Introduction
Chronic inflammation of the mammary epithelium may
result from tissue damage, leading to the sustained overexpression
of cyclooxygenase (COX) and biosynthesis of inflammatory
prostaglandins from arachidonic acid. This inflammatory environment
may stimulate key components of mammary carcinogenesis
(mitogenesis, mutagenesis, angiogenesis, reduced apoptosis,
immunosuppression and metastasis), potentially leading to the
development of breast cancer. Reciprocally, agents that inhibit COX
reduce the risk of breast cancer (1,2).
Lipoxygenase (LOX) enzymes also create an inflammatory environment
by catalyzing the conversion of arachidonic acid to inflammatory
eicosanoids, principally hydroperoxy-eicosatetraenoic acid (HPETE)
and leukotrienes. The overexpression of LOX promotes the
development of certain autoimmune conditions (rheumatoid arthritis)
and allergic reactions (asthma). Accumulating evidence from
laboratory and animal studies suggests that the overexpression of
LOX promotes carcinogenesis, and reciprocally, agents that inhibit
the LOX cascade interrupt cancer development (3-6).
Notably, a recent investigation of gene expression
data in tissue samples from 1,090 cases of invasive breast cancer
from The Cancer Genome Atlas (TCGA) revealed that genes encoding
COX-1 and COX-2, and two LOX proteins, arachidonate lipoxygenase-5
(ALOX-5) and ALOX-5-activating protein (ALOX-5AP), were expressed
in all subtypes of breast cancer. In addition, the expression
levels of these inflammatory genes were highly correlated with the
expression levels of 18 tumor promoting genes with documented
involvement in mammary carcinogenesis. Inflammatory genes were also
highly correlated with CYP-19 P-450arom (aromatase) in
all breast cancer subtypes, suggesting the importance of sustained
paracrine estrogen biosynthesis in breast cancer development
(7).
To the best of our knowledge, only one known
previous human study has reported the effects of LOX inhibitors on
breast cancer risk among women and it examined only subjects with
asthma (8). This nested cohort
study conducted in Taiwan found that among women with reported
asthma, the use of certain LOX inhibiting drugs [cysteinyl
leukotriene receptor antagonists (LTRAs)], significantly reduced
their risk of breast cancer as well as their overall cancer risk.
The investigators reported that, at a given time, for every 31
cancer cases identified in the LTRA treatment group, there were 69
in the untreated group. Their findings for breast cancer were
stronger. Stratifying on the type of cancer, they found that for
every 9 breast cancer cases among the LTRA-treated group, there
were 91 among the untreated group [hazard ratio (HR), 0.09; 95%
confidence interval (CI), 0.03-0.26] (8).
An emerging consensus in the field of
chemoprevention is that the complexities of the carcinogenic
process will require a combination of agents targeting multiple
pathways involved in the inflammogenesis of breast cancer to
achieve optimal efficacy. Nevertheless, there are no known human
studies on the effects of LOX inhibitors or the combined use of COX
and LOX inhibitors on breast cancer in the general female
population. Therefore, herein, an epidemiological case-control
study was conducted to investigate the association of breast cancer
risk with exposure to compounds that modulate either the COX or LOX
cascades, or both.
Patients and methods
Patients
A total of 611 cases of invasive breast cancer with
histological verification based on the review of the pathology
records, and 615 group-matched controls with no personal history of
cancer and no current breast disease based on screening mammography
were examined. Cases were interviewed at the time of their
diagnosis during 2003 through September, 2004 at The Arthur G.
James Cancer Hospital and Richard J. Solove Research Institute,
Columbus, Ohio. The controls were patients at the mammography
service of the cancer hospital during the same time period and
frequency matched to the cases by 5-year age interval, race and
place (county) of residence. Screening mammography findings were
normal for all controls. The study was approved by The Ohio State
University Medical Center Institutional Review Board (IRB) and
conducted in full compliance with ethical standards of the US
National Institutes of Health for human medical research. Written
informed consent was obtained from each study participant to
provide information for analysis and publication of results.
Information on COX- and LOX-modulating agents and
other factors was obtained utilizing medical records and a
standardized risk factor questionnaire. The questionnaires were
administered in person by trained medical personnel prior to
definitive surgery or treatment for the cases and at the time of
screening mammography for controls. The data variables collected
consisted of demographic characteristics, height, weight, menstrual
and pregnancy history, family history of breast and ovarian cancer,
comprehensive information on cigarette smoking, alcohol intake,
pre-existing medical conditions (arthritis, chronic headache,
cardiovascular conditions including hypertension, angina, ischemic
attacks, stroke and myocardial infarction, lung disease, and
diabetes mellitus), and medication history including over the
counter and prescription agents.
The usage patterns of COX and LOX modulating agents
(frequency, dose and duration) and the type were recorded for each
participant. The COX inhibitors included compounds selective for
COX-2 (celecoxib, rofecoxib and meloxicam) and non-selective (COX-1
or COX-2 or both) non-steroidal anti-inflammatory drugs (NSAIDs),
such as aspirin, ibuprofen, naproxen and indomethacin. The
LOX-modulating agents prescribed for the treatment of asthma
included zileuton, an inhibitor of 5-lipoxygenase, bestatin, an
inhibitor of leukotriene B4, the leukotriene receptor antagonists,
pranlukast, montelukast and zafirlukast, as well as theophylline
and cromolyn sodium. Since the frequency of using LOX-modulating
medications was low (approximately 10%), exposure to these
compounds was investigated by pooling the use of any of them.
Statistical analysis
Case-control differences in means and frequencies
were examined for statistical significance by t-tests and
Chi-square tests, respectively. Logistic regression was used to
estimate odds ratios (ORs) to quantify the association between the
pre-diagnostic use of COX and LOX inhibitors, separately and
together, with breast cancer risk. The ORs were adjusted for age
and classic breast cancer risk factors (parity, family history,
body mass, menopausal status, chronic smoking and regular alcohol
intake). Estimates of the independent effects of COX or LOX
inhibitors also were adjusted for each other (9,10).
Results
The pertinent characteristics of the cases and
controls are presented in Table
I. The cases exhibited higher frequencies of nulliparity, a
family history of breast or ovarian cancer and estrogen replacement
therapy in post-menopausal subjects. As expected, (due to group
matching), the cases and controls had similar distributions of age,
race and education.
 | Table ICharacteristics of breast cancer cases
and controls. |
Table I
Characteristics of breast cancer cases
and controls.
|
Characteristica | Cases (n=611)
(%) | Controls (n=615)
(%) |
|---|
| Age (years) |
|
<50 | 19 | 20 |
|
50-65 | 55 | 52 |
|
>65 | 26 | 28 |
|
Mean
(SEM) | 55.8 (0.8) | 55.2 (0.4) |
| Race |
|
Caucasian | 91 | 89 |
|
All
other | 9 | 11 |
| Education |
|
<12
years | 12 | 12 |
|
12
years | 53 | 55 |
|
>12
years | 31 | 33 |
| Parity |
|
Nulliparous | 6 | 4 |
|
First
pregnancy <30 years | 83 | 89 |
|
First
pregnancy >30 years | 11 | 7 (P<0.05) |
| Family history |
|
Positive | 32 | 17 |
|
Negative | 68 | 83 (P<0.01) |
| Body mass |
|
BMI
<22 | 23 | 21 |
|
BMI
22-28 | 35 | 39 |
|
BMI
>28 | 42 | 40 |
|
Mean
(SEM) | 27.5 (0.9) | 27.1 (0.7) |
| Menopausal
status |
|
Premenopausal | 41 | 47 |
|
Postmenopausal | 52 | 53 |
| Postmenopausal
ERT | 38 | 31 (P<0.05) |
| Smoking |
|
Never
smoker | 35 | 32 |
|
Ex-smoker | 38 | 40 |
|
Current
smoker | 27 | 28 |
| Alcohol intake |
|
None | 47 | 45 |
|
1-2 drinks
per week | 36 | 35 |
|
>2 drinks
per week | 17 | 20 |
The comparative frequencies of the COX and LOX
modulating agents under study with adjusted ORs and their 95 CIs
are shown in Table II. In the
table, the numbers of cases and controls using both COX and LOX
inhibitors are shown separately from those using only individual
compounds.
 | Table IIOdds ratios with 95% confidence
intervals for breast cancer and COX inhibitors, LOX inhibitors, and
the combined use of COX and LOX (COX/LOX) inhibitors. |
Table II
Odds ratios with 95% confidence
intervals for breast cancer and COX inhibitors, LOX inhibitors, and
the combined use of COX and LOX (COX/LOX) inhibitors.
| Compound | Cases | Controls | Adjusted
ORb (95% CI) |
|---|
|
Referencea | 480 | 353 | 1.00 |
| COX Inhibitors | 95 | 200 | 0.38 (0.27-0.54)
(P<0.001) |
| LOX Inhibitors | 27 | 39 | 0.52 (0.32-0.82)
(P<0.01) |
| COX/LOX
Inhibitors | 9 | 23 | 0.26 (0.11-0.64)
(P<0.01) |
| Totals | 611 | 615 | |
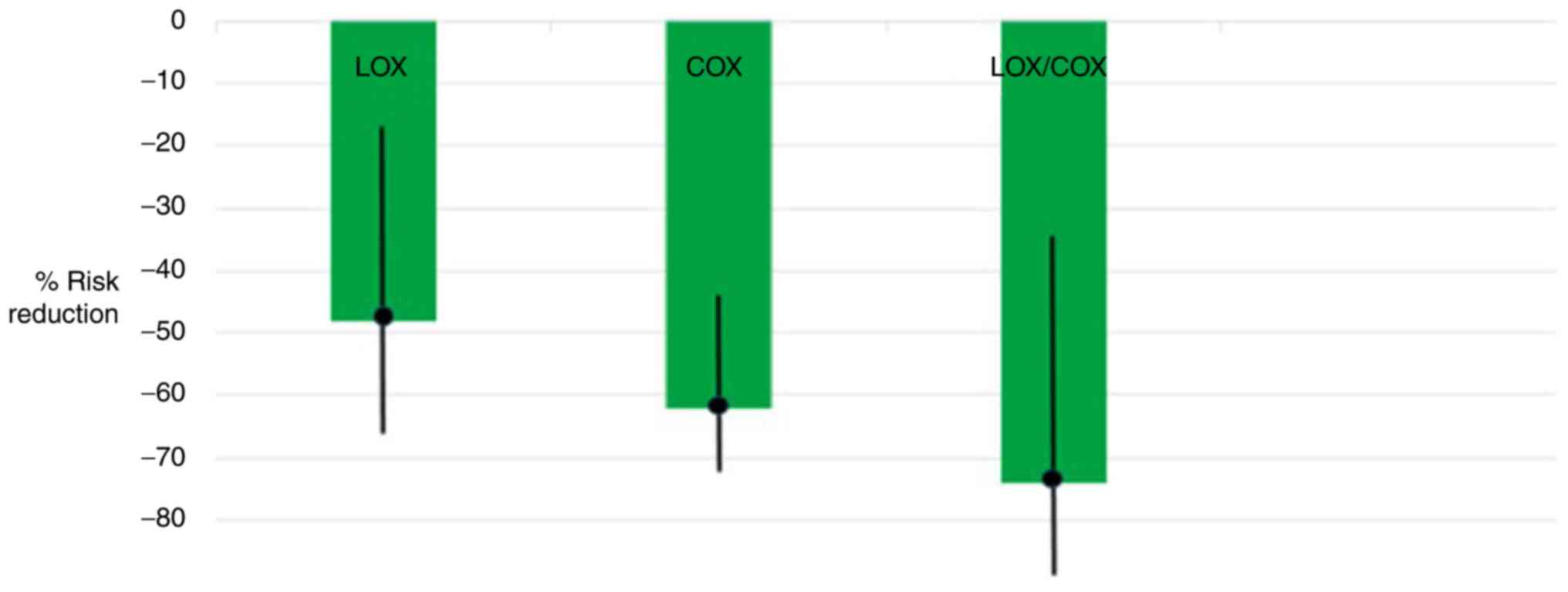
Risk reductions were found for COX (OR, 0.38; 95%
CI, 0.27-0.54; P<0.001) and LOX inhibitors (OR, 0.52; 95% CI,
0.32-0.82; P<0.01). Notably, the combined use of COX and LOX
inhibitors produced the greatest risk reduction (OR, 0.26; 95% CI,
0.11-0.64; P<0.01). These results are illustrated in Fig. 1. In Fig. 1, the ORs with 95% CIs were
converted to percentage risk reductions by subtracting estimates
from 1.0 and multiplying by 100. Estimates were similar with and
without adjustment for potential confounders and among subgroups by
menopausal status, the use of estrogen replacement therapy and
family history.
Discussion
In this case control study, it was found that the
separate use of either COX or LOX inhibitors reduces breast cancer
risk. In addition, in this first known study to evaluate the joint
effects of these anti-inflammatory agents, their combined use
produced a greater effect than their individual use.
The findings of this study for LOX inhibitors are
consistent with those of previous molecular studies, suggesting
that the overexpression of leukotrienes and leukotriene receptors
are associated with prominent features of mammary carcinogenesis,
including cell proliferation, angiogenesis, reduced apoptosis and
metastasis (11,12). For example, leukotriene B4 (LBT4)
is a major inflammatory factor in the ALOX-5 cascade that modulates
inflammatory and carcinogenic effects through two cell membrane
receptors, BLT1 and BLT2. Elevated levels of LBT4 and its receptors
have been observed in cancerous tissues of breast cancer, as well
as in numerous other human malignancies including colon cancer,
prostate cancer, ovarian cancer, renal cancer, pancreatic cancer,
esophageal cancer, lung cancer and neuroblastoma (13-25).
The results of this study are also supported by
those of previous preclinical studies demonstrating that
leukotriene inhibitors, a therapeutic category that includes the
asthma medications montelukast (Singulair®), zafirlukast
(Accolate®), zileuton (Zyflo®) and ubenimex
(bestatin), inhibit tumors of the lung, esophagus and colon
(26-29).
Finally, to the best of our knowledge, the only known human study
to examine the effects of leukotriene inhibiting asthma medication
found an overall reduced risk of cancer, in general, as well as of
breast cancer, in particular; however, this study was limited to
asthmatic patients (8).
The breast cancer risk-reducing properties of COX
inhibitors observed herein are consistent with a large body of
previous literature demonstrating the cancer risk-reducing
properties of these agents (1-4).
The results for COX inhibitors in the present study have been
previously reported in more detail (30). In brief, effects were observed for
selective inhibitors of COX-2, such as celecoxib (OR, 0.15; 95% CI,
0.08-0.28) and non-selective over the counter agents, such as
aspirin and ibuprofen (OR, 0.43; 95% CI, 0.25-0.55).
This study has two limitations. First, this study
did not include the conditions for which the COX and LOX
medications were used. This information is necessary to determine
whether the findings are attributable to the medication per se or
the causes for which the medication was administered. For example,
it has been shown that allergies and hay fever, which are related
to asthma risk, are associated with a reduced risk of breast cancer
(31). Possibly, of greater
concern for interpreting the findings of this study, is that the
same study found an inverse association between asthma and breast
cancer risk among premenopausal women. The second limitation is
that the sample size in this study was too small to evaluate the
effects of individual medications. Further studies using larger
sample sizes are required to investigate independent and joint
effects of these inflammation-inhibiting compounds.
In conclusion, this study observed significant
reductions in the risk of human breast cancer with the intake of
agents that inhibit the COX and LOX inflammatory cascades. The
greatest risk reduction (74%) was observed with combined use of
agents that inhibit both COX and LOX. The findings of this study
suggest that combining agents that inhibit both the COX and LOX
inflammatory cascades has strong potential for breast cancer
chemoprevention. Nevertheless, the risk-benefit ratio of combining
COX and LOX inhibitors for chemoprevention has yet to be determined
and the results must therefore be judiciously considered. The novel
findings of this study thus require replication.
Acknowledgements
The authors would like to thank Elvira M. Garofalo,
Program Manager of the James Cancer Mammography Unit, and Julie M.
Coursey, Assistant Director of the James Cancer Medical Records
Registry, for their assistance in the conduct of this
investigation.
Funding
This study was supported in part by a grant from
Pfizer, New York, NY, and grant P30 CA16058 from the National
Cancer Institute, Bethesda, MD, USA.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
REH designed and directed the study. JB assisted in
study design, coordinated data collection and quality control, and
assisted in the interpretation of results. JAS assisted in study
design and the analysis and interpretation of results.
Ethics approval and consent to
participate
The study was approved by The Ohio State University
Medical Center Institutional Review Board (IRB) and conducted in
full compliance with ethical standards of the US National
Institutes of Health for human medical research. Written informed
consent was obtained from each study participant to provide
information for analysis and publication of results.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harris RE: Cyclooxygenase-2 (cox-2)
blockade in the chemoprevention of cancers of the colon, breast,
prostate, and lung. Inflammopharmacology. 17:1–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harris RE, Casto BC and Harris ZM:
Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J
Clin Oncol. 5:677–692. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Steele VE, Holmes CA, Hawk ET, Kopelovich
L, Lubet RA, Crowell JA, Sigman CC and Kelloff GJ: Lipoxgenase
inhibitors as potential cancer chemopreventives. Cancer Epidemiol
Biomarkers Prev. 8:467–483. 1999.PubMed/NCBI
|
|
4
|
Wang D and Dubois RN: Eicosanoids and
cancer. Nat Rev Cancer. 10:181–193. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Schneider C and Pozzi A: Cyclooxygenases
and lipoxygenases in cancer. Cancer Metastasis Rev. 30:277–294.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wisastra R and Dekker FJ: Inflammation,
cancer and oxidative lipoxygenase activity are intimately linked.
Cancers (Basel). 6:1500–1521. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kennedy BM and Harris RE: Cyclooxygenase
and lipoxygenase gene expression in the inflammogenesis of breast
cancer. Inflammopharmacology. 26:909–923. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tsai MJ, Wu PH, Sheu CC, Hsu YL, Chang WA,
Hung JY, Yang CJ, Yang YH, Kuo PL and Huang MS: Cysteinyl
leukotriene receptor antagonists decrease cancer risk in asthma
patients. Sci Rep. 7(23979)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Schlesselman JJ: Case control studies.
Oxford University Press, New York, NY, 1982.
|
|
10
|
Harrell F: Logistic regression procedure.
Statistical Analysis System (SAS), 2005.
|
|
11
|
Jiang WG, Douglas-Jones AG and Mansel RE:
Aberrant expression of 5-lipoxygenase-activating protein (5-LOXAP)
has prognostic and survival significance in patients with breast
cancer. Prostaglandins Leukot Essent Fatty Acids. 74:125–134.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang J, John EM and Ingles SA:
5-lipoxygenase and 5-lipoxygenase-activating protein gene
polymorphisms, dietary linoleic acid, and risk for breast cancer.
Cancer Epidemiol Biomarkers Prev. 17:2748–2754. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Choi JA, Lee JW, Kim H, Kim EY, Seo JM, Ko
J and Kim JH: Pro-survival of estrogen receptor-negative breast
cancer cells is regulated by a BLT2-reactive oxygen species-linked
signaling pathway. Carcinogenesis. 31:543–551. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim H, Choi JA, Park GS and Kim JH: BLT2
up-regulates interleukin-8 production and promotes the invasiveness
of breast cancer cells. PLoS One. 7(e49186)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Haeggström JZ and Funk CD: Lipoxygenase
and leukotriene pathways: Biochemistry, biology, and roles in
disease. Chem Rev. 111:5866–5898. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang WG, Douglas-Jones A and Mansel RE:
Levels of expression of lipoxygenases and cyclooxygenase-2 in human
breast cancer. Prostaglandins Leukot Essent Fatty Acids.
69:275–281. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Soumaoro LT, Iida S, Uetake H, Ishiguro M,
Takagi Y, Higuchi T, Yasuno M, Enomoto M and Sugihara K: Expression
of 5-lipoxygenase in human colorectal cancer. World J
Gastroenterol. 12:6355–6360. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kleinstein SE, Heath L, Makar KW, Poole
EM, Seufert BL, Slattery ML, Xiao L, Duggan DJ, Hsu L, Curtin K, et
al: Genetic variation in the lipoxygenase pathway and risk of
colorectal neoplasia. Genes Chromosomes Cancer. 52:437–449.
2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ghosh J and Myers CE: Arachidonic acid
stimulates prostate cancer cell growth: Critical role of
5-lipoxygenase. Biochem Biophys Res Commun. 235:418–423.
1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rocconi RP, Kirby TO, Seitz RS, Beck R,
Straughn JM Jr, Alvarez RD and Huh WK: Lipoxygenase pathway
receptor expression in ovarian cancer. Reprod Sci. 15:321–326.
2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Faronato M, Muzzonigro G, Milanese G,
Menna C, Bonfigli AR, Catalano A and Procopio A: Increased
expression of 5-lipoxygenase is common in clear cell renal cell
carcinoma. Histol Histopathol. 22:1109–1118. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tong WG, Ding XZ, Talamonti MS, Bell RH
and Adrian TE: LTB4 stimulates growth of human pancreatic cancer
cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res
Commun. 335:949–956. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hennig R, Grippo P, Ding XZ, Rao SM,
Buchler MW, Friess H, Talamonti MS, Bell RH and Adrian TE:
5-Lipoxygenase, a marker for early pancreatic intraepithelial
neoplastic lesions. Cancer Res. 65:6011–6016. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen X, Wang S, Wu N, Sood S, Wang P, Jin
Z, Beer DG, Giordano TJ, Lin Y, Shih WC, et al: Overexpression of
5-lipoxygenase in rat and human esophageal adenocarcinoma and
inhibitory effects of zileuton and celecoxib on carcinogenesis.
Clin Cancer Res. 10:6703–6709. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sveinbjörnsson B, Rasmuson A, Baryawno N,
Wan M, Pettersen I, Ponthan F, Orrego A, Haeggström JZ, Johnsen JI
and Kogner P: Expression of enzymes and receptors of the
leukotriene pathway in human neuroblastoma promotes tumor survival
and provides a target for therapy. FASEB J. 22:3525–3536.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rioux N and Castonguay A: Inhibitors of
lipoxygenase: A new class of cancer chemopreventive agents.
Carcinogenesis. 19:1393–1400. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mashima R and Okuyama T: The role of
lipoxygenases in pathophysiology: New insights and future
perspectives. Redox Biol. 6:297–310. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Moore GY and Pidgeon GP: Cross-talk
between cancer cells and the tumour microenvironment: The role of
the 5-lipoxygenase pathway. Int J Molecular Sciences.
18(236)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao S, Yao K, Li D, Liu K, Jin G, Yan M,
Wu Q, Chen H, Shin SH, Bai R, et al: Inhibition of LTA4H by
bestatin in human and mouse colorectal cancer. EBioMedicine.
44:361–374. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Harris RE, Beebe-Donk J and Alshafie GA:
Reduction in the risk of human breast cancer by selective
cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer.
6(27)2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lowcock EC, Cotterchio M and Ahmad N:
Association between allergies, asthma, and breast cancer risk among
women in Ontario, Canada. Cancer Causes Control. 24:1053–1056.
2013.PubMed/NCBI View Article : Google Scholar
|