J, Qu S and Si Y: Efficacy and safety of endoscopic
nasopharyngectomy combined with low-dose radiotherapy for primary
T1-2 nasopharyngeal carcinoma. Technol Cancer Res Trea. Apr
26–2021.(Epub ahead of print).
|
|
97
|
Weng JJ, Wei JZ, Li M, Zhang SJ, Wei YZ,
Wang HW, Qin DX, Lu JL, Jiang H and Qu SH: Effects of surgery
combined with chemoradiotherapy on short- and long-term outcomes of
early-stage nasopharyngeal carcinoma. Cancer Manag Res.
12:7813–7826. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Su SF, Han F, Zhao C, Chen CY, Xiao WW, Li
JX and Lu TX: Long-term outcomes of early-stage nasopharyngeal
carcinoma patients treated with intensity-modulated radiotherapy
alone. Int J Radiat Oncol Biol Phys. 82:327–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu LR, Liu YT, Jiang N, Fan YX, Wen J,
Huang SF, Guo WJ, Bian XH, Wang FJ, Li F, et al: Ten-year survival
outcomes for patients with nasopharyngeal carcinoma receiving
intensity-modulated radiotherapy: An analysis of 614 patients from
a single center. Oral Oncol. 69:26–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Niu X, Xue F, Liu P, Hu C and He X:
Long-term outcomes of nasopharyngeal carcinoma patients with T1-2
stage in intensity-modulated radiotherapy era. Int J Med Sci.
19:267–273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Chan KA, Woo JK, King A, Zee BC, Lam WJ,
Chan SL, Chu SW, Mak C, Tse IO, Leung SY, et al: Analysis of plasma
Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl
J Med. 377:513–522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zheng XH, Li XZ, Tang CL, Zhang YM, Zhou
T, Yang XJ, Liao Y, He YQ, Wang TM, Xue WQ and Jia WH: Detection of
Epstein-Barr virus DNA methylation as tumor markers of
nasopharyngeal carcinoma patients in saliva, oropharyngeal swab,
oral swab, and mouthwash. MedComm (2020). 5:e6732024. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Sun J, Yong J and Zhang H: microRNA-93,
upregulated in serum of nasopharyngeal carcinoma patients, promotes
tumor cell proliferation by targeting PDCD4. Exp Ther Med.
19:2579–2587. 2020.PubMed/NCBI
|
|
104
|
Siak PY, Khoo ASB, Leong CO, Hoh BP and
Cheah SC: Current status and future perspectives about molecular
biomarkers of nasopharyngeal carcinoma. Cancers. 13:34902021.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
He Q, Zhou Y, Zhou J, Zhao D, Li L, Li X,
Huang Y, Wang Q, Zou H, Zhang K, et al: Clinical relevance of
plasma EBV DNA as a biomarker for nasopharyngeal carcinoma in
non-endemic areas: A multicenter study in southwestern China. Clin
Chim Acta. 541:1172442023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lee VHF, Adham M, Kridis WB, Bossi P, Chen
MY, Chitapanarux I, Gregoire V, Hao SP, Ho C, Ho GF, et al:
International recommendations for plasma Epstein-Barr virus DNA
measurement in nasopharyngeal carcinoma in resource-constrained
settings: Lessons from the COVID-19 pandemic. Lancet Oncol.
23:e544–e551. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lam WKJ and Lo YMD: Plasma epstein-barr
virus DNA analysis for personalised management of nasopharyngeal
carcinoma-current opportunities and challenges. Ann Nasopharynx
Cancer. 6:1–8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Economopoulou P, Lianidou E and Psyrri A:
Epstein-Barr virus DNA detection by targeted sequencing in
post-treatment plasma samples and prognosis of locally advanced
nasopharyngeal cancer: Implications for clinical research. Ann
Oncol. 33:747–749. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Chen AY, Frankowski R, Bishop-Leone J,
Hebert T, Leyk S, Lewin J and Goepfert H: The development and
validation of a dysphagia-specific quality-of-life questionnaire
for patients with head and neck cancer: The MD Anderson dysphagia
inventory. Arch Otolaryngol Head Neck Surg. 127:870–876.
2001.PubMed/NCBI
|
|
110
|
Bjordal K, Hammerlid E, Ahlner-Elmqvist M,
de Graeff A, Boysen M, Evensen JF, Biorklund A, de Leeuw JRJ,
Fayers PM, Jannert M, et al: Quality of life in head and neck
cancer patients: Validation of the European Organization for
research and treatment of cancer quality of life
questionnaire-H&N35. J Clin Oncol. 17:1008. 1999. View Article : Google Scholar : PubMed/NCBI
|