1. The need for a change in the current
scientific paradigm
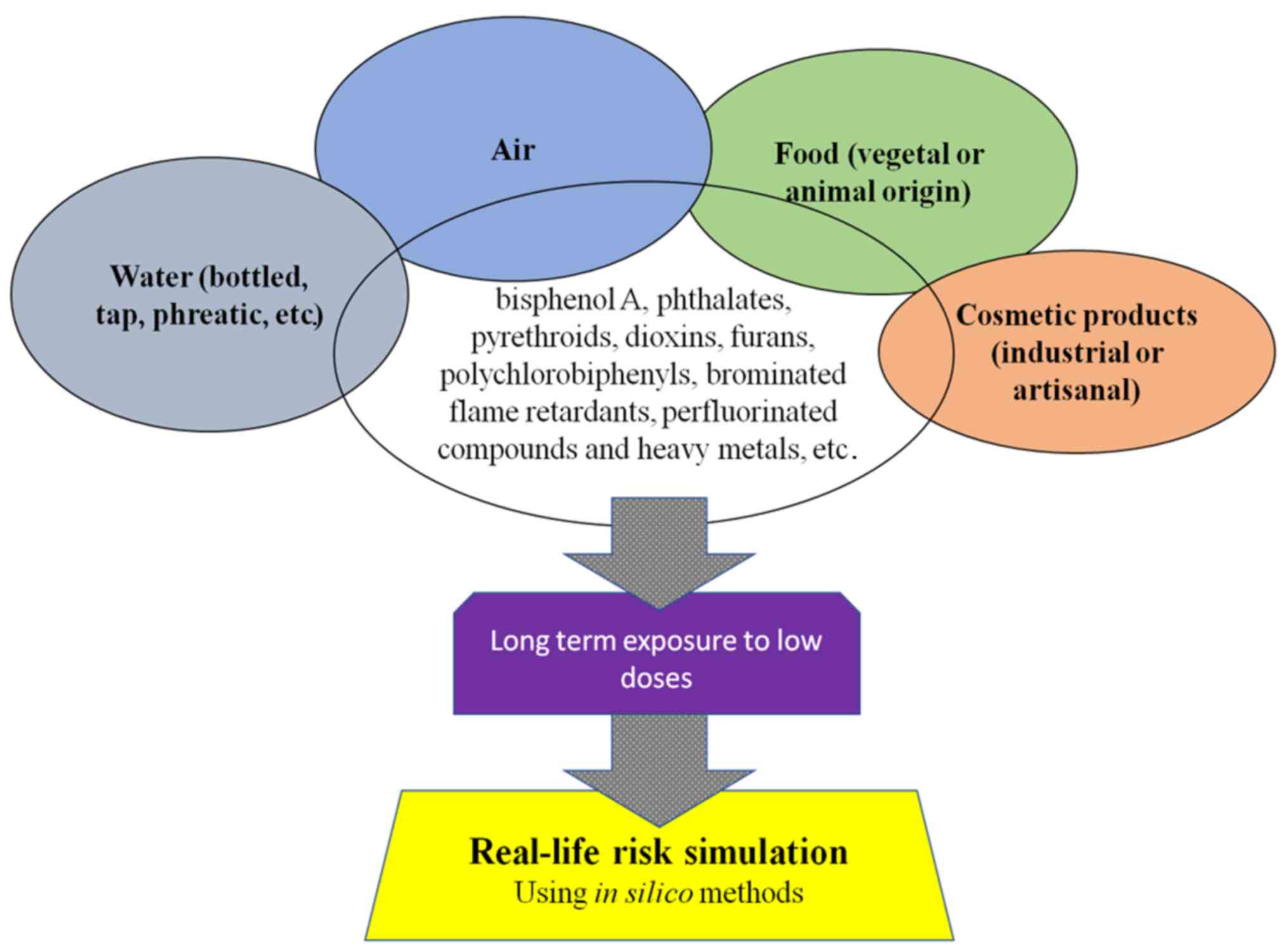
Human populations come into contact, on a daily
basis, with a large range of chemical mixtures, at low levels of
exposure, from virtually every product that is used, from the food
consumed (raw or processed, either vegetal, containing soil
originating substances, or animal), drinking water (tap or
bottled), the air that is breathed, consumer products (cosmetics,
either artisanal or industrial), etc. The results from a
nation-wide survey of environmental contaminants among 4,145
pregnant women in France in 2011 indicated that bisphenol A,
phthalates, pesticides (such as pyrethroids), dioxins, furans,
polychlorobiphenyls, brominated flame retardants, perfluorinated
compounds and heavy metals are quantifiable in virtually all
individuals (1). Over the past 15
years, researchers from different disciplines (toxicologists,
biochemists, chemists, medical doctors and molecular biologists)
have made constant efforts to unravel the possible effects induced
by the long-term exposure to low doses of chemicals on human
physiology (2) (Fig. 1).
The realization that humans are exposed to a large
number of substances through different routes in their everyday
lives has led to a change in scientific paradigms (2). To date, the majority of studies, and
in particular regulatory toxicology studies, have focused on
testing multiple outcomes resulting from the administration of a
single substance at medium-high doses to laboratory animals
(3). Also, biomonitoring studies
(carried out mostly in urine samples) have confirmed exposure to
different chemicals. Although the presence of chemicals (or their
metabolites) in biological samples does not constitute a proof of
the occurrence of adverse health effects in humans, it represents a
source of concern. However, there is growing evidence of the
effects of chemical mixtures at concentrations for which individual
components failed to elicit adverse effects when tested
individually (4,5) (Fig.
2).
Data on the effects of chemical mixtures are
increasingly taken into consideration by the scientific community
and regulatory agencies worldwide to issue regulations and
guidelines to understand better the long-term effects of exposure
to environmental (or dietary) mixtures of chemicals in real-life
exposure scenarios and to protect/preserve the human health.
Although humans are not exposed simultaneously to all existing
chemicals; the assessment of an infinite number of potential
chemical combinations in not feasible from a regulatory point of
view. Hence, the most representative chemical mixtures, and their
risk drivers, should be identified at first, and then validated and
internationally accepted tools can be applied to assess their
potential combined effects.
Toxicological studies testing combinations of
chemicals at low doses, around or below their no observed adverse
effect level (NOAEL), mimicking real-life scenarios, under the
framework of real-life risk simulation (RLRS), are imperative to
evaluate the effects induced by these chemical mixtures in humans
(6-13).
Another area of research for future studies
investigating the effects of mixtures of environmental pollutants
is the role of the gut microbiome. Communities of microorganisms
inhabiting the human gastrointestinal tract have the ability to
metabolize a large range of chemicals and affect their therapeutic
efficacy or their toxicity (14).
Since the potential chemical metabolism of the gut microbiome
remains largely uncharacterized, recent reviews have encouraged the
conduct of studies simulating real-life exposure to mixtures using
laboratory animals or simulators of the human gut microbiome
ecosystem (15). Machine learning
algorithms could also help developing reliable approaches to
simulate gut microbiome metabolism and its consequence on human
health in future studies (16).
2. Relevance of exposure to mixtures of
endocrine disruptor chemicals in the context of 21st century
research
One of the most important areas of concern regards
the potential health effects of exposure to low doses of mixtures
of endocrine disruptors (EDs) also known as endocrine-disrupting
chemicals (EDCs). Modern lifestyles result in ubiquitous daily
exposures to a combination of environmental mixtures of EDs that
can accumulate in the body tissues and fluids. Human exposure,
particularly at very low-doses, is continuous and occurs in
different mixtures with potential effects that may not be
predictable when evaluating individual compounds. Thus, the
assessment of potential human risks resulting from exposure to
mixtures of EDs is crucial for consumer safety (17). Moreover, recent evidence indicates
that exposure to these chemicals during development can affect not
only the exposed individuals, but also their offspring and future
generations as a result of epigenetic modifications (18).
Specifically, synthetic compounds can contain
polycyclic aromatic structures, resembling the structure of
endogenous hormones. By interacting on specific receptors, and
depending on their concentration, affinity and potency, they can
elicit effects by mimicking natural hormones. For this reason, such
chemicals can also exert effects even at very low concentrations
(e.g., steroid hormones, such as dehydroepiandrosterone sulphate
(DHEAS) can have effects at femtomolar concentrations) (19). By interfering with physiological
endogenous systems, EDs impair the hormone balance and disrupt
normal function, ultimately inducing toxicological effects.
Exposure to such substances is of particular concern in sensitive
periods, such as the prenatal period, as these exposures can lead
to irreversible changes in the developing organs and increase the
susceptibility to develop diseases later in life. Nevertheless,
there is still controversy concerning the possible role of exposure
at real-life concentrations to environmental chemicals and certain
endocrine-related human diseases, such as hormone-related cancers,
reproductive disorders, obesity, diabetes and neurodevelopment
disorders (20). Certainly, EDs
interfere with brain development through changes in thyroid hormone
levels that are essential for the development of the nervous
system.
Different International Organizations and Agencies
have provided a similar definition for EDs. WHO defines an ED as
‘an exogenous substance or mixture that alters function(s) of the
endocrine system and consequently causes adverse health effects in
an intact organism, or its progeny, or (sub)populations’ (21). This is also the working definition
adopted by the European Commission (EC) (22). The European Food Safety Authority
(EFSA) inserted the term ‘Endocrine Active Substances (EASs)’
defined as ‘any chemical that can interact directly or indirectly
with the endocrine system, and subsequently result in an effect on
the endocrine system, target organs and tissues’ (23). The reason for inserting this term
was to discriminate between chemicals that may interfere with the
endocrine or hormone systems without inducing adverse outcomes.
The Environmental Protection Agency (US-EPA) defined
EDs as ‘exogenous agents that interfere with the production,
release, transport, metabolism, binding, action, or elimination of
the natural hormones in the body responsible for the maintenance of
homeostasis and the regulation of developmental processes’
(24). EDs have been linked from
fertility disturbances to a number of highly prevalent human
pathologies, such as obesity, cancer and diabetes mellitus
(23-32).
Trasande et al (2015) estimated that EDs contribute at least
€157 billion per year to the cost of human disease in the European
Union (EU) (33). In the US, the
estimated figure is even larger, reaching $340 billion per annum
(34).
There are some points to be considered when
discussing EDs in the context of RLRS. Firstly, there is the
incredible chemical diversity of EDs. These can include natural
substances from plants and/or fungi (such as phytoestrogens),
pharmacologically active molecules (such as contraceptive hormones
or molecules used in hormone-responsive malignancies), chemicals
used as additives, preservatives in food/cosmetics, pesticides,
solvents, lubricants, fungicides and other types. Chemical
structures also vary considerably, some of them being clustered
based on their common structure, such as polychlorinated
derivatives, bisphenols, dioxins, phthalates, or diethylstilbestrol
(35,36).
Existing assays are currently focused on the
estrogen, androgen, thyroid and steroidogenesis (EATS) pathways and
less on non-EATS modalities. However, standard chronic apical
toxicity tests are capable of detecting most downstream effects of
perturbation of the non-EATS pathways (20).
A useful toxicological tool for EDs is the Endocrine
Disruptor Knowledge Base (EDKB), an online library available at the
US FDA, containing experimental data for >3,200 chemical
compounds and serves as a resource for both research and regulatory
scientists (37). Based on the
EDKB, the National Center for Toxicological Research (NCTR) of the
US is currently developing methods and models for the computational
prediction of endocrine-related risks.
Similarly, the Endocrine Active Substances
Information System (EASIS) was developed in the EU. EASIS can be
used to search for results from scientific studies on chemicals
related to endocrine activity. Currently, it contains information
on >500 different chemicals based on in vitro and in
vivo assays in various species. However, the presence of a
substance in the database does not mean necessarily that it is an
ED. A new and improved version, EASIS 2.0, is anticipated to be
published soon (38).
The Organization for Economic Cooperation and
Development (OECD), in 2018, updated the document entitled ‘Revised
Guidance Document 150 on Standardized Test Guidelines for
Evaluating Chemicals for Endocrine Disruption’ as a standard for
the assay to be used for the identification of new EDs based on
endocrine signaling pathways (39). When data are lacking, the document
advises the use of quantitative structure-activity relationship
(QSAR) models, analogue, category and read-across approaches for
hazard identification. On its website, the OECD made available a
free QSAR Toolbox that can be used as standalone software or for a
better interpretation of the mechanisms underlying in vivo
results.
The EC requested EFSA and ECHA to develop a common
harmonized guidance to ensure that the endocrine disruptor criteria
adopted by the EU in 2017 are applied consistently for the
assessment of biocides and pesticides. For drafting this guidance,
the Joint Research Centre (JRC), the EC's science and knowledge
service provided its support due to its expertise in the area and
previous reports development (23).
The US EPA has a dedicated Endocrine Disruptor
Screening Program (EDSP) to identify substances that have the
potential to interact with the estrogen, androgen, or thyroid
hormone systems and to establish a dose-effect relationship. The
program uses two major exposure models, the first being
‘off-the-shelf’ chemicals released into the environment by the
industry, and the second concerns consumer and in-home chemicals
ingredients (40). Recent studies
have shown that the ToxCast database can be profitably used to
elucidate the mechanisms of action of chemicals acting as
obesogens, such as neonicotinoids (41), or as estrogen receptor agonists,
such as bisphenol A alternatives (42).
3. Future directions in real-life risk
simulations of EDs
EDs mixtures used in experimental studies are very
simple and consist of unrealistic mixtures compared to the
real-world scenario. As such, the net effect in humans of a mixture
of numerous EDs with diverse activities is unpredictable and
requires further developments. Computational methods are an
essential tool in the drug discovery process, and they are
intensively used for the identification of new EDs, considering the
time and cost consuming efforts to test all household and
industrial chemical ingredients. Computational methods are an
important complementary tool for in vitro and in vivo
toxicity tests with a high predictive potential that can contribute
to identify and assessing risks, and ultimately to reduce animal
testing, cost and time (43). The
application of machine learning methods on toxicological ‘big data’
has already been shown to outperform animal test reproducibility
(36). This has also been proven
to be a successful strategy for determining the effects of chemical
mixtures, such as those comprised by EDs. For example, a recent
study identified that both the pharmacological estrogen,
17α-ethinylestradiol, and the pesticide, trans-Nonachlor, were not
able to activate the pregnane X receptor (PXR) individually;
however, when combined, they were efficacious. A biophysical
analysis complemented by structural bioinformatics analysis
revealed that these compounds formed supramolecular ligands,
allowing the combined chemical structure to fit into and activate
the ligand binding pocket of the PXR (44).
There is a wide range of computational models,
varying from read across, chemical categories, absorption,
distribution, metabolism, and elimination (ADME) predictive models,
physiologically-based pharmacokinetic (PBPK) models, quantitative
structure activity relationships (QSARs), docking and molecular
dynamics that are currently used to identify new EDs and to predict
their mechanisms of action (45).
The predictive power of these methods depends on their selectivity
and specificity (46). For
example, some studies have suggested that molecular docking methods
are not the best choice to evaluate androgen receptor antagonists,
while the results of QSAR analyses and molecular dynamics
simulations have acceptable sensitivities and specificities
(46,47). Kar et al developed a QSAR
model to evaluate mixtures of perfluoroalkyl substances (PFASs), an
important class of ED pollutants, based on zebrafish embryos
development data. The predicted chemicals mixtures displayed a
concentration addition pattern suggesting a similar mode of toxic
action and non-interaction (48).
In the case of estrogen receptors, a large range of methods has
been applied in large-scale modeling projects (49). Molecular dynamics simulations have
also been used to study the interaction between glyphosate and
estrogen receptor alpha (42). It
is crucial that researchers and all regulatory agencies understand
the drawbacks, limitations and confidence limits of each method. As
an example, pharmacophore models work very well for estrogen and
androgen receptors, although they are limited in the case of
various enzymes that control hormone metabolism (50,51).
There is no single tool available with which to
identify all types of potentially active groups; thus, several
methods need to be used. The accumulation of biological data from
several types of assays on EDs will increase the accuracy of the
computational models and will certainly expand their usefulness. In
addition, with 48 nuclear hormone receptors (52), many more peptide receptors and an
unknown number of signaling pathways as potential targets for these
chemicals, the conceivable effects on human biological pathways is
massive.
Another important point is the difficulty
encountered in the extrapolation of the effects induced by the EDs
from an in vivo tested dose to a RLRS model, based on the
fact that the dose-response curves for such chemicals are under an
intense debate (53,54). As an example, for a number of
years, the regulation of pesticides has been based on the paradigm
that larger doses (above the NOAEL) result in larger effects, i.e.,
‘the dose makes the poison’. However, studies published over the
last 15 years have demonstrated toxic effects of combinations of
chemicals at concentrations lower than the NOAEL that can disrupt
biological systems (55),
suggesting that this area requires further scrutiny (56). The cumulative risk assessment of
chemicals in mixtures should be considered in addition to the
evaluation of their individual effects (57). Another area of interest that
represents a relevant challenge to human health is the
non-monotonic dose-response relationships (NMDR). Under this
hypothesis, the effects at low doses cannot be predicted from
effects at high doses and, if confirmed, chemical testing would
need to be changed to protect human health. One review of 51
studies identified 170 non-monotonic dose-response relationships
(58); nevertheless, the majority
of data comes from in vitro studies.
Evaluating the effects induced by mixtures of
chemicals, only considering the ED class, can be very challenging
due to the multitude of possible complex combinations of
chemicals/chemical classes that humans can come in contact with.
Sarigiannis et al proposed a comprehensive framework for
addressing this challenge (59).
The identification of an effective model to test the
above-mentioned effects, is the first step in this type of
research. There are, of course, two criteria to be met: to find the
mixture of molecules that mimics best real-life situations and to
find a way to evaluate the effects induced in vivo. Since
the doses are low, the realization of chronic feeding studies is
preferable, but complex and costly. In this context, molecular
modeling may constitute a solid first step in such an endeavor.
It is practically impossible to test all the
combinations of EDs, even for binary mixtures. The groundbreaking
work of Bliss identified three categories of joint action in
mixtures (60). In the first
pattern, the combined effect is the sum of the components, their
toxic effect being independent, even if the toxicological mechanism
is the same or not. It is the simplest case, as the proportions of
each component do not alter their combined effect. In the second
case, the toxic effect is not independent and it can be greater
than that of each constituent in the case of a synergistic action
or lower in the case of antagonistic effects. In the particular
case of ED mixtures, the synergistic action is the most important
and several models have been developed to address this problem
(61). However, at low exposure
levels (around the NOAEL), synergism or antagonism are considered
to be unlikely or toxicologically insignificant.
The generalized concentration addition is a
mathematical model that evaluates the interaction between mixtures
components using a response function independent of the response
functions of each individual constituent. The receptor-oriented
approach in cumulative risk assessment changes the paradigm from
the traditional source-oriented approach, focusing on the exposure
assessment of humans to EDs, coupled with effect assessment
considering a time variable exposure.
Acknowledging
the infinite possible combination of mixtures, the
development of hazard estimation approaches fit for purpose,
instead of the “umbrella” approaches to cover all grounds, might be
more appropriate in certain cases (62). With a view to alleviate
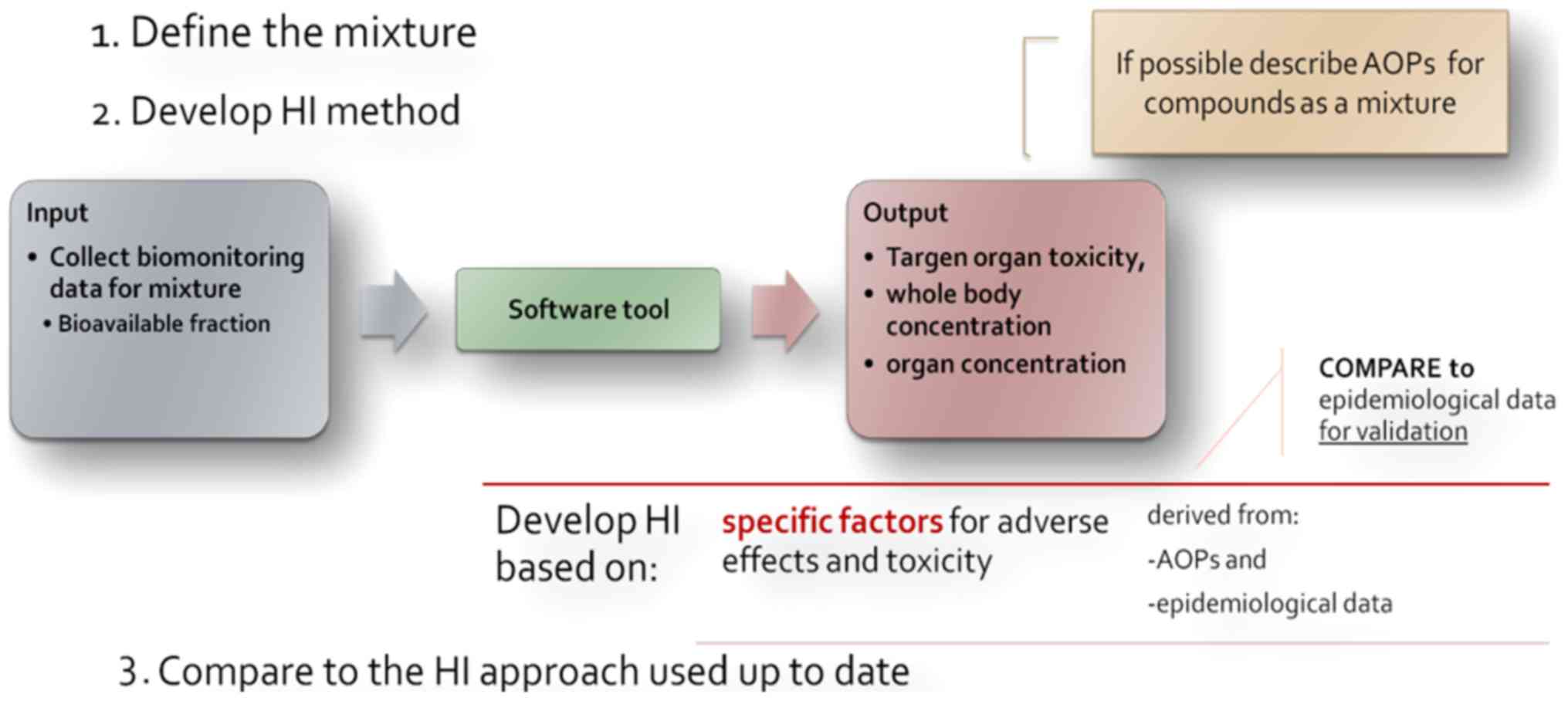
uncertainties, a method outline for defined mixtures is proposed
(Fig. 3). A three-step process is
described, where firstly the mixture in question has to be defined.
The mixture (components and portions) is meant to reflect real
exposures [e.g., measuring the occurrence and concentration of
substances in drinking water of a specific area for the development
of a specific hazard index (HI)].
Moving to the second step, the basic notion for the
development of the HI is to collect human biomonitoring data on the
substances determined and via a software tool, which assesses
aggregated (e.g., INTEGRA) or cumulative exposure, to obtain data
on certain adverse effects' markers, e.g., biomarkers of target
organ toxicity. The data obtained from the in silico model could be
integrated with data from other lines of evidence (in vivo,
in vitro, epidemiology) concerning the same compounds. This
could help i) fill the gaps in describing adverse outcome oathways
(AOPs) for the specific mixture and not individual substances; and
ii) develop an adversity specific HI which is to be compared with
the currently applied HI and evaluated accordingly, as the last
step of the proposed process.
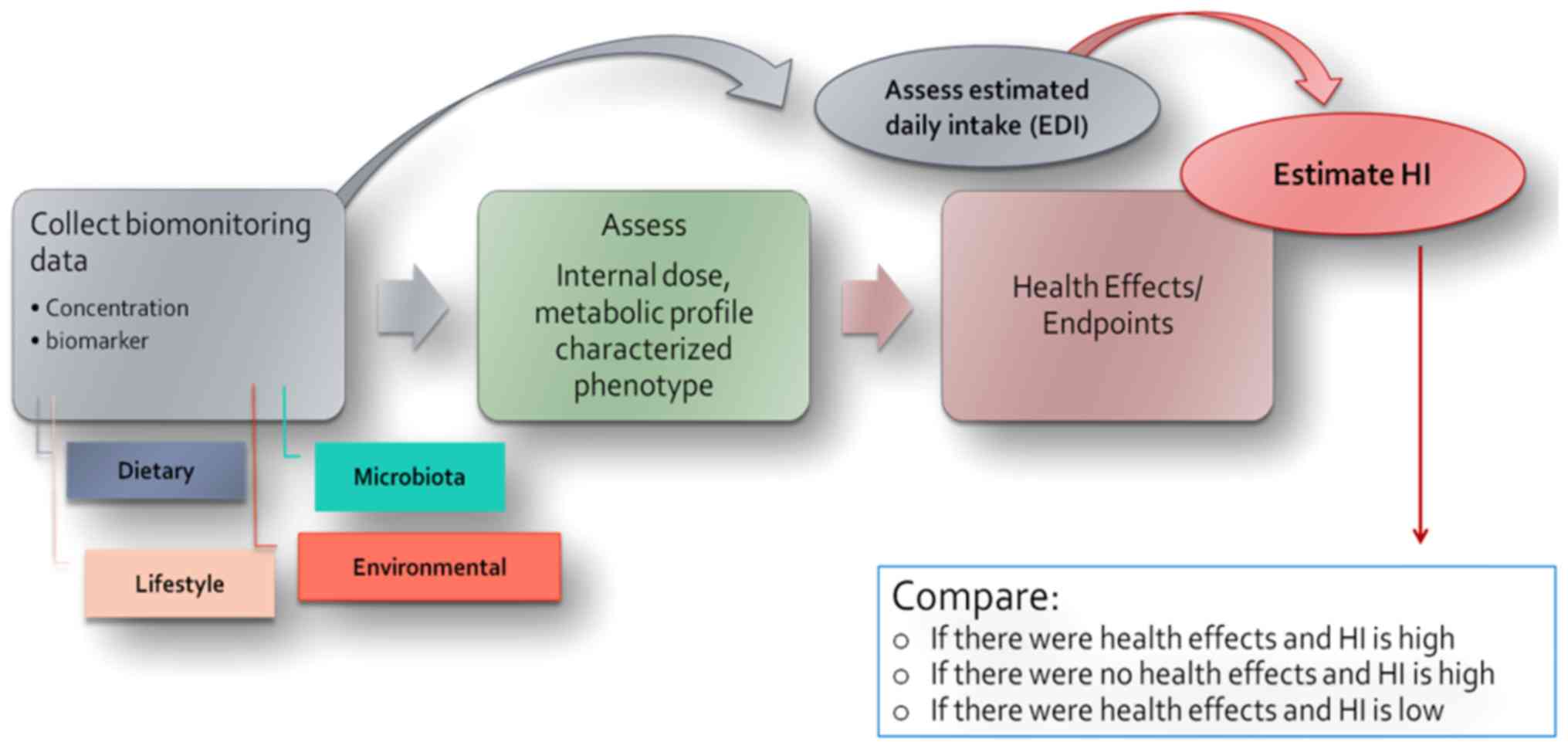
Furthermore, aiming to reach more realistic risk
characterization methods, an extension of the internal dose
approach is proposed (Fig. 4).
This approach is based on collecting human biomonitoring data
regarding cumulative exposure (dietary, lifestyle, environmental
and microbiota) in order to assess the internal dose for the
compounds of a specific mixture or characterize the metabolic
profile phenotype. Following the internal dose assessment, health
effects and toxicity endpoints can be determined. The biomonitoring
data could additionally be used for assessing the estimated daily
intake (EDI) (63) and
subsequently estimating the HI. A comparison between described
health effects and the HI estimated could serve as an evaluation of
the method (64).
4. Conclusions
Endocrine-mediated adverse effects of chemical
mixtures cannot be always identified in standard toxicological
studies performed to comply with regulatory requirements.
Therefore, supplementary and more focused mechanistic studies may
be necessary to further investigate an endocrine mode of action.
Despite all limitations, it can be considered that the use of in
silico methods to evaluate complex RLRS models will have a great
impact and such methods will become a powerful toxicological tool.
Those methods can contribute to the identification of potential new
EDs and to the prediction of their toxicological targets, thus
becoming an effective method to concentrate on similar toxicity
pathways and mechanisms of action.
Acknowledgements
Not applicable.
Funding
Michael Aschner was supported by National Institute
of Health (NIH) R01 ES10563, R01 ES07331 and R01 ES020852. This
study was also partially supported by the Special Research Account
of University of Crete (ELKE No. 4920), the University of Crete
spin-off ToxPlus S.A.
Availability of data and materials
Not applicable.
Authors' contributions
DM and AU wrote sub-sections 1 and 2. GMN, RM, MG,
DASa and EAR contributed to sub-sections 2 and 3. DM, AU, GMN, RM,
MG, DASa and EAR also contributed to the search of the literature
for this review and to the selection of appropriate references for
citation. MA, DASp, AFH and AT performed the critical review of the
manuscript and contributed to the conception and design of the
study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dereumeaux C, Saoudi A, Pecheux M, Berat
B, de Crouy-Chanel P, Zaros C, Brunel S, Delamaire C, le Tertre A,
Lefranc A, et al: Biomarkers of exposure to environmental
contaminants in French pregnant women from the Elfe cohort in 2011.
Environ Int. 97:56–67. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tsatsakis A, Goumenou M, Liesivuori J,
Dekant W and Hernández AF: Toxicology for real-life risk simulation
- Editorial preface to this special issue. Toxicol Lett. 309:33–34.
2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kar S and Leszczynski J: Exploration of
Computational Approaches to Predict the Toxicity of Chemical
Mixtures. Toxics. 7(7)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
da Fonseca TG, Abessa DMS and Bebianno MJ:
Effects of mixtures of anticancer drugs in the benthic polychaete
Nereis diversicolor. Environ Pollut. 252 (Pt B):1180–1192.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thrupp TJ, Runnalls TJ, Scholze M,
Kugathas S, Kortenkamp A and Sumpter JP: The consequences of
exposure to mixtures of chemicals: Something from ‘nothing’ and ‘a
lot from a little’ when fish are exposed to steroid hormones. Sci
Total Environ. 619-620:1482–1492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tsatsakis AM, Docea AO, Calina D, Buga AM,
Zlatian O, Gutnikov S, Kostoff RN and Aschner M: Hormetic
Neurobehavioral effects of low dose toxic chemical mixtures in
real-life risk simulation (RLRS) in rats. Food Chem Toxicol.
125:141–149. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsatsakis AM, Docea AO and Tsitsimpikou C:
New challenges in risk assessment of chemicals when simulating real
exposure scenarios; simultaneous multi-chemicals' low dose
exposure. Food Chem Toxicol. 96:174–176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tsatsakis AM, Kouretas D, Tzatzarakis MN,
Stivaktakis P, Tsarouhas K, Golokhvast KS, Rakitskii VN, Tutelyan
VA, Hernandez AF, Rezaee R, et al: Simulating real-life exposures
to uncover possible risks to human health: A proposed consensus for
a novel methodological approach. Hum Exp Toxicol. 36:554–564.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kostoff RN, Goumenou M and Tsatsakis A:
The role of toxic stimuli combinations in determining safe exposure
limits. Toxicol Rep. 5:1169–1172. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Docea AO, Calina D, Goumenou M, Neagu M,
Gofita E and Tsatsakis A: Study design for the determination of
toxicity from long-term-low-dose exposure to complex mixtures of
pesticides, food additives and lifestyle products. Toxicol Lett.
258(S179)2016. View Article : Google Scholar
|
|
11
|
Docea AO, Gofita E, Goumenou M, Calina D,
Rogoveanu O, Varut M, Olaru C, Kerasioti E, Fountoucidou P,
Taitzoglou I, et al: Six months exposure to a real life mixture of
13 chemicals' below individual NOAELs induced non monotonic
sex-dependent biochemical and redox status changes in rats. Food
Chem Toxicol. 115:470–481. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Docea AO, Goumenou M, Calina D, Arsene AL,
Dragoi CM, Gofita E, Pisoschi CG, Zlatian O, Stivaktakis PD,
Nikolouzakis TK, et al: Adverse and hormetic effects in rats
exposed for 12 months to low dose mixture of 13 chemicals: RLRS
part III. Toxicol Lett. 310:70–91. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Renieri EA, Goumenou M, Kardonsky DA,
Veselov VV, Alegakis AΚ, Buha A, Tzatzarakis MN, Nosyrev AE,
Rakitskii VN, Kentouri M, et al: Indicator PCBs in farmed and wild
fish in Greece - Risk assessment for the Greek population. Food
Chem Toxicol. 127:260–269. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mesnage R, Antoniou MN, Tsoukalas D,
Goulielmos GN and Tsatsakis A: Gut microbiome metagenomics to
understand how xenobiotics impact human health. Curr Opin Toxicol.
11-12:51–58. 2018.
|
|
15
|
Tsiaoussis J, Antoniou MN, Koliarakis I,
Mesnage R, Vardavas CI, Izotov BN, Psaroulaki A and Tsatsakis A:
Effects of single and combined toxic exposures on the gut
microbiome: Current knowledge and future directions. Toxicol Lett.
312:72–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Espinoza JL: Machine learning for tackling
microbiota data and infection complications in immunocompromised
patients with cancer. J Intern Med. 284:189–192. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ribeiro E and Ladeira C and Viegas S: EDCs
Mixtures: A Stealthy Hazard for Human Health? Toxics.
5(E5)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Street ME, Angelini S, Bernasconi S,
Burgio E, Cassio A, Catellani C, Cirillo F, Deodati A, Fabbrizi E,
Fanos V, et al: Current knowledge on endocrine disrupting chemicals
(EDCs) from animal biology to humans, from pregnancy to adulthood:
Highlights from a national italian meeting. Int J Mol Sci.
19(E1647)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Munn S and Goumenou M: Thresholds for
Endocrine Disrupters and Related Uncertainties. Report of the
Endocrine Disrupters Expert Advisory Group. Publications Office of
the European Union, 2013. https://publications.europa.eu/en/publication-detail/-/publication/a1a68b31-c013-484a-b4f0-ce089822aad8/language-en.
Accessed July 11. 2014.
|
|
20
|
Day P, Green RM, Gross M, Weltje L and
Wheeler JR: Endocrine Disruption: Current approaches for regulatory
testing and assessment of plant protection products are fit for
purpose. Toxicol Lett. 296:10–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
International Programme on Chemical
Safety: Global assessment on the state of the science of endocrine
disruptors. World Health Organization, Geneva, 2002. https://apps.who.int/iris/handle/10665/67357.
|
|
22
|
Munn S and Goumenou M: Key scientific
issues relevant to the identification and characterisation of
endocrine disrupting substances. Report of the Endocrine Disrupters
Expert Advisory Group. Publications Office of the European Union,
2013. https://publications.europa.eu/en/publication-detail/-/publication/4b84ccc2-422d-4bd1-97da-1f414ad52c27/language-en.
Accessed April 2. 2013.
|
|
23
|
European Chemicals Agency (ECHA) and
European Food Safety Authority (EFSA) with support from the Joint
Research Centre (JRC): Guidance for the identification of endocrine
disruptors in the context of Regulations (EU) No 528/2012 and (EC)
No 1107/2009. EFSA J 16: e05311, 2018.
|
|
24
|
Schug TT, Johnson AF, Birnbaum LS, Colborn
T, Guillette LJ Jr, Crews DP, Collins T, Soto AM, Vom Saal FS,
McLachlan JA, et al: Minireview: Endocrine Disruptors: Past Lessons
and Future Directions. Mol Endocrinol. 30:833–847. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Darbre PD: Endocrine Disruptors and
Obesity. Curr Obes Rep. 6:18–27. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ravichandran G, Lakshmanan DK, Raju K,
Elangovan A, Nambirajan G, Devanesan AA and Thilagar S: Food
advanced glycation end products as potential endocrine disruptors:
An emerging threat to contemporary and future generation. Environ
Int. 123:486–500. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee YM, Jacobs DR Jr and Lee DH:
Persistent Organic Pollutants and Type 2 Diabetes: A Critical
Review of Review Articles. Front Endocrinol (Lausanne).
9(712)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lind PM and Lind L: Endocrine-disrupting
chemicals and risk of diabetes: An evidence-based review.
Diabetologia. 61:1495–1502. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Idowu O, Semple KT, Ramadass K, O'Connor
W, Hansbro P and Thavamani P: Beyond the obvious: Environmental
health implications of polar polycyclic aromatic hydrocarbons.
Environ Int. 123:543–557. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Di Nisio A and Foresta C: Water and soil
pollution as determinant of water and food quality/contamination
and its impact on male fertility. Reprod Biol Endocrinol.
17(4)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rahmani S, Pour Khalili N, Khan F, Hassani
S, Ghafour-Boroujerdi E, Abdollahi M and Bisphenol A: Bisphenol A:
What lies beneath its induced diabetes and the epigenetic
modulation? Life Sci. 214:136–144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Petrakis D, Vassilopoulou L, Mamoulakis C,
Psycharakis C, Anifantaki A, Sifakis S, Docea AO, Tsiaoussis J,
Makrigiannakis A and Tsatsakis AM: Endocrine Disruptors Leading to
Obesity and Related Diseases. Int J Environ Res Public Health.
14(1282)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Trasande L, Zoeller RT, Hass U, Kortenkamp
A, Grandjean P, Myers JP, DiGangi J, Bellanger M, Hauser R, Legler
J, et al: Estimating burden and disease costs of exposure to
endocrine-disrupting chemicals in the European union. J Clin
Endocrinol Metab. 100:1245–1255. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Attina TM, Hauser R, Sathyanarayana S,
Hunt PA, Bourguignon JP, Myers JP, DiGangi J, Zoeller RT and
Trasande L: Exposure to endocrine-disrupting chemicals in the USA:
A population-based disease burden and cost analysis. Lancet
Diabetes Endocrinol. 4:996–1003. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
De Coster S and van Larebeke N:
Endocrine-disrupting chemicals: Associated disorders and mechanisms
of action. J Environ Public Health. 2012(713696)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Luechtefeld T, Marsh D, Rowlands C and
Hartung T: Machine Learning of Toxicological Big Data Enables
Read-Across Structure Activity Relationships (RASAR) Outperforming
Animal Test Reproducibility. Toxicol Sci. 165:198–212.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ding D, Xu L, Fang H, Hong H, Perkins R,
Harris S, Bearden ED, Shi L and Tong W: The EDKB: An established
knowledge base for endocrine disrupting chemicals. BMC
Bioinformatics. 11 (Suppl 6)(S5)2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Castello P, Wittwehr C and Goumenou MP:
Endocrine Active Substances Information System (EASIS) Software
Implementation Plan. Technical Report. EU PUBSY No. JRC76923.
2012.
|
|
39
|
OECD: Revised Guidance Document 150 on
Standardised Test Guidelines for Evaluating Chemicals forEndocrine
Disruption OECD Series on Testing and Assessment. OECD Publishing,
Paris, 2018. https://doi.org/10.1787/9789264304741-en.
|
|
40
|
Browne P, Noyes PD, Casey WM and Dix DJ:
Application of adverse outcome pathways to U.S. EPA's endocrine
disruptor screening program. Environ Health Perspect.
125(096001)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mesnage R, Biserni M, Genkova D,
Wesolowski L and Antoniou MN: Evaluation of neonicotinoid
insecticides for oestrogenic, thyroidogenic and adipogenic activity
reveals imidacloprid causes lipid accumulation. J Appl Toxicol.
38:1483–1491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mesnage R, Phedonos A, Arno M, Balu S,
Corton JC and Antoniou MN: Editor's Highlight: Transcriptome
Profiling Reveals Bisphenol A Alternatives Activate Estrogen
Receptor Alpha in Human Breast Cancer Cells. Toxicol Sci.
158:431–443. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Raies AB and Bajic VB: In silico
toxicology: Computational methods for the prediction of chemical
toxicity. Wiley Interdiscip Rev Comput Mol Sci. 6:147–172.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Delfosse V, Dendele B, Huet T, Grimaldi M,
Boulahtouf A, Gerbal-Chaloin S, Beucher B, Roecklin D, Muller C,
Rahmani R, et al: Synergistic activation of human pregnane X
receptor by binary cocktails of pharmaceutical and environmental
compounds. Nat Commun. 6(8089)2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sarigiannis DA and Gotti A: Biology-based
dose-response models for health risk assessment of chemical
mixtures. Fresenius Environ Bull. 17:1439–1451. 2008.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Vuorinen A, Odermatt A and Schuster D: In
silico methods in the discovery of endocrine disrupting chemicals.
J Steroid Biochem Mol Biol. 137:18–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wahl J and Smieško M: Endocrine Disruption
at the Androgen Receptor: Employing Molecular Dynamics and Docking
for Improved Virtual Screening and Toxicity Prediction. Int J Mol
Sci. 19(1784)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kar S, Ghosh S and Leszczynski J: Single
or mixture halogenated chemicals? Risk assessment and developmental
toxicity prediction on zebrafish embryos based on weighted
descriptors approach. Chemosphere. 210:588–596. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mansouri K, Abdelaziz A, Rybacka A,
Roncaglioni A, Tropsha A, Varnek A, Zakharov A, Worth A, Richard
AM, Grulke CM, et al: CERAPP: Collaborative Estrogen Receptor
Activity Prediction Project. Environ Health Perspect.
124:1023–1033. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kaserer T, Beck RK, Akram M, Odermatt A
and Schuster D: Pharmacophore Models and Pharmacophore-Based
Virtual Screening: Concepts and Applications Exemplified on
Hydroxysteroid Dehydrogenases. Molecules. 20:22799–22832.
2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ruiz P, Sack A, Wampole M, Bobst S and
Vracko M: Integration of in silico methods and computational
systems biology to explore endocrine-disrupting chemical binding
with nuclear hormone receptors. Chemosphere. 178:99–109.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Robinson-Rechavi M, Carpentier A-S,
Duffraisse M and Laudet V: How many nuclear hormone receptors are
there in the human genome? Trends Genet. 17:554–556.
2001.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang Z, Liu H and Liu S: Low-Dose
Bisphenol A Exposure: A Seemingly Instigating Carcinogenic Effect
on Breast Cancer. Adv Sci (Weinh). 4(1600248)2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Nohynek GJ, Borgert CJ, Dietrich D and
Rozman KK: Endocrine disruption: Fact or urban legend? Toxicol
Lett. 223:295–305. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schneider S, Fussell KC, Melching-Kollmuss
S, Buesen R, Gröters S, Strauss V, Jiang X and van Ravenzwaay B:
Investigations on the dose-response relationship of combined
exposure to low doses of three anti-androgens in Wistar rats. Arch
Toxicol. 91:3961–3989. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Roszko MŁ, Kamińska M, Szymczyk K,
Piasecka-Jóźwiak K and Chabłowska B: Endocrine disrupting potency
of organic pollutant mixtures isolated from commercial fish oil
evaluated in yeast-based bioassays. PLoS One.
13(e0197907)2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Seeger B, Klawonn F, Nguema Bekale B and
Steinberg P: Mixture Effects of Estrogenic Pesticides at the Human
Estrogen Receptor α and β. PLoS One. 11(e0147490)2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Vandenberg LN, Colborn T, Hayes TB,
Heindel JJ, Jacobs DR Jr, Lee DH, Shioda T, Soto AM, vom Saal FS,
Welshons WV, et al: Hormones and endocrine-disrupting chemicals:
Low-dose effects and nonmonotonic dose responses. Endocr Rev.
33:378–455. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Sarigiannis D, Gotti A, Cimino Reale G and
Marafante E: Reflections on new directions for risk assessment of
environmental chemical mixtures. Int J Risk Assess Manag.
13:216–241. 2009. View Article : Google Scholar
|
|
60
|
Bliss CI: The calculation of the
dosage-mortality curve. Ann Appl Biol. 22:134–167. 1935. View Article : Google Scholar
|
|
61
|
Sarigiannis DAHU and Hansen U: Considering
the cumulative risk of mixtures of chemicals - a challenge for
policy makers. Environ Health. 11 (Suppl 1)(S18)2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Goumenou M and Tsatsakis A: Proposing new
approaches for the risk characterisation of single chemicals and
chemical mixtures: The source related Hazard Quotient (HQS) and
Hazard Index (HIS) and the adversity specific Hazard Index (HIA).
Toxicol Rep. 6:632–636. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Katsikantami I, Colosio C, Alegakis A,
Tzatzarakis MN, Vakonaki E, Rizos AK, Sarigiannis DA and Tsatsakis
AM: Estimation of daily intake and risk assessment of
organophosphorus pesticides based on biomonitoring data - The
internal exposure approach. Food Chem Toxicol. 123:57–71.
2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Papadakis GZ, Karantanas AH, Tsiknakis M,
Tsatsakis A, Spandidos DA and Marias K: Deep learning opens new
horizons in personalized medicine. Biomed Rep. 10:215–217.
2019.PubMed/NCBI View Article : Google Scholar
|